Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LUMOS PHARMA, INC. | nlnk-20180416x8k.htm |
| EX-99.1 - PRESS RELEASE - LUMOS PHARMA, INC. | nlnk-20180416x8kxex991.htm |

American Association of Cancer Research (AACR) 2018
Theodore S. Johnson, MD, PhD
Georgia Cancer Center – Augusta University
April 15, 2018
Front-line Therapy of DIPG Using IDO Pathway Inhibitor Indoximod
in Combination with Radiation and Chemotherapy

Cautionary Note Regarding Forward-Looking Statements
This presentation contains forward-looking statements of NewLink Genetics that involve substantial risks and
uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-
looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words
"anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek"
or the negative of these terms or other similar expressions are intended to identify forward-looking statements,
although not all forward-looking statements contain these identifying words. These forward-looking statements
include, among others, statements about NewLink Genetics' financial guidance for 2018; results of its clinical trials for
product candidates; its timing of release of data from ongoing clinical studies; its plans related to execution of clinical
trials; plans related to moving additional indications into clinical development; NewLink Genetics' future financial
performance, results of operations, cash position and sufficiency of capital resources to fund its operating
requirements; and any other statements other than statements of historical fact. Actual results or events could differ
materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink
Genetics makes due to a number of important factors, including those risks discussed in "Risk Factors" and
elsewhere in NewLink Genetics' Annual Report on Form 10-K for the year ended December 31, 2017 and other
reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this
presentation represent NewLink Genetics' views as of the date of this presentation. NewLink Genetics anticipates
that subsequent events and developments will cause its views to change. However, while it may elect to update
these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You
should, therefore, not rely on these forward-looking statements as representing NewLink Genetics' views as of any
date subsequent to the date of this presentation.
2

IDO Pathway a Key Immuno-oncology Target
3
Treg, regulatory T cell; IDO, indoleamine 2,3-dioxygenase; MDSC, myeloid-derived suppressor cell; CTL, cytotoxic T lymphocyte.
1. Metz R. Oncoimmunology. 2012;1(9):1460-1468. 2. Johnson TS. Immunol Invest. 2012;41(6-7):765-797.
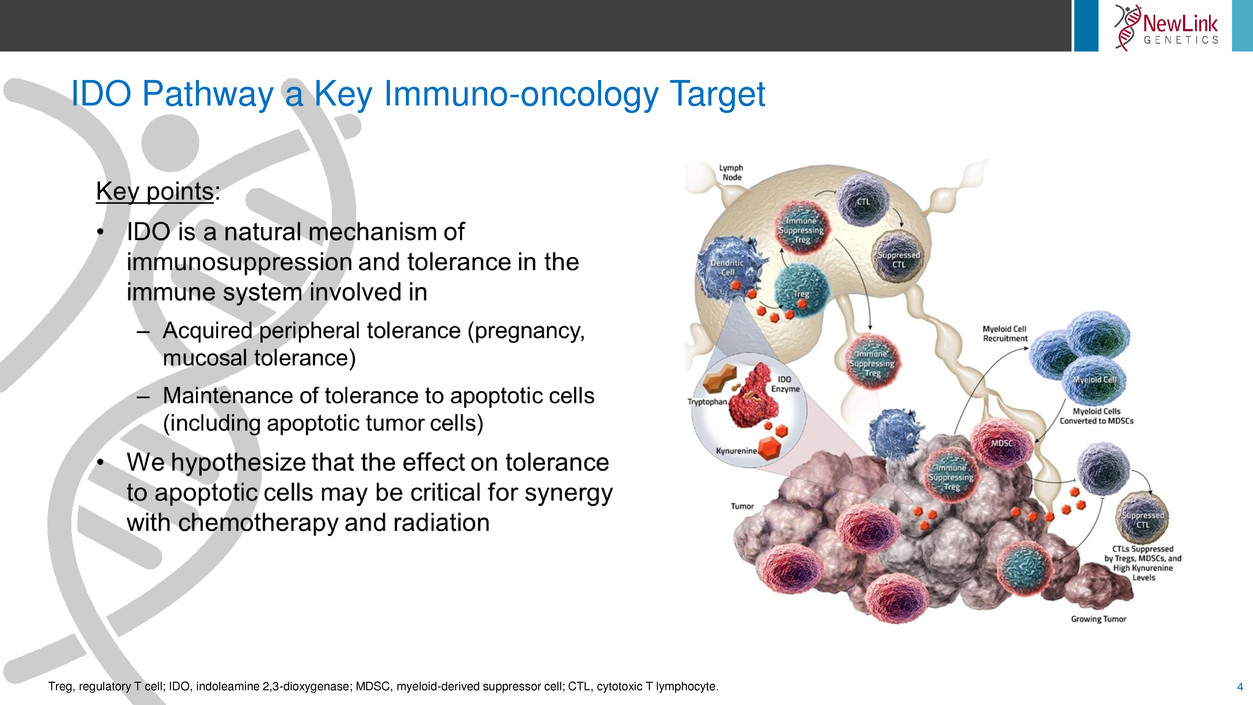
IDO Pathway a Key Immuno-oncology Target
4Treg, regulatory T cell; IDO, indoleamine 2,3-dioxygenase; MDSC, myeloid-derived suppressor cell; CTL, cytotoxic T lymphocyte.

IDO Expression in Certain Tumors is Associated with Poor Patient Outcomes
5
IDO, indoleamine 2,3-dioxygenase; LN, lymph node; NSCLC, non-small cell lung cancer; DLBCL, diffuse large B-cell lymphoma;
RCC, renal cell carcinoma; TCC, transitional cell carcinoma;
TNBC, triple-negative breast cancer. Munn DH, et al. J Clin Invest. 2004;114(2):280-290.
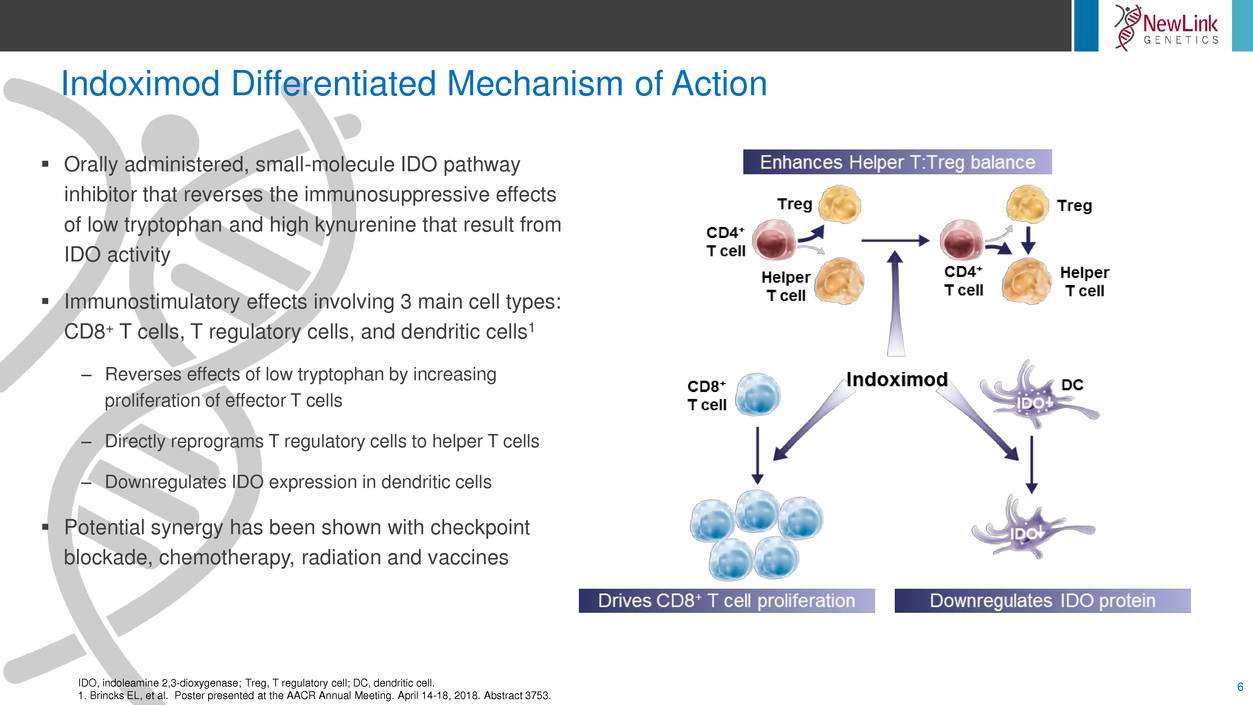
Indoximod Differentiated Mechanism of Action
6IDO, indoleamine 2,3-dioxygenase; Treg, T regulatory cell; DC, dendritic cell.
1. Brincks EL, et al. Poster presented at the AACR Annual Meeting. April 14-18, 2018. Abstract 3753.
Orally administered, small-molecule IDO pathway
inhibitor that reverses the immunosuppressive effects
of low tryptophan and high kynurenine that result from
IDO activity
Immunostimulatory effects involving 3 main cell types:
CD8+ T cells, T regulatory cells, and dendritic cells1
– Reverses effects of low tryptophan by increasing
proliferation of effector T cells
– Directly reprograms T regulatory cells to helper T cells
– Downregulates IDO expression in dendritic cells
Potential synergy has been shown with checkpoint
blockade, chemotherapy, radiation and vaccines
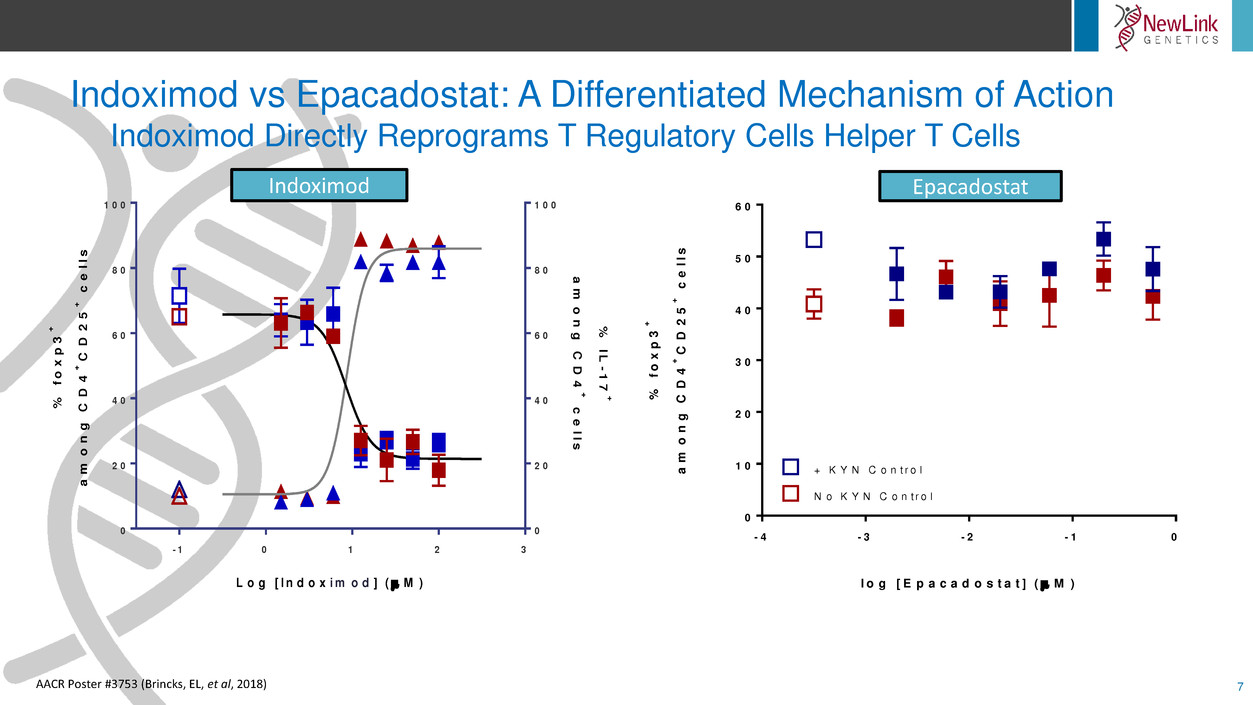
Indoximod vs Epacadostat: A Differentiated Mechanism of Action
Indoximod Directly Reprograms T Regulatory Cells Helper T Cells
7
- 1 0 1 2 3
0
2 0
4 0
6 0
8 0
1 0 0
0
2 0
4 0
6 0
8 0
1 0 0
L o g [ I n d o x i m o d ] ( M )
%
f
o
x
p
3
+
a
m
o
n
g
C
D
4
+
C
D
2
5
+
c
e
ll
s
%
IL
-1
7
+
a
m
o
n
g
C
D
4
+
c
e
lls
- 4 - 3 - 2 - 1 0
0
1 0
2 0
3 0
4 0
5 0
6 0
l g [ E p a c a d o s t a t ] ( M )
%
f
o
x
p
3
+
a
m
o
n
g
C
D
4
+
C
D
2
5
+
c
e
ll
s
+ K Y N C o n t r o l
N o K Y N C o n t r o l
AACR Poster #3753 (Brincks, EL, et al, 2018)
Indoximod Epacadostat

Designing Multimodal Chemo-radio-immunotherapy
8
Hypothesis
– Immune activation (immunotherapy) can allow responsiveness to chemotherapy and
radiation in patients who would otherwise be refractory
However, this synergy with chemotherapy/radiation requires targeting the
antigen-presenting step and creating a
pro-inflammatory (immunogenic) tumor milieu
– Essentially, it must break tolerance to the dying/apoptotic tumor cells
– This antigen cross-presentation step lies upstream of the conventional
T-cell checkpoints
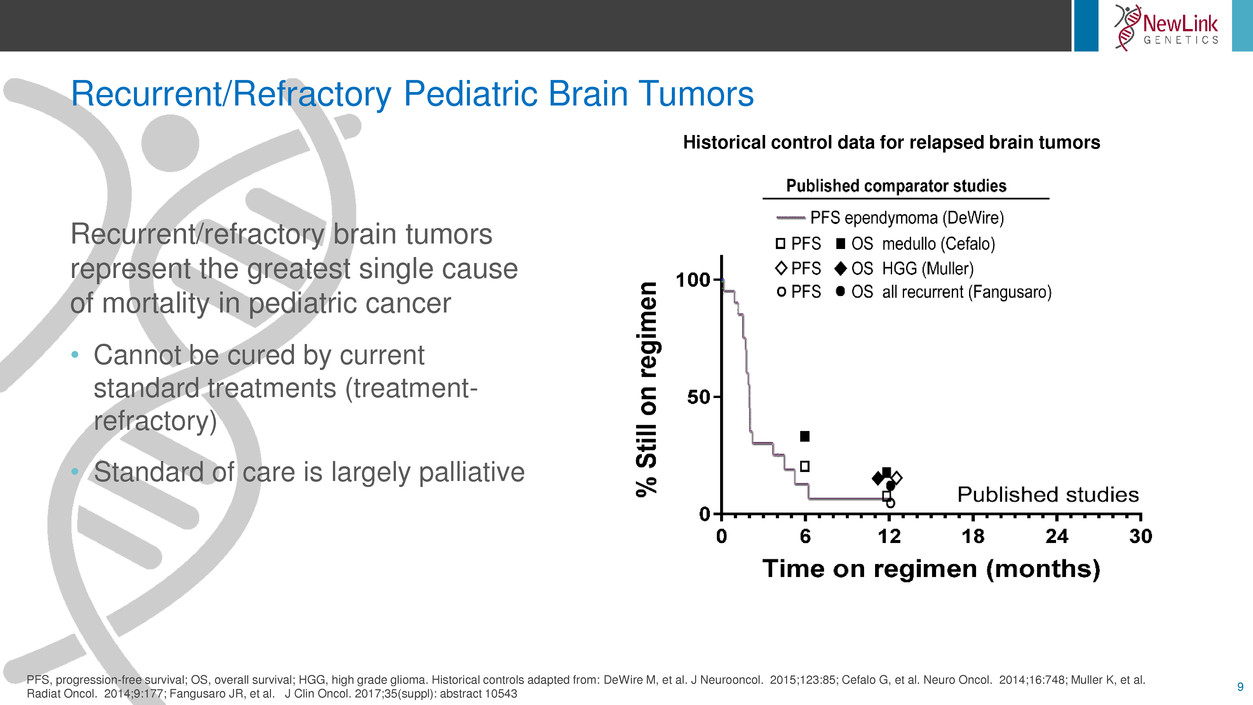
Recurrent/Refractory Pediatric Brain Tumors
9
Recurrent/refractory brain tumors
represent the greatest single cause
of mortality in pediatric cancer
• Cannot be cured by current
standard treatments (treatment-
refractory)
• Standard of care is largely palliative
PFS, progression-free survival; OS, overall survival; HGG, high grade glioma. Historical controls adapted from: DeWire M, et al. J Neurooncol. 2015;123:85; Cefalo G, et al. Neuro Oncol. 2014;16:748; Muller K, et al.
Radiat Oncol. 2014;9:177; Fangusaro JR, et al. J Clin Oncol. 2017;35(suppl): abstract 10543
Historical control data for relapsed brain tumors
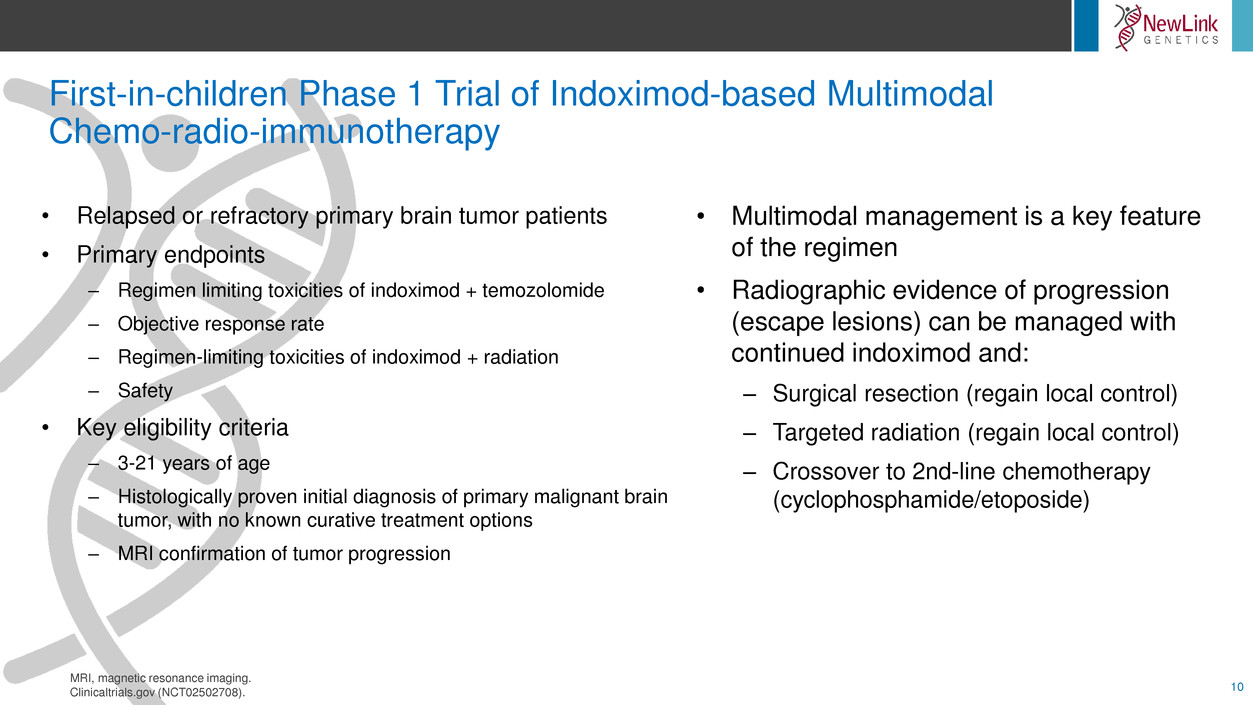
First-in-children Phase 1 Trial of Indoximod-based Multimodal
Chemo-radio-immunotherapy
10
• Multimodal management is a key feature
of the regimen
• Radiographic evidence of progression
(escape lesions) can be managed with
continued indoximod and:
– Surgical resection (regain local control)
– Targeted radiation (regain local control)
– Crossover to 2nd-line chemotherapy
(cyclophosphamide/etoposide)
• Relapsed or refractory primary brain tumor patients
• Primary endpoints
– Regimen limiting toxicities of indoximod + temozolomide
– Objective response rate
– Regimen-limiting toxicities of indoximod + radiation
– Safety
• Key eligibility criteria
– 3-21 years of age
– Histologically proven initial diagnosis of primary malignant brain
tumor, with no known curative treatment options
– MRI confirmation of tumor progression
MRI, magnetic resonance imaging.
Clinicaltrials.gov (NCT02502708).
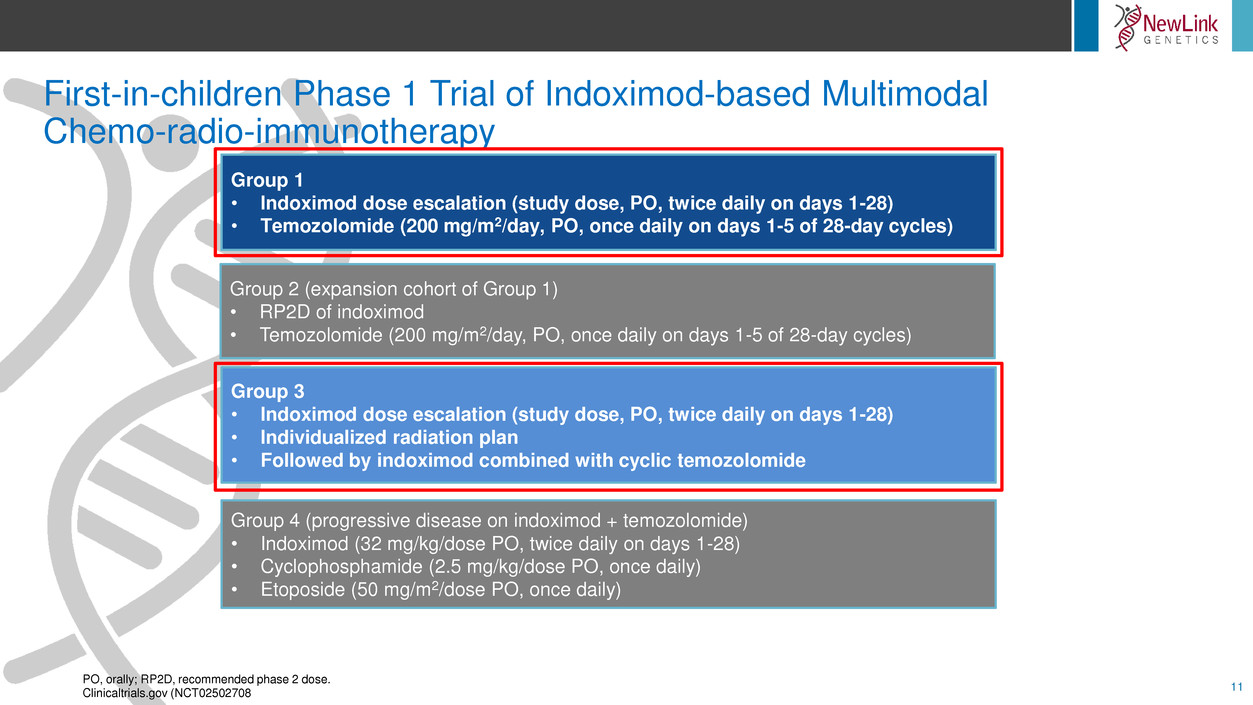
First-in-children Phase 1 Trial of Indoximod-based Multimodal
Chemo-radio-immunotherapy
11
Group 1
• Indoximod dose escalation (study dose, PO, twice daily on days 1-28)
• Temozolomide (200 mg/m2/day, PO, once daily on days 1-5 of 28-day cycles)
Group 2 (expansion cohort of Group 1)
• RP2D of indoximod
• Temozolomide (200 mg/m2/day, PO, once daily on days 1-5 of 28-day cycles)
Group 3
• Indoximod dose escalation (study dose, PO, twice daily on days 1-28)
• Individualized radiation plan
• Followed by indoximod combined with cyclic temozolomide
Group 4 (progressive disease on indoximod + temozolomide)
• Indoximod (32 mg/kg/dose PO, twice daily on days 1-28)
• Cyclophosphamide (2.5 mg/kg/dose PO, once daily)
• Etoposide (50 mg/m2/dose PO, once daily)
PO, orally; RP2D, recommended phase 2 dose.
Clinicaltrials.gov (NCT02502708
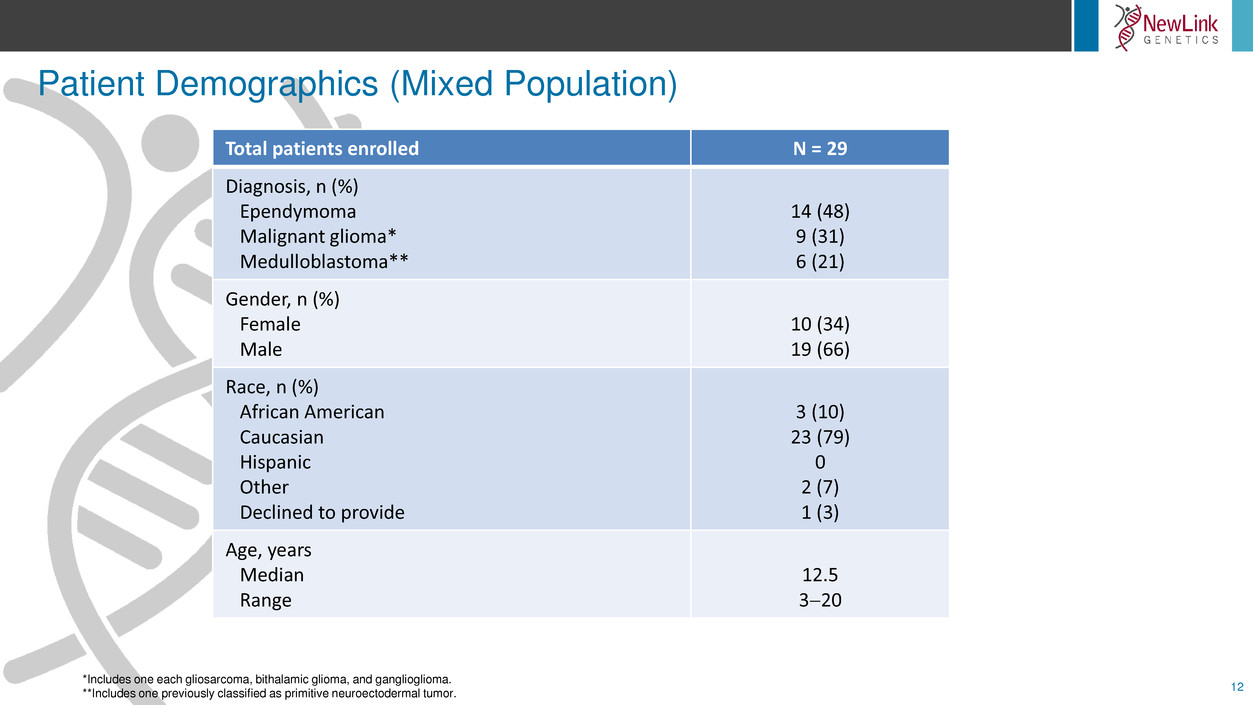
Patient Demographics (Mixed Population)
12
*Includes one each gliosarcoma, bithalamic glioma, and ganglioglioma.
**Includes one previously classified as primitive neuroectodermal tumor.
Total patients enrolled N = 29
Diagnosis, n (%)
Ependymoma
Malignant glioma*
Medulloblastoma**
14 (48)
9 (31)
6 (21)
Gender, n (%)
Female
Male
10 (34)
19 (66)
Race, n (%)
African American
Caucasian
Hispanic
Other
Declined to provide
3 (10)
23 (79)
0
2 (7)
1 (3)
Age, years
Median
Range
12.5
320

Patient 001: Example of Multimodal Management Chemo-radio-immunotherapy
13
(TTRF)
Continuing indoximod-
based therapy
Progressing disease
Responding/stable disease
Chemo regimen 1
Chemo regimen 2
Chemo regimen 3
Surgery
PR PD PR PDMRI
Pt 001
SD
Surgery
Chemo 1 Chemo 2 Chemo 3
0 6 12 18 24
Continuous Indoximod (months)
Partial RT
(SRS)
Low-dose outpatient
chemo
Partial RT
(low-dose)
RT RT RT
• 7-year-old with ependymoma: prolonged disease responsiveness
• Indoximod-based multimodal regimen is well tolerated
SRS, stereotactic radiosurgery; RT, radiation therapy; PR, partial response; PD, progressive disease; SD, stable disease.
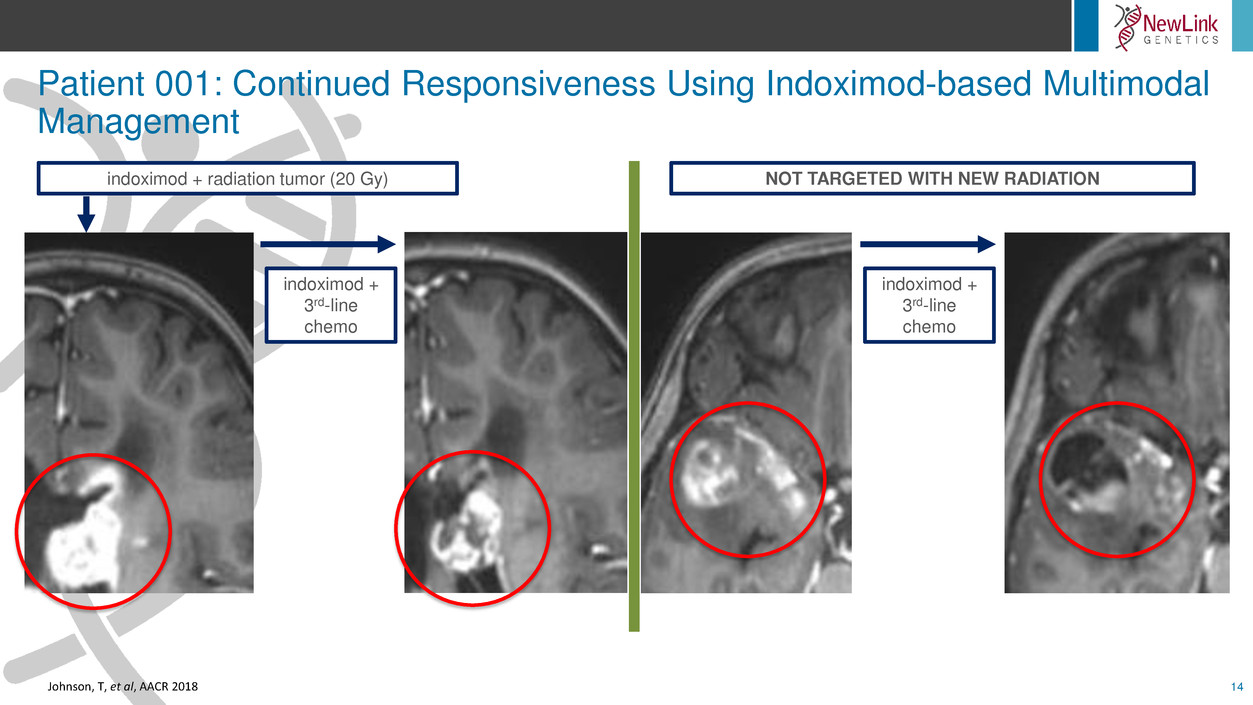
Patient 001: Continued Responsiveness Using Indoximod-based Multimodal
Management
14
indoximod + radiation tumor (20 Gy)
indoximod +
3rd-line
chemo
indoximod +
3rd-line
chemo
NOT TARGETED WITH NEW RADIATION
Johnson, T, et al, AACR 2018

Radio-Immunotherapy Improves Time to Regimen Failure (TTRF)
15
Historical controls adapted from: DeWire M, et al. J Neurooncol 2015;123:85. RT, radiation therapy Fangusaro JR, et al. J Clin Oncol, 2017;35(suppl): abstract 10543.
Cefalo G, et al. Neuro Oncol 2014;16:748.
Muller K, et al. Radiat Oncol 2014;9:177.
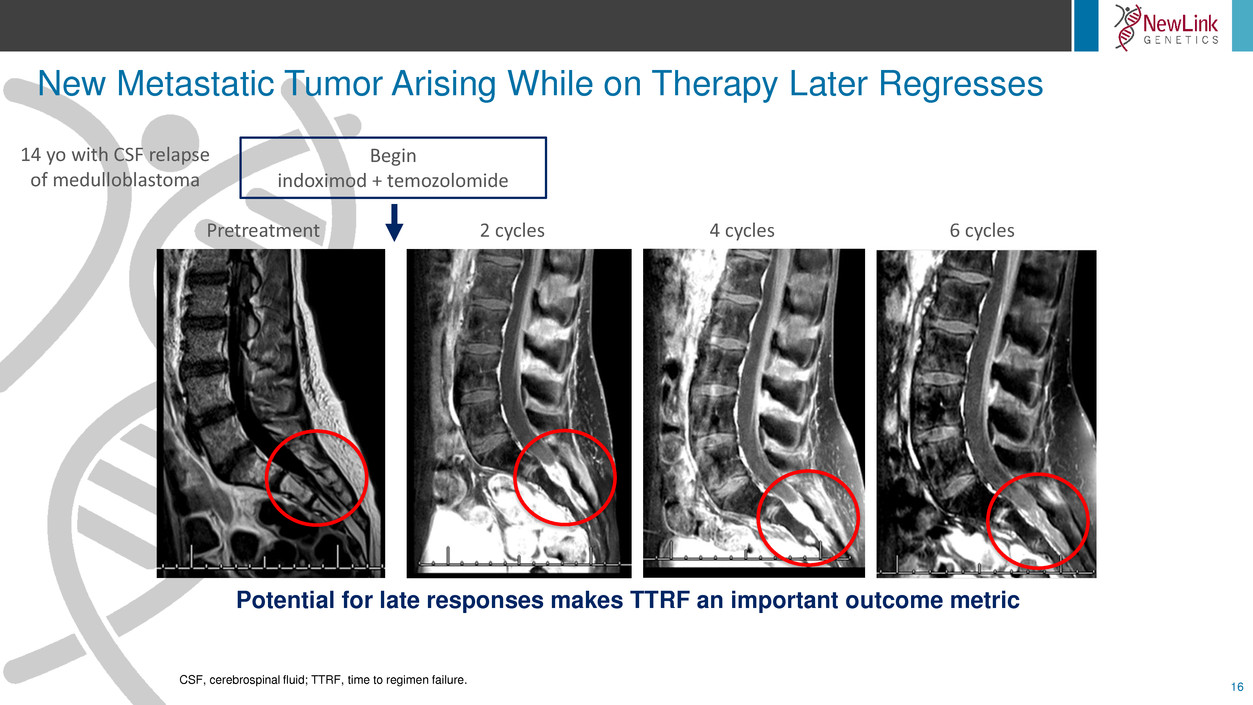
New Metastatic Tumor Arising While on Therapy Later Regresses
16
CSF, cerebrospinal fluid; TTRF, time to regimen failure.
Pretreatment 2 cycles 4 cycles 6 cycles
Begin
indoximod + temozolomide
14 yo with CSF relapse
of medulloblastoma
Potential for late responses makes TTRF an important outcome metric

Indoximod-based Multimodal Regimen is Well Tolerated
17
In the 29 patients included in the study, SAEs possibly related to indoximod
included 1 case each of:
– Febrile neutropenia
– Hemiparesis
– Hydrocephalus
– Spinal cord compression
– Status epilepticus
– Urinary tract infection
Overall, indoximod did not worsen the toxicity of the base treatment
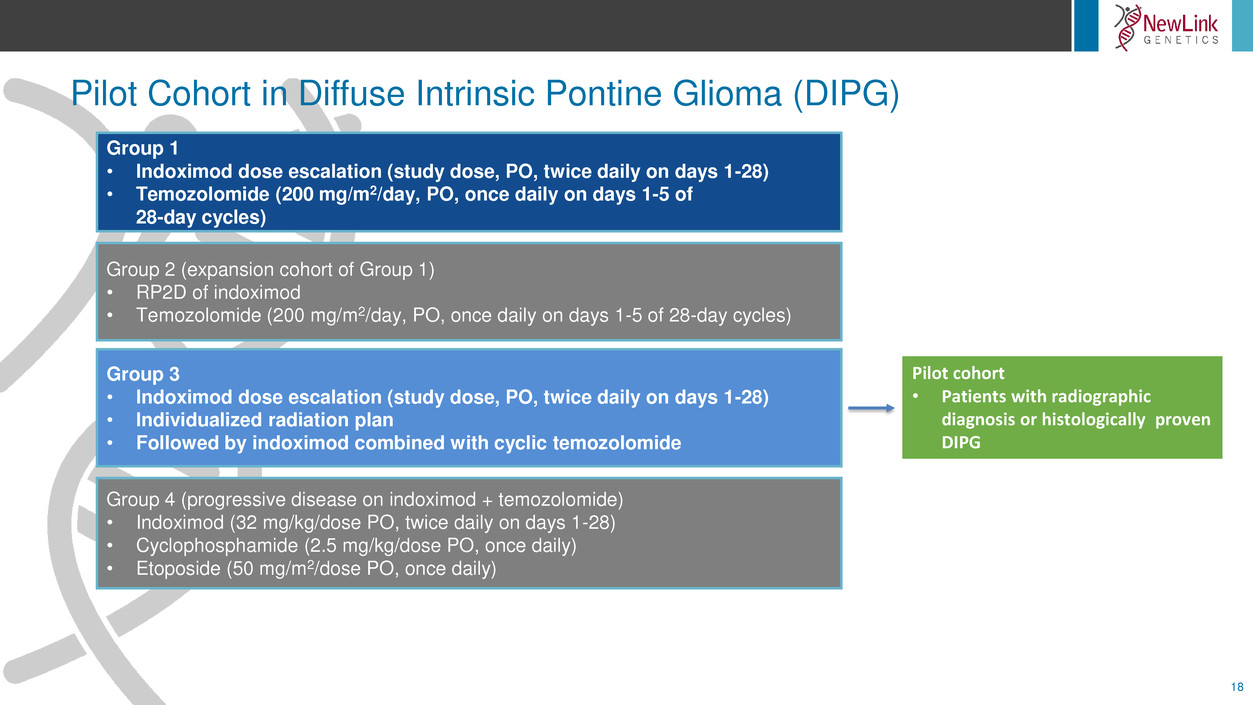
Pilot Cohort in Diffuse Intrinsic Pontine Glioma (DIPG)
18
Group 1
• Indoximod dose escalation (study dose, PO, twice daily on days 1-28)
• Temozolomide (200 mg/m2/day, PO, once daily on days 1-5 of
28-day cycles)
Group 2 (expansion cohort of Group 1)
• RP2D of indoximod
• Temozolomide (200 mg/m2/day, PO, once daily on days 1-5 of 28-day cycles)
Group 3
• Indoximod dose escalation (study dose, PO, twice daily on days 1-28)
• Individualized radiation plan
• Followed by indoximod combined with cyclic temozolomide
Group 4 (progressive disease on indoximod + temozolomide)
• Indoximod (32 mg/kg/dose PO, twice daily on days 1-28)
• Cyclophosphamide (2.5 mg/kg/dose PO, once daily)
• Etoposide (50 mg/m2/dose PO, once daily)
Pilot cohort
• Patients with radiographic
diagnosis or histologically proven
DIPG
DIPG Is Rapidly Fatal
19
DIPG has the worst prognosis of any pediatric cancer
Median time to progression after radiation is ~6 months1
At progression, patients follow a rapidly declining course
– Median OS is 10-12 months2
–Uniformly fatal
DIPG, diffuse intrinsic pontine glioma; OS, overall survival.
1. Wolff JE, et al. J Neurooncol. 2012;106(2):391-397. 2. Cohen KJ, et al. Neuro Oncol. 2011;13(4):410-416.

Effective Treatments for DIPG are Lacking
20
Standard-of-care treatment is palliative radiation (usually 54 Gy)
Chemotherapy has no proven benefit
Thus far, trials have not shown clinical benefit from currently
available chemotherapy, radiosensitizing drugs, or biologics
Due to their location in the brainstem, DIPGs cannot be surgically
removed
DIPG, diffuse intrinsic pontine glioma.

Multimodal Chemo-radio-immunotherapy for DIPG Pilot Cohort
21
First question: Could DIPG patients tolerate the indoximod immunotherapy
regimen?
– DIPG patients are often highly symptomatic
Pilot cohort of 6 newly diagnosed DIPG patients
– All 6 patients have finished upfront radiation combined with indoximod
– All 6 patients showed initial improvement in symptoms
– 3/6 later developed inflammatory symptoms (eg, waxing/waning, migratory)
• 2 of these occurred during first cycle of temozolomide with indoximod
.
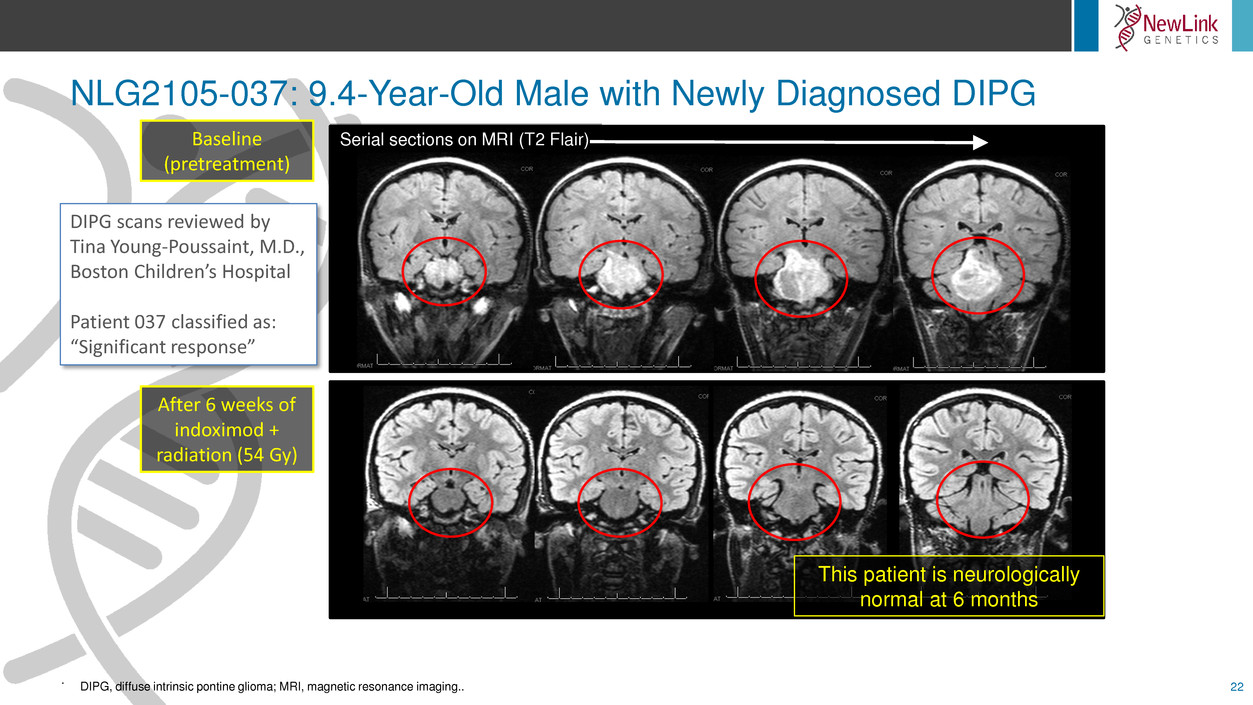
NLG2105-037: 9.4-Year-Old Male with Newly Diagnosed DIPG
22
.
Serial sections on MRI (T2 Flair)Baseline
(pretreatment)
After 6 weeks of
indoximod +
radiation (54 Gy)
This patient is neurologically
normal at 6 months
DIPG scans reviewed by
Tina Young-Poussaint, M.D.,
Boston Children’s Hospital
Patient 037 classified as:
“Significant response”
DIPG, diffuse intrinsic pontine glioma; MRI, magnetic resonance imaging..
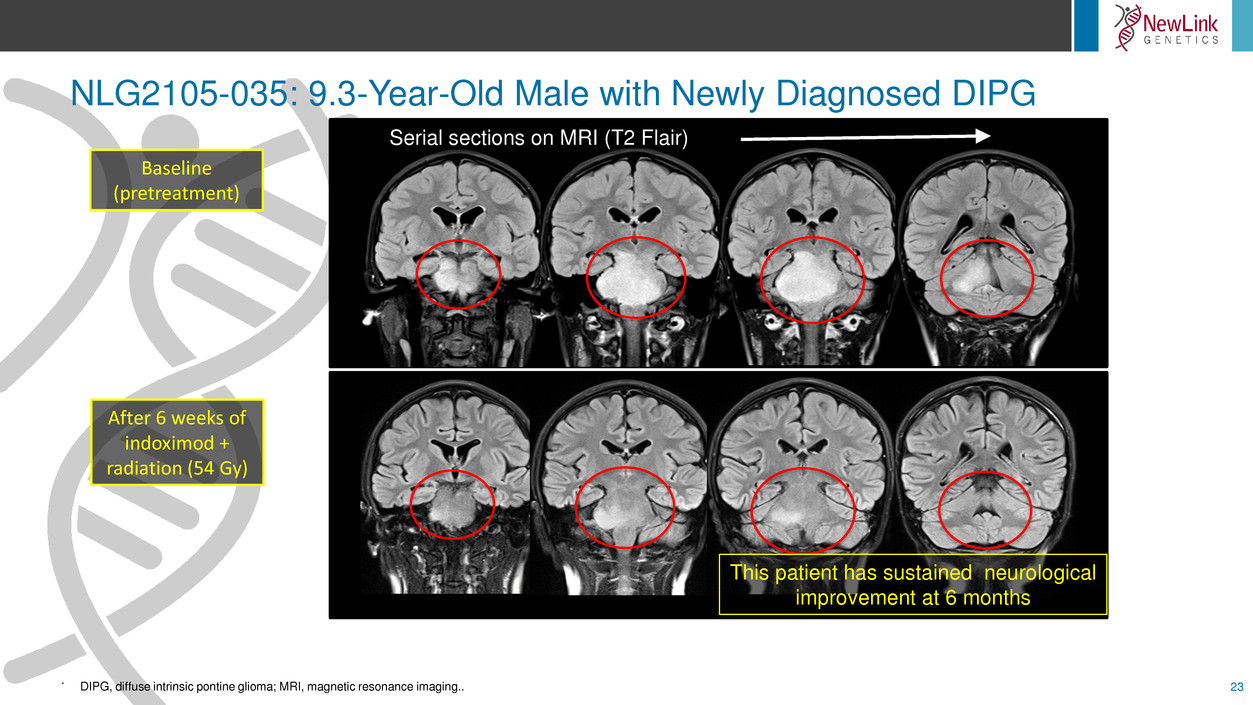
NLG2105-035: 9.3-Year-Old Male with Newly Diagnosed DIPG
23
.
DIPG, diffuse intrinsic pontine glioma; MRI, magnetic resonance imaging..
This patient has sustained neurological
improvement at 6 months
Serial sections on MRI (T2 Flair)
Baseline
(pretreatment)
After 6 weeks of
indoximod +
radiation (54 Gy)
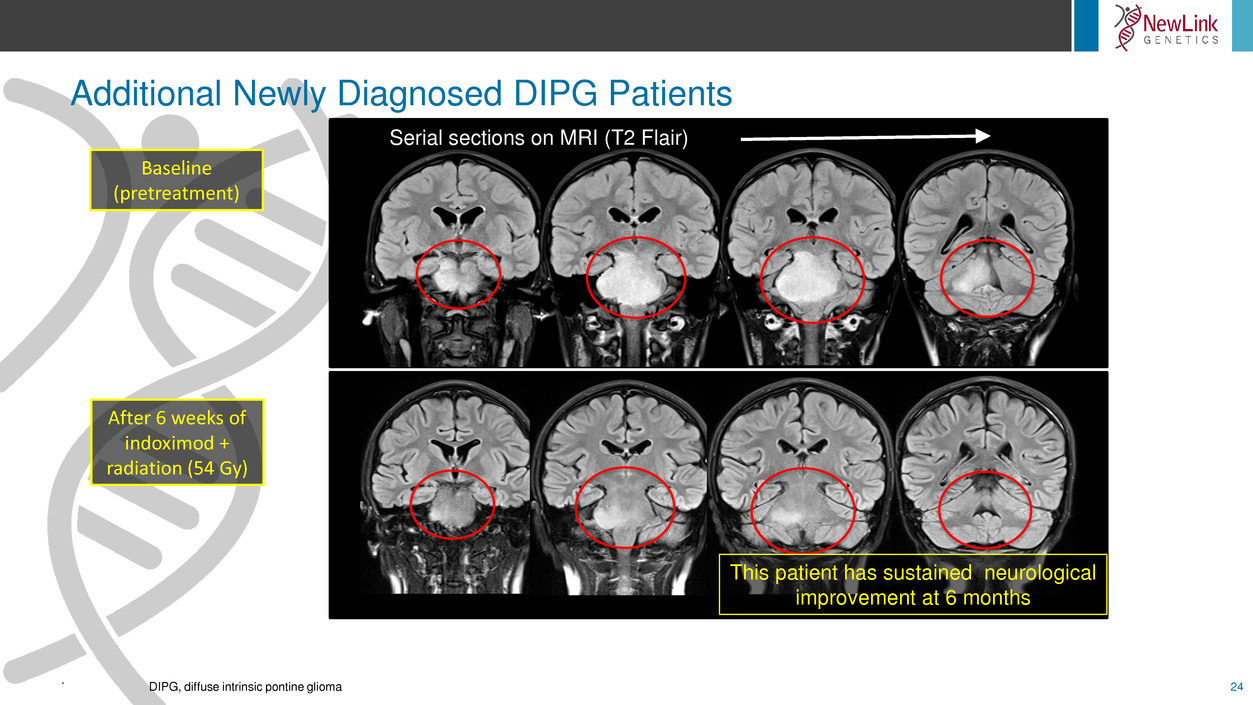
Additional Newly Diagnosed DIPG Patients
24
.
DIPG, diffuse intrinsic pontine glioma
This patient has sustained neurological
improvement at 6 months
Serial sections on MRI (T2 Flair)
Baseline
(pretreatment)
After 6 weeks of
indoximod +
radiation (54 Gy)
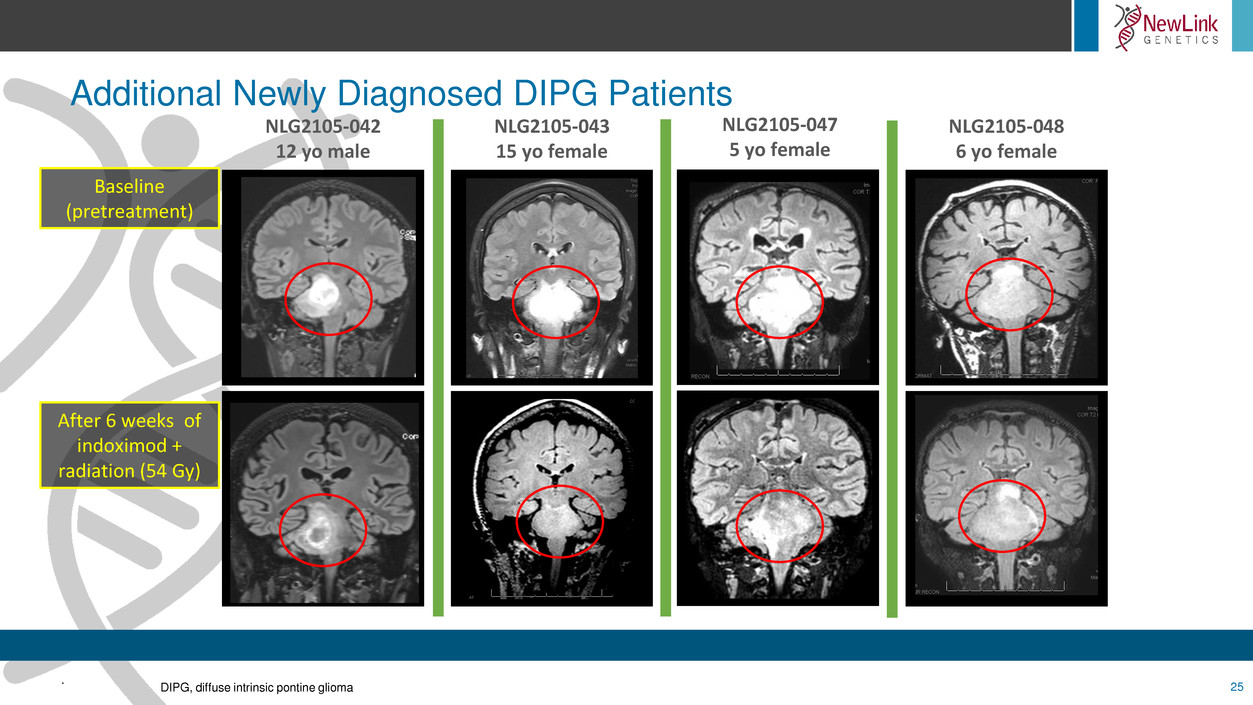
Additional Newly Diagnosed DIPG Patients
25
.
DIPG, diffuse intrinsic pontine glioma
Baseline
(pretreatment)
After 6 weeks of
indoximod +
radiation (54 Gy)
NLG2105-042
12 yo male
NLG2105-043
15 yo female
NLG2105-047
5 yo female
NLG2105-048
6 yo female

Conclusions and Future Directions
26
Phase 1 data suggest that indoximod-based immunotherapy can allow
disease responsiveness to conventional therapy (radiation,
chemotherapy)
Pilot cohort is under way applying this approach to newly diagnosed
DIPG patients
Phase 2 trial is planned
.
