Attached files
| file | filename |
|---|---|
| EX-32.1 - SECTION 906_ALBERT F. HUMMEL - Obagi Medical Products, Inc. | exhibit32_1.htm |
| EX-23.1 - PWC CONSENT - Obagi Medical Products, Inc. | exhibit23_1.htm |
| EX-31.1 - SECTION 302_ALBERT F. HUMMEL - Obagi Medical Products, Inc. | exhibit31_1.htm |
| EX-31.2 - SECTION 302_PRESTON S. ROMM - Obagi Medical Products, Inc. | exhibit31_2.htm |
| EX-32.2 - SECTION 906_PRESTON S. ROMM - Obagi Medical Products, Inc. | exhibit32_2.htm |
| EX-10.38 - 2011 PERFORMANCE INCENTIVE PLAN - Obagi Medical Products, Inc. | exhibit10_38.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
(Mark One)
|
||
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the fiscal year ended December 31, 2010
|
||
|
OR
|
||
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from to
|
||
OBAGI MEDICAL PRODUCTS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
22-3904668
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
3760 Kilroy Airport Way, Suite 500, Long Beach, CA 90806
|
||
|
(Address of principal executive offices)
|
||
(562) 628-1007
Securities registered pursuant to Section 12(b) of the Act:
|
Title of class
|
Name of each exchange on which registered
|
|
|
Common Stock, par value $0.001 per share
|
Nasdaq Global Market
|
Securities registered pursuant to Section 12(g) of the Act:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company, as defined in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer ý
|
|
Non-accelerated filer o
(Do not check if a smaller reporting company)
|
Smaller reporting company o
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No ý
The aggregate market value of the registrant’s common stock, $0.001 par value per share, held by non-affiliates of the registrant on June 30, 2010, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $203.0 million (based on the closing sales price of the registrant’s common stock on that date). Shares of the registrant’s common stock held by each officer, director and each person known to the registrant to own 10% or more of the outstanding common stock of the registrant have been excluded in that such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not a determination for other purposes. As of March 2, 2011, there were 18,499,939 shares of the Registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference from the definitive proxy statement for the Annual Meeting of Stockholders to be held on or about June 7, 2011.
OBAGI MEDICAL PRODUCTS, INC.
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
Obagi®, Obagi Blue Peel®, Obagi CLENZIderm®, Condition & Enhance®, ELASTIderm®, Nu-Derm®, Obagi-C®, Rosaclear®, Blue Peel RADIANCE™, ELASTILash™, Penetrating Therapeutics™ and SoluCLENZ™ are among the trademarks of Obagi Medical Products, Inc. and/or its affiliates in the United States and certain other countries. Botox® is a registered trademark of Allergan, Inc. Refissa™ is a trademark of Spear Pharmaceuticals Inc. Any other trademarks or trade names mentioned are the property of their respective owners.
1
We have made forward-looking statements in this Annual Report on Form 10-K ("Report"), including the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations" and "Business,” that are based on our management’s beliefs and assumptions and information currently available to us. Forward-looking statements include the information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, the effects of future regulations, litigation and competition. Forward-looking statements include all statements that are not historical facts and can be identified by the use of forward-looking terminology such as the words “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate” or similar expressions.
Forward-looking statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, our actual results may differ materially and adversely from those expressed in any forward-looking statement. We do not have any intention to update forward-looking statements after this Report is filed. You should understand that many important factors, in addition to those discussed under the section entitled “Risk Factors” in Item 1A and elsewhere in this Report, could cause our results to differ materially and adversely from those expressed in the forward-looking statements.
PART I
Corporate Information
The Company was founded as WorldWide Product Distribution, Inc. (“Worldwide”) in 1988. OMP Acquisition Corporation was incorporated in California in October 1997 to purchase substantially all of the assets and to assume the accounts payable and related operating liabilities of WorldWide and subsequently changed its name to Obagi Medical Products, Inc. in December 1997. OMP, Inc. (“OMP”) was incorporated in Delaware in November 2000; in January 2001 Obagi Medical Products, Inc. was merged into OMP, with OMP as the surviving corporation. In December 2004, the stockholders of OMP exchanged their shares of OMP for an equal number of shares in a newly formed holding company incorporated in Delaware, Obagi Medical Products, Inc., which became the parent holding company for all existing operations of OMP. Unless the context indicates otherwise, in this Report we use the terms “Obagi,” “we,” “us” and “our” to refer to Obagi Medical Products, Inc. and its subsidiaries on a consolidated basis.
Our principal executive offices are located at 3760 Kilroy Airport Way, Suite 500, Long Beach, California 90806, and our telephone number at that location is (562) 628-1007. Our website address is www.obagi.com. Copies of our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission (“SEC”) can be obtained free of charge as soon as reasonably practicable after such materials are electronically filed with, or furnished to, the SEC by clicking the SEC Filings link from the Investor Relations page on our website at www.obagi.com. Members of the public may also read and copy any materials we file with, or furnish to, the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. To obtain information on the operation of the Public Reference Room, please call the SEC at 1-800-SEC-0330. The SEC also maintains an Internet site at www.sec.gov that contains the reports, proxy and information statements, and other information that we file electronically with the SEC. Our website and the information contained thereon or connected thereto are not intended to be incorporated into this Report.
Our common stock trades on the Nasdaq Global Market under the symbol “OMPI.”
Overview
We are a specialty pharmaceutical company that develops, markets and sells, and are a leading provider of, proprietary topical aesthetic and therapeutic prescription-strength skin care systems and related products in the physician-dispensed market. Obagi systems and products are designed to prevent and improve the most common and visible skin disorders in adult skin, including premature aging, photodamage, hyperpigmentation (irregular or patchy discoloration of the skin), acne, sun damage, facial redness, and soft tissue deficits, such as fine lines and wrinkles. Our products have been developed to enhance the underlying health of patients’ skin, and clinical studies have demonstrated that the use of our systems results in skin that looks and acts younger and healthier.
We sell our products through a direct sales force in the United States and internationally through distribution partners in over 45 countries across North America, Central America, Europe, the Middle East and Asia. Our domestic sales force and foreign distributors predominantly sell our products directly to dermatologists, plastic surgeons and other physicians who are focused on aesthetic and therapeutic skin care. These physicians then dispense our products in-office, directly to their patients. We believe that we are the market leader in this growing physician-dispensed skin care channel, according to a 2010 study by Kline & Co., an independent market research firm. Our net sales have grown from approximately $35.6 million in 2001 to $112.8 million for the year ended December 31, 2010.
2
Over the years, we have developed numerous prescription-strength skin care products and systems for the enhancement of skin health. Using our Penetrating Therapeutics technologies, our products are designed to improve the penetration of prescription and over-the-counter (“OTC”) cosmetic agents across the skin barrier to address the most common and visible skin conditions in adult skin.
Our leading product line, launched in 1988, is the Obagi Nu-Derm System. We believe the Nu-Derm System is the leading clinically-proven, prescription-based topical skin health system on the market that has been shown to enhance the skin’s overall health by correcting photodamage using drugs that, by definition, work at the cellular level. This results in a reduction of the visible signs of aging. In addition, we offer several other skin care systems and related products, including: (i) the Obagi-C Rx System, introduced in 2004, a prescription-strength system that reduces the early effects of sun damage and evens skin tone through the use of a Vitamin C serum combined with 4% hydroquinone; (ii) our Professional-C series of products, introduced in 2005, which consist of high potency antioxidant Vitamin C serums that help to counteract the effects of ultraviolet radiation and other environmental influences; (iii) the Condition & Enhance System, launched in 2006, which was developed to enhance the results of physician-delivered surgical and non-surgical cosmetic procedures, such as Botox, injectable fillers, chemical peels, microdermabrasion and laser resurfacing treatments; (iv) the ELASTIderm family of products, first introduced in 2006, which includes treatments developed to restore skin elasticity and collagen production, and reduce fine lines and wrinkles, around the eyes and décolletage; (v) our CLENZIderm M.D. Acne Therapeutic Systems, introduced in 2007, featuring a solubilized formulation of benzoyl peroxide (“BPO”) to treat and prevent acne at its root; (vi) the Rosaclear System, launched in 2009, which is a complete prescription-based system developed specifically for the treatment and prevention of the signs and symptoms of facial redness; (vii) ELASTILash Eyelash Solution, a member of the ELASTIderm family launched in 2010, designed to help acheive the appearance of visibly thicker, fuller-looking eyelashes; and (viii) Blue Peel RADIANCE, launched in 2011, a gentle salicylic acid-based peel that utilizes a unique blend of acids and other soothing ingredients for a potent combination that exfoliates, evens out skin tone and improves overall complexion, with little-to-no downtime. We also offer: (i) tretinoin, a generic equivalent to Retin-A that is among the most widely used acne treatments, as well as Refissa, a 0.05% strength tretinoin, approved by the United States Food and Drug Administration (“FDA”), with an emollient base that has a broad indication for treatment of fine facial lines, hyperpigmentation and tactile roughness; (ii) metronidazole topical gel, a generic equivalent to Metrogel, for use in conjunction with our Rosaclear System; and (iii) Obagi Blue Peel Essential Kit, which has been cited by Kline & Co. as one of the most well known brands for use in physician-strength facial peel procedures.
We also advance our development objectives through product and license agreements with third parties. These agreements may include patent and technology licenses, product licenses and new product collaboration agreements. We compete in the Japanese retail skin care markets through strategic licensing agreements with a Japanese pharmaceutical manufacturer and distributor that sells a series of OTC products under the Obagi brand name in the Japanese drug and variety store channels. In addition, we have other licensing arrangements in Japan to market and sell OTC product systems under the Obagi brand in the aesthetic spa channel, both for in-office use in facial procedures, as well as for sale as a take-home product kit. Our net licensing revenue from skin health systems and products in Japan was approximately $4.4 million for the year ended December 31, 2010.
The Skin Care Market
According to the American Society of Plastic Surgeons, there were 13.1 million cosmetic procedures performed in the United States in 2010, representing a 5% increase over 2009 and a 77% increase over 2000. We believe this reflects a growing desire and acceptance among the population to seek assistance from physicians to improve their appearance, including the appearance of their skin. A key driver of this trend is the aging of the “baby boomer” segment of the U.S. population. In addition, life expectancy in the United States has extended in recent years, leading to a further increase in the average age of the country’s population. Because healthcare needs, including the treatment of skin disorders, tend to increase with age, we expect the demand for skin care products to continue to increase over time. In particular, women tend to demonstrate a higher motivation than men to improve their personal appearances. The number of women between the ages of 35 and 65, the primary users of our products, was estimated by the U.S. Census Bureau to have grown 44.7% between 1990 and 2010. With this segment’s strong desire to reduce the signs of premature aging, we expect the aging female population to continue to increase the market opportunity for skin care products.
Most of the cosmetic skin care products available today are designed to mask the effects of aging and skin disorders, rather than treat the underlying health of the skin. As a result, consumers may see temporary skin surface improvements, but underlying skin restoration often does not occur because the active agents in these products lack the ability to effectively penetrate the skin barrier. In addition, most traditional approaches to skin care are not comprehensive programs designed to integrate complementary products. Individual products, even those that are widely used by consumers (such as facial soaps or sunscreens), are not generally designed to work together, and therefore may cause unintended side effects or reduced effectiveness when used in combination.
For these reasons, consumers have increasingly turned to their physicians for products and simple in-office procedures that can provide better results than consumer cosmetics. For example, physician-recommended cosmetic products and commonly performed cosmetic procedures such as Botox injections, injectable fillers, laser hair removal and microdermabrasion, have experienced substantial growth as consumers learn that they can achieve positive cosmetic results with minimally invasive techniques. Beyond anti-aging and aesthetic treatments, there is also significant market demand for effective products that treat skin diseases such as acne, rosacea, psoriasis, and eczema (dermatitis), as well as for eyelash enhancement and effective sunscreen products. While a number of therapies,
3
treatments and products exist for such diseases or conditions, most treatments or products consist of either topical applications with limited efficacy, or systemic (oral) applications that carry significant potential side effects.
According to Kline & Co., in 2010, there were approximately 25,600 physicians practicing dermatology and plastic surgery in the United States, of which more than 11,400 dispensed skin care products directly to their patients. Based on our experience with physicians who have opened accounts with us, we believe a growing number of general practice, family practice, internal medicine, dental and obstetrics and gynecology (“OBGYN”) physicians are also dedicating resources in their practices to skin care. We believe that these physicians are responding to the rapid increase in consumer demand for non-invasive skin care treatments. Furthermore, according to Kline & Co., more than 6,700 of these physicians dispensed prescription and non-prescription skin care products directly to their patients in 2010.
Outside of the United States, the physician-dispensed skin care market varies by country due to cultural differences and regulatory requirements. Cultural desires for skin with lighter and more even pigmentation have created large and growing aesthetic skin care demands throughout Asia, particularly Japan, China and Korea. European and certain South American countries such as Brazil also present large skin care markets due to the complementary growth in cosmetic procedures and willingness on the part of their consumers to spend discretionary income on aesthetic enhancements. We believe that the growth in major international markets will also be driven by cultural desires to lessen the appearance of skin darkening caused by exposure to sun, aging populations and a heightened awareness and availability of aesthetic products and procedures. While physician dispensing is common in most countries, certain countries prohibit or limit the types of products that can be dispensed from a physician’s office, requiring physicians to either partner with a retail pharmacy or drug store, or to simply forgo dispensing.
Our Obagi Systems and Related Products
We believe the effects of aging and skin disorders are best addressed at a deeper level, where the skin’s natural cell regeneration processes occur, rather than at the surface of the skin. Thus, a number of our systems and related products have been designed to improve the overall health of the skin by improving cellular processes such as collagen and elastin production, keratinocyte clearing, and melanocyte regulation, using drugs that, by definition, work at the cellular level. We have developed comprehensive skin care systems and products that incorporate a range of individual prescription and non-prescription therapeutic agents, as well as cosmetic ingredients. The individual components of each system and related products have been specifically formulated to complement one another, enhancing the effectiveness of a particular system as a whole and allowing the physician to tailor the treatment program to the specific needs of the patient. The design of our systems and products is generally proprietary to us, and we are the sole licensee of both provisional and issued U.S. patents and have patent applications pending for the composition of most of these products.
4
|
System and related products
|
Segment/product category
|
Description
|
Applications
|
Launch date
|
|
Obagi Nu-Derm System
|
Physician-Dispensed/ Nu-Derm
|
Comprehensive system of six products including prescription and OTC drugs
|
Fine lines, wrinkles, acne, photo damage, hyperpigmentation, melasma, laxity, skin sallowness
|
1988
|
|
Obagi Condition & Enhance Systems
|
Physician-Dispensed/ Nu-Derm
|
Line extension of Nu-Derm designed for use before and after surgical and non-surgical cosmetic procedures; prescription-based
|
Enhances patient outcomes and patient satisfaction
|
2006
|
|
Obagi-C Rx System
|
Physician-Dispensed/ Vitamin C
|
Highly stable Vitamin C serum with 4% hydroquinone system; prescription-based
|
Fine lines, wrinkles, hyperpigmentation, skin sallowness
|
2004
|
|
Professional-C
|
Physician-Dispensed/ Vitamin C
|
Highly stable Vitamin C serums; non-prescription
|
Antioxidant protection, fine lines, wrinkles, hyperpigmentation
|
2005
|
|
ELASTIderm Eye and Décolletage
|
Physician-Dispensed/ Elasticity
|
System of skin health products built around a novel formulation of a bi-mineral complex; prescription and non-prescription based
|
Increase elasticity and skin tone of eyes, face, neckline and chest
|
2006/2008
|
|
ELASTILash Eyelash Solution
|
Physician-Dispensed/ Elasticity
|
Eyelash enhancing solution; non-prescription
|
Enhance the appearance of thickness and fullness of eyelashes
|
2010
|
|
CLENZIderm M.D. Systems
|
Physician-Dispensed/ Therapeutic
|
Systems for acne treatment built around a novel formulation of BPO; non-prescription
|
Acne
|
2007
|
|
Obagi Rosaclear System
|
Physician-Dispensed/ Therapeutic
|
System for rosacea treatment that reduces redness and flushing, along with treating papules and pustules; prescription based
|
Rosacea
|
2009
|
|
Tretinoin
|
Physician-Dispensed/ Other
|
Generic equivalent of Retin-A available in the United States; requires prescription
|
Acne
|
2002
|
|
Refissa
|
Physician-Dispensed/ Other
|
Branded Retin-A available in the United States; requires prescription
|
Fine facial lines, hyperpigmentation and tactile roughness
|
2009
|
|
Metronidazole
|
Physician-Dispensed/ Other
|
Generic equivalent of Metrogel; requires prescription
|
Rosacea
|
2009
|
|
Obagi Blue Peel Essential Kit
|
Physician-Dispensed/ Other
|
Topical system to aid in the application of TCA (trichloroacetic acid) chemical peels
|
Fine lines, wrinkles, hyperpigmentation
|
1988
|
|
Blue Peel RADIANCE
|
Physician-Dispensed/ Other
|
Salicylic acid-based superficial chemical peel
|
Fine facial lines, hyperpigmentation and tactile roughness
|
2011
|
5
We further differentiate our skin care systems and related products by supporting their safety and efficacy with randomized controlled and comparative clinical studies conducted by leading market experts. We believe that these clinical studies provide added scientific credibility to our products in the physician-dispensed market. We have initiated 86 clinical studies since 2003, involving patients in the areas of acne, aesthetics and elasticity to analyze the efficacy, side effects and tolerability of our systems and products, as well as to compare them to other commonly used regimens. We currently intend to launch approximately 17 additional clinical studies in 2011 to assess other therapeutic indications for new and existing products.
Obagi Nu-Derm System
Our Obagi Nu-Derm System was initially launched in 1988, and since that time, we have made substantial enhancements to the system through the application of our Penetrating Therapeutics technologies. We believe that the Nu-Derm System is the leading clinically proven, prescription-based topical skin health system currently available that has been shown to enhance the skin’s overall health by correcting photodamage using ingredients including pharmaceutical agents that, by definition, work at the cellular level.
The Obagi Nu-Derm System consists of a combination of six prescription, OTC therapeutic agents and adjunctive cosmetic skin care products to treat visible skin conditions such as photodamage and hyperpigmentation resulting from extrinsic damage and intrinsic changes to the skin. The Nu-Derm cosmetic skin care products include cleansers and exfoliating creams. Three of these products contain hydroquinone, in a 4% prescription concentration, which acts as a bleaching agent that is designed to correct skin pigmentation problems by normalizing the production of new melanin in the epidermis. Physicians may also prescribe the drug tretinoin as a complement to the system, in various concentrations, depending on the physician’s judgment of patient need. We believe that the use of these prescription therapeutic agents, the ability of such agents to penetrate the skin’s surface and the order of application distinguishes our Nu-Derm System from other commonly prescribed regimens. While we have designed the Nu-Derm System to include products that patients can use in a systematic treatment regimen, we also make the component products available for individual sale. We believe that physicians who dispense the Obagi Nu-Derm System generally encourage their patients to use the component products together in a systematic treatment regimen. However, we also believe that some patients elect to use the products that make up the system individually. Products that are used individually at times include the sunscreen products and Obagi Nu-Derm Clear, which physicians may dispense on occasion to address localized pigmentation problems. Side effects from use of the products may include redness, mild to moderate irritation and/or excessive flaking or sloughing of the outer layers of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use.
The Obagi Nu-Derm System, including the Obagi Condition & Enhance System (described below), accounted for approximately 52% and 54% of our consolidated net sales for the years ended December 31, 2010 and 2009, respectively. Although the volume of sales for each individual product within the system varies, we believe the majority of our sales of each component product is due to the fact that they are sold as part of the system.
Obagi Condition & Enhance
To address the growing market for cosmetic procedures, in July 2006, we launched Obagi Condition & Enhance, a line extension of our Nu-Derm System targeted for use with surgical and non-surgical cosmetic procedures, such as Botox, injectable fillers, chemical peels, microdermabrasion and laser resurfacing treatments. This system provides adjunctive therapy both before and after these procedures, and has been clinically proven to enhance aesthetic outcomes and improve overall patient satisfaction.
Like our Nu-Derm System, the Condition & Enhance System consists of a combination of six prescription and OTC therapeutic agents and adjunctive cosmetic skin care products to treat visible skin conditions such as photodamage and hyperpigmentation resulting from extrinsic damage and intrinsic changes to the skin. Condition & Enhance cosmetic skin care products include cleansers and exfoliating creams. Three of these products contain hydroquinone, in a 4% prescription concentration, which acts as a bleaching agent that is designed to correct skin pigmentation problems by normalizing the production of new melanin in the epidermis. While we have designed Obagi Condition & Enhance to include products that patients can use in a systematic treatment regimen, we also make the component products available for individual sale. We believe that physicians who dispense Obagi Condition & Enhance generally encourage their patients to use the component products together in a systematic treatment regimen. Side effects from use of the products may include redness, mild to moderate irritation and/or excessive flaking or sloughing of the outer layers of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use.
Obagi-C Rx Systems
The Obagi-C Rx Systems consist of a combination of four prescription and OTC drugs and adjunctive cosmetic skin care products to treat skin conditions resulting from sun damage and the oxidative damage of free radicals. The central ingredients in the systems are hydroquinone, in a 4% concentration, and Vitamin C. This combination distinguishes Obagi-C Rx from other Vitamin C based products available in the physician office, and Obagi-C Rx products have been shown in clinical studies to penetrate all levels of
6
skin better than the leading Vitamin C competitor. We are the sole licensee of certain patents of Avon Products, Inc. (“Avon”) relating to these products. Two products in each of the Obagi-C Rx Systems contain hydroquinone, in a 4% concentration, which is designed to correct skin pigmentation problems by normalizing the production of new melanin in the epidermis. The Obagi-C Rx Systems include cosmetic skin care cleansers and exfoliating lotions. When combined in a system, we believe hydroquinone, Vitamin C and a sunscreen provide correction of, and protection against, premature skin aging. As with the Obagi Nu-Derm System, the products that make up the Obagi-C Rx Systems are generally used together in a coordinated regimen by the majority of patients. Side effects from use of these products may include redness and/or mild to moderate irritation of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use.
Obagi Professional-C
Obagi Professional-C products are a complete line of proprietary, non-prescription products, that consist of Vitamin C serums used to reduce the appearance of damage to the skin caused by ultraviolet radiation and other environmental influences. Vitamin C (L-ascorbic acid) acts as a potent antioxidant. The Professional-C line consists of a 5% serum for the area around the eyes and 10%, 15% and 20% serums for the face, neck and chest. Obagi Professional-C products are sold individually and are used on their own, or in combination with other Obagi system products. These products are classified as cosmetics and side effects are not generally associated with their use; however certain sensitive individuals may experience mild irritation of the skin where product is applied. These products have been shown in clinical studies to penetrate all levels of skin better than the leading Vitamin C competitor.
ELASTIderm
As skin ages, it loses its elasticity and begins to sag, particularly around the eyes, on the neck and on the hands. Our ELASTIderm product line includes products for the treatment of skin laxity featuring patent pending bi-mineral complexes that may help the body’s own natural ability to increase epidermal thickness, augment hypodermal fat and increase elastin levels by supplying increased local concentrations of natural mineral actives to the relevant tissue, thereby improving elasticity and skin tone. We believe this is the first clinically proven skin health system to aid in the regeneration of elastin, which we believe is a new and novel topical application for anti-aging. Based on the results of our clinical studies, which showed significant improvements in measured collagen and elastin in under eye skin treated over an eight-week period, we launched an eye cream product under the Obagi ELASTIderm brand name in October 2006. In February 2007, we launched an eye gel, which is the second product under the Obagi ELASTIderm brand, and in February 2008, we launched ELASTIderm Décolletage, a system to treat skin conditions resulting from sun damage and improve the elasticity and skin tone for the neck and chest area. Side effects from use of these products may include redness and/or mild to moderate irritation of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use.
In response to the growing market for eyelash enhancers, in 2010, we introduced ELASTILash Eyelash Solution, a member of the ELASTIderm family. The ELASTILash Eyelash Solution contains a peptide (believed to achieve the appearance of visibly thicker, fuller-looking eyelashes) and other cosmetic ingredients, and does not require a prescription. The product is dermatologist- and ophthalmologist-tested, non-allergenic and has not resulted in any eyelid darkening or changes in iris pigmentation.
We are continuing to evaluate the use of future ELASTIderm products on other areas of the body. These areas may include the hands, full face, arms and other areas of the body. However, we cannot assure you that additional products will be successfully developed in the future.
CLENZIderm M.D.
Current formulations of BPO are emulsions, comprised of large, highly insoluble particles that do not pass easily into the skin follicle to treat the underlying causes of acne. As a result, the efficacy of existing formulations of currently marketed BPO products is limited. We have developed a system of products for acne treatment featuring a novel formulation of BPO that our clinical studies show penetrates more readily into the skin follicle than current creams or gels because the solubilized particle size is substantially smaller than that of other brands. We believe that our solution-based acne system is more effective treatment for acne because a greater amount of the active ingredient, BPO, in a solubilized form will penetrate the hair follicle to act on propionibaceterium acne (“P. acne”). We conducted several clinical studies that demonstrated that our novel BPO alone achieved greater intrafollicular and skin surface bactericidal activity (or kill) against P. acnes than both a prescription generic BPO formulation and a prescription BPO/antibiotic combination product. We believe these studies show the efficacy of our novel BPO formulations in reducing acne and clearing visible acne lesions on the skin. Based on the results of these studies, we launched the CLENZIderm M.D. System for normal to oily skin in February 2007 and for normal to dry skin in July 2007. Side effects from use of these products may include redness and/or mild to moderate irritation of the treated skin. Side effects generally resolve upon discontinuance of use.
7
Obagi Rosaclear
According to the National Rosacea Society, rosacea is a very common skin condition, affecting more than 16 million Americans. Our Rosaclear System is the first complete prescription-based system developed specifically for treating the signs and symptoms of rosacea, or facial redness. The all-in-one system is designed, and has been clinically proven, to effectively reduce redness and flushing, along with treating papules and pustules, to help rosacea patients achieve a clearer, calmer and more balanced-looking complexion. The system consists of a gentle cleanser, metronidazole topical gel 0.75%, and a Hydrating Complex Corrector designed to protect and moisturize irritated skin as well as minimize redness and flushing. A chemical-free, non-comedogenic Skin Balancing Sun Protection SPF 30 sunscreen was also developed for use in conjunction with the system. This sunscreen was designed to provide broad-spectrum UVA/UVB protection and is tinted to help reduce the appearance of facial redness and irritation. Side effects can include tearing of the eyes, burning, skin irritation, dryness, transient redness, metallic taste, tingling or numbness of the extremities and nausea in certain sensitive individuals. If these effects occur, the medication should either be used less frequently or discontinued.
Metronidazole
Metronidazole, an antibiotic, is often used in a topical gel form for the treatment of rosacea in the United States. Topical metronidazole helps to treat the inflammatory papules and pustules associated with rosacea. While physicians can, and in many cases do, write a prescription for metronidazole to be filled by a pharmacy in conjunction with our Rosaclear System, we also offer a FDA-approved metronidazole topical gel 0.75% to provide physicians the option of dispensing metronidazole directly from the office along with the Rosaclear System. Side effects can include tearing of the eyes, burning, skin irritation, dryness, transient redness, metallic taste, tingling or numbness of the extremities and nausea in certain sensitive individuals. If these effects occur, the medication should either be used less frequently or discontinued.
Tretinoin and Refissa
Tretinoin creams and related adjunctive acne care products are used for the topical treatment of acne in the United States. Tretinoin, the active ingredient in the prescription acne drug Retin-A, is a Vitamin A derivative and has been the primary prescription acne therapy for approximately 25 years. Topical tretinoin normalizes the growth rate of skin cells, disrupting the onset of acne. We offer FDA-approved formulations of tretinoin through exclusive licenses in the physician-dispensed skin care channel. Our tretinoin line is available in concentrations of 0.1%, 0.05% and 0.025%. In September 2009, we also began offering Refissa, a FDA-approved 0.05% strength tretinoin with an emollient base that has a broad indication for treatment of fine facial lines, hyperpigmentation and tactile roughness. These products are sold individually and are used by doctors as a single therapy, in combination with Obagi Nu-Derm and Condition & Enhance or in combination with other acne therapies. Side effects include excessively red, edematous, blistered, or crusted skin for certain sensitive individuals. If these effects occur, the medication should either be discontinued until the integrity of the skin is restored, or the medication should be adjusted to a level the patient can tolerate. To date, adverse effects generally have been reversed upon discontinuation of therapy.
Obagi Blue Peel Essential Kit
Obagi Blue Peel Essential Kit is a topical system to aid in the application of trichloroacetic acid (“TCA”) chemical peels used to smooth the surface of skin, improve skin tone and color, diminish wrinkles and shrink pore sizes and is sold for professional use only. While the Obagi Blue Peel Essential Kit is not dispensed for daily home use in a system and is therefore not a significant source of our revenue, it is used to aid physicians in skin peeling activities. Chemical peels are in-office procedures performed either by a physician or a member of a physician’s staff, depending on the skin depth of the peel. During the procedure, acidic solutions are combined in our delivery system and applied to the face to remove the thin surface layers of aged and damaged skin. After removal, the body will naturally replace the removed skin layers with new, healthy skin cells. The Obagi Blue Peel Essential Kit provides for an even application and slows the penetration of the solution into the skin, allowing physicians to more accurately monitor and control the depth of the peel. This produces a more uniform and consistent application, which reduces the risk of complications. We believe that the Obagi Blue Peel Essential Kit is especially effective as a complementary treatment to our Nu-Derm System. The Obagi Blue Peel Essential Kit products have no known side effects in and of themselves. Patients treated with Obagi Blue Peel Essential Kit products as part of an acid peel procedure can expect to experience side effects that are associated with such chemical peel procedures. We do not manufacture, supply or recommend the source for the TCA generally used in these chemical peel procedures.
Blue Peel RADIANCE
Blue Peel RADIANCE, launched in January 2011, is a salicylic-acid based superficial chemical peel product that is sold for professional use only. The product consists of a unique blend of acids and other soothing ingredients for a potent combination that can be used to improve a variety of skin disorders, including acne scarring, photodamage and melasma (uneven pigmentation). As compared to deeper TCA chemical peels, Blue Peel RADIANCE may be applied in an in-office procedure that takes a matter of minutes
8
and offers little-to-no downtime. However, because it is a more gentle peel product, generally a series of four to six peel procedures provides the best overall results. During and after a peel procedure using Blue Peel RADIANCE, the following may be experienced: stinging, itching, burning, mild pain, tightness, peeling and scabbing of the superficial layers of the skin. These sensations should gradually diminish over the course of a few weeks as the skin returns to normal. However, some patients may react differently. In unusual and severe cases, the skin may turn very red, blister, swell and later scab and crust. The skin may be uncomfortable and look like a bad sunburn. The peeling usually lasts about three to seven days, although it may last longer. Following a peel procedure, adequate sunscreen should be used for at least a week to allow for proper healing of the skin.
Areas of Future Growth
Our product development strategy is to enhance the efficacy of established, widely prescribed, FDA-approved dermatology products (both prescription and OTC) by developing mechanisms that significantly enhance the penetration of these agents across the skin’s protective barrier into the deeper layers of the skin, where therapeutic benefits are realized. We describe this enabling technology as Penetrating Therapeutics, in which the individual chemical characteristics of both active and inactive agents are addressed and then combined in a systematic manner based on their pharmacokinetic properties.
We believe that our Penetrating Therapeutics technology may be applicable in other skin health areas. We are currently evaluating additional therapeutic formulations in related areas, some of which could result in new product introductions.
We seek to demonstrate through clinical studies the improved efficacy of various topical agents when used as part of our systems. We are conducting and plan to initiate numerous clinical studies to demonstrate the advantages of our products in both blinded, randomized controlled studies and direct comparative studies that will provide both clinically and statistically significant results. We will work with leading researchers and clinicians in their respective fields of applications and markets. We intend to use these results in marketing our products.
Sales and Marketing
Domestic
In the United States, we sell our systems and related products directly to physicians through our internal sales force. Physicians then dispense our products in-office, directly to their patients, a distribution method commonly referred to as the “physician-dispensed” channel. We believe that the physician-dispensed distribution model ultimately results in higher patient satisfaction because it is better suited to the provision of system-based skin care than traditional drug distribution channels. Our physician customer base consists primarily of plastic surgeons and dermatologists, but also includes physicians from other practice areas, such as general practice, family practice, internal medicine, dental and OBGYNs who are adding skin care to their practices.
Based on a 2010 study by Kline & Co., we are the leading skin health company in the physician-dispensed channel, with an estimated 30.5% market share, nearly two times the market share of the next largest competitor. As of December 31, 2010, we had approximately 6,500 active accounts in the United States. Each account has at least one licensed physician on-site and we estimate that there are over 12,300 physicians in total practicing under these active accounts. This includes physicians on-site at a small but growing number of medical spas.
The U.S. market accounted for 84% and 82% of our net sales for the years ended December 31, 2010 and 2009, respectively. As of December 31, 2010, we had 145 sales, marketing and education specialists, including 107 dedicated sales representatives and managers. We believe that we have sufficient sales representation to enable us to effectively target the plastic surgeons, dermatologists and aesthetic skin care physicians who dispense skin care products in the United States. In addition to effective systems, we also offer turn-key practice building programs and patient events that help these physicians grow their practices. These resources help physicians improve their recurring patient visits and revenue streams.
Our marketing efforts have a dual focus. First, we market directly to physicians in an attempt to create treatment awareness and encourage the use of our products by both new and existing physician clients. To support this effort, we use medical journal advertising, sales aids, videos, CDs and slide demonstrations, physician-to-physician speaker presentations, trade shows, reminder items, educational and training support, internet resources, and telemarketing. Second, we attempt to create and then build upon the relationships with our physician customers by marketing our products to their patients. We accomplish this through in-office materials in our physician customers’ medical practices; consumer engagement through our website, beauty blogs and social media outlets; public relations efforts to create brand awareness; and product maintenance programs to increase patient loyalty. In addition, we provide patient education booklets and videos, funding for cooperative advertising, training and direct assistance in patient seminars and other programs, continuous product education and public relations programs for patient referrals.
In addition, our research and development staff is designing and directing clinical studies to support the application of our existing product lines for new markets and to enhance our current marketing efforts. We have conducted numerous studies with the
9
leading academic institutions and key experts in dermatology and aesthetic medicine, and will continue to expand our collaborations with leading dermatologists and institutions to coordinate additional clinical studies for new product applications and formulations. We believe evidence from our clinical studies will validate the performance of our existing products and provide a platform for the development of new products and product applications.
International
International markets accounted for 16% and 18% of our net sales for the years ended December 31, 2010 and 2009, respectively. We address international markets through 20 international distribution and two licensing partners that have sales and marketing activities in approximately 45 countries outside of the United States, and three trademark and know-how license agreements for the drug store and aesthetic spa channels in Japan. We target distribution partners who are capable and willing to mirror our sales and distribution model in the United States and who have an established business and reputation with physicians. The products that we sell internationally are generally the same formulations as those sold in the United States; however, in some instances, formulations have been modified to comply with the regulatory requirements of certain countries.
These distributors use a model similar to our business model in the United States, addressing their territories through direct sales representatives who sell to physicians, or through alternative distribution channels, depending on regulatory requirements and industry practices. The sale of skin health and restoration products through physician offices is not as widely established internationally as it is in the United States. For example, some of our more successful international distribution partners include our partners in Southeast Asia, the Middle East and North America (excluding the United States). In many of these territories, our partners utilize Obagi-branded medical centers to employ trained physicians for patient sales, as well as a training center to provide product and sales training to regional physicians and their staff. In addition, some of our distribution partners, such as our distributors in Mexico and Canada, leverage their existing complementary product offerings to gain rapid account penetration for Obagi Systems in their territories. International sales are diversified, and our largest individual medical channel distribution partner purchased approximately $1.9 million of products from us during the year ended December 31, 2010.
We intend to continue expanding our international presence by entering into strategic relationships in key locations such as Asia, Europe and South America. We believe that there is potential for significant sales growth of our products in international markets due to cultural emphasis on overall skin health and appearance, and the continued development and acceptance of surgical and non-surgical cosmetic procedures throughout many countries of the world. In 2011, we intend to invest in efforts to assess international market opportunities and to review and optimize our current international markets.
Licensing
In areas where the physician-dispensed skin care channel is underdeveloped, we look for alternative models to build a presence and brand awareness for our products. For example, in Japan we have pursued three separate distribution channels for our brand and product concepts. In 2002, we launched our first formal long-term relationship in Japan by entering into a trademark and know-how license agreement with Rohto Pharmaceutical Co., Ltd. (“Rohto”) to market and sell our Obagi-developed products in Japan. Rohto is a Japanese pharmaceutical manufacturer and distributor. Under our current agreement, Rohto is licensed to manufacture and sell a series of OTC products developed by it under the Obagi brand name, as well as Obagi-C products, in the Japanese drug store channel, for which it pays us a license fee. Rohto’s Obagi branded products achieved retail annual sales of approximately $75.0 million in 2010 through approximately 6,300 high-end drug and variety stores. In 2008, we expanded that relationship to provide for collaboration on the development of new products and to pursue the higher end department store channel in Japan. In addition, at the end of 2008 we entered into a License Distribution Agreement with Rohto for sales of products using our bi-mineral complex technology, for which we receive royalty payments on net sales. We have additional licensing arrangements in Japan to market and sell OTC product systems under the Obagi brand in the aesthetic spa channel, both for in-office use in facial procedures, as well as for sale as a take-home product kit. Separately, we have entered into a distribution agreement with a partner, who has exclusive rights to distribute the Obagi Systems in the physician-dispensed channel in Japan. Our strategic partners in Japan have engaged in aggressive direct-to-consumer advertising, which we believe has raised consumer demand in Japan, creating greater brand awareness in the physician channel to the benefit of our core prescription lines. During the year ended December 31, 2010, our distribution channels in Japan generated approximately $4.4 million in license fees.
We will continue to look for credible partners to address new geographies, and to evaluate alternative channel opportunities in other countries to drive brand awareness and accelerate overall market penetration.
Manufacturing
We believe our manufacturing processes provide us with a competitive advantage, which we have developed through years of experience formulating skin care products. We maintain manufacturing scalability and flexibility by maintaining manufacturing with several qualified independent third-party contract manufacturers. Our main contract manufacturers during 2010 included Arizona Natural Resources, Inc., Bay Cities Container Corporation, Denison Pharmaceuticals Inc., PureTek Corporation and Swiss-American Products
10
Inc., and we continue to explore and establish relationships with additional third-party manufacturers. However, we do not have long-term contracts with most of these parties. For all of our proprietary product concepts, we believe we own the related manufacturing processes, methods and formulations.
We use FDA-compliant manufacturers who specialize in the manufacture of prescription and OTC pharmaceutical and/or cosmetic products. These parties manufacture products pursuant to our specifications. All of these manufacturers are required by law and by our manufacturing standards to comply with current Good Manufacturing Practice (“cGMP”). We pre-qualify and continually monitor our manufacturers for quality and compliance. We also require documentation of compliance and quality from those manufacturers for whom we act as representative in connection with the promotion and sale of their products. For most of our key products, we have two or more qualified manufacturers. Although certain products, including our hydroquinone and some of our sun protection products, are currently supplied by a single source, we intend to qualify additional manufacturers for such products.
In addition, while physicians can and in many cases do write a prescription to be filled by a pharmacy for tretinoin for use in conjunction with our Obagi Nu-Derm and Condition & Enhance Systems or for metronidazole for use in conjunction with our Rosaclear System, we also purchase such products directly from third parties in order to provide physicians the option of dispensing these products directly from the office along with Obagi products. These products are currently purchased under exclusive product supply agreements with two manufacturers of tretinoin and one manufacturer of metronidazole that expire in 2014, 2014 and 2015, respectively. While there are several other manufacturers of generic tretinion and metronidazole, the termination of those agreements or any loss of services under those agreements could be difficult for us to replace.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
We have pursued an aggressive trademark registration policy as a means to achieve brand recognition and product differentiation in the market. We own various U.S. and foreign trademark registrations and applications and common law marks. In connection with developing new products and product applications, we have filed, or have obtained an exclusive license to, 37 U.S. provisional and non-provisional patent applications since 2004.
We have licensed certain patent applications owned by JR Chem LLC (“JR”) covering our ELASTIderm, CLENZIderm and Rosaclear Systems. We have also relied on services provided by JR in the development of new products to address acne, skin elasticity and facial redness, under a five-year contract. Although that contract expired in September 2010, we believe the license and royalty obligations of the parties under the contract survived termination. See “Certain Material Agreements – Jose Ramirez and JR” below. In addition, we have licensed the rights to four patents owned by Avon, one of which expired in 2008 while the remaining three expire between 2013 and 2018, covering methods and formulations for stabilizing Vitamin C in a serum for facial skin benefits, which is in our Obagi-C Rx C-Clarifying serum, Professional-C 5% serum, Professional-C 10% serum, Professional-C 15% serum and Professional-C 20% serum. We entered into the license agreement with Avon in June 2003, for an initial three-year term, which is renewed year to year thereafter, at our option, through the life of the last patent to expire. The last patent will expire in September 2018.
We have also acquired rights to market, distribute, sell and, in some cases, make products pursuant to license agreements with other third parties. Such agreements contain provisions that require us to meet certain specified requirements, such as quantity purchase and payment obligations, in order to maintain the rights granted under the agreements.
Certain Material Agreements
Triax
Under a product supply agreement with Triax Pharmaceuticals, LLC (“Triax”), originally entered into on December 8, 2005 and subsequently amended and restated on August 24, 2009, we have exclusive rights to sell certain tretinoin products in 0.1%, 0.05% and 0.025% concentrations and, at our request, any other concentrations for which Triax receives abbreviated new drug application (“ANDA”) approvals from the FDA, in the physician-dispensed skin care channel in the United States (including all territories of the United States). In addition, we have rights to sell such products on a non-exclusive basis outside of the United States. If we do not purchase a minimum number of units of products each year in any combination of concentrations, we will lose our exclusive selling rights in the United States. During the years ended December 31, 2010 and 2009, we met the minimum purchase requirements. Under the terms of the agreement, we are required to pay Triax a fixed price for the products, subject to volume discounts we may receive if we purchase a certain volume of products and those products are sold to our customers within a specified period of time. These volume
11
discounts are based on calendar year performance and applied to cumulative quarterly purchases. The initial term of the amended and restated agreement is five years, with an automatic renewal for a successive five-year term unless written notice is provided by either party at least 12 months before the end of the current term. Each party has the right to terminate the agreement upon prior written notice in the case of material breach and failure to take action to cure such a breach within a specified period. Each party also has the option to terminate the agreement by written notice if the other party ceases to carry on its business or becomes the subject of any proceeding under state or federal law for the relief of debtors or otherwise becomes insolvent, bankrupt or makes an assignment for the benefit of creditors, or upon the appointment of a receiver for the other party or the reorganization of the other party for the benefit of creditors.
Jose Ramirez and JR
Pursuant to a consultant services and confidentiality agreement with Jose Ramirez and JR (the “JR Agreement”), we hired JR to perform research and development activities including product formulation, product development and regulatory work, as detailed in various statements of work. We agreed to pay JR a minimum annual fee of $0.1 million per year for five years, commencing on January 1, 2005, plus reasonable and customary expenses incurred at our request, in connection with the provision of such services. We paid consulting fees and expenses totaling $0.9 million, $1.6 million and $1.6 million under the agreement for the years ended December 31, 2010, 2009 and 2008, respectively.
We had a right of first refusal for the exclusive license of any and all of JR’s inventions that he introduced to us related to skin healthcare that were developed or reduced to practice by JR during the term of the agreement, subject to certain exceptions. In the event that we exercised this right and obtained such an exclusive license, we agreed to pay a tiered royalty to JR. We are entitled, however, to credit against such royalty payments a portion of the expenses we have incurred to develop and commercialize any product subject to the exclusive license. We paid JR an aggregate of approximately $0.4 million, $0.3 million and $0.2 million in royalty fees under the agreement for the years ended December 31, 2010, 2009 and 2008, respectively. To the extent that we sublicense any of our rights under an exclusive license in exchange for only a license fee, we have agreed to pay JR a portion of any such license fee that we receive, subject to an annual cap and to our ability to credit against any amounts due to JR a portion of the research and development expenses we incurred in connection with the sublicense. Any such sublicense would be subject to JR’s prior approval, which approval may not be unreasonably withheld. We have negotiated a separate royalty arrangement for sales of our bi-mineral complex with Rohto. Pursuant to our arrangement with Rohto, in December 2008, we amended the JR Agreement to modify the royalty rates paid to JR based on Rohto’s sales of products containing the bi-mineral technology.
The term of the JR Agreement was for five years, which began in January 2005. Between January 1, 2010 and April 2010, at which time we reached what we believed was an agreement in principle for amending the JR Agreement, we continued to operate under the original terms of the JR Agreement. Although that amendment was never executed, the parties operated under the agreement, as amended, from June 2010 through September 2010. The JR Agreement terminated in September 2010 with respect to services, and accordingly our obligations to JR, except for certain royalty obligations, ceased at that time. However, many obligations of JR survived such termination in a manner in which we believe will not impact our rights to the technology.
We are currently in discussions with JR on a variety of matters. We have been advised by JR that it believes that certain of our products, including the ELASTIderm line of products, and CLENZIderm and Rosaclear, are covered by both issued and pending patents which JR contends are owned by it and which it believes were licensed to us under the JR Agreement. JR contends that such license and our right to continue to sell such products terminated when the JR Agreement terminated. We disagree with JR’s position on this issue and are seeking to resolve this and other issues in our ongoing negotiations with JR. Should we fail to reach agreement with respect to these current discussions, we could end up in litigation with JR regarding the ownership rights to these technologies, and our business, results of operations and financial condition could be adversely affected. See “Risk Factors – Risks Related to Intellectual Property - If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights and - We and our manufacturers and suppliers license certain technologies and patents from third parties. If these licenses are breached, terminated or disputed, our ability to commercialize products dependent on these technologies and patents may be compromised” in Part I, Item 1A of this Report.
Avon
Under a license agreement with Avon, dated June 26, 2003, we have an exclusive worldwide license to manufacture and sell skin care products containing an ascorbic acid component directly to physicians and medical spas. Under the terms of the agreement, we also have a non-exclusive license to manufacture and sell such products directly to drug stores outside of the United States. We paid Avon a non-refundable license issue fee. Additionally, we pay a non-refundable annual renewal fee. The original term of the agreement expired in 2006 and we have continued to annually renew the agreement each year since that time. Our annual option to renew the agreement survives until the last of the licensed patents expires on September 10, 2018. Each party, at its option, may
12
terminate the agreement upon prior written notice in the case of default in the performance of any obligation under the agreement by the other party and failure to take action to cure such a default within a specified period. If we become bankrupt or insolvent, or file a petition for bankruptcy, or if our business is placed in the hands of a receiver, assignee or trustee from which we cannot extract ourselves within 120 days, or if we are liquidated or substantially all of our assets or shares are sold, exchanged or transferred, or in the event of a merger or consolidation to which we are a party and to which Avon reasonably objects, the agreement shall immediately terminate without notice.
Rohto
On September 13, 2002, we entered into a strategic licensing agreement with Rohto, a Japanese pharmaceutical manufacturer and distributor. Under the agreement, Rohto is licensed to manufacture and sell a series of OTC products developed by it under the Obagi brand name, as well as Obagi-C products, in the Japanese drug and variety store channels and we receive a royalty based upon sales of Obagi branded products in Japan by Rohto.
On December 4, 2008, we entered into an amendment (the “Amendment”) to the original agreement with Rohto. The Amendment extends the term of agreement to December 4, 2017 and permits extension of the Territory (as defined) to areas outside Japan, subject to separate written agreement(s). The Channel (as defined) has been expanded to include department stores if certain conditions are met. To date, these conditions have not been met. Rohto and Obagi have also agreed to work together to up-brand the Obagi products sold in the various channels and work cooperatively on messaging, branding and product imaging. Under the Amendment, Obagi has also agreed to provide an exclusive royalty-bearing license to sell and/or manufacture certain products developed by Obagi, Rohto or jointly developed by Obagi and Rohto.
On December 4, 2008, we entered into a license and supply agreement with Rohto (the “Bi-mineral Agreement”) whereby we granted Rohto an exclusive right to manufacture, market and sell our bi-mineral collagen and elastin enhancing products in all channels (other than the aesthetic and spa channels) in Japan. Rohto also has the right to develop improvements to such products or new products related to the products. The initial term of the Bi-mineral Agreement is for five years, through December 4, 2013, with an optional five-year extension. The Bi-mineral Agreement calls for certain annual sales volume and expense commitments on behalf of Rohto. If such commitments are not met, we have the right to terminate such agreement or render it non-exclusive. As consideration for the exclusive license, Rohto will pay a development fee to us in equal installments over a five-year period as well as quarterly royalty fees based on product sales. If the Bi-Mineral Agreement should be terminated by either party before all five installments of the development fee have been paid or in the event of early termination, then any unpaid installments will become due and payable to us ten days before the effective termination date. The royalty rate is scaled over the term of the agreement.
DDN
On July 1, 2009, we entered into a services agreement with DDN/Obergfel LLC (“DDN”) under which DDN has agreed to serve as our exclusive third-party logistics provider for Obagi products sold or distributed in states east of the Mississippi in the United States. Under the agreement, DDN has agreed to receive, warehouse and ship on our behalf any Obagi products sold in such territories and to provide standard order fulfillment and other shipping information related to those products. In return, we have agreed to pay DDN specified warehousing and distribution fees and, if we do not meet certain minimum billing requirements, an additional management fee. We have also agreed to pay specified additional fees for certain order adjustments, processing and handling of returned goods, access to DDN’s information systems and account management services. The agreement has an initial term of 36 months, and will automatically renew for successive 12-month terms unless written notice is provided by either party a specified period of time before the end of the current term. Each party has the right to terminate the agreement upon prior written notice in the case of a breach of the agreement by the other party and failure to cure such a breach within a specified period. In addition, we may terminate the agreement at any time upon prior written notice to DDN and payment of an early termination fee. In the event that DDN raises its fee rates more than a certain amount, then we may terminate the agreement without payment of any early termination fee upon written notice to DDN provided within a specified period after receiving notice of the fee rate adjustment.
Competition
The market for skin health and restoration is highly competitive with many established manufacturers, suppliers and distributors engaged in all phases of the business. We believe that we face strong competition. Competitive factors in our market include:
|
•
|
product efficacy and uniqueness;
|
|
•
|
brand awareness and recognition;
|
13
|
•
|
product quality, reliability of performance and convenience of use;
|
|
•
|
cost-effectiveness;
|
|
•
|
breadth of product offerings;
|
|
•
|
sales and marketing capabilities and methods of distribution;
|
|
•
|
resources devoted to product education and technical support; and
|
|
•
|
speed of introducing new competitive products and existing product upgrades.
|
We face and will continue to face intense competition. A number of our competitors have greater research and development and marketing capabilities, more diverse distribution channels and greater financial resources than we do. These competitors may have developed, or could in the future develop, new technologies that compete with our products or render our products obsolete. We are also likely to encounter increased competition as we enter new markets and as we attempt to further penetrate existing markets. Some of our competitors have in the past and may in the future compete by lowering prices on their products. We may respond by lowering our prices, exiting the market, expanding into other markets, or investing in the development of new, improved products.
We believe our direct competitors in the physician-dispensed skin care channel include BioMedic from La-Roche Posay, SkinMedica, Inc., Kinerase from Valeant Pharmaceuticals International, various products from SkinCeuticals, a division of L’Oreal S.A., Allergan, Inc., Vitamin C and various products from IS Clinical and Neova from PhotoMedex, Inc.
We believe our indirect competitors that generally sell skin care products directly to consumers consist primarily of large cosmetic companies, including, but not limited to The Estee Lauder Companies Inc., Helene Curtis Industries, Inc., L’Oreal S.A., Matrix Essentials, Inc., a division of L’Oreal, Procter & Gamble Company, Neutrogena, a division of Johnson & Johnson, Revlon, Inc. and Unilever N.V.
Our acne products compete with Triaz from Medicis Pharmaceutical Corporation, Benzaclin from Dermik Laboratories, Inc., Brevoxyl and Duac from Stiefel Laboratories Inc., and Benzac from Galderma Laboratories, L.P. Our acne products also indirectly compete with OTC anti-acne products.
Our elasticity products have demonstrated clinical evidence of aiding the restoration of elasticity and mature elastin in aged skin. We currently know of no other products that have clinical evidence to support this claim. However, there are several products that claim to enhance elastin.
Our products also compete with current and future medical devices, such as lasers, which are or will be positioned for a variety of skin enhancements such as facial rejuvenation, dermal thickening, acne and other uses. Competitors in this market include Candela Corp., CoolTouch Corporation, Cutera, Inc., Cynosure, Inc., Lumenis Ltd., Reliant Technologies, Inc., Syneron Medical Ltd. and Thermage, Inc. However, we believe, when used as a pre/post treatment, our Obagi Systems are complementary to many of these procedures.
Government Regulation
Federal, state and local governmental authorities in the United States and other countries regulate, among other things, the testing, production, distribution, advertising promotion and sale of prescription and OTC drugs and cosmetics. In the United States, the FDA, acting under the Food Drug and Cosmetic Act (“FDCA”), and other federal statutes (including the Public Health Service Act, Administrative Procedure Act, Federal Trade Commission Act, Fair Packaging and Labeling Act, Lanham Act and Homeland Security Act) and agencies implementing regulations, regulate our products primarily on the basis of their intended use, as determined by the labeling claims or other representations made for the product.
FDA regulation of cosmetics
The FDCA defines cosmetics as products and their components intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance. Cosmetic products are not subject to FDA pre-market approval authority, although the FDA can take enforcement action under the Code of Federal Regulations (“CFR”) for marketed cosmetic products that are adulterated or misbranded, including violations of product safety requirements, use and quantity of ingredients, labeling and promotion and methods of manufacture. Additionally, the FDA monitors compliance of cosmetic products through random inspections of cosmetic company websites, importers, manufacturers and distributors. The labeling of cosmetic products is subject to the requirements of the FDCA, the Fair Packaging and Labeling Act and other FDA regulations.
14
We believe that many of the products in the Obagi Nu-Derm, Condition & Enhance, Obagi-C Rx, Professional-C, ELASTIderm, CLENZIderm M.D. and Obagi Rosaclear Systems and other product lines, as labeled and intended for use, fall within the FDA definition of cosmetics and therefore do not require pre-market review and approval. Cosmetics may be sold both OTC and through a physician’s office, subject to state laws governing the commercial practices of physicians.
FDA regulation of drug products
The FDCA defines drugs as products intended to cure, mitigate, treat or prevent a disease or to affect the structure or any function of the human body. Drug products are subject to more comprehensive safety and efficacy requirements of the FDCA and its implementing regulations. In general, products falling within the FDCA’s definition of “new drugs” require pre-marketing clearance by the FDA. Products falling within the FDCA’s definition of “drugs” that are not “new drugs” because they are generally recognized as “safe and effective” for the indication for which they are being marketed and have been used to a material extent and for a material time under those conditions generally do not require pre-market review and approval from the FDA. In addition, certain other drugs that have been in existence for a specified period of time are not currently subject to FDA pre-market review and approval. The official legal status of many of these drug products has often not been determined or finalized. Others are marketed under the FDA's regulatory discretion. Such drug products are commonly commercialized under the FDA Compliance Policy Guide (“CPG”) entitled “Marketed New Drugs Without Approved New Drug Applications” and are subject to compliance with FDA regulations concerning manufacture, labeling, distribution, and recordkeeping for drug products.
Brief History of the Development of the FDCA
The original Federal Food and Drugs Act was adopted in 1906 and prohibited the sale of adulterated or misbranded drugs, but did not require that drugs be approved by the FDA prior to marketing. In 1938, Congress enacted the FDCA, which required that all “new drugs” be approved for safety through a New Drug Application (“NDA”). Drugs that were on the market prior to the enactment of the FDCA were exempt from “new drug” status under a grandfather clause, and did not require an NDA. If a new drug was approved by the FDA between 1938 and 1962, the FDA generally allowed identical, related or similar (“IRS”) drugs to be marketed without independent approval on the grounds that the active ingredient(s) was generally recognized as safe. In 1962, Congress amended the FDCA to require that all “new drugs” be demonstrated to be effective as well as safe to obtain FDA approval. However, under a grandfather clause in the 1962 amendment, a drug was exempt from the effectiveness requirement if, before the amendment was enacted, it was: (i) used or sold commercially in the United States; (ii) not considered a “new drug;” and (iii) not covered by an effective application.
The Drug Efficacy Study Implementation (“DESI”) was a program begun by FDA in 1962 after the amendments to the FDCA were adopted. The DESI program was intended to classify all pre-1962 drugs that were already on the market and approved based on safety only as either effective, ineffective, or needing further study. The DESI program evaluated over 3,000 separate products and over 16,000 therapeutic claims. By 1984, final action had been completed on 3,443 products; of these, 2,225 were found to be effective, 1,051 were found not effective, and 167 were pending. The DESI program remains uncompleted because the FDA turned its regulatory priorities to more pressing regulatory matters, (the OTC Drug Review (the "OTC Review") in the 1970's, the generic drug scandal in the late 1980's, the so-called drug lag in the 1990's, and the backlog in applications for generic drugs since the turn of the century with the need for increased inspections for raw material suppliers and applicants). The interactions of these mixed programs and priorities have created gray legal areas for drug products that have been marketed for decades in accordance with the FDA's CPG.
Drugs that did not have pre-1962 approvals or that were not IRS to drugs with pre-1962 approvals were not subject to DESI. Some of these products are not formally exempt from the FDA pre-marketing “new drug” approval today, but may continue to be marketed and sold under a CPG that allows certain drug products to continue to be sold until the FDA determines that a NDA must be filed or otherwise concludes that such product is unsafe or ineffective.
FDA regulation of “new drug” products
Today, the steps required before a “new drug” may be marketed in the United States include: (i) pre-clinical laboratory and animal testing; (ii) submission to the FDA of an Investigational New Drug (“IND”), application, which must become effective before clinical trials may commence; (iii) adequate and well controlled clinical trials to establish the safety and efficacy of the drug; (iv) submission to the FDA of a NDA; and (v) FDA approval of the NDA prior to any commercial sale or shipment of the drug. An ANDA is required to be submitted and approved when the drug product intended for commercialization is bioequivalent to an already approved NDA product; an ANDA application does not need to repeat the clinical trials that were required for approval of the NDA, or reference listed drug. In addition to obtaining FDA approval for each product, each domestic drug-manufacturing establishment must be registered with the FDA. Drug product manufacturing establishments located in California also must be licensed by the State of California in compliance with separate regulatory requirements.
Pre-clinical testing is generally conducted on laboratory animals to evaluate the potential safety and the efficacy of a drug. The results of these studies are submitted to the FDA as a part of an IND, which must be approved before clinical trials in humans can begin. Typically, clinical evaluation involves a time consuming and costly three-phase process. In Phase I, clinical trials are conducted with a small number of subjects to determine the early safety profile, the pattern of drug distribution and metabolism. In Phase II, clinical trials are conducted with groups of patients afflicted with a specific disease to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In Phase III, large-scale, multi-center, comparative trials are conducted with patients afflicted with a target disease to provide sufficient data to demonstrate the efficacy and safety required by the FDA. The FDA closely
15
monitors the progress of each of the three phases of clinical trials and may, at its discretion, re-evaluate, alter, suspend or terminate the testing based upon the data that have been accumulated to that point and its assessment of the risk/benefit ratio to the patient.
We in-license three ANDA products, tretinoin, Refissa and metronidazole, all prescription drug product offerings. Each supplier holds FDA approval of ANDAs for its respective products.
FDA Enforcement Discretion for Marketed Unapproved Drug Products
Products falling within the FDCA’s definition of “drugs” that are not “new drugs” and that are generally recognized as “safe and effective” for the indication for which they are being marketed or that fall within the 1938 or 1962 grandfather clauses or within the CPG have not been required to obtain pre-market review and approval from the FDA.
We market a number of products containing 4% hydroquinone that we believe are not “new drug” products subject to FDA pre-market approval and that are governed by the CPG. The Obagi Nu-Derm Clear, Blender and Sunfader products and the Obagi-C Rx C-Clarifying Serum, C-Night Therapy and ELASTIderm Décolletage Skin Lightening Complex products contain the active ingredient hydroquinone at a 4% concentration and are marketed as prescription drugs under the FDA CPG entitled “Marketed New Drugs Without Approved NDAs.” These hydroquinone products must be administered under the supervision of a physician but are not currently subject to prior FDA approval when formulated and labeled in accordance with guidelines for prescriber information under direction of a physician.
Hydroquinone, as a skin bleaching agent has been subject to FDA regulation as an OTC drug since the initiation of the OTC Review in the early 1970's. The OTC Review was created to replicate the DESI Review by evaluating the safety and effectiveness of the data available for the active ingredients (drugs) in the hundreds of thousands of OTC drug products that were on the market. Drugs that are subject to the OTC Review are and have been in a regulatory safe harbor for the uses covered by that multi-step process pending completion of the monograph applicable to the intended use under the OTC Review and the CPG.
To date, the FDA has not required NDA or other approval for the continued marketing of hydroquinone. In August 2006, the FDA issued a proposed rule that stated that OTC skin bleaching products containing hydroquinone and currently marketed outside the physician channel at 2% concentrations or less, were not generally recognized as safe and effective, were misbranded, and are new drugs within the meaning of the FDCA. The FDA proposed that because of the carcinogenic and ochronosis potential of hydroquinone, its use in skin bleaching drug products should be restricted to prescription use only, and users of such products should be closely monitored under medical supervision. The FDA also withdrew a tentative proposed monograph that concluded that hydroquinone products with concentrations of 2% or less were generally recognized as safe and effective and stated its intent to require NDA approval for continued marketing of prescription hydroquinone products at the time of publication of the final rule. In the proposed rule, the FDA recommended that additional studies be conducted to determine if there is a risk to humans from the use of hydroquinone. The FDA nominated hydroquinone for further study by the National Toxicology Program (the “NTP”), and in December 2009 the NTP Board of Scientific Counselors approved the nomination. As a result, the following three NTP studies will be conducted:
|
•
|
Comparative metabolism studies in rats and mice by oral and dermal routes;
|
|
•
|
Reproductive toxicity study in rats and mice by oral route; and
|
|
•
|
Dermal carcinogenicity studies of hydroquinone in mice and rats.
|
There are several more steps to the nomination process before the studies will commence, and the studies themselves can take two years or more to complete. The FDA has noted that, in the interim, it believes that hydroquinone at concentrations of 2% or less should remain available as an OTC drug product. At the conclusion of the NTP studies, if the FDA does change the status of 4% hydroquinone products to require a NDA, and if the FDA does not allow us to continue to market our products while we attempt to comply with such new requirement as imposed, or if the FDA does not approve a NDA filed by us or a third party for 4% hydroquinone products, then we will no longer be able to rely on the CPG to market our Obagi Nu-Derm, Obagi-C Rx and Décolletage Systems containing 4% hydroquinone. FDA approval of a 4% hydroquinone product under a NDA filed by another party may also adversely affect our ability to continue to market our products under the CPG for a period of time. Furthermore, if the NTC studies conclude that hydroquinone is a carcinogen, we may be required to cease sales of all our products that contain hydroquinone. Should one of our contract manufacturers or any of its ingredient suppliers develop cGMP issues, the FDA could also conclude that our products no longer fall within the CPG or OTC Review safe harbor. See “Risk Factors - Risks Related to Regulatory Matters,” in Part I, Item 1A of this Report.
The OTC Monograph System
While FDA approval is generally required before a new drug product may be marketed in the U.S., many OTC drugs are exempt from the FDA’s pre-marketing approval requirements. In 1972, the FDA instituted the ongoing OTC Drug Review to evaluate the safety and effectiveness of OTC drug ingredients in the market. Through this process, the FDA issues monographs for therapeutic product categories that set forth the specific active ingredients, dosages, strengths, indications for use, warnings and labeling statements for OTC drug ingredients that the FDA will consider generally recognized as safe and effective for OTC use and therefore not subject to pre-market approval.
For most categories of OTC drugs not yet subject to a final monograph, the FDA usually permits such drugs to continue to be marketed until a final monograph becomes effective, unless the drug will pose a potential health hazard to consumers. The FDA’s
16
policy also generally applies to prescription drugs containing the same active ingredients as a marketed OTC product for the same or similar uses as the OTC product.
Drugs subject to final monographs, as well as drugs that are subject only to proposed monographs, are subject to various FDA regulations concerning, for example, manufacturing in accordance with cGMPs, general and specific labeling requirements and prohibitions against promotion for conditions other than those stated in the labeling. Drug manufacturing facilities are subject to FDA inspection, and failure to comply with applicable regulatory requirements may lead to administrative or judicially imposed penalties. State agencies also may require registration, licensure and inspection of manufacturing or distribution facilities and may require reporting of promotional activities.
We market a number of OTC products under the following monographs: Sunscreen, Acne, Skin Bleaching, Skin Protectant, External Analgesic and Topical Antibiotic. These products are regulated by the FDA as OTC drugs subject to a FDA final monograph but do not require FDA pre-market review or approval.
FDA Regulation of Our Products and Product Candidates
The following table summarizes the current status of the active ingredients in, FDA pre-marketing approval (if any) required for, FDA regulatory category of, and launch date for, our material products and product candidates:
|
Product
|
Main Functioning or Active Ingredients(1)
|
FDA Pre-Marketing Approval Required
|
Product Status(2)
|
Date Launched
|
|
Obagi Nu-Derm Gentle Cleanser(3)
|
Mild Cleansers
|
No
|
Cosmetic
|
1988
|
|
Obagi Nu-Derm Foaming Gel(3)
|
Mild Cleansers
|
No
|
Cosmetic
|
1988
|
|
Obagi Nu-Derm Toner(3)
|
Hamamelis Virginiana (Witch Hazel) Distillate
|
No
|
Cosmetic
|
1988
|
|
Obagi Nu-Derm Clear(3)
|
Hydroquinone 4%
|
No
|
DESI II
|
1988
|
|
Obagi Nu-Derm Exfoderm(3)
|
Phytic Acid
|
No
|
Cosmetic
|
1988
|
|
Obagi Nu-Derm Exfoderm Forte(3)
|
Glycolic Acid, Lactic Acid
|
No
|
Cosmetic
|
1988
|
|
Obagi Nu-Derm Blender(3)
|
Hydroquinone 4%
|
No
|
DESI II
|
1988
|
|
Obagi Nu-Derm Sun Block SPF 32(3)
|
Zinc Oxide 18.5%
|
No
|
OTC
|
2004
|
|
Obagi Nu-Derm HSP SPF 35(3)
|
Octyl Methoxycinnamate 7.5%, Zinc Oxide 9.0%
|
No
|
OTC
|
2002
|
|
Obagi Nu-Derm Sunfader
|
Hydroquinone 4% Octyl Methoxycinnamate 7.5%, Oxybenzone 5.5%
|
No
|
DESI II/OTC
|
1984
|
|
Obagi Nu-Derm Skin Balancing Toner
|
Hamamelis Virginiana (Witch Hazel) Distillate
|
No
|
Cosmetic
|
2009
|
|
Obagi Nu-Derm Blend Fx
|
Arbutin
|
No
|
Cosmetic
|
2009
|
|
Obagi Nu-Derm Clear Fx
|
Arbutin
|
No
|
Cosmetic
|
2009
|
|
Obagi Nu-Derm Eye Cream
|
Mild Moisturizers
|
No
|
Cosmetic
|
1984
|
|
Obagi Nu-Derm Tolereen
|
Hydrocortisone 0.5%
|
No
|
OTC
|
1988
|
|
Obagi Nu-Derm Sun Shield
|
Zinc Oxide 10.5%, Octin Oxate 7.5%
|
No
|
OTC
|
2011
|
|
Obagi-C Rx C-Cleansing Gel
|
Mild Cleansers
|
No
|
Cosmetic
|
2004
|
|
Obagi-C Rx C-Exfoliating Day Lotion
|
Glycolic Acid
|
No
|
Cosmetic
|
2004
|
|
Obagi-C Rx C-Clarifying Serum
|
Hydroquinone 4%
|
No
|
DESI II
|
2004
|
|
Obagi-C Rx C-Therapy Night Cream
|
Hydroquinone 4%
|
No
|
DESI II
|
2004
|
|
Obagi-C Rx C-Sunguard SPF 30
|
Octyl Methoxycinnamate 7.5%, Zinc Oxide 9.0%
|
No
|
OTC
|
2004
|
|
Obagi-C Rx System C-Balancing Toner
|
Hamamelis Virginiana (Witch Hazel) Distillate
|
No
|
Cosmetic
|
2010
|
|
Obagi-C Rx System C-Clarifying Serum Normal to Oily skin
|
Hydroquinone
|
No
|
DESI II
|
2009
|
17
|
Product
|
Main Functioning or Active Ingredients(1) |
FDA Pre-Marketing Approval Required
|
Product Status(2)
|
Date Launched
|
|
Obagi Professional-C Serum
|
L Ascorbic Acid (Vitamin C) 5% to 20% concentrations
|
No
|
Cosmetic
|
2005
|
|
Obagi Tretinoin(4)
|
Tretinoin 0.025%, 0.05%, and 0.1%
|
Yes
|
ANDA
|
2006
|
|
Refissa Tretinoin
|
Tretinoin 0.05%
|
Yes
|
ANDA
|
2009
|
|
Obagi CLENZIderm M.D. Daily Care Foaming Cleanser
|
Salicylic Acid 2%
|
No
|
OTC
|
2007
|
|
Obagi CLENZIderm M.D. Pore Therapy
|
Salicylic Acid 2%
|
No
|
OTC
|
2007
|
|
Obagi CLENZIderm M.D. Serum Gel
|
Benzoyl Peroxide 5%
|
No
|
OTC
|
2007
|
|
Obagi ELASTIderm Eye Cream
|
Mineral complexes
|
No
|
Cosmetic
|
2006
|
|
Obagi ELASTIderm Eye Gel
|
Mineral complexes
|
No
|
Cosmetic
|
2007
|
|
Obagi ELASTIderm Décolletage Fx Wrinkle Reducing lotion
|
Mineral complexes
|
No
|
Cosmetic
|
2009
|
|
Obagi ELASTIderm Décolletage Fx Skin Lightening Complex
|
Arbutin
|
No
|
Cosmetic
|
2009
|
|
Obagi ELASTILash Eyelash Solution
|
Peptide
|
No
|
Cosmetic
|
2010
|
|
Obagi CLENZIderm M.D. Therapeutic Lotion
|
Benzoyl Peroxide 5%
|
No
|
OTC
|
2007
|
|
Obagi CLENZIderm M.D. Therapeutic
|
Glyceryn 20% Dimethieme 1%
|
No
|
OTC
|
2007
|
|
Obagi CLENZIderm M.D. Daily Care Cream Cleanser
|
Mild Cleansers
|
No
|
Cosmetic
|
2007
|
|
Obagi Décolletage Chest and Neck Akin Lightening Complex
|
Hydroquinone 4%
|
No
|
DESI II
|
2008
|
|
Obagi Décolletage Chest and Neck Wrinkle Reducing Lotion
|
Mineral complexes
|
No
|
Cosmetic
|
2008
|
|
Obagi Rosaclear Metronidazole(5)
|
Metronidazole Topical Gel USP, 0.75%
|
Yes
|
ANDA
|
2009
|
|
Obagi Rosaclear Hydrating Complexion Corrector
|
Titanium Dioxide
|
No
|
Cosmetic
|
2009
|
|
Obagi Rosaclear Gentle Cleanser
|
Mild Cleansers
|
No
|
Cosmetic
|
2009
|
|
Obagi Rosaclear Skin Balancing Sun Protection SPF 30
|
Zinc Oxide 15.5%, Titanium Dioxide 2.0%
|
No
|
OTC
|
2009
|
|
Blue Peel RADIANCE
|
Salicylic acid
|
No
|
Cosmetic
|
2011
|
(1) By definition, products classified as cosmetics do not have active ingredients. For cosmetics, the main functioning ingredient is listed.
(2) Product Status Definitions: OTC, or over-the-counter, products are defined as products that are considered drugs by the FDA, but that do not require physician prescription or oversight.
DESI II products are defined as products that are considered drugs that require physician prescription, that have not been the subject of a NDA and that have not been through the DESI process. A company that first introduced a non-preapproved drug product that was on the market prior to November 13, 1984 must ensure that its basic features are the same as that used for a product that was on the market prior to November 13, 1984. If so, the FDA will generally defer enforcement action, provided that it does not present a safety problem or trigger one of the CPG’s exceptions; each of our products are the chemical equivalent to a product first introduced in 1969 and is not the subject of any CPG exception.
ANDA products are defined as drugs that have received FDA approval under an abbreviated new drug application; our three ANDA products require physician prescription.
Cosmetic products are defined as products that are not considered drugs by the FDA, are not allowed to make drug claims, and do not require FDA pre-marketing approval or physician prescription or oversight.
(3) These products are identical to the products included in our Obagi Condition & Enhance System, which was launched in 2006.
18
(4) Obagi Nu-Derm branded tretinoin is manufactured by Triax under its ANDAs, and was launched under the Obagi brand in 2006; however, we have been selling tretinoin supplied by Triax since 2003, for which we do not make any drug claims. We also began offering Refissa, a different formulation of tretinoin, manufactured by Spear, under its ANDA, in September 2009, for which we can make on-label drug claims for the treatment of fine lines, hyperpigmentation and tactile roughness consistent with the Spear ANDA.
(5) Obagi Rosaclear branded metronidazole is manufactured by Tolmar under its ANDA and was launched under the Obagi brand in 2009.
FDA Regulation of Drug Manufacturing
We and the third-party manufacturers on which we rely for the manufacture of our products are subject to requirements under 21CFR parts 210 and 211 that drugs be manufactured, packaged and labeled in conformity with cGMPs. To comply with cGMPs, manufacturers must continue to spend time, money and effort to meet requirements relating to personnel, facilities, equipment, production and process, labeling and packaging, quality control, recordkeeping and other requirements. The FDA, state agencies and foreign Ministries of Health periodically inspect drug manufacturing facilities to evaluate compliance with cGMPs.
Federal Regulation of Advertising and Promotion
The FDA regulates the advertisement of prescription drug products. The U.S. Federal Trade Commission (“FTC”), Fair Packaging and Labeling Act, and state authorities regulate the advertising of OTC drugs and cosmetics, as well as exercise general authority to prevent unfair or deceptive trade practices.
In addition to FDA restrictions on marketing of prescription products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn are used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services, reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer.
Also, as part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. This practice is overseen by the FDA, state and other governmental authorities under the Prescription Drug Marketing Act and regulations that include requirements concerning record keeping and control procedures.
Many states have also recently begun regulating the promotion of prescription drug products and require compliance with annual certification and disclosure requirements regarding our policies for drug promotion and the amount of money we spend per prescribing physician on drug promotion. Compliance with changing federal and state laws and regulations on prescription product promotion requires a great deal of time and effort.
Other Government Regulation
We and our suppliers or third-party manufacturers may also be subject to regulations under other federal, state, and local laws, including the Occupational Safety and Health Act, the Environmental Protection Act, the Clean Air Act and import, export and customs regulations as well as the laws and regulations of other countries.
19
Employees
As of December 31, 2010, we had 203 employees. Our employees include 145 in sales and marketing, 29 in product development, manufacturing and distribution and 29 in administrative functions. Our employees are all non-unionized, and we believe our relations with our employees are good.
An investment in our common stock involves a high degree of risk. You should consider carefully the following risks and other information contained in this Report before you decide whether to buy, hold or sell our common stock. If any of the events contemplated by the following discussion of risks, or any additional risk not presently known to us or that we currently deem immaterial, should occur, our business, results of operations and financial condition could suffer significantly. As a result, the market price of our common stock could decline, and you may lose all or part of the money you paid to buy our common stock.
Risks Related to Our Business
Our sales and profitability may suffer if ongoing economic uncertainties in any of our major markets inhibit people from spending their disposable income on aesthetic and skin health products.
Virtually all of our products are purchased based on consumer choice due to the fact that all of our products are considered cosmetic in nature. As a result, they are typically paid directly by the patient out of disposable income and are not subject to reimbursement by third-party payors such as health insurance organizations. Adverse changes in the economy, such as the recent recession or ongoing economic uncertainties could accordingly have a significant negative effect on consumer spending for these products. If consumers reassess their spending choices, the demand for these products could decline significantly. If demand declines significantly in our major markets, such as North America, Asia and the Middle East, it would have a material adverse effect on our sales and profitability and could lead to a decline in our stock price.
Furthermore, if we are unable to successfully sell our products against competitive products or if consumer preferences in the marketplace shift to less costly alternative treatments, we may experience a decline in demand for our products, which could also have a material adverse effect on our sales and profitability.
Recent economic trends could adversely affect our financial performance and stock price.
As widely reported, the global financial markets experienced extreme disruption in 2008 and the majority of 2009, including severely diminished liquidity and credit availability. During the year ended December 31, 2009, we saw these unprecedented global economic conditions have a negative impact on our revenue growth performance. Although we witnessed some modest signs of economic recovery in 2010, it is unclear whether the economy will show sustained growth and/or stability. Accordingly, we cannot assure you that the improvement in revenue growth that we experienced in 2010 will be sustainable. While adverse economic conditions have not materially impacted our financial position as of December 31, 2010 or liquidity for the year ended December 31, 2010, we cannot predict the timing, strength or duration of any economic recovery, and our financial condition could be negatively impacted in the future if the economy fails to sustain its recovery or if there are new events that contribute to additional deterioration in financial markets and major global economies. The recent recession also resulted in increased downward pressure on stock prices generally, including our own, which is unrelated to financial performance.
Our revenues and financial results depend significantly on sales of our Obagi Nu-Derm System. If we are unable to manufacture or sell the Nu-Derm System in sufficient quantities and in a timely manner, or maintain physician and/or patient acceptance of the Nu-Derm System, our business will be materially and adversely impacted.
To date, the majority of our revenues have resulted from sales of our principal product line, the Obagi Nu-Derm System and related products. The Nu-Derm System and related products accounted for approximately 52%, 54% and 56% of our net sales for the years ended December 31, 2010, 2009 and 2008, respectively. Although we currently offer other products such as Obagi-C Rx, Professional-C, ELASTIderm, CLENZIderm, Rosaclear, ELASTILash and Blue Peel RADIANCE, and we intend to introduce additional new products, we still expect sales of our Obagi Nu-Derm System and related products to account for a majority of our sales for the foreseeable future. Because our business is highly dependent on our Obagi Nu-Derm System and related products, factors adversely affecting the pricing of, or demand for, these products could have a material and adverse effect on our business. Sales of our Obagi Nu-Derm Systems also experience seasonality. We believe this is due to variability in patient compliance that relates to several factors such as a tendency to travel and/or engage in other disruptive activities during the summer months. Additionally, our commercial success depends in large part on our ability to sustain market acceptance of the Nu-Derm System. If existing users of our products determine that our products do not satisfy their requirements, or if our competitors develop a product that is perceived by patients or physicians to better satisfy their respective requirements, sales of the Nu-Derm System and related products may decline, and
20
our total net sales may correspondingly decline. We cannot assure you that we will be able to continue to manufacture these products in commercial quantities at acceptable costs. Our inability to do so would adversely affect our operating results and cause our business to suffer.
We face intense competition, in some cases from companies that have significantly greater resources than we do, which could limit our ability to generate sales and/or render our products obsolete.
The market for aesthetic and therapeutic skin health products is highly competitive and we expect the intensity of competition to increase in the future. We also expect to encounter increased competition as we enter new markets and as we attempt to penetrate existing markets with new products. We may not be able to compete effectively in these markets, may face significant pricing pressure from our competitors and may lose market share to our competitors. Our principal competitors are large, well established companies in the fields of pharmaceuticals, cosmetics, medical devices and health care. Our largest direct competitors generally include La-Roche Posay, SkinMedica, Inc., Valeant Pharmaceuticals International, SkinCeuticals, a division of L’Oreal S.A., Allergan, Inc., IS Clinical and PhotoMedex, Inc.
Our indirect competitors, who generally sell skin care products directly to consumers, generally consist of large well known cosmetic companies, including but not limited to, The Estee Lauder Companies Inc., Helene Curtis Industries, Inc., L’Oreal S.A., Matrix Essentials, Inc., a division of L’Oreal S.A, Procter & Gamble Company, Neutrogena, a division of Johnson & Johnson, Revlon, Inc. and Unilever N.V. We also face competition from medical device companies offering products to physicians that are used to enhance the skin’s appearance.
Many of these competitors have greater resources than we have. This enables them, among other things, to make greater research and development investments and spread their research and development costs, as well as their marketing and promotion costs, over a broader revenue base. It is also possible that developments by our competitors could make our products or technologies less competitive or obsolete. The treatment of skin conditions and the enhancement of the appearance of skin, which is what all of our products target, are the subjects of active research and development by many potential competitors, including major pharmaceutical companies and specialized biotechnology firms, such as those listed above, as well as universities and other research institutions. Competitive advances may also include the potential development of new laser or radio frequency therapies to treat hyperpigmentation and photodamaged skin. While we intend to expand our technological capabilities to remain competitive, research and development by others may result in the introduction of new products by competitors that represent substantial improvements over our existing products. If that occurs, sales of our existing products could decline rapidly. Similarly, if we fail to make sufficient investments in research and development programs, our current and planned products could be surpassed by more effective or advanced products developed by our competitors.
We may not be able to successfully expand the use of our current product lines or develop new products.
We are working to improve, extend and reformulate many of our existing products. Continued market acceptance of our products will depend on our ability to successfully develop additional applications of our existing products. The development of additional applications will require significant commitments of personnel and financial resources and we cannot assure you that we will be successful. If the attempted extensions of our product lines are not commercially successful, our business will be adversely affected.
We are also developing new product lines by applying our Penetrating Therapeutics technology to new agents. In addition, we have acquired rights to technologies described in certain patent applications relating to additional methods and formulations. New products, in various stages of development, include other applications for our acne and skin elasticity products and other new systems. These development activities, as well as clinical studies, which must be completed before these products can be marketed and sold, will require significant commitments of personnel and financial resources. We cannot assure you that we will be able to develop new products or technologies in a timely manner, or at all. Delays in the development or testing processes will cause a corresponding delay in revenue generation from those products. Regardless of whether such new products or technologies are ever released to the market, the expense of such processes, which may be considerable, will have already been incurred and we may not be able to recover such expenses.
We reevaluate our development efforts regularly to assess whether our efforts to develop a particular new product or technology are progressing at a rate that justifies our continued expenditures. On the basis of these reevaluations, we have abandoned in the past, and may abandon in the future, our efforts on a particular product or technology. In addition, new products that we develop may not be successfully commercialized. For instance, in August 2008, we entered the pharmacy channel for the first time by launching SoluCLENZ Rx Gel. However, after closely monitoring the progress of the launch and weekly sales data, we determined that the distribution of a single prescription product through the pharmacy channel and the ongoing investment to support that channel had become cost-prohibitive. Accordingly, in April 2009 we announced that we would no longer sell SoluCLENZ in the pharmacy channel. If we fail to take any other product or technology from the development stage to market on a timely basis or fail to gain successful market adoption of such product, we may incur significant expenses without a near-term or any financial return.
21
Our failure to successfully in-license or acquire additional products and technologies would impair our ability to grow.
We intend to in-license, acquire, develop and market new products and technologies. Because we have limited internal research capabilities, our business model depends in part on our ability to license patents, products and/or technologies from third parties. The success of this strategy also depends upon our ability and the ability of our third-party formulators to formulate products under such licenses, as well as our ability to manufacture, market and sell such licensed products.
We may not be able to successfully identify any new products to in-license, acquire or internally develop. Moreover, negotiating and implementing an economically viable acquisition is a lengthy and complex process. Other companies, including those with substantially greater financial, marketing and sales resources, may compete with us for the acquisition of products. We may not be able to acquire or in-license the rights to such products on terms that we find acceptable, or at all. As a result, our ability to grow our business or increase our profits could be adversely impacted.
If we lose key personnel or are unable to attract and retain other qualified personnel, we may be unable to execute our business plan and our business would be materially adversely affected.
As of December 31, 2010, we had 203 employees. Our success depends on our continued ability to attract, retain and motivate highly qualified management, business development, sales and marketing, product development and other personnel. In the future we may not be able to recruit and retain qualified personnel, particularly for senior sales and marketing and research and product development positions due to intense competition for personnel among businesses like ours. The failure to do so could have a significant negative impact on our future product sales and business results. In addition, some options granted to employees have an option price greater than our current market price. This may hinder our ability to retain qualified personnel. As previously announced, effective October 8, 2010, Steven R. Carlson resigned from his positions as our President and Chief Executive Officer and as a member of our Board of Directors. Albert F. Hummel, a member of our Board of Directors since 2005, assumed the responsibilities of President and Chief Executive Officer. Our success depends on the efforts and abilities of Mr. Hummel, Preston Romm, our Chief Financial Officer, and David Goldstein, our Executive Vice President of Global Sales and Field Marketing, as well as other members of our senior management team and our scientific and technical personnel. We may not be able to retain the services of these individuals. In addition, we do not have “key person” insurance policies on any of our executive officers that would compensate us for the loss of their services. If we lose the services of one or more of these individuals, finding a replacement could be difficult, may take an extended period of time and could significantly impede the achievement of our business objectives. This may have a material adverse effect on our results of operations and financial condition.
To grow, we will need to increase the size of our organization, and we may encounter difficulties managing our growth, which could adversely affect our results of operations.
If we are able to successfully develop additional products and extend the use of our current products, we may experience growth in the number of our employees and the scope of our operations. To the extent that we acquire and launch additional products, the resulting growth and expansion of our sales force will place a significant demand on our financial, managerial and operational resources. Since many of the new products or systems we are working on may involve new technologies or entering new markets, we may not be able to accurately forecast the number of employees required and the timing of their hire or the associated costs. The extent of any expansion we may experience will be driven largely by the success of our new products and systems. As a result, management’s ability to project the size of any such expansion and its cost to the company is limited by the following uncertainties: (i) we will not have previously sold any of the new products and technologies and the ultimate success of these new products and technologies is unknown; (ii) we will be entering new markets; and (iii) the costs associated with any expansion will be partially driven by factors that may not be fully in our control (e.g., timing of hire, market salary rates). As of December 31, 2010, due to the continued uncertainties in the economic markets, our current business plan does not anticipate that we hire a substantial number of new employees in 2011. However, since we are unable to ascertain whether the economy will continue to show signs of recovery or growth, we could be in a position to hire significant numbers of new employees on a relatively short time frame to support the launch of new product lines or increases in sales by physicians once the global markets show evidence of a sustained recovery. Due to the uncertainty surrounding the timing of new product lines or the stabilization of the global markets, our costs to hire significant numbers of new employees could be higher than anticipated. Our success will also depend on the ability of our executive officers and senior management team to continue to implement and improve our operational, information management and financial control systems, and to expand, train and manage our employee base. Our inability to manage growth effectively could cause our operating costs to grow even faster than we currently anticipate and adversely affect our results of operations.
Because we have limited research and development capabilities, we will be dependent on third parties to perform research and development and product formulation activities for us.
We have limited internal research and development capabilities and currently outsource all of our product research and development and formulation activities to third-party research labs. In particular, we have licensed technologies that may issue as patents under certain patent applications filed by JR, and in the past have relied heavily on services provided by JR in the development of
22
new products to address acne and skin elasticity. We have received sufficient support from other third-party research labs to drive our current new product development; however, our current agreement with JR expired in September 2010 and since that time we have had to locate alternative third-party research and development companies to assist us.
There are a limited number of third-party research and development companies that specialize in or have the expertise required to achieve our product development objectives. As a result, it may be difficult for us to engage research and development labs and personnel for our anticipated future needs. If we are unable to arrange for third-party research and development of our products, or to do so on commercially reasonable terms, we may not be able to develop new products or expand the application of our existing products.
Reliance on third-party research and development labs entails risks to which we would not be subject if we performed the research and development ourselves. These risks include reliance on the third party for maintaining the confidentiality of the proprietary information relating to the product being developed and for maintaining quality assurance (including adequate stability, safety and other data), the possibility of breach of the research and development agreement by the third party, and the possibility of termination or non-renewal of the agreement by the third party.
Dependence upon third parties for the research and development of our future products may limit our ability to commercialize and deliver products on a timely and competitive basis and quality assurance issues encountered with these third parties may require us to postpone a product launch or discontinue product sales until appropriate formulation adjustments and testing are performed.
Because we currently have limited commercial manufacturing capabilities, we will continue to be dependent on third parties to manufacture products for us for some time.
We have limited commercial manufacturing experience and currently outsource all of our non-BPO product manufacturing to third-party contract manufacturers. Although we have received sufficient material from our manufacturers to meet our current needs, we do not have long-term contracts with most of these third parties. Although we have two or more qualified manufacturers for most of our key products, certain products, including some of our sun protection products and our products containing hydroquinone, are currently supplied by a single source. In the event that this supplier or any of our other third-party manufacturers terminates its supply arrangement with us, experiences financial difficulties, encounters regulatory or quality assurance issues, or suffers any damage to its facilities, we may experience delays in securing sufficient amounts of our products, which could harm our business, reputation and relationships with customers. Triax and Spear are our only suppliers and manufacturers of tretinoin. We have contracts with Triax and Spear that have initial termination dates in 2014. While there are several other manufacturers of generic tretinoin, the termination of those agreements or any loss of services under those agreements could be difficult for us to replace. Refissa tretinoin manufactured by Spear is the only tretinoin currently permitted to be marketed for on-label use to treat fine lines and wrinkles. If this agreement were terminated, it would currently be impossible for us to replace with another product that would allow us to make such claims. We expect to continue to rely on third parties to produce materials required for clinical trials and for the commercial production of our products.
There are a limited number of third-party manufacturers that operate under the FDA’s current cGMP, regulations and that have the necessary expertise and capacity to manufacture our products. As a result, it may be difficult for us to locate manufacturers for our anticipated future needs. If we are unable to arrange for third-party manufacturing of our products, or to do so on commercially reasonable terms, we may not be able to complete development of, market and sell our new products.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third party for regulatory compliance and quality assurance. To the extent that any of our third-party manufacturers fails to comply with regulatory requirements or encounters quality assurance issues, we may experience an interruption in the supply of products, which could impair our customer relationships and adversely affect our business, financial condition and results of operations. In addition, reliance on third-party manufacturers subjects us to the possibility of breach of the manufacturing agreement by the third party, and the possibility of termination or non-renewal of the agreement by the third party.
We have developed a manufacturing facility dedicated to the production of our CLENZIderm M.D. Serum Gel and Therapeutic Lotion. However, we are in the process of transferring the technology and manufacturing processes of these products to our current third-party manufacturers. We cannot assure you that we will successfully transfer the technology and manufacturing processes of the these products, which may make it difficult for us to maintain and grow sales of CLENZIderm M.D. Systems, or launch other new products and compete effectively.
Dependence upon third parties for the manufacture of our products may also reduce our profit margins or the sale of our products, and may limit our ability to develop and deliver products on a timely and competitive basis.
Our products may cause undesirable side effects that could limit their use, require their removal from the market or prevent further development.
The most common side effects associated with our therapeutic products are temporary redness, stinging, burning sensation, skin peeling, flaking, acne flare-ups and photo-sensitivity normally experienced within approximately the first ten weeks of use. While these side effects generally are not severe, they may limit the use of our products, particularly if physicians or patients perceive that the risks or discomfort outweigh the benefits, or if they perceive that the side effects of competitive products are less significant.
23
Undesirable side effects caused by our products could interrupt, delay or halt our development programs, including clinical trials, and could result in adverse regulatory action by the FDA or other regulatory authorities. More severe side effects associated with our products may be observed in the future. Even if we are able to complete the development of a new product and obtain any required regulatory approval, undesirable side effects could prevent us from achieving or maintaining market acceptance of the product or could substantially increase the costs and expenses of commercializing the product. Negative publicity concerning our products, whether accurate or inaccurate, could also reduce market or regulatory acceptance of our products, which could result in decreased product demand, removal from the market or an increased number of product liability claims, whether or not such claims have merit.
The FDA issued a proposed rule that cites evidence that an active ingredient contained in some of our Obagi Nu-Derm, Obagi-C Rx and ELASTIderm Décolletage Systems may have negative side effects.
In August 2006, the FDA issued a proposed rule that cites some evidence that hydroquinone may be a carcinogen, if orally administered, and may be related to a skin condition called ochronosis, which results in the darkening and thickening of the skin, and the appearance of small bumps and grayish-brown spots. Hydroquinone is an active ingredient contained in our: (i) Obagi Clear, Obagi Blender and Obagi Sunfader products, which are part of our Nu-Derm and Condition & Enhance Systems; (ii) Obagi-C Rx C-Clarifying Serum and Obagi-C Rx C-Night Therapy products, which are part of our Obagi-C Rx Systems; and (iii) Décolletage Skin Lightening Complex, which is in the ELASTIderm product family. The FDA also concluded that it could not rule out the potential carcinogenic risk from topically applied hydroquinone. In the proposed rule, the FDA recommended that additional studies be conducted to determine if there is a risk to humans from the use of hydroquinone. The FDA nominated hydroquinone for further study by the NTP, and in December 2009 the NTP Board of Scientific Counselors approved the nomination. As a result, the following three NTP studies will be conducted:
|
•
|
Comparative metabolism studies in rats and mice by oral and dermal routes;
|
|
•
|
Reproductive toxicity study in rats and mice by oral route; and
|
|
•
|
Dermal carcinogenicity studies of hydroquinone in mice and rats.
|
There are several more steps to the nomination process before the studies will commence, and the studies themselves can take two years or more to complete. The FDA has noted that, in the interim, it believes that hydroquinone at concentrations of 2% or less should remain available as an OTC drug product. However, if the NTP studies eventually conclude that hydroquinone is a carcinogen, we could be required to suspend the marketing of our products that contain hydroquinone, conduct additional safety tests and potentially cease the sale of affected products, which would harm our business. In addition, patients who experience side effects from our products may bring product liability claims against us.
All of our products that contain hydroquinone are prescription-based. The FDA was considering regulating all hydroquinone products, including prescription-based hydroquinone products, as new drugs, and may conclude that the continued use of prescription-based hydroquinone products will require the submission and approval of a NDA. It is our understanding that the FDA is not intending to take any action with respect to regulation of products containing 4% hydroquinone until the NTP studies have been conducted. If, at the conclusion of the NTP studies, the FDA requires us to submit a NDA for our prescription-based hydroquinone products, we believe that the FDA may allow us to continue to market these products while we are preparing, submitting and waiting for approval of such NDA. However, there can be no assurance that we will be able to continue to market our products during the NDA process, and we may be required to suspend marketing of our prescription-based hydroquinone products until such time as a NDA is approved. In addition, we cannot assure you that any NDA we submit for our prescription-based hydroquinone products will be approved. If we are required to suspend or cease marketing of our prescription-based hydroquinone products, our business would be adversely affected.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
Our business exposes us to the risk of product liability claims that are inherent to the development, clinical testing and marketing of aesthetic and skin health products. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of those products. Although we maintain general liability and product liability insurance in amounts that we believe are reasonably adequate to insulate us from potential claims, this insurance may not fully cover potential liabilities. In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercial production and sale of our products, which would adversely affect our business.
24
We are subject to risks associated with doing business internationally.
Our international sales currently depend upon the marketing efforts of and sales by certain distributors and licensees, particularly Rohto, a licensee of certain of our trademarks and products for the retail drug store channel in Japan, from whom we receive royalties that accounted for approximately 4% of our net sales for both of the years ended December 31, 2010 and 2009, and approximately 4% and 5% of our gross margin for the years ended December 31, 2010 and 2009, respectively. Because incremental costs associated with this agreement are minimal, a material decline in licensing revenues from or termination of this agreement would have a material adverse effect on our net income. While no other international distribution or license partner accounted for more than 5% of our net sales for either of the years ended December 31, 2010 and 2009, our business is subject to certain risks inherent in international business, many of which are beyond our control. These risks include:
|
•
|
adverse changes in tariff and trade protection measures;
|
|
•
|
unexpected changes or differences in foreign regulatory requirements;
|
|
•
|
potentially negative consequences from changes in tax laws;
|
|
•
|
the potential business failure of one or more of our distribution partners;
|
|
•
|
changing economic conditions in countries where our products are sold or manufactured;
|
|
•
|
exchange rate risks;
|
|
•
|
potential political unrest and hostilities;
|
|
•
|
differing degrees of protection for intellectual property; and
|
|
•
|
difficulties in coordinating foreign distribution.
|
Any of these factors could adversely affect our business, financial condition and results of operations. We cannot assure you that we can successfully manage these risks or avoid their effects.
During 2010, we entered into discussions with a non-performing international distributor in an attempt to renegotiate its distribution agreement and develop a payment schedule for the distributor’s outstanding accounts receivable balance due to us related to sales in prior periods. This distributor had notified us that it believed that we had violated certain provisions of the distribution agreement. We believe such assertions were unfounded. We terminated the agreement with that distributor in November 2010, as we were unable to reach an agreement with respect to these matters. Due to the fact that we were unable to renegotiate the agreement, it is possible that either of the parties may institute litigation asserting various breach of contract claims. Although we have recorded reserves in our financial statements for the inventory held by this distributor and for the outstanding receivables owed by this distributor, if legal action is commenced, we may incur material costs in prosecuting and potentially defending such action.
Potential business combinations could require significant management attention and prove difficult to integrate with our business, which could distract our management, disrupt our business, dilute stockholder value and adversely affect our operating results.
If we become aware of potential business combination candidates that are complementary to our business, we may decide to combine with such businesses or acquire their assets in the future. Business combinations generally involve a number of additional difficulties and risks to our business, including:
|
•
|
failure to integrate management information systems, personnel, research and development and marketing, operations, sales and support;
|
|
•
|
disruption of our ongoing business and diversion of management’s attention from other business matters;
|
|
•
|
potential loss of the acquired company’s customers;
|
|
•
|
failure to develop further the acquired company’s technology;
|
25
|
•
|
unanticipated costs and liabilities; and
|
|
•
|
other accounting consequences.
|
In addition, we may not realize benefits from any business combination that we undertake in the future. If we fail to successfully integrate such businesses, or the technologies associated with such business combinations into our company, the revenue and operating results of the combined company could be adversely affected. Any integration process would require significant time and resources, and we may not be able to manage the process successfully. If our customers are uncertain about our ability to operate on a combined basis, they could delay or cancel orders for our products. We may not successfully evaluate or utilize the acquired technology or accurately forecast the financial impact of a combination, including accounting charges or volatility in the stock price of the combined entity. If we fail to successfully integrate other companies with which we may combine, our business could be adversely affected.
Risks Related to Regulatory Matters
Our ability to commercially distribute our products and our business may be significantly harmed if the regulatory environment governing our products changes, if the FDA takes enforcement action against us or our competitors marketing similar products, if a third party obtains FDA approval of a NDA for a 4% hydroquinone product for the same uses for which we market our hydroquinone products, or if our contract manufacturers fail to comply with cGMPs.
The FDA and comparable agencies of other countries regulate our products. In the United States, FDA regulations govern, among other things, the activities that we perform, including product development, product testing, product labeling, product storage, manufacturing, advertising, promotion, product sales, reporting of certain product adverse events and failures, and distribution. Individual state regulations may also govern drug product manufacturing, distribution, advertising and promotion.
The Obagi Nu-Derm, Obagi Condition & Enhance, Obagi-C Rx and ELASTIderm Décolletage Systems contain products that include 4% hydroquinone as an active ingredient and are marketed in the United States without a FDA-approved marketing application under the CPG. We believe that these products are not currently subject to FDA pre-market approval. In August 2006, the FDA issued a proposed rule that, if adopted in its current form, would establish that OTC skin bleaching drug products, such as hydroquinone, are not generally recognized as safe and effective and are misbranded, and could seek to require NDAs for new products using skin bleaching drug products, including prescription skin bleaching drug products. The FDA had indicated that upon adoption of the final rule it intended to consider all skin bleaching drug products, whether currently marketed on a prescription or OTC basis, to be new drugs requiring an approved NDA for continued marketing. In the proposed rule, the FDA recommended that additional studies be conducted to determine if there is a risk to humans from the use of hydroquinone. The FDA nominated hydroquinone for further study by the NTP, and in December 2009 the NTP Board of Scientific Counselors approved the nomination. As a result, the NTP will conduct further studies on hydroquinone. However, there are several more steps to the nomination process before the studies will commence, and the studies themselves can take two years or more to complete. The FDA has noted that, in the interim, it believes that hydroquinone at concentrations of 2% or less should remain available as an OTC drug product. It is our understanding that the FDA is no longer evaluating this proposal and is not intending to take any action with respect to regulation of products containing 4% hydroquinone until the NTP studies have been conducted. However, should the FDA reverse this position or decide upon conclusion of the NTP studies that all skin bleaching products are new drugs requiring an approved NDA, we may be required to withdraw certain products in the Nu-Derm, Condition & Enhance, Obagi-C Rx and ELASTIderm Décolletage Systems until required clinical trials are performed and new drug approvals are obtained, in effect foreclosing us from selling these products. If we are required to seek new drug approval for these products, our attention and resources will be dedicated to the process of obtaining new drug approval, which may be time-consuming and expensive. In addition, we may not successfully obtain such approval or may be delayed in obtaining such approval if one of our competitors obtains approval and nonpatent exclusivity for the same uses for which we seek approval. Other manufacturers of 4% prescription hydroquinone include SkinMedica, Inc., Taro Pharmaceuticals Inc., Valeant Pharmaceuticals, Inc. and Stiefel Laboratories, Inc. If we are unable to obtain such approval we would be prohibited from selling the Nu-Derm, Condition & Enhance and Obagi-C Rx Systems and ELASTIderm Décolletage Skin Lightening Complex, which would have a material adverse impact on our business.
As discussed above, the August 2006 proposed rule cites some evidence that hydroquinone may be a carcinogen and may be related to ochronosis. If the NTP studies conclude that hydroquinone is a carcinogen and the FDA determines that such potential health risks warrant a ban on the sale of 4% hydroquinone products, such determinations would have a material adverse effect on our sale of the Nu-Derm, Condition & Enhance, Obagi-C Rx and ELASTIderm Décolletage Systems. The FDA’s proposed rulemaking itself, and the concerns expressed therein relating to the use of hydroquinone, could have an adverse impact on the sales of these products. Certain of our competitors are attempting to use the FDA’s proposed rulemaking to convince physicians and patients not to use our products containing hydroquinone. To date, these marketing efforts by our competitors have not had a negative impact on our sales levels. However, we cannot assure you that such marketing efforts will not have a negative impact on our sales levels in the future.
26
Finally, the contract manufacturers of our hydroquinone products are also subject to regulation by the FDA, especially for compliance with cGMPs. The FDA has increased the requirements for cGMPs. Should the FDA conclude that one of our contract manufacturers or any of its ingredient suppliers is not in compliance with cGMPs, it could allege that our hydroquinone products fall outside the CPG safe harbor.
FDA and FTC regulations limit the type of marketing claims we can make about our products. If the FDA determines that any of our marketing claims are false or misleading, or suggest a clinical benefit that is not supported in the studies we have done, we may be required to cease making the challenged marketing claims, issue corrective communications, pay fines, or stop selling products until the incorrect claims have been corrected. FDA or FTC enforcement actions regarding promotional claims, including warning letters, would also divert our management’s attention and create public relations issues for our customers and opportunities for our competitors.
We are also subject to review, periodic inspection and marketing surveillance by the FDA and comparable state agencies to determine our compliance with regulatory requirements. Our manufacturing processes, any clinical trials that we perform, our distribution and our promotional activities are subject to ongoing regulatory compliance. In addition, state agencies also may require registration, licensure and inspection of manufacturing or distribution facilities and may require reporting of promotional activities. If the FDA or any state or comparable agency finds that we have failed to comply with these requirements or later discovers previously unknown problems with our products, including unanticipated adverse events of unanticipated severity or frequency, or any of our manufacturers or manufacturing processes, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, including:
|
•
|
fines, injunctions and civil penalties;
|
|
•
|
recall or seizure of our products;
|
|
•
|
restrictions on our products, manufacturing processes, or distribution systems, including operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
denial of requests for approvals of product candidates;
|
|
•
|
withdrawal of approvals already granted;
|
|
•
|
disgorgement of profits; and
|
|
•
|
criminal prosecution.
|
Any of these enforcement actions could affect our ability to commercially distribute our products in the United States and may also harm our ability to conduct the clinical trials necessary to support the marketing, clearance or approval of these products, which would materially and adversely affect our business.
New regulations could prohibit physicians from dispensing our products directly.
In our primary market, the United States, we market our products and systems directly to physicians to dispense in their offices. Most of the products and systems we sell are dispensed by physicians directly to their patients in their offices, although some patients choose to have prescriptions for our tretinoin and metronidazole products filled by pharmacies instead of the treating physician in order to obtain insurance coverage for such products. Although several of the products in our systems contain 4% hydroquinone, our products are not currently available by prescription in the pharmacy. In the event state regulations change to limit or prohibit the ability of physicians to dispense our products directly to patients in their offices or change to limit or prevent our ability to distribute products directly through physicians, patients may be unable to obtain many of our products, as they are not currently available in pharmacies. In addition, several states have recently taken action against a number of our customers who also sell our products to patients over the internet, questioning whether these practices are consistent with such states’ pharmacy licensure and physician dispensing rules. An adverse outcome by any of these states against any of these customers may result in the inability of such customers (including other customers in the state) to continue selling our products over the internet or at all. In November 2009, we received a letter (the “Letter”) from the Texas Department of State Health Services (the “Agency”). In the Letter, the Agency refers to inspections of certain retail businesses in Texas that were selling certain of our products. In that regard the Agency alleged that we are distributing unapproved new drug articles to these establishments in violation of certain Texas statutes. We submitted our response to the Agency in January 2010 indicating why we believe we are in compliance with the Texas statutes. In June 2010, we had an in-person meeting with officials from the Agency to further discuss its concerns. In August 2010, we submitted additional information to the Agency in response to the questions raised at the June meeting but have not had further communication with the Agency at this time. If patients are unable to purchase our products directly from physicians or our customers are prohibited from selling our products in their current manner or at all in Texas or other states, then both customers and patients may purchase less of our products than they otherwise would, which would harm our business.
27
Failure to obtain regulatory approvals in foreign jurisdictions would prevent us from marketing our products internationally.
We market our products outside of the United States. In order to market our products in many non-U.S. jurisdictions we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. In others, we do not have to obtain prior regulatory approval but do have to comply with other regulatory restrictions on the manufacture, marketing and sale of our products. We may be unable to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. The approval procedure varies among countries and can involve additional testing and data review. The time required to obtain approval in non-U.S. jurisdictions may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. If we get approval by the FDA, that does not ensure approval by regulatory agencies in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. The failure to obtain these approvals could harm our business.
If we or any of our third-party manufacturers do not operate in accordance with cGMPs, we could be subject to FDA enforcement actions, including the seizure of our products and the halt of production of our products.
Third-party manufacturers that we currently rely on or will rely on in the future (including ingredient suppliers that these third party manufacturers purchase from) must continuously adhere to the cGMPs set forth in the FDA’s regulations and guidance documents. In complying with cGMPs, we and our third-party manufacturers must expend significant time, money and effort in development, testing, production, record keeping and quality control to assure that our products meet applicable specifications and other regulatory requirements. The failure to comply with these specifications and other requirements could result in an FDA enforcement action, including the seizure of products and shutting down of production. Our third-party manufacturers may also be subject to comparable or more stringent regulations of foreign regulatory authorities. If our third-party manufacturers (or their ingredient suppliers) or we are unable to comply with cGMPs and applicable foreign regulatory requirements, our ability to develop, produce and sell our products could be impaired.
Risks Related to Intellectual Property
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely primarily on maintaining the confidentiality of our trade secrets and the protection of trade secret laws, as well as a combination of patent, copyright, and trademark (including common law trademark) laws, and nondisclosure, confidentiality and other contractual restrictions, to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our trade secrets may be misappropriated by current or former employees, contractors, or parties with whom we partner, or may be inadvertently disclosed or obtained by breach of a confidentiality agreement. We have recently applied for several patents both in the United States and abroad. These patent applications may not issue as patents at all, or the applications may not issue as a patent in a form that will be advantageous to us or may issue and be subsequently successfully challenged by others and invalidated or rendered unenforceable. Both the patent application process and the process of managing patent disputes can be time-consuming and expensive. Competitors may be able to design around our patents or develop products that provide outcomes comparable to ours even without misappropriating our trade secrets. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Further, the parties with whom we enter into confidentiality and intellectual property assignment agreements could dispute the ownership of intellectual property developed under these agreements. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
In order to protect or enforce our intellectual property rights, we may initiate litigation. In addition, others may initiate litigation related to intellectual property against us. Companies against whom we might initiate litigation or who might initiate litigation against us may be better able to sustain the costs of litigation because they have substantially greater resources. We may become subject to interference proceedings conducted in patent and trademark offices to determine the priority of inventions. There are numerous issued and pending patents in the skin care product field. The validity and breadth of such patents may involve complex legal and factual questions for which important legal principles may remain unresolved. If third parties file oppositions to our patent applications in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of our filed foreign patent applications. We also have worked with consultants in developing our intellectual property portfolio. To the extent any of these consultants are engaged in litigation involving intellectual property related to us, we may also become a party to such actions or otherwise be adversely affected by virtue of our relationships with the consultants. For example, JR,
28
a consultant who helped us develop CLENZIderm, and has granted us a license under certain patent applications that cover CLENZIderm, was involved in a lawsuit in which a third party plaintiff claimed that the owner of JR misappropriated the plaintiff’s trade secrets. A settlement has been reached between JR and the plaintiff, which they claim prohibits our ability to develop new delivery systems or products containing solubilized BPO, the active ingredient in the CLENZIderm products.
In January 2005, we entered into a Consulting Services and Confidentiality Agreement with JR, under which, among other things, we hired JR to perform research and development activities including product formulation, product development and regulatory work, as detailed in various statements of work. The initial term of the JR Agreement was for five years; however, in April 2010, we reached what we believed was an agreement in principle for amending the JR Agreement. Although that amendment was never executed, the parties operated under the terms of the agreement, as amended, from June 2010 to September 2010, at which time the JR Agreement terminated. We are currently in discussions with JR on a variety of matters. We have been advised by JR that it believes that certain of our products, including the ELASTIderm line of products, CLENZIderm and Rosaclear, are covered by both issued and pending patents which JR contends are owned by it and which it believes were licensed to us under the JR Agreement. JR contends that such license and our right to continue to sell such products terminated when the JR Agreement terminated. We disagree with JR’s position on this issue and are seeking to resolve this and other issues in our current negotiations with JR. Should we fail to reach agreement with respect to these current discussions, the parties could end up in litigation regarding the ownership rights to these technologies, and our business, results of operations and financial condition could be adversely effected. During the years ended December 31, 2010, 2009 and 2008, approximately 15%, 15% and 14% of our net sales, respectively, were related to products that contained technologies covered by the JR Agreement. See Part I, Item 1 “Business – Certain Material Agreements – Jose Ramirez and JR” in this Report for further information.
Litigation may be necessary for us to assert or defend against infringement claims, enforce our issued and licensed patents, protect our trade secrets or know-how or determine the enforceability, scope and validity of the proprietary rights of others. Our involvement in intellectual property claims and litigation could:
|
•
|
divert existing management, scientific and financial resources;
|
|
•
|
subject us to significant liabilities;
|
|
•
|
result in a ruling that allows our competitors to market competitive products without obtaining a license from us;
|
|
•
|
require us to enter into royalty or licensing agreements, which may not be available on terms acceptable to us, if at all; or
|
|
•
|
force us to discontinue selling or modify our products, or to develop new products.
|
If any of these events occurs, our business will be materially and adversely affected.
We and our manufacturers and suppliers license certain technologies and patents from third parties. If these licenses are breached, terminated or disputed, our ability to commercialize products dependent on these technologies and patents may be compromised.
We have licensed eighteen patents, including patents related to our Vitamin C serums that we have licensed from Avon. We entered into our license with Avon in June 2003, for an initial three-year term, and the license is renewed year to year thereafter, at our option through the life of the last patent to expire (which will be in 2018). These licensed patents contain claims that cover our Obagi-C Rx C-Clarifying serum, Professional-C 5% serum, Professional-C 10% serum, Professional-C 15% serum and Professional-C 20% serum. If one or more of our licenses with Avon or licenses that we have with other parties terminate, or if we violate the terms of our licenses or otherwise lose our rights to these patents, we may be unable to continue developing and selling our products that are covered by claims in these patents. Our licensors or others may dispute the scope of our rights under any of these licenses. The licensors under these licenses may breach the terms of their respective agreements or fail to prevent infringement of the licensed patents by third parties. Loss of any of these licenses for any reason could materially and adversely affect our financial condition and operating results. See “Risk Factors – Risks Related to Intellectual Property: If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights” and Part I, Item 1, “Business – Certain Material Agreements – Jose Ramirez and JR” in this Report for a description of current negotiations with JR.
Further, we purchase products from manufacturers and suppliers who have licensed patent rights to use and sell these products from third-party licensors, and if any dispute arises as to these licensed rights, the third-party licensors may bring legal actions against us, our respective licensees, suppliers, customers or collaborators, and claim damages and seek to enjoin the manufacturing and marketing of such products.
29
In addition, if we determine that our products do not incorporate the patented technology that we have licensed from third parties, or that one or more of the patents that we have licensed are not valid, we may dispute our obligation to pay royalties to our licensors. Any dispute with a licensor could be complex, expensive and time-consuming and an outcome adverse to us could materially and adversely affect our business and impair our ability to commercialize our patent-licensed products.
Risks Related to Our Capital Requirements and Finances
Litigation, including the Litigation and Arbitration Demand Initiated By Dr. Zein Obagi and Affiliates, Could Materially Impact our Financial Results.
Substantial, complex or extended litigation could cause us to incur large expenditures, distract our management and disrupt our business. For example, if the lawsuit initiated by Dr. Zein Obagi, ZO Skin Health, Inc. and related affiliates resulted in an unfavorable outcome it may have a material adverse effect on our financial condition, results of operations and cash flows. In addition, substantial costs will likely be incurred regardless of the ultimate outcome. See Part I, Item 3 of this Report, “Legal Proceedings” and see Note 10, “Litigation” in our Notes to Consolidated Financial Statements for information concerning this matter.
If we fail to generate sufficient cash flow from our operations, we will be unable to continue to develop and commercialize new products.
We expect capital and operating expenditures to increase over the next several years as we expand our infrastructure, and our commercialization, clinical trial, research and development and manufacturing activities. We believe that our net cash provided by operating activities and existing cash and cash equivalents will be sufficient to fund our operations for the foreseeable future. However, our present and future funding requirements will depend on many factors, including, among other things:
|
•
|
the level of research and development investment required to maintain and improve our competitive position;
|
|
•
|
the success of our product sales and related collections;
|
|
•
|
our need or decision to acquire or license complementary businesses, products or technologies;
|
|
•
|
costs relating to the expansion of the sales force, management and operational support;
|
|
•
|
competing technological and market developments; and
|
|
•
|
costs relating to changes in regulatory policies or laws that affect our operations.
|
In addition, our future liquidity may be impacted by other factors not related to the funding requirements described above. For instance, in October 2010, we announced that our Board of Directors has given us authority to repurchase up to $45.0 million of our outstanding stock depending on market conditions and other factors. Pursuant to such authority, in November 2010 we repurchased a total of 3,556,910 shares of our common stock from two former stockholders, the Stonington Capital Appreciation 1994 Fund, L.P. and the Zein and Samar Obagi Family Trust (the “selling stockholders”), for a total purchase price of $35.0 million in accordance with the provisions of the Stock Purchase Agreement, dated as of November 15, 2010, among such selling stockholders and us. Additional repurchases under this authorization may include repurchases made through open market or privately negotiated transactions.
If our net cash provided by operating activities and existing cash and cash equivalents are not sufficient to fund our operations in the future, we may need to draw down on our revolving line of credit (the “Facility”) or term loans (“Term Loans”) with Comerica Bank or raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms when needed, if at all. If we are required to draw down on our Facility and/or Term Loans, our ability to in-license new technologies, develop future products or expand our pipeline of products could all be negatively impacted, which would have an adverse effect on our ability to grow our business and remain competitive in our marketplace. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, we may be required to relinquish potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
Our quarterly operating results are variable, which may cause our stock price to decline.
Our quarterly results of operations have varied in the past and are likely to vary significantly in the future due to a number of factors, many of which are outside of our control, including:
30
|
•
|
demand for and market acceptance of our products;
|
|
•
|
the development of new competitive products by others;
|
|
•
|
changes in regulatory classifications of our products;
|
|
•
|
changes in physician or patient acceptance of the use of physician-dispensed products;
|
|
•
|
changes in treatment practices of physicians who currently prescribe our products;
|
|
•
|
reduced demand for our products during the summer months due to variability of patient compliance resulting from travel and other disruptive activities, particularly during July and August;
|
|
•
|
delays between our expenditures to acquire new product lines or businesses and the generation of revenues from those acquired products or businesses;
|
|
•
|
the timing, release and competitiveness of our products;
|
|
•
|
increases in the cost of raw materials used to manufacture our products;
|
|
•
|
the mix of products that we sell during any time period;
|
|
•
|
increased price competition; and
|
|
•
|
adverse changes in the level of economic activity in the United States and other major regions in which we do business.
|
Due to the factors summarized above, we do not believe that period-to-period comparisons of our results of operations are necessarily meaningful and should not necessarily be relied upon to predict future results of operations. In addition, due to the reduced demand for our products during the summer months as discussed above, our results of operations for the third quarter have historically been lower than our results of operations for the second quarter. We do not expect that this will change in the future. It is also possible that in future periods, our results of operations will not meet the expectations of investors or analysts, or any published reports or analyses regarding our company. In that event, the price of our common stock could decline, perhaps substantially.
Changes in, or interpretations of, accounting rules and regulations, could result in unfavorable accounting charges or require us to change our policies.
Changes to, or interpretations of, accounting methods or policies in the future may require us to reclassify, restate or otherwise change or revise our financial statements, including those contained in this Report. For example, on January 1, 2007, we adopted authoritative guidance issued by the Financial Accounting Standards Board (“FASB”), included under the Accounting Standards Codification (“ASC”) 740, Income Taxes (“ASC 740”), for uncertainties in our tax positions. We provide for uncertain tax positions when such tax positions do not meet the recognition thresholds or measurement standards prescribed by ASC 740. Changes to uncertain tax positions, including related interest and penalties, impact our effective tax rate. When particular tax matters arise, a number of years may elapse before such matters are audited and finally resolved. Favorable resolution of such matters could be recognized as a reduction to our effective tax rate in the year of resolution. Unfavorable resolution of any tax matter could increase the effective tax rate. Any resolution of a tax issue may require the use of cash in the year of resolution.
Impairment of our significant intangible assets may reduce our profitability.
The costs of our goodwill, acquired product rights, distribution rights, and trademarks are recorded as intangible assets and all, except for goodwill, are amortized over the period that we expect to benefit from the applicable assets. As of December 31, 2010, acquired net intangible assets and goodwill comprised approximately 15% of our total assets. We evaluate periodically the recoverability and the amortization period of our intangible assets. Some factors we consider important in assessing whether or not impairment exists include performance relative to expected historical or projected future operating results, significant changes in the manner of our use of the assets or the strategy for our overall business, and significant negative industry or economic trends. These factors, assumptions, and changes in them could result in an impairment of our long-lived assets. Any impairment of our intangible assets may reduce our profitability and have a material adverse effect on our results of operations and financial condition.
31
Fluctuations in demand for our products could create inventory maintenance uncertainties and could adversely affect our business.
As a result of customer buying patterns, a substantial portion of our revenues has been recognized in the last month of each quarter and the last month of the year. We schedule our inventory purchases to meet anticipated customer demand. As a result, relatively small delays in the receipt of manufactured products by us could result in revenues being deferred or lost. Our operating expenses are based upon anticipated sales levels, and a high percentage of our operating expenses are relatively fixed in the short term. Consequently, variations in the timing of sales, or reductions in sales due to ongoing global economic uncertainties, could cause significant fluctuations in operating results from period to period and may result in unanticipated periodic earnings shortfalls or losses.
If we overestimate demand, we may be required to write-off inventories and increase our reserves for product returns. If we underestimate demand, we may not have sufficient inventory of products to ship to our customers. Our products have expiration dates that range from 24 to 36 months from the date of manufacture. We establish reserves for potentially excess, dated or otherwise impaired inventories. We may not be able to accurately estimate the reserve requirement that will be needed in the future. Although our estimates are reviewed quarterly for reasonableness, our product return activity could differ significantly from our estimates. Judgment is required in estimating these reserves and we rely on data from third parties, including, but not limited to, distributor forecasts and independent market research reports. The actual amounts could be different from our estimates, and differences are accounted for in the period in which they become known. If we determine that the actual amounts exceed our reserve amounts, we will record a charge to earnings to approximate the difference. A material reduction in earnings resulting from a charge would have a material adverse effect on our net income, results of operations and financial condition.
The agreements governing our Facility and Term Loans impose restrictions on our business that may limit our business opportunities and hinder our ability to execute our business strategy.
In November 2010, we entered into a new credit facility and term loan agreement (the “Credit and Term Loan Agreement”) with Comerica Bank that superseded and replaced our former credit facility with Comerica Bank (the “Former Credit Facility”). The Credit and Term Loan Agreement contains, and other agreements we may enter into in the future may contain, covenants imposing restrictions on our business, and requires us to maintain certain financial covenants. These restrictions and covenants may affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities as they arise. These covenants place restrictions on our ability to, among other things, incur additional debt, create liens, make investments, enter into transactions with affiliates, sell assets, guarantee debt, declare or pay dividends, redeem common stock or make other distributions to stockholders, and consolidate or merge. As of January 31, 2009, we were not in compliance with the non-financial covenant under our Former Credit Facility requiring us to report monthly, a listing of our intellectual property. In February 2009, we obtained a waiver for the month of January 2009 with respect to this covenant.
Although we believe we were in compliance with all of our non-financial and financial covenants under the Credit and Term Loan Agreement as of December 31, 2010, our future ability to comply with these covenants may be affected by events beyond our control, including prevailing economic, financial, and industry conditions. In the event we ever borrow under the Credit and Term Loan Agreement, an event of default under the Credit and Term Loan Agreement would permit our lenders to declare all amounts borrowed from them to be due and payable, together with accrued and unpaid interest. If we were unable to repay any debt owed, the lenders could proceed against the collateral securing that debt.
We are subject to the requirements of Section 404 of the Sarbanes-Oxley Act. If we are unable to comply with Section 404 in a timely manner it may affect the reliability of our internal control over financial reporting.
We are subject to the requirements of Section 404 of the Sarbanes-Oxley Act and the SEC rules and regulations require an annual management report on our internal control over financial reporting, including, among other matters, management’s assessment of the effectiveness of our internal control over financial reporting, and an attestation report by our independent registered public accounting firm addressing the effectiveness of our internal control over financial reporting. Although our independent registered public accounting firm did not identify material weaknesses in connection with its audit of our internal control over financial reporting as of December 31, 2010, we cannot assure you that we will maintain an effective system of internal controls in the future. If we are not able to adequately comply with the requirements of Section 404 we may be subject to sanctions or investigation by regulatory authorities, including the SEC or the Nasdaq Global Market. Moreover, if we are unable to assert that our internal control over financial reporting is effective in any future period (or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal controls), we could lose investor confidence in the accuracy and completeness of our financial reports, which may have an a material adverse effect on us.
Our Board of Directors can issue preferred stock without stockholder approval of the terms of such stock.
Our amended and restated certificate of incorporation authorizes our Board of Directors, without stockholder approval, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges, and restrictions granted to or imposed upon the preferred stock, including voting rights, dividend rights, conversion rights, terms of redemption, liquidation
32
preference, sinking fund terms, subscription rights, and the number of shares constituting any series or the designation of a series. Our Board of Directors can issue preferred stock with voting and conversion rights that could adversely affect the voting power of the holders of common stock, without stockholder approval. As of December 31, 2010, no shares of preferred stock were outstanding and we have no present plan to issue any shares of preferred stock.
We expect that the price of our common stock will fluctuate substantially.
The market price for our common stock has been and will continue to be affected by a number of factors, some of which are beyond our control, including:
|
•
|
downturns in the global financial markets;
|
|
•
|
changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates;
|
|
•
|
quarterly variations in our or our competitors’ results of operations;
|
|
•
|
the announcement of new products or service enhancements by us or our competitors;
|
|
•
|
announcements related to litigation;
|
|
•
|
developments in our industry; and
|
|
•
|
general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors.
|
Provisions in our charter documents and Delaware law could discourage a takeover you may consider favorable or could cause current management to become entrenched and difficult to replace.
Provisions in our amended and restated certificate of incorporation and our bylaws, as well as Delaware law, could make it more difficult for other companies to acquire us, even if doing so would benefit our stockholders. Our certificate of incorporation and bylaws contain the following provisions, among others, which may inhibit an acquisition of our company by a third party:
|
•
|
advance notification procedures for matters to be brought before stockholder meetings;
|
|
•
|
a limitation on who may call stockholder meetings;
|
|
•
|
a prohibition on stockholder action by written consent; and
|
|
•
|
the ability of our Board of Directors to issue up to 10 million shares of preferred stock without a stockholder vote.
|
We are also subject to provisions of Delaware law that prohibit us from engaging in any business combination with any “interested stockholder,” meaning generally that a stockholder who beneficially owns more than 15% of our stock cannot acquire us for a period of three years from the date this person became an interested stockholder unless various conditions are met, such as approval of the transaction by our Board of Directors. Any of these restrictions could have the effect of delaying or preventing a change in control.
We do not currently intend to pay cash dividends on our common stock in the foreseeable future.
We currently anticipate that we will retain all future earnings, if any, to finance the growth and development of our business and do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will depend upon our financial condition, capital requirements, earnings and other factors deemed relevant by our Board of Directors.
None.
33
As of December 31, 2010, we leased the following facilities:
|
Function
|
Location
|
Square feet
|
Lease expiration
|
|||
|
Headquarters
|
Long Beach, CA
|
30,884 |
October 2018
|
|||
|
Distribution Center
|
Carson, CA
|
26,959 |
October 2013
|
|||
|
Manufacturing
|
Milford, CT
|
5,600 |
November 2013
|
|||
On August 6, 2008, we entered into a lease agreement with Kilroy Realty, L.P. for the lease of office space located in Long Beach, California. The new facility consists of 30,884 rentable square feet and the term of the lease is for ten years with an option to extend the lease term for five years. We took possession of the facility on July 1, 2008, and the lease term commenced in October 2008 when we took occupancy and relocated our corporate headquarters to the new facility. We believe our facilities are adequate for their intended use and are sufficient for our current level of operations.
Our activities are initiated and coordinated from our Long Beach headquarters. Our product development efforts are based at our Long Beach headquarters, with development and manufacturing facilities in Milford, Connecticut. The lease for our manufacturing facility expires in November 2013. Our skin health training center for employees is also located at our Long Beach headquarters. Our cGMP compliance training for distribution personnel and sales training for customer service representatives are provided in Long Beach.
Our distribution center is strategically located for quick, convenient and reliable distribution with access to all major carriers in Southern California.
On January 7, 2010, ZO Skin Health, Inc., a California corporation, filed a complaint in the Superior Court of the State of California, County of Los Angeles: ZO Skin Health, Inc. vs. OMP, Inc. and Obagi Medical Products, Inc. and Does 1-25; Case No. BC429414. The complaint alleges claims against us for: (i) unfair competition; (ii) intentional interference with prospective economic advantage; (iii) negligent interference with prospective economic advantage; (iv) intentional interference with contract; and (v) promissory estoppel. More specifically, the Plaintiff alleges that: we engaged in business practices, including the assertion of void and unlawful non-compete clauses in contracts with Zein Obagi, that violate California’s Unfair Competition Law and the California Business and Professional Code, Section 17200, et seq.; we intentionally and negligently interfered with plaintiff’s prospective economic advantage in relationship with Rohto and certain product distributors; we interfered with existing contracts between plaintiff and Rohto; and we allegedly did not provide certain promised assistance in connection with the alleged agreement between plaintiff and Rohto. The plaintiff seeks injunctive relief, compensatory damages “measuring in the tens of millions of dollars” and other damages in an unspecified amount, punitive damages and costs of suit including attorneys’ fees. We answered the complaint and denied the allegations in that pleading. In addition, we have asserted counterclaims against the plaintiff. Discovery in the action is active and ongoing. We are defending this action vigorously.
In addition, on or about January 7, 2010, Zein Obagi and his spouse, Samar Obagi, and the Zein and Samar Obagi Family Trust and certain other entities allegedly affiliated with Zein Obagi sent us an arbitration demand before JAMS in Los Angeles, California in which the claimants claim breaches of the 2006 Services Agreement and 2006 Separation and Release Agreement (see Note 9 in our Notes to Consolidated Financial Statements) between us and the claimants and various breaches of common law, California law, and federal law, including failure to pay certain retainer, marketing and reimbursement funds, failure to pay royalties allegedly due, failure to consult before developing and marketing new products, failure to pursue certain business strategies, threatening to invoke unenforceable and inapposite non-competition clauses in our contracts with Zein Obagi, threatening and interfering with the claimants’ business opportunities, threatening to enforce nonexistent trademark and service mark rights, and wrongfully depriving the claimants of both their rights to pursue business opportunities and also their trademark rights. Claimants seek actual and consequential damages of “millions of dollars” and other damages in an unspecified amount, restitution of monies wrongfully obtained in an unspecified amount, prejudgment interest, attorneys fees and costs, declaratory judgment and assignment of certain trademarks from us to Dr. Obagi. We have filed an answer to the Demand for Arbitration denying the allegations and asserting various counterclaims. In May 2010, the claimants in the arbitration proceeding filed an amended demand, again before JAMS in Los Angeles. The amended demand reiterates the claims asserted in the original demand. The amended demand expands the original demand's factual allegations involving our alleged interference with the claimants' rights to pursue business opportunities and with the claimants' trademark rights. Discovery in this matter is active and ongoing. We are defending the arbitration vigorously.
If the ultimate outcome is unfavorable, these matters may have a material adverse effect on our consolidated financial condition, results of operations and cash flows. Regardless of the outcome, the costs of litigation will be substantial.
34
From time to time, we are involved in other litigation and legal matters or disputes in the normal course of business. Management does not believe that the outcome of any of these other matters at this time will have a material adverse effect on our consolidated financial position, results of operations or cash flows.
35
PART II
Market Information
Our common stock is traded on the Nasdaq Global Market under the symbol “OMPI” and has been quoted since our initial public offering on December 13, 2006. The initial public offering price of our common stock on December 14, 2006 was $11.00 per share. The following table sets forth for the fiscal periods indicated the high and low sale prices for our common stock as reported by Nasdaq:
|
High
|
Low
|
|||||||
|
Fiscal 2010
|
||||||||
|
First Quarter
|
$ | 12.98 | $ | 10.21 | ||||
|
Second Quarter
|
$ | 14.16 | $ | 11.82 | ||||
|
Third Quarter
|
$ | 12.33 | $ | 9.74 | ||||
|
Fourth Quarter
|
$ | 12.68 | $ | 10.18 | ||||
|
Fiscal 2009
|
||||||||
|
First Quarter
|
$ | 7.99 | $ | 4.00 | ||||
|
Second Quarter
|
$ | 7.59 | $ | 5.22 | ||||
|
Third Quarter
|
$ | 11.60 | $ | 7.00 | ||||
|
Fourth Quarter
|
$ | 12.00 | $ | 10.12 | ||||
Holders
The approximate number of holders of our common stock was 2,139 as of February 25, 2011. Because many of the shares of our common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial owners represented by these stockholders of record.
Dividends
We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our Board of Directors, subject to compliance with certain covenants under our Credit and Term Loan Agreement, which limit our ability to declare or pay dividends, and will depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our Board of Directors may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth information regarding outstanding options and rights and shares reserved for future issuance under our equity compensation plans as of December 31, 2010:
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders
|
1,207,067 | $ | 10.63 | 1,647,254 | ||||||||
|
Equity compensation plans not approved by security holders
|
— | — | — | |||||||||
|
Total
|
1,207,067 | $ | 10.63 | 1,647,254 | ||||||||
36
Issuer Purchases of Equity Securities
On October 26, 2010, we announced that our Board of Directors authorized us to repurchase up to $45.0 million of our common stock, depending on market conditions and other factors. Share repurchases have included the repurchase of $35.0 million of shares from certain selling stockholders following completion of the registered public offering of shares of our common stock by such stockholders in November 2010 (the “secondary offering”), and may include future repurchases made through open market or privately negotiated transactions in compliance with SEC Rule 10b-18, subject to market conditions, applicable legal requirements and other factors. This authorization does not obligate us to acquire any particular amount of common stock nor does it ensure that any shares will be repurchased, and it may be suspended at any time at our discretion.
The following table provides information regarding repurchases of our common stock made by us, of our equity securities registered pursuant to Section 12 of the Exchange Act, for each month during the fourth quarter of fiscal year 2010, in the format required by SEC rules:
|
Total Number of Shares Purchased
|
Average Price Paid per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs
|
Maximum Dollar Amount of Shares (in thousands) That May Yet Be Purchased Under the Plans or Programs
|
|||||||||||||
|
October 1, 2010 - October 31, 2010
|
— | — | — | $ | 45,000 | |||||||||||
|
November 1, 2010 - November 30, 2010
|
3,556,910 | $ | 9.84 | 3,556,910 | $ | 10,000 | ||||||||||
|
December 1, 2010 - December 31, 2010
|
— | — | — | $ | 10,000 | |||||||||||
|
Total
|
3,556,910 | $ | 9.84 | 3,556,910 | $ | 10,000 | ||||||||||
The following information is not deemed to be “soliciting material” or to be “filed” with the SEC or subject to the liabilities of Section 18 of the Exchange Act, and shall not be deemed to be incorporated by reference into any prior or subsequent filing by us under the Securities Act or the Exchange Act, whether made on, before or after the date of this filing and irrespective of any general incorporation language in such filing.
The Performance Graph below represents a comparison of the total return of our common stock, the NASDAQ Market Index and the SIC—Pharmaceutical Preparations for the period between December 14, 2006 and December 31, 2010. The graph assumes $100 was invested on December 14, 2006 in stock, or November 30, 2006 in the index and peer group, and that all dividends, if any, have been reinvested. Prices and stockholder returns over the indicated periods should not be considered indicative of future stock prices or stockholder returns.
37
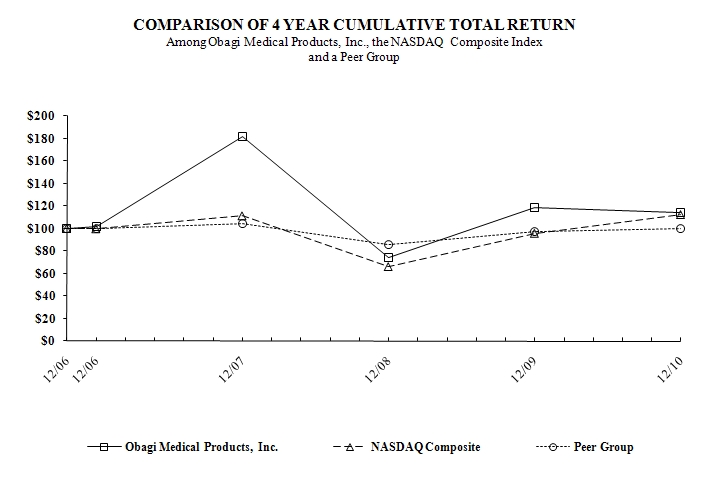
The selected consolidated financial data set forth below are derived from our audited consolidated financial statements for the fiscal years ended December 31, 2010, 2009, 2008, 2007 and 2006. The information in the following table should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this Report.
|
Year Ended December 31,
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
Consolidated statements of income data:
|
||||||||||||||||||||
|
Net sales
|
$ | 112,763 | $ | 104,096 | $ | 104,593 | $ | 102,648 | $ | 77,996 | ||||||||||
|
Net income
|
9,491 | 11,333 | 12,588 | 15,203 | 6,116 | |||||||||||||||
|
Net income attributable to common shares
|
||||||||||||||||||||
|
Basic
|
$ | 0.44 | $ | 0.52 | $ | 0.56 | $ | 0.69 | $ | 0.34 | ||||||||||
|
Diluted
|
$ | 0.44 | $ | 0.51 | $ | 0.56 | $ | 0.69 | $ | 0.34 | ||||||||||
|
December 31,
|
||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
|
Consolidated balance sheet data:
|
||||||||||||||||||||
|
Total assets
|
$ | 62,707 | $ | 87,490 | $ | 72,259 | $ | 59,763 | $ | 52,961 | ||||||||||
|
Long-term debt less current portion
|
— | — | 18 | 42 | 23,052 | |||||||||||||||
38
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business and related financing requirements, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this Report for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a specialty pharmaceutical company that develops, markets and sells, and are a leading provider of, proprietary topical aesthetic and therapeutic prescription-strength skin care systems in the physician-dispensed market. Our products are designed to prevent and improve some of the most common and visible skin disorders in adult skin, including premature aging, photodamage, skin laxity, hyperpigmentation, acne, sun damage, facial redness and soft tissue deficits, such as fine lines and wrinkles.
Current products. Our primary product line is the Obagi Nu-Derm System, which we believe is the leading clinically proven, prescription-based, topical skin health system on the market that has been shown to enhance the skin’s overall health by correcting photodamage at the cellular level, resulting in a reduction of the visible signs of aging. The primary active ingredients in this system are 4% hydroquinone and OTC skin care agents. In April 2004, we introduced the Obagi-C Rx System consisting of a combination of prescription and OTC drugs and adjunctive cosmetic skin care products to treat skin conditions resulting from sun damage and the oxidative damage of free radicals. The central ingredients in this system are 4% hydroquinone and Vitamin C. In October 2005, we launched the Obagi Professional-C products, a complete line of proprietary, non-prescription products, which consists of Vitamin C serums used to reduce the appearance of damage to the skin caused by ultraviolet radiation and other environmental influences. In July 2006, we launched our Obagi Condition & Enhance System, for use in conjunction with commonly performed surgical and non-surgical cosmetic procedures. In October 2006, we launched our first product in the ELASTIderm product line, an eye cream for improving the elasticity and skin tone around the eyes. We introduced the CLENZIderm M.D. System and a second product in the ELASTIderm product line to address acne and skin elasticity around the eye, respectively, based on positive interim clinical results, in February 2007. In July 2007, we launched our second system in the CLENZIderm M.D. line, CLENZIderm M.D. System II, which is specifically formulated for normal to dry skin. In August 2007, we launched two new Nu-Derm Condition & Enhance Systems. One is designed specifically for use with non-surgical procedures while the other has been developed for use with surgical procedures. In February 2008, we launched ELASTIderm Décolletage, a system to treat skin conditions resulting from sun damage and improve the elasticity and skin tone for the neck and chest area. In January 2009, we launched Obagi Rosaclear, a system to treat the symptoms of rosacea. In September 2009, we also began offering Refissa by Spear, a FDA-approved 0.05% strength tretinoin with an emollient base that has a broad indication for treatment of fine facial lines, hyperpigmentation and tactile roughness. In October 2010, we launched ELASTILash Eyelash Solution, a peptide-based eyelash solution that can help achieve the appearance of thicker, fuller-looking eyelashes. In January 2011, we launched Blue Peel RADIANCE, a gentle salicylic acid-based peel that utilizes a unique blend of acids and other soothing ingredients to exfoliate, even out skin tone and improve overall complexion, with little-to-no downtime. We also market tretinoin, used for the topical treatment of acne in the U.S., metronidazole, used for the treatment of facial rosacea in the U.S., and the Obagi Blue Peel Essential Kit, used to aid the physician in the application of skin peeling actives.
Future products. We focus our research and new product development activities on improving the efficacy of established prescription and OTC therapeutic agents by enhancing the penetration of these agents across the skin barrier using our proprietary technologies collectively known as Penetrating Therapeutics. However, we cannot assure you that we will be able to introduce any additional systems using these technologies.
U.S. distribution. We market all of our products through our direct sales force in the United States primarily to plastic surgeons, dermatologists and other physicians who are focused on aesthetic skin care.
Aesthetic skin care. As of December 31, 2010, we sold our products to approximately 6,500 physician-dispensing accounts in the United States, with no single customer accounting for more than 5% of our net sales. Our current products are not eligible for reimbursement from third-party payors such as health insurance organizations. We generated U.S. net sales of $94.5 million and $85.4 million during the years ended December 31, 2010 and 2009, respectively.
International distribution. We market our products internationally through 20 international distribution and two licensing partners that have sales and marketing activities in 45 countries outside of the United States. Our distributors use a model similar to our business model in the United States, selling our products through direct sales representatives to physicians, or through alternative
39
distribution channels depending on regulatory requirements and industry practices. We generated international net sales of $13.9 million and $14.0 million during the years ended December 31, 2010 and 2009, respectively.
Licensing. We market our products in the Japanese retail markets through license agreements with Rohto. Under our agreements, Rohto is licensed to manufacture and sell a series of OTC products developed by it under the Obagi brand name, as well as Obagi-C products, in the Japanese drug store channel, and we receive a royalty based upon sales of Obagi branded products in Japan by Rohto. Rohto’s Obagi branded products are sold through approximately 6,300 high-end drug stores. We have other licensing arrangements in Japan to market and sell OTC product systems under the Obagi brand, both for in-office use in facial procedures, as well as for sale as a take-home product kit in the spa channel. We receive royalties based upon these arrangements. We generated licensing revenue of $4.4 million and $4.6 million during the years ended December 31, 2010 and 2009, respectively.
Exit of Pharmacy Channel. In August 2008, we entered the pharmacy channel for the first time by launching SoluCLENZ Rx Gel, a solubilized BPO gel for the treatment of acne, which was available only by prescription. However, after closely monitoring the progress of the launch and weekly sales data, we determined that the distribution of a single prescription product through the pharmacy channel and the ongoing investment to support that channel had become cost-prohibitive. Accordingly, on April 13, 2009, we announced that we would no longer sell SoluCLENZ in the pharmacy channel.
In connection with the exit of the pharmacy channel, during the year ended December 31, 2009, we recorded charges approximating $0.8 million, related to contractual deposits, obsolete selling materials and other contract termination fees (included within “Selling, general and administrative expenses” in the Consolidated Statements of Income). In addition, during the year ended December 31, 2009, we reserved approximately $0.4 million in inventory (included within “Cost of sales” in the Consolidated Statements of Income). During the quarter ended December 31, 2009, revenue for the remaining units dispensed during the period was fully recognized as we informed our SoluCLENZ distributors that we were no longer going to issue credit for returned inventory.
Sales. Our total net sales have grown from $104.1 million for the year ended December 31, 2009 to $112.8 million for the year ended December 31, 2010. Factors contributing to our sales generation include:
|
•
|
Our professional marketing efforts to physicians. Our professional sales force targets physicians who are focused on aesthetic and therapeutic skin care and educates them on how to best provide our products to their patients. Our professional sales force also provides education to physicians, aestheticians, other staff and patients about the benefits of promoting skin health. We have increased our sales force from 96 employees as of January 1, 2006 to 131 employees as of December 31, 2010. We will continue to invest in expanding our sales force as warranted by the success of new product offerings and continued core product growth. We currently do not have plans to hire a substantial number of employees in 2011.
|
|
•
|
Strong growth in the aesthetic skin care market. We believe the market demand for cosmetic facial procedures reflects a growing desire and acceptance among the aging population to seek aesthetic facial products and procedures from their physicians. With our leading position in the physician-dispensed channel, the clinically proven aesthetic benefits of our products and the potential of our systems to enhance or complement many other facial procedures, we believe we are well positioned to meet this growing patient demand.
|
|
•
|
Conducting clinical studies on our products. We have completed 86 clinical studies since 2003 to demonstrate the efficacy of our products. We believe that these clinical studies provide our products added scientific credibility in the physician-dispensed market. We currently plan to initiate approximately 17 additional clinical studies in 2011.
|
|
•
|
International. We have formal distribution agreements with 20 distribution and two licensing partners and a trademark and product know-how license agreement in the OTC market in Japan. Currently, those distributors have sales activities in approximately 45 countries, with the most successful distributors in 2010 coming primarily from the Middle East, Europe and Southeast Asia.
|
Results of operations. We commenced operations in 1997, and as of December 31, 2010, we had accumulated earnings of $27.4 million. We reported net income of $9.5 million and $11.3 million for the years ended December 31, 2010 and December 31, 2009, respectively.
Seasonality. Sales of our products have historically been higher between September and March. We believe this is due to increased product use and patient compliance during these months. We believe this increased usage and compliance relates to several factors such as higher patient tendencies toward daily compliance inversely proportionate to their tendency to travel and/or engage in other disruptive activities during summer months. Patient travel and other disruptive activities that affect compliance are at their peak during July and August. The effects of seasonality in the past have been offset by the launch of new products. This trend was very pronounced during 2007 when we launched four new product offerings and rebranded two systems. However, we cannot assure you that we will continue to be able to offset such seasonality in the future.
40
Economy. Many treatments in which our products are used are considered cosmetic in nature, are typically paid for by the patient out of disposable income and are generally not subject to reimbursement by third-party payors such as health insurance organizations. As a result, we believe that our current and future sales growth may be influenced by the economic conditions within the geographic markets in which we sell our products. Although there are modest signs of economic recovery, it is unclear whether the economy will show sustained growth and/or stability. Accordingly, we cannot assure you that the improvement in revenue growth that we experienced in the fourth quarter of 2009 and during the year ended December 31, 2010, will be sustainable. Even with continued growth in many of our markets during this period, the recent recession could adversely impact our business in the future causing a decline in demand for our products, particularly if uncertain economic conditions are prolonged or worsen. We do believe that some of the negative impact experienced during the majority of 2009 was partially offset due to the following: (i) we are the leader in the physician-dispensed market; (ii) the aesthetic nature of our products; (iii) the lower price point of our products compared to other aesthetic products in our market; (iv) the desire to maintain a healthy and youthful appearance; and (v) the demographics of the patients who use our products.
Future growth. We believe that our future growth will be driven by increased direct sales coverage, penetration into non-core markets such as other medical specialties, ongoing marketing efforts to create increased awareness of the Obagi brand and the benefits of skin health and new product offerings. We plan to continue to invest resources on the commercialization of new applications of our current products, the continuing development of our pipeline of products and the in-licensing or acquisition of new product opportunities. However, our current business plan does not anticipate that we invest significant resources in these strategic initiatives in 2011. As a result, we believe that our ongoing profitability is primarily dependent upon the continued success of our current product offerings and certain other strategic marketing initiatives.
Critical Accounting Policies and Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, sales and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On a periodic basis, we evaluate our estimates, including those related to revenue recognition, sales returns and allowances, accounts receivable, inventory, goodwill and other intangible assets. We use authoritative pronouncements, historical experience and other assumptions as the basis for making estimates. By their nature, these estimates are subject to an inherent degree of uncertainty. As a result, it is possible that our actual results will differ significantly from these estimates. Our significant accounting policies are further described in Note 2 of Notes to Consolidated Financial Statements included elsewhere in this Report.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue recognition. We recognize revenues for our physician-dispensed segment in accordance with ASC 605, Revenue Recognition (“ASC 605”), which provides guidance on the recognition, presentation and disclosure of revenue in financial statements filed with the SEC. ASC 605 outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure related to revenue recognition policies. We recognize revenue when: (i) persuasive evidence of an arrangement exists; (ii) shipment of products has occurred or services have been rendered; (iii) the sales price charged is fixed or determinable; and (iv) collection is reasonably assured. Our shipment terms are FOB shipping point as outlined in our sales agreements.
Our domestic sales agreements do not provide for rights of return or price protection. However, we may approve returns on a case-by-case basis at our discretion. Certain international distribution agreements do provide for rights of return and price protection. Generally, such return rights are for a period of not more than 90 days after the products have been shipped to the distributors. In accordance with ASC 605-15, Revenue Recognition - Products (“ASC 605-15”), we continuously monitor and track product returns and record a provision for the estimated amount of such returns, based on historical experience and any notification we receive of pending returns. We do not grant any warranty provisions on our products. We provide for discounts and allowances based on historical experience at the time revenue is recognized as a reduction to revenue. To date, such provisions have approximated management’s estimates. Other than our allowance for sales returns, the only estimates that we have to make regarding revenue recognition pertain to the collectability of the resulting receivable, both of which are discussed below.
We grant price protection rights to certain international distributors. Such price protection rights require us to pay the distributor if there is a reduction in the list price of our products. Price protection payments would be required for the distributor’s inventory on-hand or in-transit on the date of the price reduction, for a period not to exceed 90 days prior to the date of the price reduction. We have not recorded a liability in connection with such price protection rights as we have never reduced the list prices of our products.
41
In September 2002, we entered into a licensing agreement, as amended in December 2008, with Rohto (see Note 8 of Notes to Consolidated Financial Statements), a large Japan-based company that specializes in the distribution and marketing of OTC medical oriented products in the drug store and retail channels. In January 2006, we entered into a licensing agreement with Tokyo Beauty Center, a diversified Japanese consumer products and services company, which also owns and operates a large chain of aesthetic spas in Japan. Royalty revenue is recognized as earned and is based upon a predetermined rate within the respective licensing agreement.
As noted earlier, during the year ended December 31, 2008, we entered the pharmacy channel for the first time by launching SoluCLENZ Rx Gel, which was available only by prescription. We sold SoluCLENZ to pharmaceutical wholesalers, who had the right to return purchased product prior to the units being dispensed through patient prescriptions. On April 13, 2009, we announced that we would no longer sell SoluCLENZ in the pharmacy channel. Revenue for this product was recognized in accordance with ASC 605. Among its criteria for revenue recognition from sale transactions where a buyer has a right of return, ASC 605-15 requires the amount of future returns to be reasonably estimated. As substantially all of the risks and rewards of ownership did not transfer upon shipment, we treated the sale of product under a consignment model. Under this consignment model, we did not recognize revenue upon the shipment of product with the guaranteed sale provision and accounted for inventory held by the wholesalers as consignment inventory. Due to our limited experience in the pharmacy channel, we were unable to reasonably estimate customer returns and, therefore, only recognized revenue related to SoluCLENZ units once the units had been dispensed through patient prescriptions because units dispensed to patients were not subject to return. We obtained actual prescription units dispensed based on distribution channel data provided by external, independent sources. In connection with our exit from the pharmacy channel, during the year ended December 31, 2009, revenue for the remaining units dispensed during the period was fully recognized as we informed our SoluCLENZ distributors that we were no longer going to issue credit for returned inventory.
Sales returns and allowances. When we sell our products, we reduce the amount of revenue recognized from such sales by an estimate of future product returns and other sales allowances. Sales allowances include cash discounts, rebates and sales incentives relating to products sold in the current period. Factors that are considered in our estimates of sales returns include the historical rate of returns as a percentage of net product sales, gross of returns and allowances and shipping and handling revenue, historical aging of returns and the current market conditions. Although our domestic sales agreements do not provide for a contractual right of return, we maintain a return policy that allows our customers to return product within a specified period after shipment of the product has occurred. Factors that are considered in our estimates regarding sales allowances include quality of product and recent promotional activity. If actual future experience for product returns and other sales allowances exceeds the estimates we made at the time of sale, our financial position, results of operations and cash flow would be negatively impacted. To date, such provisions have approximated management’s estimates.
Accounts receivable. We perform periodic credit evaluations of our customers and adjust credit limits based upon payment history and the customer’s current creditworthiness, as determined by our review of current credit information. Receivables are generally due within 30 days. However, the recent recession and ongoing tightening of credit in financial markets has, in some cases, adversely impacted our customers’ cash flow and ability to access sufficient credit in a timely manner, which, in turn, has impacted their ability to make timely payments to us. In light of these circumstances, and in order to remain competitive in the marketplace, for certain selected customers who we deemed to be creditworthy based upon their prior payment history; we extended our standard payment terms to net 60 days for selected product purchases made in connection with specific sales promotion programs. Such extension did not represent a permanent change to the payment terms for such customers but, rather, was applicable only to those specified purchases made by such customers in connection with the related sales promotion. We deem a receivable to be past due when it has not been paid in accordance with the terms of the applicable invoice (e.g., net 30 days or net 60 days) prepared at the time of sale. Accounts receivable, net of allowance for doubtful accounts and sales returns, were $22.7 million and $24.2 million as of December 31, 2010 and 2009, respectively. Of these amounts, 86.2%, or $19.6 million, and 81.6%, or $19.8 million, were deemed current as of December 31, 2010 and 2009, respectively. The percentage of accounts receivable deemed more than 90 days past due as of December 31, 2010 and 2009 was 0% and 2.3%, respectively. The percentage of accounts receivable deemed more than 180 days past due as of December 31, 2010 and 2009 was 0.0% for both periods.
We monitor collections and payments from our customers and maintain an allowance for doubtful accounts based upon our historical write-offs as a percentage of product sales, adjusted specifically for accounts that are past due, non-performing, in bankruptcy or otherwise identified as at risk for potential credit loss. Receivables are charged to the allowance for doubtful accounts when an account is deemed to be uncollectible, taking into consideration the financial condition of the customer and the value of any collateral. As of December 31, 2010 we increased our allowance for doubtful accounts to $2.2 million, from $1.5 million at December 31, 2009. The increase was primarily related to a non-performing international distributor (see Note 2 in our Notes to Consolidated Financial Statements). Recoveries of receivables previously charged off as uncollectible are credited to the allowance. On an annual basis, customers with significant purchases are reviewed for their creditworthiness. If the financial condition of our customers deteriorates, resulting in an impairment of their ability to make payments and if the uncollectible balances exceed our estimates as of the balance sheet date, we would need to charge additional receivables to our allowances, and our financial position, results of operation and cash flow would be negatively impacted. Our credit losses have historically been within our expectations and the allowance established.
42
Inventory. We state our inventories at the lower of cost or market, computed at standard cost on a first-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Inventory reserves are established when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. Inventory reserves are measured as the difference between the cost of inventory and estimated market value. Inventory reserves are charged to cost of sales and establish a lower cost basis for the inventory. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to changing technology, new product offerings and customer demand levels. Unfavorable changes in market conditions may result in a need for additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins. To date, actual reserve requirements have approximated management’s estimates.
Goodwill and other intangible assets. Effective January 1, 2002, we adopted authoritative guidance issued by the FASB, which requires, among other things, the use of a nonamortization approach for purchased goodwill and certain intangibles. Under a nonamortization approach, goodwill and intangibles having an indefinite life are not amortized, but instead will be reviewed for impairment at least annually or earlier if an event occurs or circumstances indicate the carrying amount may be impaired. Events or circumstances that could indicate impairment include a significant change in the business climate, economic and industry trends, legal factors, negative operating performance indicators, significant competition, changes in our strategy or disposition of a reporting unit or a portion thereof. Goodwill impairment testing is performed at the reporting unit level.
The guidance under ASC 350, Intangibles – Goodwill and Other (“ASC 350”), requires that goodwill be tested for impairment using a two-step process. The first step of the goodwill impairment test, used to identify potential impairment, compares the fair value of a reporting unit with its carrying amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test must be performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of reporting unit goodwill with the carrying amount of that goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. If the carrying amount of the reporting unit goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess.
Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to such reporting units, assignment of goodwill to such reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated using a discounted cash flow methodology. This requires significant judgments including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth for our business, the useful life over which cash flows will occur, and determination of our weighted average cost of capital. Changes in these estimates and assumptions could materially affect the determination of fair value and/or goodwill impairment for each reporting unit.
We have selected September 30 as the date on which we perform our annual goodwill impairment test. We attribute the entire balance of our goodwill of $4.6 million to our largest operating segment, which is also a reporting unit, the physician-dispensed segment. Our determination of fair value of our goodwill considered the current and future economic, market, competitive conditions, and other relevant factors. We evaluated the fair value of our goodwill using two approaches. For the first approach, we derived valuation multiples from historical earnings data of guideline companies within our industry that compete in the physician-dispensed market, and then evaluated and adjusted the multiples based on our strengths and weaknesses relative to the guideline companies. The derived multiples were then applied to our operating data to arrive at an indication of fair value. Cash-free invested capital multiples, representing a marketable, minority interest, were calculated for the guideline companies as of September 30, 2010. Revenue and earnings multiples were based on a valuation of the guideline companies’ profit margins, historical growth patterns, and a review of our own market factors, primarily related to our physician-dispensed business. The second approach, the discounted cash flow method, focused on the expected cash flows of our physician-dispensed business. We evaluated earnings projections through 2015, trending the growth down to 3% in the terminal year. The discount rate for our physician-dispensed business was determined by utilizing a weighted average cost of capital analysis, which analyzed long-term government bonds, the Moody’s Seasoned Baa bond rate, the cost of equity of similar companies relative to our physician-dispensed business, as well as our capital structure. Both methods were weighted at 50%. The analyses concluded that the fair value of our physician-dispensed segment significantly exceeded the carrying amount of our goodwill. There were no impairment triggers subsequent to September 30, 2010. As such, based on our analyses, no impairment charges were recognized for the years ended December 31, 2010, 2009 and 2008.
Other intangible assets consist of trademarks, distribution rights, covenants not-to-compete, patents, customer lists, and proprietary formulations. Other intangible assets are amortized over the expected period of benefit using the straight-line method over the following lives: trademarks (20 years); distribution rights (ten years); covenants not-to-compete (seven years); and other intangible assets (three to 17 years). In accordance with ASC 350, long-lived assets (including intangible assets that are amortized) are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, such as a significant sustained change in the business climate, during the interim periods. If an indicator of impairment exists for any
43
grouping of assets, an estimate of undiscounted future cash flows is compared to the asset group’s carrying value. If an asset is determined to be impaired, the loss is measured by the excess of the carrying amount of the asset over its fair value as determined by an estimate of discounted future cash flows. There were no indicators of impairment noted during 2010; therefore no tests were performed for long-lived assets. Any unfavorable changes in market conditions or our product lines may result in the impairment of goodwill or an intangible asset, which could adversely impact our net income.
Leases. We account for leases under the guidance of ASC 840, Leases, which requires that our leases be evaluated and classified as either operating leases or capital leases for financial reporting purposes. Minimum base rents for our operating leases, which generally have scheduled rent increases over the term of the lease, are recorded on a straight-line basis over the lease term. The initial lease term includes the period from when we are given access and control over the lease property, whether or not rent payments are due under the terms of the lease.
For leases with renewal periods at our option, we generally consider the lease term to consist of the initial lease term, as exercise of the renewal options as determined at lease inception is not considered to be reasonably assured. However, if failure to exercise a renewal option imposes an economic penalty of sufficient magnitude to us, then the renewal, at inception, is reasonably assured and will be included in the determination of the appropriate lease term.
In certain instances, we disburse cash for leasehold improvements, furnishings, fixtures and equipment to renovate leased premises. If costs are paid directly by the landlord or reimbursed to us by the landlord, we record a deferred rent liability and amortize the deferred rent liability over the lease term as a reduction to rent expense.
Stock-based compensation. We account for stock-based compensation in accordance with the guidance under ASC 718, Compensation (“ASC 718”). Determining the appropriate fair-value model and calculating the fair value of stock-based awards at the date of grant using any valuation model requires judgment. We use the Black-Scholes option-pricing model, which requires the input of highly subjective assumptions. These assumptions include estimating the length of time employees will retain their stock options before exercising them (“expected term”), the estimated volatility of our common stock price over the expected term and the number of options that will ultimately not complete their vesting requirements (“forfeitures”). We estimated our options’ expected terms in accordance with ASC 718, using our best estimate of the period of time from the grant date that we expect the options to remain outstanding. If we determine that another method to estimate expected volatility or expected term yields a more accurate estimate than our current methods, or if another method for calculating these input assumptions is prescribed by authoritative guidance, the fair value calculated for stock-based awards could change significantly. Higher volatility and expected terms result in an increase to stock-based compensation determined at the date of grant. The expected dividend rate and expected risk-free rate of return are not as significant to the calculation of fair value.
Uncertainty in Income Taxes. The provisions under ASC 740 prescribe a threshold for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Only tax positions meeting the more-likely-than-not recognition threshold at the effective date may be recognized or continue to be recognized upon adoption of this interpretation. The provisions also provide guidance on accounting for derecognition, interest and penalties, and classification and disclosure of matters related to uncertainty in income taxes. As of December 31, 2010, the liability for unrecognized tax benefits was $0.2 million.
Results of Operations
The year ended December 31, 2010 compared the year ended December 31, 2009
Net sales. The following table compares net sales by product line and certain selected products for the years ended December 31, 2010 and 2009. Our sales previously reported in the pharmacy Rx operating segment are now classified as physician-dispensed as part of the Therapeutic product category. Prior periods have been reclassified to conform to the current presentation.
44
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
Change
|
||||||||||
| Net Sales by Product Category: | ||||||||||||
|
Physician-dispensed
|
||||||||||||
|
Nu-Derm
|
$ | 59,166 | $ | 56,057 | 6 | % | ||||||
|
Vitamin C
|
16,413 | 12,428 | 32 | % | ||||||||
|
Elasticity
|
11,551 | 9,491 | 22 | % | ||||||||
|
Therapeutic
|
7,261 | 8,668 | -16 | % | ||||||||
|
Other
|
13,971 | 12,822 | 9 | % | ||||||||
|
Total
|
108,362 | 99,466 | 9 | % | ||||||||
|
Licensing fees
|
4,401 | 4,630 | -5 | % | ||||||||
|
Total net sales
|
$ | 112,763 | $ | 104,096 | 8 | % | ||||||
|
United States
|
84 | % | 82 | % | ||||||||
|
International
|
16 | % | 18 | % | ||||||||
Net sales increased by $8.7 million to $112.8 million during the year ended December 31, 2010, as compared to $104.1 million during the year ended December 31, 2009. Overall, we believe our growth during the year ended December 31, 2010 is primarily due to a larger base of active accounts, higher level of patient visits due to increased consumer confidence versus last year, the launch of new products and the average price increase of 4% on our products implemented on January 1, 2010.
Physician-dispensed sales increased $8.9 million to $108.4 million during the year ended December 31, 2010, as compared to $99.5 million during the year ended December 31, 2009. We experienced an increase in the majority of our product categories as follows: (i) an increase in Vitamin C of $4.0 million, of which $1.7 million was attributable to our normal to oily line extension of Obagi-C Rx launched in January 2010; (ii) an increase in Nu-Derm sales of $3.1 million; (iii) a $2.1 million increase in Elasticity sales, of which $1.1 million was attributable to the launch of our ELASTILash product in October 2010; and (iv) an increase in the Other category of $1.1 million. The growth in the Other category was primarily attributable to the launch of Refissa in September 2009, which accounted for $0.7 million of the increase. These increases were partially offset by a decrease of $1.4 million in the Therapeutic category. The decline in the Therapeutic category was primarily attributable to the increased promotional activity surrounding the launch of our Rosaclear product in January 2009 and our exit of the pharmacy channel in April 2009. Licensing fees decreased by $0.2 million due to a decline in unit sales by our Japanese partner Rohto during the year ended December 31, 2010, as compared to the year ended December 31, 2009.
Our aggregate sales growth was composed of $9.1 million in the U.S., partially offset by a $0.2 million decrease from our International markets and a decline in licensing fees of $0.2 million. The decline in International sales was primarily due to declines in the Therapeutic and Other product categories and was principally a result of a $0.8 million decrease in the Far East region; partially offset by: (i) a $0.4 million increase in the Middle East; (ii) a $0.1 million increase from the Americas; and (iii) a $0.1 million increase from the Europe and Other regions.
Despite the growth we have experienced during the fiscal year 2010, we believe that the ongoing economic uncertainty could negatively impact our future net sales.
Gross margin percentage. Our gross margin percentage increased slightly to 79.0% for the year ended December 31, 2010, as compared to 78.8% for the year ended December 31, 2009. The overall increase is primarily attributable to an increase in gross margin for our physician-dispensed segment, which increased to 78.3% compared to 78.0% for the same period last year. The improvement was primarily a result of: (i) an increase in sales volumes primarily in our Nu-Derm and Vitamin C product categories; (ii) an average price increase of 4% on our products implemented on January 1, 2010; and a (iii) a decrease in sales volumes in our Therapeutic products, which have a lower margin than most of our other product categories; partially offset by a $0.7 million increase in product costs, largely in our Other product category. Our gross margin for our licensing segment increased slightly to 96.9% compared to 96.8% for the same period last year. Cost of sales also includes outbound shipping and handling, work order scrap, licensing and royalty fees related to licensed intellectual property, depreciation and amortization attributable to products sold, and an inventory reserve for shrinkage and write-downs.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of salaries and other personnel-related costs, professional fees, insurance costs, stock-based compensation, depreciation and amortization not attributable to products sold, warehousing costs, marketing costs, travel expenses, general and administrative support expenses and other selling expenses. Selling, general and administrative expenses increased $10.1 million to $69.3 million during the year ended December 31,
45
2010, as compared to $59.2 million for the year ended December 31, 2009. The increase was primarily due to the following: (i) $6.0 million in expenses for matters related to Dr. Obagi, including expenses related to pending litigation of approximately $5.6 million and $0.4 million related to the termination of the agreement dated as of June 29, 2006 (“2006 Agreement”), in September 2010, which includes the write-off of prepaid rent and leasehold improvements at a Beverly Hills location leased by us; (ii) a $2.3 million increase in salaries and related expenses due to an increase in sales commissions, accrued bonus and an increase in marketing and sales force headcount; (iii) $1.0 million in severance and other related charges in connection with the departure of Steven R. Carlson, our former President and Chief Executive Officer, in October 2010; (iv) a $1.0 million increase in other marketing expenses primarily due to the launch of ELASTILash in October 2010; (v) a $1.0 million increase in promotions and training expenses; (vi) $0.8 million in expenses related to our secondary offering completed in November 2010; (vii) a $0.5 million increase in expenses related to our third party logistics provider; (viii) a $0.3 million increase in other expenses, $0.2 million of which was due to losses on disposal of fixed assets; (ix) a $0.2 million increase in volume-driven expenses; and (x) a $0.2 million increase in product development expenses. These increases were partially offset by: (i) a $2.2 million decrease in SoluCLENZ-related expenses as a result of our exit from the pharmacy channel, of which $1.4 million was due to the distribution and support of the product in the pharmacy channel and $0.8 million was due to the write-off of nonrefundable deposits and the accrual of other contract termination costs; (ii) a $0.3 million decrease in professional fees; (iii) a $0.3 million decrease in depreciation and amortization; (iv) a $0.2 million decrease in non-cash compensation; (v) a $0.1 million decrease in expenses driven by being a public company; and (vi) a $0.1 million decrease in rent expense. As a percentage of net sales, selling, general and administrative expenses in the year ended December 31, 2010 increased to 61% as compared to 57% for the year ended December 31, 2009. We hope to minimize additional operating costs in fiscal year 2011, including professional fees where possible. We intend to maintain or decrease selling, general and administrative expenses as a percentage of net sales.
Research and development. Research and development expenses decreased $0.5 million to $3.9 million for the year ended December 31, 2010, as compared to $4.4 million for the year ended December 31, 2009. During 2010 we recorded $0.5 million in accelerated advisory and related fees pursuant to the termination of the 2006 Agreement with Dr. Obagi. In addition, salaries and related expenses increased $0.3 million. These increases were offset by: (i) a $0.6 million decrease in expenses related to the development of line extensions and reformulations of existing products; (ii) a $0.5 million decrease in expenses related to the development of new products; and (iii) $0.2 million in grant funds received under the Qualifying Therapeutic Discovery Grant Program administered by the Internal Revenue Service and the Department of Health and Human Services in support of the Company’s development of Nu-Derm for the treatment of melasma, which were recorded as an offset to research and development expense. As a percentage of net sales, research and development costs in the year ended December 31, 2010 were 3% as compared to 4% in the year ended December 31, 2009.
Loss on dissolution of foreign subsidiary. On June 10, 2010, we dissolved our majority-owned subsidiary, Obagi (S) Pte Ltd. As a result of the dissolution, we recognized a loss on dissolution of foreign subsidiary of $0.1 million during the year ended December 31, 2010, which represented the accumulated other comprehensive loss on our consolidated balance sheet as of June 10, 2010.
Interest income and Interest expense. Interest income declined to $0.1 million for the year ended December 31, 2010 from $0.2 million for the year ended December 31, 2009. We earn interest income from the investment of our cash balance into higher interest-yielding certificates of deposit. Although our average cash and cash equivalents, including short-term investments, increased from $26.0 million for the year ended December 31, 2009 to $36.9 million for the year ended December 31, 2010, our weighted average interest rate decreased from 0.78% during the year ended December 31, 2009 to 0.30% during the year ended December 31, 2010. Interest expense remained fairly flat at $0.1 million during the year ended December 31, 2010, as compared to the year ended December 31, 2009. Interest expense primarily consists of amortization of debt issuance costs and commitment fees related to our Credit and Term Loan Agreement.
Income taxes. Income tax expense decreased $0.8 million to $6.3 million for year ended December 31, 2010, as compared to $7.1 million for the year ended December 31, 2009. Although our effective tax rate increased 1.4% to 40.1% for the year ended December 31, 2010, compared to 38.7% for the year ended December 31, 2009, the decline in expense is due to a decline in income before provision for income taxes of $2.6 million. As in the prior year, the effective tax rate includes the tax benefit derived from the research and development credit during the year ended December 31, 2010. The increase in the effective tax rate is primarily due to certain costs incurred during the secondary offering and stock repurchase completed in November 2010 not being deductible for tax purposes during the year ended December 31, 2010.
The year ended December 31, 2009 compared the year ended December 31, 2008
Net sales. The following table compares net sales by product line and certain selected products for the years ended December 31, 2009 and 2008. Our sales previously reported in the pharmacy Rx operating segment are now classified as physician-dispensed as part of the Therapeutic product category. Prior periods have been reclassified to conform to the current presentation.
46
|
Year Ended December 31,
|
||||||||||||
|
2009
|
2008
|
Change
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Net Sales by Product Category:
|
||||||||||||
|
Physician-dispensed
|
||||||||||||
|
Nu-Derm
|
$ | 56,057 | $ | 58,378 | -4 | % | ||||||
|
Vitamin C
|
12,428 | 12,380 | 0 | % | ||||||||
|
Elasticity
|
9,491 | 11,643 | -18 | % | ||||||||
|
Therapeutic
|
8,668 | 6,260 | 38 | % | ||||||||
|
Other
|
12,822 | 11,115 | 15 | % | ||||||||
|
Total
|
99,466 | 99,776 | 0 | % | ||||||||
|
Licensing fees
|
4,630 | 4,817 | -4 | % | ||||||||
|
Total net sales
|
$ | 104,096 | $ | 104,593 | 0 | % | ||||||
|
United States
|
82 | % | 84 | % | ||||||||
|
International
|
18 | % | 16 | % | ||||||||
Net sales decreased by $0.5 million to $104.1 million during the year ended December 31, 2009, as compared to $104.6 million during the year ended December 31, 2008. Overall, the economic conditions within the U.S. had a slightly negative impact on our revenue during the year ended December 31, 2009. We believe that the economic downturn has reduced disposable income, which we believe has led to reduced patient visits to physician offices for aesthetic products and procedures, and fewer such procedures being performed and products being purchased. The U.S. economic slowdown effect was more pronounced within the Nu-Derm and Elasticity product lines.
Physician-dispensed sales decreased $0.3 million to $99.5 million during the year ended December 31, 2009, as compared to $99.8 million during the year ended December 31, 2008. The decline was due to the following: (i) a decline in Nu-Derm sales of $2.3 million, primarily as a result of the U.S. economic slowdown; (ii) a $2.1 million decline in Elasticity sales, partially due to the increased promotional activity surrounding the launch of our ELASTIderm Décolletage product in 2008 as compared to 2009; offset in part by: (i) a total increase of $2.4 million in Therapeutic sales; and (ii) an increase of $1.7 million in the Other category. The Therapeutic category increase was primarily attributable to the launch of our Rosaclear system, which contributed $2.5 million in sales and SoluCLENZ, which contributed $0.4 million in sales, offset by a decline of approximately $0.5 million in our CLENZIderm system sales. The Other category increase was primarily attributable to the launch of Refissa, part of our tretinoin system, which contributed $1.2 million for the year ended December 31, 2009. Licensing fees decreased by $0.2 million.
Our aggregate sales decline of $2.1 million in the U.S. was partially offset by a $1.6 million increase from our International markets. International sales growth came from all product lines and primarily came from three regions: (i) $1.2 million increase from the Europe and Other region; (ii) a $1.1 million increase from the Far East; and (iii) a $0.5 million increase from the Americas; offset in part by a decrease of $1.0 million in the Middle East. Our licensing fees decreased $0.2 million.
Gross margin percentage. Our gross margin percentage decreased to 78.8%, for the year ended December 31, 2009 compared to 80.9% for the year ended December 31, 2008. The overall decline is primarily attributable to a decline in gross margin for our physician-dispensed segment, which decreased to 78.0% compared to 80.2% for the same period last year. The decline primarily resulted from: (i) an increase in discounting promotional activities of approximately $1.7 million; (ii) a change in product sales mix; and (iii) a $0.4 million reserve on SoluCLENZ inventory in connection with our exit of the pharmacy channel. Our gross margin for our licensing segment decreased to 96.8% compared to 97.2% for the same period last year.
Selling, general and administrative. Selling, general and administrative expenses decreased $0.2 million to $59.2 million during the year ended December 31, 2009, as compared to $59.4 million for the year ended December 31, 2008. The decline was primarily due to the following: (i) a $1.6 million decrease in professional fees, consisting primarily of a reduction in legal and consulting expenses; (ii) a $0.5 million decrease in other marketing, principally as a result of cost cutting initiatives; (iii) a $0.4 million decrease in expenses related to our SoluCLENZ product line, which consisted of a decrease of $1.2 million in expenses to support the pharmacy channel, offset by $0.8 million for the write-off of nonrefundable deposits and the accrual of other contract termination costs; and (iv) a $0.4 million decrease in non-cash compensation primarily due to the vesting of restricted stock for certain employees during the year ended December 31, 2009; offset in part by: (i) a $1.0 million increase in salaries and related expenses primarily due to commissions, bonus and severance of $0.4 million; (ii) a $0.5 million increase in promotional expenses; (iii) a $0.4 million increase in other expenses primarily related to taxes; (iv) a $0.4 million increase in volume driven activities; (v) a $0.2 million increase in advertising expenses; (vi) a $0.1 million increase in product development expenses; and (vii) a $0.1 million increase in depreciation and amortization primarily due to the implementation of our new Enterprise Resource Planning (“ERP”) system in early 2009. As a percentage of net sales, selling,
47
general and administrative expenses in the year ended December 31, 2009 remained flat at 57% as compared to the year ended December 31, 2008.
Research and development. Research and development expenses decreased $0.9 million to $4.4 million for the year ended December 31, 2009, as compared to $5.3 million for the year ended December 31, 2008. This was primarily due to a $0.8 million decrease related to the development costs for new indications of our existing products and a $0.1 million decrease in costs related to the development of new products. As a percentage of net sales, research and development costs in the year ended December 31, 2009 were 4% as compared to 5% in the year ended December 31, 2008.
Interest income and Interest expense. Interest income decreased $0.2 million to $0.2 million for the year ended December 31, 2009, as compared to $0.4 million for the year ended December 31, 2008. Although our average cash and cash equivalents, including short term investments, increased from $21.3 million for the year ended December 31, 2008 to $26.0 million for the year ended December 31, 2009, our weighted average interest rate decreased from 2.07% during the year ended December 31, 2008 to 0.78% during the year ended December 31, 2009. Interest expense was $0.1 million during the year ended December 31, 2009, as compared to $0.2 million for the year ended December 31, 2008. In connection with entering into our Former Credit Facility in November 2008, our Credit Agreement entered into on January 28, 2005 (as amended) was terminated. Termination of the agreement resulted in a write-off of debt issuance costs of approximately $0.1 million during the year ended December 31, 2008 and was charged to interest expense.
Income taxes. Income tax expense decreased $0.4 million to $7.1 million for year ended December 31, 2009, as compared to $7.5 million for the year ended December 31, 2008. Although our effective tax rate increased 1.3% to 38.7% for the year ended December 31, 2009, compared to 37.4% for the year ended December 31, 2008, the decline in expense is due to a decline in income before provision for income taxes of $1.6 million. The increase in the effective tax rate was primarily due to an increase in state taxes during the year ended December 31, 2009
Liquidity and Capital Resources
Trends and uncertainties affecting liquidity
Our primary sources of liquidity are our cash generated by operations and availability under our Credit and Term Loan Agreement. As of December 31, 2010 we had approximately $15.1 million in cash and cash equivalents and $35.0 million available under the Facility and Term Loans. We currently believe that our existing cash balances and cash generated by operations, together with our available credit capacity, will enable us to meet foreseeable liquidity requirements. The following has or is expected to impact liquidity:
|
•
|
in October 2010, we announced that our Board of Directors authorized us to repurchase up to $45.0 million of our common stock, depending on market conditions and other factors. During the year ended December 31, 2010, we repurchased $35.0 million of our outstanding common stock. On August 5, 2008, the Board of Directors gave us the authority to repurchase up to $10 million of our outstanding common stock, which expired on August 5, 2010. During the years ended December 31, 2009 and 2008, we purchased $1.3 million and $4.0 million of our outstanding stock, respectively; and
|
|
•
|
the legal costs associated with the litigation and arbitration demand involving us and Dr. Obagi (see Note 10 in our Notes to Consolidated Financial Statements) could be material regardless of the outcome.
|
We are operating in an uncertain and volatile economic environment, which could have unanticipated adverse effects on our business. The pharmaceutical industry has been impacted by the volatility in the financial markets, including declines in stock prices, and by uncertain economic conditions. Increases in food and fuel prices, changes in the availability of consumer credit and housing markets, actual and potential job losses among many sectors of the economy, significant declines in the stock market resulting in large losses to consumer retirement and investment accounts, and uncertainty regarding future federal tax and economic policies have all added to declines in consumer confidence and curtailed consumer spending.
The recent recession and ongoing tightening of credit in financial markets has, in some cases, adversely impacted our customers’ cash flow and ability to access sufficient credit in a timely manner, which, in turn, has impacted their ability to make timely payments to us. In light of these circumstances, and in order to remain competitive in the marketplace, for certain selected customers who we deemed to be creditworthy based upon their prior payment history, we extended our standard payment terms from net 30 days to net 60 days for selected product purchases made in connection with certain sales promotion programs. Such extension did not represent a permanent change to the payment terms for such customers but, rather, was applicable only to specified purchases made by such customers in connection with the applicable sales promotion program. Sales of products having net 60 day payment terms represented 56% of our net sales for the year ended December 31, 2010.
48
It is unclear how long the current economic environment will continue, whether there will be new events that could contribute to additional deterioration, and if so, what effect such events could have on our business in 2011. We intend to continue to moderate our growth plans and avoid substantial credit and market risk. We expect to continue to generate positive working capital through our operations.
As of December 31, 2010, we had no outstanding balance on the Facility or Term Loans. We were in compliance with all financial and non-financial covenants under the Credit and Term Loan Agreement as of December 31, 2010. We expect to be in compliance with both our non-financial and financial covenants during 2011; however economic conditions or the occurrence of any of the events discussed above under “Risk Factors” could cause noncompliance within our financial covenants.
We expect to be able to manage our working capital levels and capital expenditure amounts to maintain sufficient levels of liquidity. As of December 31, 2010 and 2009, we had approximately $36.0 million and $57.1 million, respectively, in working capital. We invested approximately $0.4 million in capital expenditures during the year ended December 31, 2010, which largely consisted of software and IT upgrades. In 2011, we expect to spend approximately $1.8 million in capital expenditures, primarily related to software and IT upgrades and disaster recovery.
Cash requirements for our business
Historically, we have generated cash from operations in excess of working capital requirements and through private and public sales of common stock. We currently invest our cash and cash equivalents in large money market funds and certificates of deposit with maturities no greater than one year. As of December 31, 2010 and 2009, we had approximately $15.1 million and $36.0 million, respectively, of cash and cash equivalents and short-term investments.
On November 3, 2010 we entered into a Credit and Term Loan Agreement with Comerica Bank. The Credit and Term Loan Agreement replaced and terminated our previous $20.0 million Former Credit Facility, dated November 21, 2008, which was scheduled to terminate on November 21, 2011. The Company did not have any outstanding balance on the Former Credit Facility at the time of termination.
Under the Credit and Term Loan Agreement, we have access to: (i) up to $20.0 million in a revolving credit facility; and (ii) one or more Term Loans in an aggregate amount of up to $15.0 million until the earliest to occur of (a) the date the aggregate outstanding principal balance of the Term Loans equals $15.0 million, (b) May 3, 2011, or (c) the date of our request to close out the Term Loans. The Facility will terminate, and any amounts outstanding under the Facility will be due on July 1, 2012 and all amounts outstanding under the Terms Loans will be due five years after the last day we request to close out the Term Loans, unless terminated earlier in accordance with the provisions of the Credit and Term Loan Agreement.
Any borrowings under the Credit and Term Loan Agreement will bear variable interest based on a margin, at our option, over prime rate or LIBOR as defined in the Credit and Term Loan Agreement. All amounts borrowed under the Credit and Term Loan Agreement are secured by a first priority security interest in all of our tangible and intangible assets. The Credit and Term Loan Agreement contains certain financial and non-financial covenants. Non-financial covenants include, among other things, monthly and quarterly reporting of a listing of our intellectual property. Financial covenants include requirements for maintaining: (i) a minimum quick ratio; (ii) a maximum ratio of total liabilities to tangible effective net worth; and (iii) a minimum EBITDA amount; as well as limitations on (a) annual capital expenditures, (b) asset- or equity-based investments, (c) stock repurchases, which may not exceed $50.3 million, (d) dividend distributions, which may not exceed 25% of net income for the fiscal year, and (e) joint venture investments, which may not exceed $2.0 million. See below for covenant compliance:
|
As of 12/31/10
|
Requirement
|
|||||
|
Facility Covenants
|
||||||
|
Minimum quick ratio
|
2.9 | 1.0 | ||||
|
Maximum ratio of total liabilities to tangible effective net worth
|
0.37 |
2.00
|
||||
|
Minimum EBITDA
|
$19.1 million
|
$12.0 million
|
||||
|
Maximum stock repurchase
|
$40.3 million
|
$50.3 million
|
||||
|
Maximum dividend distribution
|
$0 |
$2.83 million
|
||||
|
Maximum capital expenditures
|
$0.4 million
|
$3.0 million
|
As of December 31, 2010, we had no outstanding balance on the Facility or Term Loans. We were in compliance with all financial and non-financial debt covenants under the Credit and Term Loan Agreement as of December 31, 2010. If we do not draw on
49
the Term Loans on or before May 3, 2011, the $15.0 million under the Term Loans will no longer be available to us. However, we do intend to amend the Credit and Term Loan Agreement to extend the draw period of the Term Loans.
On October 26, 2010, we announced that our Board of Directors authorized us to repurchase up to $45.0 million of our common stock, depending on market conditions and other factors. Share repurchases could include the repurchase of shares from the selling stockholders in connection with the secondary offering completed in November 2010, as well as repurchases made through open market or privately negotiated transactions in compliance with SEC Rule 10b-18, subject to market conditions, applicable legal requirements and other factors. This authorization does not obligate us to acquire any particular amount of common stock nor does it ensure that any shares will be repurchased, and it may be suspended at any time at our discretion. During the fourth quarter ended December 31, 2010, we repurchased 3,556,910 shares of our outstanding common stock for a cost of $35.0 million.
On August 5, 2008, our Board of Directors authorized the repurchase of up to $10.0 million of our outstanding common, which expired on August 5, 2010. The purchases were to be made in the open market or in privately negotiated transactions from time to time as permitted by securities laws and other legal requirements. The timing manner, price and amount of any repurchases were determined by a three-person committee, consisting of members of our Board and management, at its discretion, and were subject to economic and market conditions, stock price, applicable legal requirements and other factors, and could be discontinued at any time. During the years ended December 31, 2009 and 2008, we purchased 183,664 and 627,367, shares of our outstanding stock, respectively, for a cost of $1.3 million and $4.0 million, respectively.
We continually evaluate new opportunities for products or therapeutic systems and, if and when appropriate, intend to pursue such opportunities through the acquisitions of companies, products or technologies and our own development activities. Our ability to execute on such opportunities in some circumstances may be dependent, in part, upon our ability to raise additional capital on commercially reasonable terms. There can be no assurance that funds from these sources will be available when needed or on terms favorable to us or our stockholders. If additional funds are raised by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock.
Inflation
Although at reduced levels in recent years, inflation continues to apply upward pressure on the cost of goods and services that we use. The competitive environments in many markets substantially limit our ability to fully recover these higher costs through increased selling prices. We continually seek to mitigate the adverse effects of inflation through cost containment and improved productivity and manufacturing processes.
Foreign Currency Fluctuations
Approximately 4% of our net sales in 2010 were derived from operations outside the United States and were denominated in Japanese Yen. None of our international cost structure is denominated in currencies other than the U.S. dollar. As a result, we are subject to fluctuations in sales and earnings reported in U.S. dollars due to changing currency exchange rates. We do not believe, however, that we currently have significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies.
Cash Flow
Year ended December 31, 2010. For the year ended December 31, 2010, net cash provided by operating activities was $14.5 million. The primary sources of cash were $9.5 million in net income, including the effect of: (i) adjusting for non-cash items; (ii) a decrease in inventory through an increase in our inventory turn ratio from 3.2 to 4.2; and (iii) a decrease in accounts receivable through improvement in our DSO from 71 days to 66 days; offset in part by: (i) an increase in income taxes receivable; and (ii) a net decline in accounts payable and accrued liabilities through timing of payments for operational, promotional and inventory purchases.
Net cash provided by investing activities was $5.1 million for the year ended December 31, 2010. This was due to $5.7 million in short-term investments maturing during the year ended December 31, 2010 and cash received for the disposal of property and equipment of $0.1 million, partially offset by investments in licenses and patent-related intellectual property and costs associated with software and IT upgrades during the year ended December 31, 2010. We anticipate spending approximately $1.8 million for total capital expenditures in 2011 primarily for planned investments in software and IT upgrades and disaster recovery.
Net cash used in financing activities was $34.7 million for the year ended December 31, 2010. This was primarily due to: (i) the repurchase of $35.0 million of our outstanding stock in November 2010; (ii) the purchase of vested options from Steven R. Carlson,
50
our former President and Chief Executive Officer, of $0.7 million; and (iii) principal payments made on our capital lease obligations; partially offset by $1.1 million in proceeds received from the exercise of stock options during the year ended December 31, 2010.
Year ended December 31, 2009. For the year ended December 31, 2009, net cash provided by operating activities was $18.8 million. The primary sources of cash were $11.3 million in net income, including the effect of: (i) adjusting for non-cash items; (ii) a net increase in accounts payable and accrued liabilities through timing of payments for operational, promotional and inventory purchases; (iii) a decrease in income taxes receivable and (iv) a decrease in inventory through an increase in our inventory turn ratio from 2.7 to 3.2; and (iv) a decrease in other assets; offset in part by an increase in accounts receivable from high sales volumes during the quarter ended December 31, 2009.
Net cash used in investing activities was $1.1 million for the year ended December 31, 2009. The primary uses of cash were: (i) capital invested in our new ERP system implemented in early 2009 and software upgrades; and (ii) the investment in intellectual property, primarily patents, relating to both our new product line offerings and our core product line offerings; offset by an investment in certificates of deposit during the year, which have maturity dates no greater than one year.
Net cash used in financing activities was $1.4 million for the year ended December 31, 2009. This was primarily due to repurchase of $1.3 million of our outstanding stock during the year.
Off-balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Related Party Transactions
Stock Purchase Agreement
On November 15, 2010, we entered into a Stock Purchase Agreement with the selling stockholders, under which we were obligated to purchase from the selling stockholders $35.0 million worth of our common stock, subject to certain conditions. The purchase price per share to be paid to the selling stockholders was to be the same price paid by the underwriter to purchase shares from the selling stockholders in the secondary offering completed in November 2010. However, if the purchase price to be paid was greater than $13.00, we would not purchase any shares from the selling stockholders. In addition, the purchase of the shares by us was conditioned on the sale of shares in the secondary offering.
Following the completion of the secondary offering of 2,690,244 shares on November 24, 2010, we repurchased 3,556,910 shares from the selling stockholders at a price of $9.84 per share, which was equal to the public offering price of $10.25 per share less the applicable underwriter’s discount, on November 30, 2010. Upon completion of both transactions the selling stockholders ceased to own any shares of our common stock.
Termination of 2006 Services Agreement
On September 2, 2010, we exercised our right to terminate the 2006 Agreement with Zein E. Obagi, MD Inc. (“Obagi Inc”), Zein Obagi (“Dr. Obagi” and, together with Obagi Inc., the “Obagi Entities”), Samar Obagi, the Zein and Samar Obagi Family Trust and Skin Health Properties, Inc. (“the Marketer”) (collectively, the “Dr. Obagi Parties”), effective as of October 4, 2010. In connection with the termination of the 2006 Agreement, we accelerated the payment of $0.5 million in certain fees and expenses (included within “Research and development expenses”) during the third quarter 2010, that otherwise would have been paid during the remainder of the original term, which was for five years and would have otherwise ended on June 29, 2011. No additional amounts were paid to the Dr. Obagi Parties as a result of the termination. In addition, as a result of the termination, we determined that our leasehold improvements in connection with a lease with the Marketer was impaired resulting in the write-off of $0.3 million in leasehold improvements at the Beverly Hills property and $0.2 million in prepaid rent (included within “Selling, general and administrative expenses”).
Termination of the 2006 Agreement does not relieve the Dr. Obagi Parties from their obligations to comply with certain provisions of the 2006 Agreement, particularly as they relate to a limited trademark license to Obagi Inc. and/or the Marketer, the grant to us of a license to have access to and utilize accounts, customer lists and other customer information and data developed in connection with the 2006 Agreement, the protection of our intellectual property rights and other additional confidential information related to us, nor does it affect our ownership of trademarks and other intellectual property rights.
We are currently engaged in pending litigation and other legal matters with Dr. Obagi Parties (and certain entities affiliated with the Dr. Obagi Parties). See Part I, Item 3 of this Report, “Legal Proceedings” and see Note 10, “Litigation” in our Notes to Consolidated Financial Statements for information concerning this matter.
51
Commitments and Contractual Obligations
Our major outstanding contractual obligations relate to operating leases and capital leases. These contractual obligations as of December 31, 2010 were as follows:
|
Payments due by period
|
||||||||||||||||||||
|
Total
|
Less than 1 year
|
1-3 years
|
3-5 years
|
More than 5 years
|
||||||||||||||||
| Contractual Obligations | ||||||||||||||||||||
|
Operating lease obligations
|
$ | 11,078 | $ | 1,985 | $ | 3,349 | $ | 2,279 | $ | 3,465 | ||||||||||
|
Uncertain tax liability
|
196 | 59 | 137 | — | — | |||||||||||||||
|
Total
|
$ | 11,274 | $ | 2,044 | $ | 3,486 | $ | 2,279 | $ | 3,465 | ||||||||||
Operating leases. We lease our corporate offices in Long Beach, California and distribution center in Carson, California under separate leases expiring in October 2018 and October 2013, respectively. In 2008, we entered into a lease for our new headquarters with a new landlord in Long Beach, CA. We lease our manufacturing facility in Milford, Connecticut under a lease expiring in November 2013. In addition, we lease automobiles for our sales representatives under 36-month contracts.
Uncertainty in Taxes. In connection with provisions under ASC 740, at December 31, 2010, we had approximately $0.1 million and $0.1 million of short-term and long-term liabilities, respectively, associated with uncertain tax positions.
Recent Accounting Pronouncements
New Accounting Standards
In January 2010, the FASB issued Accounting Standards Update (“ASU”) No. 2010-06, Improving Disclosures about Fair Value Measurements (“ASU No. 2010-06”), an amendment to ASC 820, Fair Value Measurements and Disclosures. This amendment requires an entity to: (i) disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers; and (ii) present separate information for Level 3 activity pertaining to gross purchases, sales, issuances, and settlements. ASU No. 2010-06 is effective for interim and annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. We adopted the provisions of ASU No. 2010-06 during the interim period ended March 31, 2010. The adoption of ASU No. 2010-06 did not have a material effect on our consolidated financial statements.
We invest our excess cash primarily in large money market funds and certificates of deposit with maturity dates no greater than one year. We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions in any material fashion. Accordingly, we believe that we are not subject to any material risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments.
Although substantially all of our sales and purchases are denominated in U.S. dollars, future fluctuations in the value of the U.S. dollar may affect the price competitiveness of our products outside the U.S. We do not believe, however, that we currently have significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies.
Interest Rate Risk
Our interest income and expense is more sensitive to fluctuations in the general level of U.S. prime rate and LIBOR interest rates than to changes in rates in other markets. Changes in U.S. LIBOR interest rates affect the interest earned on our cash and cash equivalents. At December 31, 2010, we had approximately $15.1 million of cash and cash equivalents. If the interest rates on our cash and cash equivalents were to increase or decrease by 1% for the year, annual interest income would increase or decrease by approximately $0.2 million.
52
Other Risks
The recent recession has had an adverse impact on the financial services industry, including insurance companies, some of whom provide insurance coverage to us. To the extent we have any claims in the future and such insurance providers are unable, due to their financial condition, to pay covered claims, we could experience adverse impacts on our cash flow and cash reserves. We have no way of knowing whether or not any insurance providers that are financially stable at this time will experience financial difficulties in the future that could impact their ability to pay covered claims.
The information required by this Item is incorporated herein by reference to the financial statements set forth in Item 15(a) of Part IV of this Report.
Not applicable.
Disclosure Controls and Procedures
As of the end of the period covered by this Report, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective in providing reasonable assurance that information is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules, regulations and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer and the Chief Financial Officer as appropriate, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15(d)-15(f) of the Exchange Act). Under the supervision and with the participation of management, including our Chief Executive Officer and the Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based upon the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, our management concluded that Obagi’s internal control over financial reporting was effective as of December 31, 2010.
The effectiveness of our internal control over financial reporting as of December 31, 2010 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in its Report of Independent Registered Public Accounting Firm under Part IV, Item 15 of this Report.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting during the most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
On March 8, 2011, the Compensation Committee of our Board of Directors approved the Obagi 2011 Performance Incentive Plan (the “2011 Plan”), which is an incentive compensation program for the year ending December 31, 2011 under the 2005 Stock Incentive Plan, as amended. It amends and restates and is the successor plan to the Obagi 2010 Performance Incentive Plan (the “2010 Plan”).
53
The 2011 Plan is designed to motivate, retain and reward our employees, including our executives, based on the achievement of specified corporate revenue and earnings before interest and taxes (“EBIT”) objectives, as well as individual objectives in certain cases. The Compensation Committee established target revenue and EBIT, as adjusted, to exclude the impact of non-cash charges relating to the issuance of equity securities as reported in our consolidated financial statements (“adjusted EBIT”). Assuming 100% achievement of the target revenue and adjusted EBIT objectives, the aggregate 2011 Plan pool would be funded in the amount of $2.4 million. Under the 2011 Plan, if we acheive between the minimum required targets for each objective and 100% target for each objective, then the potential bonus amount for each executive officer and non-executive employee will be reduced by 5.0% and 1.0%, respectively, for each 1% that we are below the target objective, and the bonus pool will be reduced accordingly. If the revenue and adjusted EBIT target objectives are exceeded, an increased amount will have to be funded to the 2011 Plan pool, up to 150% of the target 2011 Plan pool. Thirty percent of the 2011 Plan pool will relate to the revenue objective and 70% of the pool will relate to the adjusted EBIT objective. For the 2011 Plan pool to be funded for executives and employees to earn any bonuses, we must achieve at least 80% of the revenue objective and at least 80% of the adjusted EBIT objective. Eligible participants under the 2011 Plan are full-time employees, including executives, who do not participate in sales or other variable incentive pay plans and are employed by us on December 31, 2011.
54
The information required by this item is incorporated into this Report by reference to the definitive proxy statement for our 2011 Annual Meeting of Stockholders (the “Proxy Statement”).
The information required by this item is incorporated into this Report by reference to the Proxy Statement.
The information required by this item is incorporated into this Report by reference to the Proxy Statement.
The information required by this item is incorporated into this Report by reference to the Proxy Statement.
The information required by this item is incorporated into this Report by reference to the Proxy Statement.
55
PART IV
(a)1. Consolidated Financial Statements and Supplementary Data:
The following financial statements are included as a separate section of this report commencing on page F-1:
|
F-1
|
|
(a)2. Financial Statement Schedules:
Schedule II—Valuation and Qualifying Accounts
Other than as set forth below, all other schedules have been omitted for the reason that the required information is presented in financial statements or notes thereto, the amounts involved are not significant or the schedules are not applicable.
|
(in thousands)
|
Balance at Beginning of Period
|
Charged to Cost and Expense
|
Charged to Contra-Revenue
|
Deductions
|
Balance at End of Period
|
|||||||||||||||
|
Year ended December 31, 2008
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$ | 307 | $ | 660 | $ | — | $ | (180 | ) | $ | 787 | |||||||||
|
Allowance for sales returns
|
538 | — | (87 | ) | — | 451 | ||||||||||||||
|
Reserve for inventories
|
469 | 841 | — | (613 | ) | 697 | ||||||||||||||
|
Valuation allowance for deferred tax assets
|
57 | (5 | ) | — | — | 52 | ||||||||||||||
|
Year ended December 31, 2009
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$ | 787 | $ | 1,411 | $ | — | $ | (666 | ) | $ | 1,532 | |||||||||
|
Allowance for sales returns
|
451 | — | 41 | — | 492 | |||||||||||||||
|
Reserve for inventories
|
697 | 1,295 | — | (1,394 | ) | 598 | ||||||||||||||
|
Valuation allowance for deferred tax assets
|
52 | (5 | ) | — | — | 47 | ||||||||||||||
|
Year ended December 31, 2010
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$ | 1,532 | $ | 1,531 | $ | — | $ | (883 | ) | $ | 2,180 | |||||||||
|
Allowance for sales returns
|
492 | — | (225 | ) | — | 267 | ||||||||||||||
|
Reserve for inventories
|
598 | 1,031 | — | (1,029 | ) | 600 | ||||||||||||||
|
Valuation allowance for deferred tax assets
|
47 | — | — | (47 | ) | — | ||||||||||||||
(a)3. Exhibits:
|
Exhibit
|
Exhibit title
|
Where Located
|
||||
|
Form
|
Exhibit No.
|
Filing Date
|
Filed Herewith
|
|||
|
3.1
|
Amended and Restated Certificate of Incorporation of the Company.
|
S-1/A
|
3.1
|
11/29/06
|
||
|
3.2
|
Second Amended and Restated Bylaws of the Company.
|
8-K
|
3.1
|
2/29/08
|
||
|
4.1
|
Specimen Stock Certificate.
|
S-1/A
|
4.1
|
11/29/06
|
||
56
|
Exhibit
|
Exhibit title
|
Where Located
|
||||
|
Form
|
Exhibit No.
|
Filing Date
|
Filed Herewith
|
|||
|
10.1
|
OMP, Inc. 2000 Stock Option/Stock Issuance Plan and forms of award agreements.**
|
S-1
|
10.1
|
9/13/06
|
||
|
10.2
|
Amended and Restated Obagi Medical Products, Inc. 2005 Stock Incentive Plan and forms of award agreements.**
|
S-1/A
|
10.2
|
12/12/06
|
||
|
10.3
|
Lease Agreement between the Company and D'Amato Investments, LLC, dated December 1, 2005.
|
S-1/A
|
10.5
|
10/24/06
|
||
|
10.4
|
Distribution Agreement, by and between the Company and Cellogique Corporation, dated November 10, 2005, as amended.+
|
S-1/A
|
10.6
|
11/15/06
|
||
|
10.5
|
Know-How and Trademark License Agreement, by and between the Company and Rohto Pharmaceutical Co, Ltd., dated September 13, 2002, as amended.+
|
S-1/A
|
10.7
|
11/15/06
|
||
|
10.6
|
Agreement by and between the Company and Dr. Zein E. Obagi, Inc, dated as of June 29, 2007.
|
S-1/A
|
10.8
|
10/24/06
|
||
|
10.7
|
Separation and Release Agreement between the Company and Zein Obagi, M.D., dated June 29, 2007.
|
S-1
|
10.9
|
9/13/06
|
||
|
10.8
|
Retail Lease Agreement by and between Skin Health Properties, Inc. as Landlord and OMP, Inc. as Tenant, dated as of June 29, 2006.
|
S-1/A
|
10.10
|
10/24/06
|
||
|
10.9
|
Letter Agreement between the Company and Skin Health Properties, Inc., dated June 29, 2006.
|
S-1
|
10.11
|
9/13/06
|
||
|
10.10
|
Employment Agreement, by and between the Company and Steven R. Carlson, dated March 1, 2005.**
|
S-1
|
10.13
|
9/13/06
|
||
|
10.11
|
Amendment to Employment Agreement by and between the Company and Steven R. Carlson, dated August 6, 2007.**
|
10-K
|
10.11
|
3/15/10
|
||
|
10.12
|
Consultant Services and Confidentiality Agreement, dated July 18, 2005, by and among the Company, Jose Ramirez, and JR Chem LLC.+
|
S-1/A
|
10.20
|
11/15/06
|
||
|
10.13
|
Form of indemnification agreement.**
|
S-1
|
10.21
|
9/13/06
|
||
|
10.14
|
Patent License Agreement by and between the Company and Avon Products, Inc., dated June 26, 2003.+
|
S-1/A
|
10.23
|
11/15/06
|
||
|
10.15
|
Non-Employee Director Compensation Policy, adopted November 14, 2006, as amended.**
|
10-Q
|
10.26
|
5/8/07
|
||
|
10.16
|
Second Amendment to Employment Agreement by and between the Company and Steven Robert Carlson dated as of March 1, 2008.**
|
8-K
|
10.1
|
3/7/08
|
||
|
10.17
|
Lease Agreement between OMP, Inc. and Kilroy Realty, L.P. dated April 30, 2008.
|
8-K
|
10.1
|
5/6/08
|
||
|
10.18
|
Implementation and Support Agreement by and between the Company and Specialists in Custom Software, Inc., dated June 24, 2008.
|
10-Q
|
10.43
|
8/11/08
|
||
|
10.19
|
Lease Agreement between OMP, Inc. and Cypress-Southbay, LLC. and related construction rider, dated July 8, 2008.
|
10-Q
|
10.44
|
8/11/08
|
||
|
10.20
|
Amendment to Lease Agreement between OMP, Inc. and Kilroy Realty, L.P., dated August 6, 2008.
|
10-Q
|
10.45
|
8/11/08
|
||
|
10.21
|
Revolving Credit Agreement, by and between the Company and Comerica Bank, dated as of November 21, 2008
|
8-K
|
10.46
|
11/26/08
|
||
|
10.22
|
Amendment and Addendum to Know-How and Trademark License Agreement, by and between the Company and Rohto Pharmaceutical Co, Ltd., dated December 4, 2008.+
|
10-Q
|
10.48
|
11/6/09
|
||
|
10.23
|
License and Supply Agreement, by and between the Company and Rohto Pharmaceutical Co, Ltd., dated December 4, 2008.+
|
10-K
|
10.49
|
3/13/09
|
||
57
|
Exhibit
|
Exhibit title
|
Where Located
|
||||
|
Form
|
Exhibit No.
|
Filing Date
|
Filed Herewith
|
|||
|
10.24
|
Service Agreement, by and between the Company and Ventiv Commercial Services, LLC, dated July 1, 2008
|
10-K
|
10.50
|
3/13/09
|
||
|
10.25
|
First Amendment to Services Agreement by and between the Company and Ventiv Commercial Services, LLC, dated July 1, 2008
|
10-K
|
10.51
|
3/13/09
|
||
|
10.26
|
Amendment and Addendum to Consultant Services and Confidentiality Agreement by and between the Company and Jose Ramirez, and JR Chem LLC.+
|
10-K
|
10.52
|
3/13/09
|
||
|
10.27
|
Form of Employment Agreement by and between the Company and its executive officers**
|
8-K
|
10.53
|
6/18/09
|
||
|
10.28
|
Amended Employment Agreement by and between the Company and Preston S. Romm, dated as of June 15, 2009**
|
8-K
|
10.54
|
6/18/09
|
||
|
10.29
|
Amended Employment Agreement by and between the Company and David S. Goldstein, dated as of June 15, 2009**
|
8-K
|
10.55
|
6/18/09
|
||
|
10.30
|
Amended Employment Agreement by and between the Company and Laura B. Hunter, dated as of June 15, 2009**
|
8-K
|
10.56
|
6/18/09
|
||
|
10.31
|
Amended and Restated Product Supply Agreement between the Company and Triax Pharmaceuticals LLC dated August 24, 2009+
|
10-Q
|
10.57
|
11/6/09
|
||
|
10.32
|
Amendment No. 2 to Distribution Agreement between the Company and Cellogique Corporation, dated September 26, 2009+
|
10-Q
|
10.58
|
11/6/09
|
||
|
10.33
|
2010 Performance Incentive Plan**
|
10-Q
|
10.37
|
5/7/10
|
||
|
10.34
|
Services Agreement by and between the Company and DDN/Obergfel, LLC dated July 1, 2009+
|
10-K
|
10.34
|
3/15/10
|
||
|
10.35
|
Separation Agreement and Release dated October 15, 2010 by and between the Company and Steven R. Carlson**
|
S-1
|
10.35
|
10/25/10
|
||
|
10.36
|
Amended and Restated Revolving Credit and Term Loan Agreement dated as of November 3, 2010 between the Company, OMP, Inc., the Lenders and Comerica Bank, as Administrative Agent for the Lenders
|
10-Q
|
10.36
|
11/4/10
|
||
|
10.37
|
Stock Purchase Agreement dated as of November 15, 2010 by and among Obagi Medical Products, Inc., Stonington Capital Appreciation 1994 Fund, L.P. and The Zein and Samar Obagi Family Trust
|
8-K
|
10.1
|
11/15/10
|
||
| 10.38 | 2011 Performance Incentive Plan** | X | ||||
|
21.1
|
Subsidiaries of the Company.
|
10-K
|
21.1
|
3/15/10
|
||
|
23.1
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm.
|
X
|
||||
|
31.1
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
X
|
||||
|
31.2
|
Certification of Principal Accounting Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
X
|
||||
|
32.1
|
Certification of Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
X
|
||||
|
32.2
|
Certification of Principal Accounting Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
X
|
||||
+ Material has been omitted pursuant to a request for confidential treatment and such material has been filed separately with the Commission.
** Management contracts or compensatory plans and arrangements required to be filed pursuant to Item 601(b)(10)(ii)(A) or (iii) of Regulation S-K.
58
|
SIGNATURES
|
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
OBAGI MEDICAL PRODUCTS, INC.
|
|||
|
Date: March 10, 2011
|
By:
|
/s/ Albert F. Hummel
|
|
|
Albert F. Hummel
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Date: March 10, 2011
|
By:
|
/s/ Preston S. Romm
|
|
|
Preston S. Romm
|
|||
|
Chief Financial Officer
|
|||
|
(Principal Financial Officer)
|
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/ Albert F. Hummel
|
March 10, 2011 | |
|
Albert F. Hummel
|
Chief Executive Officer, President and Director (Principal Executive Officer)
|
|
| /s/ Preston S. Romm | March 10, 2011 | |
|
Preston S. Romm
|
Chief Financial Officer (Principal Financial and Accounting Officer)
|
|
| /s/ Albert J. Fitzgibbons III | March 10, 2011 | |
|
Albert J. Fitzgibbons III
|
Chairman of the Board of Directors
|
|
|
/s/ John A. Bartholdson
|
March 10, 2011 | |
|
John A. Bartholdson
|
Director
|
|
| /s/ John H. Duerden | March 10, 2011 | |
|
John H. Duerden
|
Director
|
|
| /s/ Edward A. Grant | March 10, 2011 | |
|
Edward A. Grant
|
Director
|
|
| /s/ Ronald P. Badie | March 10, 2011 | |
|
Ronald P. Badie
|
Director
|
|
59
To the Board of Directors and Stockholders of
Obagi Medical Products, Inc.
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Obagi Medical Products, Inc. and its subsidiaries at December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control Over Financial Reporting, appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PRICEWATERHOUSECOOPERS LLP
Los Angeles, California
March 10, 2011
F-1
Obagi Medical Products, Inc.
(Dollars in thousands, except share and per share amounts)
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Assets
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 15,139 | $ | 30,215 | ||||
|
Short-term investments
|
— | 5,743 | ||||||
|
Accounts receivable, net of allowance for doubtful accounts
|
||||||||
|
and sales returns of $2,447 and $2,025 as of December 31, 2010
|
||||||||
|
and 2009, respectively
|
22,736 | 24,240 | ||||||
|
Accounts receivable from related parties
|
— | 97 | ||||||
|
Inventories, net
|
4,625 | 6,228 | ||||||
|
Deferred income taxes
|
1,542 | 1,379 | ||||||
|
Prepaid expenses and other current assets
|
2,473 | 2,424 | ||||||
|
Income taxes receivable
|
2,393 | 730 | ||||||
|
Total current assets
|
48,908 | 71,056 | ||||||
|
Property and equipment, net
|
3,254 | 4,689 | ||||||
|
Goodwill
|
4,629 | 4,629 | ||||||
|
Intangible assets, net
|
4,592 | 4,936 | ||||||
|
Deferred income taxes
|
1,054 | 1,835 | ||||||
|
Other assets
|
270 | 345 | ||||||
|
Total assets
|
$ | 62,707 | $ | 87,490 | ||||
|
Liabilities and Stockholders' Equity
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 6,400 | $ | 7,864 | ||||
|
Current portion of long-term debt
|
— | 18 | ||||||
|
Accrued liabilities
|
6,479 | 4,801 | ||||||
|
Income taxes payable
|
— | 1,159 | ||||||
|
Amounts due to related parties
|
— | 105 | ||||||
|
Total current liabilities
|
12,879 | 13,947 | ||||||
|
Other long-term liabilities
|
1,599 | 1,555 | ||||||
|
Total liabilities
|
14,478 | 15,502 | ||||||
|
Commitments and contingencies (Note 10)
|
||||||||
|
Stockholders' equity
|
||||||||
|
Common stock, $.001 par value; 100,000,000 shares authorized,
|
||||||||
|
22,882,658 and 22,748,218 shares issued and 18,499,939
|
||||||||
|
and 21,912,857 shares outstanding at December 31, 2010
|
||||||||
|
and 2009, respectively
|
23 | 23 | ||||||
|
Additional paid-in capital
|
61,173 | 59,505 | ||||||
|
Accumulated earnings
|
27,381 | 17,890 | ||||||
|
Treasury stock, at cost; 4,367,941 and 811,031 shares at
|
||||||||
|
December 31, 2010 and 2009, respectively
|
(40,348 | ) | (5,348 | ) | ||||
|
Accumulated other comprehensive loss
|
— | (82 | ) | |||||
|
Total stockholders' equity
|
48,229 | 71,988 | ||||||
|
Total liabilities and stockholders' equity
|
$ | 62,707 | $ | 87,490 | ||||
F-2
Obagi Medical Products, Inc.
(Dollars in thousands, except share and per share amounts)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net sales
|
$ | 112,763 | $ | 104,096 | $ | 104,593 | ||||||
|
Cost of sales
|
23,686 | 22,039 | 19,931 | |||||||||
|
Gross profit
|
89,077 | 82,057 | 84,662 | |||||||||
|
Selling, general and administrative expenses
|
69,293 | 59,222 | 59,380 | |||||||||
|
Research and development expenses
|
3,883 | 4,407 | 5,284 | |||||||||
|
Income from operations
|
15,901 | 18,428 | 19,998 | |||||||||
|
Loss on dissolution of foreign subsidiary
|
(80 | ) | — | — | ||||||||
|
Interest income
|
89 | 173 | 368 | |||||||||
|
Interest expense
|
(71 | ) | (123 | ) | (243 | ) | ||||||
|
Income before provision for income taxes
|
15,839 | 18,478 | 20,123 | |||||||||
|
Provision for income taxes
|
6,348 | 7,145 | 7,535 | |||||||||
|
Net income
|
$ | 9,491 | $ | 11,333 | $ | 12,588 | ||||||
|
Net income attributable to common shares
|
||||||||||||
|
Basic
|
$ | 0.44 | $ | 0.52 | $ | 0.56 | ||||||
|
Diluted
|
$ | 0.44 | $ | 0.51 | $ | 0.56 | ||||||
|
Weighted average common shares outstanding
|
||||||||||||
|
Basic
|
21,572,870 | 21,970,491 | 22,598,474 | |||||||||
|
Diluted
|
21,816,170 | 22,022,132 | 22,607,689 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Obagi Medical Products, Inc.
(Dollars in thousands, except share and per share amounts)
|
Accumulated Other
|
|||||||||||||||||
|
Common Stock
|
Additional
|
Accumulated
|
Treasury Stock
|
Comprehensive
|
|||||||||||||
|
Shares
|
Amount
|
Paid-In Capital
|
Earnings (Deficit)
|
Shares
|
Amount
|
Loss
|
Total
|
||||||||||
|
Balances, as of December 31, 2007
|
22,643,564
|
$ 23
|
$ 55,805
|
$ (6,031
|
) |
—
|
—
|
$ (68
|
) |
$ 49,729
|
|||||||
|
Comprehensive income:
|
|||||||||||||||||
|
Translation adjustment, net of tax effect ($1 benefit)
|
—
|
—
|
—
|
—
|
—
|
—
|
(1
|
) |
(1
|
) | |||||||
|
Net income for the year ended December 31, 2008
|
—
|
—
|
—
|
12,588
|
—
|
—
|
—
|
12,588
|
|||||||||
|
Total comprehensive income
|
12,587
|
||||||||||||||||
|
Stock compensation expense
|
—
|
—
|
2,008
|
—
|
—
|
—
|
—
|
2,008
|
|||||||||
|
Issuance of vested restricted stock
|
9,785
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
|||||||||
|
Tax benefit from exercise of stock options
|
—
|
—
|
39
|
—
|
—
|
—
|
—
|
39
|
|||||||||
|
Issuance of common stock upon exercise of incentive stock options
|
18,890 | — | 174 | — | — | — | — | 174 | |||||||||
|
Repurchase of common stock
|
—
|
—
|
-
|
—
|
(627,367
|
) |
(4,016
|
) |
—
|
(4,016
|
) | ||||||
|
Balances, as of December 31, 2008
|
22,672,239
|
$ 23
|
$ 58,026
|
$ 6,557
|
(627,367
|
) |
$ (4,016
|
) |
$ (69
|
) |
$ 60,521
|
||||||
|
Comprehensive income:
|
|||||||||||||||||
|
Translation adjustment, net of tax effect ($8 benefit)
|
—
|
—
|
—
|
—
|
—
|
—
|
(13
|
) |
(13
|
) | |||||||
|
Net income for the year ended December 31, 2009
|
—
|
—
|
—
|
11,333
|
—
|
—
|
—
|
11,333
|
|||||||||
|
Total comprehensive income
|
11,320
|
||||||||||||||||
|
Stock compensation expense
|
—
|
—
|
1,619
|
—
|
—
|
—
|
—
|
1,619
|
|||||||||
|
Issuance of vested restricted stock units
|
32,500
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
|||||||||
|
Issuance of vested restricted stock
|
18,999
|
—
|
-
|
—
|
—
|
—
|
—
|
—
|
|||||||||
|
Tax expense related to share based payment arrangements
|
—
|
—
|
(140
|
) |
—
|
—
|
—
|
—
|
(140
|
) | |||||||
|
Issuance of common stock upon exercise of incentive stock options
|
150 | — | — | — | — | — | — | — | |||||||||
|
Repurchase of common stock
|
—
|
—
|
—
|
—
|
(183,664
|
) |
(1,332
|
) |
—
|
(1,332
|
) | ||||||
|
Balances, as of December 31, 2009
|
22,723,888
|
$ 23
|
$ 59,505
|
$ 17,890
|
(811,031
|
) |
$ (5,348
|
) |
$ (82
|
) |
$ 71,988
|
||||||
|
Comprehensive income:
|
|||||||||||||||||
|
Translation adjustment, net of tax effect ($1 expense)
|
—
|
—
|
—
|
—
|
—
|
—
|
2
|
2
|
|||||||||
|
Loss on dissolution of foreign subsidiary
|
—
|
—
|
—
|
—
|
—
|
—
|
80
|
80
|
|||||||||
|
Net income for the year ended December 31, 2010
|
—
|
—
|
—
|
9,491
|
—
|
—
|
—
|
9,491
|
|||||||||
|
Total comprehensive income
|
9,573
|
||||||||||||||||
|
Stock compensation expense
|
—
|
—
|
1,242
|
—
|
—
|
—
|
—
|
1,242
|
|||||||||
|
Issuance of vested restricted stock
|
24,330
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
|||||||||
|
Tax expense related to share based payment arrangements
|
—
|
—
|
94
|
—
|
—
|
—
|
—
|
94
|
|||||||||
|
Issuance of common stock upon exercise of incentive stock options
|
119,662 | — | 1,069 | — | — | — | — | 1,069 | |||||||||
|
Repurchase of vested stock options
|
—
|
—
|
(737
|
) |
—
|
—
|
—
|
—
|
(737
|
) | |||||||
|
Repurchase of common stock
|
—
|
—
|
—
|
—
|
(3,556,910
|
) |
(35,000
|
) |
—
|
(35,000
|
) | ||||||
|
Balances, as of December 31, 2010
|
22,867,880
|
$ 23
|
$ 61,173
|
$ 27,381
|
(4,367,941
|
) |
$ (40,348
|
) |
$ —
|
$ 48,229
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Obagi Medical Products, Inc.
(Dollars in thousands, except share and per share amounts)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Cash flows from operating activities
|
||||||||||||
|
Net income
|
$ | 9,491 | $ | 11,333 | $ | 12,588 | ||||||
|
Adjustments to reconcile net income to net cash provided by
|
||||||||||||
|
operating activities
|
||||||||||||
|
Depreciation and amortization
|
1,946 | 2,339 | 2,391 | |||||||||
|
Write-off of prepaids and deposits related to SoluCLENZ
|
— | 441 | — | |||||||||
|
Write-off of debt issuance costs
|
— | — | 130 | |||||||||
|
Loss on disposal of property and equipment
|
466 | 56 | — | |||||||||
|
Loss on dissolution of foreign subsidiary
|
80 | — | — | |||||||||
|
Provision for doubtful accounts
|
422 | 786 | 562 | |||||||||
|
Write-off of prepaid rent
|
153 | |||||||||||
|
Deferred income taxes
|
618 | 142 | (336 | ) | ||||||||
|
Stock compensation expense
|
1,242 | 1,619 | 2,008 | |||||||||
|
Changes in operating assets and liabilities
|
||||||||||||
|
Accounts receivable
|
1,082 | (4,378 | ) | (3,671 | ) | |||||||
|
Accounts receivable from related parties
|
97 | 421 | 295 | |||||||||
|
Income taxes receivable
|
(1,663 | ) | 1,341 | (1,512 | ) | |||||||
|
Inventories
|
1,603 | 617 | (798 | ) | ||||||||
|
Prepaid expenses and other current assets
|
(111 | ) | (33 | ) | 582 | |||||||
|
Other assets
|
56 | 463 | 173 | |||||||||
|
Cash received for reimbursable tenant improvements
|
— | — | 955 | |||||||||
|
Accounts payable
|
(1,464 | ) | 1,386 | (312 | ) | |||||||
|
Accrued liabilities
|
519 | 2,426 | 271 | |||||||||
|
Amounts due to related parties
|
(105 | ) | (64 | ) | 34 | |||||||
|
Other long-term liabilities
|
44 | 39 | 661 | |||||||||
|
Net cash provided by operating activities
|
14,476 | 18,934 | 14,021 | |||||||||
|
Cash flows from investing activities
|
||||||||||||
|
Purchases of property and equipment
|
(351 | ) | (960 | ) | (3,944 | ) | ||||||
| Disposal of property and equipment | 139 | — | — | |||||||||
|
Purchase of other intangible assets
|
(401 | ) | (446 | ) | (327 | ) | ||||||
|
Purchase of short-term investments
|
— | (2,000 | ) | (6,000 | ) | |||||||
|
Proceeds from maturity of short-term investments
|
5,743 | 2,257 | — | |||||||||
|
Net cash provided by (used in) investing activities
|
5,130 | (1,149 | ) | (10,271 | ) | |||||||
|
Cash flows from financing activities
|
||||||||||||
|
Principal payments on capital lease obligations
|
(18 | ) | (23 | ) | (30 | ) | ||||||
|
Proceeds from exercise of stock options
|
1,069 | — | 174 | |||||||||
|
Tax benefit (expense) related to share-based payment arrangements
|
94 | (140 | ) | 39 | ||||||||
|
Debt issuance costs for line of credit
|
(92 | ) | — | (32 | ) | |||||||
|
Repurchase of vested stock options
|
(737 | ) | — | — | ||||||||
|
Repurchase of common stock
|
(35,000 | ) | (1,332 | ) | (4,016 | ) | ||||||
|
Net cash used in financing activities
|
(34,684 | ) | (1,495 | ) | (3,865 | ) | ||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
2 | (13 | ) | (1 | ) | |||||||
|
Net (decrease) increase in cash and cash equivalents
|
(15,076 | ) | 16,277 | (116 | ) | |||||||
|
Cash and cash equivalents at beginning of year
|
30,215 | 13,938 | 14,054 | |||||||||
|
Cash and cash equivalents at end of year
|
$ | 15,139 | $ | 30,215 | $ | 13,938 | ||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
|
Cash paid during the year for:
|
||||||||||||
|
Interest, net of amount capitalized
|
$ | 87 | $ | 19 | $ | 83 | ||||||
|
Income taxes, net of refunds
|
$ | 8,715 | $ | 6,041 | $ | 9,418 | ||||||
|
Non cash investing and financing activities
|
||||||||||||
|
Changes in accounts payable and accrued liabilities
|
||||||||||||
|
related to purchases of property and equipment
|
$ | — | $ | — | $ | 126 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements
(Dollars in thousands, except share and per share amounts)
Obagi Medical Products, Inc. (the “Company”) is a specialty pharmaceutical company that develops, markets and sells proprietary topical aesthetic and therapeutic prescription-strength skin care systems in the physician-dispensed market. The Company is incorporated under the laws of the state of Delaware. The Company markets its products through its own sales force throughout the United States, and through 20 distribution and two licensing partners in 45 other countries in regions including North America, Europe, Asia, the Middle East and Central America. The Company also licenses certain non-prescription product concepts under the Obagi trademark to a large Japanese-based pharmaceutical company for sale through consumer distribution channels in Japan.
Secondary Public Offering
On November 24, 2010, the Company completed a registered public offering of 2,690,244 shares of its common stock by selling stockholders, at a public offering price of $10.25 per share. The Company did not receive any proceeds from the sale of shares by the selling stockholders in the secondary offering.
Following the completion of the secondary offering, the Company repurchased the remaining shares of its common stock owned by two former stockholders, the Stonington Capital Appreciation 1994 Fund, L.P. and the Zein and Samar Obagi Family Trust (the “selling stockholders”) pursuant to a Stock Purchase Agreement (see Note 9).
Note 2: Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Obagi Medical Products, Inc. and its wholly owned subsidiary, OMP, Inc. The consolidated financial statements of OMP, Inc. include the accounts of its wholly owned subsidiaries and its majority-owned subsidiary, Obagi (S) Pte Ltd. (“Obagi Singapore”) through June 10, 2010. All intercompany accounts and transactions have been eliminated in consolidation.
On June 10, 2010, the Company dissolved Obagi Singapore with the approval of the Accounting and Corporate Regulatory Authority in Singapore. As a result of the dissolution, the Company recognized a loss on dissolution of subsidiary of $80 during the year ended December 31, 2010, which represented the cumulative foreign currency translation loss on the Company’s Consolidated Balance Sheet as of June 10, 2010. As Obagi Singapore was operationally dormant and had no assets, the dissolution did not result in a liquidation of assets to external parties, nor did it trigger an impairment analysis of any of the Company’s other asset balances. The dissolution of Obagi Singapore did not have a significant impact on the Company’s consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash equivalents include demand deposits and short-term investments with a maturity of three months or less when purchased. The Company maintains its cash deposits at numerous banks located throughout the United States, which at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk. At December 31, 2010 and 2009, cash on deposit was in excess of the federally insured limit of $250.
Short-term Investments
The Company accounts for short-term investments in accordance with Accounting Standards Codification (“ASC”) 320, Investments – Debt and Equity Securities. The Company determines the appropriate classification of all short-term investments as held-to-maturity, available-for-sale or trading at the time of purchase and re-evaluates such classifications as of each balance sheet date. Short-term investments for which the Company has the positive intent and ability to hold to maturity are classified as “held-to-maturity” and reported at amortized cost. There were no investments classified as available-for-sale or trading at December 31, 2010 and 2009. Short-term investments consist of certificates of deposit with maturities of less than twelve months and are stated at amortized cost. The carrying value of the Company’s short-term investments approximates fair value due to their short maturities.
F-6
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Accounts Receivable
The Company performs periodic credit evaluations of the financial condition of its customers, monitors collections and payments from customers, and generally does not require collateral. Receivables are generally due within 30 days. However, in light of the recent recession and corresponding tightening of credit, and to remain competitive in the marketplace, for certain selected customers who the Company deemed to be creditworthy, based upon their prior payment history, the Company extended its standard payment terms to 60 days, for selected product purchases made in connection with specific sales promotions. Such extension did not represent a permanent change to the payment terms for such customers, but, rather, was applicable only to specified purchases made by such customers in connection with the relevant sales promotion.
The Company provides for the possible inability to collect accounts receivable by recording an allowance for doubtful accounts. The Company writes off an account when it is considered to be uncollectible. The Company estimates its allowance for doubtful accounts based on historical write-offs as a percentage of product sales, adjusted specifically for accounts that are past due, non-performing, in bankruptcy or otherwise identified as at risk for potential credit loss. The Company estimates its sales returns based on its historical rate of returns as a percentage of net product sales, gross of returns and allowances and shipping and handling revenue, historical aging of returns, the Company’s return policy and current market conditions. To date, losses have been within the range of management’s expectations. The allowance for doubtful accounts as of December 31, 2010 and 2009 was $2,180 and $1,532, respectively. See “Revenue Recognition” below for further discussion of allowance for sales returns.
Concentrations of credit risk, with respect to trade receivables, are limited due to the large number of customers comprising the Company’s customer base and their dispersion across different geographic regions. As of December 31, 2010 and 2009, no single customer represented more than 6% of the net accounts receivable balance.
Inventories
Inventories consist of raw materials and finished goods that are manufactured both through contracted third party manufacturers and in-house and that are purchased from third parties and are valued at the lower of cost or market. Cost is determined by the first-in, first-out method.
Inventory reserves are established when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. Inventory reserves are measured as the difference between cost of inventory and the estimated net realizable value. Provisions for inventory reserves are charged to cost of sales. The Company’s estimated inventory reserve is provided for in the consolidated financial statements and actual reserve requirements have approximated management’s estimates.
Impairment of Long-lived Assets
The Company periodically assesses potential impairments of its long-lived assets in accordance with the provisions of ASC 360, Property, Plant and Equipment (“ASC 360”). An impairment review is performed whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Factors considered by the Company include, but are not limited to, significant underperformance relative to expected historical or projected future operating results; significant changes in the manner of use of the acquired assets or the strategy for the overall business; and significant negative industry or economic trends. When the carrying value of a long-lived asset may not be recoverable based upon the existence of one or more of the above indicators of impairment, the Company will assess the recoverability of the assets by estimating the future undiscounted operating cash flows expected to result from the use of the asset and its eventual disposition. If the sum of the expected future undiscounted cash flows and eventual disposition is less than the carrying amount of the asset, the Company recognizes an impairment loss. An impairment loss is reflected as the amount by which the carrying amount of the asset exceeds the fair value of the asset, based on the fair market value if available, or discounted cash flows, if not. To date, the Company has not recognized an impairment charge related to its long-lived assets. There were no impairment triggers subsequent to September 30, 2010.
Property and equipment are stated at cost and are depreciated using the straight-line method over their estimated useful lives, as follows:
F-7
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
|
Furniture and fixtures
|
3-5 years
|
|
Computer software and equipment
|
3-5 years
|
|
Lab and office equipment
|
3 years
|
|
Leasehold improvements
|
Lesser of useful life or term of lease (3-10 years)
|
Property and equipment are stated at cost, less accumulated depreciation and amortization. Expenditures for major renewals and betterments are capitalized, while minor replacements, maintenance and repairs, which do not extend the assets’ useful lives, are charged to operations as incurred. Upon sale or disposition, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is included in operations.
Goodwill and Other Intangibles
ASC 350, Intangibles – Goodwill and Other (“ASC 350”) requires the Company to test goodwill for impairment on an annual basis and more frequently if there is reason to suspect that the value has been diminished or impaired, with any corresponding write-downs recognized as necessary.
ASC 350 requires that goodwill be tested for impairment using a two-step process. The first step of the goodwill impairment test used to identify potential impairment compares the fair value of a reporting unit with its carrying amount including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test must be performed to measure the amount of impairment loss, if any. The second step of the impairment test compares the implied fair value of reporting unit goodwill with the carrying amount of that goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. If the carrying amount of the reporting unit goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess.
The Company has selected September 30 as the date on which it performs its annual goodwill impairment test. The entire balance of goodwill of $4,629 is attributable to the Company’s largest reporting unit, which is also an operating segment, the physician-dispensed segment (see Note 12). The determination of fair value of the Company’s goodwill considered the current and future economic, market, competitive conditions, and other relevant factors. The Company evaluated the fair value of goodwill using two approaches. For the first method, the Company derived valuation multiples from historical earnings data of guideline companies within the same industry as the Company and that compete in the physician-dispensed market, and then evaluated and adjusted the multiples based on the Company’s strengths and weaknesses relative to the guideline companies. The derived multiples were then applied to the Company’s operating data to arrive at an indication of fair value. Cash-free invested capital multiples, representing a marketable minority interest, were calculated for the guideline companies as of September 30, 2010. Revenue and earnings multiples were based on a valuation of the guideline companies’ profit margins, historical growth patterns, and a review of the Company’s market factors, primarily related to its physician-dispensed business. The second method, the discounted cash flow method, focused on the expected cash flows of the Company’s physician-dispensed business. The Company evaluated its earnings projections through 2015, trending the growth down to 3% in the terminal year. The discount rate for its physician-dispensed segment was determined by utilizing a weighted average cost of capital analysis, which analyzed long-term government bonds, the Moody’s Seasoned Baa bond rate, the cost of equity of similar companies relative to its physician-dispensed business, as well as the Company’s capital structure. Both methods were weighted 50%. The analyses concluded that the fair value of the Company’s physician-dispensed segment significantly exceeded the carrying amount of its goodwill. There were no impairment triggers subsequent to September 30, 2010. As such, based on the Company’s analysis, no impairment charges were recognized for the years ended December 31, 2010, 2009 and 2008.
Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units, assignment of goodwill to reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated, in part, using a discounted cash flow methodology. This requires significant judgments including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth of the Company’s business, the useful life over which cash flows will occur, and determination of the Company’s weighted average cost of capital. Changes in these estimates and assumptions could materially affect the determination of fair value and/or goodwill impairment for each reporting unit.
Other intangible assets consist of trademarks, distribution rights, covenants not-to-compete, patents, customer lists, and proprietary formulations. Other intangible assets are amortized over the expected period of benefit using the straight-line method over the following lives: trademarks (20 years); distribution rights (ten years); covenants not-to-compete (seven years); other intangible assets (three to 17 years). In accordance with ASC 360, long-lived assets (including intangible assets that are amortized) are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, such as
F-8
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
a significant sustained change in the business climate, during the interim periods. If an indicator of impairment exists for any grouping of assets, an estimate of undiscounted future cash flows is produced and compared to its carrying value. If an asset is determined to be impaired, the loss is measured by the excess of the carrying amount of the asset over its fair value as determined by an estimate of discounted future cash flows. There were no indicators of impairment noted during the years ended December 31, 2010, 2009 and 2008; therefore no tests were performed for long-lived assets.
Intangible Assets
At December 31, 2010 and 2009, the carrying amounts and accumulated amortization of intangible assets were as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||||||||||||||||||
|
Gross Amount
|
Accumulated Amortization
|
Net Book Value
|
Gross Amount
|
Accumulated Amortization
|
Net Book Value
|
|||||||||||||||||||
|
Trademarks
|
$ | 7,530 | $ | (4,770 | ) | $ | 2,760 | $ | 7,458 | $ | (4,398 | ) | $ | 3,060 | ||||||||||
|
Distribution rights
|
— | — | — | 1,082 | (1,049 | ) | 33 | |||||||||||||||||
|
Covenant not-to-compete
|
— | — | — | 931 | (931 | ) | — | |||||||||||||||||
|
Licenses
|
1,375 | (926 | ) | 449 | 2,075 | (1,520 | ) | 555 | ||||||||||||||||
|
Other intangible assets
|
3,511 | (2,128 | ) | 1,383 | 3,785 | (2,497 | ) | 1,288 | ||||||||||||||||
| $ | 12,416 | $ | (7,824 | ) | $ | 4,592 | $ | 15,331 | $ | (10,395 | ) | $ | 4,936 | |||||||||||
During the year ended December 31, 2010, the Company wrote off expired and fully amortized licenses and other intangible assets of $3,316, as well as legal expenses associated with abandoned trademarks, resulting in a loss on disposal of $3. Amortization expense related to all intangible assets, including certain amounts reflected in cost of sales, for the years ended December 31, 2010, 2009 and 2008 was $741, $777 and $820, respectively.
Future estimated aggregate amortization expense for the next five years and thereafter is as follows:
|
Years ending December 31,
|
||||
|
2011
|
715 | |||
|
2012
|
715 | |||
|
2013
|
713 | |||
|
2014
|
687 | |||
|
2015
|
687 | |||
|
Thereafter
|
1,075 | |||
| $ | 4,592 | |||
Debt Issuance Costs
Direct costs incurred in connection with indebtedness agreements are capitalized as incurred and amortized using the effective interest method over the term of the related indebtedness. Debt issuance costs are included in “Other assets” and represent fees and other costs incurred in connection with the Credit and Term Loan Agreement with Comerica Bank entered into on November 3, 2010 (the “Credit and Term Loan Agreement”).
The Credit and Term Loan Agreement replaced and terminated the Company’s former credit facility issued under the Revolving Credit Agreement, dated as of November 21, 2008, with Comerica (the “Former Credit Facility”) which was scheduled to terminate on November 21, 2011. The Company capitalized $92 in debt issuance costs related to the Credit and Term Loan Agreement. In connection with establishing the Former Credit Facility, the Credit Agreement entered into on January 28, 2005 with Merrill Lynch Capital (the “Credit Agreement”) was terminated. Termination of the Credit Agreement resulted in a write-off of debt issuance costs of $130 during the year ended December 31, 2008. The Company capitalized $32 in debt issuance costs related to the Former Credit Facility during the year ended December 31, 2008.
The amortization and the write-offs of debt issuance costs are included as a component of “Interest expense” in the Consolidated Statements of Income. At December 31, 2010 and 2009, the Company had debt issuance costs of $103 and $25, respectively. Amortization of debt issuance costs was $21, $6 and $82 for the years ended December 31, 2010, 2009 and 2008, respectively.
F-9
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Income Taxes
Income taxes are determined using an annual effective tax rate, which generally differs from the United States federal statutory rate, primarily because of state taxes and research and development tax credits. The Company recognizes deferred tax assets and liabilities for temporary differences between the financial reporting basis and the tax basis of the Company’s assets and liabilities, along with net operating loss and credit carryforwards.
The effect on deferred taxes of a change in tax rates is recognized in income in the period that includes the enactment date. Income tax benefits credited to stockholders’ equity relate to tax benefits associated with amounts that are deductible for income purposes but do not impact net income. These benefits are principally generated from employee exercises of non-qualified stock options and the vesting of restricted stock.
Leases
The Company accounts for leases under the provisions of ASC 840, Leases, which require that the Company’s leases be evaluated and classified as either operating leases or capital leases for financial reporting purposes. Minimum base rents for the Company’s operating leases, which generally have scheduled rent increases over the term of the lease, are recorded on a straight-line basis over the lease term. The initial lease term includes the period from when the Company is given access and control over the leased property, whether or not rent payments are due under the terms of the lease.
For leases with renewal periods at the Company’s option, the Company generally considers the lease term to comprise only the initial lease term as exercise of the renewal options are not considered to be reasonably assured at lease inception. However, if failure to exercise a renewal option imposes an economic penalty of sufficient magnitude to the Company, then the renewal, at inception, is reasonably assured and will be included in the determination of the appropriate lease term.
In certain instances, the Company disburses cash for leasehold improvements, furnishings, fixtures and equipment to renovate leased premises. If costs are paid directly by the landlord or reimbursed to the Company by the landlord, the Company records a deferred rent liability and amortizes the deferred rent liability over the lease term as a reduction to rent expense.
Treasury Stock
The Company records treasury stock under the cost method, whereby the entire cost of the acquired stock is recorded as treasury stock. The Company records reissuances of treasury stock at the fair value of the underlying transactions. Differences between the fair value of the issuance and the cost basis of the treasury stock that result in gains are recorded as adjustments to additional paid-in capital. If the transaction results in a loss, the difference would be charged to additional paid-in capital to the extent of any gains previously recorded, and the remaining amount would be then charged to accumulated earnings.
Revenue Recognition
The Company recognizes revenue in the physician-dispensed segment in accordance with ASC 605, Revenue Recognition (“ASC 605”), which provides guidance on the recognition, presentation and disclosure of revenue in financial statements filed with the Securities and Exchange Commission (“SEC”). ASC 605 outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure related to revenue recognition policies. In general, the Company recognizes revenue when: (i) persuasive evidence of an arrangement exists; (ii) shipment of products has occurred; (iii) the sales price charged is fixed or determinable; and (iv) collection is reasonably assured. The Company’s shipment terms are FOB shipping point as outlined in its sales agreements.
The Company’s domestic sales agreements do not provide for rights of return or price protection. However, the Company may approve returns on a case-by-case basis at its discretion. Based on the Company’s historical experience, such returns generally approximate 1.1% of the Company’s net product sales, gross of returns and allowances and shipping and handling revenue. Certain international distribution agreements do provide for rights of return and price protection. Generally, such return rights are for a period of not more than 90 days after shipment. In accordance with ASC 605-15, Revenue Recognition - Products (“ASC 605-15”), the Company continuously monitors and tracks product returns and records a provision for the estimated future amount of such returns, based on historical experience and any notification received of pending returns. The allowance for sales returns as of December 31, 2010 and 2009 was $267 and $492, respectively, and was recorded as a reduction to revenue. The Company does not grant any warranty provisions on its products. The Company provides for discounts and allowances based on historical experience at the time revenue is recognized as a reduction to revenue. To date, actual provisions have approximated management’s estimates.
The Company grants price protection rights to certain international distributors. Such price protection rights require the Company to pay the distributor if there is a reduction in the list price of the Company’s products. Price protection payments would be required for the distributor’s inventory on-hand or in-transit on the date of the price reduction, for a period not to exceed 90 days prior to
F-10
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
the date of the price reduction. The Company has not recorded a liability in connection with such price protection rights as the Company has never reduced the list prices of its products.
In September 2002, the Company entered into a licensing agreement, as amended (as discussed in Note 8) with an international Japanese-based distributor that specializes in the distribution and marketing of over-the-counter (“OTC”) medical oriented products in the drug store and retail channels. In January 2006, the Company entered into a licensing agreement with a diversified Japanese consumer products and services company, which also owns and operates a large chain of aesthetic spas in Japan. The Company recognizes royalty revenues related to these licensing agreements based on the respective distributor’s sale of related products. These royalty revenues are recognized as earned and are based upon a predetermined rate within the respective licensing agreement.
Included in revenues are fees charged to customers for shipping and handling. Such revenues amounted to $2,146, $2,201 and $2,384 for the years ended December 31, 2010, 2009 and 2008, respectively. Shipping and handling costs incurred in a sales transaction to ship products to customers are included as a component of “Cost of sales.”
Exit of the Pharmacy Channel
During the year ended December 31, 2008, the Company entered the pharmacy channel for the first time by launching SoluCLENZ Rx Gel, a solubilized benzoyl peroxide gel for the treatment of acne, which was available only by prescription. The Company sold SoluCLENZ to pharmaceutical wholesalers, who had the right to return purchased product prior to the units being dispensed through patient prescriptions. Revenue for this product was recognized in accordance with ASC 605. Among its criteria for revenue recognition from sale transactions where a buyer has a right of return, ASC 605-15 requires the amount of future returns to be reasonably estimated.
However, after closely monitoring the progress of the launch and weekly sales data, the Company determined that the distribution of a single prescription product through the pharmacy channel and the ongoing investment to support that channel had become cost-prohibitive. Accordingly, on April 13, 2009, the Company announced that it would no longer sell SoluCLENZ in the pharmacy channel.
Prior to the Company’s exit of the pharmacy channel, during the launch period only, the Company sold product to wholesalers with a guaranteed sale provision, allowing the wholesalers to return the product within 120 days of shipment. Product shipments during the launch period were $1,569 for the year ended December 31, 2008. As substantially all of the risks and rewards of ownership did not transfer upon shipment, the Company treated the sale of product during the launch period and after the launch period under a consignment model. Under this consignment model, the Company did not recognize revenue upon the shipment of product with the guaranteed sale provision and accounted for inventory held by the wholesalers as consignment inventory. Due to the Company’s limited experience in the pharmacy channel, the Company was not able to reasonably estimate customer returns and, therefore, only recognized revenue related to SoluCLENZ units once the units were dispensed through patient prescriptions because units dispensed to patients were not subject to return. The Company obtained actual prescription units dispensed based on distribution channel data provided by external, independent sources. Gross product revenue and net product revenue (gross product revenue, net of cash discounts, rebates and patient coupons) for prescription units dispensed were $248 and $162, respectively, for the year ended December 31, 2008.
In connection with the exit of the pharmacy channel, during the year ended December 31, 2009, the Company recorded charges approximating $769, relating to contractual deposits, obsolete selling materials and other contract termination fees (included within “Selling, general and administrative expenses” in the Consolidated Statements of Income), primarily relating to the Company’s contract sales force that was dedicated to selling SoluCLENZ and the termination of certain contractual obligations related to SoluCLENZ. In addition, during the three months ended March 31, 2009, the Company reserved approximately $440 in inventory (included within “Cost of sales” in the Consolidated Statements of Income). During the three months ended December 31, 2009, the Company informed its SoluCLENZ distributors that it would no longer issue credit for returned inventory. As a result, the Company recognized $221 in revenue, previously recorded as an accrued liability to be paid to distributors for remaining inventory on hand, during the three months ended December 31, 2009. Revenues previously reported in the “Pharmacy Rx” segment are now included in the “Physician-dispensed” segment under the “Therapeutic” product category (see Note 12).
Reserves for International Distributor
During the year ended December 31, 2010, the Company recognized $662 in charges related to a non-performing international distributor. The charges are composed of a $151 reserve on inventory shipped to the distributor, for which no revenue was recognized, and a $511 reserve on the distributor’s outstanding receivable balance. The Company terminated its distribution agreement with this distributor on November 24, 2010. Sales and gross profit associated with this distributor, who solely received product from the Company’s physician-dispensed operating segment, are not significant to the Company’s physician-dispensed operating segment’s results.
F-11
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Advertising Costs
Advertising costs are expensed the first time the advertising takes place. Advertising and promotion costs incurred for the years ended December 31, 2010, 2009 and 2008 were approximately $425, $414 and $235, respectively. Advertising costs are recorded as a component of “Selling, general and administrative expense.”
Foreign Currency Translation
The financial statements of subsidiaries outside the United States are measured using the local currency as the functional currency. Assets and liabilities of these subsidiaries are translated at the rates of exchange at the balance sheet date. Revenue and expense items are translated at average monthly rates of exchange. The resultant translation adjustments are recorded in comprehensive income, a separate component of stockholders’ equity. Such adjustments amounted to an unrealized loss of $2, $13 and $1 for the years ended December 31, 2010, 2009 and 2008, respectively.
Research and Development
Research and development activities have historically focused on improving the efficacy of existing pharmaceutical ingredients by enhancing penetration. These activities primarily consist of formulation, chemistry and analytical manufacturing controls and stability work. Research and development expenses include, among other things, wages and benefits, raw materials and supplies, facilities, outside professionals and professional service provider fees. Clinical trials and certain support functions in preparing protocols, reports and other regulatory documents are performed by scientific consultants and third-party contract research organizations. Research and development costs are expensed as incurred.
In October 2010, the Company was awarded a grant of $244 under the Qualifying Therapeutic Discovery Grant Program administered by the Internal Revenue Service and the Department of Health and Human Services in support of the Company’s development of Nu-Derm for the treatment of melasma. The Company recorded the grant funds received as a component of and an offset to “Research and development expense” in the Consolidated Statements of Income.
Stock-based Compensation
Effective January 1, 2006, the Company adopted the modified prospective transition method in accordance with guidance under ASC 718, Compensation (“ASC 718”), and as a result, did not retroactively adjust results from prior periods. Under this transition method, stock-based compensation was recognized for: (i) expense related to the remaining unvested portion of all stock option awards granted during the one-year period preceding the Company’s initial public offering, based on the grant date fair value estimated in accordance with the original provisions of SFAS No. 123; and (ii) expense related to all stock option awards granted on or subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the revised provisions under ASC 718. The Company applies the Black-Scholes valuation model in determining the fair value of share-based payments to employees. The resulting compensation expense is recognized on the straight-line basis over the requisite service period, which equals the option vesting term, generally three years.
Earnings per Common Share
The Company computes earnings per share in accordance with ASC 260, Earnings per Share. Basic earnings per share are computed by dividing net income by the weighted-average number of common shares outstanding. Diluted earnings per common share are computed similar to basic earnings per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Potential common shares are excluded from the computation if their effect is anti-dilutive. The Company’s potential common shares consist of stock options, restricted stock and restricted stock units issued under the Company’s stock option plans.
Under the treasury stock method, the assumed proceeds calculation includes: (i) the actual proceeds to be received from the employee upon exercise; (ii) the average unrecognized compensation cost during the period; and (iii) any tax benefits that will be credited upon exercise to additional paid-in capital. Basic and diluted earnings per common share were calculated using the following share information for the years ended December 31, 2010, 2009 and 2008:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Weighted average shares outstanding - basic
|
21,572,870 | 21,970,491 | 22,598,474 | |||||||||
|
Effect of dilutive stock options
|
243,300 | 51,641 | 9,215 | |||||||||
|
Weighted average shares outstanding - diluted
|
21,816,170 | 22,022,132 | 22,607,689 | |||||||||
F-12
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
For the years ended December 31, 2010, 2009 and 2008, diluted earnings per share do not include the impact of common stock options and restricted stock units then outstanding of 451,000, 1,491,396 and 1,630,330, respectively, as the effect of their inclusion would be anti-dilutive.
New Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board issued Accounting Standards Update (“ASU”) No. 2010-06, Improving Disclosures about Fair Value Measurements (“ASU No. 2010-06”), an amendment to ASC 820, Fair Value Measurements and Disclosures. This amendment requires an entity to: (i) disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers; and (ii) present separate information for Level 3 activity pertaining to gross purchases, sales, issuances, and settlements. ASU No. 2010-06 is effective for interim and annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. The Company adopted the provisions of ASU No. 2010-06 during the three months ended March 31, 2010. The adoption of ASU No. 2010-06 did not have a material effect on the Company’s consolidated financial statements.
Note 3: Composition of Certain Financial Statement Captions
Inventories
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Inventories, net
|
||||||||
|
Raw materials
|
$ | 770 | $ | 1,204 | ||||
|
Finished goods
|
4,455 | 5,622 | ||||||
| 5,225 | 6,826 | |||||||
|
Less reserve for inventories
|
(600 | ) | (598 | ) | ||||
| $ | 4,625 | $ | 6,228 | |||||
Property and Equipment
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Property and equipment
|
||||||||
|
Furniture and fixtures
|
$ | 739 | $ | 733 | ||||
|
Computer software and equipment
|
4,400 | 4,357 | ||||||
|
Lab and office equipment
|
509 | 456 | ||||||
|
Leasehold improvements
|
2,658 | 4,201 | ||||||
|
Capital lease (office equipment)
|
— | 115 | ||||||
|
Construction in progress
|
10 | 199 | ||||||
| 8,316 | 10,061 | |||||||
|
Less accumulated depreciation and amortization
|
(5,062 | ) | (5,372 | ) | ||||
| $ | 3,254 | $ | 4,689 | |||||
Depreciation expense for the years ended December 31, 2010, 2009 and 2008 was $1,184, $1,556 and $1,489, respectively. At December 31, 2009, accumulated depreciation for assets under capital lease was $106.
During the year ended December 31, 2010, the Company recorded a loss on disposal of fixed assets of $463. In September 2010, the Company exercised its right to terminate the Agreement dated as of June 29, 2006 (the “2006 Agreement”) with Zein E. Obagi, MD Inc. (“Obagi Inc”), Zein Obagi (“Dr. Obagi” and, together with Obagi Inc., the “Obagi Entities”), Samar Obagi, the Zein and Samar Obagi Family Trust and Skin Health Properties, Inc. (“the Marketer”) (collectively, the “Dr. Obagi Parties”) effective as of October 4, 2010. See Note 9 for further discussion. As a result of the termination, the Company determined that its leasehold improvements in connection with a lease agreement with the Marketer for certain property located in Beverly Hills, California were impaired resulting in the write-off of $258 during the year ended December 31, 2010. The remaining loss on disposal of fixed assets of $205 related to the disposal of manufacturing and computer equipment.
F-13
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
During the year ended December 31, 2009, the Company recorded a loss on disposal of fixed assets of $56. In October 2008, the Company moved to new corporate headquarters located in Long Beach, California (see Note 10). In connection with the move, the Company wrote off $1,889 in property and equipment, primarily leasehold improvements related to its prior location. The write-off did not result in a gain or loss as the assets were fully depreciated. Losses on the disposal of fixed assets are reported as a component of “Selling, general and administrative expenses.”
|
December 31,
|
||||||||
| 2010 | 2009 | |||||||
|
Accrued liabilities
|
||||||||
|
Salaries and related benefits
|
$ | 4,748 | 3,280 | |||||
| Other | 1,731 | 1,521 | ||||||
| $ | 6,479 | $ | 4,801 | |||||
Note 4: Revolving Credit Facility and Term Loans
On November 3, 2010, the Company entered into a Credit and Term Loan Agreement with Comerica Bank. The Credit and Term Loan Agreement replaces and terminates the Company’s Former Credit Facility of $20,000 in revolving credit, dated as of November 21, 2008, which was scheduled to terminate on November 21, 2011. The Company did not have any outstanding balance on the Former Credit Facility at the time of termination.
Under the Credit and Term Loan Agreement, the Company has access to: (i) up to $20,000 in a revolving credit facility (the “Facility”); and (ii) one or more term loans in an aggregate amount of up to $15,000 (the “Term Loans”). The Company may borrow under the Term Loans until the earliest to occur of: (i) the date the aggregate outstanding principal balance of the Term Loans equals $15,000; (ii) May 3, 2011; or (iii) the date of the Company’s request to close out the Term Loans (the “Term Loan Draw Period”). The Facility will terminate, and any amounts outstanding under the Facility will be due on July 1, 2012 and all amounts outstanding under the Terms Loans will be due five years after the last day of the Term Loan Draw Period, unless terminated earlier in accordance with the provisions of the Credit and Term Loan Agreement.
Any borrowings under the Facility and Term Loans will bear variable interest based on a margin, at the Company’s option, over prime rate or LIBOR as defined in the Credit and Term Loan Agreement. All amounts borrowed under the Credit and Term Loan Agreement are secured by a first priority security interest in all of the Company’s tangible and intangible assets. Commencing January 2011, the Company must pay a revolving credit facility fee annually in arrears of $55. The Credit and Term Loan Agreement contains certain financial and non-financial covenants. Non-financial covenants include, among other things, monthly and quarterly reporting of a listing of the Company’s intellectual property. Financial covenants include requirements for maintaining: (i) a minimum quick ratio; (ii) a maximum ratio of total liabilities to net worth; and (iii) a minimum EBITDA amount; as well as limitations on (a) annual capital expenditures, (b) asset- or equity-based investments, (c) stock repurchases, which may not exceed $50,300, (d) dividend distributions, which may not exceed 25% of net income for the fiscal year, and (e) joint venture investments, which may not exceed $2,000. As of December 31, 2010, the Company was in compliance with all non-financial and financial covenants. During the year ended December 31, 2010, the Company did not draw on the Facility or the Term Loans.
As noted above, on November 21, 2008, the Company entered into the Former Credit Facility with Comerica Bank for $20,000. The Former Credit Facility had an initial term of three years, ending on November 21, 2011, unless terminated earlier in accordance with its provisions. Any borrowings under the Former Credit Facility bore variable interest based on a margin, at the Company’s option, over prime rate or LIBOR as defined in the Former Credit Facility. All amounts borrowed under the Former Credit Facility were to be secured by a first priority security interest in all of the Company’s tangible and intangible assets. The Facility contained certain financial and non-financial covenants. Non-financial covenants included, among other things, certain limitations on annual capital expenditures and asset- or equity-based investments and monthly and quarterly reporting of a listing of the Company’s intellectual property. Financial covenants included requirements for maintaining a: (i) minimum quick ratio; (ii) maximum ratio of total liabilities to net worth; (iii) no loss for two consecutive quarters; (iv) minimum level of profitability; and (v) maximum annual capital expenditures; as well as limitations on stock repurchases, which could not exceed $10,000, dividend distributions, which could not exceed 25% of net income for the fiscal year, and joint venture investments, which could not exceed $2,000. As of January 31, 2009, the Company was not in compliance with the non-financial covenant, requiring it to report monthly, a listing of its intellectual property. In February 2009, the Company obtained a waiver for January 2009 with respect to this non-financial covenant. As of December 31, 2009, the Company was in compliance with all non-financial and financial covenants. During the fiscal year 2010, through the date of termination in November 2010, and during the year ended December 31, 2009, the Company did not draw on the Former Credit Facility.
F-14
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 5: Common Stock
Stock Repurchases
On October 26, 2010, the Company announced that its Board of Directors has authorized the Company to repurchase up to $45,000 of its common stock, depending on market conditions and other factors. Share repurchases have included the repurchase of shares from the selling stockholders following completion of the secondary offering completed in November 2010 (see Note 1), and may include future repurchases made through open market or privately negotiated transactions in compliance with SEC Rule 10b-18, subject to market conditions, applicable legal requirements and other factors. This authorization does not obligate the Company to acquire any particular amount of common stock nor does it ensure that any shares will be repurchased, and it may be suspended at any time at the Company’s discretion.
During the year ended December 31, 2010, the Company repurchased 3,556,910 shares from the selling stockholders pursuant to a Stock Purchase Agreement for $35,000 at a price of $9.84 per share, which was equal to the public offering price of shares sold by the selling stockholders in the secondary offering in November 2010 of $10.25 per share less the applicable underwriter’s discount. This amount is reflected in “Treasury stock” on the Consolidated Balance Sheet. See Note 9 for further discussion.
On August 5, 2008, the Board of Directors authorized the repurchase of up to $10,000 of the Company’s outstanding common shares, which expired on August 5, 2010. The purchases were to be made in the open market or in privately negotiated transactions from time to time as permitted by securities laws and other legal requirements. The timing, manner, price and amount of any repurchases were determined by a three-person committee, consisting of members of the Company’s Board and management, at its discretion and were subject to economic and market conditions, stock price, applicable legal requirements and other factors, and could be discontinued at any time. During the year ended December 31, 2008, the Company repurchased 627,367 shares for $4,016 (including transaction costs) at an average cost of $6.37 per share. During the year ended December 31, 2009, the Company repurchased 183,664 shares for $1,332 (including transaction costs) at an average cost of $7.22 per share. These amounts are reflected in “Treasury stock” on the Consolidated Balance Sheet.
Note 6: Income Taxes
The current and deferred provision (benefit) for federal and state income taxes consists of the following:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Current
|
||||||||||||
|
Federal
|
$ | 4,620 | $ | 5,797 | $ | 6,673 | ||||||
|
State
|
1,110 | 1,206 | 1,199 | |||||||||
| 5,730 | 7,003 | 7,872 | ||||||||||
|
Deferred
|
||||||||||||
|
Federal
|
556 | 20 | (221 | ) | ||||||||
|
State
|
62 | 122 | (115 | ) | ||||||||
| 618 | 142 | (336 | ) | |||||||||
| $ | 6,348 | $ | 7,145 | $ | 7,535 | |||||||
The provision for income taxes differs from the amount that would result from applying the federal statutory rate to pre-tax income for the years ended December 31, 2010, 2009 and 2008 as follows:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Federal statutory income tax rate
|
35.0 | % | 35.0 | % | 35.0 | % | ||||||
|
State income tax, net of federal benefit
|
4.8 | % | 4.7 | % | 3.5 | % | ||||||
|
Research and development credit
|
(1.7 | )% | (1.5 | )% | (1.5 | )% | ||||||
|
Secondary offering costs and stock repurchase
|
1.6 | % | — | — | ||||||||
|
Other
|
0.4 | % | 0.5 | % | 0.4 | % | ||||||
| 40.1 | % | 38.7 | % | 37.4 | % | |||||||
The significant components of the net deferred tax assets and liabilities at December 31, 2010 and 2009 are as follows:
F-15
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred assets
|
||||||||
|
Financial statement reserves
|
$ | 899 | $ | 993 | ||||
|
State taxes
|
30 | 59 | ||||||
|
Trademark expenses
|
117 | 134 | ||||||
|
Compensation expense
|
2,105 | 1,985 | ||||||
|
Prepaid royalty
|
473 | 467 | ||||||
|
Foreign operating loss
|
— | 47 | ||||||
|
Tenant improvement allowance
|
321 | 357 | ||||||
|
Deferred revenue
|
38 | 45 | ||||||
|
Other
|
776 | 446 | ||||||
| 4,759 | 4,533 | |||||||
|
Deferred liabilities
|
||||||||
|
Prepaid expenses
|
(289 | ) | (131 | ) | ||||
|
Depreciation and amortization
|
(1,874 | ) | (1,141 | ) | ||||
| (2,163 | ) | (1,272 | ) | |||||
|
Net deferred assets
|
2,596 | 3,261 | ||||||
|
Less valuation allowance
|
— | (47 | ) | |||||
| $ | 2,596 | $ | 3,214 | |||||
The Company recorded a deferred tax asset of $0 and $47 related to the net operating loss carryover of its majority-owned subsidiary Obagi Singapore as of December 31, 2010 and 2009, respectively. A full valuation allowance was provided for the loss carryover as of December 31, 2009 as the Company believed that it was more likely than not that this deferred tax asset would not be realized. As Obagi Singapore was dissolved during the year ended December 31, 2010, the net operating loss and related valuation allowance no longer existed as of December 31, 2010.
A reconciliation of the beginning and ending amount of unrecognized tax benefits in accordance with the provisions related to uncertain tax positions under ASC 740, Income Taxes, is as follows:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Balance as of January 1
|
$ | 147 | $ | 176 | $ | 161 | ||||||
|
Additions based on tax positions related to the current year
|
78 | 24 | 30 | |||||||||
|
Reductions for tax positions of prior years due to settlements
|
(40 | ) | (53 | ) | (15 | ) | ||||||
|
Balance as of December 31
|
$ | 185 | $ | 147 | $ | 176 | ||||||
Any change in the above unrecognized tax benefits will impact the effective tax rate.
The Company does not believe there will be any material changes in its unrecognized tax benefits within the next 12 months.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. During the years ended December 31, 2010, 2009 and 2008, the Company recognized approximately $0, $6 and $14 in interest and penalties, respectively. As of December 31, 2010 and 2009, accrued interest related to uncertain tax positions was $12 for both periods.
The tax years 2006-2009 remain open to examination by the major taxing jurisdictions to which the Company is subject.
F-16
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 7: Concentrations of Credit Risk and Significant Customers
For the years ended December 31, 2010, 2009 and 2008, no single customer accounted for 5% or more of the Company’s net sales.
The Company currently has limited internal manufacturing capabilities and outsources most of its product manufacturing. Additionally, the Company does not have long term contracts with most of these third-party manufacturers. Although there are a limited number of third-party manufacturers, management believes that other suppliers could provide similar services on comparable terms. A change in suppliers, however, could cause a delay in manufacturing and a possible loss of sales, which would affect operating results adversely.
The Company currently has limited internal research and development capabilities and primarily outsources its product research and development to third-party research labs. Although there are a limited number of third-party research labs, management believes that other labs could provide similar services on comparable terms. A change in providers, however, could cause a delay in the Company’s ability to develop and deliver products on a timely and competitive basis, which could adversely affect operating results.
Note 8: Royalty Licensee Agreements
Pursuant to the patent license agreement dated June 26, 2003 with a third party, the Company licensed four interlocking method and formulation patents in exchange for non-refundable license fees for the first three years of the agreement. The agreement also calls for the Company to pay royalties on net sales of products covered by the licensed patents. Such royalties are to be paid on a quarterly basis. For the years ended December 31, 2010, 2009 and 2008, related royalties of $404, $339 and $350, respectively, have been included as a component of “Cost of sales.” The initial term of this agreement was three years but is renewable annually at the option of the Company, provided that the Company pays an annual non-refundable renewal license fee to the licensor. The patent license agreement may terminate at the licensor’s option upon a merger or acquisition of the Company that is not approved by the licensor, whose approval may not be unreasonably withheld, and may be cancelled by either party upon default.
In January 2006, the Company entered into an option and product license agreement with a third party to license certain technology, in exchange for a non-refundable fee in the amount of $250, with an additional $250 payable within 15 days of confirming successful clinical results. The non-refundable fee of $250 was to either be offset against future royalties payable under the agreement or offset against future purchases of inventory. For the first product developed under this agreement, an additional license payment was payable upon the product becoming commercially available for distribution with another license fee due in the subsequent quarter to that event. For each additional product developed, an additional license payment was due up to a maximum dollar cap for all products developed. The agreement also called for the Company to pay royalties on the net sales of products sold in a territory covered by the licensed patents, subject to minimum annual payments. In addition to the royalties, the Company was to also pay one-time milestone payments based upon achieving certain levels of net sales. The term of this agreement was for the life of any licensed patent. The option and product license agreement would terminate at the licensor’s option upon the Company’s failure to meet commercially reasonable development benchmarks. Pursuant to this agreement, the initial payment of $250 was included in long-term “Other assets” as of December 31, 2007. Royalty payments were to be expensed as incurred. As of December 31, 2008, no additional payments were made. As of December 31, 2008, there were no commercially viable products developed under this agreement. As a result, during the year ended December 31, 2008, the Company wrote off the initial payment of $250, which is included as a component of “Research and development expense.”
In March 2004, as part of an agreement with a third-party international licensor, the Company received an ongoing, non-exclusive right to market and sell any and all products marketed under the “Obagi” brand containing a specific range of Kinetin concentration, limited to existing channels of trade in Japan. Under the terms of the license agreement with the same party, the Company’s license rights are valid until the last of the related patents expire in December 2014. In exchange for this right, the Company paid, on April 2, 2004, an initial payment of $1,250, net of cash receipts in the amount of $250 from a sublicensing arrangement with an international distributor in Japan. In addition, the Company will pay royalties up to an additional maximum amount of $500 based on future sales in Japan of such skincare products. Pursuant to this agreement, the Company recorded the initial net payment as an intangible asset and is amortizing such costs on the straight-line basis over the license period. During each of the years ended December 31, 2010, 2009 and 2008, the Company recognized $106 in amortization expense. Royalty payments are expensed as incurred and have amounted to $31, $41 and $27 for the years ended December 31, 2010, 2009 and 2008, respectively. License fee amortization and royalties are included as a component of “Cost of sales.”
The Company also competes in the Japanese retail skin care markets through a strategic licensing agreement with Rohto Pharmaceutical Co., Ltd. (“Rohto”). Rohto is a Japanese pharmaceutical manufacturer and distributor. Under the agreement, Rohto is licensed to manufacture and sell a series of OTC products developed by it under the Obagi brand name, as well as Obagi-C (a Vitamin C based topical serum in various concentrations), in the Japanese drug store channel and the Company receives a royalty based upon sales of Obagi branded products in Japan by Rohto. The Company also has other licensing arrangements in Japan to market and sell OTC product
F-17
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
systems under the Obagi brand, both for in-office use in facial procedures, as well as for sale as a take-home product kit in the spa channel.
On December 4, 2008, the Company entered into an amendment to the original agreement with Rohto. The amendment extends the term of agreement to December 4, 2017 and amends the Territory (as defined) to areas outside Japan, subject to separate written agreement(s) to be developed. The Channel (as defined) has been expanded to include department stores, including mail-order and internet activities to support such expansion, provided that the parties complete a joint development plan. The parties have also agreed to work together to up-brand the Obagi products sold in the various channels and work cooperatively on messaging, branding and product imaging. Under the amendment, the Company has also agreed to provide an exclusive royalty bearing license to sell and/or manufacture certain products developed by the Company, Rohto or jointly developed by the Company and Rohto.
On December 4, 2008, the Company and Rohto entered into a License and Supply Agreement (the “Bi-mineral Agreement”) whereby the Company granted Rohto an exclusive right to manufacture, market and sell the Company’s bi-mineral collagen and elastin enhancing products in all channels (other than the aesthetic and spa channels) in Japan. Rohto also has the right to develop improvements to such products or new products related to the products. The initial term of the Bi-mineral Agreement is for five years, through December 4, 2013, with an optional five-year extension, and calls for certain annual sales volume and expense commitments on behalf of Rohto. If such commitments are not met, the Company shall have the right to terminate the agreement or render it non-exclusive.
As consideration for the exclusive license, Rohto will pay a development fee to the Company over a five-year period as well as quarterly royalty fees based on product sales. If the Bi-Mineral Agreement should be terminated by either party before all five installments of the development fee have been paid or in the event of early termination, then any unpaid installments will become due and payable to the Company ten days before the effective termination date. The royalty rate is scaled over the term of the agreement. The Company will record the installments of the development fee as deferred revenue as they are received and will amortize the development fee over the life of the agreement and any royalties received will be recognized as earned. During each of the years ended December 31, 2010, 2009 and 2008, the Company received $100 installments of the development fee and recorded the fees as deferred revenue. Net licensing revenue from skin health systems and products in Japan was approximately $4,401, $4,630 and $4,817 for the years ended December 31, 2010, 2009 and 2008, respectively.
Note 9: Related-party Transactions
Stock Purchase Agreement
On November 15, 2010, the Company entered into a Stock Purchase Agreement with the selling stockholders, under which the Company was obligated to purchase from the selling stockholders $35,000 worth of the Company’s common stock, subject to certain conditions. The purchase price per share to be paid to the selling stockholders was to be the same price paid by the underwriter to purchase shares from the selling stockholders in the secondary offering discussed in Note 1. However, if the purchase price to be paid was greater than $13.00, the Company would not purchase any shares from the selling stockholders. In addition, the purchase of the shares by the Company was conditioned on the sale of shares in the secondary offering.
Following the completion of the secondary offering of 2,690,244 shares on November 24, 2010, the Company repurchased 3,556,910 shares from the selling stockholders at a price of $9.84 per share, which was equal to the public offering price of $10.25 per share less the applicable underwriter’s discount, on November 30, 2010. Upon completion of both transactions the selling stockholders ceased to own any shares of the Company’s common stock.
Dr. Zein Obagi, M.D.
As discussed above, as of November 30, 2010, Zein Obagi, M.D., the Company’s former executive medical director and board member, ceased to own any shares of the Company’s common stock. As a result, subsequent to November 30, 2010, Dr. Obagi and his affiliates are no longer considered related parties. Dr. Obagi was granted the right to purchase products from the Company at a discount equal to the maximum discount offered to the Company’s unrelated customers.
As mentioned in Note 3, on September 2, 2010, the Company exercised its right to terminate the the 2006 Agreement with the Dr. Obagi Parties, effective as of October 4, 2010. The 2006 Agreement provided, among other things, that the Obagi Entities and/or the Marketer would promote and provide services to support the marketing of the Company’s products, including the oversight of property the Company leases in Beverly Hills, California. Additionally, the Obagi Entities would have to be available to advise and assist the Company in the formulation and clinical testing of new products and, at the Company’s request, to chair an annual symposium and up to two clinical advisory meetings per year. The 2006 Agreement also contained provisions protecting the Company’s intellectual property rights, including trademarks, and confidential information, and limiting competition by the Dr. Obagi Parties. The Company agreed to pay Obagi Inc. an annual retainer of approximately $570. In addition, the Company agreed to pay Obagi Inc. an
F-18
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
annual fee of $200 for the first two years of the 2006 Agreement for the development of Proderm products, a line of skincare products. At the end of the two years, the Company had the option to continue to sell Proderm products, in exchange for an annual royalty payment. The Company did not exercise this option. During the year ended December 31, 2008, the Company recorded $100 for the annual payment as research and development expense as the research of new products in the Proderm line was still in progress. In addition, the Company agreed to pay Obagi Inc. royalty fees for developing other products identified in the agreement equal to 5% of the Company’s net revenues from sales of those products. As there were no products commercialized under the 2006 Agreement, the Company did not pay any royalties pursuant to the 2006 Agreement during the years ended December 31, 2010, 2009 and 2008. The Company also agreed to pay certain fees and expenses for certain additional training and educational services and clinical studies, as well as 50% of all invoiced commercially reasonable marketing design and development (not production) expenses associated with the opening of the property in Beverly Hills, up to $100. As of December 31, 2009, the Company recorded a payable to Dr. Obagi for $100 related to reimbursable expenses for the opening of the property in Beverly Hills. During the year ended December 31, 2010, the Company paid the balance of $100 to Dr. Obagi. The original term of the 2006 Agreement was five years, and would have otherwise ended on June 29, 2011.
In connection with the termination of the 2006 Agreement, the Company accelerated the payment of certain fees and expenses that otherwise would have been paid during the remainder of the original term. Accordingly, the Company expensed an additional $477 in the third quarter of 2010 that it would have otherwise expensed equally in the fourth quarter of 2010 and the first and second quarters of 2011.
The termination of the 2006 Agreement does not relieve the Dr. Obagi Parties from their obligations to comply with certain provisions of the 2006 Agreement, particularly as they relate to a limited trademark license to Obagi Inc. and/or the Marketer, the grant to the Company of a license to have access to and utilize accounts, customer lists and other customer information and data developed in connection with the 2006 Agreement, the protection of the Company’s intellectual property rights and other additional confidential information of the Company, nor does it affect the Company’s ownership of trademarks and other intellectual property rights.
The Company entered into a lease agreement for the Beverly Hills property described above and a letter agreement with the Marketer as landlord dated June 29, 2006 in connection with the 2006 Agreement. The lease has a term of five years beginning August 1, 2006. The base rent under the lease is $87 per year, and could be raised at a rate of 3.5% per year thereafter. As of December 31, 2009, amounts included in “Prepaid expenses and other current assets” and “Other assets” totaled $184 and $107, respectively. As a result of the termination, the Company determined that the leasehold improvements were impaired resulting in the write-off of $258 in leasehold improvements at the Beverly Hills property and $153 in prepaid rent (included with “Selling, general and administrative expenses”). During the years ended December 31, 2010, 2009 and 2008, the Company recorded $138, $184 and $184 in rent expense related to the agreement, respectively.
The Company also entered into a letter agreement in connection with the lease agreement that relates to leasehold improvements and prepayment of rent. Under the letter agreement, the Marketer acknowledges that the Company has paid $2,197 in respect of leasehold improvements and prepayment of rent under the lease, and the Company will not be required to pay any additional amounts.
On January 9, 2008, the Company entered into an Assignment Agreement with ZSO, LP, an affiliate of Dr. Obagi, whereby the Company assigned its rights under a construction contract with Legacy Construction (“Legacy”) associated with space the Company leases for its marketing and training center.
On January 9, 2008, the Company entered into an Indemnification Agreement with the Dr. Obagi Parties and ZSO, LP (the “Indemnification Agreement”). The Indemnification Agreement provided for the Company to place approximately $340 in escrow in exchange for an indemnification for any liability arising from the underlying Legacy construction contract. By the terms of the June 29, 2006 letter agreement (the “2006 Letter Agreement”) by and among the Company and the Dr. Obagi Parties, the Dr. Obagi Parties were required to reimburse the Company for any amounts the Company spent on leasehold improvements in excess of $1,817. The $340 is above the $1,817 cap on leasehold improvements that the Company was responsible for under the 2006 Letter Agreement.
On July 14, 2008, the Company entered into a binding settlement agreement and stipulation agreement by and between Legacy, certain of the Obagi Parties and Battaglia, Inc. In this agreement, the Company was formally released from any and all remaining obligations to Legacy. In accordance with the agreement, on July 25, 2008, the Company fulfilled its obligations under the Indemnification Agreement by paying the agreed upon $340.
Pursuant to the 2006 Letter Agreement, as restated in the Indemnification Agreement, the Company was released from any future claims related to, and entitled to reimbursement for, the leasehold improvements of the marketing and training center over and above $1,817. The Company recorded $335 as a receivable due from Dr. Obagi as the amount was above the cap on leasehold improvements that the Company was responsible for. As Dr. Obagi disputed the balance due to the Company, as of December 31, 2008, the Company reserved $168 of the balance. As of December 31, 2009, the Company wrote off the remaining $167 as ZO Skin
F-19
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Health, Inc., filed a complaint in the Superior Court of the State of California, County of Los Angeles, against the Company (see Note 10). As of December 31, 2010, payments in the amount of approximately $340 over and above the $1,817 amount of leasehold improvements that the Company was responsible for have not been repaid by the Dr. Obagi Parties. Despite the amounts due from Dr. Obagi relating to the excess amount of leasehold improvements paid by the Company, the Company has not recorded a receivable due from Dr. Obagi for these amounts as of December 31, 2010 or 2009. Amounts due from Dr. Obagi as of December 31, 2010 and 2009 (see table below) solely consist of trade accounts receivable and are considered collectible.
The Company and the Dr. Obagi Parties (and certain entities affiliated with the Dr. Obagi Parties) are currently engaged in pending litigation and other legal matters. See Note 10 for a detailed description of such pending litigation and other legal matters.
Dr. Obagi was a 70% beneficial shareholder in Cellogique Corporation (“Cellogique”), the Company’s largest international distributor. Effective March 20, 2009, Dr. Obagi sold his entire interest in Cellogique. As a result, after the March 20, 2009, Cellogique is not considered a related party and no sales amounts are included in the tables below for the remainder of the year ended December 31, 2009. On November 10, 2005, the Company entered into a Distribution Agreement with Cellogique. The agreement granted Cellogique the exclusive right to promote, market, sell, distribute and sub-distribute certain specified products to customers within the Middle East. The agreement includes discounts off of the distributors’ base price based on volume purchases, and certain advertising and promotional activities. On September 26, 2009, the Company entered into an amendment of the agreement with Cellogique to, among other things; amend the pricing schedule for sales of its products to Cellogique by providing for set prices rather than certain discounts off of distributors’ base prices, and to offer certain marketing incentives to Cellogique. The agreement, as amended, has a term of 12 years, ending December 31, 2017.
Obagi Dermatology – San Gabriel Annex, Inc. (“SGA”)
Dr. Obagi is also a 75% owner of SGA, which also purchases products from the Company. Other than the common ownership interest by Dr. Obagi, the Company was otherwise unrelated to SGA. As discussed earlier, effective November 30, 2010, Dr. Obagi ceased to own any shares of the Company’s common stock. As a result, after November 30, 2010, SGA is not considered a related party and no sales amounts are included in the tables below for the remainder of the year ended December 31, 2010. SGA is located in Southern California and caters to the local Chinese communities.
Total sales made to Dr. Obagi, Cellogique and SGA, and the related cost of sales for the years ended December 31, 2010, 2009 and 2008 are included in the accompanying Consolidated Statements of Income and are as follows:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net Sales
|
$ | 380 | $ | 732 | $ | 2,947 | ||||||
|
Costs of sales
|
44 | 110 | 604 | |||||||||
Receivables due from related parties as of at December 31, 2009 are included in “Accounts receivable from related parties” in the accompanying Consolidated Balance Sheets and are as follows:
|
December 31, 2009
|
||||
|
Due from Dr. Obagi
|
$ | 65 | ||
|
Due from SGA
|
32 | |||
| $ | 97 | |||
Stonington Capital Appreciation 1994 Fund, L.P.
The Company, at times, reimburses board members affiliated with Stonington Partners, Inc., an affiliate of Stonington Fund, a former major stockholder, for expenses related to travel to and from board meetings. As discussed earlier, effective November 30, 2010, Stonington Fund ceased to own any shares of the Company’s common stock. As a result, subsequent to November 30, 2010, Stonington Fund and its affiliate, Stonington Partners, Inc. are no longer considered related parties.
F-20
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Amounts payable to Dr. Obagi for marketing fees and to other stockholders and their affiliates for other expenses as of December 31, 2009 are included in “Amounts due to related parties” in the accompanying Consolidated Balance Sheets as follows:
Note 10: Commitments and Contingencies
Lease Commitments
On August 6, 2008, the Company entered into a lease amendment with Kilroy Realty, L.P. for the lease of office space located in Long Beach, California. The facility consists of 30,884 rentable square feet and the term of the lease is for ten years from the date of initial occupancy, with an option to extend the lease term for five years. The total lease payments over the initial term will approximate $10,805. The Company took possession of the facility on July 1, 2008, and the lease term commenced in October 2008 when the Company took occupancy. Pursuant to the amendment, the Company was granted an improvement allowance of $1,028. The Company recorded the improvement allowance as a deferred rent credit, which is being amortized as a reduction to rent expense over the life of the lease. As of December 31, 2010, amounts recorded as a deferred rent credit in “Accrued liabilities” and “Other long-term liabilities” in the Consolidated Balance Sheet totaled $100 and $680, respectively. As of December 31, 2009, amounts recorded as a deferred rent credit in “Accrued liabilities” and “Other long-term liabilities” in the Consolidated Balance Sheet totaled $100 and $780, respectively. Amounts recorded as deferred rent in “Other long-term liabilities” in the Consolidated Balance Sheet totaled $723 and $674 as of December 31, 2010 and 2009, respectively. The Company leases some of its facilities and equipment under operating leases. Some of the leases require payment of property taxes and include rent escalation clauses. Future minimum operating lease commitments under non-cancelable leases are as follows as of December 31, 2010:
|
Year ending December 31,
|
Operating Leases
|
|||
|
2011
|
$ | 1,985 | ||
|
2012
|
1,844 | |||
|
2013
|
1,505 | |||
|
2014
|
1,123 | |||
|
2015
|
1,156 | |||
|
Therafter
|
3,465 | |||
|
Total minimum payments required
|
$ | 11,078 | ||
Rent expense for facilities and equipment under operating leases for the years ended December 31, 2010, 2009 and 2008 was approximately $2,514, $2,282 and $2,381, respectively.
Indemnifications
The Company is a party to a variety of agreements entered into in the ordinary course of business pursuant to which it may be obligated to indemnify the other parties for certain liabilities that arise out of or relate to the subject matter of the agreements. Some of the agreements entered into by the Company require it to indemnify the other party against losses due to property damage, including environmental contamination, personal injury, failure to comply with applicable laws, the Company’s negligence or willful misconduct, or breach of representations and warranties and covenants.
The Company provides for indemnification of directors, officers and other persons in accordance with limited liability agreements, certificates of incorporation, bylaws, articles of association or similar organizational documents, as the case may be. The Company maintains directors’ and officers’ insurance, which should enable the Company to recover a portion of any future amounts paid.
While the Company’s future obligations under certain agreements may contain limitations on liability for indemnification, other agreements do not contain such limitations and under such agreements it is not possible to predict the maximum potential amount
F-21
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
of future payments due to the conditional nature of the Company’s obligations and the unique facts and circumstances involved in each particular agreement. Historically, no payments have been made under any of these indemnities.
On September 1, 2006, the Company entered into indemnification agreements with each of its directors and executive officers that provide them with rights to indemnification and expense advancement to the fullest extent permitted under the Delaware General Corporation Law.
Steven R. Carlson Separation
Effective October 8, 2010 (the “Effective Date”), Steven R. Carlson, resigned as the Company’s President and Chief Executive Officer and as a member of its Board of Directors. In connection with Mr. Carlson’s departure, the Company entered into a Separation Agreement and Release (“Separation Agreement”) with Mr. Carlson on October 15, 2010. Pursuant to the terms of the Separation Agreement, Mr. Carlson will receive the payments and other benefits to which he would have otherwise been entitled had his employment been terminated without cause under the Employment Agreement, dated March 1, 2005, between the Company and Mr. Carlson as amended (“Employment Agreement”). As a result, on the Effective Date, Mr. Carlson received $66, representing his unpaid base salary through that date as well as accrued vacation pay. On April 11, 2011, in accordance with the terms of the Employment Agreement, Mr. Carlson will also receive: (i) $750, which is equal to 18 months of his base salary, and (ii) $221 representing the pro rata portion of amounts otherwise payable to Mr. Carlson under the Company’s 2010 Performance Incentive Plan that had been accrued as of the Effective Date. The Company will also pay for premiums for continuation of his health coverage under the Consolidated Omnibus Budget Reconciliation Act (“COBRA”) for a period of up to 18 months. In addition, the vesting of the 90,917 shares subject to options held by Mr. Carlson as of the Effective Date (equal to the number of shares subject to those options that would have vested over the 12 month period following the Effective Date) accelerated and such options became immediately exercisable with respect to those shares.
Although there were no provisions for repurchase of the options to purchase the Company’s common stock held by Mr. Carlson, under the Separation Agreement in November 2010, the Company agreed to make to Mr. Carlson a lump sum payment of $875 to extinguish all of his rights in all options to purchase shares of the Company’s common stock outstanding on the Effective Date, without regard to whether such options were then vested and exercisable, would have become exercisable or would have expired subsequent to the Effective Date. The calculation of such lump sum amount was determined based upon the difference between $11.26 per share (the 90-day trailing average closing price of the Company’s common stock as of the Effective Date) and the exercise price in effect for each of the 541,835 vested and exercisable, in-the-money options held by Mr. Carlson on the Effective Date. Upon payment of such lump sum, all of Mr. Carlson’s outstanding options were canceled, terminated and returned to the plan pool.
The Separation Agreement contains a general release by Mr. Carlson of all claims against the Company and its affiliates, a reaffirmation of Mr. Carlson’s obligations under any confidentiality, trade secrets or proprietary information and inventions assignment agreements or undertakings that he has signed with the Company (including the confidentiality and inventions assignment provisions of the Employment Agreement), as well as a reaffirmation of Mr. Carlson’s obligation under the Employment Agreement not to solicit or recommend for employment any person employed by the Company or its affiliates for a period of 18 months following the Effective Date. Mr. Carlson has also agreed to cooperate with the Company for the indefinite future in connection with certain pending litigation and other business matters in exchange for an hourly fee.
On March 11, 2010, the Compensation Committee of the Company’s Board of Directors approved an amendment to Mr. Carlson’s Employment Agreement (as discussed above), that made his agreement consistent with the amended and restated agreements of all other executive officers entered into in June 2009. The amendment provided that, in the event of a change in control in which the consideration that is paid was solely cash, all options and other equity awards issued to Mr. Carlson after January 1, 2009 would have vested in full, and all options would have become exercisable immediately prior to such change in control. In the event of a change in control where the consideration was stock or a combination of stock and cash, Mr. Carlson’s options and other equity instruments granted after January 1, 2009 would have continued to vest and become exercisable in accordance with the provisions of the existing agreements governing those awards. The amendment did not in any way affect the acceleration provisions of any options granted to Mr. Carlson prior to January 1, 2009, which continued to be governed by their provisions.
On March 3, 2008, the Company amended Mr. Carlson’s Employment Agreement. The Employment Agreement: (i) increased Mr. Carlson’s base salary to $500 per year or such greater amount as the compensation committee may have from time to time determined; (ii) increased Mr. Carlson’s annual bonus to up to 75% of his base salary based upon achievement of certain Company and individual targets; (iii) provided for the grant of non-qualified options to purchase a total of an additional 225,000 shares of the Company’s common stock, vesting over five years, at an exercise price equal to 125% of the fair market value of the Company’s common stock on the date of the grant (the date of grant being that date in 2008 on which the Company’s senior executives receive option grants, the “Option Grant”); and (iv) extended the term of Mr. Carlson’s employment agreement until the occurrence of an Event of Termination (as defined in the Employment Agreement). On May 1, 2008, upon the grant of stock options to officers of the Company, Mr. Carlson received the Option Grant at an exercise price of $10.91. Effective December 31, 2008, the Employment Agreement was amended to ensure compliance with Section 409A of the Internal Revenue Code of 1986, as amended. The revision provided that any
F-22
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
severance that would have otherwise be payable to Mr. Carlson within the first six months following a termination event giving rise to a severance payment would accrue but not be paid to Mr. Carlson until the first day of the seventh month following termination. Such amounts would continue to be paid in a lump sum.
Preston S. Romm Employment Agreement
On June 18, 2008, the Company and Preston S. Romm, the Company’s Chief Financial Officer, Executive Vice President, Finance, Operations and Administration and Treasurer, entered into an employment agreement. Under the agreement, Mr. Romm is entitled to a base salary of $320 per year; subject to annual cost of living increases or such greater increase as may be approved by the Company’s Board of Directors, and an annual bonus of up to 60% of his base salary based upon achievement of certain Company and individual targets. For fiscal year 2008, Mr. Romm received a guaranteed bonus of $50. In addition, on July 1, 2008, Mr. Romm received an option to purchase 150,000 shares of the Company’s common stock at an exercise price of $10.00 per share which was equal to 125% of the fair market value of the Company’s common stock on the date of the grant.
Transition Agreement
On March 24, 2008, the Company entered into a letter agreement with Stephen A. Garcia, the Company’s then Chief Financial Officer, regarding the transition arrangements related to his resignation (the “Transition Agreement”), pursuant to which Mr. Garcia’s employment relationship with the Company ended on July 15, 2008. During this period, Mr. Garcia continued to receive his then current salary and benefits. Because Mr. Garcia satisfied all of the conditions of the Transition Agreement, the Company paid Mr. Garcia a transition success payment equal to one year of pay at his then current annual rate of compensation of $253. This payment was payable over a period of one year (which ended on July 15, 2009) in equal installments on the Company’s normal payroll schedule, less all normal payroll deductions. In addition, Mr. Garcia’s stock options that would have vested between July 15, 2008 and December 31, 2009, vested in full and were immediately exercisable as of July 15, 2008 for a period of 90 days, 12,500 of which were exercised on September 29, 2008 at an exercise price of $8.40 per share. Pursuant to the Transition Agreement, Mr. Garcia released and discharged any claims that he may have had as of July 15, 2008 against the Company and its current and former owners, stockholders, employees, agents, attorneys, representatives, related companies, assignees as well as other related parties. The Transition Agreement also requires Mr. Garcia to maintain the confidentiality of all of the Company’s confidential and proprietary information. Pursuant to the Transition Agreement, the Company accrued $142 as of December 31, 2008 for the transition success payment. In addition, the Company recognized $19 in non cash compensation expense for the year ended December 31, 2008, related to the accelerated vesting of his outstanding stock options.
Letter of Credit Agreements
As part of the licensing requirements for four states, as of December 31, 2010, the Company issued four letters of credit to four state regulatory bodies totaling $400 to secure payment of any possible future unpaid fines or penalties levied by those states. These letters of credit are renewed annually for one-year periods unless cancelled and are secured by the Credit and Term Loan Agreement. As of December 31, 2010, the Company has not incurred any fines or penalties and none of the letters of credit have been presented for payment.
Prior to entering into the Credit and Term Loan Agreement, the letters of credit mentioned above were secured by the Former Credit Facility. During the years ended December 31, 2009 and 2008, the Company did not incur any fines or penalties, nor were any of the letters of credit presented for payment.
Litigation
On January 7, 2010, ZO Skin Health, Inc., a California corporation, filed a complaint in the Superior Court of the State of California, County of Los Angeles: ZO Skin Health, Inc. vs. OMP, Inc. and Obagi Medical Products, Inc. and Does 1-25; Case No. BC429414. The complaint alleges claims against the Company for: (i) unfair competition; (ii) intentional interference with prospective economic advantage; (iii) negligent interference with prospective economic advantage; (iv) intentional interference with contract; and (v) promissory estoppel. More specifically, the plaintiff alleges that: the Company engaged in business practices, including the assertion of void and unlawful non-compete clauses in contracts with Zein Obagi, that violate California’s Unfair Competition Law and the California Business and Professional Code, Section 17200, et seq.; the Company intentionally and negligently interfered with plaintiff’s prospective economic advantage in relationship with Rohto and certain product distributors; the Company interfered with existing contracts between plaintiff and Rohto; and the Company allegedly did not provide certain promised assistance in connection with the alleged agreement between plaintiff and Rohto. The plaintiff seeks injunctive relief, compensatory damages “measuring in the tens of millions of dollars” and other damages in an unspecified amount, punitive damages and costs of suit, including attorneys’ fees. The Company has answered the complaint and denied the allegations in that pleading. In addition, the Company has asserted counterclaims against the plaintiff. In May 2010, the claimants in the arbitration proceeding filed an amended demand, again before JAMS in Los Angeles. The amended demand reiterates the claims asserted in the original demand. The amended demand expands the original demand's factual allegations involving the Company's alleged interference with the claimants' rights to pursue business opportunities and with the claimants' trademark rights. Discovery in the action is active and ongoing. The Company is defending against this action vigorously.
F-23
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
In addition, on or about January 7, 2010, Zein Obagi and his spouse, Samar Obagi, and the Zein and Samar Obagi Family Trust and certain other entities allegedly affiliated with Zein Obagi sent the Company an arbitration demand before JAMS (formerly Judicial Arbitration and Mediation Services) in Los Angeles, California in which the claimants claim breaches of the 2006 Services Agreement and 2006 Separation and Release Agreement between the Company and the claimants and various breaches of common law, California law, and federal law, including failure to pay certain retainer, marketing and reimbursement funds, failure to pay royalties allegedly due, failure to consult before developing and marketing new products, failure to pursue certain business strategies, threatening to invoke unenforceable and inapposite non-competition clauses in the Company’s contracts with Zein Obagi, threatening and interfering with the claimants’ business opportunities, threatening to enforce nonexistent trademark and service mark rights, and wrongfully depriving the claimants of both their rights to pursue business opportunities and also their trademark rights. Claimants seek actual and consequential damages of tens of “millions of dollars” and other damages in an unspecified amount, restitution of monies wrongfully obtained in an unspecified amount, prejudgment interest, attorneys’ fees and costs, declaratory judgment and assignment of certain trademarks from the Company to Dr. Obagi. The Company has filed an answer to the Demand for Arbitration denying the allegations and asserting various counterclaims. Discovery in this matter is active and ongoing. The Company is defending against these claims vigorously.
The complaint and arbitration demand involve complex allegations, all of which are in dispute. Based on the current stage of these proceedings, the Company believes it has good faith defenses to the allegations raised. As a result, it is not possible to accurately predict the ultimate resolution of these matters. Moreover, given the current stage of the proceedings, losses, if any, associated with these matters are not reasonably estimable at the present time. If the ultimate outcome is unfavorable, these matters may have a material adverse effect on the Company’s consolidated financial position, results of operations and cash flows. Regardless of the outcome, the costs of litigation will be substantial.
From time to time, the Company is involved in other litigation and legal matters or disputes in the normal course of business. Management does not believe that the outcome of any of these other matters at this time will have a material adverse effect on the Company’s business, consolidated financial position, results of operations or cash flows.
Note 11: Stock Options
In November 2000, the Company adopted the 2000 Stock Option/Stock Issuance Plan (the “2000 Plan”). The terms of the 2000 Plan provided for the grant of options to purchase common stock to employees, consultants, and directors. The 2000 Plan included incentive stock options (“ISOs”) and nonqualified stock options (“NSOs”). The maximum number of shares of common stock that were allowed to be issued over the term of the 2000 Plan was not to exceed 375,000 shares of the Company’s common stock. In addition, the maximum number of shares of common stock issued to any person under the 2000 Plan in any calendar year was not to exceed 150,000 shares. In September 2001, the Board of Directors amended the 2000 Plan to increase the maximum number of shares of common stock that may be issued over the term of the 2000 Plan to 2,083,334 shares. The vesting period of options granted under this plan ranged from one to five years. Options expired within a period of not more than ten years from the grant date. In November 2005, the Board of Directors amended the 2000 Plan to discontinue further option grants under the plan. At December 31, 2010 and 2009, due to the aforementioned discontinuance of further option grants under the 2000 Plan, 273,815 and 273,065 shares are no longer available for the granting of additional options, respectively.
In November 2005, the Company adopted the 2005 Stock Incentive Plan (the “2005 Plan”). The 2005 Plan provides for the grant of ISOs and NSOs to employees, consultants and directors. On November 27, 2006, the Company’s Board of Directors amended the Company’s 2005 Plan. The amendments to the 2005 Plan included: (i) increasing the number of authorized shares to 1,500,000; (ii) establishing an evergreen clause that replenishes the 2005 Plan each year with a share amount equal to the lesser of 500,000 shares, 3% of outstanding shares as of the preceding December 31, or such number of shares as is determined by the Board of Directors; (iii) and other administrative provisions. The amended 2005 Plan was approved by the stockholders of the Company on November 28, 2006. On January 9, 2007, February 27, 2008, and April 15, 2009, the Board of Directors replenished the 2005 Plan with 500,000 shares on each date, available for granting of additional options. In April 2010, the Company’s Board of Directors further amended the 2005 Plan to: (i) eliminate the automatic share increase provision of the plan; (ii) provide that the authorized share reserve will be reduced by one share of the Company’s common stock for every one share subject to an option or stock appreciation right granted under the plan and one and one-half shares of the Company’s common stock for every one share subject to an award other than an option or stock appreciation right; (iii) extend the Company’s ability to grant certain performance-based awards under the plan; and (iv) effect various technical revisions. The amendment to the 2005 Plan, as previously amended, was approved by the stockholders of the Company at its 2010 Annual Meeting of Stockholders held on June 8, 2010.
Pursuant to the 2005 Plan, the exercise price per share for both ISOs and NSOs shall not be less than 100% of the fair market value per share of the Company’s common stock on the grant date. The exercise price per share for both ISOs and NSOs may not be less than 110% of the fair market value of the Company’s common stock on the grant date for an individual who, at the time of grant, owns stock representing more than 10% of the total combined voting power of all classes of the Company’s stock. The right to exercise the ISOs and the NSOs vests at a rate in accordance with the individual stock option agreements. The vesting period of the options granted under this plan typically ranges from three to five years. Options expire within a period of not more than ten years from the
F-24
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
grant date. ISOs granted to an employee, who at the time of grant owns stock representing more than 10% of the total combined voting power of all classes of stock of the Company, expire within a period of not more than five years from the grant date.
A total of 4,809,519 shares have been authorized for issuance under the 2000 Plan and the 2005 Plan (the “Plans”). Of the shares that have been authorized for issuance, 1,552,968 and 1,433,306 shares have been issued for options which have been exercised as of December 31, 2010 and 2009, respectively, and 1,207,067 and 1,769,396 shares have been reserved for options that are outstanding as of December 31, 2010 and 2009, respectively. At December 31, 2010 and 2009, there were 1,647,254 and 1,511,783 shares available for granting of additional options, respectively.
During the year ended December 31, 2010, the Company’s Board of Directors granted 359,000 options to employees of the Company, including officers, with exercise prices ranging from $11.06 to $13.54 per share, which was equal to or greater than the fair value of the underlying common stock on the date of each grant. During the year ended December 31, 2009, the Company’s Board of Directors granted 367,700 options to employees of the Company, including officers, with exercise prices ranging from $4.96 to $11.38 per share, which was equal to or greater than the fair value of the underlying common stock on the date of each grant. During the year ended December 31, 2008, the Company’s Board of Directors granted to employees of the Company, including officers, 651,200 options with exercise prices ranging from $5.94 to $16.25 per share, which was equal to or greater than the fair value of the underlying common stock on the date of each grant.
The fair value of each option granted to employees and directors is estimated using the Black-Scholes option-pricing model with the following weighted-average assumptions for the years ended December 31, 2010, 2009 and 2008:
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Expected stock price volatility
|
42.2 | % | 37.4 | % | 33.2 | % | ||||||
|
Expected dividend yield
|
0.0 | % | 0.0 | % | 0.0 | % | ||||||
|
Risk-free interest rate
|
2.6 | % | 2.0 | % | 3.1 | % | ||||||
|
Expected life of options
|
6.0 years
|
5.1 years
|
5.1 years
|
|||||||||
Expected stock price volatility is based on a combined average expected stock price volatility of publicly traded peer companies deemed to be similar entities whose share or option prices are publicly available. For fiscal years 2010, 2009 and 2008 the Company based its expected stock price volatility on the combined average stock price volatility of five publicly traded peer companies. Until such time that the Company has enough historical data, it will continue to rely on peer companies’ volatility and will ensure that the selected peer companies are still appropriate. The risk-free interest rate is based on the United States Treasury yield curve in effect at the time of grant with an equivalent remaining term. The Company does not currently plan to pay dividends in the near future. The Company has elected the simplified method for determining the expected life of options granted subsequent to the date of the shareholder transaction.
A summary of the option activity under the Plans is as follows:
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Weighted Average
|
Weighted Average
|
Weighted Average
|
||||||||||||||||||||||
|
Exercise Price
|
Exercise Price
|
Exercise Price
|
||||||||||||||||||||||
|
Shares
|
Per Share
|
Shares
|
Per Share
|
Shares
|
Per Share
|
|||||||||||||||||||
|
Outstanding, beginning of year
|
1,769,396 | $ | 10.09 | 1,592,474 | $ | 11.22 | 1,215,633 | $ | 11.21 | |||||||||||||||
|
Granted
|
359,000 | $ | 11.59 | 367,700 | $ | 5.94 | 651,200 | $ | 11.83 | |||||||||||||||
|
Exercised
|
(119,662 | ) | $ | 8.94 | (150 | ) | $ | 1.29 | (18,890 | ) | $ | 9.22 | ||||||||||||
|
Cancelled
|
(801,667 | ) | $ | 10.12 | (190,628 | ) | $ | 11.54 | (255,469 | ) | $ | 12.90 | ||||||||||||
|
Outstanding, end of year
|
1,207,067 | $ | 10.63 | 1,769,396 | $ | 10.09 | 1,592,474 | $ | 11.22 | |||||||||||||||
|
Exercisable, end of year
|
628,224 | $ | 11.17 | 966,344 | $ | 10.77 | 744,064 | $ | 10.42 | |||||||||||||||
|
Weighted average per share fair value
|
||||||||||||||||||||||||
|
of options granted during the year
|
$ | 5.12 | $ | 1.68 | $ | 3.24 | ||||||||||||||||||
The aggregate intrinsic value of stock options exercised during the years ended December 31, 2010, 2009 and 2008 was $467, $1 and $55, respectively. Upon exercise of stock options, the Company issues new shares.
A summary of options outstanding and exercisable as of December 31, 2010 is as follows:
F-25
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
| Options Outstanding | Options Exercisable | |||||||||||
|
Number of Shares
|
Weighted Average
|
Weighted Average |
|
Number of Shares
|
||||||||
| Outstanding at | Per Share |
Remaining Life
|
Exercisable at
|
Weighted Average
|
||||||||
|
Range of Exercise Price Per Share
|
December 31, 2010 | Exercise Price | (Years) | December 31, 2010 |
Exercise Price
|
|||||||
|
$4.69 to $7.00
|
233,166
|
$ |
5.81
|
8.19
|
68,172
|
$ |
$ 5.69
|
|||||
|
$7.58 to $10.00
|
218,592
|
$ |
9.70
|
7.28
|
149,727
|
$ |
$ 9.68
|
|||||
|
$10.80 to $13.00
|
584,309
|
$ |
11.22
|
7.56
|
295,810
|
$ |
$ 10.98
|
|||||
|
$13.54 to $20.34
|
171,000
|
$ |
16.37
|
7.29
|
114,515
|
$ |
$ 16.86
|
|||||
|
1,207,067
|
$ |
10.63
|
7.59
|
628,224
|
$ |
$ 11.17
|
||||||
The aggregate intrinsic value of options outstanding for the year ended December 31, 2010 was $1,935, of which $848 related to vested options as of December 31, 2010. This amount changes based on the estimated fair market value of the Company’s stock. Aggregate intrinsic value represents the total pretax intrinsic value (the difference between the Company’s estimated stock price on December 31, 2010 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all the option holders exercised their options on December 31, 2010. As of December 31, 2010, the total unrecognized stock-based compensation expense related to nonvested stock options was approximately $1,468, which is expected to be recognized over a weighted average period of approximately 1.93 years.
During the years ended December 31, 2010, 2009 and 2008, the Company issued a total of 14,778, 24,330 and 18,999 shares of restricted common stock, respectively, under the 2005 Plan to outside directors. The resulting compensation expense from the restricted stock grants is recognized on the straight-line basis over the requisite service period, which equals the restricted stock vesting term of one year. The fair market value of the restricted stock granted during the years ended December 31, 2010, 2009 and 2008 was $12.18, $7.40 and $8.68 per share, respectively, which was based upon the fair value of the Company’s common stock on the date of grant. The total fair value of restricted stock that vested during the years ended December 31, 2010, 2009 and 2008 was $180, $165 and $229, respectively.
During the year ended December 31, 2008, the Company’s Board of Directors, through its compensation committee, granted 45,000 restricted stock units under the 2005 Plan to officers of the Company. The fair market value of the restricted stock units granted was $16.25 per share, which was based upon the fair value of the Company’s common stock on the date of grant. The resulting compensation expense from the restricted stock unit grants was recognized on the straight-line basis over the requisite service period, which equaled the restricted stock unit vesting term of fifteen months. On May 31, 2009, 32,500 restricted stock units, with a total fair value of $528, vested.
Note 12: Segments
ASC 280, Segment Reporting, requires that the Company disclose certain information about its operating segments where operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. Generally, financial information is required to be reported on the basis that is used internally for evaluating segment performance and deciding how to allocate resources to segments.
As noted earlier, during the year ended December 31, 2008, the Company launched SoluCLENZ, which was the only product that the Company dispensed through the pharmacy channel. On April 13, 2009, the Company announced it would no longer sell SoluCLENZ through the pharmacy channel (see Note 2). As a result, the Company now operates its business on the basis of two reportable segments: (i) physician-dispensed; and (ii) licensing. The physician-dispensed segment produces a broad range of topical skin health systems and products that enable physicians to sell products to their patients to treat a range of skin conditions, including premature aging, photodamage, hyperpigmentation, acne, sun damage, facial redness and soft tissue deficits, such as fine lines and wrinkles. The licensing segment includes revenues generated from licensing arrangements with international distributors that specialize in the distribution and marketing of OTC medical oriented products in the drug store, retail and aesthetic spa channels. Sales and gross profit previously reported in the pharmacy Rx operating segment are now classified as physician-dispensed as part of the Therapeutic product category. Prior periods have been reclassified to conform to the current presentation.
Management evaluates its segments on a revenue and gross profit basis, which is presented below. The United States information is presented separately as the Company’s headquarters reside in the United States. United States sales represented 84% and 82% of total consolidated net sales for the year ended December 31, 2010 and 2009, respectively. No other country or single customer generates over 5% of total Company consolidated net sales.
F-26
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
All of the Company’s long-lived assets are located in the United States. The Company does not disaggregate assets on a segment basis for internal management reporting and, therefore, such information is not presented.
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net sales by segment
|
||||||||||||
|
Physician-dispensed
|
$ | 108,362 | $ | 99,466 | $ | 99,776 | ||||||
|
Licensing
|
4,401 | 4,630 | 4,817 | |||||||||
|
Net sales
|
$ | 112,763 | $ | 104,096 | $ | 104,593 | ||||||
|
Gross profit by segment
|
||||||||||||
|
Physician-dispensed
|
$ | 84,813 | $ | 77,575 | $ | 79,979 | ||||||
|
Licensing
|
4,264 | 4,482 | 4,683 | |||||||||
|
Gross profit
|
$ | 89,077 | $ | 82,057 | $ | 84,662 | ||||||
|
Geographic information
|
||||||||||||
|
United States
|
$ | 94,507 | $ | 85,432 | $ | 87,516 | ||||||
|
International
|
18,256 | 18,664 | 17,077 | |||||||||
|
Net sales
|
$ | 112,763 | $ | 104,096 | $ | 104,593 | ||||||
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Net sales by product category | ||||||||||||
|
Physician-dispensed
|
||||||||||||
|
Nu-Derm
|
$ | 59,166 | $ | 56,057 | $ | 58,378 | ||||||
|
Vitamin C
|
16,413 | 12,428 | 12,380 | |||||||||
|
Elasticity
|
11,551 | 9,491 | 11,643 | |||||||||
|
Therapeutic
|
7,261 | 8,668 | 6,260 | |||||||||
|
Other
|
13,971 | 12,822 | 11,115 | |||||||||
|
Total
|
108,362 | 99,466 | 99,776 | |||||||||
|
Licensing
|
4,401 | 4,630 | 4,817 | |||||||||
|
Total net sales
|
$ | 112,763 | $ | 104,096 | $ | 104,593 | ||||||
Note 13: 401(k) plan
On February 1, 1999, the Company established an employee savings and retirement plan and a 401(k) defined contribution retirement plan, covering substantially all full-time employees. Beginning April 2006, the Company matched 100% of employee contributions up to 2% of employee compensation. Effective January 1, 2008, the Company modified the 401(k) defined contribution retirement plan to match 100% of employee contributions up to 3% of employee compensation and 50% of employee contributions of the next 2% of employee compensation to a maximum of 4% of employee compensation. The Company may also make, at the discretion of the Board of Directors, additional contributions subject to statutory limits.
The Company contributed approximately $626, $586 and $548 for the years ended December 31, 2010, 2009 and 2008, respectively. Administrative expenses paid on behalf of the plan were nominal for each of the respective years.
Quarterly financial data for the years ended December 31, 2010 and 2009 are as follows:
F-27
Obagi Medical Products, Inc.
Notes to the Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
|
Quarter Ended
|
||||||||||||||||
|
March 31, 2010
|
June 30, 2010
|
September 30, 2010
|
December 31, 2010
|
|||||||||||||
|
Net sales
|
$ | 25,706 | $ | 28,863 | $ | 27,891 | $ | 30,303 | ||||||||
|
Gross profit
|
$ | 20,370 | $ | 22,658 | $ | 22,118 | $ | 23,931 | ||||||||
|
Net income
|
$ | 1,919 | $ | 3,087 | $ | 1,585 | $ | 2,900 | ||||||||
|
Net income attributable to common shares
|
||||||||||||||||
|
Basic
|
$ | 0.09 | $ | 0.14 | $ | 0.07 | $ | 0.14 | ||||||||
|
Diluted
|
$ | 0.09 | $ | 0.14 | $ | 0.07 | $ | 0.14 | ||||||||
|
Quarter Ended
|
||||||||||||||||
|
March 31, 2009
|
June 30, 2009
|
September 30, 2009
|
December 31, 2009
|
|||||||||||||
|
Net sales
|
$ | 22,620 | $ | 25,874 | $ | 24,899 | $ | 30,703 | ||||||||
|
Gross profit
|
$ | 17,562 | $ | 20,630 | $ | 19,649 | $ | 24,216 | ||||||||
|
Net income
|
$ | 645 | $ | 2,838 | $ | 3,045 | $ | 4,805 | ||||||||
|
Net income attributable to common shares
|
||||||||||||||||
|
Basic
|
$ | 0.03 | $ | 0.13 | $ | 0.14 | $ | 0.22 | ||||||||
|
Diluted
|
$ | 0.03 | $ | 0.13 | $ | 0.14 | $ | 0.22 | ||||||||
During the three months ended June 30, 2009, the Company recorded an out-of-period adjustment to correct an error in its vacation accrual for the three months ended March 31, 2009 that decreased income before income taxes by approximately $62 and net income by approximately $38. There was no impact of the adjustment on earnings per share. Since the error was not material to the first quarter ended March 31, 2009, the Company recorded the correction of the error during the second quarter ended June 30, 2009. The correction of the error is not material to the financial statements as of and for the year ended December 31, 2009.
On March 8, 2011, the Compensation Committee of the Company's Board of Directors approved the Obagi 2011 Performance Incentive Plan (the “2011 Plan”), which is an incentive compensation program for the year ending December 31, 2011 under the 2005 Stock Incentive Plan, as amended. It amends and restates and is the successor plan to the Obagi 2010 Performance Incentive Plan (the “2010 Plan”). The 2011 Plan is designed to motivate, retain and reward the Company's employees, including its executives, based on the achievement of specified corporate revenue and earnings before interest and taxes (“EBIT”) objectives, as well as individual objectives in certain cases. The Compensation Committee established target revenue and EBIT, as adjusted to exclude the impact of non-cash charges relating to the issuance of equity securities as reported in our consolidated financial statements (“adjusted EBIT”). Assuming 100% achievement of the target revenue and adjusted EBIT objectives, the aggregate 2011 Plan pool would be funded in the amount of $2,360. Under the 2011 Plan, if the Company acheives between the minimum required targets for each objective and 100% target for each objective, then the potential bonus amount for each executive officer and non-executive employee will be reduced by 5.0% and 1.0%, respectively, for each 1% that the Company is below the target objective, and the bonus pool will be reduced accordingly. If the revenue and adjusted EBIT target objectives are exceeded, an increased amount will have to be funded to the 2011 Plan pool, up to 150% of the target 2011 Plan pool. Thirty percent of the 2011 Plan pool will relate to the revenue objective and 70% of the pool will relate to the adjusted EBIT objective. For the 2011 Plan pool to be funded for executives and employees to earn any bonuses, the Company must achieve at least 80% of the revenue objective and at least 80% of the adjusted EBIT objective. Eligible participants under the 2011 Plan are full-time employees, including executives, who do not participate in sales or other variable incentive pay plans and are employed by the Company on December 31, 2011.
