Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a10-15566_18k.htm |
Exhibit 99.1
|
|
Developing new Treatments for GI Disorders and Diseases Gary S. Jacob, Ph.D. President & CEO August 13, 2010 |
|
|
Certain statements made in this presentation are forward-looking. Such statements are indicated by words such as “expect,” “should,” “anticipate” and similar words indicating uncertainty in facts and figures. Although Synergy believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such expectations reflected in such forward-looking statements will prove to be correct. As discussed in the Synergy Pharmaceuticals Annual Report on Form 10-K for the year ended December 31, 2009, and other periodic reports, as filed with the Securities and Exchange Commission, actual results could differ materially from those projected in the forward-looking statements as a result of the following factors, among others: uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate efficacy in larger-scale clinical trials, the risk that Synergy will not obtain approval to market its products, the risks associated with dependence upon key personnel and the need for additional financing. Forward-Looking Information |
|
|
OVERVIEW Symbol & Exchange: SGYP (OTCBB) www.synergypharma.com Proprietary Technology – GC-C Receptor Agonists Lead Drug – Plecanatide (SP-304) in Phase II trial to treat GI Disorders Experienced Management Team Headquartered in New York, NY; Research lab in Doylestown, PA More than $23 million raised last 24 months for operations |
|
|
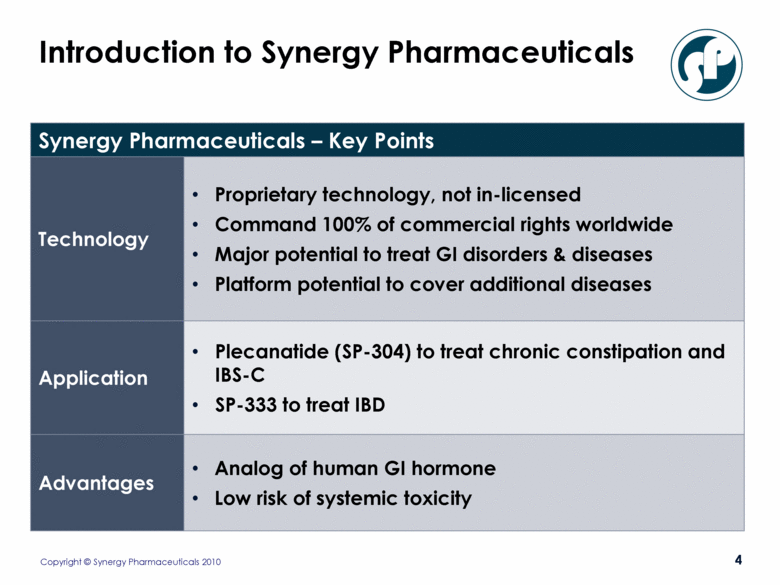
Introduction to Synergy Pharmaceuticals Synergy Pharmaceuticals – Key Points Technology Proprietary technology, not in-licensed Command 100% of commercial rights worldwide Major potential to treat GI disorders & diseases Platform potential to cover additional diseases Application Plecanatide (SP-304) to treat chronic constipation and IBS-C SP-333 to treat IBD Advantages Analog of human GI hormone Low risk of systemic toxicity |
|
|
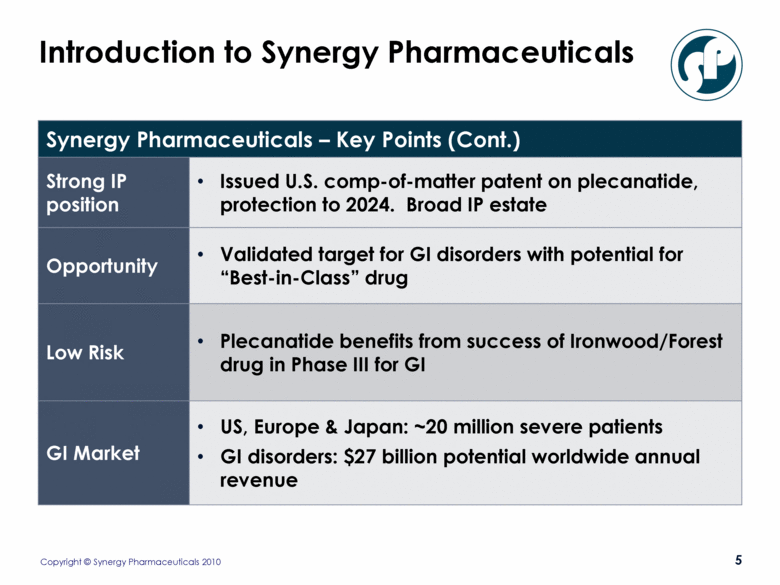
Introduction to Synergy Pharmaceuticals Synergy Pharmaceuticals – Key Points (Cont.) Strong IP position Issued U.S. comp-of-matter patent on plecanatide, protection to 2024. Broad IP estate Opportunity Validated target for GI disorders with potential for “Best-in-Class” drug Low Risk Plecanatide benefits from success of Ironwood/Forest drug in Phase III for GI GI Market US, Europe & Japan: ~20 million severe patients GI disorders: $27 billion potential worldwide annual revenue |
|
|
GI Disorders & Diseases Significant Market Opportunity |
|
|
Significant Unmet Medical Need Hard and lumpy stools, straining during defecation, a sensation of incomplete evacuation, and fewer than three bowel movements per week Significantly affects patients’ quality of life by impairing their ability to work and participate in typical daily activities Limited available treatment options Chronic Constipation (CC) Chronic abdominal pain and discomfort associated with altered bowel habits Patients can be affected physically, psychologically, socially, and economically Limited available treatment options Constipation-Predominant Irritable Bowel Syndrome (IBS-C) Affected segment of large intestine becomes inflamed and develops ulcers, causing symptoms that include bloody diarrhea, abdominal pain, and fever Limited available treatment options Ulcerative Colitis (UC) GASTROINTESTINAL DISORDERS INFLAMMATORY BOWEL DISEASES (IBD) |
|
|
The Market for a GI-Disorder Drug 35 million to 46 million people in U.S. suffer from CC and IBS-C >100 million people worldwide Severe condition: 20 million in US, Europe & Japan High prevalence: approx. 15% of U.S. population suffer from CC Estimate 6 million to 8.5 million patients in U.S. with CC sought medical care in 2007 More than $2 Billion in revenue* Synergy potential SOURCE: 2007 U.S. census data; Johanson & Kralstein, Aliment Pharmacol Ther, 25, (2007); Brandt et. al., Am. J. Gastroenterology, 100, (2005): Johanson & Kralstein, and Choung et. al., Aliment Pharmacol. Ther., 25, (2007) * Assumes conservative (4.8%) market share by 2021, and based on similar pricing to present-day branded drug |
|
|
Major “Game–Changing” Events: Marketed by Novartis 5-HT4 agonist withdrawn from market in spring 2007 due to cardio-toxicity $560 M sales in 2006, worldwide 32% growth, year on year Approved for chronic constipation and IBS-C Major impact on all future 5-HT4 agonist candidates Highlights need for new non-toxic approach to treat GI disorders Zelnorm® (tegaserod) Lotronex® (alosetron) for IBS-D & Propulsid® (cisapride) for GERD withdrawn from market due to safety concerns Source: Novartis annual reports, analyst reports |
|
|
Competitive Landscape Amitiza® (lubiprostone) Marketed by Sucampo/Takeda Chloride C-2 channel activator Approved for CC in 2006; IBS-C in 2008 $200 million annual sales in 2008 Main side effect is nausea Approx. 30% of patients Sets high price for market ~$3.80 / day retail -$3.35 / day ex-factory Source: Sucampo & Takeda annual reports, analyst reports, Movetis annual report, press releases Only Product Presently Approved in U.S. Resolor® (prucalopride) Marketed by Movetis NV 5-HT4 agonist Approved in Oct. 2009 in Europe to treat CC in women Estimated potential annual peak sales of over EUR300 million Main reported side effects: diarrhea, headache, nausea Product Recently Approved in Europe |
|
|
Linaclotide First-in-Class GC-C Agonist Co-developed by Ironwood/Forest Laboratories First-in-class GC-C receptor agonist Developed for both CC and IBS-C Currently finishing Phase III IBS-C registration trials Projected NDA filing – middle of 2011* Potential launch in 2012* *Source: Ironwood 2Q2010 Investor update, analyst reports Competitive Landscape Only late-stage drug currently in development in U.S. |
|
|
GC-C Receptor Agonists |
|
|
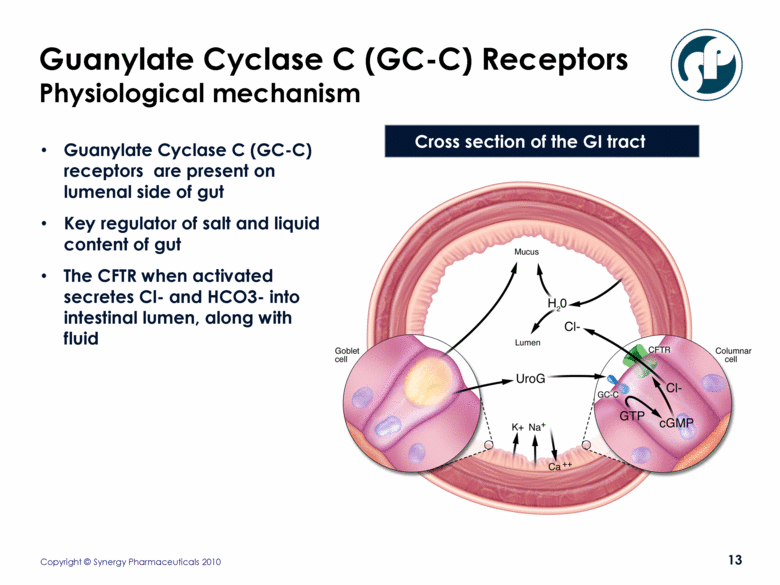
Guanylate Cyclase C (GC-C) Receptors Physiological mechanism Guanylate Cyclase C (GC-C) receptors are present on lumenal side of gut Key regulator of salt and liquid content of gut The CFTR when activated secretes Cl- and HCO3- into intestinal lumen, along with fluid Cross section of the GI tract |
|
|
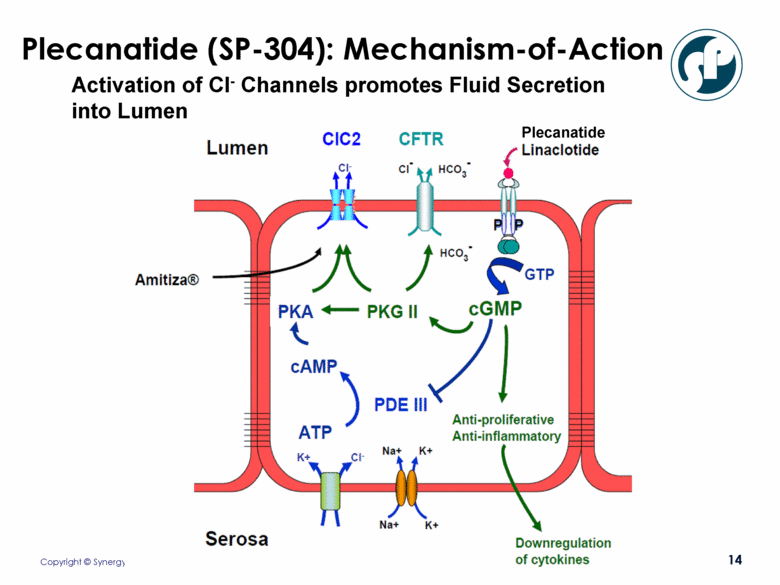
Plecanatide (SP-304): Mechanism-of-Action Activation of Cl- Channels promotes Fluid Secretion into Lumen Plecanatide |
|
|
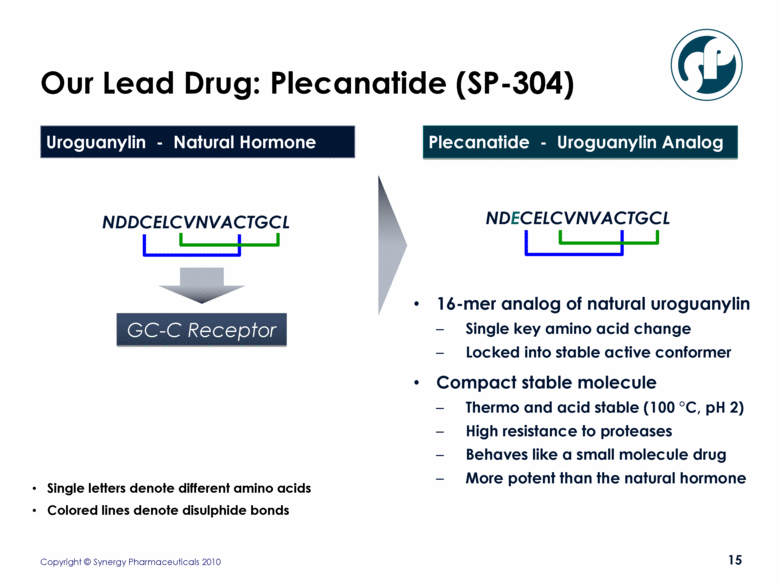
Our Lead Drug: Plecanatide (SP-304) 16-mer analog of natural uroguanylin Single key amino acid change Locked into stable active conformer Compact stable molecule Thermo and acid stable (100 °C, pH 2) High resistance to proteases Behaves like a small molecule drug More potent than the natural hormone NDDCELCVNVACTGCL NDECELCVNVACTGCL GC-C Receptor Uroguanylin - Natural Hormone Plecanatide - Uroguanylin Analog Single letters denote different amino acids Colored lines denote disulphide bonds |
|
|
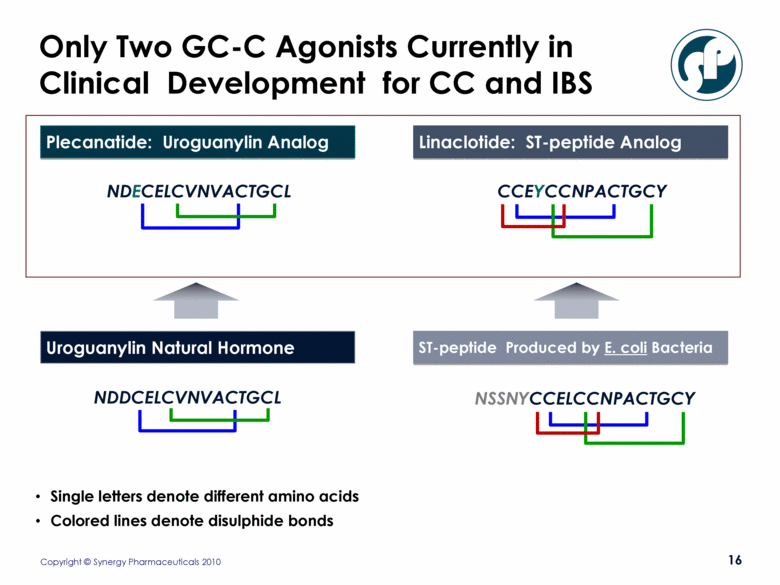
ST-peptide Produced by E. coli Bacteria Uroguanylin Natural Hormone Linaclotide: ST-peptide Analog NDECELCVNVACTGCL Plecanatide: Uroguanylin Analog Only Two GC-C Agonists Currently in Clinical Development for CC and IBS NSSNYCCELCCNPACTGCY CCEYCCNPACTGCY NDDCELCVNVACTGCL Single letters denote different amino acids Colored lines denote disulphide bonds |
|
|
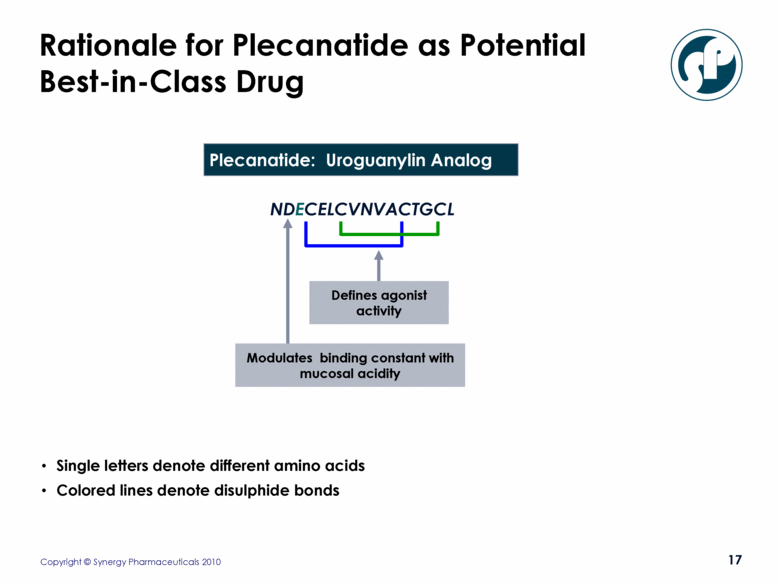
Rationale for Plecanatide as Potential Best-in-Class Drug Modulates binding constant with mucosal acidity NDECELCVNVACTGCL Plecanatide: Uroguanylin Analog Defines agonist activity Single letters denote different amino acids Colored lines denote disulphide bonds |
|
|
Plecanatide Clinical Development |
|
|
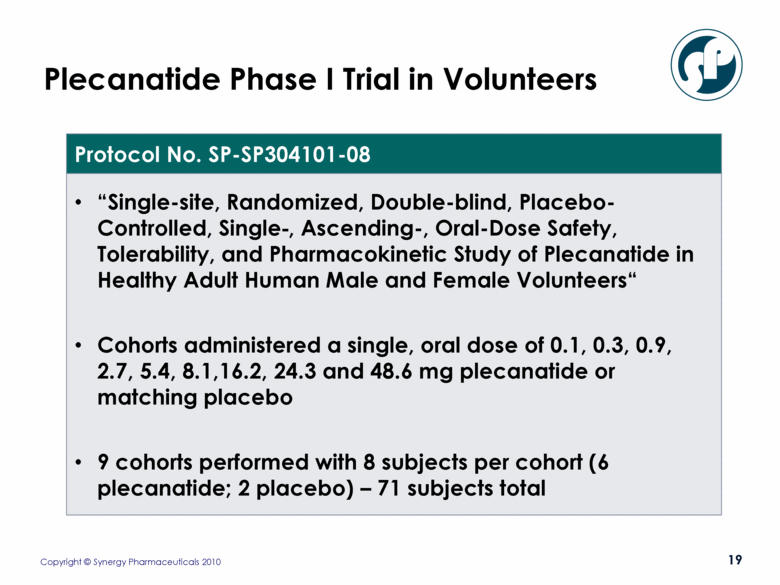
Plecanatide Phase I Trial in Volunteers Protocol No. SP-SP304101-08 “Single-site, Randomized, Double-blind, Placebo-Controlled, Single-, Ascending-, Oral-Dose Safety, Tolerability, and Pharmacokinetic Study of Plecanatide in Healthy Adult Human Male and Female Volunteers“ Cohorts administered a single, oral dose of 0.1, 0.3, 0.9, 2.7, 5.4, 8.1,16.2, 24.3 and 48.6 mg plecanatide or matching placebo 9 cohorts performed with 8 subjects per cohort (6 plecanatide; 2 placebo) – 71 subjects total |
|
|
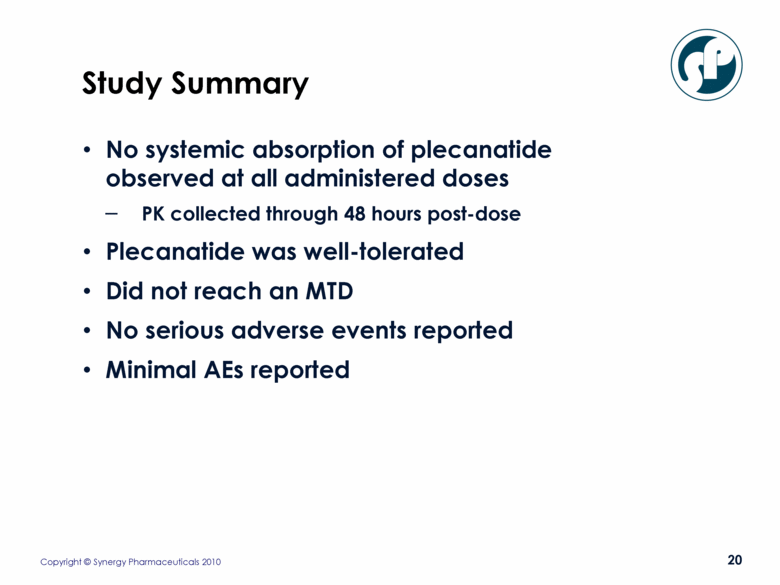
Study Summary No systemic absorption of plecanatide observed at all administered doses PK collected through 48 hours post-dose Plecanatide was well-tolerated Did not reach an MTD No serious adverse events reported Minimal AEs reported |
|
|
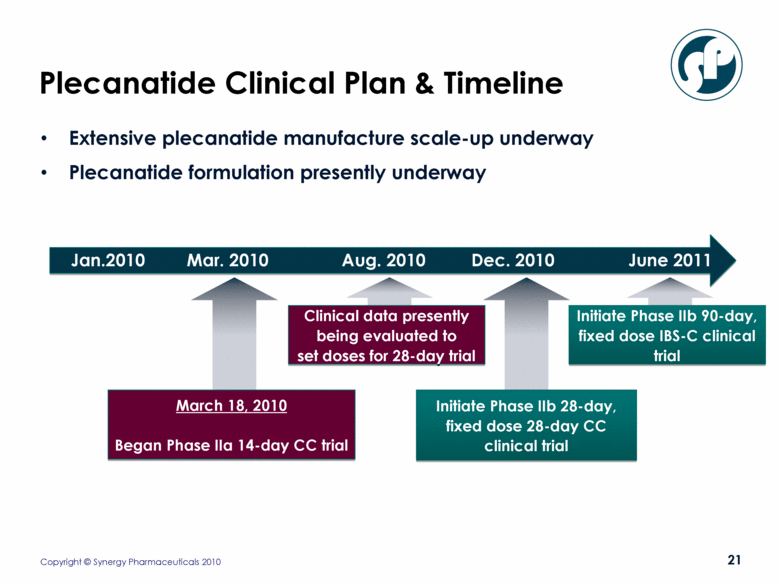
Plecanatide Clinical Plan & Timeline Extensive plecanatide manufacture scale-up underway Plecanatide formulation presently underway March 18, 2010 Began Phase IIa 14-day CC trial Initiate Phase IIb 28-day, fixed dose 28-day CC clinical trial Clinical data presently being evaluated to set doses for 28-day trial Jan.2010 Mar. 2010 Aug. 2010 Dec. 2010 June 2011 Initiate Phase IIb 90-day, fixed dose IBS-C clinical trial |
|
|
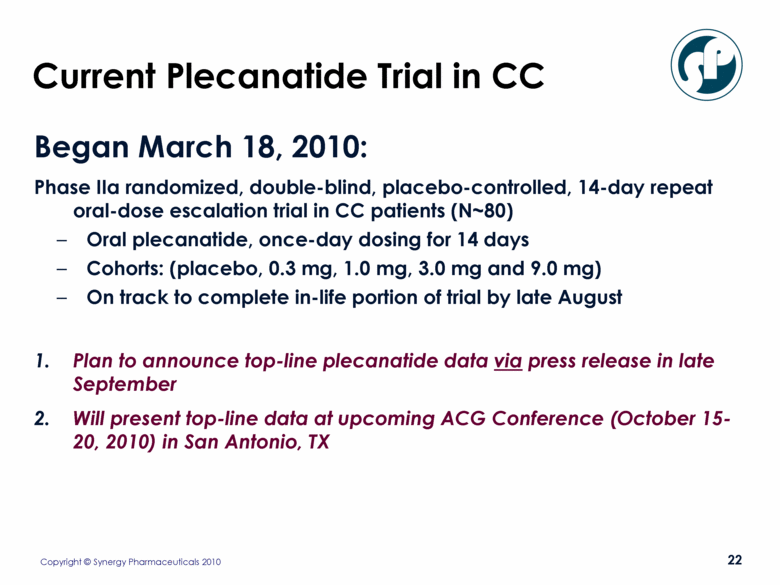
Current Plecanatide Trial in CC Began March 18, 2010: Phase IIa randomized, double-blind, placebo-controlled, 14-day repeat oral-dose escalation trial in CC patients (N~80) Oral plecanatide, once-day dosing for 14 days Cohorts: (placebo, 0.3 mg, 1.0 mg, 3.0 mg and 9.0 mg) On track to complete in-life portion of trial by late August Plan to announce top-line plecanatide data via press release in late September Will present top-line data at upcoming ACG Conference (October 15-20, 2010) in San Antonio, TX |
|
|
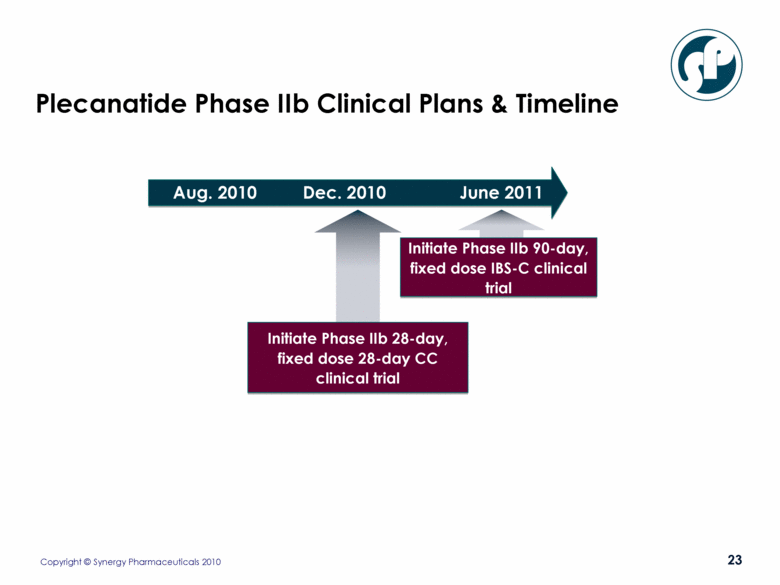
Plecanatide Phase IIb Clinical Plans & Timeline Initiate Phase IIb 28-day, fixed dose 28-day CC clinical trial Aug. 2010 Dec. 2010 June 2011 Initiate Phase IIb 90-day, fixed dose IBS-C clinical trial |
|
|
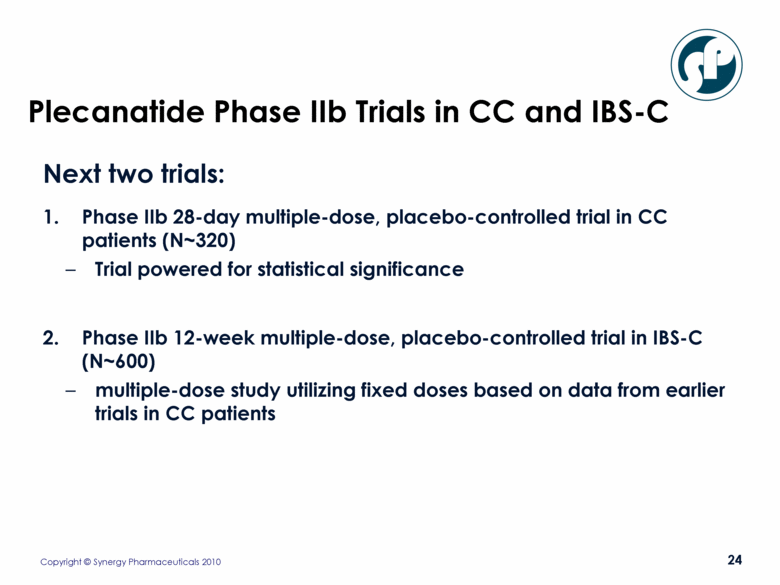
Plecanatide Phase IIb Trials in CC and IBS-C Next two trials: Phase IIb 28-day multiple-dose, placebo-controlled trial in CC patients (N~320) Trial powered for statistical significance Phase IIb 12-week multiple-dose, placebo-controlled trial in IBS-C (N~600) multiple-dose study utilizing fixed doses based on data from earlier trials in CC patients |
|
|
Plecanatide Clinical Strategic Focus Focus clinical development of plecanatide to minimize diarrhea in treated patients while maintaining efficacy Mechanistic advantage of plecanatide – “switch on/off regulation” Seek labeling and marketing advantages with use of plecanatide Note: At this stage, Synergy retains 100% worldwide commercial rights for plecanatide |
|
|
GC-C Receptor Agonists have an Established Valuation Partnership deal announced between Ironwood and Forest Laboratories for North American rights to linaclotide Forest paid $70 M upfront and $260 M in near term milestones for 50/50 rights to US, plus Canada and Mexico September 2007 May 2009 Almirall, S.A. signs deal with Ironwood for European rights to linaclotide Almirall to pay $40 M upfront and $55 M in near-term milestones plus royalties February 2010 Astellas signs deal with Ironwood for Asian rights to linaclotide Astellas pays $75 M November 2009 IRONWOOD IPO – BIGGEST IPO IN HISTORY OF BIOTECH IPO led by Morgan Stanley, J. P. Morgan and Credit Suisse Ironwood IPO (Nasdaq listing: IRWD) raises $203 million from offering and over-allotment Current Market Cap ~ $1.1 billion |
|
|
Further Validation of GI Disorder Drugs Shire (UK: SHP) offers 428 million euros ($566 million) in cash deal for Belgian specialty GI company Movetis (BE:Move) with recently approved drug Resolor for CC (*estimated potential peak sales of over EUR300 million) August 2010 Source: Shire press release |
|
|
Platform Technology SP-333 |
|
|
Synergy’s GC-C Receptor Agonist Program is a Technology Platform “Second-generation” drug positioned for ulcerative colitis (UC) GC-C agonists exhibit potent anti-inflammatory activity in animal models of inflammation Novel mechanism-of-action IND planned for 4Q2010 IP status – Key comp-of-matter patent allowed in U.S. SP-333 to treat ulcerative colitis – an IBD |
|
|
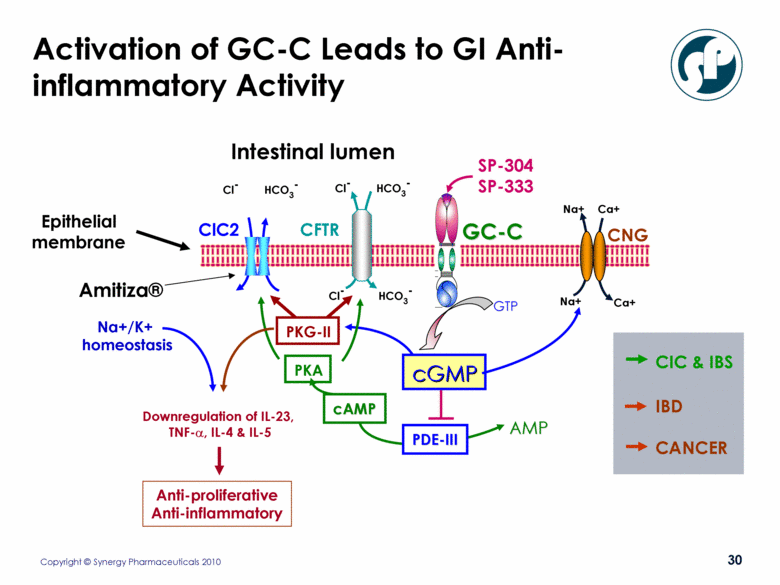
Activation of GC-C Leads to GI Anti-inflammatory Activity Epithelial membrane cAMP AMP PKA PDE-III GTP PKG-II cGMP Na+ Ca+ Na+ Ca+ Cl- HCO3- HCO3- Cl- ClC2 CFTR CNG GC-C SP-304 SP-333 Anti-proliferative Anti-inflammatory Cl- HCO3- Na+/K+ homeostasis Amitiza® CIC & IBS IBD CANCER Intestinal lumen Downregulation of IL-23, TNF-a, IL-4 & IL-5 |
|
|
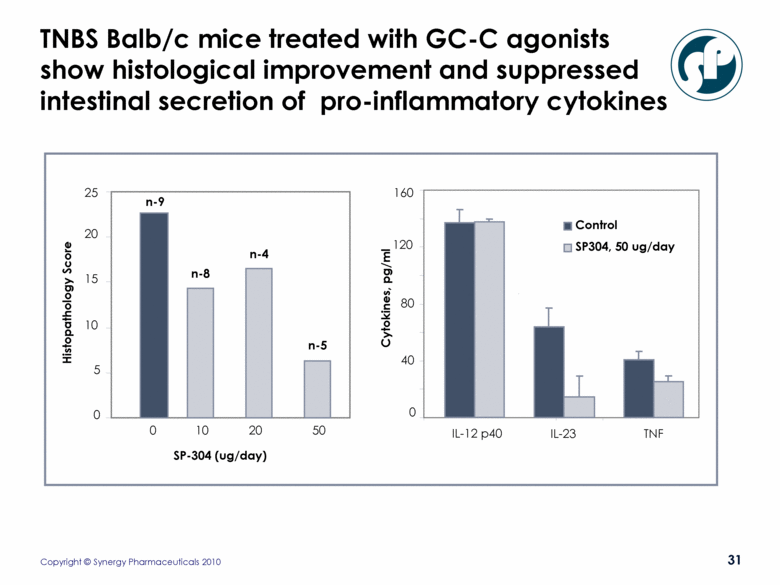
TNBS Balb/c mice treated with GC-C agonists show histological improvement and suppressed intestinal secretion of pro-inflammatory cytokines 0 40 80 120 IL-12 p40 IL-23 TNF Cytokines, pg/ml Control SP304, 50 ug/day 160 0 5 10 15 20 25 Histopathology Score SP-304 (ug/day) 0 10 20 50 n-9 n-8 n-4 n-5 |
|
|
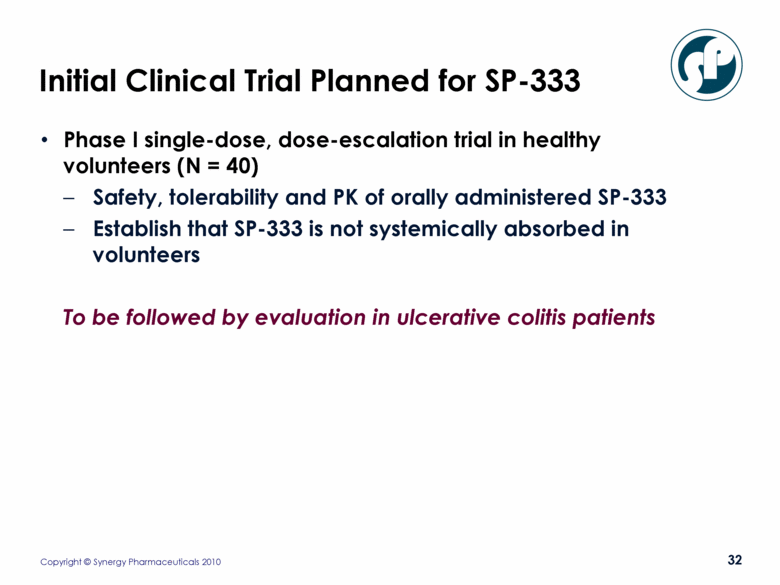
Initial Clinical Trial Planned for SP-333 Phase I single-dose, dose-escalation trial in healthy volunteers (N = 40) Safety, tolerability and PK of orally administered SP-333 Establish that SP-333 is not systemically absorbed in volunteers To be followed by evaluation in ulcerative colitis patients |
|
|
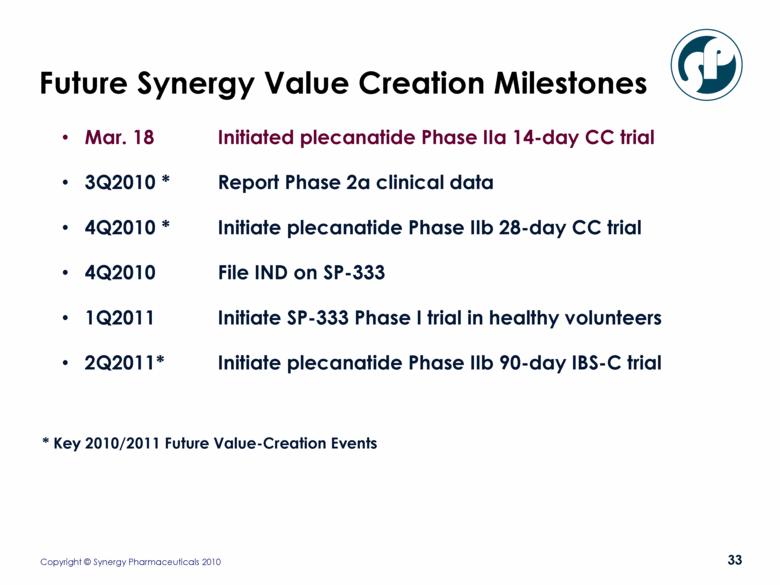
Future Synergy Value Creation Milestones Mar. 18 Initiated plecanatide Phase IIa 14-day CC trial 3Q2010 * Report Phase 2a clinical data 4Q2010 * Initiate plecanatide Phase IIb 28-day CC trial 4Q2010 File IND on SP-333 1Q2011 Initiate SP-333 Phase I trial in healthy volunteers 2Q2011* Initiate plecanatide Phase IIb 90-day IBS-C trial * Key 2010/2011 Future Value-Creation Events |
|
|
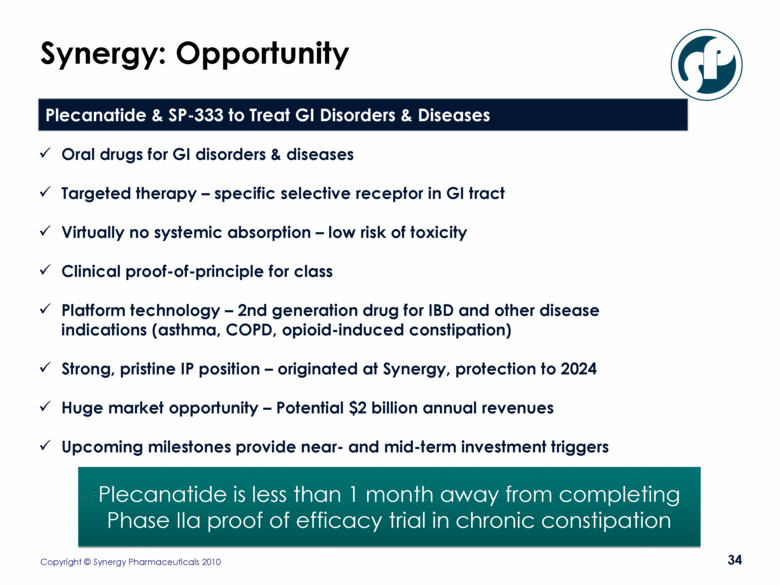
Synergy: Opportunity Plecanatide is less than 1 month away from completing Phase IIa proof of efficacy trial in chronic constipation Oral drugs for GI disorders & diseases Targeted therapy – specific selective receptor in GI tract Virtually no systemic absorption – low risk of toxicity Clinical proof-of-principle for class Platform technology – 2nd generation drug for IBD and other disease indications (asthma, COPD, opioid-induced constipation) Strong, pristine IP position – originated at Synergy, protection to 2024 Huge market opportunity – Potential $2 billion annual revenues Upcoming milestones provide near- and mid-term investment triggers Plecanatide & SP-333 to Treat GI Disorders & Diseases |
|
|
THANK YOU |