Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - CURAXIS PHARMACEUTICAL Corp | asch_8k.htm |
EXHIBIT 99.1

Changing the Trajectory of Alzheimer’s Disease:
Answering a National and Worldwide Imperative
OTCBB: CURX.OB
WEBSITE: www.curaxispharma.com
BLOG: www.blog.curaxispharma.com
WEBSITE: www.curaxispharma.com
BLOG: www.blog.curaxispharma.com

Safe Harbor
This presentation contains “forward-looking statements” within the meaning of the
“safe-harbor” provisions of the Private Securities Litigation Reform Act of 1995.
Such statements involve known and unknown risks, uncertainties and other
factors that could cause the actual results of the Company to differ materially from
the results expressed or implied by such statements, including changes from
anticipated levels of sales, future international, national or regional economic and
competitive conditions, changes in relationships with customers, access to capital,
difficulties in developing and marketing new products and services, marketing
existing products and services, customer acceptance of existing and new products
and services and other factors. Accordingly, although the Company believes that
the expectations reflected in such forward looking statements are reasonable,
there can be no assurance that such expectations will prove to be correct. The
Company has no obligation to update the forward-looking information contained in
this presentation.
“safe-harbor” provisions of the Private Securities Litigation Reform Act of 1995.
Such statements involve known and unknown risks, uncertainties and other
factors that could cause the actual results of the Company to differ materially from
the results expressed or implied by such statements, including changes from
anticipated levels of sales, future international, national or regional economic and
competitive conditions, changes in relationships with customers, access to capital,
difficulties in developing and marketing new products and services, marketing
existing products and services, customer acceptance of existing and new products
and services and other factors. Accordingly, although the Company believes that
the expectations reflected in such forward looking statements are reasonable,
there can be no assurance that such expectations will prove to be correct. The
Company has no obligation to update the forward-looking information contained in
this presentation.
2

Investment Thesis:
As a result of numerous clinical failures of AD drug
candidates based on the prevailing beta amyloid
theory, Curaxis has emerged as a leading candidate
to bring the first disease modifying drug to the multi-
billion dollar AD market.
candidates based on the prevailing beta amyloid
theory, Curaxis has emerged as a leading candidate
to bring the first disease modifying drug to the multi-
billion dollar AD market.
3

The Problem
Washington DC, May 19, 2010, A new report from the Alzheimer’s Association, “Changing the Trajectory of
Alzheimer’s Disease: A National Imperative” shows that in the absence of disease-modifying treatments, the
cumulative cost of care for people with Alzheimer’s from 2010 to 2050 will exceed $20 trillion in today’s dollars.
Alzheimer’s Disease: A National Imperative” shows that in the absence of disease-modifying treatments, the
cumulative cost of care for people with Alzheimer’s from 2010 to 2050 will exceed $20 trillion in today’s dollars.
The new report is not all bad news, however, as it shows that Medicare and Medicaid can achieve dramatic
savings - and lives could be significantly improved - with even incremental treatment improvements.
savings - and lives could be significantly improved - with even incremental treatment improvements.
Harry John’s, President & CEO of the Alzheimer’s Association said, “ Today, there are NO treatments that can
prevent, delay, slow or stop the progression of Alzheimer’s disease. While the ultimate goal is a treatment that
can completely prevent or cure Alzheimer’s, we can see that even modest improvements can have a huge
impact”
prevent, delay, slow or stop the progression of Alzheimer’s disease. While the ultimate goal is a treatment that
can completely prevent or cure Alzheimer’s, we can see that even modest improvements can have a huge
impact”
Reuters, June 12, 2010, Drug Makers to Share Data to Speed Brain Research, in an unprecedented
announcement major drug makers will share data from their clinical trials for Alzheimer's and Parkinson’s disease.
Despite decades of study, and billions of dollars of research efforts, doctors still have few effective treatments for
Alzheimer's disease.
announcement major drug makers will share data from their clinical trials for Alzheimer's and Parkinson’s disease.
Despite decades of study, and billions of dollars of research efforts, doctors still have few effective treatments for
Alzheimer's disease.
NY Times, July 13, 2010, Rules Seek to Expand Diagnosis of Alzheimer’s, for the first time in 25 years, medical
experts are proposing a major change in the criteria for Alzheimer’s disease, part of a new movement to diagnose
and, eventually, treat the disease early. If the guidelines are adopted in the fall, as expected, some experts predict
a two- to threefold increase in the number of people with Alzheimer’s disease. Many more people would be told
they probably are on their way to getting it. The changes could also help drug companies that are, for the first
time, developing new drugs to try to attack the disease earlier. So far, there are no drugs that alter the course of
the disease.
experts are proposing a major change in the criteria for Alzheimer’s disease, part of a new movement to diagnose
and, eventually, treat the disease early. If the guidelines are adopted in the fall, as expected, some experts predict
a two- to threefold increase in the number of people with Alzheimer’s disease. Many more people would be told
they probably are on their way to getting it. The changes could also help drug companies that are, for the first
time, developing new drugs to try to attack the disease earlier. So far, there are no drugs that alter the course of
the disease.
4

The Answer - www.curaxispharma.com
Watch
Video
Investor
Info
Curaxis’s - FDA Phase II Success
Vp4896 Implant
5

Investment Highlights
Huge Market
By 2050, cumulative care costs could top $20 trillion
Large Unmet Medical Need
Current predominant theory has consistently failed
Unique Solution thru Phase II and Patented
Disease modifying & dual therapy solution
Shortened Time to Commercialization
20 year safety profile & limited research needed
6
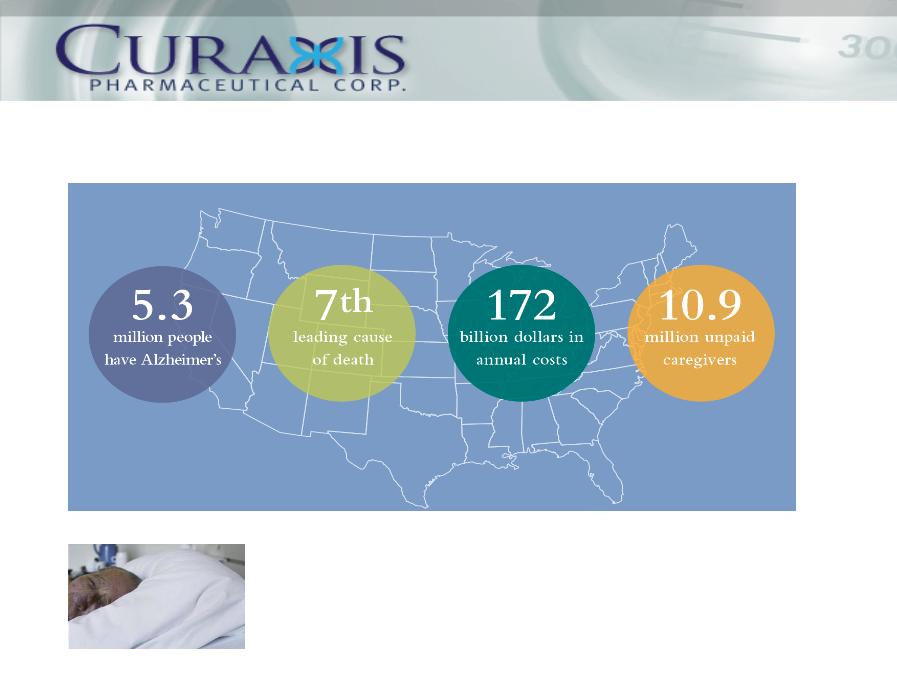
The Facts and Financial Impact
• World Wide Impact
• Significant Dollars!
• Significant People!
7
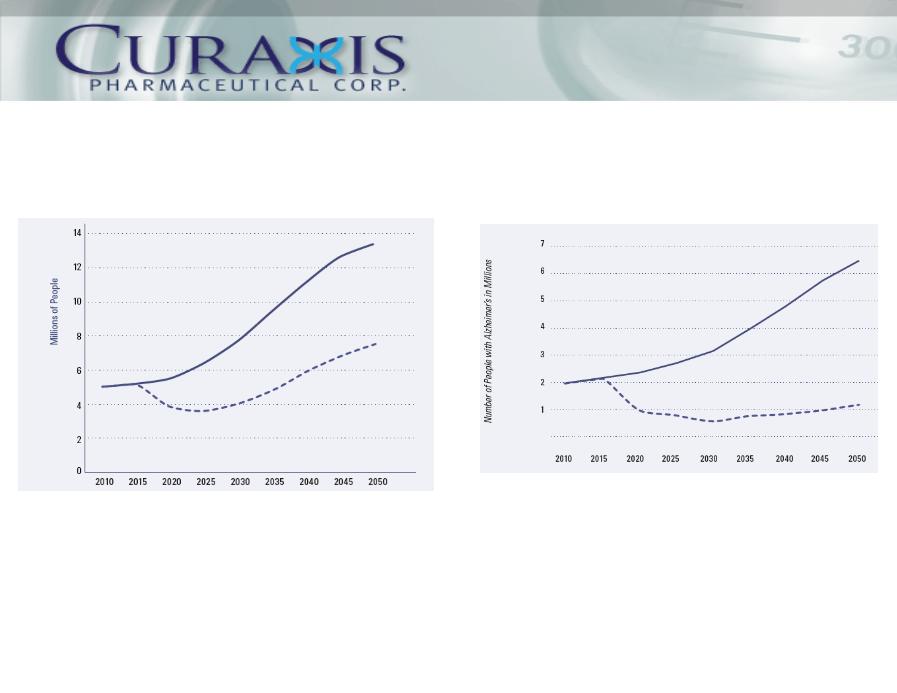
Human Impact - Delayed Disease Onset
In 2011, the first baby boomers will reach their 65th birthday. This group, totaling an estimated
70 million will have significant impact on the US healthcare system.
70 million will have significant impact on the US healthcare system.
On top of the costs paid by Medicare, in excess of $100 Billion, it is estimated that 11 million
unpaid caregivers (relatives, family, friends) spent over 12.5 billion hours in care giving with an
economic value greater than $143.9 billion in 2009. The mental and emotional impact
associated with this devastating disease is beyond estimation.
unpaid caregivers (relatives, family, friends) spent over 12.5 billion hours in care giving with an
economic value greater than $143.9 billion in 2009. The mental and emotional impact
associated with this devastating disease is beyond estimation.
Americans Age 65 and Older with Alzheimers (*)
Impact of Slowed Progression on
Severe Stage Americans Age 65 & Older
Severe Stage Americans Age 65 & Older
(*) Assumes remedy is introduced in 2015
____ Current Trajectory _ _ _ _ Delayed Progression
8

◆ Predominant scientific theory failing all efforts
◆ 6/19/2010 - in an unprecedented move major pharma companies
announced they will share research data as a last effort
announced they will share research data as a last effort
◆ Medivation (MDVN): $1.5 billion
market cap before data came out
market cap before data came out
◆ Neurochem: $750mm market cap
before Phase III failed (Phase II also
weak)
before Phase III failed (Phase II also
weak)
◆ Myraid: $1.2mm market cap before
Phase III failed
Phase III failed
$1.5B Market Cap
Pfizer invests
$200mm plus
Next . . . The Curaxis Solution
Reverse Merger
Current Status of Science & Market
9

The Curaxis Solution: Memryte
u First in class
u Small, proprietary implant
u Comprised of leuprolide acetate and
biodegradable polymer
biodegradable polymer
u Both leuprolide acetate and polymer have
long safety histories in humans
long safety histories in humans
u Simple procedure is conducted in physician’s office in roughly
10 minutes
10 minutes
u Dosed every eight weeks - assures compliance
u Easy, low cost manufacturing scale-up
10
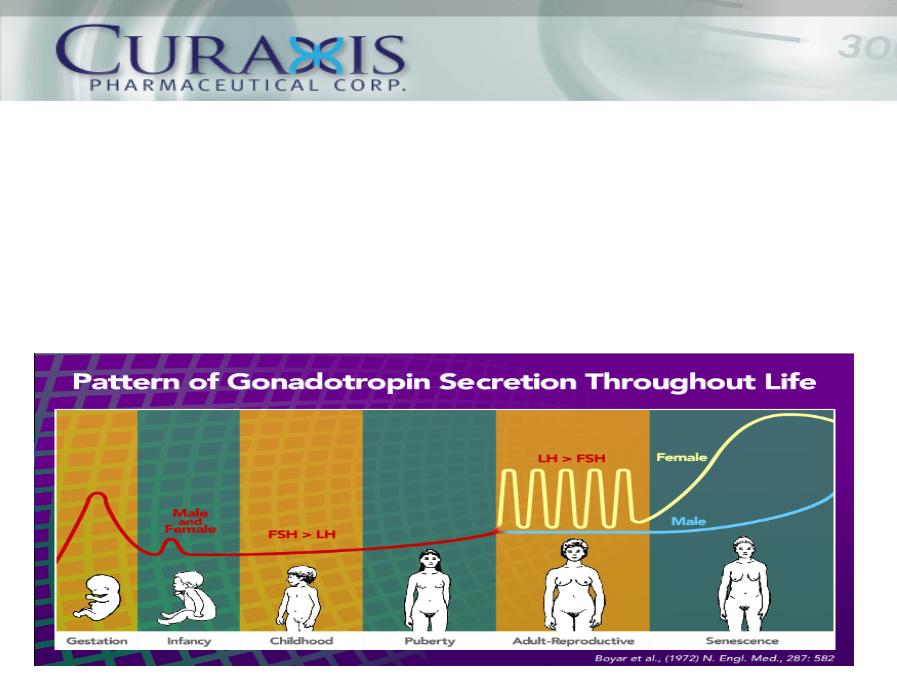
◆ Elevated Levels of luteinizing hormone (“LH”), released by pituitary
gland, associated with pathologies to lead to AD.
gland, associated with pathologies to lead to AD.
◆ Leuprolide acetate lowers LH and associated pathologies.
◆ Leads to halting of cognitive decline or improvement.
The Curaxis Solution: How it Works
11

Phase I Trial
◆ Confirming the positive release profile of the implant.
Phase II Clinical Study
◆ Results in women treated with Leuprolide and ACHEI’s show statistical
significance on primary endpoints.
significance on primary endpoints.
◆ Women represent approximately 70% of the Alzheimer’s market.
Phase III Clinical Study
◆ Study was terminated early due to financial constraints.
◆ Favorable trends were apparent on the efficacy outcome measures of the
ADAS-cog and ADCS-ADL.
ADAS-cog and ADCS-ADL.
◆ Study confirms positive signal of Phase II trial.
No further “pre-clinical” research needed
The Clinical Studies Completed
12

Phase IIb Clinical Study ($15 mm in Approximate Costs)
◆ Females Only, 200-250 patients
◆ Approximately 12 months duration, 1 active arm; 1 control arm
Phase III Clinical Study ($50mm in Approximate Costs)
◆ Recent failures in a number of Phase III studies of AD drug candidates (ie.
Neurochem, Myriad Genetics, Sanofi Aventis and Medivation (NASDAQ: MDVN))
have cast significant doubt on the Beta Amyloid hypothesis and increased receptivity
to our approach to treating Alzheimer's.
Neurochem, Myriad Genetics, Sanofi Aventis and Medivation (NASDAQ: MDVN))
have cast significant doubt on the Beta Amyloid hypothesis and increased receptivity
to our approach to treating Alzheimer's.
Go to Market
Application to Other Diseases
◆ Extensive preclinical research has been conducted to treat a number of cancers,
including hormone refractory prostate cancer, brain cancers, kidney cancer,
pancreatic cancer and non-small-cell lung cancer.
including hormone refractory prostate cancer, brain cancers, kidney cancer,
pancreatic cancer and non-small-cell lung cancer.
◆ We have demonstrated a potentially important new therapy for the treatment of a
number of cancers.
number of cancers.
The Clinical Studies Needed & Resources
13

Curaxis’ Phase II Data Compared to
Namenda’s Basis for Approval
Namenda’s Basis for Approval
|
|
Drug Type
|
Mean Diff. vs. Placebo
in ADCS-ADL |
Treatment
Period |
|
Curaxis
|
Leuprolide acetate
|
6.3
|
48 weeks
|
|
Curaxis
|
Leuprolide acetate
|
3.8
|
24 weeks
|
|
Namenda
|
NMDA antagonist
|
1.6
|
24 weeks(1)
|
Source: Namenda package insert
(1) Includes patients with moderate-to-severe Alzheimer’s disease on donepezil
14

Our Business Strategy
¨ Advance Memryte through pivotal Phase III clinical trials to
commercialization.
commercialization.
¨ Maximize value of Memryte through specialty
pharmaceutical business model.
pharmaceutical business model.
¨ Establish domestic sales force targeting high prescribing
neurologists and GPs.
neurologists and GPs.
¨ Maximize return-on-investment by outsourcing non-value
added functions.
added functions.
¨ Leverage market presence of ACIs to enhance adoption of
Memryte.
Memryte.
15

Anticipated Development Timeline & Milestones
Initiate Phase IIB Trial in Women
Continue Phase IIB Trial in Women
Complete Phase IIB Trial in Women
Enroll Phase III Trials
Conduct Patient Treatment
Complete Phase III Trials
Submit NDA
Anticipate FDA Approval
Planned Commercial Launch
2010 2011 2012 2013 2014 2015
16

“Mr. and Mrs. K from Florida heard about Curaxis in the news 8 years ago and pushed
their doctor to give it a try. Mr. K has been cognitively stable as a mild-to moderate
patient for 8 years. He is currently 84, capable of taking care of himself and carrying
on conversations. Mr. K knows his children and grandchildren. Typically , without the
trial treatment, Mr. K would have been hospitalized 5 years ago.”
their doctor to give it a try. Mr. K has been cognitively stable as a mild-to moderate
patient for 8 years. He is currently 84, capable of taking care of himself and carrying
on conversations. Mr. K knows his children and grandchildren. Typically , without the
trial treatment, Mr. K would have been hospitalized 5 years ago.”
“Mr. B, whose brother is a long time supporter of the Company, contacted Curaxis
several years ago. He began therapy using our platform after convincing his physician
of the scientific merit of our treatment. Mr. B has been treated for 6 years and has
suffered no decline in his cognitive abilities. Mr. B has continued to maintain an active
life and social interactions.”
several years ago. He began therapy using our platform after convincing his physician
of the scientific merit of our treatment. Mr. B has been treated for 6 years and has
suffered no decline in his cognitive abilities. Mr. B has continued to maintain an active
life and social interactions.”
“Mr. P was originally enrolled in our Phase III trial. His improvement was impressive.
Upon termination of the trial, he and his wife were devastated, and sought therapy
through his physician. After the trial ended and prior to re-instating his therapy
Upon termination of the trial, he and his wife were devastated, and sought therapy
through his physician. After the trial ended and prior to re-instating his therapy
(approximately 6 months) his condition worsened. Upon re-instatement of the therapy,
his cognitive skills returned to previous levels. Mr. P has been stable for almost 5 years.
Mr. and Mrs. P are now shareholders!”
his cognitive skills returned to previous levels. Mr. P has been stable for almost 5 years.
Mr. and Mrs. P are now shareholders!”
Personal Stories
17

Experienced Management
Patrick S. Smith - President, CEO and Chairman of the Board
◆Co-Founder who has lead Curaxis team since inception in 2001.
◆Founder of Critical Care America, an alternative healthcare company.
◆35+ years experience as leader in healthcare/drug development industry.
David J. Corcoran - CFO and Executive Vice President
◆Co-founder who has strategically directed the operations of Curaxis since inception
◆Co-founder of MetNet Affiliates, a provider of office-based orthopedic services.
◆General Counsel to Critical Care America.
◆35+ years experience in healthcare/drug development industry.
Judith S. T. Geaslen - VP Finance
◆Active manager within the Curaxis team since 2004.
◆10+ years experience with internationally recognized public accounting firm.
◆Served as Corporate Controller for large financial holding company.
◆20+ years experience in the accounting and financial services profession.
18

Experienced Directors
Note: Curaxis intends to add additional independent directors to the Board as certain milestones are met.
|
Patrick S. Smith
|
Chairman of the Board
|
◆ President and CEO
|
|
David J. Corcoran
|
Secretary
|
◆ Executive Vice President and CFO
|
|
Sheldon L. Goldberg
|
Director
|
◆ Former Senior Vice President of Corporate
Development for Curaxis. ◆ Former CEO of the Alzheimer's Association.
|
|
Father William E. McConville
|
Director
|
◆ Member of the Order of Friars Minor and former
Professor of Religious Studies at Sienna College. ◆ Served as a member of the board of directors and
board of trustees for various educational and charitable institutions. |
|
Ronald V. Pompeo
|
Director
|
◆ Long-time shareholder of Curaxis.
◆ President and CEO of general construction
contractor based in Weymouth, Massachusetts. |
|
Bert A. Spilker, PhD, MD,
FCP, FFPM
|
Director
|
◆ Independent consultant to Curaxis who has
worked in clinical trial design, drug development and FDA matters for over 30 years. ◆ Most recently the Senior Vice President of
Scientific and Regulatory Affairs for PhRMA. (Pharmaceutical Research and Manufacturers of America) |
19

Experienced Advisors
|
Tommy J. Thompson
|
Advisor to the
Chairman |
◆ Former Secretary of the Department of Health and Human Services
and former Governor of the State of Wisconsin. ◆ Partner in Washington D. C. Law firm.
|
|
Mark Smith, PhD, FRCPath
|
Key Consultant
|
◆ Professor of Pathology and Director of Basic Science Research of
the University Memory and Cognition Center, Case Western Reserve University. ◆ Editor-in-Chief, Journal of Alzheimer’s Disease.
◆ Executive Director, American Aging Association.
◆ Distinguished as one of top 3 in world by peers in Alzheimer’s
Disease Researchers |
|
Kevin Keim, PhD., MSc.
|
Key Consultant
|
◆ Chief Development Officer, INC Research
◆ 40 years of research and discovery, clinical research and regulatory
experience. |
|
Chengjie Xiong
|
Key Consultant
|
◆ Research Associate Professor of Biostatistics at Washington
University School of Medicine, Department of Biostatistics. |
|
Felix Theeuwes, DSc
|
Manufacturing
Partner |
◆ Chairman, Co-Founder and Chief Scientific Officer of Durect
Corporation |
|
George Perry
|
Key Consultant
|
◆ Dean and Professor of Biology, University of Texas at San Antonio
◆ Distinguished as one of top 5 in the world by peers in Alzheimer’s
Disease Researchers with over 800 publications. |
|
Christopher W. Gregory,
PhD |
Key Consultant
|
◆ Senior Director, Clinical Development, Clinsys Clinical Research.
◆ Former Vice President of Research, Curaxis
|
20

The Simple Math:
Although existing Alzheimer’s drugs are ineffective for an extended period they still sell $5 billion per year!
Although existing Alzheimer’s drugs are ineffective for an extended period they still sell $5 billion per year!
|
Company
|
Drug
|
FDA Approval
|
Sales - First Year
|
Sales - Most
Recent Fiscal Year |
|
Forest Labs
|
Namenda®
(memantine)
|
October 2003
|
$333 million
|
$1.1 billion
|
|
Ortho-McNeill
Neurologics |
Razadyne®
(galantamine)
|
February 2001
(patent expired
12-28-08) |
$ ? million
|
$130 million
|
|
Novartis
|
Exelon®
(rivastigmine)
|
April 2000 (Oral)
July 2007(Patch)
|
$367 million
|
$954 million
|
|
Eisai
|
Aricept®
(donepezil)
|
November 1996
|
$180 + million
|
$3.5 billion
|
Note: Per the 2004 Medicare Current Beneficiary Survey, the Average Annual Per Person Payments for Healthcare and Long-Term Cares
Services with Alzheimer’s totaled $33,007 compared to $10,603 per beneficiary with no Alzheimer's or reported Dementia.
Services with Alzheimer’s totaled $33,007 compared to $10,603 per beneficiary with no Alzheimer's or reported Dementia.
|
Curaxis
|
Memrtye
(leuprolide
acetate) |
Estimated: 2015
|
$5 Billion
|
Assumes 1 million
users at $5,000 per year |
21

Capital Structure:
|
|
Shares
|
%
|
|
Officers & Directors
|
16,425,064
|
23%
|
|
Other Insiders (5% owners)
|
10,162,560
|
14%
|
|
Other (Lockup for 12 months from merger)
|
29,060,395
|
40%
|
|
Public Float
|
16,238,279
|
23%
|
|
TOTAL SHARES OUTSTANDING
|
71,886,298
|
100%
|
|
|
|
|
|
Current Stock Price ($1.10) x Total Shares
|
$79,074,927
|
|
22

Mean $4.3 billion
Price $59.95
23

Medivation
(MDVN)
(MDVN)
Mkt Cap
($’s in
mm’s):
($’s in
mm’s):
◆Big-Pharma in desperate search of new solution -
◆ 8/2010 - Alectose Therapeutics - Merck to invest up to $289mm pre - Phase I
◆Neurochem: $750mm mkt pre Phase III failure(Phase II clinical data also weak)
◆Myraid: $1.2 billion market cap before Phase III failed
Public Merger
$50mm
Mkt Cap
Pfizer Invests
$200mm-$500mm
$200mm-$500mm
Mkt Cap
Market Cap grows
to $1.5b on
questionable results
questionable results
Fact that Clinical trials
were conduct in Russia
always questionable
were conduct in Russia
always questionable
24

IN THE NEWS:
ØREUTERS - With the failures of all Beta-
Amyloid Phase IIIs’, in a unprecedented
event Big-Pharma announce plan to share
results.
Amyloid Phase IIIs’, in a unprecedented
event Big-Pharma announce plan to share
results.
Ø BIOWORLD - “No 1 public health
challenge of 21st Century”
challenge of 21st Century”
Ø BUSINESS JOURNAL- Quintles takes
stake in Australian Co to help fund
Alzheimers initiative
stake in Australian Co to help fund
Alzheimers initiative
Ø 10/20- BLOOMBERG - Big-Pharma in
desparate need of “Pipeline”-Amylin, Lilly,
Alkermes Shares Fall as FDA reject
Diabetes Drug.
desparate need of “Pipeline”-Amylin, Lilly,
Alkermes Shares Fall as FDA reject
Diabetes Drug.
Ø 10/19 - NY TIMES -Alzheimers hits
Front Page
Front Page
Market Awareness - Key to Next Steps:
CURAXIS - IN THE NEWS:
ØSmith “For 10 years we’ve maintained
that Beta Amyloid is a symptom not a
cause”
that Beta Amyloid is a symptom not a
cause”
Ø Announces addition of Bert Spiker to the
Board of Directors
Board of Directors
Ø Announces funding commitment from
Southridge for Phase IIB clinical testing
Southridge for Phase IIB clinical testing
Ø Announces appointment of Tommy
Thompson as Special Advisor
Thompson as Special Advisor
Ø Investor & Public Relations Calendar
Continuously Updated
Continuously Updated
25

• Current Shareholder Presentation/Updates and Referrals
• Dedicated IR Managers - Giannini/Robinson(Southridge) Tag Team
• Road shows - US and European
• Press Releases
• Trader and Market Maker Awareness
• Direct Mailers
• Message Boards/Online Presence Monitoring -Search Engines
• Social Media Programs -Facebook, Twitter, LindedIn
• Corporate Profiling Websites
• Mainstream and Industry Specific Media Exposure ( PR Agency
Provided By Southridge)
Provided By Southridge)
Market Awareness Campaigns:
26
