Attached files
| file | filename |
|---|---|
| 8-K - ADEONA PHARMACEUTICALS--FORM 8K - Synthetic Biologics, Inc. | s22-9533_8k.htm |
| EX-99.2 - PRESS RELEASE, JANUARY 12, 2010 - Synthetic Biologics, Inc. | s22-9533_ex992.htm |
| EX-99.3 - PRESS RELEASE, JANUARY 8, 2010 - Synthetic Biologics, Inc. | s22-9533_ex993.htm |

AMEX: AEN
OneMedForum 2010
January 13, 2010

Forward Looking Statements
AMEX: AEN

the leader addressing
subclinical zinc deficiency
and
chronic copper toxicity
in the
mature population
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN
NYSE-Amex Listed: AEN
Founded 2001
$6MM Personally Invested by Chairman
Went Public October 2006
$21MM @$2.00/share 2006-2007
Current Market Cap: $25MM @ $1.19 share
Daily Volume 725,000 shares/day
21MM Primary Outstanding Shares
+3MM Warrants & Options ($1.51 Avg. Exercise Price/Share)
$3,600,000 cash on hand 9/30/09
Average Burn Rate/Qtr. ~$790K
Adeona Corporate Overview

Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN
Launch
Date/Status
HartLab: CLIA Certified Reference Lab 7/10/09
CopperProof™ Diagnostic Panel 11/9/09
Zinthionein™ ZC GS-150 Q1 2010
Zinc-Monocysteine GRAS
Process
Oral Estriol for MS Phase
II/III
Oral dnaJP1 for RA Phase II Completed
Oral Flupirtine for FMS Phase II IND Approved
Product Pipeline

Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

David A. Newsome, M.D.
President, HealthMine Subsidiary
President, HealthMine Subsidiary
Training:
Duke University, A.B.
Columbia University, M.D.
Harvard Medical School, Ophthalmology Residency
(Massachusetts Eye and Ear Infirmary)
Harvard Medical School, Retina Disease and Surgery Fellowship
Bascom Palmer Eye Institute, Retina Disease and Surgery Fellowship
Research & Faculty Experience:
– National Eye Institute (NIH)
• Chief, Retinal Disease Section
– Johns Hopkins, Wilmer Eye Institute, Associate
Professor
– LSU Eye Center, Professor
• Author, 150 Peer-Reviewed Publications and 2 books
– Originated Zinc Hypothesis for Age-Related
Macular Degeneration
– 1988, Published First Clinical Trial, “Oral
Zinc Supplementation for Age Related
Macular Degeneration”
Macular Degeneration”
– Currently marked as “Preservision”,
“Ocuvite” “iCaps”
• 2008 Sales ~ $300MM, B&L and Alcon
AMEX: PP
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

George J. Brewer, M.D.
Co-Founder, Chair SAB
Co-Founder, Chair SAB
• Chairman Emeritus, Department of Human Genetics, University of
Michigan
Michigan
• Foremost Authority on Chronic Copper Toxicity - Wilson’s disease,
>500 Publications
>500 Publications
• Member, EPA-Convened National Academy of Sciences -
Committee on Copper in Drinking Water (2000)
Committee on Copper in Drinking Water (2000)
• Drawbacks of Galzin®/Wilzin®:
– Nausea in 90% of Patients, G.I. Irritation
in 30% of Patients
– 3X/day dosing away from meals
• Co-inventor, Adeona Modified-Release Oral Zinc Technology
AMEX: PP, Inc.
AMEX: AEN
Galzin is a registered trademark of Gate Pharmaceuticals Inc.

Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN
Diseases of Confirmed or Suspected
Copper/Zinc Dyshomeostasis
Copper/Zinc Dyshomeostasis
|
DISEASE |
U.S. PERSONS |
|
Wilson’s Disease |
10,000 |
|
Dry Age-Related Macular Degeneration |
17,000,000 |
|
Alzheimer’s Disease |
5,800,000 |
|
Mild Cognitive Impairment |
15,000,000 |
|
Parkinson’s Disease |
400,000 |
|
Complications of Diabetes |
23,000,000 |
|
Estimated Total U.S. Persons |
50,000,000 |

Copper’s
Involvement
in
Alzheimer’s
and Mild
Cognitive
Impairment
Involvement
in
Alzheimer’s
and Mild
Cognitive
Impairment
(COPPER)
(Copper)
(Copper)
(Copper)
(Copper)
(Copper)
Courtesy: NIA;
“Copper” Text Added.


5.3 Million Miles of Copper Tube in U.S.

Current EPA Limit of Copper in Water:
1.3mg/L
1.3mg/L
Average Home has About 1/10th the EPA Limit



Copper-Containing Multivitamins and
Cognitive Decline in the CHAP Study (2006)
Cognitive Decline in the CHAP Study (2006)
• Chicago Health and Aging Project (CHAP)
• 3718 Chicago Residents over Age 65
• Cognition Tested at 0, 3 and 6 Years
• Copper-Containing Multivitamin Consumption
Noted and Compared
Noted and Compared
• Consumers of Copper-Containing
Multivitamins (i.e. 2mg/day) with a High Fat
Diet had 6.51 Times the Rate of Cognitive
Decline than Persons not taking Copper-
Containing Multivitamins
Multivitamins (i.e. 2mg/day) with a High Fat
Diet had 6.51 Times the Rate of Cognitive
Decline than Persons not taking Copper-
Containing Multivitamins
AMEX: AEN

Morris MC et. al., Arch. Neurol. Vol. 63;1085-8 (Aug 2006)
Chicago Health and Aging Project (CHAP)
AMEX: AEN

Serum Copper Dyshomeostasis in AD & MCI
|
Free Copper Ions |
0 |
0.0001 |
|
Homocysteine |
<10 |
<0.5% |
|
Ferroxidase II |
<10 |
<0.5% |
|
Extracellular SOD |
<10 |
<0.5% |
|
Factors V and VIII |
<5 |
<0.5% |
|
Metallothionein |
<1 |
<0.1% |
|
15-60kDa Proteins |
40 |
<0.5% |
|
Small Peptides |
35 |
<0.5% |
|
Albumin |
150 |
15% |
|
Ceruloplasmin |
700 |
70% |
%
µg/L
Copper
Bound
To
Cerulo-
Plasmin
(Cp)
Free
Copper
Copper
Bound
To
Cerulo-
Plasmin
(Cp)
Free
Copper
Normal
Wilson’s
AMEX: PP
Copyright © 2007 Pipex Pharmaceuticals, Inc.
Free
Copper
Copper
Bound
To
Cerulo-
Plasmin
(Cp)
Alzheimer’s
& MCI
& MCI
Linder et. al.

Serum Copper/Zinc Dyshomeostasis in
Alzheimer’s Disease
Alzheimer’s Disease
• Elevated Percent Serum Free Copper in AD
– Distinguishes AD from Normals (Squitti
et. al. 2005)
– Prognostic for Cognitive Decline (Squitti
et. al Jan. 2009)
– Correlates with Cognitive Loss (Arnal
N, et. al. Dec. 2009)
• Defective Ceruloplasmin (Squitti et. al. Jan. 2008)

CopperProof-1 Study
• 90 Subject Prospective, Observational, IRB-Approved
Randomized Comparative Study
Randomized Comparative Study
• Sponsored by Adeona
• The Alzheimer’s Center at Albany Medical Center - P.I. Earl
Zimmerman, M.D.
Zimmerman, M.D.
• 30 Alzheimer’s, 30 Parkinson’s, 30 Age-Matched Normals
• Patients Recruited 2007-2008. Analysis Q1 2009
• Results Presented at ICAD (International Conference on
Alzheimer’s Disease) July 15, 2009
Alzheimer’s Disease) July 15, 2009

• Acquisition by Adeona - July 10, 2009
• CLIA-Certified Laboratory, Chicago
• Launched CopperProof™ Panel - Nov. 2009
• Serum-Based Copper/Zinc Diagnostic Panel
• Diagnose and Grade Patients with
Copper/Zinc Dyshomeostasis (AMD, AD, MCI,
PD etc.)
Copper/Zinc Dyshomeostasis (AMD, AD, MCI,
PD etc.)
• Dx-Rx Strategy - Zinthionein™ ZC GS-150

Zinthionein™ ZC GS-150 Oral Zinc
Gastroretentitive/Sustained Release Oral High Dose Zinc
Preparation
Preparation
Convenient Once-a-Day Dosing (vs. 3X Galzin® or 2X
AREDS)
AREDS)
Broad Patent Protection Anticipated
100% GRAS Ingredients
Highest Single Dose Bioavailability of Oral Zinc in Humans
Extended Sustained Serum Zinc Levels Maintained
Proximal Metallothionein Induction
Intended Lower Instance of Nausea and Gastric Irritation
(vs. 90% for Galzin® at 1/3 Dose)
(vs. 90% for Galzin® at 1/3 Dose)
Planned Launch this Quarter

• Medical Foods:
– Legislation Section 5 of Orphan Drug Act
– GRAS Ingredients “Intended fro the
Dietary
Management of Disease or Condition not
Satisfied by Normal Diet Alone”
Management of Disease or Condition not
Satisfied by Normal Diet Alone”
– Exempt from NDA Requirements
– Must be Sold Under Doctors’ Supervision
• Prescription Product
• Distributed through Pharmacy Distribution
Chain
Chain
– Reimbursement Generally Available
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

CopperProof-2 Clinical Trial
• First Controlled Clinical Trial of Zinc therapy for AD or MCI
• 60 Patient, Two Part, Double Blind, Randomized Placebo Controlled
Trial of Once-Daily Zinthionein™ ZC GS-150 in AD and MCI Patients with
Impaired Serum Copper/Zinc Status
Trial of Once-Daily Zinthionein™ ZC GS-150 in AD and MCI Patients with
Impaired Serum Copper/Zinc Status
• P.I. Diana Pollack, Alzheimer’s Center - Clearwater Hospital, Florida
• IRB Approved November 2nd, 2009
• 30 Day Washout Period for Copper Supplements
• Part I: Single Day Comparison to Galzin® and Placebo
– Endpoint: Improved bioavailability and tolerability
• Part II: 6 Month, Double Blind Randomized Placebo Controlled
Study
– Endpoint: Cognition, MMSE and ADAS-Cog at
3 and 6 months
• Initial Data Expected Next Month (February)
* Zinthionein™ ZC contains 150mg elemental zinc
and does not contain zinc-monocysteine.
and does not contain zinc-monocysteine.

- Issued Composition of Matter Patents
• U.S. Patent 7,164,035, Issued Jan. 16, 2007
• U.S. Patent 6,586,611, Issued July 1, 2003
– Additional pending patents pending
– Modified Release Zinc - GR, SR, High Dose,
Once-Daily
– Combination formulas
– Copper-free formulas
– Pill dispenser that incorporates eye self-test
– Serum based diagnostic assays
– Trademarks:
• Zinthionein®, Eyedaily®, (888) EYE-DAILY,
www.eyedaily.com
www.eyedaily.com
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

AMEX: AEN
ADDITIONAL CLINICAL PROGRAMS
FOR WHICH ADEONA IS SEEKING
BUSINESS DEVELOPMENT
OPPORTUNITIES
FOR WHICH ADEONA IS SEEKING
BUSINESS DEVELOPMENT
OPPORTUNITIES

TRIMESTATM (oral estriol) for Multiple Sclerosis
• Approved in Europe & Asia for 40 years (No U.S.)
• Oral Version of Endogenous Pregnancy Hormone
– Estriol or E3
• First Potential Oral Treatment for MS
• Developed by Rhonda Voskuhl M.D.
– Chair UCLA MS Neurology Center
• Exclusively Licensed from UCLA
• Believed to be Responsible for:
– Fetal Immune Privilege
– Spontaneous Remission of Autoimmune Diseases
• Completed 22 Month Phase IIa Clinical Trial under
funding from the NMSS
funding from the NMSS
AMEX: PP
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

79% Reduction in MRI Lesions @ 3 Months (p=0.02)
Lower number of lesions and lower lesion volume while on Trimesta
New Enhancing Lesion Numbers
New Enhancing Lesion Volumes (mm3)
TRIMESTA (19-22)
Post Tx (16-18)
Post Tx (13-15)
Pre Tx (1-6)
*
*
*
*
*
Months Tx
Copyright © 2008 Pipex Pharmaceuticals, Inc.
AMEX: AEN

Improvement In Cognitive Function By
14% in 6 months (p=0.04)
14% in 6 months (p=0.04)
6 RR Trimesta Tx (month 12)
6 RR Pre Tx (month 3)
4 SP Trimesta Tx (month 12)
4 SP Pre Tx (month 3)
All 10 Trimesta Tx (month 12)
All 10 Pre Tx (month 3)
*
AMEX: AEN

TRIMESTA™ Phase II/III Clinical Trial
• Phase II/III Clinical Trial In MS
– 150 Patients, 71 Enrolled
– 16 U.S. Centers
– Double-Blind, 1:1 Placebo Design with Copaxone
as Standard of Care
– Female Relapsing-Remitting Patients
– Funded by $5 Million NIH/National MS Society
Grant
• Largest Clinical Grant Ever Awarded by NMSS
• Recent Additional $860,000 Grant under ARRA
– Primary Endpoint
• Relapse Rate at 2 Years with Interim Analysis
at 1 Year
– Secondary Endpoints
• Cognitive Tests-PASAT
• NAA levels
• MRI
• Hippocampus volume
AMEX: AEN

AMEX: AEN
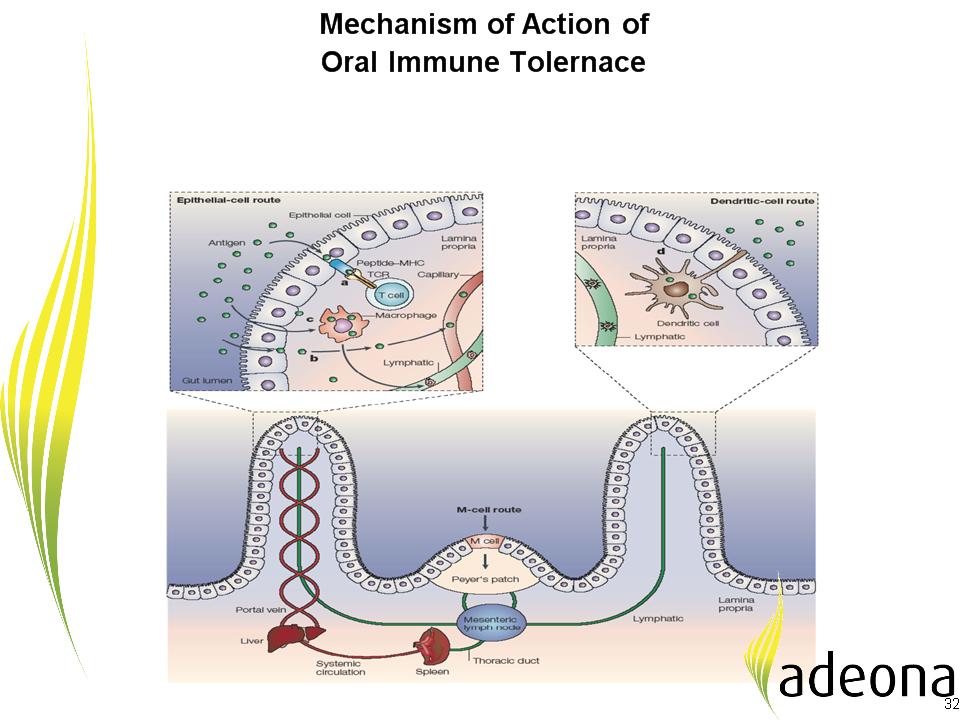
Oral mucosal route induces immune tolerance
to the epitope-specific antigens
AMEX: AEN

•Double-Blind, Placebo-Controlled Phase II Clinical Trial
•Active RA patients
•Background Therapy Medications Allowed
•11 Clinical Centers
•Stanford, UCSD, U. Arizona, John Hopkins, Mayo Clinic
•Daily oral administration, 6 months
•160 patients: 1:1 randomization
•Enrollment criteria based on presence of both immunological reactivity to HSP-
dnaJP1 and clinically active disease
dnaJP1 and clinically active disease
•Endpoints:
•Clinical Endpoints: ACR 20, ACR50
• ACR20: FDA approvable endpoint
•Immunologic Endpoints
AMEX: AEN

Published Results of Phase II Study
• dnaJP1 appeared to be safe and well-tolerated.
• There was a significant reduction in the percentage of T cells producing the
proinflammatory cytokine tumor necrosis factor alpha (TNF-alpha) (p < 0.0007).
proinflammatory cytokine tumor necrosis factor alpha (TNF-alpha) (p < 0.0007).
• The primary efficacy end point (meeting the American College of Rheumatology
20% improvement criteria at least once on day 112, 140, or 168) showed a
difference between treatment groups (p=0.09) that became significant in post hoc
analysis using generalized estimating equations (p=0.04).
20% improvement criteria at least once on day 112, 140, or 168) showed a
difference between treatment groups (p=0.09) that became significant in post hoc
analysis using generalized estimating equations (p=0.04).
• Differences in clinical responses were also found between treatment groups on
day 140 and at follow up, indicative a durable response following discontinuation
of therapy.
day 140 and at follow up, indicative a durable response following discontinuation
of therapy.
Arthritis & Rheumatism, Nov. 2009

• Oral flupirtine
– Developed by Andrew Stoll M.D.
• Experimental Pharmacology, McLean Hospital/Harvard
University
– Oral flupirtine, marketed in Europe since
1984
• Potassium Channel Modulator
• In-direct NMDA receptor antagonist
• Non-opioid, centrally acting analgesic compound
• New mechanism of action
– Issued U.S. Patent, pending international
patents
– Pilot human results in refractory fibromyalgia
patients
• ~24 patients in fibromyalgia
• ~150 patient clinical results in glaucoma,
retinitis pigmentosa, diabetic retinopathy
– Phase II ready clinical trial in refractory
fibromyalgia
• IND Approved
• IRB Approval Received
AMEX: AEN

Steve H. Kanzer, CPA, JD
Chairman
Adeona Pharmaceuticals, Inc.
3930 Varsity Drive
Ann Arbor, Michigan 48108
Phone: (734) 332-7800
Fax: (734) 332-7878
www.adeonapharma.com
Copyright © 2010 Adeona Pharmaceuticals, Inc.
AMEX: AEN
