Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ReShape Lifesciences Inc. | d8k.htm |
 Canaccord
Genuity Presentation August 11, 2011
Exhibit 99.1 |
 Safe Harbor
Statement 2
This
presentation
contains
forward-looking
statements
about
EnteroMedics
Inc.
Our
actual
results
could
differ
materially
from
those
discussed
due
to
known
and
unknown
risks,
uncertainties
and
other
factors
including
our
limited
history
of
operations;
our
losses
since
inception
and
for
the
foreseeable
future;
our
lack
of
regulatory
approval
for
our
Maestro®
System
for
the
treatment
of
obesity;
our
preliminary
findings
from
our
EMPOWER™
pivotal
trial;
our
ability
to
comply
with
the
NASDAQ
continued
listing
requirements;
our
ability
to
commercialize
our
Maestro
System;
our
dependence
on
third
parties
to
initiate
and
perform
our
clinical
trials;
the
need
to
obtain
regulatory
approval
for
any
modifications
to
our
Maestro
System;
physician
adoption
of
our
Maestro
System
and
VBLOC®
vagal
blocking
therapy;
our
ability
to
obtain
third
party
coding,
coverage
or
payment
levels;
ongoing
regulatory
compliance;
our
dependence
on
third
party
manufacturers
and
suppliers;
the
successful
development
of
our
sales
and
marketing
capabilities;
our
ability
to
raise
additional
capital
when
needed;
international
commercialization
and
operation;
our
ability
to
attract
and
retain
management
and
other
personnel
and
to
manage
our
growth
effectively;
potential
product
liability
claims;
potential
healthcare
fraud
and
abuse
claims;
healthcare
legislative
reform;
and
our
ability
to
obtain
and
maintain
intellectual
property
protection
for
our
technology
and
products.
These
and
additional
risks
and
uncertainties
are
described
more
fully
in
the
Company's
filings
with
the
Securities
and
Exchange
Commission,
particularly
those
factors
identified
as
"risk
factors"
in
our
Annual
Report
on
Form
10-K
filed
with
the
Securities
and
Exchange
Commission
on
March
7,
2011.
We
are
providing
this
information
as
of
the
date
of
this
presentation
and
do
not
undertake
any
obligation
to
update
any
forward-
looking
statements
contained
in
this
document
as
a
result
of
new
information,
future
events
or
otherwise. |
 EnteroMedics
3
Leader in neuroblocking with strong IP
Data in >400 patients support VBLOC therapy as safe and
effective in obesity. Promising results in type 2 diabetes
–
Clinically significant weight loss
–
Improvement in glycemic control
–
Reduction in blood pressure in hypertensive patients
–
Excellent safety, including cardiac
ReCharge Pivotal Trial underway and enrolling
–
FDA encouragement to file IDE
–
High investigator enthusiasm
Commercialization process started in Europe and Australia
–
CE Mark |
 The Obesity
Epidemic in the US 1/3 of US adults are obese
–
More than 72 million people in the US (Body Mass Index “BMI”
>30)
–
1 in 8 deaths in the US are caused by an overweight/obesity related illness
–
CDC estimates an overall economic cost of obesity of approximately $150 billion
Approximately 26 million surgical candidates in the US (BMI>35)
About 1% of eligible patients seek surgery
–
220,000 bariatric procedures completed in the US in 2010
•
Bypass accounts for about 55% of these procedures
High priority for US government and major strategic players
4 |
 Current
Treatments Less Invasive
More Invasive
Pharmaceuticals
Bariatric Surgery
Serious safety concerns, esp. cardiac
Adverse side-effects
Less effective for morbid obesity
–
Limited weight loss
–
Unsustained effect
Duration of use restrictions
Bypass & sleeve surgery irreversible and risky
All result in long-term complications and major lifestyle
changes, e.g., dietary restrictions and nutritional
deficiencies
Adjustable gastric bands have added long-term follow-up
burdens (e.g. vomiting, quarterly adjustments)
5 |
 Role of the
Vagus Nerve Vagus nerve controls:
–
Sensation of hunger
–
Expansion, fullness and emptying
of stomach
–
Digestive enzyme secretion
Severing the vagus nerve
(vagotomy) causes:
–
Reduced appetite
–
Delayed stomach emptying
–
Prevention of weight gain
The effects of vagotomy are not
sustainable
–
The problem —
accommodation,
or “work around”, of permanent
interruption
–
The
solution —
EnteroMedics’
proprietary intermittent block
6
80% of vagus
nerve fibers
send messages
to the brain
20% of vagus
nerve fibers send
instructions from
the brain |
 VBLOC
Therapy Delivered via the Maestro System
VBLOC Therapy
–
First in class –
non-punitive; direct effect on mechanism of metabolic disease
–
Intermittent neuroblocking
technology blocks vagus
nerve signals, therefore
reducing hunger feelings and promoting earlier fullness
–
Subcutaneously implanted, pacemaker-like device with leads placed
laparoscopically
on the intra-abdominal vagal
trunks
7
RC
Commercial
Device
The implantation procedure and usage of the Maestro System carry some risks, such as the risk
generally associated with laparoscopic procedures and those related to treatment as
described in the EMPOWER clinical study informed consent. |
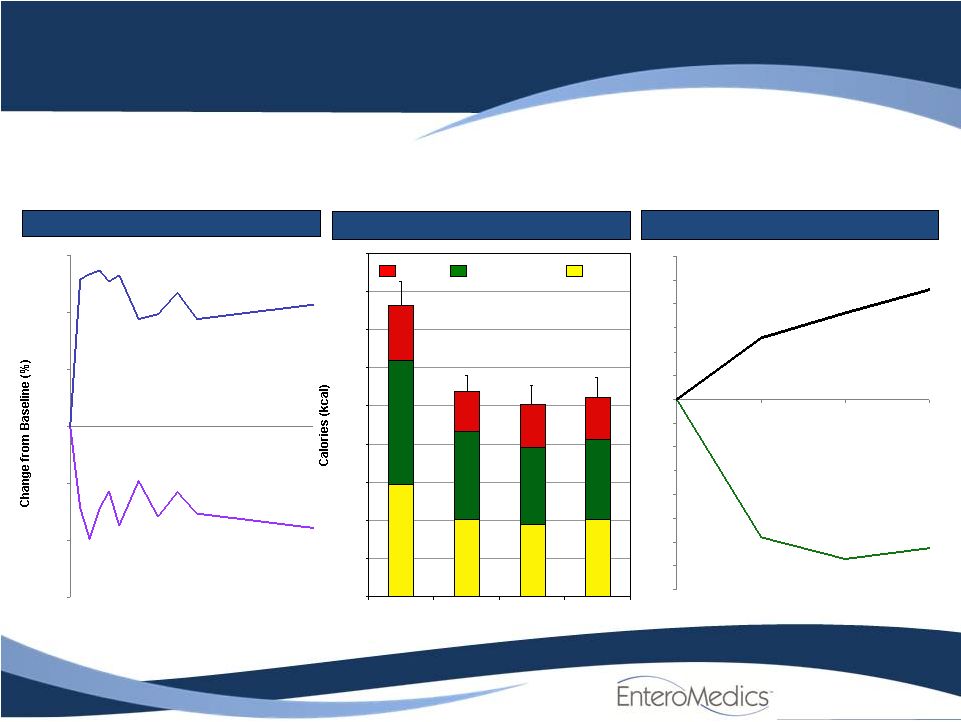 Protein
Carbohydrates
Fat
0
250
500
750
1000
1250
1500
1750
2000
2250
Pre-implant
(n=10)
4 weeks
(n=10)
12 weeks
(n=8)
6 months
(n=9)
20%
43%
37%
20%
44%
36%
23%
41%
22%
40%
38%
Reduced Portion Size
(40%)
(35%)
(30%)
(25%)
(20%)
(15%)
(10%)
(5%)
0%
5%
10%
15%
20%
25%
30%
% EWL from
Implant
Calories
(kcal %)
Reduced Calories
Pre-implant
4 weeks
12 weeks
6 months
36%
Source: VBLOC-I Sub-study., Flinders UMC, Adelaide, Australia
Compelling Proof of Concept
Earlier Fullness and Less Hunger
(90)%
(60)%
(30)%
0%
30%
60%
90%
Reduced
hunger
Therapy
Initiation
4 weeks
12 weeks
6 months
Earlier
fullness
Significant impact on hunger and fullness drives successful weight loss
8 |
 Study
Location
# Patients
~400 Overall
Study
Duration (yrs)
Efficacy
~ %EWL
First Generation Maestro RF System
VBLOC-1
OUS
31
0.5
14 (6
months)
VBLOC-RF2
OUS
38
3
23 (2 years)
(1)
EMPOWER
US
294
2/5
20 (2.5 years)
(2)
Second Generation Maestro RC System
VBLOC-RC1
OUS
5
1/5
26 (1 year)
VBLOC-DM2
OUS
28
1/5
25 (1.5 year)
(3)
ReCharge
US
234
1/5
Enrolling
Note: Conducted gastric function study as well in 12 patients
1) 18 patients
2) 107 patients
3) 22 patients
Safety
No therapy related SAEs; Low overall SAE rate
Positive safety profile, including CV
Efficacy
Clinically significant weight-loss
Control of major co-morbidities
–
Diabetes and hypertension
Broad acceptance by surgeons and patients
The Maestro System
Clinical Experience
9 |
 Pivotal
Trials EMPOWER
–
294 subjects
Double blind, placebo controlled randomized trial
BMI range 35 to 39.9 with co-morbidity; 40 to 45 with or without
–
Endpoints
Primary efficacy: Greater efficacy in treated arm versus control arm
Secondary efficacy: Greater proportion of treated subjects versus control reach
>25% EWL
Safety: Estimate procedure and safety adverse events
ReCharge
–
Approximately 234 Subjects
–
Currently enrolling, completion 4Q11
10 |
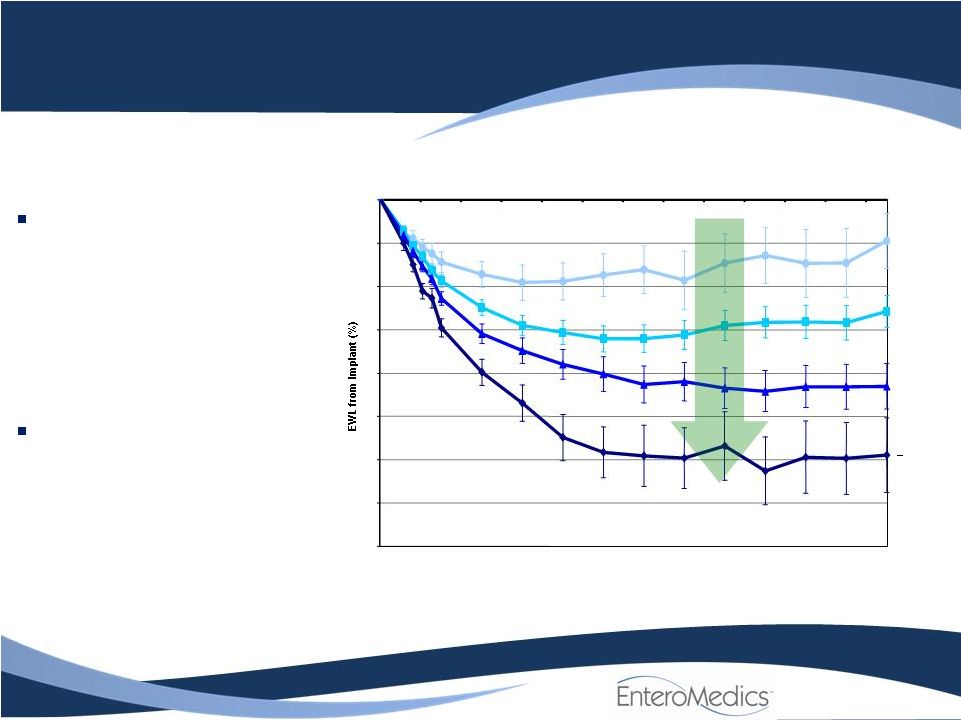 Maestro
System Usage equals RESULTS
-40
-35
-30
-25
-20
-15
-10
-5
0
Implant
4 weeks
3 months
6 months
9 months
12 months
More treatment = More weight loss
< 6hr
(n = 25)
6 -
9hr
(n = 61)
9 -
12hr
(n = 63)
>12hr
(n = 16)
EMPOWER Trial Outcome
Mean Excess Weight Loss (EWL) by Hours of Use in Treatment Group
11
“Dose Effect”
shows clear
correlation between
average EWL and the
number of hours of device
use
–
Demonstrates efficacy
–
Important to FDA
Placebo group result was
nearly identical due to
unanticipated therapeutic
effect |
 EMPOWER Trial
Summary Both groups experienced significant, dose-dependent Excess Weight Loss
(EWL) –
EWL greater than 20% in the prescribed use group of both arms
–
An unanticipated therapeutic effect was seen in the placebo arm
Safety endpoint met
–
No deaths, low 1-yr surgical revision rate and low serious adverse event rate
–
No therapy related serious adverse events
–
Excellent cardiac safety
Long-term follow-up data continue to demonstrate that VBLOC Therapy works
–
At 24 months, >
9 hours daily use patients have an average EWL of ~23% (n=71)
–
Over two-thirds of patients remained in trial at two years
–
At 30 month, all patients, irrespective of hours of device use,
have reached an
average EWL of ~20% (n=107)
FDA subsequently approved a second pivotal trial (ReCharge)
12 |
 US RECHARGE
Trial Pivotal Trial for US Approval
Use next generation implantable device
–
More convenient
–
Hours of use controlled by device
Placebo group receives non-active device
–
No charge delivered
Approximately 234 morbidly obese subjects
–
2:1 randomization
–
Treated group “on”
for ~12 hrs per day
Key trial end points at 12 months
–
Efficacy
–
Safety
Enrolling
13 |
 VBLOC
–DM2 ENABLE Trial Diabetes and Hypertension
Design:
prospective, open-label, multi-center, 12 month trial
Cohort:
28 patients with obesity and type 2 diabetes, 18 with
hypertension
Inclusion criteria:
–
BMI 30 to 40 kg/m
–
NIDDM, <12 yrs duration
–
HbA1c levels >7% to <10%
–
Absence of significant diabetic complications (e.g., gastroparesis).
Data collection:
weight loss (EWL), glycemic (FPG, HbA1c) and blood
pressure control
Analysis:
weeks 1, 4 and 12; and 6, 12 and 18 months
14
2 |
 VBLOC
–DM2 ENABLE Trial %EWL Results
15
*N represents subjects using the device for 12 hours or more
35
25
20
15
-10
5
0
n=26
n=24
n=24
n=22
Implant
3 Months
-
-
-
-
-
6 Months
12 Months
18 Months
-30 |
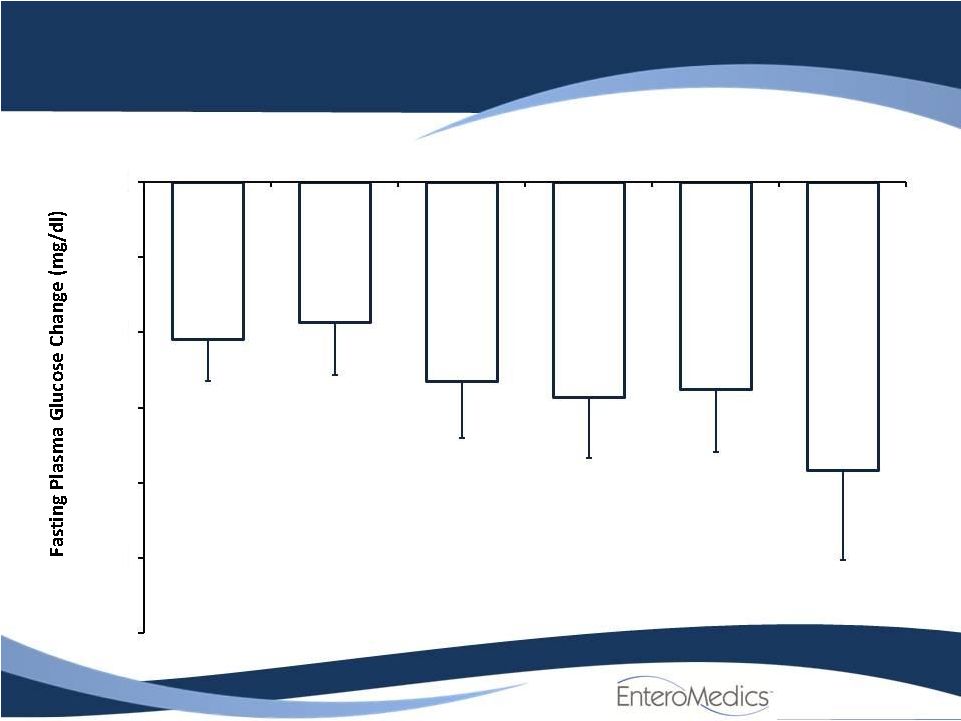 Change in
Fasting Glucose Week 1
Week 4
Week 12
6 Months
12 Months
18 Months
At all time points, changes are significant with p<0.01
Baseline
=
151.4
±
6.5 mg/dl
-60
-50
-40
-30
-20
-10
0
*
n=27
*
n=26
*
n=25
*
n=25
*
n=12
*
n=28 |
 Change in
HbA1c % 17
Week 1
Week 4
Week 12
6 Months
12 Months
18 Months |
 Change in
Diastolic Blood Pressure in Patients with Elevated DBP
-20
-15
-10
-5
0
1 Wk
4 Wk
6 Mo
12 Mo
18 Mo
At all time points statistically significant with p<.01
Baseline
=
87.5
±
2.2
mmHg
Week 1
Week 4
6 Months
12 Months
18 Months
n= 12
n= 12
n= 10
n= 10
n= 11 |
 Change in Mean
Arterial Pressure in Patients with Hypertension
Week 1
Week 12
6 Months
12 Months
18 Months
n= 15
n= 14
n= 13
n= 13
n= 14
At all time points statistically significant with p=.04
Baseline=
100.1
±
2.4
mmHg
-20
-15
-10
-5
0 |
 No Change in
Blood Pressure in Normotensive Patients
-10
-8
-6
-4
-2
0
2
4
6
8
10
Week 1
Week 4
Week 12
6 Months
12 Months
Baseline MAP = 89.0 ±1.1 mmHg
-1.0
n=10
0.5
n=10
-1.8
n=10
-3.2
n=10
3.2
n=4
Week 1
Week 4
Week 12
6 Months
12 Months |
 Significant
Promise in Treatment of Metabolic Disease Diabetes and Hypertension
Clinically significant effect of VBLOC on two major co-morbidities
–
Type 2 Diabetes Mellitus
•
About 26 million people in US and more than 220 million people worldwide have diabetes
–
Cardiovascular / blood pressure
•
About 74 million people in the US and 1 billion worldwide are effected by hypertension
Improvements were immediate and sustained
–
Diabetes:
•
HbA1c reduced to below 7.0%
Diabetes control level set by the American Diabetes Association
–
Blood pressure:
•
~10mmHg reduction in mean arterial pressure and diastolic blood pressure
•
Durable through 18 months
Excellent cardiovascular safety
–
Heart rate reduction
–
Blood pressure
21 |
 Commercialization in Europe and Australia
Australia
–
Historical leadership with new obesity treatments
–
Extensive clinical experience with the Maestro System
•
Australian Institute of Weight Control (AIWC)
–
Device Technologies Australia
•
Distributor
•
Regulatory and reimbursement support
–
TGA approval and first revenue targeted for 2H 2011
Europe
–
Clinical experience in two European centers
–
CE Mark approval for RC System
–
Commercialization activities are progressing in select European markets
22 |
 Financial
Summary Balance Sheet Data
As of June 30, 2011
Cash and cash equivalents $27.4
million Total invested
capital $171 million
NASDAQ: ETRM
Diluted Shares Outstanding
As of June 30, 2011
Common
Shares
Warrants
Options
2.0
million
Diluted
Shares
Outstanding
52.1
million
23
27.9
million
22.2
million |
 EnteroMedics
Leader in neuroblocking with strong IP
Data in >400 patients support VBLOC therapy as safe and
effective in obesity. Promising results in type 2 diabetes
–
Clinically significant weight loss
–
Improvement in glycemic control
–
Reduction in blood pressure in hypertensive patients
–
Excellent safety, including cardiac
ReCharge Pivotal Trial underway and enrolling
–
FDA encouragement to file IDE
–
High investigator enthusiasm
Commercialization process started in Europe and Australia
–
CE Mark
24 |
 |
