Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vanda Pharmaceuticals Inc. | w84243e8vk.htm |
| EX-99.2 - EX-99.2 - Vanda Pharmaceuticals Inc. | w84243exv99w2.htm |
Exhibit 99.1

| Vanda Pharmaceuticals Inc. 2011 Corporate Presentation |

| Forward-Looking Statement Various statements in this release are "forward-looking statements" under the securities laws. Words such as, but not limited to, "believe," "expect," "anticipate," "estimate," "intend," "plan," "targets," "likely," "will," "would," and "could," or the negative of these terms and similar expressions or words, identify forward-looking statements. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. Important factors that could cause actual results to differ materially from those reflected in the company's forward-looking statements include, among others: the extent and effectiveness of the development, sales and marketing and distribution support Fanapt(r) receives; Vanda's ability to successfully commercialize Fanapt(r) outside of the U.S. and Canada; delays in the completion of Vanda's clinical trials; a failure of Vanda's products, product candidates or partnered products to be demonstrably safe and effective; Vanda's failure to obtain regulatory approval for its products, product candidates or partnered products or to comply with ongoing regulatory requirements; a lack of acceptance of Vanda's products, product candidates or partnered products in the marketplace, or a failure to become or remain profitable; Vanda's expectations regarding trends with respect to its costs and expenses; Vanda's inability to obtain the capital necessary to fund additional research and development activities; Vanda's failure to identify or obtain rights to new products or product candidates; Vanda's failure to develop or obtain sales, marketing and distribution resources and expertise or to otherwise manage its growth; limitations on Vanda's ability to utilize some or all of its prior net operating losses and research and development credits; a loss of any of Vanda's key scientists or management personnel; losses incurred from product liability claims made against Vanda; a loss of rights to develop and commercialize Vanda's products or product candidates under its license and sublicense agreements and other factors that are described in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's most recent annual or quarterly report filed with the SEC and available on the SEC's website at www.sec.gov. In addition to the risks described above and in Vanda's annual and quarterly reports, other unknown or unpredictable factors also could affect Vanda's results. There can be no assurance that the actual results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on Vanda. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this release is provided only as of the date of this release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. 2 |
| Development and Commercial Capabilities Address Unmet Medical Needs CNS Specialty Company Vanda Company Vision 3 |
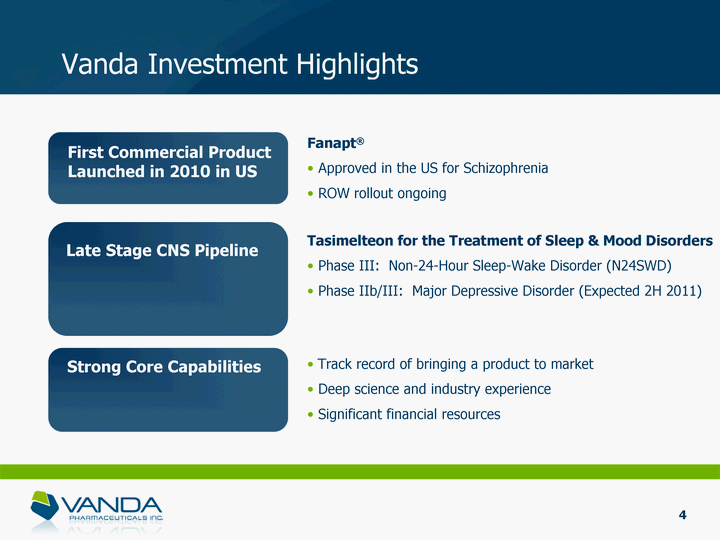
| Vanda Investment Highlights First Commercial Product Launched in 2010 in US Late Stage CNS Pipeline Strong Core Capabilities Fanapt(r) Approved in the US for Schizophrenia ROW rollout ongoing Tasimelteon for the Treatment of Sleep & Mood Disorders Phase III: Non-24-Hour Sleep-Wake Disorder (N24SWD) Phase IIb/III: Major Depressive Disorder (Expected 2H 2011) Track record of bringing a product to market Deep science and industry experience Significant financial resources 4 |
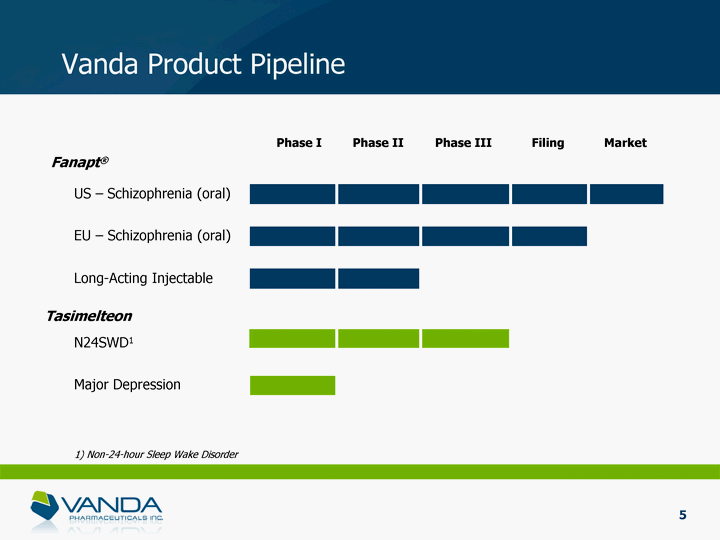
| Vanda Product Pipeline Phase I Phase II Phase III Filing US - Schizophrenia (oral) Long-Acting Injectable N24SWD1 Fanapt(r) Tasimelteon Major Depression Market EU - Schizophrenia (oral) 5 1) Non-24-hour Sleep Wake Disorder |
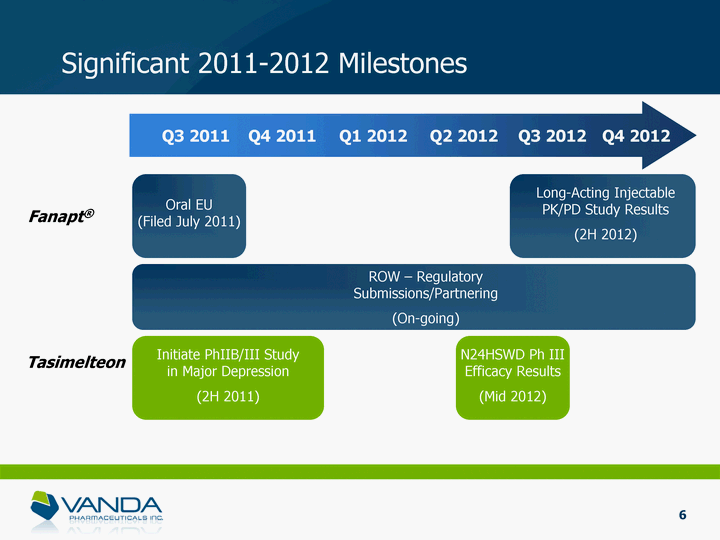
| Significant 2011-2012 Milestones Q3 2011 Q4 2011 Q1 2012 Q2 2012 Q3 2012 Q4 2012 Fanapt(r) Tasimelteon Oral EU (Filed July 2011) Long-Acting Injectable PK/PD Study Results (2H 2012) ROW - Regulatory Submissions/Partnering (On-going) Initiate PhIIB/III Study in Major Depression (2H 2011) N24HSWD Ph III Efficacy Results (Mid 2012) 6 |

| Fanapt(r) (iloperidone) |

| Fanapt(r) Overview Fanapt(r) oral formulation Fanapt(r) long-acting injectable formulation Fanapt(r) franchise Intellectual Property An atypical antipsychotic agent for the treatment of schizophrenia in adults 8 |
| Fanapt(r) Oral Formulation Status US Clinical Regulatory Status US FDA approval: May 6, 2009 Indication: Schizophrenia in adults US Commercial Status Partnered with Novartis for US and Canada Launched January 2010 On preferred drug list (PDL) or unrestricted in >90% of state formularies 9 |
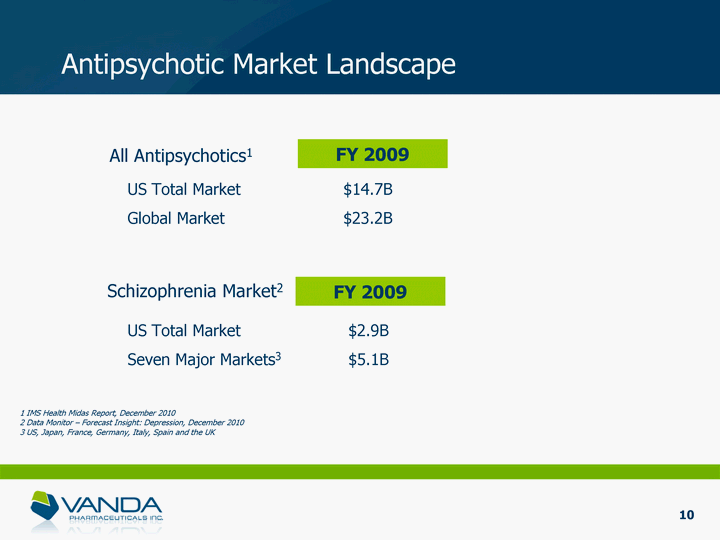
| Antipsychotic Market Landscape FY 2009 US Total Market $14.7B Global Market $23.2B All Antipsychotics1 US Total Market $2.9B Seven Major Markets3 $5.1B Schizophrenia Market2 FY 2009 1 IMS Health Midas Report, December 2010 2 Data Monitor - Forecast Insight: Depression, December 2010 3 US, Japan, France, Germany, Italy, Spain and the UK 10 |
| Fanapt(r) U.S. Update Novartis Agreement Vanda receives a low double digit royalty on US & Canada net sales $265M in potential outstanding development and sales based milestones 11 Prescription results1 through Q2 2011 Over 110,000 prescriptions have been written since launch in January 2010 Q2 2011 prescription growth of 17% compared to Q1 2011 1 IMS Health |
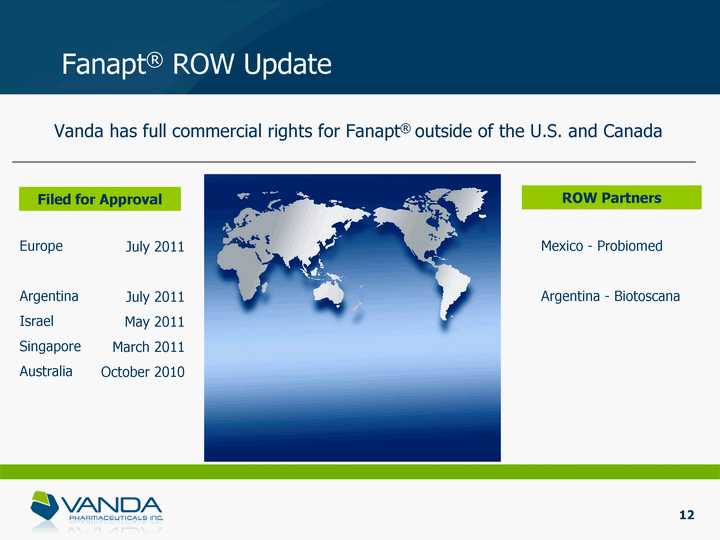
| Fanapt(r) ROW Update Filed for Approval Europe Argentina Israel Singapore Australia ROW Partners Mexico - Probiomed Argentina - Biotoscana 12 July 2011 July 2011 May 2011 March 2011 October 2010 Vanda has full commercial rights for Fanapt(r) outside of the U.S. and Canada |
| Fanapt(r) Long-Acting Injectable Long-acting Injectable Formulation Once a month formulation to address non-compliance Prior Phase I/II study supports further development Less crowded commercial market as compared to oral market Novartis responsible for development and US/Canada Vanda retained rights for ROW 13 |
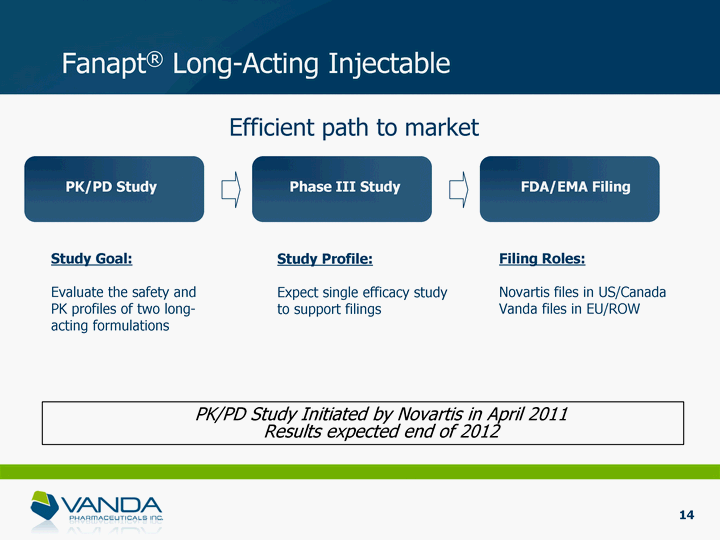
| Fanapt(r) Long-Acting Injectable Efficient path to market Study Goal: Evaluate the safety and PK profiles of two long- acting formulations Study Profile: Expect single efficacy study to support filings Filing Roles: Novartis files in US/Canada Vanda files in EU/ROW PK/PD Study Phase III Study FDA/EMA Filing PK/PD Study Initiated by Novartis in April 2011 Results expected end of 2012 14 |
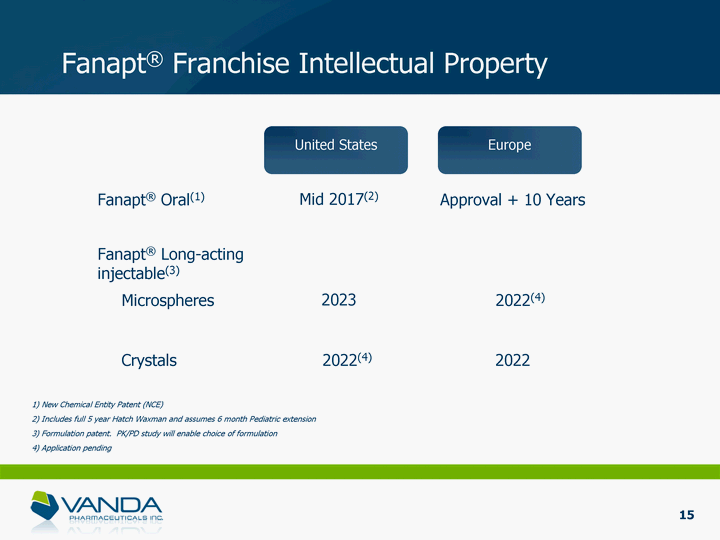
| Fanapt(r) Franchise Intellectual Property Fanapt(r) Oral(1) Mid 2017(2) Approval + 10 Years Microspheres 2023 2022(4) Fanapt(r) Long-acting injectable(3) Crystals 2022 1) New Chemical Entity Patent (NCE) 2) Includes full 5 year Hatch Waxman and assumes 6 month Pediatric extension 3) Formulation patent. PK/PD study will enable choice of formulation 4) Application pending 2022(4) United States Europe 15 |

| Tasimelteon A Circadian Regulator A Circadian Regulator |
| Tasimelteon - Mechanism of Action Unique Molecular Mechanism of Action Melatonin 1 (MT1) agonist Melatonin 2 (MT2) agonist Circadian Regulator Circadian Phase Shift effects Soporific effects Mood restoring effects 17 |
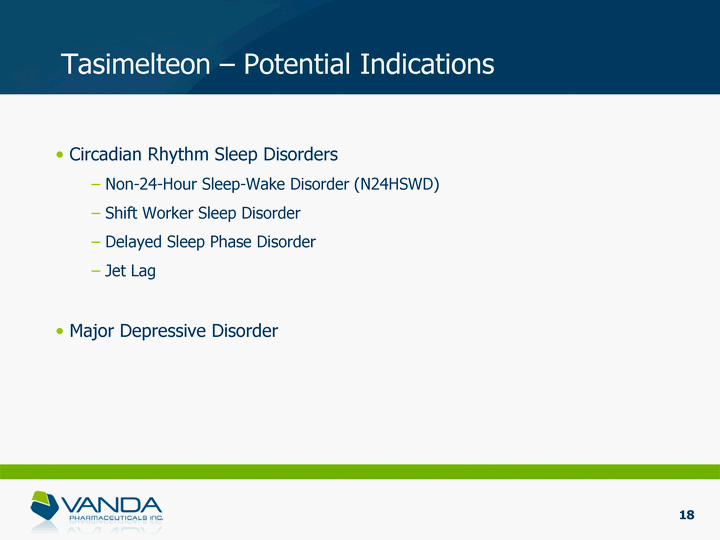
| Tasimelteon - Potential Indications Circadian Rhythm Sleep Disorders Non-24-Hour Sleep-Wake Disorder (N24HSWD) Shift Worker Sleep Disorder Delayed Sleep Phase Disorder Jet Lag Major Depressive Disorder 18 |

| Tasimelteon N24HSWD |

| N24HSWD Facts A Circadian Rhythm Sleep Disorder Affects 65,000 - 95,000 people in the US Occurs almost entirely in blind subjects with no light perception Person affected will "free run" slightly longer than 24 hours without environmental input, causing a phase delay in the body clock each day No approved treatment 20 |
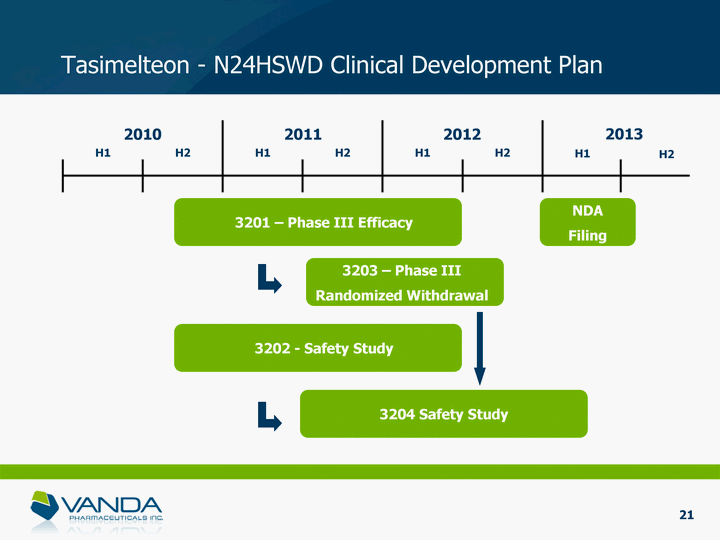
| Tasimelteon - N24HSWD Clinical Development Plan 2010 H1 H2 2011 2012 H1 H2 H1 H2 2013 H1 H2 3201 - Phase III Efficacy NDA Filing 3203 - Phase III Randomized Withdrawal 3202 - Safety Study 3204 Safety Study 21 |
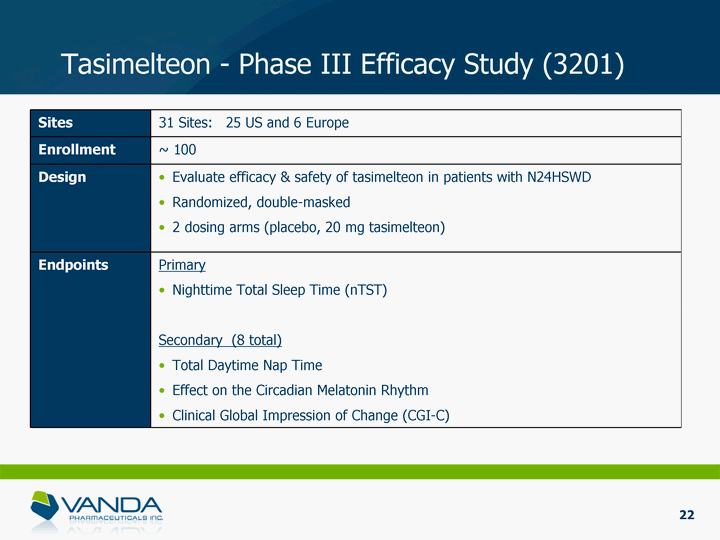
| Sites 31 Sites: 25 US and 6 Europe Enrollment ~ 100 Design Evaluate efficacy & safety of tasimelteon in patients with N24HSWD Randomized, double-masked 2 dosing arms (placebo, 20 mg tasimelteon) Endpoints Primary Nighttime Total Sleep Time (nTST) Secondary (8 total) Total Daytime Nap Time Effect on the Circadian Melatonin Rhythm Clinical Global Impression of Change (CGI-C) Tasimelteon - Phase III Efficacy Study (3201) 22 |
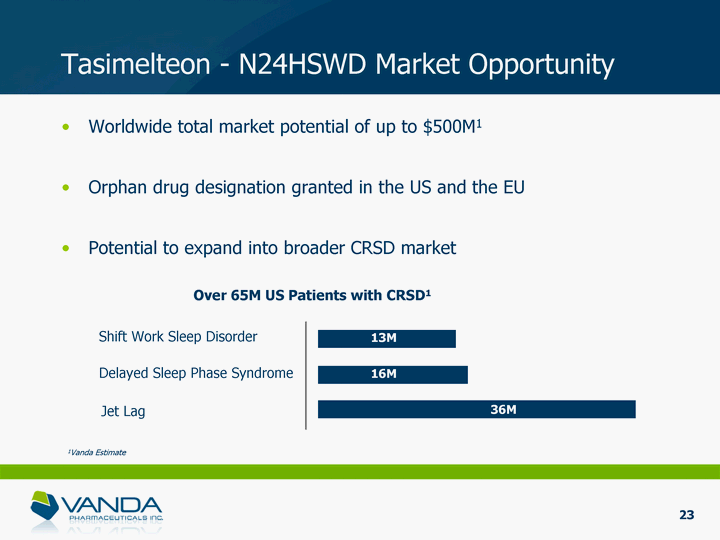
| Tasimelteon - N24HSWD Market Opportunity Worldwide total market potential of up to $500M1 Orphan drug designation granted in the US and the EU Potential to expand into broader CRSD market Shift Work Sleep Disorder Delayed Sleep Phase Syndrome Jet Lag Over 65M US Patients with CRSD1 13M 16M 36M 1Vanda Estimate 23 |

| Tasimelteon Major Depressive Disorder Major Depressive Disorder |
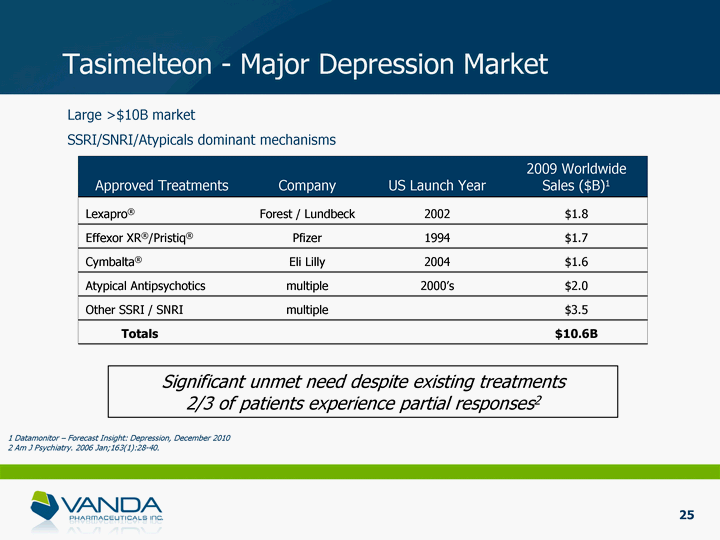
| Tasimelteon - Major Depression Market Approved Treatments Company US Launch Year 2009 Worldwide Sales ($B)1 Lexapro(r) Forest / Lundbeck 2002 $1.8 Effexor XR(r)/Pristiq(r) Pfizer 1994 $1.7 Cymbalta(r) Eli Lilly 2004 $1.6 Atypical Antipsychotics multiple 2000's $2.0 Other SSRI / SNRI multiple $3.5 Totals $10.6B Large >$10B market SSRI/SNRI/Atypicals dominant mechanisms Significant unmet need despite existing treatments 2/3 of patients experience partial responses2 25 1 Datamonitor - Forecast Insight: Depression, December 2010 2 Am J Psychiatry. 2006 Jan;163(1):28-40. |

| Tasimelteon - Major Depressive Disorder Circadian Mechanism Suspected in Major Depressive Disorder Phase advance theory of depression Diurnal variation of symptoms Light therapy in seasonal affective disorder Tasimelteon: Proof of concept - Positive forced swim test Valdoxan(r): MT1/MT2 agonist approved in EU for MDD 26 |
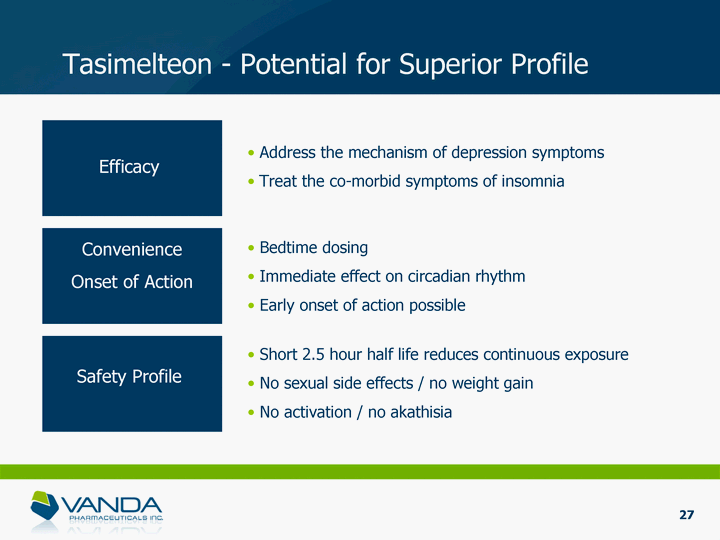
| Tasimelteon - Potential for Superior Profile Bedtime dosing Immediate effect on circadian rhythm Early onset of action possible Short 2.5 hour half life reduces continuous exposure No sexual side effects / no weight gain No activation / no akathisia Address the mechanism of depression symptoms Treat the co-morbid symptoms of insomnia Efficacy Convenience Onset of Action Safety Profile 27 |
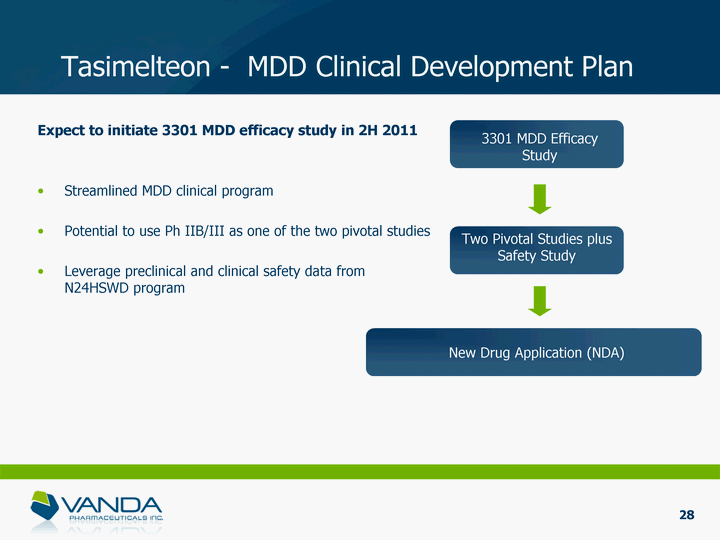
| Expect to initiate 3301 MDD efficacy study in 2H 2011 Streamlined MDD clinical program Potential to use Ph IIB/III as one of the two pivotal studies Leverage preclinical and clinical safety data from N24HSWD program Two Pivotal Studies plus Safety Study New Drug Application (NDA) Tasimelteon - MDD Clinical Development Plan 3301 MDD Efficacy Study 28 |
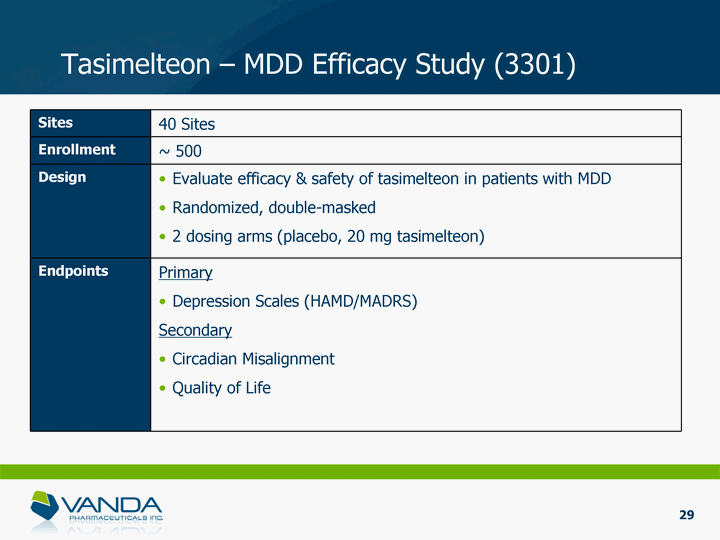
| Sites 40 Sites Enrollment ~ 500 Design Evaluate efficacy & safety of tasimelteon in patients with MDD Randomized, double-masked 2 dosing arms (placebo, 20 mg tasimelteon) Endpoints Primary Depression Scales (HAMD/MADRS) Secondary Circadian Misalignment Quality of Life Tasimelteon - MDD Efficacy Study (3301) 29 |
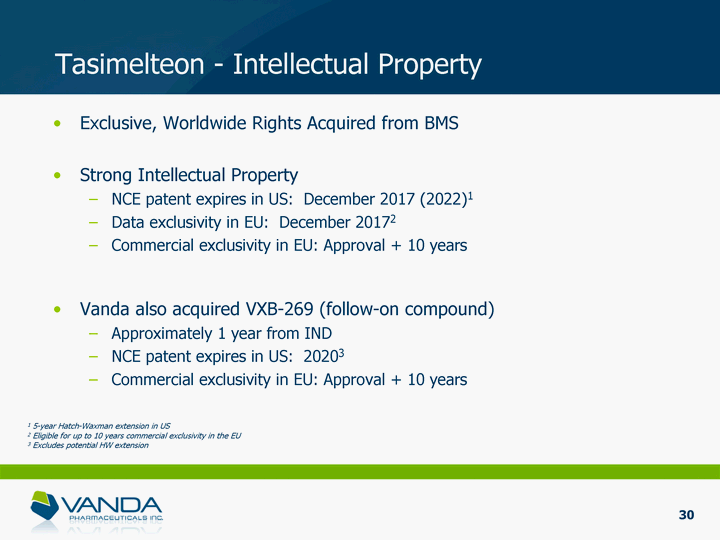
| Tasimelteon - Intellectual Property Exclusive, Worldwide Rights Acquired from BMS Strong Intellectual Property NCE patent expires in US: December 2017 (2022)1 Data exclusivity in EU: December 20172 Commercial exclusivity in EU: Approval + 10 years Vanda also acquired VXB-269 (follow-on compound) Approximately 1 year from IND NCE patent expires in US: 20203 Commercial exclusivity in EU: Approval + 10 years 1 5-year Hatch-Waxman extension in US 2 Eligible for up to 10 years commercial exclusivity in the EU 3 Excludes potential HW extension 30 |

| Financial Summary |
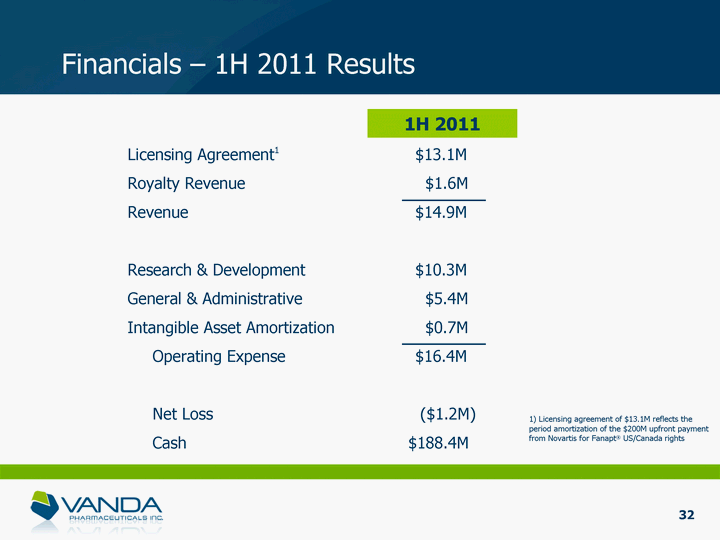
| Financials - 1H 2011 Results Licensing Agreement1 $13.1M Royalty Revenue $1.6M Revenue $14.9M Research & Development $10.3M General & Administrative $5.4M Intangible Asset Amortization $0.7M Operating Expense $16.4M Net Loss ($1.2M) Cash $188.4M 1H 2011 1) Licensing agreement of $13.1M reflects the period amortization of the $200M upfront payment from Novartis for Fanapt(r) US/Canada rights 32 |
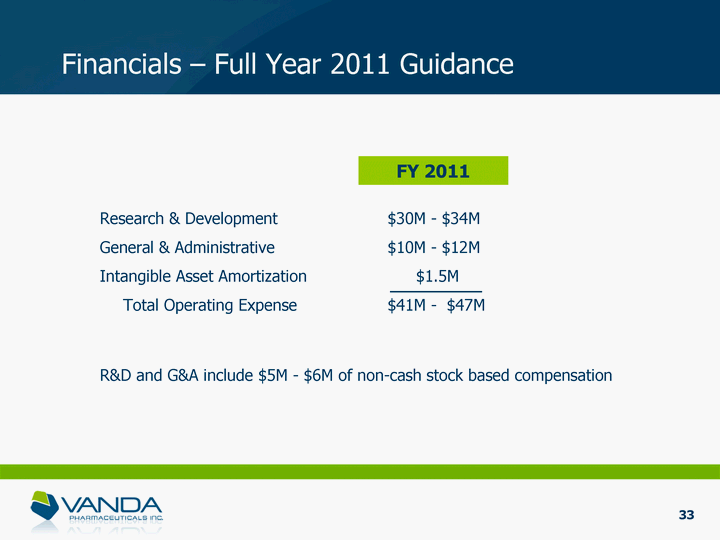
| Financials - Full Year 2011 Guidance Research & Development $30M - $34M General & Administrative $10M - $12M Intangible Asset Amortization $1.5M Total Operating Expense $41M - $47M FY 2011 R&D and G&A include $5M - $6M of non-cash stock based compensation 33 |
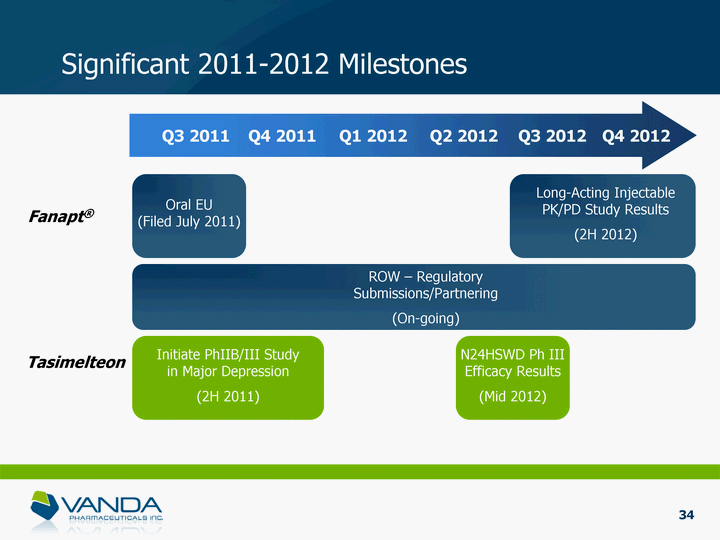
| Significant 2011-2012 Milestones Q3 2011 Q4 2011 Q1 2012 Q2 2012 Q3 2012 Q4 2012 Fanapt(r) Tasimelteon Oral EU (Filed July 2011) Long-Acting Injectable PK/PD Study Results (2H 2012) ROW - Regulatory Submissions/Partnering (On-going) Initiate PhIIB/III Study in Major Depression (2H 2011) N24HSWD Ph III Efficacy Results (Mid 2012) 34 |

| Vanda Pharmaceuticals Inc. |
