Attached files
| file | filename |
|---|---|
| EX-32.1 - Biostar Pharmaceuticals, Inc. | ex32-1.htm |
| EX-31.2 - Biostar Pharmaceuticals, Inc. | ex31-2.htm |
| EX-32.2 - Biostar Pharmaceuticals, Inc. | ex32-2.htm |
| EX-31.1 - Biostar Pharmaceuticals, Inc. | ex31-1.htm |
| EX-10.27 - Biostar Pharmaceuticals, Inc. | ex10-27.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
| For the fiscal year ended December 31, 2009 |
OR
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
| For the transition period from to |
Commission file number: 333-147363
BIOSTAR PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Maryland
|
20-5101287
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
No. 588 Shiji Xi Avenue
Xianyang, Shaanxi Province
The People’s Republic of China
|
712046
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number: 011-86-29-33686638
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, no par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes þ No o
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes o No þ
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained herein, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o Accelerated filer o
Non-accelerated filer o Smaller reporting company þ
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No þ
As of June 30, 2009, the aggregate market value of the voting and non-voting common stock held by non-affiliates of the Registrant was approximately $35.6 million based on a closing price of $2.50 per share of common stock as reported on the Over-the-Counter Bulletin Board on such date.
On March 24, 2010, we had 25,820,119 shares of common stock issued and outstanding.
TABLE OF CONTENTS
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED DECEMBER 31, 2009
|
PART I
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
3 | |
|
Item 1B.
|
25 | |
|
Item 2.
|
41 | |
|
Item 3.
|
42 | |
| 42 | ||
|
PART II
|
||
|
Item 5.
|
43 | |
|
Item 6.
|
44 | |
|
Item 7.
|
44 | |
|
Item 7A.
|
51 | |
|
Item 8.
|
51 | |
|
Item 9.
|
51 | |
|
Item 9A.
|
52 | |
|
Item 9B.
|
52 | |
|
PART III
|
||
|
Item 10.
|
53 | |
|
Item 11.
|
57 | |
|
Item 12.
|
61 | |
|
Item 13.
|
63 | |
|
Item 14.
|
64 | |
|
PART IV
|
||
|
Item 15.
|
65 | |
| 68 | ||
CAUTION REGARDING FORWARD-LOOKING INFORMATION
In this annual report, references to “Biostar Pharmaceuticals,” “Biostar,” “BSPM,” “the Company,” “we,” “us,” and “our” refer to Biostar Pharmaceuticals, Inc.
This Annual Report on Form 10-K (including the section regarding Management's Discussion and Analysis or Plan of Operation) contains forward-looking statements regarding our business, financial condition, results of operations and prospects. Words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," "estimates" and similar expressions or variations of such words are intended to identify forward-looking statements, but are not deemed to represent an all-inclusive means of identifying forward-looking statements as denoted in this Annual Report on Form 10-K. Additionally, statements concerning future matters are forward-looking statements.
Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our Management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading "Risks Related to Our Business" below, as well as those discussed elsewhere in this Annual Report on Form 10-K. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We file reports with the Securities and Exchange Commission ("SEC"). We make available on our website under "Investor Relations/SEC Filings," free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such materials with or furnish them to the SEC. Our website address is www.sunwaytech.com.cn/. You can also read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this Annual Report on Form 10-K, except as required by law. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this Annual Report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
PART I
ITEM 1. BUSINESS
Overview
Biostar Pharmaceuticals is a holding company that, through our wholly-owned subsidiary Shaanxi Biostar Biotech, Ltd. (“Shaanxi Biostar”) and our variable interest entity ("VIE") Shaanxi Aoxing Pharmaceutical Co., Ltd. (“Aoxing Pharmaceutical”), develops, manufactures and markets pharmaceutical products for a variety of diseases and conditions in the People’s Republic of China (the “PRC” or “China”).
Corporate Organization and History
Biostar was incorporated in the State of Maryland on March 27, 2007. Through the steps described immediately below, we became the indirect holding company for Aoxing Pharmaceutical, a medical and pharmaceutical developer, manufacturer and marketer in the PRC on November 1, 2007.
On June 15, 2007, we formed Shaanxi Biostar Biotech, Ltd. (“Shaanxi Biostar”) in the PRC as our wholly-owned subsidiary. Because Shaanxi Biostar is wholly-owned by Biostar, a U.S. company, it is deemed a wholly foreign-owned enterprise, or WFOE, under PRC laws.
Aoxing Pharmaceutical was formed on May 8, 1997, as a limited liability company under the laws of the PRC. Its current registered address is No. 588 Shiji Xi Road, Xianyang, Shaanxi Province, PRC, and its registered capital is 61,800,000 Renminbi (“RMB”).
On November 1, 2007, Shaanxi Biostar and Aoxing Pharmaceutical entered into a series of agreements (collectively the “Contractual Arrangements”) pursuant to which we have acquired control over Aoxing Pharmaceutical and which allows us to consolidate the profits and losses of Aoxing Pharmaceutical under U.S. Generally Accepted Accounting Principles (“GAAP”):
Management Entrustment Agreement. Pursuant to the management entrustment agreement, Aoxing Pharmaceutical and its shareholders agreed to transfer control, or entrust, the operations and management of Aoxing Pharmaceutical’s business to Shaanxi Biostar. Shaanxi Biostar manages the operations and assets of Aoxing Pharmaceutical, controls all of the cash flow of Aoxing Pharmaceutical through a bank account controlled by Shaanxi Biostar, is entitled to all of the net profits of Aoxing Pharmaceutical as a management fee, and is obligated to pay all payables and loan payments of Aoxing Pharmaceutical. In addition, Shaanxi Biostar has been granted certain rights which include, in part, the right to appoint and terminate members of Aoxing Pharmaceutical’s board of directors, hire management and administrative personnel and control decisions relating to entering and performing customer contracts and other instruments. We anticipate that Aoxing Pharmaceutical will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Shaanxi Biostar exercises its option. Global Law Office, our PRC counsel, has advised us that in their opinion the management entrustment agreement is legal and enforceable under PRC law.
In exchange for causing Aoxing Pharmaceutical to enter into the management entrustment agreement, we issued an aggregate of 19,832,311 shares our common stock to the shareholders of Aoxing Pharmaceutical, which was allocated based on their respective pro rata ownership of Aoxing Pharmaceutical.
On May 6, 2008, Shaanxi Biostar entered into an amended and restated management entrustment agreement with Aoxing Pharmaceutical and its shareholders in order to remove a provision that allows the management entrustment agreement to be terminated at a mutually agreed date. As amended and restated, the management entrustment agreement, and all of the attendant rights of Shaanxi Biostar, remains in effect until such time that Shaanxi Biostar acquires all of the assets or equity of Aoxing Pharmaceutical under the terms of the exclusive option agreement as more fully described below, or until Aoxing Pharmaceutical ceases its business operations.
Voting Proxy Agreement. In order to give us further control over Aoxing Pharmaceutical, Aoxing Pharmaceutical’s shareholders entered into a voting proxy agreement with Shaanxi Biostar, whereby these shareholders irrevocably and exclusively appointed the members of Shaanxi Biostar's board of directors as their proxies to vote on all Aoxing Pharmaceutical matters that require shareholder approval, including, without limitation, the right to appoint members of Aoxing Pharmaceutical’s board of directors. The voting proxy agreement further provides that Shaanxi Biostar will appoint all members of Biostar’s board of directors to Aoxing Pharmaceutical’s board of directors. As the composition of Biostar’s board of directors changes, Shaanxi Biostar must accordingly remove and appoint new members to Aoxing Pharmaceutical’s board of directors. The voting proxy agreement terminates upon the exercise of the option by Shaanxi Biostar to purchase the shares of Aoxing Pharmaceutical as described below, and is governed by the laws of the PRC.
Exclusive Option Agreement. In order to permit Aoxing Pharmaceutical to become an indirectly wholly-owned subsidiary of Biostar when permitted under PRC law, Aoxing Pharmaceutical and its shareholders entered into an exclusive option agreement with Shaanxi Biostar, whereby Aoxing Pharmaceutical’s shareholders granted Shaanxi Biostar an irrevocable and exclusive purchase option (the “Option”) to acquire Aoxing Pharmaceutical's equity and/or remaining assets, but only to the extent that the acquisition does not violate limitations imposed by PRC law on such transactions. Current PRC law does not specifically provide for the equity of a non-PRC entity to be used as consideration for the purchase of a PRC entity's assets or equity unless the value of the shares are equal to or greater than the value of the enterprise acquired. In addition, there is a lengthy appraisal process which must be approved by the provincial PRC government entities. The consideration for the exercise of the Option is to be determined by the parties and memorialized in future definitive agreements setting forth the kind and value of such consideration.
We will consider exercising the Option under such circumstances we believe will be in the best interests of the Company and our shareholders, and the exclusive option agreement has been drafted to give us such flexibility. In considering whether or not we will exercise the Option, we may consider such factors as: (1) if the exercise price can be lower than the appraised value under current PRC law, (2) availability of funds, (3) any relevant tax considerations at the time, (4) any other relevant PRC laws that may exist at the time, (5) the value of our shares that were previously paid to Aoxing Pharmaceutical’s shareholders, and (6) whether or not the exercise of the Option will provide any other additional benefits to us or our shareholders. Upon exercise of the Option, the parties will prepare transfer documents to be submitted for governmental approval and work together to obtain all approvals and permits. The exclusive option agreement may be terminated by the agreement of all parties or by Shaanxi Biostar with 30 days notice, and is governed by the laws of the PRC.
Share Pledge Agreement. In order to further solidify our control over Aoxing Pharmaceutical, Shaanxi Biostar and Aoxing Pharmaceutical’s shareholders entered into a share pledge agreement, whereby Aoxing Pharmaceutical’s shareholders pledged all of their equity interests in Aoxing Pharmaceutical, including the proceeds thereof, to guarantee the performance by the shareholders of all of the agreements they entered into with Shaanxi Biostar. Upon breach by any shareholder of any of the Contractual Arrangements, Shaanxi Biostar is entitled by operation of law to become the beneficial owner of the shareholders’ equity interests of Aoxing Pharmaceutical. Prior to termination of the share pledge agreement, the pledged equity interests of Aoxing Pharmaceutical cannot be transferred without Shaanxi Biostar's prior written consent. The share pledge agreement will not terminate until agreed to by all of the parties in writing, and is governed by the laws of the PRC.
The Contractual Arrangements described above were utilized instead of a direct acquisition of the assets, common stock or a share exchange because we could not pay cash to directly or indirectly acquire Aoxing Pharmaceutical or its assets. PRC law permits the purchase of equity interests, or assets of a PRC entity by a non-PRC entity for cash. The purchase price must be based on the appraised value of the equity or assets. Because we did not have sufficient cash to pay the estimated full value of all of the assets of Aoxing Pharmaceutical, we, through Shaanxi Biostar, entered into the Contractual Arrangements in exchange for the right to exercise functional control over Aoxing Pharmaceutical, and we obtained substantially the same result as a direct share exchange with Aoxing Pharmaceutical.
Shaanxi Biostar’s control over Aoxing Pharmaceutical under the Contractual Arrangements requires us to consolidate its financial statements pursuant to the FASB Interpretation 46, “Consolidation of Variable Interest Entities (VIEs)” (“FIN 46R”), an Interpretation of Accounting Research Bulletin No. 51, because Aoxing Pharmaceutical is considered a VIE of Shaanxi Biostar. FIN 46R requires a VIE to be consolidated by any company that is subject to a majority of the risk of loss for the variable interest entity or is entitled to receive a majority of the variable interest entity’s residual returns. Since Shaanxi Biostar is the primary and only beneficiary of Aoxing Pharmaceutical (the VIE), FIN 46R requires the consolidation of its financial statements with Shaanxi Biostar and ultimately consolidated with Shaanxi Biostar’s parent company, Biostar.
On March 18, 2010, we entered into an agreement to acquire 100% of Xi’an Meipude Biotech Co., Ltd., a Xi’an-based medical equipment and nutrients manufacturer (“Meipude”) for RMB 7.85 million ($1.1million). We officially took control over the operations and the assets of Meipude on March 29, 2010, including its facilities, corporate documents and records, and business licenses and certificates. The Administration for Industry and Commerce of Xi’an Hi-Tech District has also accepted our application to change Meipude’s ownership registration, and we expect to receive the certificate attesting to such change shortly. Meipude manufactures and distributes topical hernia treatment belts, also manufactures a nutraceutical product for treatment of gynecological inflammation in young and middle-aged women. Meipude has been manufacturing and selling its products in Shaanxi and an adjacent province since 2004.
The following diagram illustrates our current corporate structure:
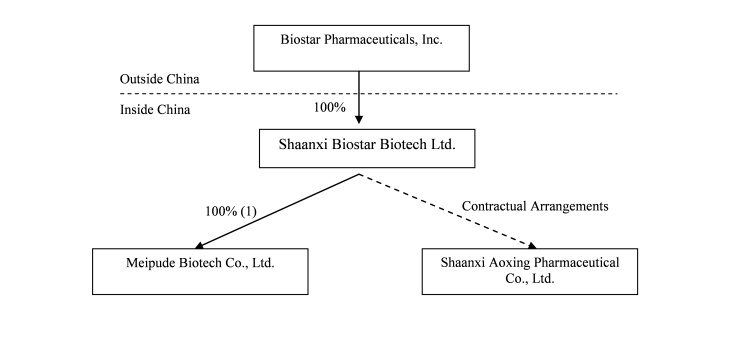
|
(1)
|
Subject to our receipt of certificate from the Administration for Industry and Commerce of the Xi’an Hi-Tech District certifying our ownership registration.
|
When we sell our equity or borrow funds, we expect the proceeds will be forwarded to Aoxing Pharmaceutical and accounted for as a loan to Aoxing Pharmaceutical and eliminated during consolidation. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt.
Neither Biostar nor Shaanxi Biostar has any operations or plans to have any operations in the future other than acting as a holding company and management company for Aoxing Pharmaceutical and raising capital for its operations. However, we reserve the right to change our operating plans regarding Biostar and Shaanxi Biostar.
Our Business
We develop, manufacture and market pharmaceutical products in the PRC for a variety of diseases and conditions. Our most popular product is the Xin Ao Xing Oleanolic Acid Capsule, an over-the- counter (“OTC”) medicine for chronic hepatitis B, a disease affecting approximately 10% of the Chinese population (Source: PRC Ministry of Health). Our current product line also includes 2 other OTC products and 2 prescription-based pharmaceuticals. We are also licensed to produce and sell13 nutrient products, although we did not produce or sell these products in 2009.
Our products are derived from medicinal herbs that are either grown at our own facility or purchased from our suppliers. We rely on approximately 10 suppliers for our raw materials. For fiscal year 2009, we purchased all our raw materials from suppliers because the herbs planted at our facility were not yet ready for harvest and use.
We devote substantial resources to the research and development of new products that must be approved by the regulatory agencies. We currently have 10 products under development to complement our existing product line, 3 of which are currently awaiting approval from the State Food and Drug Administration of the PRC (the “SFDA”). We have adopted international manufacturing standards and currently hold one patent, with two additional patents pending approval. We are subject to extensive government regulation which is discussed in detail in the section below called “Government Regulation.” In the event that a new product is not approved or it is found in violation of these laws and regulations, it could have a materially adverse effect on the prospects of our business operations.
Our products are currently being sold in over 24 provinces in the PRC through 20 distributors and an established network of more than 250 dedicated sales people. In addition, we are enhancing our marketing efforts with the launch of our internet-based China Hepatitis Internet Hospital (www.zggbyy.com, “CHIH”) in June 2009. The multi-function website is designed to be a one-stop portal for HBV patients, providing current and relevant information on HBV and treatment options as well as a convenient method to purchase our HBV medicine. Registered users can secure a membership card for a fee of 200 RMB (approximately $25). Members are entitled to a 20% discount on diagnosis and medical services provided on CHIH, free expert diagnosis and free medicine delivery, and a wide range of inquiry, instruction and other complementary services. Registered users can also seek medical advice from a pool of HBV health professionals without having to go to the hospital. CHIH will facilitate our ability to provide customer service and add purchasing convenience for our consumers.
Our Products
The table below summarizes our current pharmaceutical products approved for sale by the SFDA:
|
Name
|
Treatment
|
Benefits and Side Effects
|
SFDA Classification
|
|||
|
Xin Ao Xing
Oleanlic Acid
Capsule
|
Hepatitis B
|
Relieves hepatic injury, reduce glutamic-pyruvic transaminase activity, reduce r-GLO. Believed to promote hepatic cell regeneration, to be effective in hepatic coma treatment, to inhibit fibrous hyperplasia and prevent hepatocirrhosis. Used to reduce hepatic damage caused by HBV regeneration.
|
OTC
|
|||
|
Ganwang
Compound
Paracetamol &
Amantadine
Hydrochloride Capsule
|
Colds, runny nose, sore throat pain, headache and fever
|
Relieves the symptoms of the common cold, including runny nose, sniffles and sneezing. Some patients experience symptoms of anorexia, queasiness and upset stomach after use.
|
OTC
|
|||
|
Danshen Granule
|
Coronary heart disease, myocarditis and angina pectoris
|
Believed to stimulate circulation to end stasis, regulating the flow of qi (vital energy) to alleviate pain. There are no known side effects.
|
Prescription
|
|||
|
Taohuasan
Pediatric Medicine
|
Bronchial congestion and coughs
|
Used for the treatment for children’s cough and respiratory tract infection. There are no known side effects.
|
Prescription
|
|||
|
Tianqi
Dysmenorrhea
Capsule
|
Dysmenorrhea
|
Traditional Chinese medicine used for treatment of pain and other symptoms associated with menstruation. There are no known side effects.
|
OTC
|
Xin Ao Xing Oleanolic Acid Capsule, also known as Ao Xing Liver Cure, is the only non-prescription drug currently being sold on the market that has been approved by the SFDA for the treatment of chronic hepatitis B virus ("HBV"), which is prevalent in the PRC. It is estimated that more than 130 million people are infected with HBV, or 10% of the population (some estimates are as high as 15% of the population) in the PRC. According to the World Health Organization, approximately about 1 million people die from hepatic failure, hepatocirrhosis and primary hepatoma caused by HBV infection per year; however, it was not until December 2, 2005, that the Chinese government first issued an HBV prevention manual for the general public. (Source: www.chinagan.com)
There are two kinds of medicine typically used for antivirotic treatment: interferon and ribonucleotide analog, both of which do not kill the HBV directly, but inhibit the metabolizing of HBV replication. Their side effects, however, include damage to normal healthy cells, and they require prolonged treatment periods of more than one year and high costs. (Source: Pharmacopoeia of the People's Republic of China)
Our Xin Ao Xing Oleanolic Acid Capsule is a pentacyclic triterpenoid which contains extracts from natural plants, Fructus Ligustri Lucidi and Hemsleya, and is the only SFDA-approved product to be manufactured as an OTC hepatitis B medicine in the PRC. It is also certified by the Chinese Medical Association as a specific product for hepatitis B treatment. Its pharmacological actions include the relief of hepatic injury, reduction of glutamic-pyruvic transaminase activity, promotion of hepatic cell regeneration, the inhibition of fibrous hyperplasia and prevention of hepatocirrhosis.
We estimate the demand for medicines treating hepatitis B amount to approximately $7.8 billion annually. We believe that we are well-positioned to become a leader in the sale of OTC medicines for the treatment of hepatitis B as our Xin Ao Xing Oleanolic Acid Capsule is the only oral OTC drug approved by the SFDA for such treatment. We continue to aggressively advertise this product and has actively maintained a program where consumers can try a one-month supply of the drug for free.
The table below summarizes our current nutrient products approved for sale by the SFDA, although we did not produce or sell these products in 2009:
|
Name
|
Primary Ingredients
|
Function and Benefits
|
SFDA Classification
|
|||
|
Ao Xing No.1
|
Egg yolk lecithin, vitamin E, vitamin C
|
Believed to promote health of the elderly, development of children's intelligence, and improve memory.
|
Nutrient, OTC
|
|||
|
Yanshuang
|
Sterculia scaphigera, chrysanthemum, honeysuckle and mint
|
For the relief of pharyngeal discomfort and chronic pharyngitis.
|
Nutrient, OTC
|
|||
|
NewChagederi
|
Agastache, perilla, licorice and mint
|
Believed to prevent and relive symptoms of rhinitis, sinusitis, headache congestion and running nose.
|
Nutrient, OTC
|
|||
|
Tiantianle
|
Folicartemisiae, chrysanthemum, honeysuckle, agastache and mint
|
Believed to strengthen immune system against influenza and certain viruses from sexually transmitted diseases and general detoxification.
|
Nutrient, OTC
|
|||
|
AoXing Ganbao
|
Polygonum cuspidatum, cordyceps and ginseng
|
Believed to promoting blood circulation by removing blood stasis, strengthen the spleen and stomach, detoxify, and act as a cholagogic purgative and lower transaminase.
|
Nutrient, OTC
|
|||
|
Fengningdan
|
Safflower, osthol, cork, dragles, dracorhodin, borneol and angelica
|
Believed to assist in elimination of toxins associated with gynecological inflammation of young and middle-aged women.
|
Nutrient, OTC
|
|||
|
Heigen
|
Black sesame seeds and medla
|
Believed to treat premature graying of beard and hair, phalacrosis caused by excess fat, and postpartum hair loss.
|
Nutrient, OTC
|
|||
|
Baitongning
|
Bulleyacontitin, myrrh and centipede
|
Believed to relieve rheumatism, hyperplasia, cervical spondylosis, lumbar lesion, osteoporosis and rheumatoid arthritis.
|
Nutrient, OTC
|
|||
|
Sukang Capsule
|
Agkistrodon, cinnamon, safflower and hawthorn
|
Believed to improve cardiovascular and cerebrovascular complications, such as, dizziness headache, amnesia, kidney deficiency, coronary heart disease, and Atherosclerosis, in the elderly.
|
Nutrient, OTC
|
|||
|
Aoxing Ointment
|
Walnut meat and camphor
|
Used to treat psoriasis, vitiligo and various dermatitis.
|
Nutrient, OTC
|
|||
|
Yizi Capsule
|
Tibetan snow lotus, ejiao, cinnamon, mulberry, raspberry
|
Believed to aid infertility and helps in fetal development during pregnancy
|
Nutrient, OTC
|
|||
|
Tangning Capsule
|
Rhodiola, bitter, pueraria, white bud root, polygonatum, spirulina
|
Believed to treat type II diabetes
|
Nutrient, OTC
|
|||
|
Shengjing Capsule
|
Tibetan ginseng, yak organ, extract of deer organ, cinnamon, gorgon fruit, raspberry
|
Believed to replenish kidney function
|
Nutrient, OTC
|
The table below summarizes our products that are in various stages of regulatory approval and their classification by the SFDA:
|
Name
|
Functions
|
Type
|
STAGE
|
|||
|
Gansukang
|
For the treatment of acute and chronic hepatitis treatment, and removal of toxins from the body
|
Prescription
|
Awaiting approval permit
|
|||
|
Niaosaitong
|
A traditional Chinese medicine used for regulating vital energy, dredging collaterals and eliminating stagnation and ischuria
|
Prescription
|
Undergoing SFDA examination phase
|
|||
|
Azithromycin
|
A chemical prescription medicine used for the treatment of tympanitis, pharyngitis, bronchitis, pneumonia and anti-infection
|
Prescription
|
Undergoing SFDA examination phase
|
|||
|
Shenrong
|
Used for strengthening immunity, preventative cancer care and restraining growth of tumor, sedating and resting pain, anti-fatigue, anti-aging, strengthen sexual function and desire
|
Prescription
|
Clinical test has been completed. Now in phase of application for preliminary permit
|
|||
|
Zhixuening
|
For stopping bleeding, relieving swelling and expelling blood stasis. Used in the treatment of uterine bleeding, acute vaginal bleeding and hematockezia in women , nose bleeding, blood regurgitation
|
Prescription
|
Clinic test has been completed. Now in the phase of application for preliminary permit.
|
|||
|
Xiao'aiping
|
For the treatments of tumors, and mainly on esophagus cancer, stomach cancer and lung cancer
|
Prescription
|
Clinic test has been completed. Now in phase of application for preliminary permit.
|
|||
|
Zhenbao Wan
|
A type of traditional Chinese medicine. It is mainly used in the treatment of thrombus and coronary heart disease, hemiplegia, muscle and tendon atrophy, kidney impairment and meridian damage, murrain and pyreticosis
|
Prescription
|
Clinic test has been completed. Now in phase of application for preliminary permit.
|
|||
|
Huazhi Pian
|
Used to reduce temperature, clean blood, reduce bleeding, activate “Qi” flow and eliminate stasis to activate blood circulation
|
Prescription
|
Clinic test has been completed. Now in phase of application for preliminary permit.
|
|||
|
KunLing Wan
|
Used to regulate menstruation and nourish blood, dissipate blood stasis and generate new blood, treatment of irregular menses, abnormal menstrual blood volume, menstrual cramps, uterus cold, infertility, leucorrhea with red and white discharge, metrorrhagia and metrostaxis, deficiency of vital energy due to a long illness, deficiency of kidney and lower back pain
|
OTC
|
Clinic test has been completed. Now in phase of application for preliminary permit.
|
|||
|
Zushima
|
An aerosol pain suppressant and blood coagulant, which has already been approved by the Chinese Military SFDA for clinic trial
|
OTC
|
Undergoing SFDA examination phase
|
Market for our Products
Based on data that we have compiled from the business intelligence service DataMonitor, over the past decade, the Chinese medicine and pharmaceutical industry has developed at an annual growth rate of over 16%, making it one of the fastest growing industries in the Chinese economy. Worldwide, the PRC is among the ten largest medicine manufacturing countries and medical raw materials exporting countries.
With approximately one-fifth of the world's population and a fast-growing gross domestic product, the PRC presents significant potential for the pharmaceutical industry. The relative performance of the market is forecast to decelerate, with an anticipated CAGR of 14.6% for the five-year period 2006-2011 expected to drive the market to a value of $29.9 billion by the end of 2011, according to DataMonitor. However, this growth is still significantly higher as compared to the rest of the world, where growth of the pharmaceutical industry is projected to be at a CAGR of 5.0% to 8.0% between 2004 and 2009 according to IMS Health.
The following table sets forth the projected pharmaceutical market trends in the PRC in terms of demand from 2006 to 2011:

We believe that the burgeoning market provides many interesting opportunities for us. We are pursuing opportunities in several sectors that we believe will experience high growth and that we can address with our manufacturing and distribution expertise. The following is a brief overview of these potential sectors:
Hepatitis
We estimate that there are approximately 120 million hepatitis patients in the PRC. Currently, the most common way to establish an effective treatment protocol is through a doctor or hospital. As many patients have chronic HBV, ailments are prevalent and typically become more severe if not properly treated. However, HBV patients in the PRC also bear substantial psychological pressure, since it is very contagious. Infected patients are often fearful that their relatives, friends and coworkers will become aware of their circumstances and wind up soliciting treatment in secret, if at all. (Source: www.mdcn.com.cn) In addition to producing a medicine to treat HBV, we have launched CHIH, an internet portal designed to promote our product while providing HBV patients with current and relevant treatment information at the same time. We are positioning ourselves as a leader in HBV treatment.
Coronary Disease
According to the World Heart Federation, cardiovascular disease is the leading cause of death in the developing world (with the exception of sub-Saharan Africa). Its rise is linked to the increase in prevalence of risk factors such as tobacco use and relative lack of access to interventions to managing the ensuing disease. In the PRC, annual direct costs are estimated at (euro) 30.76 billion or 4% percent of gross national income. The PRC is facing an increase in cardiac disease on two fronts. We believe that in urban and upscale areas, heart disease is on the rise as their lifestyles increasingly imitate western culture, i.e., higher stress levels associated with a `privatized" economy, poorer nutrition, decreased physical activity and increase in tobacco use. Within the rural provinces, we believe that impoverishment is also contributing to the rise in coronary disease as most villages have no or limited access to medical help. Our Danshen Granule has been accepted as an effective product for the treatment of coronary heart disease, myocarditis and angina pectoris and we are marketing the product aggressively within the rural and urban markets. (Source: www.heart999.cn)
Dysmenorrhe
There are an estimated 400 million "pre-menopausal" women in the PRC. (Source: www.women.org.cn) As the PRC continues to develop, the demand by women for products to treat their health concerns will continue to rise. We believe that our Tianqi Dysmenorrhea Capsule is positioned to take a leading role in this sector.
Influenza
Influenza is one of the most common recurring diseases in the PRC. It has been estimated that there is an annual market of $6.25 billion for flu-related healthcare in the PRC, 85% of which is in the form of OTC consumption (source: www.pharmatech.org.cn). Some of our pharmaceutical and nutrient products are designed to relieve symptoms associated with the flu.
The Rural Market
"Modern" medicine is not yet established in much of rural PRC. Frequently-occurring respiratory, digestive, and infectious diseases (such as hepatitis) often result in far more severe symptoms than would occur with proper treatment. Patients in remote areas are often lucky to be tended to by a technical school graduate at a village "clinic" with treatments passed down from generation to generation; professional doctors are few and far between. According to a Hai Tong Securities Industry Research report, median family incomes in many parts of western PRC are less than $100 per year, yet a day in the hospital can cost $25 and when medicines, procedures and other services are added this can exceed $50.
As the PRC government works to improve the overall health of its population, the rural markets represent a significant opportunity for growth. This sector has typically been neglected by the PRC's pharmaceutical and medicine industry, as there is minimal healthcare infrastructure or standardized health care service in much of rural PRC. As part of a strategy to improve rural healthcare, the PRC's central government has initiated its "New Rural Medical Care Cooperative Program" which will be launched in 2008, with the intention of achieving full coverage of all rural citizens by 2010. With an estimated 900 million rural farmers throughout the nation, the implementation of this program provides substantial opportunity for market expansion in this sector, where expenditures are estimated at nearly US$ 5.6 billion in the next 3 years - with 80% of that budget to be paid by the regional provincial governments in mid and western PRC. Of these rural markets the provinces of Shaanxi, Sichuan, Chongqing, Gansu, Henan, Hubei, and Hunan are expected to comprise 30% of the market, or $US 1.7 billion. (Source: China State Council Rules of Rural Cooperative Medical System.). We believe that we are well established within the rural marketplace and have developed a targeted, aggressive sales and marketing campaign designed to expand our presence of all of our products in this sector.
Pediatric Medicine
The PRC’s fifth national census (published in 2001) indicated that with a population of 296,500,000, the 14 and under age group represented 22.89% of her population. A lack of education within the population results in children often given treatments created for adults and without direction from a doctor, often resulting adverse reactions to the child. Even aspirin, taken by a baby can result in gastric mucous membrane erosion. Other "adult" medicines can affect a child's hearing, cause trauma of bones and joints, and even induce permanent deafness and renal damage.
Increased access to information through education programs and the general promotion of good health within the PRC are helping to generate demand for products designed specifically for children. Furthermore, as the PRC continues to advocate the one child per family policy, parents' demands for quality children's medicines are increasing, as is the interest in brand differentiation. However, at present, few manufacturing plants specialize in pediatric medicine and there is no leading national brand. Approximately 90% of general pharmaceuticals and medicines utilized in the PRC have no corresponding pediatric formula for their drugs, leaving substantial opportunity for growth. We plan to introduce new products to address these issues. In particular, we plan to enhance production of our pediatric medicines and market our pediatric cough medication.
Respiratory Disease
With the aggravation of air pollution and worsening environmental conditions, the incidence of respiratory diseases remains high in the PRC. Influenza is one of the most common diseases in the PRC, and according to the Ministry of Health of the PRC an estimated 75% of the population suffers from influenza every year and 5.5% suffer from tracheitis caused by influenza. This rate is more than 15% for senior citizens, who often suffer from influenza more than 3 times per year.
As is shown in the related statistics in the National Health Care Department in the PRC, the percentage of the population suffering from some form of respiratory diseases in the PRC is approximately 6.94%, or approximately 80,000,000 people suffering from respiratory diseases every year.
The four common respiratory diseases - acute nasopharyngitis, influenza, tonsillar tracheitis, and chronic bronchitis - account for 80% of the respiratory diseases in the PRC.
Our Taohuasan Pediatrics Medicine is used to treat respiratory disease in children.
Industry Consolidation
In 2003, the Chinese government issued “The Medicine Management Law”, “Pharmaceutical Manufacturing Quality Management Specifications” and implemented the Good Manufacturing Practices (“GMP”). This action has, and will continue to result in, industry consolidation as those companies without GMP certificates and without qualified facilities, capital or management expertise necessary to secure approval are forced to find strategic alternatives or cease operations.
Since 2003, the number of pharmaceutical companies in the PRC has decreased rather significantly, from 6,700 to approximately 3,600. (Source: Research and Markets, “China Pharmaceutical Industry Report (Merger and Reorganization) 2006-2007”) This trend has also resulted in significant opportunity for us, as we plan to identify companies that have similar products or other assets, but an inability to bring them to market.
Our Customers
Approximately 75.7% of our sales are made directly to pharmacies, while approximately 24.3% of sales are made through distributors. Our largest customer, Guangdong Run Tai Medicine Wholesale Co., Ltd., accounted for approximately 4.3%, 6.1% and 11.8% our sales for the years ended December 31, 2009, 2008 and 2007, respectively. No customer accounts for 10% or more of our total sales for fiscal year 2009.
Below is a summary of sales of our five products for the year ended December 31, 2009, through our direct sales and through our distributors:
|
Product
|
Sales through Distributors
|
Sales by Company
|
||
|
Xin Ao Xing Oleanlic Acid Capsule
|
6.7%
|
62.1%
|
||
|
Ganwang Compound Paracetamol & Amantadine Hydrochloride Capsule
|
5.3%
|
2.9%
|
||
|
Danshen Granule
|
3.3%
|
2.8%
|
||
|
Taohuasan Pediatric Medicine
|
4.5%
|
3.6%
|
||
|
Tianqi Dysmenorrhea Capsule
|
4.5%
|
4.4%
|
||
|
Total
|
24.3%
|
75.8%
|
Competition
The pharmaceutical industry both within the PRC and globally is intensely competitive and is characterized by rapid and significant technological progress. Our competitors, both domestic and international, include large pharmaceutical companies, universities, and public and private research institutions that currently engage in or may engage in efforts related to the discovery and development of new pharmaceuticals. Many of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than us, as well as more experience in research and development, clinical trials, regulatory matters, manufacturing, marketing and sales.
The following table lists the primary competitors for each of our current product offerings as well as the nutrient products that we are licensed to produce:
|
Product
|
Competitors
|
|
|
Xin Ao Xing Oleanlic Acid Capsule
|
Wulanhaote Zhong Meng pharmaceutical Co., Ltd., Yang Ling Mai Di Sen Pharmaceutical Co., Ltd. and other suppliers of prescription medicines that are used for hepatitis treatment
|
|
|
Ganwang Compound Paracetamol & Amantadine Hydrochloride Capsule
|
Jiang Xi Ren He Pharmaceuticals, Inc. and Hainan Asia Pharmaceuticals, Inc.
|
|
|
Danshen Granule
|
Yun Nan Yong An Pharmaceuticals, Inc. and Hai Nan Min Hai Pharmaceuticals, Ltd.
|
|
|
Taohuasan Pediatric Medicine
|
Shandong Bai Cao Pharmaceuticals, Ltd., and Chang Chun Ren Min Pharmaceuticals, Ltd.
|
|
|
Tianqi Dysmenorrhea Capsule
|
Yun Nan Yu Xi City Wei He Pharmaceutical, Ltd., and Shandong Phoenix Pharmaceuticals, Ltd.
|
|
|
Nutrient Products
|
Wulanhaote Zhong Meng Pharmaceutical Co., Ltd. and and Yang Ling Mai Di Sen Pharmaceutical Co., Ltd. and other traditional Chinese medicine suppliers
|
Of these companies, our three major competitors are Wulanhaote Zhong Meng Pharmaceutical Co., Ltd., Yang Ling Mai Di Sen Pharmaceutical Co., Ltd., and Inner Mongolia Ku Lun Pharmaceutical, Co., Ltd. because some of their products are sold in the same markets as ours. Additionally, only Shan Dong Phoenix Pharmaceutical Inc., Yun Nan Yu Xi Wei He Pharmaceutical, Ltd., Yang Ling Mai Di Sen Pharmaceutical Co., Ltd., and Yun Nan Yong An Pharmaceuticals, Co., Ltd. hold GMP certificates.
Sources and Availability of Raw Materials and Principal Suppliers
Our principal raw materials are the active ingredients for each of our products. We will soon have the ability to source certain raw materials internally, while other raw materials, as well as packaging materials, are sourced from various independent suppliers in the PRC.
Third party vendors are selected based on a number of factors, including quality, timely delivery, cost and technical capability. Management also conducts periodic onsite reviews of our suppliers’ facilities. The vast majority of our raw material needs are readily available. We try to maintain relationships with at least two vendors for each major raw material in order to ensure a reliable supply at reasonable prices.
We rely on approximately 10 suppliers for our products. Approximately 25.9% and 20.9% of our supplies are purchased from Xi’an Chinese Medicine Herbal Tablets Factory and Xianyang Wenlin, respectively.
Set forth below is the names of our suppliers and the amount of materials purchased from them during fiscal year 2009.
|
Name of Supplier
|
Amount Purchased ($)
|
Percentage of Total Purchase
|
||
|
Xi’an Chinese Medicine Herbal Tablets Factory
|
3,541,819
|
25.9%
|
||
|
Xianyang Wenlin
|
2,858,843
|
20.9%
|
||
|
Sichuankangfulai Pharmaceutical Group Ltd.
|
1,899,558
|
13.9%
|
||
|
Zhejiang Xinchang Yaofeng
|
1,615,816
|
11.8%
|
||
|
Sichuan Xieli Pharmaceutical Co., Ltd.
|
1,443,709
|
10.5%
|
||
|
Shaanxi Hengqing Pharmaceutical Co., Ltd.
|
933,013
|
6.8%
|
||
|
Xi’an Jinqiao Plastics Plant
|
531,627
|
3.9%
|
||
|
Xi’an Xinping Industry &Trade Co., Ltd.
|
394,268
|
2.9%
|
||
|
Xianyang Si Fang Carton Co., Ltd.
|
263,857
|
1.9%
|
We have also been cultivating herbs since October 2008, including salvia miltiorrhiza, priclklyash peel, eucommia bark, gingko, honeysuckle, shizandra berry, scutellaeria baicalensis georgi, milk veteh and radix codonopsitis, and we are expecting our first harvest in April 2010. We are also constructing a processing plant nearby, which we anticipate to complete in April 2010.Once completed, we will be able to process these herbs into raw materials for our products. We will also be able to sell excesses on the market as raw materials.
Intellectual Property
We rely on a combination of trademark, patent and trade secret protection laws in the PRC, as well as confidentiality procedures and contractual provisions to protect our intellectual property. We also require our employees to execute confidentiality and trade secret agreements.
We currently hold one patent for the production method of our Aoxing Ganbao product, with two additional patents pending approval, and 12 registered trademarks in the PRC, and own the rights to the internet domain names www.biostarpharmaceuticals.com and www.aoxing-group.com. Our patent, patent number ZL2007100180930, was approved on September 16, 2009, and is valid for twenty years.
Below is a list of our trademarks:
|
Trademark
|
International Class
|
Registration
|
Issuing Authority
|
Term
|
|
“Bai Ying Shen” & device
(Certificate No. 1196275)
|
5
|
Aoxing Pharmaceutical
Note: This trademark was first registered by Shaanxi Xianyang Pharmaceutical Baiyingshen Drug Centre, and was transferred to us on March 14, 2001. The transfer was duly recorded in the Trademark Certificate.
|
Trademark Bureau of State Administration for Industry and Commerce
|
From August 7, 1998 to August 6, 2008 and is in the process of renewal
|
|
“Yi Wen Ling” & device
(Certificate: No. 1008816)
|
5
|
Aoxing Pharmaceutical
Note: This trade mark was first registered by Shaanxi Xianyang Pharmaceutical Baiyingshen Drug Centre, and then was transferred to the Company on April 12, 2002. The transfer was duly recorded in the Trademark Certificate.
|
Trademark Bureau of State Administration for Industry and Commerce
|
From May 21, 2007 to May 20, 2017
|
|
“Zhong Ao” & device. (Certificate: No. 1728599)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From March 14, 2002 to March 13, 2012
|
|
“Xin Tai Ke” & device (Certificate:
No. 1908333)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From September 28, 2002 to September 27, 2012
|
|
“Gan Wang” & device
(Certificate: No. 3001006)
|
5
|
Aoxing Pharmaceutical
Note: This trade mark was first registered by Xianyang Aotong Baojian Technology Limited, and then was transferred to us on August 14, 2003. The transfer was duly recorded in the Trademark Certificate.
|
Trademark Bureau of State Administration for Industry and Commerce
|
From November 14, 2002 to November 13, 2012
|
|
“Hei Gen” (Certificate: No. 3168882)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From July 7, 2003 to July 6, 2013
|
|
“Shi Li Ming” (Certificate: No. 3180355)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From August 7, 2003 to August 6, 2013
|
|
“Aoxing No.1” (Certificate: No. 3168883)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From February 21, 2004 to February 20, 2014
|
|
“Dan Shen” (Certificate: No. 1908335)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From October 7, 2002 to October 6, 2012
|
|
“Cha Ge De Ri” & device (Certificate: No. 4770095)
|
5
|
Aoxing Pharmaceutical
Note: This trademark was first registered by Wulanhaote Zhongmeng Pharmaceutical Limited, and then was transferred to us on May 30, 2002. The transfer was duly recorded in the Trademark Certificate.
|
Trademark Bureau of State Administration for Industry and Commerce
|
From Auguast 28, 2001 to August 27 2011
|
|
“Mei Bi De” & device (Certificate: No. 1330246 )
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From November 7, 1999 to November 6, 2009 and is in the process of renewal
|
|
“Ao Xing Xin Le” & device (Certificate: No. 4319027)
|
5
|
Aoxing Pharmaceutical
|
Trademark Bureau of State Administration for Industry and Commerce
|
From November 28, 2007 to November 27, 2017
|
Bio-pharmaceutical companies are at times involved in litigation based on allegations of infringement or other violations of intellectual property rights. Furthermore, the application of laws governing intellectual property rights in China and abroad is uncertain and evolving and could involve substantial risks to us.
Government Regulation
The testing, approval, manufacturing, labeling, advertising and marketing, post-approval safety reporting, and export of our products are extensively regulated by governmental authorities in the PRC. We are also subject to the Drug Administration Law of the PRC, which governs the licensing, manufacturing, marketing and distribution of pharmaceutical products in the PRC and sets penalties for violations of the law. We are also subject to various other regulations and permit systems by the Chinese government. These regulations and their impact on our business are set forth in more detail below.
|
Drug Administration Law of the PRC was promulgated by the Standing Committee of National People’s Congress on February 28, 2001 and effective as of December 1, 2001, and its implementing guidelines were promulgated by the State Council on August 4, 2004 and effective as of September 15, 2002. According to Drug Administration Law of the PRC and its implementing guidelines, a pharmaceutical manufacturer is required to obtain a Pharmaceutical Manufacturing Permit and Drug Approval Number for each manufactured drug from the relevant SFDA’s provincial branch, which will be valid for five years and is renewable upon application before expiration. Accordingly, we are required to apply for these approvals and any extensions thereof for each of our products.
|
|
Administration Regulations for Drug Registration was promulgated by the SFDA on July 10, 2007, and was effective as of October 1, 2007. The Administration Regulations for Drug Registration specifies the requirements and procedure for obtaining a Drug Approval Number for a new drug. It includes the requirements for clinical trial of new drugs, procedure for registering imported medicine and reporting and approval procedure for generic medicine. The Drug Approval Number is valid for five years and can be re-registered upon expiration. We are required to obtain a Drug Approval Number for each of our new drugs and reapply for an extension prior to the expiration date the drugs.
|
|
Good Manufacturing Practices (GMP) for Pharmaceutical Products, as revised in 1998 was promulgated by the SFDA on June 18, 1999 and became effective as of August 1, 1999, and the Authentication Regulations for Drug GMP was promulgated by the SFDA on September 7, 2005 and became effective on October 1, 2005. A pharmaceutical manufacturer must meet the GMP standards and obtain the GMP Certificate with a five-year validity period from SFDA. Before the GMP Certification expires, the pharmaceutical manufacturer must apply again and complete the relevant procedures, which may take about 120 working days, to obtain a new GMP Certificate. On October 24, 2007, the SFDA issued new guidelines for authentication standards of GMP, effective as of January 1, 2008. The new guideline may result in a rise of cost for a pharmaceutical manufacturer to meet the new standards in order to maintain the GMP qualification. If a pharmaceutical manufacturer fails to obtain or maintain GMP Certification and still carries on production of its drugs, it will be fined and its Pharmaceutical Manufacturing Permit may be revoked under serious circumstances. We are required to apply for a GMP certificate for each of our products and reapply prior to the expiration date and maintain our Pharmaceutical Manufacturing Permit.
|
|
Administration Regulations for Drug Call-back was promulgated by the SFDA on December 10, 2007 and effective on the same day. According to the Administration Regulations for Drug Call-back, the pharmaceutical manufacturer should establish a drug call-back system and collect information regarding the drug safety. If a manufacturer discovers any unreasonable danger of drug that threatens people’s safety and health, it should immediately stop the manufacturing and sale of such drug, notify the distributors and report to the branch of the SFDA. This regulation also stipulates the procedures of drug call-back and danger valuation standards established and maintain a drug call back system in conformance the regulations.
|
|
Administration Regulations for Drug Instructions and Labels was promulgated by the SFDA on March 15, 2006 and was effective as of June 1, 2006. According to Administration Regulations for Drug Instructions and Labels, the contents of instructions and labels of each drug must be approved by the SFDA, and the smallest packing unit of drug shall be attached with instruction. We have developed, received approval and maintain drug labeling in conformance with the regulations for our existing products and must do so for new products.
|
|
Supervision Administration Regulations for Drug Distribution was promulgated by the SFDA on January 31, 2007 and effective as of May 1, 2007. According to Supervision Administration Regulations for Drug Distribution, a pharmaceutical manufacturer can only sell drugs produced by itself, and it shall not sell drugs produced by other manufacturers or produced by itself but for commissioning manufacturing purposes. We do not resell drugs from any other pharmaceutical manufacturers.
|
|
Regulations for Drug Advertisement Censoring was promulgated by the SFDA and State Administration for Industry and Commerce (the “SAIC”) on March 13, 2007 and effective as of May 1, 2007. The Standards for Drug Advertisement Censoring and Publication was promulgated by the SFDA and the SAIC on March 3, 2007 and made effective as of May 1, 2007. According to Regulations for Drug Advertisement Censoring, a pharmaceutical manufacturer must obtain a Drug Advertisement Approval Number from the provincial branch of the SFDA which is valid period of one year if the drug advertisement describes the functions or benefits of a drug. However, if an over the counter drug advertisement in any media, or a prescription drug advertisement in professional medical magazine, only refers to the name of the drug, including the general name and commercial name, without any other addition promotional information, the advertisement does not need to be censored or approved. We have obtained a Drug Advertisement Approval Number for all our drugs and review all of our OTC drug advertisements so that they are in conformance with the regulations relating to advertising these products.
|
|
|
Food Hygiene Law and Rules on Food Hygiene Certification mandates that a distributor of nutritional supplements and other food products must obtain a food hygiene certificate from relevant provincial or local health regulatory authorities. The grant of such certificate is subject to an inspection of the distributor’s facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. The food hygiene certificate is valid for four years, and the holder must apply for renewal of the certificate within six months prior to its expiration.
|
|
We have enjoyed a sound, cooperative working relationship with the Shaanxi People's Government and related government departments since our founding. Adjustments to our operating strategies and long-term business plans have been unanimously approved by relevant departments and by provincial-level government entities.
The SFDA
The application and approval procedure in the PRC for a newly developed drug has numerous steps. For each new product, we prepare documentation covering pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to the provincial SFDA. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial SFDA, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation and tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years.
The SFDA and the China Traditional Medicine Administration Bureau regulate the process for new drug approval and licensing in the PRC, which can involve many levels of authority, lacking in transparency, and presents one of the greatest obstacles for companies to introduce new drugs into the market. One of the preliminary aspects of the application process involves a review of the Chinese market's need for a particular drug. If the SFDA determines that the market niche for a particular drug is saturated, the drug will not receive further consideration and the licensing application will be denied. According to industry analysts, eighty-five percent of applications for new drugs licensing is determined by SFDA to be in saturated markets and thus are not considered for approval. Only fifteen percent of new-to-market drug applications are considered for approval by the SFDA.
Furthermore, only companies that meet the GMP standard may apply for new drug approvals with the SFDA. The SFDA estimates that less than 20% out of the 6,000 pharmaceutical companies in the PRC currently meet the GMP standard.
We estimate that the cost to receive approval from the SFDA for a new product will range from 1 million RMB to 4.5 million RMB.
Our receipt of a GMP certificate and approval by the SFDA of our prescription and OTC drugs represent a significant competitive advantage as these approvals present a significant barrier to entry by new companies hoping to enter the pharmaceutical drug industry.
Nevertheless, the new drugs we seek to bring to market are regulated by the SFDA and the China Traditional Medicine Administration Bureau and are estimated to now cost between 1.1 million RMB (approximately $158,000) to 4.15 RMB (approximately $593,000) per product which must be provided through our cash flow or from financing activities as new products are introduced. In addition, our new products may not pass the clinical review and testing process which can negatively affect our cash flow and income.
We are subject to possible administrative and legal proceedings and actions by these various regulatory bodies. Such actions may include product recalls, seizures and other civil and criminal sanctions which could have a materially adverse effect on our prospects.
Environmental Regulation
Our operations and facilities are subject to environmental laws and regulations stipulated by the national and the local environment protection bureaus in the PRC. Relevant laws and regulations include provisions governing air emissions, water discharge and the management and disposal of hazardous substances and wastes. The PRC regulatory authorities require pharmaceutical companies to carry out environmental impact studies before engaging in new construction projects to ensure that their production processes meet the required environmental standards.
We maintain controls at our production facilities to facilitate compliance with environmental rules and regulations. We are not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor have we been subject to any action by any environmental administration authorities of the PRC. To our knowledge, our operations meet or exceed the existing requirements of the PRC.
Advertising Laws
Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements (tentative) from State Council provide guidelines for advertising prescription and OTC drugs and nutrients. The rules limit where advertisements may be placed and govern the claims that may be made by the manufacturer.
Product Liability and Consumers Protection
Product liability claims may arise if the products sold have any harmful effect on the consumers. The injured party may make a claim for damages or compensation. The General Principles of the Civil Law of the PRC, which became effective in January 1987, state that manufacturers and sellers of defective products causing property damage or injury shall incur civil liabilities for such damage or injuries.
The Product Quality Law of the PRC was enacted in 1993 and amended in 2000 to strengthen the quality control of products and protect consumers’ rights and interests. Under this law, manufacturers and distributors who produce or sell defective products may be subject to confiscation of earnings from such sales, revocation of business licenses and imposition of fines, and in severe circumstances, may be subject to criminal liability.
The Law of the PRC on the Protection of the Rights and Interests of Consumers was promulgated on October 31, 1993 and became effective on January 1, 1994 to protect consumers’ rights when they purchase or use goods or services. All business operators must comply with this law when they manufacture or sell goods and/or provide services to customers. In extreme situations, pharmaceutical product manufacturers and distributors may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties.
Circular 106
On May 31, 2007, China’s State Administration of Foreign Exchange (“SAFE”) issued an official notice known as “Circular 106”, which requires the owners of any Chinese companies to obtain SAFE’s approval before establishing any offshore holding company structure in so-called “round-trip” investment transactions for foreign financing as well as subsequent acquisition matters in China. Likewise, the “Provisions on Acquisition of Domestic Enterprises by Foreign Investors”, issued jointly by Ministry of Commerce (“MOFCOM”), State-owned Assets Supervision and Administration Commission, State Taxation Bureau, State Administration for Industry and Commerce, China Securities Regulatory Commission and SAFE in September 2006, impose approval requirements from MOFCOM for “round-trip” investment transactions, including acquisitions in which equity was used as consideration.
Dividend Distribution
The principal laws, rules and regulations governing dividends paid by our PRC affiliated entities include the Company Law of the PRC (1993), as amended in 2006, Wholly Foreign Owned Enterprise Law (1986), as amended in 2000, and Wholly Foreign Owned Enterprise Law Implementation Rules (1990), as amended in 2001. Under these laws and regulations, each of our consolidated PRC entities, including wholly foreign owned enterprises, or WFOEs, and domestic companies in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, each of our consolidated PRC entities, including WFOEs and domestic companies, is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its statutory surplus reserve fund until the accumulative amount of such reserve reaches 50% of its respective registered capital. These reserves are not distributable as cash dividends. As of December 31, 2009, the accumulated balance of our statutory reserve funds reserves amounted to RMB 20.6 million (US$2.9 million) and the accumulated profits of our consolidated PRC entities that were available for dividend distribution amounted to RMB 166.5 million (US$23.3 million).
Taxation
The recently enacted PRC Enterprise Income Tax Law, or the EIT Law, and the implementation regulations for the EIT Law issued by the PRC State Council, became effective as of January 1, 2008. The EIT Law provides that enterprises established outside of China whose “de facto management bodies” are located in China are considered “resident enterprises” and are generally subject to the uniform 25% enterprise income tax rate as to their worldwide income. Under the implementation regulations for the EIT Law, “de facto management body” is defined as a body that has material and overall management and control over the manufacturing and business operations, personnel and human resources, finances and treasury, and acquisition and disposition of properties and other assets of an enterprise. Although substantially all of our operational management is currently based in the PRC, it is unclear whether PRC tax authorities would treat us as a PRC resident enterprise.
Under the EIT Law and implementation regulations, PRC income tax at the rate of 10% is applicable to dividends payable to investors that are “non-resident enterprises,” which do not have an establishment or place of business in the PRC, or which have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC. Similarly, any gain realized on the transfer of shares by such investors is also subject to 10% PRC income tax if such gain is regarded as income derived from sources within the PRC. If we are considered a PRC “resident enterprise,” it is unclear whether dividends we pay with respect to our common shares, or the gain you may realize from the transfer of our common shares, would be treated as income derived from sources within the PRC and be subject to PRC income tax. It is also unclear whether, if we are considered a PRC “resident enterprise,” holders of our common shares might be able to claim the benefit of income tax treaties entered into between China and other countries.
Price Controls
The retail prices of some pharmaceutical products sold in China, primarily those included in the national and provincial medical insurance catalogs and those pharmaceutical products whose production or distribution are deemed to constitute monopolies, are subject to price controls in the form of fixed prices (for non-profit medical institutions) or price ceilings. Manufacturers or distributors cannot freely set or change the retail price for any price-controlled product above the applicable price ceiling or deviate from the applicable fixed price imposed by the PRC government. The prices of medicines that are not subject to price controls are determined freely at the discretion of the respective pharmaceutical companies, subject to notification to the provincial pricing authorities.
The retail prices of medicines that are subject to price controls are administered by the Price Control Office of the National Development and Reform Commission (“NDRC”), and provincial and regional price control authorities. The retail price, once set, also effectively determines the wholesale price of that medicine. From time to time, the NDRC publishes and updates a list of medicines that are subject to price control. Fixed prices and price ceilings on medicine are determined based on profit margins that the relevant government authorities deem reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicine and the extent of the manufacturer’s compliance with the applicable Good Manufacturing Practice (“GMP”) standards. The NDRC directly regulates the pricing of a portion of the medicine on the list, and delegates to provincial and regional price control authorities the authority to regulate the pricing of the rest of the medicine on the list. Provincial and regional price control authorities have discretion to authorize price adjustments based on the local conditions and the level of local economic development. Currently, approximately 2,014 pharmaceutical products are subject to price controls. The price controls of all of those pharmaceutical products are administered by the NDRC.
Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine, and it must either apply to the provincial price control authorities in the province where it is incorporated, if the medicine is provincially regulated, or to the NDRC, if the medicine is NDRC regulated. For a provincially regulated medicine, in cases where provincial price control authorities approve an application, manufacturers must file the newly approved price with the NDRC for record and thereafter the newly approved price will become binding and enforceable across China.
Since May 1998, the PRC government has been ordering reductions in the retail prices of various pharmaceutical products. The latest price reduction occurred in October 2008. As of December 31, 2009, only one of our pharmaceutical products was subject to price controls. Price controls, however, have had no significant impact on our operations as our price points have historically been substantially below such government-imposed ceilings.
The NDRC may grant premium pricing status to certain pharmaceutical products that are under price control. The NDRC may set the retail prices of pharmaceutical products that have obtained premium pricing status at a level that is significantly higher than comparable products.
Research and Development
We currently have 10 potential products in the research and development pipeline. Identified compounds are currently being tested for indications related to neoplastic disease, central nervous system disease, an anti-infection medicine, kidney tonifying medicine and sterility. We anticipate we will be able to introduce three to five new products to market each year.
In addition to the work being done in our in-house research department, we are working with prestigious Chinese universities and research institutes in the PRC to develop effective, high margin products. Specifically:
On June 13, 2006, Aoxing Pharmaceutical entered into a 2-year technological cooperation agreement with Shaanxi University of Science and Technology (“Shaanxi University”) under which Shaanxi University agreed to provide interns to assist with our product development for payment from us of RMB 600 per month to the interns. In fiscal year 2008, we paid the interns a total of 3,000 RMB in the form of subsidies. Additionally, Shaanxi University agreed to provide advisory educational services to improve our pharmaceutical production techniques. We are authorized to use the education material in our production process but do not own the educational materials. Shaanxi University also agreed to assist us in developing improved production techniques for new drugs, the ownership of which shall be held by Aoxing Pharmaceutical. The fees to be paid to Shaanxi University for new drug development will be made under a separate agreement, although there is currently no funding requirement. On June 18, 2008, we extended the technological cooperation agreement with Shaanxi University for two more years.
On September 10, 2006, Aoxing Pharmaceuticals entered into a 2-year technological cooperation agreement with the College of Life Sciences of Northwest University (“Northwest University”), pursuant to which we agreed to make our facilities available for practical studies for interns from Northwest University, and to hire between 30 to 40 interns at a cost of RMB 600 per month per intern, not to exceed a total of RMB 4,000 per month. In return, Northwest University agreed to assign its personnel to teach our staff various agricultural sciences associated with growing plants and herbs used in traditional Chinese medicines (“TCM”). We are authorized to use the education material in our production process but do not own the educational materials. In addition, the parties agreed to cooperate on the development of new TCM, the ownership of which will be held by us. The fees to be paid Northwest University for new drug development will be made under a separate agreement, although we have currently not entered into any such agreement. On September 18, 2008, we renewed the technological cooperation agreement with Northwest University for two more years.
On January 5, 2007, Aoxing Pharmaceutical entered into a cooperation agreement with Xianyang Material Medical Institute (“Xianyang Institute”) for the development of a new drug called Zenbaowan Capsule. Under the agreement, Xianyang Institute is responsible for the research and development of the new drug in compliance with the PRC Drug Administration Law and the Administration Regulations for Drug Registration, as well as the SFDA application process for, the new drug. In addition, the parties agreed to long term technical cooperation on products mutually identified in the future under the terms of separate agreements. Any product developed by Xianyang Institute under this agreement, and the intellectual property rights related thereto, will be owned by us. We agreed to pre-pay all application expenses and to pay Xianyang Institute the aggregate consideration of RMB 180,000 (approximately $24,290), of which 50% will be paid on the first day that Zenbaowan Capsule passes the first materials and production site examinations by the SFDA, and 50% upon accreditation and receipt of the drug approval number from the SFDA. The agreement can be terminated by either party without notice. No payments have been made to date.
We spent approximately 220,001 RMB in fiscal year 2008 and 3,500,000 RMB in fiscal year 2009 for research and development. We anticipate spending approximately 5,000,000 RMB for research and development in fiscal year 2010.
Employees
As of March 24, 2010, we had a total of about 530 full time employees who receive labor insurance. These employees are organized into a union under the labor laws of the PRC and can bargain collectively with us. We maintain good relations with our employees.
We are required to contribute a portion of our employees' total salaries to the Chinese government's social insurance funds, including medical insurance and unemployment insurance and to purchase job injury insurance for employees, in accordance with relevant regulations. The government's social insurance funds account for 20% of employees' total salaries. The job injury insurance premium is about RMB 50 (approximately US$7) per person. We expect the amount of our contributions to the government's social insurance funds and the cost related to job injury insurance to increase in the future as we expand our workforce and operations.
Seasonality of Sales
Sales in the first quarter are usually lower due to people traveling and taking vacations during the traditional Chinese New Year and Chinese Spring Festival holidays. Sales in the fourth quarter are usually higher. Sales in the first and fourth quarters of fiscal 2006 to 2008 averaged approximately 15.5% and 32.3% of total sales for those periods, respectively, and approximately 14.0% and 32.0% for those periods in fiscal 2009, respectively. This reflects the seasonal nature of our sales.
ITEM 1A. RISK FACTORS
Risks Relating to Our Business
Our operating history may not serve as an adequate basis to judge our future prospects and results of operations.
Aoxing Pharmaceutical commenced its current line of business operations in 1997 and received its Good Manufacturing Practices (“GMP”) certification in January 2006, which must be renewed every five years for Aoxing Pharmaceutical to stay in business. Aoxing Pharmaceutical’s operating history may not provide a meaningful basis on which to evaluate its business. We cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties frequently experienced by companies at a similar stage of development, including our potential failure to:
|
·
|
raise adequate capital for expansion and operations;
|
|
·
|
implement our business model and strategy and adapt and modify them as needed;
|
|
·
|
increase awareness of our brand name, protect our reputation and develop customer loyalty;
|
|
·
|
manage our expanding operations and service offerings, including the integration of any future acquisitions;
|
|
·
|
maintain adequate control of our expenses; or
|
|
·
|
anticipate and adapt to changing conditions in the medical over the counter, pharmaceutical and nutritional supplement markets in which we operate as well as the impact of any changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
|
If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
The loss of Aoxing Pharmaceutical as our operating business would have a material adverse effect on our business and the price of our common stock.
We have no equity ownership interest in Aoxing Pharmaceutical. Our ability to control Aoxing Pharmaceutical and consolidate its financial results is through a series of contractual arrangements between it and our wholly owned subsidiary Shaanxi Biostar. Management of Aoxing Pharmaceutical is an affiliate of us and of Shaanxi Biostar and the stockholders of Aoxing Pharmaceutical are also our stockholders. Thus the contractual arrangements were not entered into as a result of arms’ length negotiations because the parties to such agreements are under common control. Mr. Wang, our chief executive officer and chairman, holds approximately 45.31% of the shares of Aoxing Pharmaceutical and 38.58% of our common stock. While we have been advised by our PRC counsel that the contractual arrangements are legal and enforceable under PRC law, these affiliates control the parties to the contractual arrangements, and it could be possible for them to cause Aoxing Pharmaceutical and its shareholders to breach the contractual arrangements, in which event our unaffiliated investors would have little or no recourse because of the inherent difficulties in enforcing their rights since all our assets are located in the PRC. (See, Risk Factor “The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.”) In the event that management of Aoxing Pharmaceutical decides to cause a breach the contractual arrangements, the risk of loss for the affiliated shareholders of Aoxing Pharmaceutical could be lower than that for the unaffiliated investors, and the interests of the management and shareholders of Aoxing Pharmaceutical would be in conflict with the interest of our other stockholders.
Our failure to compete effectively may adversely affect our ability to generate revenue.
We compete with other companies, many of whom are developing or can be expected to develop products similar to ours. Many of our competitors are also more established than we are, and have significantly greater financial, technical, marketing and other resources than we presently possess. Some of our competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
We may not be able to effectively control and manage our growth.
If our business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. An expansion would increase demands on existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect our operations, cause delay in production and delivery of our products, and increase administrative inefficiencies.
We may require additional financing in the future and a failure to obtain such required financing will inhibit our ability to grow.
The continued growth of our business may require additional funding from time to time, which we expect to raise in private placements of our equity or debt securities with accredited investors or by offering our securities for sale pursuant to an effective registration statement on a market where our common stock is traded. The proceeds of these funding will be forwarded to Aoxing Pharmaceutical and accounted for as a loan to Aoxing Pharmaceutical and eliminated during consolidation. The proceeds would be used for general corporate purposes of Aoxing Pharmaceutical, which could include acquisitions, investments, repayment of debt and capital expenditures among other things. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Obtaining additional funding would be subject to a number of factors including market conditions, operating performance and investor sentiment, many of which are outside of our control. These factors could make the timing, amount, terms and conditions of additional funding unattractive or unavailable to us. Our management believes that we currently have sufficient funds from working capital to meet our current operating costs over the next 12 months.
The terms of any future financing may adversely affect your interest as stockholders.
If we require additional financing in the future, we may be required to incur indebtedness or issue equity securities, the terms of which may adversely affect your interests in us. For example, the issuance of additional indebtedness may be senior in right of payment to your shares upon our liquidation. In addition, indebtedness may be under terms that make the operation of Aoxing Pharmaceutical's business more difficult because the lender's consent could be required before we take certain actions. Similarly the terms of any equity securities we issue may be senior in right of payment of dividends to your common stock and may contain superior rights and other rights as compared to your common stock. Further, any such issuance of equity securities may dilute your interest in us.
We may engage in future acquisitions that could dilute the ownership interests of our stockholders, cause us to incur debt and assume contingent liabilities.
We may review acquisition and strategic investment prospects that we believe would complement our current product offerings, augment our market coverage or enhance our technical capabilities, or otherwise offer growth opportunities. From time to time we review investment opportunities in new businesses and we expect to make investments in, and to acquire, businesses, products, or technologies in the future. We expect that when we raise funds from investors for any of these purposes we will be either the issuer or the primary obligor while the proceeds will be forwarded to Aoxing Pharmaceutical and accounted for as a loan to Aoxing Pharmaceutical and eliminated during consolidation. In the event of any future acquisitions, we could:
|
·
|
issue equity securities which would dilute current stockholders’ percentage ownership;
|
|
·
|
incur substantial debt;
|
|
·
|
assume contingent liabilities; or
|
|
·
|
expend significant cash.
|
These actions could have a material adverse effect on our operating results or the price of our common stock. Moreover, even if we do obtain benefits in the form of increased sales and earnings, there may be a lag between the time when the expenses associated with an acquisition are incurred and the time when we recognize such benefits. Acquisitions and investment activities also entail numerous risks, including:
|
·
|
difficulties in the assimilation of acquired operations, technologies and/or products;
|
|
·
|
unanticipated costs associated with the acquisition or investment transaction;
|
|
·
|
the diversion of management’s attention from other business concerns;
|
|
·
|
adverse effects on existing business relationships with suppliers and customers;
|
|
·
|
risks associated with entering markets in which Aoxing Pharmaceutical has no or limited prior experience;
|
|
·
|
the potential loss of key employees of acquired organizations; and
|
|
·
|
substantial charges for the amortization of certain purchased intangible assets, deferred stock compensation or similar items.
|
We cannot ensure that we will be able to successfully integrate any businesses, products, technology, or personnel that we might acquire in the future, and our failure to do so could have a material adverse effect on our business, operating results and financial condition.
We are responsible for the indemnification of our officers and directors.
Our certificate of incorporation provides for the indemnification and/or exculpation of our directors, officers, employees, agents and other entities which deal with it to the maximum extent provided, and under the terms provided, by the laws and decisions of the courts of the state of Maryland. Since we do not hold any indemnification insurance, these indemnification provisions could result in substantial expenditures, which we may be unable to recoup, which could adversely affect our business and financial conditions. Ronghua Wang, our chairman, president and chief executive officer, Elaine Zhao, our chief financial officer, Shuang Gong, our corporate secretary, Amei Zhang, our chief operating officer and Qinghua Liu, Haipeng Wu, Zibing Pan and Zhongyang Shang, our directors, are key personnel with rights to indemnification under our certificate of incorporation.
We may not have adequate internal accounting controls. While we have certain internal procedures in our budgeting, forecasting and in the management and allocation of funds, our internal controls may not be adequate.
We are constantly striving to improve our internal accounting controls. We expect to continue to improve our internal accounting control for budgeting, forecasting, managing and allocating our funds and to better account for them as we grow. There is no guarantee that such improvements will be adequate or successful or that such improvements will be carried out on a timely basis. If we do not have adequate internal accounting controls, we may not be able to appropriately budget, forecast and manage our funds, we may also be unable to prepare accurate accounts on a timely basis to meet our continuing financial reporting obligations and we may not be able to satisfy our obligations under US securities laws.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require every public company to include a management report on such company’s internal controls over financial reporting in its annual report, which contains management’s assessment of the effectiveness of our internal controls over financial reporting. The standards that must be met for management to assess the internal control over financial reporting as effective are new and complex, and require significant documentation, testing and possible remediation to meet the detailed standards. Some members of our management team have limited or no experience operating a public company, or subject to SEC rules and requirements, including SEC reporting practices and requirements that are applicable to a public company. While we are in the process of engaging a consulting firm to evaluate and assist us with implementing a viable internal control system, our lack of familiarity with Section 404 may nevertheless unduly divert management's time and resources in executing the business plan. Effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent fraud. So far, our external auditors have not reported to our board of directors any significant weakness on our internal control and provided recommendations accordingly. Nevertheless, our failure to achieve and maintain effective internal controls over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business and negatively impact the trading price of our stock. Furthermore, we anticipate that we will incur considerable costs and use significant management time and other resources in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
We are dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and pharmaceutical factory operational expertise of key personnel. We are dependent upon the services of Mr. Wang, our president, chief executive officer and chairman, for the continued growth and operation of our Company because of his experience in the industry and his personal and business contacts in the PRC. We do not have an employment agreement with Mr. Wang and do not anticipate entering into an employment agreement in the foreseeable future. Although we have no reason to believe that Mr. Wang will discontinue his services with us or Aoxing Pharmaceutical, the interruption or loss of his services would adversely affect our ability to effectively run our business and pursue our business strategy as well as our results of operations.
Additionally, Elaine Zhao, our chief financial officer, Amei Zhang, our chief operating officer, Shuang Gong, our corporate secretary, Yuan Jian, general manager and chief engineer of Aoxing Pharmaceutical, perform key functions in the operation of our business. There can be no assurance that we will be able to retain these officers after the term of their employment contracts expire. The loss of these officers could have a material adverse effect upon our business, financial condition, and results of operations. We do not carry key man life insurance for any of our key personnel or personnel nor do we foresee purchasing such insurance to protect against a loss of key personnel and the key personnel.
We may not be able to hire and retain qualified personnel to support its growth and if it is unable to retain or hire these personnel in the future, its ability to improve its products and implement its business objectives could be adversely affected.
We must attract, recruit and retain a sizeable workforce of technically competent employees. Competition for senior management and senior personnel in the PRC is intense, the pool of qualified candidates in the PRC is very limited, and we may not be able to retain the services of our senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. This failure could materially and adversely affect our future growth and financial condition. We expect to hire additional sales and plant personnel throughout fiscal year 2010 in order to accommodate our growth.
If we fail to increase our brand recognition, we may face difficulty in obtaining new customers and business partners.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future products and services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and ability to provide reliable and useful products and services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we will incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition, would be materially adversely affected.
Our operating results may fluctuate as a result of factors beyond our control.
Our operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are beyond our control. These factors include:
|
·
|
the costs of pharmaceutical products and development;
|
|
·
|
the relative speed and success with which we can obtain and maintain customers, merchants and vendors for our products;
|
|
·
|
capital expenditure for equipment;
|
|
·
|
marketing and promotional activities and other costs;
|
|
·
|
changes in our pricing policies, suppliers and competitors;
|
|
·
|
the ability of our suppliers to provide products in a timely manner to their customers;
|
|
·
|
changes in operating expenses;
|
|
·
|
increased competition in the pharmaceutical markets; and
|
|
·
|
other general economic and seasonal factors.
|
We face risks related to product liability claims.
We presently do not maintain product liability insurance. We face the risk of loss because of adverse publicity associated with product liability lawsuits, whether or not such claims are valid. We may not be able to avoid such claims. Although product liability lawsuits in the PRC are rare, and we have not, to date, experienced significant failure of our products, there is no guarantee that we will not face such liability in the future. This liability could be substantial and the occurrence of such loss or liability may have a material adverse effect on our business, financial condition and prospects.
We face marketing risks.
Newly developed drugs and technology may not be compatible with market needs. Because markets for drugs differentiate geographically inside the PRC, we must develop and manufacture our products to accurately target specific markets to ensure product sales. If we fail to invest in extensive market research to understand the health needs of consumers in different geographic areas, we may face limited market acceptance of our products, which could have material adverse effect on our sales and earnings.
We face risks relating to difficulty in defending intellectual property rights from infringement.
Our success depends on protection of our current and future technology and products and our ability to defend our intellectual property rights. We have filed for trademark protection for the various names and brands of our products sold in the PRC. We have also filed for patent protection on three of our products, one of which has been approved. However, it is possible for its competitors to develop similar competitive products even thought it has taken steps to protect its intellectual property. If we fail to protect our intellectual property adequately, competitors may manufacture and market products similar to ours. We expect to file patent applications seeking to protect newly developed technology and products in various countries, including the PRC. Some patent applications in the PRC are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of our discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for our products. Patents that are issued may be challenged, invalidated or circumvented by our competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
We face risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may be required to commence or defend against charges relating to the infringement of patent or proprietary rights. Any such litigation could:
|
·
|
require us to incur substantial expense, even if covered by insurance or are successful in the litigation;
|
|
·
|
require us to divert significant time and effort of our technical and management personnel;
|
|
·
|
result in the loss of our rights to develop or make certain products; and
|
|
·
|
require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties.
|
Although patent and intellectual property disputes within the pharmaceutical industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling some of our products or increase our costs to market these products.
In addition, when seeking regulatory approval for some of our products, we may be required to certify to regulatory authorities, including the SFDA that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against us. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
Our launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to us. If we are found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of our operations and financial condition.
We face risks related to research and the ability to develop new drugs.
Our growth and survival depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technology and platforms. As such, if we fail to make sufficient investments in research, be attentive to consumer needs or does not focus on the most advanced technology, our current and future products could be surpassed by more effective or advanced products of other companies.
We rely on a small number of important customers for a large portion of our sales.
Sales to Guangdong Run Tai Medicine Wholesale Co., Ltd. accounted for 11.8%, 6.1% and 4.3% of our gross sales for fiscal 2007, 2008 and 2009, respectively, If such major customer or others were to become unable or unwilling to continue purchasing our products on the scale of their recent purchases, our revenue and competitive position could be harmed.
Risk Related To the PharmaceuticalIndustry
Our certificates, permits, and licenses related to our pharmaceutical operations are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
Aoxing Pharmaceutical is subject to various PRC laws and regulations pertaining to the pharmaceutical industry. Aoxing Pharmaceutical has attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC.
In 1998, the State Food and Drug Administration of the PRC (“SFDA”) introduced the Good Manufacturing Practice (GMP) Certificate in order to promote quality and safety of pharmaceutical production. The Good Manufacturing Practices were revised in July and October, 2004. We and our competitors are required to meet GMP standards in order to continue manufacturing pharmaceutical products and health foods. For each new product, Aoxing Pharmaceutical prepares documentation of pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to provincial food and drug administration. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial food and drug administration, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation and tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years
Aoxing Pharmaceutical initially obtained pharmaceutical products and health food production permits by submitting its manufacturing processes and product tests to the SFDA who verified that its production processes and products met the standards by onsite inspections, review of test results and a determination that the market was not saturated by its products. The production permits are permanent once issued as long as they are renewed by the expiration date.
The GMP certificate is valid for a term of five years, the pharmaceutical products production permits are subject to renewal every five years, and the health food production permits are valid for three year terms, and each must be renewed before its expiration, if applicable. Aoxing Pharmaceutical originally obtained its GMP certificate in January 2006, and it is valid until January 23, 2011. The GMP certificate applies to products described as medicinal tablets, granules, capsules, soft capsules, powder, and ointment. If the GMP certificate expires without renewal, Aoxing Pharmaceutical will not be able to continue production of pharmaceutical products, which will cause its operations to terminate. We intend to renew the GMP certificate before its current expiration date.
Aoxing Pharmaceutical holds five production permits for its five OTC and prescription drugs, which expire as follows:
|
Pharmaceutical Products
|
Expires on
|
|
|
Xin Ao Xing Oleanlic Acid Capsule
|
January 28, 2013
|
|
|
Ganwang (Compound Paracetamol Capsule)
|
October 15, 2012
|
|
|
Danshen granule
|
October 15, 2012
|
|
|
Taohuasan (Pediatric Medicine)
|
September 12, 2012
|
|
|
Tianqi Dysmenorrhea Capsule
|
September 12, 2012
|
Aoxing Pharmaceutical also holds numerous production permits for its nutrient products, which expire as follows:
|
Nutrient Products
|
Expires on
|
|
|
Ao Xing No.1
|
August 24, 2010
|
|
|
Yanshuang
|
August 4, 2010
|
|
|
New Chakederi
|
October 16, 2010
|
|
|
Tiantianle
|
June 28, 2010
|
|
|
AoXing Gan Bao
|
June 28, 2010
|
|
|
Fengningdan
|
April 8, 2011
|
|
|
Heigen
|
May 28, 2010
|
|
|
Baitongning
|
July 14, 2010
|
|
|
Sukang Capsule
|
July 28, 2010
|
|
|
Aoxing Ointment
|
September 14, 2010
|
We intend to apply for renewal of these health food production permits prior to expiration. During the renewal process, Aoxing Pharmaceutical will be re-evaluated by the appropriate governmental authorities and must comply with the then prevailing standards and regulations which may change from time to time. In the event that it is not able to renew the certificates, permits and licenses, all or part of its operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of its operations, it may adversely affect its operation and our profitability.
According to Drug Administration Law of the PRC and its implementing rules, the SFDA approvals, including Pharmaceutical Manufacturing Permit and Drug Approval Numbers, may be suspended or revoked prior to the expiration date under circumstances that include:
|
·
|
producing counterfeit medicine;
|
|
·
|
producing inferior quality products;
|
|
·
|
failing to meet the drug GMP standards;
|
|
·
|
purchasing medical ingredients used in the production of products sources that do not have Pharmaceutical Manufacturing Permit or Pharmaceutical Trade Permit;
|
|
·
|
fraudulent reporting of results or product samples in application process;
|
|
·
|
failing to meet drug labeling and direction standards;
|
|
·
|
bribing doctors or hospital personnel to entice them to use products,
|
|
·
|
producing pharmaceuticals for use or resale by companies that are not approved by the SFDA, or
|
|
·
|
the approved drug has a serious side effect.
|
If our pharmaceutical products fail to receive regulatory approval or are severely limited in these products' scope of use, we may be unable to recoup considerable research and development expenditures.
Our research and development of pharmaceutical products is subject to the regulatory approval of the SFDA. The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of resources not currently available; in such an event, it may be necessary for us to abandon our application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use. If approval of our product is denied, abandoned, or severely limited in terms of the scope of products use, it may result in the inability to recoup considerable research and development expenditures. Currently, three of our products, Niao Sai Tong, Gan Fu Kang and Azithromycin Dispersible Tablets, have pending applications with the SFDA. Phase II clinical testing is currently occurring for six other products (Shenrong Capsules, Zhixuening Pian, Xiao'aiping Dispersible Tablets, Zhenbao Wan Capsules, Hua Zhi Pian, and KunLing Wan Capsules), which is expected to be completed sometime in 2012 to 2015. After phase II clinical test, these products will need to go through a phase III clinical test before they can be submitted for SFDA approval. We expect phase III clinical test for all six products will be completed sometime in 2015 to 2016. If we do not receive timely approval for any of these drugs, then production will be delayed and sales of the products cannot be planned for.
Price control regulations may decrease our profitability.
The laws of the PRC provide for the government to fix and adjust prices. The prices of certain medicines we distribute, including those listed in the Chinese government's catalogue of medications that are reimbursable under the PRC’s social insurance program, or the Insurance Catalogue, are subject to control by the relevant state or provincial price administration authorities. The PRC establishes price levels for products based on market conditions, average industry cost, supply and demand and social responsibility. In practice, price control with respect to these medicines sets a ceiling on their retail price. The actual price of such medicines set by manufacturers, wholesalers and retailers cannot historically exceed the price ceiling imposed by applicable government price control regulations. Although, as a general matter, government price control regulations have resulted in drug prices tending to decline over time, there has been no predictable pattern for such decreases.
For the years ended December 31, 2008 and 2009, our Danshen Granule is the only product subject to price controls which did not affect our gross profit, gross margin and net income in a material respect. It is possible that additional products may be subject to price control, or that price controls may be increased in the future. To the extent that our products are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and we may face no limitation on our costs. Further, if price controls affect both our revenue and costs, our ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
If the medicines we produce are replaced by other medicines or are removed from the PRC's insurance catalogue in the future, our revenue may suffer.
Under Chinese regulations, patients purchasing medicine listed by the central and/or provincial governments in the insurance catalogue may be reimbursed, in part or in whole, by a social medicine fund. Accordingly, pharmaceutical distributors prefer to engage in the distribution of medicine listed in the insurance catalogue. Currently, our main prescription products are listed in the insurance catalogue. The content of the insurance catalogue is subject to change by the PRC Ministry of Labor and Social Security, and new medicine may be added to the insurance catalogue by provincial level authorities as part of their limited ability to change certain medicines listed in the insurance catalogue. If the medicine we produce are replaced by other medicines or removed from the insurance catalogue in the future, our revenue may suffer.
Adverse publicity associated with our products, ingredients or network marketing program, or those of similar companies, could harm our financial condition and operating results.
The results of our operations may be significantly affected by the public's perception of our product and similar companies. This perception is dependent upon opinions concerning:
|
·
|
the safety and quality of our products and ingredients;
|
|
·
|
the safety and quality of similar products and ingredients distributed by other companies; and
|
|
·
|
our sales force.
|
Adverse publicity concerning any actual or purported failure to comply with applicable laws and regulations regarding product claims and advertising, good manufacturing practices, or other aspects of our business, whether or not resulting in enforcement actions or the imposition of penalties, could have an adverse affect on our goodwill and could negatively affect our sales and ability to generate revenue.
In addition, our consumers' perception of the safety and quality of products and ingredients as well as similar products and ingredients distributed by other companies can be significantly influenced by media attention, publicized scientific research or findings, widespread product liability claims and other publicity concerning our products or ingredients or similar products and ingredients distributed by other companies. Adverse publicity, whether or not accurate or resulting from consumers' use or misuse of our products, that associates consumption of our products or ingredients or any similar products or ingredients with illness or other adverse effects, questions the benefits of our or similar products or claims that any such products are ineffective, inappropriately labeled or have inaccurate instructions as to their use, could negatively impact our reputation or the market demand for our products.
If we fail to develop new products with high profit margins, and our high profit margin products are substituted by competitor's products, our gross and net profit margins will be adversely affected.
There is no assurance that we will be able to sustain our profit margins in the future. The pharmaceutical industry in the PRC is very competitive, and there may be pressure to reduce sale prices of products without a corresponding decrease in the price of raw materials. In addition, new products are constantly being introduced to the market. In order to increase our sales and expand our market share, we may be forced to reduce prices in the future, leading to a decrease in gross profit margin. The research and development of new products and technology is costly and time consuming, and there are no assurances that our research and development of new products will either be successful or completed within the anticipated timeframe, if ever at all. There is no assurance that our competitors' new products, technology, and processes will not render our existing products obsolete or non-competitive. To the extent that we fail to develop new products with high profit margins and our high profit margin products are substituted by competitors' products, our gross profit margins will be adversely affected.
The commercial success of our products depends upon the degree of market acceptance among the medical community and failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
The commercial success of our products depends upon the degree of market acceptance by the PRC medical community, such as hospitals and physicians. Even if our products are approved by the SFDA, there is no assurance that physicians will prescribe or recommend our products to patients. Furthermore, a product's prevalence and use at hospitals may be contingent upon its relationship with the medical community. Currently, Danshen Granule and Taohausan are only available by medical prescription. The acceptance of our products by the PRC medical community may depend upon several factors, including but not limited to, the product's acceptance by physicians and patients as a safe and effective treatment, cost effectiveness, potential advantages over alternative treatments, and the prevalence and severity of side effects. Failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
Risks Related To Doing Business In The PRC
Changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
Our business operations may be adversely affected by the current and future political environment in the PRC. The PRC has operated as a socialist state since the mid-1900s and is controlled by the PRC’s Communist Party. The Chinese government exerts substantial influence and control over the manner in which we and it must conduct our business activities. The PRC has only permitted provincial and local economic autonomy and private economic activities since 1988. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy, particularly the pharmaceutical industry, through regulation and state ownership. Our ability to operate in the PRC may be adversely affected by changes in Chinese laws and regulations, including those relating to taxation, import and export tariffs, raw materials, environmental regulations, land use rights, property and other matters. Under current leadership, the government of the PRC has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the government of the PRC will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
The PRC's economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case.
A change in policies by the PRC government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the PRC government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC's political, economic and social life.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. The PRC’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are considered a foreign person or foreign funded enterprise under PRC laws, and as a result, we are required to comply with PRC laws and regulations. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on its businesses. If the relevant authorities find that we are in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
|
·
|
levying fines;
|
|
·
|
revoking Aoxing Pharmaceutical’s business and other licenses;
|
|
·
|
requiring that we restructure our ownership or operations; and
|
|
·
|
requiring that we discontinue any portion or all of our business.
|
Among the material laws of the PRC that we are subject to are (i) the Medicine Management Law, governing the management of pharmaceutical companies, medicine production procedure and packaging, prices, (ii) the Advertisement Law , the Rules of Medicine Advertisements Management implemented by the State Administration for Industry and Commerce, and the Regulations on Control of Advertisements from the State Council, governing rules on advertising, (iii) the Standardization of the Management on the Quality of Medicine Production issued by the SFDA, providing standards for staff, plants, equipment, materials, environment and production management, (iv) the Price Law, (v) the Measurement Law, (vi) the Tax Law, (vii) the Environmental Protection Law, (viii) the Contract Law, (ix) the Patent Law, (x) the Accounting Laws and (xi) the Labor Law.
A slowdown, inflation or other adverse developments in the PRC economy may harm our customers and the demand for our services and products.
All of our operations are conducted in the PRC and all of our revenue is generated from sales in the PRC. Although the PRC economy has grown significantly in recent years, we cannot assure you that this growth will continue. A slowdown in overall economic growth, an economic downturn, a recession or other adverse economic developments in the PRC could significantly reduce the demand for our products and harm our business.
While the PRC economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth could lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may harm our profitability. In order to control inflation in the past, the PRC government has imposed controls on bank credit, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In October 2004, the People's Bank of China, the PRC's central bank, raised interest rates for the first time in nearly a decade and indicated in a statement that the measure was prompted by inflationary concerns in the Chinese economy. Repeated rises in interest rates by the central bank would likely slow economic activity in the PRC which could, in turn, materially increase its costs and also reduce demand for its products.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of the Chinese currency, the Renminbi (“RMB”), into foreign currencies and, in certain cases, the remittance of currency out of the PRC. We receive substantially all of our revenue in RMB, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of the PRC to pay capital expenses such as the repayment of bank loans denominated in foreign currencies.
The PRC government may also in the future restrict access to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
The fluctuation of the Renminbi may harm your investment.
The value of the RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC's political and economic conditions. According to the website www.xe.com, as of March 29, 2010, $1 was equal to RMB 6.82670. As we rely entirely on revenue earned in the PRC, any significant revaluation of the RMB may materially and adversely affect our cash flows, revenue and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from an offering of our securities into RMB for Aoxing Pharmaceutical’s operations, appreciation of the RMB against the U.S. dollar would diminish the value of the proceeds of the offering and this could harm Aoxing Pharmaceutical’s business, financial condition and results of operations because it would reduce the proceeds available to us for capital investment in proportion to the appreciation of the RMB. Thus if we raise 1,000,000 dollars and the RMB appreciates against the U.S. dollar by 15%, then the proceeds will be worth only RMB 5,802,695 as opposed to RMB 6,826,700 prior to the appreciation. Conversely, if we decide to convert our RMB into U.S. dollars for the purpose of making payments for dividends on our common shares or for other business purposes and the U.S. dollar appreciates against the RMB; the U.S. dollar equivalent of the RMB we convert would be reduced in proportion to the amount the U.S. dollar appreciates. In addition, the depreciation of significant RMB denominated assets could result in a charge to our income statement and a reduction in the dollar value of these assets. Thus if Aoxing Pharmaceutical has RMB 1,000,000 in assets and RMB is depreciated against the U.S. dollar by 15%, then the assets will be valued at $127,377 as opposed to $146,484 prior to the depreciation.
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the RMB to the U.S. dollar. Under the new policy, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an approximately 14.5% appreciation of the RMB against the U.S. dollar as of June 2, 2008. While the international reaction to the RMB revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the RMB against the U.S. dollar.
The State Administration of Foreign Exchange of the PRC ("SAFE") regulations regarding offshore financing activities by PRC residents which may increase the administrative burden we face. The failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
In October 2005, SAFE issued a public notice effective from November 1, 2005, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice or SAFE #75, which requires PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of the PRC, referred to as an "offshore special purpose company," for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in the PRC. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
Certain of our shareholders who may be subject to the foregoing registration requirement (including certain members of our management) have submitted their registration applications to the relevant SAFE authority as well as notified the local authority where we are domiciled of such applications. We have been advised by such SAFE authority, however, that it is unable to issue SAFE registration due to current internal policy, but may issue a confirmation acknowledging receipt of our applications in lieu thereof, and issue the SAFE registration at a later time when internal policy changes. There is no assurance, however, that we will receive such confirmation or that such confirmation, when issued, would be sufficient for compliance purpose with the SAFE notice. Additionally, we do not know when the internal policy of the relevant SAFE authority will change, if at all, and there is no assurance that when such policy changes, we will be issued SAFE registration. As such, we or our PRC resident shareholders may nevertheless be deemed in violation of SAFE #75 despite our attempt at compliance.
In the event that we or our PRC resident shareholders are deemed to be in violation of SAFE #75 despite our attempt at compliance, Shaanxi Biostar could lose the ability to remit monies outside of the PRC and would therefore be unable to pay dividends or make other distributions. Our PRC resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the PRC Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
The PRC's legal and judicial system may not adequately protect our business and operations and the rights of foreign investors.
The PRC legal and judicial system may negatively impact foreign investors. In 1982, the National People's Congress amended the Constitution of the PRC to authorize foreign investment and guarantee the "lawful rights and interests" of foreign investors in the PRC. However, the PRC's system of laws is not yet comprehensive. The legal and judicial systems in the PRC are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the PRC lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the PRC judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The PRC's legal system is based on the civil law regime, that is, it is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in Chinese enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the PRC's political, economic or social life, will not affect the PRC government's ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on our business and prospects.
The practical effect of the PRC legal system on our business operations in the PRC can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the Foreign Invested Enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate Articles and contracts to Foreign Invested Enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are qualitatively different from the general corporation laws of the United States. Similarly, the PRC accounting laws mandate accounting practices, which are not consistent with U.S. generally accepted accounting principles. PRC's accounting laws require that an annual "statutory audit" be performed in accordance with PRC accounting standards and that the books of account of Foreign Invested Enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People's Republic of China Wholly Foreign-Owned Enterprise Law requires a wholly foreign-owned enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. While the enforcement of substantive rights may appear less clear than United States procedures, the Foreign Invested Enterprises and Wholly Foreign-Owned Enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution. Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the Chinese legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of Foreign Invested Enterprises
Any recurrence of severe acute respiratory syndrome, or SARS, or another widespread public health problem, could harm our operations.
A renewed outbreak of SARS or another widespread public health problem (such as bird flu) in the PRC, where all of our revenue is derived, could significantly harm our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some of our offices that would adversely disrupt our operations. Any of the foregoing events or other unforeseen consequences of public health problems could significantly harm our operations.
Because our principal assets are located outside of the United States and most of our directors and officers reside outside of the United States, it may be difficult for you to enforce your rights based on U.S. Federal Securities Laws against us and our officers or to enforce U.S. Court Judgments against us or them in the PRC
Most of our directors and, excepting our chief financial officer, all of our officers reside outside of the United States. In addition, our operating company is located in the PRC and substantially all of our assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the U.S. Federal securities laws against us in the courts of either the U.S. or the PRC and, even if civil judgments are obtained in U.S. courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the U.S. Federal securities laws or otherwise.
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
Risks Related to our Common Stock
Our officers and directors control us through their positions and stock ownership and their interests may differ from other stockholders.
As of March 24, 2010, there were 25,820,119 shares of our common stock issued and outstanding. Our officers and directors own approximately 34.87% of our issued and outstanding common stock. Mr. Ronghua Wang, our chairman, owns approximately 34.73%of our common stock. As a result, he is able to influence the outcome of stockholder votes on various matters, including the election of directors and extraordinary corporate transactions including business combinations. Yet Mr. Wang's interests may differ from those of other stockholders. Furthermore, ownership of 34.87% of our common stock by our officers and directors reduces the public float and liquidity, and may affect the market price, of our common stock when and if our common stock becomes eligible to trade on the NASDAQ.
The full conversion and exercise of certain outstanding series B convertible preferred stock and related warrants could result in the substantial dilution of the company in terms of a particular percentage ownership in our Company as well as the book value of the common shares. The sale of a large amount of common shares received upon exercise of the warrants on the public market to finance the exercise price or to pay associated income taxes, or the perception that such sales could occur, could substantially depress the prevailing market prices for our shares.
As of March 24, 2010, there are a total of 1,467,317 series B convertible preferred stock, and 500,000 warrants outstanding with a weighted average exercise price of $3.00. In the event of conversion or exercise of these securities, a stockholder could suffer substantial dilution of his, her or its investment in terms of the percentage ownership in us as well as the book value of the common shares held. Full conversion and exercise of the outstanding series B convertible preferred stock and warrants would increase the outstanding common shares as of March 24, 2010 by approximately 7.62% to approximately 27.8 million shares.
We are not likely to pay cash dividends in the foreseeable future.
We intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate. Should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions.
Our common shares are thinly traded and, you may be unable to sell at or near ask prices or at all if you desire to liquidate your shares.
We cannot predict the extent to which an active public market for its common stock will develop or be sustained. We have applied for listing of our common stock on the NASDAQ Capital Market, but cannot assure you that this listing or listing on any other exchange will ever occur.
Our common shares have historically been sporadically or “thinly-traded” on the OTC Bulletin Board, meaning that the number of persons interested in purchasing our common shares at or near bid prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
The market price for our common stock is particularly volatile given our status as a relatively small company with a small and thinly traded “float” and lack of current revenues that could lead to wide fluctuations in our share price. The price at which you purchase our common stock may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common stock at or above your purchase price if at all, which may result in substantial losses to you.
The market for our common shares is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as noted above, our common shares are sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of our common shares are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative or “risky” investment due to our fluctuating level of revenues or profits to date and uncertainty of future market acceptance for our current and potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. The following factors may add to the volatility in the price of our common shares: actual or anticipated variations in our quarterly or annual operating results; adverse outcomes; the termination of our contractual arrangements with Aoxing Pharmaceutical; and additions or departures of our key personnel, as well as other items discussed under this “Risk Factors” section, as well as elsewhere in this registration statement. Many of these factors are beyond our control and may decrease the market price of our common shares, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common shares will be at any time, including as to whether our common shares will sustain their current market prices, or as to what effect that the sale of shares or the availability of common shares for sale at any time will have on the prevailing market price.
Stockholders should be aware that the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence of these patterns or practices could increase the volatility of our share price.
Volatility in our common share price may subject us to securities litigation.
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
Investors may have difficulty liquidating their investment because our common stock is subject to the penny Stock" rules, which require delivery of a schedule explaining the penny stock market and the associated risks before any sale.
Our common stock may be subject to regulations prescribed by the SEC relating to "penny stocks." The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price (as defined in such regulations) of less than $5 per share, subject to certain exceptions. These regulations impose additional sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 and individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 (individually) or $300,000 (jointly with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of these securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Legal remedies, which may be available to the investor, are as follows:
|
·
|
If penny stocks are sold in violation of the investor's rights listed above, or other federal or state securities laws, the investor may be able to cancel his purchase and get his money back;
|
|
·
|
If the stocks are sold in a fraudulent manner, the investor may be able to sue the persons and firms that caused the fraud for damages;
|
|
·
|
If the investor has signed an arbitration agreement, however, s/he may have to pursue a claim through arbitration.
|
If the person purchasing the securities is someone other than an accredited investor or an established customer of the broker-dealer, the broker-dealer must also approve the potential customer's account by obtaining information concerning the customer's financial situation, investment experience and investment objectives. The broker-dealer must also make a determination whether the transaction is suitable for the customer and whether the customer has sufficient knowledge and experience in financial matters to be reasonably expected to be capable of evaluating the risk of transactions in such securities. Accordingly, the SEC's rules may limit the number of potential purchasers of the shares of our common stock and stockholders may have difficulty selling their securities.
A large number of shares will be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them.
Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
We are authorized to issue "blank check" preferred stock, which, if issued without stockholders approval, may adversely affect the rights of holders of our common stock.
We are authorized to issue 10,000,000 shares of preferred stock, of which 5,000,000 shares have been designated as series A preferred stock, and 5,000,000 as series B preferred stock. As of March 24, 2010, there were no shares of series A preferred stock, and 1,464,317 shares of series B preferred stock, issued and outstanding. Our board of directors is authorized under our Articles of Incorporation, as amended, to provide for the issuance of additional shares of preferred stock by resolution, and to fix the designation, powers, preferences and rights of the shares of each such series and the qualifications, limitations or restrictions thereof without any further vote or action by the stockholders. Any shares of preferred stock so issued are likely to have priority over the common stock with respect to dividend or liquidation rights. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control, which could have the effect of discouraging bids for our company and thereby prevent stockholders from receiving the maximum value for their shares. We have no present intention to issue any shares of our preferred stock in order to discourage or delay a change of control. However, there can be no assurance that preferred stock will not be issued at some time in the future.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. DESCRIPTION OF PROPERTY
The table below provides a general description of our offices and facilities:
|
Location
|
Principal Activities
|
Area (sq. meters)
|
Land Use Right/Lease Expiration Date
|
|||
|
No. 588 Shiji Xi Road
Xianyang, Shaanxi Province
PRC 712000
|
Headquarters, production facilities, R&D, quality control
|
52,264
|
50-year land use right expiring in June 2056
|
|||
|
Wuquan Village Jiangcun Town Hu Country Xi'an City
|
Herb cultivation
|
343,983
|
40-year land lease expiring on May 4, 2049
|
|||
|
Zouan Town Xi'an City
|
Processing plant and laboratory*
|
34,803
|
50-year land use right, certificate pending
|
* Under construction, anticipated to be completed April 2010.
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants landholders a land use right in exchange for a purchase price for such right. The land use right allows its holder the right to use the land for a specified long-term period of time and enjoys all the incidents of ownership of the land.
The land use right for the site of our headquarters was acquired in 2006 for a total of RMB 51.9 million ($7.6 million), including land confiscation fee, settlement compensation, ground structure compensation, city construction fitting fee, land reclamation fee, agriculture land fund, water construction fund, agricultural tax, land use fee, and land leasing fee. No additional payment will be needed to retain this right.
The land lease for our cultivation site was entered into in 2009 for a total of RMB 8 million ($1.2 million).
The land use right for the site of our processing facility currently under construction was acquired in 2009 for a total of RMB 20.0 million ($2.9 million), including land confiscation fee, settlement compensation, ground structure compensation, city construction fitting fee, land reclamation fee, agriculture land fund, water construction fund, agricultural tax, land use fee, and land leasing fee. No additional payment will be needed to retain this right. We are currently waiting for the certificate of this land use right to be issued.
ITEM 3. LEGAL PROCEEDINGS
None.
PART II
ITEM 5. MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock, par value $0.001 per share, is traded on the OTC Bulletin Board under the symbol “BSPM.OB”. There was no active trading market for the common stock before December 2008. The following table sets forth, for the periods indicated, the reported high and low closing bid quotations for our common stock as reported on the OTC Bulletin Board. The bid prices reflect inter-dealer quotations, do not include retail markups, markdowns or commissions and do not necessarily reflect actual transactions.
Common Stock
|
Quarter Ended
|
High Bid
|
Low Bid
|
||||||
|
March 31, 2010*
|
$ | 4.75 | $ | 3.14 | ||||
|
December 31, 2009
|
4.55 | 1.85 | ||||||
|
September 30, 2009
|
3.99 | 0.67 | ||||||
|
June 30, 2009
|
2.10 | 0.25 | ||||||
|
March 31, 2009
|
1.01 | 0.25 | ||||||
* Through March 29, 2010
As of March 24, 2010, we had 25,820,119 shares of common stock and 1,467,317 shares of series “B” convertible preferred stock issued and outstanding, as well as warrants to purchase up to 500,000 shares of common stock.
Holders
As of March 24, 2010, we had 242 record holders of our common stock based upon a shareholder list provided by our transfer agent, and 15 record holders of our Series B preferred stock. Our transfer agent is Empire Stock Transfer Inc. located at 1859 Whitney Mesa, Henderson, Nevada 89014, and their telephone number is (702) 818-5898.
Dividends
We have not declared or paid any cash dividends on our common stock during either of our last two fiscal years. The payment of dividends, if any, is at the discretion of the Board of Directors and is contingent on the Company's revenue and earnings, capital requirements, financial conditions. We currently intend to retain all earnings, if any, for use in business operations. Accordingly, we do not anticipate declaring any dividends in the near future.
Securities Authorized for Issuance under Equity Compensation Plans
Please see the discussion in Item 12 titled “Equity Compensation Plan Information” below.
Sales of Unregistered Securities
The following sets forth recent sales by the Company of unregistered securities during the three months ended December 31, 2009:
On November 2, 2009, we entered into a securities purchase agreement with certain accredited investors, pursuant to which we sold and issued to these investors an aggregate of 2,060,000 shares of series “B” convertible preferred stock (the “Series B Preferred”) with attached warrants to purchase up to 500,000 shares of our common stock, for an aggregate purchase price of $3,605,000. The shares of Series B Preferred and warrants were offered and sold to these investors in a private placement transaction made in reliance upon exemptions from registration pursuant to Section 4(2) under the Securities Act and Rule 506 of Regulation D promulgated thereunder. The Investors are accredited investors as defined in Rule 501 of Regulation D.
On November 18, 2009, we entered into a securities purchase agreement with certain accredited investors, pursuant to which we sold and issued an aggregate of 1,000,000 shares of the Series B Preferred to these investors for an aggregate purchase price of $2,120,000. We used the net proceeds of the sale for the purchase of assets through our wholly-owned subsidiary, Shaanxi Biostar. The Series B Preferred were offered and sold to these investors in a private placement transaction made in reliance upon exemptions from registration pursuant to Section 4(2) under the Securities Act of 1933 and Rule 506 of Regulation D promulgated thereunder. The investors are accredited investors as defined in Rule 501 of Regulation D.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our financial statements and the notes thereto which appear elsewhere in this report. The results shown herein are not necessarily indicative of the results to be expected in any future periods. This discussion contains forward-looking statements based on current expectations, which involve uncertainties. In some cases, you can identify forward-looking statements by terminology such as "anticipate," "estimate," "plan," "project," "predict," "potential," "continue," "ongoing," "expect," "believe," "intend," "may," "will," "should," "could," or the negative of these terms or other comparable terminology. All forward-looking statements included in this document are based on information available to the management on the date hereof. Actual results and the timing of events could differ materially from the forward-looking statements as a result of a number of factors. Readers should also carefully review factors set forth in other reports or documents that we file from time to time with the Securities and Exchange Commission.
Overview
Biostar Pharmaceuticals, Inc. (“We”, the “Company” or “Biostar”) was incorporated on March 27, 2007, under the laws of the State of Maryland. Our business operation is conducted in China primarily through our variable interest entity (“VIE”), Shaanxi Aoxing Pharmaceutical Co., Ltd. (“Aoxing Pharmaceutical”), which we control through contractual arrangements between Aoxing Pharmaceutical and our wholly-owned subsidiary, Shaanxi Biostar Biotech Ltd. (“Shaanxi Biostar”).
Our current product line includes 3 over-the-counter (OTC) medicines and 2 prescription-based pharmaceuticals, which are sold and distributed in over 24 provinces by an established network of sales people and by distributors. We are also licensed to produce and sell 13 nutrient products, although we did not produce or sell these products in 2009.
Our best-selling product, Xin Aoxing Oleanolic Acid Capsule (“Xin Aoxing”), is the only state-approved OTC drug currently available on the market for treatment of Hepatitis B. Xin Aoxing meets the requirements of China’s “Chronic Hepatitis Prevention Guide” and is listed as a designated quality product for Hepatitis B in China.
Recent Development
On March 18, 2010, we entered into an agreement to acquire 100% of Xi’an Meipude Bio-Technology Co., Ltd., a Xi’an-based medical equipment and nutrients manufacturer (“Meipude”) for RMB 7.85 million ($1.1million). We officially took control over the operations and the assets of Meipude on March 29, 2010, including its facilities, corporate documents and records, and business licenses and certificates. The Administration for Industry and Commerce of Xi’an Hi-Tech District has also accepted our application to change Meipude’s ownership registration, and we expect to receive the certificate attesting to such change shortly. Meipude manufactures and distributes topical hernia treatment belts, also manufactures a nutraceutical product for treatment of gynecological inflammation in young and middle-aged women. Meipude has been manufacturing and selling its products in Shaanxi and an adjacent province since 2004.
Liquidity and Capital Resources
On November 2, 2009, we issued an aggregate of 2,060,000 shares of Series B convertible preferred stock (the “Series B Preferred”) with attached warrants to purchase a total of 500,000 shares of our common stock in consideration of an aggregate purchase price of $3.6 million. On November 18, 2009, we issue an aggregate of 1,000,000 shares of the Series B Preferred for an aggregate purchase price of $2,120,000. As the Series B Preferred does not require redemption by us, upon issuance, we recorded a one-time deemed dividend of $2.7 million for the beneficial conversion feature (the "BCF") embedded in the Series B Preferred. Between May 29, 2007 and June 4, 2007, we raised $0.7 million in a private placement of our Series A convertible preferred stock (the “Series A Preferred”) at a purchase price of $10.00 per share which we issued together with an aggregate of 1,088,588 warrants to purchase our common stock at a purchase price of $1.00 per share. On October 20, 2008, the Series A Preferred were converted into 1,088,588 shares of common stock. In connection with the conversion, the Company recorded a deemed dividend of $1.5 million for the BCF. Both deemed dividends were charged to retained earnings.
As of December 31, 2009, we had cash and cash equivalents of approximately $8.6 million. We believe our existing cash and cash equivalents will be sufficient to maintain our operations at present level for at least the next twelve months.
Net cash provided by operating activities for the year ended December 31, 2009 was $5.7 million. This was primarily due to the net income of $10.5 million, adjusted by non-cash related expenses including depreciation and amortization of $0.6 million, loss on disposal of building of $0.4 million and stock-based compensation of $1.0 million, offset by a net decrease in working capital items of $6.8 million. The net decrease in working capital items was mainly due to an increase in accounts receivable resulting from increase in sales. Increase in prepayments for research and development and advertising expense also contributed to the net decrease in working capital items. The net decrease in working capital items was partially offset by the increases in accounts payable and accrued expenses, VAT tax payable and income tax payable.
Net cash provided by operating activities for the year ended December 31, 2008 was $2.1 million. This was primarily due to net income of $6.7 million, adjusted by non-cash related expenses including depreciation and amortization of $0.6 million, offset by a net decrease in working capital items of $5.2 million. The net decrease in working capital items was mainly due to an increase in accounts receivable, which resulted from increase in revenue during the year, the longer credit term provided to customers as part of sales promotion (for customers with two years or more business relationship, the payment term may be extended to six months as opposed to the general payment term of three months), and an increase in inventories in preparation of sales promotion. The net decrease in working capital items was partially offset by the increase in accounts payable and accrued expenses, VAT tax payable, and income tax payable.
Net cash used in investing activities for the year ended December 31, 2009 was $3.5 million, primarily due to capital expenditure of $2.3 million on acquisition of land use right for and construction in progress related to the raw material processing plant, and deposit of $1.3 million for acquiring a medical equipment and nutrients manufacturer and a medicine packaging manufacturer.
Net cash used in investing activities for the year ended December 31, 2008, was $3.2 million. This was primarily due to capital expenditures on equipment and vehicles of $0.05 million and on land use right of $0.3 million, and deposit paid for another land use right of $2.9 million for building a raw material processing plant.
Net cash provided by financing activities for the year ended December 31, 2009, was $5.7 million, our net proceeds from the issuance of the Series B Preferred in November 2009.
Net cash used in financing activities for the year ended December 31, 2008, was $0.5 million, representing the repayment of the short-term bank loan.
Results of Operations
Net Sales
|
Year Ended December 31,
|
% of
|
|||||||||||||||||||
|
2009
|
2008
|
change
|
||||||||||||||||||
|
Xin Aoxing Oleanolic Acid Capsule
|
$ | 36,703,857 | 69 | % | $ | 18,742,952 | 55 | % | 96 | % | ||||||||||
|
Taohuasan Pediatrics Medicine
|
4,306,220 | 8 | % | 4,315,558 | 13 | % | 0 | % | ||||||||||||
|
Gan Wang Compound Paracetamol Capsule
|
4,352,902 | 8 | % | 3,671,955 | 11 | % | 19 | % | ||||||||||||
|
Tianqi Dysmenorrhea Capsule
|
4,748,206 | 9 | % | 3,815,790 | 11 | % | 24 | % | ||||||||||||
|
Danshen Granule
|
3,207,559 | 6 | % | 3,364,667 | 10 | % | -5 | % | ||||||||||||
|
Total net sales
|
$ | 53,318,744 | 100 | % | $ | 33,910,922 | 100 | % | 57 | % | ||||||||||
For the year ended December 31, 2009, total net sales increased by approximately $19.4 million or 57% compared to the same period of 2008. This was primarily due to increased sales in three of our five state-approved drugs, Xin Aoxing, Tianqi Dysmenorrhea Capsule (”Tianqi”), and Gan Wang Compound Paracetamol Capsule (“Gan Wang”). The sales of Xin Aoxing increased because we expanded our sales into Tianjing municipality, Fujian province and Xinjiang province during 2009 by establishing our own sales offices selling directly to local retail pharmacies at higher retail price. The sales of Tianqi and Gan Wang increased mainly due to increased sales through existing sales network as well as sales generated from the “new rural cooperative medical supply network plan”, which markets directly to consumers in China’s rural area through retail pharmacies at higher retail price. Domestic Chinese customers still accounted for 100% of total sales.
Cost of Sales
|
Year Ended December 31,
|
% of
|
|||||||||||||||||||
|
2009
|
2008
|
change
|
||||||||||||||||||
|
Xin Aoxing Oleanolic Acid Capsule
|
$ | 6,293,000 | 45 | % | $ | 5,441,750 | 39 | % | 16 | % | ||||||||||
|
Taohuasan Pediatrics Medicine
|
1,502,427 | 10 | % | 1,468,654 | 10 | % | 2 | % | ||||||||||||
|
Gan Wang Compound Paracetamol Capsule
|
2,345,918 | 16 | % | 2,138,676 | 15 | % | 10 | % | ||||||||||||
|
Tianqi Dysmenorrhea Capsule
|
1,695,956 | 12 | % | 1,504,485 | 11 | % | 13 | % | ||||||||||||
|
Danshen Granule
|
2,477,475 | 17 | % | 3,505,778 | 25 | % | -29 | % | ||||||||||||
|
Total cost of sales
|
$ | 14,314,776 | 100 | % | $ | 14,059,343 | 100 | % | 2 | % | ||||||||||
Compared to the same period of 2008, cost of sales increased about $0.3 million or 2% for the year ended December 31, 2009. The increase in the cost of sales of Xin Aoxing, Tianqi, and Gan Wang was primarily due to increased sales volumes of these products. Such increase was offset by the decrease in the cost of sales of Danshen Granule (“Danshen”) resulting from a decrease in its raw material prices, and resulted in only a 2% increase in our overall cost of sales.
Gross Profit
|
Year Ended December 31,
|
||||||||||||||||||||
|
2009
|
2008
|
|||||||||||||||||||
|
gross profit
|
gross profit
|
% of
|
||||||||||||||||||
|
margin
|
margin
|
change
|
||||||||||||||||||
|
Xin Aoxing Oleanolic Acid Capsule
|
$ | 30,410,857 | 83 | % | $ | 13,301,202 | 71 | % | 129 | % | ||||||||||
|
Taohuasan Pediatrics Medicine
|
2,803,793 | 65 | % | 2,846,904 | 66 | % | -2 | % | ||||||||||||
|
Gan Wang Compound Paracetamol Capsule
|
2,006,984 | 46 | % | 1,533,279 | 42 | % | 31 | % | ||||||||||||
|
Tianqi Dysmenorrhea Capsule
|
3,052,250 | 64 | % | 2,311,305 | 61 | % | 32 | % | ||||||||||||
|
Danshen Granule
|
730,084 | 23 | % | (141,111 | ) | -4 | % | n/a | ||||||||||||
|
Total
|
$ | 39,003,968 | 73 | % | $ | 19,851,579 | 59 | % | 96 | % | ||||||||||
Gross profit increased $19.2 million or 96% for the year ended December 31, 2009 compared to the same period of 2008. The increase in gross profit was due primarily to the increase in net sales of Xin Aoxing, Tianqi and Gan Wang. The decrease in the raw material prices of Xin Aoxing and Danshen also contributed to the increase in gross profits.
The overall gross profit margin increased 14 percentage points for the year ended December 31, 2009 compared to the same period of 2008 due primarily to increase in average selling price which was higher than increase in the average cost.
Selling, General And Administrative Expenses
|
Year Ended December 31,
|
||||||||||||||||||||
|
2009
|
2008
|
|||||||||||||||||||
|
% of total
|
% of total
|
% of
|
||||||||||||||||||
|
net sales
|
net sales
|
change
|
||||||||||||||||||
|
Selling, general and administrative expenses
|
$ | 22,873,250 | 43 | % | $ | 12,089,937 | 36 | % | 89 | % | ||||||||||
The year-over-year increase in selling, general and administrative expenses in dollar amount and as a percentage of total net sales for the year ended December 31, 2009 was mainly due to increase in promotional and advertising expenditures of $5.4 million, increase in sales commissions and sales personnel expenses of $3.2 million, and increase in travel expenses of $0.7 million, all resulting from ongoing marketing expansion, as well as due to increase in sales related tax levy of $0.3 million from our increased net sales. The increase in research and development expenses of $0.5 million also contributed to the increase in selling, general and administrative expenses for the year ended December 31, 2009.
Stock-based compensation
Our board of directors adopted a stock option incentive plan in August, 2009. The values of these options are expensed over the term of the respective vesting periods. The stock awards are valued using the market price on or around the date the shares were awarded and included as a period compensation expense. Consequently, we incurred $1.0 million stock-based compensation for the year ended December 31, 2009.
Interest Expense
We did not incur interest expenses for the year ended December 31, 2009, compared to $0.04 million for the same period of 2008. The year-over-year decrease resulted from payoff of short-term bank loan at September 30, 2008.
Loss on Disposal of Building
In the year ended December 31, 2009, we recorded a loss on disposal of building of $0.4 million from the sale of a building we used prior to moving to our current location. The disposal was consummated in July 2009.
Provision for Income Taxes
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Provision for income taxes
|
$ | 4,250,922 | $ | 1,033,402 | ||||
|
Effective tax rate
|
29 | % | 13 | % | ||||
Our effective tax rates increased for the year ended December 31, 2009 as compared to the same period of 2008, primarily due to the expiration on December 31, 2008 of a preferential 50% income tax reduction enjoyed by Aoxing Pharmaceutical. With such expiration, the statutory income tax rate for Aoxing Pharmaceutical increased to 25%. The effective tax rate for the year ended December 31, 2009 was higher than 25% because general and administrative expenses and stock-based compensation expense incurred by us in the United States and by Shaanxi Biostar are not tax deductible against Aoxing Pharmaceutical’s taxable income. This resulted in a higher overall effective tax rate as compared to what would have been expected because income tax was calculated based on Aoxing Pharmaceutical’s taxable income alone without taking our and Shaanxi Biostar’s expenses into account.
Critical Accounting Policies
We believe the following critical accounting policies, among others, affect management’s more significant judgments and estimates used in the preparation of the financial statements:
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. The allowance for doubtful accounts is based on specific identification of customer accounts and management’s best estimate of the likelihood of potential loss, taking into account such factors as the financial condition and payment history of major customers. Management evaluates the collectability of the receivables at least quarterly. If the financial condition of the customers were to deteriorate further, resulting in an impairment of their ability to make payments, additional allowances may be required. The differences could be material and could significantly impact cash flows from operating activities.
The following table sets out the aging of our accounts receivable for each balance sheet periods presented.
|
Accounts Receivable Aging
|
Total
|
1-30 days
|
31-60 days
|
61-90 days
|
91-120 days
|
121-365 days
|
> 365 days
|
|||||||||||||||||||||
|
As of December 31, 2009
|
$ | 19,937,154 | $ | 7,238,448 | $ | 6,598,953 | $ | 6,093,151 | - | $ | 6,602 | - | ||||||||||||||||
|
As of December 31, 2008
|
$ | 11,835,630 | $ | 3,627,709 | $ | 4,102,305 | $ | 2,807,852 | $ | 887,760 | $ | 408,638 | $ | 1,366 | ||||||||||||||
The following table presents the number of days that sales were outstanding, calculated based on sales and accounts receivables in RMB term for the years ended December 31, 2009 and 2008.
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Days sales outstanding
|
135 | 124 | ||||||
The number of days that sales were outstanding increased to 135 days for the year ended December 31, 2009 from 124 days for the same period in 2008, due to slower payments from customers impacted by economic slowdown and from less collection efforts during the Chinese New Year period all during the first quarter of 2009. In the first quarter of 2009, the number of days that sales were outstanding was 169 days.
We take the following steps in collecting accounts receivable:
Step 1: After the payment term has been exceeded, Aoxing Pharmaceutical stops taking orders from delinquent customer and allows the responsible sales person three to six months to collect the accounts receivable. Most of the accounts receivable will be collected in this step because the sales person’s compensation is tied to sales receipts.
Step 2: If the sales person’s collection efforts are not successful, Aoxing Pharmaceutical hires a collection agent and allows the agent another three to six months to collect the accounts receivable.
Step 3: If the collection agent’s efforts are not successful, Aoxing Pharmaceutical will commence legal action to collect the accounts receivable.
Our policies for writing off accounts receivable are as follows:
|
1.
|
If after taking legal action, it appears that an accounts receivable is not likely to become collectible, such accounts receivable will be written off if it is more than two years old.
|
|
2.
|
If during the collection period, a customer provides bankruptcy or other insolvency documentation, the corresponding accounts receivable will be written off.
|
|
3.
|
If we are no longer able to locate a particular customer in order for us to take any collection or legal actions, the accounts receivable for such customer will be written off if more than two years old.
|
Inventory
We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand, future pricing and market conditions. If actual future demands, future pricing or market conditions are less favorable than those projected by management, additional inventory write-downs may be required and the differences could be material. Such differences might significantly impact cash flows from operating activities.
Property and Equipment
Property and equipment are stated at historical cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Judgment is required to determine the estimated useful lives of assets, especially for computer equipment, including determining how long existing equipment can function and when new technologies will be introduced at cost-effective price points to replace existing equipment. Changes in these estimates and assumptions could materially impact the financial position and results of operations.
Stock-Based Compensation
Our stock-based compensation expense is estimated at the grant date based on the award’s fair value as calculated by the Black-Scholes-Merton (BSM) option-pricing model and is recognized as expense over the requisite service period. The BSM model requires various highly judgmental assumptions including expected volatility and option life. Changes in these assumptions could materially impact the financial position and results of operations.
Valuation of Intangibles
From time to time, we acquire intangible assets that are beneficial to our product development processes. Management periodically evaluates the carrying value of intangibles, including the related amortization periods. In evaluating acquired intangible assets, management determines whether there has been impairment by comparing the anticipated undiscounted cash flows from the operation and eventual disposition of the product line with its carrying value. If the undiscounted cash flows are less than the carrying value, the amount of the impairment, if any, will be determined by comparing the carrying value of each intangible asset with its fair value. Fair value is generally based on either a discounted cash flows analysis or market analysis. Future operating income is based on various assumptions, including regulatory approvals, patents being granted, and the type and nature of competing products. If regulatory approvals or patents are not obtained or are substantially delayed, or other competing technologies are developed and obtain general market acceptance or market conditions otherwise change, our intangibles may have a substantially reduced value, which could be material.
Income Taxes
We use the asset and liability method of accounting for income taxes. Under this method, income tax expense is recognized for the amount of taxes payable or refundable for the current year. In addition, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities and for operating losses and tax credit carry-forwards. Management must make assumptions, judgments and estimates to determine the current provision for income taxes and the deferred tax assets and liabilities and any valuation allowance to be recorded against a deferred tax asset. Management’s judgments, assumptions and estimates relative to the current provision for income tax take into account current tax laws, management’s interpretation of current tax laws and possible outcomes of current and future audits conducted by foreign and domestic tax authorities. Changes in tax law or management’s interpretation of tax laws and the resolution of current and future tax audits could significantly impact the amounts provided for income taxes in the financial statements. Management’s assumptions, judgments and estimates relative to the value of a deferred tax asset take into account predictions of the amount and category of future taxable income, such as income from operations. Actual operating results and the underlying amount and category of income in future years could render management’s current assumptions, judgments and estimates of recoverable net deferred taxes inaccurate. Any of the assumptions, judgments and estimates mentioned above could cause our actual income tax obligations to differ from the estimates, thus materially impact the financial position and results of operations.
Foreign Currency
Our functional currency is the U.S. dollar and our subsidiary and our VIE in China use their respective local currencies as their functional currencies, i.e. the Chinese Yuan Renminbi (RMB). An entity’s functional currency is the currency of the primary economic environment in which the entity operates. Management must use judgment in determining an entity’s functional currency, assessing economic factors including cash flow, sales price, sales market, expense, financing and inter-company transactions and arrangements. Impact from exchange rate changes related to transactions denominated in currencies other than the functional currency is recorded as a gain and loss in the statements of operations, while impact from exchange rate changes related to translating a foreign entity’s financial statements from the functional currency to its reporting currency, the U.S. dollar, is disclosed and accumulated in a separate component under the equity section of the balance sheets. Different judgments or assumptions resulting in a change of functional currency may materially impact our financial position and results of operations.
Contractual Obligations
We are obligated to pay research and development expense of $0.6 million for the clinical trial of two new drugs.
Inflation
Management believes that inflation has not had a material effect on our results of operations.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, as defined in Regulation S-K Section 303(a)(4).
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS
The Consolidated Financial Statements and Financial Statement Schedule are included in Part III, Item 15 (a) (1) and (2) of this Annual report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
As reported in our Current Report on Form 8-K filed with the SEC on July 6, 2009, on June 30, 2009, Morgenstern, Svoboda & Baer CPA resigned as our independent registered public accounting firm, and we appointed Acquavella, Chiarelli, Shuster, Berkower & Co., LLP (“ACSB”) in its place. As reported in our Current Report on Form 8-K/A filed with the SEC on January 28, 2010, we dismissed ACSB as our independent registered public accounting firm on January 22, 2010, and appointed Mazars CPA Limited in its place.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company maintains a set of disclosure controls and procedures designed to ensure that information required to be disclosed by the Company in the reports filed under the Securities Exchange Act, is recorded, processed, summarized and reported within the time periods specified by the SEC's rules and forms. Disclosure controls are also designed with the objective of ensuring that this information is accumulated and communicated to the Company's management, including the Company's chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Based upon their evaluation as of the end of the period covered by this report, the Company's chief executive officer and chief financial officer concluded that, the Company's disclosure controls and procedures are effective to ensure that information required to be included in the Company's periodic SEC filings is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms.
Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control system was designed to provide reasonable assurance to our management and Board of Directors regarding the preparation and fair presentation of published financial statements.
The Company’s management assessed the effectiveness of our internal control over financial reporting as of December 31, 2009. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework. Based on its assessment the Company’s management believes that, as of December 31, 2009, the Company’s internal control over financial reporting is effective based on those criteria.
This annual report does not include an attestation report of the Company’s registered accounting firm regarding internal control over financial reporting. The management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission.
Changes in Internal Control over Financial Reporting
No changes in the Company's internal control over financial reporting have come to management's attention during the Company's last fiscal quarter that have materially affected, or are likely to materially affect, the Company's internal control over financial reporting.
Limitations on Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following are our officers and directors as of the date of this report. Some of our officers and directors are residents of the PRC and, therefore, it may be difficult for investors to effect service of process within the U.S. upon them or to enforce judgments against them obtained from the U.S. courts.
|
Name
|
Position
|
Age
|
Date of Appointment
|
|||
|
Ronghua Wang
|
Chairman of the Board of Directors, President, Chief Executive Officer
|
55
|
November 1, 2007
|
|||
|
|
||||||
|
Qinghua Liu
|
Director
|
43
|
November 1, 2007
|
|||
|
|
||||||
|
Haipeng Wu
|
Director
|
53
|
July 1, 2007
|
|||
|
|
||||||
|
Zibing Pan
|
Director
|
41
|
December 30, 2009
|
|||
|
|
||||||
|
Zhongyang Shang
|
Director
|
57
|
December 30, 2009
|
|||
|
Elaine Zhao
|
Chief Financial Officer
|
36
|
July 1, 2007
|
|||
|
Shuang Gong
|
Secretary
|
43
|
April 1, 2008
|
|||
|
|
||||||
|
Amei Zhang
|
Chief Operating Officer
|
36
|
November 1, 2007
|
Business Experience Descriptions
Ronghua Wang has been our Chairman and chief executive officer since our inception and Chairman of Aoxing Pharmaceutical since September of 2006 and a director since 1997. He has served as Aoxing Pharmaceutical’s chief executive officer since1997 and its President since 2007. Beginning 1997, he was Aoxing Pharmaceutical’s manager in charge of sales, management and manufacturing. Prior to 2006, Mr. Wang’s experience in management of other companies was at Geological Research Institute, Drugs Research Institute, General Contractor from 1985 to 1994. In 2001, Mr. Wang was named a “top ten most important person” in the Shaanxi pharmaceutical industry by News Department. In December 1997, he acquired 45.3%. Aoxing Pharmaceutical and began to serve as its general manager. He graduated from Northwest University, with a Bachelor’s degree in Geology.
Qinghua Liu has been our director since 2007. Ms. Liu also serves as chief financial officer of Aoxing Pharmaceutical, a position she has held since 2006. She began working at Aoxing Pharmaceutical in 1996 as the manager of its finance department. Prior to that, Ms. Liu served as an accountant at Xing Ping Paper Mill and at a traditional Chinese medicine research academy. Ms. Liu graduated from Northwest Light Industry College in Shaanxi, PRC in 1990 with an Associate’s Degree in financial management.
Haipeng Wu has been our director since July 2007, and is also the chairman of the board of directors’ nominating committee. From 2001 until now, Mr. Wu worked at Automobile Repairing Department as manager and chief executive officer. He graduated from Northwest University in Xi’an, PRC, in 1982.
Zibing Pan has been our director since December 30, 2009, and is also the chairman of the board of directors’ audit committee. Mr. Pan is a Certified Public Accountant, certified by the Oklahoma State Board of Accountancy and member of American Institute of Certified Public Accountant (AICPA) and Oklahoma Society of Certified Public Accountants (OSCPA). Mr. Pan is currently chief financial officer of China Education Alliance, Inc., to which he was appointed in August 2009. Prior to that position, Mr. Pan was an audit manager with Eide Bailly CPAs & Business Advisors (“Eide Bailly”) at its Oklahoma City office. Mr. Pan had been working at Eide Bailly since September 2005. From September 1998 to September 2005, Mr. Pan was a statistical analyst and economist with the State of Oklahoma. From 1994 to 1996, Mr. Pan worked as a loan project officer for Asian Development Bank Loan Management Office in Anhui, China. From 1988 to 1994, Mr. Pan was an associate professor at Anhui University, China, teaching English language. Mr. Pan graduated with a Master of Business Administration from the University of Central Oklahoma in 1999. He obtained his Bachelor of Arts from Anhui University, China in 1988.
Zhongyang Shang has been our director since December 30, 2009, and is also the chairman of the board of directors’ compensation committee. Mr. Shang is currently the director of Shaanxi Province Administration of Industry and Commerce’s Bureau of Fair Trading, a position he has held since 2006. From 1996 to 2006, Mr. Shang was the director of the Administration of Industry and Commerce for the municipalities of Tongchuan and Xianyang in Shaanxi Province. Mr. Shang was the deputy director of Tongchuan’s Foreign Trade Bureau from 1993 to 1996, and the director of Tongchuan’s Transportation Department from 1984 to 1992. From 1980 to 1983, Mr. Shang was an editor and reporter with the Shaanxi Daily News. Mr. Shang is a graduate of the Central Party College of Economics and Management.
Elaine Zhao has been our chief financial officer since July 1, 2007. In 2005, she founded ELZ Accountancy Corp., a Los Angeles based accounting and financial advisory firm, and she has served as its president since that time. Ms. Zhao continues to work for ELZ. In her work with ELZ, Ms. Zhao has served clients including privately owned and publicly traded company in various industries and has worked with banks in financing small businesses. Ms. Zhao has held Series 7 and 66 licenses as a broker at a national brokerage firm and is an independent financial advisor. From October 2000 to October 2005, Ms. Zhao worked as accountant and auditor at Liang & Company Accountancy Corp. in Los Angeles. Ms. Zhao is a co-founder of the Southern California Chinese Professional Association. She holds an MS in Finance from the Kelley School of Business at Indiana University and is a Certified Public Accountant.
Shuang Gong has been corporate secretary of Aoxing Pharmaceutical since 2006. She is also Administration Manager of Aoxing Pharmaceutical. From 1998 to 2000, Ms. Gong served as Assets Operation Manager of West Securities and Assistant Economist at West Securities; she currently serves as Assistant Office Director of Aoxing Pharmaceutical. Ms. Gong graduated from Xi’an Institute of Technology in Xi’an, PRC, with a bachelor’s degree in machine and electricity integration and earned a second bachelor’s degree in business management from Provincial Party College in Xi’an, China in 2001.
Amei Zhang has been chief operating officer of Aoxing Pharmaceutical since July 2007. From 1999 until now she has served in various capacities at Aoxing Pharmaceutical. Ms. Zhang graduated from North-West University of China with a major of law in 1999, and received a bachelor’s degree in Economics from The Central Party School in 2005 in Xianyang, PRC.
Family Relationships
There are no family relationships between or among any of our current directors, executive officers or persons nominated or charged by the Company to become directors or executive officers. There are no family relationships among our officers and directors and the officers and directors of our direct and indirect subsidiaries.
Involvement in Certain Legal Proceedings
|
|
None of our directors or executive officers has, during the past ten years:
|
|
·
|
Had any petition under the federal bankruptcy laws or any state insolvency law filed by or against, or had a receiver, fiscal agent, or similar officer appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
|
|
·
|
Been convicted in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
·
|
Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
|
|
|
(i)
|
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
|
|
|
(ii)
|
Engaging in any type of business practice; or
|
|
|
(iii)
|
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of federal or state securities laws or federal commodities laws;
|
|
·
|
Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any federal or state authority barring, suspending, or otherwise limiting for more than 60 days the right of such person to engage in any activity described in (i) above, or to be associated with persons engaged in any such activity;
|
|
·
|
Been found by a court of competent jurisdiction in a civil action or by the SEC to have violated any federal or state securities law, where the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated; or
|
|
·
|
Been found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any federal commodities law, where the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended, or vacated.
|
Compliance with Section 16(a) of the Exchange Act
Not applicable.
Code of Ethics
We have adopted a code of ethics that applies to our officers, directors and employees, including our chief executive officer, senior executive officers, principal accounting officer, and other senior financial officers. Our code of ethics is available on our website at www.biostarpharmaceuticals.com. A copy of our code of ethics will also be provided to any person without charge, upon written request sent to us at our offices located at No. 588 Shiji Avenue, Xianyang City, Shaanxi Province, People’s Republic of China 712046.
Audit Committee
Although we are not a “listed company” under SEC rules and are therefore not required to have an audit committee, we have an audit committee comprised of independent directors. We established our audit committee in December 2009, which is consist of Haipeng Wu, Zibing Pan and Zhongyang Shang. Zibing Pan, chairman of the audit committee, is an “audit committee financial expert” as defined under Item 407(d) of Regulation S-K. The purpose of the audit committee is to represent and assist our board of directors in its general oversight of our accounting and financial reporting processes, audits of the financial statements and internal control and audit functions. The audit committee’s responsibilities include:
|
•
|
The appointment, replacement, compensation, and oversight of work of the independent auditor, including resolution of disagreements between management and the independent auditor regarding financial reporting, for the purpose of preparing or issuing an audit report or performing other audit, review or attest services.
|
|
•
|
Reviewing and discussing with management and the independent auditor various topics and events that may have significant financial impact on our Company or that are the subject of discussions between management and the independent auditors.
|
The board of directors has adopted a written charter for the audit committee. A copy of the audit committee charter is posted on our corporate website at: www.biostarpharmaceuticals.com.
Material Changes to the Procedures by which Security Holders May Recommend Nominees to the Board of Directors
There have been no material changes to the procedures by which security holders may recommend nominees to the Board of Directors.
Compensation Committee
We established our compensation committee in December 2009. The compensation committee consists of Haipeng Wu and Zhongyang Shang, each of whom is an independent director. Zhongyang Shang is the chairman of the compensation committee. The compensation committee is responsible for the design, review, recommendation and approval of compensation arrangements for our directors, executive officers and key employees, and for the administration of our equity incentive plans, including the approval of grants under such plans to our employees, consultants and directors. The compensation committee also reviews and determines compensation of our executive officers, including our chief executive officer. The board of directors has adopted a written charter for the compensation committee. A copy of the compensation committee charter is posted on our corporate website at: www.biostarpharmaceuticals.com.
Nominating Committee
We established our nominating committee in December 2009. The nominating committee consists of Haipeng Wu and Zhongyang Shang, each of whom is an independent director. Haipeng Wu is the chairman of the nominating committee. The nominating committee assists in the selection of director nominees, approves director nominations to be presented for stockholder approval at our annual general meeting and fills any vacancies on our board of directors, considers any nominations of director candidates validly made by stockholders, and reviews and considers developments in corporate governance practices. The board of directors has adopted a written charter for the nominating committee. A copy of the nominating committee charter is posted on our corporate website at: www.biostarpharmaceuticals.com.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table reflects the compensation for the two fiscal years ended December 31, 2009 and 2008, paid to our principal executive officers and each of our other two highest paid executives whose total compensation exceeded $100,000 during these fiscal years (if any).
|
Name and
Principal
Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock Awards ($)
|
Option
Awards ($)
|
Non-Equity Incentive Plan Compensation ($)
|
Non-
Qualified Deferred Compensation Earnings
($)
|
All Other Compensation ($)
|
Total
($)
|
|||||||||||||||
|
Ronghua Wang
chief executive officer, president (1)
|
2009
2008
|
8,771
8,640
|
—
13,000
|
—
—
|
412,050 —
|
—
—
|
—
—
|
—
—
|
420,821
21,640
|
|||||||||||||||
|
Elaine Zhao
chief financial officer (2)
|
2009
2008
|
47,917
45,000
|
—
—
|
—
—
|
306,851
—
|
—
—
|
—
—
|
—
—
|
354,768
45,000
|
|||||||||||||||
|
(1)
|
Mr. Ronghua Wang was appointed our president and chief executive officer on November 1, 2007. Mr. Wang received the compensation set forth above from Aoxing Pharmaceutical and he received no compensation from Biostar in 2008 or 2009. Mr. Wang’s cash compensation was paid in RM which, for reporting purposes, has been converted to U.S. dollars at the conversion rate of 6.9444 RMB to one U.S. dollar for 2008, and 6.8409 RMB to one U.S. dollars for 2009.
|
|
(2)
|
Mrs. Elaine Zhao was appointed our chief financial officer on July 1, 2007. Mrs. Zhao received the compensation set forth above from Biostar.
|
Grants of Plan-Based Awards
|
All Other
|
All Other
|
|||||||||||||||||||||||||||||
|
Stock
|
Option
|
Exercise
|
||||||||||||||||||||||||||||
|
Awards:
|
Awards:
|
or Base
|
Grant Date
|
|||||||||||||||||||||||||||
|
Estimated Future Payouts Under
|
Estimated Future Payouts Under
|
Number
|
Number of
|
Price of
|
Fair Value
|
|||||||||||||||||||||||||
|
Non-Equity Incentive Plan Awards
|
Equity Incentive Plan Awards
|
of Shares
|
Securities
|
Option
|
of Stock
|
|||||||||||||||||||||||||
|
Grant
|
Thres-
|
Target
|
Max-
|
Thres-
|
Target
|
Max-
|
of Stock
|
Underlying
|
Awards
|
and Option
|
||||||||||||||||||||
|
Name
|
Date
|
hold ($)
|
($)
|
$ |
imum
|
hold ($)
|
($)
|
$ |
imum
|
or Units (#)
|
Options (#)(2)
|
($/Sh)
|
Awards (1)
|
|||||||||||||||||
| Ronghua Wang | 10/22/09 | $ | ― | $ | ― | $ | ― |
$
|
― | $ | ― |
$
|
― | ― | 220,000 | $ | 2.60 | $ | 160,242 | |||||||||||
| Elaine Zhao | 10/22/09 | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | ― | 200,000 | $ | 2.60 | $ | 119,331 | |||||||||||
|
(1)
|
Reflects dollar amount expensed by the Company during the applicable fiscal year for financial statement reporting purposes pursuant to FAS 123R.
|
|
(2)
|
The vesting schedule for these options is as follows: 33.33% on the grant date and each of the 1st and 2nd-year anniversary of the grant date.
|
Outstanding Equity Awards at 2009 Fiscal Year End
|
Option Awards
|
Stock Awards
|
||||||||||||||||||||||||||||||||
|
Name
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#)
|
Option
Exercise
Price ($)
|
Option
Expiration
Date
|
Number
of Shares
or Units
of Stock
That
Have Not
Vested
(#)
|
Market
Value of
Shares or
Units of
Stock That
Have Not
Vested ($)
|
Equity
Incentive
Plan
Awards:
Number of
Unearned
Shares,
Units, or
Other
Rights
That
Have Not
Vested (#)
|
Equity
Incentive
Plan Awards:
Market or
Payout
Value of
Unearned
Shares, Units,
or Other
Rights That
Have Not
Vested (#)
|
||||||||||||||||||||||||
|
Ronghua Wang
|
73,334
|
(1)
|
146,666
|
(1)
|
―
|
$
|
2.60
|
10/21/14
|
―
|
―
|
―
|
―
|
|||||||||||||||||||||
|
Elaine Zhao
|
66,667
|
(1)
|
133,333
|
(1)
|
―
|
$
|
2.60
|
10/21/14
|
―
|
―
|
―
|
―
|
|||||||||||||||||||||
|
(1)
|
33.33% of this named executive officer’s options vest(ed) on October 22, 2009, October 22, 2010 and October 22, 2011.
|
Employment Agreements
Except as described below, we currently have no employment agreements with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s responsibilities following a change-in-control.
On August 1, 2009, we entered into a financial services agreement with ELZ Accountancy Corp. (“ELZ”), for the services of ELZ’s employee, Elaine Zhao, to continue her services as our chief financial officer through May 31, 2011 (the “Term”). Pursuant to the agreement, ELZ shall receive a service fee at the rate of $50,000 per year. ELZ shall also receive $1,000 and $2,000 in cash or stock during the first and second years of the Term, respectively, to be paid in the first week of January each year, as well as a discretionary additional service fee based upon the profits of the Company. During the Term, Ms. Zhao is entitled to participate in all benefit plans that may be in effect for senior management, including dental, medical and disability insurance, in accordance with the Company’s policies established and in effect from time to time. Ms. Zhao’s employment with the Company may be terminated at any time, with or without just cause. In the event that Ms. Zhao’s employment is terminated by the Company without just cause, ELZ is entitled to a termination payment of three months’ service fee plus a pro-rated additional service fee, as well as any previously declared additional service fee issued pursuant to the agreement.
Employment Agreements of Aoxing Pharmaceutical
Aoxing Pharmaceutical has employment agreements with Shuang Gong, who serves as corporate secretary of both Aoxing Pharmaceutical and Biostar, and Amei Zhang, who is chief operating officer for both Aoxing Pharmaceutical and Biostar. The employment agreements of Ms. Gong, Mr. Yuan and Ms. Zang have the same material terms. Their employment agreements provide for a term of 5 years, year-end bonuses based on profitability of Aoxing Pharmaceutical, a salary increases based on performance, and health and insurance benefits. Aoxing Pharmaceutical may terminate the employment agreements for cause by reason of serious neglect, criminal charges, or violation of the Aoxing Pharmaceutical’s rules by the employee. On the other hand, the employee may terminate the employment agreement on 30-day notice and may terminate without notice in the event Aoxing Pharmaceutical violates health and safety regulations, fails to provide labor protection or fails to pay the employee.
Director Compensation
The following table provides compensation information for our directors during the fiscal year ended December 31, 2009:
|
Name
|
|
Fees
Earned or
Paid in Cash
($)
|
Stock
Awards
($)(1)
|
Option
Awards ($)(1)
|
Non-Equity
Incentive Plan
Compensation ($)
|
Non-Qualified
Deferred
Compensation
Earnings ($)
|
All Other
Compensation
($)
|
Total
($)
|
|
|||||||||||||||||||
|
Ronghua Wang (2)
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||
|
|
||||||||||||||||||||||||||||
|
Qinghua Liu
|
$
|
5,262
|
$
|
-
|
$
|
47,732
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
52,994
|
||||||||||||||
|
|
||||||||||||||||||||||||||||
|
Haipeng Wu
|
$
|
3,508
|
$
|
-
|
$
|
11,933
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
15,441
|
||||||||||||||
|
|
||||||||||||||||||||||||||||
|
Ziping Pan
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||
|
Zhongyang Shang
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||
|
(1)
|
Reflects dollar amount expensed by the Company during the applicable fiscal year for financial statement reporting purposes pursuant to FAS 123R. FAS 123R requires the Company to determine the overall value of the stock award as of the date of grant, and to then expense that value over the service period over which the stock award becomes exercisable (vested). As a general rule, for time in service based stock awards, the Company will immediately expense any stock award or portion thereof that is vested upon grant, while expensing the balance on a pro rata basis over the remaining vesting term of the stock award.
|
|
(2)
|
This individual’s compensation is reflected in the Summary Compensation Table on page 57 above.
|
Agreements with Directors
Under our agreement with Mr. Pan, he is appointed for one year or until the next annual shareholders’ meeting, and will be entitled to receive annual compensation of 120,000 Renminbi (“RMB”) for his services rendered as a member of the board of directors and as chairman of the audit committee, payable in quarterly installments and subject to his continuous service on the board of directors. Mr. Pan is additionally granted options under our 2009 Incentive Stock Plan (the “Plan”) to purchase up to 50,000 shares of Common Stock, and in connection therewith, Mr. Pan will enter into a nonstatutory stock option agreement with us. Additionally, Mr. Pan will be reimbursed for his expenses incurred in connection with the performance of his duties, including travel expenses. We have also agreed to obtain directors’ and officers’ liability insurance, and to maintain such insurance during Mr. Pan’s appointment on the board of directors. Mr. Pan’s appointment terminates immediately if he: (a) resigns for any reason; (b) is removed or not re-elected at the next annual meeting of shareholders; (c); is declared bankrupt; (d) is disqualified from acting as a director; (e) dies; or (f) is ordered to resign by a court of competent jurisdiction.
Under our agreement with Mr. Shang, he is appointed for one year or until the next annual shareholders’ meeting, and will be entitled to receive annual compensation of RMB 20,000 for his services rendered as a member of the board of directors and as chairman of the compensation committee and member of the audit and nominating committees, payable in quarterly installments and subject to his continuous service on the board of directors. Mr. Shang is additionally granted options under the Plan to purchase up to 50,000 shares of Common Stock, and in connection therewith, Mr. Shang will enter into a nonstatutory stock option agreement with us. Additionally, Mr. Shang will be reimbursed for his expenses incurred in connection with the performance of his duties, including travel expenses. We have also agreed to obtain directors’ and officers’ liability insurance, and to maintain such insurance during Mr. Shang’s appointment on the board of directors. Mr. Shang’s appointment terminates immediately if he: (a) resigns for any reason; (b) is removed or not re-elected at the next annual meeting of shareholders; (c); is declared bankrupt; (d) is disqualified from acting as a director; (e) dies; or (f) is ordered to resign by a court of competent jurisdiction.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation plans
|
||||||
|
Equity compensation plans
approved by security holders
|
—
|
—
|
—
|
||||||
|
Equity compensation plans
not approved by security holders
|
1,080,000
|
$2.77
|
920,000
|
||||||
|
TOTAL
|
1,080,000
|
$2.77
|
920,000
|
||||||
|
On August 25, 2009, our board of directors approved a stock incentive plan for officers, directors, employees, and consultants entitled “Biostar Pharmaceuticals, Inc. 2009 Incentive Stock Plan” (hereinafter the “2009 Plan”). The maximum number of shares that may be issued under the 2009 Plan is 2,000,000 shares of our common stock. The 2009 Plan was approved by written consent of shareholders holding a majority of our issued and outstanding common stock; however, we have not notified all of our shareholders of such approval as required under applicable Maryland law. Under this Plan, the Company may issue common stock and/or options to purchase common stock to certain officers, directors and employees and consultants of the Company and its subsidiaries. The 2009 Plan is administered either by the compensation committee or a committee appointed by the Board, which is comprised of a combination of two or more officers and/or members of the Board. The committee has full and complete authority, in its discretion, but subject to the express provisions of the Plan to approve the eligible persons nominated by the management of the Company to be granted awards of common stock (“Awards”) or stock options, to determine the number of Awards or stock options to be granted to an eligible person; to determine the time or times at which or stock options shall be granted; to establish the terms and conditions upon which Awards or Stock Options may be exercised; to remove or adjust any restrictions and conditions upon Awards or Stock Options; to specify, at the time of grant, provisions relating to exercisability of Stock Options and to accelerate or otherwise modify the exercisability of any Stock Options; and to adopt such rules and regulations and to make all other determinations deemed necessary or desirable for the administration of the Plan. As of December 31, 2009, there are 920,000 shares of our common stock remaining available for future issuance under the 2009 Plan.
|
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of March 24, 2010, by (i) any person or group with more than 5% of our voting securities, (ii) each director and executive officer and (iii) all executive officers and directors as a group. In general, a person is deemed to be a “beneficial owner” of a security if that person has or shares the power to vote or direct the voting of such security, or the power to dispose or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which the person has the right to acquire beneficial ownership within 60 days. Shares of common stock subject to options, warrants or convertible securities exercisable or convertible within 60 days of March 24, 2010, are deemed outstanding for computing the percentage of the person or entity holding such options, warrants or convertible securities but are not deemed outstanding for computing the percentage of any other person.
|
Title of
Class
|
Name and
Address of
Beneficial Owner (1)
|
Amount of
Beneficial
Owner (2)
|
Percent of
Class (2)
|
|||||
|
Common Stock
|
Ronghua Wang
chairman of Board, president and chief executive officer
|
9,040,308
|
(3)
|
35.01
|
% |
(3)
|
||
|
|
||||||||
|
Common Stock
|
Qinghua Liu
Director
|
29,667
|
(4)
|
*
|
% |
(4)
|
||
|
Common Stock
|
Haipeng Wu
Director
|
6,667
|
(5)
|
*
|
% |
(5)
|
||
|
|
||||||||
|
Common Stock
|
Zibing Pan (6)
Director
|
0
|
-
|
|||||
|
|
||||||||
|
Common Stock
|
Zhongyang Shang
Director
|
0
|
-
|
|||||
|
|
||||||||
|
Common Stock
|
Elaine Zhao (7)
chief financial officer
|
69,907
|
(7)
|
*
|
%
|
|
||
|
Common Stock
|
Amei Zhang
chief operating officer
|
49,667
|
(8)
|
*
|
% |
(8)
|
||
|
Common Stock
|
Shuang Gong
corporate secretary
|
36,334
|
(9)
|
*
|
% |
(9)
|
||
|
Common Stock
|
All Directors and Officers of the Company as a group
|
9,232,550
|
35.76
|
%
|
|
|||
|
Common Stock
|
Andrew Barron Worden
|
2,556,629
|
(10)
|
9.90
|
% |
(10)
|
||
* Less than 1%
|
(1)
|
Unless otherwise noted, the address for each of the named beneficial owners is: No. 588 Shiji Avenue, Xianyang City, Shaanxi province, PRC, 712046.
|
|
(2)
|
Unless otherwise noted, the number and percentage of outstanding shares of common stock of Biostar is based upon 25,820,119 shares outstanding as of March 24, 2010. Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of shares of common stock actually outstanding.
|
|
(3)
|
Includes 73,334 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(4)
|
Includes 26,667 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(5)
|
Includes 6,667 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(6)
|
Zibing Pan’s address is: 1813 NW 176th PL Edmond, OK 73012.
|
|
(7)
|
Elaine Zhao’s address is: 20955 Pathfinder Road, Suite 100, Diamond Bar, California 91765. Includes 66,66 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(8)
|
Includes 26,667 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(9)
|
Includes 33,334 shares of common stock issuable upon exercise of stock options that were granted to this stockholder on October 22, 2009.
|
|
(10)
|
Andrew Barron Worden’s address is 730 Fifth Avenue, 25th Floor, New York, New York 10019. The number of shares reported herein as beneficially owned by Mr. Worden includes 1,690,634 shares held by a group of shareholders to which Mr. Worden has been granted trading authorization to and share dispositive power of.
|
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Certain Relationships and Related Transactions
As described in “Business – Our History and Corporate Structure” above, we have the right, through our wholly-owned subsidiary Shaanxi Biostar, to appoint the officers and directors of Aoxing Pharmaceutical. The transactions described in “Business – Our History and Corporate Structure” involve officers and directors of Shaanxi Biostar and Aoxing Pharmaceutical, some of whom are also our officers and directors. To understand these relationships and these transactions, you should review the discussion in this prospectus under “Business – Our History and Corporate Structure.”
Other than the above transactions or otherwise set forth in any reports filed by the Company with the SEC, the Company and its subsidiaries have not entered into any material transactions with any director, executive officer, and nominee for director, beneficial owner of five percent or more of its common stock, or family members of such persons. The Company is not a subsidiary of any company.
Director Independence
Based upon information submitted by Haipeng Wu, Zibing Pan and Zhongyang Shang, the board of directors has determined that each of them is considered independent under Rule 5605(a)(2) of the NASDAQ Listing Rules, even though such definition does not currently apply to us because we are not listed on the NASDAQ Capital Market. All members of the audit, compensation and nominating committees satisfy the “independence” standards applicable to members of each such committee.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Morgenstern, Svoboda & Baer, CPAs, P.C. (“MSB”) served as our independent registered public accounting firm for our fiscal year ended December 31, 2008. On June 30, 2009, MSB resigned and we appointed Acquavella, Chiarelli, Shuster, Berkower & Co., LLP (“”ACSB”) as our new independent registered public accounting firm for our fiscal year ended December 31, 2009. On January 22, 2010, we dismissed ACSB and appointed Mazars CPA Limited (“Mazars”) as our new independent registered public accounting firm for our fiscal year ended December 31, 2009. The following table shows the fees that were billed for audit and other services provided by these firms during the 2009 and 2008 fiscal years:
|
Fiscal Year Ended December 31,
|
||||||||||||||||
|
2009
|
2008
|
|||||||||||||||
|
Billing Firm
|
Mazars
|
ACSB
|
MSB
|
MSB
|
||||||||||||
|
Audit Fees (1)
|
$
|
105,000
|
$
|
16,000
|
$
|
44,000
|
$
|
76,707
|
||||||||
|
Audit-related Fees (2)
|
-
|
-
|
-
|
-
|
||||||||||||
|
Tax Fees (3)
|
-
|
-
|
-
|
-
|
||||||||||||
|
All Other Fees (4)
|
-
|
-
|
-
|
-
|
||||||||||||
|
Total
|
$
|
105,000
|
$
|
16,000
|
$
|
44,000
|
$
|
76,707
|
||||||||
|
(1)
|
Audit Fees – This category includes the audit of our annual financial statements, review of financial statements included in our Quarterly Reports on Form 10-Q, and services that are normally provided by independent auditors in connection with statutory and regulatory filings or the engagement for fiscal years. This category also includes advice on audit and accounting matters that arose during, or as a result of, the audit or the review of interim financial statements.
|
|
(2)
|
Audit-Related Fees – This category consists of assurance and related services by our independent auditors that are reasonably related to the performance of the audit or review of our financial statements and are not reported above under "Audit Fees." The services for the fees disclosed under this category include consultation regarding our correspondence with the SEC.
|
|
(3)
|
Tax Fees – This category consists of professional services rendered by our independent auditors for tax compliance and tax advice. The services for the fees disclosed under this category include tax return preparation and technical tax advice.
|
|
(4)
|
All Other Fees – This category consists of fees for other miscellaneous items.
|
ITEM 15. EXHIBITS
(1) Financial Statements
The following consolidated financial statements of Biostar are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2009 and 2008
Consolidated Statements of Operations for the Years Ended December 31, 2009 and 2008
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2009 and 2008
Consolidated Statements of Cash Flows for the Years Ended December 31, 2009 and 2008
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
(3) Exhibits
EXHIBIT INDEX
|
Exhibit
Number
|
Description
|
|||||
|
3.1
|
Articles of Incorporation filed with the corporate secretary of State of the State of Maryland on March 27, 2007 (1)
|
|||||
|
3.2
|
Articles of Amendment. filed with the corporate secretary of State of the State of Maryland on August 1, 2007 (1)
|
|||||
|
3.3
|
Articles of Amendment filed with the corporate secretary of State of the State of Maryland on September 14, 2007 (1)
|
|||||
|
3.4
|
Certificate of Designation for the Series B Convertible Preferred Stock as filed with the corporate secretary of State of Maryland on November 2, 2009 (8)
|
|||||
|
3.4
|
Bylaws (1)
|
|||||
|
4.1
|
Form of $1.00 Common Stock Warrant (1)
|
|||||
|
4.2
|
Form of $3.00 Common Stock Warrant (8)
|
|||||
|
10.1
|
Amended and Restated Entrusted Management Agreement by and among Shaanxi Aoxing Pharmaceutical Co., Ltd., its shareholders and Shaanxi Biostar Biotech Ltd. dated May 6, 2008 (4)
|
|||||
|
10.2
|
Form of Preferred Stock and Warrant Purchase Agreement between the Company and the purchasers of the Series A Convertible Preferred Stock (1)
|
|||||
|
10.3
|
Form of Amendment No. 1 to the Preferred Stock and Warrant Purchase Agreement between the Company and the purchasers of the Series A Convertible Preferred Stock (1)
|
|||||
|
10.4
|
Exclusive Option Agreement by and among Shaanxi Biostar Biotech Ltd., Wang Ronghua, Wang Yan, Wang Rongfa, Wang Rangmei, Cao Xuezhu, Wang Yuxing, An Xiaoru, Ao Quanfang, Tang Wenying, Qin Hongxia, Wu Gang, Wu Weiping, Bai Rong, Wu Jin and Shaanxi Aoxing Pharmaceutical Co., Ltd. (2)
|
|||||
|
10.5
|
Share Pledge Agreement by and among Wang Ronghua, Wang Yan, Wang Rongfa, Wang Rangmei, Cao Xuezhu, Wang Yuxing, An Xiaoru, Ao Quanfang, Tang Wenying, Qin Hongxia, Wu Gang, Wu Weiping, Bai Rong, Wu Jin, and Shaanxi Biostar Biotech Ltd. (2)
|
|||||
|
10.6
|
Shareholders’ Voting Proxy Agreement by and among Shaanxi Biostar Biotech Ltd., Wang Ronghua, Wang Yan, Wang Rongfa, Wang Rangmei, Cao Xuezhu, Wang Yuxing, An Xiaoru, Ao Quanfang, Tang Wenying, Qin Hongxia, Wu Gang, Wu Weiping, Bai Rong and Wu Jin (2)
|
|||||
|
10.7
|
Cooperation Agreement by and between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Xianyang Material Medical Institute (3)
|
|||||
|
10.8
|
Technological Cooperation Agreement between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Shaanxi University of Science and Technology (3)
|
|||||
|
10.9
|
Drug Supply and Marketing Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Guangdong Runtai Pharmaceutical Co., Ltd. (2)
|
|||||
|
10.10
|
Purchase Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Xi'an Chemical Industry Medicine Supply and Marketing Company (2)
|
|||||
|
10.11
|
Labor Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Shuang Gong dated June 10, 2006 (2)
|
|||||
|
10.12
|
Labor Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Jianmin Du dated January 2, 2006 (2)
|
|||||
|
10.13
|
Labor Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Ame Zhang dated July 7, 2007 (2)
|
|||||
|
10.14
|
Corporate Finance Advisory Agreement between Friedland Capital Inc. and Shaanxi Aoxing Pharmacy Co., Ltd. dated March 8 , 2007 (2)
|
|||||
|
10.15
|
Entrust Agreement for Land Acquisition (2)
|
|||||
|
10.16
|
Land Use Right (2)
|
|||||
|
10.17
|
Purchase Contract between Shaanxi Aoxing Pharmaceutical Co., Ltd. and Xi’an Chinese Drug Tablet Factory (2)
|
|||||
|
10.18
|
Loan Agreement between Shaanxi Aoxing Pharmaceutical Co., Ltd and Qindu District Rural Credit Cooperative (Fengxi Branch) dated February 13, 2008 (3)
|
|||||
|
10.19
|
Technological Cooperation Agreement between Shaanxi Aoxing Pharmaceutical Co., Ltd and College of Life Sciences of Northwest University dated September 10, 2006 (3)
|
|||||
|
10.20
|
Preliminary Employment Agreement between Biostar Pharmaceuticals, Inc. and Elaine Zhao dated June 25, 2007 (5)
|
|||||
|
10.21
|
Stock Purchase Agreement by and between the Company and purchasers of Series B Convertible Preferred Stock dated November 2, 2009 (8)
|
|||||
|
10.22
|
Make Good Escrow Agreement by and among the Company, purchasers of Series B Convertible Preferred Stock, and Sichenzia Ross Friedman Ference LLP dated November 2, 2009 (8)
|
|||||
|
10.23
|
Closing Escrow Agreement by and among the Company, purchasers of Series B Convertible Preferred Stock and Sichenzia Ross Friedman Ference LLP dated November 2, 2009 (8)
|
|||||
|
10.24
|
Stock Purchase Agreement by and between the Company and purchasers of Series B Convertible Preferred Stock dated November 18, 2009 (9)
|
|||||
|
10.25
|
Financial Services Agreement by and among the Company, ELZ Accountancy Corp., and Elaine Zhao dated August 1, 2009 (7)
|
|||||
|
10.26
|
Form of Director Offer Letter with Zibing Pan and Zhongyang Shang (10)
|
|||||
|
10.27
|
||||||
|
14
|
Code of Ethics (10)
|
|||||
|
16.1
|
Letter from Morgenstern, Svoboda & Baer CPA (6)
|
|||||
|
16.2
|
Letter from Acquavella, Chiarelli, Shuster, Berkower & Co., LLP (11)
|
|||||
|
21
|
Subsidiaries (1)
|
|||||
| 31.1 | ||||||
| 31.2 | ||||||
| 32.1 | ||||||
| 32.2 | ||||||
|
|
*
|
Filed herewith
|
|
|
(1)
|
Previously filed as an exhibit to the Company’s Registration Statement on Form SB-2 (File No. 333-147363) filed with the SEC on November 13, 2007.
|
||
|
(2)
|
Previously filed as an exhibit to the Company’s Registration Statement on Form S-1A (File No. 333-147363) filed with the SEC on February 25, 2008.
|
||
|
(3)
|
Previously filed as an exhibit to the Company’s Registration Statement on Form S-1A (File No. 333-147363) filed with the SEC on May 9, 2008.
|
||
|
(4)
|
Previously filed as an exhibit to the Company’s Registration Statement on Form S-1A (File No. 333-147363) filed with the SEC on June 12, 2008.
|
||
|
(5)
|
Previously filed as an exhibit to the Company’s Registration Statement on Form S-1A (File No. 333-147363) filed with the SEC on June 27, 2008.
|
||
|
(6)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K filed with the SEC on July 6, 2009.
|
||
|
(7)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K filed with the SEC on August 6, 2009.
|
||
|
(8)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K filed with the SEC on November 3, 2009.
|
||
|
(9)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2009.
|
||
|
(10)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K filed with the SEC on January 5, 2010.
|
||
|
(11)
|
Previously filed as an exhibit to the Company’s Current Report on Form 8-K/A filed with the SEC on January 28, 2010.
|
||
Pursuant to the requirements of section 13 or 15 (d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
||||||||
|
BIOSTAR PHARMACEUTICALS, INC.
(Registrant)
|
||||||||
|
Date: March 31, 2010
|
|
By: /s/ Ronghua Wang
|
||||||
|
Ronghua Wang
Chief Executive Officer
(Principal Executive Officer)
|
||||||||
|
Date: March 31, 2010
|
By: /s/ Elaine Zhao
|
|||||||
|
Elaine Zhao
Chief Financial Officer
(Principal Financial Officer)
|
||||||||
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Name
|
Title
|
Date
|
||
|
/s/ Ronghua Wang
|
Chief Executive Officer/Director
|
March 31, 2010
|
||
|
Ronghua Wang
|
||||
|
/s/ Elaine Zhao
|
Chief Financial Officer
|
March 31, 2010
|
||
|
Elaine Zhao
|
||||
|
/s/ Qinghua Liu
|
Director
|
March 31, 2010
|
||
|
Qinghua Liu
|
||||
|
/s/ Haipeng Wu
|
Director
|
March 31, 2010
|
||
|
Haipeng Wu
|
||||
|
/s/ Zibing Pan
|
Director
|
March 31, 2010
|
||
|
Zibing Pan
|
||||
|
/s/ Zhongyang Shang
|
Director
|
March 31, 2010
|
||
|
Zhongyang Shang
|
|
Page(s)
|
|
|
Financial Statements
|
|
|
F-1
|
|
|
F-2
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
Report of Independent Registered Public Accounting Firm
To the Audit Committee, Board of Directors and Stockholders
Biostar Pharmaceuticals, Inc
We have audited the accompanying consolidated balance sheet of Biostar Pharmaceuticals, Inc. (“Biostar”) and its subsidiaries (the “Company”) as of December 31, 2009, and the related consolidated statement of operation, consolidated statement of stockholders’ equity and consolidated statement of cash flows for the year then ended. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing auditing procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audits also included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2009, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Mazars CPA Limited
Mazars CPA Limited
Certified Public Accountants
Hong Kong
March 31, 2010
MORGENSTERN, SVOBODA & BAER, CPA’s, P.C.
CERTIFIED PUBLIC ACCOUNTANTS
40 Exchange Place, Suite 1820
New York, N.Y.10005
TEL: (212) 925-9490
FAX: (212) 226-9134
E-MAIL: MSBCPAS@gmail.com
Board of Directors and Stockholders of
Biostar Pharmaceutical, Inc.
We have audited the accompanying consolidated balance sheets of Biostar Pharmaceutical, Inc. as of December, 31 2008, and the related consolidated statements of income, stockholders’ equity and comprehensive income, and cash flows for the year ended December 31, 2008. Biostar Pharmaceutical, Inc’s management is responsible for these financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Biostar Pharmaceutical, Inc and subsidiaries as of December 31, 2008, and the results of their operations and their consolidated cash flows for the year ended December 31, 2008 in conformity with accounting principles generally accepted in the United States of America.
/s/ Morgenstern, Svoboda & Baer CPA’s P.C.
Certified Public Accountants
New York, N.Y.
March 25, 2009
|
BIOSTAR PHARMACEUTICALS, INC.
|
||||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||||
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$ | 8,577,704 | $ | 758,316 | ||||
|
Accounts receivable
|
19,803,434 | 11,700,841 | ||||||
|
Inventories
|
340,078 | 315,745 | ||||||
|
Prepaid expenses and other receivables
|
1,500,327 | 8,753 | ||||||
|
Total Current Assets
|
30,221,543 | 12,783,655 | ||||||
|
Deposits
|
1,316,328 | 2,917,919 | ||||||
|
Property and equipment, net
|
4,340,917 | 5,930,467 | ||||||
|
Intangible assets, net
|
11,131,681 | 7,365,765 | ||||||
|
Total Assets
|
$ | 47,010,469 | $ | 28,997,806 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable and accrued expenses
|
$ | 3,559,281 | $ | 2,228,296 | ||||
|
Customer and other deposits
|
- | 2,556,097 | ||||||
|
Value-added tax payable
|
1,050,051 | 527,103 | ||||||
|
Income tax payable
|
1,481,266 | 413,205 | ||||||
|
Total Current Liabilities
|
6,090,598 | 5,724,701 | ||||||
|
Commitment and contingencies
|
||||||||
|
Stockholders' Equity
|
||||||||
|
Series B, convertible preferred stock, $0.001 par value, 5,000,000 shares
|
||||||||
|
authorized, 3,060,000 shares and Nil issued and outstanding at December 31, 2009 and 2008
|
3,060 | - | ||||||
|
Common stock, $0.001 par value, 100,000,000 shares authorized,
|
||||||||
|
23,374,799 and 23,240,899 shares issued and outstanding at December 31, 2009 and 2008
|
23,375 | 23,241 | ||||||
|
Additional paid-in capital
|
19,801,366 | 10,430,168 | ||||||
|
Statutory reserve
|
2,860,685 | 1,585,383 | ||||||
|
Retained earnings
|
17,548,676 | 10,996,655 | ||||||
|
Accumulated other comprehensive income
|
682,709 | 237,658 | ||||||
|
Total Stockholders' Equity
|
40,919,871 | 23,273,105 | ||||||
|
Total Liabilities and Stockholders' Equity
|
$ | 47,010,469 | $ | 28,997,806 | ||||
The accompanying notes are an integral part of these financial statements.
|
BIOSTAR PHARMACEUTICALS, INC.
|
||||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS
|
||||||||
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Sales, net
|
$ | 53,318,744 | $ | 33,910,922 | ||||
|
Cost of sales
|
14,314,776 | 14,059,343 | ||||||
|
Gross profit
|
39,003,968 | 19,851,579 | ||||||
|
Operating expenses:
|
||||||||
|
Selling, general and administrative expenses
|
22,873,250 | 12,089,937 | ||||||
|
Stock-based compensation
|
1,029,875 | - | ||||||
|
Total operating expenses
|
23,903,125 | 12,089,937 | ||||||
|
Income from operations
|
15,100,843 | 7,761,642 | ||||||
|
Other Income (Expense)
|
||||||||
|
Interest income
|
2,899 | 2,917 | ||||||
|
Interest expense
|
- | (40,615 | ) | |||||
|
Loss on disposal of building
|
(357,789 | ) | - | |||||
|
Foreign exchange loss
|
2,809 | - | ||||||
|
Total other Income (Expense)
|
(352,081 | ) | (37,698 | ) | ||||
|
Income before income taxes
|
14,748,762 | 7,723,944 | ||||||
|
Provision for income taxes
|
4,250,922 | 1,033,402 | ||||||
|
Net income
|
$ | 10,497,840 | $ | 6,690,542 | ||||
|
Deemed dividend from beneficial conversion feature of preferred stock
|
(2,670,517 | ) | (1,462,240 | ) | ||||
|
Net income applicable to common stockholders
|
$ | 7,827,323 | $ | 5,228,302 | ||||
|
Net income per common stock
|
||||||||
|
Basic
|
$ | 0.34 | $ | 0.23 | ||||
|
Diluted
|
$ | 0.32 | $ | 0.22 | ||||
|
Weighted average number of common stocks outstanding
|
||||||||
|
Basic
|
23,255,391 | 22,369,434 | ||||||
|
Diluted
|
24,338,471 | 23,257,470 | ||||||
The accompanying notes are an integral part of these financial statements.
|
BIOSTAR PHARMACEUTICALS, INC.
|
||||||||||||||||||||||||||||||||||||
|
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
|
||||||||||||||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||||||||||||||
|
Additional
|
Other
|
Total
|
||||||||||||||||||||||||||||||||||
|
Capital Stock
|
Preferred Stock
|
Paid-in
|
Statutory
|
Retained
|
Comprehensive
|
Stockholders'
|
||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Reserve
|
Earnings
|
Income (Loss)
|
Equity
|
||||||||||||||||||||||||||||
|
BALANCE, JANUARY 1, 2008
|
22,152,311 | $ | 22,152 | 72,500 | $ | 725,000 | $ | 8,244,017 | $ | 902,113 | $ | 6,451,623 | $ | (53,517 | ) | $ | 16,291,388 | |||||||||||||||||||
|
Conversion of Series A preferred stock
|
1,088,588 | 1,089 | (72,500 | ) | (725,000 | ) | 723,911 | - | - | - | - | |||||||||||||||||||||||||
|
Deemed dividend on Series A preferred stock
|
- | - | - | - | 1,462,240 | - | (1,462,240 | ) | - | - | ||||||||||||||||||||||||||
|
Transfer to statutory reserve
|
- | - | - | - | - | 683,270 | (683,270 | ) | - | - | ||||||||||||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||||||
|
Net income
|
- | - | - | - | - | - | 6,690,542 | - | 6,690,542 | |||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
- | - | - | - | - | - | - | 291,175 | 291,175 | |||||||||||||||||||||||||||
|
Total comprehensive income
|
- | - | - | - | - | - | - | - | 6,981,717 | |||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2008
|
23,240,899 | $ | 23,241 | - | $ | - | $ | 10,430,168 | $ | 1,585,383 | $ | 10,996,655 | $ | 237,658 | $ | 23,273,105 | ||||||||||||||||||||
|
Issuance of Series B preferred stock
|
- | - | 3,060,000 | 3,060 | 5,670,940 | - | - | - | 5,674,000 | |||||||||||||||||||||||||||
|
Deemed dividend on Series B preferred stock
|
- | - | - | - | 2,670,517 | - | (2,670,517 | ) | - | - | ||||||||||||||||||||||||||
|
Stock-based compensation
|
133,900 | 134 | - | - | 1,029,741 | - | - | - | 1,029,875 | |||||||||||||||||||||||||||
|
Transfer to statutory reserve
|
- | - | - | - | - | 1,275,302 | (1,275,302 | ) | - | - | ||||||||||||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||||||
|
Net income
|
- | - | - | - | - | - | 10,497,840 | - | 10,497,840 | |||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
- | - | - | - | - | - | - | 445,051 | 445,051 | |||||||||||||||||||||||||||
|
Total comprehensive income
|
- | - | - | - | - | - | - | - | 10,942,891 | |||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2009
|
23,374,799 | $ | 23,375 | 3,060,000 | $ | 3,060 | $ | 19,801,366 | $ | 2,860,685 | $ | 17,548,676 | $ | 682,709 | $ | 40,919,871 | ||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
|
BIOSTAR PHARMACEUTICALS, INC.
|
||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net income
|
$ | 10,497,840 | $ | 6,690,542 | ||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
provided by operating activities:
|
||||||||
|
Depreciation and amortization
|
607,649 | 645,846 | ||||||
|
Loss on disposal of building
|
357,789 | - | ||||||
|
Stock-based compensation
|
1,029,875 | - | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(8,069,157 | ) | (7,187,746 | ) | ||||
|
Inventories
|
(23,535 | ) | (92,443 | ) | ||||
|
Prepaid expenses and other receivables
|
(1,490,765 | ) | 10,528 | |||||
|
Accounts payable and accrued expenses
|
1,175,997 | 1,593,165 | ||||||
|
Other deposits
|
- | 17,236 | ||||||
|
VAT tax payable
|
521,357 | 205,829 | ||||||
|
Income tax payable
|
1,066,459 | 210,249 | ||||||
|
Net cash provided by operating activities
|
5,673,509 | 2,093,206 | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Purchase of property and equipment
|
(16,561 | ) | (51,770 | ) | ||||
|
Construction in progress
|
(1,169,440 | ) | - | |||||
|
Acquisition of land use right
|
(1,169,440 | ) | (276,531 | ) | ||||
|
Deposit paid for acquisition of land use right
|
- | (2,872,635 | ) | |||||
|
Proceeds from disposal of property and equipment
|
143,256 | - | ||||||
|
Deposit paid for acquisition of business
|
(1,315,620 | ) | - | |||||
|
Net cash used in investing activities
|
(3,527,805 | ) | (3,200,936 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Repayment of short-term bank loan
|
- | (545,801 | ) | |||||
|
Proceeds from issuance of preferred stock
|
5,674,000 | - | ||||||
|
Net cash provided by (used in) financing activities
|
5,674,000 | (545,801 | ) | |||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(316 | ) | 125,428 | |||||
|
Net increase (decrease) in cash and cash equivalents
|
7,819,388 | (1,528,103 | ) | |||||
|
Cash and cash equivalents, beginning balance
|
758,316 | 2,286,419 | ||||||
|
Cash and cash equivalents, ending balance
|
$ | 8,577,704 | $ | 758,316 | ||||
|
SUPPLEMENTAL DISCLOSURES:
|
||||||||
|
Interest payments
|
$ | - | $ | 40,615 | ||||
|
Income tax payments
|
$ | 3,184,462 | $ | 823,153 | ||||
|
SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
||||||||
|
Conversion of preferred stock to common stock
|
$ | - | $ | 725,000 | ||||
|
Prior year deposit received for disposed building
|
$ | 2,561,074 | $ | - | ||||
|
Prior year deposit paid for acquisition of land use right
|
$ | 2,923,600 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 - ORGANIZATION
Biostar Pharmaceuticals, Inc. (“Biostar” or “the Company”) was incorporated in the State of Maryland on March 27, 2007. On June 15, 2007, Biostar formed Shaanxi Biostar Biotech Ltd. (“Shaanxi Biostar”). Shaanxi Biostar is a wholly-owned subsidiary of Biostar and a limited liability company organized under the laws of the People's Republic of China (the "PRC").
On November 1, 2007, Shaanxi Biostar entered into a series of agreements including a Management Entrustment Agreement, a Shareholders’ Voting Proxy Agreement, an Exclusive Option Agreement and a Share Pledge Agreement (collectively the “Agreements”) with Shaanxi Aoxing Pharmaceutical Co., Ltd. ("Aoxing Pharmaceutical") and its shareholders (the “Transaction”). Aoxing Pharmaceutical is a corporation formed under the laws of the PRC. According to these Agreements, Shaanxi Biostar acquired management control of Aoxing Pharmaceutical whereby Shaanxi Biostar is entitled to all of the net profits of Aoxing Pharmaceutical as a management fee, and is obligated to fund Aoxing Pharmaceutical’s operations and pay all of the debts. In exchange for entering into the Agreements, on November 1, 2007, the Company issued 19,832,311 shares of its common stock to Aoxing Pharmaceutical owners, representing approximately 90% of the Company’s common stock outstanding after the Transaction. Consequently, the owners of Aoxing Pharmaceutical own a majority of the Company's common stock immediately following the Transaction. Therefore, the Transaction is being accounted for as a reverse acquisition, and Aoxing Pharmaceutical is deemed to be the accounting acquirer in the reverse acquisition.
The Agreements provide that Shaanxi Biostar has controlling interest in Aoxing Pharmaceutical as defined by Financial Accounting Standard Board (“FASB”) Interpretation No. 46R “Consolidation of Variable Interest Entities” (“FIN 46R”), included in the FASB Accounting Standards Codification (”Codification”) as Accounting Standards Codification (“ASC”) 810, Consolidation, an Interpretation of Accounting Research Bulletin (“ARB”) No. 51, included in the Codification as ASC 810, Consolidation, which requires Shaanxi Biostar to consolidate the financial statements of Aoxing Pharmaceutical and ultimately consolidate with its parent company, Biostar (see Note 2 “Principles of Consolidation”).
The Company, through its subsidiary and the Agreements with Aoxing Pharmaceutical, is engaged in the business of discovering, developing, manufacturing and marketing of over-the-counter (“OTC”) and prescription pharmaceutical products as well as nutrient products for a variety of diseases and conditions such as hepatitis, gynecopathy and various male diseases in the PRC.
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“US GAAP”).
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted FIN 46R which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In determining Aoxing Pharmaceutical is the VIE of Shaanxi Biostar, the Company considered the following indicators, among others:
|
·
|
Shaanxi Biostar has the full right to control and administrate the financial affairs and daily operation of Aoxing Pharmaceutical and has the right to manage and control all assets of Aoxing Pharmaceutical. The equity holders of Aoxing Pharmaceutical as a group have no right to make any decision about Aoxing Pharmaceutical’s activities without the consent of Shaanxi Biostar.
|
|
·
|
Shaanxi Biostar was assigned all voting rights of Aoxing Pharmaceutical and has the right to appoint all directors and senior management personnel of Aoxing Pharmaceutical. The equity holders of Aoxing Pharmaceutical possess no substantive voting rights.
|
|
·
|
Shaanxi Biostar will provide financial support if Aoxing Pharmaceutical requires additional funds to maintain its operations and to repay its debts.
|
|
·
|
Shaanxi Biostar is entitled to a management fee equal to Aoxing Pharmaceutical’s net profits and is obligated to assume all operation risks and bear all losses of Aoxing Pharmaceutical. Therefore, Shaanxi Biostar is the primary beneficiary of Aoxing Pharmaceutical.
|
Aoxing Pharmaceutical is wholly owned by the majority stockholders of the Company. The further capital provided to Aoxing Pharmaceutical by the Company was recorded as interest-free loan to Aoxing Pharmaceutical. There was no written note to this loan and the loan is not interest bearing and was eliminated during consolidation. Under the terms of the Agreements, the shareholders of Aoxing Pharmaceutical are required to transfer their ownership of Aoxing Pharmaceutical to the Company’s subsidiary in China when permitted by PRC laws and regulations or to designees of the Company at any time when the Company considers it is necessary to acquire Aoxing Pharmaceutical. In addition, the shareholders of Aoxing Pharmaceutical have pledged their shares in Aoxing Pharmaceutical as collateral to secure these Agreements.
Foreign Currency
The Company’s reporting currency is the U.S. dollar (“$”). The Company’s operation in China uses Chinese Yuan Renminbi (“RMB”) as its functional currency. The financial statements of the subsidiary are translated into U.S. dollars in accordance with Statement of Financial Accounts Standards (“SFAS”) No. 52, Foreign Currency Translation, included in the Codification as ASC 830, Foreign Currency Matters. According to the topic, all assets and liabilities were translated at the current exchange rate, stockholders equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with SFAS No. 130, Reporting Comprehensive Income as a Component of Shareholders Equity, included in the Codification as ASC 220, Comprehensive Income. Foreign exchange transaction gains and losses are reflected in the income statement. During the year ended December 31, 2009, the foreign currency translation adjustments to the Company’s other comprehensive income were $445,051.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s management evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Terms of the sales vary. Reserves are recorded primarily on a specific identification basis. Allowance for doubtful accounts amounted to $133,720 and $134,789 as at December 31, 2009 and 2008, respectively.
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market. The Management compares the cost of inventories with the market value and allowance is made for writing down the inventories to market value, if lower. Inventories consist of the following:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Raw materials
|
$ | 261,868 | $ | 29,387 | ||||
|
Work in process
|
41,010 | 8,597 | ||||||
|
Finished goods
|
37,200 | 277,761 | ||||||
| $ | 340,078 | $ | 315,745 | |||||
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Real property
|
30 years
|
|
Machinery & equipment
|
5-10 years
|
|
Leasehold improvements
|
30 years
|
|
Computers & office equipment
|
5-10 years
|
Property, plant & equipment consist of the following:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Construction in progress
|
$ | 1,169,440 | $ | - | ||||
|
Real property
|
1,528,543 | 4,393,371 | ||||||
|
Machinery & equipment
|
542,195 | 541,063 | ||||||
|
Leasehold improvements
|
1,956,289 | 1,946,831 | ||||||
|
Furniture & fixtures
|
63,420 | 62,982 | ||||||
|
Vehicle
|
24,970 | 19,437 | ||||||
| 5,284,857 | 6,963,684 | |||||||
|
Less: Accumulated depreciation
|
(943,940 | ) | (1,033,217 | ) | ||||
| $ | 4,340,917 | $ | 5,930,467 | |||||
At December 31, 2009, expenditures of $1,169,440 had been incurred for construction of raw material processing plant.
In July 2009, we consummated a disposal of a building we used prior to moving to our current location for RMB 18,500,000 ($2,705,786), about RMB 17,520,000 ($2,562,452) of the sales proceeds was received in advance in year 2007, with the remaining balance being received in year 2009. Consequently, in the year ended December 31, 2009, we recorded a loss on disposal of building of $357,789.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Intangible Assets
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from ten to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No impairments of intangible assets have been identified during any of the periods presented. The land use rights will expire between year 2056 and year 2060. The proprietary technologies were contributed by four stockholders of the Company and relate to the production of the Company's five state approved drugs. All of the Company’s intangible assets are subject to amortization with estimated lives of:
|
Land use right
|
50 years
|
|
Proprietary technologies
|
10 years
|
The components of finite-lived intangible assets are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Land use right
|
$ | 10,571,810 | $ | 6,478,769 | ||||
|
Proprietary technologies
|
1,511,544 | 1,511,544 | ||||||
| 12,083,354 | 7,990,313 | |||||||
|
Less: Accumulated amortization
|
(951,673 | ) | (624,548 | ) | ||||
| $ | 11,131,681 | $ | 7,365,765 | |||||
The estimated future amortization expenses related to intangible asset as of December 31, 2009 are as follows:
|
Years Ending December 31,
|
|
|
2010
|
$ 327,200
|
|
2011
|
327,200
|
|
2012
|
327,200
|
|
2013
|
327,200
|
|
2014
|
327,200
|
|
Thereafter
|
9,495,681
|
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Long-Lived Assets
Effective January 1, 2002, the Company adopted SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, included in the Codification as ASC 360, Property, Plant, and Equipment, which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of, and the accounting and reporting provisions of APB Opinion No. 30, Reporting the Results of Operations for a Disposal of a Segment of a Business, included in the Codification as ASC 225, Income Statement. The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with ASC 360. ASC 360 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. During the year ended December 31, 2009, there was $357,789 loss on disposal of a plant building.
Fair Value of Financial Instruments
SFAS No. 107, Disclosures about Fair Value of Financial Instruments, included in the Codification as ASC 825, Financial Instruments, requires that the Company discloses estimated fair values of financial instruments. The carrying amounts reported in the balance sheets for current assets and current liabilities qualifying as financial instruments are a reasonable estimate of fair value.
Value Added Tax Payable
The Company is subject to a value added tax rate of 17% on product sales by the PRC. Value added tax payable is computed net of value added tax paid on purchases for all sales in the PRC.
Revenue Recognition
The Company’s revenue recognition policies are in compliance with Staff Accounting Bulletin (“SAB”) 104, included in the Codification as ASC 605, Revenue Recognition. Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist and collectability is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue.
The Company does not allow its customers to return products. The Company’s customers can exchange products only if they are damaged in transportation.
Stock-Based Compensation
The Company has elected to use the Black-Scholes-Merton (“BSM”) pricing model to determine the fair value of stock options on the dates of grant. Also, the Company recognizes stock-based compensation using the straight-line method.
The Company values stock awards using the market price on or around the date the shares were awarded and includes the amount of compensation as a period compensation expense.
For the years ended December 31, 2009 and 2008, the Company recognized stock-based compensation of $1,029,875 and $0, respectively.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Advertising
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company expenses all advertising costs as incurred. For the years ended December 31, 2009 and 2008, the Company incurred advertising expense of $9,947,551 and $5,051,404, respectively.
Research and Development
Remuneration of research and development staff and material costs incurred for internal research and development activities and payments made to third parties in connection with research and development collaborations prior to regulatory approval are expensed as incurred. Payments made to third parties subsequent to regulatory approval are capitalized and amortized over the shorter of the remaining license period or product patent life. In the years ended December 31, 2009 and 2008, the Company incurred research and development expense of $511,630 and $31,599, respectively.
Income Taxes
The Company utilizes SFAS No. 109, Accounting for Income Taxes, included in the Codification as ASC 740, Income Taxes, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
On January 1, 2007, The Company adopted the provisions of FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes (“FIN 48”), included in the Codification as ASC 740, Income Taxes. The topic addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on derecognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
Net Income Per Share
Basic net income per share is computed on the basis of the weighted average number of common stocks outstanding during the period.
Diluted net income per share is computed on the basis of the weighted average number of common stocks and common stock equivalents outstanding. Dilutive securities having an anti-dilutive effect on diluted net income per share are excluded from the calculation.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Dilution is computed by applying the treasury stock method for options and warrants. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
Dilution is computed by applying the if-converted method for convertible preferred stocks. Under this method, the convertible preferred stocks are assumed to be converted at the beginning of the period (or at the time of issuance, if later), and preferred dividends (if any) shall be added back to determine income applicable to common stock. The shares issuable upon conversion shall be added to weighted average number of common stock outstanding. Conversion shall be assumed only if it reduces earnings per share (or increases loss per share).
Comprehensive income
Comprehensive income is defined as the change in equity of a company during a period from transactions and other events and circumstances excluding transactions resulting from investments from owners and distributions to owners. For the Company, comprehensive income for the periods presented includes net income, foreign currency translation adjustments.
Statement of Cash Flows
In accordance with SFAS No. 95, Statement of Cash Flows, included in the Codification as ASC 230, Statement of Cash Flows, cash flows from the Company’s operations is based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited.
Segment Reporting
SFAS No. 131, Disclosure about Segments of an Enterprise and Related Information, included in the Codification as ASC 280, Segment Reporting, requires use of the management approach model for segment reporting. The management approach model is based on the way a company's management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Recent accounting pronouncements
Effective 1 July 2009, the Company adopted ASC Topic 105, “the FASB Accounting Standards Codification” (“Codification”) (formerly SFAS No. 168). Codification will become the source of authoritative US GAAP recognized by the FASB to be applied by nongovernmental entities. Once the Codification is in effect, all of its content will carry the same level of authority. The adoption of this statement does not have a material effect on the Company's financial statements. However, because the Codification completely replaces existing standards, it will affect the way US GAAP is referenced within the Company’s financial statements and accounting policies.
Effective April 1, 2009, the Company adopted (i) ASC Topic 320, “Recognition of Presentation of Other-Than-Temporary Impairments” (formerly FASB Staff Positions (“FSP”) FAS No. 115-2 and FAS No. 124-2), (ii) ASC Topic 825, “Interim Disclosures about Fair Value of Financial Instruments” (formerly FSP FAS No. 107-1 and Accounting Principles Board Opinion (“APB”) No. 28-1), and (iii) ASC Topic 820, “Determining the Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” (formerly FSP FAS No. 157-4). ASC Topic 320 amends the other-than-temporary impairment guidance in the US GAAP for debt securities to modify the requirement for recognizing other-than-temporary impairments, change the existing impairment model, and modify the presentation and frequency of related disclosures. ASC Topic 825 requires disclosures about fair value of financial instruments for interim reporting periods as well as in annual financial statements. ASC Topic 820 provides additional guidance for estimating fair value in accordance with ASC Topic 820, “Fair Value Measurements” (formerly SFAS No. 157). The adoption of these topics does not have a material effect on the Company’s financial statements.
Effective April 1, 2009, the Company adopted ASC Topic 855, “Subsequent Events” (formerly SFAS No. 165), which establishes standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. It requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date, i.e., whether that date represents the date the financial statements were issued or were available to be issued. Effective February 24, 2010, the FASB issued Accounting Standards Update (“ASU”) 2010-09 which amend guidance on subsequent events to remove the requirement for Securities and Exchange Commission (“SEC”) filers (as defined in ASU 2010-09) to disclose the date through which an entity has evaluated subsequent events. This change alleviates potential conflicts with current SEC guidance. An SEC filer is still required to evaluate subsequent events through the date financial statements are issued, but disclosure of that date is no longer required. The amendments in ASU 2010-09 became effective upon issuance of the guidance. The adoption of this topic does not have a material effect on the Company’s financial statements.
Effective January 1, 2009, the Company adopted ASC Topic 260, “Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities” (formerly SFAS No. 141). ASC Topic 260 addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share. The adoption of this topic does not have a material effect on the Company’s financial statements.
In August 2009, the FASB issued ASC Topic 820, “Measuring Liabilities at Fair Value”, with respect to the fair value measurement of liabilities. ASC Topic 820 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following techniques: (1) the quoted price of the identical liability when traded as an asset, (2) the quoted prices for similar liabilities or similar liabilities when traded as assets, and (3) another valuation technique (e.g., a market approach or income approach) including a technique based on the amount an entity would pay to transfer the identical liability, or a technique based on the amount an entity would receive to enter into an identical liability. This topic will be effective for periods beginning after October 1, 2009 with early adoption permitted. The Company has not elected to early adopt this topic and is evaluating the impact that this topic will have on the Company’s financial statements.
In June 2009, the FASB issued ASC Topic 860, “Accounting for Transfers of Financial Assets - an amendment of FASB Statement No. 140” (formerly SFAS No. 166). ASC Topic 860 amends the de-recognition accounting and disclosure guidance relating to SFAS 140. ASC Topic 860 eliminates the exemption from consolidation for qualifying special-purpose entity (“QSPE”), it also requires a transferor to evaluate all existing QSPE to determine whether it must be consolidated in accordance with ASC Topic 810. This topic will be effective for periods beginning after November 15, 2009 with early adoption permitted. The Company has not elected to early adopt thus topic and is evaluating the impact that this topic will have on the Company’s financial statements.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In June 2009, the FASB issued ASC Topic 810, “Amendments to FASB Interpretation No. 46(R)” (formerly SFAS No. 167), which amends FASB Interpretation No. 46 (revised December 2003) to address the elimination of the concept of a qualifying special purpose entity. ASC Topic 810 also replaces the quantitative-based risks and rewards calculation for determining which enterprise has a controlling financial interest in a variable interest entity with an approach focused on identifying which enterprise has the power to direct the activities of a variable interest entity and the obligation to absorb losses of the entity or the right to receive benefits from the entity. Additionally, ASC Topic 810 provides more timely and useful information about an enterprise’s involvement with a variable interest entity. This topic will be effective for periods beginning after November 15, 2009 with early adoption permitted. The Company has not elected to early adopt this topic and is evaluating the impact that this topic will have on the Company’s financial statements.
Note 3 – PREPAID EXPENSES AND OTHER RECEIVABLES
Prepaid expenses and other receivables include deposits for research and development, prepaid advertising expenses and professional fee. As of December 31, 2009, the Company prepaid $767,785 for research and development and $702,042 for advertising expenses.
Note 4 – DEPOSITS
At December 31, 2009, deposits include $438,776 for acquiring a medical equipment and nutrients manufacturer and $877,552 refundable deposit for acquiring a medicine packaging manufacturer.
Note 5 – STOCKHOLDERS’ EQUITY
Series B Convertible Preferred Stock
On November 2, 2009 (the “Closing Date”), the Company entered into and closed on a securities purchase agreement with certain accredited investors (the “Investors”) pursuant to which the Investors purchased 2,060,000 shares of its series B convertible preferred stock (“Series B Preferred Stock”) with attached warrants to purchase a total of 500,000 shares of its common stock (the “Warrants”) for an aggregate purchase price of $3,605,000. The Series B Preferred Stock is convertible into 2,060,000 shares of the Company’s common stock.
On November 18, 2009, the Company entered into and closed on a securities purchase agreement with certain accredited investors pursuant to which the investors purchased 1,000,000 shares of its Series B Preferred Stock for an aggregate purchase price of $2,120,000. The Series B Preferred Stock is convertible into 1,000,000 shares of the Company’s common stock.
The Series B Preferred Stock does not pay annual dividends and shall not have any voting rights except as required by law. No dividends shall be declared or payable with respect to the Company’s common stock while the Series B Preferred Stock is outstanding. The Company shall not redeem or purchase any shares of common stock or any other class or series of capital stock which is junior to or on parity with the Series B Preferred Stock while the Series B Preferred Stock is outstanding.
The Series B Preferred Stock is subject to full ratchet and anti-dilution adjustment for subsequent lower price issuances by the Company and both the conversion price of the Series B Preferred Stock and the exercise price of the Warrants are subject to customary adjustments provisions for stock splits, stock dividends, recapitalizations and the like. The full ratchet and anti-dilution protection provided for in the Series B Preferred Stock for subsequent lower price issuances shall be null and void and shall have no further force or effect if EITF 07-5, as such may amended, supplemented or modified by any accounting guidance and/or announcement(s) issued by the Financial Accounting Standards Board, the Emerging Issues Task Force or any other regulatory authority, will adversely effect the Company’s financial condition as a result of such provision.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company has agreed that if its common stock is not listed on a national securities exchange within one hundred and forty-five (145) days of the Closing Date, the Company shall pay the Investors as liquidated damages and not as a penalty, an amount equal to twelve percent (12%) per annum, based on the lesser of (i) the Purchase Price or (ii) that percentage of the Purchase Price which the shares of common stock issuable upon conversion of the Series B Preferred Stock and issuable upon exercise of the Warrants bears to the number of shares of common stock initially issuable upon conversion of the Series B Preferred Stock; provided, however, such payment of liquidated damages shall not accrue until the Company fulfills all of its requirements for listing on a national securities exchange.
As the Series B Preferred Stock does not require redemption by the Company, upon issuance, the Company recorded a one-time deemed dividend and as an increase in additional paid-in capital, the intrinsic value of the beneficial conversion feature (the "BCF") of $2,670,517. The intrinsic value of the BCF is the difference between the fair value of the common stock underlying the Series B Preferred Stock at issuance and the amount of proceeds to be allocated to the preferred stock. The proceeds of $3,605,000 from the November 2, 2009 private placement were allocated to the preferred stock and warrants based on their relative fair values. The warrants were valued using the BSM model and recorded in additional paid-in capital.
Series A Convertible Preferred Stock
Between May 29, 2007 and June 4, 2007, the Company raised $725,000 from two investors in a private placement of its series A convertible preferred stock ($0.001 par value) (the “Series A Preferred Stock”) at a purchase price of $10.00 per unit, for a unit consisting of one share of preferred stock and one warrant.
The Series A Preferred Stock was not entitled to any dividends. The Series A Preferred Stock has no voting rights except that the approval of the holders of at least 51% of the Series A Preferred Stock is required for the authorization, creation, or issuance, or any increase in the authorized or issued amount, of any class or series of stock ranking equal or prior to the Series A Preferred Stock; or the amendment, alteration, or repeal of any of the provisions of the Articles of Incorporation of the corporation which would alter or change the powers, preferences, or special rights of the shares of the Series A Preferred Stock so as to affect them adversely. On liquidation the holders are entitled to receive $10 per share (out of available assets) before any distribution or payment can be made to the holders of any junior securities.
Each share of Series A Preferred Stock is convertible into shares of common stock at the option of the holder at a conversion price of $0.67 per share. The aggregate number of shares of common stock which may be issued upon conversion of the Series A Preferred Stock shall be no more than 2 million shares.
On October 20, 2008, the 72,500 shares of Series A Preferred Stock issued and outstanding on that date were converted into 1,088,588 shares of common stock. In connection with the conversion, the Company recorded a deemed dividend of $1,462,240 for the BCF embedded in the Series A Preferred Stock for the year ended December 31, 2008. The $725,000 proceeds received from the Series A Preferred Stock were allocated to the preferred stock and warrants based on their relative fair values. The warrants were valued using the BSM model and recorded in additional paid-in capital.
F-15
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Common stock
At December 31, 2009, the Company has 100,000,000 shares of common stock authorized and 23,374,799 shares issued and outstanding at par value $0.001 per share.
Warrants
The Company has issued warrants to purchase its common stock in the 2007 private placement of Series A Preferred Stock. The warrants are exercisable for three years from the effective date of October 10, 2007 on which a conversion price is first determinable, at an exercise price of $1.00.
The Warrants issued in connection with the November 2, 2009 private placement of Series B Preferred Stock are exercisable for a period of five years from the date of issuance at an initial exercise price of $3.00. The Company shall have the right at any time, on at least forty-five (45) day written notice, to redeem the outstanding Warrants at a price of one cent ($0.01) per share provided the market price of the Company’s common stock shall equal or exceed $4.50 on each trading day for the consecutive twenty (20) trading days in the period ending on the trading day prior to the date that the Company intends to redeem the Warrants.
The warrants are classified as equity and amounts attributable to the warrants are classified within additional paid-in capital.
The following table summarizes the activities for the warrants for the years ended December 31, 2009 and 2008.
|
Weighted
|
||||||||
|
Underlying
|
Average
|
|||||||
|
Shares
|
Exercise Price
|
|||||||
|
Warrants outstanding, January 1, 2008
|
1,088,588 | $ | 1.00 | |||||
|
Issued
|
- | |||||||
|
Warrants outstanding, December 31, 2008
|
1,088,588 | $ | 1.00 | |||||
|
Issued
|
500,000 | $ | 3.00 | |||||
|
Warrants outstanding, December 31, 2009
|
1,588,588 | $ | 1.63 | |||||
|
Exercisable as of December 31, 2009
|
1,588,588 | $ | 1.63 | |||||
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Stock Options
The Company’s board of directors approved its 2009 stock plan (“Stock Plan”) under which it may grant incentive and nonqualified stock options, stock awards or restricted stocks to eligible participants. Options are generally granted for a term of 5 years. Except for the options granted to the Company’s existing management on October 22, 2009, options granted under the Stock Plan generally vest annually in 3 equal installments, the first being on the first anniversary of the grant contingent upon employment with the Company on the vesting date. Options granted on October 22, 2009 vest annually in 3 equal installments, the first being on the grant date.
Under the Stock Plan, the Company also issued stock awards. For the year ended December 31, 2009, the Company issued 121,900 shares of common stock to its PRC employees and 12,000 shares to a former director.
The following table presents the weighted-average assumptions used to estimate the fair values of the stock options granted in the period presented:
|
2009
|
|
|
Risk-free interest rate (%)
|
1.7%
|
|
Expected dividend yield (%)
|
-
|
|
Expected option life (years)
|
3.4
|
|
Expected volatility (%)
|
93.3%
|
|
Weighted average grant date fair value
|
$1.70
|
The following table summarizes the activities for the Company’s options for the year ended December 31, 2009:
|
Options Outstanding
|
||||||||||||
|
Number of Shares
|
Weighted-Average Exercise Price
|
Weighted-Average Remaining Life (in years)
|
||||||||||
|
Balance at January 1, 2009
|
- | |||||||||||
|
Granted
|
1,080,000 | $ | 2.77 | |||||||||
|
Balance at December 31, 2009
|
1,080,000 | $ | 2.77 | 4.7 | ||||||||
|
Vested and exercisable as of December 31, 2009
|
326,667 | $ | 2.60 | 4.8 | ||||||||
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table summarizes additional information regarding outstanding, and exercisable and vested stock options at December 31, 2009:
|
Exercise Price
|
Options Outstanding
|
Weighted-Average Remaining Life (in years)
|
Options Exercisable and Vested
|
|||||||||||
| $ | 2.60 | 980,000 | 4.8 | 326,667 | ||||||||||
| $ | 4.45 | 100,000 | 4.0 | - | ||||||||||
| 1,080,000 | 4.7 | 326,667 | ||||||||||||
NOTE 6 - EARNINGS PER SHARE
The following table sets forth the computation of basic and diluted earnings per share of common stock:
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Basic net income per share:
|
||||||||
|
Numerator:
|
||||||||
|
Net income
|
$ | 10,497,840 | $ | 6,690,542 | ||||
|
Deemed dividend from beneficial conversion feature of preferred stock
|
(2,670,517 | ) | (1,462,240 | ) | ||||
|
Net income applicable to common stockholders
|
$ | 7,827,323 | $ | 5,228,302 | ||||
|
Denominator:
|
||||||||
|
Weighted average number of common stock outstanding
|
23,255,391 | 22,369,434 | ||||||
|
Basic net income per share
|
$ | 0.34 | $ | 0.23 | ||||
|
Diluted net income per share:
|
||||||||
|
Numerator:
|
||||||||
|
Net income
|
$ | 10,497,840 | $ | 6,690,542 | ||||
|
Deemed dividend from beneficial conversion feature of preferred stock
|
(2,670,517 | ) | (1,462,240 | ) | ||||
|
Net income applicable to common stockholders
|
$ | 7,827,323 | $ | 5,228,302 | ||||
|
|
||||||||
|
Denominator:
|
||||||||
|
Weighted average number of common stock outstanding
|
23,255,391 | 22,369,434 | ||||||
|
Weighted average effect of dilutive securities:
|
||||||||
|
Convertible preferred stocks
|
461,918 | 871,465 | ||||||
|
Stock warrants
|
604,638 | 16,571 | ||||||
|
Make good shares
|
16,524 | - | ||||||
|
Shares used in computing diluted net income per share
|
24,338,471 | 23,257,470 | ||||||
|
Diluted net income per share
|
$ | 0.32 | $ | 0.22 | ||||
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As an inducement for the Investors to purchase the Series B Preferred Stock, on November 2, 2009, the Company entered into a Make Good Securities Escrow Agreement with the Investors and Sichenzia Ross Friedman Ference LLP (the “Escrow Agent”) whereby the Company has agreed to deliver to the Escrow Agent (i) resolutions executed by the Board of Directors of the Company and (ii) irrevocable instructions of the Company’s transfer agent executed by the Company for the issuance of up to an additional 2,000,000 shares of common stock and/or Series B Preferred Stock (the “Make Good Shares”), at the option of the Investors, in the event the Company fails to achieve certain financial performance thresholds for the 12-month periods ended December 31, 2009 and December 31, 2010.
The financial performance threshold for the year ended December 31, 2009 is income from operations at no less than $15,900,000. As the Company’s year 2009 actual income from operations is $15,100,843 which is about 5% shortfall of the $15,900,000 threshold, the Company will have to deliver about 100,523 Make Good Shares to the Investors. The weighted average number of the 100,523 Make Good Shares was included in the diluted earnings per share calculation.
Note 7 - INCOME TAXES
The Company was incorporated in the United States of America (“USA”) and has operations in two tax jurisdictions - the USA and the PRC. The Company generated substantially all of its net income from its PRC operations for the years ended December 31, 2009 and 2008 and has recorded income tax provision for the periods.
The provision for income taxes consists of the following:
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current:
|
||||||||
|
Domestic
|
$ | - | $ | - | ||||
|
Foreign
|
4,250,922 | 1,033,402 | ||||||
|
Deferred
|
- | - | ||||||
|
Provision for income taxes
|
$ | 4,250,922 | $ | 1,033,402 | ||||
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The reconciliation of USA statutory income tax rate to the Company’s effective income tax rate is as follows:
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Income tax at USA statutory rate (34%)
|
$ | 5,014,579 | $ | 2,626,141 | ||||
|
State tax, net of federal effect
|
- | - | ||||||
|
Foreign rate differential
|
(1,234,130 | ) | (1,641,074 | ) | ||||
|
Change in valuation allowance
|
470,473 | 48,335 | ||||||
|
Provision for income taxes
|
$ | 4,250,922 | $ | 1,033,402 | ||||
As of December 31, 2009, the Company had federal and state net operating loss carryforwards of approximately $1,238,499 available to offset future taxable income in the USA. The net operating loss carryforwards will expire, if unused, in varying amounts through the year ending December 31, 2029. The deferred tax assets for the USA operation at December 31, 2009 consists mainly of net operating loss carryforwards and for which a full valuation allowance has been provided, as the management believes it is more likely than not that these assets will not be realized in the future.
The Company’s subsidiary and VIE were incorporated in the PRC which is governed by the Income Tax Law of the PRC and various local income tax laws (the “Income Tax Laws"). Effective January 1, 2008, China adopted a uniform tax rate of 25% for all enterprises (including foreign-invested enterprises).
Under the PRC Income Tax Laws, the Company’s VIE was granted a tax exemption from income tax from January 1, 2005 to December 31, 2006 and followed by a 50% reduction in the tax rate from January 1, 2007 to December 31, 2008. The deferred tax assets for the Company’s China subsidiary and VIE were immaterial at December 31, 2009.
Uncertain Tax Positions
Interest associated with unrecognized tax benefits are classified as income tax and penalties are classified in selling, general and administrative expenses in the statements of operations.
For the years ended December 31, 2009 and 2008, the Company had no unrecognized tax benefits and related interest and penalties expenses. Currently, the Company is not subject to examination by major tax jurisdictions.
Note 8 - STATUTORY RESERVES
The Company’s subsidiary and VIE in the PRC are required to make appropriations to certain non-distributable reserve funds. In accordance with the laws and regulations applicable to China’s Foreign Investment Enterprises and the China Company Laws, an enterprise’s income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves. The proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund, not to exceed 50 percent of registered capital.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of December 31, 2009 and 2008, the Company’s VIE had allocated $2,860,685 and $1,585,383 to these non-distributable reserve funds, respectively.
Note 9 - OTHER COMPREHENSIVE INCOME
Balance of related after-tax components comprising accumulated other comprehensive income (loss) included in stockholders’ equity at December 31, 2009 and 2008 were as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Accumulated other comprehensive income (loss), beginning of period
|
$ | 237,658 | $ | (53,517 | ) | |||
|
Change in cumulative translation adjustment
|
445,051 | 291,175 | ||||||
|
Accumulated other comprehensive income, end of period
|
$ | 682,709 | $ | 237,658 | ||||
Note 10 - COMMITMENT
Research and Development (“R&D”) Agreement
On November 15, 2009 and December 19, 2009, the Company entered into two agreements with Fourth Military Medical University Xijing Hospital State Drug Clinical Research Center (“Fourth Military”) to conduct clinical trial for two new drugs. Pursuant to these agreements, the Company paid Fourth Military $767,785 in the fourth quarter of 2009 as deposit for clinical trial expenses and is obligated to pay Fourth Military additional $601,196 upon completion of the clinical trial.
Note 11 - SEGMENT INFORMATION
During the years ended December 31, 2009 and 2008, revenues of the Company represented net sales of pharmaceutical and nutrient products. No financial information by business segment is presented. Furthermore, as all revenues are derived from the PRC, no geographic information by geographical segment is presented. In addition, all tangible and intangible assets are located in the PRC.
BIOSTAR PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 12- CURRENT VULNERABILITY DUE TO CERTAIN RISK FACTORS
The Company’s major operations are carried out in the PRC, therefore the Company is subject to the risks not typically associated with entities operating in the United States of America. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC's economy. All of the following risks may impair the Company’s business operations. If any of the following risks actually occurs, the Company’s business, financial condition or results of operations could be materially adversely affected. In such case, investor may lose all or part of the investment. Additional risks include:
|
§
|
The Company may not be able to adequately protect and maintain its intellectual property.
|
|
§
|
The Company may not be able to obtain regulatory approvals for its products.
|
|
§
|
The Company may have difficulty competing with larger and better financed companies in the same sector. New legislative or regulatory requirements may adversely affect the Company’s business and operations. The Company is dependant on certain key existing and future personnel.
|
|
§
|
The Company’s growth is dependent on its ability to successfully develop, market, or acquire new drugs. The Company may be subject to product liability claims in the future.
|
|
§
|
Changes in the laws and regulations in the PRC may adversely affect the Company’s ability to conduct its business.
|
|
§
|
The Company may experience barriers to conducting business due to governmental policy.
|
|
§
|
Capital outflow policies in the PRC may hamper the Company’s ability to remit income to the United States.
|
|
§
|
Fluctuation of the Renminbi could materially affect the Company’s financial condition and results of operations.
|
|
§
|
The Company may face obstacles from the communist system in the PRC.
|
|
§
|
The Company may have difficulty establishing adequate management, legal and financial controls in the PRC.
|
|
§
|
Trade barriers and taxes may have an adverse affect on the Company’s business and operations. There may not be sufficient liquidity in the market for the Company’s securities in order for investors to sell their securities.
|
Note 13 – RECLASSIFICATION
Certain amounts in the prior year have been reclassified to conform to the current year’s presentation.
Note 14 – SUBSEQUENT EVENT
On March 18, 2010, the Company entered into an agreement to acquire 100% of Xi’an Meipude Bio-Technology Co., Ltd., a Xi’an-based medical equipment and nutrients manufacturer (“Meipude”) for RMB 7,850,000 ($1,148,131). The Company officially took control over the operations and the assets of Meipude on March 29, 2010, including its facilities, corporate documents and records, and business licenses and certificates. Meipude manufactures and distributes topical hernia treatment belts, also manufactures a nutraceutical product for treatment of gynecological inflammation in young and middle-aged women. Meipude has been manufacturing and selling its products in Shaanxi and an adjacent province since 2004. The initial accounting for the acquisition is not yet completed upon approval of these financial statements because financial information of the Meipude is pending for final verification and therefore, certain disclosures as required by ASC Topic 805, Business Combinations, have not been made.
