Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Orexigen Therapeutics, Inc. | d8k.htm |
 1
Investor Presentation
Investor Presentation
December 2010
December 2010
Exhibit 99.1 |
 2
Forward-Looking Statements
Forward-Looking Statements
This
presentation
contains
forward-looking
statements
about
Orexigen
Therapeutics,
Inc.
Words
such
as
“believes,”
“anticipates,”
“plans,”
“expects,”
“will,”
“intends,”
“potential,”
“assuming”
and
similar
expressions
are
intended
to
identify
forward-looking
statements.
These
statements
are
based
on
Orexigen’s
current
beliefs
and
expectations.
These
forward-looking
statements
include
statements
regarding
the
potential
for,
and
timing
of,
approval
of
the
Contrave
®
New
Drug
Application
(NDA)
and
an
NDA
submission
for
Empatic™;
the
efficacy
and
safety
of
Contrave
and
Empatic;
Orexigen's
belief
that
Contrave
and
Empatic
may
be
important
and
effective
therapeutic
options
in
the
treatment
of
obesity;
the
potential
for,
timing
of,
cost
and
design
of
a
post-
approval
study
to
evaluate
safety
risks
of
Contrave;
the
potential
milestone
and
royalty
payments
under
the
collaboration
agreement
with
Takeda
Pharmaceutical
Company
Limited;
the
potential
strength
of
Orexigen’s
market
entry
with
Contrave,
if
approved;
and
Orexigen’s
plans
to
commence
strategic
collaboration
and/or
co-promotion
discussions
with
respect
to
Empatic
and
outside
of
the
United
States,
Contrave.
The
inclusion
of
forward-looking
statements
should
not
be
regarded
as
a
representation
by
Orexigen
that
any
of
its
plans
will
be
achieved.
Actual
results
may
differ
from
those
set
forth
in
this
presentation
due
to
the
risk
and
uncertainties
inherent
in
Orexigen’s
business,
including,
without
limitation:
the
potential
for
the
U.S.
Food
and
Drug
Administration
(FDA)
to
delay
the
scheduled
Prescription
Drug
User
Fee
Act
action
date
of
January
31,
2011
due
to
the
FDA’s
internal
resource
constraints
or
other
reasons;
the
uncertainty
of
the
FDA
approval
process
and
other
regulatory
requirements;
the
uncertainty
of
the
process
with
the
FDA
to
agree
on
and
finalize
a
design
for
a
post-approval
study
to
evaluate
safety
risks
of
Contrave;
the
FDA
may
not
agree
with
Orexigen’s
interpretation
of
efficacy
and
safety
results;
the
FDA
may
require
Orexigen
to
complete
additional
clinical,
non-clinical
or
other
requirements
prior
to
the
approval
of
the
Contrave
NDA
or
the
submission
of
an
NDA
for
Empatic;
the
therapeutic
and
commercial
value
of
Contrave
and
Empatic;
reliance
on
third
parties
to
assist
with
the
development
of
Contrave
and
Empatic;
the
potential
for
adverse
safety
findings
relating
to
Contrave
or
Empatic
to
delay
or
prevent
regulatory
approval
or
commercialization;
Orexigen’s
ability
to
enter
into
additional
collaboration
or
co-promotion
arrangements;
and
other
risks
described
in
Orexigen’s
filings
with
the
Securities
and
Exchange
Commission
(SEC),
including
those
detailed
under
the
heading
“Risk
Factors”
in
Orexigen’s
periodic
filings
with
the
SEC.
You
are
cautioned
not
to
place
undue
reliance
on
these
forward-looking
statements,
which
speak
only
as
of
the
date
hereof,
and
Orexigen
undertakes
no
obligation
to
revise
or
update
this
presentation
to
reflect
events
or
circumstances
after
the
date
hereof.
All
forward-looking
statements
are
qualified
in
their
entirety
by
this
cautionary
statement.
This
caution
is
made
under
the
safe
harbor
provisions
of
Section
21E
of
the
Private
Securities
Litigation
Reform
Act
of
1995
and
Section
27A
of
the
Securities
Act
of
1933,
as
amended.
Information
included
herein
is
based
on
clinical
data
the
Company
has
received
to
date
and
its
evaluation
of
such
data.
All
conclusions
contained
herein
are
subject
to
and
contingent
upon
additional
clinical
data
being
generated
by
the
Company
as
well
as
the
evaluation
of
such
data
by
the
FDA
and
other
regulatory
agencies. |
 3
Realizing the Vision
Industry Leader in Obesity Therapeutics
PRODUCTS
Contrave
®
–
NDA under
review by FDA
Empatic™
–
Preparing
for Phase III
STRATEGY/EXECUTION
Successful Execution
on All Major Milestones
to Date
Gain High Quality
Partners for Global
Commercialization
RESOURCES
Powerful Partner for
Contrave
in
North America
Strong Balance Sheet
Experienced Team |
 4
2010 –
A Year of Strong Execution
2010 –
A Year of Strong Execution
Q1
Q2
Q3
Q4
2010
Filed NDA
NDA Accepted
by FDA
Clinical Results
Published in
The Lancet
and
Obesity
Signed Takeda
Partnership
Positive Advisory
Committee
Meeting |
 5
Obesity Epidemic Plus Lack of Solutions Have
Created a Perfect Storm
Obesity Epidemic Plus Lack of Solutions Have
Created a Perfect Storm
Increase in Obesity
Source: http://www.cdc.gov; http://www.fightchronicdisease.org;
Flegal KM, et al. 2010 U.S.
103M
16%
34%
43%
Serious Disease Attributable
to Obesity
Treatment Options Needed
T2DM, Driven by Obesity, Is an Epidemic
-
1 in 3 Adults in the U.S. May Have Diabetes by 2050 (CDC)
-
Associated with Premature Death & Physical/Psychosocial Consequences
Weight
Loss
of
5
10%
Confers
Significant
Benefits
Lifestyle Change is Critical, but Weight Loss Difficult to
Achieve/Maintain
Large Gaps in Current Treatment Paradigm
75M |
       6
Corporate Objectives for 2011
Corporate Objectives for 2011
Pursue
Additional
Partnerships
Launch
Contrave
with Takeda
Obtain
Approval for
Contrave
Address FDA
Input |
 7
Key Results from Advisory Committee
Key Results from Advisory Committee
13:7 on Approvability
11:8 –
Outcomes Trial should be Run
Post-Approval (Not Pre-approval)
Major Takeaways
Efficacy Met FDA Guidance
Strong Support for Comprehensive Risk Mitigation Strategy
Desire for a Post-Approval Outcomes Study
Risk/Benefit Deemed Acceptable for Approval |
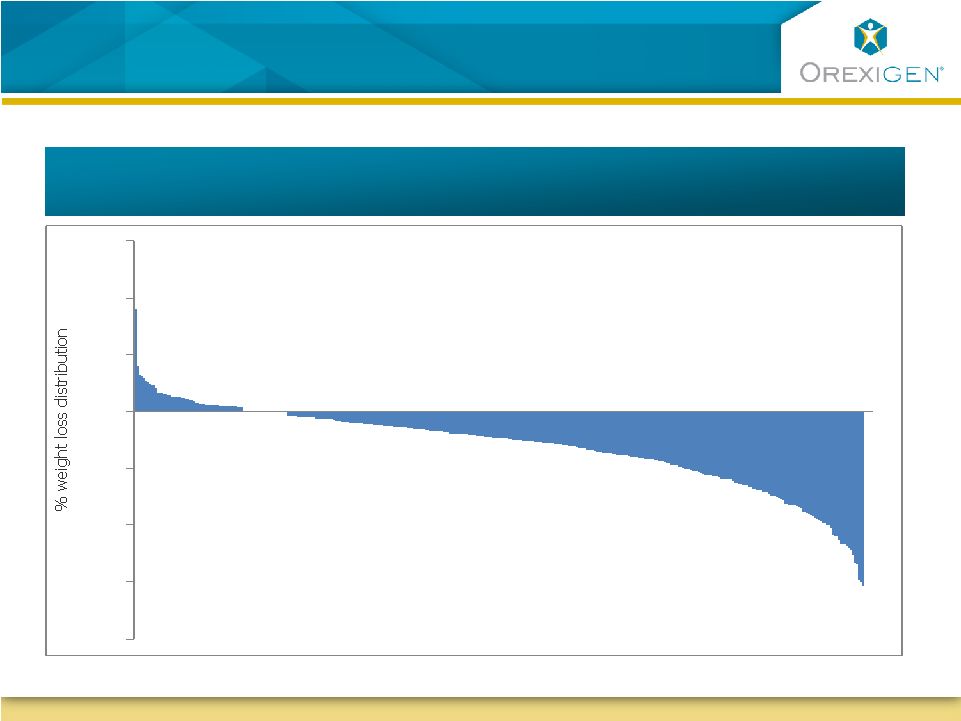 8
-40
-30
-20
-10
0
10
20
30
Typical Response to Weight Loss Therapy
is not Uniform
Typical Response to Weight Loss Therapy
is not Uniform
COR-I Weight Loss Distribution (Contrave32) |
 9
-40
-30
-20
-10
0
10
20
30
Our Recommended Treatment Algorithm Focuses on
Patients who Achieve a Meaningful Response
Our Recommended Treatment Algorithm Focuses on
Patients who Achieve a Meaningful Response
Our Focus: Patients
Who Lose
5%
<5% weight loss
COR-I Weight Loss Distribution (Contrave32) |
     10
Treatment Algorithm: Identify Early Responders
for Continued Long-term Therapy
Treatment Algorithm: Identify Early Responders
for Continued Long-term Therapy
Appropriate BMI
Motivated to Adhere
to Lifestyle Change
Inadequate
Response to Diet &
Exercise
Select Responders
for Continued
Treatment
Reinforce Adherence
to Appropriate Use
Evaluate Adherence
to Appropriate Use
Guidance
Assess Response
to Therapy (>5%)
Screening
16-Week
Therapeutic Trial
Long-Term
Therapy |
 11
Contrave
Early Responders Go on to Achieve
Substantial Long Term Weight Loss
Contrave
Early Responders Go on to Achieve
Substantial Long Term Weight Loss
Represents
one
year
data
for
responders
(patients
with
5%
weight
loss
at
week
16
per
recommended
treatment
protocol)
Weight Loss Distribution
0% -
5%
Weight loss
10% -
15%
Weight Loss
Avg
Wt Loss =
26.7 lbs
>15% Wt Loss
Avg
Wt Loss =
43.9 lbs
Weight Gain
5% -
10%
Weight Loss
Avg
Wt Loss =
17.0 lbs |
 12
Contrave
Responders Achieve Improvement across
Multiple Cardiometabolic
Parameters
Contrave
Responders Achieve Improvement across
Multiple Cardiometabolic
Parameters
Improvement
(Standardized Mean Change from Baseline)
Contrave32
Contrave32
N=1038
N=1038
Placebo
N=254
-11.3%
-8.6%
-9.5 cm
-7.2 cm
5.2 mg/dL
2.9 mg/dL
-2.5 mg/dL
1.7 mg/dL
-16.0%
-9.2%
-38.9%
-36.2%
14.7
12.3
-1.0%
-0.6%
-0.9 mm Hg
-2.8 mm Hg
-1.6 mm Hg
-2.6 mm Hg
0.2 bpm
-2.3 bpm
Proportion w/
5% Wt Loss Wk 16
51%
19%
Contrave32 Standardized Mean
Change from Baseline at 1 Year
Change from Baseline at 1 Year
Body Weight
Waist Circ.
HDL
LDL
Triglycerides
hsCRP
IWQOL
HbA1c
SBP
DBP
Heart Rate |
 13
Contrave: Well Characterized Safety Profile
Contrave: Well Characterized Safety Profile
Delivers Safety Experience Consistent with
20+ Year History of Underlying Agents
Known Areas of Focus –
CV Effects, Seizure, Psychiatric Effects, Creatinine
Meets FDA Guidance for Patient Exposure for Evaluation
of Safety and Tolerability with >4,500 Patients
Long Term Evaluation of Benefits and Outcomes
Proposed for Post-approval |
     14
Label
Appropriate Selection
and Management of
Patients
REMS
Medication Guide,
Communication Plan
and Assessment
Post-Marketing
Data Capture
Prospective
Cohort Study
Outcomes Trial
Comprehensive Risk Mitigation Plan Designed
to Drive Appropriate Use
Comprehensive Risk Mitigation Plan Designed
to Drive Appropriate Use
Appropriate Use Program: Delivers Tools for Screening,
Evaluation and Comprehensive
Weight Management |
 15
Post-approval Outcomes Trial Represents Significant
Opportunity for Orexigen
Post-approval Outcomes Trial Represents Significant
Opportunity for Orexigen
Beneficial
outcomes
predicted
based
on
cardiometabolic
improvements
--
Demonstrating
outcomes
benefit
could
be
transformational
for
Contrave
and
the obesity field
Proposing
large
simple
trial
(LST)
--
Streamlined,
practical,
affordable
trial
design targeting per-patient cost at a fraction of typical outcomes trials
Aligned with principles of FDA-Duke Clinical Trial Transformation Initiative
(CTTI) |
 16
SCALE
Top 15 Pharma
Significant U.S. Presence
Primary Care Focus
COMMITMENT
TO OBESITY
Partnership
with Amylin
Stated
Strategy
DOMAIN
EXPERTISE
Leader in
Cardiometabolic
Care
Excellence in
Life Cycle
Management
POWERFUL
BRAND BUILDER
Actos ~$3B in U.S.
Sales in 2009
Prevacid ~$3B in U.S.
Sales in 2009
Takeda Is the Ideal Partner for Contrave
Takeda Is the Ideal Partner for Contrave |
 17
Competitive Product Profile Attractive to Patients
Competitive Product Profile Attractive to Patients
% of
Patients Losing
10% of Body
Weight after
Completing 1 year
of Treatment
Women Dominate the Market
1/3 to 1/2 Experienced Market-Meaningful Weight Loss
No Addictive Concerns
Avg
Loss
34 lbs
Avg
Loss
35 lbs
Avg
Loss
39 lbs
No Abuse Liability
No DEA Limitations on
Distribution Expected
73%
73%
Sources:
Orexigen
Quantitative
Market
Research
2009;
IMS
NDTI
Audit
Data,
6
Months Ending 3/09
Female, Often
Childbearing Age |
 18
Contrave: Keys to Commercial Success
Contrave: Keys to Commercial Success
Source: CDC (2009)
103M
US Obesity Rate Growth
Obesity
Rate
Blockbuster Market Size
Market Growth
Significant Unmet Need –
Limited Competition
Diet & Lifestyle Modifications
Contrave: Differentiated Profile
Takeda: Heavily Resourced
BMI
Distribution
of US Pop:
Morbidly Obese
Obese
Overweight
~ 113K People
Treated w/ Surgery
~ 2 Million People
Treated w/ Rxs
Majority of Obese are Untreated in an Environment of Limited Treatment Options
US Population
~ 1 in 3 People
in the US
Are Obese |
 19
We Partnered Early for the Significant Activities
Required for a Quality Launch
We Partnered Early for the Significant Activities
Required for a Quality Launch
Sales Force
Sales Aids
Direct Mail
Physician Ed Brochure
Sales Training
Professional
Advisory Boards
KOL Identification
Peer-to-Peer Programs
HCP Website
Patient
Patient Ed Brochure
Patient Support Program
Websites/Digital Ads
Pharmacy Programs
Brand
Development
Market Research
Positioning / Targeting
Customer Segmentation
Sample Strategy
Market
Development
KOL Mapping
Medical Education
Publications
Health Outcomes
Managed Markets;
Government Affairs
Employer Segmentation
Health Outcomes Research
Pricing/Contracting
Advocacy/Policy |
      20
Seeking High Quality Partner for Contrave
Commercialization in ROW
Seeking High Quality Partner for
Contrave Commercialization in ROW 8 to 10
Attractive
Potential
Partners
Commitment to Cardiometabolic
Disease in Target Markets
Strong Regulatory Track Record
Powerful Commercialization Capacities
Motivated to Build Late Stage Pipeline |
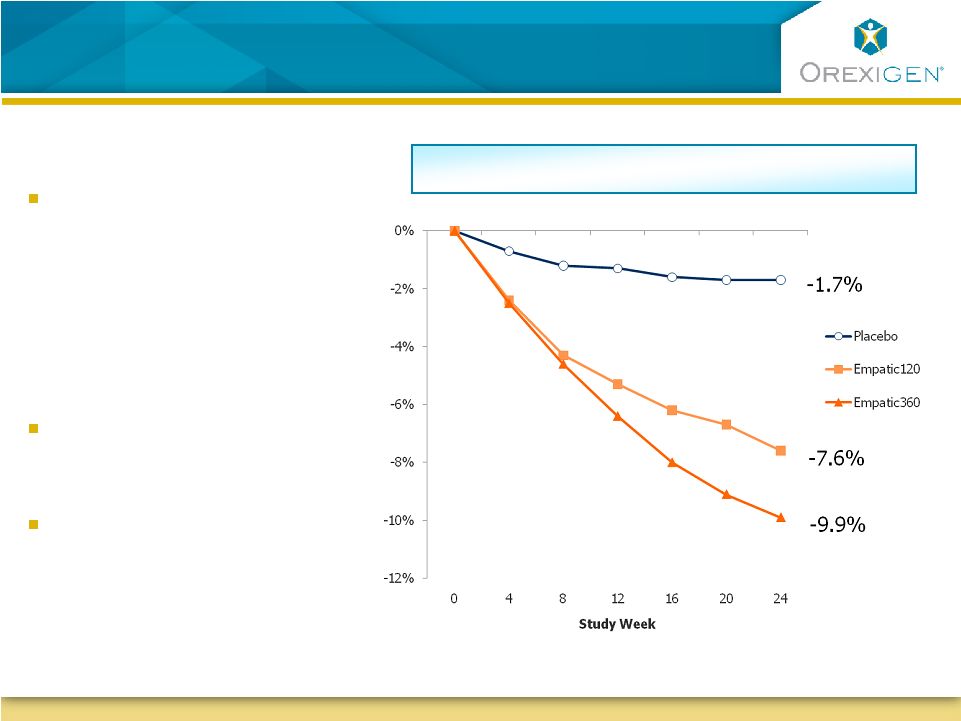 21
Empatic: Second Late Stage Obesity Program with
Complementary Profile
Empatic: Second Late Stage Obesity Program with
Complementary Profile
Observed Case Analysis
Key to Multi-Solution
Approach
-
High Efficacy
-
Different MOA than
Contrave
-
Helps Orexigen
Meet
Differing Patients Needs
Safety Profile Appears
Consistent with Underlying
Constituents
Phase II Completed;
Exploring Partnership
Options for Phase III
Note
:
All
differences
between
Empatic
and
placebo
statistically
significant
at
all
time
points.
Data
are
LS
mean. |
   22
2011
2012
Contrave
PDUFA
Potential Launch
with Takeda in
United States
Partnership
for Ex-NA
Multiple Near-Term High-Value Catalysts
Multiple Near-Term High-Value Catalysts
Empatic
Phase III Planning
End of Phase II Meeting
Partnership
Phase III Initiation
Lifecycle
Management
Opportunities |
 23
No Foreseeable Need to Finance
No Foreseeable Need to Finance
$50M Payment from Takeda in Q3
$100M of Cash and Investments as of 9/30/10
Strong Balance Sheet
$100M in Milestones Achievable Between Now and
Product Launch
Additional Milestones for New Potential Collaborations
Near-Term Milestones
Takeda Responsible for All Commercialization Expenses in NA
Post-Approval Development Costs Shared
Ex-NA and Empatic
Activities Focused on Selecting Partner
Run-rate Opex
under $60M
Low Burn Rate |
 24
Realizing the Vision
Industry Leader in Obesity Therapeutics
PRODUCTS
Contrave
®
-
NDA under
review by FDA
Empatic™
–
Preparing
for Phase III
STRATEGY/EXECUTION
Successful Execution
on All Major Milestones
to Date
Gain High Quality
Partners for Global
Commercialization
RESOURCES
Powerful Partner for
Contrave
in
North America
Strong Balance Sheet
Experienced Team |
 25 |
