Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MEDICINOVA INC | d8k.htm |
 ©
MediciNova, Inc. 2011
Accelerating
the global development
and commercialization of
innovative pharmaceuticals
Exhibit 99.1 |
 ©
MediciNova, Inc. 2011
Statements
in
this
presentation
that
are
not
historical
in
nature
constitute
forward-looking
statements
within
the
meaning
of
the
safe
harbor
provisions
of
the
Private
Securities
Litigation
Reform
Act
of
1995.
These
forward-looking
statements
include
statements
regarding
MediciNova’s
clinical
trials
supporting
the
safety
and
efficacy
of
its
product
candidates
and
the
potential
novelty
of
such
product
candidates
as
treatments
for
disease,
plans
and
objectives
for
clinical
trials
and
product
development,
strategies,
future
performance,
expectations,
assumptions,
financial
condition,
liquidity
and
capital
resources.
These
forward-looking
statements
may
be
preceded
by,
followed
by
or
otherwise
include
the
words
"believes,"
"expects,"
"anticipates,"
"intends,"
"estimates,"
"projects,"
"can,"
"could,"
"may,"
“will,”
"would,"
or
similar
expressions.
Actual
results
or
events
may
differ
materially
from
those
expressed
or
implied
in
any
forward-looking
statements
due
to
various
factors,
including
the
risks
and
uncertainties
inherent
in
clinical
trials
and
product
development
and
commercialization,
such
as
the
uncertainty
in
results
of
clinical
trials
for
product
candidates,
the
uncertainty
of
whether
the
results
of
clinical
trials
will
be
predictive
of
results
in
later
stages
of
product
development,
the
risk
of
delays
or
failure
to
obtain
or
maintain
regulatory
approval,
the
risk
of
failure
of
the
third
parties
upon
whom
MediciNova
relies
to
conduct
its
clinical
trials
and
manufacture
its
product
candidates
to
perform
as
expected,
the
risk
of
increased
cost
and
delays
due
to
delays
in
the
commencement,
enrollment,
completion
or
analysis
of
clinical
trials
or
significant
issues
regarding
the
adequacy
of
clinical
trial
designs
or
the
execution
of
clinical
trials
and
the
timing,
cost
and
design
of
future
clinical
trials
and
research
activities;
the
timing
of
expected
filings
with
the
FDA;
MediciNova’s
failure
to
execute
strategic
plans
or
strategies
successfully;
MediciNova’s
collaborations
with
third
parties;
MediciNova’s
ability
to
realize
the
anticipated
strategic
and
financial
benefits
from
its
acquisition
of
Avigen,
Inc.,
to
integrate
the
two
ibudilast
development
programs
and
to
pursue
discussions
with
potential
partners
to
secure
a
strategic
collaboration
to
advance
the
clinical
development
of
the
combined
development
program;
the
availability
of
funds
to
complete
product
development
plans
and
MediciNova’s
ability
to
raise
sufficient
capital
when
needed,
or
at
all;
MediciNova’s
ability
to
comply
with
the
covenants
in
its
financing
agreements;
intellectual
property
or
contract
rights;
and
the
other
risks
and
uncertainties
described
in
MediciNova’s
filings
with
the
Securities
and
Exchange
Commission,
including
MediciNova’s
annual
report
on
Form
10-K
for
the
year
ended
December
31,
2009
and
its
subsequent
periodic
reports
on
Forms
10-Q
and
8-K.
You
are
cautioned
not
to
place
undue
reliance
on
these
forward-looking
statements,
which
speak
only
as
of
February
1,
2011.
MediciNova
disclaims
any
intent
or
obligation
to
revise
or
update
these
forward-looking
statements.
Forward-Looking Statements |
 ©
MediciNova, Inc. 2011
3
MediciNova
Overview:
•
Founded in September 2000
•
Headquartered in San Diego, CA
•
Additional office in Tokyo, Japan
•
Dual-listing on NasdaqGM
as MNOV
and Osaka Securities Exchange as 4875
•
$62.2 million Market Cap (NasdaqGM) as of 2/01/2011
Development Company Focused on Differentiated Product Candidates
•
Unique access to differentiated, potentially high-value assets primarily from
Japanese alliances (Kyorin, Kissei, Mitsubishi Tanabe Pharma, Meiji)
New Approaches to Treat Serious Medical Conditions:
•
MN-221: Intravenous (IV) acute asthma and COPD candidate
•
Potential
$1
billion+
combined
market
opportunity
worldwide*
•
Ibudilast: Neuropathic pain, progressive multiple sclerosis, drug addiction
candidate Corporate Overview:
MediciNova, Inc.
*Source: Internal MediciNova
projections |
 ©
MediciNova, Inc. 2011
4
In-License:
•
Novel, small-molecule product candidates with significant
clinical or preclinical data packages
and attractive market opportunities Conduct Proof-of-Concept Clinical
Trials: •
Conduct Phase I and Phase II clinical trials to demonstrate
safety and efficacy of compound
Two Pathways After Phase II:
1.
Continue internal development of compound towards
commercialization
2.
Seek partnership for further development of compound
Business
Model:
Return On Investment |
          ©
MediciNova, Inc. 2011
5
Leadership
Years
Experience
Background
Yuichi Iwaki, MD, PhD
Yuichi Iwaki, MD, PhD
CEO & President
35
Professor at USC, formerly Professor at
University of Pittsburgh; Advisor to JAFCO,
Tanabe
Michael Coffee
Michael Coffee
Chief Business
Officer;
Interim Chief Financial Officer
26
Avigen, Amarin
Corp., Elan
Pharmaceuticals,
N.A., Athena Neurosciences
Kirk Johnson, Ph.D.
Kirk Johnson, Ph.D.
Chief Scientific Officer
21
Avigen, Genesoft
Pharmaceuticals, Chiron
Corporation
Masatsune
Masatsune
Okajima, CMA
Okajima,
CMA , CMA
VP, Head of Japanese Office
19
Daiwa Securities SMBC, Sumitomo Capital
Securities, Sumitomo Bank
Management Team with
Global Experience |
 ©
MediciNova, Inc. 2011
6
Upcoming Near-Term Business Milestones:
1.
Secure a global partnership for Ibudilast
(MN-166/AV411)
2.
Secure a strategic partnership for MN-221
Upcoming Clinical Milestones:
1.
MN-221-CL-007 Phase II Study for Acute Exacerbations of Asthma
•
Anticipated completion in 2H, 2011*
Completed Milestones in 2010:
1.
Announced Positive MN-221-CL-010 Phase Ib
Study Results in
Moderate-to-Severe COPD Patients on March 17, 2010
3.
Secured $15M Debt Financing from Oxford Finance Corporation on May 10, 2010
4.
Announced Positive Safety and Efficacy data for Ibudilast
(MN-166/AV411) Phase Ib/2a Study
Results for Opioid
Withdrawal and Analgesia on December 13, 2010
Investment Highlights
*Anticipated completion dates based on current projections
|
 Ibudilast:
•
•
Neuropathic Pain
•
Multiple Sclerosis
•
Addiction |
 ©
MediciNova, Inc. 2011
8
Ibudilast
(MN-166/AV411)
•
Oral administration
•
Safe and well-tolerated (approved in Japan/Korea with over 3.2 million
patient exposures)
•
Mechanism(s) of Action primarily Inhibition of Macrophage
Migration Inhibitor Factor
(MIF), PDE-4,10 inhibition; Attenuation of Glial
Cell Activation
Clinical Safety & Preliminary Efficacy
•
Completed Phase 2 Multiple Sclerosis Proof-of-Concept study (30 and 60 mg/d,
predominately RRMS pts.)
•
Completed Phase 1b/2a trial in Diabetic Neuropathic Pain (40 and
80 mg/d)
•
Completed Phase 1b/2a clinical trial in Opioid
Withdrawal & Analgesia (40 and 80 mg/d)
(Columbia Univ/NYSPI via NIDA funding)
•
Ongoing Phase 1b Methamphetamine interaction trial (UCLA via NIDA funding)
•
Additional Supporting Data
•
3 completed Phase 1 clinical trials
•
Dosing up to 100 mg single dose & 100 mg daily (50 mg twice/day)
•
~400 subjects treated with MN-166/AV411 to date (safe &
well-tolerated) Ibudilast
for the Treatment of MS,
Neuropathic Pain, & Drug Addiction |
 ©
MediciNova, Inc. 2011
9
Status for Chronic Pain:
•
MN-166/AV411 is enabled to go directly to Phase 2b clinical development
•
MN-166/AV411 mechanism of action is novel and thus complimentary to
current pain treatments,
and has both stand-alone and adjunctive utilities
•
Majority of potential pharma
partners are strategically committed to new pain therapies
•
MN-166/AV411 has an attractive development timeline and long term
exclusivity Status for Drug Addiction/Opioid
Withdrawal:
•
Announced positive safety/efficacy results from Phase 1b/2a study in Opioid
Withdrawal (12/10)
•
UCLA initiated Phase Ib
study for Methamphetamine Addiction (9/10)
Status for Multiple Sclerosis:
•
MN-166/AV411 requires significant funding for future trials
•
Phase 2 data were at doses that are below maximum utility
•
Most attractive option may be Progressive MS which would require
an additional Phase 2b
clinical trial
Ibudilast
(MN-166/AV411):
Status for Each Indication |
 ©
MediciNova, Inc. 2011
10
Ibudilast
Neuropathic Pain
Market Opportunity
Drug
Company
Total Rxs
in 2009
(US)
Lyrica
®
Pfizer
9.1 Million
Cymbalta
®
Eli Lilly
14.7 Million
Neurontin
®
(Gabapentin)
Pfizer
23.4 Million
Total
47.1 Million
Neuropathic Pain Annual Market
Opportunity:
~$8.0 Billion
†
•
Prevalence is approximately 4.2
million neuropathic pain patients in
the U.S. and 40
million worldwide
•
MN-166 has a different
mechanism of action than currently
marketed neuropathic pain
therapies
•
MN-166 has potential to capture
substantial market share in the
neuropathic pain market
*Source: SDI/Verispan, Lilly and Pfizer Quarterly Reports
Approved
indications:
Lyrica:
Neuropathic
pain
associated
with
diabetic
peripheral
neuropathy,
post
herpetic
neuralgia,
partial
onset
seizures,
fibromyalgia;
Neurontin:
postherpetic
neuralgia,
partial
seizures
;
Cymbalta:
Major
Depressive
Disorder,
Generalized
Anxiety
Disorder,
Diabetic
Peripheral
Neuropathic
Pain,
Fibromyalgia
†
Market Value Calculated at Branded Prices |
 ©
MediciNova, Inc. 2011
11
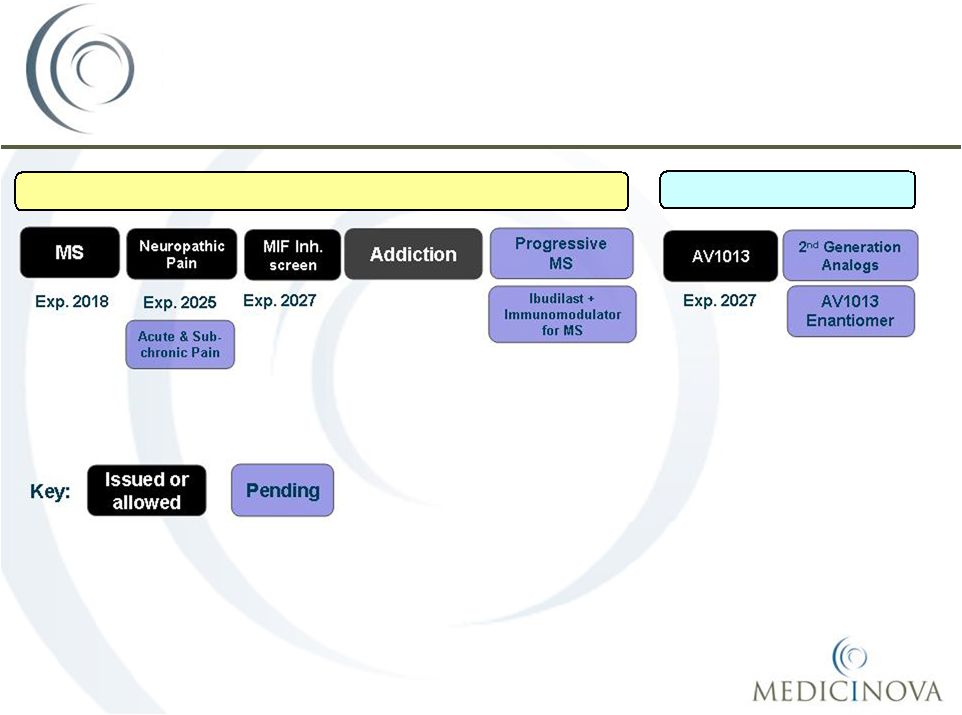
Patent/Commercial Overview
Method of Use
Composition of Matter |
 ©
MediciNova, Inc. 2011
•
Collaboration Structure with Pharma
Partner:
1.
Shared Risk
2.
All indications; Ibudilast
+ Analogues
3.
Option Agreement around Phase 2b Diabetic Peripheral Neuropathic
Pain
and/or
Progressive
MS
trial
with
Exclusive
License,
Development Milestones, Royalties, Sales Milestones.
•
Sustain NIDA-sponsored Drug Addiction development
•
Consider Investigator-sponsored Neurological Trials
12
Most Likely Scenario for
Ibudilast’s
Development |
 MN-221:
•
Acute Exacerbations of Asthma
•
Exacerbations of Chronic Obstructive
Pulmonary Disease (COPD) |
 ©
MediciNova, Inc. 2011
14
Acute Exacerbations of Asthma
Definition:
•
Long-lasting and severe asthma episode that is not responsive to initial
bronchodilator or corticosteroid therapy
Market Opportunity*:
•
Approximately 2 million annual emergency room visits in the US
•
~500,000 annual hospitalizations in the US
•
Average length of stay for asthma hospitalization is 3.3 days
•
Average cost for asthma hospitalization is $6,477
•
Approximately 2.7 million annual emergency room visits in
UK/Spain/Germany/France/Italy •
~560,000 annual hospitalizations in UK/Spain/Germany/France/Italy
Current Standard of Care (SOC):
•
Inhaled Beta agonists, inhaled anticholinergics, and IV or oral corticosteroids
*Source: National Center for Health Statistics / CDC, WHO website, “Core
Health indicators”, 2007 National Hospital Discharge Survey, IMS
Health’s Disease and Condition Benchmarks – PharMetrics
Integrated
Database, 1/2007 –
12/2008 |
 ©
MediciNova, Inc. 2011
15
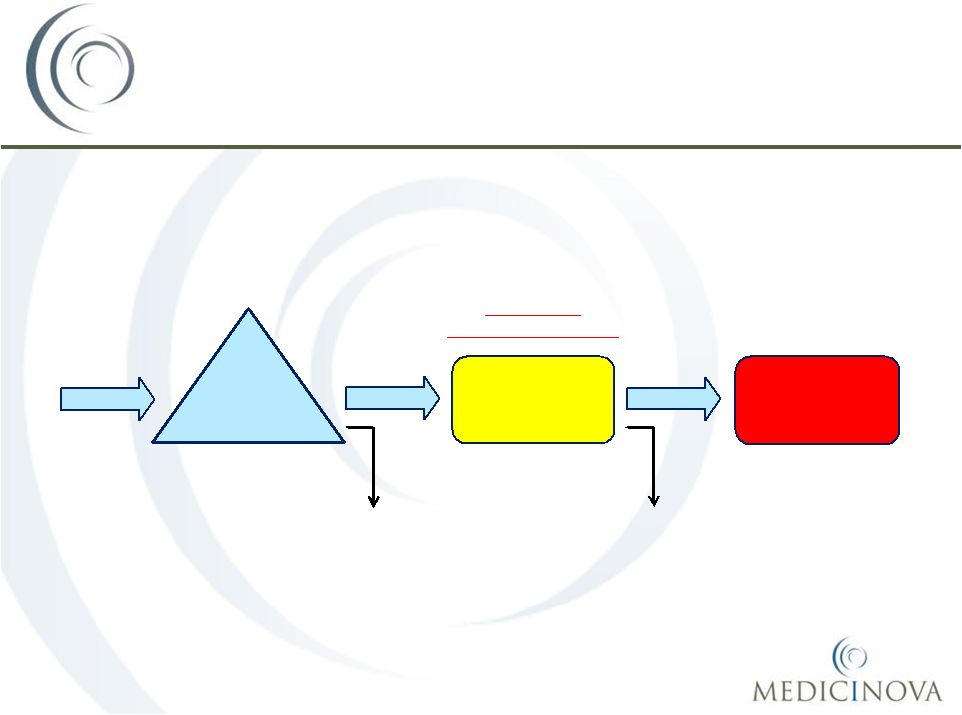
Acute Asthma Treatment Flow in
Emergency Departments (EDs) in the U.S.
965,000
935,000
935,000
410,000
525,000
525,000
Input:
1,900,000 patients
with acute
exacerbations of
asthma present at
U.S.
EDs
annually
1
st
line therapy in ED:
Patients receive SOC,
many while in the
waiting room
2
nd
line therapy in ED:
Patients who do not
initially respond
continuing receiving SOC
Large Market
Opportunity for MN-221
1,900,000
1,900,000
Hospitalization:
Patients who do not
respond to SOC are
eventually hospitalized
Patients who respond
to initial therapy and
are discharged
Patients who
eventually respond to
standard therapy and
are discharged
Source: Weber, Silverman et al,
American Journal of Medicine, 2002,
Volume 113; pp 371 |
 ©
MediciNova, Inc. 2011
16
Limitations of Current Therapies
What are the limitations of current therapies for acute exacerbations of
asthma?
Limitations of Inhaled Therapies:
•
Bronchoconstriction:
inflammation
and
bronchoconstriction
result
in
insufficient
air
flow to get good drug deposition in the lungs
•
Mucus Plug Formation:
mucus secretion and the formation of thick mucus plugs
can cause persistent airflow limitation
•
Albuterol
Non-Responders:
not
all
patients
benefit
from
albuterol
Limitations of Current Intravenous Therapies:
•
Safety:
currently
available
options
(e.g.
epinephrine,
terbutaline)
have
significant
cardiovascular
risks
at
doses
used |
 ©
MediciNova, Inc. 2011
17
MN-221: Target Product Profile
MN-221
Indication:
Treatment
of
bronchospasms
in
patients
with
acute
exacerbations
of
asthma
or
COPD.
It
is
administered
adjunctive
to
standard
of
care
by
intravenous
infusion.
•A
well-tolerated,
potent,
selective
ß
2
-agonist
which
is
only
a
partial
agonist
at
ß
1
.
•A
bronchodilating
duration
of
action
that
is
longer
than
Short-Acting
Beta
Agonists
(SABAs)
and
shorter
than
Long-Acting
Beta
Agonists
(LABAs).
•Provides
additional
bronchodilation
when
used
in
addition
to
the
standard
treatments
of
inhaled
albuterol,
inhaled
ipratropium,
and
steroids.
•Reduces
the
hospitalization
rate
among
patients
treated
with
MN-221.
•No
clinical
adverse
effects
when
added
to
standard
of
care. |
 ©
MediciNova, Inc. 2011
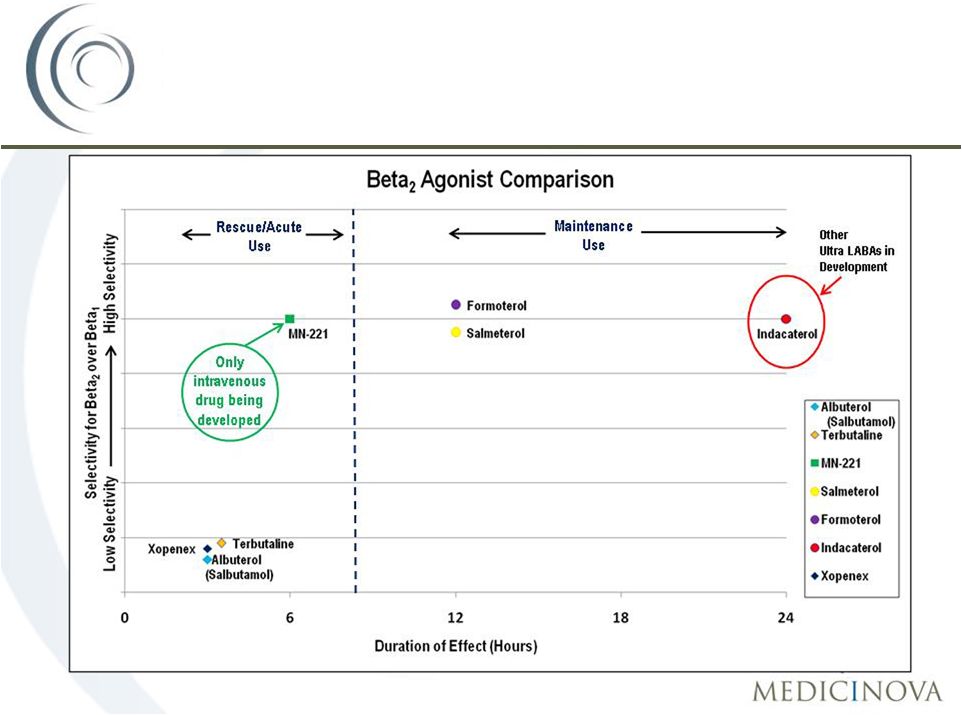
MN-221:
A
novel,
highly
selective
ß
2
-
adrenergic
receptor
agonist
Three potential advantages over current therapy:
1.
Improved Efficacy
•
Route of Administration (IV v. Inhalation)
2.
Improved Safety
•
Higher
selectivity
for
ß
2
receptor
than
ß
1
•
Partial
agonist
for
ß
1
receptor
3.
Reduced Health Care Expenses
18
MN-221: A New Approach to Treating
Acute Exacerbations of Asthma |
 ©
MediciNova, Inc. 2011
Beta
2
agonist U.S. Market Overview
19 |
 ©
MediciNova, Inc. 2011
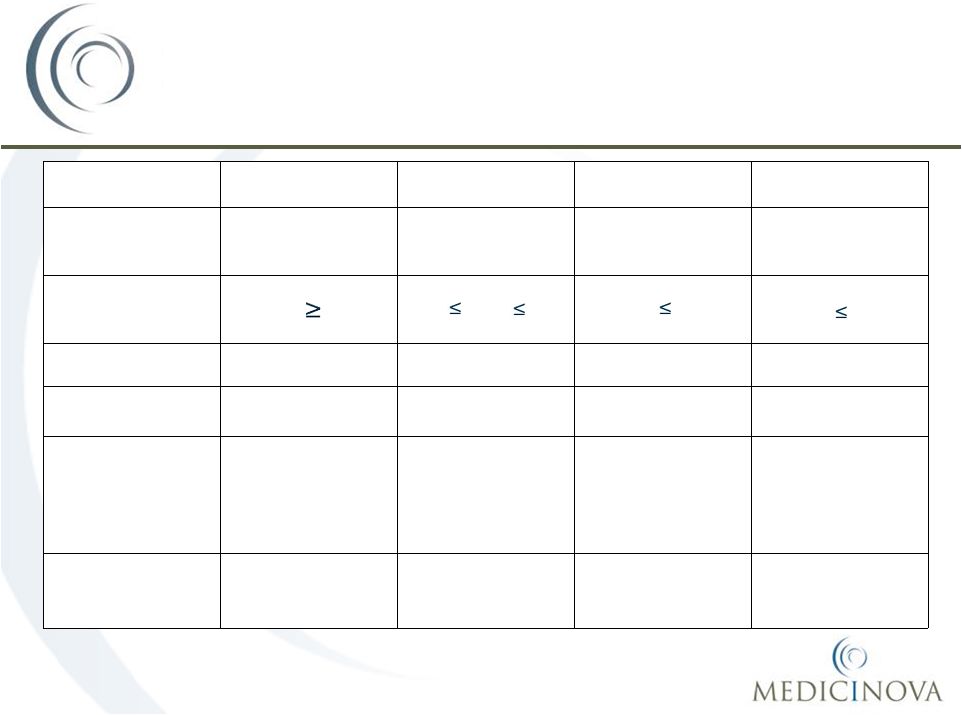
MN-221 Phase II Study Designs
in Asthma Indication
20
MN-221-CL-004
MN-221-CL-005
MN-221-CL-006
MN-221-CL-007
Type of
Asthma
Stable
mild-to-moderate
Stable
moderate-to-severe
Acute
Exacerbations
Acute
Exacerbations
FEV
1
(Entry Criteria)
FEV
1
60%
40%
FEV
1
75%
FEV
1
55%
FEV
1
50%
Number Patients
23
17
29
200 projected
Number Sites
4
4
8
20 projected
Doses compared
to Placebo
5.25, 15, 52.5, 150,
240, 450, 900 µg
over 15 min
1080 µg over 2-hr;
1,125 µg over 1-hr
240, 450 µg
over 15 min;
1080 µg over 2-hr
1200 µg over 1-hr
Concurrent
Therapy
None
None
Standard of care
Standard of care |
 ©
MediciNova, Inc. 2011
Completed Phase IIa
trial in the Emergency Dept.
•
Randomized, placebo-controlled, single-blind, dose escalation study
•
29 patients with acute exacerbations of asthma (FEV
1
55% predicted) at
8 Emergency Department sites
•
Doses:
•
16
µg/min for 15 minutes (240 µg)
•
30
µg/min for 15 minutes (450 µg)
•
16 µg/min for 15 minutes; 8 µg/min for 105 minutes (1,080 µg)
•
Patients received Standard of Care (SOC) treatment in addition to
adjunctive treatment with MN-221 or placebo
•
Outcome measures –
descriptive statistics only –
FEV
1
, PK, safety
MN-221-CL-006:
Study Design
21 |
 ©
MediciNova, Inc. 2011
MN-221-CL-006
Mean Change in FEV
1
and
Differences in Hospitalization Rate
22
Mean change in FEV
1
from baseline was 5.3%
higher in the MN-221 dose groups versus the
placebo group
MN-221 reduced the hospitalization rate by 45% |
 ©
MediciNova, Inc. 2011
23
What did we learn from MN-221-CL-006?
•
There were no safety concerns with adding MN-221 to
the standard of care.
•
There was a reduction in the hospitalization rate
among patients treated with MN-221.
•
Overall,
improvement
in
FEV
1
was
greater
for
patients
receiving MN-221 than placebo.
•
A dose of 1,200 µg
of MN-221 administered over one
hour was selected for the MN-221-CL-007 trial.
MN-221-CL-006:
What have we learned? |
 ©
MediciNova, Inc. 2011
•
Randomized, placebo-controlled, double-blind, multi-center Phase II
clinical trial •
Up
to
200
patients
with
severe,
acute
exacerbations
of
asthma
(FEV
1
50%
predicted)
at multiple Emergency Department sites in the United States
•
Dose Groups (up to 100 patients/group):
•
1,200
µg
of
MN-221
over
1
hour
(600
µg
in
15
minutes;
600
µg
in
next
45
minutes)
•
Placebo
•
Patients
will
receive
SOC
treatment
in
addition
to
adjunctive
treatment
with
MN-221
or
placebo
•
Primary
efficacy
endpoint
will
be
improvement
in
FEV
1
(%
predicted)
at
3
hours
•
The study is designed to have 80% power to detect a treatment difference of
5 percentage points in FEV1
(% predicted) when comparing MN-221 + SOC
to
Placebo
+
SOC
at
a
two
sided
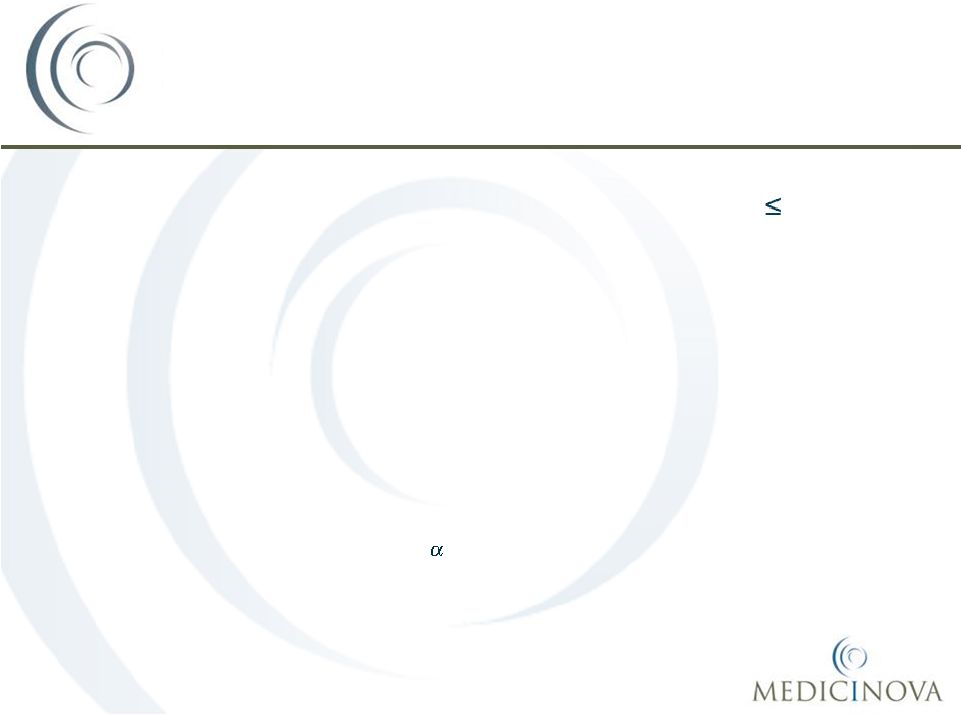
-level
of
0.05.
•
Anticipated completion in 2H, 2011*
MN-221-CL-007:
Study Design
24
Note: Development plans / timelines for MN-221 clinical trials are subject to
change *Anticipated completion date based on current projections
|
 ©
MediciNova, Inc. 2011
MN-221 Safety Summary
Safety Database:
•
MN-221 has been tested in almost 300 subjects in the US and Europe
to date
•
No serious adverse events related to MN-221 were reported in any
studies completed to date
•
No clinically significant cardiovascular, ECG, or vital sign changes, or
other safety concerns have been reported
•
Doses up to 3,840 micrograms have been tested at different infusion
rates
•
Subjects tested have included healthy volunteers, healthy pregnant
women, and asthmatics
25 |
 ©
MediciNova, Inc. 2011
MN-221 Patent Summary
26
•
MediciNova
has rights to a portfolio of patents and know-how related to
MN-221, including composition of matter.
•
The U.S. patent for MN-221 has composition of matter and method of use
claims and is set to expire no earlier than February
2017.
•
U.S. patent expiration does not include Waxman-Hatch patent term
restoration (industry average = 4.5 years).
•
Corresponding composition of matter patents in various other countries are
set to expire no earlier than February 2017.
•
Waxman-Hatch grants 5 years of exclusivity from approval in the U.S.
Exclusivity in Europe is 10 years for first approval of new chemical entities.
|
 MN-221
Potential Development Opportunity:
•Exacerbations of
COPD |
 ©
MediciNova, Inc. 2011
28
Chronic Obstructive Pulmonary Disease
•
Chronic obstructive pulmonary disease (COPD) is a progressive
disease that causes airflow blockage and breathing-related problems.
•
COPD includes two main conditions:
emphysema and chronic
obstructive bronchitis.
•
Cigarette smoking is the leading cause of COPD.
•
An estimated 10 million adults had a diagnosis of COPD in the U.S. in
the year 2000.
•
The prevalence and age-adjusted death rate for COPD increased
more than 30 percent since 1980.
•
The direct/indirect costs related to COPD amounted to approximately
$42.6 billion in the U.S. in 2007.
Source:
CDC, CDC COPD surveillance in U.S. 2000; US Census; American Lung Association
website |
 ©
MediciNova, Inc. 2011
29
A COPD exacerbation
is a sustained worsening of the
patient's condition, from the stable state and beyond
normal day-to-day variations, that is acute in onset.
COPD exacerbations are associated with a significant
increase in mortality, hospitalization and healthcare
utilization.
1.5 million hospital emergency department visits
765,000 hospitalizations
Average length of stay 7.4 days*
Average cost ~$32,000*
119,000 deaths
COPD Exacerbations
COPD patients are generally more ill than asthmatics with
overall higher hospitalizations and mortality.
Source:
CDC, CDC COPD surveillance in U.S. 2000; US Census; American Lung Association
website *For
COPD
pts.
with
anemia;
for
pts.
w/o
anemia
the
stay
is
~5.8
days
at
a
cost
of
~$25K
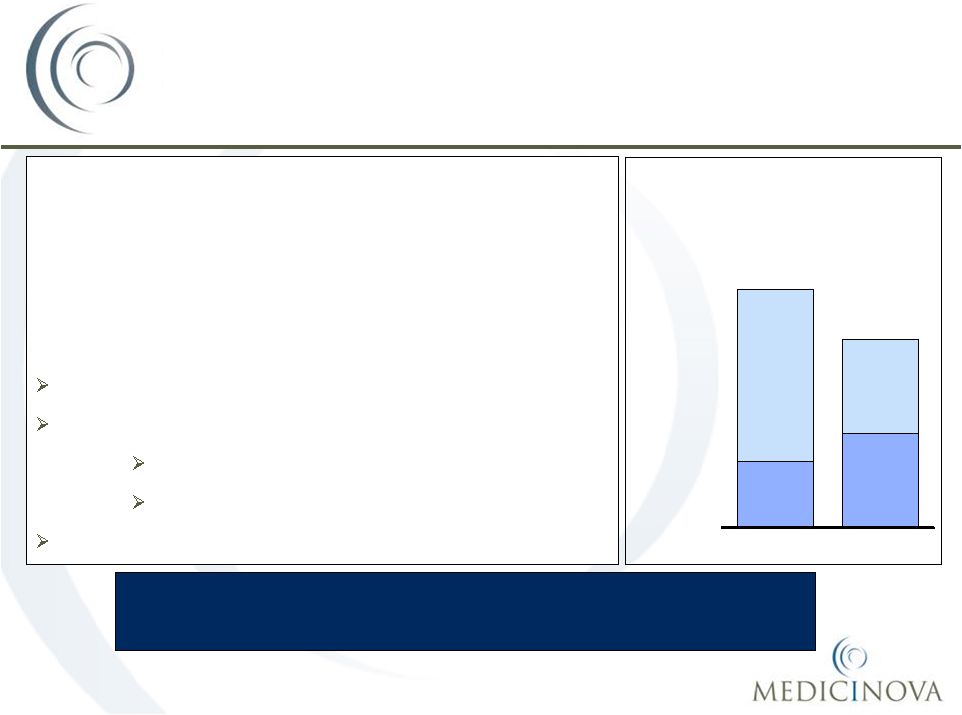
COPD
Discharged
Hospitalized
72%
28%
1,900
Asthma
52%
48%
1,500
Hospitalization rates amongst
Asthma and COPD patients
Thousands |
 ©
MediciNova, Inc. 2011
In patients who
respond to
theophylline: IV
aminophylline
(used rarely)
Noninvasive
intermittent
ventilation (NIV)
(e.g. bipap
mask)
Source: Global Initiative for Chronic Obstructive Lung Disease 2007; team
analysis 30
Represents leading drugs
currently used
History and
physical
Pulse oximetry
Arterial blood
gas (ABG),
Chest X-ray
(CXR)
Other physical
and clinical
evaluation to
assess severity
Hospitalization
and
resumption of
first line
therapy
ICU admission
-
Intubation/
mechanical
ventilation
-
Resumption
of first line
therapy
Initial assessment
Standard treatments
Other treatments
(not commonly used)
Initial treatment failure
and subsequent
hospitalization
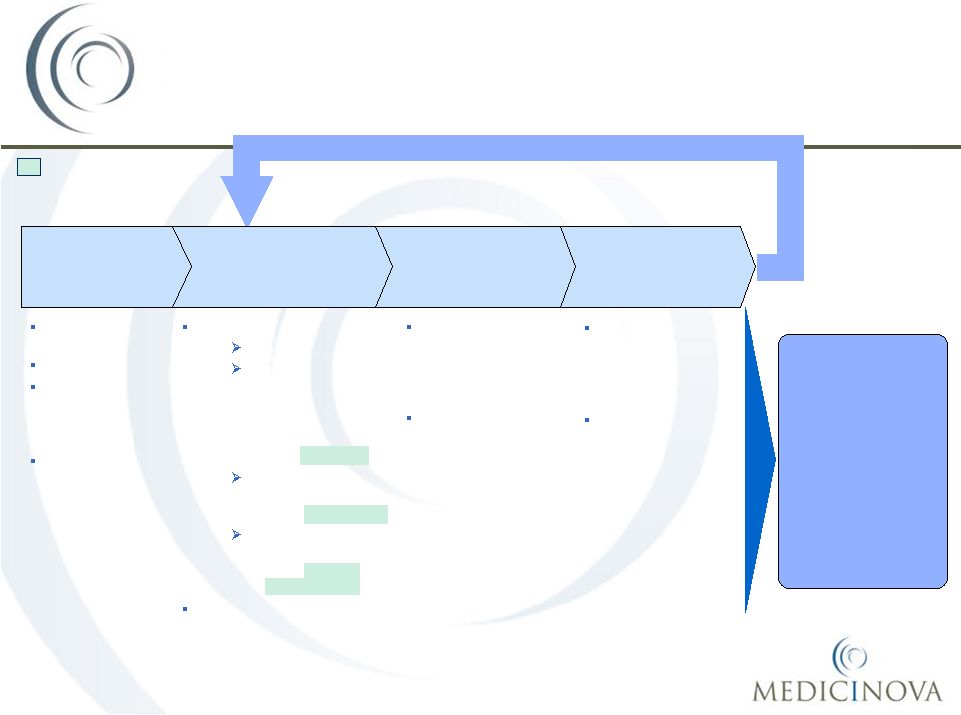
COPD:
Current
treatment
paradigm
in
emergency
department
and
hospital
settings
Hospitalized patients follow the same
treatment paradigm as in ER
•
COPD
management in the
hospital and ICU
settings mirrors
the ER approach
•
There are few
treatment options
beyond the first
line of therapy
Concurrently:
Low flow oxygen
Intermittent or
continuous
nebulized
short-
acting
ß2-agonist
(SABA)
(e.g., Albuterol)
Nebulized
anticholinergic
(e.g., Ipratropium)
IV or oral systemic
steroids
(e.g., Methyl-
prednisolone)
Antibiotics |
 ©
MediciNova, Inc. 2011
31
COPD Exacerbation Treatment Flow in
Emergency Departments in the U.S.
225,000
1,275,000
1,275,000
510,000
765,000
765,000
Input:
1,500,000 patients
with acute
exacerbations of
asthma present at
U.S. EDs
annually
1
st
line
therapy
in
ED:
Patients receive SOC,
many while in the
waiting room
2
nd
line
therapy
in
ED:
Patients who do not
initially respond
continuing receiving SOC
Large Market
Opportunity for MN-221
1,500,000
1,500,000
Hospitalization:
Patients who do not
respond to SOC are
eventually hospitalized
Patients who respond
to initial therapy and
are discharged
Patients who
eventually respond to
standard therapy and
are discharged
Source:
CDC COPD surveillance in U.S. 2000; Expert interviews, team analysis
|
 ©
MediciNova, Inc. 2011
Study Design
•
Randomized, double-blind, placebo-controlled dose escalation study
•
48 subjects with stable moderate-to-severe Chronic Obstructive Pulmonary
Disease
(COPD)
(FEV
1
30%
<
80%
and
FEV
1
/FVC
ratio
<
0.7)
at
6
sites
•
Doses:
•
10 µg/min for 15 minutes followed by 3.3 µg/min for 45 minutes (1-hour
infusion with a total dose of 300 µg) or placebo
•
20 µg/min for 15 minutes followed by 6.67 µg/min for 45 minutes (1-hour
infusion with a total dose of 600 µg) or placebo
•
40 µg/min for 15 minutes followed by 13.3 µg/min for 45 minutes (1-hour
infusion with a total dose of 1,200 µg) or placebo
•
Outcome measures –
descriptive statistics only –
FEV
1
, PK, safety
MN-221-CL-010 (COPD)
32 |
 ©
MediciNova, Inc. 2011
MN-221-CL-010:
Mean Change in FEV
1
33
Baseline:
50.31%
Baseline:
46.7%
Baseline:
46.6%
Baseline:
47.9%
p = 0.0012
p = 0.0147 |
 Addendum
|
 ©
MediciNova, Inc. 2011
Study Design
•
Randomized, placebo-controlled, double-blind, dose escalation study
•
23 subjects with mild-to-moderate stable asthma (FEV
1
60% predicted)
at 4 sites
•
Patients are randomized to one of four different treatment groups (25% of
patients on placebo for every dose level)*
•
Each
treatment
sequence
consist
of
placebo
and
escalating
doses
of
MN-221
(5.25µg,
15.0µg,
52.5µg,
150µg,
240µg,
450µg,
900µg)
over
15
minutes
•
Primary endpoint -
mean change in FEV
1
(forced expiratory volume
in 1 second) from baseline (start of infusion) to 15 minutes (end of
infusion)
•
Outcome measures –
inferential statistics –
FEV
1
, pharmacokinetic (PK),
safety and tolerability
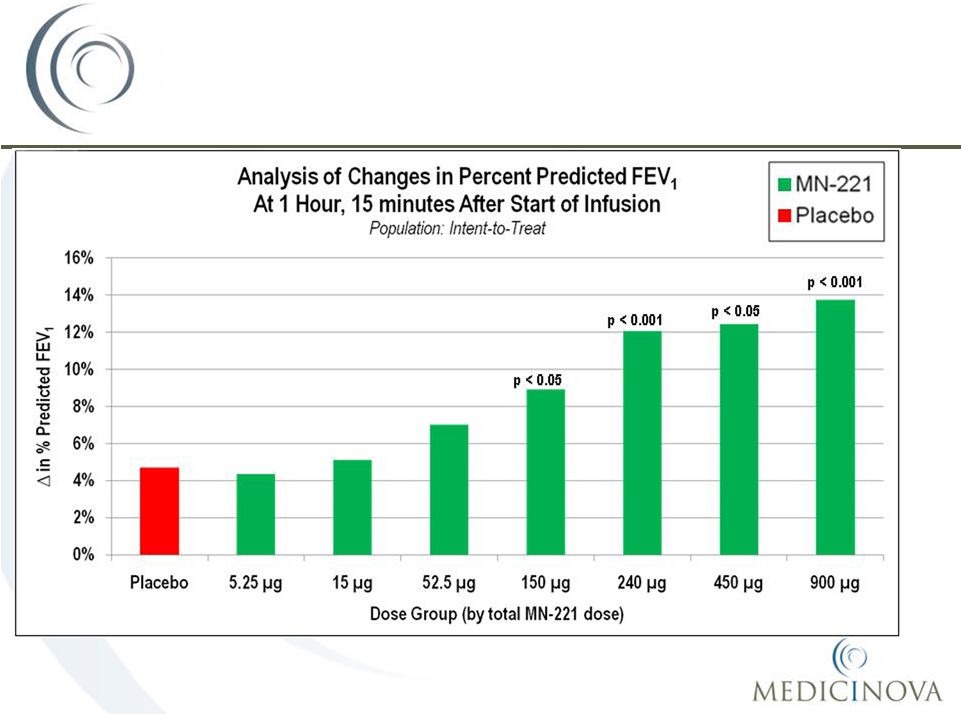
MN-221-CL-004
35 |
 ©
MediciNova, Inc. 2011
MN-221-CL-004:
Mean Change in FEV
1
36 |
 ©
MediciNova, Inc. 2011
Study Design
•
Randomized, placebo-controlled, single-blind, dose rate escalation
study
•
17 subjects with moderate-to-severe stable asthma (FEV
1
40%,
but
75% predicted) at 4 sites
•
Doses:
•
16
µg/min for 15 minutes followed by 8 µg/min for 105
minutes (2-hour infusion with a total dose of 1,080
µg) or placebo •
30
µg/min for 15 minutes followed by 15 µg/min for 45
minutes (1-hour infusion with a total dose of 1,125
µg) or placebo •
Outcome measures –
descriptive statistics only –
FEV
1
, PK, safety
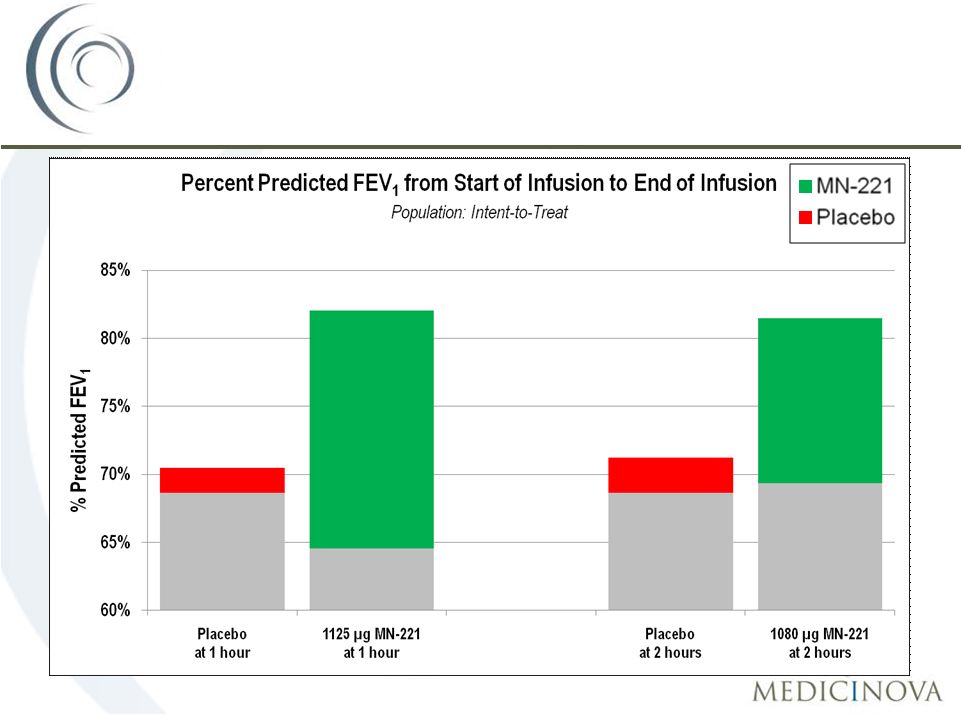
MN-221-CL-005
37 |
 ©
MediciNova, Inc. 2011
MN-221-CL-005:
Mean Change in FEV
1
38
Baseline:
64.6%
Baseline:
69.4%
1 Hour:
82.0%
2 Hours:
81.5%
Baseline:
68.6%
Baseline:
68.6%
1 Hour:
70.5%
2 Hours:
71.2% |
 ©
MediciNova, Inc. 2011
Published evidence
•
It has been demonstrated that airway abnormalities extend from the proximal to the most
distal airways in asthmatics, and in severe stable asthmatics it has been
postulated that one reason that they are difficult to control
is
that
inhaled
particle
(drug)
deposition
in
the
distal
airways
is
impaired
(1).
•
The bronchoconstriction, inflammation, and mucus plugging that occur during an acute
exacerbation of asthma will magnify this problem. Modeling of airflow patterns
in patients with acute asthma demonstrates that
airway
resistance
is
twice
as
high
during
the
exacerbation
than
after
recovery.
Furthermore,
the
airflow
is more profoundly affected in regions where the effects of asthma are significant
(2). •
Chronic
Mucus
Plug
Formation:
In
severe
asthma,
mucus
secretion
and
the
formation
of
inspissated
mucus
plugs can cause persistent airflow limitation (3).
•
Taken together, delivery of aerosolized medications to the distal airways is negatively
impacted during an acute asthma exacerbation.
Anecdotal evidence
•
The emergency room doctors in our studies and key opinion leaders we have spoken to all
believe in the concept
of
"intravenous
beta
2
agonist"
to
treat
acute
exacerbations
of
asthma.
They
have
all
cited
the
fact
that if
a patient is having difficulty breathing, the patient cannot fully inhale
medicine. MN-221 may improve efficacy over current
standard of care due to its route of
administration
39
(1)
Veen
et
al.
Recurrent
exacerbations
in
severe
asthma
are
associated
with
enhanced
airway
closure
during
stable
episodes.
Am
J
Respir
Crit
Car
Med
2000:161(6):1902-06)
(2)
Inthavong
et
al.
Comparative
study
of
the
effects
of
acute
asthma
in
relation
to
a
recovered
airway
tree
on
airflow
patterns.
In:
13th
International
conference
on
Biomedical
Engineering
2009 V. 23.
ISBM 978-3-540-92841-6 (online)
(3)
University
of
California,
San
Diego,
School
of
Medicine,
Division
of
Medical
Education
https://meded.ucsd.edu/isp/1998/asthma/html/naep.html |
 ©
MediciNova, Inc. 2011
•
MediciNova
has preclinical data and clinical data which demonstrates
the safety of MN-221.
•
In summary, we have not seen clinically significant safety concerns
with MN-221.
•
According to interviews of emergency room physicians, less-selective
injectable
beta agonists such as epinephrine and terbutaline
are not
commonly used to treat acute asthma.
The main reason they are not
used more often is due to safety concerns, particularly cardiovascular
side effects.
MN-221 may result in fewer cardiovascular
side effects than the current standard of care
40 |
 ©
MediciNova, Inc. 2011
•
Since the average hospitalization cost is $6,477 in the US, the payor
would save this amount for each hospitalization prevented.
•
Since US hospitals lose money on the typical asthma hospitalization
due to low reimbursements from Medicaid and HMOs, hospitals
would also make more money for each asthma hospitalization
prevented.
MN-221 may reduce health care expenses
by reducing the hospitalization rate
41 |
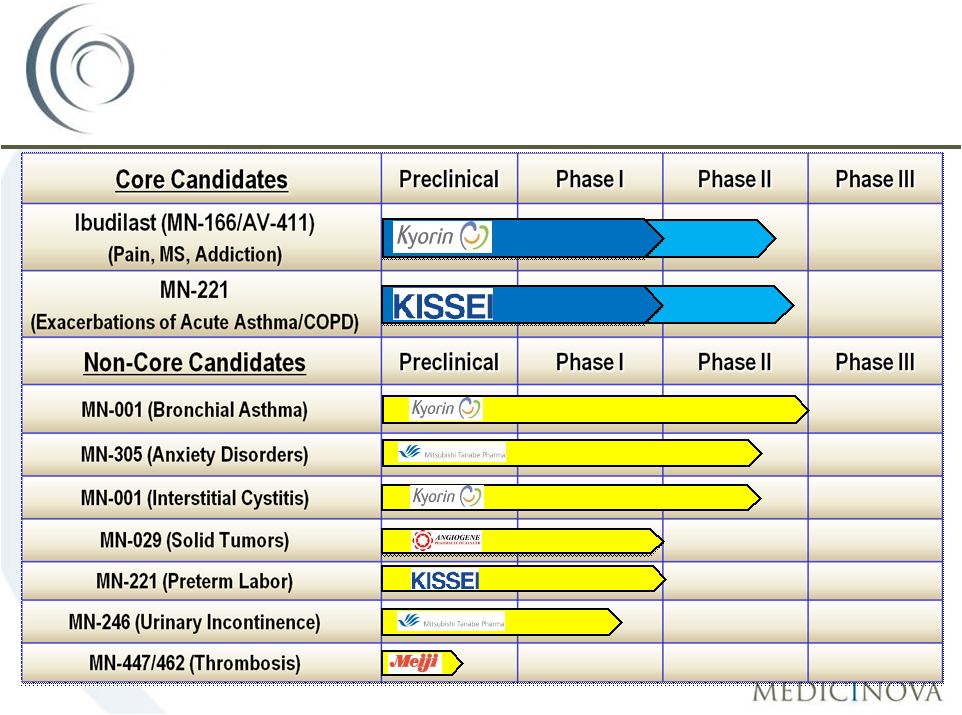 ©
MediciNova, Inc. 2011
42
Commercially-Attractive
Diversified Portfolio
COPD
COPD
Asthma
Asthma
Pain/MS
Pain/MS
Addiction
Addiction |
