Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a6388439.htm |
Exhibit 99.1

Corporate Update 1 August 6, 2010

2 Forward-Looking Statement Forward-looking statements contained in this presentation are based on Sucampo’s assumptions and expectations concerning future events. They are subject to significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those reflected in the forward-looking statements. Sucampo’s forward-looking statements could be affected by numerous foreseeable and unforeseeable events and developments such as regulatory delays, the failure of clinical trials, the inability to fund drug development initiatives, competitive products and other factors identified in the “Risk Factors” section of Sucampo’s Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. While Sucampo may elect to update these statements at some point in the future Sucampo specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise. In light of the significant uncertainties inherent in the forward-looking information in this presentation, you are cautioned not to place undue reliance on these forward-looking statements.

3 Sucampo Pharmaceuticals, Inc. -- A Biopharmaceutical Company Amitiza®First FDA approved drug for chronic idiopathic constipation (CIC) in adults of all ages (Amitiza 24 mcg)Only FDA approved drug for irritable bowel syndrome with constipation (IBS-C) in women 18 years and older (Amitiza 8 mcg)Marketing approval in Switzerland for CIC received; pricing review ongoingPhase 3 efficacy trial for CIC in Japan met endpoint (p<0.001), and reported positive interim data from companion long-term phase 3 safety trialWill conduct another US phase 3 trial in opioid-induced bowel dysfunction (OBD) A pipeline of prostone-based product opportunities Rescula ® FDA-approved for glaucoma and ocular hypertension; promising data for additional indicationsCobiprostone for prevention of NSAID-induced gastric ulcers in phase 2SPI-017 for peripheral arterial disease going into phase 2Additional prostones in preclinical development Strong financial position$114.4 million in cash and investments (as of June 30, 2010)No debt
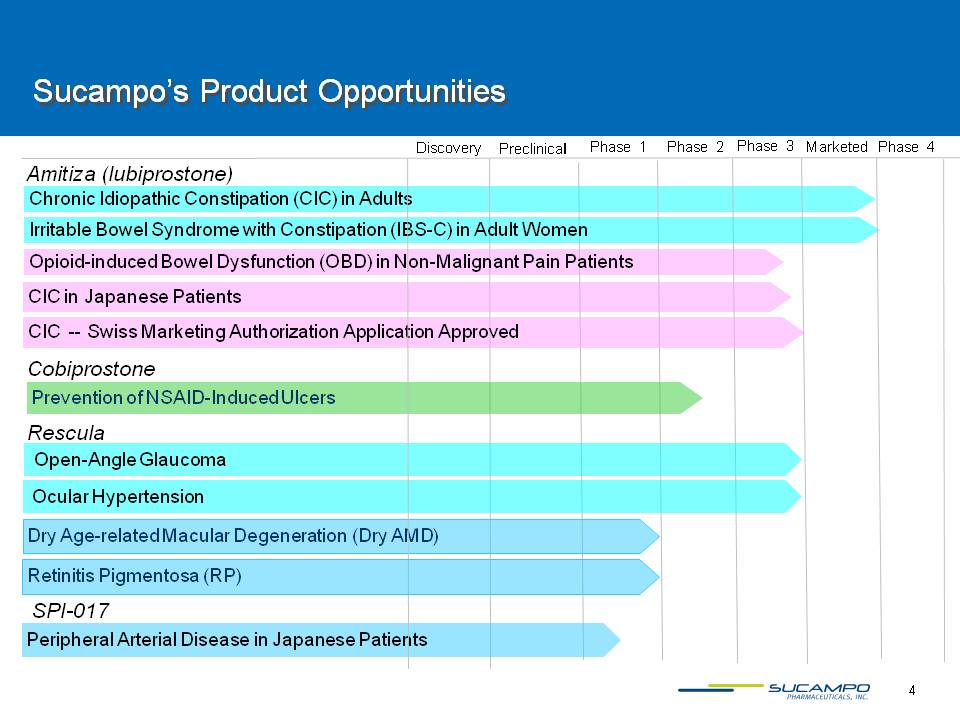
4 Sucampo’s Product Opportunities Chronic Idiopathic Constipation (CIC) in Adults Preclinical Phase 2 Phase 3 Phase 1 Phase 4 Marketed Amitiza (lubiprostone) Discovery Opioid-induced Bowel Dysfunction (OBD) in Non-Malignant Pain Patients Cobiprostone SPI-017 Prevention of NSAID-Induced Ulcers Peripheral Arterial Disease in Japanese Patients Irritable Bowel Syndrome with Constipation (IBS-C) in Adult Women CIC in Japanese Patients CIC -- Swiss Marketing Authorization Application Approved Open-Angle Glaucoma Dry Age-related Macular Degeneration (Dry AMD) Rescula Ocular Hypertension Retinitis Pigmentosa (RP)

5 Amitiza Answers Unmet Medical Needs in the Gastro-Intestinal Market Proven safety and efficacy for long-term usageEfficacy and tolerability similar for both genders and across age groups for CIC90% of nausea events diminish after first weekCompeting products recommended for short-term use onlyQuick and predictable relief of symptoms Up to 63% of CIC patients respond within 24 hoursIBS-C patients were twice as likely to achieve overall response than those receiving placeboA major market opportunityEstimated U.S. market of 12 million patients with CICEstimated more than 10 million patients with IBS-C in the U.S. More than 14 million (CIC and IBS-C) new diagnoses each year Unique mechanisms of actionIn CIC, Amitiza activates chloride ion channels, promoting fluid secretion In IBS-C, Amitiza activates chloride ion channels and promotes mucosal barrier protection
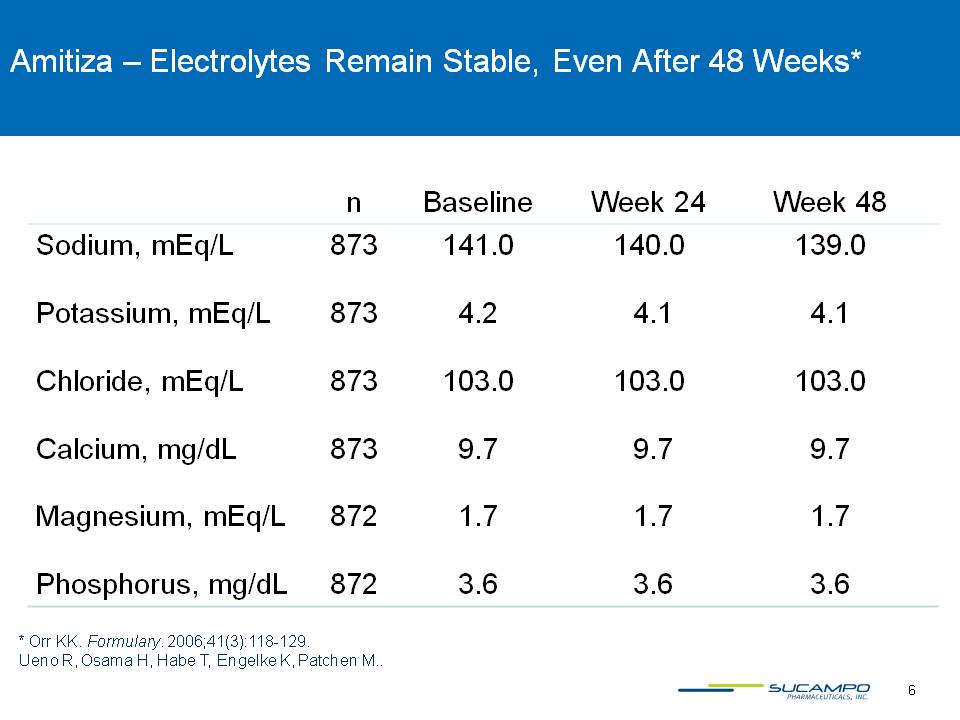
6 * Orr KK. Formulary. 2006;41(3):118-129.Ueno R, Osama H, Habe T, Engelke K, Patchen M. Gastroenterology. 2004;126 (suppl 2):A-100. Amitiza – Electrolytes Remain Stable, Even After 48 Weeks*
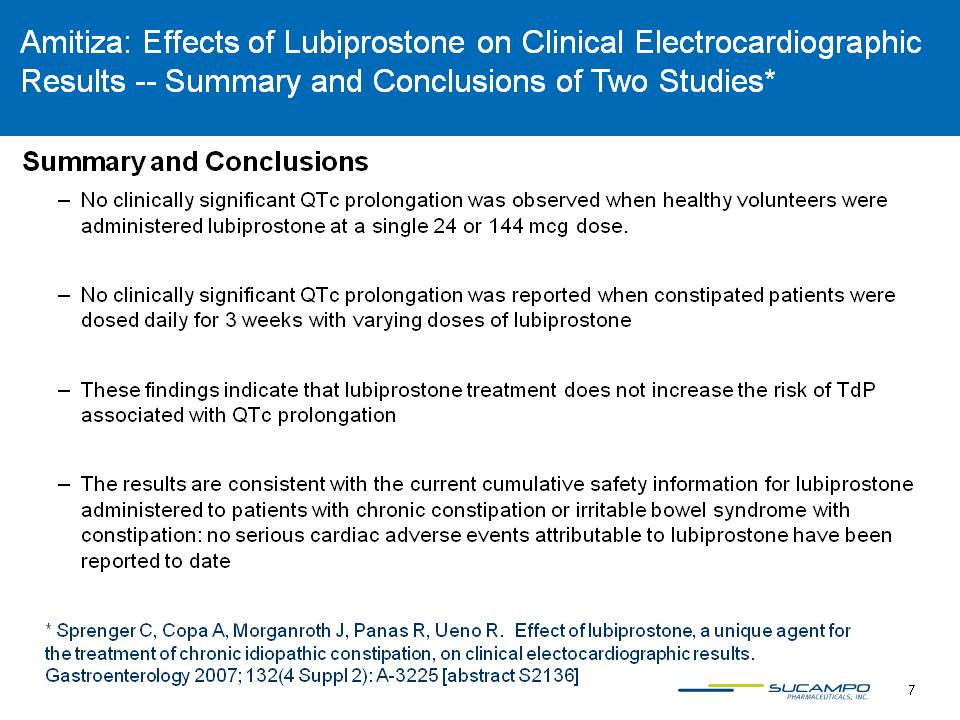
7 Amitiza: Effects of Lubiprostone on Clinical Electrocardiographic Results -- Summary and Conclusions of Two Studies* Summary and Conclusions No clinically significant QTc prolongation was observed when healthy volunteers were administered lubiprostone at a single 24 or 144 mcg dose.No clinically significant QTc prolongation was reported when constipated patients were dosed daily for 3 weeks with varying doses of lubiprostoneThese findings indicate that lubiprostone treatment does not increase the risk of TdP associated with QTc prolongationThe results are consistent with the current cumulative safety information for lubiprostone administered to patients with chronic constipation or irritable bowel syndrome with constipation: no serious cardiac adverse events attributable to lubiprostone have been reported to date * Sprenger C, Copa A, Morganroth J, Panas R, Ueno R. Effect of lubiprostone, a unique agent for the treatment of chronic idiopathic constipation, on clinical electocardiographic results. Gastroenterology 2007; 132(4 Suppl 2): A-3225 [abstract S2136]
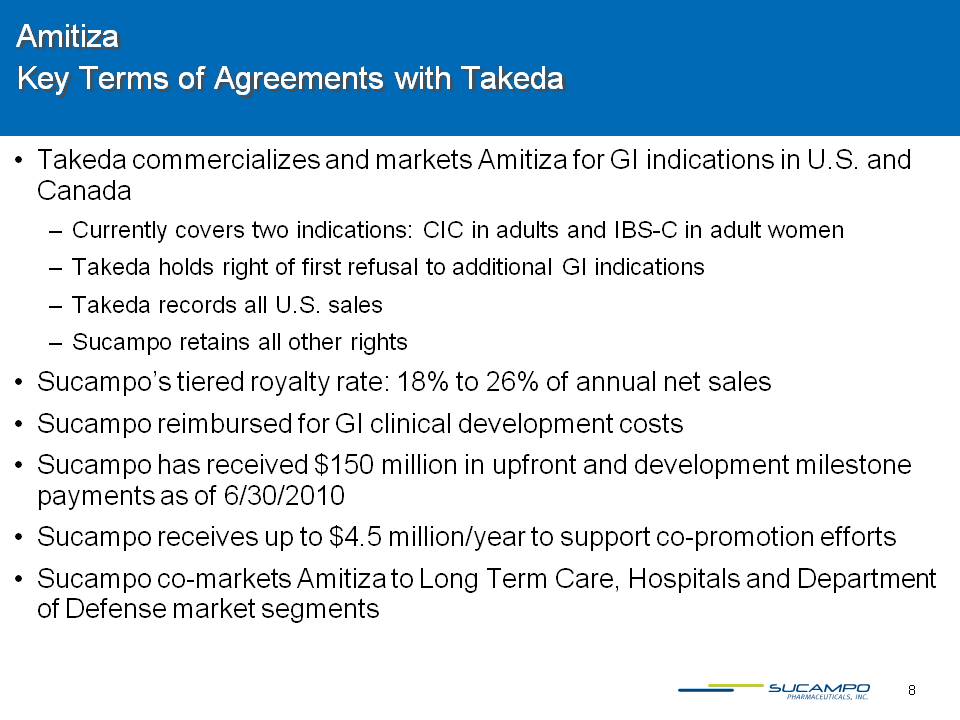
8 Amitiza Key Terms of Agreements with Takeda Takeda commercializes and markets Amitiza for GI indications in U.S. and Canada Currently covers two indications: CIC in adults and IBS-C in adult women Takeda holds right of first refusal to additional GI indications Takeda records all U.S. sales Sucampo retains all other rights Sucampo’s tiered royalty rate: 18% to 26% of annual net sales Sucampo reimbursed for GI clinical development costs Sucampo has received $150 million in upfront and development milestone payments as of 6/30/2010 Sucampo receives up to $4.5 million/year to support co-promotion efforts Sucampo co-markets Amitiza to Long Term Care, Hospitals and Department of Defense market segments

9 Amitiza – Design of Successful Phase 3 Trial for Opioid-induced Bowel Dysfunction (OBD) A total of 443 OBD patients received two 24-mcg capsules of lubiprostone or placebo each day for 12 weeks (1 capsule, twice a day) in a randomized placebo controlled phase 3 trial Conducted at multiple sites in the U.S. and Canada Primary endpoint: change from baseline in spontaneous bowel movement (SBM) frequency at Week 8 in patients without reduction in dose of study pain medication Concomitant pain medications: fentanyl, methadone, morphine and oxycontin Trial ‘632 did not hit endpoint
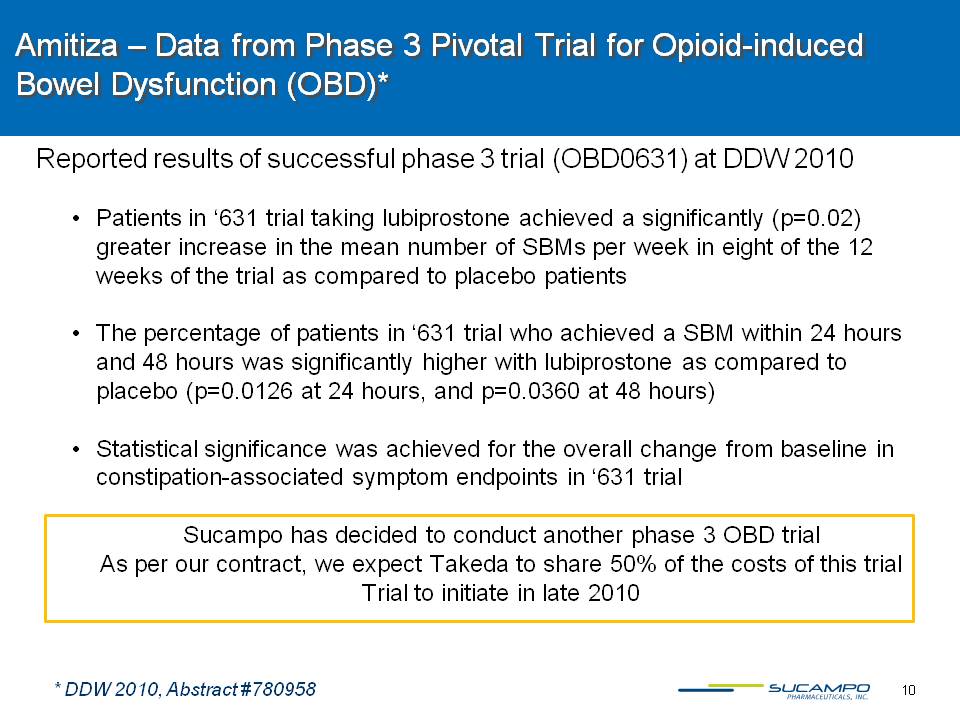
10 Amitiza – Data from Phase 3 Pivotal Trial for Opioid-induced Bowel Dysfunction (OBD)* Reported results of successful phase 3 trial (OBD0631) at DDW 2010 Patients in ‘631 trial taking lubiprostone achieved a significantly (p=0.02) greater increase in the mean number of SBMs per week in eight of the 12 weeks of the trial as compared to placebo patientsThe percentage of patients in ‘631 trial who achieved a SBM within 24 hours and 48 hours was significantly higher with lubiprostone as compared to placebo (p=0.0126 at 24 hours, and p=0.0360 at 48 hours) Statistical significance was achieved for the overall change from baseline in constipation-associated symptom endpoints in ‘631 trial Sucampo has decided to conduct another phase 3 OBD trial As per our contract, we expect Takeda to share 50% of the costs of this trialTrial to initiate in late 2010 * DDW 2010, Abstract #780958
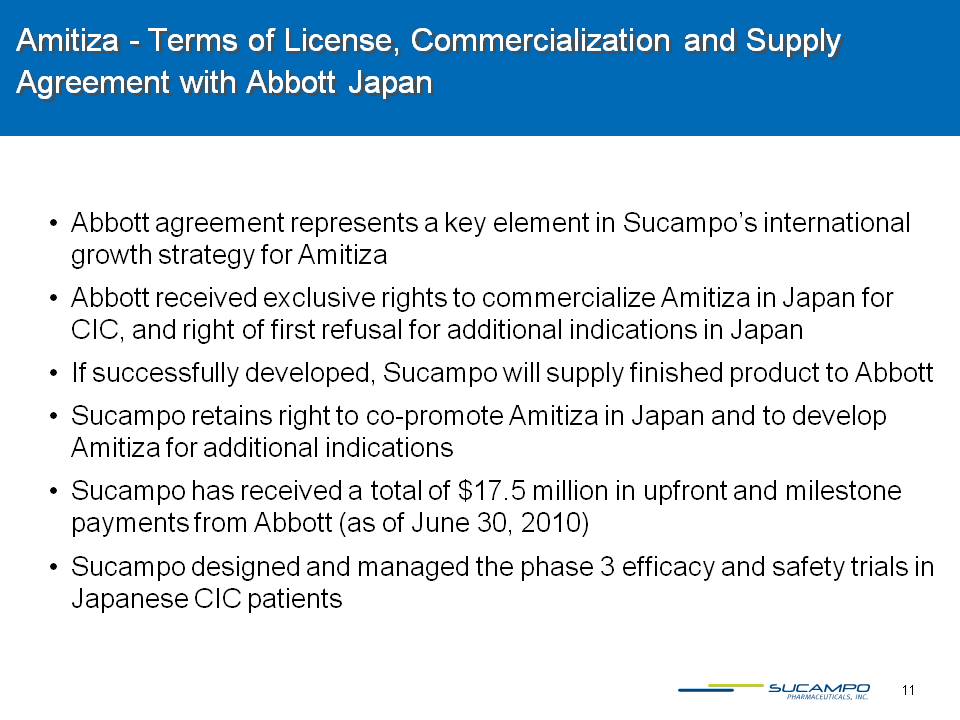
11 Amitiza - Terms of License, Commercialization and Supply Agreement with Abbott Japan Abbott agreement represents a key element in Sucampo’s international growth strategy for Amitiza Abbott received exclusive rights to commercialize Amitiza in Japan for CIC, and right of first refusal for additional indications in Japan If successfully developed, Sucampo will supply finished product to Abbott Sucampo retains right to co-promote Amitiza in Japan and to develop Amitiza for additional indicationsSucampo has received a total of $17.5 million in upfront and milestone payments from Abbott (as of June 30, 2010) Sucampo designed and managed the phase 3 efficacy and safety trials in Japanese CIC patients
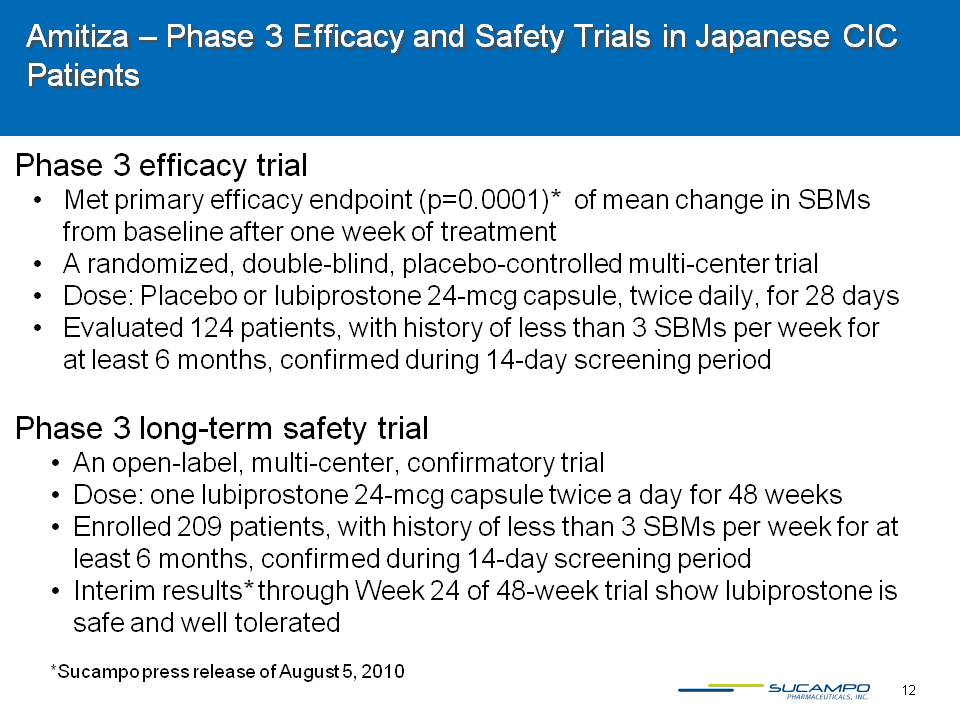

13 Rescula – Agreement with R-Tech Ueno Ltd. covers U.S. and Canada Sucampo’s first non-GI therapeutic area drug, licensed from R-Tech Ueno, Ltd. Rescula is a prostone-based drug with a unique mechanism of actionFDA-approved in 2000 for lowering of intra-ocular pressure (IOP) in glaucoma and ocular hypertension patients, not currently marketed in U.S. or Canada Activates BK channels in retinal cells Lowers IOP by increased outflow of aqueous humor through trabecular meshwork Proven to increase ocular blood flow to optic nerve and in the choroid Demonstrated to maintain visual field, to inhibit apoptosis and to inhibit changes in an ischemic optic nerve head These data were developed after Rescula’s first approval in 2000 Sucampo filed these data in an sNDA in August 2009; awaiting decision
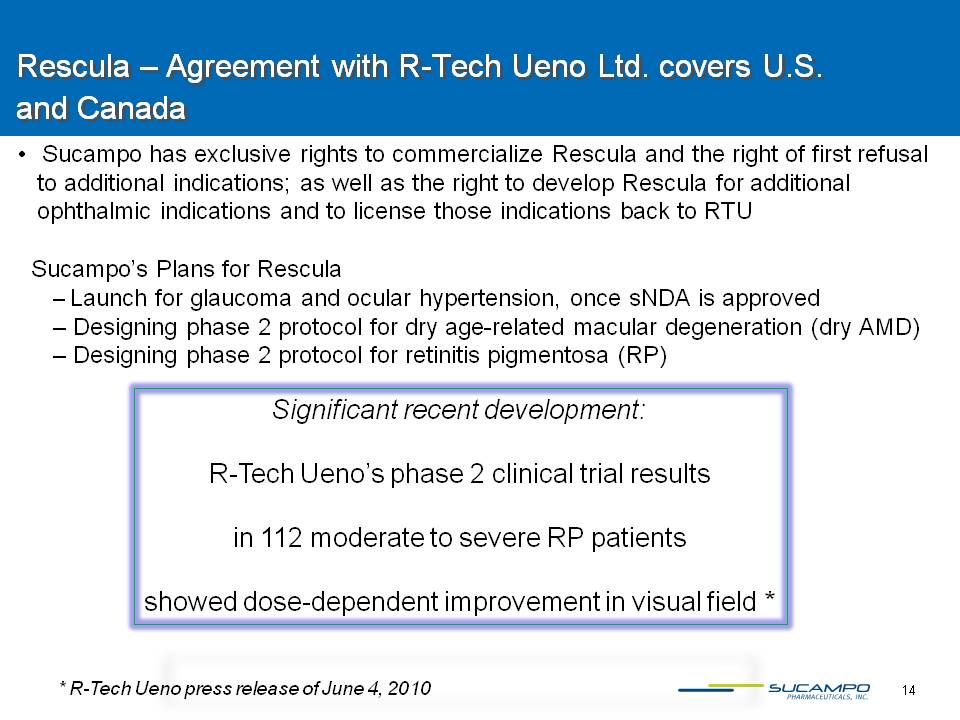
14 Rescula – Agreement with R-Tech Ueno Ltd. covers U.S. and Canada Sucampo has exclusive rights to commercialize Rescula and the right of first refusal to additional indications; as well as the right to develop Rescula for additional ophthalmic indications and to license those indications back to RTU Sucampo’s Plans for Rescula Launch for glaucoma and ocular hypertension, once sNDA is approved Designing phase 2 protocol for dry age-related macular degeneration (dry AMD) Designing phase 2 protocol for retinitis pigmentosa (RP) Significant recent development:R-Tech Ueno’s phase 2 clinical trial results in 112 moderate to severe RP patients showed dose-dependent improvement in visual field * * R-Tech Ueno press release of June 4, 2010
15 Cobiprostone -- Phase 2 Trial Design Cobiprostone is a locally acting chloride channel activator with potent activity in the GI tract Purpose of phase 2 trial: to assess the safety and protective effects of cobiprostone compared to placebo in patients taking chronic NSAID therapy for osteoarthritis and/or rheumatoid arthritis Three dose levels of cobiprostone: 18-mcg once, twice or three times a day All subjects received 500-mg naproxen twice a day Baseline endoscopy without gastric and duodenal ulcers or = 2 gastric and/or duodenal erosions Primary endpoint: overall incidence of gastric ulcers during 12 week treatment period Addresses the risk of C dificile infections as well as constipation associated with PPI-NSAID combinations
16 Cobiprostone -- Phase 2 Trial Results in Prevention of NSAID-Induced GI Injury* Subjects in high-dose cobiprostone cohort experienced 50% reduction in overall incidence of gastric ulcers when compared to placebo Cobiprostone showed an overall statistically significant reduction in gastric erosions through the 12 week treatment period and reductions in gastric erosions through Week 12 were dose dependent, middle and high dose cohorts showed statistical significance Time-to-onset of all ulcer or erosion development was delayed in cobiprostone cohorts with overall statistical significance across the 12 weeks *DDW 2010, oral presentation 780837
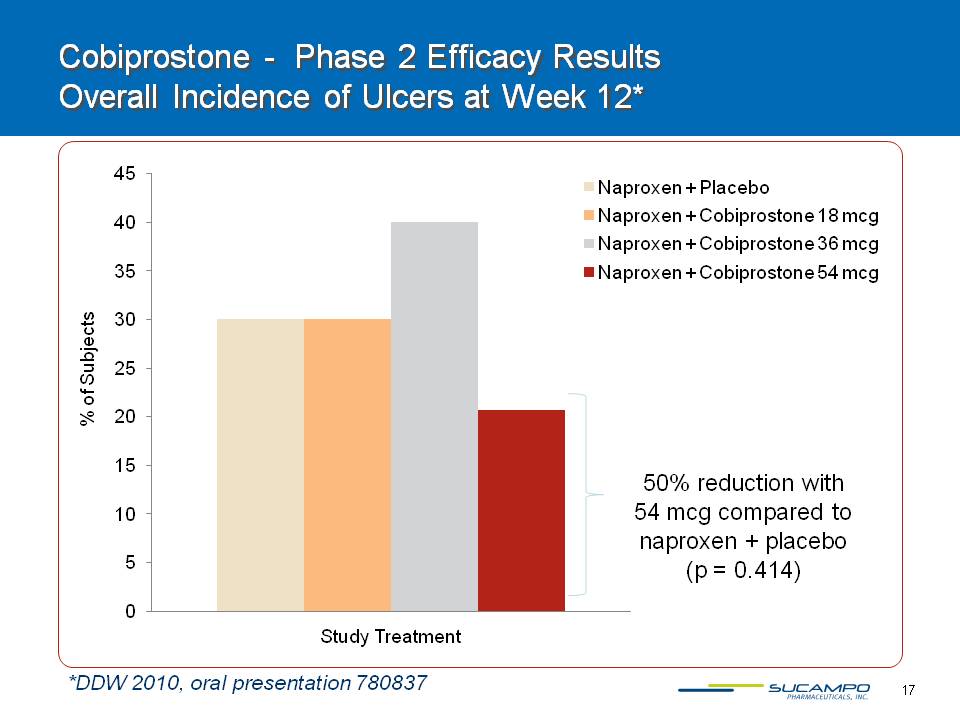
17 Cobiprostone - Phase 2 Efficacy Results Overall Incidence of Ulcers at Week 12* % of Subjects *DDW 2010, oral presentation 780837
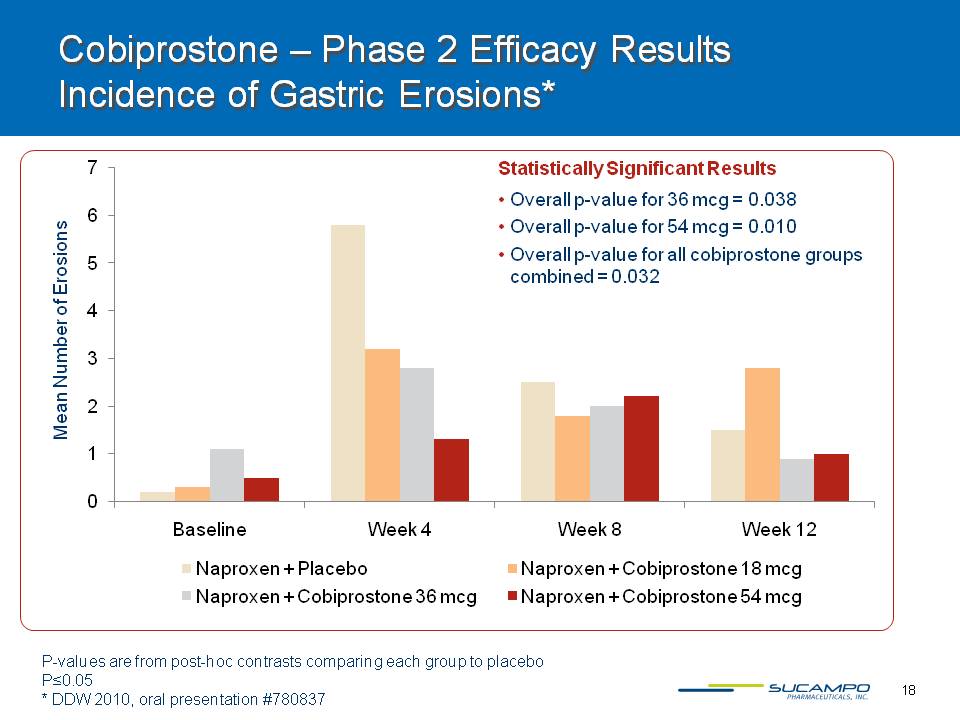
18 Mean Number of Erosions P-values are from post-hoc contrasts comparing each group to placeboP=0.05* DDW 2010, oral presentation #780837 Statistically Significant ResultsOverall p-value for 36 mcg = 0.038Overall p-value for 54 mcg = 0.010Overall p-value for all cobiprostone groups combined = 0.032 Cobiprostone – Phase 2 Efficacy Results Incidence of Gastric Erosions*
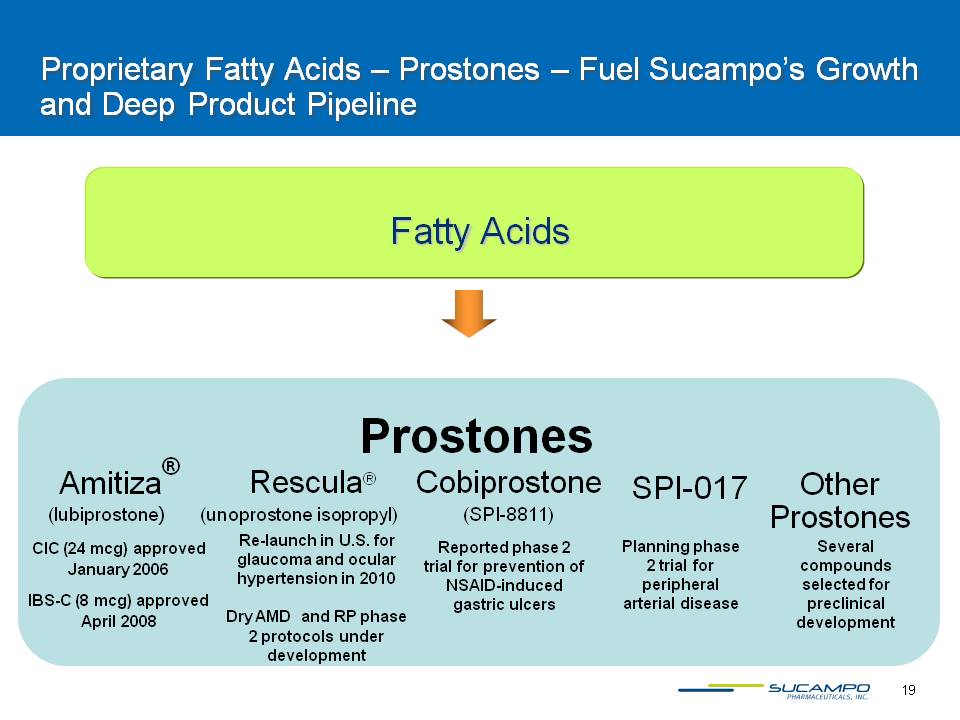
19 Proprietary Fatty Acids – Prostones – Fuel Sucampo’s Growth and Deep Product Pipeline Fatty Acids Prostones Amitiza® Cobiprostone SPI-017 (SPI-8811) CIC (24 mcg) approved January 2006IBS-C (8 mcg) approved April 2008 (lubiprostone) Other Prostones Six compounds selected for preclinical development Reported phase 2 trial for prevention of NSAID-induced gastric ulcers Planning phase 2 trial for peripheral arterial disease Rescula® Re-launch in U.S. for glaucoma and ocular hypertension in 2010Dry AMD and RP phase 2 protocols under development (unoprostone isoprophyl)
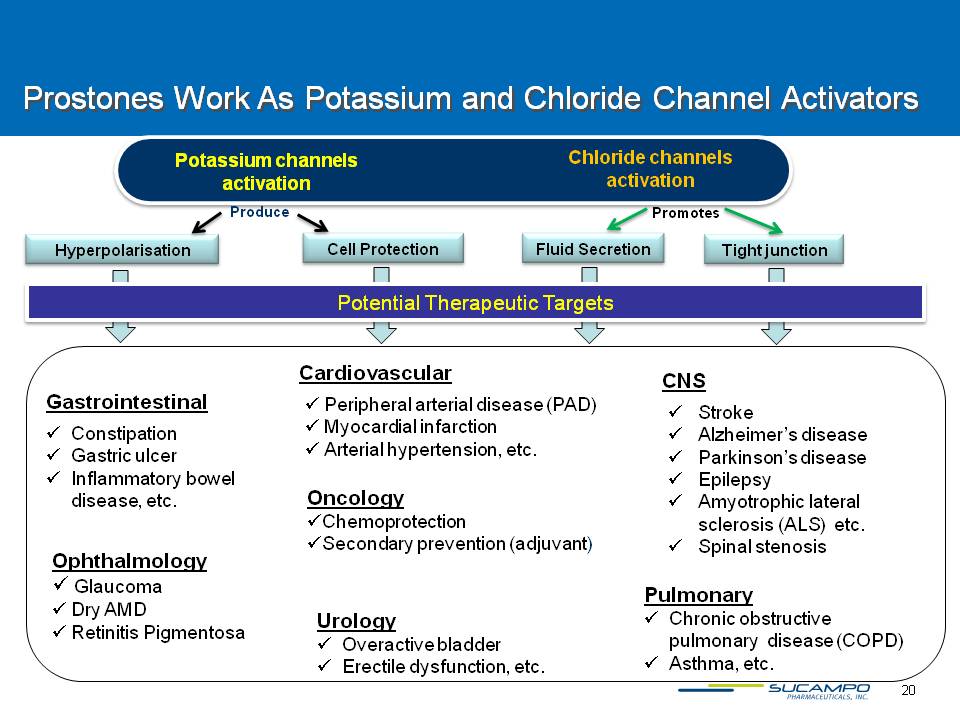
20 Prostones Work As Potassium and Chloride Channel Activators Chloride channels activation Potassium channels activation Potential Therapeutic Targets GastrointestinalConstipationGastric ulcerInflammatory bowel disease, etc. CardiovascularPeripheral arterial disease (PAD)Myocardial infarctionArterial hypertension, etc. CNS Stroke Alzheimer’s disease Parkinson’s disease Epilepsy Amyotrophic lateral sclerosis (ALS) etc. Spinal stenosis Pulmonary Chronic obstructive pulmonary disease (COPD)Asthma, etc. Urology Overactive bladder Erectile dysfunction, etc. Hyperpolarisation Cell Protection Fluid Secretion Tight junction Produce Promotes Ophthalmology Glaucoma Dry AMD Retinitis Pigmentosa Oncology Chemoprotection Mucositis/Stomatitis Secondary prevention (adjuvant)
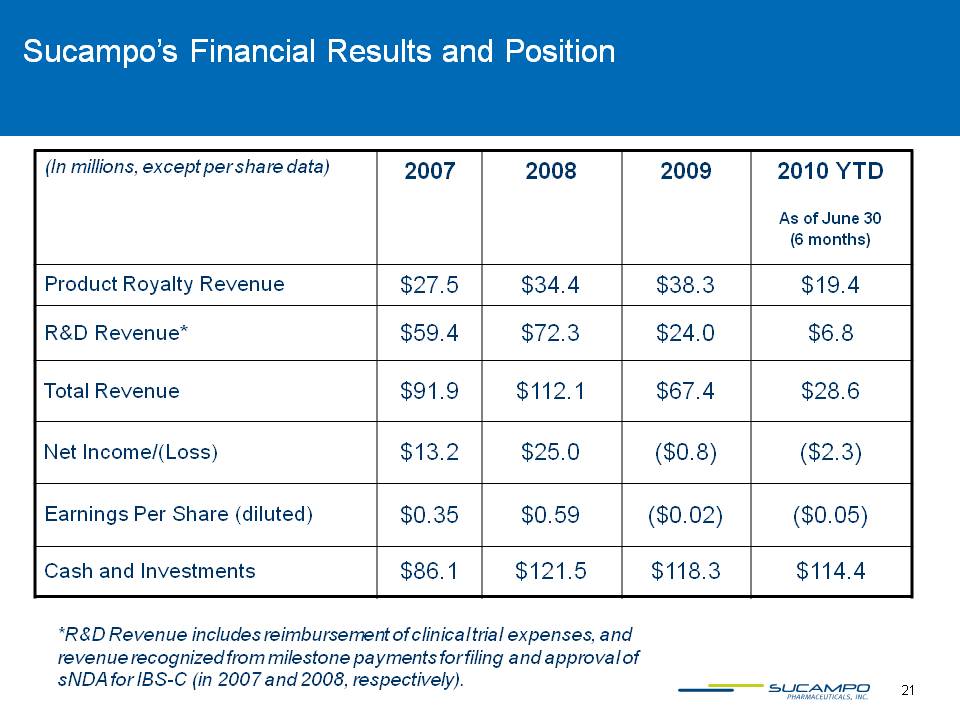
21 Sucampo’s Financial Results and Position *R&D Revenue includes reimbursement of clinical trial expenses, and revenue recognized from milestone payments for filing and approval of sNDA for IBS-C (in 2007 and 2008, respectively).
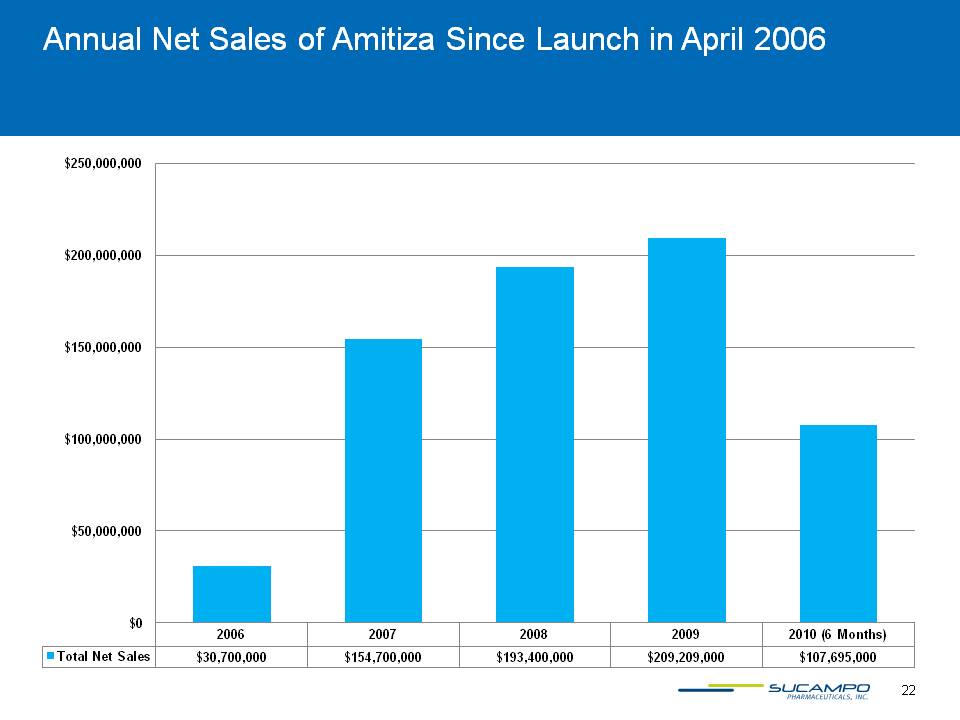
22 Annual Net Sales of Amitiza Since Launch in April 2006

23 Sucampo’s 2010 Milestones Plan commercialization of Amitiza in Switzerland, based on pricing negotiations with Swiss authoritiesv Report phase 3 efficacy trial results of Amitiza in Japanese CIC patients Initiate Amitiza phase 3 trial in OBD patientsInitiate phase 2 trial of Rescula in dry AMD Continue phase 1 trial of SPL-017 for peripheral arterial disease (PAD) in Japanese patientsComplete Amitiza for OBD phase 3 follow-on safety extension trial

24 Corporate Update. August 6, 2010
