Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - MyoKardia, Inc. | myok-ex991_35.htm |
| 8-K - 8-K - MyoKardia, Inc. | myok-8k_20200511.htm |

May 11, 2020 Investor Call: EXPLORER-HCM Topline Data Supplemental Materials Exhibit 99.2

forward-looking statement Statements we make in this presentation may include statements which are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements regarding the clinical and therapeutic potential of mavacamten, our plans to present detailed EXPLORER-HCM results at an upcoming cardiovascular medical meeting, to hold a pre-NDA meeting with the U.S. Food and Drug Administration (FDA), to submit a New Drug Application (NDA) for marketing approval of mavacamten in the U.S. and to initiate discussions with EMA and align on a registrational plan for mavacamten in Europe, and the timing of these events, as well as the requirements for registration of the Company's product candidates, reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, risks associated with the development and regulation of our product candidates and the effects of the ongoing COVID-19 pandemic, as well as those set forth in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, and our other filings with the SEC. Except as required by law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. [Company Logo] 2
introduction Patients on treatment experienced meaningful results: Improvement of symptoms Improvement in functional status Reduction or elimination of LVOT gradient Mavacamten’s safety results were comparable to placebo Mavacamten was well tolerated; primary and all secondary endpoints were met with high statistical significance (p<0.0006) [Company logo]3
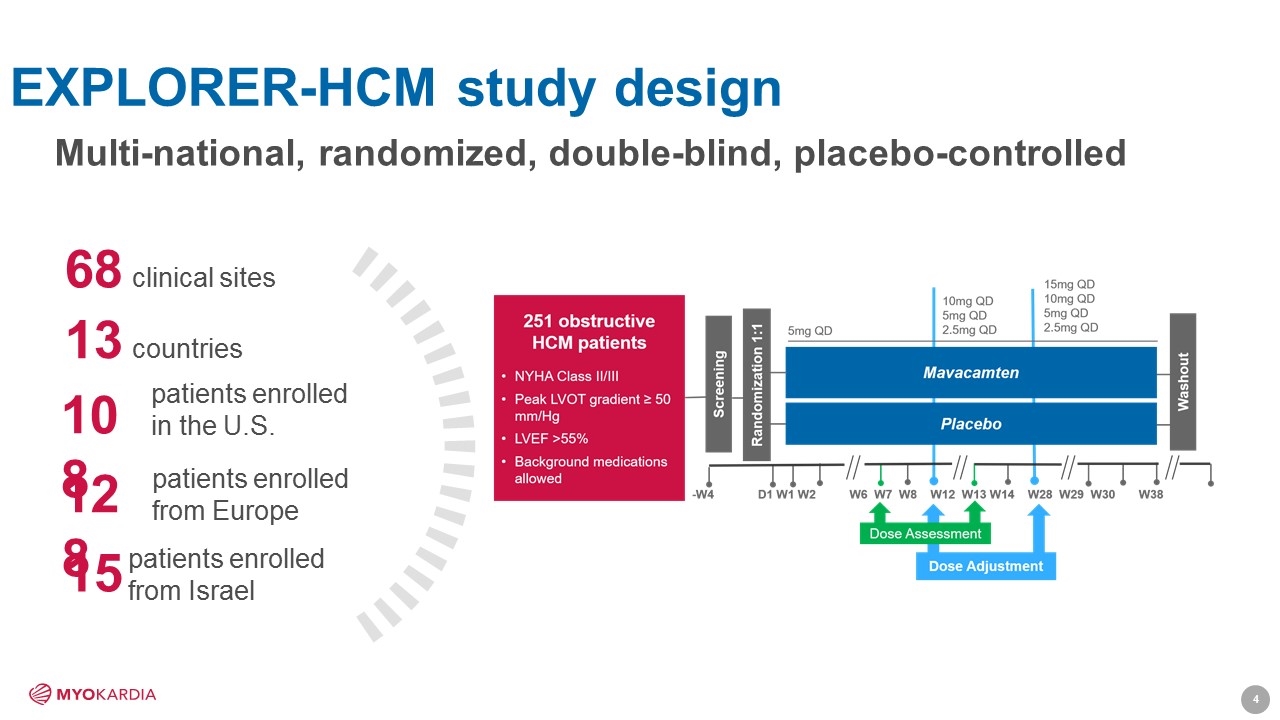
EXPLORER-HCM study design Multi-national, randomized, double-blind, placebo-controlled 68 clinical sites 13 countries 108 128 patients enrolled from Europe patients enrolled in the U.S. 15 patients enrolled from Israel [Company logo] 4
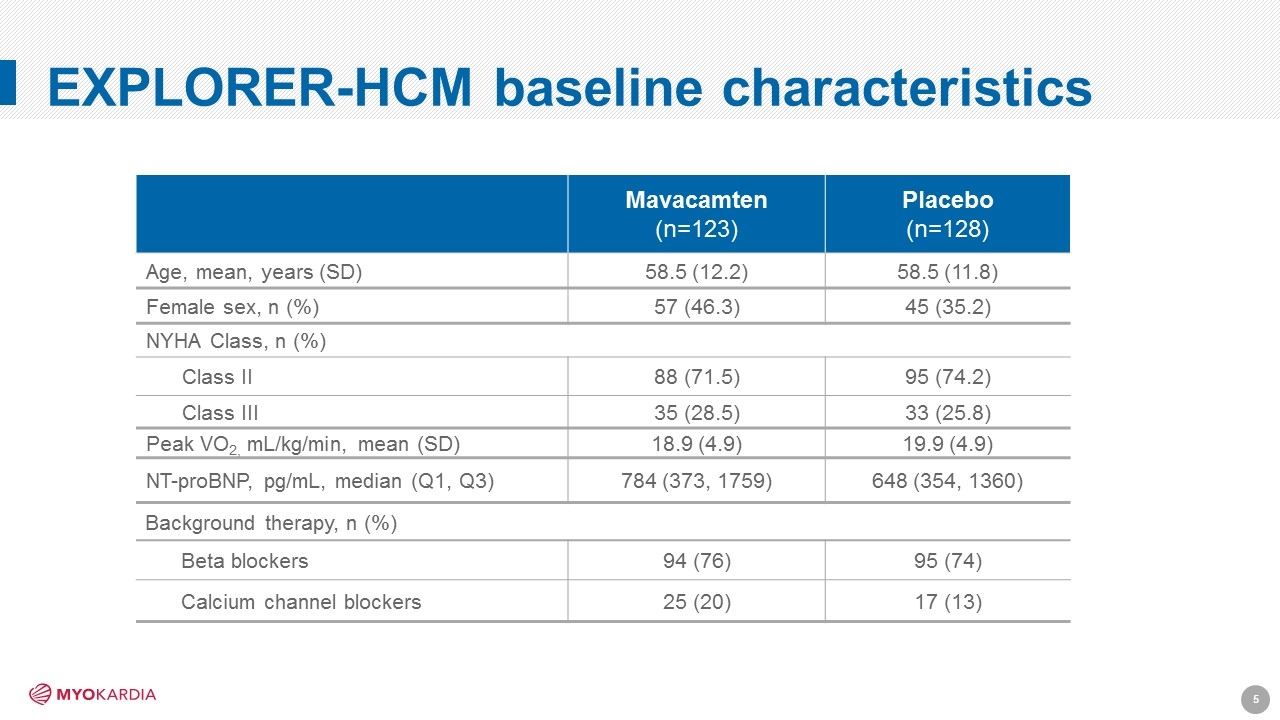
EXPLORER-HCM baseline characteristics Mavacamten (n=123) Placebo (n=128) Age, mean, years (SD) 58.5 (12.2) 58.5 (11.8) Female sex, n (%) 57 (46.3) 45 (35.2) NYHA Class, n (%) Class II 88 (71.5) 95 (74.2) Class III 35 (28.5) 33 (25.8) Peak VO2, mL/kg/min, mean (SD) 18.9 (4.9) 19.9 (4.9) NT-proBNP, pg/mL, median (Q1, Q3) 784 (373, 1759) 648 (354, 1360) Background therapy, n (%) Beta blockers 94 (76) 95 (74) Calcium channel blockers 25 (20) 17 (13) [Company logo]5
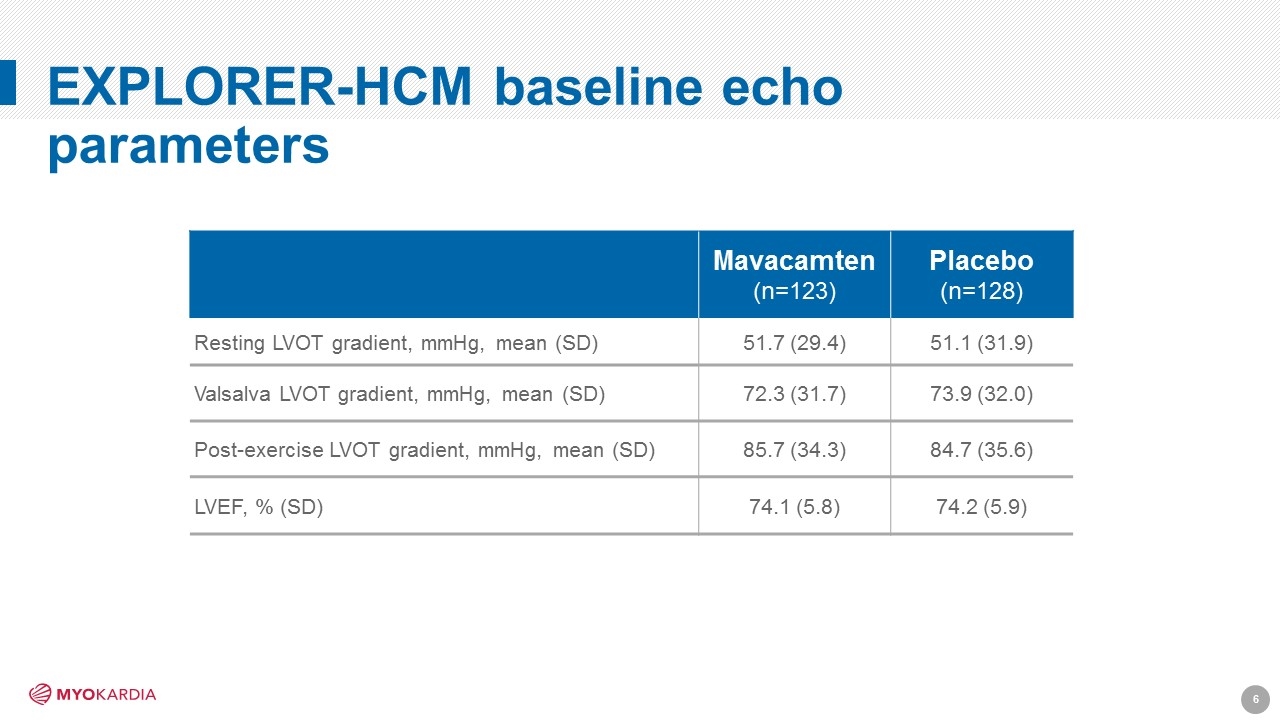
EXPLORER-HCM baseline echo parameters Mavacamten (n=123) Placebo (n=128) Resting LVOT gradient, mmHg, mean (SD) 51.7 (29.4) 51.1 (31.9) Valsalva LVOT gradient, mmHg, mean (SD) 72.3 (31.7) 73.9 (32.0) Post-exercise LVOT gradient, mmHg, mean (SD) 85.7 (34.3) 84.7 (35.6) LVEF, % (SD) 74.1 (5.8) 74.2 (5.9) [Company logo]6
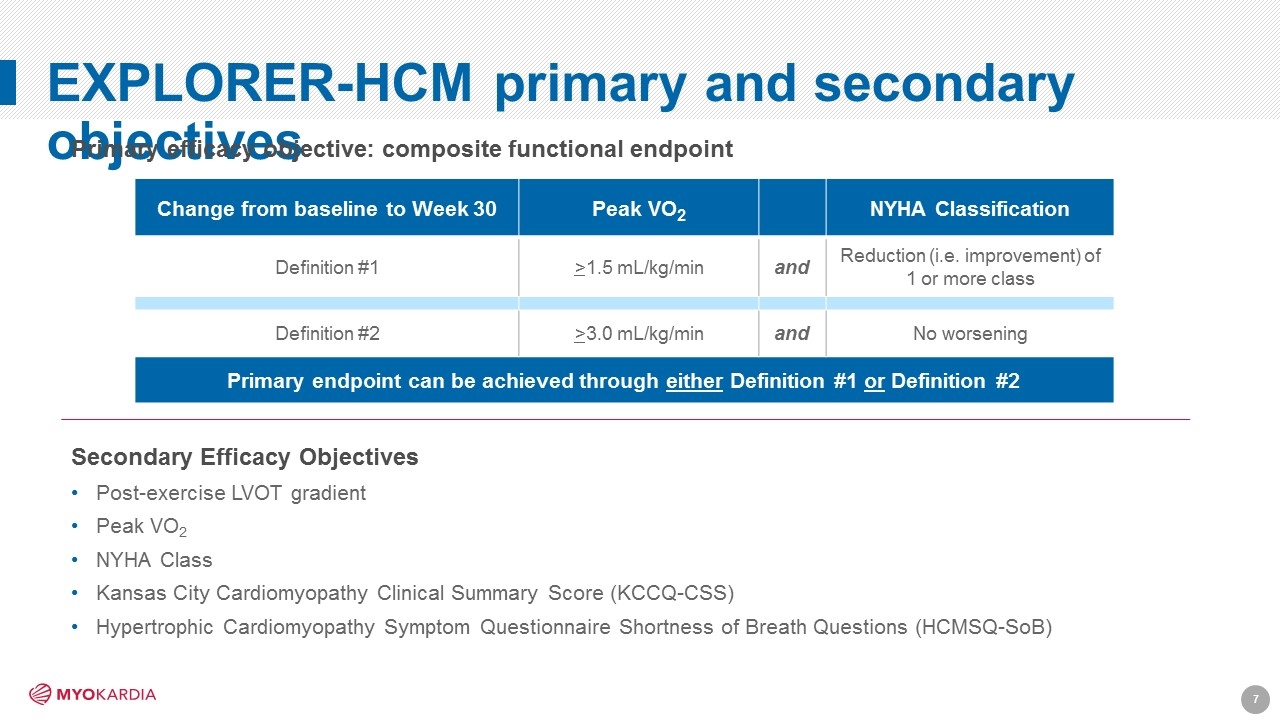
EXPLORER-HCM primary and secondary objectives Secondary Efficacy Objectives Post-exercise LVOT gradient Peak VO2 NYHA Class Kansas City Cardiomyopathy Clinical Summary Score (KCCQ-CSS) Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness of Breath Questions (HCMSQ-SoB) Change from baseline to Week 30 Peak VO2 NYHA Classification Definition #1 >1.5 mL/kg/min and Reduction (i.e. improvement) of 1 or more class Definition #2 >3.0 mL/kg/min and No worsening Primary endpoint can be achieved through either Definition #1 or Definition #2 Primary efficacy objective: composite functional endpoint [Company logo]7
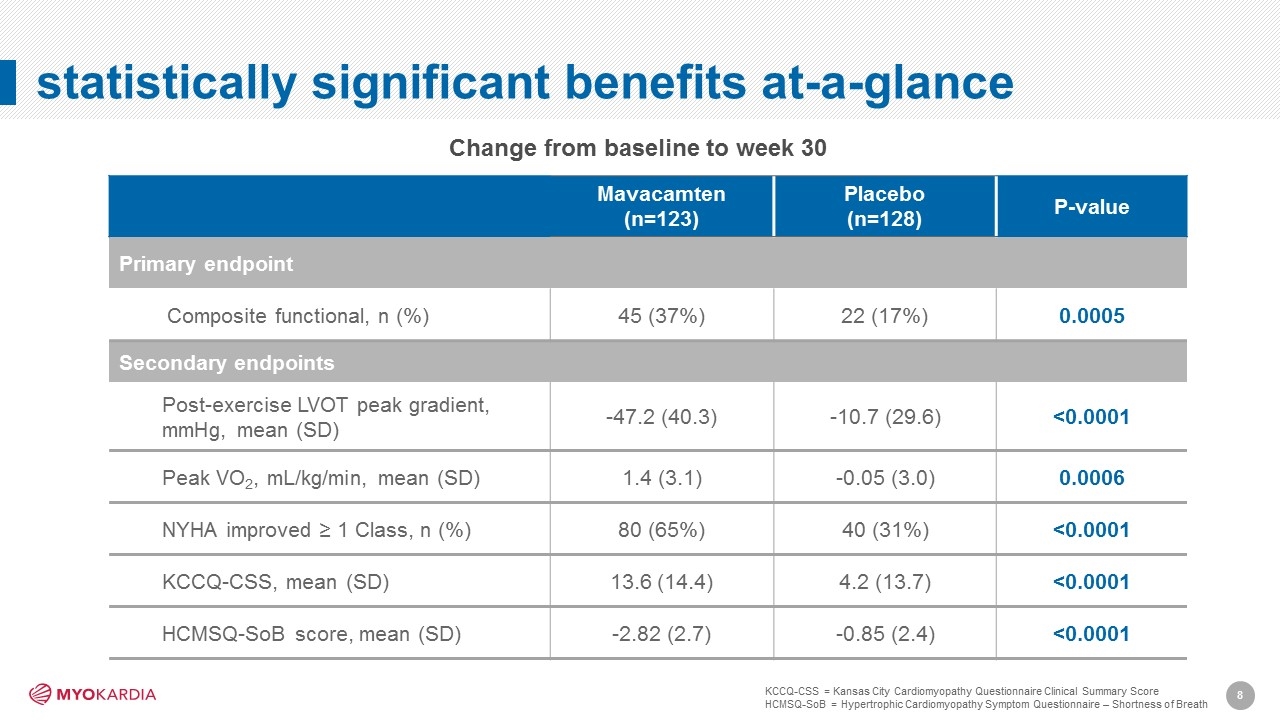
statistically significant benefits at-a-glance Mavacamten (n=123) Placebo (n=128) P-value Primary endpoint Composite functional, n (%) 45 (37%) 22 (17%) 0.0005 Secondary endpoints Post-exercise LVOT peak gradient, mmHg, mean (SD) -47.2 (40.3) -10.7 (29.6) <0.0001 Peak VO2, mL/kg/min, mean (SD) 1.4 (3.1) -0.05 (3.0) 0.0006 NYHA improved ≥ 1 Class, n (%) 80 (65%) 40 (31%) <0.0001 KCCQ-CSS, mean (SD) 13.6 (14.4) 4.2 (13.7) <0.0001 HCMSQ-SoB score, mean (SD) -2.82 (2.7) -0.85 (2.4) <0.0001 Change from baseline to week 30 KCCQ-CSS = Kansas City Cardiomyopathy Questionnaire Clinical Summary Score HCMSQ-SoB = Hypertrophic Cardiomyopathy Symptom Questionnaire – Shortness of Breath [Company logo]8
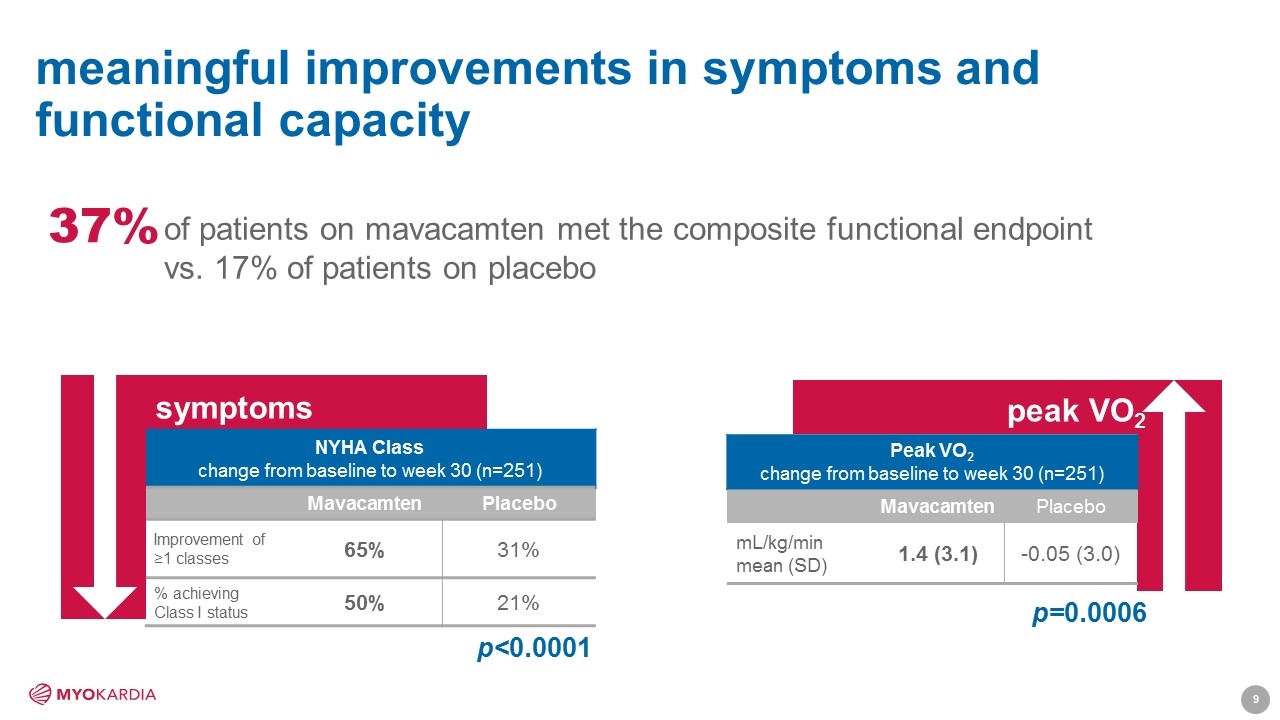
meaningful improvements in symptoms and functional capacity peak VO2 Peak VO2 change from baseline to week 30 (n=251) Mavacamten Placebo mL/kg/min mean (SD) 1.4 (3.1) -0.05 (3.0) p=0.0006 NYHA Class change from baseline to week 30 (n=251) Mavacamten Placebo Improvement of ≥1 classes 65% 31% % achieving Class I status 50% 21% symptoms p<0.0001 of patients on mavacamten met the composite functional endpoint vs. 17% of patients on placebo 37% [Company logo]9
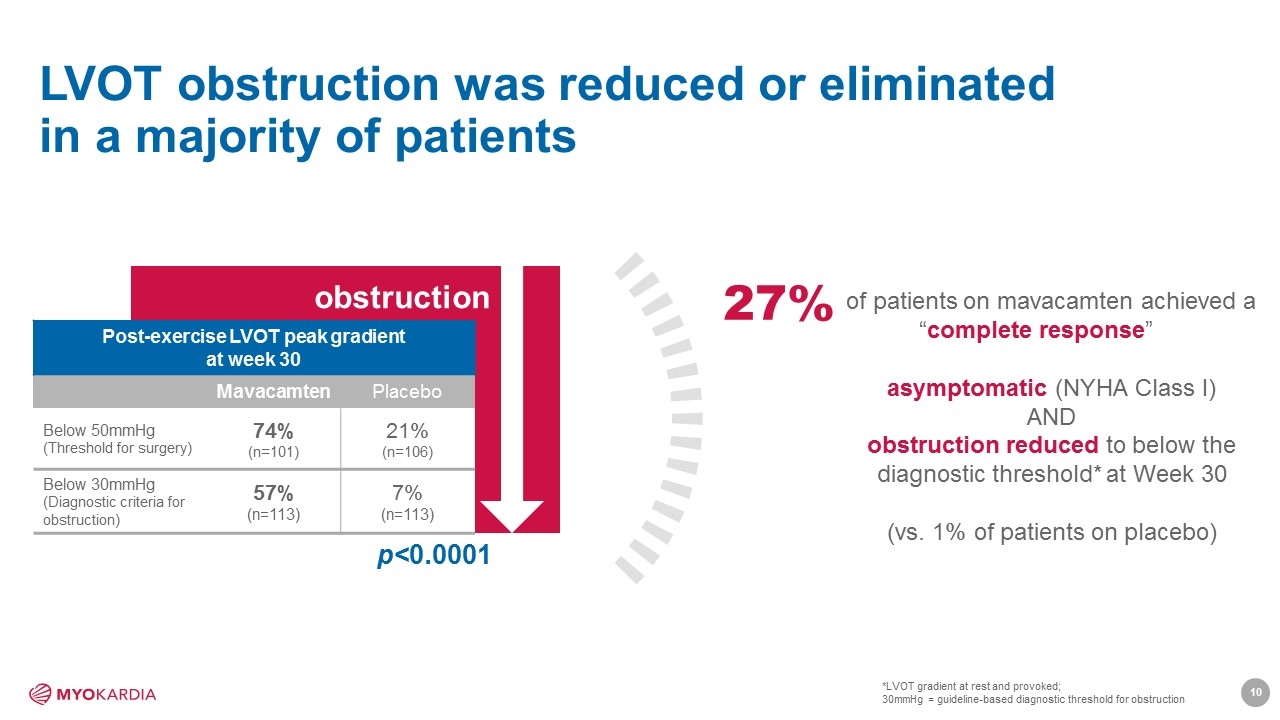
LVOT obstruction was reduced or eliminated in a majority of patients obstruction Post-exercise LVOT peak gradient at week 30 Mavacamten Placebo Below 50mmHg (Threshold for surgery) 74% (n=101) 21% (n=106) Below 30mmHg (Diagnostic criteria for obstruction) 57% (n=113) 7% (n=113) p<0.0001 of patients on mavacamten achieved a “complete response” asymptomatic (NYHA Class I) AND obstruction reduced to below the diagnostic threshold* at Week 30 (vs. 1% of patients on placebo) 27% *LVOT gradient at rest and provoked; 30mmHg = guideline-based diagnostic threshold for obstruction [Company logo]10
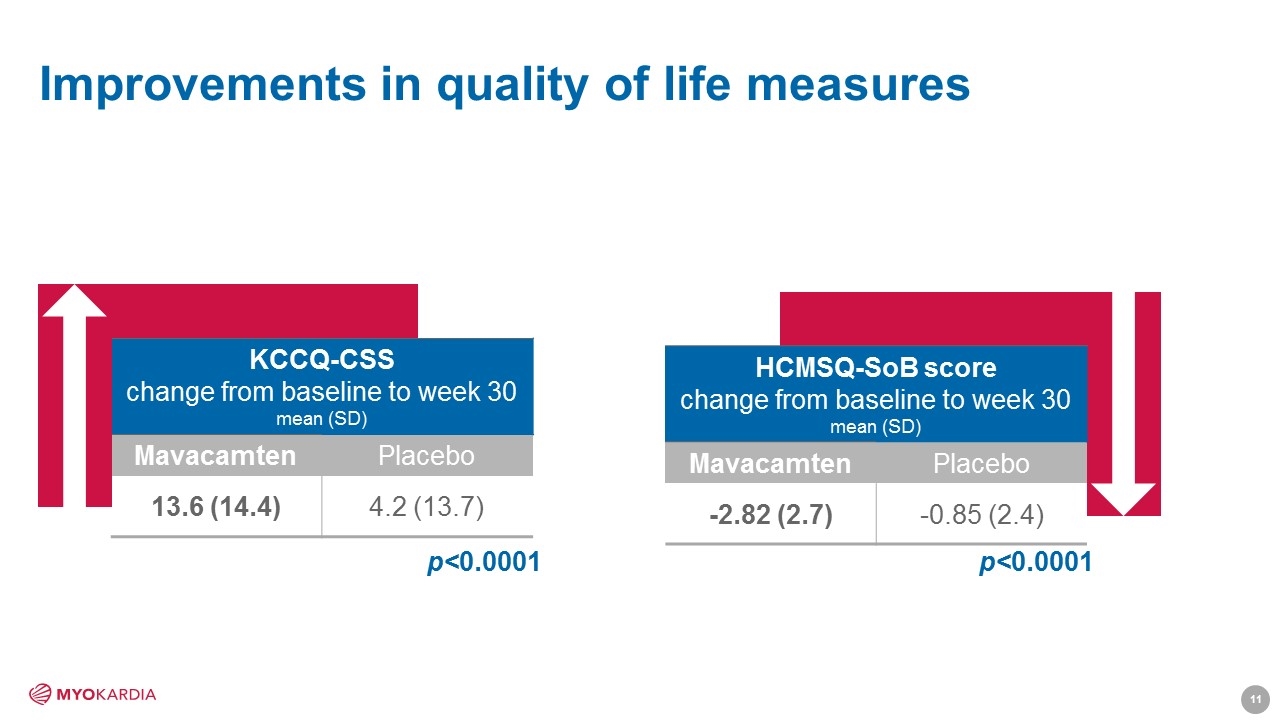
Improvements in quality of life measures HCMSQ-SoB score change from baseline to week 30 mean (SD) Mavacamten Placebo -2.82 (2.7) -0.85 (2.4) p<0.0001 KCCQ-CSS change from baseline to week 30 mean (SD) Mavacamten Placebo 13.6 (14.4) 4.2 (13.7) p<0.0001 [Company logo]11
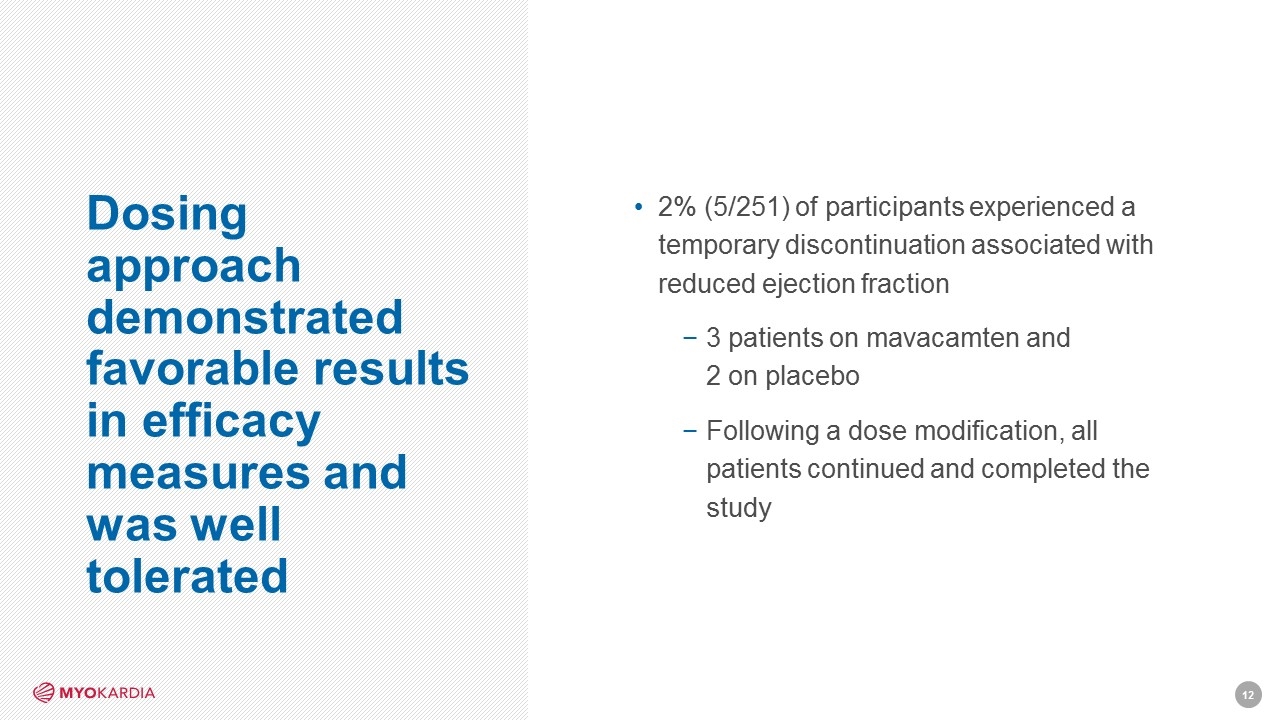
Dosing approach demonstrated favorable results in efficacy measures and was well tolerated 2% (5/251) of participants experienced a temporary discontinuation associated with reduced ejection fraction 3 patients on mavacamten and 2 on placebo Following a dose modification, all patients continued and completed the study [Company logo]12

251 mavacamten was well tolerated low drop out rate (2%) 3 due to adverse events (2 mavacamten, 1 placebo) 2 withdrew for other reasons (1 mavacamten, 1 placebo) no participants withdrew due to reduced EF or symptoms of heart failure serious adverse events Occurred in 10 (8.1%) participants on mavacamten vs. 11 (8.6%) on placebo Higher number of SAEs occurred on placebo (20) vs. mavacamten (12) cardiac SAEs Occurred in 4 participants on mavacamten vs. 4 participants on placebo One sudden death occurred in the placebo arm of the study atrial fibrillation Events occurred in 8 (6.5%) participants on mavacamten vs. 9 (7.0%) participants on placebo 2 participants on mavacamten (1.6%) vs. 4 participants on placebo (3.1%) experienced atrial fibrillation SAEs treatment emergent AEs TEAEs occurred in 108 (87.8%) participants on mavacamten vs. 101 (78.9%) participants on placebo [Company logo]13
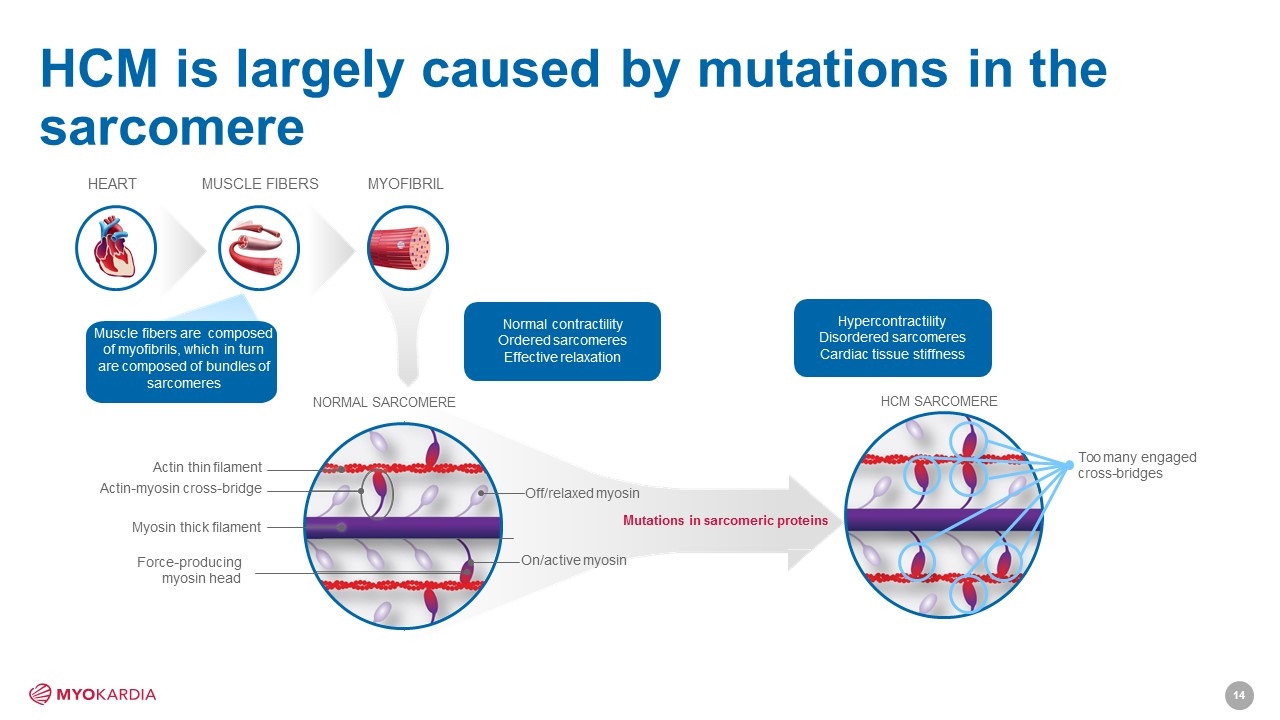
HCM is largely caused by mutations in the sarcomere MYOFIBRIL On/active myosin Off/relaxed myosin Muscle fibers are composed of myofibrils, which in turn are composed of bundles of sarcomeres HEART MUSCLE FIBERS Actin thin filament Actin-myosin cross-bridge Myosin thick filament Force-producing myosin head Mutations in sarcomeric proteins Normal contractility Ordered sarcomeres Effective relaxation HCM SARCOMERE Hypercontractility Disordered sarcomeres Cardiac tissue stiffness Too many engaged cross-bridges NORMAL SARCOMERE [Company logo]14
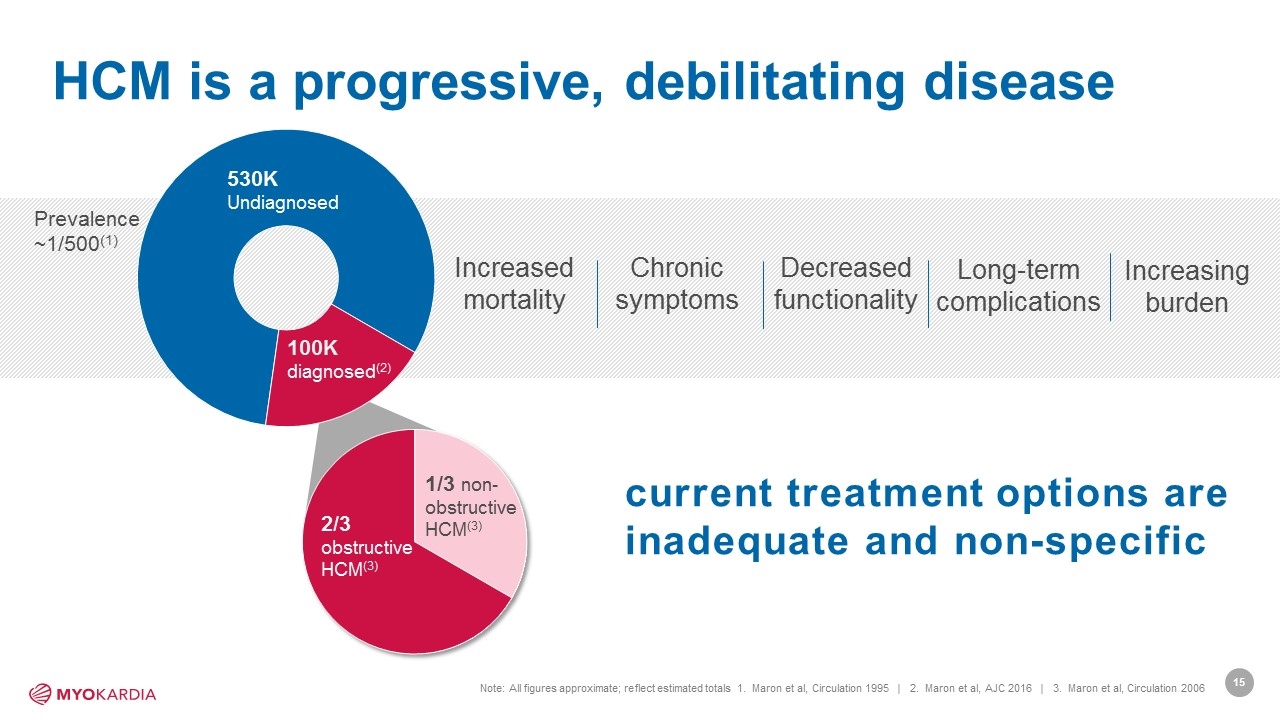
HCM is a progressive, debilitating disease Note: All figures approximate; reflect estimated totals 1. Maron et al, Circulation 1995 | 2. Maron et al, AJC 2016 | 3. Maron et al, Circulation 2006 current treatment options are inadequate and non-specific 100K diagnosed(2) 530K Undiagnosed Increased mortality Chronic symptoms Decreased functionality Long-term complications Increasing burden 1/3 non-obstructive HCM(3) 2/3 obstructive HCM(3) Prevalence ~1/500(1) [Company logo]15
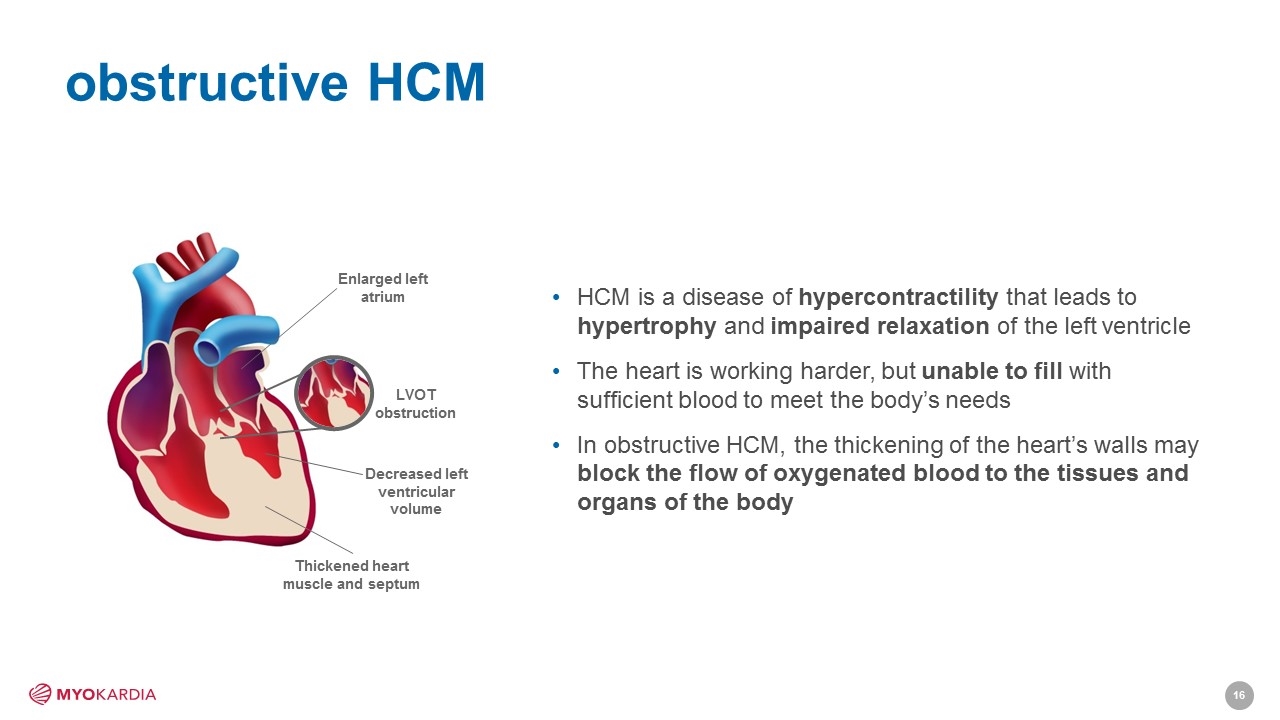
obstructive HCM HCM is a disease of hypercontractility that leads to hypertrophy and impaired relaxation of the left ventricle The heart is working harder, but unable to fill with sufficient blood to meet the body’s needs In obstructive HCM, the thickening of the heart’s walls may block the flow of oxygenated blood to the tissues and organs of the body Thickened heart muscle and septum Decreased left ventricular volume Enlarged left atrium LVOT obstruction [Company logo]16

planned next steps on path to registration Present detailed EXPLORER-HCM results at an upcoming cardiovascular medical meeting (2H 2020) Pre-NDA meeting with FDA anticipated (Q3 2020) Submit NDA for marketing approval in the US (Q1 2021) Initiate process with EMA to discuss EXPLORER-HCM study data and align on registrational plan for Europe (2H 2020) [Company logo]17
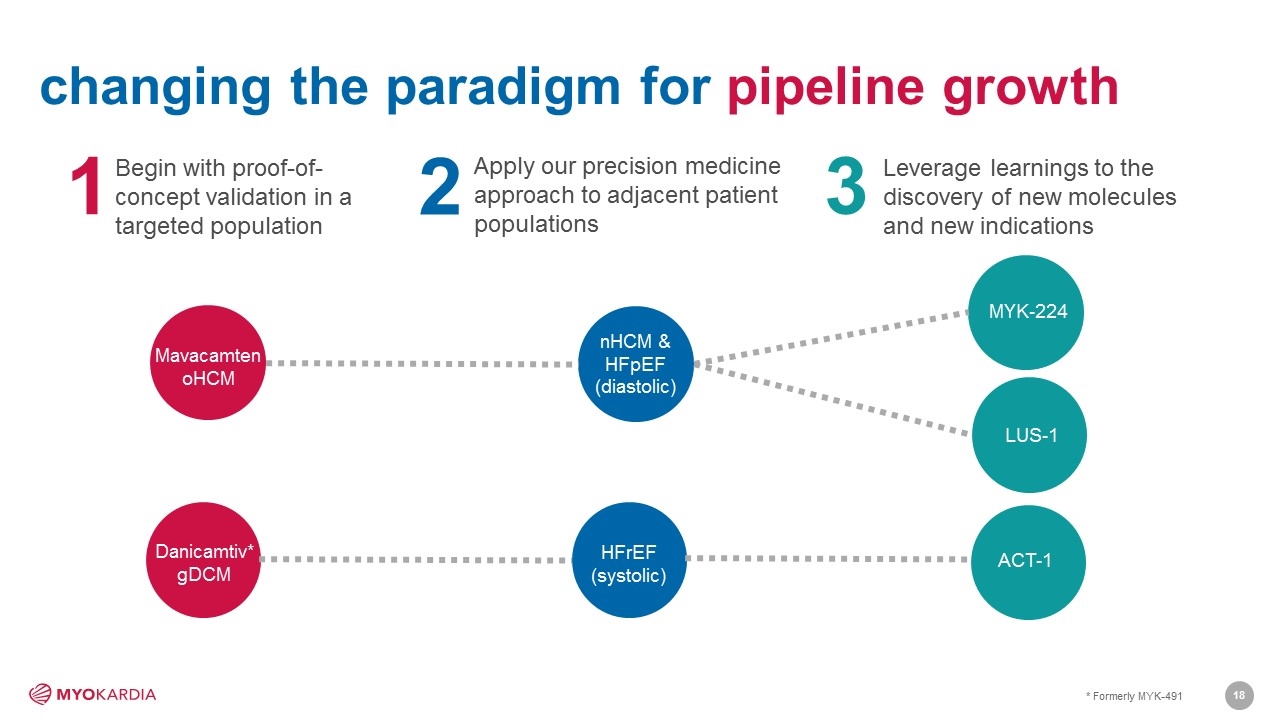
3 2 MYK-224 nHCM & HFpEF (diastolic) HFrEF (systolic) LUS-1 Danicamtiv* gDCM Begin with proof-of-concept validation in a targeted population Apply our precision medicine approach to adjacent patient populations Leverage learnings to the discovery of new molecules and new indications 1 2 3 Mavacamten oHCM ACT-1 changing the paradigm for pipeline growth * Formerly MYK-491 [Company logo]18

today Q2 2020 Platform strategy for aggressive value creation starting with mavacamten and danicamtiv Positive Phase 3 data for mavacamten in obstructive HCM Growing body of data fueling pipeline – new indications (HFpEF) AND new discoveries Global rights to entire portfolio World-class team focused on our mission and vision [Company logo]19

Driven by the heart
