Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ASENSUS SURGICAL, INC. | c02146e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - ASENSUS SURGICAL, INC. | c02146exv99w1.htm |
Exhibit 99.2

| Where Safety Meets Innovation Endoscopic and Minimally Invasive Surgical Devices www.SafeStitch.com OTCBB: SFES 1 June 2010 |

| This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaningregarding product development and commercialization efforts and other non-historical facts about our expectations, beliefs or intentions regarding our business, technologies and products, financial condition, strategies or prospects. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements. These factors include those described in our filings with the Securities and Exchange Commission, as well as risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments. In addition, forward-looking statements may also be adversely affected by general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, patent positions and litigation, among other factors. We do not undertake any obligation to update forward-looking statements. We intend that all forward- looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. looking statements be subject to the safe-harbor provisions of the PSLRA. 2 June 2010 |

| Charles J. Filipi, M.D. Professor of Surgery at Creighton University School of Medicine; Past President of the American Hernia Society; Fellow of the American College of Surgeons Phillip Frost, M.D. Chairman, CEO and President of OPKO Health; Chairman of Teva Pharmaceuticals; Former Chairman and CEO of IVAX Corporation Jane Hsiao, Ph.D. Chairman of Non-Invasive Monitoring Systems; Vice- Chairman and CTO of OPKO Health; Former Vice-Chairman of IVAX Corporation Jeffrey G. Spragens Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine Co-founder and former Director of North American Vaccine 3 June 2010 |

| Jeffrey G. Spragens - Chief Executive Officer and President Co-Founder and former Director of North American Vaccine Corporation; Co- Founder and Director of Foundation for Peace; Co-Founder of Mint Management and Development Stewart B. Davis, M.D. - Chief Operating Officer Managing Partner and Medical Director of Parasol International; Former Asst. Medical Director of Innovia LLC, InnFocus LLC, InnoGraft LLC and InnCardia LLC Adam S. Jackson - Chief Financial Officer Chief Financial Officer of Non-Invasive Monitoring Systems, Inc.; Former Senior Vice President, Finance and Senior Vice President, Controller of Levitt Corporation; Former Chief Financial Officer of Romika-USA, Inc. Joshua Weingard - Chief LegalOfficer CLO of Non-Invasive Monitoring Systems, Inc. and Cardo Medical, Inc.; Former EVP and CLO of NationsHealth Inc; Sr. Corporate and Securities Counsel for Andrx Corporation Charles J. Filipi, M.D. - Chief Medical Officer Professor of Surgery at Creighton University School of Medicine; Former President of the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society the American Hernia Society 4 June 2010 |
| Jane Hsiao Ph.D. - Chairman of the Board Jeffrey G. Spragens Charles J. Filipi, M.D. Chao Chen, Ph.D. Chief Operating Officer of UniMed VM, Former Vice President of TaiMed Inc., Formerly with Cordis and Ethicon, subsidiaries of Johnson & Johnson Richard Pfenniger, Jr. CEO and President of Continucare Corporation (AMEX: CNU) Steven Rubin Executive Vice President of OPKO Health (AMEX: OPK); Former Senior Vice President, General Counsel and Secretary of IVAX Corporation Kevin Wayne, D.B.A. Associate Professor of Business Administration at Rivier College; Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. Co-founder and Former Vice President of Onux Medical, Inc. 5 June 2010 |

| Para Chondrosoma, M.D. University of Southern California (Chairman of Pathology); Esophageal Pathology Tom DeMeester, M.D. University of Southern California (Chairman of Surgery); Esophageal Surgery; Expert in Barrett's Esophagus Glen Lehman, M.D. Indiana University; Gastroenterology; Expert in Endoscopic GERD Treatment and ERCP Jeff Peters, M.D. University of Rochester (Chairman of Surgery); GERD Surgery; expert in Barrett's and esophageal dilation Raul Rosenthal, M.D. Cleveland Clinic; Bariatric Surgery; Chairman of the Bariatric Centers of Excellence Accreditation Committee Richard Rothstein, M.D. Dartmouth University; Gastroenterology; Expert in GERD, Barrett's Esophagus and NOTES Scott Shikora, M.D. Tufts University Medical Center (Chief of Bariatrics); Bariatric Surgery; Former President of the American Bariatric Society Lee Swanstrom, M.D. Oregon Clinic; Laparoscopic and Endoscopic Surgery; First Surgeon to perform Transoral NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US NOTES Cholecystectomy in the US 6 June 2010 |
| February 2009 - FDA clearance for SMART DilatorTM November 2009 - FDA clearance for AMID StaplerTM February 2010 - CE Mark for AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM June 2010 - Commercial Launch of AMID StaplerTM 7 June 2010 |
| Miami Headquarters Development Lab Distribution Warehouse Omaha Office Development Lab Development Lab Development Lab Development Lab Development Lab Development Lab Development Lab Development Lab 8 June 2010 |

| Ready to launch sales - June 2010 510(k) cleared by FDA - November 2009 CE Mark received - February 2010 Sales and Marketing Team in place Led by industry veterans, Caron D'Ambruso and J.C. Campbell Campbell Campbell Campbell Campbell Campbell Campbell June 2010 9 |
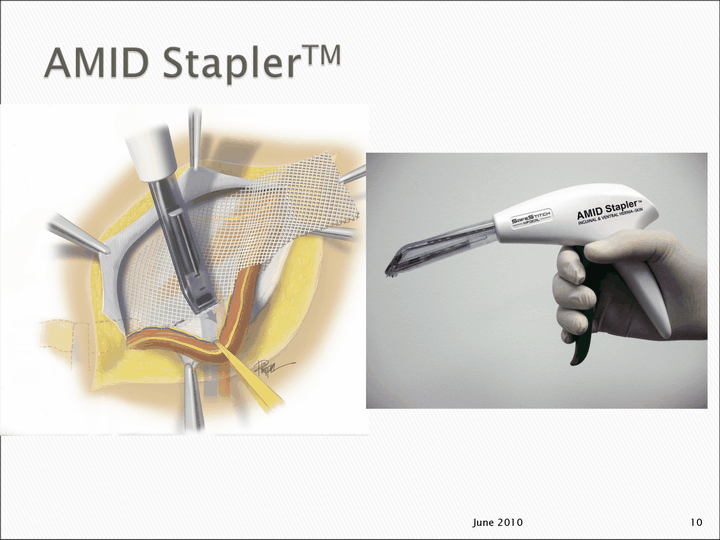
| June 2010 10 |
| Up to 1 million inguinal hernia cases per year in the U.S. Approximately 80% of inguinal hernia repairs worldwide utilize the Lichtenstein method of affixing mesh to the herniated abdominal wall Popularized by UCLA's Parviz Amid, M.D. Hand suturing Significant retraction Approximately 100,000 ventral hernia cases per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. per year in the U.S. 11 June 2010 |
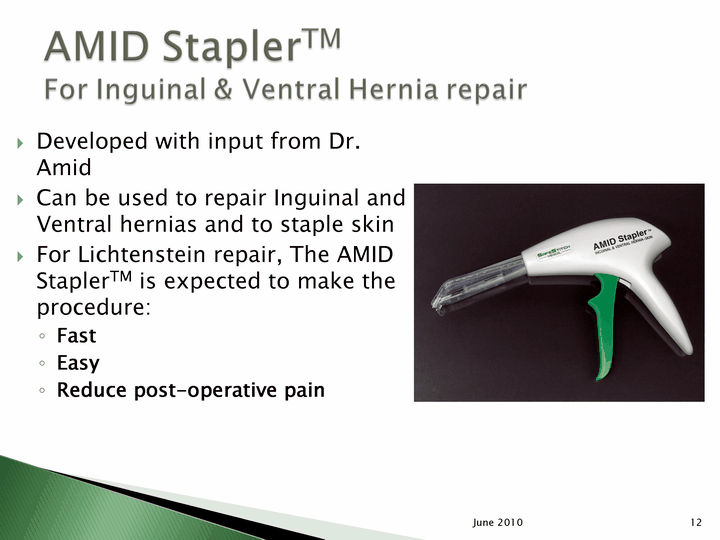
| Developed with input from Dr. Amid Can be used to repair Inguinal and Ventral hernias and to staple skin For Lichtenstein repair, The AMID StaplerTM is expected to make the procedure: Fast Easy Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain Reduce post-operative pain 12 June 2010 |

| NOVEL DEVICES and GI Complementary Products Intraluminal Gastroplasty Kit For Obesity For GastroEsophageal Reflux Disorder (GERD) Status - Ready for IDE Intraluminal Fixation Device Status - 510(k) submitted Intraluminal Excision Device Status - 510(k) ready to submit June 2010 Barrett's Excision and Strip Mucosal Device Status - Prototype in development SMART DilatorTM Status - 510(k) cleared by FDA Retention Bite Block - 510(k) exempt Airway Bite Block - 510(k) exempt T Fastener Status - Prototype in development Novel Devices for Natural Orifice Transluminal Endoscopic Surgery (NOTES) Status - IP filed 13 June 2010 June 2010 |

| Closely linked to heart disease, diabetes, hypertension and other major health issues ~27% of U.S. population may be obese Treated with diet, exercise, prescription drugs and/or surgery Surgery is most effective treatment Current surgical procedures have become household terms (gastric bypass, stomach stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) stapling, lap band) 14 June 2010 |
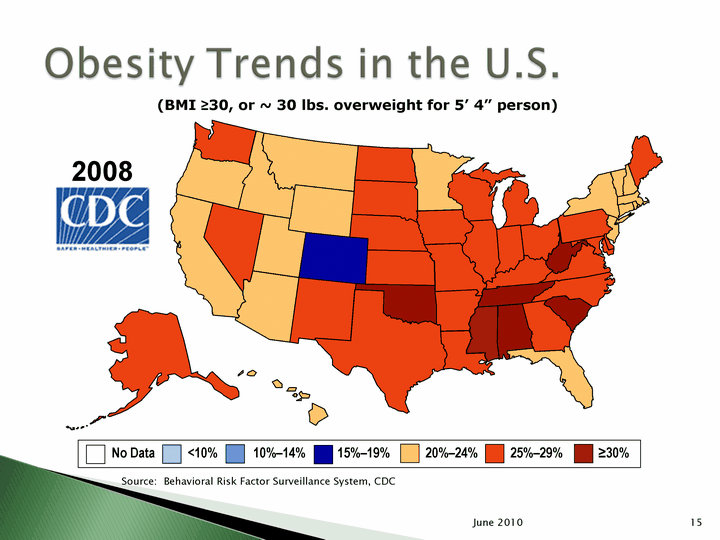
| 15 June 2010 No Data <10% 10%-14% 15%-19% 20%-24% 25%-29% ^30% (BMI ^30, or ~ 30 lbs. overweight for 5' 4" person) 2008 Source: Behavioral Risk Factor Surveillance System, CDC |

| 350,000 - 400,000 bariatric procedures are currently performed worldwide each year Existing procedures cost $15,000 to $70,000 Existing procedures involve open abdominal surgery High risk patients, with high morbidity and mortality for these procedures mortality for these procedures mortality for these procedures mortality for these procedures mortality for these procedures mortality for these procedures mortality for these procedures mortality for these procedures 16 June 2010 |

| Advantages: Outpatient procedure Safer Faster recovery Durability of effect Significant cost reduction Clinical trials expected to begin in Q3 2010 17 June 2010 |

| GastroEsophageal Reflux Disorder Heartburn, indigestion Multibillion dollar market in pharmaceuticals alone Recent New England Journal of Medicine papers discuss increased risk of bone fractures with chronic PPI use GERD can lead to Barrett's Esophagus, which may lead to cancer 200,000 to 250,000 GERD procedures are performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year performed worldwide each year 18 June 2010 |
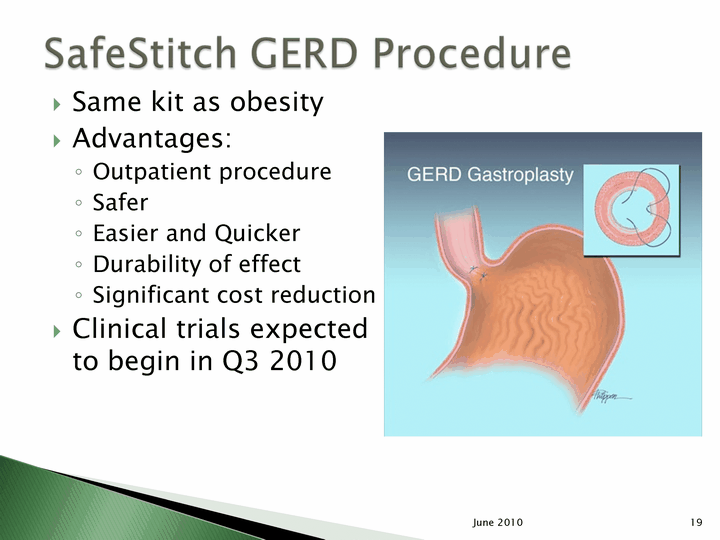
| Same kit as obesity Advantages: Outpatient procedure Safer Easier and Quicker Durability of effect Significant cost reduction Clinical trials expected to begin in Q3 2010 19 June 2010 |

| 20 EXCISION SUTURE June 2010 KNOTTER |

| Intraluminal Fixation Device Status - 510(k) submitted Market - Pouch reduction, Outlet reduction, Gastro-gastro fistula closure, NOTES gastrostomy closure, Esophageal perforation closure, Post colonoscopy rectal sigmoid colon perforation closure, Post endoscopic gastric perforation closure, Gastric sleeve revision, Prosthetic weight loss sleeve fixation to stomach, Gastric ulcer perforation closure, NOTES intraperitoneal applications, Enterotomy closure, Bowel perforation closure, Reinforce sutured closures Intraluminal Excision Device Status - 510(k) ready to submit June 2010 Market - Pouch reduction, Outlet reduction, Gastro-gastro fistula closure, Gastric sleeve revision, Biopsy of gastric ulcers suspicious for gastric cancer in US and Asia, Removal of sessile gastric polyps, Biopsy of gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas gastric leiomyomas June 2010 21 |

| Condition in which the esophagus changes so that some of its lining is replaced by a type of tissue similar to that normally found in the intestine, intestinal metaplasia GERD is most common cause May lead to esophageal cancer May lead to esophageal cancer May lead to esophageal cancer May lead to esophageal cancer May lead to esophageal cancer May lead to esophageal cancer May lead to esophageal cancer 22 June 2010 |

| Approximately 11 million patients worldwide Advantages: Large piece of tissue excised; can be sent for histology Clean margins Easier and Quicker than EMR and RF Ablation Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction Cost reduction 23 June 2010 |

| 24 June 2010 |
| 800,000 dilations of esophageal strictures are performed annually in the U.S.; ~2 million Worldwide Perforation Rate: ~1.1% Important Features Visual feedback force gauge handle Tapered tip Endoscope channel through entire device Disposable: other disposable dilators sell for $150 - $250 Ready to Launch Sales of GI complementary products 25 June 2010 |
| Approximately 18 - 20M upper endoscopies done per year worldwide and all procedures need bite blocks to prevent injury to endoscopes and the patients' teeth Class I, 510(k) exempt device Advantages: Harder to expel from mouth Softer material Superior crush resistance Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter 26 June 2010 |
| Approximately 5M upper endoscopies done per year worldwide on obese patients Many obese patients have airway problems during procedures which require intervention Class I, 510(k) exempt device Advantages: Novel design integrating Retention Bite Block and Oral-Pharyngeal airway Softer Material Superior crush resistance Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter Larger working inner diameter 27 June 2010 |

| T Fastener For Upper GI Bleeding and closing gastrotomies following NOTES T Fastener can be placed using multi-firing endoscopic device High mortality rate due to Upper GI bleeding Needs to be treated quickly and effectively Novel Devices for NOTES IP for multiple devices: Magnetic retractors Device for closing gastrotomies following NOTES NOTES access platforms 28 June 2010 June 2010 |
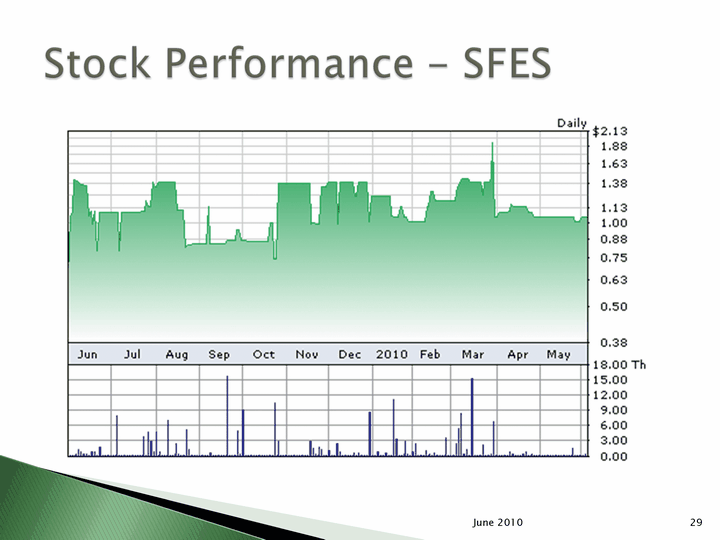
| June 2010 29 |

| Large, important and growing markets Obesity, GERD, Hernia and Barrett's Esophagus Multibillion dollar markets Unique devices Outpatient and minimally invasive Increased safety for patients Reduce procedural costs Reduce societal costs from obesity and obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases obesity-related diseases OTCBB: SFES 30 June 2010 |

| Where Safety Meets Innovation Contact: Jeffrey Spragens - CEO & President Stewart Davis M.D. - COO Adam Jackson - CFO 4400 Biscayne Boulevard Miami, FL 33137 (305) 575-4600 (305) 575-4130 Fax www.SafeStitch.com OTCBB: SFES 31 June 2010 |
