Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ENDO HEALTH SOLUTIONS INC. | d622983d8k.htm |
| EX-99.1 - EX-99.1 - ENDO HEALTH SOLUTIONS INC. | d622983dex991.htm |
| EX-99.2 - EX-99.2 - ENDO HEALTH SOLUTIONS INC. | d622983dex992.htm |
 Endo Health
Solutions 3Q 2013 Earnings Report
and
A Compelling Combination:
Endo Health Solutions
and
Paladin Labs
November 5, 2013
©2013 Endo Pharmaceuticals Inc. All rights reserved.
Exhibit 99.3 |
 Offer Language
Disclosures ©2013 Endo Pharmaceuticals Inc. All rights
reserved. This
communication
is
not
intended
to
and
does
not
constitute
an
offer
to
sell
or
the
solicitation
of
an
offer
to subscribe for or buy or an invitation to purchase or subscribe for any securities or the
solicitation of any vote
or
approval
in
any
jurisdiction
pursuant
to
the
acquisition
or
otherwise,
nor
shall
there
be
any
sale,
issuance or transfer of securities in any jurisdiction in contravention of applicable law. No
offer of securities shall be made except by means of a prospectus meeting the
requirements of Section 10 of the Securities Act of 1933, as amended.
1 |
 Endo Forward
Looking Statements; Non-GAAP Financial Measures
©2013 Endo Pharmaceuticals Inc. All rights reserved.
2
This presentation contains forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995. Statements including words such as
“believes,” “expects,” “anticipates,” “intends,” “estimates,” “plan,” “will,” “may,” “look
forward,” “intend,” “guidance,” “future” or similar
expressions are forward-looking statements. These forward-looking statements may
include, without limitation, statements regarding the completion of the proposed transaction
and other statements that are not historical facts. Although Endo and Paladin each
believe its forward-looking statements are reasonable, they are subject to important risks and
uncertainties. Those include, without limitation, the failure to receive, on a timely basis or
otherwise, the required approvals by Endo and Paladin shareholders, the Superior Court
of Québec and applicable government and regulatory authorities, the terms of those approvals,
the risk that a condition to closing contemplated by the arrangement agreement may not be
satisfied or waived, the inability to realize expected synergies or cost savings or
difficulties related to the integration of Endo and Paladin operations, the ability of the combined
company to retain and hire key personnel and maintain relationships with customers, suppliers
or other business partners, or other adverse events, changes in applicable laws or
regulations, competition from other pharmaceutical companies, and other risks disclosed in
Endo and Paladin's public filings, any or all of which could cause actual results to differ
materially from future results expressed, projected or implied by the
forward-looking statements. The forward-looking statements in this presentation are qualified by these risk factors. As a
result of these risks and uncertainties, the proposed transaction could be modified,
restructured or not be completed, and actual results and events may differ materially
from the results and events contemplated in these forward-looking statements and from historical
results. Neither Endo nor Paladin assumes any obligation to publicly update any
forward-looking statements, except as may be required under applicable securities
laws, or to comment on expectations of, or statements made by the other party or third parties in respect of
the proposed transaction. These forward-looking statements are not guarantees of
future performance, given that they involve risks and uncertainties. Investors
should not assume that any lack of update to previously issued forward-looking statement constitutes a
reaffirmation of that statement. Continued reliance on forward-looking statements is
at investors’ own risk.
This presentation may refer to non-GAAP financial measures, including adjusted diluted
EPS, that are not prepared in accordance with accounting principles generally accepted
in the United States and that may be different from non-GAAP financial measures used by other
companies. Investors are encouraged to review Endo’s current report on Form 8-K filed
with the SEC for Endo’s reasons for including thos non-GAAP financial measures
in this presentation. No reconciliation to GAAP amounts has been provided because the majority of the
amounts excluded from the comparable GAAP amounts are not currently possible to estimate with
a reasonable degree of accuracy. |
 Today’s
Agenda Review of Third Quarter Accomplishments and 2013 Financial Results
Provide Updated 2013 Financial Guidance
Overview of Acquisition of Paladin Labs
•
Deal Rationale and Terms
•
Overview of Paladin Business
•
Endo Overview
•
New Endo Operating Model
•
Post Transaction Structure -
Domicile as an Irish plc
Q&A
©2013 Endo Pharmaceuticals Inc. All rights reserved.
3 |
 Third Quarter
2013 Progress on Near-Term Priorities Enhance operational focus on organic growth
drivers Pursuing accretive, value-creating M&A opportunities
•
Announced $225M acquisition of Boca Pharmacal
•
Expected to be immediately accretive to adjusted diluted EPS upon close
•
Value creating
Exploring strategic alternatives for HealthTronics
Sharpen R&D focus on near-term opportunities
•
Aveed PDUFA date set for February 28, 2014
•
Completed interim analysis of BEMA Buprenorphine
Strengthen talent and organization
•
Announced new CFO and COO, Pharmaceuticals
©2013 Endo Pharmaceuticals Inc. All rights reserved.
4 |
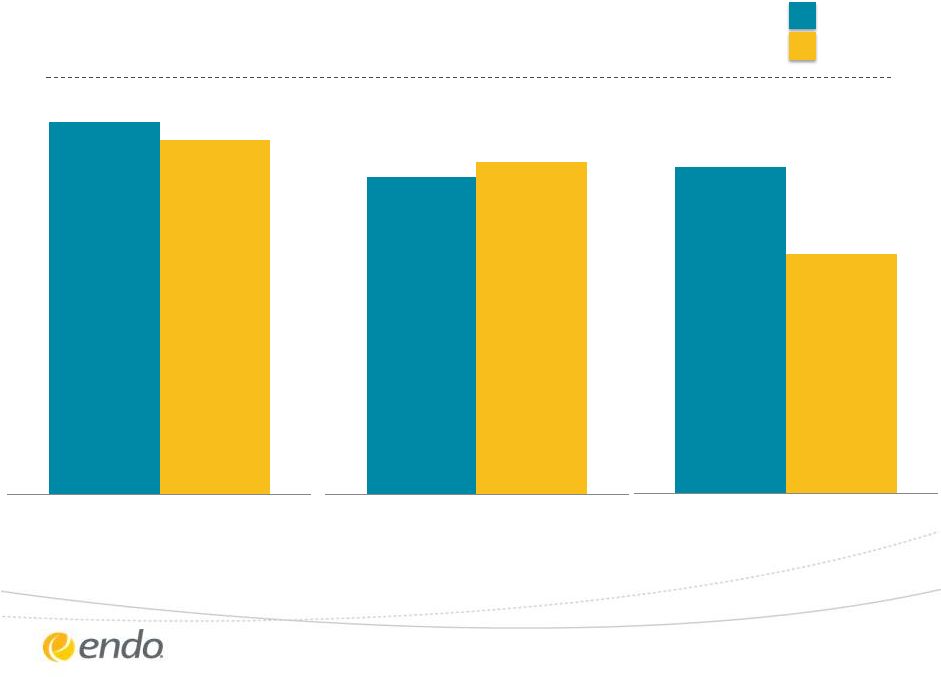 Q3 2013
Financial Performance ©2013 Endo Pharmaceuticals Inc. All rights
reserved. Q3 2012
Q3 2013
5
$1.28
$1.34
Adjusted Diluted EPS
$0.45
$0.33
Reported Diluted (GAAP) EPS
$750
$715
Revenues ($M) |
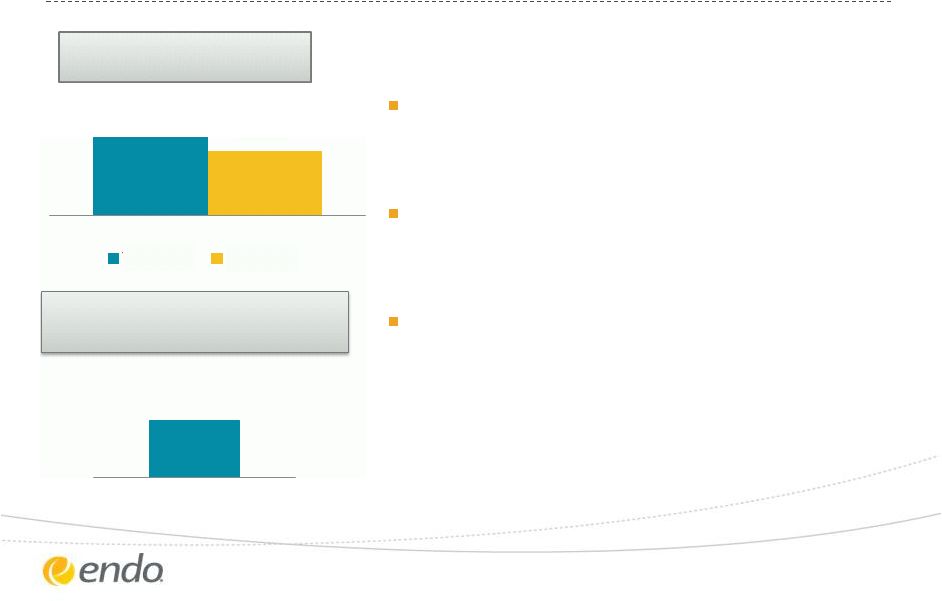 Drive Organic
Growth – Qualitest
©2013 Endo Pharmaceuticals Inc. All rights reserved.
6
13%
11%
Total Revenue Growth vs.
Same Period 2012
YTD 2013
Q3 2013
Contribution of New Products
to YTD Growth
On-track for double-digit growth for
full-year
Demand driven growth led by new
products and oral contraceptives
Planning for efficient integration of
Boca Pharmacal
60% |
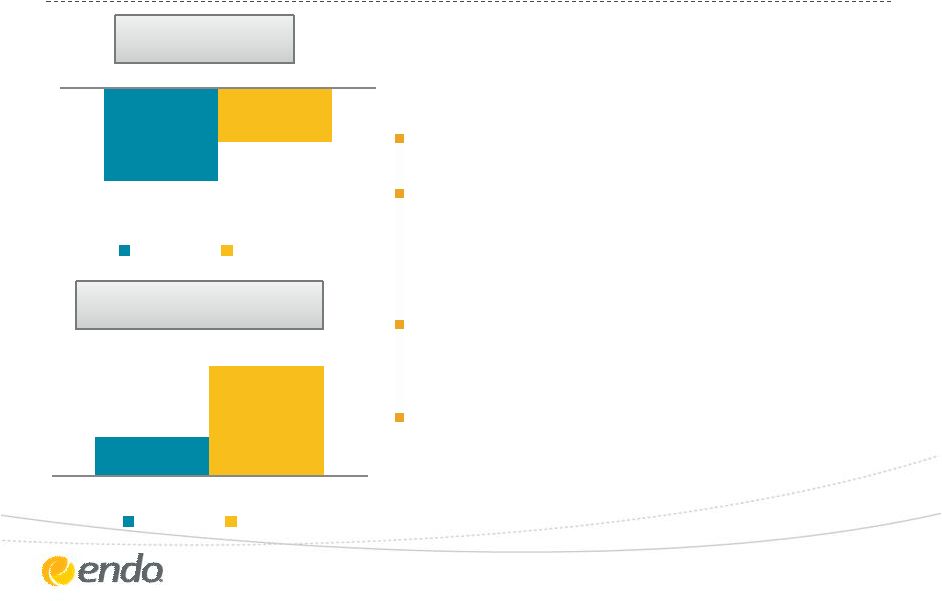
 Drive Organic
Growth – Endo Pharmaceuticals
Managing LIDODERM
®
LOE
Continuing to support ADF
technologies and assert IP covering
OPANA
®
ER
Commercial support for OPANA
®
ER
through specialty promotion
Focused on driving performance of
growth assets: SUPPRELIN
®
LA,
Voltaren
®
Gel and FORTESTA
®
Gel
©2013 Endo Pharmaceuticals Inc. All rights reserved.
7
-12%
10%
All Products
All Products (ex -
LIDODERM/OPANA
ER/Actavis Royalty)
Q3 2013 Revenue Growth
vs. Q3 2012 |
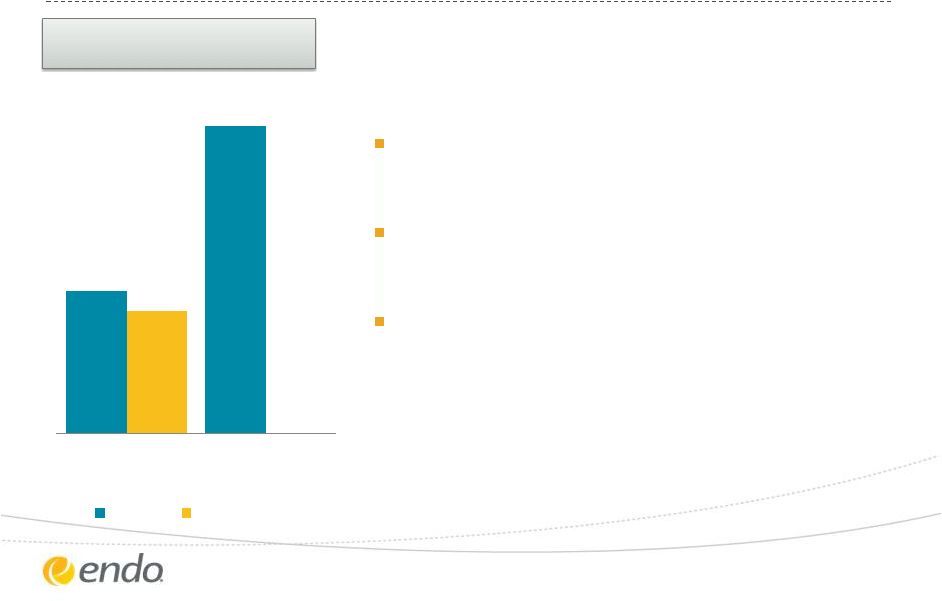
 Drive Organic
Growth – AMS
©2013 Endo Pharmaceuticals Inc. All rights reserved.
8
-3%
-2%
YTD 2013
Q3 2013
Total Revenue Growth
vs. Same Period 2012
1%
4%
YTD 2013
Q3 2013
Total (ex-WH) Revenue Growth
vs. Same Period 2012
Continued strength in Men’s Health
Continued strength in GreenLight
supported by medical education
focused on GOLIATH results
Managing impact of market decline in
Women’s Health
Launch of MiniArc
Pro Single-
Incision Sling System for Treatment of
Female Stress Urinary Incontinence
Key drivers of turnaround efforts:
TM
TM |
 Implement
Lean
Operating
Model
–
Restructuring
Efforts
On-Track
Run-rate by mid-
2014E
2013E
©2013 Endo Pharmaceuticals Inc. All rights reserved.
9
$150
$325
$129
Target
Achieved to date
Reductions Announced Relative
to 2012A ($M)
Key actions taken:
All US headcount reductions
communicated on June 5
External spend reduction under
way
Ongoing focus on gaining
additional efficiencies |
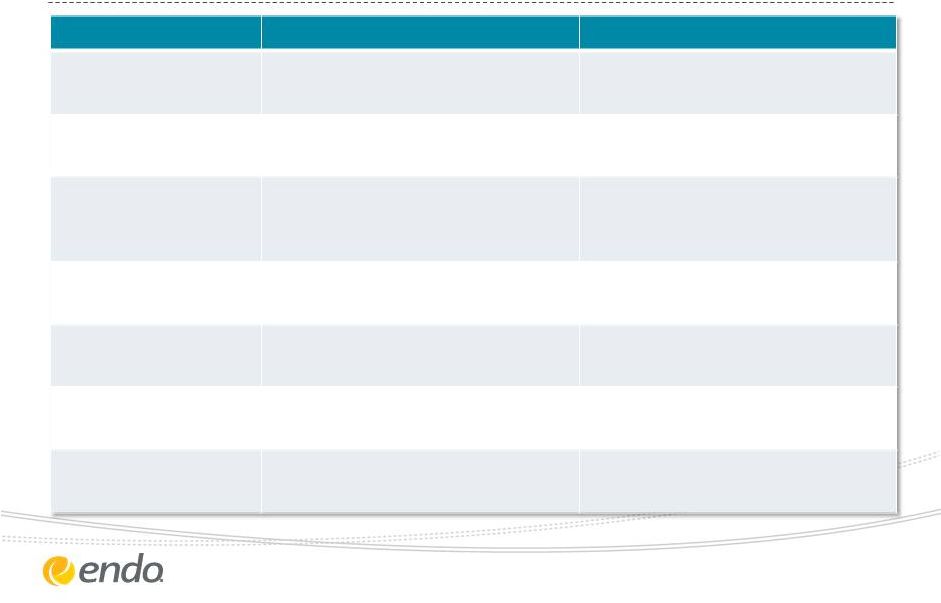 2013 Revised
Financial Guidance ©2013 Endo Pharmaceuticals Inc. All rights
reserved. 10
Measure
Previous 2013 Guidance
Revised 2013 Guidance
Revenues
$2.70B -
$2.80B
$2.75B -
$2.80B
Adjusted Gross
Margin
64% to
66%
Adjusted
Operating
Expenses
Adjusted Diluted EPS
$4.25 to $4.55
$4.60 to $4.75
Adjusted
Effective
Tax Rate
28.5% to 29.5%
28% to 28.5%
Diluted Shares
Outstanding
~117M
~118M
Capital Expenses
~$80M
~$80M
Reduced by approximately $150
million, which represents a 15%
decline versus 2012
Reduced by approximately $150
million, which represents a 15%
decline versus 2012
64% to 66% |
 A Compelling
Combination: Endo Health Solutions
and
Paladin Labs
©2013 Endo Pharmaceuticals Inc. All rights reserved.
|
 Transformational Opportunity
New Endo
©2013 Endo Pharmaceuticals Inc. All rights reserved.
12
Together creating a top tier specialty healthcare organization |
 Accelerates
transformation into a leading specialty healthcare company •
Creates international specialty pharmaceutical business
•
Immediately accretive to adjusted diluted earnings per share
•
Enhances cash flow and earnings sustainability while further diversifying revenues in
pharmaceutical segment
•
Product portfolio and geography complementary across companies
•
Highly diversified revenue streams in Canada
•
Access
to
attractive
emerging
markets
–
South
Africa
and
Latin
America
Focused operating model to maximize organic growth potential and
cash flow
generation supplemented with an active M&A agenda
•
Net debt to adjusted EBITDA 2.4x upon close with rapid de-levering
•
Platform for organic growth with broader options for future M&A
•
Operational and tax synergies resulting in at least $75M after tax savings annually
•
Improved cash conversion leading to enhanced capital structure
Transaction Benefits
©2013 Endo Pharmaceuticals Inc. All rights reserved.
13
beneficial financial platform to facilitate future growth
Domicile as an Irish plc-- |
 Combination
Creates Value Paladin shareholders and employees
•
Monetize outstanding track record of Paladin value creation
•
Direct access to U.S. market and opportunity to accelerate growth through a larger
platform
•
Retain Paladin name for Canadian business
Strategic value for Endo
•
Expanded geographic footprint
•
Diversify revenue base
•
Advantaged platform for growth with future acquisitions
Financially sound
•
Stable and growing cash flows
•
Immediately accretive to adjusted EPS
•
Enables inversion transaction
•
Combined company steady state tax rate of approximately 20%
©2013 Endo Pharmaceuticals Inc. All rights reserved.
14 |
 Proposed
Transaction Terms $77
(CAD)
per
share
represents
a
20%
premium
to
Paladin
Labs’
closing
price
of
$63.91 as of
November 4, 2013 and values the transaction at approximately $1.6 billion (USD)
Paladin shareholders will receive:
•
1.6331 shares of New Endo for each share of Paladin Labs owned
•
$1.16 (CAD) in cash for each share of Paladin Labs Paladin owned
–
Cash consideration to be received by Paladin Labs shareholders will be
increased if Endo’s average share price declines more than 7% during a pre-
specified period
•
1 share of Knight Therapeutics for each share of Paladin Labs owned
Pro Forma ownership
•
Endo Shareholders to own 77.5% of New Endo
•
Paladin Shareholders to own 22.5% of New Endo
Endo has secured committed financing that will be used to refinance certain elements of the
company’s existing indebtedness and the early repurchase of our convertible notes
due April 2015, subject to market conditions
©2013 Endo Pharmaceuticals Inc. All rights reserved.
15 |
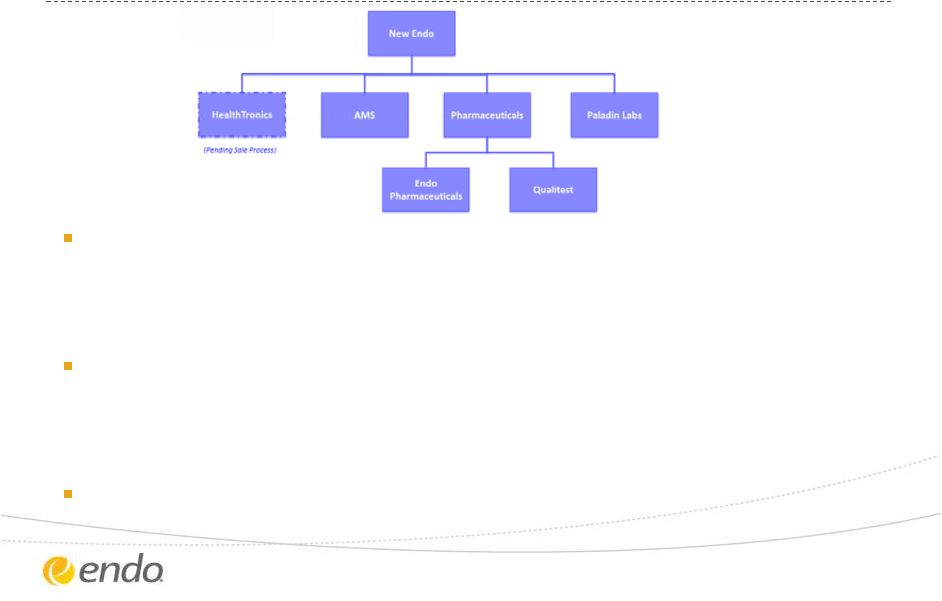 New Endo
Structure and Trading Endo management to lead combined company
•
Jonathan
Ross
Goodman
will
serve
as
an
advisor
to
the
Endo
Board
of
Directors
Existing management team will continue to operate Paladin
•
Mark Beaudet will continue as President, Paladin reporting to Rajiv De
Silva
New Endo will trade on NASDAQ
©2013 Endo Pharmaceuticals Inc. All rights reserved.
16 |
 Overview of
Paladin Labs Paladin Labs is a specialty pharmaceutical company focused on acquiring or
in-licensing innovative pharmaceutical products for the Canadian and select
international markets
Strong diversified revenue growth
•
5 year revenue growth CAGR of 27%
•
Proven partner for the Canadian market
•
Demonstrated track record of profitable growth
•
Financial strength to drive expansion
Strong, near-term pipeline for growth
•
Solid late stage development pipeline including multiple products currently under
regulatory review
•
Zohydro
ER, Seralaxin
•
Over 15 products in development
©2013 Endo Pharmaceuticals Inc. All rights reserved.
17
TM |
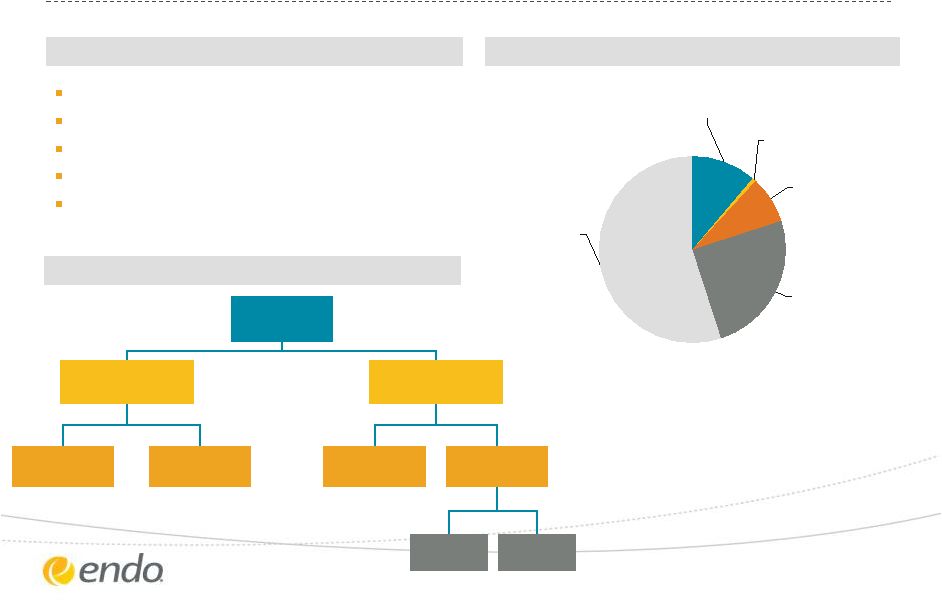 Paladin Labs
Structure Company Information
Structure
Adjusted Revenue Mix (prop. consolidation)
Canada Rx, 55%
Litha, 25%
Base Paladin Intl,
8%
Isodiagnostika,
1%
Canada OTC, 11%
1996
Montreal, Canada
Jonathan
Ross
Goodman
Mark Beaudet
127
©2013 Endo Pharmaceuticals Inc. All rights reserved.
18
Paladin
Labs
Base Paladin
International
platforms
Canada
Other
Litha
Latin
America
Mexico
Brazil
Founded:
Headquarters:
Chairman:
Interim CEO:
Employees:
Source: Paladin Labs Inc. management presentation |
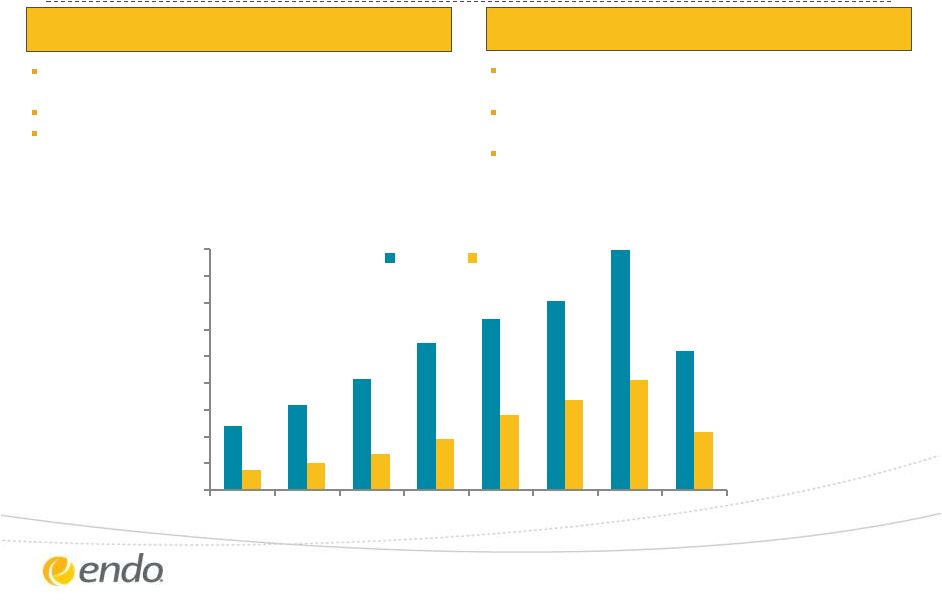 Paladin’s
Year-Over-Year Consecutive Growth ($ CAD) REVENUE
EBITDA
*Adjusted refers to the proportional consolidation of Litha’s results effective July 2,
2012 and Paladin Mexico’s results effective January 1, 2013 Source:
Paladin Labs Inc. Q2 2013 Investor Relations Presentation YTD
$M CAD
©2013 Endo Pharmaceuticals Inc. All rights reserved.
19
0
20
40
60
80
100
120
140
160
180
2006
2007
2008
2009
2010
2011
2012*
2013*
Revenue
EBITDA
Adjusted* revenue for 2012 of $179.0 million, up 27%
over 2011
Guidance for 2013 of $190 million adjusted* revenues
Adjusted* revenues for Q2 2013 of $52.3 million, up
41% versus Q2 2012
9 consecutive years of record EBITDA with EBITDA
representing 39% of revenues in 2012
Adjusted* EBITDA for 2012 of $79.0 million for 2012,
up 17% versus 2011
Adjusted* EBITDA for Q2 2013 of $22.7, up 32% versus
Q2 2012 |
 Aspire to be
a leading specialty healthcare company Continue our commitment to serving our patients
and customers
Participate in specialty areas offering above average growth
and favorable margins
Transform our operating model to maximize growth
potential and cash flow generation
Endo Overview
©2013 Endo Pharmaceuticals Inc. All rights reserved.
20
Allows Endo to maximize shareholder value by adapting to market
dynamics and portfolio changes |
 Recent Endo
Restructuring Actions ©2013 Endo Pharmaceuticals Inc. All rights
reserved. 21
•
Endo Pharmaceuticals
•
Qualitest
•
AMS
Drive organic growth through our core businesses
Implement lean operating model
Pursue accretive, value-creating acquisition opportunities
Explore strategic alternatives for HealthTronics
Sharpen R&D focus on near-term opportunities
Strengthen talent and organization |
 New Endo
Operating Model Lean, efficient operating model
Focused, de-risked R&D
Streamlined and diversified organization with quick decision making
Performance metrics aligned with shareholder interests
Agnostic on therapeutic areas, but with focus in specialty areas
M&A as an important component of building and growing the business long term
©2013 Endo Pharmaceuticals Inc. All rights reserved.
22 |
 New Endo
International Strategy Utilize new operating structure to drive international
expansion and growth
Focus on emerging markets with above average growth
characteristics
Invest in areas with growing healthcare infrastructure and
expanding economies
•
Proven local operators, competency in core business functions
•
International management expertise
Expand presence in markets with favorable reimbursement
environment or a large cash pay component
©2013 Endo Pharmaceuticals Inc. All rights reserved.
23 |
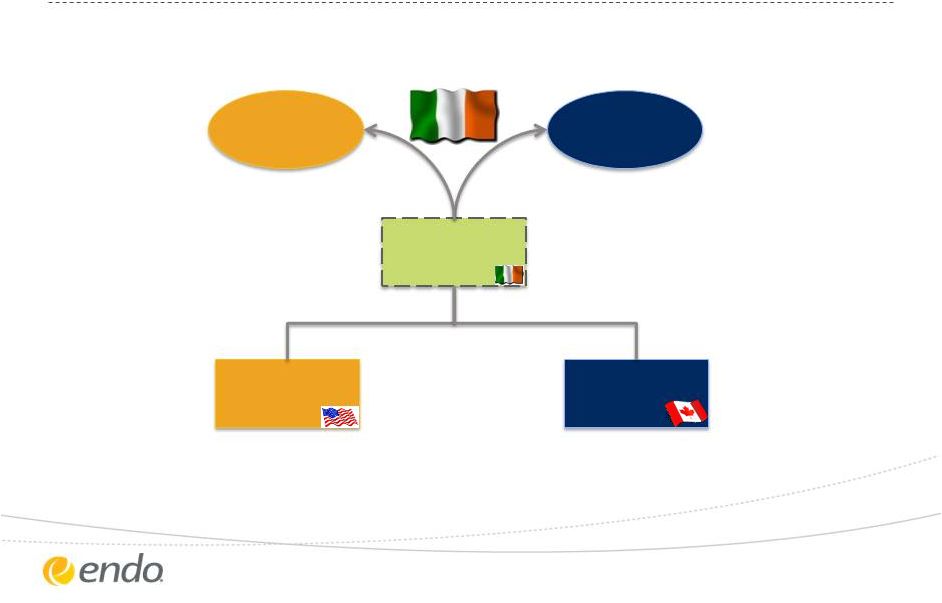 Corporate
Redomicile Endo
Shareholders
Paladin Labs
Shareholders
Paladin Labs
Shareholders
Endo
(& Subs)
Paladin Labs
(& Subs)
Paladin Labs
(& Subs)
Irish New Endo
77.5%
22.5%
24
©2013 Endo Pharmaceuticals Inc. All rights reserved.
|
 New Endo
- Capital Structure
Proforma capital structure will continue to support the long
term strategy of Endo
Secured committed financing that will be used to refinance
certain elements of existing indebtedness and the early
repurchase of our convertible notes due April 2015
Proforma leverage ratio is expected to be in line with
current levels
Immediate accretion and improved cash flow conversion will
lead to rapid de-levering
©2013 Endo Pharmaceuticals Inc. All rights reserved.
25 |
 Approvals and
Timing Standard U.S. merger process for Endo
•
Proxy/registration statement
•
Majority approval by Endo shareholders
2/3 vote required by Paladin Labs shareholders
•
Customary regulatory approvals, including Investment Canada
•
Shareholders representing 34% of Paladin Labs outstanding shares
have agreed to vote in favor of deal
Expected closing 1
st
half 2014
Transaction not currently expected to be taxable to U.S.
shareholders of Endo, as structured, but will be taxable for
shareholders of Paladin
©2013 Endo Pharmaceuticals Inc. All rights reserved.
26 |
 Accelerates
transformation into a leading specialty healthcare company •
Creates international specialty pharmaceutical business
•
Immediately accretive to adjusted diluted earnings per share
•
Enhances cash flow and earnings sustainability while further diversifying revenues in
pharmaceutical segment
•
Product portfolio and geography complementary across companies
•
Highly diversified revenue streams in Canada
•
Access
to
attractive
emerging
markets
–
South
Africa
and
Latin
America
Focused operating model to maximize organic growth potential and
cash flow
generation supplemented with an active M&A agenda
•
Net debt to adjusted EBITDA 2.4x upon close with rapid de-levering
•
Platform for organic growth with broader options for future M&A
•
Operational and tax synergies resulting in at least $75M after tax savings annually
•
Improved cash conversion leading to enhanced capital structure
Transaction Benefits
©2013 Endo Pharmaceuticals Inc. All rights reserved
27
beneficial financial platform to facilitate future growth
Domicile as an Irish plc– |
 Endo Health
Solutions 3Q 2013 Earnings Report
and
A Compelling Combination:
Endo Health Solutions
and
Paladin Labs
November 5, 2013
©2013 Endo Pharmaceuticals Inc. All rights reserved.
|
 Appendix
29
©2013 Endo Pharmaceuticals Inc. All rights reserved.
|
 Reconciliation
of Non-GAAP Measures ©2013 Endo Pharmaceuticals Inc. All
rights reserved. Three Months Ended September 30, 2013
(unaudited) Actual
Reported
(GAAP)
Adjustments
Non-GAAP
Adjusted
REVENUES
$
714,954
$
—
$
714,954
COSTS AND EXPENSES:
Cost of revenues
287,970
(46,105)
(1)
241,865
Selling, general and administrative
199,719
(30,069)
(2)
169,650
Research and development
38,080
(10,005)
(3)
28,075
Litigation-related and other contingencies
30,895
(30,895)
(4)
—
Asset impairment charges
38,807
(38,807)
(5)
—
Acquisition-related and integration items, net
2,207
(2,207)
(6)
—
OPERATING INCOME
$
117,276
$
158,088
$
275,364
INTEREST EXPENSE, NET
43,150
(5,704)
(7)
37,446
OTHER (INCOME) EXPENSE, NET
(17,292)
17,293
(8)
1
INCOME BEFORE INCOME TAX
$
91,418
$
146,499
$
237,917
INCOME TAX
36,803
26,008
(9)
62,811
CONSOLIDATED NET INCOME
$
54,615
$
120,491
$
175,106
Less: Net income attributable to noncontrolling interests
14,392
—
14,392
NET INCOME ATTRIBUTABLE TO ENDO HEALTH
SOLUTIONS INC.
$
40,223
$
120,491
$
160,714
DILUTED EARNINGS PER SHARE
$
0.33
$
1.34
DILUTED WEIGHTED AVERAGE SHARES
120,261
120,261
30
Notes to reconciliation of our GAAP statements of operations to our
adjusted statements of operations: (1) To exclude amortization of
commercial intangible assets related to marketed products of $44,105 and accruals for
milestone payments to partners of $2,000.
(2)
To exclude certain separation benefits and other costs incurred in
connection with continued efforts to enhance the company's
operations of $13,616, amortization of customer relationships of $2,748 and mesh litigation-related defense
costs of $13,705.
(3)
To exclude milestone payments to partners of $1,092 and certain
separation benefits and other costs incurred in connection with
continued efforts to enhance the company's operations of $8,913.
(4)
To exclude the net impact of accruals related to mesh -related
product liability. (5)
To exclude asset impairment charges.
(6)
To exclude integration costs of $2,144 and a loss of $63 recorded to
reflect the change in fair value of the contingent consideration
associated with the Qualitest acquisition. (7) To exclude additional interest
expense as a result of the prior adoption of ASC 470 -20.
(8)
To exclude $(14,628) related to patent litigation settlement income and
$(2,665) for a gain on sale of business. To reflect the
cash tax savings results from our acquisitions and the tax effect of the pre -tax adjustments above at applicable tax
rates.
|
 Reconciliation
of Non-GAAP Measures 31
©2013 Endo Pharmaceuticals Inc. All rights reserved.
Three Months Ended September 30, 2012 (unaudited)
Actual
Reported
(GAAP)
Adjustments
Non
-GAAP
Adjusted
REVENUES
$
750,482
$
—
$
750,482
COSTS AND EXPENSES:
Cost of revenues
294,267
(52,762)
(1)
241,505
Selling, general and administrative
210,446
(10,480)
(2)
199,966
Research and development
48,952
(6,421)
(3)
42,531
Patent litigation settlement, net
(46,238)
46,238
(4)
—
Litigation-related and other contingencies
82,600
(82,600)
(5)
—
Asset impairment charges
11,163
(11,163)
(6)
—
Acquisition-related and integration items, net
5,776
(5,776
(7)
—
OPERATING INCOME
$
143,516
$
122,964
$
266,480
INTEREST EXPENSE, NET
45,505
(8)
40,296
LOSS ON EXTINGUISHMENT OF DEBT
1,789
(1,789)
(9)
—
OTHER INCOME, NET
(250)
—
(250)
INCOME BEFORE INCOME TAX
$
96,472
$
129,962
$
226,434
INCOME TAX
28,287
30,678
(10)
58,965
CONSOLIDATED NET INCOME
$
68,185
$
99,284
$
167,469
Less: Net income attributable to noncontrolling interests
14,376
—
14,376
NET INCOME ATTRIBUTABLE TO ENDO HEALTH
SOLUTIONS INC.
$
53,809
$
99,284
$
153,093
DILUTED EARNINGS PER SHARE
$
0.45
$
1.28
DILUTED WEIGHTED AVERAGE SHARES
119,579
119,579
Notes to reconciliation of our GAAP statements of operations to our
adjusted statements of operations: (1) To exclude amortization of
commercial intangible assets related to marketed products of $55,999, net
milestone payments and receipts of $1,440, an adjustment to the accrual
for the payment to Impax related to sales of OPANA ER of
$(6,000) and certain separation benefits and other costs incurred in connection
with continued efforts to enhance the company’s operations of
$1,323. (2)
To exclude certain separation benefits and other costs incurred in
connection with continued efforts to enhance the company’s
operations of $7,744 and amortization of customer relationships of $2,736.
(3)
To exclude milestone payments to partners of $3,898 and certain
separation benefits and other costs incurred in connection with
continued efforts to enhance the company’s operations of $2,523.
(4)
To exclude the net impact of the Actavis (Watson) litigation
settlement. (5)
To exclude the net impact of accruals for litigation-related and
other contingencies. (6)
To exclude asset impairment charges.
(7)
To exclude acquisition-related and integration costs of $5,680 and
a loss of $96 recorded to reflect the change in fair value of
the contingent consideration associated with the Qualitest Pharmaceuticals acquisition.
(8)
To exclude additional interest expense as a result of the prior
adoption of ASC 470-20.
(9)
To exclude the unamortized debt issuance costs written off and recorded
as a loss on extinguishment of debt upon our third quarter 2012
prepayments on our Term Loan indebtedness. (10) To reflect the cash tax savings
results from our acquisitions and the tax effect of the pre-tax adjustments above at
applicable tax rates.
(5,209) |
 Reconciliation
of Non-GAAP Measures 32
©2013 Endo Pharmaceuticals Inc. All rights reserved.
Nine Months Ended September 30, 2013 (unaudited)
Actual
Reported
(GAAP)
Adjustments
Non
-GAAP
Adjusted
REVENUES
$
2,189,982
$
—
$
2,189,982
COSTS AND EXPENSES:
Cost of revenues
883,063
(149,045)
(1)
734,018
Selling, general and administrative
689,436
(117,485)
(2)
571,951
Research and development
113,740
(19,187)
(3)
94,553
Litigation-related and other contingencies
159,098
(159,098)
(4)
—
Asset
impairment charges
46,994
(46,994)
(5)
—
Acquisition-related and integration items, net
6,165
(6,165)
(6)
—
OPERATING INCOME
$
291,486
$
497,974
$
789,460
INTEREST EXPENSE, NET
129,939
(16,816)
(7)
113,123
LOSS ON EXTINGUISHMENT OF DEBT
11,312
(11,312)
(8)
—
OTHER (INCOME) EXPENSE, NET
(51,873)
54,113
(9)
2,240
INCOME BEFORE INCOME TAX
$
202,108
$
471,989
$
674,097
INCOME TAX
72,779
112,260
(10)
185,039
CONSOLIDATED NET INCOME
$
129,329
$
359,729
$
489,058
Less: Net income attributable to noncontrolling interests
38,758
—
38,758
NET INCOME ATTRIBUTABLE TO ENDO HEALTH
SOLUTIONS INC.
$
90,571
$
359,729
$
450,300
DILUTED EARNINGS PER SHARE
$
0.77
$
3.85
DILUTED WEIGHTED AVERAGE SHARES
116,890
116,890
Notes to reconciliation of our GAAP statements of operations to our
adjusted statements of operations: (1) To exclude amortization of
commercial intangible assets related to marketed products of $140,355, certain separation
benefits and other costs incurred in connection with continued efforts
to enhance the company's operations of $2,690 and accruals for
milestone payments to partners of $6,000. (2) To exclude certain separation
benefits and other costs incurred in connection with continued efforts to enhance the company's
operations of $74,363, amortization of customer relationships of
$8,251and mesh litigation-related defense costs of $34,871.
(3)
To exclude milestone payments to partners of $5,064 and certain
separation benefits and other costs incurred in connection with
continued efforts to enhance the company's operations of $14,123 .
(4)
To exclude the net impact of accruals primarily for mesh-related
product liability. (5)
To exclude asset impairment charges.
(6)
To exclude integration costs of $6,002 and a loss of $163
recorded to reflect the change in fair value of the contingent
consideration associated with the Qualitest acquisition.
(7)
To exclude additional interest expense as a result of the prior
adoption of ASC 470-20.
(8)
To exclude the unamortized debt issuance costs written off and recorded
as a loss on extinguishment of debt upon our March 2013
prepayment on our Term Loan indebtedness as well as upon the amendment and restatement of our existing credit
facility.
(9)
To exclude $(50,400) related to patent litigation settlement
income, $(2,665) for a gain on sale of business and other income
of $(1,048). To reflect the cash tax savings results
from our acquisitions and the tax effect of the pre-tax adjustments above at applicable tax rates. |
 Reconciliation
of Non-GAAP Measures 33
©2013 Endo Pharmaceuticals Inc. All rights reserved.
Nine Months Ended September 30, 2012 (unaudited)
Actual
Reported
(GAAP)
Adjustments
Non
-GAAP
Adjusted
REVENUES
$
2,226,303
$
—
$
2,226,303
COSTS AND EXPENSES:
Cost of revenues
953,657
(272,857)
(1)
680,800
Selling, general and administrative
698,522
(30,044)
(2)
668,478
Research and development
183,067
(56,201)
(3)
126,866
Patent litigation settlement, net
85,123
(85,123)
(4)
—
Litigation-related and other contingencies
82,600
(82,600)
(5)
—
Asset impairment charges
54,163
(54,163)
(6)
—
Acquisition-related and integration items, net
16,580
(16,580)
(7)
—
OPERATING INCOME
$
152,591
$
597,568
$
750,159
INTEREST EXPENSE, NET
138,386
(15,354)
(8)
123,032
LOSS ON EXTINGUISHMENT OF DEBT
7,215
(7,215)
(9)
—
OTHER EXPENSE, NET
498
(300
(10)
198
INCOME BEFORE INCOME TAX
$
6,492
$
620,437
$
626,929
INCOME TAX
(9,263)
182,820
(11)
173,557
CONSOLIDATED NET INCOME
$
15,755
$
437,617
$
453,372
Less: Net income attributable to noncontrolling interests
39,826
—
39,826
NET (LOSS) INCOME ATTRIBUTABLE TO ENDO HEALTH
SOLUTIONS INC.
$
(24,071)
$
437,617
$
413,546
DILUTED (LOSS) EARNINGS PER SHARE
$
(0.21)
$
3.42
DILUTED WEIGHTED AVERAGE SHARES
116,688
121,083
Notes to reconciliation of our GAAP statements of operations to our
adjusted statements of operations: (1) To exclude amortization of
commercial intangible assets related to marketed products of $162,414, the impact of
inventory step-up recorded as part of acquisition accounting of
$880, the accrual for the payment to Impax related to sales of
OPANA ER of $104,000, net milestone payments to partners of $2,927 and certain separation benefits and other
costs incurred in connection with continued efforts to enhance the
company’s operations of $2,636. (2) To exclude certain separation
benefits and other costs incurred in connection with continued efforts to enhance the
company’s operations of $21,799 and amortization of customer
relationships of $8,245. (3)
To exclude milestone payments to partners of
$53,678 and certain separation benefits and other costs incurred in
connection with continued efforts to enhance the company’s
operations of $2,523. (4)
To exclude the net impact of the Actavis (Watson)
litigation settlement. (5)
To exclude the net impact of accruals for
litigation-related and other contingencies. (6) To exclude asset impairment
charges. (7)
To exclude acquisition-related and integration costs of $16,552
and a loss of $28 recorded to reflect the change in fair value
of the contingent consideration associated with the Qualitest Pharmaceuticals acquisition.
(8)
To exclude additional interest expense as a result of the prior
adoption of ASC 470-20.
(9)
To exclude the unamortized debt issuance costs written off and
recorded as a loss on extinguishment of debt upon our 2012
prepayments on our Term Loan indebtedness. (10) To exclude milestone and upfront
payments to partners. To reflect the cash tax savings results
from our acquisitions and the tax effect of the pre-tax adjustments above at applicable tax rates. |
 Reconciliation
of Non-GAAP Measures 34
©2013 Endo Pharmaceuticals Inc. All rights reserved.
Endo Health Solutions Inc. Net Revenues (unaudited) (in thousands)
Three Months Ended September 30,
Percent
Growth
Nine Months Ended September 30,
Percent
Growth
2013
2012
2013
2012
Endo Pharmaceuticals:
LIDODERM®
$
149,946
$
238,282
(37)%
$
566,626
$
676,302
(16)%
OPANA®
ER
59,936
62,232
(4)%
174,214
236,731
(26)%
Voltaren®
Gel
45,044
35,483
27%
123,937
79,173
57%
PERCOCET®
26,250
24,209
8%
78,818
73,413
7%
FROVA®
16,027
15,706
2%
44,116
45,352
(3)%
FORTESTA®
Gel
15,025
8,823
70%
47,156
21,526
119%
SUPPRELIN®
LA
14,105
14,534
(3)%
44,128
42,777
3%
VANTAS®
3,039
4,114
(26)%
10,013
12,352
(19)%
VALSTAR®
6,024
8,394
(28)%
16,327
20,717
(21)%
Other Branded Products
508
933
(46)%
1,833
1,788
3%
Royalty and Other
30,232
3,935
668%
32,204
12,874
150%
Total Endo Pharmaceuticals
$
366,136
$
416,645
(12)%
$
1,139,372
$
1,223,005
(7)%
Total Qualitest
$
183,939
$
166,070
11%
$
532,722
$
471,310
13%
American Medical Systems:
Men's Health
61,536
58,316
6%
197,185
192,728
2%
Women's Health
24,200
29,399
(18)%
80,470
95,763
(16)%
BPH Therapy
25,508
25,589
—%
82,212
83,110
(1)%
Total AMS
111,244
113,304
(2)%
359,867
371,601
(3)%
HealthTronics
53,635
54,463
(2)%
158,021
160,387
(1)%
Total Revenue
714,954
750,482
(5)%
2,189,982
2,226,303
(2)% |
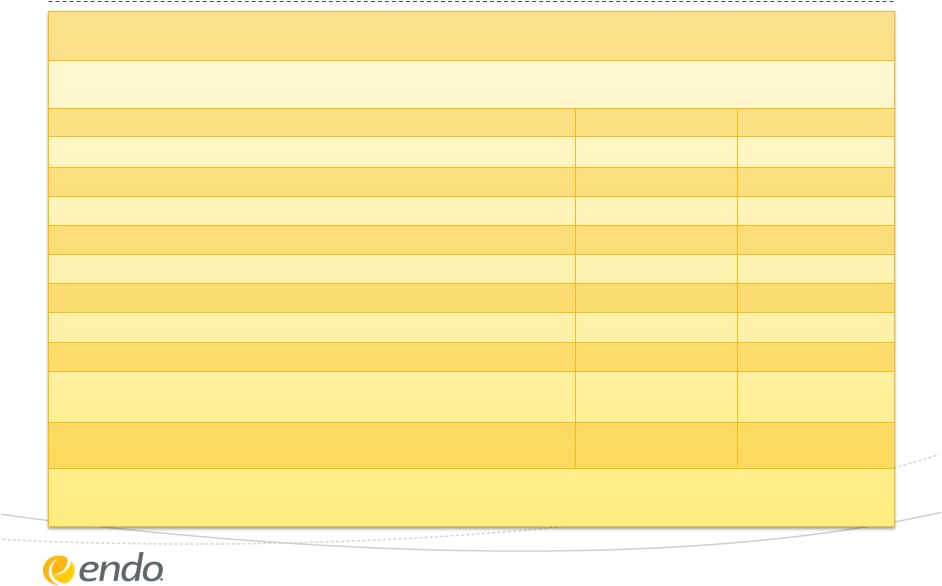 Reconciliation
of Non-GAAP Measures For an explanation of Endo’s reasons for using non-GAAP
measures, see Endo’s Current Report on Form 8-K filed today with the
Securities and Exchange Commission Reconciliation of Projected GAAP Diluted Earnings Per
Share to Adjusted Diluted Earnings Per Share Guidance for the Year Ending December 31,
2013 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$0.95
$1.10
Upfront and milestone-related payments to partners
$0.20
$0.20
Amortization of commercial intangible assets and inventory step-up
$1.64
$1.64
Integration and Restructuring Charges
$0.86
$0.86
Charges for Litigation and other legal matters
$1.79
$1.79
Asset Impairment Charges
$0.41
$0.41
Actavis (Watson) litigation settlement
($0.44)
($0.44)
Interest expense adjustment for ASC 470-20 and other treasury items
$0.29
$0.29
Tax effect of pre-tax adjustments at the applicable tax rates and certain
other expected cash tax savings as a result of recent acquisitions
($1.10)
($1.10)
Diluted adjusted income per common share guidance
$4.60
$4.75
The company's guidance is being issued based on certain assumptions including:
•Certain of the
above amounts are based on estimates and there can be no assurance that Endo will achieve these results
•Includes all
completed business development transactions as of November 5, 2013
35
©2013 Endo Pharmaceuticals Inc. All rights reserved.
|
