Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - PROGENITY, INC. | prog-ex991_7.htm |
| 8-K - 8-K - PROGENITY, INC. | prog-8k_20201109.htm |

Business Update and Third Quarter 2020 Financial Results November 9, 2020 Exhibit 99.2

Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, which statements are subject to substantial risks and uncertainties and are based on estimates and assumptions. All statements, other than statements of historical facts included in this presentation, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, financing needs, plans or intentions relating to product candidates, estimates of market size, estimates of market growth, business trends, expected testing supply and demand, the anticipated timing, design and conduct of our planned clinical trials, the development of our product candidates, including the timing and likelihood of regulatory filings and approvals for our product candidates, our ability to commercialize our product candidates, if approved, the pricing and reimbursement of our product candidates, if approved, the potential to develop future product candidates, the potential benefits of strategic collaborations and our intent to enter into any strategic arrangements, the timing and likelihood of success, plans and objectives of management for future operations and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this presentation, including those described in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, and elsewhere in such filings and in other subsequent disclosure documents filed with the U.S. Securities and Exchange Commission (SEC). We cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. Forward-looking statements are not historical facts, and reflect our current views with respect to future events. Given the significant uncertainties, you should evaluate all forward-looking statements made in this presentation in the context of these risks and uncertainties and not place undue reliance on these forward-looking statements as predictions of future events. All forward-looking statements in this presentation apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this presentation. We disclaim any intent to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances, except as required by law. Industry and Market Data: We obtained the industry, market, and competitive position data used throughout this Presentation from our own internal estimates and research, as well as from industry and general publications, and research, surveys, and studies conducted by third parties. Internal estimates are derived from publicly available information released by industry analysts and third-party sources, our internal research and our industry experience, and are based on assumptions made by us based on such data and our knowledge of the industry and market, which we believe to be reasonable. In addition, while we believe the industry, market, and competitive position data included in this prospectus is reliable and based on reasonable assumptions, we have not independently verified any third-party information, and all such data involve risks and uncertainties and are subject to change based on various factors. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.

Q3 2020 Progenity Corporate Highlights Grew total tests 12%; reported ~84,000 tests in Q3 2020, mostly from COVID-19 testing. Continued expansion of COVID-19/SARS CoV-2 diagnostic testing to broader geographies within our channel to support unmet need. Continuing improvements in revenue cycle management to maximize revenues. Achieved an important analytical verification milestone for our Preeclampsia rule-out LDT, tradename PreecludiaTM. Work progressing well under precision medicine pharma collaboration; continued engagement with pharma for further potential partnerships. Achieved Innatal 4 development milestone: demonstrated ability to quantify fetal fraction. Added access for 60M health plan members with Multiplan contract. Two abstracts, including category award winner, related to our PIL Dx capsule presented at American College of Gastroenterology (ACG) meeting in October 2020.
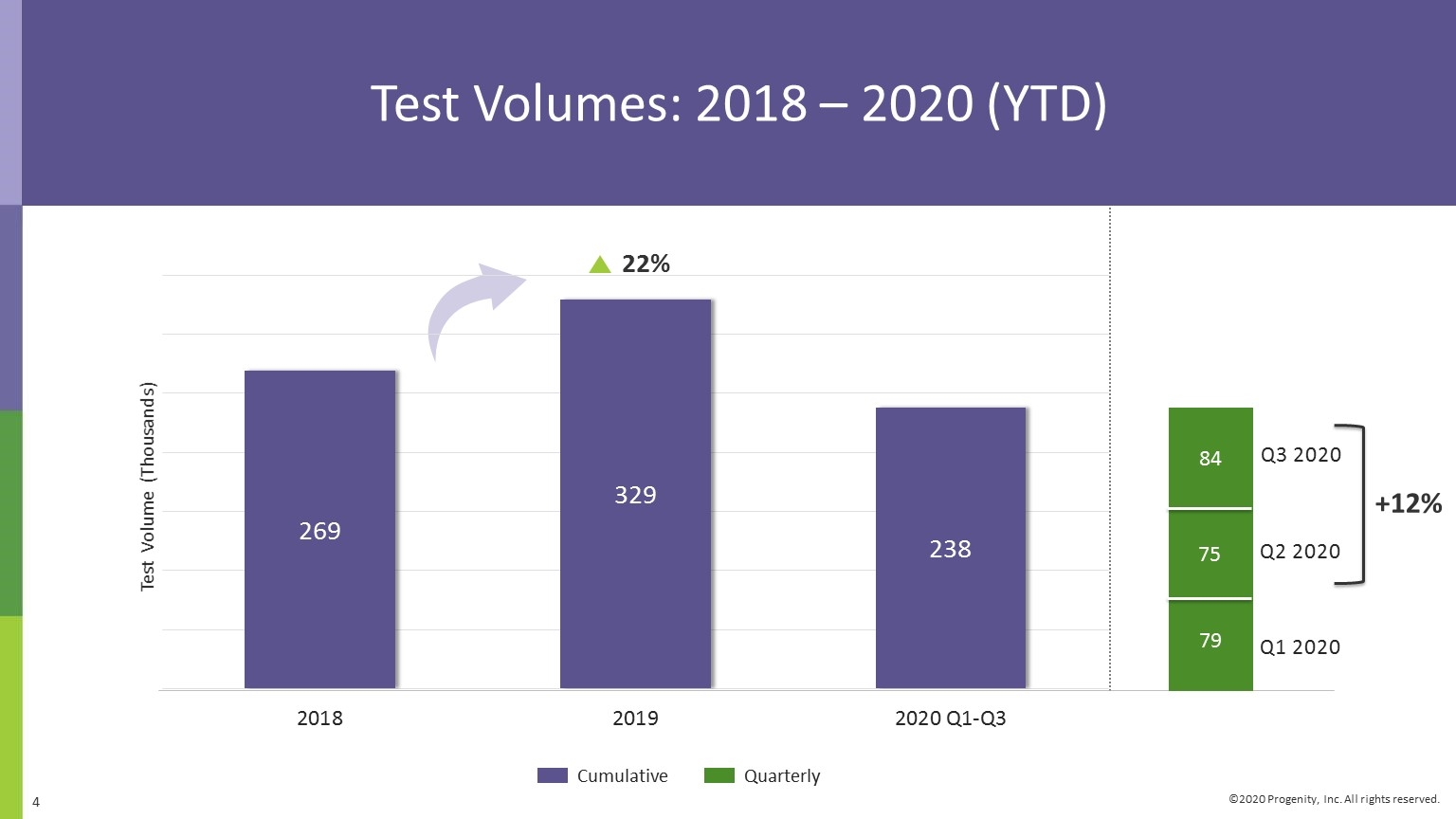
Test Volumes: 2018 – 2020 (YTD) Strong History of Volume Growth Cumulative Quarterly Test Volume (Thousands) Q2 2020 Q1 2020 22% Q3 2020 79 75 84 +12%
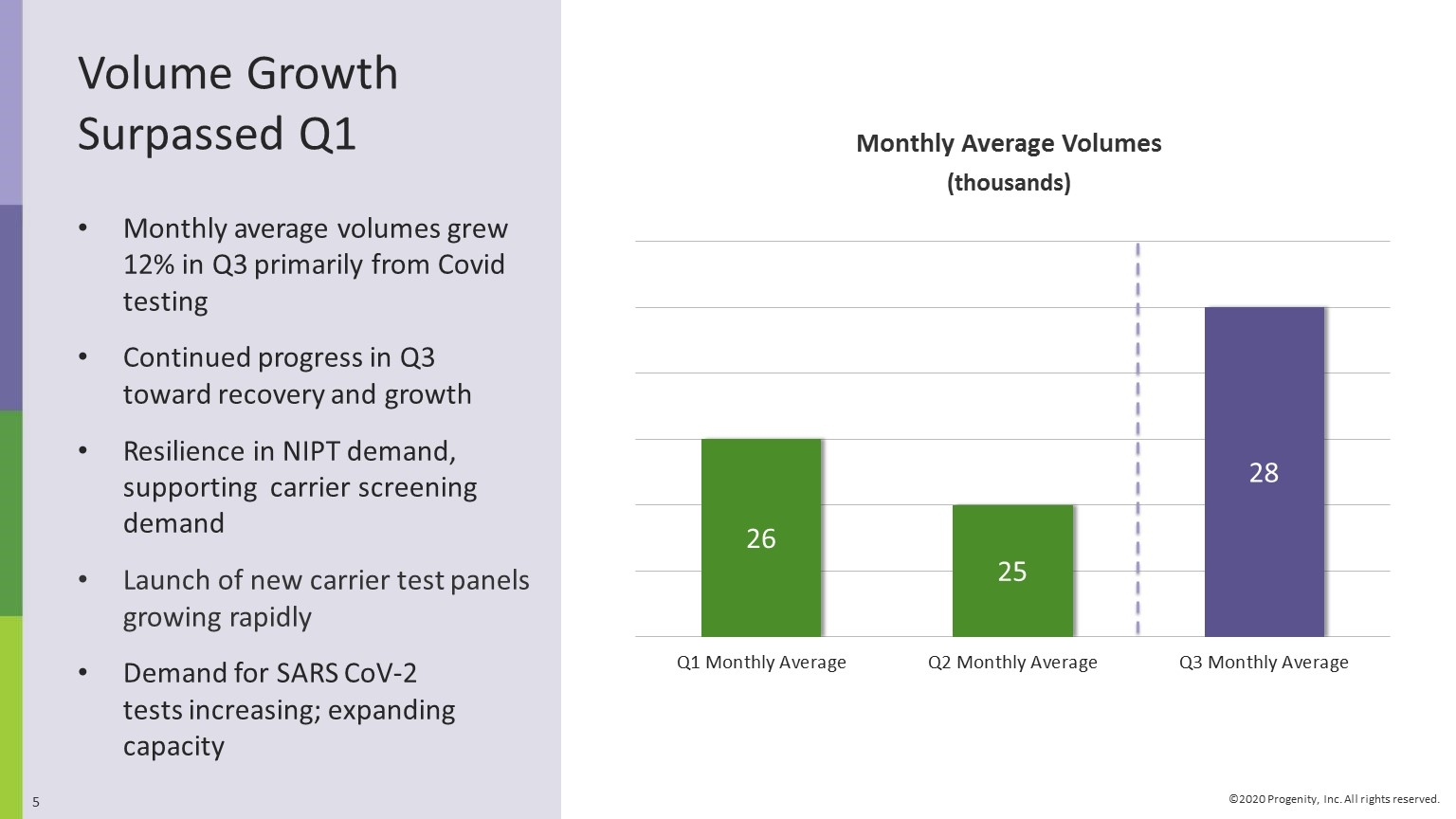
Volume Growth Surpassed Q1 Monthly average volumes grew 12% in Q3 primarily from Covid testing Continued progress in Q3 toward recovery and growth Resilience in NIPT demand, supporting carrier screening demand Launch of new carrier test panels growing rapidly Demand for SARS CoV-2 tests increasing; expanding capacity Monthly Average Volumes (thousands)

Expanding the In-Network Footprint New Multiplan contract; 60M health plan members have access to Multiplan services Added 1.5 million regional plan covered lives in Q3 Aetna covering average risk NIPT through end of 2020 Centene, Humana and some state Medicaid plans also began covering NIPT for average risk In-Network Lives – Progenity (millions) 2019 2020 Additions + + + Total In-Network Lives 2020 Additions: Aetna/Cigna/Other (1) Does not include Multiplan; some overlap with current in-network lives 2019 1

R&D Pipeline Update
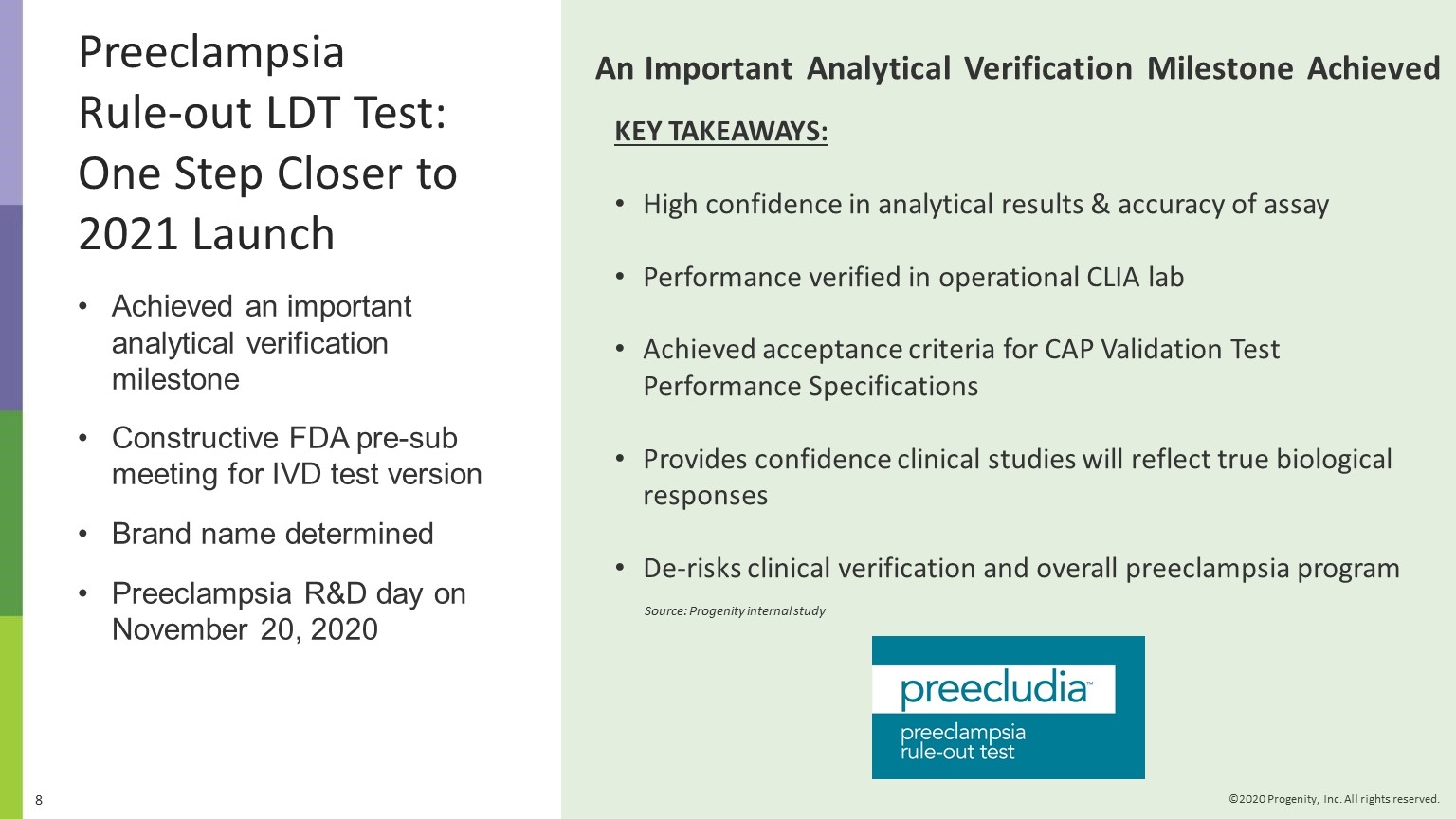
Achieved an important analytical verification milestone Constructive FDA pre-sub meeting for IVD test version Brand name determined Preeclampsia R&D day on November 20, 2020 An Important Analytical Verification Milestone Achieved Source: Progenity internal study Preeclampsia Rule-out LDT Test: One Step Closer to 2021 Launch KEY TAKEAWAYS: High confidence in analytical results & accuracy of assay Performance verified in operational CLIA lab Achieved acceptance criteria for CAP Validation Test Performance Specifications Provides confidence clinical studies will reflect true biological responses De-risks clinical verification and overall preeclampsia program
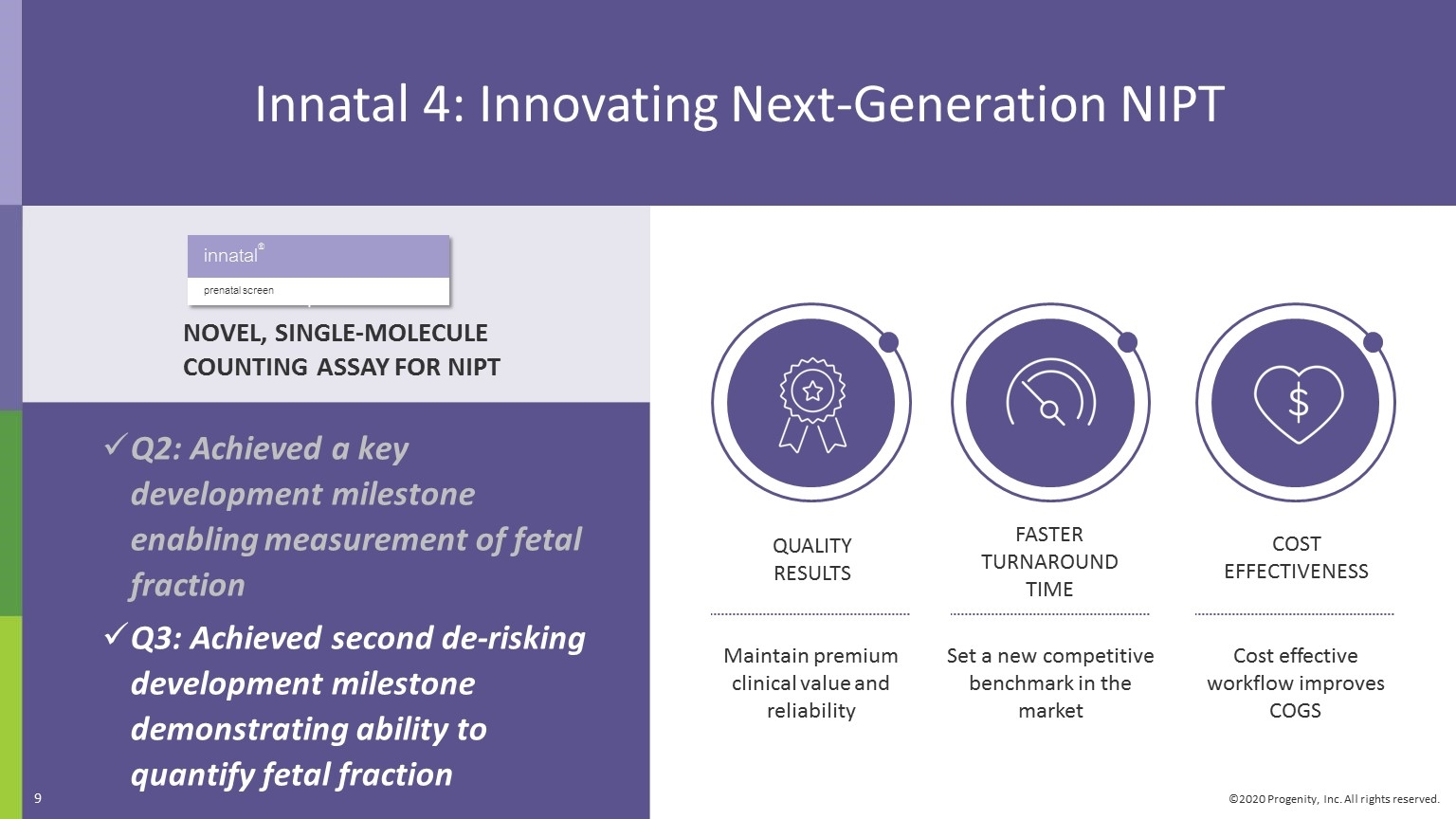
Innatal 4: Innovating Next-Generation NIPT COST EFFECTIVENESS QUALITY RESULTS Maintain premium clinical value and reliability FASTER TURNAROUND TIME Set a new competitive benchmark in the market Cost effective workflow improves COGS Q2: Achieved a key development milestone enabling measurement of fetal fraction Q3: Achieved second de-risking development milestone demonstrating ability to quantify fetal fraction NOVEL, SINGLE-MOLECULE COUNTING ASSAY FOR NIPT innatal® prenatal screen 9 4
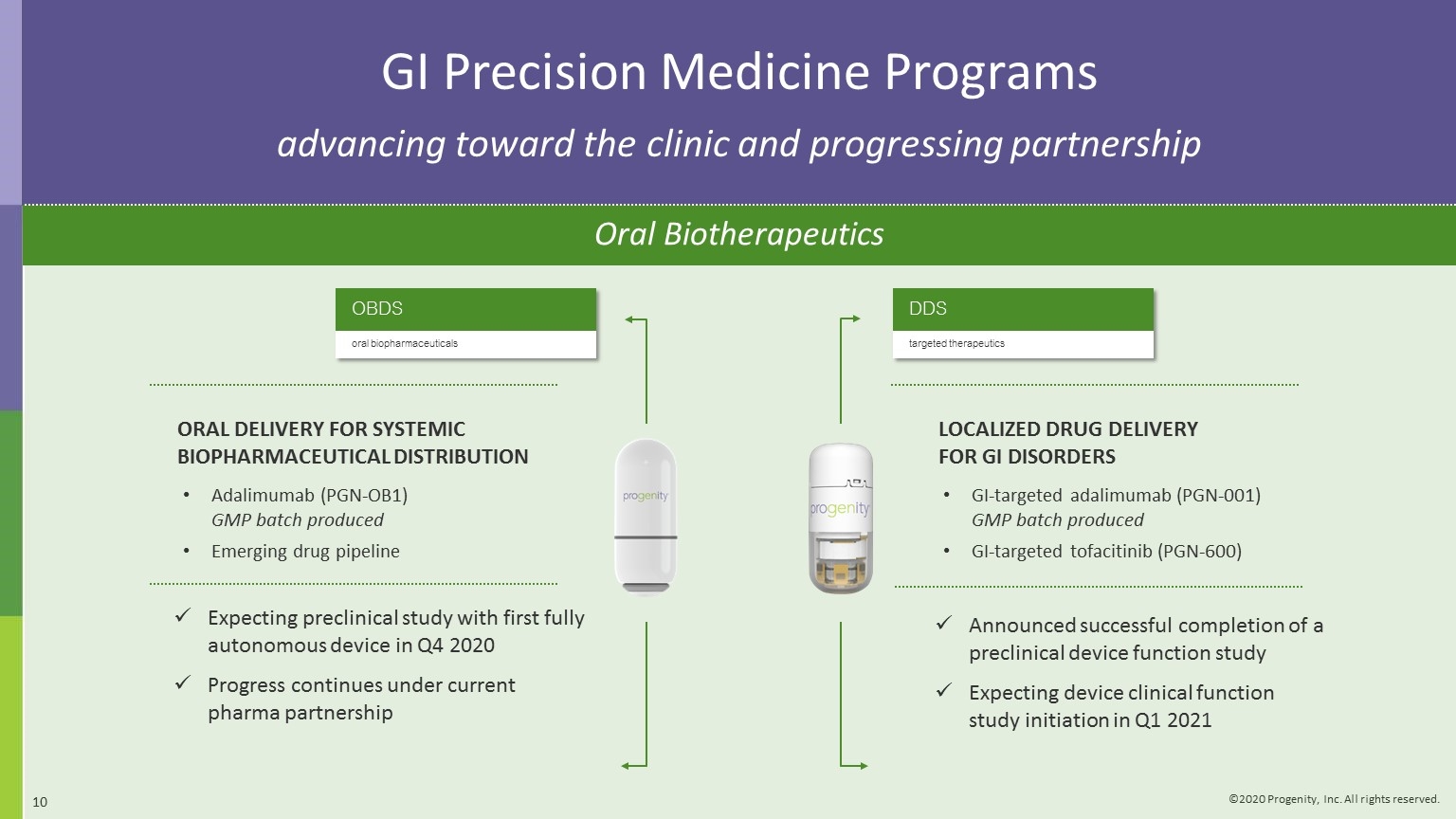
Expecting preclinical study with first fully autonomous device in Q4 2020 Progress continues under current pharma partnership OBDS oral biopharmaceuticals ORAL DELIVERY FOR SYSTEMIC BIOPHARMACEUTICAL DISTRIBUTION Adalimumab (PGN-OB1) GMP batch produced Emerging drug pipeline Announced successful completion of a preclinical device function study Expecting device clinical function study initiation in Q1 2021 DDS targeted therapeutics LOCALIZED DRUG DELIVERY FOR GI DISORDERS GI-targeted adalimumab (PGN-001) GMP batch produced GI-targeted tofacitinib (PGN-600) GI Precision Medicine Programs advancing toward the clinic and progressing partnership Oral Biotherapeutics
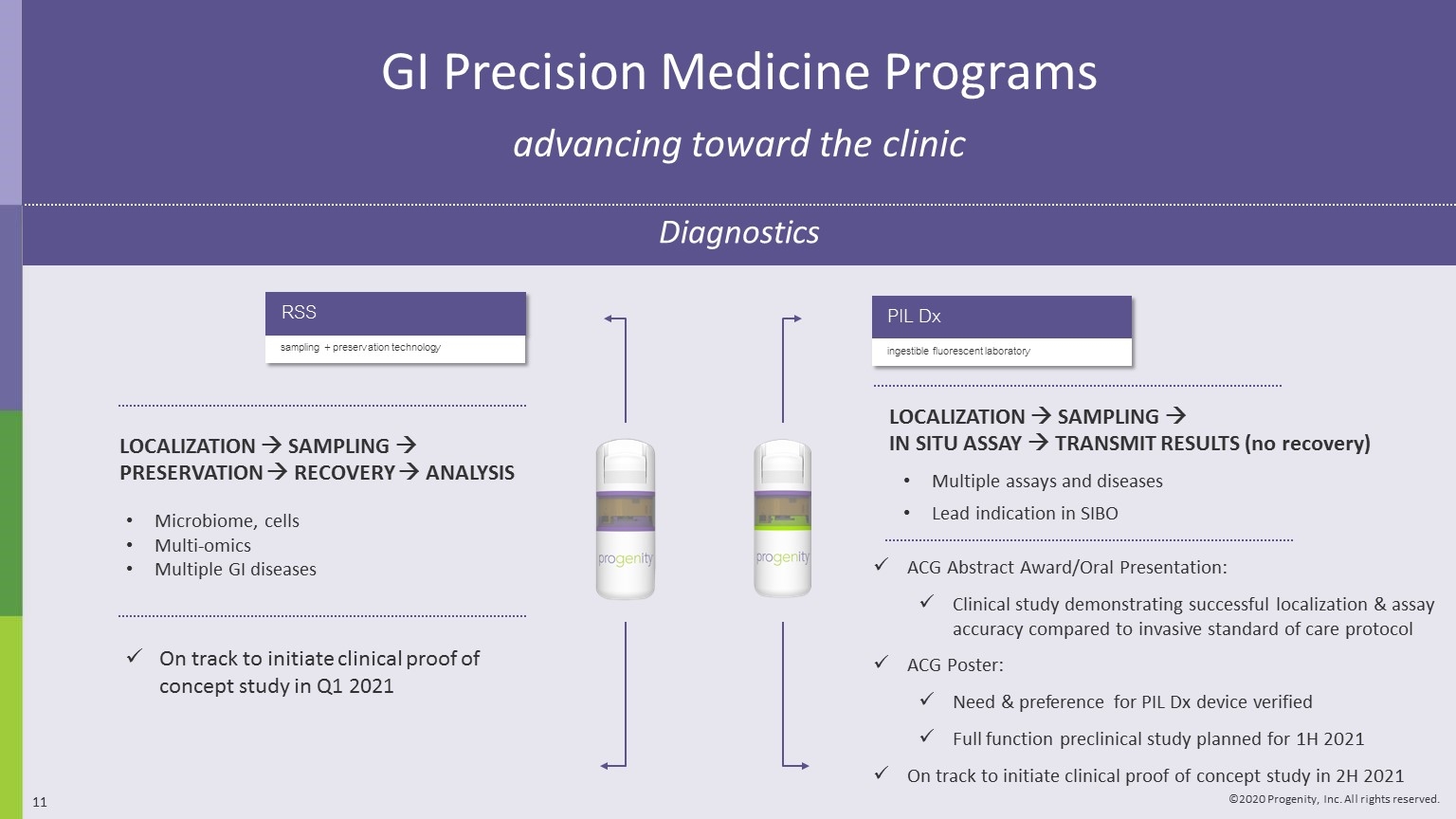
PIL Dx ingestible fluorescent laboratory On track to initiate clinical proof of concept study in Q1 2021 LOCALIZATION à SAMPLING à PRESERVATION à RECOVERY à ANALYSIS Microbiome, cells Multi-omics Multiple GI diseases ACG Abstract Award/Oral Presentation: Clinical study demonstrating successful localization & assay accuracy compared to invasive standard of care protocol ACG Poster: Need & preference for PIL Dx device verified Full function preclinical study planned for 1H 2021 On track to initiate clinical proof of concept study in 2H 2021 LOCALIZATION à SAMPLING à IN SITU ASSAY à TRANSMIT RESULTS (no recovery) GI Precision Medicine Programs advancing toward the clinic Diagnostics RSS sampling + preservation technology Multiple assays and diseases Lead indication in SIBO

Third Quarter Financial Results
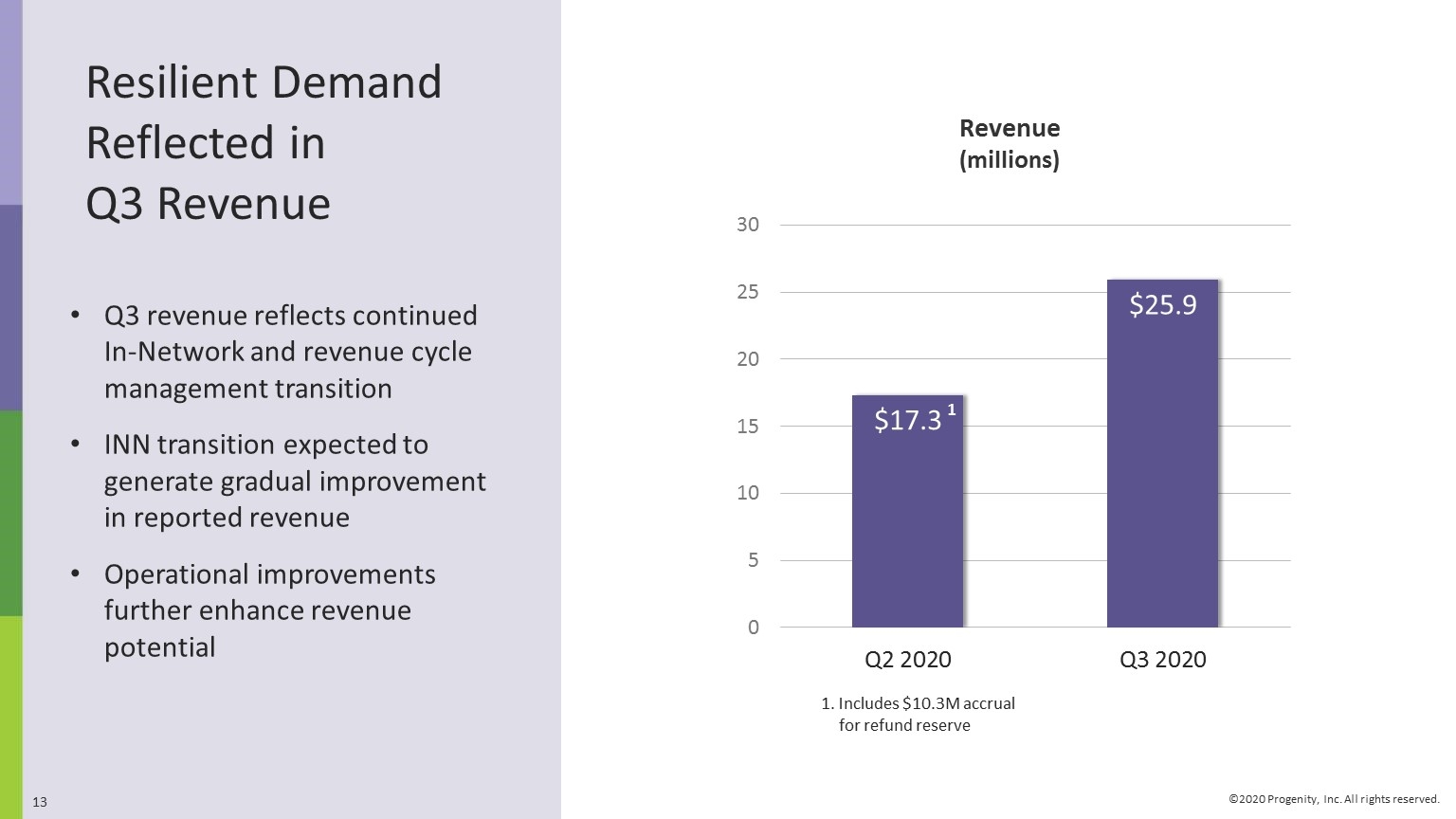
Resilient Demand Reflected in Q3 Revenue Q3 revenue reflects continued In-Network and revenue cycle management transition INN transition expected to generate gradual improvement in reported revenue Operational improvements further enhance revenue potential 1. Includes $10.3M accrual for refund reserve Revenue (millions)
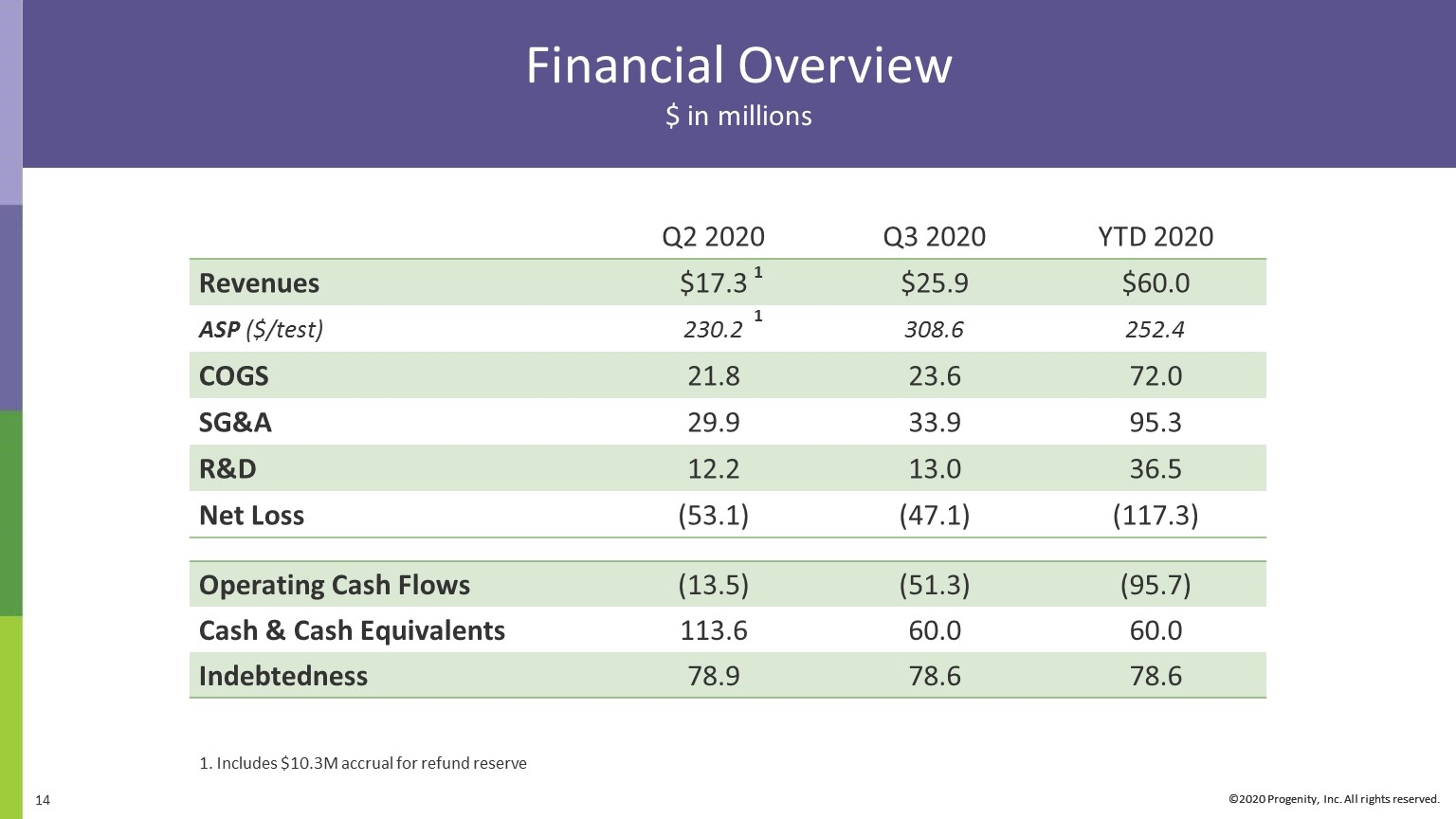
Financial Overview $ in millions ©2020 Progenity, Inc. All rights reserved. Q2 2020 Q3 2020 YTD 2020 Revenues $17.3 $25.9 $60.0 ASP ($/test) 230.2 308.6 252.4 COGS 21.8 23.6 72.0 SG&A 29.9 33.9 95.3 R&D 12.2 13.0 36.5 Net Loss (53.1) (47.1) (117.3) Operating Cash Flows (13.5) (51.3) (95.7) Cash & Cash Equivalents 113.6 60.0 60.0 Indebtedness 78.9 78.6 78.6 Includes $10.3M accrual for refund reserve 1 1
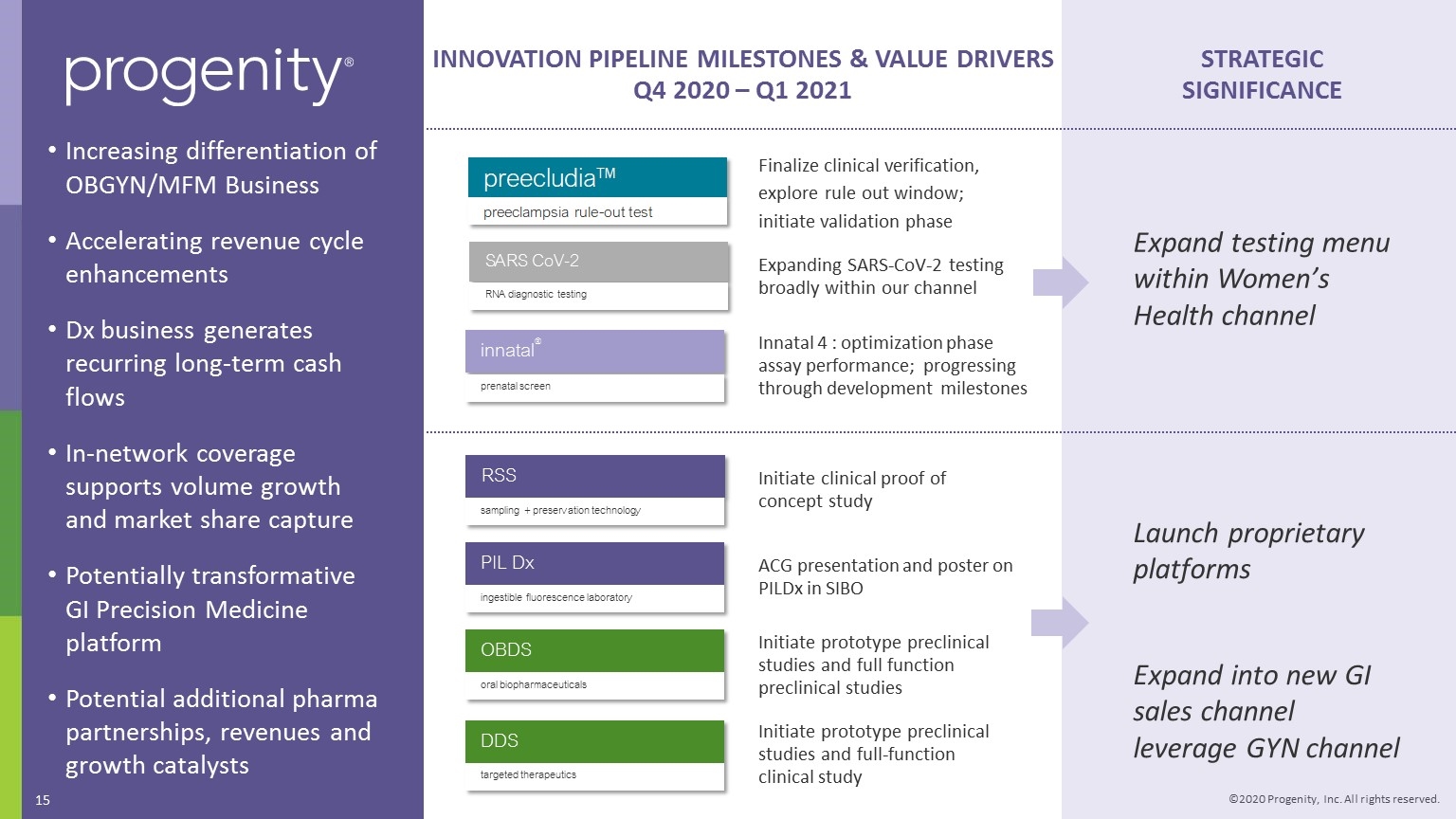
INNOVATION PIPELINE MILESTONES & VALUE DRIVERS Q4 2020 – Q1 2021 Expand testing menu within Women’s Health channel STRATEGIC SIGNIFICANCE Increasing differentiation of OBGYN/MFM Business Accelerating revenue cycle enhancements Dx business generates recurring long-term cash flows In-network coverage supports volume growth and market share capture Potentially transformative GI Precision Medicine platform Potential additional pharma partnerships, revenues and growth catalysts Initiate clinical proof of concept study RSS sampling + preservation technology SARS CoV-2 RNA diagnostic testing Expanding SARS-CoV-2 testing broadly within our channel Finalize clinical verification, explore rule out window; initiate validation phase innatal® prenatal screen Innatal 4 : optimization phase assay performance; progressing through development milestones PIL Dx ingestible fluorescence laboratory ACG presentation and poster on PILDx in SIBO OBDS oral biopharmaceuticals Initiate prototype preclinical studies and full function preclinical studies Initiate prototype preclinical studies and full-function clinical study DDS targeted therapeutics Launch proprietary platforms Expand into new GI sales channel leverage GYN channel

Q&A Session
