Attached files
| file | filename |
|---|---|
| EX-10.1 - STOCK OPTION AGREEMENT - INDESTRUCTIBLE 1, INC | f10k2010ex10i_dongsheng.htm |
| EX-32.1 - CERTIFICATION - INDESTRUCTIBLE 1, INC | f10k2010ex32i_dongsheng.htm |
| EX-31.1 - CERTIFICATION - INDESTRUCTIBLE 1, INC | f10k2010ex31i_dongsheng.htm |
| EX-3.3 - BYLAWS - INDESTRUCTIBLE 1, INC | f10k2010ex3iii_dongsheng.htm |
| EX-32.2 - CERTIFICATION - INDESTRUCTIBLE 1, INC | f10k2010ex32ii_dongsheng.htm |
| EX-31.2 - CERTIFICATION - INDESTRUCTIBLE 1, INC | f10k2010ex31ii_dongsheng.htm |
| EX-10.3 - EMPLOYMENT AGREEMENT WITH DI WU. - INDESTRUCTIBLE 1, INC | f10k2010ex10iii_dongsheng.htm |
| EX-10.2 - EMPLOYMENT AGREEMENT WITH XIAODONG ZHU. - INDESTRUCTIBLE 1, INC | f10k2010ex10ii_dongsheng.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED JUNE 30, 2010
o TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Dongsheng Pharmaceutical International Co., Ltd.
(Name of Registrant as specified in its charter)
|
Delaware
|
333-154787
|
26-2603989
|
|
(State or other Jurisdiction of Incorporation)
|
(Commission File Number)
|
(IRS Employer Identification No.)
|
China Bing’qi Plaza, Floor 17, No 69,
Zi Zhu Yuan Rd, Hai’dian District, Beijing
People’s Republic of China 100089
(Address of principal executive offices)
+86-10-88580708
(Telephone number, including area code)
Securities registered under Section 12(b) of the Exchange Act: None
Securities registered under Section 12(g) of the Exchange Act:
Common Stock, $0.0001 par value
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 the Securities Act. Yes ¨ No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ý No ¨
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the last 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes ¨ No ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer
|
¨
|
Accelerated Filer
|
¨
|
|
Non-Accelerated Filer
|
¨
|
Smaller reporting company
|
ý
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No ý
Issuer’s revenue for its most recent fiscal year was approximately $20.5 million. The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant on September 20, 2010, based on a closing price of $3.00 on June 14, 2010, was approximately $16,161,000. As of September 20, 2010, the registrant had 17,000,000 shares of its common stock, par value $0.0001 per share, outstanding.
Documents Incorporated By Reference: None.
TABLE OF CONTENTS
|
PART I
|
PAGE
|
|
| 2 | ||
| 9 | ||
| 16 | ||
| 16 | ||
| 16 | ||
| 16 | ||
|
PART II
|
||
| 17 | ||
| 17 | ||
| 17 | ||
| 22 | ||
| 22 | ||
| 23 | ||
| 23 | ||
| 23 | ||
|
PART III
|
||
| 24 | ||
| 25 | ||
| 26 | ||
| 28 | ||
| 28 | ||
|
PART IV
|
||
| 29 | ||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934. These statements relate to future events or our future financial performance. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “expects,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predict,” “should” or “will” or the negative of these terms or other comparable terminology. These statements are only predictions. Uncertainties and other factors, including the risks outlined under Risk Factors contained in Item 1A of this Form 10-K, may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels or activity, performance or achievements expressed or implied by these forward-looking statements.
A variety of factors, some of which are outside our control, may cause our operating results to fluctuate significantly. They include:
|
·
|
the availability and cost of drugs from our suppliers;
|
|
·
|
changes in end-user demand for the drugs distributed to our customers;
|
|
·
|
general and cyclical economic and business conditions, domestic or foreign, and, in particular, those in China’s pharmaceutical industries;
|
|
·
|
the rate of introduction of new products by our customers;
|
|
·
|
changes in our pricing policies or the pricing policies of our competitors or suppliers;
|
|
·
|
our ability to retain key employees;
|
|
·
|
our ability to compete effectively with our current and future competitors;
|
|
·
|
our ability to manage our growth effectively, including possible growth through acquisitions;
|
|
·
|
our ability to enter into and renew key corporate and strategic relationships with our customers and suppliers;
|
|
·
|
our ability to certain key employees.
|
|
·
|
our implementation of share-based compensation plans;
|
|
·
|
changes in the favorable tax incentives enjoyed by our PRC operating companies;
|
|
·
|
foreign currency exchange rates fluctuations;
|
|
·
|
adverse changes in the securities markets; and
|
|
·
|
legislative or regulatory changes in China.
|
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Our expectations are as of the date this Form 10-K is filed, and we do not intend to update any of the forward-looking statements after the filing date to conform these statements to actual results, unless required by law.
We file annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy and information statements and amendments to reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended. You may read and copy these materials at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding us and other companies that file materials with the SEC electronically. You may also obtain copies of reports filed with the SEC, free of charge, via a link included on our website at http://www.dongshengpharm.com.
1
PART I
Item 1. Business.
As used in this annual report, “we,” “us,” “our,” “Dongsheng,” or the “Company” refers to Dongsheng Pharmaceutical International Co., Ltd.
Overview
Dongsheng Pharmaceutical International Co., Ltd. is an established national primary pharmaceutical wholesaler in the People’s Republic of China (PRC or China), which specializes in the wholesale and distribution of high quality, high margin and high demand drugs, mainly to secondary and third tier regional drug wholesalers in China. Our objective is to source the highest quality drug products to the medical community.
Our major suppliers are pharmaceutical manufacturers. We contract with them to obtain exclusive sales rights whether regionally or nationally in China to sell their products in exclusive territories. Our performance is measured by sales quotas and market development performance set up in individual contracts with each manufacturer.
Our major customers are independent secondary or third tier regional drug wholesalers who then sell the drugs to hospitals, medical institutions and retail pharmacies. We carefully select our customers, set up sales quotas and periodically measure each customer’s performance against such quotas. As of September 2010, we have about 250 independent secondary or third tier drug wholesalers, whose sales network in aggregate covers approximately 2,500 hospitals in all 32 providences and municipal cities in the PRC.
Our main pharmaceutical products primarily cover the following therapeutic areas: Parkinson’s disease, cardiovascular, immunology and infectious diseases (See “Major Products”).
We believe that our experienced management team will continue to leverage our current position to market and distribute high quality, high margin, and high demand drugs. Our senior management team shares a common vision to build Dongsheng into a global pharmaceutical company with capacities in manufacturing, sales & distribution as well as proprietary drug research & development.
Our principal executive office is located in Beijing, China. Our primary operation is located in Chengdu City, Sichuan Providence, China.
Dongsheng Pharmaceutical’s common stock is publicly traded on the Over-the Counter Bulletin Board (“OTCBB”) under the symbol “DNGH.”
Corporate History
The Company’s core operational subsidiary is Sichuan Xintai Pharmaceutical Co., Ltd. (“Xintai”), which was incorporated in 1999, as a small pharmaceutical sales company in Chengdu City, Sichuan Providence, PRC. On July 15th, 2006, Zhu Xiaodong, an experienced drug wholesaler, and his sister, Zhu Xiaomei, acquired 100% of Xintai for approximately $260,000. Under Mr. Zhu’s leadership, Xintai soon became a well-known licensed pharmaceutical wholesale company, specializing in the sales of prescription and over-the-counter medicines.
On January 11, 2010, Sichuan Provincial Government approved Dynamic Bhorizon Limited (“DBL”), a company incorporated in the Cayman Islands on April 8, 2009, to acquire 100% of Xintai. On January 21, 2010, the Sichuan Administration for Industry and Commerce issued new business license to Xintai. With the new license, Xintai has been transformed from a domestic Chinese company to a wholly foreign owned enterprise (“WFOE”).
Dongsheng Pharmaceutical International Co., Ltd. (the “Company” or “Dongsheng”) was incorporated in the state of Delaware on March 25, 2008, originally under the name ‘Indestructible I.” On March 25, 2010, the Company entered into a share exchange agreement and acquired all of the outstanding capital stock of DBL. Pursuant to the share exchange agreement, DBL became a wholly-owned subsidiary of the Company and Mr. Zhu was appointed as the Company’s Chief Executive Officer.
In connection with the acquisition of DBL, the Company issued a total of 15,830,000 shares of common stock to the shareholders of DBL, their designees or assigns, in exchange for all of the capital stock of DBL. Upon the completion of the share exchange, the stockholders of DBL owned, in aggregate, approximately 93.12% of the issued and outstanding capital stock of the Company.
The acquisition was accounted for as a reverse merger under the purchase method of accounting since there was a change of control. Accordingly, DBL and its subsidiary, Xintai, will be treated as the continuing entity for accounting purposes.
On April 5, 2010, Indestructible I, Inc. changed its name to Dongsheng Pharmaceutical International Co., Ltd. to better reflect the Company’s business.
2
Organization Structure
Dongsheng’s organizational structure was developed to allow foreign capital infusion under the laws of the PRC and to maintain an efficient tax structure, as well as to maintain internal organizational efficiencies. The Company’s current organization structure is summarized in the table below:
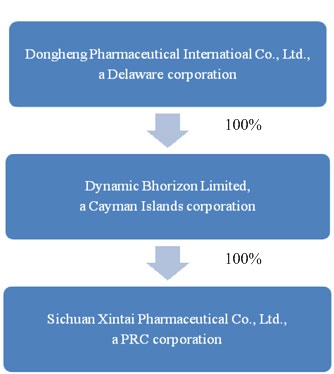
Industry Overview
Dongsheng operates in the pharmaceutical wholesale and distribution sector of the larger pharmaceutical industry in the PRC. Over the years, the pharmaceutical wholesale and distribution sector has become the vital link between pharmaceutical manufacturers and pharmaceutical retailers.
There are two primary ways for pharmaceutical manufacturers to sell drugs in China. The traditional way is to sell drugs through a proprietary sales network. During the past 20 years, burdened with rising raw material prices, ongoing capital needs for manufacturing facility upgrades, as well as significant time and money spent on maintaining a “Good Manufacturing Practice” (“GMP”) license, most pharmaceutical manufacturers have realized they cannot afford the cost of capital and other resources to keep their own sales force intact. GMP guidelines define the standards for the pharmaceutical manufacturing process in order to reduce the possibility of contamination errors. The Chinese government issued its own GMP standards in 1988, followed with two sets of revisions, the most recent in 1999. In China, pharmaceutical manufacturers must have a GMP certificate in order to legally produce drug.
The second way for pharmaceutical manufacturers to sell drugs in China is to contract with a primary drug wholesaler and grant them sale authorization in exclusive territories. Over the years, pharmaceutical manufacturers have found it more efficient to leverage a sales network of drug wholesalers to achieve its sales target. The biggest wholesalers are positioning themselves as indispensable intermediaries in the supply chain and staking out a powerful position in high-growth channels.
Market Overview
The growing demand for drugs in the PRC is driving the rapid growth of the pharmaceutical wholesale and distribution market. However, in its current state, the market is highly competitive and fragmented. We believe mass consolidation in the near future is highly likely.
Fast Growing Market Size
China accounts for 20% of the world’s population. It is expected to become the world’s third-largest prescription drug market in 2011, according to a report released by pharmaceutical market research firm IMS Health (“IMS”). IMS says pharmaceutical revenue in China is growing at a rapid pace, and the potential PRC market could double by the year 2013 as the country improves its health care infrastructure and aims for near-universal health coverage. IMS predicts that drug revenue in the PRC will grow by $40 billion through the year 2013. Such fast growing drug demand is driving the rapid growth of the pharmaceutical wholesale and distribution market in the PRC.
Fragmentation and the Trend for Consolidation
3
The pharmaceutical wholesale and distribution market is highly fragmented and competitive. According to recent statistics provided by the Chinese Medical Association, in 2009, there were approximately 13,000 drug wholesalers and distributors in the PRC. Measured by sales revenue in 2009, only eight drug wholesalers and distributors have exceeded RMB 10 billion (equivalent of USD 1.5 billion), while less than 5% of all 13,000 players have exceeded RMB 50 million (equivalent of USD 7.3 million). Total market share of the three largest Chinese wholesaler and distributors (China National Pharmaceutical Group Corporation, Shanghai Pharmaceutical Co., Ltd., and China Jointown Group) in 2009, was only 20% of the entire market.
To improve overall efficiency, the Chinese government has been encouraging mass consolidation and targets to support two to three largest wholesalers and distributors achieving a sales target of RMB 100 billion (equivalent of USD 15 billion), and top 20 largest wholesalers achieving a sales target of RMB 10 billion (equivalent of USD 1.5 billion), by 2015. This consolidation tide has stimulated most players in this sector to strategically seek collaborative partners.
Business Model
Licensed with a pharmaceutical wholesale permit, Dongsheng can lawfully sell pharmaceuticals within China. Our current business model is to negotiate exclusive wholesale and distribution agreements with pharmaceutical manufacturers for products we believe we can sell. We purchase drugs at a discount from pharmaceutical manufacturers who grant us authorization to sell their drugs in exclusive territories and then sell these drugs to secondary or third tier regional drugs wholesalers at competitive prices.
Our major suppliers are large pharmaceutical manufacturers. We contract with them to obtain authorizations to sell their products in exclusive territories. Our major customers are independent secondary or third tier regional drug wholesalers who then sell drugs to end users, such as hospitals, medical institutions and pharmacies.
We specialize in high quality, high margin and high demand drugs, which primarily cover the following therapeutic areas: Parkinson’s disease, cardiovascular, immunology and infectious diseases. (See “Major products”).
Core Competencies
Dongsheng (through its wholly owned subsidiaries) operates in the pharmaceutical wholesale sector in the PRC which is highly competitive. However, with a number of core competencies, Dongsheng has established itself as a highly regarded industry player. Our main core competencies that distinguish us from our competitors are:
|
·
|
Extensive Nationwide Sales Network – Dongsheng contracts with about 250 independent secondary or third tier drug wholesalers across China to sell drugs. It carefully selects them and periodically measures their performance against a sales quota system. Over the years, the contractual relationship with these secondary and third tier drug wholesalers has evolved to become a stable and strong sales network, through which, Dongsheng can make its point of sales throughout all of 32 provinces and municipal cities in China, covering over approximately 2,500 hospitals and medical institutions. We manage this sales network through our sales management department, which is composed of approximately 30 employed sales professionals. (See “Sales Network Management”). Compared to owning our proprietary scaled nationwide sales network, we believe this contractual binding sales network creates lower overall risk and tends to be more cost effective.
|
|
·
|
Professional and Experienced Sales Personnel – Each of the 250 independent secondary or third tier wholesalers employs about eight to ten sales representatives. In total, there are approximately 2,000 professional pharmaceutical sales representatives within our sales network. These sales representatives do not have employment contractual relationships with the Company. Rather, they are employed by our customers. Most of them are compensated through a commission arrangement with no or minimum base salary.
|
All sales representatives have educational backgrounds or training in the following therapeutic areas: Parkinson’s disease, cardiovascular, immunology and infectious diseases, which are the main markets our pharmaceutical drugs target to sell. They have also established long term relationships with many hospitals and doctors who are the main channels to move our products.
|
·
|
High Quality and Personalized Customer Services – To maintain and expand our sales network, Dongsheng provides high quality and personalized customer services to our customers, who are those secondary or third tier drug wholesalers. The services begin with pre-contractual support and conclude with post sales support.
|
|
o
|
Pre-contractual support – Before entering into contracts with secondary or third tier drug wholesalers, we carefully validate their drug sale license and their qualifications based on a number of factors, including, but not limited to, historical sales performance, covered regions and their relationships with regional hospitals, medical institutions and pharmacies. This screening process ensures that we select the best secondary and third tier drug wholesalers to be part of our sales network.
|
|
o
|
Marketing services – To assist our customers to achieve or exceed sales quotas, we employ various marketing campaigns to promote our products, such as advertising in major academic journals and press and hosting academic promotion conferences (see “Marketing Strategy”).
|
4
|
o
|
Post sales support – We provide high quality post sales support as well, such as fast drug delivery and return policy. We also host large gala events every year to honor our VIP customers.
|
|
·
|
Dedicated Focus on High Value and High Growth Drugs – Dongsheng carefully chooses its product lines. We specialize in authorized exclusive dealerships for such popular medicines that have comprehensive usage, large consumption per patient, and relative high value and high profit margins. Our main pharmaceutical products cover the following therapeutic areas: Parkinson’s disease, cardiovascular, immunology and infectious diseases (See “Major Products”). This strategy ensures that we achieve both top line and bottom line growth, which eventually distinguishes us from other wholesalers with limited or no product focus.
|
|
·
|
Good Relationship with Chinese Medical Governance Body and Pharmaceutical Manufacturers. Over the years, Dongsheng has built strong collaborative relationships with the Chinese medical governance body and many pharmaceutical manufacturers.
|
|
o
|
Most Chinese pharmaceutical manufacturers don’t have their own zoning distribution channels or have limited marketing capabilities. Due to our historical sales record, we are able to gain and secure exclusive authorizations from some of the pharmaceutical manufacturers to sell their high demand and high growth products.
|
|
o
|
Once we are under contract with pharmaceutical manufacturers, we generally assist them to tender such drugs to be part of the “Chinese Basic Medicine Insurance Coverage List” at both the national and provincial levels, which not only helps to greatly increase sales volume of the drugs, but also improves the brand of such pharmaceutical manufacturers.
|
Marketing and Pricing Strategies
Marketing Strategy: We use various marketing campaigns to promote our products, such as advertising in major academic journals, press conferences, and academic promotion conferences.
|
·
|
Academic conferences: Dongsheng has four different academic expert teams, each comprised of well known professionals from therapeutic areas such as Parkinson’s disease, cardiovascular, immunology and infectious diseases. Whenever Dongsheng launches a new product, an expert team gives a lecture or hosts forums across the country. We invite doctors, pharmacists and our customers to the conferences so they get a better understanding of our products.
|
Price Strategy: In China, drug prices are primarily guided by national and local medical regulatory bodies. Along with this guidance, we offer our high quality drugs to our customers at competitive prices through various sales discounts.
Sales Network Management
Our sales network is built on various contractual relationships with independent secondary or third tier regional pharmaceutical wholesalers. We manage this network through seven of our full time regional sales directors, who are supported by approximately twenty full time sales managers. Regions are geographically divided. Sale directors and their managers are responsible for managing the relationship with the secondary and third tier pharmaceutical wholesalers.
|
·
|
Customer screening: Regional sales directors carefully select secondary or third tier pharmaceutical wholesalers based on a number of factors, such as historical sales performance, covered regions and their relationships with regional hospitals, medical institutions and pharmacies. This screening process ensures that we select the best secondary or third tier drug wholesalers to be part of our sales network.
|
|
·
|
Performance measurement: Regional sales directors periodically measure the sales performance of secondary or third tier wholesalers against our sales quota system.
|
|
·
|
Customer Appreciations: The Company has been focused on developing approximately 100 VIP clients. The Company will give discounts monthly or quarterly as customer loyalty incentives.
|
|
·
|
Incentive Mechanism: Sales directors and sales managers are compensated with a combination of base salary and bonuses. The bonus is tied to the sales target, market development and the quality of customer management.
|
Distribution Channel Management
Dongsheng has distribution capacity in Sichuan Providence, where our core operation Xintai is located. However it does not currently own its national drug distribution channel. Once sales are made to customers out of Sichuan providence, we contract with local retail distribution companies to distribute the drugs to hospitals, medical institutions and pharmacies. Each regional sales director and manager works with at least ten retail distribution companies. Therefore, at any one time, there are at least 200 retail distributors working closely with Dongsheng.
5
Inventory Management
Xintai, the Company’s core operational subsidiary, leases warehouses in Chengdu City, Sichuan Providence. Since 80% of our sales require advance payments from our customers, we are able to use customer advances to purchase drugs from our suppliers. We then either temporarily keep the drugs delivered by our suppliers in our warehouse before we ship the drugs to our customers, or we can instruct our suppliers to ship the drugs directly to our customers. This model allows us to keep inventory and storage costs low.
Major Suppliers
Measured by purchase cost in fiscal year 2010, Dongsheng’s top three suppliers include Aode Pharmaceutical, Hebei Furuide Pharmaceutical, and Beijing Guorun Pharmaceutical Co. These three supplies accounted for 35.88%, 29.97% and13.47%, respectively, of Dongsheng’s total purchases during fiscal year 2010.
Major Customers
Measured by sales revenue in fiscal year 2010, Dongsheng’s top three customers are Hezhong Huitong Pharmaceutical (in Henan Province), Jieshou City Medical Co., and Sanmenxia Huawei Mecial Co. They accounted for 5.43%, 4.57% and 4.54%, respectively, of our total sales during fiscal year of 2010.
Major Products
Dongsheng carefully chooses its product lines. We specialize in the wholesale and distribution of high quality, high margin and high demand drugs. Our main pharmaceutical products cover the following therapeutic areas: Parkinson’s disease, cardiovascular, immunology and infectious diseases.
GANGLIOSIDE
Product Indication and Pharmacology:
Ganglioside is indicated for improving symptoms, delaying disease progression, and restoring damaged brain cells in Parkinson’s disease patients.
Gangliosides are complex glycolipid molecules which are natural components of cellular membranes. Numerous gangliosides have been identified and have been found to be particularly abundant in nerve tissue, especially in brain tissue. Gangliosides stimulate the remaining brain cells to sprout new nerve endings and rescue other cells that might otherwise die. Parkinson's disease is caused by a loss of dopamine-secreting cells in the brain, which results in impaired motor skills, speech, and other functions. Abnormal accumulation of the protein α-synuclein may contribute to this neuronal cell death. Treatment with gangliosides reversed the lysosomal disruption, which suggests that gangliosides protect against the lysosomal damage of a-synuclein accumulation.
Market Analysis
According to a 2010 research report issued by a medical research firm based in Guangzhou, China, Guangzhou Biaodian Pharmaceutical Information Co., 2009 Chinese senile dementia drug sales reached over $15 billion, a 29% increase over 2008. Ganglioside by far is the second largest senile dementia drug with 28% market share and over $3 billion sales revenue in 2009.
Currently there are eleven authorized Ganglioside manufacturers in China. Among them, six are new market entrants while the other five are seasoned players. Among all Ganglioside manufacturers in China, only two of them, Qilu Pharmaceutical and Harbin Medical University Pharmaceutical, are granted with Ganglioside medicine raw material production permit by State Food and Drug Administration (“SFDA”), which ensures a stable supply. Harbin Medical University Pharmaceutical, through its affiliates, provides us steady Ganglioside drug supply.
Sales Performance
In 2007, we obtained an exclusive wholesale contract from Aode Pharmaceutical, an affiliate with Harbin Medical University Pharmaceutical, to sell Ganglioside within 11 providences in China. Since then, our sales revenue of Ganglioside has been steadily increasing. In fiscal year 2010, our revenue from Gangioside sale reached $19.3 million, which represented a 24% increase on constant dollar basis over 2009 and accounted for 94% of our total sales in 2010. Ganglioside is now admitted into the” China Provincial Base Medicine Insurance Coverage list” of a number of providences that we cover.
In September 2010, we renewed such contract with the expiration date extended to Aug 31, 2018.
EDARAVONE
Product Indication and Pharmacology:
6
Edaravone is a neuro-protectiveagent, and is indicated for relief of neurological symptoms associated with acute cerebral infarction, and for enhancing routine activity and alleviating functional disorders.
Edaravone acts as a potent antioxidant and strongly scavenges free radicals, protecting against oxidative stress and neuronal apoptosis. It has been shown to attenuate methamphetamine- and 6-OHDA-induced dopaminergic neurotoxicity in the striatum and substantial nigra, and does not affect methamphetamine-induced dopamine release or hyperthermia. It has also been demonstrated to protect against MPTP-mediated dopaminergic neurotoxicity to the substantial nigra.
Market Analysis
According to the same research report mentioned above (see details in “Galioside”) , Edaravone accounted for 7.8% market share of China senile dementia drug with sales revenue reaching $1.2 billion in 2009.
Currently there are six authorized Edaravone manufacturers in China. The one with the largest market share is Simcere Pharmaceutical Group (Product name: “Bicun”).
Edaravone is now admitted into “China National Base Medicine Insurance Coverage list Type B”
Sales Performance
On December 30, 2009, we obtained a one-year exclusive wholesale contract with Jilin Boda Pharmaceutical (Product name: “Yidasheng”) and China National Medicines Guorui Pharmaceutical Co., Ltd., to sell Edaravone in four providences in China. The contract is subject to renew on December 30 of every year.
Our sales from Edaravone are still in ramp up period. In fiscal year 2010, our revenue from Edaravone sale reached $0.6 million, which accounted for 3% of our total sales in 2010.
SALVIANOLATE INJECTION
Product Indication and Pharmacology:
Salvianolate, a cardiovascular pharmaceutical product, is a purified and isolated Chinese traditional medicine. With magnesium acetate as the main component of Salvia poly-phenol salts, Salvianolate is considered as one of the most important active ingredients for the treatment of cardiovascular diseases among the Salvia group products. It significantly improves blood circulation.
Salvianolate injection is indicated for the treatment of steady angina pectoris of Coronary heart disease with stagnation of heart-blood syndromes at stage I to II. Specifically it relieves symptoms associated with the heart-blood syndromes, such as chest pain, short of breath and palpitation.
Market Analysis
According to a report released by pharmaceutical market research firm IMS Health (“IMS”), Salvianolate market realized sales of $1.7 billion in 2009, an increase of 9.97% over 2007.
The Salvianolate is the successful product of 13-year academic research by Shanghai Institute of Pharmacology, a branch of Chinese Academy of Sciences. On May 25, 2005, Salvianolate was granted with New Medicine Grade B Certificate by China State Food and Drug Administration. Currently it is covered by Chinese National Basic Medicine Insurance Coverage list.
Sales Performance
We obtained a one year exclusive contract with Shanghai Leiyunshang Green Valley Pharmaceutical Co to sell the Salvanolate they produce in Heilongjiang providence. The contract is effective from January 14, 2010 and is subject to renew on yearly basis.
Currently our sales from Salvianolate are in ramp up period and expect to grow steadily in future years.
SULBENICILLIN INJECTION
Product Indication and Pharmacology:
Sulbenicillin Injection is a penicillin antibiotic. Sulbenicillin is a broad-spectrum semi synthetic penicillin that has actions and uses similar to those of carbenicillin. It belongs to the class of penicillins with extended spectrum.
Sulbenicillin Injection is indicated for the treatment of pneumonia, urinary tract infection, skin/soft tissue infections and septicaemia, caused by Pseudomonas aeruginosa and nonpenicillinase-producing strains of Proteus spp. Sulbenicillin Injection is also effective in treating abdominal/pelvic infections caused by sensitive bacteria when it is used in conjunction with anti anaerobic bacteria drugs.
7
Market Analysis
Sulbenicillin Injection is covered under “China National Basic Medicine Insurance Coverage List Category B)
In China, SFDA has granted manufacturing permits for producing Sulbenicillin Injection to four pharmaceutical manufactuers, Shandong Ruiyang Pharmaceutical, Shenyang Meiluo, Harbin General Pharmaceutical Factory and Hunan Xiangyao Pharmaceutical Co., Ltd. Among them, only Shandong Ruiyang Pharmaceutical and Hunan Xiangyao Pharmaceutical Co., Ltd are equipped with adequate manufacturing capacity.
Sales Performance
On December 16, 2009, we obtained a three year exclusive authorization from Hunan Xiangyao Pharmaceutical Co. Ltd to sell their Sulbenicillin Injection in six providences in China.
Currently our sales from Sulbenicillin Injection are in ramp up period and expect to grow steadily in future years.
IMIPENEM/CILASTATIN INJECTION
Product Indication and Pharmacology:
Imipenem/cilastatin Injection is a broad spectrum beta-lactam antibiotic containing equal quantities of imipenem and cilastatin. It is related to the penicillin/cephalosporin family of antibiotics but is classified as belonging to the carbapenem class. Imipenem inhibits bacterial cell wall synthesis. Cilastatin prevents metabolism of imipenem, resulting in increased urinary recovery and decreased renal toxicity.
Imipenem/cilastatin Injection is indicated for treatment of serious infections of lower respiratory tract and urinary tract, intra-abdominal and gynecologic infections, bacterial septicemia, bone and joint infections, skin and skin structure infections, endocarditis, and polymicrobic infections due to susceptible microorganisms.
Market Analysis
Imipenem/cilastatin Injection is covered by China National Basic Medicine Coverage list.
In China, there are currently five domestic Imipenem/cilastatin Injection manufacturers, including Shenzhen Haibin Pharmaceutical Co., Ltd, Zhejiang Hisum Pharmaceutical Co., Ltd, China National Medicines Guorui Pharmaceutical Co., Ltd, Shandong New Era Pharmaceutical Co, and Zhuhai United Pharmaceutical Co. Compared to the imported Imipenem/cilastatin Injection, the domestic-made Imipenem/cilastatin Injection competes in lower price with the annual sales increasing rate of 30%; Tienam from Merck & Co., Inc has been dominating imipenem market for long time. Along with the entering of new companies recent years, the market share of Merck & Co., Inc has been gradually dropping though.
Sales Performance
On April 8, 2008, we obtained a five year exclusive authorization from China National Medicines Guorui Pharmaceutical Co., Ltd to sell their Imipenem/cilastatin Injection in all 32 providences in China.
Research & Development
Currently, we do not maintain our own R & D department. Instead, we conduct collaborated work with some of the high end medical research institutions in China, such as Academy of Military Medical Sciences, China Pharmaceutical University and Chinese Academy of Sciences. Through the collaborated work, our drug Research and Development strategy aims at: (1) copying after off-patent drugs that tend to be high demand and high quality; (2) developing new drug formulations and obtaining patent protection for those drugs with greater market potentials
Future Development Plan
Dongsheng aims to become a leading global pharmaceutical company with comprehensive capabilities in pharmaceutical manufacturing, sales and distribution, as well as proprietary drug research & development. Within the next three to five years, we are targeting on achieving the followings:
Wholesaler and distributor alliance: In responsive to the mass consolidation trend in Chinese pharmaceutical wholesaler and distribution sector, we are looking to build strong alliance and strategic partnership with other highly regarded wholesalers and distributors.
8
Sign up for higher value-added product dealerships: To maximize the usage of our sales network and improve profit margins, we are looking to obtain t additional exclusive sales dealerships by adding two to three cardiovascular products, one to two anti-tumor products and two to three antibiotics drugs into our product list.
Increase Production Capability by Acquiring Pharmaceutical Manufacturers: To ensure stable supply and steadily increase product lines, we are looking to acquire one to two pharmaceutical manufacturers.
Acquire R&D institutes and increase proprietary R&D capability: Dongsheng intends to acquire quality pharmaceutical companies with valuable R&D assets and promising projects which have great potential developing into products.
Overseas Expansion: In addition to the Chinese market, we have built a sales representative office in Hanoi, Vietnam. To maximize our sales revenue and profit margin, we are looking to build more overseas presences.
Overall, our vision is to build our wholesale and distribution alliance, add more high value-added product lines, build our own manufacturing facility, put more focus on proprietary R&D and expand our overseas presences. We believe this development plan maximizes the Company’s long term competitiveness in the pharmaceutical market.
Employees
Dongsheng currently has a total of sixty nine employees, which includes ten senior management personnel, seven regional sales directors, sixteen regional sales managers and other staffs
WHERE YOU CAN FIND MORE INFORMATION
You are advised to read this Form 10-K in conjunction with other reports and documents that we file from time to time with the SEC. In particular, please read our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that we file from time to time. You may obtain copies of these reports directly from us or from the SEC at the SEC’s Public Reference Room at 100 F. Street, N.E. Washington, D.C. 20549, and you may obtain information about obtaining access to the Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains information for electronic filers at its website http://www.sec.gov.
Item 1A. Risk Factors.
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated herein that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, you may lose all or part of your investment.
Risks Relating to Our Business
SUBSTANTIALLY ALL OF OUR BUSINESS, ASSETS AND OPERATIONS ARE LOCATED IN THE PRC.
Substantially all of our business, assets and operations are located in the PRC. The economy of PRC differs from the economies of most developed countries in many respects. The economy of PRC has been transitioning from a planned economy to a market-oriented economy. Although in recent years, the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in PRC is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry by imposing industrial policies. It also exercises significant control over PRC’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Some of these measures benefit the overall economy of PRC, but may have a negative effect on us.
OUR MANAGEMENT HAS NO EXPERIENCE IN MANAGING AND OPERATING A PUBLIC COMPANY. ANY FAILURE TO COMPLY OR ADEQUATELY COMPLY WITH FEDERAL SECURITIES LAWS, RULES OR REGULATIONS COULD SUBJECT US TO FINES OR REGULATORY ACTIONS, WHICH MAY MATERIALLY ADVERSELY AFFECT OUR BUSINESS, RESULTS OF OPERATIONS AND FINANCIAL CONDITION.
Our current management has no experience managing and operating a public company and relies in many instances on the professional experience and advice of third parties including its attorneys and accountants. Failure to comply or adequately comply with any laws, rules, or regulations applicable to our business may result in fines or regulatory actions, which may materially adversely affect our business, results of operation, or financial condition and could result in delays in the development of an active and liquid trading market for our stock.
OUR PLANS TO EXPAND OUR PRODUCT LINES, TO ACQUIRE A PHARMACEUTICAL FACTORY, TO BUILD A RESEARCH AND DEVELOPMENT CENTER AND TO IMPROVE AND UPGRADE OUR INTERNAL CONTROL AND MANAGEMENT SYSTEM WILL REQUIRE CAPITAL EXPENDITURES IN 2010 AND FUTURE YEARS
9
Our plans to expand our product lines, to acquire a pharmaceutical factory, to build a research and development center and to improve and upgrade our internal control and management system will require capital expenditures in 2010 and future years. We may also need further funding for working capital, investments, potential acquisitions and other corporate requirements. We cannot assure you that cash generated from our operations will be sufficient to fund these development plans, or that our actual capital expenditures and investments will not significantly exceed our current planned amounts. If either of these conditions arises, we may have to seek external financing to satisfy our capital needs. Our ability to obtain external financing at reasonable costs is subject to a variety of uncertainties. Failure to obtain sufficient external funds for our development plans could adversely affect our business, financial condition and operating performance.
WE DERIVE ALL OF OUR REVENUES FROM SALES IN THE PRC AND ANY DOWNTURN IN THE CHINESE ECONOMY COULD HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS AND FINANCIAL CONDITION.
Currently all of our revenues are generated from sales in the PRC. We anticipate that revenues from sales of our products in the PRC will continue to represent the substantial portion of our total revenues in the near future. Our success is influenced by changes in government regulations on pharmaceutical industry and a number of economic factors which affect disposable consumer income, such as employment levels, business conditions, interest rates, oil and gas prices and taxation rates. Adverse changes in government regulations on pharmaceutical industry and these economic factors, among others, may restrict consumer spending, thereby negatively affecting our sales and profitability.
OUR PLANNED EXPANSION COULD BE DELAYED OR ADVERSELY AFFECTED BY, AMONG OTHER THINGS, DIFFICULTIES IN OBTAINING SUFFICIENT FINANCING, TECHNICAL DIFFICULTIES, OR HUMAN OR OTHER RESOURCE CONSTRAINTS.
Our planned expansion could be delayed or adversely affected by, among other things, difficulties in obtaining sufficient financing, technical difficulties, or human or other resource constraints. Moreover, the costs involved in these projects may exceed those originally contemplated. Costs savings and other economic benefits expected from these projects may not materialize as a result of any such project delays, cost overruns or changes in market circumstances. Failure to obtain intended economic benefits from these projects could adversely affect our business, financial condition and operating performances.
WE ENCOUNTER SUBSTANTIAL COMPETITION IN OUR BUSINESS AND ANY FAILURE TO COMPETE EFFECTIVELY COULD ADVERSELY AFFECT OUR RESULTS OF OPERATIONS.
As described in the industry and market overviews as well as products section, for every product we sell, we encounter strong competitors. We anticipate that our competitors will continue to expand and seek to obtain additional market share with competitive price and performance characteristics. Aggressive expansion of our competitors or the entrance of new competitors into our markets could have a material adverse effect on our business, results of operations and financial condition.
OUR LIMITED OPERATING HISTORY MAY NOT SERVE AS AN ADEQUATE BASIS TO JUDGE OUR FUTURE PROSPECTS AND RESULTS OF OPERATIONS.
Our limited operating history in the pharmaceutical industry may not provide a meaningful basis for evaluating our business. Dongsheng entered into its current line of business in 2006, although its wholly owned core operational subsidiary - Xintai was incorporated in 1999. Although its revenues have grown rapidly since its inception, we cannot guaranty that we will maintain profitability or that we will not incur net losses in the future. We will continue to encounter risks and difficulties that companies at a similar stage of development frequently experience, including the potential failure to:
|
·
|
obtain sufficient working capital to support our expansion;
|
|
·
|
expand our product offerings and maintain the high quality of our products;
|
|
·
|
manage our expanding operations and continue to fill customers’ orders on time;
|
|
·
|
maintain adequate control of our expenses allowing us to realize anticipated income growth;
|
|
·
|
implement our product development, sales, and acquisition strategies and adapt and modify them as needed;
|
|
·
|
successfully integrate any future acquisitions; and
|
|
·
|
anticipate and adapt to changing conditions in the pharmaceutical industry resulting from changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
|
If we are not successful in addressing any or all of the foregoing risks, our business may be materially and adversely affected.
10
WE NEED TO MANAGE GROWTH IN OPERATIONS TO MAXIMIZE OUR POTENTIAL GROWTH AND ACHIEVE OUR EXPECTED REVENUES AND OUR FAILURE TO MANAGE GROWTH WILL CAUSE A DISRUPTION OF OUR OPERATIONS RESULTING IN THE FAILURE TO GENERATE REVENUES AT LEVELS WE EXPECT.
In order to maximize potential growth in our current and potential markets, we believe that we must expand our operations. This expansion will place a significant strain on our management and our operational, accounting, and information systems. We expect that we will need to continue to improve our financial controls, operating procedures, and management information systems. We will also need to effectively train, motivate, and manage our employees. Our failure to manage our growth could disrupt our operations and ultimately prevent us from generating the revenues we expect.
WE CANNOT ASSURE THAT OUR GROWTH STRATEGY WILL BE SUCCESSFUL WHICH MAY RESULT IN A NEGATIVE IMPACT ON OUR GROWTH, FINANCIAL CONDITION, RESULTS OF OPERATIONS AND CASH FLOW.
One of our strategies is to grow through acquiring a pharmaceutical factory and increase product lines. However, there are many obstacles to achieve this strategy. We cannot, therefore, assure you that we will be able to successfully overcome such obstacles and establish our products in any additional markets. Our inability to implement this growth strategy successfully may have a negative impact on our growth, future financial condition, results of operations or cash flows.
IF WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF OUR OPERATIONS.
If adequate additional financing is not available on reasonable terms, we may not be able to undertake acquisitions of a pharmaceutical factory or to build a research and development center, and we would have to modify our business plans accordingly. There is no assurance that additional financing will be available to us.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the level of our investment in research and development; and (iii) the amount of our capital expenditures, including acquisitions and product lines expansions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
In recent years, the securities markets in the United States have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, our securities can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If we need additional funding we will, most likely, seek such funding in the United States (although we may be able to obtain funding in the PRC) and the market fluctuations affect on our stock price could limit our ability to obtain equity financing.
If we cannot obtain additional funding, we may be required to: (i) limit our expansion; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures. Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions that are favorable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to the shares of our common stock. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
WE MAY NOT BE ABLE TO ATTRACT AND RETAIN SKILLED QUALIFIED PERSONNEL TO SUPPORT THE EXAPNSION OF OUR BUSINESS. .
The Company’s future success also depends upon its continuing ability to attract and retain highly qualified personnel. Expansion of the Company’s business and operation will require additional managers and employees with industry experience, and the success of the Company will be highly dependent on the Company’s ability to attract and retain skilled management personnel and other employees. There can be no assurance that the Company will be able to attract or retain highly qualified personnel. Competition for skilled personnel in the pharmaceutical industry is significant. This competition may make it more difficult and expensive to attract, hire and retain qualified managers and employees.
THE LOSS OF THE SERVICES OF OUR KEY EMPLOYEES, PARTICULARLY THE SERVICES RENDERED BY XIAODONG ZHU, OUR DIRECTOR, DI WU, OUR DEVELOPMENT OFFICER, AND JIANPING CHEN, OUR CHIEF FINANCIAL OFFICER COULD HARM OUR BUSINESS.
Our success depends to a significant degree on the services rendered to us by our key employees. If we fail to attract, train and retain sufficient numbers of these qualified people, our prospects, business, financial condition and results of operations will be materially and adversely affected. In particular, we are heavily dependent on the continued services of Xiaodong Zhu, our director, Di Wu, our development officer, and Jianping Chen, our Chief Financial Officer. The loss of any key employees, including members of our senior management team, and our inability to attract highly skilled personnel with sufficient experience in our industry could harm our business.
11
OUR FAILURE TO COMPLY WITH INCREASINGLY STRINGENT ENVIRONMENTAL REGULATIONS AND RELATED LITIGATION COULD RESULT IN SIGNIFICANT PENALTIES, DAMAGES AND ADVERSE PUBLICITY FOR OUR BUSINESS.
In recent years, the government of China has become increasingly concerned with the degradation of China’s environment that has accompanied the country’s rapid economic growth. In the future, we expect that our manufacturers’ operations and properties will be subject to extensive and increasingly stringent laws and regulations pertaining to, among other things, the discharge of materials into the environment and the handling and disposition of wastes (including solid and hazardous wastes) or otherwise relating to protection of the environment. While, the laws of PRC protect distributors from claims solely related to manufacturing defects, failure by our manufacturers to comply with any laws and regulations and future changes to them may result in significant consequences to us, including civil and criminal penalties, liability for damages and negative publicity in the future. We cannot assure you that additional environmental issues will not require currently unanticipated investigations, assessments or expenditures, or that requirements applicable to us will not be altered in ways that will require us to incur significant additional costs.
WE WILL INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS.
We will incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Risks Relating To Our Industry
WE MAY BE SUBJECT TO SUBSTANTIAL LIABILITY SHOULD THE CONSUMPTION OF ANY OF OUR PRODUCTS CAUSE PERSONAL INJURY OR ILLNESS. UNLIKE MOST PHARMACEUTICAL COMPANIES IN THE UNITED STATES, WE DO NOT MAINTAIN PRODUCT LIABILITY INSURANCE TO COVER OUR POTENTIAL LIABILITIES.
The sale of drug products for human consumption involves an inherent risk of injury to consumers. Such injuries may result from tampering by unauthorized third parties or product contamination or degeneration, including the presence of foreign contaminants, chemical substances or other agents or residues during the various stages of production process. We cannot assure you that consumption of our products will not cause a health-related illness in the future, or that we will not be subject to claims or lawsuits relating to such matters if the laws of the PRC change.
Even if a product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding any assertions that our products caused personal injury or illness could adversely affect our reputation with customers and our corporate and brand image. Unlike most pharmaceutical companies in the United States, but in line with industry practice in the PRC, we do not maintain product liability insurance. Furthermore, the products we sold could potentially suffer from product tampering, contamination or degeneration or be mislabeled or otherwise damaged. Under certain circumstances, we may be required to recall products. Even if a situation does not necessitate a product recall, we cannot assure you that product liability claims by our distributors will not be asserted against us as a result. A product liability judgment against us or a product recall could have a material adverse effect on our revenues, profitability and business reputation.
IF THE PHARMACEUTICAL INDUSTRY IN THE PRC DOES NOT GROW AS WE EXPECT, OUR RESULTS OF OPERATIONS AND FINANCIAL CONDITION WILL BE ADVERSELY AFFECTED.
We believe drug products have strong growth potential in the PRC and, accordingly, we have continuously increased our sales of drug products. However, the market for pharmaceutical industry in the PRC has grown in recent years due to the increased wealth of the average resident of China and increasing health cautiousness, which has been the result of double-digit annual growth in the Chinese economy. Due to the worldwide recession, the growth of the Chinese economy has slowed. If the pharmaceutical industry in the PRC does not grow as we expect, our business will be harmed, we will need to adjust our growth strategy, and our results of operation will be adversely affected.
12
SALES OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT MEDICATION IN THE PRC WHICH NEGATIVELY IMPACTING OUR PROFITABILITY.
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. Exact data are impossible to collect, but the FBI believes that more than half of the pharmaceuticals sold in PRC are counterfeit. Examples of the seriousness of the problem include: six months after Viagra was introduced in 2002, state media reported that some 90 percent of little blue pills sold in Shanghai were counterfeit; and 192,000 Chinese patients were reported to have died in 2001 from fake drugs. Counterfeit products shrink markets for legitimate goods. This situation affects the Company and other major domestic and foreign drug manufacturers in PRC, especially for products marketed over-the-counter rather than through hospitals. However, we believe the Chinese authorities are becoming increasingly vigilant against counterfeiting, because, in 2001, the authorities closed approximately 1,300 factories while investigating approximately 480,000 cases of counterfeit drugs. Currently, active pharmaceutical ingredients are governed only by chemical regulations. We believe that a major step towards controlling the problem would be taken should the Chinese State Food and Drug Administration be given oversight over PRC’s bulk chemicals producers. However, our ability to increase sales as rapidly as we would like, and our profitability, could be affected if this problem persists or worsens.
Risks Relating to the People’s Republic of China
CERTAIN POLITICAL AND ECONOMIC CONSIDERATIONS RELATING TO THE PRC COULD ADVERSELY AFFECT OUR COMPANY.
The PRC is transitioning from a planned economy to a market economy. While the PRC government has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms carried out by the PRC government are unprecedented or experimental, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC’s economic and social conditions as well as by changes in the policies of the PRC government, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the interest rate or method of taxation, and the imposition of restrictions on currency conversion in addition to those described below.
THE RECENT NATURE AND UNCERTAIN APPLICATION OF MANY PRC LAWS APPLICABLE TO US CREATE AN UNCERTAIN ENVIRONMENT FOR BUSINESS OPERATIONS AND THEY COULD HAVE A NEGATIVE EFFECT ON US.
The PRC legal system is a civil law system. Unlike the common law system, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects.
CURRENCY CONVERSION COULD ADVERSELY AFFECT OUR FINANCIAL CONDITION.
The PRC government imposes control over the conversion of Renminbi into foreign currencies. Under the current unified floating exchange rate system, the People’s Bank of China publishes an exchange rate, which we refer to as the PBOC exchange rate, based on the previous day’s dealings in the inter-bank foreign exchange market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of Renminbi into foreign exchange by Foreign Investment Enterprises, or FIEs, for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC. Conversion of Renminbi into foreign currencies for capital account items, including direct investment, loans, and security investment, is still under certain restrictions. On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
13
Furthermore, the Renminbi is not freely convertible into foreign currencies nor can it be freely remitted abroad. Under the PRC’s Foreign Exchange Control Regulations and the Administration of Settlement, Sales and Payment of Foreign Exchange Regulations, Foreign Invested Enterprises are permitted either to repatriate or distribute its profits or dividends in foreign currencies out of its foreign exchange accounts, or exchange Renminbi for foreign currencies through banks authorized to conduct foreign exchange business. The conversion of Renminbi into foreign exchange by Foreign Invested Enterprises for recurring items, including the distribution of dividends to foreign investors, is permissible. The conversion of Renminbi (Chinese currency) into foreign currencies for capital items, such as direct investment, loans and security investment, is subject, however, to more stringent controls.
Our operating company is a FIE to which the Foreign Exchange Control Regulations are applicable. Accordingly, we will have to maintain sufficient foreign exchange to pay dividends and/or satisfy other foreign exchange requirements.
EXCHANGE RATE VOLATILITY COULD ADVERSELY AFFECT OUR FINANCIAL CONDITION.
Since 1994, the exchange rate for Renminbi against the United States dollar has remained relatively stable, most of the time in the region of approximately RMB8.28 to $1.00. However, in 2005, the Chinese government announced that it would begin pegging the exchange rate of the Chinese Renminbi against a number of currencies, rather than just the U.S. dollar and, the exchange rate for the Renminbi against the U.S. dollar became RMB8.02 to $1.00. If we decide to convert Chinese Renminbi into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese Renminbi we convert would be reduced. There can be no assurance that future movements in the exchange rate of Renminbi and other currencies will not have an adverse effect on our financial condition.
SINCE OUR ASSETS ARE LOCATED IN THE PRC, ANY DIVIDENDS OF PROCEEDS FROM LIQUIDATION ARE SUBJECT TO THE APPROVAL OF THE RELEVANT CHINESE GOVERNMENT AGENCIES.
Our operating assets are located inside the PRC. Under the laws governing Foreign Invested Enterprises in the PRC, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to the relevant government agency’s approval and supervision as well as the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
IT MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY RESIDE OUTSIDE THE UNITED STATES.
As our operations are presently based in the PRC and most of our director and officer resides in the PRC, service of process on our company and such director and officer may be difficult to effect within the United States. Also, our main assets are located in the PRC and any judgment obtained in the United States against us may not be enforceable outside the United States.
DUE TO VARIOUS RESTRICTIONS UNDER PRC LAWS ON THE DISTRIBUTION OF DIVIDENDS BY OUR PRC OPERATING COMPANIES, WE MAY NOT BE ABLE TO PAY DIVIDENDS TO OUR STOCKHOLDERS.
The Wholly-Foreign Owned Enterprise Law (1986), as amended and The Wholly-Foreign Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law of the PRC (2006) contain the principal regulations governing dividend distributions by wholly foreign owned enterprises. Under these regulations, wholly foreign owned enterprises may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. Additionally, such companies are required to set aside a certain amount of their accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends except in the event of liquidation and cannot be used for working capital purposes. The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the Company’s profits.
Furthermore, if our subsidiaries in China incur debt on their own in the future, the instruments governing the debt may restrict its ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our common stock.
Risks Related To Our Securities
IN ORDER TO RAISE SUFFICIENT FUNDS TO EXPAND OUR OPERATIONS, WE MAY HAVE TO ISSUE ADDITIONAL SECURITIES AT PRICES WHICH MAY RESULT IN SUBSTANTIAL DILUTION TO OUR SHAREHOLDERS.
If we raise additional funds through the sale of equity or convertible debt, our current stockholders’ percentage ownership will be reduced. In addition, these transactions may dilute the value of our common shares outstanding. We may have to issue securities that may have rights, preferences and privileges senior to our common stock. We cannot provide assurance that we will be able to raise additional funds on terms acceptable to us, if at all. If future financing is not available or is not available on acceptable terms, we may not be able to fund our future needs, which would have a material adverse effect on our business plans, prospects, results of operations and financial condition.
14
OUR ARTICLES OF INCORPORATION PROVIDE FOR INDEMNIFICATION OF OFFICERS AND DIRECTORS AT OUR EXPENSE AND LIMIT THEIR LIABILITY WHICH MAY RESULT IN A MAJOR COST TO US AND HURT THE INTERESTS OF OUR SHAREHOLDERS BECAUSE CORPORATE RESOURCES MAY BE EXPENDED FOR THE BENEFIT OF OFFICERS AND/OR DIRECTORS.
Our articles of incorporation and applicable Delaware law provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on our behalf. We will also bear the expenses of such litigation for any of our directors, officers, employees, or agents, upon such person’s written promise to repay us if it is ultimately determined that any such person shall not have been entitled to indemnification. This indemnification policy could result in substantial expenditures by us which we will be unable to recoup.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification for liabilities arising under federal securities laws, other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding, is asserted by a director, officer or controlling person in connection with the securities being registered, we will (unless in the opinion of our counsel, the matter has been settled by controlling precedent) submit to a court of appropriate jurisdiction, the question whether indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. The legal process relating to this matter if it were to occur is likely to be very costly and may result in us receiving negative publicity, either of which factors is likely to materially reduce the market and price for our shares, if such a market ever develops.
OUR PRINCIPAL STOCKHOLDER HAS SIGNIFICANT VOTING POWER AND MAY TAKE ACTIONS THAT MAY NOT BE IN THE BEST INTEREST OF ALL OTHER STOCKHOLDERS.
The Company’s Chairman and Chief Executive Officer controls approximately 42.135% of the Company’s current outstanding shares of voting common stock. He may be able to exert significant control over our management and affairs requiring stockholder approval, including approval of significant corporate transactions. This concentration of ownership may expedite approvals of Company decisions, or have the effect of delaying or preventing a change in control, adversely affect the market price of our common stock, or be in the best interests of all our stockholders.
CURRENTLY, THERE IS NO PUBLIC MARKET FOR OUR SECURITIES, AND THERE CAN BE NO ASSURANCES THAT ANY PUBLIC MARKET WILL EVER DEVELOP OR THAT OUR COMMON STOCK WILL BE QUOTED FOR TRADING AND, EVEN IF QUOTED, IT IS LIKELY TO BE SUBJECT TO SIGNIFICANT PRICE FLUCTUATIONS.
We have a trading symbol for our common stock, DNGH, which permits our shares to be quoted on the OTCBB. However, our stock has been thinly traded since approval of our quotation on the over-the-counter bulletin board by FINRA. Consequently, there can be no assurances as to whether:
|
·
|
any market for our shares will develop;
|
|
·
|
the prices at which our common stock will trade; or
|
|
·
|
the extent to which investor interest in us will lead to the development of an active, liquid trading market. Active trading markets generally result in lower price volatility and more efficient execution of buy and sell orders for investors.
|
In addition, our common stock is unlikely to be followed by any market analysts, and there may be few institutions acting as market makers for our common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these risk factors, investor perception of us and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our common stock.
WE ARE NOT LIKELY TO PAY CASH DIVIDENDS IN THE FORESEEABLE FUTURE.
We currently intend to retain any future earnings for use in the operation and expansion of our business. Accordingly, we do not expect to pay any cash dividends in the foreseeable future, but will review this policy as circumstances dictate. Should we determine to pay dividends in the future, our ability to do so will depend upon the receipt of dividends or other payments from our PRC operating subsidiary may, from time to time, be subject to restrictions on its ability to make distributions to us, including restrictions on the conversion of RMB into U.S. dollars or other hard currency and other regulatory restrictions.
15
WE MAY BE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE OUR SECURITIES MORE DIFFICULT TO SELL.
We may be subject in the future to the SEC’s “penny stock” rules if our securities sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings and reduce the trading activity for our securities. As long as our securities are subject to the penny stock rules, the holders of such securities may find it more difficult to sell their securities.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Description of Property.
We currently lease approximately 11,288 square feet of furnished office space at China Bing’qi Plaza, Floor 17, No 69, Zi Zhu Yuan Rd, Hai’dian District Beijing, China, 100089 for $14,818 per month on a month-to-month basis.
We currently lease approximately 1,882 square feet of furnished office space at Room 10, 9th Floor, Building No. 5, 9 Gaoshengqiao Road, Dayi Louver Plaza, Chengdu, Sichuan Province, and People’s Republic of China 610041 for $1,414 per month on a month-to-month basis.
We currently lease approximately 6,240 square feet of warehouse located at suburban area of Chengdu city for $1,359 per month on a month-to-month basis.
Item 3. Legal Proceedings.
In January 2010, Chongqing Yidong Pharmaceuticals Co., Ltd. (“Yidong”) filed a lawsuit against the Company in the People’s Court of Yuzhong District, Chongqing City, China. Yidong claims compensation in an amount of RMB 1,520,040 (equivalent to USD 222,648) along with court fees for its alleged losses. On July 27, 2010, the judge issued a judicial authentication order on the signature on the contract that Dongsheng had signed with Yidong. As of September 15, 2010, the judge has not announced any results on judicial authentication or other judgment order related to this case. The Company believes that the case will likely be decided in its favor.
Other than described above, we are currently not involved in any litigation that we believe could have a materially adverse effect on our financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our company or any of our subsidiaries, threatened against or affecting our company, our common stock, any of our subsidiaries or of our company’s or our company’s subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
Item 4. (Removed and Reserved).
16
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
MARKET INFORMATION
On April 5, 2010, we changed our name to Dongsheng Pharmaceutical International Co., Ltd. as a result of the reverse merger that closed on March 25, 2010. Accordingly, our symbol was changed to “DNGH.OB.” Prior to April 5, 2010, the Company traded under the symbol “IBLE.OB.” Since the Company has been cleared for trading, there have been minimum trades of the Company’s stock. There are no assurances that a market will ever develop for the Company’s stock.
HOLDERS OF OUR COMMON STOCK
As of September 15, 2010, a total of 17,000,000 shares of the Company’s common stock are currently outstanding held by approximately 117 shareholders of record.
DIVIDENDS
We have not declared or paid any dividends on our common stock and intend to retain any future earnings to fund the development and growth of our business. There are no restrictions on our present ability to pay dividends to stockholders of our common stock, other than those prescribed by Delaware law. Payment of future dividends, if any, will be at the discretion of the board of directors of the Company after taking into account various factors, including current financial condition, operating results, current and anticipated cash needs and regulations governing dividend distributions.
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
Dongsheng Pharmaceutical International Co., Ltd. 2010 Equity Incentive Plan
The Company’s board of directors adopted the Dongsheng Pharmaceutical International Co., Ltd. 2010 Equity Incentive Plan (the “2010 Plan”) on September 3, 2010. The 2010 Plan is administered by the board of directors. The 2010 Plan provides for the grant of qualified and non-qualified stock options. In no event shall the aggregate number of shares of the Company’s common stock that may be issued pursuant to incentive stock options exceed nine hundred thousand (900,000) shares. Further, the maximum number of shares granted hereunder to any one 2010 Plan participant may not exceed thirty percent (30%) of the total shares subject to the 2010 Plan.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.
The following discussion should be read in conjunction with the Consolidated Financial Statements and Notes thereto appearing elsewhere in this Form 10-K. The following discussion contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 relating to future events or our future performance. Actual results may materially differ from those projected in the forward-looking statements as a result of certain risks and uncertainties set forth in this prospectus. Although management believes that the assumptions made and expectations reflected in the forward-looking statements are reasonable, there is no assurance that the underlying assumptions will, in fact, prove to be correct or that actual results will not be different from expectations expressed in this report.
Overview
We operate in the pharmaceutical wholesale business in the PRC. Since Mr. Xiaodong Zhu purchased our core operational subsidiary, Xintai, in July 2006, our business has been growing steadily, measured by annual sales revenue. Compared to fiscal year of 2009, both our sales and net income have improved due to our expanded sales network, more binding contractual relationships with our suppliers and customers, as well as devoted promotions and product line expansion strategies.
17
Results of Operations for the Years Ended June 30, 2010 and 2009
|
2010
US$
|
2009
US$
|
$
Change
|
%
Change
|
|||||
|
Revenues
|
20,479,212
|
17,127,474
|
3,351,738
|
19.57%
|
||||
|
Cost of Sales
|
(15,168,626)
|
|
(12,278,936)
|
2,889,690
|
23.53%
|
|||
|
Gross Profit
|
5,310,586
|
4,848,538
|
462,048
|
9.53%
|
||||
|
Operating Expenses
|
(200.528)
|
1,647,050
|
(1,847,578)
|
(112.17%)
|
||||
|
Operating Income
|
5,511,115
|
3,201,488
|
2,309,627
|
72.14%
|
||||
|
Other Income (Expenses)
|
(98.706)
|
(9,809)
|
(88,897)
|
906.28%
|
||||
|
Income Before Income Tax
|
5,412,409
|
3,191,679
|
2,220,730
|
69.58%
|
||||
|
Income Tax Provision
|
1,354,922
|
800,084
|
554,838
|
69.35%
|
||||
|
Net Income
|
4,057,487
|
2,391,595
|
1,665,892
|
69.66%
|
||||
Revenues:
Revenues for the year ended June 30, 2010, increased by approximately $3.4 million or 19.57% to $20.5 million as compared to $17.1 million for the year ended June 30, 2009. Our sales growth was mainly driven by a 24% revenue increase on constant dollar basis over 2009 contributed by one of our major products, Ganglioside Injection, resulting from higher market demand as well as our more aggressive pricing strategy.
Gross Profit:
We achieved gross profits of $5.3 million for the year ended June 30, 2010, compared to $4.8 million for the same period of the previous year, representing a 9.5% year to year increase. In order to boost sales, we implemented a more aggressive pricing strategy by offering pricing discount to VIP customers, which largely resulted in an overall gross profit margin reduction of 2.4% from 28.3% for the year ended June 30, 2009, to 25.9% for the year ended June 30, 2010.
Operating Expenses:
Our operating expenses, consisting of selling, general and administrative expenses, decreased by approximately $1.8 million to ($0.2) million for the year ended June 30, 2010, from $1.6 million for the same period of the previous year. This decrease is largely due to a $1.4 million recovery of bad debt allowance in 2010 compared to a $0.9 million provision for bad debt allowance in 2009. The decrease is partially offset by approximately $0.5 million increase in promotion expenses and travelling expenses and other expenses relating to our growth in sales.
Other Income (Expenses):
Other income (expenses), consisting primarily of interest expenses and other miscellaneous expenses, increased by around $89,000 to $98,700 expense for the fiscal year ended June 30, 2010 from $9,809 expense for the previous year.
Income Tax Provision:
Our provisions for income taxes for the years ended June 30, 2010 and 2009 were $1.4 million and $0.8 million, respectively, an increase of $0.6 million or 69.4 % from period to period. The increase in the income tax provision was in proportion to our increase in operating income.
Net Income:
Net income for the fiscal year ended June 30, 2010, increased by approximately $1.7 million to $4.1 million as compared to $2.4 million for the previous year, representing a 69.66% increase year-to-year. This increase was mainly attributable to our increase in revenues and operating income, coupled with our efforts to recover doubtful accounts.
18
Liquidity and Capital Resources
We have historically financed our operations and capital expenditures through cash flows from operations. As of June 30, 2010, we had approximately $4.4 million in cash, up $3.8 million from $0.6 million at June 30, 2009. The following table summarizes our cash flows for each of the periods indicated:
|
For The Years Ended
June 30,
|
||||||||
|
2010
US$
|
2009
US$
|
|||||||
|
Cash provided by (used in):
|
||||||||
|
Operating Activities
|
1,242,671
|
2,957,297
|
||||||
|
Investing Activities
|
2,369,920
|
(2,564,775)
|
||||||
|
Financing Activities
|
132,430
|
(134,624)
|
||||||
Operations
Cash provided by operating activities totaled $1,240,463 for the year ended June 30, 2010, as compared to $2,957,297 for the previous year. The decrease of $1,716,834 in our cash provided by operations was primarily due to a decrease in account receivables and payables due to our efforts to settle with our business partners.
Investments
Cash provided by investing activities was $2,369,920 for the year ended June 30, 2010, as compared to cash used in $2,564,775 for the same period of prior year. The change was mainly due to our efforts to collect loans that were lent to outside parties in fiscal year 2009.
Financing
We collected loans from related parties of $132,430 during the year ended June 30, 2010, as compared to obtaining $134,624 during the year ended June 30, 2009.
Off-Balance Sheet Arrangements
We do not have off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities.”
Critical Accounting Policies
While our significant accounting policies are more fully described in Note 2 to our financial statements, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Principles of Consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America.
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, DBL and Xintai. All significant inter-company balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires Management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes, and disclosure of contingent liabilities at the date of the financial statements. Estimates are used for, but not limited to, the selection of the useful lives of property and equipment, allowance for doubtable accounts receivables, depreciation, fair values, revenue recognition, and taxes. Management believes that the estimates utilized in preparing its financial statements are reasonable and prudent. Actual results could differ from these estimates.
19
Long-lived Assets
The Company applies the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, which was subsequently codified within Financial Accounting Standard Board (“FASB”) Accounting Standards Codification (“ASC”) No. 360, “Property, Plant and Equipment”. ASC 360 requires long-lived assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable through the estimated undiscounted cash flows expected to result from the use and eventual disposition of the assets. Whenever any such impairment exists, an impairment loss will be recognized for the amount by which the carrying value exceeds the fair value.
The Company tests long-lived assets, including property and equipment, for recoverability at least annually or more frequently upon the occurrence of an event or when circumstances indicate that the net carrying amount is greater than its fair value. Assets are grouped and evaluated at the lowest level for their identifiable cash flows that are largely independent of the cash flows of other groups of assets. The Company considers historical performance and future estimated results in its evaluation of potential impairment and then compares the carrying amount of the asset to the future estimated cash flows expected to result from the use of the asset. If the carrying amount of the asset exceeds the estimated undiscounted future cash flows, the Company measures the amount of impairment by comparing the carrying amount of the asset to its fair value. The estimation of fair value is generally measured by discounting expected future cash flows as the rate the Company utilizes to evaluate potential investments. The Company estimates fair value based on the information available in making whatever estimates, judgments and projections are considered necessary. There was no impairment of long-lived assets for years ended June 30, 2010 and 2009.
Advance from Customers
Advance from customers consist of amounts received from customers relating to the sales of pharmaceuticals. The Company recognizes these funds as a current liability until the related sale can be recognized.
Income taxes
The Company accounts for income taxes using an asset and liability approach which allows for the recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the Company is able to realize their benefits, or that future deductibility is uncertain. (Please see Note 10 for details on valuation allowance taken in fiscal year 2010).
Income tax returns for the years prior to 2007 are no longer subject to examination by tax authorities.
Revenue recognition
The Company recognizes revenue when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price to the customer is fixed or determinable and (iv) collection of the resulting receivable is reasonably assured. Revenue is not recognized until title and risk of loss is transferred to the customer, which occurs upon delivery of goods, and objective evidence exists that customer acceptance provisions have been met. Deposits or advance payments from customers prior to delivery of goods and passage of title of goods are recorded as advance from customers.
20
Fair Value of Financial Instruments
The Company adopted the provisions of Accounting Standards Codification (“ASC”) 820, Fair Value Measurements and Disclosures. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
|
·
|
Level 1 – Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
|
|
·
|
Level 2 – Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
|
|
·
|
Level 3 – Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
|
The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, advances to vendors, other receivables, accounts payable, advance from customers, taxes payable, other payables and accrued liabilities, approximate their fair value due the short-term nature of these items. The Company uses Level 3 method to measure fair value of its long-term assets and liabilities. The carrying amount of bank loan approximates the fair value based on the Company's expected borrowing rate with similar remaining maturities and comparable risk in market. The Company did not identify any other assets or liabilities that are required to be presented on the consolidated balance sheets at fair value in accordance with ASC 820.
Foreign Currency Translation
The Company’s financial information is presented in US dollars. The functional currency of the Company is Renminbi (“RMB”), the currency of the PRC. Transactions at the Company which are denominated in currencies other than RMB are translated into RMB at the exchange rate quoted by the People’s Bank of China prevailing at the dates of the transactions. Exchange gains and losses resulting from transactions denominated in a currency other than that RMB are included in statements of operations as exchange gains. The financial statements of the Company have been translated into U.S. dollars in accordance with ASC 830, “Foreign Currency Matters”. The financial information is first prepared in RMB and then is translated into U.S. dollars at period-end exchange rates as to assets and liabilities and average exchange rates as to revenue and expenses. Capital accounts are translated at their historical exchange rates when the capital transactions occurred. The effects of foreign currency translation adjustments are included as a component of accumulated other comprehensive income in shareholders’ equity.
The RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of accounts receivable and other receivables. The Company does not require collateral or other security to support these receivables. The Company conducts periodic reviews of its clients’ financial condition and customer payment practices to minimize collection risk on accounts receivable.
Risks and Uncertainties
The operations of the Company are located in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy.
The Company’s operations in the PRC are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes in the political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
21
Recent accounting pronouncements
In January 2010, FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure as follows: 1) Transfers in and out of Levels 1 and 2. A reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. 2) Activity in Level 3 fair value measurements. In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than as one net number). This update provides amendments to Subtopic 820-10 that clarifies existing disclosures as follows: 1) Level of disaggregation. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities. A class is often a subset of assets or liabilities within a line item in the statement of financial position. A reporting entity needs to use judgment in determining the appropriate classes of assets and liabilities. 2) Disclosures about inputs and valuation techniques. A reporting entity should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3. The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company has determined the adoption of this ASU does not have a material impact on its financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We do not hold any derivative instruments and do not engage in any hedging activities.
Item 8. Financial Statements.
Our consolidated financial statements are contained in pages F-1 through F-19 which appear at the end of this annual report.
22
DONGSHENG PHARMACEUTICAL INTERNATIONAL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| PAGE NUMBER | |
| REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMS | F-1 - F-2 |
| CONSOLIDATED BALANCE SHEETS AT JUNE 30, 2010 AND 2009 | F-3 |
| CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME FOR THE YEARS ENDED JUNE 30, 2010 AND 2009 | F-4 |
| CONSOLDIATED STATEMENTS OF STOCKHOLDERS’ EQUITY FOR THE YEARS ENDED JUNE 30, 2010 AND 2009 | F-5 |
| CONSOLDIATED STATEMENTS OF CASH FLOWS FOR THE YEARS ENDED JUNE 30, 2010 AND 2009 | F-6 |
| NOTES TO CONSOLIDATED FINANCIAL STATEMENTS | F-7 |
BAGELL, JOSEPHS, LEVINE & COMPANY, L.L.C.
Certified Public Accountants
406 Lippincott Drive, Ste. J
Marlton, NJ 08053-4168
(856) 355 5900 Fax (856) 396 0022
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and
Stockholders of Sichuan Xintai Pharmaceutical Co., Ltd.
We have audited the accompanying balance sheet of Sichuan Xintai Pharmaceutical Co., Ltd. (“the Company”) as of June 30, 2009 and the related statements of income and other comprehensive income, stockholders’ equity, and cash flows for the year ended June 30, 2009. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2009 and the results of its operations and cash flows for the year ended June 30, 2009 in conformity with accounting principles generally accepted in the United States of America.
/s/ Bagell, Josephs, Levine & Company, L.L.C.
Bagell, Josephs, Levine & Company, L.L.C.
Marlton, New Jersey
October 30, 2009
F-1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Dongsheng Pharmaceutical International Co., Ltd.
We have audited the accompanying consolidated balance sheet of Dongsheng Pharmaceutical International Co., Ltd. (“the Company”) as of June 30, 2010, and the related consolidated statements of income, cash flows and changes in stockholders' equity for the year ended June 30, 2010. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial statements are free of material misstatement. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of June 30, 2010, and the consolidated results of its operations and its cash flows for the year ended June 30, 2010 in conformity with accounting principles generally accepted in the United States of America.
/s/Friedman LLP
New York, NY
September 22, 2010
F-2
DONGSHENG PHARMACEUTICAL INTERNATIONAL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
CONSOLIDATED BALANCE SHEETS
(IN US DOLLARS)
|
June 30
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 4,395,515 | $ | 620,596 | ||||
|
Accounts receivable, net of allowance for doubtful accounts of $-0- and $1,318,555
|
72,021 | 3,582,261 | ||||||
|
Other receivables, net of allowance for doubtful accounts of $-0- and $122,652
|
191,961 | 39,877 | ||||||
|
Inventories
|
152,500 | 59,693 | ||||||
|
Advance to vendors
|
465,183 | 1,077,677 | ||||||
|
Deferred tax assets
|
— | 360,302 | ||||||
|
VAT tax receivable - current
|
200,893 | — | ||||||
|
Due from related parties
|
— | 111,155 | ||||||
|
Total current assets
|
5,478,073 | 5,851,561 | ||||||
|
Property and equipment, net
|
112,655 | 91,259 | ||||||
|
Long-term loans to outside parties
|
— | 1,129,944 | ||||||
|
Long-term loans to related party
|
— | 1,358,843 | ||||||
|
Distribution deposit
|
412,894 | — | ||||||
|
VAT tax receivable - non-current
|
— | 1,452,664 | ||||||
|
Total Assets
|
$ | 6,003,622 | $ | 9,884,271 | ||||
|
|
||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 200,076 | $ | 8,123,101 | ||||
|
Advance from customers
|
691,411 | 752,784 | ||||||
|
Other payables and accrued liabilities
|
419,235 | 96,254 | ||||||
|
Taxes payable
|
257,415 | 499,285 | ||||||
|
Due to related parties
|
21,366 | — | ||||||
|
Total current liabilities
|
1,589,503 | 9,471,424 | ||||||
|
Long-term liabilities
|
— | 86,199 | ||||||
|
Total Liabilities
|
1,589,503 | 9,557,623 | ||||||
|
Stockholders’ equity
|
||||||||
|
Preferred stock, $0.0001 par value, 50,000,000 shares authorized, -0- shares issued and outstanding
|
— | — | ||||||
|
Common stock, $0.0001 par value, 200,000,000 shares authorized, 17,000,000 and 15,830,000 shares issued and outstanding as of June 30, 2010 and 2009, respectively
|
1,700 | 1,583 | ||||||
|
Additional paid-in capital
|
249,273 | 249,390 | ||||||
|
Retained earnings
|
4,578,936 | 521,449 | ||||||
|
Accumulated other comprehensive loss
|
(415,790 | ) | (445,774 | ) | ||||
|
Total stockholders’ equity
|
4,414,119 | 326,648 | ||||||
|
Total Liabilities and Stockholders’ Equity
|
$ | 6,003,622 | $ | 9,884,271 | ||||
The accompanying notes are an integral part of these consolidated financial statements
F-3
DONGSHENG PHARMACEUTICAL INTERNATIONAL CO., LTD
(FORMERLY INDESTRUCTIBLE I, INC.)
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
(IN US DOLLARS)
|
For the Years Ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenues
|
$ | 20,479,212 | $ | 17,127,474 | ||||
|
Cost of sales
|
15,168,626 | 12,278,936 | ||||||
|
Gross profit
|
5,310,586 | 4,848,538 | ||||||
|
Operating expenses
|
||||||||
|
Selling and distribution expenses
|
599,284 | 457,938 | ||||||
|
Provision for/(Recovery of) doubtful accounts
|
(1,441,889 | ) | 924,451 | |||||
|
General and administrative expenses
|
642,077 | 264,661 | ||||||
|
Total operating expenses
|
(200,528 | ) | 1,647,050 | |||||
|
Operating income
|
5,511,115 | 3,201,488 | ||||||
|
Other income (expenses)
|
||||||||
|
Interest expenses
|
(4,540 | ) | (1,480 | ) | ||||
|
Other expenses
|
(94,165 | ) | (8,329 | ) | ||||
|
Total other income (expenses)
|
(98,706 | ) | (9,809 | ) | ||||
|
Income before income taxes
|
5,412,409 | 3,191,679 | ||||||
|
Provision for income taxes
|
1,354,922 | 800,084 | ||||||
|
Net income
|
$ | 4,057,487 | $ | 2,391,595 | ||||
|
Other comprehensive income (loss)
|
||||||||
|
Foreign currency translation adjustment
|
29,984 | (6,737 | ) | |||||
|
Comprehensive income
|
$ | 4,087,471 | $ | 2,384,858 | ||||
|
Basic and diluted income per common share
|
||||||||
|
Basic
|
$ | 0.25 | $ | 0.15 | ||||
|
Diluted
|
$ | 0.25 | $ | 0.15 | ||||
|
Weighted average common shares outstanding
|
||||||||
|
Basic
|
16,140,932 | 15,830,000 | ||||||
|
Diluted
|
16,140,932 | 15,830,000 | ||||||
F-4
DONGSHENG PHARMACEUTICAL INTERNATIONAL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
FOR THE YEARS ENDED JUNE 30, 2010 AND 2009
(IN US DOLLARS)
|
Preferred Stock
Shares
|
Amount
|
Common Stock
Shares
|
Amount
|
Additional
Paid-in-
Capital
|
Retained
Earnings
|
Accumulated Other
Comprehensive
Loss
|
Total
|
|||||||||||||||||||
|
Balance at June 30, 2008
|
—
|
$
|
—
|
15,830,000
|
$
|
1,583
|
$
|
249,390
|
$
|
(1,870,146
|
)
|
$
|
(439,037
|
)
|
$
|
(2,058,210
|
)
|
|||||||||
|
Net income for the year
|
2,391,595
|
2,391,595
|
||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
(6,737
|
)
|
(6,737
|
)
|
||||||||||||||||||||||
|
Balance at June 30, 2009
|
—
|
—
|
15,830,000
|
1,583
|
249,390
|
521,449
|
(445,774
|
)
|
326,648
|
|||||||||||||||||
|
Acquisition of Indestructible I, Inc.
|
1,170,000
|
117
|
(117
|
)
|
—
|
|||||||||||||||||||||
|
Net income for the year
|
4,057,487
|
4,057,487
|
||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
29,984
|
29,984
|
||||||||||||||||||||||||
|
Balance at June 30, 2010
|
—
|
$
|
—
|
17,000,000
|
$
|
1,700
|
$
|
249,273
|
$
|
4,578,936
|
$
|
(415,790
|
)
|
$
|
4,414,119
|
|||||||||||
F-5
DONGSHENG PHARMACEUTICAL INTERNATIONAL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(IN US DOLLARS)
|
For The Years Ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net income
|
$ | 4,057,487 | $ | 2,391,595 | ||||
|
Adjustments to reconcile net income to net cash provided by (used in) operating activities:
|
||||||||
|
Depreciation
|
13,212 | 8,484 | ||||||
|
Provision for/(Recovery of) doubtful accounts
|
(1,441,889 | ) | 924,451 | |||||
|
Changes in assets and liabilities:
|
||||||||
|
(Increase) decrease in -Account receivables
|
4,831,600 | (904,692 | ) | |||||
|
Other receivables
|
(28,060 | ) | (39,594 | ) | ||||
|
Inventories
|
(91,750 | ) | 338,326 | |||||
|
Advance to vendors
|
616,142 | (966,746 | ) | |||||
|
Prepaid expenses
|
— | 17,359 | ||||||
|
Deferred tax asset
|
360,472 | 421,336 | ||||||
|
VAT tax receivable
|
1,253,813 | 1,413,458 | ||||||
|
Distribution deposit
|
(410,109 | ) | — | |||||
|
Increase (decrease) in -
|
||||||||
|
Accounts payable
|
(7,928,217 | ) | (1,033,336 | ) | ||||
|
Advance from customers
|
(66,393 | ) | (181,845 | ) | ||||
|
Other payables and accrued liabilities
|
320,205 | 71,791 | ||||||
|
Taxes payable
|
(243,842 | ) | 496,710 | |||||
|
Net cash provided by operating activities
|
1,242,671 | 2,957,297 | ||||||
|
Cash flows from investing activities
|
||||||||
|
Purchase of property and equipment
|
(33,805 | ) | (77,883 | ) | ||||
|
Collection from (loans to) outside parties
|
1,044,239 | (1,129,084 | ) | |||||
|
Collection from (loans to) related parties
|
1,359,486 | (1,357,808 | ) | |||||
|
Net cash provided by (used) in investing activities
|
2,369,920 | (2,564,775 | ) | |||||
|
Cash flows from financing activities
|
||||||||
|
Proceeds from (loans to) related parties
|
132,430 | (134,624 | ) | |||||
|
Net cash provided by (used in) financing activities
|
132,430 | (134,624 | ) | |||||
|
Effect of exchange rate changes on cash and cash equivalents
|
29,898 | 1,698 | ||||||
|
Net increase in cash and cash equivalents
|
3,774,919 | 259,596 | ||||||
|
Cash and cash equivalents, beginning of year
|
620,596 | 361,000 | ||||||
|
Cash and cash equivalents, end of year
|
4,395,515 | 620,596 | ||||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Interest paid
|
$ | 4,600 | $ | 1,480 | ||||
|
Income taxes paid
|
$ | 1,218,692 | $ | 4,249 | ||||
F-6
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. ORGANIZATION AND BASIS OF PRESENTATION
Dongsheng Pharmaceutical International Co., Ltd. (the “Company”) was incorporated in the state of Delaware as of March 25, 2008 originally under the name “Indestructible I, Inc”. On March 25, 2010, the Company entered into a share exchange agreement and acquired all of the outstanding capital stock of Dynamic Bhorizon Limited, (“DBL”), a company incorporated in the Cayman Islands on April 8, 2009. Pursuant to the Exchange Agreement, DBL became a wholly-owned subsidiary of the Company. DBL has not carried on any substantive operations of its own. Instead, the Company owns 100% of Sichuan Xintai Pharmaceutical Co., Ltd. (“Xintai”), a company incorporated in Chengdu City, Sichuan Province, People’s Republic of China (“PRC”) in November 1999. Xintai has a registered capital in amount of RMB 2,000,000 (equivalent to US$250,973) and is primarily engaged in pharmaceutical technology promotion, trading and warehousing of traditional Chinese medicine and bio-chemistry products.
In connection with the transaction, the Company issued a total of 15,830,000 shares of common stock to the DBL Shareholders, their designees or assigns in exchange for all of the capital stock of DBL (the “Share Exchange”). Upon the completion of the Share Exchange, the stockholders of DBL own, in aggregate, 93.12% of the issued and outstanding capital stock of the Company.
The acquisition was accounted for as a reverse merger under the purchase method of accounting since there was a change of control. Accordingly, DBL and its subsidiary, Xintai, will be treated as the continuing entity (the accounting acquirer) for accounting purposes.
On April 5, 2010, Indestructible I, Inc. changed its name to Dongsheng Pharmaceutical International Co., Ltd. to better reflect the Company’s business.
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America.
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, DBL and Xintai. All significant inter-company balances and transactions have been eliminated in consolidation.
F-7
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires Management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes, and disclosure of contingent liabilities at the date of the financial statements. Estimates are used for, but not limited to, the selection of the useful lives of property and equipment, allowance for doubtable accounts receivables, depreciation, fair values, revenue recognition, and taxes. Management believes that the estimates utilized in preparing its financial statements are reasonable and prudent. Actual results could differ from these estimates.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities of three months or less when purchased to be cash equivalents. The Company maintains bank accounts in the PRC and in United States. Total cash at June 30, 2010 and 2009 amounted to US$4,395,515 and US$620,596, respectively, of which no deposits are covered by insurance. The Company has not experienced any losses in such accounts and management believes it is not exposed to any risks on its cash in bank accounts.
Accounts receivable
Accounts receivable consists of balances due from customers for the sale of pharmaceutical products. Accounts receivable are recorded at net realizable value consisting of the carrying amount less an allowance for uncollectible amounts.
The Company performs periodical reviews as to whether the carrying values of accounts have become impaired. The assets are considered to be impaired if the collectability of the balances become doubtful, accordingly, the management estimates the valuation allowance for anticipated uncollectible receivable balances. When facts subsequently become available to indicate that the valuation allowance provided requires an adjustment, then the adjustment will be classified as provision for or recovery of doubtful accounts. As of June 30, 2010 and 2009, the allowance for doubtful accounts was $0 and US$1,318,555, respectively.
Inventories
The Company’s inventories consist of pharmaceutical products. Inventories are stated at the lower of cost (determined on a weighted average basis) or market. Management compares the cost of inventories with the fair market value and an allowance is made for writing down the inventories to fair market value, if lower than the cost. As of June 30, 2010 and 2009, no allowance for writing down the inventories to their fair market value is considered necessary.
F-8
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Advances to vendors
Advances to vendors consist of balances paid for pharmaceuticals that have not been provided or received. Advances to vendors are reviewed periodically to determine whether their carrying value has become impaired. The Company considers the assets to be impaired if the collectability of the services and materials become doubtful. The Company determines that no reserve is necessary at June 30, 2010 and 2009.
Loans to outside parties
Loans to outside parties consist of various cash advances to unrelated companies with which the Company has business relationships.
Loans to outside parties are reviewed periodically as to whether their carrying value has become impaired. The Company considers the assets to be impaired if the collectability of the balances becomes doubtful. The Company determines that no reserve is necessary at June 30, 2010 and 2009.
Property and equipment
Property and equipment are recorded at cost less accumulated depreciation and any impairment losses. The cost of an asset comprises of its purchase price and any directly attributable costs of bringing the asset to its working condition and location for its intended use. Expenditure incurred after the fixed assets have been put into operation, such as repairs and maintenance and overhaul costs, is normally charged to the profit and loss account in the year in which it is incurred.
In situations where it can be clearly demonstrated that the expenditure has resulted in an increase in the future economic benefits expected to be obtained from the use of the asset beyond its originally assessed standard of performances, the expenditure is capitalized as an additional cost of the asset.
Any gain or loss on disposal or retirement of a fixed asset is recognized in the profit and loss account and is the difference between the net sales proceeds and the carrying amount of the relevant asset. When property and equipment are retired or otherwise disposed of, the assets and accumulated depreciation are removed from the accounts and the resulting profit or loss is reflected in income.
Expenditures for maintenance and repairs are charged to expense as incurred. Additions, renewals and betterments are capitalized.
F-9
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Long-lived assets
The Company applies the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, which was subsequently codified within Financial Accounting Standard Board (“FASB”) Accounting Standards Codification (“ASC”) No. 360, “Property, Plant and Equipment”. ASC 360 requires long-lived assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable through the estimated undiscounted cash flows expected to result from the use and eventual disposition of the assets. Whenever any such impairment exists, an impairment loss will be recognized for the amount by which the carrying value exceeds the fair value.
The Company tests long-lived assets, including property and equipment, for recoverability at least annually or more frequently upon the occurrence of an event or when circumstances indicate that the net carrying amount is greater than its fair value. Assets are grouped and evaluated at the lowest level for their identifiable cash flows that are largely independent of the cash flows of other groups of assets. The Company considers historical performance and future estimated results in its evaluation of potential impairment and then compares the carrying amount of the asset to the future estimated cash flows expected to result from the use of the asset. If the carrying amount of the asset exceeds the estimated undiscounted future cash flows, the Company measures the amount of impairment by comparing the carrying amount of the asset to its fair value. The estimation of fair value is generally measured by discounting expected future cash flows as the rate the Company utilizes to evaluate potential investments. The Company estimates fair value based on the information available in making whatever estimates, judgments and projections are considered necessary. There was no impairment of long-lived assets for years ended June 30, 2010 and 2009.
Advance from customers
Advance from customers consist of amounts received from customers relating to the sales of pharmaceuticals. The Company recognizes these funds as a current liability until the related sale can be recognized.
Income taxes
The Company accounts for income taxes using an asset and liability approach which allows for the recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the
F-10
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Company is able to realize their benefits, or that future deductibility is uncertain. (Please see Note 10 for details on valuation allowance taken in fiscal year 2010).
Income tax returns for the years prior to 2007 are no longer subject to examination by tax authorities.
Revenue recognition
The Company recognizes revenue when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price to the customer is fixed or determinable and (iv) collection of the resulting receivable is reasonably assured. Revenue is not recognized until title and risk of loss is transferred to the customer, which occurs upon delivery of goods, and objective evidence exists that customer acceptance provisions have been met. Deposits or advance payments from customers prior to delivery of goods and passage of title of goods are recorded as advance from customers.
Comprehensive income
Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income arose from the changes in foreign currency exchange rates.
Fair Value of Financial Instruments
The Company adopted the provisions of Accounting Standards Codification (“ASC”) 820, Fair Value Measurements and Disclosures. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
F-11
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The carrying amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, advances to vendors, other receivables, accounts payable, advance from customers, taxes payable, other payables and accrued liabilities, approximate their fair value due the short-term nature of these items. The Company uses Level 3 method to measure fair value of its long-term assets and liabilities. The carrying amount of bank loan approximates the fair value based on the Company’s expected borrowing rate with similar remaining maturities and comparable risk in market. The Company did not identify any other assets or liabilities that are required to be presented on the consolidated balance sheets at fair value in accordance with ASC 820.
Earnings per share
The Company computes earnings per share (“EPS’) in accordance with ASC 260, “Earning Per Share”. ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
Foreign currency translation
The Company’s financial information is presented in US dollars. The functional currency of the Company is Renminbi (“RMB”), the currency of the PRC. Transactions at the Company which are denominated in currencies other than RMB are translated into RMB at the exchange rate quoted by the People’s Bank of China prevailing at the dates of the transactions. Exchange gains and losses resulting from transactions denominated in a currency other than that RMB are included in statements of operations as exchange gains. The financial statements of the Company have been translated into U.S. dollars in accordance with ASC 830, “Foreign Currency Matters”. The financial information is first prepared in RMB and then is translated into U.S. dollars at period-end exchange rates as to assets and liabilities and average exchange rates as to revenue and expenses. Capital accounts are translated at their historical exchange rates when the capital transactions occurred. The effects of foreign currency translation adjustments are included as a component of accumulated other comprehensive income in shareholders’ equity.
|
June 30, 2010
|
June 30, 2009
|
|||||||
|
Year end RMB: US$exchange rate
|
6.7814 | 6.8307 | ||||||
|
Average yearly RMB: US$exchange rate
|
6.8275 | 6.8591 | ||||||
F-12
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation.
Concentration of credit risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of accounts receivable and other receivables. The Company does not require collateral or other security to support these receivables. The Company conducts periodic reviews of its clients’ financial condition and customer payment practices to minimize collection risk on accounts receivable.
Shipping costs
Shipping costs are expensed as incurred. Shipping costs were included in selling expenses and amounted to $17,101 and $11,709 for the years ended June 30, 2010 and 2009, respectively.
Advertising
Advertising is expensed as incurred. Advertising expenses were included in selling expenses and amounted to $47,701 and $25,995 for the years ended June 30, 2010 and 2009, respectively.
Risks and uncertainties
The operations of the Company are located in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy.
The Company’s operations in the PRC are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes in the political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
F-13
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Risks of Losses
The Company is potentially exposed to risks of losses that may result from business interruptions, injury to others (including employees) and damage to property. These losses may be uninsured, especially due to the fact that the Company’s operations are in China, where business insurance is not readily available. If: (i) information is available before the Company’s financial statements are issued or are available to be issued indicates that such loss is probable and (ii) the amount of the loss can be reasonably estimated, an estimated loss will be accrued by a charge to income. If such loss is probable but the amount of loss cannot be reasonably estimated, the loss shall be charged to the income of the period in which the loss can be reasonably estimated and shall not be charged retroactively to an earlier period. As of June 30, 2010 and 2009, the Company has not experienced any uninsured losses from injury to others or other losses.
Recent accounting pronouncements
In January 2010, FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure as follows: 1) Transfers in and out of Levels 1 and 2. A reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. 2) Activity in Level 3 fair value measurements. In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than as one net number). This update provides amendments to Subtopic 820-10 that clarifies existing disclosures as follows: 1) Level of disaggregation. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities. A class is often a subset of assets or liabilities within a line item in the statement of financial position. A reporting entity needs to use judgment in determining the appropriate classes of assets and liabilities. 2) Disclosures about inputs and valuation techniques. A reporting entity should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3. The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company has determined the adoption of this ASU does not have a material impact on its financial statements.
F-14
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 3. ADVANCE TO VENDORS
The Company periodically makes advances to certain vendors for purchases of pharmaceutical products, and records those advances as advance to vendors. Advances to vendors as of June 30, 2010 and 2009 amounted to $465,183 and $1,077,677, respectively.
Note 4. PROPERTY AND EQUIPMENT
As of June 30, 2010 and 2009, property and equipment was as follows:
|
June 30
|
|||||||||
|
Useful Lives
|
2010
|
2009
|
|||||||
|
Office equipment
|
5 years
|
$ | 111,717 | $ | 77,122 | ||||
|
Automobiles
|
10 years
|
30,071 | 29,854 | ||||||
| 141,788 | 106,976 | ||||||||
|
Less: accumulated depreciation
|
(29,133 | ) | (15,717 | ) | |||||
|
Property and equipment, net
|
$ | 112,655 | $ | 91,259 | |||||
Depreciation expense for the years ended June 30, 2010 and 2009 was $13,212 and $8,484, respectively.
Note 5. LOANS TO OUTSIDE PARTIES
As of June 30, 2010 and June 30, 2009, the loans to outside parties included the following:
|
Names of Borrowers
|
Loan Term
|
Annual
Interest Rate |
Loan Balance as of
|
||||||||
|
June 30, 2010
|
June 30, 2009
|
||||||||||
|
Harbin Maidesen Artistic Design Co., Ltd.
|
From Jul. 24, 2009 to Dec. 31, 2011
|
0.1
|
%
|
$
|
—
|
$
|
46,599
|
||||
|
Beijing Anpudi Science & Technology Co., Ltd.
|
From Jun. 6, 2008 to Dec. 31, 2011
|
0.1
|
%
|
—
|
351,355
|
||||||
|
Harbin Maidesen Artistic Design Co., Ltd.
|
From Jan. 31, 2009 to Dec. 31, 2010
|
0.1
|
%
|
—
|
731,990
|
||||||
|
Total
|
$
|
—
|
$
|
1,129,944
|
|||||||
The above loans were unsecured and were all paid off as of June 30, 2010.
Note 6. RELATED PARTY TRANSACTIONS
As of June 30, 2010 and 2009, the Company had receivables from and payables to related parties as follows:
F-15
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 6. RELATED PARTY TRANSACTIONS (Continued)
|
June 30,
|
||||||||
|
2010
|
2009 | |||||||
|
Beijing Dongsheng Kexin Biology Curative Co.,Ltd
|
$ | — | $ | 1,358,843 | ||||
|
Zhu Xiaomei
|
(21,366 | ) | 111,155 | |||||
|
Total
|
$ | (21,366 | ) | $ | 1,469,998 | |||
Beijing Dongsheng Kexin Biology Curative Co., Ltd. (“Dongshen Kexin”) is also owned by the Mr. Xiaodong Zhu, CEO and major shareholder of the Company. The balance with Dongshen Kexin was paid off as of June 30, 2010.
Ms. Xiaomei Zhu is the sister of Mr. Xiaodong Zhu. The balance due to Ms. Zhu is unsecured and bears no interest and has no fixed repayment date.
Note 7. DISTRIBUTION DEPOSIT
Distribution deposit represents the deposit paid to one major vendor of the Company for a three-year exclusive distribution contract signed on December 25, 2009. This contract enables the Company to maintain long-term relationships with such vendor and purchase products. As of June 30, 2010 and 2009, the Company had distribution deposit of $412,894 and $-0-, respectively.
Note 8. VALUE ADDED TAX RECEIVABLE
Value added tax receivable represents value added taxes (“VAT”) paid on purchases made with the relevant supporting invoices (input VAT), which are to be used in the following years (see Note 10 b). As of June 30, 2010 and 2009, the Company’s unused input VAT totaled $200,893 and $1,452,664, respectively.
Note 9. LONG-TERM LOAN
The long-term loan represents working capital loan from Shenyang Zhonghai Bio-Chemical Technology Co., Ltd., an unrelated company. The loan has been paid off as of June 30, 2010.
Note 10. TAXES
(a) Corporation income tax (“CIT”)
Under the Income Tax Laws of PRC, Chinese companies are generally subject to an income tax at an effective rate on income reported in the statutory financial statements after appropriate tax adjustments. The PRC local government has provided various incentives to companies in order to encourage economic development. Such incentives include reduced tax rates, loss carry-forward and other measures.
F-16
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 10. TAXES (Continued)
The Company did not generate any taxable income outside of the PRC for the years ended June 30, 2010 and 2009. The Company’s operational subsidiary “Xintai” was incorporated in Sichuan Providence, China. The Management does not expect to repatriate Xintai’s net income back to US in the near future, therefore Xintai is governed by the Income Tax Law of the People’s Republic of China concerning the private-run enterprises, which are generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriate tax adjustments.
On March 16, 2007, the National People’s Congress of China approved the Corporate Income Tax Law of the People’s Republic of China (the New CIT Law), which is effective from January 1, 2008. Under the current law, the corporate income tax rate applicable to all Companies, including both domestic and foreign-invested companies, will be 25%, replacing the previous applicable tax rate of 33%. However, pending the detailed implementation rulings from the tax authorities, some of the tax concession granted to eligible companies prior to the new CIT laws may be grandfathered. The provision for income taxes for the two years ended June 30, 2010 and 2009 are as follows:
|
For the years ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Current
|
$ | 994,450 | $ | 378,748 | ||||
|
Deferred
|
360,472 | 421,336 | ||||||
|
Total
|
$ | 1,354,922 | $ | 800,084 | ||||
As of June 30, 2010 and 2009, the Company had taxes payable as follows:
|
As of June 30,
|
|||||||
|
2010
|
2009
|
||||||
|
VAT payable
|
$
|
98,929
|
$
|
118,429
|
|||
|
Income tax payable
|
154,846
|
377,864
|
|||||
|
Other taxes and fees
|
3,640
|
2,992
|
|||||
|
Total
|
$
|
257,415
|
$
|
499,285
|
|||
For the year ended June 30, 2009, the allowance for doubtful accounts gave rise to the deferred tax assets. These assets were fully used for the year ended June 30, 2010 due to the recovery of the doubtful accounts.
F-17
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 10. TAXES (Continued)
The Company was incorporated in the United States. It is governed by the Income Tax law of United States. It incurred a net operating loss for U.S. income tax purposes for the year ended June 30, 2010. The net operating loss carry-forward, which was resulted from administrative expenses, for United States income tax purposes amounted to $7,242 as of June 30, 2010. There was no net operating loss carry-forward as of June 30, 2009. This loss carry forward, which may be available to reduce future periods’ taxable income, will expire, if not utilized, in 2030. Management believes that the realization of the benefits arising from this loss appear to be uncertain due to Company’s limited operating history and continuing losses for United States income tax purposes. Accordingly, the Company has provided a 100% valuation allowance at June 30, 2010 for the temporary difference related to the loss carry-forward. Management reviews this valuation allowance periodically and makes adjustments as warranted. The valuation allowance increased $2,462 during the fiscal year ended June 30, 2010.
As of June 30, 2010 and 2009, the deferred tax assets and the related valuation allowance were as follows:
|
June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Current Deferred Tax Assets
|
||||||||
|
Allowance for doubtful accounts
|
$ | — | $ | 360,302 | ||||
|
Net operating loss carry forwards
|
2,462 | — | ||||||
|
Less: Valuation Allowance
|
(2,462 | ) | — | |||||
|
Net Deferred Tax Assets
|
$ | — | $ | 360,302 | ||||
(b) Value added tax
The Company is subject to VAT for trading and warehousing pharmaceutical products. The applicable VAT tax rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (“output VAT”) less VAT paid on purchases made with the relevant supporting invoices (“input VAT”). Under the commercial practice of the PRC, the Company paid VAT based on tax invoices issued. The tax invoices may be issued subsequent to the date on which revenue is recognized, and there may be a considerable delay between the date on which the revenue is recognized and the date on which the tax invoice is issued. Such timing difference may result in VAT receivables.
F-18
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 11. SHAREHOLDERS’ EQUITY
On March 25, 2010, the Company entered into share exchange transaction which has been accounted for as a reverse merger under the purchase method of accounting since there has been a change of control. In connection with this transaction, the Company issued 15,830,000 shares of its common stock to DBL shareholders. Prior to the Share Exchange, the Company had 16,700,000 shares of common stock issued and outstanding at $.0001 per share. Before the closing of the Share Exchange transaction, the Company retired 15,530,000 shares of common stock.
As a result of the reverse merger, the equity accounts of the Company, prior to the share exchange date, has been retroactively restated so that the ending outstanding share balance as of the share exchange date is equal to the number of post share-exchange shares.
The Company computes the weighted-average number of common shares outstanding in accordance with ASC 805, which states that in calculating the weighted average shares when a reverse merger takes place in the middle of the year, the number of common shares outstanding from the beginning of that period to the acquisition date shall be computed on the basis of the weighted-average number of common shares of the legal acquiree (the accounting acquirer) outstanding during the period multiplied by the exchange ratio established in the merger agreement. The number of common shares outstanding from the acquisition date to the end of that period shall be the actual number of common shares of the legal acquirer (the accounting acquiree) outstanding during that period.
Note 12. CONCENTRATION
Three major vendors provided approximately 79.32% of the Company’s purchases of inventories for the year ended June 30, 2010, with each vendor individually accounting for approximately 35.88%, 29.97% and 13.47%.
Three major vendors provided approximately 85.23% of the Company’s purchases of inventories for the year ended June 30, 2009, with each vendor individually accounting for 38.91%, 24.13% and 22.19%.
None of sales to any individual customer exceeds 10% of the Company’s total sales for the years ended June 30, 2010 and 2009.
The sales revenue from one of our major products provided approximately 93.63% and 90.32% of the Company’s total revenue for the year ended June 30, 2010 and 2009, respectively.
F-19
DONGSHENG PHARMACEUTICAL INTERNATIOANL CO., LTD.
(FORMERLY INDESTRUCTIBLE I, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 13. COMMITMENTS
The commitments are for the rental for the Company’s office space and warehouse. As of June 30, 2010, the commitments related to the above rental are as follows:
|
Years Ending June 30,
|
$ |
US
|
||
|
2011
|
208,073
|
|||
|
2012
|
120,035
|
|||
|
2013
|
6,796
|
|||
|
Thereafter
|
—
|
|||
|
$
|
334,904
|
Note 14. LITIGATION
In January 2010, Chongqing Yidong Pharmaceuticals Co., Ltd. (“Yidong”) filed a lawsuit against the Company at the People’s Court of Yuzhong District, Chongqing City, China. Yidong claims compensation in an amount of RMB1,520,040 (equivalent to US$ 222,648) along with court fees for its alleged losses. Although the Company believes the plaintiff’s allegation is based on a falsified sales contract, a judgment order has not been issued and the Company cannot assure the judgment will be given in the Company’s favor. On July 27, 2010, the Judge issued a judicial authentication order on the signature on the contract that Dongsheng had signed with Yidong. If the signature proves to be fraudulently added by Yidong, the Company will prevail. However, there is a remote possibility that the judicial authentication of the signature fails, which may result judgment order for Dongsheng to compensate Yidong. The Company believes this possibility is remote and therefore no accrual has been provided. As of September 15, 2010, the judge has not announced any results on judicial authentication or other judgment order related to this case.
Note 15. SUBSEQUENT EVENT
On September 5, 2010, the Board of Directors of the Company approved the issuance of stock options to one of the Company’s employees to purchase up to 150,000 shares of the Company’s common stock, with an exercise price of $1.00 per share. One-third of the options will vest yearly over a three-year period commencing from May 14, 2011 to May 15, 2013 with the exercise price at $1.00 per share.
F-20
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
(a)
|
Dismissal of Previous Independent Registered Public Accounting Firm.
|
|
(i)
|
On March 25, 2010, we dismissed Gately & Associates, LLC (“Gately”) as our independent registered public accounting firm. The board of directors of the Company approved such resignation on March 25, 2010.
|
|
(ii)
|
The Company’s board of directors participated in and approved the decision to change our independent registered public accounting firm.
|
|
(iii)
|
Gately’s reports on the financial statements of the Company for the years ended December 31, 2009 and 2008 did not contain an adverse opinion or a disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles.
|
|
(iv)
|
In connection with the audit and review of the financial statements of the Company through March 25, 2010, there were no disagreements on any matter of accounting principles or practices, financial statement disclosures, or auditing scope or procedures, which disagreements if not resolved to their satisfaction would have caused them to make reference in connection with Gately’s opinion to the subject matter of the disagreement.
|
|
(v)
|
In connection with the audited financial statements of the Company for the years ended December 31, 2009 and 2008, there have been no reportable events with the Company as set forth in Item 304(a)(1)(v) of Regulation S-K.
|
|
(vi)
|
The Company provided Gately with a copy of this Current Report on Form 8-K and requested that Gately furnished it with a letter addressed to the SEC stating whether or not they agree with the above statements. The Company has received the requested letter from Gately, and a copy of such letter is filed as Exhibit 16.1 to this Current Report Form 8-K.
|
|
(b)
|
Engagement of New Independent Registered Public Accounting Firm.
|
|
(i)
|
On March 25, 2010, the Board appointed Friedman LLP (“Friedman”) as the Company’s new independent registered public accounting firm. The decision to engage Friedman was approved by the Company’s board of directors on March 25, 2010.
|
|
(ii)
|
Prior to March 25, 2010, the Company did not consult with Friedman regarding (1) the application of accounting principles to a specified transactions, (2) the type of audit opinion that might be rendered on the Company’s financial statements, (3) written or oral advice was provided that would be an important factor considered by the Company in reaching a decision as to an accounting, auditing or financial reporting issues, or (4) any matter that was the subject of a disagreement between the Company and its predecessor auditor as described in Item 304(a)(1)(iv) or a reportable event as described in Item 304(a)(1)(v) of Regulation S-K.
|
Item 9A. Controls and Procedures
This Annual Report on Form 10-K does not contain (i) management’s assessment of the internal control over financial reporting for the year ended June 30, 2010 or (ii) an attestation report by the Company’s independent auditors for the year ended June 30, 2010, due to a transition period established by rules of the U.S. Securities and Exchange Commission. The Company completed a reverse acquisition transaction on March 25, 2010, and the management of the Company is currently in the process of finalizing its procedures for internal controls over financial reporting.
Item 9B. Other Information.
None.
23
Item 10. Directors, Executive Officers and Corporate Governance.
The following table and biographical summaries set forth information, including principal occupation and business experience, about our directors and executive officers at September 20, 2010. Executive officers are elected annually by our board of directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
|
NAME
|
AGE
|
POSITION
|
||
|
Xiaodong Zhu
|
35
|
President, Chief Executive Officer and Chairman
|
||
|
Jianping Chen
|
36
|
Chief Financial Officer
|
||
|
Di Wu
|
43
|
Chief Development Officer
|
Mr. Xiaodong Zhu, President, Chief Executive Officer and Chairman of the board of directors, age 35, has 11 years of sales experience in pharmaceutical industry. From July 2006 to present, he serves as CEO in Sichuan Dongsheng Pharmaceutical Co., Ltd. From November 2004 to July 2006, Mr. Zhu served as founder and CEO in Dongsheng Kexin Biology Curative Co., Ltd. From August 2002 to November 2004, Mr. Zhu served as Regional Sales Manager in Pharmaceutical Factory of Norman Bethune University of Medical Science. From June 2000 to March 2004, Mr. Zhu also worked as Regional Sales Manager in Yunnan Laoboyuntang Pharmaceutical. Mr. Xiaodong Zhu graduated from Harbin Normal University with Bachelor degree of Science in 1999.
Ms. Jianping Chen, Chief Financial Officer, age 36, is a Certified Public Accountant in the United States since 2001. Before joining the Company, she served as finance manager at several global financial service companies in both United States and China. Ms. Chen was graduated from Iowa State University with a Master Degree of Accountancy in 2001 and she obtained an MBA degree from the Wharton School of the University of Pennsylvania in 2008.
Mr. Di Wu, Chief Development Officer, age 43, is the President and Founder in GoodeePharma Suzhou, China since 2008. From 2002 to 2008, Mr. Wu served as Core Management in the Division of Medical Genetics in University of Pennsylvania, Philadelphia, PA and focused on DMPK, Histology and Morphology. From 2000 to 2002, he was the Post Doctor in the Department of Molecular Genetics, The Wistar Institute, and Philadelphia, PA and focused on Molecular Biology. Mr. Wu graduated from the School of Medicine of Soochow University as a Medicine Doctor in 1991.
Board of Directors
All actions of the board of directors require the approval of a majority of the directors in attendance at a meeting at which a quorum is present. Each director is expected to participate, either in person or via teleconference, in meetings of our board of directors and meetings of committees of our board of directors in which each director is a member, and to spend the time necessary to properly discharge such director’s respective duties and responsibilities. We do not have a written policy with regard to directors’ attendance at annual meetings of stockholders; however, all directors are encouraged to attend the annual meeting.
Audit Committee Financial Expert
Our board of directors currently acts as our audit committee. We currently do not have a member who qualifies as an “audit committee financial expert” as defined in Item 401(e) of Regulation S-K and is “independent” as the term is used in Item 7(d) (3) (iv) of Schedule 14A under the Securities Exchange Act. Our board of directors is in the process of searching for a suitable candidate for this position.
Audit Committee
We have not yet appointed an audit committee. At the present time, we believe that the members of board of directors are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
Employment Agreements
The Company has executed employment agreements with Xiaodong Zhu, the Company’s Chief Executive Officer and Di Wu, the Company’s Chief Development Officer.
Zhu's Employment Agreement
Dongsheng’s employment agreement with Xiaodong Zhu is effective from September 11, 2010 to September 10, 2013. Mr. Zhu’s monthly salary is RMB 50,000.
24
Wu's Employment Agreement
Dongsheng’s employment agreement with Di Wu is effective from January 1, 2010 to January 1, 2011. Mr. Wu’s monthly salary is RMB 20,000.
Family Relationships
There are no family relationships between any of our directors or executive officers and any other directors or executive officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers have been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past five years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Compliance with Section 16(A) of the Exchange Act
Section 16(a) of the Exchange Act requires the Company’s officers and directors, and persons who beneficially own more than 10% of a registered class of the Company’s equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission and are required to furnish copies to the Company. To the best of the Company’s knowledge, any reports required to be filed were timely filed in fiscal year ended June 30, 2010.
Item 11. Executive Compensation.
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officers paid by us during the fiscal years ended June 30, 2010 and 2009, in all capacities for the accounts of our executives.
SUMMARY COMPENSATION TABLE
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock Awards
($)
|
Option Awards
($)
|
Non-Equity Incentive Plan Compensation ($)
|
Non-Qualified Deferred Compensation Earnings
($)
|
All Other Compensation
($)
|
Totals
($)
|
||||||||||||||||||||
|
Xiaodong Zhu, CEO and Chairman (1)
|
2010
|
$
|
14,220
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
||||||||||||||||||
|
Xicai Su, former Chief Operating Officer (2)
|
2010
|
$
|
44,117
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
||||||||||||||||||
|
2009
|
$
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
|||||||||||||||||||
|
Di Wu, Chief Development Officer (3)
|
2010
|
$
|
17,647
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
||||||||||||||||||
|
Jianping Chen, Chief Financial Officer (4)
|
2010
|
$
|
12,867
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
||||||||||||||||||
|
(1)
|
Mr. Zhu was appointed as the Chief Executive Officer of the Company on March 25, 2010.
|
|
(2)
|
Mr. Su resigned from his position as Chief Operating Officer of the Company on August 19, 2010.
|
|
(3)
|
Mr. Wu was appointed as the Chief Development Officer of the Company on March 25, 2010.
|
|
(4)
|
Ms. Chen was appointed as the Chief Financial Officer of the Company on May 15, 2010.
|
Outstanding Equity awards at Fiscal Year End
There are no outstanding equity awards as of June 30, 2010.
25
Director Compensation
Our directors will not receive a fee for attending each board of directors meeting or meeting of a committee of the board of directors. All directors will be reimbursed for their reasonable out-of-pocket expenses incurred in connection with attending board of director and committee meetings.
Option Grants
As of June 30, 2010, we did not maintain any equity incentive or stock option plan. On September 3, 2010, the board of directors of the Company adopted the Dongsheng Pharmaceutical International Co., Ltd. 2010 Equity Incentive Plan (the “2010 Plan”). The 2010 Plan is administered by the board of directors. The 2010 Plan provides for the grant of qualified and non-qualified stock options. In no event shall the aggregate number of shares of the Company’s common stock that may be issued pursuant to incentive stock options exceed nine hundred thousand (900,000) shares. Further, the maximum number of shares granted hereunder to any one 2010 Plan participant may not exceed thirty percent (30%) of the total shares subject to the 2010 Plan.
Certain Relationships and Related Transactions
We will present all possible transactions between us and our officers, directors or 5% stockholders, and our affiliates to the board of directors for their consideration and approval. Any such transaction will require approval by a majority of the disinterested directors and such transactions will be on terms no less favorable than those available to disinterested third parties.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
As of September 20, 2010, our authorized capitalization was 250,000,000 shares of capital stock, consisting of 200,000,000 shares of common stock, $0.0001 par value per share and 50,000,000 shares of preferred stock, $0.0001 par value per share. As of June 30, 2010, there were 17,000,000 shares of our common stock outstanding, all of which were fully paid, non-assessable and entitled to vote. Each share of our common stock entitles its holder to one vote on each matter submitted to the stockholders.
The following table sets forth, as of September 20, 2010, the number of shares of our common stock owned by (i) each person who is known by us to own of record or beneficially five percent (5%) or more of our outstanding shares, (ii) each of our directors, (iii) each of our executive officers and (iv) all of our directors and executive officers as a group. Unless otherwise indicated, each of the persons listed below has sole voting and investment power with respect to the shares of our common stock beneficially owned.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Unless otherwise indicated, the stockholders listed in the table have sole voting and investment power with respect to the shares indicated. Unless otherwise noted, the principal address of each of the stockholders, directors and officers listed below is c/o Dongsheng Pharmaceutical International, Co., Ltd., Room 10, 9th Floor, Building No. 5, 9 Gaoshengqiao Road, Dayi Louver Plaza, Wuhou District, Chengdu, Sichuan Province, People’s Republic of China 610041.
All share ownership figures include shares of our Common Stock issuable upon securities convertible or exchangeable into shares of our Common Stock within sixty (60) days of September 20, 2010, which are deemed outstanding and beneficially owned by such person for purposes of computing his or her percentage ownership, but not for purposes of computing the percentage ownership of any other person.
|
Title of Class
|
Name of Beneficial Owner (1)(2)
|
Number of
shares
|
Percent of
Class (3)(4)
|
||||
|
Common
|
Xiaodong Zhu, Chairman and Chief Executive Officer
|
7,163,000
|
(5)
|
42.135
|
%
|
||
|
Common
|
Di Wu, Chief Development Officer
|
0
|
|
0
|
%
|
||
|
Common
|
Jianping Chen, Chief Financial Officer
|
0
|
0
|
%
|
|||
|
Common
|
Shimin Cai
|
2,950,000
|
17.353
|
%
|
|||
|
Common
|
Weihua Zhao
|
1,500,000
|
8.824
|
%
|
|||
|
All officers and directors as a group (3 persons)
|
7,163,000
|
42.135
|
%
|
||||
|
All officers, directors and 5% holders as a group (5 persons)
|
11,613,000
|
68.312
|
%
|
||||
|
(1)
|
Pursuant to Rule 13d-3 under the Exchange Act, a person has beneficial ownership of any securities as to which such person, directly or indirectly, through any contract, arrangement, undertaking, relationship or otherwise has or shares voting power and/or investment power or as to which such person has the right to acquire such voting and/or investment power within 60 days.
|
26
|
(2)
|
Unless otherwise stated, each beneficial owner has sole power to vote and dispose of the shares and the address of such person is c/o the Company, at 9 Gaoshengqiao Road, Dayi Louver Plaza, Wuhou District, Chengdu, Sichuan Province, P.R. China 610041.
|
|
(3)
|
Figures may not add up due to rounding of percentages.
|
|
(4)
|
Based on 17,000,000 shares of common stock issued and outstanding as of September 15, 2010.
|
|
(5)
|
All 7,163,000 shares were beneficially owned through Super Deal International Limited where Mr. Xiaodong Zhu is the Executive Director. Mr. Zhu has voting and control power over the shares held by Super Deal International Limited.
|
Changes in Control
There are no arrangements, known to the Company, including any pledge by any person of securities of the Company the operation of which may, at a subsequent date, result in a change in control of the Company.
Description of Securities
The Company is authorized to issue 200,000,000 shares of common stock, par value $0.0001 per share and 50,000,000 shares of preferred stock, par value $0.0001 per share. As of September 20, 2010, 17,000,000 shares of common stock and no shares of preferred stock were issued and outstanding.
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of common stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of common stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The common stock is not entitled to preemptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of common stock are entitled to share ratably in all assets remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the common stock. The outstanding shares of common stock are fully paid and non-assessable.
Preferred Stock
The board of directors is empowered to designate and issue from time to time one or more classes or series of preferred stock and to fix and determine the relative rights, preferences, designations, qualifications, privileges, options, conversion rights, limitations and other special or relative rights of each such class or series so authorized. Such action could adversely affect the voting power and other rights of the holders of the Company’s capital shares or could have the effect of discouraging or making difficult any attempt by a person or group to obtain control of the Company.
Market for Common Equity
Our common stock, par value $0.0001 per share, has a trading symbol (“DNGH.OB”) but has been thinly traded on the Over-The-Counter Bulletin Board (“OTCBB”). Our common stock has been listed on the OTCBB since July 17, 2009.
The market price of our common stock is subject to significant fluctuations in response to variations in our quarterly operating results, general trends in the market, and other factors, over many of which we have little or no control. In addition, broad market fluctuations, as well as general economic, business and political conditions, may adversely affect the market for our common stock, regardless of our actual or projected performance.
Holders
As of September 20, 2010, 17,000,000 shares of common stock are issued and outstanding. There are approximately 117 shareholders of our common stock.
Transfer Agent and Registrar
The Transfer Agent for our common stock is American Registrar & Transfer Co., at 342 East 900 South, Salt Lake City 84111. Its telephone number is 801-363-9065.
27
Penny Stock Regulations
The SEC has adopted regulations, which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share. Our Common Stock, when and if a trading market develops, may fall within the definition of penny stock and be subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 individually, or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser’s prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the “penny stock” rules may restrict the ability of broker-dealers to sell our Common Stock and may affect the ability of investors to sell their Common Stock in the secondary market.
Dividend Policy
Any future determination as to the declaration and payment of dividends on shares of our common stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of common stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in the PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from and of Dongsheng. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Dongsheng Pharmaceutical Co., Ltd.
As of June 30, 2010 and June 30, 2009, the Company had payables to Ms. Xiaomei Zhu, Mr. Xiaodong Zhu’s sister, for a total of $21,366 and receivables from Xiaomei Zhu in amount of $111,155, respectively. The balance with Ms. Zhu is unsecured and bears no interest and has no fixed repayment date. The Company expects the entire amount of the loan be settled within six months.
Director Independence
Our determination of independence of directors is made using the definition of “independent director” contained in Rule 4200(a) (15) of the Marketplace Rules of the NASDAQ Stock Market (“NASDAQ”), even though such definitions do not currently apply to us because we are not listed on NASDAQ. We have determined that Mr. Zhu, our sole director, is not “independent” within the meaning of such rules because he currently serves as our Chief Executive Officer.
Indemnification of Officers and Directors
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act.
Our certificate of incorporation provides that we shall indemnify our directors to the full extent permitted by the provisions of Section 102(b) (7) and Section 145 of the Delaware General Corporation Law (the “DGCL”) as the same may be amended and supplemented. Section 102(b) (7) of the DGCL, relating to indemnification is hereby incorporated herein by reference. Notwithstanding the above, our certificate of incorporation provides that a director shall be liable to the extent provided by applicable law, (i) for breach of the director’s duty of loyalty to us; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL, or (iv) for any transaction from which the director derived an improper personal benefit.
At present, there is no pending litigation or proceeding involving any of our director, officer or employees in which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
Item 14. Principal Accountant Fees and Services.
|
(a)
|
Audit Fees: Aggregate fees billed by Friedman for professional services rendered for the audit of our annual financial statements and review of our financial statements included in Form 10-K for the year ended June 30, 2010, were approximately $67,000.
|
|
(b)
|
Aggregate fees billed by Gately for professional services rendered for the audit of our annual financial statements and review of our financial statements included in Form 10-K for the years ended June 30, 2010 and December 31, 2009 were approximately $0 and $1,500, respectively.
|
|
(c)
|
Audit-Related Fees: No fees were billed for assurance and related services reasonably related to the performance of the audit or review of our financial statements and not reported under “Audit Fees” above in the years ended June 30, 2010.
|
|
(d)
|
Tax Fees: Aggregate fees billed by Gately for tax services for the years ended June 30, 2010 and December 31, 2009 were $0 and $0, respectively.
|
|
(e)
|
All Other Fees: Aggregate fees billed for professional services other than those described above were $0 in the year ended June 30, 2010.
|
28
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) Financial Statements.
Audited Financial Statements for the year ended June 30, 2010
(b) Exhibits Required by Item 601 of Regulation S-K.
|
Exhibit No.
|
Description
|
|
2.1
|
Share Exchange Agreement by and between the Company and Dynamic Bhorizon Limited, dated March 25, 2010 (as filed on Form 8-K with the SEC on March 25, 2010).
|
|
3.1
|
Articles of Incorporation (as filed as Exhibit 3.1 on Form S-1 with the SEC on October 28, 2010).
|
|
3.2
|
Amendment to the Articles of Incorporation (as filed as Exhibit 3.1 on Form 8-K with the SEC on May 27, 2010)
|
|
3.3
|
Bylaws
|
|
10.1
|
Stock Option Agreement, granted pursuant to the terms of the Dongsheng Pharmaceutical International Co., Ltd. Equity Incentive Plan, by and between the Company and Jianping Chen, dated September 3, 2010.
|
|
10.2
|
Employment Agreement, by and between Dongsheng Pharmaceutical International Co., Ltd. and Xiaodong Zhu.
|
| 10.3 |
Employment Agreement, by and between Dongsheng Pharmaceutical International Co., Ltd. and Di Wu.
|
|
31.1
|
Certification of Xiaodong Zhu pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
31.2
|
Certification of Jianping Chen pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
32.1
|
Certification of Xiaodong Zhu pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
32.2
|
Certification of Jianping Chen pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
29
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
DONGSHENG PHARMACEUTICAL
INTERNATIONAL CO., LTD.
|
||
|
Dated: September 22, 2010
|
By:
|
/s/ Xiaodong Zhu
|
|
Xiaodong Zhu
|
||
|
Chief Executive Officer
|
||
30
