Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d8k.htm |
 February 2011
February 2011
A specialty pharmaceutical company focused on the development
and commercialization of proprietary products to address important
therapeutic needs in the field of neuroscience
Exhibit 99.1 |
 Forward
looking statements Forward looking statements
This presentation contains forward-looking statements that involve substantial risks and
uncertainties. All statements, other than statements of historical facts, included in
this presentation are forward-looking statements. Examples of such statements
include our expectation regarding the FDA having a favorable view
of
the
Intermezzo
®
residual
effect
profile;
the
sufficiency
of
the
Intermezzo
®
NDA
to
gain
FDA
approval;
that
Intermezzo
®
will
become
the
first
prescription
sleep
aid
indicated
for
use
in
the
middle
of
the
night
and
the
first
strongly
differentiated
insomnia
treatment
strategy
since
Ambien
®
launch;
the
potential
market
size
for
a
middle
of
the
night
sleep
aid;
plans
to
commercialize
Intermezzo
®
under
favorable
market
conditions
through
the
Purdue
collaboration
and
the
receipt
by
Transcept
of
payments
from
Purdue
thereunder;
reimbursement
coverage
for
Transcept
product
candidates;
intellectual
property
protection
being
obtained
and
maintained
for
Transcept
product
candidates;
and
plans
to
begin
a
Phase 2
study
of
TO-2061
in
Q1
2011
and
report
results
therefrom
in
H1
2012.
Transcept
may
not
actually meet
these expectations and carryout these plans. Various important factors that could cause
actual events to differ materially from such forward-looking statements include
FDA deemed insufficiencies in our
Intermezzo
®
NDA
resubmission,
competitive
product
commercialization;
adverse
patent
decisions
at
the
USPTO
or
in
court;
the
willingness
of
Purdue
to
commercialize
Intermezzo
®
and
its
ability
to
do
so
successfully;
payer
opinion
of
Intermezzo
®
,
if
approved;
dependence
on
third
parties
to
manufacture,
and
carry-out
the
planned
study
of
TO-2061,
and
variability
in
the
business
of
Transcept
generally.
These
and
other
risks
are
described
in
greater
detail
in
the
"Risk
Factors"
section
of
Transcept
periodic
reports filed with the Securities and Exchange Commission. Forward-looking statements do
not reflect the potential impact of any future in-licensing, collaborations,
acquisitions, mergers, dispositions, joint ventures,
or
investments
Transcept
may
enter
into
or
make.
Transcept
does
not
assume
any
obligation
to
update any forward-looking statements, except as may be required by law.
2 |
 U.S.
primary care partnership: Purdue Pharma Co-promote
option
1
yr
post
Intermezzo
®
launch
9/30/10: $74M cash, equivalents and investments
No debt
Neuroscience / psychiatry
Intermezzo
®
:
middle
of
the
night
awakenings
Transcept: preparing for Intermezzo
®
commercialization
Transcept: preparing for Intermezzo
®
commercialization
3
Intermezzo
®
PDUFA
date
July
14,
2011
TO-2061
Phase
2
results:
anticipated
H1
2012
Large markets,
unmet needs
Therapeutic focus
Commercial
platform
Strong balance
sheet
Near term
catalysts
: treatment resistant OCD
TO-2061 |
 Intermezzo
®
(zolpidem
tartrate
sublingual tablet)
Intermezzo
®
(zolpidem
tartrate
sublingual tablet)
Proposed
indication
statement:
Intermezzo
®
is
indicated
for use as needed for the treatment of insomnia when a
middle of the night awakening is followed by
difficulty returning to sleep |
 Middle of the
night (MOTN) awakening: A major unmet medical need in the insomnia category
Middle of the night (MOTN) awakening:
A major unmet medical need in the insomnia category
Large U.S. insomnia market
–
$2.1
billion
(ex-factory)
(1)
–
78
million
new
and
refill
prescriptions
(2)
Insomnia is an under-treated condition
–
11
million
patients
receive
Rx
(3)
–
4x
to
6x
more
are
not
diagnosed
or
treated
by
a
physician
(3)(4)
MOTN awakening: the most prevalent insomnia symptom
(5)
–
35%
of
Americans
suffer
from
MOTN
awakenings
at
least
3x
/
week
(5)
–
>90% report awakenings persist more than six months;
50%
report
awakenings
persist
more
than
five
years
(6)
5
(1) IMS
NSP
2009;
(2)
Wolters
Kluwer,
PHAST
2010;
(3)
BluePrint
Research
Group;
(4)
Institutes
of
Medicine
-
Sleep
disorders
and
sleep
deprivation
Apr. 2006;
(5)
Ohayon,
Nocturnal
awakenings
and
comorbid
disorders
in
the
American
general
population.
J
of
Psych
Research
(2009);
(6)
Ohayon,
Difficulty
in
resuming
or
inability
to
resume
sleep
and
the
links
to
daytime
impairment,
J
of
Psych
Research
(2009). |
 No product
currently indicated for prn treatment at the
time of a middle of the night awakening
No product currently indicated for prn
treatment at the
time of a middle of the night awakening
MOTN awakenings typically do not occur every night
7-8 hr sleep aids (Ambien
®
, Ambien
CR
®
, Lunesta
®
) require
bedtime prophylactic dosing to prevent awakenings
An ideal therapeutic would:
–
Be used only at the time patients need help returning to sleep,
not every night in advance of a problem that may not occur
–
Return patients to sleep rapidly
–
Use a low dose to avoid next day residual effects
6 |
 Sleep lab,
objective data: 14 minute sleep onset with 3.5 mg dose
No residual next-morning effects vs. placebo in
sleep lab and outpatient studies
Driving study primary statistical analysis: no
significant next morning effect vs. placebo 4 hrs
after dosing, consistent with proposed label
Sublingual tablet dissolves in ~ 2 minutes
pH
drives
zolpidem
base,
rapidly
absorbed
72%
lower
dose
than
Ambien
CR
®
Intermezzo
®
: anticipated to be the first sleep aid for
use in the middle of the night at the time of awakening
Intermezzo
®
: anticipated to be the first sleep aid for
use in the middle of the night at the time of awakening
7
Favorable residual
effects profile
Fast acting
Low dose
Novel formulation |
 Intermezzo
®
3.5 mg delivered greater early zolpidem
bioavailability
than
a
~3x
higher
Ambien
®
dose
Intermezzo
®
3.5 mg delivered greater early zolpidem
bioavailability
than
a
~3x
higher
Ambien
®
dose
8
PK comparison study:
Intermezzo
®
3.5mg vs. Ambien
10mg PO n=33
Time (min)
9.3x
2.9x
1.4x
Intermezzo
®
3.5 mg
Ambien
®
10 mg
0
1
2
3
4
0
5
10
15
20 |
 Intermezzo
®
commercial
opportunity
Intermezzo
®
commercial
opportunity |
 Estimating
the MOTN market size at branded prices Estimating the MOTN market size at branded
prices Ohayon, et al., Nocturnal Awakenings in the American
General Population: Prevalence and Consequences
235 M U.S. adults
(3)
x Ohayon
MOTN sufferers at least 3x week
35.5%
(1)
=
83 million
…with difficulty returning to sleep
43.0%
(1)
=
36
…without difficulty initiating sleep
62.6%
(1)
=
22
…who seek MD treatment
16.0%
(2)
=
x mean length of therapy, currently marketed Rx sleep aids
108 to 169 days
(4)
x per tablet branded pricing, currently marketed Rx sleep aids
$5.05 to $5.67
(5)
= 100% market estimate: treatment seeking MOTN patients
$1.9 B to $3.4 B
(1)
Ohayon,
et
al.,
Nocturnal
awakenings
in
the
American
general
population:
Prevalence
and
consequences.
World
Sleep
Conference
2007;
(2)
Estimated
based
on
percentage
applied
to
adults
with
difficulty
returning
to
sleep,
with
and
without
difficulty
initiating
sleep
in
Ohayon,
et
al.,
Using
difficulty resuming sleep to define nocturnal awakenings. Sleep Medicine 11 (2010); (3) U.S.
Census data, 2010, adults 18 and over; (4) IMS: Length of
therapy
in
the
sleep
disorder
market,
March
2007.
Products
evaluated
are
indicated
for
bedtime
use:
Ambien
®
,
Ambien
CR
®
,
Lunesta
®
,
Restoril
®
,
Rozerem
®
,
Sonata
®
,
Desyrel
®
;
(5)
Wolters
Kluwer
WAC
pricing
(branded).
Jan
2011.
Products
evaluated:
Ambien
®
,
Ambien
CR
®
,
Lunesta
®
,
Silenor
®
.
Ambien
®
and
Ambien
CR
®
are
currently
available
in
generic
form.
10
3.6 |
 Leading
pain therapeutic franchise 2009 rev: >$2.5 billion
Branded products include:
OxyContin
®
, MSContin
®
, Dilaudid
®
, Butrans
®
Sales force of >500 field reps calling on primary care
physicians and high prescribing specialists
High value pain prescribers tend to be significant insomnia
prescribers
Multi-year sales and marketing agreement with psychiatry
co-promote option, royalties and milestone payments
Commercialization partnership with Purdue Pharma
Commercialization partnership with Purdue Pharma
11 |
 Commercialization agreement: key Transcept
benefits
Commercialization agreement: key Transcept
benefits
Co-promote option: foundation for a commercial future
–
Transcept
option: co-promote to psychiatrists as early as the first
anniversary after Purdue launch
Milestone payments
–
Upfront license fee: $25M received August 2009
–
FDA approval milestone of $30M, reduced by $2M each 30-day period
after June 30, 2010 ($6M if approved on July 14, 2011 PDUFA date)
–
Up to $90M additional upon the achievement of certain patent
milestones and net sales targets, including $10M for first formulation
patent listed in Orange Book
Royalty structure
–
Base royalty: double digit up to the mid 20% level on net sales
–
Co-promote royalty: additional double digit royalty on psychiatrist Rx
net sales
12 |
 Intermezzo
®
partnership structure enables
significant Transcept
marketing efficiency
Intermezzo
®
partnership structure enables
significant Transcept
marketing efficiency
Purdue responsibilities
–
U.S. product launch
–
Primary care sales and marketing activities
–
Managed care and formulary placement
–
Manufacturing and distribution
–
Post marketing studies, if required
–
Book revenues
Transcept
responsibilities
–
U.S. product approval
–
Psychiatry co promote option 1 yr after commercial launch
13 |
 Favorable
market
conditions
for
Intermezzo
®
launch
Favorable
market
conditions
for
Intermezzo
®
launch
Intermezzo
®
: anticipated to be the first strongly
differentiated insomnia treatment strategy since
launch of Ambien
®
in 1993
Continuing decline in sales presentations to
physicians in insomnia segment
Significantly reduced DTC spending
14 |
 Intermezzo
®
managed markets research: broad
formulary access expected
Intermezzo
®
managed markets research: broad
formulary access expected
Research conducted with Pharmacy and Medical Directors
from health plans covering 170M lives
Statement of unmet medical need generally well accepted by
payers
–
Unique insomnia indication
–
Low dose used less often
–
Delivery technology drives rapid bioavailability
Tier 3 formulary placement anticipated, utilization
management criteria consistent with other hypnotic brands
Intermezzo
®
brand parity pricing not viewed as a major
barrier with managed markets
Purdue managed markets team to drive formulary access
15 |
 Intermezzo
®
NDA resubmission
Intermezzo
®
NDA resubmission |
 FDA Complete
Response Letter (October 2009) and subsequent discussions
FDA Complete Response Letter (October 2009) and
subsequent discussions
FDA indicated Transcept
has submitted substantial evidence
of
effectiveness
for
Intermezzo
®
in
its
intended
indication
FDA
recognized
that
the
Intermezzo
®
Phase
3
data
did
not
indicate significant next morning residual effects at 4 hrs
However, given the unique MOTN indication, FDA requested:
–
additional data on next morning driving effects
–
demonstration that inadvertent dosing errors can be minimized,
or that the consequences of such errors are acceptable
•
inadvertent re-dosing in a single night
•
inadvertent dosing with < 4 hrs of bedtime remaining
–
arguments against the conduct of an in-use study
17 |
 Intermezzo
®
NDA: PDUFA date July 14, 2011
Intermezzo
®
NDA: PDUFA date July 14, 2011
18
Driving study primary statistical analysis: no significant next
morning effect when dosed per proposed label
Comparative analysis vs. previous driving study data: even when
dosed
off
label
(<
4hrs
prior
to
driving),
Intermezzo
®
next
day
effects are relatively small
New epidemiology study demonstrates widespread middle of the
night (off-label) use of 7-8 hour sleep aids
–
Results suggest ~11% of all hypnotic users sometimes take their sleep
aid in the middle of the night in order to return to sleep
Packaging changes, patient tools and instructions to address FDA
concerns re: dosing errors |
 Redesigned
unit dose packaging for NDA resubmission Redesigned unit dose packaging for NDA
resubmission 19
Prototype
package:
Intermezzo
®
is
not
approved
by
the
U.S.
FDA |
 Intermezzo
®
highway driving study overview
Intermezzo
®
highway driving study overview
Approximately 40 healthy adult volunteers
Highway driving over a one-hour period
Single-center (Maastricht),
double-blind,
randomized,
placebo-controlled
crossover design
Key comparisons:
–
Intermezzo
®
3.5mg vs. placebo, dosed 4 hours prior to driving
–
Intermezzo
®
3.5mg vs. placebo, dosed 3 hours prior to driving
–
Zopiclone 7.5mg vs. placebo (positive control)
Key measure of driving performance: standard
deviation of lateral position (variability in lane position)
as compared to placebo
20 |
 Intermezzo
®
highway driving study results
Intermezzo
®
highway driving study results
4 hrs after MOTN Intermezzo
®
dosing
–
Test condition consistent with proposed label
–
Primary analysis (symmetry): no statistically significant effect
–
Secondary analysis (mean): statistically significant but small
effect of 0.8 cm
SDLP
3 hrs after MOTN Intermezzo
®
dosing
–
Test condition included to characterize risk profile of
Intermezzo
®
when not used according to label
–
Primary analysis (symmetry): statistically significant effect
–
Secondary analysis (mean): statistically significant but small
effect of 1.5 cm
SDLP
–
One drive discontinued due to excessive drowsiness
FDA may consider other statistical analyses
21 |
 0
1
2
3
4
5
6
7
8
4h
post-dose
3h
post-dose
9h
post-dose
10-11h
post-dose
10-11h
post-dose
10-11h
post-dose
10-11h
post-dose
10-11h
post-dose
Effect on driving produced by various hypnotics and
blood alcohol concentrations: cross study comparison
Effect on driving produced by various hypnotics and
blood alcohol concentrations: cross study comparison
Intermezzo
®
highway driving study
Zopiclone
and US-marketed hypnotics
Brookhuis
K.
Hum.
Psychopharmacol.
Clin.
Exp.
1998;13:S64–S69.
0.05%
EU
0.08%
USA
DUI blood
alcohol
concentration
22
Note: The cross study comparisons reflected above should be viewed with caution. Cross study
meta-analyses are subject to interpretational and other risks due to a
number of factors, including unaccounted for variables that may render comparisons difficult or invalid, and potential inconsistencies in methods of
normalizing study results. Additional studies from the Brookhuis publication
reflecting data from drugs not approved in the United States are not reflected above.
The above collection of studies is not presented as a complete presentation of highway
driving studies analyzing hypnotic drugs approved in the United States. |
 Intermezzo
®
:
intellectual property
Intermezzo
®
:
intellectual property |
 Intermezzo
®
: two formulation patents issued; method
of use patents pending
Intermezzo
®
: two formulation patents issued; method
of use patents pending
Formulation
for
transmucosal
absorption
–
Two issued U.S. patents -
7,682,628 and 7,658,945
–
Patents expire no sooner than February 2025
–
Low dose zolpidem, ~1mg to ~5mg
–
Formulation
with
buffer
system
for
transmucosal
absorption
Method of treating MOTN awakenings
–
Priority date: May 2006
–
Low dose zolpidem, ~1mg to ~5mg
–
Treatment of middle of the night awakenings
Administration as needed after the subject awakens
–
Proposed claims cover multiple dosage forms
24 |
 TO-2061
(ultra low dose ondansetron)
TO-2061
(ultra low dose ondansetron)
25
Proposed indication: adjunctive therapy in adult patients
with obsessive compulsive disorder not adequately
responsive to SSRI treatment |
 Significant
unmet medical need: OCD patients not responding adequately to SSRI treatment
Significant unmet medical need: OCD patients not
responding adequately to SSRI treatment
Obsessive Compulsive Disorder
–
Intrusive thoughts and repetitive actions to reduce distress
–
Affects 1% to 2% of U.S. adult population, 40% to 50% seek treatment
–
Significantly impacts everyday life activities of patients and their families
–
40% to 60% of OCD patients do not respond adequately to first-line
SSRI
treatment
(e.g.,
Prozac
®
,
Luvox
®
,
Paxil
®
,
Zoloft
®
)
No FDA approved treatment for SSRI resistant OCD
–
Atypical antipsychotics are often used off-label to augment SSRIs
•
~68% of SSRI resistant OCD patients do not respond
•
Frequently reported adverse events: weight gain, metabolic disorder
26 |
 Pilot
studies: ultra low dose adjunctive ondansetron therapy in SSRI resistant OCD
Pilot studies: ultra low dose adjunctive ondansetron
therapy in SSRI resistant OCD
Ondansetron
(5-HT
3
antagonist approved as Zofran
®
)
–
Affects serotonin and dopamine pathways
–
Typical daily Zofran
®
doses of 16 mg to 24 mg for chemotherapy-
induced nausea and vomiting
Two 12 week open-label adjunctive therapy studies
with ondansetron
titrated to 0.5 mg BID dose
–
Pilot
Study
A
(1)
:
Adjunctive
ondansetron
therapy
in
patients
who
responded
poorly
to
at
least
12
weeks
of
SSRIs
combined
with
an
atypical antipsychotic, n=14
–
Pilot
Study
B
(2)
:
Adjunctive
ondansetron
therapy
in
patients
who
responded poorly to at least 12 weeks of SSRI treatment, n=21
27
1) S. Pallanti, S. Bernardi, S. Antonini, N. Singh, E. Hollander: Ondansetron augmentation
in Treatment-Resistant Obsessive-Compulsive Disorder, CNS Drugs (2009); (2)
S. Pallanti, S. Bernardi, E. Hollander: Ondansetron augmentation in Treatment-Resistant OCD (TR-OCD): Relapse in Y-BOCS symptoms
th
following
discontinuation
of
ondansetron,
Poster
presentation,
American
College
of
Neuropsychopharmacology
49
Annual
Conference,
December 2010. |
 Improvement:
measured as a % decrease over baseline on the Yale Brown Obsessive Compulsive Scale
(YBOCS) Improvement: measured as a % decrease over baseline on the
Yale Brown Obsessive Compulsive Scale (YBOCS)
28
All Patients: n=21
26.3% improvement at 12 weeks
Responders: n=12 of 21 (57%)
44.3% improvement at 12 weeks
Pilot Study B, n=21
Week
2
Week
4
Week
6
Week
8
Week
10
Week
12
50%
40%
30%
20%
10%
0%
All patients
Week
2
Week
4
Week
6
Week
8
Week
10
Week
12
50%
40%
30%
20%
10%
0%
Responders
Non-responders |
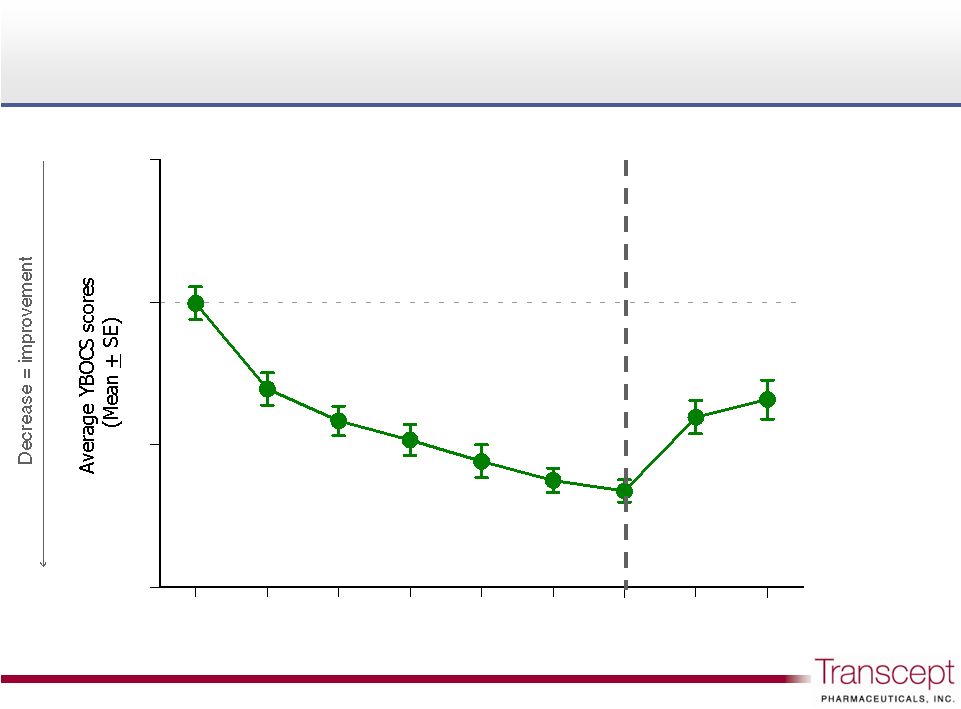 Week
0
Week
2
Week
4
Week
6
Week
8
Week
10
Week
12
Week
14
Week
16
10
20
30
40
Relapse following discontinuation in responders
Relapse following discontinuation in responders
29
38.3%
YBOCS
worsening
from Week 12
Phase 1
Ondansetron
augmentation
Phase 2
Discontinuation
Pilot Study B, n=12 of 21 (responders only) |
 TO-2061: development overview
TO-2061: development overview
505b2 NDA pathway
Phase 2 study, n
150
–
To start Q1 2011, top-line results 2012
–
Approx. $10M investment through end of Phase 2 study
Intellectual property
–
Method of use patent application filed, priority date May 19, 2009
–
Ondansetron, up to ~1.5 mg/day
–
Pending claims for treating SSRI resistant OCD with ondansetron
augmentation
Managed care survey (~108M lives): unmet medical need
acknowledged, Tier 3 formulary placement expected
Strategic fit: psychiatry
–
~87% of patient visits for OCD were to psychiatrists in 2009
*
–
Complementary to Intermezzo
®
psychiatry co-promote option with Purdue
30
* SDI Physician Drug and Diagnosis Audit |
 Financial
overview Financial overview |
 Financial
position: September 30, 2010 Financial position: September 30, 2010
Cash & investments:
$74.2 M
Q3 2010 cash burn rate:
$ 1.6 M / month
Shares outstanding:
13.4 M
Options / warrants / other:
2.5
Total:
15.9 M
Employees:
31
32 |
 Key
Transcept activities and goals
Key Transcept
activities and goals
Completed Intermezzo
®
US marketing partnership: July 2009
Patent coverage: two Intermezzo
®
formulation patents issued
Highway driving study data: Oct 2010
Intermezzo
®
NDA resubmitted and accepted: Jan 2011
Begin TO-2061 Phase 2 study Q1 2011 Intermezzo
®
NDA action date: July 14, 2011
Intermezzo
®
pre-launch planning
33 |
 Intermezzo
®
is a registered trademark of Transcept
Pharmaceuticals, Inc.
Ambien
®
and Ambien
CR
®
are registered trademarks of sanofi-aventis
Lunesta
®
is a registered trademark of Sunovion
Pharmaceuticals Inc.
Zofran
®
and
Paxil
®
are registered trademarks of The GlaxoSmithKline Group of Companies
Prozac
®
is a registered trademark of Eli Lilly & Co.
Luvox
®
is a registered trademark of Solvay Pharmaceuticals, Inc.
Zoloft
®
is a registered trademark of Pfizer Inc. |
