Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Frequency Therapeutics, Inc. | d924569d8k.htm |
Exhibit 99.1
| A letter from David L. Lucchino
Chief Executive Officer
| ||

|
Dear fellow shareholders,
This past year was one of tremendous progress for Frequency Therapeutics, and I could not be more proud to lead an organization that may redefine the landscape for regenerative therapeutics and transform the treatment paradigm for those suffering from the most common form of hearing loss.
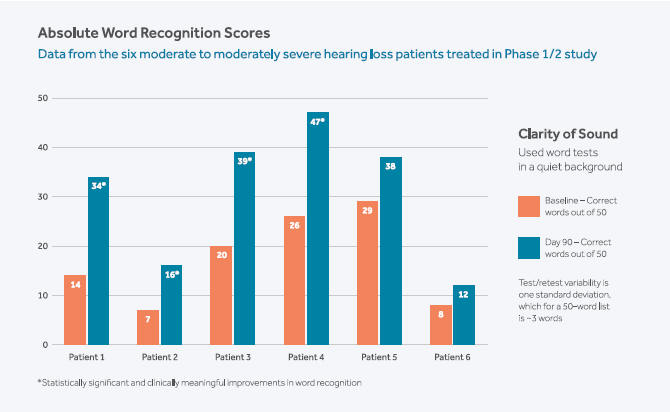
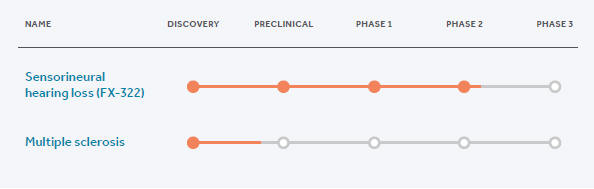
We achieved several major milestones in 2019, advancing our hearing program, executing a global licensing and collaboration agreement, expanding our organization for the demands of future growth and taking the company public. We reported data from our Phase 1/2 study of FX-322, our lead product candidate for the treatment of sensorineural hearing loss (SNHL), in which we observed a hearing signal and the potential return of function in patients with hearing loss. We believe these results have never before been seen in hearing research and have positioned Frequency to advance what may be the first restorative, disease-modifying treatment for the millions of patients with SNHL. Our approach may potentially transform how this debilitating condition is treated beyond the device-based standard of care.
We entered into a global licensing and collaboration agreement for FX-322 with Astellas Pharma Inc. in a deal worth more than $600 million, including an $80 million up-front payment. Astellas has the rights to develop and commercialize FX-322 outside of the U.S., while Frequency maintains rights in the U.S. market.
Our Phase 2a trial of FX-322 commenced dosing in October 2019 and was followed by the U.S. Food and Drug Administration (FDA) granting FX-322 Fast Track designation, increasing our ability to engage with the agency regarding our ongoing development efforts. |
1
2

3
4

5

