Attached files
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-Q
(Mark
One)
|
S
|
QUARTERLY
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
quarter ended: November 30, 2009
|
o
|
TRANSITION
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
transition period from ___________ to ___________
Commission
file number: 333-137888
GLOBAL
HEALTH VENTURES INC.
(Exact
name of registrant as specified in its charter)
|
Nevada
|
N/A
|
|
(State
or other jurisdiction of incorporation
or organization)
|
(I.R.S.
Employer Identification No.)
|
|
409
Granville Street, Suite 1023, Vancouver, British Columbia
Canada
|
V6C
1T2
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
(604)
324-4844
(Registrant’s
telephone number, including area code)
Indicate
by checkmark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months
(or for such shorter period that the registrant was required to file such
reports), and (2) has been subject to such filing requirements for the past 90
days. Yes x No
o
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes o No o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company filer.
See definition of “accelerated filer” and “large accelerated filer” in
Rule 12b-2 of the Exchange Act (Check one):
|
Large
Accelerated Filer o
|
Accelerated
Filer o
|
Non-Accelerated
Filer o
|
Smaller
Reporting Company x
|
Indicate
by check mark whether the registrant is a shell company as defined in Rule 12b-2
of the Exchange Act. Yes No x
State the
number of shares outstanding of each of the issuer’s classes of common equity,
as of January 15, 2010: 68,055,400 shares of common stock.
GLOBAL
HEALTH VENTURES INC. AND SUBSIDIARIES
FORM
10-Q
___________________
TABLE
OF CONTENTS
___________________
|
Page
|
||
|
PART
I - FINANCIAL INFORMATION
|
||
|
Item
1.
|
Consolidated
Financial Statements
|
F- |
|
Consolidated
Balance Sheets
|
F-1 | |
|
Consolidated
Statements of Loss and Comprehensive Loss
|
F-2 | |
|
Consolidated
Statements of Cash Flows
|
F-3 | |
|
Notes
to Consolidated Financial Statements
|
F-5 | |
|
Item
2.
|
Management’s
Discussion & Analysis or Plan of Operation
|
|
|
Item
3
|
Quantitative
and Qualitative Disclosures About Market Risk
|
6 |
|
Item
4.
|
Controls
and Procedures
|
7 |
|
PART
II – OTHER INFORMATION
|
||
|
Item
1.
|
Legal
Proceedings
|
8 |
|
Item
1A
|
Risk
Factors
|
8 |
|
Item
2.
|
Unregistered
Sales of Equity securities and Use of Proceeds
|
8 |
|
Item
3.
|
Defaults
Upon Senior Securities
|
8 |
|
Item
4.
|
Submission
of Matters to a Vote of Security Holders
|
8 |
|
Item
5
|
Other
Information
|
8 |
|
Item
6.
|
Exhibits
|
8 |
|
Signatures
|
9 |
GLOBAL
HEALTH VENTURES INC.
(a
development stage company)
FINANCIAL
STATEMENTS
(EXPRESSED IN U.S.
DOLLARS)
NOVEMBER
30, 2009
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
November
30, 2009
(unaudited)
| Index | |
| Consolidated Balance Sheets | F-1 |
| Consolidated Statements of Operations | F-2 |
| Consolidated Statements of Cash Flows | F-3 |
| Consolidated Statement of Stockholders’ Deficit | F-4 |
| Notes to the Consolidated Financial Statements |
F-5
|
F-
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Consolidated
Balance Sheets
(Expressed
in US Dollars)
|
November
30,
|
May
31,
|
|||||||
|
2009
|
2009
|
|||||||
|
(unaudited)
|
||||||||
| $ | $ | |||||||
|
ASSETS
|
||||||||
|
Current
Assets
|
||||||||
|
Cash
and cash equivalents
|
597,387 | 332,716 | ||||||
|
Accounts
receivable
|
3,385 | - | ||||||
|
Prepaid
expenses
|
11,230 | 15,892 | ||||||
|
Due
from shareholder
|
23,120 | - | ||||||
| 635,122 | 348,608 | |||||||
|
Property,
Plant and Equipment
|
||||||||
|
Laboratory
equipment
|
9.730 | 3,769 | ||||||
|
Accumulated
depreciation
|
(923 | ) | (377 | ) | ||||
|
Computer
Hardware
|
2,772 | - | ||||||
|
Accumulated
depreciation
|
(109 | ) | - | |||||
| 11,470 | 3,392 | |||||||
|
Intangible
Assets
|
||||||||
|
Website
development costs
|
2,509 | 2,509 | ||||||
|
Accumulated
amortization
|
(1,673 | ) | (418 | ) | ||||
|
Patents
|
275 | - | ||||||
| 1,111 | 2,091 | |||||||
|
Total
Assets
|
647,703 | 354,091 | ||||||
|
LIABILITIES
AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current
Liabilities
|
||||||||
|
Accounts
payable
|
13,138 | 3,788 | ||||||
|
Accrued
liabilities (Note 3)
|
205,854 | 67,063 | ||||||
|
Due
to related party (Note 4)
|
32,686 | 1,499 | ||||||
| 251,678 | 72,350 | |||||||
|
Commitments
and Subsequent Events (Note 4(b) & 9)
|
||||||||
|
Stockholders’
Equity
|
||||||||
|
Preferred
Stock: 80,000,000 shares authorized, $0.0001 par value
No
shares issued and outstanding
|
- | - | ||||||
|
Common
Stock: 196,000,000 shares authorized, $0.0001 par value
63,522,000
shares issued and outstanding (May 31, 2009 – 62,722,000
shares)
|
6,352 | 6,272 | ||||||
|
Additional
Paid-In Capital
|
1,366,762 | 567,478 | ||||||
|
Donated
Capital
|
2,474,000 | 2,474,000 | ||||||
|
Accumulated
other comprehensive income (loss)
|
37,352 | 41,632 | ||||||
|
Deficit
accumulated during the development stage
|
(3,488,441 | ) | (2,807,641 | ) | ||||
| 396,025 | 281,741 | |||||||
|
Total
Liabilities and Stockholders’ Equity
|
647,703 | 354,091 | ||||||
(The
accompanying notes are an integral part of these consolidated financial
statements)
F-1
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Consolidated
Statements of Operations (Unaudited)
(Expressed
in US Dollars)
|
|
Accumulated
from
April 25, 2006
|
|||||||||||||||||||
|
Three
Months Ended
|
Three
Months Ended
|
Six
Months Ended
|
Six
Months Ended
|
(Date
of inception)
|
||||||||||||||||
|
November
30, 2009
|
November
30, 2008
|
November
30, 2009
|
November
30, 2008
|
to
November 30, 2009
|
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
|
Revenue
|
– | – | – | – | – | |||||||||||||||
|
Expenses
|
||||||||||||||||||||
|
Amortization
|
628 | – | 1,255 | – | 1,673 | |||||||||||||||
|
Depreciation
|
402 | – | 655 | – | 1,032 | |||||||||||||||
|
General
and administrative:
|
||||||||||||||||||||
|
Stock-based
compensation
|
33,091 | – | 42,965 | – | 42,965 | |||||||||||||||
|
Incurred
|
84,134 | (1,388 | ) | 105,823 | (515 | ) | 189,118 | |||||||||||||
|
Interest
expense
|
– | 52 | – | 147 | 905 | |||||||||||||||
|
Professional
fees:
|
||||||||||||||||||||
|
Stock-based
compensation
|
8,273 | – | 10,741 | – | 10,741 | |||||||||||||||
|
Incurred
|
17,448 | 20,708 | 31,566 | 37,883 | 172,986 | |||||||||||||||
|
Research
and development (Note 4(b))
|
86,247 | – | 179,584 | – | 212,917 | |||||||||||||||
|
Salaries
and wages:
|
||||||||||||||||||||
|
Stock-based
compensation
|
115,104 | – | 145,658 | – | 145,658 | |||||||||||||||
|
Incurred
|
78,413 | – | 162,553 | – | 211,446 | |||||||||||||||
|
Total
Expenses
|
423,740 | 19,372 | 680,800 | 37,515 | 989,441 | |||||||||||||||
|
Net
Loss
|
(423,740 | ) | (19,372 | ) | (680,800 | ) | (37,515 | ) | (989,441 | ) | ||||||||||
|
Other
Comprehensive Income
|
||||||||||||||||||||
|
Foreign
currency translation adjustment
|
42 | – | (4,280 | ) | – | 37,352 | ||||||||||||||
|
Comprehensive
Loss
|
(423,698 | ) | (19,372 | ) | (685,080 | ) | (37,515 | ) | (952,089 | ) | ||||||||||
|
Net
Loss Per Share – Basic and Diluted
|
– | – | – | – | ||||||||||||||||
|
Weighted
Average Shares Outstanding
|
63,020,901 | 60,657,000 | 62,870,634 | 60,590,000 | ||||||||||||||||
(The
accompanying notes are an integral part of these consolidated financial
statements)
F-2
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Consolidated
Statements of Cash Flows (Unaudited)
(Expressed
in US Dollars)
|
Accumulated
from April 25, 2006
|
||||||||||||||||||||
|
Three
Months Ended
|
Three
Months Ended
|
Six
Months Ended
|
Six
Months Ended
|
(Date
of inception)
|
||||||||||||||||
|
November
30, 2009
|
November
30, 2008
|
November
30, 2009
|
November
30, 2008
|
to
November 30, 2009
|
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
|
Operating
Activities
|
||||||||||||||||||||
|
Comprehensive
loss
|
(423,698 | ) | (19,372 | ) | (685,080 | ) | (37,515 | ) | (952,089 | ) | ||||||||||
|
Adjustment
to reconcile net loss to net cash used in operating
activities:
|
||||||||||||||||||||
|
Donated
services
|
- | 2,250 | - | 4,500 | 24,000 | |||||||||||||||
|
Amortization
|
628 | - | 1,255 | - | 1,673 | |||||||||||||||
|
Depreciation
|
403 | - | 655 | - | 1,032 | |||||||||||||||
|
Stock
based compensation
|
156,468 | - | 199,364 | - | 199,364 | |||||||||||||||
|
Change
in operating assets and liabilities:
|
||||||||||||||||||||
|
Accounts
receivable
|
(3,385 | ) | - | (3,385 | ) | - | (3,385 | ) | ||||||||||||
|
Prepaid
expenses
|
(430 | ) | - | 4,662 | - | (11,230 | ) | |||||||||||||
|
Due
from shareholder
|
(23,120 | ) | - | (23,120 | ) | (23,120 | ) | |||||||||||||
|
Accounts
payable and accrued liabilities
|
67,409 | (12,892 | ) | 148,140 | 333 | 218,991 | ||||||||||||||
|
Due
to related party
|
26,128 | 15,621 | 31,187 | 15,621 | 64,572 | |||||||||||||||
|
Net
Cash Used In Operating Activities
|
(199,597 | ) | (14,393 | ) | (326,322 | ) | (17,061 | ) | (480,192 | ) | ||||||||||
|
Investing
Activities
|
||||||||||||||||||||
|
Purchase
of equipment
|
(6,753 | ) | - | (8,732 | ) | - | (12,501 | ) | ||||||||||||
|
Website
development costs
|
- | - | - | - | (2,509 | ) | ||||||||||||||
|
Patents
|
(275 | ) | - | (275 | ) | - | (275 | ) | ||||||||||||
|
Net
Cash Used in Investing Activities
|
(7,028 | ) | - | (9,007 | ) | - | (15,285 | ) | ||||||||||||
|
Financing
Activities
|
||||||||||||||||||||
|
Payment
of share offering costs
|
- | 1,000 | - | 1,000 | (28,400 | ) | ||||||||||||||
|
Advances
from a related party
|
- | (23 | ) | - | 17,684 | 165,261 | ||||||||||||||
|
Repayments
to a related party
|
- | (1,707 | ) | - | (1,707 | ) | (81,147 | ) | ||||||||||||
|
Proceeds
from issuance of common stock
|
600,000 | - | 600,000 | - | 1,037,150 | |||||||||||||||
|
Proceeds
from loan payable
|
- | - | - | - | 6,234 | |||||||||||||||
|
Repayments
of loan payable
|
- | (4,441 | ) | - | (4,857 | ) | (6,705 | ) | ||||||||||||
|
Net
Cash Provided by (Used In) Financing Activities
|
600,000 | (5,171 | ) | 600,000 | 12,120 | 1,092,393 | ||||||||||||||
|
Effect
of exchange rate changes on cash
|
- | 269 | - | - | 471 | |||||||||||||||
|
Increase
(decrease) in Cash
|
393,375 | (19,295 | ) | 264,671 | (4,941 | ) | 597,387 | |||||||||||||
|
Cash
– Beginning of Period
|
204,012 | 20,080 | 332,716 | 5,726 | - | |||||||||||||||
|
Cash
- End of Period
|
597,387 | 785 | 597,387 | 785 | 597,387 | |||||||||||||||
|
Supplemental
Disclosures
|
||||||||||||||||||||
|
Interest
paid
|
– | – | – | 52 | 902 | |||||||||||||||
|
Income
taxes paid
|
– | – | – | – | – | |||||||||||||||
|
Non-cash
Financing Transactions
|
||||||||||||||||||||
|
Shares
issued in settlement of advances from related party
|
– | – | – | – | 116,000 | |||||||||||||||
(The
accompanying notes are an integral part of these consolidated financial
statements)
F-3
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Consolidated
Statement of Stockholders’ Deficit (Unaudited)
For the
Period from April 25, 2006 (Date of Inception) to November 30, 2009
(Expressed
in US Dollars)
| Common Stock |
|
Accumulated
Other
|
Deficit
Accumulated
During
the
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
Additional Paid-In
Capital |
Donated
Capital
|
Comprehensive
Income
|
Development
Stage
|
Total
|
|||||||||||||||||||||
| # | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||
|
Balance
– April 25, 2006 (Date of inception)
|
– | – | – | – | – | – | – | ||||||||||||||||||||
|
April
26, 2006 – common shares issued for cash at $0.0001 per
share
|
140,000,000 | 14,000 | (13,000 | ) | – | – | – | 1,000 | |||||||||||||||||||
|
Donated
services and rent
|
– | – | – | 750 | – | – | 750 | ||||||||||||||||||||
|
Net
loss for the period
|
– | – | – | – | – | (9,040 | ) | (9,040 | ) | ||||||||||||||||||
|
Balance
– May 31, 2006
|
140,000,000 | 14,000 14,000 | (13,000 | ) | 750 | – | (9,040 | ) | (7,290 | ) | |||||||||||||||||
|
April
2, 2007 – common shares issued for cash at $0.0036 per
share
|
14,322,000 | 1,432 | 49,718 | – | – | – | 51,150 | ||||||||||||||||||||
|
Share
issuance costs
|
(28,400 | ) | – | (28,400 | ) | ||||||||||||||||||||||
|
Donated
services and rent
|
– | – – | – | 9,000 | – | – | 9,000 | ||||||||||||||||||||
|
Net
loss for the year
|
– | – | – | – | – | (61,174 | ) | (61,174 | ) | ||||||||||||||||||
|
Balance
– May 31, 2007
|
154,322,000 | 15,432 | 8,318 | 9,750 | (70,214 | ) | (36,714 | ) | |||||||||||||||||||
|
Cancellation
of shares
|
(93,800,000 | ) | (9,380 | 9,380 | – | – | – – | – | |||||||||||||||||||
|
Donated
services and rent
|
– | – | – | 9,000 | – | – | 9,000 | ||||||||||||||||||||
|
Net
loss for the year
|
– | – | – | – | – | (65,168 | ) | (65,168 | ) | ||||||||||||||||||
|
Balance
– May 31, 2008
|
60,522,000 | 6,052 | 17,698 | 18,750 | – | (135,382 | ) | (92,882 | ) | ||||||||||||||||||
|
Donated
services and rent
|
– | – | – | 5,250 | – | – | 5,250 | ||||||||||||||||||||
|
Sep
30, 2008 – common shares issued at $0.0001 per share in loan
settlement
|
10,000,000 | 1,000 | 2,499,000 | – | – | (2,499,000 | ) | 1,000 | |||||||||||||||||||
|
Sep
30. 2008 – common shares returned to treasury
|
(9,800,000 | ) | (980 | (2,449,020 | ) | 2,450,000 | – | – | – | ||||||||||||||||||
|
Jan
20, 2009 – common shares issued at $0.25 per share in loan
settlement
|
460,000 | 46 | 114,954 | – | – | – | 115,000 | ||||||||||||||||||||
|
Jan
20, 2009 - common shares issued for cash at $0.25 per
share
|
1,540,000 | 154 | 384,846 | – | – | – | 385,000 | ||||||||||||||||||||
|
Foreign
currency translation adjustment
|
– | – | – | – | 41,632 | – | 41,632 | ||||||||||||||||||||
|
Net
loss for the year
|
(173,259 | ) | (173,259 | ) | |||||||||||||||||||||||
|
Balance
– May 31, 2009
|
62,722,000 | 6,272 | 567,478 | 2,474,000 | 41,632 | (2,807,641 | ) | 281,741 | |||||||||||||||||||
|
Oct
28, 2009 - common shares issued for cash at $0.75 per share (Note
6)
|
133,333 | 13 | 99,987 | – | – | – | 100,000 | ||||||||||||||||||||
|
Oct
28, 2009 - common shares issued for cash at $0.75 per share (Note
6)
|
666,667 | 67 | 499,933 | – | – | – | 500,000 | ||||||||||||||||||||
|
Stock-based
compensation
|
– | – | 199,364 | – | – | – | 199,364 | ||||||||||||||||||||
|
Foreign
currency translation adjustment
|
– | – | – | – | (4,280 | ) | – | (4,280 | ) | ||||||||||||||||||
|
Net
loss for the period
|
– | – | – | – | – | (680,800 | ) | (680,800 | ) | ||||||||||||||||||
|
Balance
– November 30, 2009
|
63,522,000 | 6,352 | 1,366,762 | 2,474,000 | 37,352 | (3,488,441 | ) | 396,025 | |||||||||||||||||||
(See Note
6 for return and issuance of common shares)
(The
accompanying notes are an integral part of these consolidated financial
statements)
F-4
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
1. Development
Stage Company
Global
Health Ventures Inc. (the “Company”) was incorporated in the State of Nevada on
April 25, 2006 under the name Acting Scout Inc. The Company changed its name to
Goldtown Investments Corp. on September 20, 2007 and on October 6, 2008 changed
its name to Global Health Ventures Inc. The Company is located in British
Columbia, Canada. The Company is a healthcare technology financial institution
that is in the business of acquiring and licensing current outstanding and
promising healthcare related technologies for further development and
re-licensing to major pharmaceutical companies. The Company is
a Development Stage Company, as defined by Statement of Financial Accounting
Standard (“SFAS”) No.7 “Accounting and Reporting for
Enterprises in the Development Stage”.
These
consolidated financial statements have been prepared on a going concern basis,
which implies the Company will continue to realize its assets and discharge its
liabilities in the normal course of business. The Company has not generated
significant revenues since inception and has never paid any dividends and is
unlikely to pay dividends or generate significant earnings in the immediate or
foreseeable future. The continuation of the Company as a going concern is
dependent upon the continued financial support from its shareholders, the
ability of the Company to obtain necessary equity financing to continue
operations and the attainment of profitable operations. These factors raise
substantial doubt regarding the Company’s ability to continue as a going
concern. As at November 30, 2009, the Company has never generated any
significant revenue and has accumulated losses of $3,451,089 since inception.
These consolidated financial statements do not include any adjustments to the
recoverability and classification of recorded asset amounts and classification
of liabilities that might be necessary should the Company be unable to continue
as a going concern.
The
Company’s common shares trade on the Over the Counter Bulletin Board (“OTCBB”)
under the symbol “GHLV”.
2. Summary
of Significant Accounting Policies
|
a)
|
Basis
of Presentation
|
These
consolidated financial statements and related notes are presented in accordance
with accounting principles generally accepted in the United States, and are
expressed in U.S. dollars. These consolidated financial statements include the
accounts of the Company and its inactive wholly-owned subsidiary, Global Health
(BC) Ventures Inc.
|
b)
|
Interim
Financial Statements
|
The
interim unaudited financial statements have been prepared on the same basis as
the annual financial statements and in the opinion of management, reflect all
adjustments, which include only normal recurring adjustments, necessary to
present fairly the Company’s financial position, results of operations and cash
flows for the periods shown. The results of operations for such
periods are not necessarily indicative of the results expected for a full year
of for any future period. These financial statements should be used
in conjunction with our annual audited financial statements.
|
c)
|
Use
of Estimates
|
The
preparation of financial statements in accordance with United States generally
accepted accounting principles requires management to make estimates and
assumptions that affect the reported amounts of assets and liabilities at the
date of the financial statements and the reported amounts of revenue and
expenses in the reporting period. The Company regularly evaluates estimates and
assumptions related to deferred income tax asset valuation allowance. The
Company bases its estimates and assumptions on current facts, historical
experience and various other factors that it believes to be reasonable under the
circumstances, the results of which form the basis for making judgments about
the carrying values of assets and liabilities and the accrual of costs and
expenses that are not readily apparent from other sources. The actual results
experienced by the Company may differ materially and adversely from the
Company’s estimates. To the extent there are material differences between the
estimates and the actual results, future results of operations will be
affected.
F-5
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
2. Summary
of Significant Accounting Policies (continued)
d) Basic and
Diluted Net Income (Loss) Per Share
The
Company computes net income (loss) per share in accordance with SFAS No. 128,
"Earnings per Share".
SFAS No. 128 requires presentation of both basic and diluted earnings per share
(EPS) on the face of the income statement. Basic EPS is computed by dividing net
income (loss) available to common shareholders (numerator) by the weighted
average number of shares outstanding (denominator) during the period. Diluted
EPS gives effect to all dilutive potential common shares outstanding during the
period using the treasury stock method and convertible preferred stock using the
if-converted method. In computing diluted EPS, the average stock price for the
period is used in determining the number of shares assumed to be purchased from
the exercise of stock options or warrants. Diluted EPS excludes all dilutive
potential shares if their effect is anti dilutive.
e) Revenue
Recognition
The
Company recognizes revenue in accordance with Securities and Exchange Commission
Staff Accounting Bulletin No. 104 (“SAB 104”), “Revenue Recognition in Financial
Statements”. The Company has never generated any revenue since
inception.
f) Comprehensive
Loss
SFAS No.
130, “Reporting Comprehensive
Income,” establishes standards for the reporting and display of
comprehensive loss and its components in the financial statements.
g) Cash
and Cash Equivalents
The
Company considers all highly liquid instruments with maturity of three months or
less at the time of issuance to be cash equivalents.
h) Property,
Plant and Equipment
Property,
plant and equipment are recorded at cost. Depreciation is provided
annually at rates calculated to write off the assets over their estimated useful
lives as follows:
| Laboratory equipment | 20% diminishing balance |
| Computer hardware | 45% diminishing balance |
In the
year of acquisition, these rates are reduced by one-half.
i) Long-Lived
Assets
In
accordance with SFAS No. 144, “Accounting for the Impairment or
Disposal of Long-Lived Assets”, the Company tests long-lived assets or
asset groups for recoverability when events or changes in circumstances indicate
that their carrying amount may not be recoverable. Circumstances which could
trigger a review include, but are not limited to: significant decreases in the
market price of the asset; significant adverse changes in the business climate
or legal factors; accumulation of costs significantly in excess of the amount
originally expected for the acquisition or construction of the asset; current
period cash flow or operating losses combined with a history of losses or a
forecast of continuing losses associated with the use of the asset; and current
expectation that the asset will more likely than not be sold or disposed
significantly before the end of its estimated useful life.
Recoverability
is assessed based on the carrying amount of the asset and its fair value which
is generally determined based on the sum of the undiscounted cash flows expected
to result from the use and the eventual disposal of the asset, as well as
specific appraisal in certain instances. An impairment loss is recognized when
the carrying amount is not recoverable and exceeds fair value.
F-6
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
2. Summary
of Significant Accounting Policies (continued)
j) Website
Development Costs
The
Company capitalizes website development costs in accordance with the American
Institute of Certified Public Accountants (“AICPA”) Statement of Position
(“SOP”) No. 98-1, “Accounting
for the Costs of Computer Software Developed or Obtained for Internal
Use” and Emerging Issues Task Force (EITF) No. 00-2, “Accounting for Website Development
Costs”, whereby costs related to the preliminary project stage of
development are expensed and costs related to the application development stage
are capitalized. Any additional costs for upgrades and enhancements
which result in additional functionality will be
capitalized. Capitalized costs will be amortized based on their
estimated useful life over three years. Internal costs related to the
development of website content are charged to operations as
incurred.
k) Financial
Instruments
The fair
value of financial instruments, which include cash, accounts payable and amounts
due to a related party, were estimated to approximate their carrying values due
to the immediate or short-term maturity of these financial instruments. The
Company’s operations will be in Canada and the United States, resulting in
exposure to market risks from changes in foreign currency rates. The financial
risk is the risk to the Company’s operations that arise from fluctuations in
foreign exchange rates and the degree of volatility of these rates. Currently,
the Company does not use derivative instruments to reduce its exposure to
foreign currency risk.
l) Income
Taxes
The
Company follows the asset and liability method of accounting for income taxes.
Under this method, current taxes are recognized for the estimated income taxes
payable for the current period.
Deferred
income taxes are provided based on the estimated future tax effects of temporary
differences between financial statement carrying amounts of assets and
liabilities and their respective tax bases as well as the benefit of losses
available to be carried forward to future years for tax purposes.
Deferred
tax assets and liabilities are measured using enacted tax rates that are
expected to apply to taxable income in the years in which those temporary
differences are expected to be covered or settled. The effect of deferred tax
assets and liabilities of a change in tax rates is recognized in operations in
the period that includes the enactment date. A valuation allowance is recorded
for deferred tax assets when it is more likely than not that such deferred tax
assets will not be realized.
|
m)
|
Foreign
Currency Translation
|
The
functional currency of the Company is the Canadian dollar with the reported
amounts being stated in the United States dollar. In accordance with the
Statement of Financial Accounting Standards (“SFAS”) No. 52, Foreign Currency
Translation, assets and liabilities are translated at the rates of exchange at
the balance sheet dates. Income and expense items are translated at average
annual rates of exchange. The resulting translation adjustments are included in
accumulated other comprehensive income (loss), a separate component of
stockholders’ equity.
|
n)
|
Research
and development costs
|
Research
costs are expensed in the period incurred. Development costs are
expensed in the period incurred unless the Company believes a development
project meets generally accepted accounting criteria for deferral and
amortization. No such costs have been deferred as at November 30, 2009 and
2008.
F-7
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
2. Summary
of Significant Accounting Policies (continued)
o) Stock-based
Compensation
In
accordance with SFAS 123R, “Share Based Payments”, the
Company accounts for share-based payments using the fair value
method. Common shares issued to third parties for non-cash
consideration are valued based on the fair market value of the services provided
or the fair market value of the common stock on the measurement date, whichever
is more readily determinable
|
p)
|
Recent
Accounting Pronouncements
|
In May
2009, the FASB issued FAS No. 165, “Subsequent
Events”. This pronouncement establishes standards for
accounting for and disclosing subsequent events (events which occur after the
balance sheet date but before financial statements are issued or are available
to be issued). FAS 165 requires an entity to disclose the date
subsequent events were evaluated and whether that evaluation took place on the
date financial statements were issued or were available to be
issued. It is effective for interim and annual periods ending after
June 15, 2009. The adoption of FAS 165 did not have a material impact
on the Company’s financial condition or results of operation.
In June
2009, the FASB issued FAS 166, “Accounting for Transfers of
Financial Assets”, an amendment of FAS 140. FAS 140 is
intended to improve the relevance, representational faithfulness, and
comparability of the information that a reporting entity provides in its
financial statements about a transfer of financial assets: the
effects of a transfer on its financial position, financial performance and cash
flows: and a transferor’s continuing involvement, if any, in transferred
financial assets. This statement must be applied as of the beginning
of each reporting entity’s first annual reporting period that begins after
November 15, 2009. The Company does not expect the adoption of
FAS 166 to have an impact on the Company’s results of operations, financial
condition or cash flows.
In June
2009, the FASB issued FAS 167, “Amendments to FASB Interpretation
No. 46(R)”. FAS 167 is intended to (1) address the effects on
certain provisions of FASB Interpretation No. 46 (revised December 2003), Consolidation of Variable Interest
Entities, as a result of the elimination of the qualifying
special-purpose entity concept in FAS 166, and (2) constituent concerns about
the application of certain key provisions of Interpretation 46R, including those
in which the accounting and disclosures under the Interpretation do not always
provide timely and useful information about an enterprise’s involvement in a
variable interest entity. This statement must be applied as of the
beginning of each reporting entity’s first annual reporting period that begins
after November 15, 2009. The Company does not expect the adoption of
FAS 167 to have an impact on the Company’s results of operations, financial
condition or cash flows.
In June 2009, the FASB issued FAS
168, “The FASB Accounting
Standards Codification and the Hierarchy of General Accepted Accounting
Principles”. FAS 168 will become the source of authoritative
U.S. general accepted accounting principles (GAAP) recognized by the FASB to be
applied by nongovernmental entities. Rules and interpretive releases
of the Securities and Exchange Commission (SEC) under authority of federal
securities laws are also sources of authoritative GAAP for SEC
registrants. On the effective date of this Statement, the
Codification will supersede all then-existing non-SEC accounting and reporting
standards. All other non-grandfathered non-SEC accounting literature
not included in the Codification will become non-authoritative. This
statement is effective for financial statements issued for interim and annual
periods ending after September 15, 2009. The Company does not expect
the adoption of FAS 168 to have an impact on the Company’s results of
operations, financial condition or cash flows.
In
September 2009, the FASB issued ASC 820-10 Measuring Liabilities at Fair Value
(“ASC 820-10”). ASC 820-10 provides additional guidance on how
companies should measure liabilities at fair value. Specifically, the
fair value of a liability is not adjusted to reflect the impact of contractual
restrictions that prevent its transfer. ASC 820-10 will be adopted by
the Company in the second quarter of fiscal year 2010. The Company is
currently evaluating the impact of ASC 820-10, but does not expect the adoption
to have a material impact on its financial position, results of operations, and
cash flows.
F-8
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
3. Accrued
Liabilities
|
November
30,
2009
$
|
May
31,
2009
$
|
|||||||
|
Accrued
interest
|
- | - | ||||||
|
Professional
fees
|
- | 10,830 | ||||||
|
Research
and development
|
33,333 | 33,333 | ||||||
|
Salaries
|
172,521 | 22,900 | ||||||
| 205,854 | 67,063 | |||||||
4. Related
Party Transactions
|
a)
|
During
the six month period ended November 30, 2009, the President of the Company
advanced $Nil (2008 - $17,684) to the Company, was repaid $4,557 (2008 -
$Nil) by the Company and incurred $10,754 (2008 - $10,651) of expenses on
behalf of the Company. During the six month period ended
November 30, 2009, the Company loaned the President $75,712, of which
$47,234 has been repaid. The balance of the loan is repayable,
bears no interest and can be offset by expenses the President incurs on
behalf of the Company.
|
|
b)
|
On
March 15, 2009, the Company entered into a research contract with Globe
Laboratories Inc. (“Globe”), a company controlled by 2 individuals related
to the President of the Company, to engage Globe for research on the
sublingual technologies developed by Globe. The Company agreed to pay
$50,000 per quarter to Globe from April 1, 2009 until the technology is
put into commercial production, or the technologies are sold or
sublicensed. To date, $133,333 in research costs have been
accrued under this agreement, of which $100,000 has been paid to
Globe.
|
|
c)
|
During
the six month period ended November 30, 2009, a company controlled by the
President advanced $32,686 (2008 - $Nil) to the
Company.
|
|
d)
|
On
March 25, 2009, the Company signed a letter of intent with Posh
Cosmeceuticals Inc. (“Posh”), a company controlled by the President of the
Company, whereby the Company has agreed to purchase all of the issued and
outstanding capital stock of Posh from its shareholders. The
Company completed the acquisition of Posh in December
2009.
|
5. Income
Taxes
The
Company accounts for income taxes using the liability method of tax allocation.
Deferred income taxes are recognized for the future income tax consequences
attributable to differences between the carrying values of assets and
liabilities and their respective income tax bases. Deferred income tax assets
are evaluated periodically and if realization is not considered likely, a
valuation allowance is provided.
a) Deferred
tax assets and liabilities
|
November
30, 2009
$
|
May
31, 2009
$
|
|||||||
|
Operating
loss carry forwards
|
268,000 | 92,400 | ||||||
|
Valuation
allowance
|
(268,000 | ) | (92,400 | ) | ||||
|
Net
future tax asset
|
- | - | ||||||
Management
believes that it is not more likely than not that it will create sufficient
taxable income sufficient to realize its deferred tax assets. Due to this, the
Company has no income tax expense.
F-9
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
5. Income
Taxes (continued)
b) Loss
carry forwards
The
Company has estimated accumulated non-capital losses of approximately $765,000
which will expire as follows:
|
2026
|
$ | 9,000 | ||
|
2027
|
52,000 | |||
|
2028
|
56,000 | |||
|
2029
|
168,000 | |||
|
2030
|
480,000 | |||
| $ | 765,000 | |||
6. Warrants
On
January 20, 2009, pursuant to the completion of a 2,000,000 units private
placement, the Company issued 2,000,000 share purchase warrants exercisable to
acquire 2,000,000 common shares of the Company at $0.40 per share, expiring
January 20, 2011. At November 30, 2009, the 2,000,000 warrants issued
are still outstanding.
On
October 28, 2009, pursuant to the completion of a total of 800,000 units private
placement, the Company issued 800,000 share purchase warrants exercisable to
acquire 800,000 common shares of the Company at $1.00 per share, expiring
October 28, 2011. At November 30, 2009, the 800,000 warrants issued
are still outstanding.
7. Stock
Option Plan
The
Company may grant options to purchase 2,000,000 common shares of the
Company. Options may be issued under the stock option plan as
determined at the sole discretion of the Company’s board of
directors. Options may be issued for a term of up to 10 years at an
exercise price of $0.70. All options vest at a rate of four per cent
of the total number of Options granted to an Optionee vesting each month on a
monthly basis during the two-year period and the total remainder of such Options
vesting on the second anniversary of the date of grant.
A summary
of stock options outstanding is presented below:
|
Number
of
Options
|
Weighted
Average Exercise Price
$
|
|||||||
|
Options
outstanding at June 1, 2009
|
- | - | ||||||
|
Granted
|
2,000,000 | $ | 0.70 | |||||
|
Expired
|
- | - | ||||||
|
Options
outstanding at November 30, 2009
|
2,000,000 | $ | 0.70 | |||||
During
the six months ended November 30, 2009, the Company granted 2,000,000
options. The fair value of the options of $199,364 has been expensed.
At November 30, 2009, 320,000 of the options are exercisable.
The
Company has estimated the fair value of each option on the date of grant using
the Black-Scholes Options Pricing Model with the following weighted average
assumptions:
| Risk-free interest rate | 2.95% |
| Expected life of options | 5-10 years |
| Expected volatility in the market price of the shares | 150% |
| Expected dividend yield | 0.0% |
F-10
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
8. Fair
Value Measures
SFAS 157
requires an entity to maximize the use of observable inputs and minimize the use
of unobservable inputs when measuring fair value. SFAS 157 establishes a fair
value hierarchy based on the level of independent, objective evidence
surrounding the inputs used to measure fair value. A financial instrument’s
categorization within the fair value hierarchy is based upon the lowest level of
input that is significant to the fair value measurement. SFAS 157 prioritizes
the inputs into three levels that may be used to measure fair
value:
Level
1
Level 1
applies to assets or liabilities for which there are quoted prices in active
markets for identical assets or liabilities.
Level
2
Level 2
applies to assets or liabilities for which there are inputs other than quoted
prices that are observable for the asset or liability such as quoted prices for
similar assets or liabilities in active markets; quoted prices for identical
assets or liabilities in markets with insufficient volume or infrequent
transactions (less active markets); or model-derived valuations in which
significant inputs are observable or can be derived principally from, or
corroborated by, observable market data.
Level
3
Level 3
applies to assets or liabilities for which there are unobservable inputs to the
valuation methodology that are significant to the measurement of the fair value
of the assets or liabilities.
Our
financial instruments consist principally of cash and accounts payable. Pursuant
to SFAS No. 157, Fair Value Measurements, or
SFAS 157, the fair value of our cash is determined based on “Level 1”
inputs, which consist of quoted prices in active markets for identical assets.
We believe that the recorded values of all of our other financial instruments
approximate their current fair values because of their nature and respective
maturity dates or durations.
9. Commitments
Pursuant
to an agreement dated November 12, 2009 the Company has entered into a
non-exclusive agreement with an investment bank to raise funds for the Company
on a “best efforts” basis. Under the terms of this agreement, the
investment bank (“Agent”) will receive a success fee subject to the following
fee structure:
|
i.
|
8%
of the amount of debt or equity
raised.
|
|
ii.
|
50%
(i) above of the Aggregate Consideration received by the Company from any
transactions closed, including multiple successive transactions, with an
investor candidate or a strategic candidate (or upon closing a transaction
with a covered party, including multiple successive transactions, within
twelve months after the termination date), which amount will be paid when
the Company receives proceeds from the
transaction.
|
|
iii.
|
Warrants
to purchase that number of shares of the Company’s common stock equal to
10% of the value of such transactions for successful common stock equity
raised at the closing of such transaction for a period of 1 year, and/or
to grant the Agent warrants to purchase that number of shares of the
Company’s common stock equal to 10% of the value of such
transactions for successful preferred stock, debt, hybrid debt of any kind
or debt and equity combination raised at the closing of such transaction
for a period of 1 year. These stocks shall be delivered in
cashless exercise and issuable from the investment closing date up to no
more than 5 years from the date and upon exercise thereof shall be fully
paid and non-assessable. The stock obtainable upon exercise of
such warrants shall carry unlimited “piggyback” registration rights of the
Company.
|
F-11
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
10. Subsequent
Events
Effective
December 8, 2009, the Company issued an aggregate of 533,333 shares of common
stock and 533,333 warrants to one investor pursuant to a private placement
subscription agreement dated October 28, 2009 for gross proceeds of
$400,000. Each warrant entitles the investor to purchase one
additional share of common stock of the Company at a price of $1.00 per share
until December 8, 2011. The Company issued the securities to one
non-U.S. person (as that term is defined in Regulation S of the Securities Act
of 1933) in an offshore transaction relying on Regulation S and/or Section 4(2)
of the Securities Act of 1933.
Effective
December 11, 2009, the Company issued an aggregate of 4,000,000 shares of its
common stock to the shareholders of Posh Cosmeceuticals Inc. pursuant to share
exchange agreements dated June 12, 2009. The Company issued the
securities to twenty-seven non-U.S. persons (as that term is defined in
Regulation S of the Securities Act of 1933) in an offshore transaction relying
on Regulation S and/or Section 4(2) of the Securities Act of 1933.
11. Merger
Agreement
a) Unaudited
Condensed Combined Pro Forma Financial Statements
Pursuant
to the terms of a "Merger (Share Exchange) Agreement" (Non Binding Letter of
Intent) dated March 25, 2009 between Global Health Ventures Inc. ("Global") and
Posh Cosmeceuticals Inc. ("Posh"), Global agreed to purchase all of the issued
and outstanding capital stock of Posh from its shareholders.
Global
completed the acquisition of Posh in December 2009. Global and Posh are
considered to be related parties as they have common controlling shareholders,
officers and directors. As they are related parties, the values of the assets
and liabilities acquired from Posh are recorded at Posh's carrying
value.
The
following unaudited pro forma financial information gives effect to Global's
acquisition of 40,286,676 shares of common stock (100%) of Posh . The pro forma
financial information is presented for illustrative purposes only and is not
necessarily indicative of the future results of operations of Global after its
investment in Posh.
F-12
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
11.
a) Unaudited Condensed Combined Pro Forma Financial Statements
(continued)

F-13
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
11.
a) Unaudited Condensed Combined Pro Forma Financial Statements
(continued)
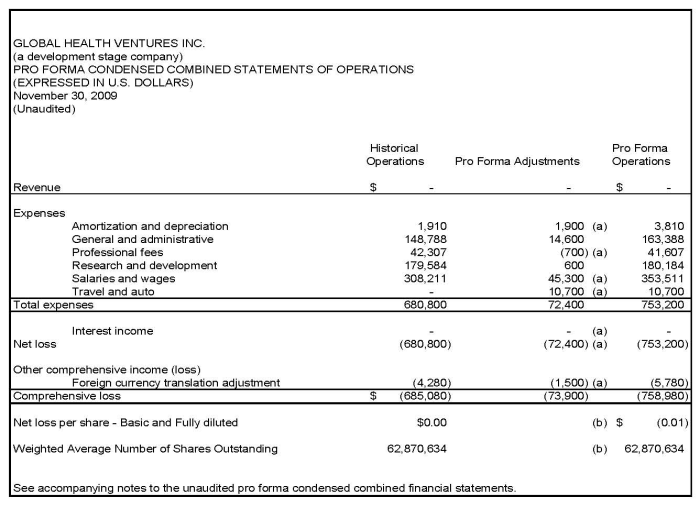
b) Notes
to the Unaudited Condensed Combined Pro Forma Financial Statements
|
i.
|
Basis
of Presentation
|
The
following table sets forth the allocation of the purchase price for the
investment in Global included in the pro forma financial
information:
|
Working
capital acquired
|
$ | 93,900 | ||
|
Property,
Plant and Equipment
|
10,100 | |||
|
Patents
and rights
|
22,200 | |||
|
Other
long-term assets
|
21,500 | |||
| $ | 147,700 |
| Consideration: | ||||
|
Common
shares of the Company
|
$ | 400 | ||
| Related party adjustment on purchase charged to deficit | 147,300 | |||
| $ | 147,700 |
F-14
Global
Health Ventures Inc.
(formerly
Goldtown Investments Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements (Unaudited)
November
30, 2009
11. b)
Notes to the Unaudited Condensed Combined Pro Forma Financial Statements
(continued)
|
i.
|
Basis
of Presentation
|
The pro
forma combined financial statements included herein have been prepared by Global
without audit, pursuant to the rules and regulations of the Securities and
Exchange Commission. These pro forma combined statements should be read in
conjunction with the financial statements and the notes thereto included in
Global's Form 10-K for the fiscal year ended May 31, 2009 and the unaudited
financial statements and notes thereto included in Global's Form 10-Q for the
six months ended November 30, 2009.
The pro
forma condensed combined balance sheet assumes the acquisition took place on
November 30, 2009. The unaudited pro forma condensed combined balance sheet
reflects the appropriate pro forma adjustments to record the acquisition using
the purchase method of accounting. The pro forma condensed statement of
operations for the fiscal quarter ending November 30, 2009, assume the
acquisition took place as of June 1, 2009, and combines the historical
operations of Global with the historical operations of Posh for the
corresponding period, with pro forma adjustments.
|
ii.
|
Pro
Forma Adjustments
|
(i)The
pro forma condensed combined balance sheet reflects the following
adjustments:
|
|
(a)
To record the issuance of 4,000,000 common shares to acquire Posh at its
carrying value.
|
(ii) The
pro forma combined statements of operations reflects the following
adjustments:
(a)Adjustment
to record Posh's operating results for the quarter ended November 30, 2009 upon
consolidation as if the purchase of Posh had occurred at the beginning of the
pro forma period.
(b) Basic
and diluted net loss per share is calculated by dividing the pro forma net loss
by the common shares used to calculate net loss per share in the historical
period of Global plus the effect of the common stock of Global which was issued
under the "Merger (Share Exchange) Agreement".
F-15
ITEM 2. MANAGEMENT’S
DISCUSSION OR PLAN OF OPERATION
FORWARD-LOOKING
STATEMENTS
This
quarterly report contains forward-looking statements as that term is defined in
applicable securities laws. These statements relate to future events or our
future financial performance. In some cases, you can identify forward-looking
statements by terminology such as “may”, “should”, “expects”, “plans”,
“anticipates”, “believes”, “estimates”, “predicts”, “potential” or “continue” or
the negative of these terms or other comparable terminology. These statements
are only predictions and involve known and unknown risks, uncertainties and
other factors, including the risks in the section entitled “Risk Factors”, that
may cause our or our industry’s actual results, levels of activity, performance
or achievements to be materially different from any future results, levels of
activity, performance or achievements expressed or implied by these
forward-looking statements. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee future
results, levels of activity, performance or achievements. Except as required by
applicable law, including the securities laws of the United States, we do not
intend to update any of the forward-looking statements to conform these
statements to actual results.
Financial
information contained in this quarterly report and in our unaudited interim
consolidated financial statements are stated in United States dollars and are
prepared in accordance with United States generally accepted accounting
principles. The following discussion should be read in conjunction with our
unaudited interim consolidated financial statements and the related notes that
appear elsewhere in this quarterly report.
As used
in this quarterly report, and unless otherwise indicated, the terms “we”, “us”
and “our” mean Global Health Ventures Inc., unless otherwise
indicated.
Corporate
History
We were
incorporated on April 25, 2006 under the name “Acting Scout Inc.”, pursuant to
the laws of the state of Nevada.
On
September 10, 2007, we filed an Amendment to our Articles of Incorporation with
the Secretary of State of Nevada to be effective as of September 10, 2007 to
decrease our authorized capital from 80,000,000 common shares to 14,000,000
common shares.
On
September 20, 2007, we changed our name to Goldtown Investments Corp. to better
reflect our new business model. In addition, effective as of September 20, 2007,
we also filed a Certificate of Change to increase our issued and outstanding,
and authorized, capital, on a basis of fourteen (14) new common shares for every
one (1) existing common share, from 11,023,000 issued and outstanding common
shares into 154,322,000 issued and outstanding common shares, and from
14,000,000 authorized common shares to 196,000,000 authorized common
shares.
On
September 29, 2008, Blair Law resigned as our president, secretary, treasurer,
chief executive officer and chief financial officer. On September 29, 2008,
Hassan Salari was appointed as our president, secretary, treasurer, chief
executive officer and chief financial officer.
Effective
October 6, 2008, we changed our name from “Goldtown Investments Corp.” to
“Global Health Ventures Inc.” as a result of a merger with Global Health
Ventures Inc., our wholly-owned subsidiary that was incorporated solely to
effect the name change. Our common shares trade on the Over The Counter Bulletin
Board (OTCBB) under the symbol “GHLV”.
On March
15, 2009, we entered into a research contract with Globe Laboratories Inc., a
company controlled by two individuals related to the president of our company,
to engage Globe for research on the sublingual technologies developed by Globe.
We agreed to pay $50,000 per quarter to Globe from April 1, 2009 until the
technologies are put into commercial production, or the technologies are sold or
sublicensed.
On May
14, 2009, Dr. David Filer and Christian Bezy were appointed to our board of
directors. Also on May 14,
2009, Audrey
Lew was appointed as our chief financial officer and Hassan Salari resigned from
this position.
Current
Business
We are a
specialty pharmaceutical company developing proprietary platform technology that
delivers drugs via the sublingual (under the tongue) route. This unique method
delivers drugs to the bloodstream quickly and with minimal drug breakdown in the
liver and gastro-intestinal system, a process that can greatly reduce side
effects while also requiring a lower dosage than conventional oral drugs while
generally still producing the same results. Additionally, we are also developing
oral formulations of drugs which are intended to cause fewer stomach side
effects than formulations of such drugs previously marketed by other
pharmaceutical companies. Our drug formulations are specifically designed to
deliver drugs to the blood stream rapidly and more efficiently than traditional
drug formulations but with fewer side effects.
1
We intend
to develop our products to the final stage of marketing. If we need to carry out
further clinical trials to support regulatory filings we will do so in-house. We
plan to market the products through direct consumer sources such as
advertisements on the internet, television, radio and in magazines. We also plan
to sign up co-marketing partners, which typically will be larger specialty
pharmaceutical companies and distributors. In the latter case, we plan to share
the revenue on a pre-arranged royalty basis structure. We anticipate our
products and any new product will require substantial funding. There can be no
assurance, however, that we will be able to acquire the financing necessary to
enable us to pursue our plan of operation. If our company requires additional
financing and we are unable to acquire such funds, our business may
fail.
Management
of our company believes that there are perceived benefits to being a reporting
company with a class of publicly-traded securities. These are commonly thought
to include: (i) the ability to use registered securities to acquire assets or
businesses; (ii) increased visibility in the financial community; (iii) the
facilitation of borrowing from financial institutions; (iv) improved trading
efficiency; (v) stockholder liquidity; (vi) greater ease in subsequently raising
capital; (vii) compensation of key employees through stock options; (viii)
enhanced corporate image; and (ix) a presence in the United States capital
market.
RESULTS
OF OPERATIONS
The
following discussion of our financial condition and results of operations should
be read together with the unaudited interim financial statements and the notes
to the unaudited interim financial statements included in this quarterly report.
This discussion contains forward-looking statements that reflect our plans,
estimates and beliefs. Our actual results may differ materially from those
anticipated in these forward-looking statements.
For
the three month periods ended November 30, 2009 and November 30,
2008
Our
interim consolidated financial statements report a net loss of $423,740 for the
three month period ended November 30, 2009 as compared to a net loss of $19,372
for the three month period ended November 30, 2008.
During
the three months ended November 30, 2009, we incurred expenses of $423,740
compared to $19,372 during the three months ended November 30, 2008. The
increase in expenses during the three months ended November 30, 2009 was largely
due to increases in general and administrative expenses, research and
development expenses and salaries and wages.
For
the six month periods ended November 30, 2009 and November 30, 2008
Our
interim consolidated financial statements report a net loss of $680,800 for the
six month period ended November 30, 2009 as compared to a net loss of $37,515
for the six month period ended November 30, 2008.
During
the six months ended November 30, 2009, we incurred expenses of $680,800
compared to $37,515 during the six months ended November 30, 2008. The increase
in expenses during the six months ended November 30, 2009 was largely due to
increases in general and administrative expenses, research and development
expenses and salaries and wages.
As of
November 30, 2009, we had a working capital of $383,444. Our total liabilities
as of November 30, 2009 were $251,678, as compared to total liabilities of
$72,350 as of May 31, 2009. The change was due primarily to an increase in
accrued liabilities of $138,791.
Cash
Flow Used in Operating Activities
Operating
activities used cash of $199,597 for the three month period ended November 30,
2009, compared to using cash of $14,393 for the three month period ended
November 30, 2008. The increase in cash used during the three month
period ended November 30, 2009 was
commensurate with an increase in the amount due to related parties of $26,128
and an increase in accounts payable and accrued liabilities of
$67,409.
2
Cash
Flow Provided by Financing Activities
Financing
activities provided cash of $600,000 for the three month period ended November
30, 2009 compared to using cash of $5,171 for the three month period ended
November 30, 2008.
Cash
Flow Provided by Investing Activities
Investing
activities used cash of $7,028 for the three month period ended November 30,
2009 compared to using $Nil for the three month period ended November 30,
2008.
Liquidity
and Capital Resources
We had
cash and cash equivalents of $597,387 and current liabilities of $251,678 as of
November 30, 2009. We had working capital of $383,444 as of November 30,
2009.
To date,
we have had negative cash flows from operations and we have been dependent on
sales of our equity securities and debt financing to meet our cash requirements.
We expect this situation to continue for the foreseeable future. We anticipate
that we will have negative cash flows during the next twelve month period ended
November 30, 2010.
On
January 2, 2009, we agreed to settle the outstanding amount of $115,000 owed to
our president by the issuance of 460,000 units of our company at a price of
$0.25 per unit. Each unit consisted of one common share and one common share
purchase warrant exercisable to acquire an additional 460,000 common shares at
an exercise price of $0.40 per share and having an expiry date of January 20,
2011. We issued the 460,000 units to our president on January 20,
2009.
Also on
January 20, 2009, we completed a private placement of 1,540,000 units of our
company at a price of $0.25 per unit for gross proceeds of $385,000. Each unit
consisted of one common share and one common share purchase warrant exercisable
to acquire an additional 1,540,000 common shares at an exercise price of $0.40
per share and having an expiry date of January 20, 2011.
On
October 28, 2009, pursuant to the completion of a total of 800,000 units private
placement, the Company issued 800,000 share purchase warrants exercisable to
acquire 800,000 common shares of the Company at $1.00 per share, expiring
October 28, 2011. At November 30, 2009, the 800,000 warrants issued
are still outstanding.
We
incurred a loss of $423,740 for the three month period ended November 30, 2009.
As indicated below, our estimated working capital requirements and projected
operating expenses for the next twelve month period total $1,300,000. Although
we have sufficient funds to carry out our operations for the next twelve month
period, we may attempt to raise additional funds through the issuance of equity
securities or through debt financing. There can be no assurance that we will be
successful in raising the required capital or that actual cash requirements will
not exceed our estimates. We intend to fulfill any additional cash requirement
through the sale of our equity securities.
We expect
to require a total of approximately $1,300,000, as set out below to carry out
our business plan over the twelve months beginning September 2009. Our
expenditures for the next twelve months include:
|
Our
cost to
|
|||
|
complete
|
|||
|
Description
|
$
|
||
|
Equipment
|
250,000
|
||
|
Leasehold
Improvement/rent
|
200,000
|
||
|
Research
|
200,000
|
||
|
Packaging
|
100,000
|
||
|
Wages
|
300,000
|
||
|
Professional
Fees
|
100,000
|
||
|
Travel
|
50,000
|
||
|
Overhead
|
50,000
|
||
|
Administration
|
50,000
|
||
|
Total
|
1,300,000
|
We do not anticipate generating positive
internal operating cash flow until we can generate substantial revenues, which
may take the next few years to realize. There is no assurance we will achieve
profitable operations in the future. We have historically financed our
operations primarily by cash flows generated from the sale of our equity
securities and through cash infusions from officers and affiliates in exchange
for debt and/or common stock. No officer or affiliate has made any commitment or
is obligated to continue to provide cash through loans or purchases of
equity.
3
We intend
to meet the balance of our cash requirements for the next 12 months through a
combination of debt financing and equity financing through private placements.
Currently we are active in contacting broker/dealers in Canada and elsewhere
regarding possible financing arrangements. However, we currently do not have any
arrangements in place for the completion of any further private placement
financings and there is no assurance that we will be successful in completing
any further private placement financings. If we are unsuccessful in raising
sufficient funds through future capital raising efforts, we may review other
financing options.
We have
generated no revenues and incurred significant operating losses from operations.
Since we anticipate we will expand operational activities in the future, we may
continue to experience net negative cash flows from operations and will be
required to obtain additional financing to fund operations through equity
securities offerings and bank borrowings to the extent necessary to provide
working capital. Our continuation as a going concern is dependent upon our
ability to generate sufficient cash flow from stockholders or other outside
sources to sustain operations and meet our obligations on a timely basis and
ultimately to attain profitability. We have limited capital with which to pursue
our business plan. There can be no assurance that our future operations will be
significant and profitable or that we will have sufficient resources to meet our
objectives.
There are
no assurances that we will be able to obtain funds required for our continued
operation. There can be no assurance that additional financing will be available
to us when needed or, if available, that it can be obtained on commercially
reasonable terms. If we are not able to obtain additional financing on a timely
basis, we will not be able to meet our other obligations as they become due and
we will be forced to scale down or perhaps even cease the operation of our
business.
There is
substantial doubt about our ability to continue as a going concern as the
continuation of our business is dependent upon obtaining further financing. The
issuance of additional equity securities by us could result in a significant
dilution in the equity interests of our current stockholders. Obtaining
commercial loans, assuming those loans would be available, will increase our
liabilities and future cash commitments.
Product
Research and Development
During
the twelve month period ending November 30, 2010, we intend to work on the
formulation of our drugs. The rate of blood absorption will be investigated.
Further, we intend to evaluate the dose ratio between active chemicals and other
ingredients. We want to improve the drug bioavailability and maximize
absorption. We also intend to set out a formula for drug to body mass ratio.
Research will also be conducted on other ingredients which can be used for
enhancement of skin penetration.
Purchase
of Significant Equipment
During
the twelve month period ending November 30, 2010, we intend to purchase a tablet
maker which will produce at least 100 tablets a minute. This tablet maker will
cost approximately $50,000. We also intend to purchase an automated tablet
packager. The automated tablet packager will automatically drop a certain number
of tablets into each bottle. The bottles then go for sealing, capping and
labeling. Once the sealing, capping and labeling are completed, the automated
tablet packager will produce a complete packed bottle. The cost of this fully
automated equipment is approximately $200,000.
As of
November 30, 2009, our company had no off-balance sheet arrangements, including
any outstanding derivative financial statements, off-balance sheet guarantees,
interest rate swap transactions or foreign currency contracts. Our company does
not engage in trading activities involving non-exchange traded
contracts.
Employees
Our
company is currently operated by Hassan Salari as our president, secretary,
treasurer and chief executive officer and Audrey Lew as our chief financial
officer. Currently we have four employees. Our company may hire employees when
circumstances warrant, however we do not anticipate hiring additional employees
in the near future. We presently conduct our business through agreements with
consultants and arms-length third parties.
4
Going
Concern
Due to
the uncertainty of our ability to meet our current operating and capital
expenses, in their report on our annual financial statements for the year ended
May 31, 2009, our independent auditors included an explanatory paragraph
regarding concerns about our ability to continue as a going
concern.
APPLICATION
OF CRITICAL ACCOUNTING POLICIES
The
preparation of financial statements in conformity with United States generally
accepted accounting principles requires our management to make estimates and
assumptions that affect the reported amount of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenues and expenses during the
reporting period. Actual results could differ from those estimates. Our
management routinely makes judgments and estimates about the effects of matters
that are inherently uncertain.
We have
identified certain accounting policies, described below, that are most important
to the portrayal of our current financial condition and results of
operations.
Long-Lived
Assets
In
accordance with SFAS No. 144, “Accounting for the Impairment or
Disposal of Long-Lived Assets”, our company tests long-lived assets or
asset groups for recoverability when events or changes in circumstances indicate
that their carrying amount may not be recoverable. Circumstances which could
trigger a review include, but are not limited to: significant decreases in the
market price of the asset; significant adverse changes in the business climate
or legal factors; accumulation of costs significantly in excess of the amount
originally expected for the acquisition or construction of the asset; current
period cash flow or operating losses combined with a history of losses or a
forecast of continuing losses associated with the use of the asset; and current
expectation that the asset will more likely than not be sold or disposed
significantly before the end of its estimated useful life.
Recoverability
is assessed based on the carrying amount of the asset and its fair value which
is generally determined based on the sum of the undiscounted cash flows expected
to result from the use and the eventual disposal of the asset, as well as
specific appraisal in certain instances. An impairment loss is recognized when
the carrying amount is not recoverable and exceeds fair value.
Website Development
Costs
We
capitalize website development costs in accordance with the American Institute
of Certified Public Accountants (“AICPA”) Statement of Position (“SOP”) No.
98-1, “Accounting for the
Costs of Computer Software Developed or Obtained for Internal Use” and
Emerging Issues Task Force (EITF) No. 00-2, “Accounting for Website Development
Costs”, whereby costs related to the preliminary project stage of
development are expensed and costs related to the application development stage
are capitalized. Any additional costs for upgrades and enhancements which result
in additional functionality will be capitalized. Capitalized costs will be
amortized based on their
estimated
useful life over three years. Internal costs related to the development of
website content are charged to operations as incurred.
Research and Development
Costs
Research
costs are expensed in the period incurred. Development costs are expensed in the
period incurred unless we believe a development project meets generally accepted
accounting criteria for deferral and amortization. No such costs had been
deferred as at November 30, 2009 and 2008.
Foreign Currency
Translation
Our
company’s functional currency is the Canadian dollar with the reported amounts
being stated in the United States dollar. In accordance with the Statement of
Financial Accounting Standards (“SFAS”) No. 52, Foreign Currency Translation,
assets and liabilities are translated at the rates of exchange at the balance
sheet dates. Income and expense items are translated at average annual rates of
exchange. The resulting translation adjustments are included in accumulated
other comprehensive income (loss), a separate component of stockholders’
equity.
5
Stock-based
Compensation
In
accordance with SFAS 123R, “Share Based Payments”, our
company accounts for share-based payments using the fair value method. Common
shares issued to third parties for non-cash consideration are valued based on
the fair market value of the services provided or the fair market value of the
common stock on the measurement date, whichever is more readily
determinable.
RECENT
ACCOUNTING PRONOUNCEMENTS
In May
2009, the FASB issued FAS No. 165, “Subsequent
Events”. This pronouncement establishes standards for
accounting for and disclosing subsequent events (events which occur after the
balance sheet date but before financial statements are issued or are available
to be issued). FAS 165 requires an entity to disclose the date
subsequent events were evaluated and whether that evaluation took place on the
date financial statements were issued or were available to be
issued. It is effective for interim and annual periods ending after
June 15, 2009. The adoption of FAS 165 did not have a material impact
on the Company’s financial condition or results of operation.
In June
2009, the FASB issued FAS 166, “Accounting for Transfers of
Financial Assets”, an amendment of FAS 140. FAS 140 is
intended to improve the relevance, representational faithfulness, and
comparability of the information that a reporting entity provides in its
financial statements about a transfer of financial assets: the
effects of a transfer on its financial position, financial performance and cash
flows: and a transferor’s continuing involvement, if any, in transferred
financial assets. This statement must be applied as of the beginning
of each reporting entity’s first annual reporting period that begins after
November 15, 2009. The Company does not expect the adoption of
FAS 166 to have an impact on the Company’s results of operations, financial
condition or cash flows.
In June
2009, the FASB issued FAS 167, “Amendments to FASB Interpretation
No. 46(R)”. FAS 167 is intended to (1) address the effects on
certain provisions of FASB Interpretation No. 46 (revised December 2003), Consolidation of Variable Interest
Entities, as a result of the elimination of the qualifying
special-purpose entity concept in FAS 166, and (2) constituent concerns about
the application of certain key provisions of Interpretation 46R, including those
in which the accounting and disclosures under the Interpretation do not always
provide timely and useful information about an enterprise’s involvement in a
variable interest entity. This statement must be applied as of the
beginning of each reporting entity’s first annual reporting period that begins
after November 15, 2009. The Company does not expect the adoption of
FAS 167 to have an impact on the Company’s results of operations, financial
condition or cash flows.
In June
2009, the FASB issued FAS 168, “The FASB Accounting Standards
Codification and the Hierarchy of General Accepted Accounting
Principles”. FAS 168 will become the source of authoritative
U.S. general accepted accounting principles (GAAP) recognized by the FASB to be
applied by nongovernmental entities. Rules and interpretive releases
of the Securities and Exchange Commission (SEC) under authority of federal
securities laws are also sources of authoritative GAAP for SEC
registrants. On the effective date of this Statement, the
Codification will supersede all then-existing non-SEC accounting and reporting
standards. All other non-grandfathered non-SEC accounting literature
not included in the Codification will become non-authoritative. This
statement is effective for financial statements issued for interim and annual
periods ending after September 15, 2009. The Company does not expect
the adoption of FAS 168 to have an impact on the Company’s results of
operations, financial condition or cash flows.
In
September 2009, the FASB issued ASC 820-10 Measuring Liabilities at Fair Value
(“ASC 820-10”). ASC 820-10 provides additional guidance on how
companies should measure liabilities at fair value. Specifically, the
fair value of a liability is not adjusted to reflect the impact of contractual
restrictions that prevent its transfer. ASC 820-10 will be adopted by
the Company in the second quarter of fiscal year 2010. The Company is
currently evaluating the impact of ASC 820-10, but does not expect the adoption
to have a material impact on its financial position, results of operations, and
cash flows.
OFF
BALANCE SHEET TRANSACTIONS
We have
no off-balance sheet arrangements.
ITEM
3. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISKS
The
Company is not subject to certain market risks, including changes in interest
rates and currency exchange rates.
6
ITEM
4. CONTROLS AND PROCEDURES
Disclosure
Controls and Procedures
We
maintain disclosure controls and procedures that are designed to ensure that
information required to be disclosed in our reports filed under the Securities
Exchange Act of 1934, as amended, is recorded, processed, summarized and
reported within the time periods specified in the Securities and Exchange
Commission’s rules and forms, and that such information is accumulated and
communicated to our management, including our president (who is also our chief
executive officer, secretary and treasurer) and our chief financial officer to
allow for timely decisions regarding required disclosure. In designing and
evaluating our disclosure controls and procedures, our management recognizes
that any controls and procedures, no matter how well designed and operated, can
provide only reasonable assurance of achieving the desired control objectives,
and our management is required to apply its judgment in evaluating the
cost-benefit relationship of possible controls and procedures.
As of
November 30, 2009, the period covered by this report, we carried out an
evaluation, under the supervision and with the participation of our management,
including our president (who is also our chief executive officer, secretary and
treasurer) and our chief financial officer, of the effectiveness of the design
and operation of our disclosure controls and procedures. Based on the foregoing,
our president and chief financial officer concluded that our disclosure controls
and procedures were effective as of the end of the period covered by this
quarterly report.
Changes
in Internal Control Over Financial Reporting
There
have been no changes in our internal controls over financial reporting that
occurred during the three month period ended November 30, 2009 that have
materially or are reasonably likely to materially affect, our internal controls
over financial reporting.
7
We are
involved in routine litigation incidental to the conduct of our business. There
are currently no material pending litigation proceedings to which we are a party
or to which any of our property is subject.
ITEM
1A. RISK FACTORS.
There are
no material changes to the risk factors disclosed in our annual report filed on
Form 10-K/A dated September 23, 2009.
Effective
December 8, 2009, the Company issued an aggregate of 533,333 shares of common
stock and 533,333 warrants to one investor pursuant to a private placement
subscription agreement dated October 28, 2009 for gross proceeds of
$400,000. Each warrant entitles the investor to purchase one
additional share of common stock of the Company at a price of $1.00 per share
until December 8, 2011. The Company issued the securities to one
non-U.S. person (as that term is defined in Regulation S of the Securities Act
of 1933) in an offshore transaction relying on Regulation S and/or Section 4(2)
of the Securities Act of 1933.
Effective
December 11, 2009, the Company issued an aggregate of 4,000,000 shares of its
common stock to the shareholders of Posh Cosmeceuticals Inc. pursuant to share
exchange agreements dated June 12, 2009. The Company issued the
securities to twenty-seven non-U.S. persons (as that term is defined in
Regulation S of the Securities Act of 1933) in an offshore transaction relying
on Regulation S and/or Section 4(2) of the Securities Act of
1933.
As of
January 15, 2010, the Company has 68,055,400 issued and outstanding common
shares.
None.
None
None
|
(a)
|
Exhibits
|
|
Exhibit
Number
|
Description
of Exhibit
|
|
|
31.1
|
Certification
of Principal Executive Officer pursuant to Rule 13a-14 and Rule 15d-14(a),
promulgated under the Securities Exchange Act of 1934, as
amended
|
|
|
31.2
|
Certification
of Principal Financial Officer pursuant to Rule 13a-14 and Rule 15d-14(a),
promulgated under the Securities Exchange Act of 1934, as
amended
|
|
|
32.1
|
Certification
of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002
|
|
|
32.2
|
Certification
of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002
|
8
SIGNATURES
In
accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused
this report to be signed on its behalf by the undersigned, there unto duly
authorized.
|
Date: January
15, 2010
|
GLOBAL
HEALTH VENTURES INC.
|
|
/s/ Hassan
Salari
|
|
|
By:
Hassan Salari, President, Secretary,
|
|
|
Treasurer,
Chief Executive Officer and Director
|
|
|
(Principal
Executive Officer)
|
|
|
/s/ Audrey
Lew
|
|
|
By:
Audrey Lew, Chief Financial Officer
|
|
|
(Principal
Financial Officer and
|
|
|
Principal
Accounting Officer)
|
9
