Attached files
| file | filename |
|---|---|
| EX-2.1 - SHARE EXCHANGE AGREEMENT BY AND BETWEEN THE COMPANY AND DYNAMIC BHORIZON LIMITED, DATED MARCH 25, 2010 - INDESTRUCTIBLE 1, INC | f8k032510ex2i_indestruc1.htm |
| EX-99.1 - THE AUDITED CONSOLIDATED FINANCIAL STATEMENTS OF XINTAI AS OF JUNE 30, 2009 AND 2008 - INDESTRUCTIBLE 1, INC | f8k032510ex99i_indestruc1.htm |
| EX-16.1 - LETTER FROM GATELY & ASSOCIATES, LLC, DATED MARCH 25, 2010 - INDESTRUCTIBLE 1, INC | f8k032510ex16i_indestruc1.htm |
| EX-99.2 - THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS OF XINTAI AS OF DECEMBER 31, 2009 AND 2008 - INDESTRUCTIBLE 1, INC | f8k032510ex99ii_indestruc1.htm |
| EX-99.3 - THE UNAUDITED PRO FORMA FINANCIAL INFORMATION OF INDESTRUCTIBLE - INDESTRUCTIBLE 1, INC | f8k032510ex99iii_indestruc1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): March 25, 2010
| INDESTRUCTIBLE I, INC. |
| (Exact name of registrant as specified in its charter) |
|
Delaware
|
333-154787
|
26-2603989
|
||
|
(State or other jurisdiction of incorporation)
|
(Commission File Number)
|
(I.R.S. Employer Identification No.)
|
|
Room 10, 9th Floor, Building No. 5
9 Gaoshengqiao Road, Dayi Louver Plaza
Wuhou District, Chengdu, Sichuan Province
People’s Republic of China 610041
|
|
(Address of principal executive offices) (Zip Code)
|
|
+86 (028) 8506-8768
|
|
(Registrant’s telephone number, including area code)
|
|
50 West Broadway, 10th Fl.
Salt Lake City, Utah 84101
|
|
(Former name or former address, if changed since last report)
|
––––––––––––––––
Copies to:
Joseph M. Lucosky, Esq.
Yarona Y. Liang, Esq.
Anslow & Jaclin, LLP
195 Route 9 South, Suite 204
Manalapan, New Jersey 07726
(732) 409-1212
––––––––––––––––
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
1
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
The Current Report on Form 8-K contains forward looking statements that involve risks and uncertainties, principally in the sections entitled "Description of Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” All statements other than statements of historical fact contained in this Current Report on Form 8-K, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or elsewhere in this Current Report on Form 8-K, which may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any forward-looking statements.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short term and long term business operations, and financial needs. These forward-looking statements are subject to certain risks and uncertainties that could cause our actual results to differ materially from those reflected in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this Current Report on Form 8-K, and in particular, the risks discussed below and under the heading “Risk Factors” and those discussed in other documents we file with the Securities and Exchange Commission that are incorporated into this Current Report on Form 8-K by reference. The following discussion should be read in conjunction with our annual report on Form 10-K and our quarterly reports on Form 10-Q incorporated into this Current Report on Form 8-K by reference, and the consolidated financial statements and notes thereto included in our annual and quarterly reports. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this Current Report on Form 8-K may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statement.
You should not place undue reliance on any forward-looking statement, each of which applies only as of the date of this Current Report on Form 8-K. Before you invest in our common stock, you should be aware that the occurrence of the events described in the section entitled “Risk Factors” and elsewhere in this Current Report on Form 8-K could negatively affect our business, operating results, financial condition and stock price. Except as required by law, we undertake no obligation to update or revise publicly any of the forward-looking statements after the date of this Current Report on Form 8-K to conform our statements to actual results or changed expectations.
2
Item 1.01Entry into a Material Definitive Agreement
As more fully described in Item 2.01 below, Indestructible I, Inc. (“we,” “Indestructible” or the “Company”) acquired a pharmaceutical company in accordance with a share exchange agreement dated March 25, 2010 (the “Exchange Agreement”) by and among the Company, Dynamic Bhorizon Limited, a Cayman Islands company (“DBL”), and the shareholders of DBL (the “DBL Shareholders”). The closing of the transaction (the “Closing”) took place on March 25, 2010 (the “Closing Date”). On the Closing Date, pursuant to the terms of the Exchange Agreement, we acquired all of the outstanding shares (the “Interests”) of DBL from the DBL Shareholders; and the DBL Shareholders transferred and contributed all of their Interests to us. In exchange, we issued to the DBL Shareholders, their designees or assigns, 15,830,000 shares (the “Exchange Shares”) or 93.12% of the shares of common stock of the Company issued and outstanding after the Closing (the “Share Exchange”). In addition, the shareholders of DBL agreed to pay $325,000 in cash to the shareholders of the Company.
Pursuant to the terms of the Exchange Agreement, Patrick Day, the majority shareholder of the Company, cancelled a total of 12,000,000 shares of common stock of the Company. A copy of the Exchange Agreement is included as Exhibit 2.1 to this Current Report and is hereby incorporated by reference. All references to the Exchange Agreement and other exhibits to this Current Report are qualified, in their entirety, by the text of such exhibits.
Pursuant to the Exchange Agreement, DBL became a wholly-owned subsidiary of the Company. The sole director of the Company has approved the Exchange Agreement and the transactions contemplated under the Exchange Agreement. The directors of DBL have approved the Exchange Agreement and the transactions contemplated hereunder.
As a further condition of the Share Exchange, Patrick Day and Richard M. Day, Jr. resigned as the officers and directors of the Company. Mr. Xiaodong Zhu, Mr. Xicai Su and Mr. Di Wu were appointed as the new officers of the Company, and Mr. Xiaodong Zhu was appointed as the new director of the Company effectively immediately.
The Share Exchange transaction is discussed more fully in Section 2.01 of this Current Report. The information therein is hereby incorporated in this Section 1.01 by reference.
Item 2.01Completion of Acquisition or Disposition of Assets
CLOSING OF EXCHANGE AGREEMENT
As described in Item 1.01 above, on March 25, 2010, we acquired DBL, which is in the business of drug wholesale in the People’s Republic of China (“China” or the “PRC”), in accordance with the Exchange Agreement. The closing of the transaction took place on March 25, 2010. On the Closing Date, pursuant to the terms of the Exchange Agreement, we acquired all the Interests of DBL from the DBL Shareholders; and the DBL Shareholders transferred and contributed all of their Interests to us. In exchange, we issued a total of 15,830,000 shares of common stock to the DBL Shareholders, their designees or assigns, which totals 93.12% of the issued and outstanding shares of common stock of the Company on a fully-diluted basis as of and immediately after the Closing of the Share Exchange.
Pursuant to the terms of the Exchange Agreement, Patrick Day cancelled a total of 12,000,000 shares of common stock of the Company. Following the Share Exchange, there are 17,000,000 shares of common stock issued and outstanding.
DBL was incorporated in Cayman Islands on April 8, 2009. It owns 100% of the issued and outstanding capital stock of Sichuan Xintai Pharmaceutical Co., Ltd. (“Xintai”), which was incorporated as a limited liability company on November 25, 1999 under the laws of China. On January 11, 2010, Sichuan Provincial Government approved DBL to acquire 100% of Xintai. On January 21, 2010, Sichuan Administration for Industry and Commerce issued the new business license of Xintai. With the approval and license, Xintai has been transformed from a domestic company to a wholly foreign owned enterprise (“WFOE”). Pursuant to the Exchange Agreement, DBL became a wholly-owned subsidiary of the Company. DBL and Xintai are collectively referred to herein as “DBL.”
Overview
DBL, through Xintai, is a pharmaceutical wholesale company in the PRC. Xintai was incorporated in 1999 as a small pharmaceutical sales company. On July 15th, 2006, Zhu Xiaodong and Zhu Xiaomei acquired 100% of Xintai for approximately $250,973. Xintai soon became a well-known licensed pharmaceutical wholesale company, specializing in the sales of prescription and OTC medicines. Xintai is also involved in research and development (R&D) of new drugs and medicine registration fields.
3
Our revenue increased approximately 126% for the fiscal year ended June 30, 2009 compared to the fiscal year ended June 30, 2008. We generated over $17.1 million in sales, and approximately $2.4 million in after-tax net income for the year ending June 30, 2009. Our revenue increased approximately 27% for the six months ended December 31, 2009 compared to the six months ended December 31, 2008. We generated over $9.6 million in sales, and approximately $1.77 million in after-tax net income for the six months ended December 31, 2009.
Historical Sales & Income Summary
Because Indestructible existed as a shell prior to the merger, the transaction is being accounted for as a reverse acquisition, so DBL is treated as the continuing reporting entity that acquired Indestructible. As a result, all historical financial information being presented is that of DBL and Xintai.
|
(Amounts expressed in USD)
|
Fiscal Year Ended
June 30,
|
%
|
6 Months
Ended December 31,
(Unaudited)
|
%
|
||||||||||||||||||||
|
2009
|
2008
|
Growth
|
2009
|
2008
|
Growth
|
|||||||||||||||||||
|
Revenue
|
$ | 17,127,474 | $ | 7,576,098 | 126.07 | % | $ | 9,653,544 | $ | 7,557,227 | 27.74 | % | ||||||||||||
|
Gross Profit
|
4,848,538 | 2,010,689 | 141.14 | % | 2,617,236 | 1,679,918 | 55.80 | % | ||||||||||||||||
|
Net Income
|
2,391,595 | 935,008 | 155.78 | % | 1,777,194 | 992,078 | 79.14 | % | ||||||||||||||||
Organization & Subsidiaries
DBL’s organizational structure was developed to allow foreign capital infusion under the laws of the PRC and to maintain an efficient tax structure, as well as to maintain internal organizational efficiencies. The Company’s organization structure post-Share Exchange is summarized in the table below:
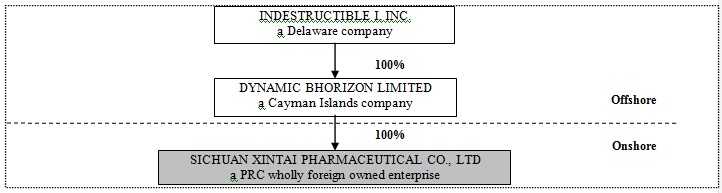
DBL was incorporated in the Cayman Islands on April 8, 2009. It owns 100% of the issued and outstanding capital stock of Xintai, which was incorporated as a limited liability company on November 25, 1999 under the laws of China. On January 11, 2010, Sichuan Provincial Government approved DBL to acquire 100% of Xintai. On January 21, 2010, Sichuan Administration for Industry and Commerce issued the new business license to Xintai. With the approval and the new business license, Xintai has been transformed from a Chinese domestic company to a WFOE.
Business
Xintai was incorporated in Chengdu City, Sichuan Province, China in November 1999. Xintai is in the business of prescription medicines sales, OTC medicines sales and medicine R&D. Effective March 25, 2010, DBL entered into a Purchase Agreement to be acquired by us.
Industry and Market Analysis
Industry Overview
According to the research conducted by Intercontinental Marketing Services (“IMS’) (website http://www.imshealth.com), the total revenue for prescription medicines in the world reached 745 billion US dollars in 2008. The prescription medicines sales in developing countries accounted for 25% of the market shares, which increased by 25.7% compared to 2007.
4
China’s pharmaceutical market is highly fragmented and inefficient. As of 2007, China had around 3,000 to 6,000 domestic pharmaceutical manufacturers and approximately 14,000 domestic pharmaceutical distributors. A lack of protection of intellectual property rights, a lack of visibility for drug approval procedures, a lack of effective governmental incentives and poor corporate support for drug research make their lives even harder.
The severe competition in China’s pharmaceutical market and market environment results in the fact that wholesalers and distributors play a major role in the value chain. Those pharmaceutical manufacturers who work closely with wholesalers with strong sales network sell more drugs. Therefore, further market consolidation is highly expected in China.
Market Analysis
According to IMS’s report dated October 8, 2009, the value of the global pharmaceutical market in 2010 is expected to grow by 4% – 6% on a constant-dollar basis, exceeding $825 billion US dollars, driven by stronger near-term growth. The forecast also predicts global pharmaceutical market sales to grow at a 4% - 7% compound annual growth rate through 2013, and takes into account the impact of the global macro economy, the changing mix of innovative and mature products, and the rising influence of healthcare access and funding on market demand. Global pharmaceutical market value is expected to expand to more than $975 billion US dollars by 2013. China’s pharmaceutical market is expected to continue to grow at more than 20% pace annually, and contribute 21 percent of overall global growth through 2013.
In addition, the PRC government announced that by 2020, the national medical insurance will cover over 90% of the total population. China’s changing health-care environment is designed to extend basic health insurance to a larger portion of the population and give individuals greater access to products and services. Following this period of change, medical insurance is now more widespread and the pharmaceutical industry is expected to continue its expansion.
Core Competencies
Since its incorporation in 1999, Xintai has become a rapidly growing pharmaceutical wholesale company. Its rapid development is built on a number of core competencies:
Professional and Experienced Sales Personnel. Currently, we have more than 2,000 contracted professional pharmaceutical sales representatives. In addition to the strong sales team, Xintai has an expert team providing post-sales support. Xintai has its point of sales throughout all of 32 provinces and municipal cities in China. This extensive sales network enables the company to make efficient product distribution and sales. All the sales representatives had educational background or training in neurological, cardio/cerebrovascular, antineoplastic medicines or antibiotics, which are some of the drugs we are specialized in marketing. They have also established long term relationship with many hospitals and doctors who are the main channels of our products. Most of our sales representatives are commission-based only, meaning they only receive payment when they make successful sales. Xintai relies on this practice to significantly reduce risk in marketing.
Extensive Sales Network. We also work with both local pharmaceutical sales companies and local authorized distribution companies. Xintai’s products are currently sold in more than 2,500 hospitals in China.
Ability to Obtain Exclusive Dealership of High Value New Medicines. Xintai carefully chooses its product lines. In light of the highly competitive market in China, Xintai focuses on authorized dealership on such popular medicines that have comprehensive usage, large consumption per patient and relative high value and high profit margin. Xintai’s competitive advantages are:
|
·
|
Xintai continues to collect market information from partners, hospitals, doctors and patients and carefully analyzes its sales data. Therefore, Xintai is able to develop market trend ahead of many of our competitors
|
|
·
|
Xintai’s suppliers are pharmaceutical manufacturers. Most domestic pharmaceutical manufacturers don’t have their own zoning distribution channels or have limited marketing capabilities. Xintai is able to secure dealership of high value-added products due to its impressive sales record and good reputation in pharmaceutical distribution.
|
|
·
|
Xintai focuses on high margin products, such as cardiovascular and antineoplastic drugs. Those medicines have long-term and stable demand. Xintai also employs other promotion channels, such as advertising on major academic journals, press conference, academic promotion conference and regional promotion to sell the company’s products.
|
Products and Competitive Advantages
The company is dedicated to the distribution and sales of prescription medicines to hospitals and medical institutions. Xintai is certified by the Provincial Food and Drug Administration for Drug Supply License and Drug Supply Certificate of Quality Management Standards. Focusing on cardiac, cerebral and vascular products, Xintai’s main products are cardiac, cerebral and vascular, oncoma and anti-infective medicines, for example ganglioside, edaravone, polydanshinolate vial, sulbenicillin sodium, imipenem and cilastatin sodium and arsenious acid.
5
Major Products sold in China:
Below is a chart which shows the major products of our company sold in China:
|
Product
|
Category
|
Covered by Medical Insurance
|
Estimated Market Share in China (2009)
|
Expected Growth Rate
|
|
Ganglioside Injection
|
Cardio/cerebrovascular
|
No
|
25%
|
25%
|
|
Edaravone Injection Injection
|
Cardio/cerebrovascular
|
Yes
|
5%
|
35%
|
|
Magnesium Lithospermate B for Injection
|
Cardio/cerebrovascular
|
Yes
|
0.5%
|
45%
|
|
Imipenem and Cilastatin Sodium for Injection
|
Antibiotics
|
Yes
|
1%
|
20%
|
Ganglioside
Product feature
The product is the medicine for repairing nerve damages with the most affirmative clinical effect, currently with no substitute. Its market share is expected to continue to increase in the next three to five years.
Market size
More than 7 million patients with central nerve damage from brain stroke are in need of treatment in China. About 8 million patients with other nerve damage by bone injury are also in need of treatment. Calculated according to the treatment cycle of three weeks, the annual Market size is around 7.3 billion US dollars; the current realized market sales of the product is 440 million US dollars. It is estimated that in the next three to five years, the market sales will reach to 2.93 billion US dollars.
Competition analysis
Currently there are five manufacturers who make this product. Only two manufacturers have the Approval for Raw Material Production, therefore ensuring a stable supply—Qilu Pharmaceutical and Harbin Medical University Pharmaceutical, while the other three manufacturers can’t maintain a stable supply due to the lack of the raw materials. Xintai’s cooperative manufacturer partner--Harbin Medical University Pharmaceutical is one of the two manufacturers with the Approval for Raw Material Production. At present, Xintai’s main competitor—Qilu Pharmaceutical (“Qilu”) employs non-agency, direct sales practice. Although Qilu entered the market earlier and has a larger market share, Xintai outperforms Qilu in terms of rapid market building, low-cost operation and academic marketing.
Edaravone Injection
Product feature
Edaravone, one of the second grade new medicines, is a protective medicine to neuronal injury. The product was listed on the 2009 National Medical Insurance List in China, having a substantial increase in market share.
Market size
The market size for Edaravone is estimated to be 1.46 billion US dollars in China. Currently, the actual sales of “Bicun” products amounted to near 87.9 million US dollars, and the sale of “Yidasheng” was 29.3 million US dollars. After the product enters the National Medical Insurance List, it is expected to expand its market share by 30% annually in the next three to five years.
Competition analysis
Our main competitive manufacturers are: 1) Simcere Pharmaceutical Group. Product name: “Bicun”. 2) Jinlin Boda Pharmaceutical. Product name: “Yidasheng”. By November 2008, SFDA had approved five pharmaceutical companies to manufacture this product. The other three are: 3) China National Medicines Guorui Pharmaceutical Co., Ltd, 4) Kunming Jida Pharmaceutical Co., Ltd, and 5) Jilin Huinan Changlong Bio-chemical Pharmaceutical Co., Ltd. Our cooperative manufacturer partner is Jilin Boda Pharmaceutical. “Yidasheng” is of exclusive specification, with certain extent of monopoly-sized market share.
6
Polydanshinolate Vial
Product feature
The Polydanshinolate Vial was bestowed with the New Medicine Certificate from China State Food and Drug Administration on May 25th 2005, classified as the second grade national new medicine, and category B national medical insurance medicine. So far it is the Danshen (Salvia miltiorrhiza Bge) formulations with the clearest and highest content of active ingredients. The relative preparation techniques are patented in China and in the US as well. It is the first Chinese medical product with pharmacokinetic parameters in human body and adopting exercise stress test to evaluate the therapeutic effect. Through large amount of clinical trials, it has been proved to have significant therapeutic effect, medicinal safety and stable quality on the treatment of coronary heart disease and angina. At present, the pharmacological activity and importance of magnesium danshinoacetate has been confirmed by the authority of Pharmacopoeia Commission of P.R.C. And magnesium danshinoacetate is confirmed to be the control index of the quality standard for Danshen pills.
The polydanshinolate vial is the successful product of 13-year academic research by Shanghai Institute of Pharmacology, a branch of Chinese Academy of Sciences. The research on the effective ingredients of Danshen water-solubility has found that the magnesium danshinoacetate dominate polyphenol niobate is the most important and most effective active ingredient in treating cardiac and vascular disease, starting from which, advanced manufacturing technologies are adopted to introduce the hi-tech finger prints technology. With the polydanshinolate content in the product reaching 100% and the content of magnesium danshinoacetate reaching 80%, the product is manufactured into frozen dry powder injection.
Market size
As the traditional Chinese medicine promoting blood circulation by removing blood stasis, Danshen has been used to treat coronary heart disease, angina, ischemic stroke, etc. The consumption of injection alone in China is over 3 billion vials. The 2008 sale volume of polydanshinolate vial was 1 million vials, with the sales revenues of 20.2 million US dollars. It is estimated the market will grow by 40% annually in the next three years.
Competition analysis
The polydanshinolate vial is the only Chinese medicine product with 100% of effective ingredient in Chinese market. It has positive clinical effect, its market advantages cannot be compared by any other products in the same category, and its clinical application is irreplaceable.
Sulbenicillion Sodium
Product feature
Sulbenicillion sodium is one of the penicillin antibiotics, applicable to sensitive pseudomonas aeruginosa, some bacillus proteus and other sensitive Gram-positive bacteria caused pneumonia, urinary track infection, CSSSI and septicemia.
Market size
In 2009, the company signed an (3+2) agency agreement with Hunan Xiangyao Pharmaceutical Co., Ltd. According to the different economic conditions, population situations in six provinces, we formulate corresponding investment inviting policies. The sale of Sulbenicillion Sodium will reach 4 million vials in the first year, and the returned money for the bottom price is nearly 5.86 million US dollars. It is estimated that the market will grow by 30% annually.
Competition analysis
There are four sulbenicillion sodium manufacturers with Manufacture Approvals in China currently, which are Shandong Reyong Pharmaceutical, Shenyang Meiluo, Harbin General Pharmaceutical Factory and Hunan Xiangyao Pharmaceutical Co., Ltd. At present, only Shandong Reyong Pharmaceutical and Hunan Xiangyao Pharmaceutical Co., Ltd are equipped with manufacturing ability. Our company represents the 1g sulbenicillion sodium from Hunan Xiangyao.
Sulbenicillion sodium has entered the category B in the adjusted National Medical Insurance in 2009, with the exceptional advantages of few manufacturers, small competition, and listed medical insurance product. Given the national restriction on the antibiotics use especially the cephems antibiotics, our product has won the unanimous appraisal from all drug agencies and medical staff. The marketing prospective is exceptionally broad.
7
Imipenem and Cilastatin Sodium
Product feature
Imipenem and cilastatin sodium is characterized by high efficiency, broad spectrum and no cross-resistance with other β-lactam antibiotics. It has good enzyme-resistance, with relatively less cross-resistance with other β-lactam antibiotics, and is still effective to the infections caused by many cephalosporin resisted bacteria. The product is mainly applicable to sensitive bacteria caused lower respiratory infection, abdominal infection, gynecological genital tract infection, urinary and reproductive system infection, skin and soft tissue infection, bone and joint infection, septicemia, and endocarditis, etc. It is also used in preoperative infection prevention and post-operative infection prevention.
Market size
Penem medicine (carbopenems) is a dark horse in antibiotics arising in recent years. It came out in 1980s, belonging to untypical β-lactam antibiotics with the broadest antimicrobial spectrum, also characterized by strong antibiotic activity. To date, there are altogether seven to eight penem medicines around the world. In 2008, the total sales volume in the globe reached to US dollars 2.5 billion, taking up around 10% of the antibiotics market.
From 2005 to 2008, the consumption by hospitals averaged a growing rate of 24.13%. In 2008, the amount for procurement of penem medicines reached 329 million US dollars.
Imipenem has the second largest sales among penem medicines. Meiluo Penem is in leading position in penem medicine market. The increasing rate in recent years was over 33.34%. In 2008, Meiluo Penem Hospital consumption scale was around 170.4 million US dollars. The growth in the next three years is expected to exceed 30% annually.
Competition analysis
There are four manufacturers importing imipenem and cilastatin sodium vial, including Shenzhen Haibin Pharmaceutical Co., Ltd, Zhejiang Hisum Pharmaceutical Co., Ltd, and China National Medicines Guorui Pharmaceutical Co., Ltd in China. The domestic imipenem enjoys price advantage, with the annual increasing rate of 30%; Tienam from Merck & Co., Inc (including Hangzhou Merck & Co., Inc) has been dominating the whole imipenem market for long. Along with the entering of new companies recent years, the market share of Merck & Co., Inc has been gradually dropping.
Research & Development
Currently, we do not maintain our own R & D department. Instead, we conduct collaborated work with some of the most prestigious medical research institutions in China, such as Academy of Military Medical Sciences, China Pharmaceutical University and Chinese Academy of Sciences. Through the collaborated work, our strategy aims at: (1) imitating popular drugs that are off-patent protection; (2) developing new drug formulations and obtaining patent protection for those drugs with greater market potentials. In October 2009, we founded a R&D center together with Dr. Di Wu, to focus on research and development of new drugs.
At present, Our Company, through collaborated work with other academic institutions, has the following key drugs under research and development:
Clopidogrel Bisulfate Tablets
Product features
It belongs to platelet aggregation inhibitor, which combines with the blood platelet through the selective inhibition of adenosine diphosphate (ADP) and then ADP mediates the activation of glycoprotein complex. It applies to the patients with stroke and myocardial infarction who have recent attack, and those who have been diagnosed with peripheral arteriosclerosis. Clopidogrel can reduce the occurrence of atherosclerosis events (such as myocardial infarction, stroke and vascular death).
Market size
The trade name of the leading brand for this product in 2008 is Plavix, whose annual sales volume is about 3.5 billion US dollars worldwide and around 73.2 million US dollars in China alone. Another top-selling product in Chinese market is called “Jiatai”, whose annual sales volume in China is about 29.3 million US dollars in 2009. Because of the exclusiveness of the patented technology and the particularity of the technique, there has not been any third pharmaceutical enterprise declaring this kind of new drug. It is predicted that there will be over 40% market growth in the following 3-5 years.
8
Competition analysis
Currently there are two similiar drugs market in China. One is Plavix produced by Sanofi-Aventis Pharmaceuticals and the other is Jiatai produced by Shenzhen Salubris Pharmaceuticals Co., Ltd in China. The curative effect of Plavix is affirmative, its price is high and it enjoys about 2/3 market share; while Jiatai has the price advantage, but the clinical popularization is insufficient, the recognition from the doctors has yet to be improved and its market share is about 1/3. The other product Aggrastat® (tirofiban HCl) which is produced by NovaMed Pharmaceuticals Inc in China, NovaMed has exclusive right from Iroko selling non US countries should also be noticed, Aggrastat® is a fast acting, reversible GP IIb/IIIa inhibitor that disaggregates platelet thrombi and improve myocardial perfusion. Aggrastat is proven to be significantly effective in improving the results of primary coronary angioplasty in patients with myocardial infarction. This drug is in the process of regulatory filing. Our clopidogrel development team possesses extensive experience working at some of the world’s most famous drug enterprises and the technology we developed has the comparative advantages over the domestic enterprises. We will develop and manufacture the drug domestically, the cost can be much lower compared to that of Plavix and we will have huge price advantage over Plavix. Our impressive marketing team has extensive and mature experience in the academic promotion of cardiovascular products and our clinical academic promotion ability can rival Plavix’s promotion techniques. Therefore, in the next 2-3 years, we expect that we will take over at least 1/3 market share. Moreover, we will focus on to further improve our production standard after reinforcing the strength of our company and aim to enter the U.S. market with the product as early as possible.
Time schedule for development
Through the collaborated work with some of the top academic institutions, we believe that we have already developed the necessary production process and we will start to apply for the Federal Manufacture Approval and apply for Clinical Verification in March – April, 2010. The predicted mass production will be by the end of 2013.
Marketing and Sales Strategy
Xintai’s revenues are mainly from authorized medicine sales. Xintai is the market leader for some medicines. For example, Xintai’s Ganglioside Injection sales accounts for 30% of the total market share in China. Overall, Xintai’s products can reach 65% of China’s upper first-class hospitals. By 2010, this number is expected to be increased to 80%.
Marketing Model
Xintai has Pharmaceutical Trade License and is approved by Good Supply Practice (GSP) to lawfully sell medicine within China. Our headquarter is located in Chengdu city of Sichuan province in China. We have a sales representative office in Hanoi, Vietnam. Xintai also has contracted dealership with certain pharmaceutical manufacturers which produce top selling drugs for neurological disorder, cardio/cerebrovascular disease, as well as antibiotics and anti-tumor medicine. Xintai’s differentiated marketing strategy is combining targeting specific hospitals with academic promotion, this strategy help to build sale channel of the products in a long term. Xintai’s suppliers are pharmaceutical manufacturers and its customers are retail companies. Xintai does not directly sell to hospitals and doctors in order to prevent business fraud and brides in the sales chain.
Academic Promotion Team
Xintai has four different academic promotion teams which consist of experts from neurological, Cardio/cerebrovascular, infectious diseases and anti-tumor areas. When Xintai starts selling new drugs, these teams will give lecture and hold forums in the cities around country by means such as road shows. These experts who are influential in the field will have positive influence on the images of Xintai’s new products since they are well known professionals. After the good reputation and image are built,, Xintai’s experts will invite local senior doctors to attend product launching meetings so that these doctors will learn the products better and build relationship with expert.
Sales Network
Xintai’s sales force has two key advantages: many years experience professional and strong structure. Three sales directors with solid medicine sales background are responsible for launching and marketing the products. 15 regional sales managers are leading 256 city-level sales managers. And another 2000 sales representatives are behind city-level sales managers who have build long term relationship with hospitals and doctors and they all have amazing sales record.
Xintai also has an exclusive database of more than 5000 “would-be” part-time sales representatives who are motivated when new products are launched. Our marketing team consists of 1 marketing director, 6 product managers and 20 promotion specialists, which are able to organize tailored marketing campaigns for specific products.
9
Distribution Channel
Xintai sales channel is structured through retail distributor companies to hospitals. These companies are legally authorized medicine distributors in China. Xintai sets up minimum sales target. If distributors cannot meet such minimum sales target, Xintai will move on to better performed retail companies with wide coverage and selling power. Each regional sales manager works with at least 10 retail companies, therefore at any point of time, there are at least 200 retail companies are selling products wholesaled through Xintai.
Marketing and Sales Strategies
Product Strategy: focusing on newly developed medicine targeting main disease area and selected the top selling drugs with high profit margin. R&D team will try to develop off-patent drugs.
Price Strategy: selling price is primarily determined by local medical insurance regulatory bodies or public bidding. Xintai can usually sell below these set prices since the profit margins are still high.
Distributor Strategy: Xintai is not relying on one major distributor; instead, it has at least 3 distributors for each region which will ensure that distribution channels are always functional at all time.
Promotion Strategy: The key members of academic promotion teams are comprise of 10 leading experts of each field. They will recruit 30-50 key field leaders who can be academics or physicians and another 100-300 promotion specialists at each time when the new products are launched or when periodic marketing campaigns are scheduled. They will attend every local seminar or forum organized by Xintai and build connections with local doctors. These teams will also promote Xintai’s products through public channel.
Government Affair Strategy: Xintai maintained long-term good relationships with Medicare, Tendering and Pricing authorities so all the latest information or policies can be obtained at a timely manner.
Incentive Mechanism: Build up the incentive policy combined with cash and bonus. The management team implement performance appraisal on sales team monthly and quarterly. Revenue received is correlated with cash bonus and also combines with profit target, market development and customer management and etc. The senior and middle management team will be allocated with bonus option.
Customer Appreciations: Focus on developing 100 VIP clients, the Company will give discounts monthly or quarterly as customer loyalty incentives; also host customer appreciation party for VIP clients quarterly;
Suppliers
The Chinese domestic pharmaceutical market is highly fragmented and inefficient. Many of the manufacturers are making similar products which are resulting in vicious competition. Xintai’s suppliers are those who produce best sell medicines with greater returns. Xintai’s longtime suppliers include Aode Pharmaceutical, Kaide Medical R&D Center, and Furuide Pharmaceutical. These three supplies account for 38.91%, 24.13% and 22.19% of Xintai’s total purchase as of June 2009.
Major Customer
Xintai usually receives payment first and then makes the delivery. When customers place the order, Xintai will deliver the products after the payments are received.
Three main customers for Xintai are Kangtai Pharmaceutical (in Anhui Province), Shandong Pharmaceutical (in Shandong Province) and Hezhonghuitong Pharmaceutical (in Henan Province). They accounts for 7.76%, 7.45% and 6.3% of our total sales respectively as of June 2009.
Development Plan
Xintai aims to expand the company’s business and maximize its profit by acquiring and merging with R&D institutes and pharmaceutical manufacturers in the next five years. The company will emphasize more on R&D, add product lines, and focus on high value-added products. This business model will maximize the company’s competitiveness in the market. Xintai plans to aggressively grow medicine import and export business with countries in South-East Asia, Africa, Mid-East, Mid-Asia, and East-Europe region, in addition to growing the domestic market. The company also intends to acquire pharmaceutical manufacturers in South-East Asia, and take the advantages of their relative low cost operation and favorable government policies to take international market shares. Medicine, medical equipments and medical supplies will become the company’s three major product lines.
10
Sign up for higher value-added product dealerships: Xintai plans to sign up for more dealerships by adding 2-3 cardiovascular products, 1-2 anti-tumor products and 2-3 antibiotics.
Increase product lines: Xintai intends to develop 3-5 prescription drugs, 5-10 OTC drugs and 10-30 generic drugs by outsourcing manufacture or manufacturing in acquired pharmaceutical manufacturers.
Acquire R&D institutes and increase self R&D capability: Xintai intends to acquire upper stream good quality pharmaceutical companies with valuable R&D assets and promising projects which have great potential developing into products so that Xintai’s developing speed could be accelerated.
Increase Production Capability by Acquiring Pharmaceutical Manufacturers: Xintai is to increase manufacturing capability by acquiring pharmaceutical companies which hold medicine production permits.
Overseas Expansion: From 2010 to 2014, Xintai plan to register local offices in Jakarta, New Delhi, Cairo, and Astana to make medicine registration and sales.
Properties
As of December 31, 2009 and June 30, 2009, the detail of property, plant and equipments was as follows:
|
As of
|
||||||||
|
December 31, 2009
|
June 30, 2009
|
|||||||
|
(Unaudited)
|
||||||||
|
Office equipment
|
$ | 29,870 | $ | 77,122 | ||||
|
Automobiles
|
110,971 | 29,854 | ||||||
|
Sub-total
|
140,841 | 106,976 | ||||||
|
Less: accumulated depreciation
|
(23,390 | ) | (15,717 | ) | ||||
|
Property, plant and equipment, net
|
$ | 117,451 | $ | 91,259 | ||||
Depreciation expense for the six months ended December 31, 2009 and 2008 was $7,662 and $2,767, respectively.
Employees
Xintai currently has senior management team of 35 people, 3 sales directors, 15 regional sales managers, 256 city-level sales managers and more than 2000 commission-based sales representatives.
Xintai’s employment agreement with Xiaodong Zhu is effective from September 10, 2009 to September 10, 2010. Monthly salary is 1,465 US dollars.
Xintai’s employment agreement with Xicai Su is effective from September 10, 2009 to September 10, 2010. Monthly salary is 879 US dollars.
Xintai’s employment agreement with Di Wu is effective from January 1, 2010 to January 1, 2011. Monthly salary is 2,930 US dollars.
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated herein that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, you may lose all or part of your investment.
11
Risks Relating to Our Business
Substantially all of our business, assets and operations are located in the PRC.
Substantially all of our business, assets and operations are located in the PRC. The economy of PRC differs from the economies of most developed countries in many respects. The economy of PRC has been transitioning from a planned economy to a market-oriented economy. Although in recent years the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in PRC is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry by imposing industrial policies. It also exercises significant control over PRC’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Some of these measures benefit the overall economy of PRC, but may have a negative effect on us.
Our management has no experience in managing and operating a public company. Any failure to comply or adequately comply with federal securities laws, rules or regulations could subject us to fines or regulatory actions, which may materially adversely affect our business, results of operations and financial condition.
Our current management has no experience managing and operating a public company and relies in many instances on the professional experience and advice of third parties including its attorneys and accountants. Failure to comply or adequately comply with any laws, rules, or regulations applicable to our business may result in fines or regulatory actions, which may materially adversely affect our business, results of operation, or financial condition and could result in delays in the development of an active and liquid trading market for our stock.
Our plans to expand our product lines, to acquire a pharmaceutical factor, to build a research and development center and to improve and upgrade our internal control and management system will require capital expenditures in 2010.
Our plans to expand our product lines, to acquire a pharmaceutical factor, to build a research and development center and to improve and upgrade our internal control and management system will require capital expenditures in 2010. We may also need further funding for working capital, investments, potential acquisitions and other corporate requirements. We cannot assure you that cash generated from our operations will be sufficient to fund these development plans, or that our actual capital expenditures and investments will not significantly exceed our current planned amounts. If either of these conditions arises, we may have to seek external financing to satisfy our capital needs. Our ability to obtain external financing at reasonable costs is subject to a variety of uncertainties. Failure to obtain sufficient external funds for our development plans could adversely affect our business, financial condition and operating performance.
We derive all of our revenues from sales in the PRC and any downturn in the Chinese economy could have a material adverse effect on our business and financial condition.
All of our revenues are generated from sales in the PRC. We anticipate that revenues from sales of our products in the PRC will continue to represent the substantial portion of our total revenues in the near future. Our sales and earnings can also be affected by changes in the general economy since purchases of pork products are generally discretionary for consumers. Our success is influenced by a number of economic factors which affect disposable consumer income, such as employment levels, business conditions, interest rates, oil and gas prices and taxation rates. Adverse changes in these economic factors, among others, may restrict consumer spending, thereby negatively affecting our sales and profitability.
Our planned expansion could be delayed or adversely affected by, among other things, difficulties in obtaining sufficient financing, technical difficulties, or human or other resource constraints.
Our planned expansion could be delayed or adversely affected by, among other things, difficulties in obtaining sufficient financing, technical difficulties, or human or other resource constraints. Moreover, the costs involved in these projects may exceed those originally contemplated. Costs savings and other economic benefits expected from these projects may not materialize as a result of any such project delays, cost overruns or changes in market circumstances. Failure to obtain intended economic benefits from these projects could adversely affect our business, financial condition and operating performances.
We encounter substantial competition in our business and any failure to compete effectively could adversely affect our results of operations.
As described in the products section, for every product we sell, we encounter strong competitors. We anticipate that our competitors will continue to expand and seek to obtain additional market share with competitive price and performance characteristics. Aggressive expansion of our competitors or the entrance of new competitors into our markets could have a material adverse effect on our business, results of operations and financial condition.
12
Our limited operating history may not serve as an adequate basis to judge our future prospects and results of operations.
Our limited operating history in the pharmaceutical industry may not provide a meaningful basis for evaluating our business. Xintai entered into its current line of business in 2006, although it was incorporated in 1999. Although its revenues have grown rapidly since its inception, we cannot guaranty that we will maintain profitability or that we will not incur net losses in the future. We will continue to encounter risks and difficulties that companies at a similar stage of development frequently experience, including the potential failure to:
|
·
|
obtain sufficient working capital to support our expansion;
|
|
·
|
expand our product offerings and maintain the high quality of our products;
|
|
·
|
manage our expanding operations and continue to fill customers’ orders on time;
|
|
·
|
maintain adequate control of our expenses allowing us to realize anticipated income growth;
|
|
·
|
implement our product development, sales, and acquisition strategies and adapt and modify them as needed;
|
|
·
|
successfully integrate any future acquisitions; and
|
|
·
|
anticipate and adapt to changing conditions in the pharmaceutical industry resulting from changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
|
If we are not successful in addressing any or all of the foregoing risks, our business may be materially and adversely affected.
We need to manage growth in operations to maximize our potential growth and achieve our expected revenues and our failure to manage growth will cause a disruption of our operations resulting in the failure to generate revenues at levels we expect.
In order to maximize potential growth in our current and potential markets, we believe that we must expand our producing operations. This expansion will place a significant strain on our management and our operational, accounting, and information systems. We expect that we will need to continue to improve our financial controls, operating procedures, and management information systems. We will also need to effectively train, motivate, and manage our employees. Our failure to manage our growth could disrupt our operations and ultimately prevent us from generating the revenues we expect.
We cannot assure you that our growth strategy will be successful which may result in a negative impact on our growth, financial condition, results of operations and cash flow.
One of our strategies is to grow through acquiring a pharmaceutical factory and increase product lines. However, there are many obstacles to expand our product lines. We cannot, therefore, assure you that we will be able to successfully overcome such obstacles and establish our products in any additional markets. Our inability to implement this organic growth strategy successfully may have a negative impact on our growth, future financial condition, results of operations or cash flows.
If we need additional capital to fund our growing operations, we may not be able to obtain sufficient capital and may be forced to limit the scope of our operations.
If adequate additional financing is not available on reasonable terms, we may not be able to undertake acquisitions of a pharmaceutical factory or to build a research and development center, and we would have to modify our business plans accordingly. There is no assurance that additional financing will be available to us.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
13
In recent years, the securities markets in the United States have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, our securities can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If we need additional funding we will, most likely, seek such funding in the United States (although we may be able to obtain funding in the PRC) and the market fluctuations affect on our stock price could limit our ability to obtain equity financing.
If we cannot obtain additional funding, we may be required to: (i) limit our expansion; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures. Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are favorable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to the Units. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
Need for additional employees.
The Company’s future success also depends upon its continuing ability to attract and retain highly qualified personnel. Expansion of the Company’s business and operation will require additional managers and employees with industry experience, and the success of the Company will be highly dependent on the Company’s ability to attract and retain skilled management personnel and other employees. There can be no assurance that the Company will be able to attract or retain highly qualified personnel. Competition for skilled personnel in the construction industry is significant. This competition may make it more difficult and expensive to attract, hire and retain qualified managers and employees.
The loss of the services of our key employees, particularly the services rendered by Xiaodong Zhu, our director, and Xicai Su and Di Wu, our officers, could harm our business.
Our success depends to a significant degree on the services rendered to us by our key employees. If we fail to attract, train and retain sufficient numbers of these qualified people, our prospects, business, financial condition and results of operations will be materially and adversely affected. In particular, we are heavily dependent on the continued services of Xiaodong Zhu, Our director, and Xicai Su and Di Wu, our officers. The loss of any key employees, including members of our senior management team, and our inability to attract highly skilled personnel with sufficient experience in our industry could harm our business.
Our failure to comply with increasingly stringent environmental regulations and related litigation could result in significant penalties, damages and adverse publicity for our business.
In recent years, the government of China has become increasingly concerned with the degradation of China’s environment that has accompanied the country’s rapid economic growth. In the future, we expect that our operations and properties will be subject to extensive and increasingly stringent laws and regulations pertaining to, among other things, the discharge of materials into the environment and the handling and disposition of wastes (including solid and hazardous wastes) or otherwise relating to protection of the environment. Failure to comply with any laws and regulations and future changes to them may result in significant consequences to us, including civil and criminal penalties, liability for damages and negative publicity. We cannot assure you that additional environmental issues will not require currently unanticipated investigations, assessments or expenditures, or that requirements applicable to us will not be altered in ways that will require us to incur significant additional costs.
We will incur significant costs to ensure compliance with United States corporate governance and accounting requirements.
We will incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
14
We may not be able to meet the internal control reporting requirements imposed by the Securities and Exchange Commission resulting in a possible decline in the price of our common stock and our inability to obtain future financing.
As directed by Section 404 of the Sarbanes-Oxley Act, as amended by SEC Release No. 33-8934 on June 26, 2008, the Securities and Exchange Commission adopted rules requiring each public company to include a report of management on the company’s internal controls over financial reporting in its annual reports. In addition, the independent registered public accounting firm auditing a company’s financial statements must also attest to and report on management’s assessment of the effectiveness of the company’s internal controls over financial reporting as well as the operating effectiveness of the company’s internal controls. Commencing with its annual report for the year ending June 30, 2010, we will be required to include a report of management on its internal control over financial reporting. The internal control report must include a statement
|
·
|
Of management’s responsibility for establishing and maintaining adequate internal control over its financial reporting;
|
|
·
|
Of management’s assessment of the effectiveness of its internal control over financial reporting as of year end; and
|
|
·
|
Of the framework used by management to evaluate the effectiveness of our internal control over financial reporting.
|
Furthermore, in the following year, our independent registered public accounting firm is required to file its attestation report separately on our internal control over financial reporting on whether it believes that we have maintained, in all material respects, effective internal control over financial reporting.
While we expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404 of the Sarbanes-Oxley Act, there is a risk that we may not be able to comply timely with all of the requirements imposed by this rule. In the event that we are unable to receive a positive attestation from our independent registered public accounting firm with respect to our internal controls, investors and others may lose confidence in the reliability of our financial statements and our stock price and ability to obtain equity or debt financing as needed could suffer.
In addition, in the event that our independent registered public accounting firm is unable to rely on our internal controls in connection with its audit of our financial statements, and in the further event that it is unable to devise alternative procedures in order to satisfy itself as to the material accuracy of our financial statements and related disclosures, it is possible that we would be unable to file our Annual Report on Form 10-K with the Securities and Exchange Commission, which could also adversely affect the market price of our securities and our ability to secure additional financing as needed.
The transaction involves a reverse merger of a foreign company into a domestic shell company, so that there is no history of compliance with United States securities laws and accounting rules.
In order to be able to comply with United States securities laws, DBL prepared its financial statements for the first time under U.S. generally accepted accounting principles and recently had its initial audit of its financial statements in accordance with Public Company Accounting Oversight Board (United States). As the Company does not have a long term familiarity with U.S. generally accepted accounting principles, it may be more difficult for it to comply on a timely basis with SEC reporting requirements than a comparable domestic company.
Risks Relating To Our Industry
We may be subject to substantial liability should the consumption of any of our products cause personal injury or illness. Unlike most pharmaceutical companies in the United States, we do not maintain product liability insurance to cover our potential liabilities.
The sale of drug products for human consumption involves an inherent risk of injury to consumers. Such injuries may result from tampering by unauthorized third parties or product contamination or degeneration, including the presence of foreign contaminants, chemical substances or other agents or residues during the various stages of production process. We cannot assure you that consumption of our products will not cause a health-related illness in the future, or that we will not be subject to claims or lawsuits relating to such matters.
Even if a product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding any assertions that our products caused personal injury or illness could adversely affect our reputation with customers and our corporate and brand image. Unlike most pharmaceutical companies in the United States, but in line with industry practice in the PRC, we do not maintain product liability insurance. Furthermore, the products we sold could potentially suffer from product tampering, contamination or degeneration or be mislabeled or otherwise damaged. Under certain circumstances, we may be required to recall products. Even if a situation does not necessitate a product recall, we cannot assure you that product liability claims by our distributors will not be asserted against us as a result. A product liability judgment against us or a product recall could have a material adverse effect on our revenues, profitability and business reputation.
15
If the pharmaceutical industry in the PRC does not grow as we expect, our results of operations and financial condition will be adversely affected.
We believe drug products have strong growth potential in the PRC and, accordingly, we have continuously increased our sales of drug products. However, the market for pharmaceutical industry in the PRC has grown in recent years due to the increased wealth of the average resident of China and increasing health cautious, which has been the result of double-digit annual growth in the Chinese economy. Due to the worldwide recession, the growth of the Chinese economy has slowed. If the pharmaceutical industry in the PRC does not grow as we expect, our business will be harmed, we will need to adjust our growth strategy, and our results of operation will be adversely affected.
Sales of our products could be harmed by the widespread presence of counterfeit medication in the PRC which negatively impacting our profitability.
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. Exact data are impossible to collect, but the FBI believes that more than half of the pharmaceuticals sold in PRC are counterfeit. Examples of the seriousness of the problem include: six months after Viagra was introduced in 2002, state media reported that some 90 percent of little blue pills sold in Shanghai were counterfeit; and 192,000 Chinese patients were reported to have died in 2001 from fake drugs. Counterfeit products shrink markets for legitimate goods. This situation affects Benda and other major domestic and foreign drug manufacturers in PRC, especially for products marketed through the OTC rather than hospital channel. However, we believe the Chinese authorities are becoming increasingly vigilant against counterfeiting because in 2001 the authorities closed 1,300 factories while investigating 480,000 cases of counterfeit drugs. Currently, active pharmaceutical ingredients are governed only by chemical regulations. We believe that a major step towards controlling the problem would be taken should the SFDA be given oversight over PRC’s bulk chemicals producers. However, our ability to increase sales as rapidly as we would like, and our profitability, could be affected if this problem persists or worsens.
Risks Relating to the People's Republic of China
Certain political and economic considerations relating to the PRC could adversely affect our company.
The PRC is transitioning from a planned economy to a market economy. While the PRC government has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms carried out by the PRC government are unprecedented or experimental, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC’s economic and social conditions as well as by changes in the policies of the PRC government, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the interest rate or method of taxation, and the imposition of restrictions on currency conversion in addition to those described below.
The recent nature and uncertain application of many PRC laws applicable to us create an uncertain environment for business operations and they could have a negative effect on us.
The PRC legal system is a civil law system. Unlike the common law system, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects.
Currency conversion could adversely affect our financial condition.
The PRC government imposes control over the conversion of Renminbi into foreign currencies. Under the current unified floating exchange rate system, the People’s Bank of China publishes an exchange rate, which we refer to as the PBOC exchange rate, based on the previous day’s dealings in the inter-bank foreign exchange market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
16
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of Renminbi into foreign exchange by Foreign Investment Enterprises, or FIEs, for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC. Conversion of Renminbi into foreign currencies for capital account items, including direct investment, loans, and security investment, is still under certain restrictions. On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
Furthermore, the Renminbi is not freely convertible into foreign currencies nor can it be freely remitted abroad. Under the PRC’s Foreign Exchange Control Regulations and the Administration of Settlement, Sales and Payment of Foreign Exchange Regulations, Foreign Invested Enterprises are permitted either to repatriate or distribute its profits or dividends in foreign currencies out of its foreign exchange accounts, or exchange Renminbi for foreign currencies through banks authorized to conduct foreign exchange business. The conversion of Renminbi into foreign exchange by Foreign Invested Enterprises for recurring items, including the distribution of dividends to foreign investors, is permissible. The conversion of Reminbi into foreign currencies for capital items, such as direct investment, loans and security investment, is subject, however, to more stringent controls.
Our operating company is a FIE to which the Foreign Exchange Control Regulations are applicable. Accordingly, we will have to maintain sufficient foreign exchange to pay dividends and/or satisfy other foreign exchange requirements.
Exchange rate volatility could adversely affect our financial condition.
Since 1994, the exchange rate for Renminbi against the United States dollar has remained relatively stable, most of the time in the region of approximately RMB8.28 to $1.00. However, in 2005, the Chinese government announced that it would begin pegging the exchange rate of the Chinese Renminbi against a number of currencies, rather than just the U.S. dollar and, the exchange rate for the Renminbi against the U.S. dollar became RMB8.02 to $1.00. If we decide to convert Chinese Renminbi into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese Renminbi we convert would be reduced. There can be no assurance that future movements in the exchange rate of Renminbi and other currencies will not have an adverse effect on our financial condition.
Since our assets are located in the PRC, any dividends of proceeds from liquidation are subject to the approval of the relevant Chinese government agencies.
Our operating assets are located inside the PRC. Under the laws governing Foreign Invested Enterprises in the PRC, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to the relevant government agency’s approval and supervision as well as the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
It may be difficult to affect service of process and enforcement of legal judgments upon our company and our officers and directors because they reside outside the United States.
As our operations are presently based in the PRC and our director and officer resides in the PRC, service of process on our company and such director and officer may be difficult to effect within the United States. Also, our main assets are located in the PRC and any judgment obtained in the United States against us may not be enforceable outside the United States.
Due to various restrictions under PRC laws on the distribution of dividends by our PRC Operating Companies, we may not be able to pay dividends to our stockholders.
The Wholly-Foreign Owned Enterprise Law (1986), as amended and The Wholly-Foreign Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law of the PRC (2006) contain the principal regulations governing dividend distributions by wholly foreign owned enterprises. Under these regulations, wholly foreign owned enterprises may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. Additionally, such companies are required to set aside a certain amount of their accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends except in the event of liquidation and cannot be used for working capital purposes. The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the Company’s profits.
17
Furthermore, if our subsidiaries in China incur debt on their own in the future, the instruments governing the debt may restrict its ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our common stock.
Risks Related To Our Securities
In order to raise sufficient funds to expand our operations, we may have to issue additional securities at prices which may result in substantial dilution to our shareholders.
If we raise additional funds through the sale of equity or convertible debt, our current stockholders’ percentage ownership will be reduced. In addition, these transactions may dilute the value of our common shares outstanding. We may have to issue securities that may have rights, preferences and privileges senior to our common stock. We cannot provide assurance that we will be able to raise additional funds on terms acceptable to us, if at all. If future financing is not available or is not available on acceptable terms, we may not be able to fund our future needs, which would have a material adverse effect on our business plans, prospects, results of operations and financial condition.
Our articles of incorporation provide for indemnification of officers and directors at our expense and limit their liability which may result in a major cost to us and hurt the interests of our shareholders because corporate resources may be expended for the benefit of officers and/or directors.
Our articles of incorporation and applicable Delaware law provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on our behalf. We will also bear the expenses of such litigation for any of our directors, officers, employees, or agents, upon such person's written promise to repay us if it is ultimately determined that any such person shall not have been entitled to indemnification. This indemnification policy could result in substantial expenditures by us which we will be unable to recoup.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification for liabilities arising under federal securities laws, other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding, is asserted by a director, officer or controlling person in connection with the securities being registered, we will (unless in the opinion of our counsel, the matter has been settled by controlling precedent) submit to a court of appropriate jurisdiction, the question whether indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. The legal process relating to this matter if it were to occur is likely to be very costly and may result in us receiving negative publicity, either of which factors is likely to materially reduce the market and price for our shares, if such a market ever develops.
Currently, there is no public market for our securities, and there can be no assurances that any public market will ever develop or that our common stock will be quoted for trading and, even if quoted, it is likely to be subject to significant price fluctuations.
We have a trading symbol for our common stock, IBLE, which permits our shares to be quoted on the OTCBB. However, our stock has been thinly traded since approval of our quotation on the over-the-counter bulletin board by FINRA. Consequently, there can be no assurances as to whether:
|
§
|
any market for our shares will develop;
|
|
§
|
the prices at which our common stock will trade; or
|
|
§
|
the extent to which investor interest in us will lead to the development of an active, liquid trading market. Active trading markets generally result in lower price volatility and more efficient execution of buy and sell orders for investors.
|
In addition, our common stock is unlikely to be followed by any market analysts, and there may be few institutions acting as market makers for our common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these risk factors, investor perception of us and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our common stock.
18
We are not likely to pay cash dividends in the foreseeable future.
We currently intend to retain any future earnings for use in the operation and expansion of our business. Accordingly, we do not expect to pay any cash dividends in the foreseeable future, but will review this policy as circumstances dictate. Should we determine to pay dividends in the future, our ability to do so will depend upon the receipt of dividends or other payments from our PRC operating subsidiary may, from time to time, be subject to restrictions on its ability to make distributions to us, including restrictions on the conversion of RMB into U.S. dollars or other hard currency and other regulatory restrictions.
We may be subject to the penny stock rules which will make our securities more difficult to sell.
We may be subject in the future to the SEC’s “penny stock” rules if our securities sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings and reduce the trading activity for our securities. As long as our securities are subject to the penny stock rules, the holders of such securities may find it more difficult to sell their securities.
Our controlling shareholders may take actions that conflict with your interests.
All of our officers and directors beneficially own approximately 59.49% of our common stock pursuant to the terms of the Exchange Agreement. In this case, all of our officers and directors will be able to exercise control over all matters requiring shareholder approval, including the election of directors, amendment of our certificate of incorporation and approval of significant corporate transactions, and they will have significant control over our management and policies. The directors elected by these shareholders will be able to significantly influence decisions affecting our capital structure. This control may have the effect of delaying or preventing changes in control or changes in management, or limiting the ability of our other shareholders to approve transactions that they may deem to be in their best interest. For example, our controlling shareholders will be able to control the sale or other disposition of our operating businesses and subsidiaries to another entity.
MANAGEMENT’S DISCUSSION AND ANALYSISOF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of the consolidated financial condition and results of operations should be read with our consolidated financial statements and related notes appearing elsewhere in this Current Report. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those set forth under “Risk Factors” and elsewhere in this Current Report.
Overview
We are in the business of pharmaceutical wholesale in the People’s Republic of China (“PRC” or “China”).
Sichuan Xintai Pharmaceutical Co., Ltd. (“Xintai”) was incorporated as a limited liability company on November 25, 1999 under the laws of China. On January 11, 2010, Sichuan Provincial Government approved DBL to acquire 100% shares of Xintai. On January 21, 2010, Sichuan Administration for Industry and Commerce issued the new business license to Xintai. With the approval and the new business license, Xintai became a Wholly-Foreign-Owned Enterprise (“WFOE”).
Since its inception, Xintai has become a well-known licensed pharmaceutical wholesale company in China, specializing in the sales of prescription and Over-The-Counter medicines.
19
Because Indestructible existed as a shell prior to the merger, the transaction is being accounted for as a reverse acquisition, so DBL is treated as the continuing reporting entity that acquired Indestructible. As a result, all historical financial information being presented is that of DBL and Xintai.
Results of Operations for the Years Ended June 30, 2009 and 2008
|
Years Ended June 30,
|
||||||||||||||||
|
2009
US$
|
2008
US$
|
$
Change
|
%
Change
|
|||||||||||||
|
Revenues
|
17,127,474 | 7,576,098 | 9,551,376 | 126 | % | |||||||||||
|
Cost of Sales
|
(12,278,936 | ) | (5,565,409 | ) | (6,713,527 | ) | 121 | % | ||||||||
|
Gross Profit
|
4,848,538 | 2,010,689 | 2,837,849 | 141 | % | |||||||||||
|
Operating Expenses
|
1,647,050 | 676,269 | 970,781 | 144 | % | |||||||||||
|
Operating Income
|
3,201,488 | 1,334,420 | 1,867,068 | 140 | % | |||||||||||
|
Income Before Income Tax
|
3,191,679 | 1,332,668 | 1,859,011 | 139 | % | |||||||||||
|
Income Tax Provision
|
800,084 | 397,660 | 402,425 | 101 | % | |||||||||||
|
Net Income
|
2,391,595 | 935,008 | 1,456,587 | 156 | % | |||||||||||
Revenues:
Revenues for the year ended June 30, 2009 increased by approximately $9.55 million or 126% to $17.13 million as compared to $7.58 million for the year ended June 30, 2008. Our sales growth was driven by expansion of our customer bases and new products.
Gross Profit:
We achieved gross profits of $4.85 million for the year ended June 30, 2009, compared to $2.01 million for the same period of the previous year, representing a 141 % year to year increase. Our overall gross profit margin as a percentage of revenue grew by 1.8% from 28.3% for the year ended June 30, 2009 compared to 26.5% for the year ended June 30, 2008.
Operating Expenses:
Our operating expenses, consisting of selling, general and administrative expenses, increased by approximately $971,000, to $1.6 million, for the year ended June 30, 2009 from $676,000 for the same period of the previous year. This 144 % increase is mainly attributable to the increase in promotion expenses and travelling expenses and other expenses relating to our growth in sales. The overall increase in our operating expenses was in proportion to our increase in sales.
Income Tax Provision:
Our provisions for income taxes for the years ended June 30, 2009 and 2008 were $800,084 and $397,660, respectively, an increase of $402,425 or 101 % from period to period. The increase in the income tax provision was in proportion to our increase in sales and operating income.
|
For The Years Ended
June 30,
|
||||||||
|
2009
US$
|
2008
US$
|
|||||||
|
Cash provided by (used in):
|
||||||||
|
Operating Activities
|
|
470,405
|
183,617
|
|||||
|
Investing Activities
|
|
(77,883)
|
(13,389)
|
|||||
|
Financing Activities
|
|
(134,624)
|
103,046
|
|||||
20
Operations
Cash provided by operating activities totaled $470,405 for the year ended June 30, 2009 as compared to $183,617 for the previous year. The increase in our cash provided by operations was primarily due to the increase in our net income.
Investments
We invested $77,883 in fixed assets for the years ended June 30, 2009. This compares to $13,389 in the same period of the prior year.
We repaid $134,624 of shareholder loans during the year ended June 30, 2009 as compared to obtaining $103,046 during the year ended June 30, 2008.
Off-Balance Sheet Arrangements
We do not have off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities.”
Selected Quarterly Results of Operations
The following table sets forth our unaudited selected quarterly results of operations for the six months ended December 31, 2009. You should read the following table in conjunction with our audited financial statements and related notes included elsewhere in this Form 8K. We have prepared the unaudited financial information on the same basis as our audited financial statements. The unaudited financial information includes all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of our financial position and operating results for the quarters presented.
Because Indestructible existed as a shell prior to the merger, the transaction is being accounted for as a reverse acquisition, so DBL is treated as the continuing reporting entity that acquired Indestructible. As a result, all historical financial information being presented is that of DBL and Xintai.
Results of Operations for the Six Months Ended December 31, 2009 and 2008
|
Six Months Ended
December 31,
(Unaudited)
|
||||||||||||||||
|
2009
US$
|
2008
US$
|
$
Change
|
%
Change
|
|||||||||||||
|
Revenues
|
9,653,544 | 7,557,227 | 2,096,318 | 27.7 | % | |||||||||||
|
Cost of Sales
|
(7,036,308 | ) | (5,877,309 | ) | (1,158,999 | ) | 19.7 | % | ||||||||
|
Gross Profit
|
2,617,236 | 1,679,918 | 937,318 | 55.8 | % | |||||||||||
|
Operating Expenses
|
545,506 | 355,057 | 190,450 | 53.6 | % | |||||||||||
|
Operating Income
|
2,071,730 | 1,324,861 | 746,869 | 56.4 | % | |||||||||||
|
Income Before Income Tax
|
2,375,161 | 1,322,770 | 1,052,391 | 79.6 | % | |||||||||||
|
Income Tax Provision
|
597,967 | 330,692 | 267,275 | 80.8 | % | |||||||||||
|
Net Income
|
1,777,194 | 992,078 | 785,116 | 79.1 | % | |||||||||||
Revenues:
Revenues for the six months ended December 31, 2009 increased by approximately $2.10 million or 27.7% to $9.65 million as compared to $7.56 million for the six months ended December 31, 2008. Our sales growth was driven by expansion of our customers and new products.
21
Gross Profit:
We achieved gross profits of $2.62 million for the six months ended December 31, 2009, compared to $1.68 million for the same period of the previous year, representing a 55.8 % period to period increase. Our overall gross profit margin as a percentage of revenue grew by 4.9% from 27.1% for the six months ended December 31, 2009 compared to 22.2% of the same period prior year.
Operating Expenses:
Our operating expenses, consisting of selling, general and administrative expenses, increased by approximately $190,450, to $545,506, for the six months ended December 31, 2009 from $355,057 for the same period of the previous year. This 53.6% increase is mainly attributable to the increase in promotion and travelling expenses and other expenses relating to our growth in sales. The overall increase in our operating expenses was in proportion to our increase in sales.
Income Tax Provision:
Our provisions for income taxes for the six months ended December 31, 2009 and 2008 were $597,967 and $330,692 respectively, an increase of $267,265 or 80.8 % from period to period. The increase in the income tax provision was in proportion to our increase in sales and operating income.
Liquidity and Capital Resources
|
For The Six Months Ended
December 31,
(Unaudited)
|
||||||||
|
2009
US$
|
2008
US$
|
|||||||
|
Cash provided by (used in):
|
||||||||
|
Operating Activities
|
|
(770,164)
|
803,145
|
|||||
|
Investing Activities
|
|
(33,796)
|
(49,805)
|
|||||
|
Financing Activities
|
|
287,462
|
(1,041,116)
|
|||||
Operations
Cash used in operating activities totaled $770,164 for the six months ended December 31, 2009 as compared to $803,145 provided by the same period of the previous year. The decrease in our cash used in operations was primarily due to the increase in our account receivable in proportion to our increase in sales.
Investments
We invested $33,796 in fixed assets for the six months ended December 31, 2009. This compares to $49,805 in the same period of the prior year.
Financing
We obtained $287,462 proceeds from shareholder loans for the six months ended December 31, 2009 as compared to paying off $1,041,116 loans to the related party and shareholders during the six months ended December 31, 2008.
Off-Balance Sheet Arrangements
We do not have off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities.”
Critical Accounting Policies
While our significant accounting policies are more fully described in Note 2 to our financial statements, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes, and disclosure of contingent liabilities at the date of the financial statements. Estimates are used for, but not limited to, the selection of the useful lives of property and equipment, provision necessary for contingent liabilities, fair values, revenue recognition, taxes, budgeted costs and other similar charges. Management believes that the estimates utilized in preparing its financial statements are reasonable and prudent. Actual results could differ from these estimates.
22
Property and equipment
Property and equipment are recorded at cost less accumulated depreciation and any impairment losses. The cost of an asset comprises of its purchase price and any directly attributable costs of bringing the asset to its working condition and location for its intended use. Expenditure incurred after the fixed assets have been put into operation, such as repairs and maintenance and overhaul costs, is normally charged to the profit and loss account in the year in which it is incurred.
In situations where it can be clearly demonstrated that the expenditure has resulted in an increase in the future economic benefits expected to be obtained from the use of the asset beyond its originally assessed standard of performances, the expenditure is capitalized as an additional cost of the asset.
Depreciation is computed using the straight-line method over the estimated useful lives of the assets, less any estimated residual value. Estimated useful lives of the assets are as follows:
| Office equipments | 5 years |
| Vehicle | 10 years |
Any gain or loss on disposal or retirement of a fixed asset is recognized in the profit and loss account and is the difference between the net sales proceeds and the carrying amount of the relevant asset. When property and equipment are retired or otherwise disposed of, the assets and accumulated depreciation are removed from the accounts and the resulting profit or loss is reflected in income.
Expenditures for maintenance and repairs are charged to expense as incurred. Additions, renewals and betterments are capitalized.
Long-lived assets
The Company applies the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, which was subsequently codified within Financial Accounting Standard Board (“FASB”) Accounting Standards Codification (“ASC”) No. 360, “Property, Plant and Equipment”. ASC 360 requires long-lived assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable through the estimated undiscounted cash flows expected to result from the use and eventual disposition of the assets. Whenever any such impairment exists, an impairment loss will be recognized for the amount by which the carrying value exceeds the fair value.
The Company tests long-lived assets, including property, plant and equipment and other assets, for recoverability at least annually or more frequently upon the occurrence of an event or when circumstances indicate that the net carrying amount is greater than its fair value. Assets are grouped and evaluated at the lowest level for their identifiable cash flows that are largely independent of the cash flows of other groups of assets. The Company considers historical performance and future estimated results in its evaluation of potential impairment and then compares the carrying amount of the asset to the future estimated cash flows expected to result from the use of the asset. If the carrying amount of the asset exceeds estimated expected undiscounted future cash flows, the Company measures the amount of impairment by comparing the carrying amount of the asset to its fair value. The estimation of fair value is generally measured by discounting expected future cash flows as the rate the Company utilizes to evaluate potential investments. The Company estimates fair value based on the information available in making whatever estimates, judgments and projections are considered necessary. There was no impairment of long-lived assets for the years ended June 30, 2009 and 2008 and six months ended December 31, 2009 and 2008.
Advance from customers
Advance from customers consist of amounts received from customers relating to the sales of pharmaceuticals. The Company recognizes these funds as a current liability until the revenue can be recognized.
Income taxes
The Company accounts for income taxes using an asset and liability approach which allows for the recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the Company is able to realize their benefits, or that future deductibility is uncertain.
23
The Company records a valuation allowance for deferred tax assets, if any, based on its estimates of its future taxable income as well as its tax planning strategies when it is more likely than not that a portion or all of its deferred tax assets will not be realized. If the Company is able to utilize more of its deferred tax assets than the net amount previously recorded when unanticipated events occur, an adjustment to deferred tax assets would increase the Company’s net income when those events occur.
Commencing January 1, 2008, the PRC’s new Enterprise Income Tax ("EIT") law replaced the laws for Domestic Enterprises ("DES") and Foreign Invested Enterprises ("FIEs"). The new standard EIT rate of 25% replaced the 33% rate formerly applicable to both DES and FIEs.
Revenue recognition
The Company’s revenue policies are in compliance with the United States Accounting Guide. The Company recognizes revenue when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price to the customer is fixed or determinable and (iv) collection of the resulting receivable is reasonably assured. Revenue is not recognized until title and risk of loss is transferred to the customer, which occurs upon delivery of goods, and objective evidence exists that customer acceptance provisions have been met. Deposits or advance payments from customers prior to delivery of goods and passage of title of goods are recorded as advance from customers.
Cost of sales
Costs of sales include costs of the pharmaceuticals sold and used, inbound freight costs, cost of direct labor and overhead. Write-down of inventory to lower of cost or market is also recorded in cost of sales.
Selling, general and administrative costs
Selling, general and administrative costs consist primarily of salaries and commissions for sales representatives, salaries for administrative staffs, rent expenses, depreciation expense and employee benefits for administrative staffs.
Comprehensive income
Statement of Financial Accounting Standards ("FAS") No. 130, “Reporting Comprehensive Income”, which was subsequently codified within ASC 220, “Comprehensive Income”, requires disclosure of all components of comprehensive income and loss on an annual and interim basis. Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income arose from the changes in foreign currency exchange rates.
Statement of Cash Flows
In accordance with SFAS 95, "Statement of Cash Flows," which was subsequently codified within ASC 230, “Statement of Cash Flows”, cash flows from the Company's operations is calculated based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statements of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Fair Value of Financial Instruments
The Company’s financial instruments include cash and cash equivalents, accounts receivable, advances to suppliers, other receivables, accounts payable, accrued expenses, taxes payable, notes payable and other loans payable. Management has estimated that the carrying amounts approximate their fair value due to the short-term nature.
Subsequent Events
The Company has evaluated subsequent events that have occurred through March 25, 2010, the date of this financial statement issuance.
Earnings Per Share
The Company computes earnings per share (“EPS’) in accordance with SFAS 128, “Earnings Per Share”, which was subsequently codified within ASC 260, “Earning Per Share”. ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
24
Foreign currency translation
The Company’s financial information is presented in US dollars. The functional currency of the Company is Renminbi (“RMB”), the currency of the PRC. Transactions at the Company which are denominated in currencies other than RMB are translated into RMB at the exchange rate quoted by the People’s Bank of China prevailing at the dates of the transactions. Exchange gains and losses resulting from transactions denominated in a currency other than that RMB are included in statements of operations as exchange gains. The financial statements of the Company have been translated into U.S. dollars in accordance with Statement of Financial Accounting Standard (“SFAS”) No. 52, “Foreign Currency Translation”, which was subsequently codified within ASC 830, “Foreign Currency Matters”. The financial information is first prepared in RMB and then is translated into U.S. dollars at period-end exchange rates as to assets and liabilities and average exchange rates as to revenue and expenses. Capital accounts are translated at their historical exchange rates when the capital transactions occurred. The effects of foreign currency translation adjustments are included as a component of accumulated other comprehensive income in shareholders’ equity.
The RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation
Concentration of credit risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of accounts receivable and other receivables. The Company does not require collateral or other security to support these receivables. The Company conducts periodic reviews of its clients' financial condition and customer payment practices to minimize collection risk on accounts receivable.
Risks and uncertainties
The operations of the Company are located in the PRC. Accordingly, the Company's business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy.
The Company’s operations in the PRC are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes
in the political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
Recent accounting pronouncements
In April 2009, the FASB updated the accounting standards to provide guidance on estimating the fair value of a financial asset or liability when the trade volume and level of activity for the asset or liability have significantly decreased relative to historical levels. The standard requires entities to disclose the inputs and valuation techniques used to measure fair value and any changes in valuation inputs or techniques. In addition, debt and equity securities as defined by GAAP shall be disclosed by major category. This standard is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009, and is to be applied prospectively. The adoption did not have a material effect on the Company's results of operations and financial condition.
In May 2009, the FASB issued guidance related to subsequent events under ASC 855-10, Subsequent Events. This guidance sets forth the period after the balance sheet date during which management or a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date, and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. It requires disclosure of the date through which an entity has evaluated subsequent events and the basis for that date, whether that date represents the date the financial statements were issued or were available to be issued. This guidance is effective for interim and annual periods ending after June 15, 2009. We have included the required disclosures in our consolidated condensed financial statements.
25
In June 2009, the FASB issued an amendment to ASC 810-10, Consolidation. This guidance amends ASC 810-10-15 to replace the quantitative-based risks and rewards calculation for determining which
enterprise has a controlling financial interest in a VIE with a primarily qualitative approach focused on identifying which enterprise has the power to direct the activities of a VIE that most significantly impact the entity’s economic performance. It also requires ongoing assessments of whether an enterprise is the primary beneficiary of a VIE and requires additional disclosures about an enterprise’s involvement in VIEs. This guidance is effective as of the beginning of the reporting entity’s first annual reporting period that begins after November 15, 2009 and earlier adoption is not permitted. We are currently evaluating the potential impact, if any, of the adoption of this guidance will have on our consolidated condensed financial statements.
In June 2009, the FASB issued Accounting Standards Update No. 2009-01 which amends ASC 105, Generally Accepted Accounting Principles. This guidance states that the ASC will become the source of authoritative U.S. GAAP recognized by the FASB to be applied by nongovernmental entities. Once effective, the Codification’s content will carry the same level of authority. Thus, the U.S. GAAP hierarchy will be modified to include only two levels of U.S. GAAP: authoritative and non-authoritative. This is effective for financial statements issued for interim and annual periods ending after September 15, 2009. We adopted ASC 105 as of September 30, 2009 and thus have incorporated the new Codification citations in place of the corresponding references to legacy accounting pronouncements.
In August 2009, the FASB issued Accounting Standards Update No. 2009-05, Measuring Liabilities at Fair Value, which amends ASC 820, Fair Value Measurements and Disclosures. This Update provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure the fair value using one or more of the following techniques: a valuation technique that uses the quoted price of the identical liability or similar liabilities when traded as an asset, which would be considered a Level 1 input, or another valuation technique that is consistent with ASC 820. This Update is effective for the first reporting period (including interim periods) beginning after issuance. Thus, we adopted this guidance as of September 30, 2009, which did not have a material impact on our consolidated condensed financial statements.
In September 2009, the Financial Accounting Standards Board (FASB) amended existing authoritative guidance to improve financial reporting by enterprises involved with variable interest entities and to provide more relevant and reliable information to users of financial statements. The amended guidance is effective for fiscal annual reporting periods beginning after November 15, 2009, for interim periods within that first annual reporting period, and for interim and annual reporting periods thereafter. The Company is currently assessing the impact, if any, adoption may have on its financial statements or disclosures.
MANAGEMENT
Appointment of New Directors and Officers
At the Closing Date of the Exchange Agreement, Patrick Day and Richard M. Day, Jr., our directors and officers, resigned from all their positions and Mr. Xiaodong Zhu was appointed as our director. In addition, Mr. Xiaodong Zhu, Mr. Xicai Su and Mr. Di Wu were appointed as the new officers of the Company effective immediately at the Closing.
The following table sets forth the names, ages, and positions of our new executive officer and director. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
|
NAME
|
AGE
|
POSITION
|
||
|
Xiaodong Zhu
|
35
|
President, Chief Executive Officer and Chairman of the Board of Directors
|
||
|
Xicai Su
|
45
|
Chief Operating Officer
|
||
|
Di Wu
|
43
|
Chief Development Officer
|
A brief biography of each officer and director is more fully described in Item 5.02(c). The information therein is hereby incorporated in this section by reference.
26
Audit Committee Financial Expert
Our board of directors currently acts as our audit committee. We currently do not have a member who qualifies as an “audit committee financial expert” as defined in Item 401(e) of Regulation S-K and is “independent” as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act. Our board of directors is in the process of searching for a suitable candidate for this position.
Audit Committee
We have not yet appointed an audit committee. At the present time, we believe that the members of board of directors are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
Employment Agreements
We currently do not have employment agreement with any our directors and executive officers.
Family Relationships
There are no family relationships between any of our directors or executive officers and any other directors or executive officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers have been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past five years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Code of Ethics
We have adopted a code of ethics that applies to our officers, employees and directors, including our CEO and other senior executives.
EXECUTIVE COMPENSATION
Indestructible Executive Compensation Summary
Summary Compensation Table
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officers paid by us during the period ended December 31, 2009 in all capacities for the accounts of our executives, including the Chief Executive Officer (CEO) and Chief Financial Officer (CFO):
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock Awards
($)
|
Option Awards
($)
|
Non-Equity Incentive Plan Compensation ($)
|
Non-Qualified Deferred Compensation Earnings
($)
|
All Other Compensation
($)
|
Totals
($)
|
|||||||||||||||||||||||||
|
Patrick Day, CEO, CFO and Chairman (1)
|
2009
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
0
|
|||||||||||||||||||||||
|
Richard M. Day, Jr., Secretary (1)
|
2009
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
0
|
|||||||||||||||||||||||
|
(1)
|
On the Closing Date, Patrick Day and Richard M. Day, Jr. tendered their resignations from all positions held in the Company, effective immediately.
|
Outstanding Equity awards at Fiscal Year End
There are no outstanding equity awards as of the date hereof.
27
Director Compensation
Our directors will not receive a fee for attending each board of directors meeting or meeting of a committee of the board of directors. All directors will be reimbursed for their reasonable out-of-pocket expenses incurred in connection with attending board of director and committee meetings.
Option Grants
We do not maintain any equity incentive or stock option plan. Accordingly, we did not grant options to purchase any equity interests to any employees or officers, and no stock options are issued or outstanding to any officers. We do, however, anticipate adopting a non-qualified stock option plan where we will be granting our officers options to purchase our common stock pursuant to the terms of their employment agreements. But, no such plan has been finalized or adopted.
Certain Relationships and Related Transactions
We will present all possible transactions between us and our officers, directors or 5% stockholders, and our affiliates to the Board of Directors for their consideration and approval. Any such transaction will require approval by a majority of the disinterested directors and such transactions will be on terms no less favorable than those available to disinterested third parties.
Xintai Executive Compensation Summary
The table below sets forth the positions and compensations for each officer and director of Xintai for the year ended June 30, 2009 and 2008.
|
Name
|
Title
|
06/30/2009 Fiscal Year
Annual Salary (US$)
|
06/30/2008 Fiscal Year
Annual Salary (US$)
|
||||||
|
Xiaodong Zhu (1)
|
Chief Executive Officer
|
|
0
|
|
0
|
||||
|
Xicai Su (1)
|
Chief Operating Officer
|
|
0
|
|
0
|
||||
|
Di Wu (1)
|
Chief Development Officer
|
|
0
|
|
0
|
||||
|
(1)
|
In connection with the Closing of the Share Exchange, Mr. Xiaodong Zhu was appointed as the Chairman and Chief Executive Officer, Xicai Su was appointed as Chief Operating Officer and Di Wu was appointed as Chief Development Officer, effective immediately at the Closing.
|
PRINCIPAL STOCKHOLDERS
Pre-Share Exchange
The following table sets forth certain information regarding our securities beneficially owned as of the date hereof, for (i) each stockholder known to be the beneficial owner of 5% or more of the Company’s outstanding shares of common stock, (ii) each executive officer and director, and (iii) all executive officers and directors as a group, on a pro forma basis prior to the Closing of the Share Exchange.
|
Name and Address of Beneficial Owner
|
Amount of
Beneficial Ownership
|
Percentage
of Class
|
||||||
|
Patrick Day
|
12,000,000 | 71.86 | % | |||||
|
50 West Broadway, 10th Fl
|
||||||||
|
Salt Lake City, Utah 84101
|
||||||||
|
All Executive Officers and
|
||||||||
|
Directors as a Group (1 Person)
|
12,000,000 | 100 | % | |||||
Post-Share Exchange
The following table sets forth certain information regarding our securities beneficially owned on the Closing Date, for (i) each stockholder known to be the beneficial owner of 5% or more of the Company’s outstanding shares of common stock, (ii) each executive officer and director, and (iii) all executive officers and directors as a group.
28
As of the date of filing, we have 17,000,000 shares of common stock issued and outstanding.
|
Name of Beneficial Owner (1)(2)
|
Amount and Nature of Beneficial Ownership
|
Percent of Class (3)
|
||||||
|
Xiaodong Zhu (4)
|
7,163,000 | 42.14 | % | |||||
|
Xicai Su
|
0 | 0 | ||||||
|
Di Wu
|
0 | 0 | ||||||
|
Shimin Cai
|
2,950,000 | 17.35 | % | |||||
|
Weihua Zhao
|
1,500,000 | 8.82 | % | |||||
|
All Executive Officers and Directors as a group (3 persons)
|
7,163,000 | 42.14 | % | |||||
|
(1)
|
Pursuant to Rule 13d-3 under the Exchange Act, a person has beneficial ownership of any securities as to which such person, directly or indirectly, through any contract, arrangement, undertaking, relationship or otherwise has or shares voting power and/or investment power or as to which such person has the right to acquire such voting and/or investment power within 60 days.
|
|
(2)
|
Unless otherwise stated, each beneficial owner has sole power to vote and dispose of the shares and the address of such person is c/o the Company, at 9 Gaoshengqiao Road, Dayi Louver Plaza, Wuhou District, Chengdu, Sichuan Province, P.R. China 610041.
|
|
(3)
|
Based on 17,000,000 shares of common stock issued and outstanding as of the date hereof.
|
|
(4)
|
All 7,163,000 shares were beneficially owned through Super Deal International Limited where Mr. Xiaodong Zhu is the Executive Director. Mr. Zhu has voting and control power over the shares held by Super Deal International Limited.
|
DESCRIPTION OF SECURITIES
The Company is authorized to issue 200,000,000 shares of common stock, par value $0.0001 per share and 50,000,000 shares of preferred stock, par value $0.0001 per share. As of the date hereof, 17,000,000 shares of common stock and no shares of preferred stock were issued and outstanding.
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of common stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of common stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The common stock is not entitled to preemptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of common stock are entitled to share ratably in all assets remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the common stock. The outstanding shares of common stock are, and the shares of common stock to be issued upon exercise of the Warrants will be, fully paid and non-assessable.
Preferred Stock
The Board of Directors is empowered to designate and issue from time to time one or more classes or series of preferred stock and to fix and determine the relative rights, preferences, designations, qualifications, privileges, options, conversion rights, limitations and other special or relative rights of each such class or series so authorized. Such action could adversely affect the voting power and other rights of the holders of the Company’s capital shares or could have the effect of discouraging or making difficult any attempt by a person or group to obtain control of the Company.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common stock, par value $0.0001 per share, has a trading symbol (“IBLE.OB”) but has been thinly traded on the Over-The-Counter Bulletin Board (“OTCBB”). Our common stock has been listed on the OTCBB since July 17, 2009.
The market price of our common stock is subject to significant fluctuations in response to variations in our quarterly operating results, general trends in the market, and other factors, over many of which we have little or no control. In addition, broad market fluctuations, as well as general economic, business and political conditions, may adversely affect the market for our common stock, regardless of our actual or projected performance.
29
Holders
As of the date hereof, 17,000,000 shares of common stock are issued and outstanding. There are approximately 100 shareholders of our common stock.
Transfer Agent and Registrar
The Transfer Agent for our common stock is American Registrar & Transfer Co., at 342 East 900 South, Salt Lake City 84111. Its telephone number is 801-363-9065.
Penny Stock Regulations
The SEC has adopted regulations which generally define "penny stock" to be an equity security that has a market price of less than $5.00 per share. Our Common Stock, when and if a trading market develops, may fall within the definition of penny stock and be subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 individually, or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the "penny stock" rules may restrict the ability of broker-dealers to sell our Common Stock and may affect the ability of investors to sell their Common Stock in the secondary market.
Dividend Policy
Any future determination as to the declaration and payment of dividends on shares of our common stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of common stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in the PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from and of Xintai. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
Equity Compensation Plan Information
None.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Sichuan Xintai Pharmaceutical Co., Ltd.
As of June 30, 2009, the Company had receivables from Beijing Dongsheng Kexin Biology Curative Co., Ltd. (“Dongsheng Kexin”) a total of $ 1,358,843. Dongsheng Kexin is also owned by Mr. Xiaodong Zhu, CEO of the Xintai, who holds 95% of Xintai’s total shares. The balance with Dongsheng Kexin has a term from July 3, 2008 to December 31, 2011. The loan is unsecured and bears an annual interest of 0.1%.
As of June 30, 2009 and June 30, 2008, the Company had receivables from Ms. Xiaomei Zhu, Mr. Xiaodong Zhu’s sister, for a total of $111,155 and payables to Xiaomei Zhu in amount of$23,474, respectively. Ms. Xiaomei Zhu holds 5% of the company’s total shares. The balance with Ms. Zhu is unsecured and bears no interest and has no fixed repayment date. The company expects the entire amount of the loan be settled within one year.
30
LEGAL PROCEEDINGS
Currently there are no legal proceedings pending or threatened against us. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act.
Our certificate of incorporation provides that we shall indemnify our directors to the full extent permitted by the provisions of Section 102(b)(7) and Section 145 of the Delaware General Corporation Law (the “DGCL”) as the same may be amended and supplemented. Section 102(b)(7) of the DGCL, relating to indemnification is hereby incorporated herein by reference. Notwithstanding the above, our certificate of incorporation provides that a director shall be liable to the extent provided by applicable law, (i) for breach of the director’s duty of loyalty to us; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL, or (iv) for any transaction from which the director derived an improper personal benefit.
At present, there is no pending litigation or proceeding involving any of our director, officer or employees in which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS
Gately & Associates, LLC (“Gately”) served as our independent auditor in connection with the audits of the Company’s financial statements for the fiscal years ended December 31, 2009 and 2008. In connection with the Share Exchange, our board of directors recommended and approved the appointment of Friedman LLP (“Friedman”) as the independent auditor for the Company and DBL.
During the fiscal years ended December 31, 2009 and 2008 and through the date hereof, neither us nor anyone acting on our behalf consulted Friedman with respect to (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report was provided to us or oral advice was provided that Friedman concluded was an important factor considered by us in reaching a decision as to the accounting, auditing or financial reporting issue; or (ii) any matter that was the subject of a disagreement or reportable events set forth in Item 304(a)(1)(v) of Regulation S-K.
For a more detailed discussion of our change in auditor, please refer to Item 4.01 below.
Item 3.02Unregistered Sales of Equity Securities.
Pursuant to the Exchange Agreement, on March 25, 2010, we issued 15,830,000 shares of common stock to individuals and entities as designated by the DBL Shareholders in exchange for 100% of the outstanding shares of DBL. Such securities were not registered under the Securities Act. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance securities by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which we sold a high number of securities to a high number of investors. In addition, these shareholders had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such securities are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these securities would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
Item 4.01 Changes in Registrant’s Certifying Accountant.
(a) Dismissal of Previous Independent Registered Public Accounting Firm.
|
i
|
On March 25, 2010, we dismissed Gately & Associates, LLC (“Gately”) as our independent registered public accounting firm. The Board of Directors of the Company approved such resignation on March 25, 2010.
|
|
ii
|
The Company’s Board of Directors participated in and approved the decision to change our independent registered public accounting firm.
|
31
|
iii
|
Gately’s reports on the financial statements of the Company for the years ended December 31, 2009 and 2008 did not contain an adverse opinion or a disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles.
|
|
iv
|
In connection with the audit and review of the financial statements of the Company through March 25, 2010, there were no disagreements on any matter of accounting principles or practices, financial statement disclosures, or auditing scope or procedures, which disagreements if not resolved to their satisfaction would have caused them to make reference in connection with Gately’s opinion to the subject matter of the disagreement.
|
|
v
|
In connection with the audited financial statements of the Company for the years ended December 31, 2009 and 2008, there have been no reportable events with the Company as set forth in Item 304(a)(1)(v) of Regulation S-K.
|
|
vi
|
The Company provided Gately with a copy of this Current Report on Form 8-K and requested that Gately furnished it with a letter addressed to the SEC stating whether or not they agree with the above statements. The Company has received the requested letter from Gately, and a copy of such letter is filed as Exhibit 16.1 to this Current Report Form 8-K.
|
(b) Engagement of New Independent Registered Public Accounting Firm.
|
i
|
On March 25, 2010, the Board appointed Friedman LLP (“Friedman”) as the Company’s new independent registered public accounting firm. The decision to engage Friedman was approved by the Company’s Board of Directors on March 25, 2010.
|
|
ii
|
Prior to March 25, 2010, the Company did not consult with Friedman regarding (1) the application of accounting principles to a specified transactions, (2) the type of audit opinion that might be rendered on the Company’s financial statements, (3) written or oral advice was provided that would be an important factor considered by the Company in reaching a decision as to an accounting, auditing or financial reporting issues, or (4) any matter that was the subject of a disagreement between the Company and its predecessor auditor as described in Item 304(a)(1)(iv) or a reportable event as described in Item 304(a)(1)(v) of Regulation S-K.
|
Item 5.01Changes in Control of Registrant.
As explained more fully in Item 2.01, in connection with the Exchange Agreement, on March 25, 2010, we issued 15,830,000 shares of common stock to individuals and entities as designated by DBL in exchange for 100% of the outstanding shares of DBL to us. As such, immediately following the Closing of the Share Exchange, the individuals and entities designated by DBL hold approximately 93.12% of the total voting power of our common stock entitled to vote.
In connection with the Closing of the Share Exchange, and as explained more fully in the above Item 2.01 under the section titled “Management” and below in Item 5.02 of this Current Report on Form 8-K, Patrick Day and Richard M. Day, Jr. resigned from all their positions with the Company effective immediately. Further, Mr. Xiaodong Zhu was appointed as the director. Mr. Xiaodong Zhu, Mr. Xicai Su, and Mr. Di Wu were appointed as the new officers of the Company effective immediately at the Closing.
Item 5.02Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers
(a) Resignation of Director and Officer
At the Closing, Patrick Day resigned as the sole member of our board of directors, President, Chief Executive Officer, and Chief Financial Officer, and Richard M. Day, Jr. resigned as our Secretary, effective immediately. There were no disagreements between Patrick Day, Richard M. Day, Jr. and the Company.
(b) Appointment of Directors and Officers
At the Closing, the following persons were appointed as our new directors and officers:
|
NAME
|
AGE
|
POSITION
|
||
|
Xiaodong Zhu
|
35
|
President, Chief Executive Officer and Chairman of the Board of Directors
|
||
|
Xicai Su
|
45
|
Chief Operating Officer
|
||
|
Di Wu
|
43
|
Chief Development Officer
|
The business background descriptions of the newly appointed directors and officers are as follows:
32
Mr. Xiaodong Zhu, President, Chief Executive Officer and Chairman of the Board of Directors, age 35, has 11 years of sales experience in pharmaceutical industry. From July 2006 to present, he serves as CEO in Sichuan Xintai Pharmaceutical Co., Ltd. From November 2004 to July 2006, Mr. Zhu served as founder and CEO in Dongsheng Kexin Biology Curative Co., Ltd. From August 2002 to November 2004, Mr. Zhu served as Regional Sales Manager in Pharmaceutical Factory of Norman Bethune University of Medical Science. From June 2000 to March 2004, Mr. Zhu also worked as Regional Sales Manager in Yunnan Laoboyuntang Pharmaceutical. Mr. Xiaodong Zhu graduated from Harbin Normal University with Bachelor degree of Science in 1999.
Mr. Xicai Su, Chief Operating Officer, age 45, has more than 15 years of marketing and sales experience. Since September 2009, Mr. Su is working as COO of Sichuan Xintai Pharmaceutical Co., Ltd. From March 2008 to September 2009, he was the Head of Sales Department & Vice General Manager of Shenzhen Ausa Pharmed Co., Ltd. From March 2004 to December 2007, Mr. Su served as Vice President & the Head of Marketing and Sales Department in Beijing Wharf Pharmaceutical. From June 2000 to March 2004, he served as the Head of Marketing and Sales Department in Shanghai Yudan Pharmaceutical. Mr. Su graduated from Jilin Medical College with the Bachelor degree of Medicine in 1988 and he obtained a Master degree of Philosophy from Nankai University in 1994.
Mr. Di Wu, Chief Development Officer, age 43, is the President and Founder in GoodeePharma Suzhou, China since 2008. From 2002 to 2008, Mr. Wu served as Core Management in the Division of Medical Genetics in University of Pennsylvania, Philadelphia, PA and focused on DMPK, Histology and Morphology. From 2000 to 2002, he was the Post Doctor in the Department of Molecular Genetics, The Wistar Institute, Philadelphia, PA and focused on Molecular Biology. Mr. Wu graduated from the School of Medicine of Soochow University as a Medicine Doctor in 1991.
(c) Family Relationships
There are no family relationships between the officers or directors of the Company.
(d) Employment Agreements of the Executive Officers
We currently did not enter into any employment agreement with our executive officers.
(e) Related Party Transactions
There are no related party transactions reportable under Item 5.02 of Form 8-K and Item 404(a) of Regulation S-K.
Item 5.03Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
On March 25, 2010, pursuant to the Exchange Agreement, the Board of Directors adopted a resolution by unanimous written consent changing our name to “Dongsheng Pharmaceutical International Co., Ltd.” to better reflect our business plan.
On March 25, 2009, pursuant to the Exchange Agreement, the Board of Directors adopted a resolution by unanimous written consent changing its fiscal year end from December 31 to June 30. This change was made to be consistent with the fiscal year of Xintai, which is now our wholly-owned subsidiary and the operating company.
Item 5.06Change In Shell Company Status
As explained more fully in Item 2.01 above, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act) immediately before the Closing of the Share Exchange. As a result of the Share Exchange, DBL became our wholly owned subsidiary and became our main operational business. Consequently, we believe that the Share Exchange has caused us to cease to be a shell company. For information about the Share Exchange, please see the information set forth above under Item 2.01 of this Current Report on Form 8-K which information is incorporated herein by reference.
Item 9.01Financial Statement and Exhibits.
(a) Financial Statements of Business Acquired.
The Audited Consolidated Financial Statements of Xintai as of June 30, 2009 and 2008 are filed as Exhibit 99.1 to this current report and are incorporated herein by reference.
The Unaudited Consolidated Financial Statements of Xintai as of December 31, 2009 and 2008 are filed as Exhibit 99.2 to this current report and are incorporated herein by reference.
33
(b) Pro Forma Financial Information.
The unaudited pro forma condensed consolidated balance sheet as of December 31, 2009 and the unaudited pro forma condensed consolidated statement of operations for the year ended December 31, 2009 concerning the acquisition of the business operation of DBL are filed as Exhibit 99.3 to this current report and are incorporated herein by reference.
(c) Shell Company Transactions.
Reference is made to Items 9.01(a) and 9.01(b) and the exhibits referred to therein which are incorporated herein by reference.
(d) Exhibits.
|
Exhibit No.
|
Description
|
|
|
2.1
|
Share Exchange Agreement by and between the Company and Dynamic Bhorizon Limited, dated March 25, 2010
|
|
|
3.1
|
Certificate of Incorporation (1)
|
|
|
3.2
|
By Laws (1)
|
|
|
16.1
|
Letter from Gately & Associates, LLC, dated March 25, 2010
|
|
|
99.1
|
The Audited Consolidated Financial Statements of Xintai as of June 30, 2009 and 2008
|
|
|
99.2
|
The Unaudited Consolidated Financial Statements of Xintai as of December 31, 2009 and 2008
|
|
|
99.3
|
The Unaudited Pro Forma Financial Information of Indestructible
|
(1) Incorporated herein by reference to the Form S-1 Registration Statement filed on October 28, 2008.
34
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned hereunto duly authorized.
|
INDESTRUCTIBLE I, INC.
|
|||
|
Dated: March 30, 2010
|
By:
|
/s/ Xiaodong Zhu
|
|
|
Xiaodong Zhu
|
|||
|
President, CEO and Chairman of the Board
|
|||
