Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50065440.htm |
Exhibit 99.1

Applying scientific and medical leadership in prostones to develop and commercialize therapeutics for an aging population Corporate Update Stanley G. Miele Senior Vice President, Sales & Marketing November 10, 2011

Forward-Looking Statements Forward-looking statements contained in this presentation are based on Sucampo’s assumptions and events expectations concerning future events. They are subject to significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those reflected in the forward-looking statements. Sucampo’s forward-looking statements could be affected by numerous foreseeable and unforeseeable events and developments such as regulatory delays, the failure of clinical trials, the inability to fund drug development initiatives, competitive products and other factors identified in the “Risk Factors” section of Sucampo’s Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. While Sucampo may elect to update these statements at some point in the future Sucampo specifically disclaims any obligation to do so, whether as a result of new information, future events or otherwise. In light of the significant uncertainties inherent in the forward-looking information in this presentation, you are cautioned not to place undue reliance on 2 these forward-looking statements.

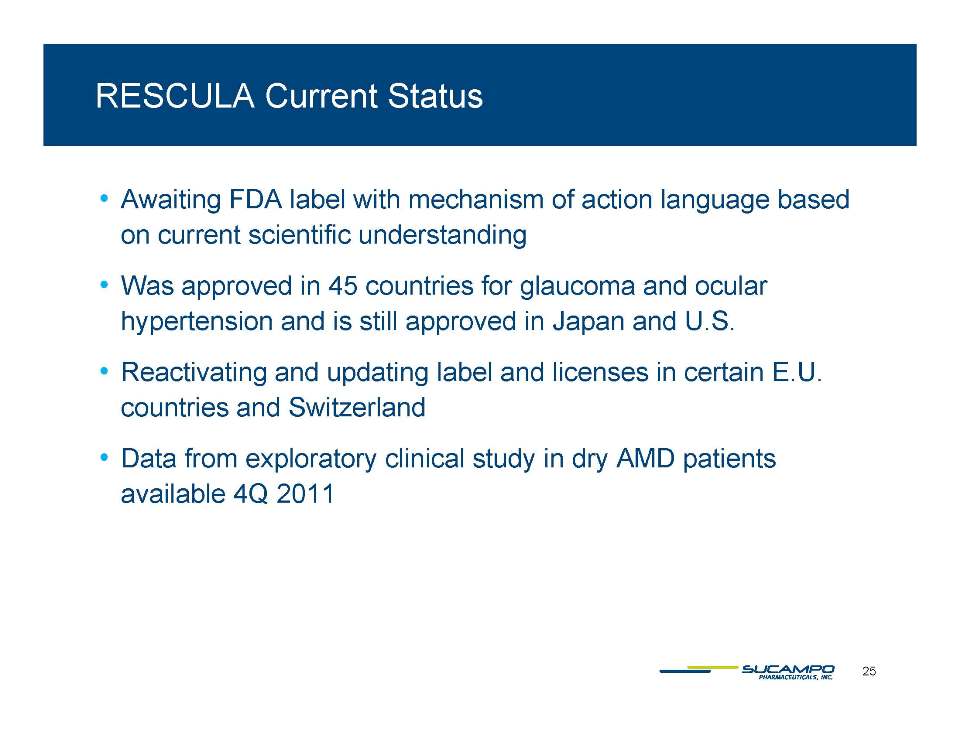
Sucampo Investment Highlights Proven technology: Leadership in prostones key ion channel activators with validated clinical utility Commercial products focused on large patient populations AMITIZA in the U S.; RESCULA was approved in the U.S. and was approved in 45 countries; on market in Japan Clinical expansion: AMITIZA label expansion strategies underway in the U.S., Europe and Asia; RESCULA label expansion strategies underway in the U.S. and Europe Robust pipeline Revenue stream fueling advances in proprietary prostone-based portfolio
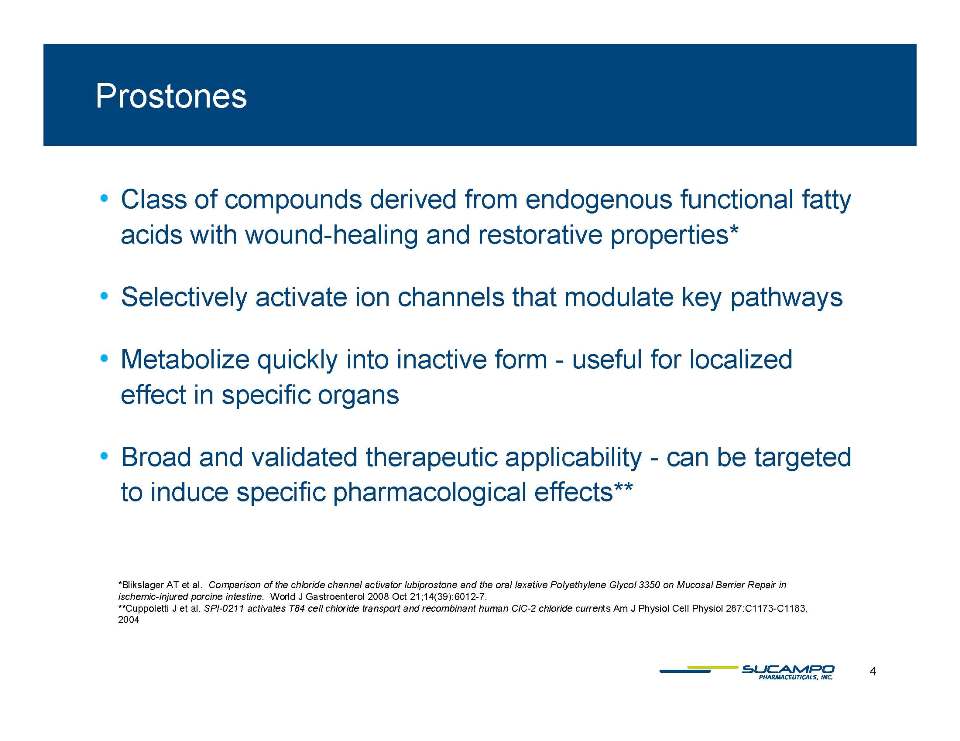
Class of compounds derived from endogenous functional fatty wound acids with wound-healing and restorative properties Selectively activate ion channels that modulate key pathways Metabolize quickly into inactive form - useful for localized effect in specific organs Broad and validated therapeutic applicability can be targeted to induce specific pharmacological effects Blikslager AT et al. Comparison of the chloride channel activator lubiprostone and the oral laxative Polyethylene Glycol 3350 on Mucosal Barrier Repair in ischemic-injured porcine intestine. World J Gastroenterol 2008 Oct 21;14(39):6012-7. 4 Cuppoletti J et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents Am J Physiol Cell Physiol 287:C1173-C1183, 2004
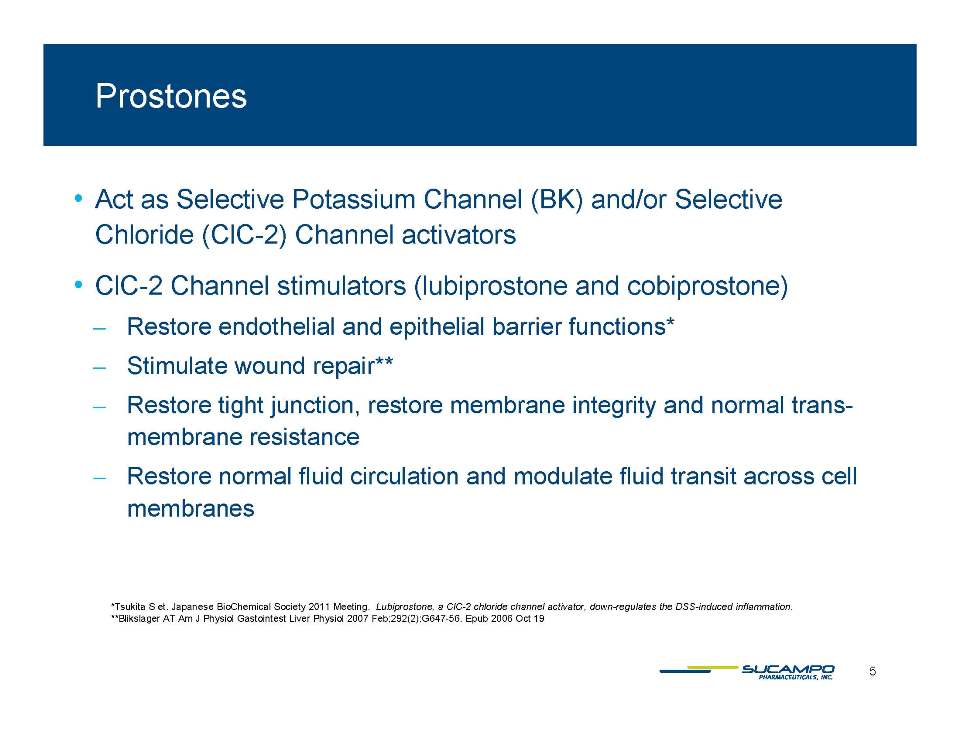
Act as Selective Potassium Channel (BK) and/or Selective Chloride ClC 2) Channel (ClC-activators • ClC-2 Channel stimulators (lubiprostone and cobiprostone) ̶ Restore endothelial and epithelial barrier functions* ̶ Stimulate wound repair** ̶ Restore tight junction, restore membrane integrity and normal trans-membrane resistance ̶ Restore normal fluid circulation and modulate fluid transit across cell membranes Tsukita S et. Japanese BioChemical Society 2011 Meeting. Lubiprostone, a ClC-2 chloride channel activator, down-regulates the DSS-induced inflammation. **Blikslager AT Am J Physiol Gastointest Liver Physiol 2007 Feb;292(2):G647-56. Epub 2006 Oct 19
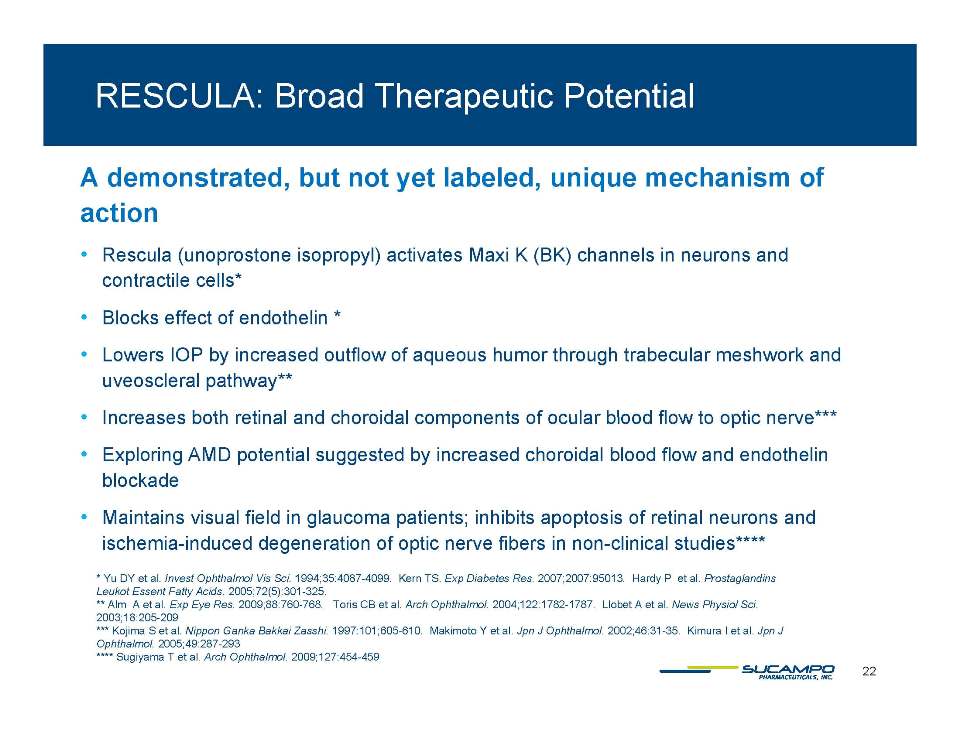
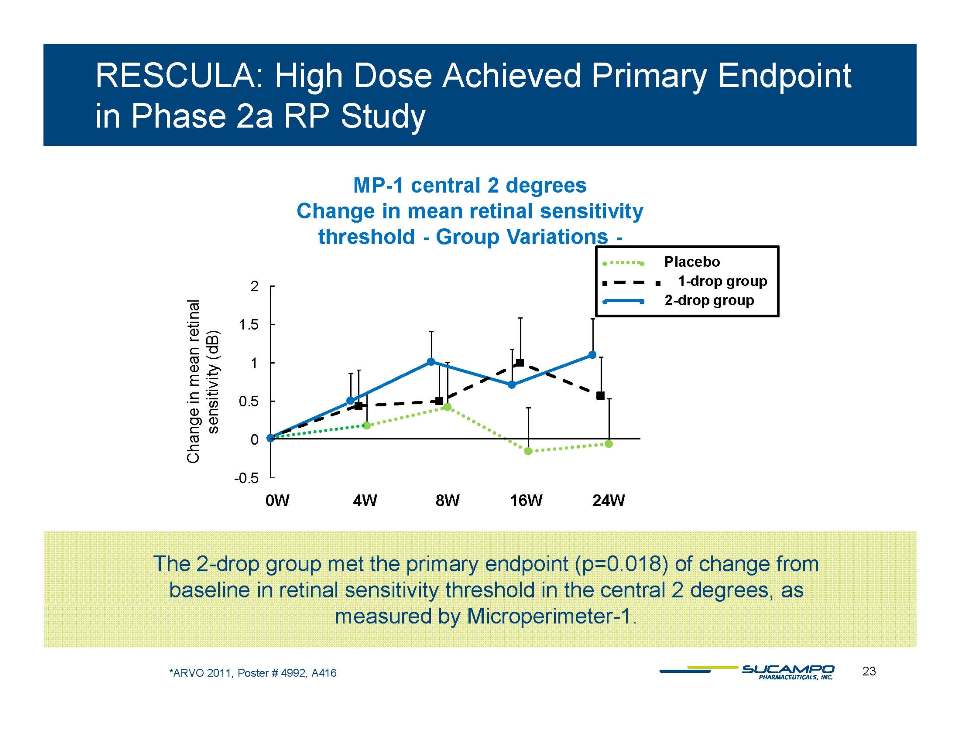
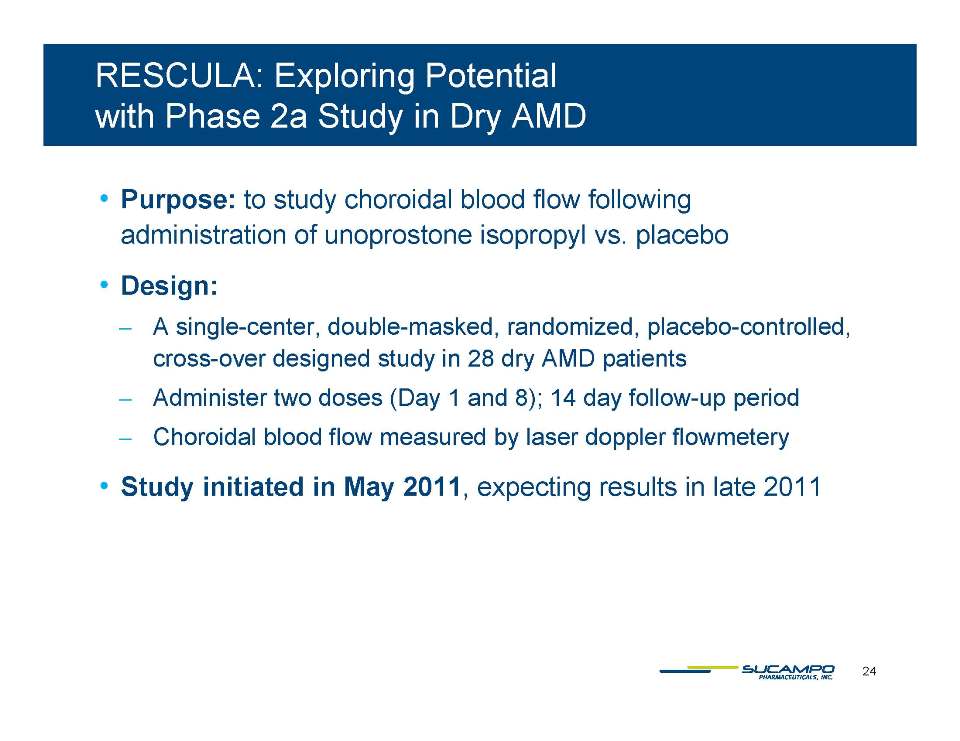
BK Channel Stimulators (unoprostone isopropyl) ̶ Down-regulate inflammation, hypoxia and edema* Block pro-apoptotic and excito-toxic effects** ̶ Block the vaso-constrictive and pro-inflammatory effects of endothelin in the microvasculature ̶ Reduce normal fluid pressure in the eye*** ̶ Demonstrate neuroprotective effects in preclinical ophthalmology models (light-induced injury)**** ̶ Demonstrate dose-dependent neuroprotective effects in clinical studies glaucoma (head to head vs in longitudinal studies vs. latanoprost***** in Japan) and retinitis pigmentosa****** (phase 2b in Japan) * Yu DY et al. Invest Ophthalmol Vis Sci 1994: 35:4087-4099. Kern TS. Exp Diabetes Res. 2007: 2007: 95013. Hardy P et al. Prostaglandins Leukot Essent Acids 301 325 6 Fatty Acids. 2005: 72(5): 301-**Sugiyama T et al. Arch Ophthalmol. 2009;127:454-459 *** Inoue K et al. Clinical Ophthalmology 2011:5 1003-1005 ****Hayami K et al. Ophthalmic Res. 2001 Jul-Aug;33(4):203-9 and Melamed S. Drugs Exp Clin Res 2002;28(2-3):63-73. *****Ishida T al. Topical Monotherapy for Normal Tension Glaucoma-Comparison of Long-term Monotherapies in Maintaining Visual Field Ophthalmology 47:1107-1112,2005. ******ARVO 2011, Poster#4992, A416
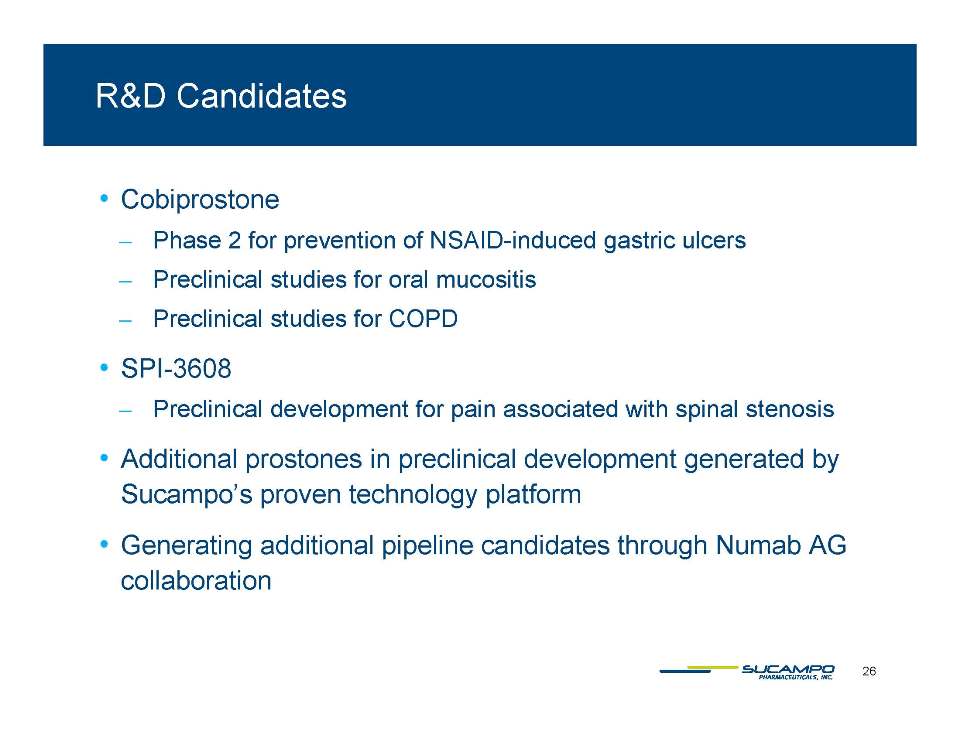
Vast therapeutic targeting potential ̶ GI* ̶ Ophthalmology** ̶ CV ̶ Oncology *** ̶ Urology ̶ CNS ̶ Pulmonary**** *Cryer B et al Cobiprostone demonstrates protective effects against non-steroidal anti-inflammatory drug-induced gastrointestinal injury. Gastroenterology 138(5 Suppl 1):S-64 [abstract 475F]. ** Cuppoletti J et al. Cellular and molecular effects of unoprostone as a BK channel activator. BioChem Biophys Acta 1768(5):1083-92. ***Cuppoletti J et al. Lubiprostone suppresses growth of colon cancer cells in vitro and in vivo. Am J Gastroenterol 105 (Suppl 1):S76-77 [abstract 203]. **** Cuppoletti J. et al. SPI-8811 activates human bronchial epithelial cell chloride transport and recombinant human ClC-2 chloride currents. Pediatr Pulmonol 38(Suppl 27):245-6 [ abstract 167].
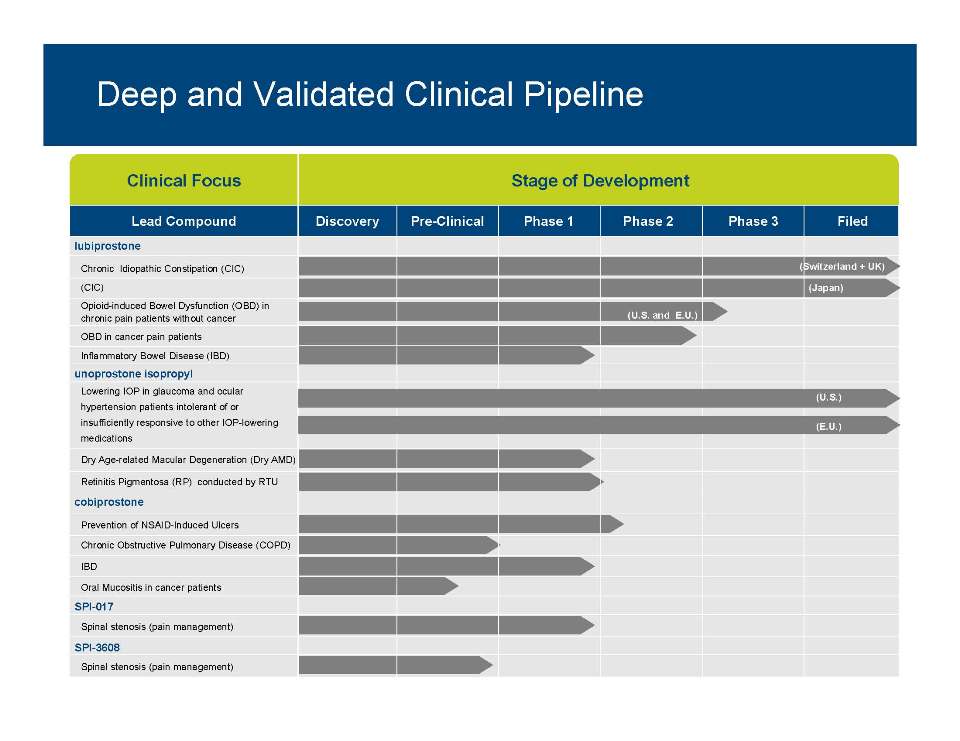
Clinical Focus Stage of Development Lead Compound Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Filed lubiprostone Chronic Idiopathic Constipation (CIC) (CIC) Opioid-induced Bowel Dysfunction (OBD) in chronic pain patients without cancer (U.S. and E.U.) (Japan) (Switzerland + UK) unoprostone isopropyl OBD in cancer pain patients Inflammatory Bowel Disease (IBD) Lowering IOP in glaucoma and ocular hypertension patients intolerant of or (U.S.) cobiprostone insufficiently responsive to other IOP-lowering medications Dry Age-related Macular Degeneration (Dry AMD) Retinitis Pigmentosa (RP) conducted by RTU (E.U.) SPI 017 Prevention of NSAID-Induced Ulcers Chronic Obstructive Pulmonary Disease (COPD) IBD Oral Mucositis in cancer patients 8 SPI-Spinal stenosis (pain management) SPI-3608 Spinal stenosis (pain management)
AMITIZA (lubiprostone) 24 mcg for CIC 8 mcg for IBS-C
A differentiated mechanism of action • Small secretion: Chloride intestine fluid ions enter intestinal lumen following CLC-2 activation • Small intestine fluid secretion: Sodium ions follow chloride ions into lumen to maintain isoelectric neutrality • Small intestine fluid secretion: Ion transport also draws water into lumen to maintain osmotic neutrality • Lubiprostone activates intestinal CLC-2 channels: Works through ‘facilitated diffusion’ and/or ‘passive transport’ • Increases tight junction integrity and function to maintain normal transepithelial resistance 10 resistance. Basavappa S., et al Journal of Cellular Physiology 2005;202(1):21-31
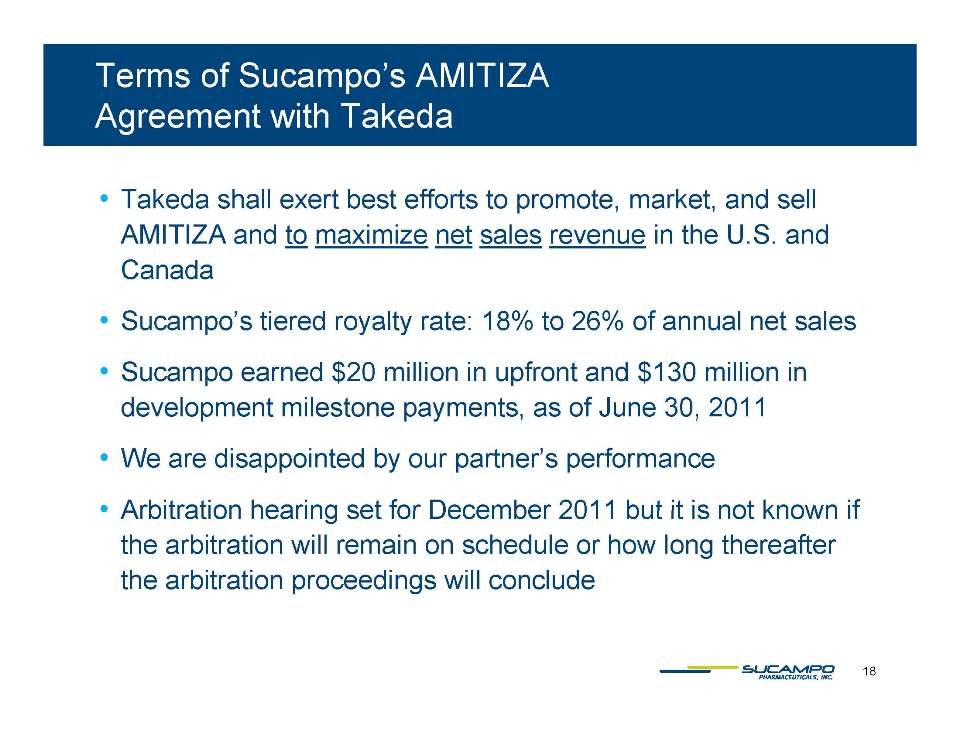
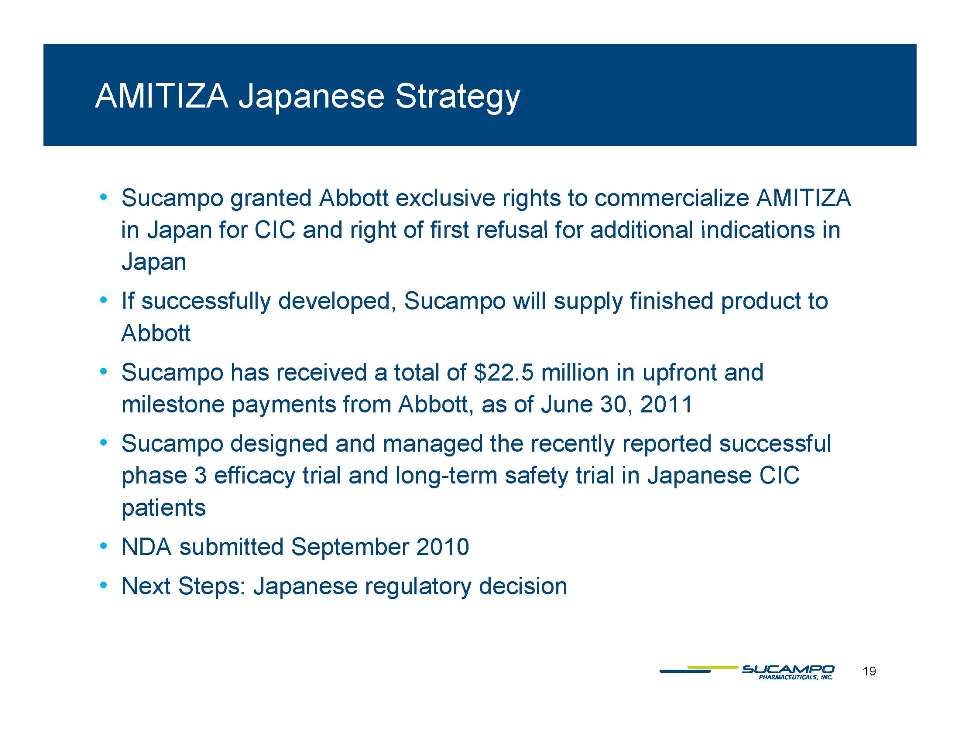
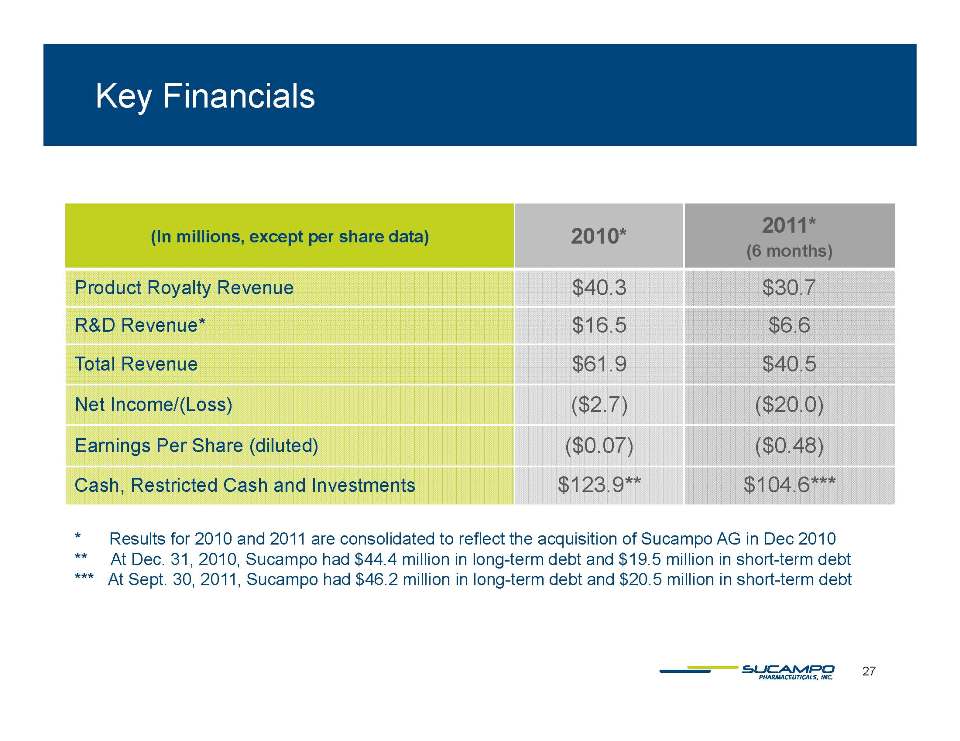
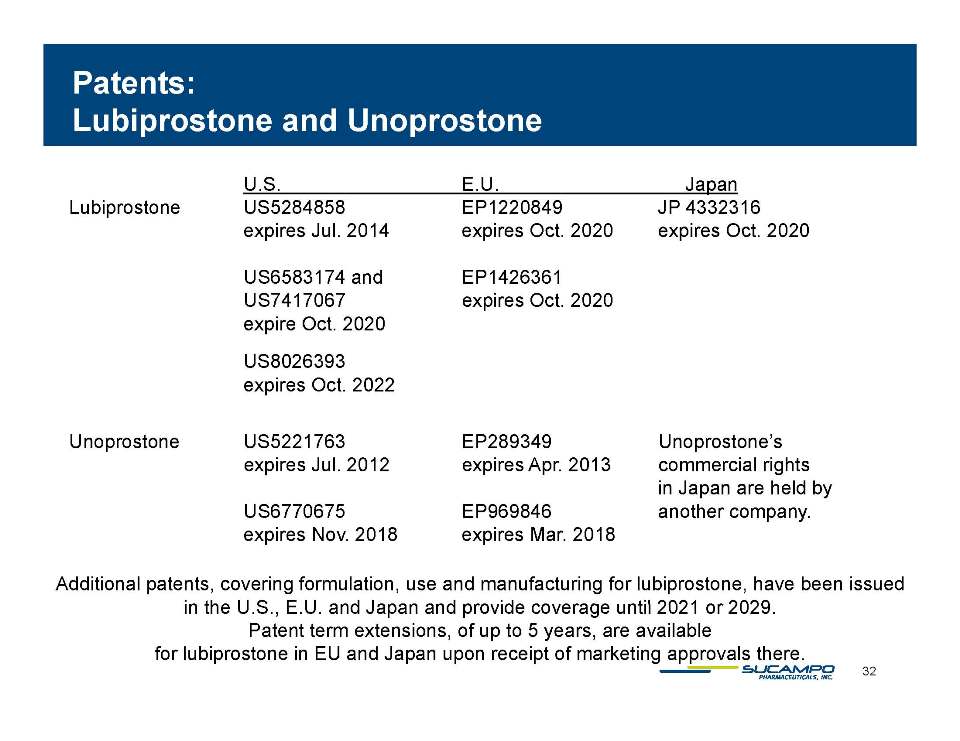
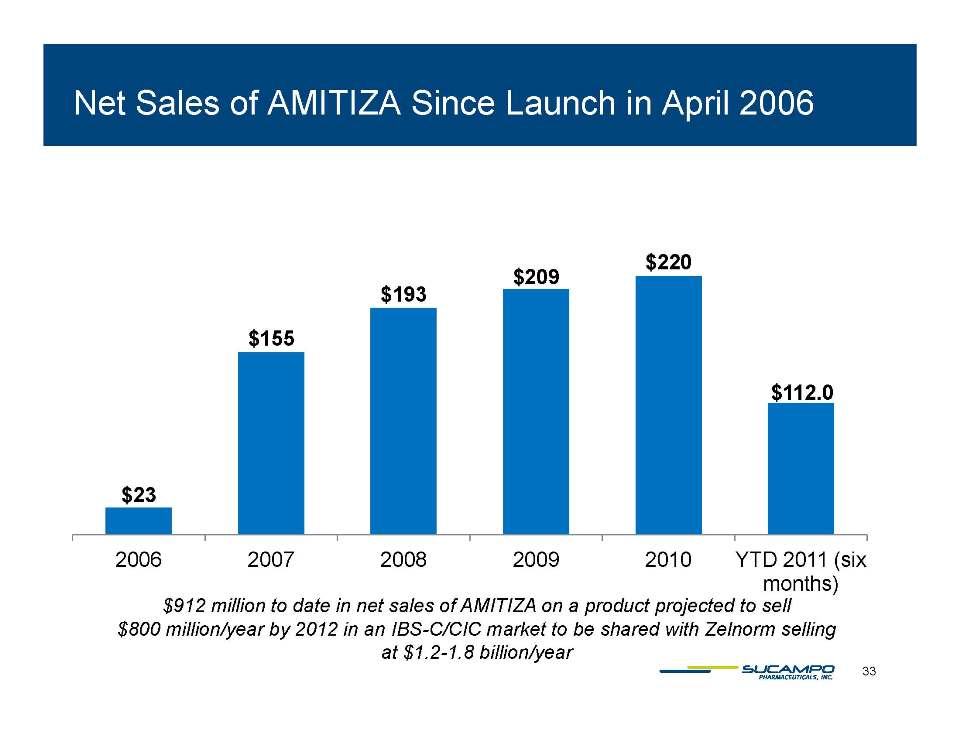
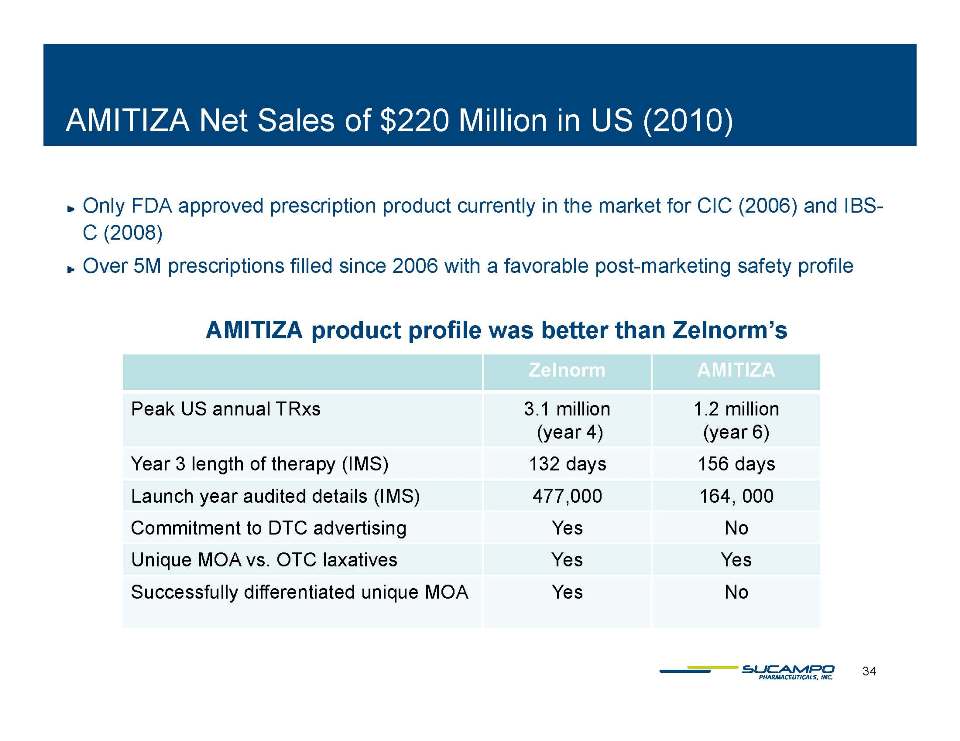
Indications: Approved by FDA for the treatment of IBS-C in women aged 18 years and older and for the treatment of CIC in adults Global status: Marketed by Takeda in US, Abbott holds marketing rights to CIC in Japan, ROW owned by Sucampo U.S. Sales (net): $220 million in 2010 Patent Life: May extend effective exclusivity to 2022
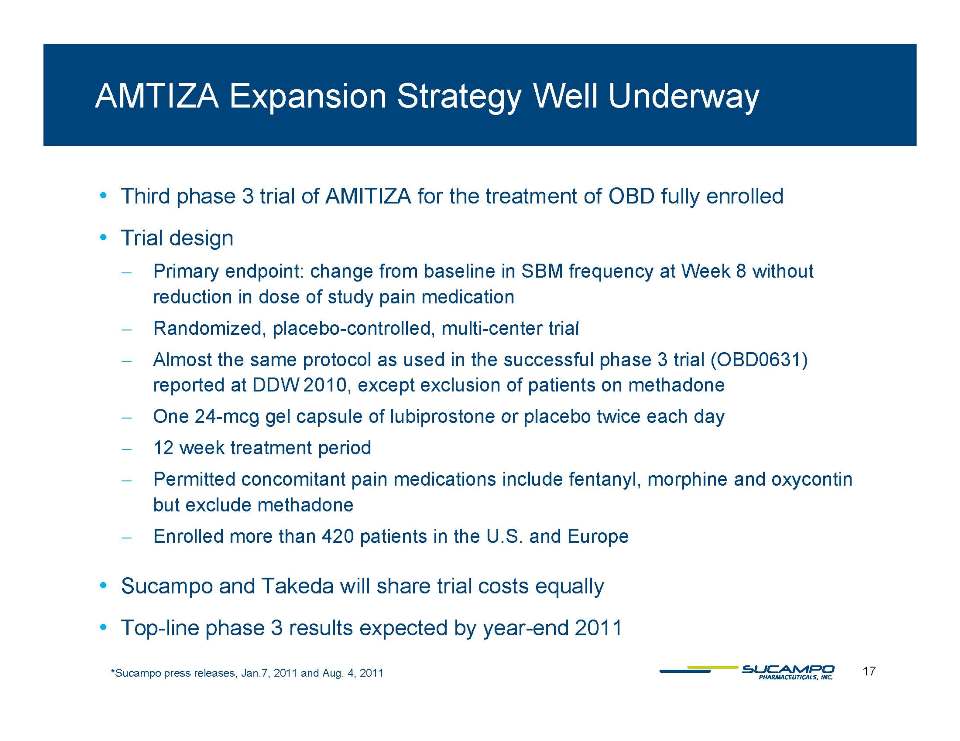
Key Events Label expansion Phase 3 OBD trial results Swiss pricing Japanese NDA decision MAA approval in the U.K. E.U. pricing Japanese pricing Decision in Takeda arbitration
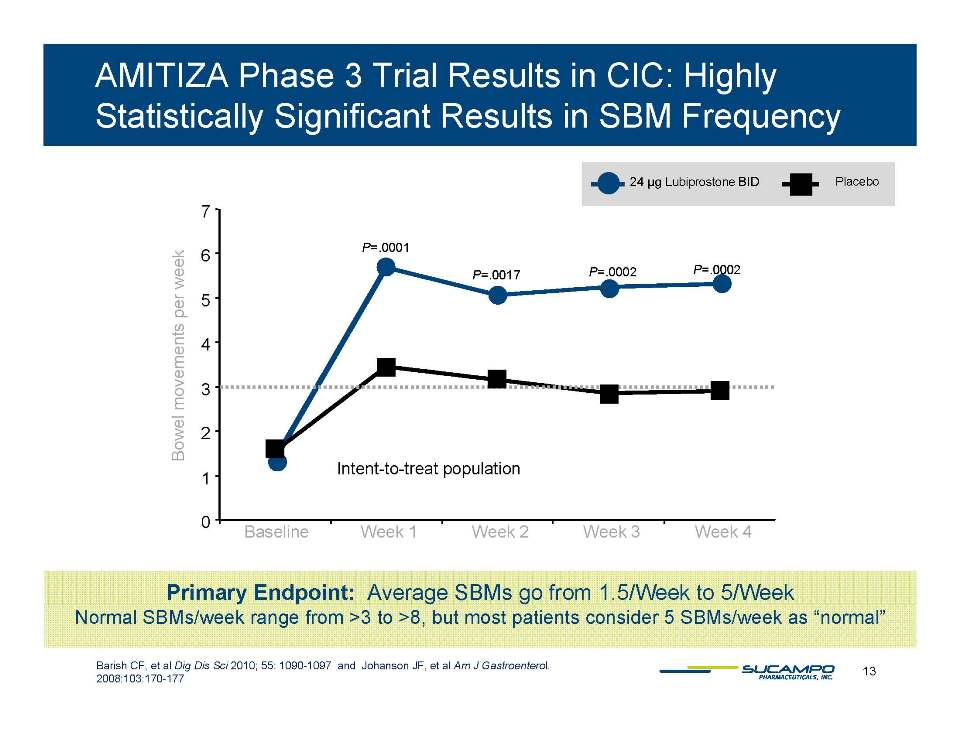
Primary Endpoint: Average SBMs go from 1.5/Week to 5/Week 13 Barish CF, et al Dig Dis Sci 2010; 55: 1090-1097 and Johanson JF, et al Am J Gastroenterol. 2008:103:170-177 Normal SBMs/week range from >3 to >8, but most patients consider 5 SBMs/week as “normal”
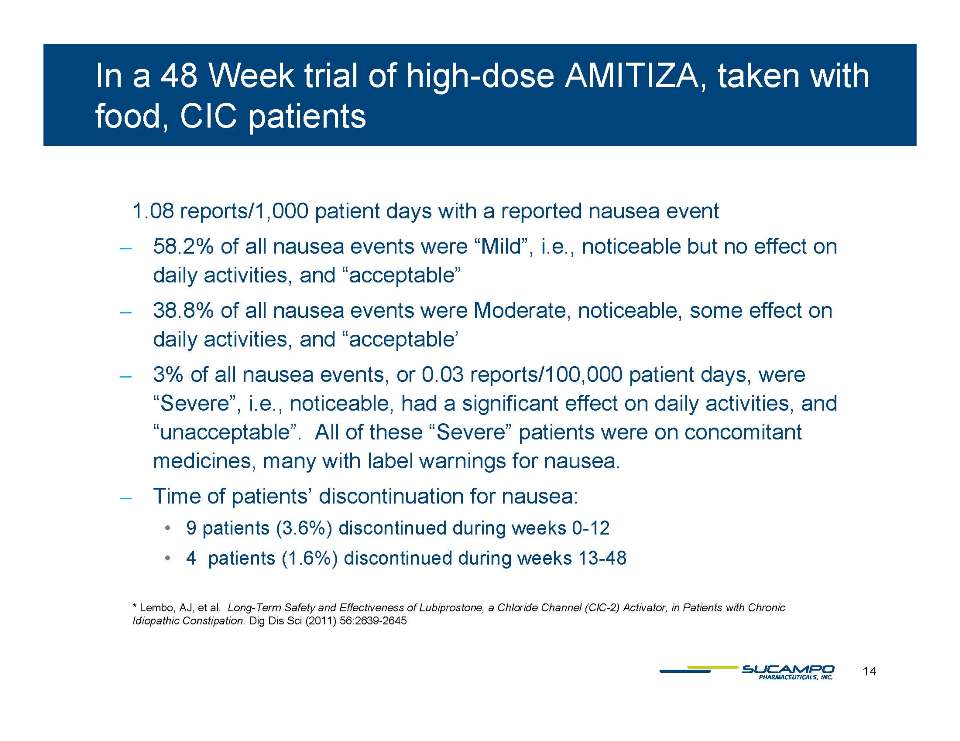
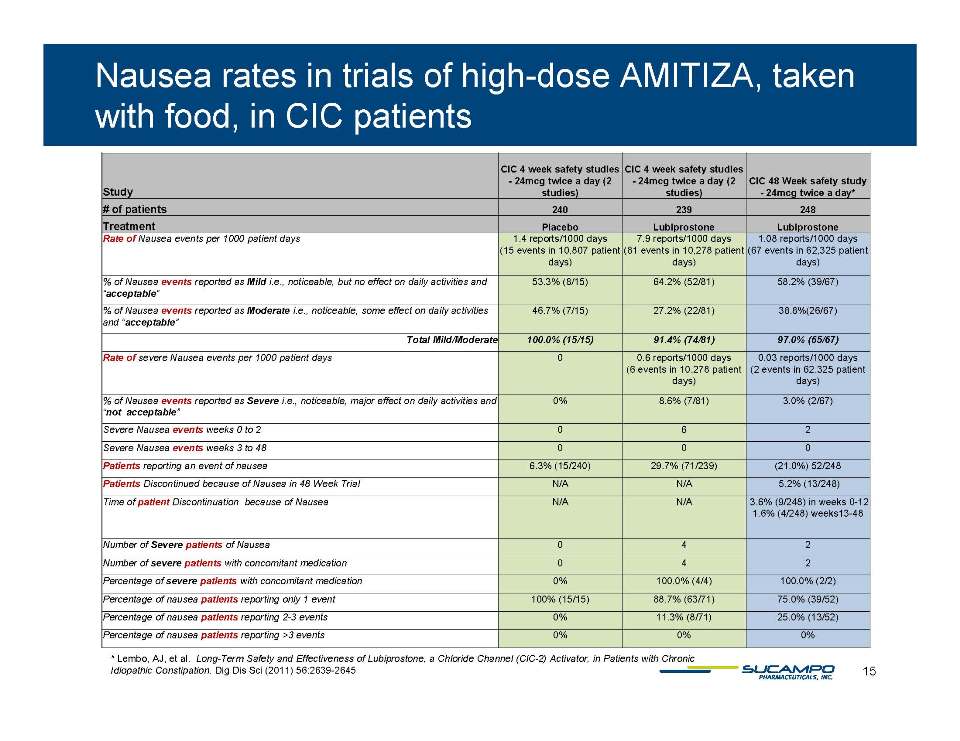
In a 48 week trial of high-dose amitiza taken with food cic patients 1.08 reports/1,000 patient days with a reported nausea event ̶ 58.2% of all nausea events were “Mild”, i.e., noticeable but no effect on daily activities, and “acceptable” ̶ 38.8% of all nausea events were Moderate, noticeable, some effect on daily activities, and “acceptable’ ̶ 3% of all nausea events, or 0.03 reports/100,000 patient days, were “Severe”, i.e., noticeable, had a significant effect on daily activities, and “unacceptable”. All of these “Severe” patients were on concomitant medicines, many with label warnings for nausea. ̶ Time of patients’ discontinuation for nausea: 9 patients (3.6%) discontinued during weeks 0-12 4 patients (1.6%) discontinued during weeks 13-48 Lembo, AJ, et al. Long-Term Safety and Effectiveness of Lubiprostone, a Chloride Channel (ClC-Activator, in Patients with Chronic Idiopathic Constipation. Dig Dis Sci (2011) 56:2639-2645