Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Hepion Pharmaceuticals, Inc. | tm207150-1_8k.htm |
Exhibit 99.1

1 C ORP OR A T EP RE S E N T A T I ON•2 0 2 0 Nasdaq: HEPA Nasdaq: HEPA

Forward-Looking Statements 2 This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such forward-looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated in these forward-looking statements. We believe that it is important to communicate future expectations to investors. However, there may be events in the future that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger-scale clinical trials, the risk that we will not obtain approval to market our products, the risks associated with dependence upon key personnel and the need for additional financing. We do not assume any obligation to update forward-looking statements as circumstances change. This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Hepion Pharmaceuticals or its affiliates. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. Nasdaq: HEPA

Corporate Overview Lead Asset CRV431: Novel, high-potency, cyclophilin inhibitor that targets multiple stages of liver disease, including NASH 3 • • • • • • Anti-fibrotic, anti-viral, and anti-cancer properties (pleiotropic) Strong preclinical proof of concept Strong safety profile in preclinical and Phase 1 clinical studies Orally active, once daily Robust IP Built upon 30 years' experience in this very specific field of chemistry • Core team that founded Aurinia Pharmaceuticals (Nasdaq:AUPH, >$2 Billion USD), and discovered and developed voclosporin through to phase 2 Voclosporin met all primary and secondary endpoints in a Phase 3 trial for lupus nephritis (NDA submission, H1 2020) • Nasdaq: HEPA
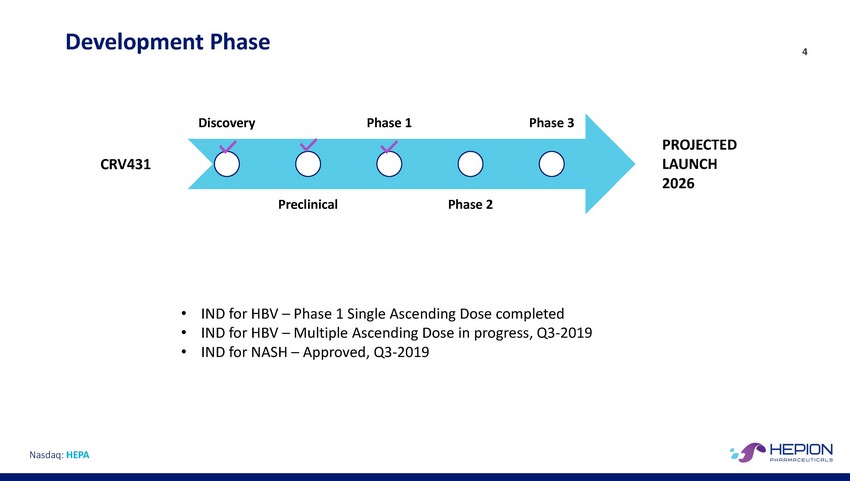
Development Phase 4 Discovery Phase 1 Phase 3 PROJECTED LAUNCH 2026 CRV431 Preclinical Phase 2 • • • IND for HBV – Phase 1 Single Ascending Dose completed IND for HBV – Multiple Ascending Dose in progress, Q3-2019 IND for NASH – Approved, Q3-2019 Nasdaq: HEPA
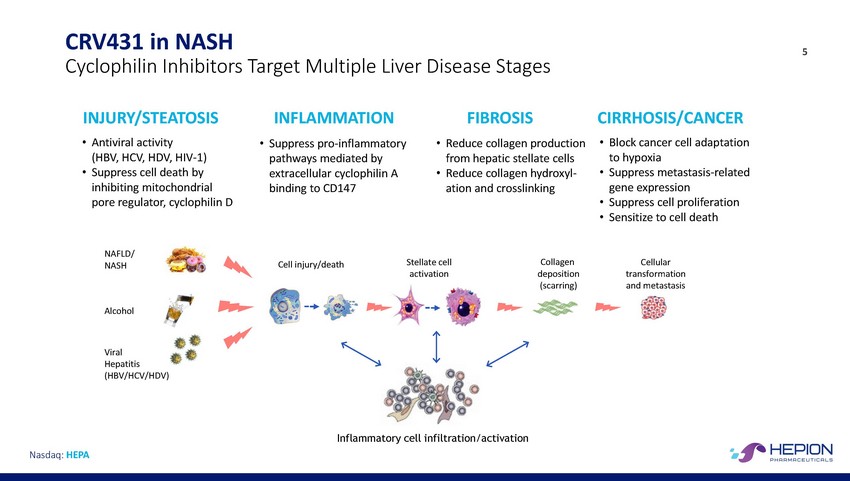
CRV431 in NASH Cyclophilin Inhibitors 5 Target Multiple Liver Disease Stages INJURY/STEATOSIS INFLAMMATION Suppress pro-inflammatory pathways mediated by extracellular cyclophilin A binding to CD147 FIBROSIS Reduce collagen production from hepatic stellate cells Reduce collagen hydroxyl-ation and crosslinking CIRRHOSIS/CANCER • • Antiviral activity (HBV, HCV, HDV, HIV-1) Suppress cell death by inhibiting mitochondrial pore regulator, cyclophilin D • • Block cancer cell adaptation to hypoxia Suppress metastasis-related gene expression Suppress cell proliferation Sensitize to cell death • • • • • NAFLD/ NASH Stellate cell activation Collagen deposition (scarring) Cellular transformation and metastasis Cell injury/death Alcohol Viral Hepatitis (HBV/HCV/HDV) Inflammatory cell infiltration/activation Nasdaq: HEPA
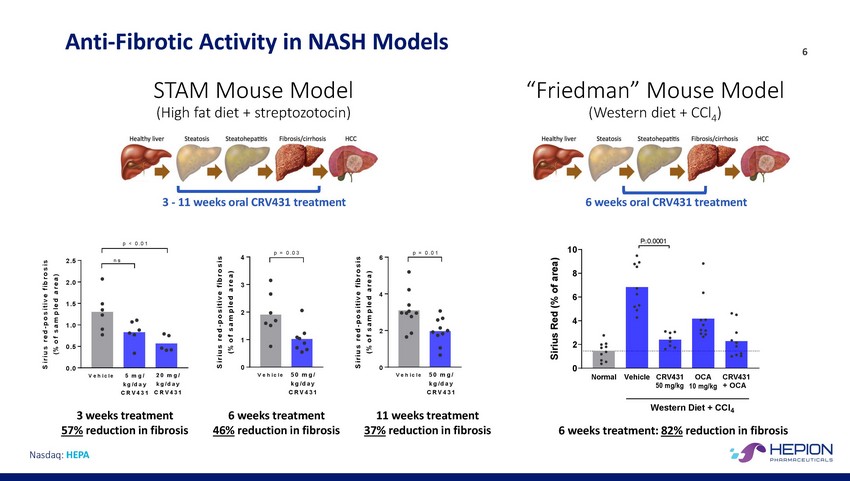
Anti-Fibrotic Activity in NASH STAM Mouse Model (High fat diet + streptozotocin) Models 6 “Friedman” Mouse Model (Western diet + CCl4) 3 - 11 weeks oral CRV431 treatment 6 weeks oral CRV431 treatment P≤0.0001 p < 0. 0 1 10 p = 0. 0 3 p = 0. 0 1 4 6 2 . 5 n s 8 2 . 0 3 4 6 1 . 5 2 4 1 . 0 2 1 2 0 . 5 0 0 0 0 . 0 V e h ic le 50 m g / k g/ da y C R V 431 V e h ic le 50 m g / k g/ da y C R V 431 V e h ic le 5 m g / k g/ da y C R V 431 20 m g / k g/ da y C R V 431 Normal Vehicle CRV431 50 m g/kg OCA CRV431 + OCA 10 m g/kg Western Diet + CCl4 6 weeks treatment: 82% reduction in fibrosis 3 weeks treatment 57% reduction in fibrosis 6 weeks treatment 46% reduction in fibrosis 11 weeks treatment 37% reduction in fibrosis Nasdaq: HEPA S ir i u s r e d - p o s i t iv e f ib r o s is ( % o f s am p l ed ar ea ) S ir i u s r e d - p o s i t iv e f ib r o s is ( % o f s am p l ed ar ea ) S ir i u s r e d - p o s i t iv e f ib r o s is ( % o f s am p l ed ar ea ) Sirius Red (% of area)
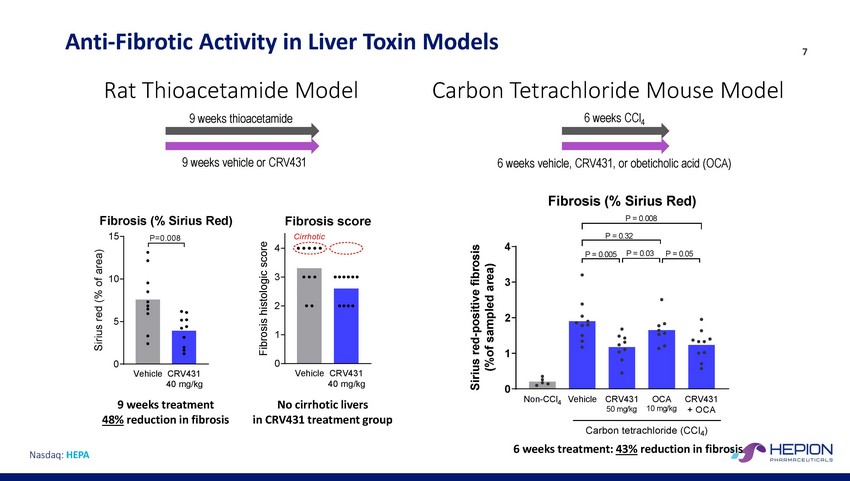
Anti-Fibrotic Activity in Liver Toxin Rat Thioacetamide Model 9 weeks thioacetamide Models Carbon Tetrachloride Mouse Model 6 weeks CCl4 7 9 weeks vehicle or CRV431 6 weeks vehicle, CRV431, or obeticholic acid (OCA) Fibrosis (% Sirius Red) P = 0.008 Fibrosis (% Sirius Red) Fibrosis score 15 P = 0.32 4 4 3 10 3 2 2 5 1 1 0 0 Vehicle CRV431 40 mg/kg Vehicle CRV431 40 mg/kg 0 Non-CCl4 Vehicle CRV431 50 mg/kg OCA 10 mg/kg CRV431 + OCA 9 weeks treatment 48% reduction in fibrosis No cirrhotic livers in CRV431 treatment group Carbon tetrachloride (CCl4) 6 weeks treatment: 43% reduction in fibrosis Nasdaq: HEPA Sirius red (% of area) Fibrosis histologic score Sirius red-positive fibrosis (%of sampled area) Cirrhotic P = 0.005 P = 0.03 P = 0.05 P=0.008
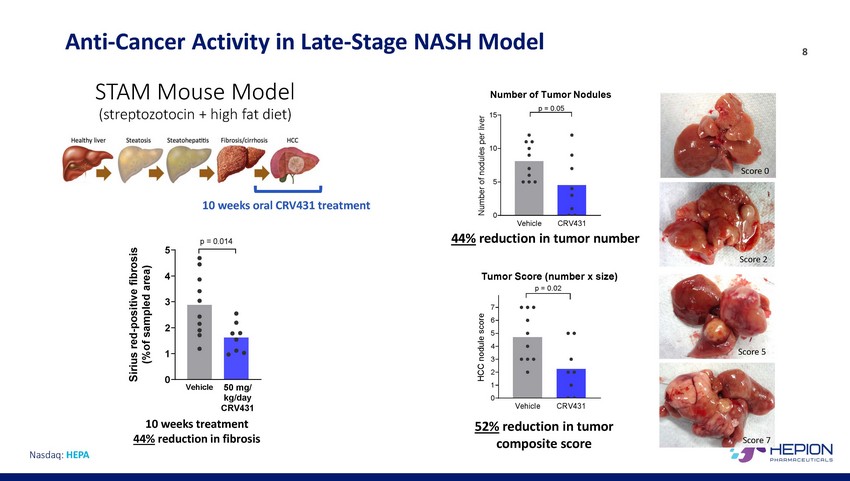
Anti-Cancer Activity in STAM Mouse Model (streptozotocin + high fat diet) Late-Stage NASH Model 8 Number of Tumor Nodules p = 0.05 15 10 Score 0 5 10 weeks oral CRV431 treatment 0 Vehicle CRV431 44% reduction in tumor number p = 0.014 5 Score 2 4 Tumor Score (number x size) p = 0.02 3 7 6 5 4 3 2 1 0 2 Score 5 1 0 50 mg/ kg/day CRV431 Vehicle Vehicle CRV431 10 weeks treatment 44% reduction in fibrosis 52% reduction in tumor composite score Score 7 Nasdaq: HEPA Sirius red-positive fibrosis (%of sampled area) HCC nodule score Number of nodules per liver
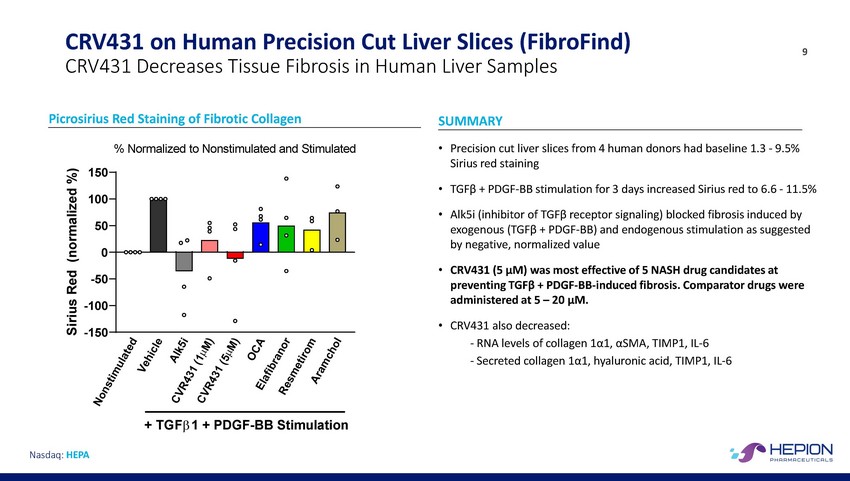
CRV431 on Human Precision Cut Liver Slices (FibroFind) 9 CRV431 Decreases Tissue Fibrosis in Human Liver Samples Picrosirius Red Staining of Fibrotic Collagen SUMMARY • % Normalized to Nonstimulated and Stimulated Precision cut liver slices from 4 human donors had baseline 1.3 - 9.5% Sirius red staining 150 • TGFβ + PDGF-BB stimulation for 3 days increased Sirius red to 6.6 - 11.5% 100 • Alk5i (inhibitor of TGFβ receptor signaling) blocked fibrosis induced by exogenous (TGFβ + PDGF-BB) and endogenous stimulation as suggested by negative, normalized value 50 0 • CRV431 (5 μM) was most effective of 5 NASH drug candidates at preventing TGFβ + PDGF-BB-induced fibrosis. Comparator drugs were administered at 5 – 20 μM. CRV431 also decreased: - RNA levels of collagen 1α1, αSMA, TIMP1, IL-6 - Secreted collagen 1α1, hyaluronic acid, TIMP1, IL-6 -50 -100 • -150 + TGFβ 1 + PDGF-BB Stimulation Nasdaq: HEPA Sirius Red (normalized %)
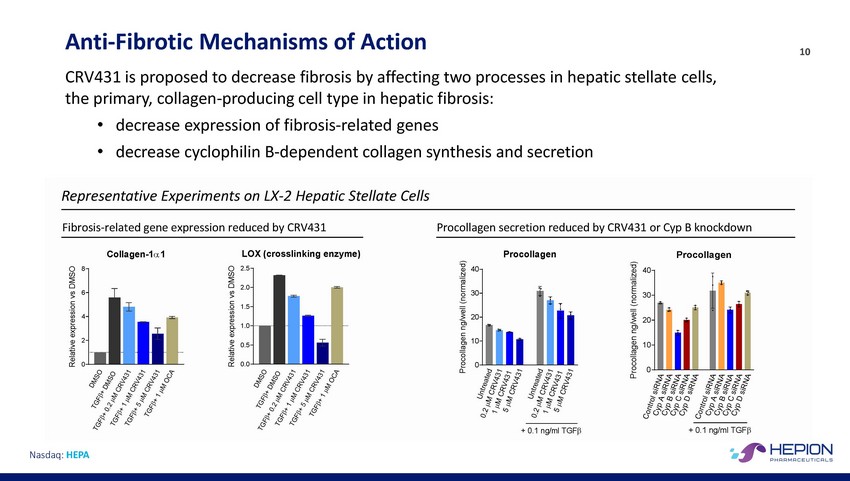
Anti-Fibrotic Mechanisms of Action CRV431 is proposed to decrease fibrosis by affecting two processes in hepatic stellate the primary, collagen-producing cell type in hepatic fibrosis: 10 cells, • • decrease expression of fibrosis-related genes decrease cyclophilin B-dependent collagen synthesis and secretion 6 30 30 1.0 Nasdaq: HEPA Relative expression vs DMSO Relative expression vs DMSO Procollagen ng/well (normalized) Procollagen ng/well (normalized) Representative Experiments on LX-2 Hepatic Stellate Cells Fibrosis-related gene expression reduced by CRV431Procollagen secretion reduced by CRV431 or Cyp B knockdown Collagen-1α 1 LOX (crosslinking enzyme)ProcollagenProcollagen 82.540 40 2.0 1.5 420 20 20.510 10 00.00 0 + 0.1 ng/ml TGFβ + 0.1 ng/ml TGFβ
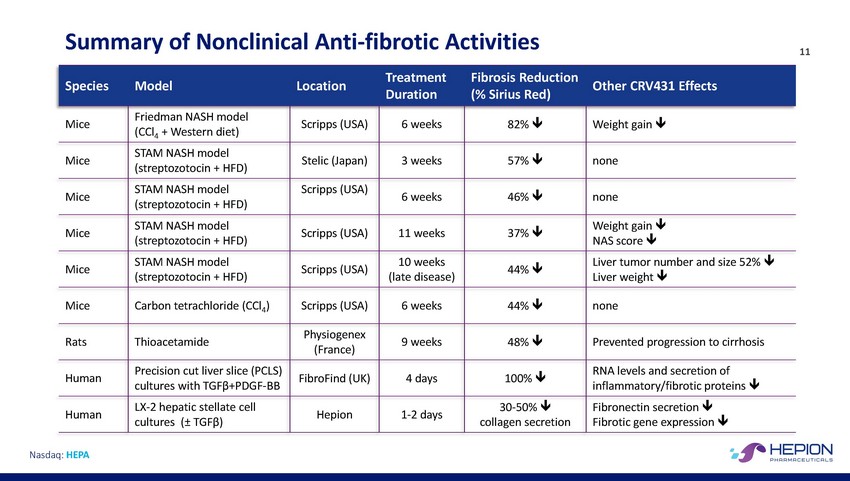
Summary of Nonclinical Anti-fibrotic Activities 11 Other CRV431 Effects Duration (% Sirius Red) NAS score Liver weight inflammatory/fibrotic proteins Fibrotic gene expression Nasdaq: HEPA SpeciesModelLocationTreatmentFibrosis Reduction Mice Friedman NASH model (CCl4 + Western diet) Scripps (USA) 6 weeks 82% Weight gain Mice STAM NASH model (streptozotocin + HFD) Stelic (Japan) 3 weeks 57% none Mice STAM NASH model (streptozotocin + HFD) Scripps (USA) 6 weeks 46% none Mice STAM NASH model (streptozotocin + HFD) Scripps (USA) 11 weeks 37% Weight gain Mice STAM NASH model (streptozotocin + HFD) Scripps (USA) 10 weeks (late disease) 44% Liver tumor number and size 52% Mice Carbon tetrachloride (CCl4) Scripps (USA) 6 weeks 44% none Rats Thioacetamide Physiogenex (France) 9 weeks 48% Prevented progression to cirrhosis Human Precision cut liver slice (PCLS) cultures with TGFβ+PDGF-BB FibroFind (UK) 4 days 100% RNA levels and secretion of Human LX-2 hepatic stellate cell cultures (± TGFβ) Hepion 1-2 days 30-50% collagen secretion Fibronectin secretion

Clinical Pharmacology Single Ascending Dose (SAD) Study (CTRV-CRV431-101) 12 Objectives • • To evaluate the safety and tolerability of single oral doses of CRV431 at increasing dose levels To evaluate the pharmacokinetics of CRV431 Design • Randomized, Partially blinded, Placebo-controlled, sequential SAD Study in healthy volunteers CRV431 375 mg CRV431 225 Healthy Subjects CRV431 75 mg 32 (24 CRV431; 8 Placebo) Nasdaq: HEPA SAD CRV431525 mg mg N =

Clinical Pharmacology: Safety Profile and Conclusions SAD Study 13 Safety Profile • • • • No SAE’s were reported in the SAD Study AE’s from the SAD study have been mild to moderate and mostly unrelated to study drug There were no Grade 3 or Grade 4 laboratory abnormalities Vital signs and ECGs were unremarkable Conclusions • • In the SAD doses were tested up to 525 mg with no concerns The collective data from the SAD demonstrate a favorable pharmacological, pharmacokinetic, and safety profile for CRV431 with acceptable safety margins that support the proposed clinical development program Nasdaq: HEPA
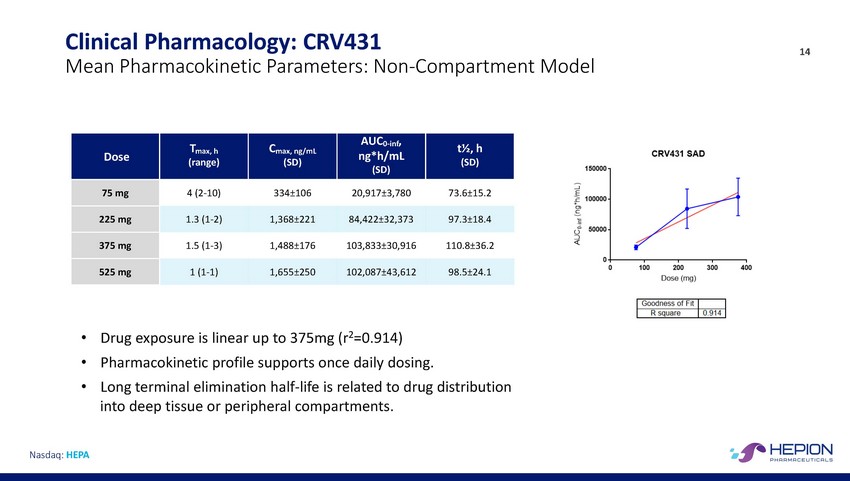
Clinical Pharmacology: CRV431 14 Mean Pharmacokinetic Parameters: Non-Compartment Model (SD) • • • Drug exposure is linear up to 375mg (r2=0.914) Pharmacokinetic profile supports once daily dosing. Long terminal elimination half-life is related to drug distribution into deep tissue or peripheral compartments. Nasdaq: HEPA Dose Tmax, h (range) Cmax, ng/mL (SD) AUC0-inf, ng*h/mL (SD) t½, h 75 mg 4 (2-10)334±10620,917±3,78073.6±15.2 225 mg 1.3 (1-2)1,368±22184,422±32,37397.3±18.4 375 mg 1.5 (1-3)1,488±176103,833±30,916110.8±36.2 525 mg 1 (1-1)1,655±250102,087±43,61298.5±24.1
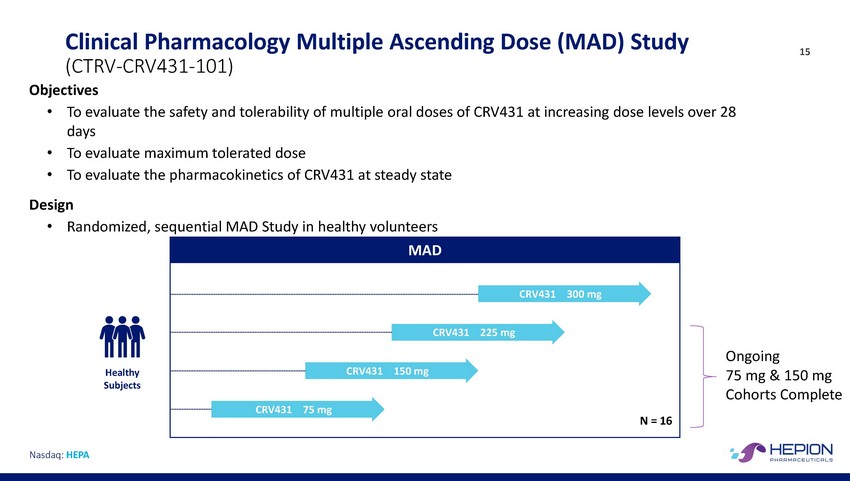
Clinical Pharmacology Multiple Ascending Dose (MAD) Study (CTRV-CRV431-101) Objectives 15 • To evaluate the safety and tolerability of multiple oral doses of CRV431 at increasing dose levels over 28 days To evaluate maximum tolerated dose To evaluate the pharmacokinetics of CRV431 at steady state • • Design • Randomized, sequential MAD Study in healthy volunteers CRV431 225 mg Ongoing 75 mg & 150 mg Cohorts Complete RV431 150 Healthy Subjects CRV431 75 mg Nasdaq: HEPA MAD CRV431300 mg N = 16 mg C
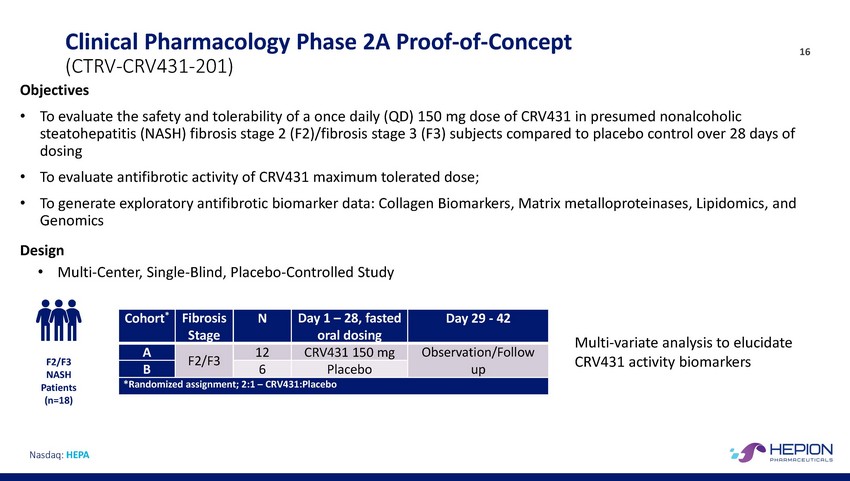
Clinical Pharmacology Phase 2A Proof-of-Concept (CTRV-CRV431-201) Objectives 16 • To evaluate the safety and tolerability of a once daily (QD) 150 mg dose of CRV431 in presumed nonalcoholic steatohepatitis (NASH) fibrosis stage 2 (F2)/fibrosis stage 3 (F3) subjects compared to placebo control over 28 days of dosing To evaluate antifibrotic activity of CRV431 maximum tolerated dose; To generate exploratory antifibrotic biomarker data: Collagen Biomarkers, Matrix metalloproteinases, Lipidomics, and Genomics • • Design • Multi-Center, Single-Blind, Placebo-Controlled Study Multi-variate analysis to elucidate CRV431 activity biomarkers F2/F3 NASH Patients (n=18) Nasdaq: HEPA Cohort* Fibrosis Stage N Day 1 – 28, fasted oral dosing Day 29 - 42 A F2/F3 12 CRV431 150 mg Observation/Follow up B 6 Placebo *Randomized assignment; 2:1 – CRV431:Placebo
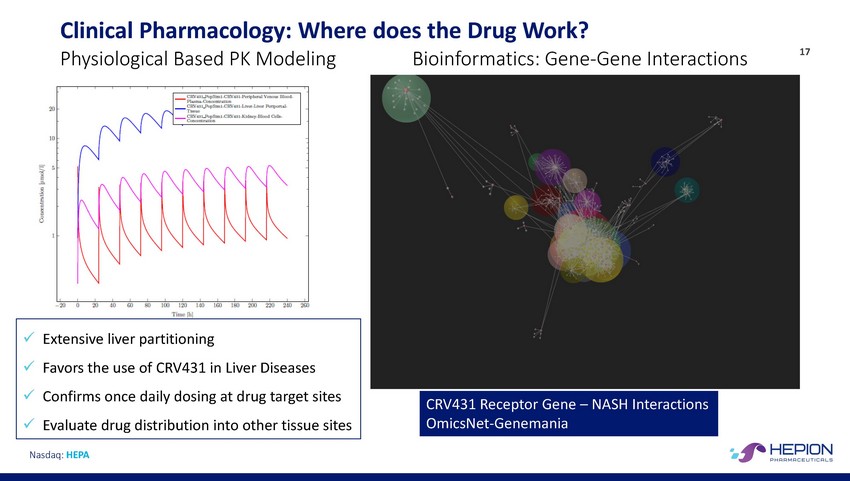
Clinical Pharmacology: Where does the Drug Work? 17 Physiological Based PK Modeling Bioinformatics: Gene-Gene Interactions Nasdaq: HEPA CRV431 Receptor Gene – NASH Interactions OmicsNet-Genemania x Extensive liver partitioning x Favors the use of CRV431 in Liver Diseases x Confirms once daily dosing at drug target sites x Evaluate drug distribution into other tissue sites
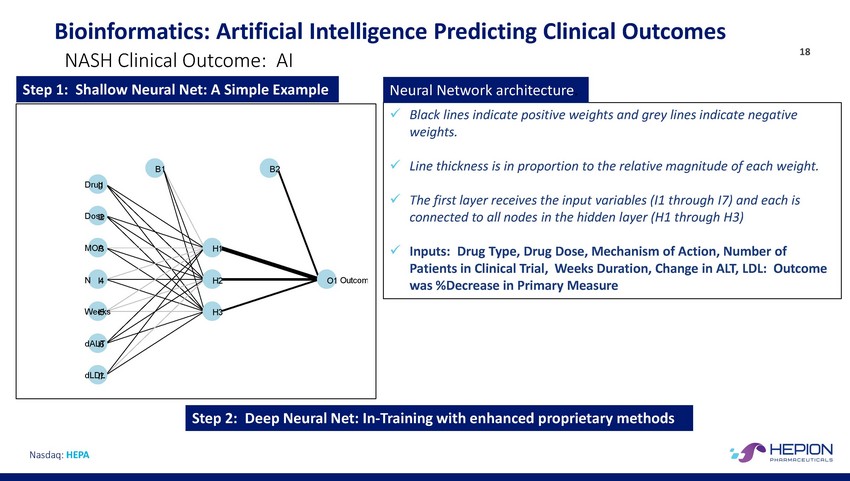
Bioinformatics: Artificial Intelligence Predicting Clinical Outcomes 18 NASH Clinical Outcome: AI was %Decrease in Primary Measure Nasdaq: HEPA Step 2: Deep Neural Net: In-Training with enhanced proprietary methods Neural Network architecture. xBlack lines indicate positive weights and grey lines indicate negative weights. xLine thickness is in proportion to the relative magnitude of each weight. xThe first layer receives the input variables (I1 through I7) and each is connected to all nodes in the hidden layer (H1 through H3) xInputs: Drug Type, Drug Dose, Mechanism of Action, Number of Patients in Clinical Trial, Weeks Duration, Change in ALT, LDL: Outcome Step 1: Shallow Neural Net: A Simple Example B1B2 DruIg1 DosI2e MOAI3H1 N I4H2O1 Outcom WeeI5ksH3 dALI6T dLDI7L
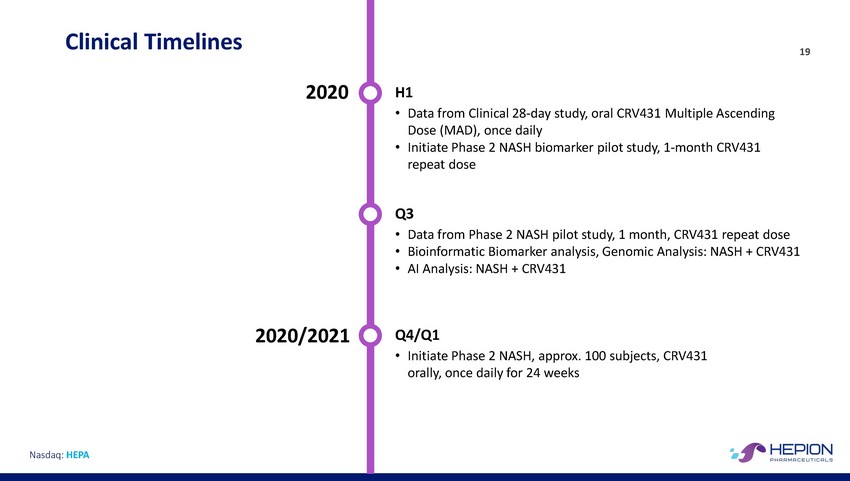
Clinical Timelines 19 2020 H1 • Data from Clinical 28-day study, oral CRV431 Multiple Ascending Dose (MAD), once daily • Initiate Phase 2 NASH biomarker pilot study, 1-month CRV431 repeat dose Q3 • • • Data from Phase 2 NASH pilot study, 1 month, CRV431 repeat dose Bioinformatic Biomarker analysis, Genomic Analysis: NASH + CRV431 AI Analysis: NASH + CRV431 2020/2021 Q4/Q1 • Initiate Phase 2 NASH, approx. 100 subjects, CRV431 orally, once daily for 24 weeks Nasdaq: HEPA
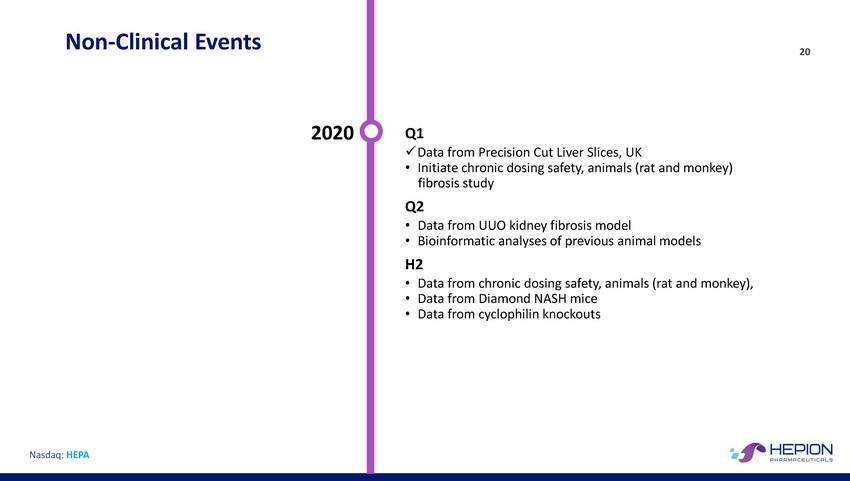
Non-Clinical Events 20 2020 Q1 x Data from Precision Cut Liver Slices, UK • Initiate chronic dosing safety, animals (rat and monkey) fibrosis study Q2 • Data from UUO kidney fibrosis model • Bioinformatic analyses of previous animal models H2 • • • Data from chronic dosing safety, animals (rat and monkey), Data from Diamond NASH mice Data from cyclophilin knockouts Nasdaq: HEPA
21 Thank You Investor Relations: Stephen Kilmer skilmer@hepionpharma.com Tel: 646.274.3580 Corporate Contact: Robert Foster rfoster@hepionpharma.com Nasdaq: HEPA
