Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - AveXis, Inc. | a17-28377_1ex99d2.htm |
| 8-K - 8-K - AveXis, Inc. | a17-28377_18k.htm |
2 This presentation contains forward-looking statements, including statements about: the timing, progress and results of preclinical studies and clinical trials for AVXS-101, including statements regarding the potential of AVXS-101 to positively impact quality of life and alter the course of disease in patients with SMA Type 1 and SMA Type 2, the timing of initiation of studies or trials, our expectations regarding timing for meetings with regulatory agencies, our ability to meet future commercial demand for AVXS-101 through our manufacturing facility, our manufacturing strategy and developments, key regulatory and development milestones and our research and development programs. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward-looking statements include the risks described in the “Risk Factors” sections of the Company’s Annual Report on Form 10-K for the year ended December 31, 2016 and the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2017, as well as those set forth from time to time in the Company’s other SEC filings, available at http://www.sec.gov. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Disclaimers

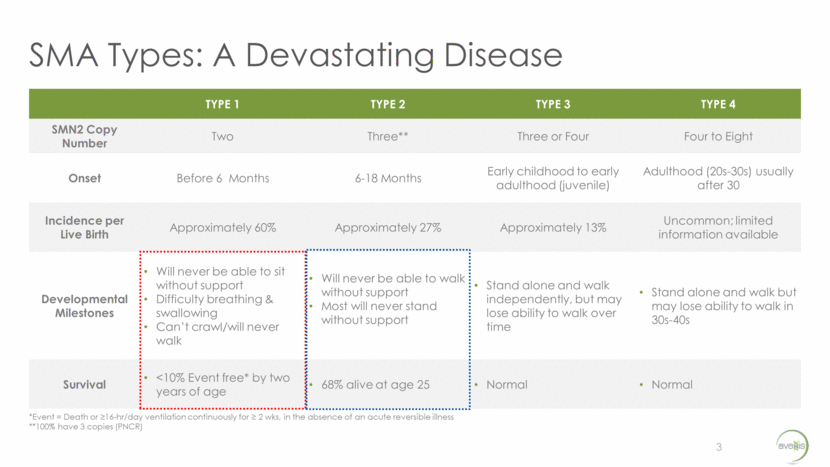
SMA Types: A Devastating Disease 3 TYPE 1 TYPE 2 TYPE 3 TYPE 4 SMN2 Copy Number Two Three** Three or Four Four to Eight Onset Before 6 Months 6-18 Months Early childhood to early adulthood (juvenile) Adulthood (20s-30s) usually after 30 Incidence per Live Birth Approximately 60% Approximately 27% Approximately 13% Uncommon; limited information available Developmental Milestones Will never be able to sit without support Difficulty breathing & swallowing Can’t crawl/will never walk Will never be able to walk without support Most will never stand without support Stand alone and walk independently, but may lose ability to walk over time Stand alone and walk but may lose ability to walk in 30s-40s Survival <10% Event free* by two years of age 68% alive at age 25 Normal Normal *Event = Death or >16-hr/day ventilation continuously for > 2 wks, in the absence of an acute reversible illness **100% have 3 copies (PNCR)
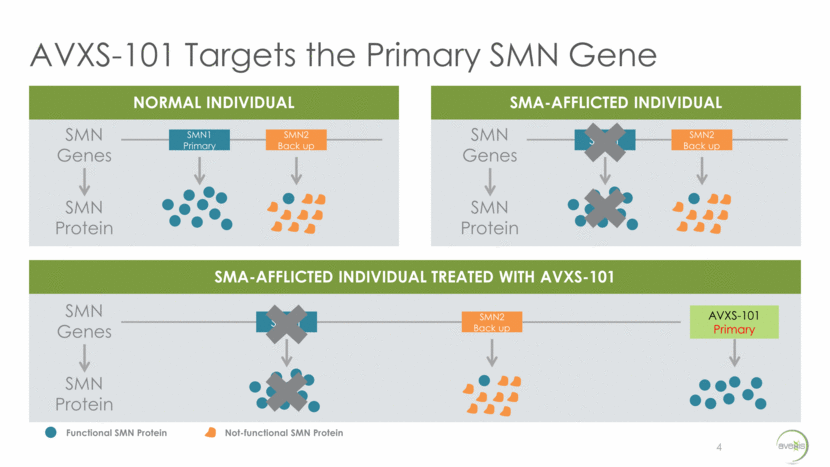
AVXS-101 Targets the Primary SMN Gene 4 NORMAL INDIVIDUAL SMA-AFFLICTED INDIVIDUAL SMA-AFFLICTED INDIVIDUAL TREATED WITH AVXS-101 SMN Genes SMN Protein SMN1 Primary SMN2 Back up SMN Genes SMN Protein SMN1 SMN2 Back up SMN Genes SMN Protein SMN1 SMN2 Back up AVXS-101 Primary Functional SMN Protein Not-functional SMN Protein
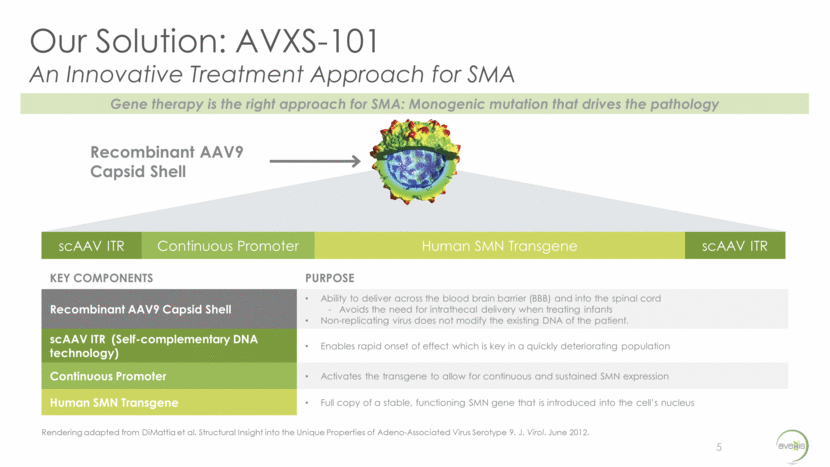
Our Solution: AVXS-101 An Innovative Treatment Approach for SMA 5 scAAV ITR Continuous Promoter Human SMN Transgene scAAV ITR KEY COMPONENTS PURPOSE Recombinant AAV9 Capsid Shell Ability to deliver across the blood brain barrier (BBB) and into the spinal cord - Avoids the need for intrathecal delivery when treating infants Non-replicating virus does not modify the existing DNA of the patient. scAAV ITR (Self-complementary DNA technology) Enables rapid onset of effect which is key in a quickly deteriorating population Continuous Promoter Activates the transgene to allow for continuous and sustained SMN expression Human SMN Transgene Full copy of a stable, functioning SMN gene that is introduced into the cell’s nucleus Recombinant AAV9 Capsid Shell Rendering adapted from DiMattia et al. Structural Insight into the Unique Properties of Adeno-Associated Virus Serotype 9. J. Virol. June 2012. Gene therapy is the right approach for SMA: Monogenic mutation that drives the pathology
Phase 1 Trial of AVXS-101 in SMA Type 2 6 Route of Administration One-time intrathecal dose Prednisolone 1 mg/kg 1 day Pre-GT Trial Design 27-patient, Dose-comparison AVXS-101 PHASE 1 TRIAL OVERVIEW – SMA TYPE 2 Study Sites 11 centers in U.S. Safety Safety and Tolerability of intrathecal administration of AVXS-101 Determination of optimal dose SAFETY OBJECTIVE Primary Ability to stand alone for at least 3 seconds Secondary Ability to walk without assistance, defined as taking at least 5 steps independently displaying coordination and balance Exploratory Hammersmith Functional Motor Scale – Expanded Change from baseline in fine and gross motor components EFFICACY OBJECTIVES: PATIENTS LESS THAN 24 MONTHS* Primary Change from baseline in Hammersmith Functional Motor Scale – Expanded Secondary Ability to walk without assistance, defined as taking at least 5 steps independently displaying coordination and balance Exploratory Change from baseline in fine and gross motor components EFFICACY OBJECTIVES: PATIENTS AT LEAST 24 MONTHS* *Additionally, compelling, demonstrable, documented evidence of efficacy as determined by changes in developmental abilities as captured during videotaping sessions during site visits and/or captured/provided by parent/legal guardian will be collected for all patients in all age groups.
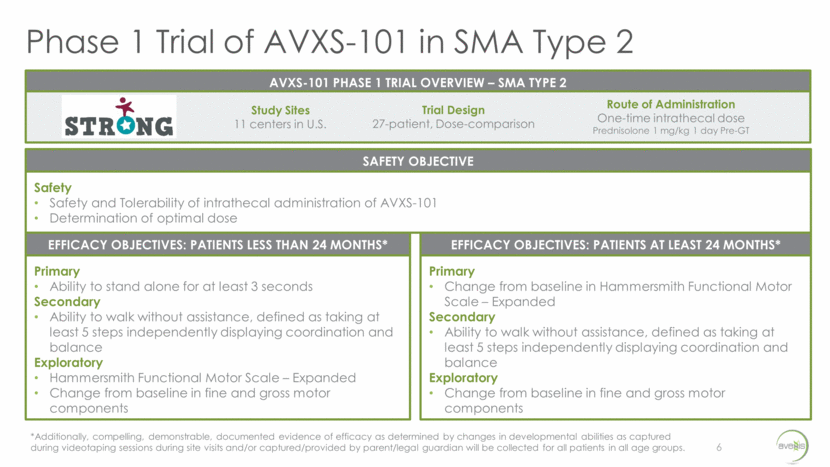
Phase 1 Trial: Key Enrollment Criteria 7 Inclusion 60 months of age and younger at day of vector infusion as defined by the following features: Bi-allelic SMN1 gene deletion 3 copies of SMN2 gene Ability to sit unassisted for 10 or more seconds Negative gene testing for SMN2 gene modifier mutation (c.859G>C) Onset of clinical signs and symptoms consistent with SMA at less than 12 months of age Exclusion Historical or current ability to stand or walk Use of invasive ventilatory support Use or requirement of non-invasive ventilatory support for 12 or more hours daily over the two weeks prior to dosing Key Enrollment Criteria Kaufman et al. Prospective cohort study of spinal muscular atrophy types 2 and 3; Neurology. 2012 Oct 30; 79(18): 1889–1897.
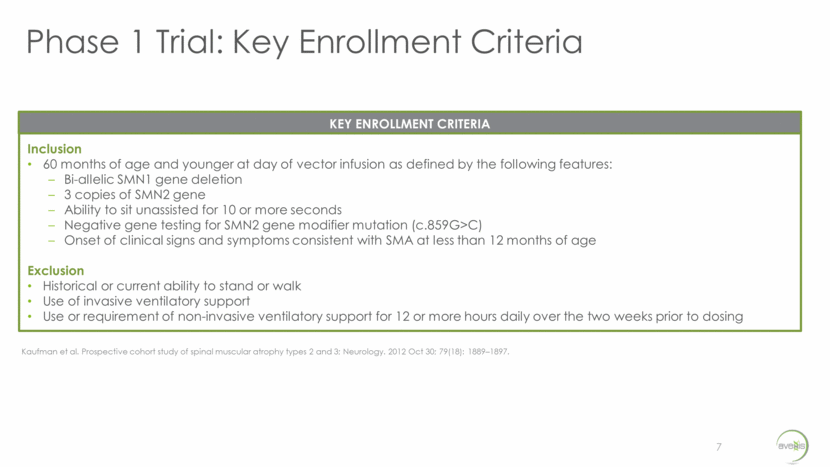
Phase 1 Trial: Dose Comparison & Methodology 8 Dose Comparison Stratified into two groups based on age at time of dosing: Less than 24 months of age (n=15) At least 24 months and less than 60 months (n=12) Methodology Cohort 1 (n=3) – Lower Dose/Dose A (6.0 X 1013 vg) Patients less than 24 months of age 4 week interval after each patient to review safety data prior to continuation If safety is established by the DSMB, advance to Dose B Cohort 2 (n=3) – Higher Dose/Dose B (1.2 X 1014 vg) All ages up to 60 months 4 week interval after each patient to review safety data prior to continuation If safety is established by the DSMB, Cohort 2 will be expanded to include 21 additional participants Expanded Cohort 2 (n=21) – Higher Dose/Dose B (1.2 X 1014 vg) Enrollment continues until 12 patients less than 24 months, and 12 patients at least 24 months and less than 60 months, receive Dose B Dose comparison & methodology
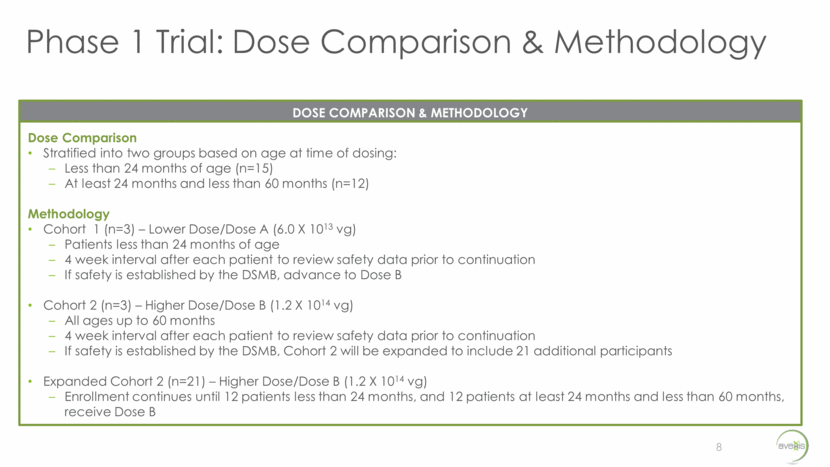
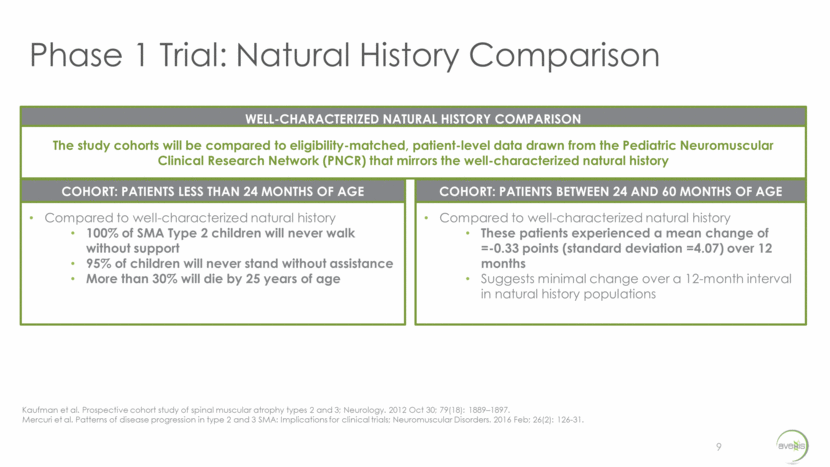
Phase 1 Trial: Natural History Comparison 9 Compared to well-characterized natural history 100% of SMA Type 2 children will never walk without support 95% of children will never stand without assistance More than 30% will die by 25 years of age COHORT: PATIENTS LESS THAN 24 MONTHS OF AGE Compared to well-characterized natural history These patients experienced a mean change of =-0.33 points (standard deviation =4.07) over 12 months Suggests minimal change over a 12-month interval in natural history populations COHORT: PATIENTS BETWEEN 24 AND 60 MONTHS OF AGE The study cohorts will be compared to eligibility-matched, patient-level data drawn from the Pediatric Neuromuscular Clinical Research Network (PNCR) that mirrors the well-characterized natural history WELL-CHARACTERIZED NATURAL HISTORY COMPARISON Kaufman et al. Prospective cohort study of spinal muscular atrophy types 2 and 3; Neurology. 2012 Oct 30; 79(18): 1889–1897. Mercuri et al. Patterns of disease progression in type 2 and 3 SMA: Implications for clinical trials; Neuromuscular Disorders. 2016 Feb; 26(2): 126-31.
Looking Ahead: Upcoming Milestones 10 SMA Type 1 end-of-Phase 1 Meeting with FDA: On December 5, 2017, AveXis had an end-of-Phase 1 meeting with FDA with respect to AVXS-101 for SMA Type 1. The company anticipates providing an update on feedback from FDA following the receipt of the final meeting minutes in early January. SMA Type 1 EU Trial: AveXis incorporated EU specific Scientific Advice from the EMA into the protocol design and expects to initiate a pivotal trial of AVXS-101 in SMA Type 1 in the EU in the first half of 2018. Rett Syndrome and Genetic ALS Programs: AveXis anticipates providing an update on the progress and timelines of its early-stage programs in Rett Syndrome and genetic ALS in the first quarter of 2018. Upcoming milestones

Q&A