Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d8k.htm |
| EX-99.1 - PRESENTATION DATED APRIL 8, 2010 - Paratek Pharmaceuticals, Inc. | dex991.htm |
EXHIBIT 99.2
Absence of Rebound Effects With Low-Dose Zolpidem Tartrate
Sublingual Tablet 3.5 mg As-Needed Use: Preliminary Analysis
Russell Rosenberg, Ph.D.1, Timothy Roehrs, Ph.D.2, Nikhilesh N. Singh, Ph.D.3, Frank Steinberg, D.O.4, Thomas Roth, Ph.D.2
1. NeuroTrials Research Inc., Atlanta, GA, 2. Henry Ford Hospital, Detroit, MI, 3. Transcept Pharmaceuticals, Inc., Pt. Richmond, CA, 4. Chief Medical Consultant, Transcept Pharmaceuticals, Inc., Pt. Richmond, CA
INTRODUCTION
Insomnia characterized by difficulty returning to sleep after a middle-of-the-night (MOTN) awakening is a common occurrence. Dosing only when needed (prn) at the time of the awakening decreases patients’ overall exposure to hypnotics. It is not known, however, if prn MOTN dosing results in rebound insomnia on nights medication was not taken. This analysis is the first of its kind.
BACKGROUND
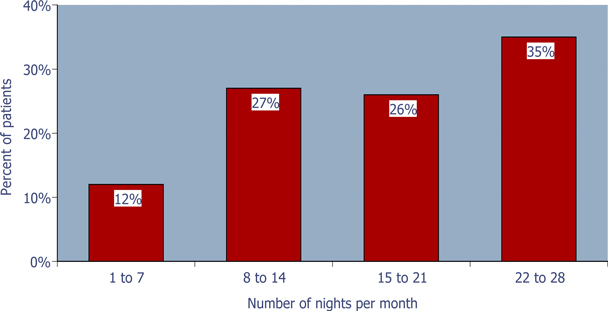
A proprietary binary buffer-containing 1.75 and 3.5 mg zolpidem tartrate sublingual tablet (ZST) for elderly and non-elderly adult patients respectively, is under FDA review for the prn treatment of insomnia when a middle-of-the-night (MOTN) awakening is followed by difficulty returning to sleep. ZST was developed to be used only after MOTN awakening when patients have difficulty returning to sleep and still have at least 4 hours of time remaining in bed. Recently, the 3.5 mg sublingual tablet was shown to be safe and effective in reducing the time to sleep onset when taken after MOTN awakening in a 4-week outpatient study in primary insomnia adult patients with a documented history of MOTN awakenings (described in Poster ID #101). As expected most patients did not take the drug every night of the study (Figure 1).
Figure 1: Pattern of ZST use during the study
65% of the patients used ZST £ 75% of the nights during the 4-week outpatient study
ZST NON-DOSING NIGHTS & REBOUND INSOMNIA
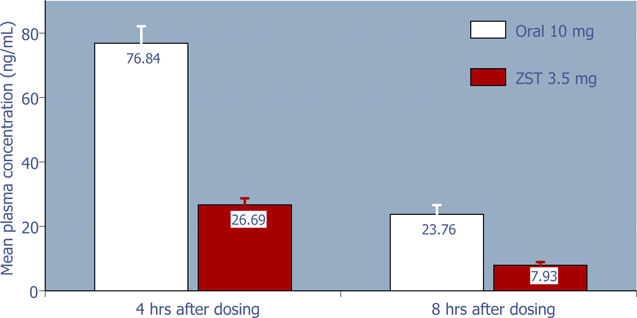
Rebound is typically defined as the effect after a medication is discontinued, when the symptoms being treated by that medication return with greater severity than prior to first taking the medication. On the basis of the relatively low dose of zolpidem in ZST, and hence low residual plasma concentration compared to oral zolpidem 10 mg (Figure 2), it was hypothesized that intermittent use of ZST should not produce rebound insomnia on non-dosing nights. Subsequent analysis of the sleep parameters on non-dosing nights from the 4-week outpatient study supports the hypothesis that on average, ZST does not adversely affect sleep on non-dosing nights (Figures 3 – 6).
Figure 2: 4-hour and 8-hour zolpidem mean plasma concentrations following 3.5 mg ZST and 10 mg oral zolpidem (Ambien®) in adults (Based on data from a separate study of the pharmacokinetics of ZST 3.5 mg compared to oral zolpidem tartrate 10 mg tablet)
SLEEP ON NON-DOSING NIGHTS
Figure 3: Time to sleep onset at bedtime on non-dosing nights
ZST had no effect on bedtime sleep onset on non-dosing nights.*
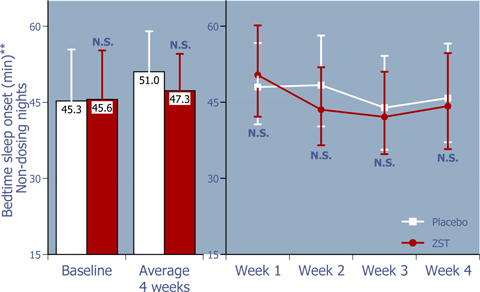
| * | Baseline values were compared using 2-way ANOVA with p value set at 0.05, whereas the remaining p values were based on ANCOVA using the corresponding baseline values as the covariate. |
| ** | LS Mean (95% Confidence Interval) |
Figure 4: Total sleep time from bedtime on non-dosing nights
ZST had no effect on total sleep time on non-dosing nights.*
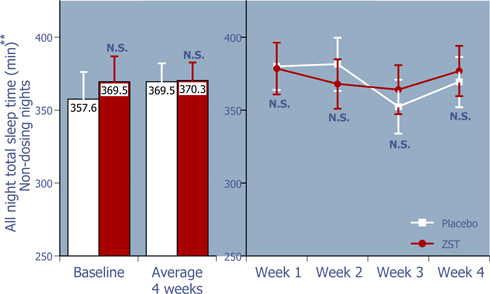
| * | Baseline values were compared using 2-way ANOVA with p value set at 0.05, whereas the remaining p values were based on ANCOVA using the corresponding baseline values as the covariate. |
| ** | LS Mean (95% Confidence Interval) |
SLEEP ON NON-DOSING NIGHTS (continued)
Figure 5: Next morning sleepiness/alertness scores on non-dosing nights
ZST had no negative effects on mornings after non-dosing nights.*
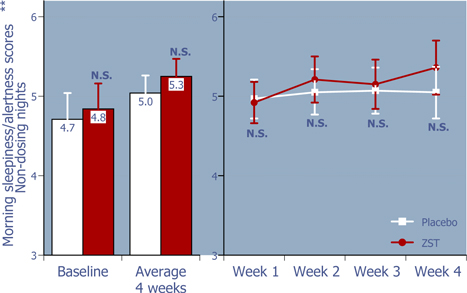
| * | Baseline values were compared using 2-way ANOVA with p value set at 0.05, whereas the remaining p values were based on ANCOVA using the corresponding baseline values as the covariate. |
| ** | LS Mean (95% Confidence Interval) |
Figure 6: Sleep quality on non-dosing nights
ZST had no adverse effect on the quality of sleep on non-dosing nights.*
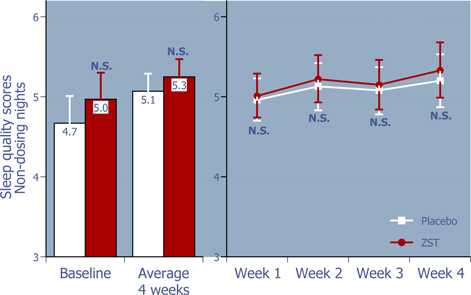
| * | Baseline values were compared using 2-way ANOVA with p value set at 0.05, whereas the remaining p values were based on ANCOVA using the corresponding baseline values as the covariate. |
| ** | LS Mean (95% Confidence Interval) |
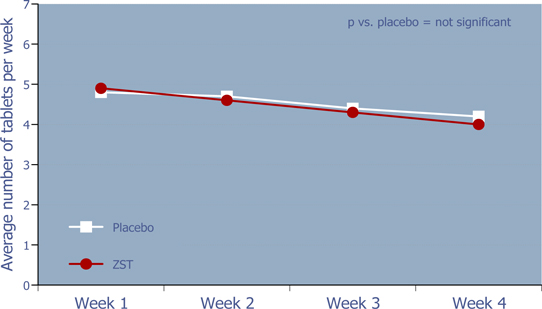
WEEKLY RATE OF DRUG USE
Figure 7: Weekly rate of drug use
There was no evidence of increased utilization over time for ZST or placebo.
CONCLUSIONS
| • | Sleep data from non-dosing nights, which as per the outpatient study protocol are non-MOTN awakening nights, suggest absence of rebound effects on those nights. |
| • | Absence of rebound effects of MOTN use of 3.5 mg zolpidem tartrate sublingual tablet could be explained by relatively less drug exposure due to the low dose of zolpidem and less frequent use. |
For further information on this study, please see Poster ID # 101, “As-Needed Treatment of Insomnia Following Middle-of-the-Night (MOTN) Awakening: Clinical Efficacy of Low-Dose Zolpidem Tartrate Sublingual Tablet,” presented at Association of Professional Sleep Societies, June 7, 2009.
Supported by funding from Transcept Pharmaceuticals, Inc.
[Poster originally presented at SLEEP 2009 23rd Annual Meeting of the Associated Professional Sleep Societies in Seattle, Washington, on June 8, 2009.]
