Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - QUESTCOR PHARMACEUTICALS INC | d429669d8k.htm |
| EX-99.2 - TRANSCRIPT OF CONFERENCE CALL - QUESTCOR PHARMACEUTICALS INC | d429669dex992.htm |
| EX-99.1 - PRESS RELEASE - QUESTCOR PHARMACEUTICALS INC | d429669dex991.htm |
 1
1
Third Quarter 2012
Conference Call
Third Quarter 2012
Conference Call
NASDAQ:
QCOR
Exhibit 99.3 |
 2
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
•
Telephone replay is available by dialing:
–
U.S.: (855) 859-2056.
–
International: (404) 537-3406.
–
Passcode: 39696380
•
By webcast: At Questcor's investor relations website:
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
•
Telephone replay is available by dialing:
–
U.S.: (855) 859-2056.
–
International: (404) 537-3406.
–
Passcode: 39696380
•
By webcast: At Questcor's investor relations website:
Conference Call Logistics
2
http://ir.questcor.com/
http://ir.questcor.com/events.cfm |
 3
Safe Harbor Statement
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future
financial performance. In some cases, you can identify forward-looking statements by terminology such as
"believes," "continue," "could," "estimates," "expects,"
"growth," "may," "plans," "potential," “remain,” "should,"
"substantial" or "will" or the negative of such terms and other comparable
terminology. These statements are only predictions. Actual events or results may differ
materially. Factors that could cause or contribute to such differences include, but are
not limited to, the following: Our reliance on Acthar for substantially all of our net sales and profits; Reductions in
vials used per prescription resulting from changes in treatment regimens by physicians or
patient compliance with physician recommendations; The complex nature of our
manufacturing process and the potential for supply disruptions or other business
disruptions ;The lack of patent protection for Acthar; and the possible FDA approval and market introduction of
competitive products; Our ability to continue to generate revenue from sales of Acthar to treat
on-label indications associated with NS, and our ability to develop other therapeutic
uses for Acthar; Research and development risks, including risks associated with
Questcor's work in the area of NS and potential work in the area of Rheumatology, and our reliance on
third-parties to conduct research and development and the ability of research and
development to generate successful results; Our ability to comply with federal and state
regulations, including regulations relating to pharmaceutical sales and marketing
practices; The results of any pending or future litigation, investigations or claims, including with respect to the
investigation by the United States Attorney’s Office for the Eastern District of
Pennsylvania regarding the Company’s promotional practices; Regulatory changes or
other policy actions by governmental authorities and other third parties in connection
with U.S. health care reform or efforts to reduce federal and state government deficits; Our ability to receive high
reimbursement levels from third party payers; An increase in the proportion of our Acthar unit
sales comprised of Medicaid- eligible patients and government entities; Our ability to
estimate reserves required for Acthar used by government entities and
Medicaid-eligible patients and the impact that unforeseen invoicing of historical Medicaid prescriptions may have upon
our results; Our ability to effectively manage our growth, including the expansion of our sales
forces, and our reliance on key personnel; The impact to our business caused by economic
conditions; Our ability to protect our proprietary rights; The risk of product liability
lawsuits; Unforeseen business interruptions and security breaches; Volatility in Questcor's monthly and
quarterly Acthar shipments, estimated channel inventory, and end-user demand, as well as
volatility in our stock price; and Other risks discussed in Questcor's annual report on
Form 10-K for the year ended December 31, 2011 as filed with the Securities and
Exchange Commission, or SEC, on February 22, 2012, and other documents filed with the SEC.
The risk factors and other information contained in these documents should be considered in
evaluating Questcor's prospects and future financial performance. |
 4
•
335 paid NS scripts
•
1,291 paid MS scripts
•
102 paid IS scripts
•
Financial results
–
5,590 vials shipped, up 92% YOY
–
$140.3M in net sales, up 135% YOY
–
$0.91 GAAP EPS (diluted), up 160% YOY
•
335 paid NS scripts
•
1,291 paid MS scripts
•
102 paid IS scripts
•
Financial results
–
5,590 vials shipped, up 92% YOY
–
$140.3M in net sales, up 135% YOY
–
$0.91 GAAP EPS (diluted), up 160% YOY
3Q-12 Results |
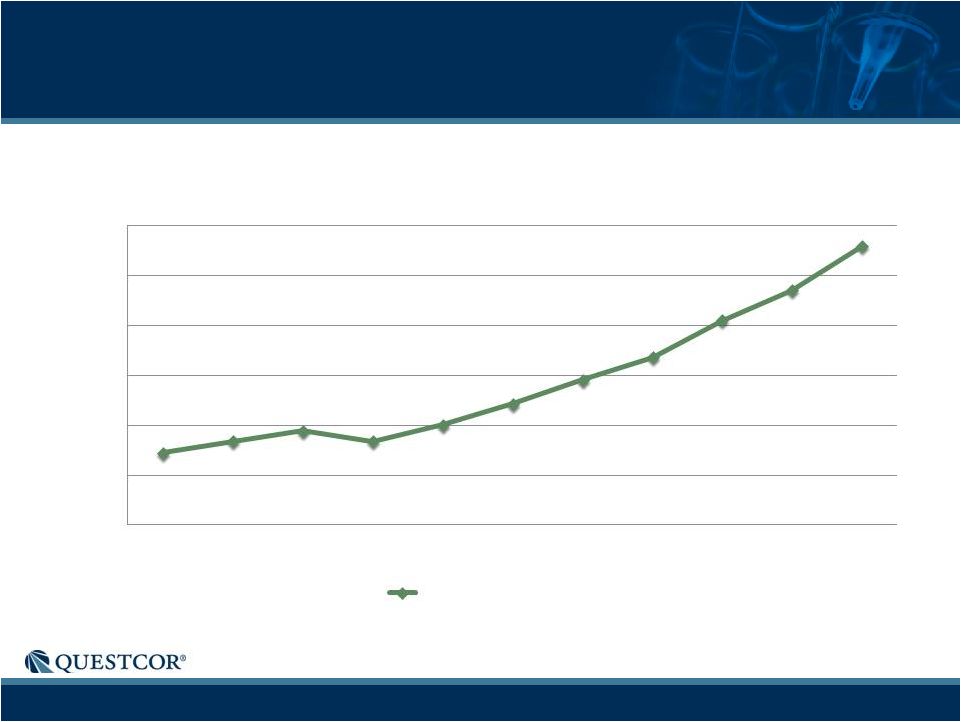 5
Growth in Shipped Vials
0
1,000
2,000
3,000
4,000
5,000
6,000
Q1-10
Q2-10
Q3-10
Q4-10
Q1-11
Q2-11
Q3-11
Q4-11
Q1-12
Q2-12
Q3-12
Shipped Vials
Shipped Vials |
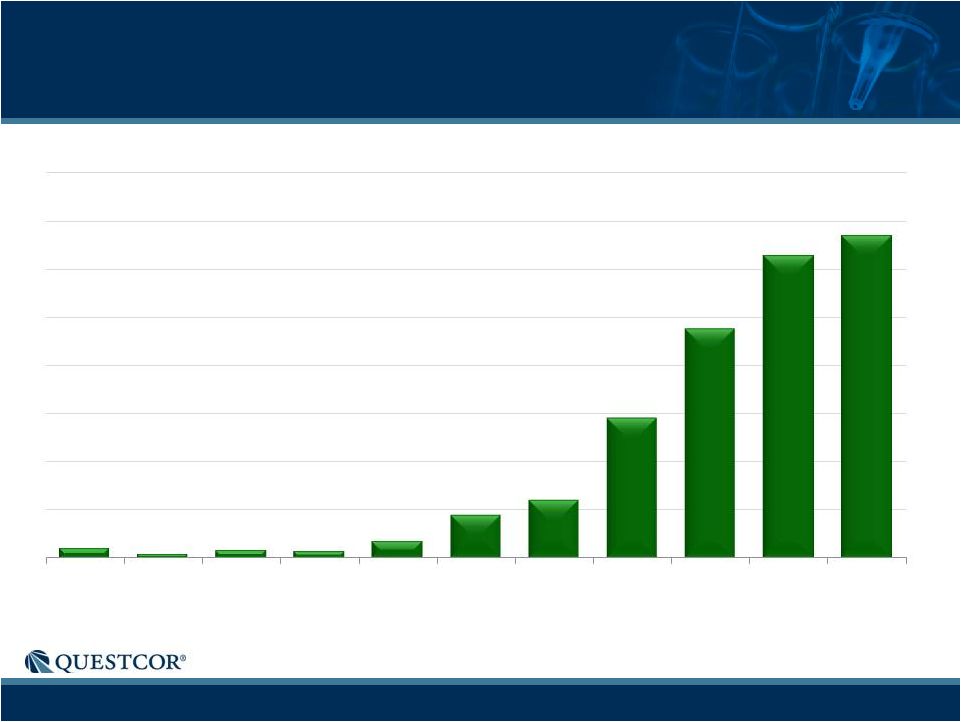 6
Paid Rxs
Paid Rxs
NS Scripts-Strong Continued Growth
11
4
8
7
18
45
60
146
238
314
335
Q1 '10
Q2 '10
Q3 '10
Q4 '10
Q1 '11
Q2 '11
Q3 '11
Q4 '11
Q1 '12
Q2 '12
Q3 '12
Notes: Historical trend information is not necessarily indicative of future results.
Chart includes "Related Conditions" - diagnoses that are either alternative
descriptions of the condition or are closely related to the medical condition which is the focus of the chart. |
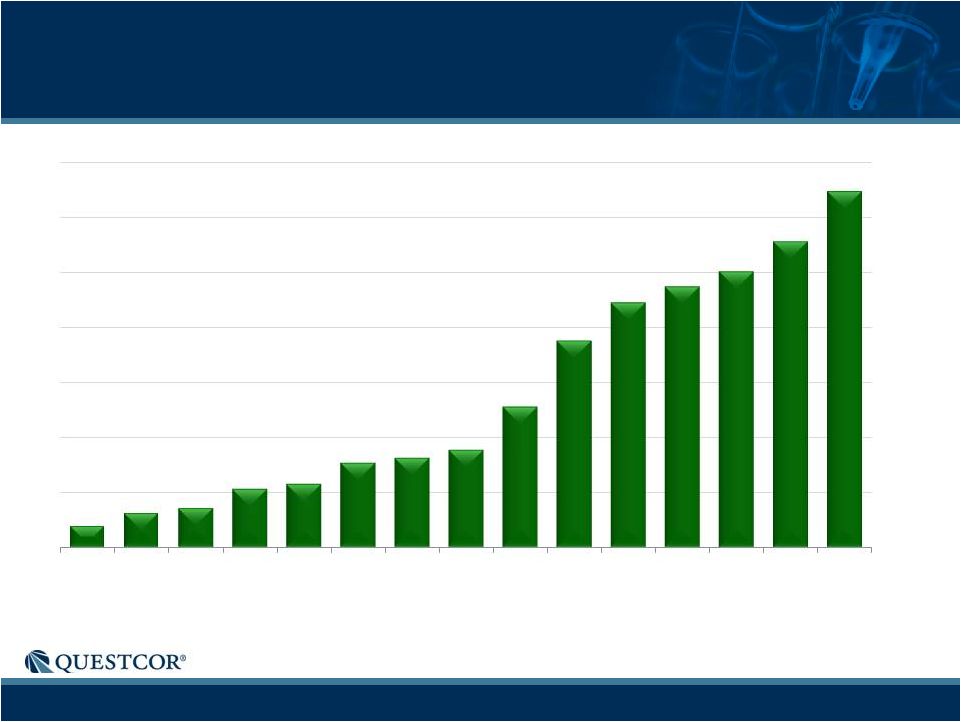 7
Notes: Historical trend information is not necessarily indicative of future
results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. Notes:
Historical trend information is not necessarily indicative of future results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. MS Scripts-Record of
Consistent Growth Paid Rxs
78
124
141
213
231
304
323
354
508
751
886
945
1,000
1,110
1,291
Q1 '09
Q2 '09
Q3 '09
Q4 '09
Q1 '10
Q2 '10
Q3 '10
Q4 '10
Q1 '11
Q2 '11
Q3 '11
Q4 '11
Q1 '12
Q2 '12
Q3 '12 |
 8
•
Understanding the biological properties of Acthar
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Further research related to on-label indications
•
Possible new indications to explore
•
Understanding the biological properties of Acthar
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Further research related to on-label indications
•
Possible new indications to explore
The Emerging Science Behind Acthar
Preclinical and Clinical Studies |
 9
•
335 paid NS scripts
•
1,291 paid MS scripts
•
102 paid IS scripts
•
Financial results
–
5,590 vials shipped, up 92% YOY
–
$140.3M in net sales, up 135% YOY
–
$0.91 GAAP EPS (diluted), up 160% YOY
•
335 paid NS scripts
•
1,291 paid MS scripts
•
102 paid IS scripts
•
Financial results
–
5,590 vials shipped, up 92% YOY
–
$140.3M in net sales, up 135% YOY
–
$0.91 GAAP EPS (diluted), up 160% YOY
3Q-12 Results |
 10
10
Third Quarter 2012
Conference Call
Third Quarter 2012
Conference Call
NASDAQ:
QCOR |
