Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MERIDIAN BIOSCIENCE INC | c94597e8vk.htm |
Exhibit 99.1

| VIVO CJS Securities, Inc. 10th Annual "New Ideas for the New Year" Investor Conference January 14, 2010 |

| Forward Looking Statements The Private Securities Litigation Reform Act of 1995 provides a safe harbor from civil litigation for forward-looking statements accompanied by meaningful cautionary statements. Except for historical information, this report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, which may be identified by words such as "estimates", "anticipates", "projects", "plans", "seeks", "may", "will", "expects", "intends", "believes", "should" and similar expressions or the negative versions thereof and which also may be identified by their context. Such statements, whether expressed or implied, are based upon current expectations of the Company and speak only as of the date made. The Company assumes no obligation to publicly update or revise any forward-looking statements even if experience or future changes make it clear that any projected results expressed or implied therein will not be realized. These statements are subject to various risks, uncertainties and other factors that could cause actual results to differ materially, including, without limitation, the following: Meridian's continued growth depends, in part, on its ability to introduce into the marketplace enhancements of existing products or new products that incorporate technological advances, meet customer requirements and respond to products developed by Meridian's competition. While Meridian has introduced a number of internally developed products, there can be no assurance that it will be successful in the future in introducing such products on a timely basis. Ongoing consolidations of reference laboratories and formation of multi-hospital alliances may cause adverse changes to pricing and distribution. Recessionary pressures on the economy and the markets in which our customers operate, as well as adverse trends in buying patterns from customers can change expected results. Costs and difficulties in complying with laws and regulations administered by the United States Food and Drug Administration can result in unanticipated expenses and delays and interruptions to the sale of new and existing products. Changes in the relative strength or weakness of the U.S. dollar can also change expected results. One of Meridian's main growth strategies is the acquisition of companies and product lines. There can be no assurance that additional acquisitions will be consummated or that, if consummated, will be successful and the acquired businesses successfully integrated into Meridian's operations. The Company cannot predict the possible effects of potential healthcare reform in the United States and similar initiatives in other countries on its results of operations. In addition to the factors described in this paragraph, Part I, Item 1A Risk Factors of our Form 10-K contains a list and description of uncertainties, risks and other matters that may affect the Company. VIVO |
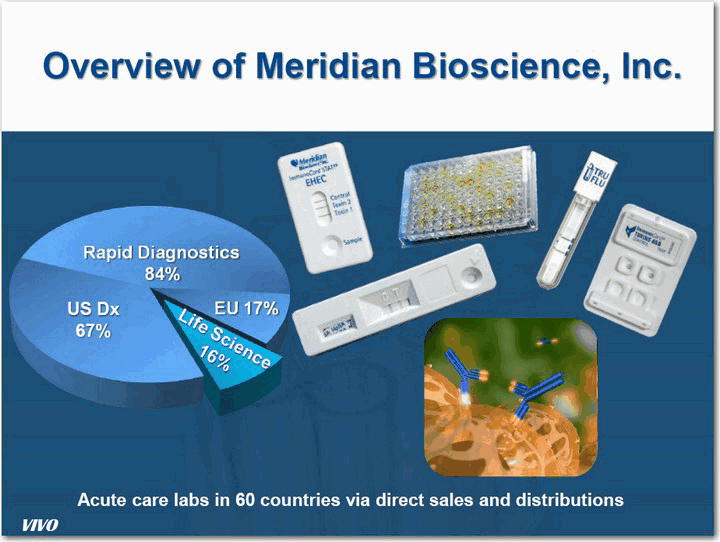
| Overview of Meridian Bioscience, Inc. Acute care labs in 60 countries via direct sales and distributions US Dx 67% VIVO |
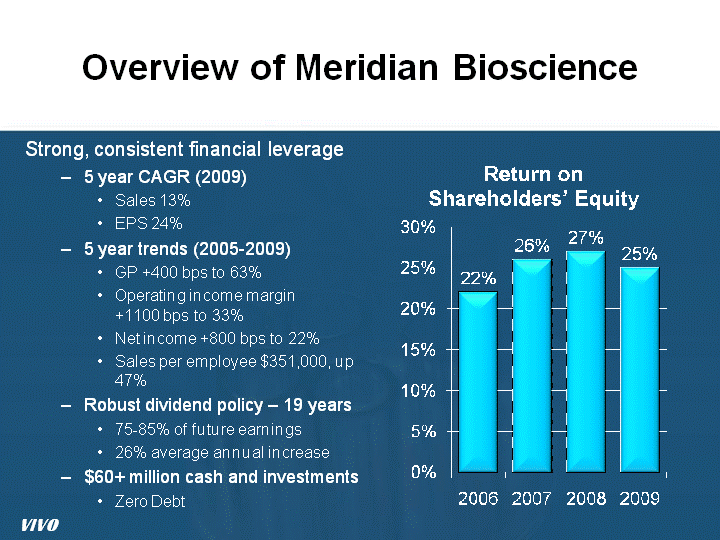
| Overview of Meridian Bioscience Strong, consistent financial leverage 5 year CAGR (2009) Sales 13% EPS 24% 5 year trends (2005-2009) GP +400 bps to 63% Operating income margin +1100 bps to 33% Net income +800 bps to 22% Sales per employee $351,000, up 47% Robust dividend policy - 19 years 75-85% of future earnings 26% average annual increase $60+ million cash and investments Zero Debt VIVO |
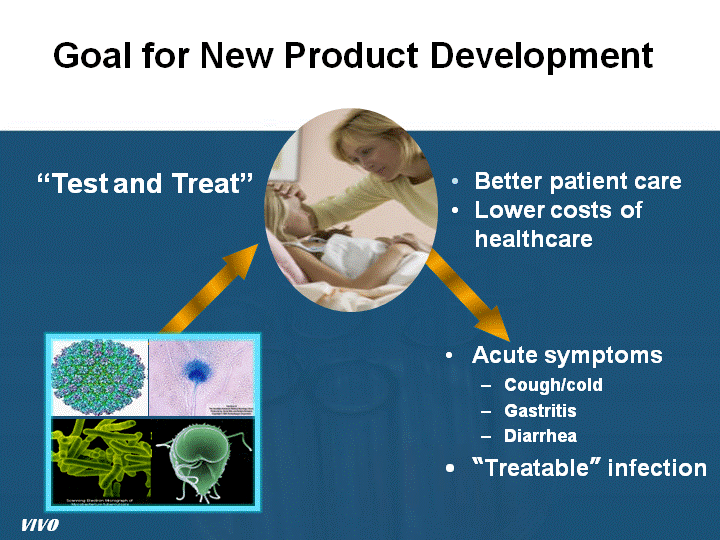
| Goal for New Product Development Acute symptoms Cough/cold Gastritis Diarrhea "Treatable" infection VIVO "Test and Treat" Better patient care Lower costs of healthcare |

| Fiscal 2010 Dx Growth Drivers Food Borne Diseases Stomach Ulcers Upper Respiratory Infections C. difficile Disease VIVO |
| Meridian Life Science Leading producer of viral proteins for Rubella, Mumps, Measles, Toxoplasma, VZV, CMV, Herpes, Epstein-Barr Virus, and Hepatitis A Virus Leading supplier of antibodies, antigens & related components to major diagnostics manufacturers Custom process development and manufacturing services, including cGMP manufacturing capability High-value biological components and technologies for diagnostic and biopharmaceutical manufacture VIVO |
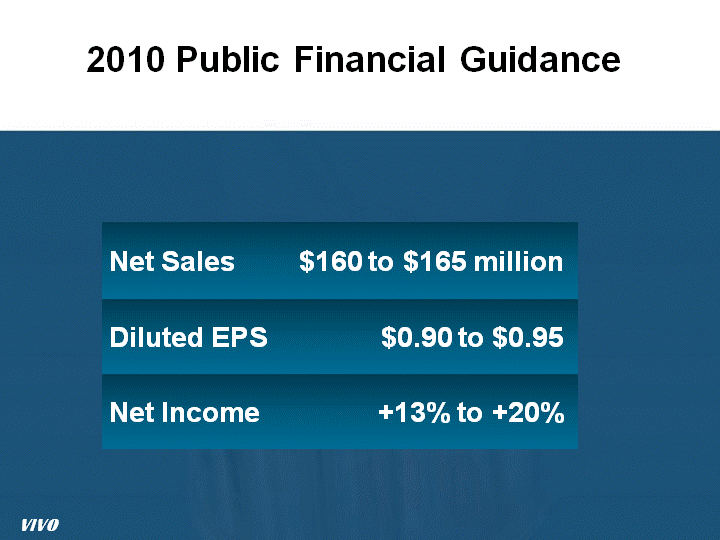
| 2010 Public Financial Guidance 2010 Public Financial Guidance VIVO |
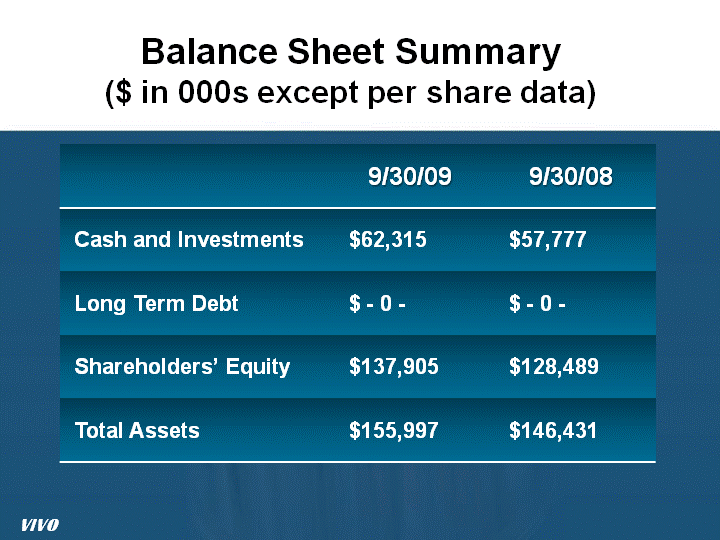
| Balance Sheet Summary ($ in 000s except per share data) ($ in 000s except per share data) ($ in 000s except per share data) VIVO |
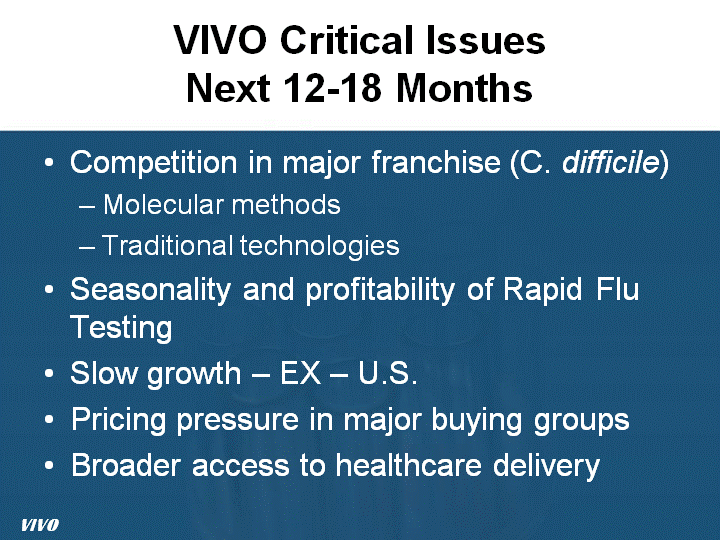
| VIVO Critical Issues Next 12-18 Months Competition in major franchise (C. difficile) Molecular methods Traditional technologies Seasonality and profitability of Rapid Flu Testing Slow growth - EX - U.S. Pricing pressure in major buying groups Broader access to healthcare delivery VIVO |
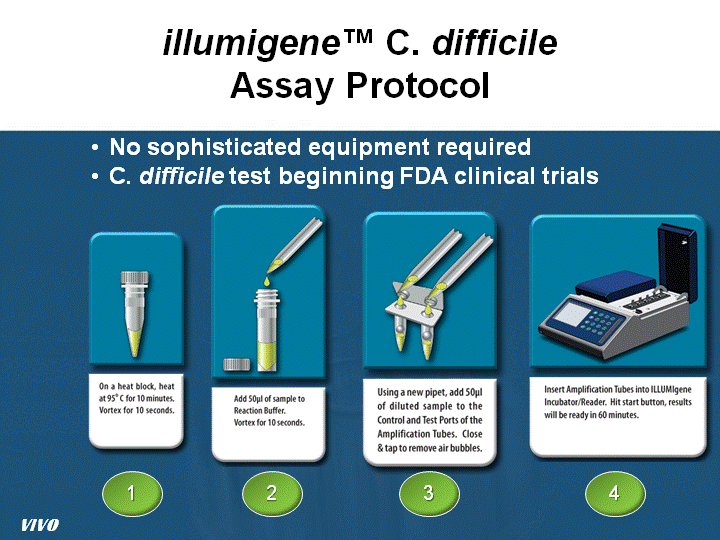
| illumigene(tm) C. difficile Assay Protocol 1 2 3 4 No sophisticated equipment required C. difficile test beginning FDA clinical trials VIVO |

| Near Term Sources of Growth Recent new product activity Premier(tm) CAMPY and ImmunoCard STAT! (r) CAMPY highly accurate tests for a serious stool pathogen TRU FLU test for Influenza A&B TRU RSV test for Respiratory Syncytial Virus New illumigene(tm) molecular diagnostic technology beginning FDA clinical trials VIVO |
