Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a6507677.htm |
Exhibit 99.1

Credit Suisse 2010 Healthcare Conference James J. Egan Chief Operating Officer November 12, 2010 1

Forward-Looking Statement Forward-looking statements contained in this presentation are based on Sucampo’s assumptions and expectations concerning future events. They are subject to significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those reflected in the f dl ki t t t S ’ f dl ki t t t ldb forward-looking statements. Sucampo’s forward-looking statements could be affected by numerous foreseeable and unforeseeable events and developments such as regulatory delays, the failure of clinical trials, the inability to fund drug development initiatives initiatives, competitive products and other factors identified in the “Risk Factors” section of Sucampo’s Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. While Sucampo may elect to update these statements at some point in the future Sucampo specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise. In light of the significant uncertainties inherent in the forward-looking information in this presentation, you are cautioned not to place undue reliance on these forward-looking statements. 2
Sucampo: A Biopharmaceutical Company Rescula® FDA approved for lowering intra-ocular pressure (IOP) in glaucoma and ocular hypertension in patients who are intolerant of or insufficiently responsive to other IOP lowering medications In-licensed US + Canadian development and marketing rights in April 2009 Awaiting FDA approval of label-enhancing supplemental NDA (sNDA) to re-launch in U.S. Designing trials for additional indications, based on partner’s breakthrough clinical results Amitiza® Only FDA approved drug for chronic idiopathic constipation (CIC) in adults Only FDA approved drug for irritable bowel syndrome with constipation (IBS-C) in adult women Marketing authorization approved (Nov in Switzerland for CIC indication Phase 3 trial in opioid-induced bowel dysfunction (OBD) to initiate late 2010 U.S + Canadian commercial rights held by Takeda, commercial rights in Japan held by Abbott A deep pipeline leveraging prostone technology, expertise Cobiprostone for prevention of NSAID-induced gastric ulcers in Phase 2 SPI-017 for peripheral arterial disease going into Phase 2 Additional prostones in preclinical development, such as SPI-3608 Strong financial position $110.7 million in cash and investments as of Sept. 30, 2010 3
Rescula: In-Licensed from R-Tech Ueno Sucampo licensed Rescula’s US and Canada rights from R-Tech Ueno (RTU) in April 2009 Sucampo gained exclusive rights to commercialize Rescula in the U.S. and Canada for approved indication and right of first refusal to additional indications for which RTU develops Rescula Also received the right to develop Rescula for additional ophthalmic indications RTU is responsible for clinical and commercial supply of Rescula to Sucampo Sucampo paid an upfront payment of $3 million to RTU and is responsible for additional milestone payments Sucampo responsible for development, regulatory and commercialization activities and expenses in the U.S. and Canada 4
Rescula: Phase 2 Clinical Trial Design --Retinitis Pigmentosa Design of Phase 2 Trial •A multi-center, randomized, double-blind, three parallel group, placebo-controlled trial •Enrolled 112 mid-to late-stage Retinitis Pigmentosa (RP) patients with visual acuity of 0.5 or more in a narrow visual field •Conducted at 6 sites in Japan •Patients received either one or two drops of active drug or placebo twice a day for 24 weeks •Primary endpoint : change from baseline in the mean retinal sensitivity of the central 2-degrees of the ocular fundus as measured with an MP-1 microperimeter •Secondary endpoints included –Retina sensitivity measured by Humphrey perimeter (10-2) –Visual acuity –Contrast sensitivity –Health related Quality of Life (measured by VFQ-25)
Rescula: Current Status •Rescula eye-drops are a prostone-based drug, not a prostaglandin •FDA-approved for lowering of intra-ocular pressure (IOP) in primary open-angle glaucoma (POAG) and ocular hypertension patients who are intolerant of or are insufficiently responsive to other IOP lowering medications; not currently available in U.S. •Sucampo submitted data developed after Rescula’s FDA approval in 2000 in an sNDA (August 2009) •Will complete label discussions with FDA before finalizing US launch plans

Amitiza Answers Unmet Medical Needs •Represents a major market opportunity–More than 14 million (CIC and IBS-C) office visits each year in U.S. –4.5 million U.S. office visits seeking relief of opioid-induced bowel dysfunction (OBD) •Offers proven safety and efficacy for long-term usage–Efficacy + tolerability are similar for both genders + across age groups for CIC –90% of nausea events diminish after first week of use –Competing products recommended for short-term use only •Provides quick and predictable relief of symptoms –Between 57%-63% of CIC patients respond within 24 hours and remain responsive –IBS-C patients were twice as likely to achieve overall response than those receiving placebo •Differentiated mechanisms of action–In CIC, Amitiza activates chloride ion channels, promoting fluid secretion –In IBS-C, Amitiza activates chloride ion channels and promotes mucosal barrier protection
Amitiza: Chronic Idiopathic Constipation* Phase 3 pivotal trial design •2 multicenter trials, both randomized, parallel-group, enrolled 479 patients •Administered 24 mcg gel capsule of Amitiza or placebo twice daily •4 week treatment period preceded by 2 week baseline period •Entry criteria: modified Rome II criteria for functional constipation •Primary efficacy endpoint: change from baseline in number of spontaneous bowel movements (SBMs) after 1 week of treatment •Secondary endpoints included:•SBMs at weeks 2, 3 and 4 •Percentage of patients with a SBM within 24 hours of first dose •Time to first SBM •BarishCF. Dig DisSci2010; 55: 1090-1097 •JohansonJF et al Am J Gastroenterol. 2008:103:170-177
Amitiza: Chronic Idiopathic Constipation* Phase 3 Trials Results Amitiza met the primary endpoint with statistical significance (p<0.0001), as Amitiza patients experienced statistically significantly greater mean numbers of SBMs at Week 1 as compared to placebo patients (5.5 / 5.9 vs. 3.5 / 4.0) Secondary endpoint results: •In each week of the trials, Amitiza patients had significantly higher frequency of SBMs at all weeks except week 2 •Significantly higher percentage of Amitiza patients experienced a SBM within 24 hours of first dose as compared to placebo (57-61.3% vs. 32-37%) •Time to first SBM was significantly shorter in Amitiza patients than with placebo * Barish CF, et al Dig DisSci2010; 55: 1090-1097 and JohansonJF, et al Am J Gastroenterol. 2008:103:170-177 Amitiza approved by FDA for treatment of CIC in adults with no upper age limit in January 2006
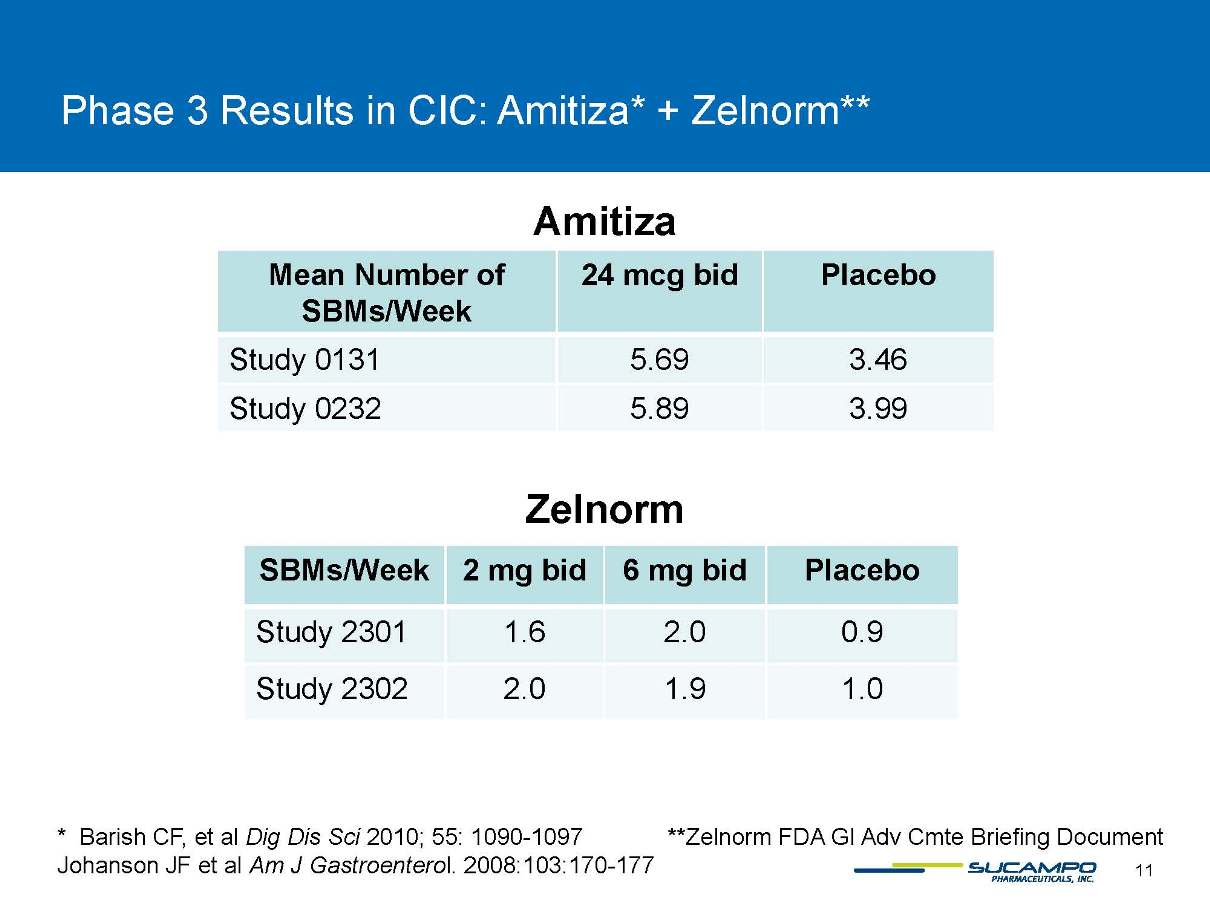
Phase 3 Results in CIC: Amitiza* + Zelnorm** Amitiza Zelnorm * BarishCF, et al Dig DisSci2010; 55: 1090-1097 **ZelnormFDA GI Adv CmteBriefing Document JohansonJF et al Am J Gastroenterol. 2008:103:170-177 Mean Number of SBMs/Week 24 mcg bid Placebo Study 0131 5.69 3.46 Study 0232 5.89 3.99 SBMs/Week 2 mgbid 6 mg bid Placebo Study 2301 1.6 2.0 0.9 Study 2302 2.0 1.9 1.0
Amitiza: Irritable Bowel Syndrome with Constipation* Design of two Phase 3 trials •2 multicenter trials identically designed, randomized, parallel-groups •1,171 U.S. patients received 8 mcg Amitiza gel capsule or placebo twice daily •12 week treatment period following a 2 week baseline period •Entry criteria: all patients met Rome II criteria for Constipation-Predominant IBS •To measure relief, patients responded to a weekly question: “How would you rate your relief of IBS symptoms over the past week compared to how you felt before you entered the study?” •7-point scale used to rate relief: “significantly relieved,” “moderately relieved,” “a little bit relieved,” “unchanged,” “a little bit worse,” “moderately worse,” “significantly worse” Endpoint •Primary endpoint was percentage of overall responders in drug and placebo groups •An overall responder was a monthly responder for at least 2 of the 3 months of the study * DrossmanDA, Chey WD, JohansonJF et al, Aliment PharmacolTher2009 Feb;29(3):329-41
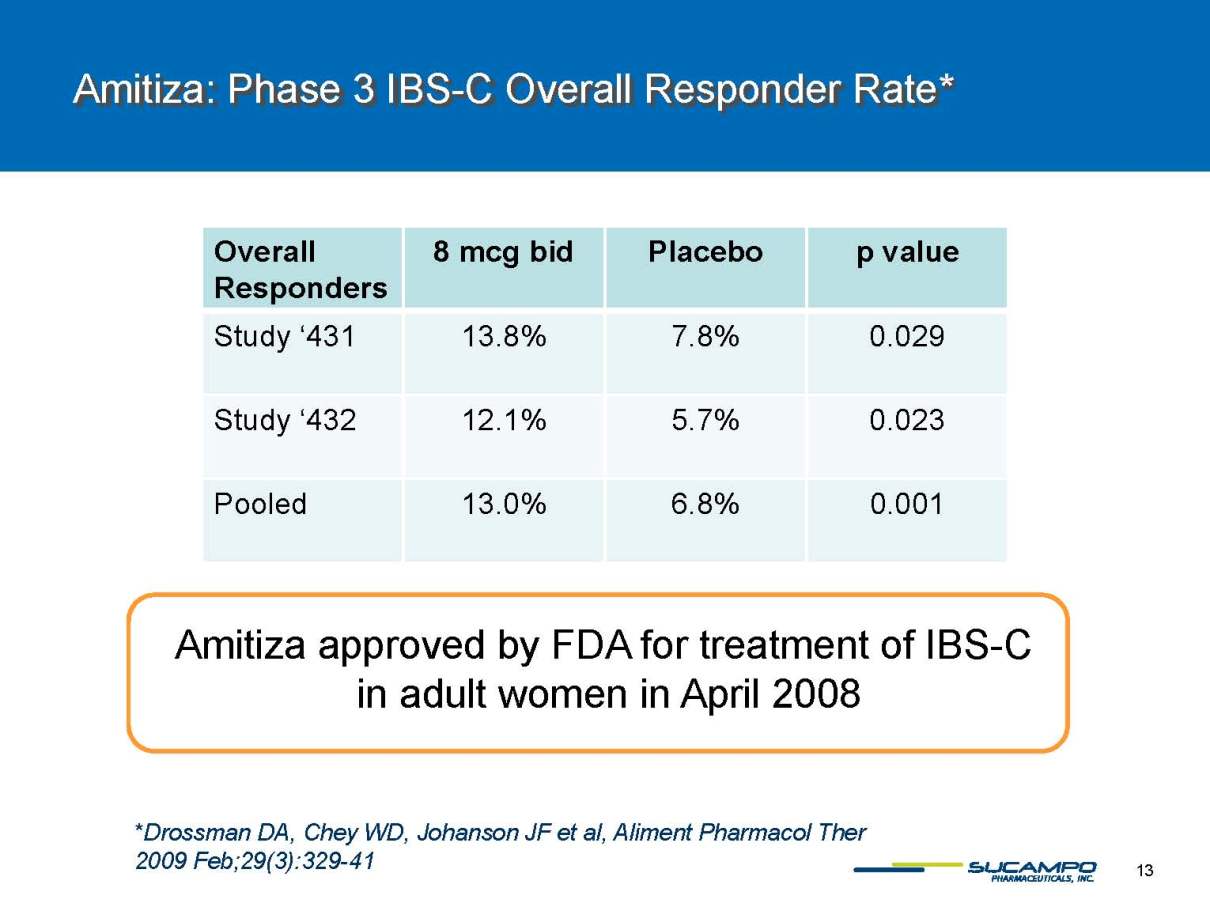
Amitiza: Phase 3 IBS-C Overall Responder Rate* *Drossman DA, Chey WD, JohansonJF et al, Aliment PharmacolTher2009 Feb;29(3):329-41 Amitiza Overall Responders 8 mcg bid Placebo p value Study ‘431 13.8% 7.8% 0.029 Study ‘432 12.1% 5.7% 0.023 Pooled 13.0% 6.8% 0.001 Amitiza approved by FDA for treatment of IBS-C in adult women in April 2008
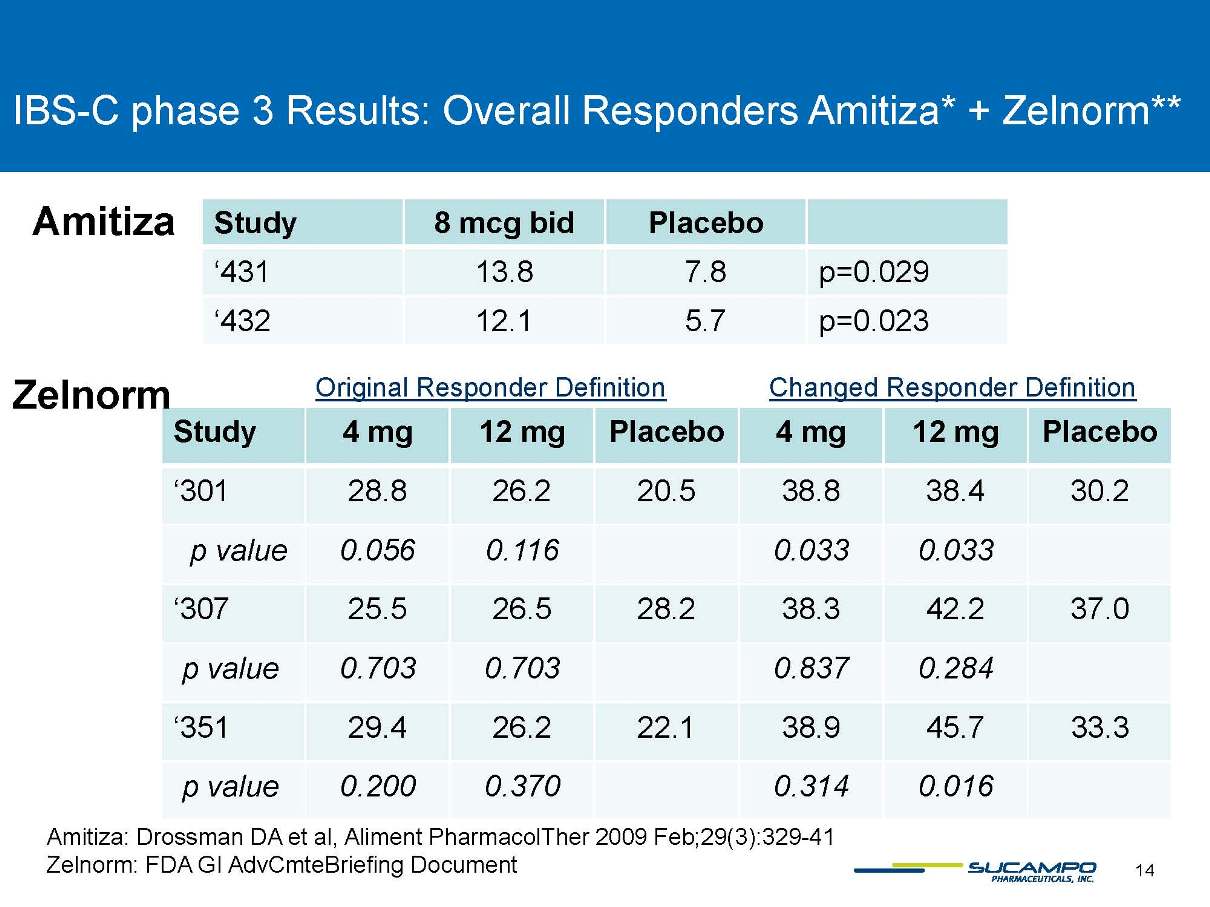
IBS-C phase 3 Results: Overall Responders Amitiza* + Zelnorm** Amitiza: DrossmanDA et al, Aliment PharmacolTher2009 Feb;29(3):329-41 Zelnorm: FDA GI AdvCmteBriefingDocument Study 8 mcg bid Placebo ‘431 13.8 7.8 p=0.029 ‘432 12.1 5.7 p=0.023 Study 4 mg 12 mg Placebo 4 mg 12 mg Placebo ‘301 28.8 26.2 20.5 38.8 38.4 30.2 p value 0.056 0.116 0.033 0.033 ‘307 25.5 26.5 28.2 38.3 42.2 37.0 p value 0.703 0.703 0.837 0.284 ‘351 29.4 26.2 22.1 38.9 45.7 33.3 p value 0.200 0.370 0.314 0.016
Amitiza -Further Opportunities: OBD Management of Opioid-induced Bowel Dysfunction in non-malignant pain (OBD) patients •4.5 million office visits in U.S. annually, seeking relief from OBD •Sucampo conducted two phase 3 trials, one reached statistical significance for primary endpoint •Sucampo to conduct another phase 3 trial, Takeda to share costs •Design of successful Phase 3 trial: •Randomized, placebo-controlled, multi-center trial in 443 OBD patients •One 24-mcg gel capsule of lubiprostone or placebo twice each day •12 week treatment period •Permitted concomitant pain medications included: fentanyl, methadone, morphine and oxycontin •Primary endpoint: change from baseline in SBM frequency at Week 8 without reduction in dose of study pain medication
Amitiza: Data from Successful Phase 3 Pivotal OBD Trial * Management of Opioid-induced Bowel Dysfunction in non-malignant pain (OBD) patients •Reported results phase 3 trial (OBD0631) at DDW 2010 •Patients in ‘631 trial taking lubiprostone achieved a statistically significant (p=0.02) greater increase in the mean number of SBMs per week in 8 of the 12 weeks of the trial as compared to placebo patients •The percentage of patients in ‘631 trial who achieved a SBM within 24 hours and 48 hours was significantly higher with lubiprostone as compared to placebo (p=0.0126 at 24 hours, and p=0.0360 at 48 hours) •Statistical significance was achieved for the overall change from baseline in constipation-associated symptom secondary endpoints in ‘631 trial * DDW 2010, Abstract #780958
Amitiza: Solid Cardiovascular Safety Data* •No clinically significant QTc prolongation was observed when healthy volunteers were administered lubiprostone at a single 24 or 144 mcg dose •No clinically significant QTc prolongation was reported when constipated patients were dosed daily for 3 weeks with varying doses of lubiprostone •These findings indicate that lubiprostone treatment does not increase the risk of TdP associated with QTc prolongation •Cumulative safety information for lubiprostone administered to patients with chronic constipation or irritable bowel syndrome with constipation are consistent * Sprenger C, Copa A, Morganroth J, Panas R, Ueno R. Effect of lubiprostone, a unique agent for the treatment of chronic idiopathic constipation, on clinical electocardiographic results. Gastroenterology 2007; 132(4 Suppl 2): A-3225 [abstract S2136] No serious cardiac adverse events attributable to lubiprostone have been reported to date
Key Terms of Agreements with Takeda •Takeda shall exert best efforts to commercialize and market Amitiza in the U.S. and Canada to maximize net sales of Amitiza–Currently covers two indications: CIC in adults and IBS-C in adult women –Takeda holds right of first refusal to additional GI indications –Takeda records all U.S. sales, Sucampo receives a royalty –Sucampo retains all other rights –Takeda also has rights to Amitiza in Canada, but not yet launched •Sucampo’s tiered royalty rate: 18% to 26% of annual net sales •Sucampo reimbursed for majority of GI clinical development costs •Sucampo has received a total of $150 million in upfront and development milestone payments as of Sept. 30, 2010
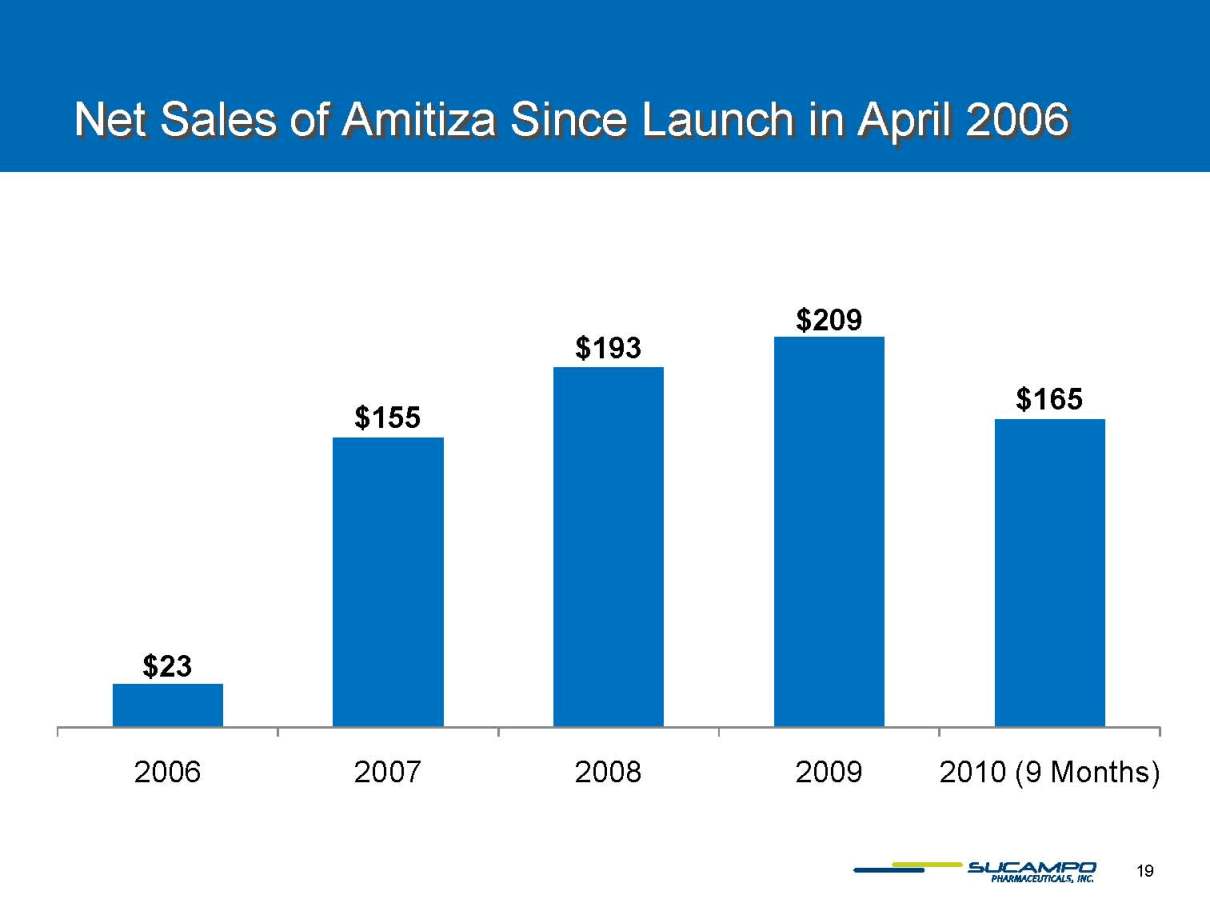
Net Sales of Amitiza Since Launch in April 2006

Amitiza: Agreement with Abbott Japan •A key element in Sucampo’s international growth strategy for Amitiza •Abbott received exclusive rights to commercialize lubiprostone in Japan for CIC, and right of first refusal for additional indications in Japan •If successfully developed, Sucampo will supply finished product to Abbott •Sucampo retains right to co-promote Amitiza in Japan and to develop Amitiza for additional indications •Sucampo has received a total of $22.5 million in upfront and milestone payments from Abbott, as of Sept. 30, 2010 •Sucampo designed and managed the recently reported successful phase 3 efficacy trial and interim data from long-term safety trial in Japanese CIC patients

Amitiza: Future Opportunities in Japan Japanese Phase 3 efficacy trial •Primary efficacy endpoint reached statistical significance (p=0.001)*•Double-blind, placebo-controlled multi-center trial, evaluated 124 patients •Dose: Placebo or lubiprostone 24-mcg soft gel capsule, twice daily, for 28 days•Results filed with Japanese authorities in marketing application (Sept. 2010)Japanese Phase 3 long-term safety trial*•An open-label, multi-center trial with 209 patients•Dose: one lubiprostone 24-mcg gel capsule twice a day for 48 weeks•Interim results through Week 24 of 48-week trial show lubiprostone is safe and well tolerated•Interim safety results included in Japanese marketing application
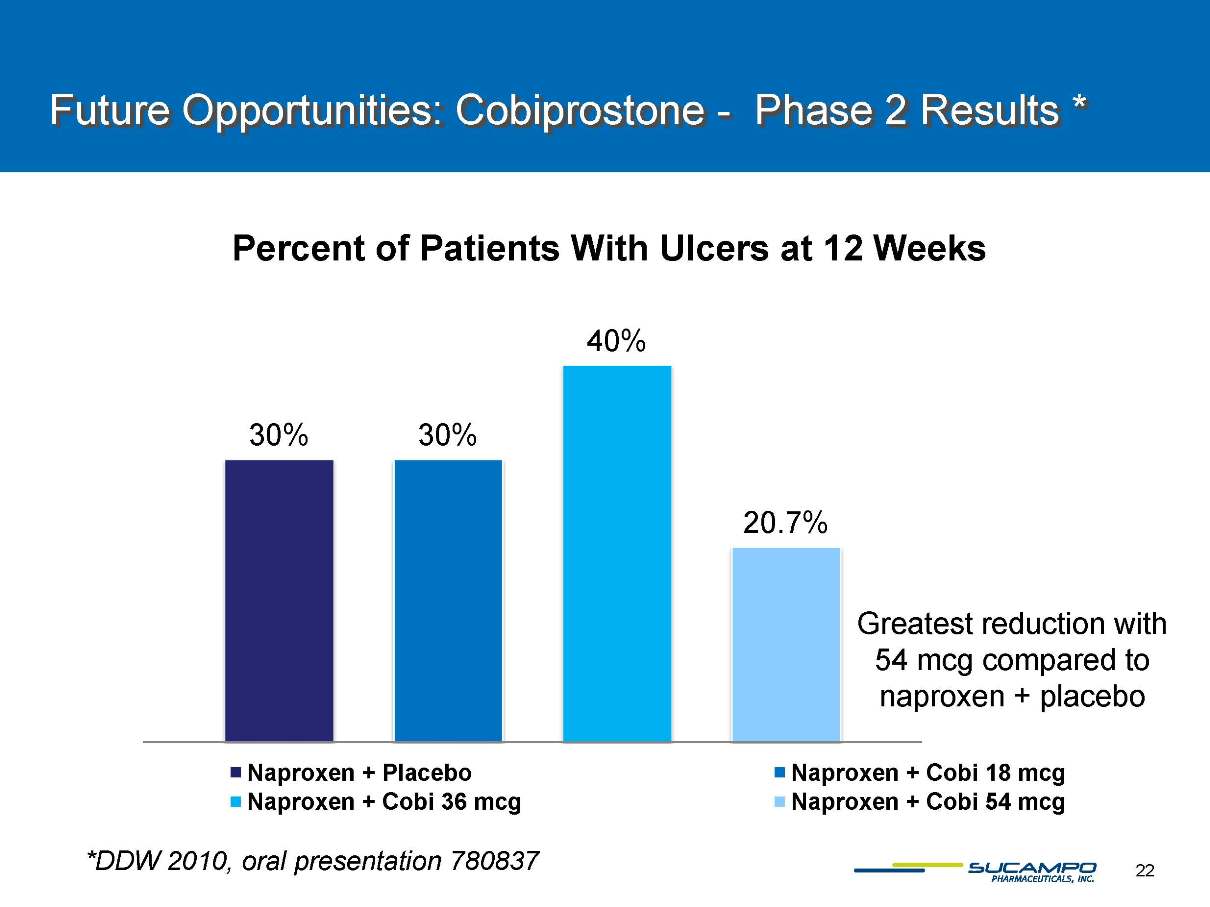
Future Opportunities: Cobiprostone -Phase 2 Results * Percent of Patients With Ulcers at 12 WeeksNaproxen + PlaceboNaproxen + Cobi 18 mcgNaproxen + Cobi 36 mcgNaproxen + Cobi 54 mcgGreatest reduction with54 mcg compared to naproxen + placebo
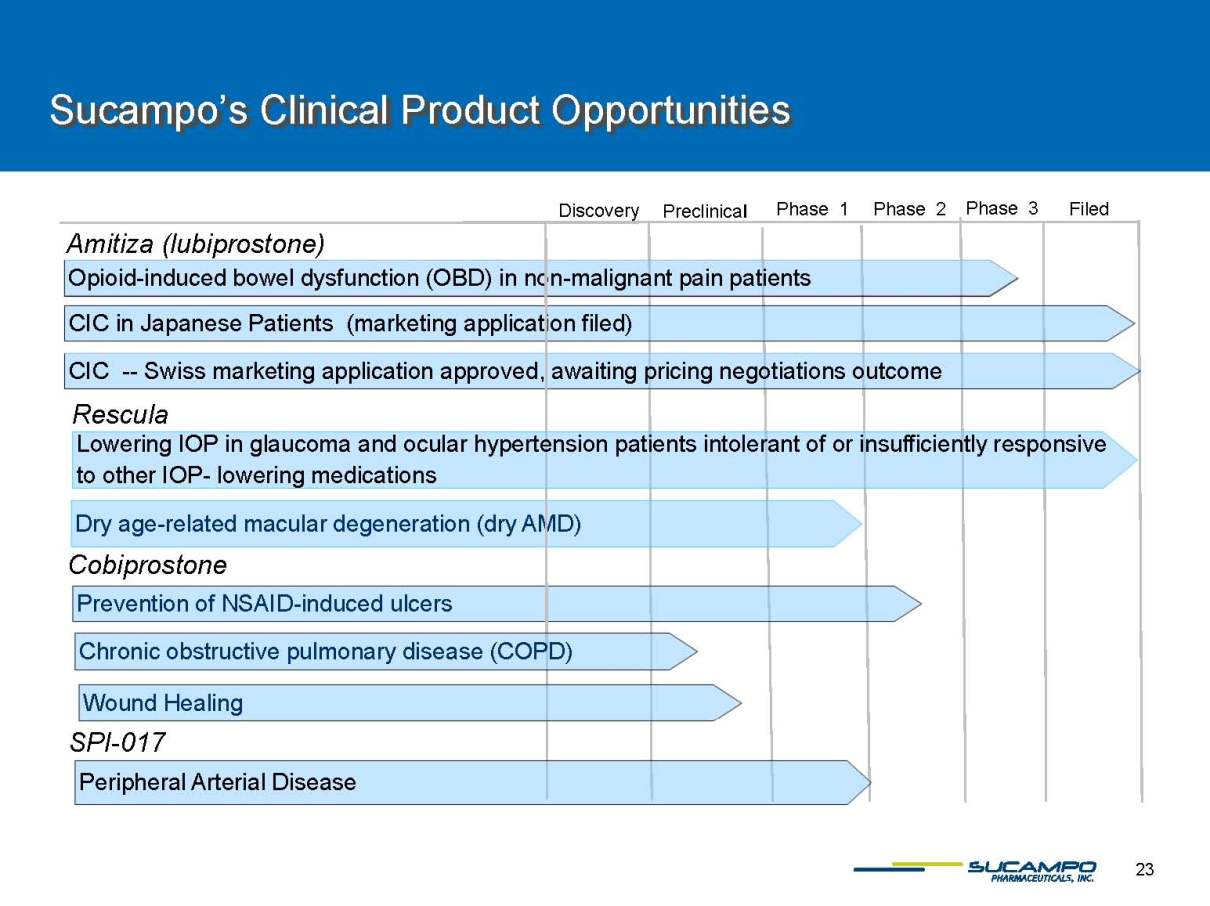
Sucampo’s Clinical Product Opportunities Phase 3 Preclinical Phase 2 Phase 1 Filed Amitiza (lubiprostone) Discovery Opioid-induced bowel dysfunction (OBD) in non-malignant pain patients Cobiprostone SPI-017 Prevention of NSAID-induced ulcers CIC in Japanese Patients (marketing application filed) CIC --Swiss marketing application approved, awaiting pricing negotiations outcome Dry age-related macular degeneration (dry AMD) Rescula Peripheral Arterial Disease Chronic obstructive pulmonary disease (COPD) Wound Healing Lowering IOP in glaucoma and ocular hypertension patients intolerant of or insufficiently responsive to other IOP-lowering medications
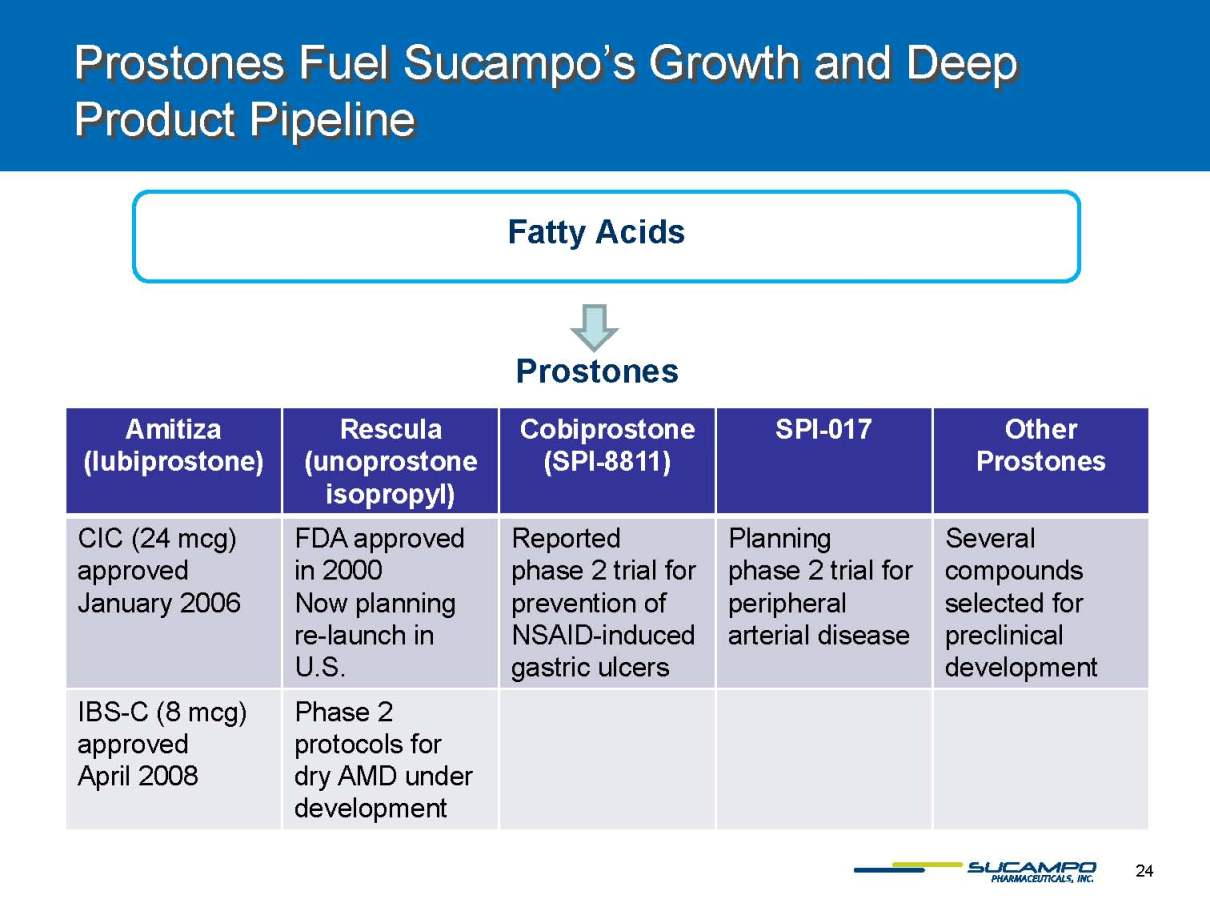
Prostones Fuel Sucampo’s Growth and Deep Product Pipeline Fatty Acids Amitiza (lubiprostone) Rescula (unoprostoneisopropyl) Cobiprostone (SPI-8811) SPI-017 Other Prostones CIC (24 mcg) approved January 2006 FDAapproved in 2000 Now planning re-launch in U.S. Reported phase 2 trial for prevention of NSAID-induced gastric ulcers Planning phase 2 trial for peripheral arterial disease Several compounds selected for preclinicaldevelopment IBS-C (8 mcg) approved April 2008 Phase 2 protocols for dryAMDunder development
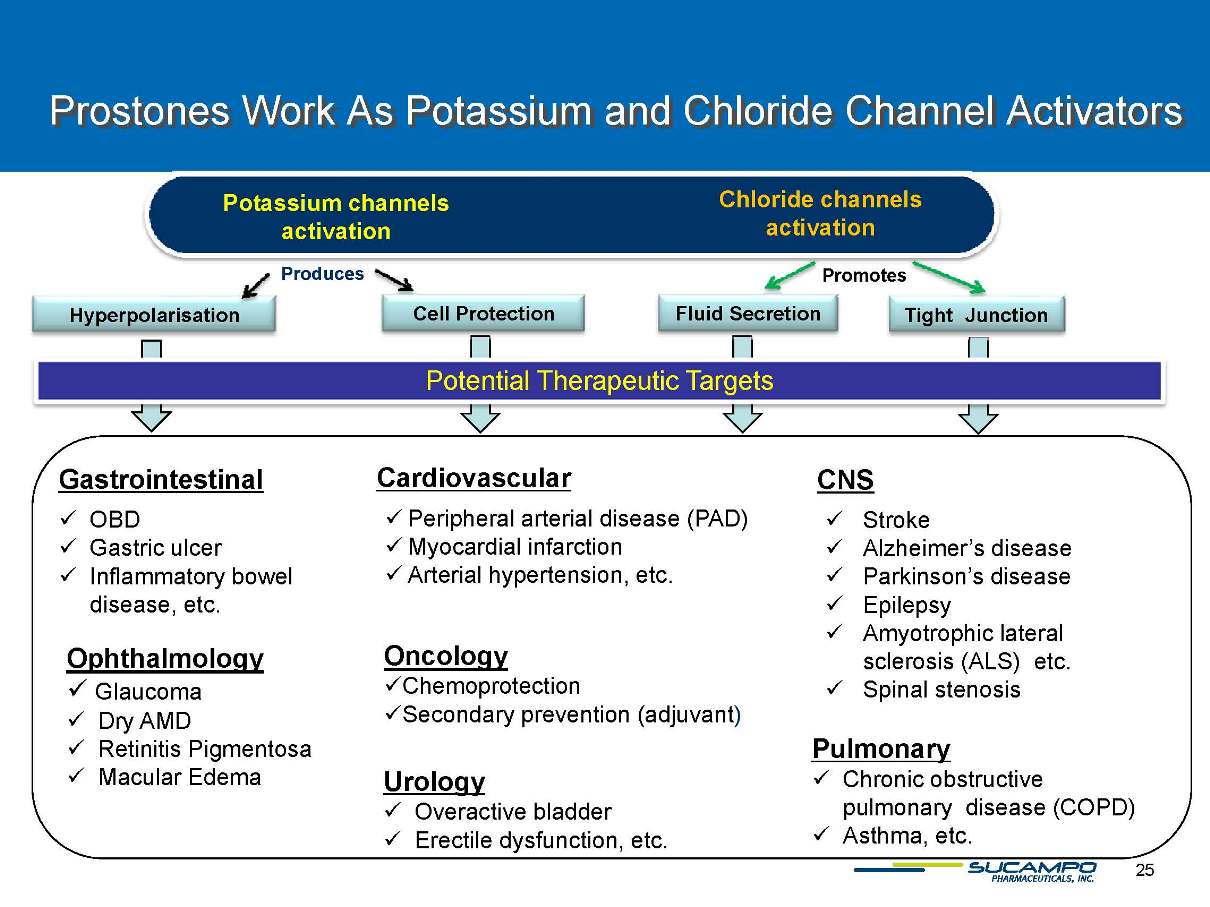
Prostones Work As Potassium and Chloride Channel Activators Chloride channels activation Potassium channels activation Potential Therapeutic Targets Gastrointestinal ��OBD ��Gastric ulcer ��Inflammatory bowel disease, etc. Cardiovascular ��Peripheral arterial disease (PAD) ��Myocardial infarction ��Arterial hypertension, etc. CNS ��Stroke ��Alzheimer’s disease ��Parkinson’s disease ��Epilepsy ��Amyotrophic lateral sclerosis (ALS) etc. ��Spinal stenosis Pulmonary ��Chronic obstructive pulmonary disease (COPD) ��Asthma, etc. Urology ��Overactive bladder ��Erectile dysfunction, etc. Hyperpolarisation Cell Protection Fluid Secretion Tight Junction Produces Promotes Ophthalmology ��Glaucoma ��Dry AMD ��Retinitis Pigmentosa ��Macular Edema Oncology ��Chemoprotection ��Secondary prevention (adjuvant)
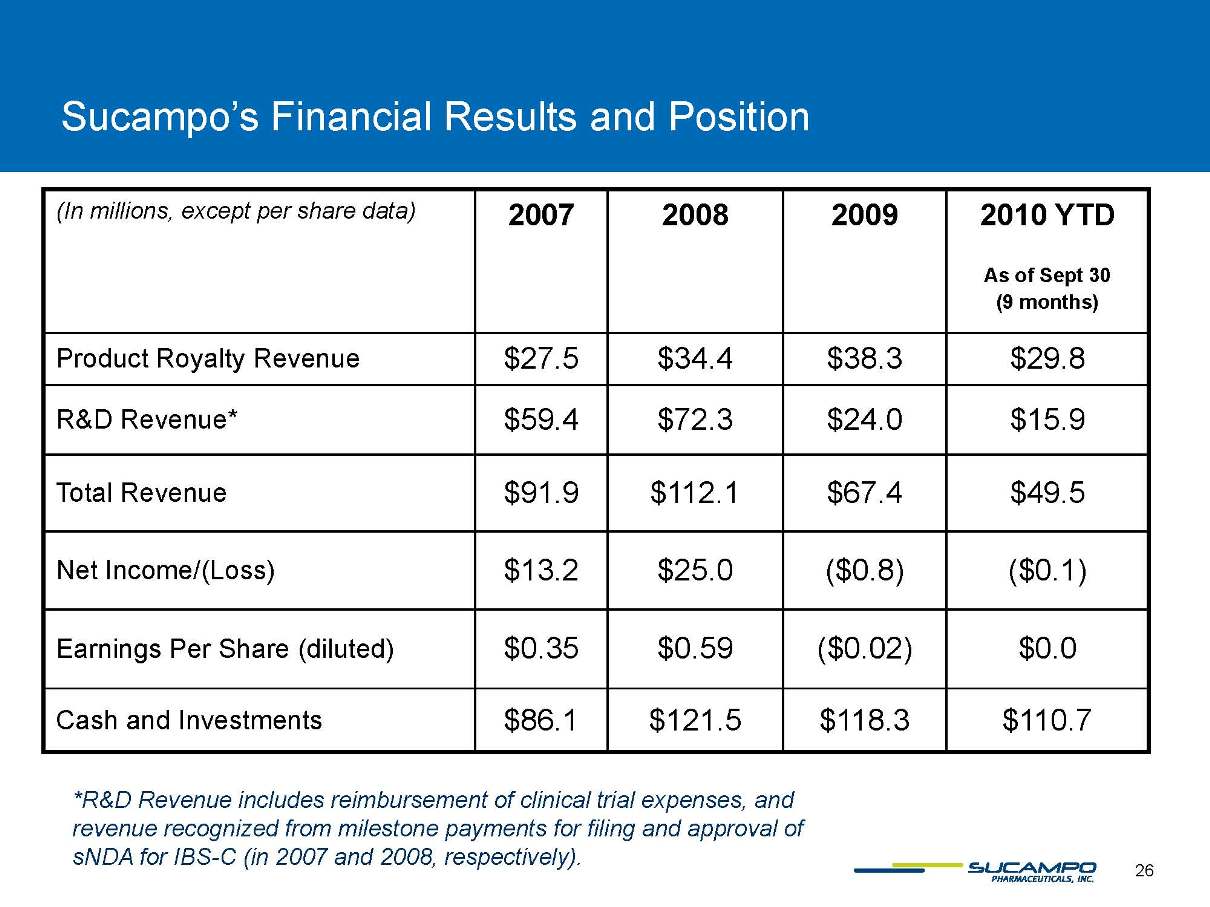
Sucampo’s Financial Results and Position (In millions, except per share data) 2007 2008 2009 2010 YTD As of Sept 30 (9 months) Product Royalty Revenue $27.5 $34.4 $38.3 $29.8 R&D Revenue* $59.4 $72.3 $24.0 $15.9 Total Revenue $91.9 $112.1 $67.4 $49.5 Net Income/(Loss) $13.2 $25.0 ($0.8) ($0.1) Earnings Per Share (diluted) $0.35 $0.59 ($0.02) $0.0 Cash and Investments $86.1 $121.5 $118.3 $110.7 *R&D Revenue includes reimbursement of clinical trial expenses, and revenue recognized from milestone payments for filing and approval of sNDA for IBS-C (in 2007 and 2008, respectively).

Sucampo’s 2010 Milestones √Submit NDA in Japan with results to date of Amitiza in CIC program √Report phase 3 efficacy trial results of Amitiza in Japanese CIC patients √Complete phase 1 trial of SPL-017 for PAD in Japanese patients •Initiate third pivotal phase 3 trial of Amitiza in OBD patients •Initiate phase 2 trial of Rescula in dry AMD •Plan Amitiza’sSwiss commercialization based on outcome of pricing negotiations

Credit Suisse2010 Healthcare Conference James J. Egan Chief Operating Officer November 12, 2010
