Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_earningsrelease.htm |
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8-k93017.htm |

Third Quarter 2017 Conference Call
Nasdaq: SELB
November 7, 2017

Safe Harbor / Disclaimer
2
Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation, the
progress of the Phase 1/2 clinical program of SEL-212, the potential of SEL-212 to treat severe gout patients and resolve their debilitating symptoms, the ability of
SVP-Rapamycin to induce immune tolerance against pegsiticase, the ability of SEL-212 to improve acute symptoms during a short induction cycle, the ability of SEL-
212 to be re-administered if severe gout symptoms recur, whether the company will determine an appropriate dose of SEL-212 for a Phase 3, whether the company
will advance to a Phase 3 for SEL-212 at all, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate immune response and create better
therapeutic outcomes, the potential treatment applications for products utilizing the SVP platform in areas such as enzyme therapy, gene therapy, oncology therapy,
vaccines and treatments for allergies and autoimmune diseases, the potential of the company’s two gene therapy product candidates to enable repeat administration,
whether the SEL-212 program informs the development of other product candidates, the contributions of employees, the company's expectations about receiving
additional payments from Spark Therapeutics, Inc. under the license agreement, the sufficiency of the company’s cash, cash equivalents, investments, and restricted
cash and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,”
“predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation
Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but
not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes, the availability and timing
of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results
of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology,
potential delays in enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its product
candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations or licenses, its inability to protect its proprietary
technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating
expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, a significant portion of the company’s total outstanding shares
have recently become eligible to be sold into the market, and other important factors discussed in the “Risk Factors” section of the company’s Quarterly Report on
Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 11, 2017, and in other filings that the company makes with the SEC. In addition,
any forward-looking statements included in this presentation represent the company’s views only as of the date of its publication and should not be relied upon as
representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-looking statements included in this
presentation.

3
IMAGINE IF WE COULD…
1. Effectively treat many more
patients with existing biologics
2. Enable a new generation of novel
non-immunogenic biologics for
rare and serious diseases

Spark Therapeutics License Agreement
4
• December 2016 agreement provides Spark Therapeutics with
exclusive worldwide rights to Selecta's SVP technology for up
to five gene therapy targets
• Initial focus on combination of SVP with Spark’s Hemophilia A
gene therapy
• Among the largest gene therapy and SMID-cap to SMID-cap biotech deals announced to
date
• Subject to the terms of the license agreement, Spark agreed to pay to Selecta:
- $30 million of initial cash payments and investments in Selecta equity; final $7.5 million received on Oct. 31, 2017
- Up to $430 million in milestone payments for each target
- Mid-single to low-double-digit royalties on worldwide annual net sales of any resulting commercialized gene therapy

SEL-212: Advancing a Potential New Treatment Option
for Chronic Severe Gout Patients Toward Phase 3
5
Rare and Serious Disease
• ~160,000 adults with chronic severe gout treated by U.S. rheumatologists
• Debilitating flares and joint-damaging arthritis caused by uric acid deposits; risk of renal and cardiovascular disease
Immunogenicity Barrier
• Uricases are highly effective in breaking down uric acid deposits, but are foreign to the human immune system, causing
immunogenicity that can negate efficacy and present safety risks
Clear Clinical Path
• Serum uric acid level reduction – a robust FDA/EMA primary endpoint for approval – can be seen rapidly upon dosing, easy
to measure, maintenance strongly correlated with low/negative ADA titers
• Adult patient population with rapid enrollment potential
Ownership
• In-licensed pegsiticase in 2014; combined with SVP-Rapamycin to form SEL-212
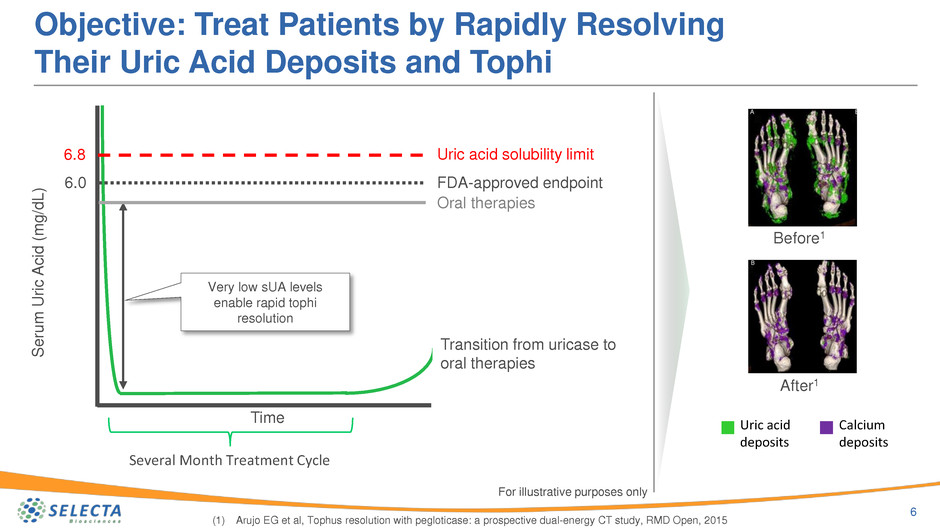
Objective: Treat Patients by Rapidly Resolving
Their Uric Acid Deposits and Tophi
6
(1) Arujo EG et al, Tophus resolution with pegloticase: a prospective dual-energy CT study, RMD Open, 2015
Before1
After1
Uric acid
deposits
Calcium
deposits
6.8
6.0
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
Time
Transition from uricase to
oral therapies
Very low sUA levels
enable rapid tophi
resolution
Several Month Treatment Cycle
Uric acid solubility limit
FDA-approved endpoint
Oral therapies
For illustrative purposes only
Dose (mg/kg):
Advancing SEL-212 Toward Phase 3
7
Phase 1a
Single Dose
0.1 0.2 0.4 0.8 1.2
= Pegsiticase; = SVP-Rapamycin

Dose (mg/kg):
Advancing SEL-212 Toward Phase 3
8
Phase 1a
Single Dose
0.1 0.2 0.4 0.8 1.2
Dose (mg/kg):
Phase 1b
Single Dose
0.4
Dose (mg/kg): 0.100.03 0.15 0.30
= Pegsiticase; = SVP-Rapamycin
0 1 2 3 4 5
0
5 0
1 0 0
0 1 2 3 4 5
0
5 0
1 0 0
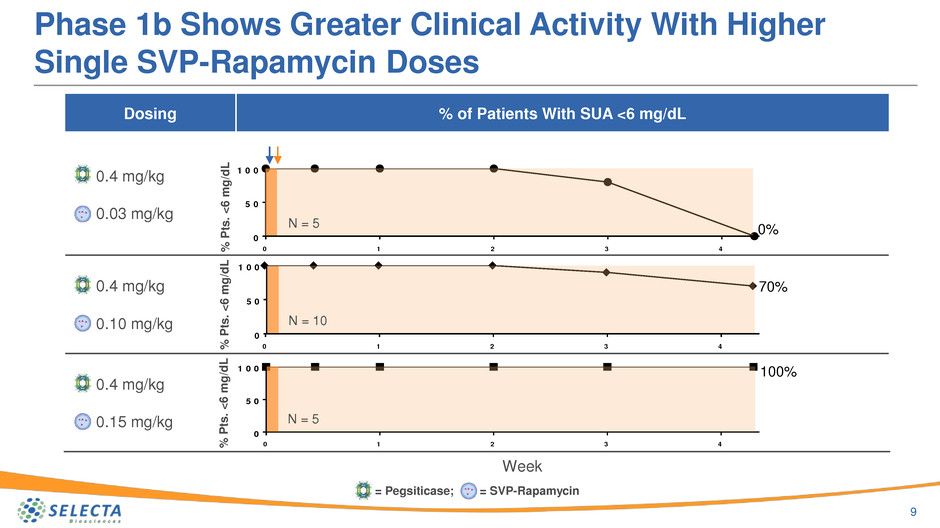
Phase 1b Shows Greater Clinical Activity With Higher
Single SVP-Rapamycin Doses
9
Week
0 1 2 3 4 5
0
5 0
1 0 0
%
Pt
s
.
<
6
m
g
/d
L
%
Pt
s
.
<
6
m
g
/d
L
= Pegsiticase; = SVP-Rapamycin
%
Pt
s
.
<
6
m
g
/d
L
N = 5
N = 10
N = 5
0%
70%
100%
Dosing % of Patients With SUA <6 mg/dL
0.4 mg/kg
0.03 mg/kg
0.4 mg/kg
0.10 mg/kg
0.4 mg/kg
0.15 mg/kg
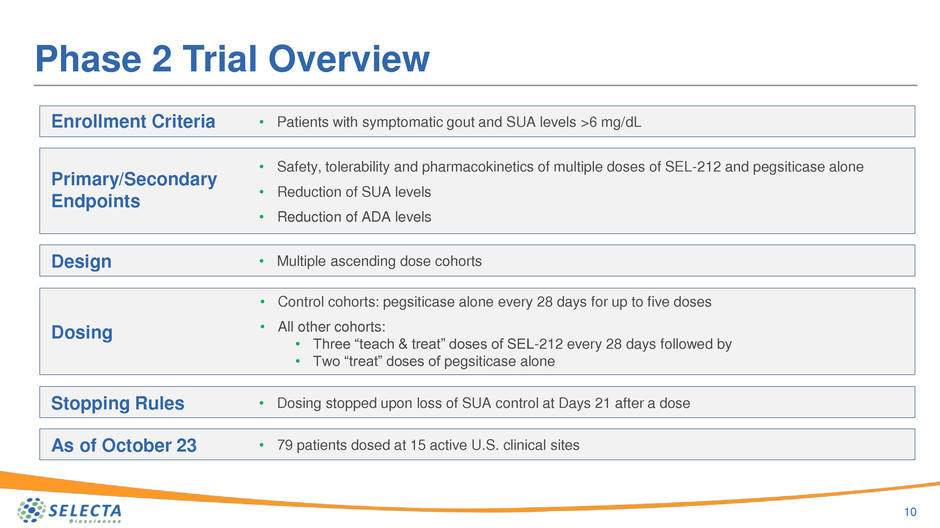
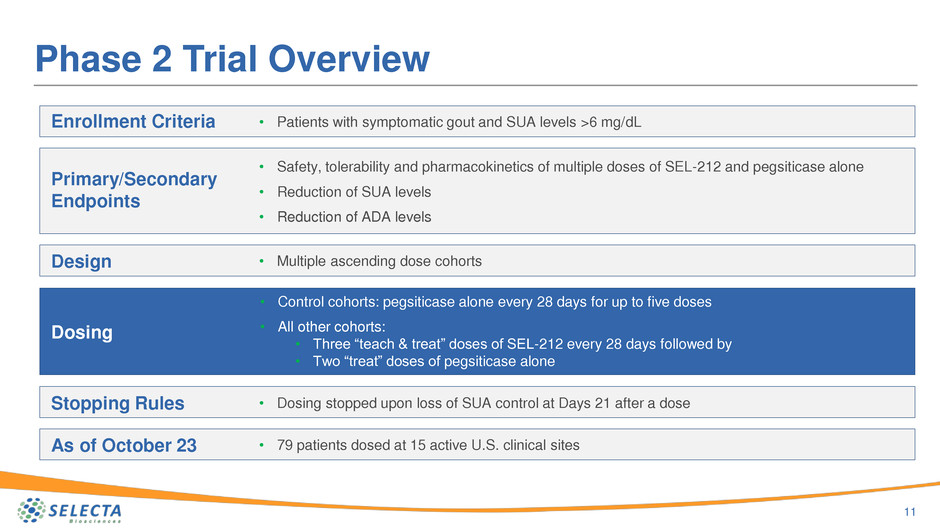
Phase 2 Trial Overview
10
Enrollment Criteria
Primary/Secondary
Endpoints
Design
Dosing
Stopping Rules
As of October 23
• Patients with symptomatic gout and SUA levels >6 mg/dL
• Safety, tolerability and pharmacokinetics of multiple doses of SEL-212 and pegsiticase alone
• Reduction of SUA levels
• Reduction of ADA levels
• Multiple ascending dose cohorts
• Control cohorts: pegsiticase alone every 28 days for up to five doses
• All other cohorts:
• Three “teach & treat” doses of SEL-212 every 28 days followed by
• Two “treat” doses of pegsiticase alone
• Dosing stopped upon loss of SUA control at Days 21 after a dose
• 79 patients dosed at 15 active U.S. clinical sites
Phase 2 Trial Overview
11
Enrollment Criteria
Primary/Secondary
Endpoints
Design
Dosing
Stopping Rules
As of October 23
• Patients with symptomatic gout and SUA levels >6 mg/dL
• Safety, tolerability and pharmacokinetics of multiple doses of SEL-212 and pegsiticase alone
• Reduction of SUA levels
• Reduction of ADA levels
• Multiple ascending dose cohorts
• Control cohorts: pegsiticase alone every 28 days for up to five doses
• All other cohorts:
• Three “teach & treat” doses of SEL-212 every 28 days followed by
• Two “treat” doses of pegsiticase alone
• Dosing stopped upon loss of SUA control at Days 21 after a dose
• 79 patients dosed at 15 active U.S. clinical sites
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0
3
6
9
1 2
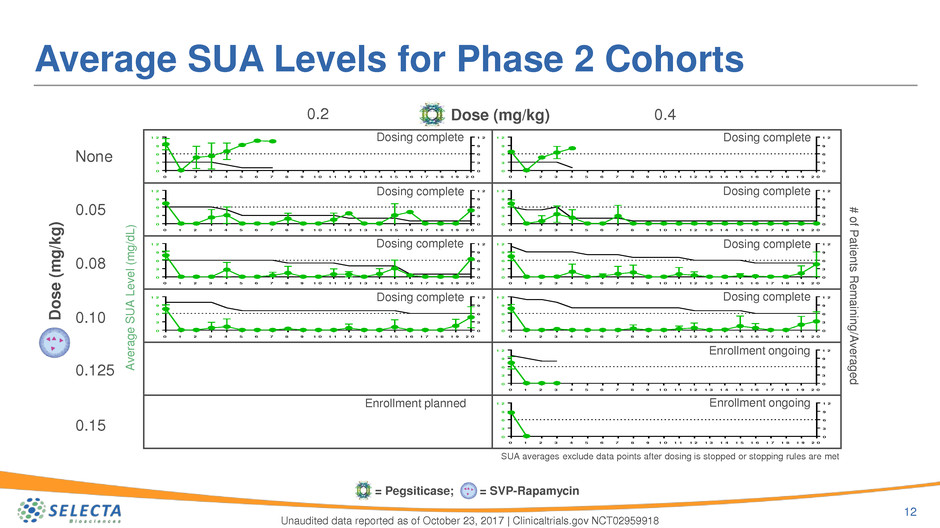
Average SUA Levels for Phase 2 Cohorts
12
SUA averages exclude data points after dosing is stopped or stopping rules are met
0.2
None
0.4Dose (mg/kg)
D
o
s
e
(m
g
/k
g
)
0.05
0.08
0.10
0.125
0.15
#
o
f P
a
tie
n
ts
Re
m
a
in
in
g
/A
v
e
ra
g
e
d
A
v
e
ra
g
e
SUA
L
e
v
e
l
(m
g
/d
L
)
Dosing complete Dosing complete
Dosing completeDosing complete
Dosing complete Dosing complete
Dosing completeDosing complete
Unaudited data reported as of October 23, 2017 | Clinicaltrials.gov NCT02959918
Enrollment ongoing
Enrollment ongoing
= Pegsiticase; = SVP-Rapamycin
Enrollment planned
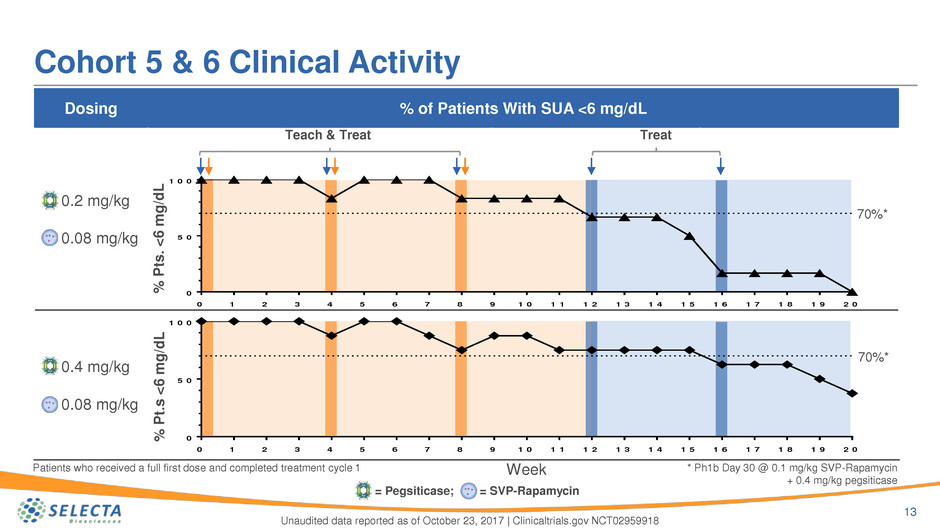
Dosing % of Patients With SUA <6 mg/dL
0.2 mg/kg
0.08 mg/kg
0.4 mg/kg
0.08 mg/kg
Cohort 5 & 6 Clinical Activity
13
Unaudited data reported as of October 23, 2017 | Clinicaltrials.gov NCT02959918
TreatTeach & Treat
Week
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
5 0
1 0 0
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
5
1 0 0
%
P
ts.
<6
m
g
/d
L
%
P
t.
s
<6
mg
/d
L
Patients who received a full first dose and completed treatment cycle 1
70%*
* Ph1b Day 30 @ 0.1 mg/kg SVP-Rapamycin
+ 0.4 mg/kg pegsiticase
70%*
= Pegsiticase; = SVP-Rapamycin
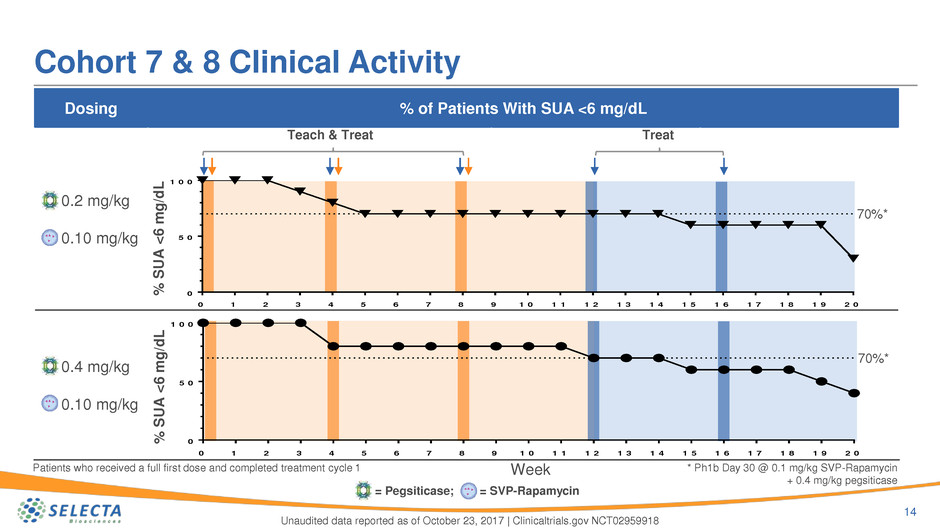
Dosing % of Patients With SUA <6 mg/dL
0.2 mg/kg
0.10 mg/kg
0.4 mg/kg
0.10 mg/kg
Cohort 7 & 8 Clinical Activity
14
Week
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
5 0
1 0 0
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
5 0
1 0 0
Unaudited data reported as of October 23, 2017 | Clinicaltrials.gov NCT02959918
%
S
U
A
<6
m
g
/d
L
%
S
U
A
<6
mg
/d
L
Patients who received a full first dose and completed treatment cycle 1
70%*
* Ph1b Day 30 @ 0.1 mg/kg SVP-Rapamycin
+ 0.4 mg/kg pegsiticase
70%*
TreatTeach & Treat
= Pegsiticase; = SVP-Rapamycin
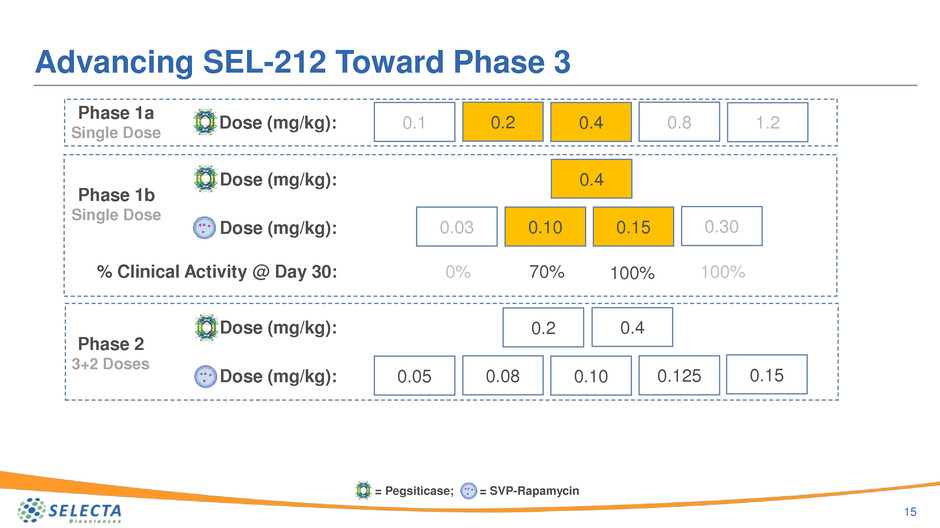
Dose (mg/kg):
Dose (mg/kg):
Dose (mg/kg):
Dose (mg/kg):
Advancing SEL-212 Toward Phase 3
15
Phase 1a
Single Dose
0.1 0.2 0.4 0.8 1.2
Phase 1b
Single Dose
0.4
0.100.03 0.15 0.30
Phase 2
3+2 Doses
0.4
0.080.05 0.10 0.125
0.2
0.15Dose (mg/kg):
% Clinical Activity @ Day 30: 0% 70% 100% 100%
= Pegsiticase; = SVP-Rapamycin
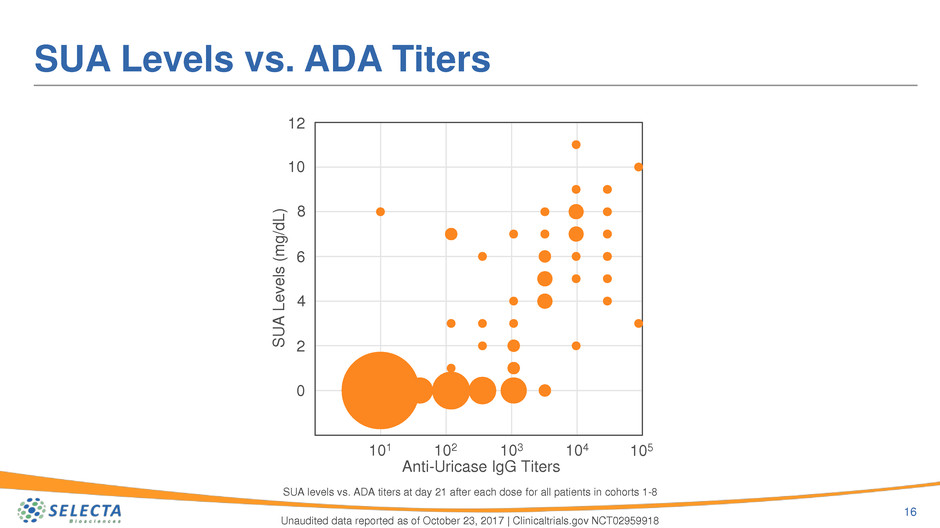
SUA Levels vs. ADA Titers
16
Unaudited data reported as of October 23, 2017 | Clinicaltrials.gov NCT02959918
101 102 103 104 105
0
2
4
6
8
10
12
Anti-Uricase IgG Titers
S
UA
L
e
v
e
ls
(
m
g
/d
L
)
SUA levels vs. ADA titers at day 21 after each dose for all patients in cohorts 1-8

1 1 2 3 4 5
0
2 0
4 0
6 0
Data Continue to Suggest Reduction in Flare
Frequency During SEL-212 Therapy
17
• Data indicate SEL-212 lowers flares initially and over time during treatment
• Urate lowering therapies typically increase the incidence of flares at the beginning of therapy
Unaudited data as of October 23, 2017 | Clinicaltrials.gov NCT02959918
% o
f
P
at
ie
n
ts
w
/Fl
a
re
Pegsiticase
Alone
% of Patients Experiencing Flares by Month
SEL-212
Month

SEL-212 Generally Well Tolerated at Clinically
Active Doses
18
• SEL-212 has been generally well tolerated at clinically active doses following >200
administrations
• SAEs reported in the Phase 2 trial:
- Four were reported not to be or unlikely to be related to study drug:
- Two patients with a history of gall stones experienced cholecystitis (inflammation of gall bladder caused by impacted gall stones);
(reported not to be related to study drug)
- One patient experienced a post-Micturition autonomic response during administration (reported not to be related to study drug)
- One patient experienced peripheral edema (reported as unlikely to be related to study drug)
- Seven infusion reactions:
- Four in cohorts receiving pegsiticase alone or pegsiticase in combination with the lowest dose of SVP-Rapamycin, as anticipated
- Two due to protocol deviations related to dosing errors
- One during a repeat dose of SEL-212 in a higher dose cohort
- Each of these SAEs occurred prior to Selecta’s June data report
- None occurred after treatment period 2
• All SAEs were successfully treated and resolved without further issues
Unaudited data as of October 23, 2017 | Clinicaltrials.gov NCT02959918
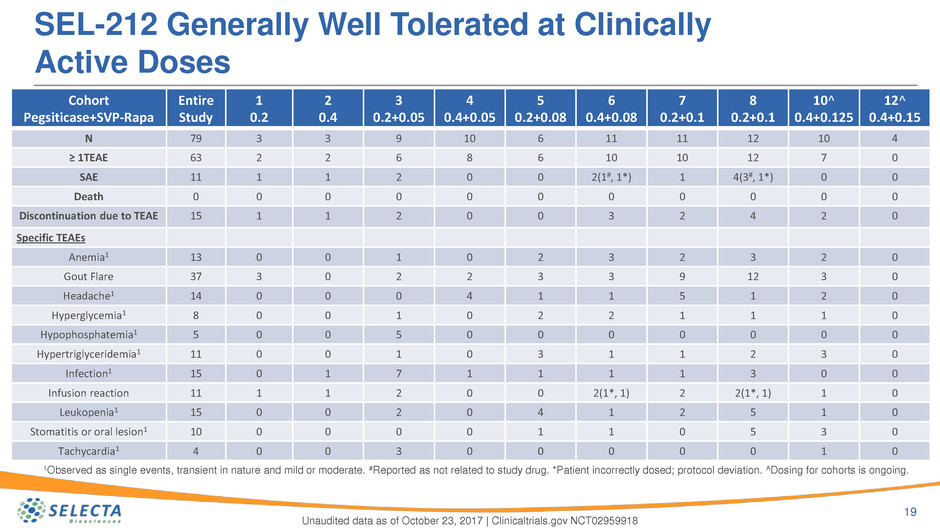
SEL-212 Generally Well Tolerated at Clinically
Active Doses
19
Unaudited data as of October 23, 2017 | Clinicaltrials.gov NCT02959918
Cohort
Pegsiticase+SVP-Rapa
Entire
Study
1
0.2
2
0.4
3
0.2+0.05
4
0.4+0.05
5
0.2+0.08
6
0.4+0.08
7
0.2+0.1
8
0.2+0.1
10^
0.4+0.125
12^
0.4+0.15
N 79 3 3 9 10 6 11 11 12 10 4
≥ 1TEAE 63 2 2 6 8 6 10 10 12 7 0
SAE 11 1 1 2 0 0 2(1#, 1*) 1 4(3#, 1*) 0 0
Death 0 0 0 0 0 0 0 0 0 0 0
Discontinuation due to TEAE 15 1 1 2 0 0 3 2 4 2 0
Specific TEAEs
Anemia1 13 0 0 1 0 2 3 2 3 2 0
Gout Flare 37 3 0 2 2 3 3 9 12 3 0
Headache1 14 0 0 0 4 1 1 5 1 2 0
Hyperglycemia1 8 0 0 1 0 2 2 1 1 1 0
Hypophosphatemia1 5 0 0 5 0 0 0 0 0 0 0
Hypertriglyceridemia1 11 0 0 1 0 3 1 1 2 3 0
Infection1 15 0 1 7 1 1 1 1 3 0 0
Infusion reaction 11 1 1 2 0 0 2(1*, 1) 2 2(1*, 1) 1 0
Leukopenia1 15 0 0 2 0 4 1 2 5 1 0
Stomatitis or oral lesion1 10 0 0 0 0 1 1 0 5 3 0
Tachycardia1 4 0 0 3 0 0 0 0 0 1 0
1Observed as single events, transient in nature and mild or moderate. #Reported as not related to study drug. *Patient incorrectly dosed; protocol deviation. ^Dosing for cohorts is ongoing.
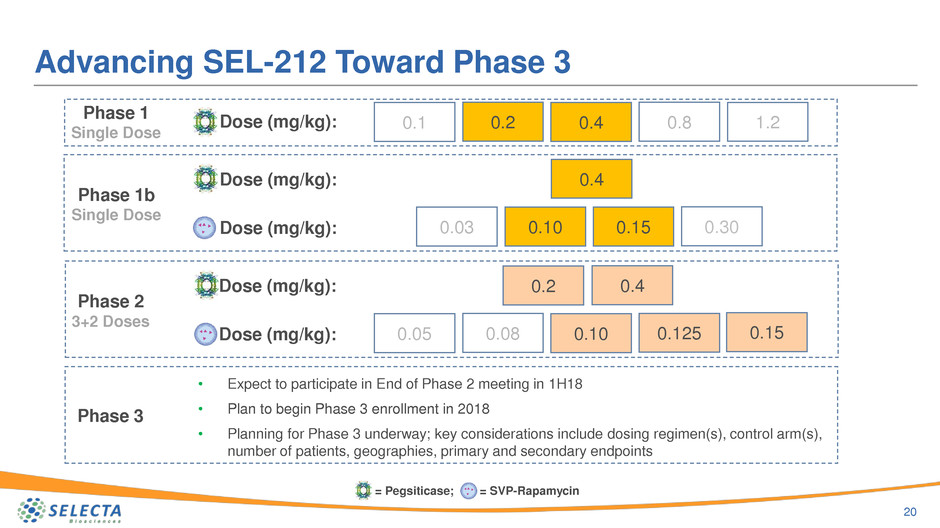
Dose (mg/kg):
Dose (mg/kg):
Dose (mg/kg):
Dose (mg/kg):
Dose (mg/kg):
Advancing SEL-212 Toward Phase 3
20
Phase 1
Single Dose
0.1 0.2 0.4 0.8 1.2
Phase 1b
Single Dose
0.4
0.100.03 0.15 0.30
Phase 2
3+2 Doses
0.4
0.080.05 0.10 0.125
0.2
0.15
Phase 3
• Expect to participate in End of Phase 2 meeting in 1H18
• Plan to begin Phase 3 enrollment in 2018
• Planning for Phase 3 underway; key considerations include dosing regimen(s), control arm(s),
number of patients, geographies, primary and secondary endpoints
= Pegsiticase; = SVP-Rapamycin
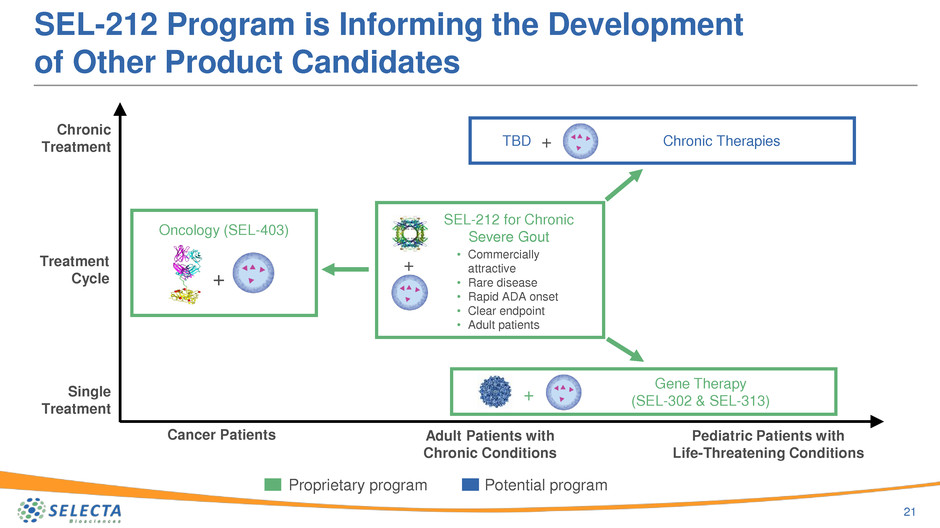
SEL-212 Program is Informing the Development
of Other Product Candidates
21
Chronic Therapies+
Proprietary program Potential program
Single
Treatment
Chronic
Treatment
Adult Patients with
Chronic Conditions
Cancer Patients Pediatric Patients with
Life-Threatening Conditions
Oncology (SEL-403)
Treatment
Cycle +
Gene Therapy
(SEL-302 & SEL-313)+
SEL-212 for Chronic
Severe Gout
+
• Commercially
attractive
• Rare disease
• Rapid ADA onset
• Clear endpoint
• Adult patients
TBD

Important Additions to Selecta’s Management
22
John Leaman, M.D.
Chief Financial Officer &
Head of Corporate Strategy
• 15+ years of financial, operations, corporate strategy and
M&A experience at life sciences companies
• Most recently served as Head of Corporate Development at
InfaCare Pharmaceutical Corp., a specialty pharmaceutical
company that was acquired by Mallinckrodt plc
• Previously was Chief Financial Officer of Medgenics, Inc., a
publicly traded biotech company, and held senior roles at
Shire plc and Devon Park Bioventures, a life sciences VC
firm
• Began career at McKinsey & Company
Stephen Smolinski
Chief Commercial Officer
• Deep commercial expertise and knowledge of the
immunology and rheumatology spaces
• Most recently served as VP and Head of Sanofi/Genzyme’s
North American Rheumatology Business Unit, leading the
development of commercialization plans for KEVZARA®
• Previously served as Group VP of Immunology &
Inflammation, Global Strategic Unit at Sanofi and held
senior commercial roles at Roche-Genentech, Bristol-Myers
Squibb, Johnson & Johnson and Savient Pharmaceuticals,
Inc.
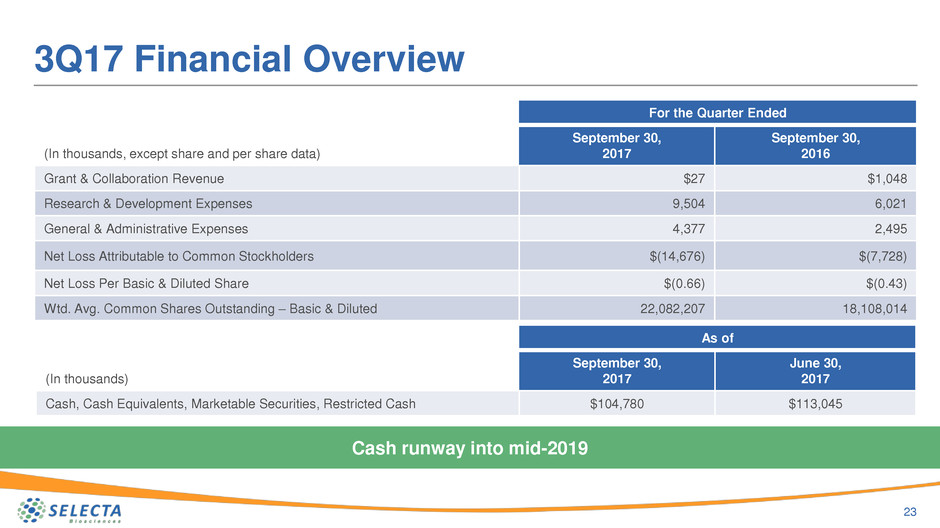
3Q17 Financial Overview
23
For the Quarter Ended
(In thousands, except share and per share data)
September 30,
2017
September 30,
2016
Grant & Collaboration Revenue $27 $1,048
Research & Development Expenses 9,504 6,021
General & Administrative Expenses 4,377 2,495
Net Loss Attributable to Common Stockholders $(14,676) $(7,728)
Net Loss Per Basic & Diluted Share $(0.66) $(0.43)
Wtd. Avg. Common Shares Outstanding – Basic & Diluted 22,082,207 18,108,014
As of
(In thousands)
September 30,
2017
June 30,
2017
Cash, Cash Equivalents, Marketable Securities, Restricted Cash $104,780 $113,045
Cash runway into mid-2019

Thank You
