Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GILEAD SCIENCES INC | d259746d8k.htm |
| EX-99.1 - JOINT PRESS RELEASE OF GILEAD AND PHARMASSET - GILEAD SCIENCES INC | d259746dex991.htm |
 Gilead to Acquire Pharmasset
November 21, 2011
Exhibit 99.2 |
 Slide 2
Safe Harbor Disclaimer
Statements included in this presentation that are not historical in nature are
“forward-looking statements” within the meaning of the Private Securities
Litigation Reform Act of 1995. Gilead cautions readers that forward-looking
statements are subject to certain risks and uncertainties, which could cause actual results to differ
materially. These risks and uncertainties include: Gilead’s ability to consummate the
acquisition of Pharmasset and advance Pharmasset’s product pipeline as planned;
Gilead’s ability to successfully develop its HCV franchise; Gilead’s ability to
develop an all oral antiviral regimen for HCV; and Gilead’s ability to achieve its anticipated
full year 2011 financial results. Gilead directs readers to its Quarterly Report on Form
10-Q for the third quarter ended September 30, 2011 and subsequent current reports on Form
8-K. Gilead claims the protection of the Safe Harbor contained in the Private Securities
Litigation Reform Act of 1995 for forward-looking statements. All forward-looking statements are based on
information currently available to Gilead, and Gilead assumes no obligation to update any such
forward-looking statements.
This announcement is neither an offer to purchase nor a solicitation of an offer to sell shares of
Pharmasset. At the time the offer is commenced, Gilead will file a Tender Offer Statement
on Schedule TO with the U.S. Securities and Exchange Commission, and Pharmasset will file a
Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the offer.
Pharmasset stockholders and other investors are urged to read the tender offer materials
(including an Offer to Purchase, a related Letter of Transmittal and certain other offer documents)
and the Solicitation/Recommendation Statement because they will contain important information
which should be read carefully before any decision is made with respect to the tender
offer. The Offer to Purchase, the related Letter of Transmittal and certain other offer
documents, as well as the Solicitation/Recommendation Statement, will be made available to all
stockholders of Pharmasset at no expense to them. The Tender Offer Statement and the
Solicitation/Recommendation Statement will be made available for free at the Commission's web
site at www.sec.gov. |
 Slide 3
Agenda
1.
Pharmasset Acquisition Highlights
2.
HCV Landscape
3.
Overview of Pharmasset HCV Portfolio
4.
Gilead’s Combined HCV Opportunity
Page
4
8
16
22 |
 Slide 4
Pharmasset Acquisition Highlights |
 Slide 5
Terms of the Agreement
Cash tender offer at $137 per share
Transaction value of approximately $11 billion
Tender offer expected to close within Q1 12
–
Subject to minimum tender requirement
–
Hart-Scott-Rodino (antitrust) clearance
–
Other customary conditions |
 Slide 6
Strategic Rationale
Accelerates Gilead’s strategy to develop the first all-oral
regimen for the treatment of HCV
–
PSI-7977 is the most potent, advanced nucleotide in clinical development
–
Phase 3 clinical studies of PSI-7977 in combination with ribavirin open to
enrollment
–
Targeted U.S. FDA approval in 2014
Since HCV can be cured, it will be important to be first to
market with an all-oral, well-tolerated regimen
Opportunity for significant revenue growth and diversification in
2014 and beyond
–
Composition of matter patent protection for PSI-7977 into 2029
Pharmasset is a strategic fit with Gilead’s areas of operational
excellence |
 Slide 7
Fit with Gilead’s Areas of Operational Excellence
Established expertise and commitment to liver disease
Preclinical, clinical development and regulatory expertise
–
Developed and commercialized 5 HIV and 2 hepatitis products
–
Viread regulatory approvals in approximately 140 countries
–
Established team: Quad NDA filing in 6 weeks after last patient, last
visit Expertise in process research and development, and
established worldwide API and product manufacturing network
–
Developed 1 fixed-dose combination and 3 single-tablet regimens in
HIV –
Manufacture tenofovir DF at >110 metric tons per year
Established commercial operations in 23 countries worldwide,
with expanding presence in Asia |
 Slide 8
HCV Landscape |
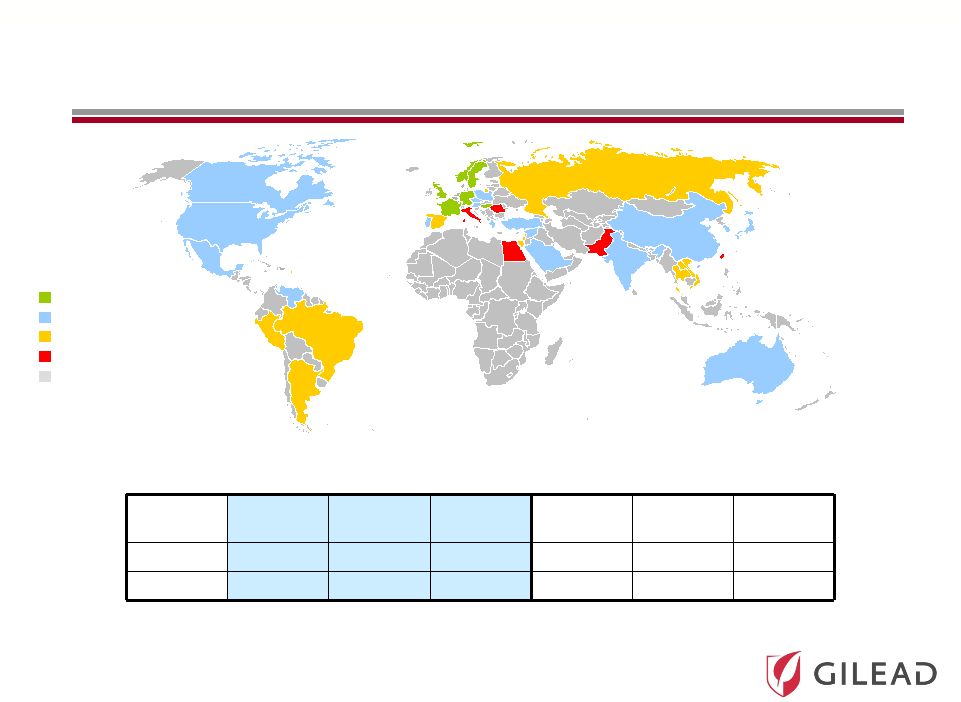 Slide 9
<1%
1-1.9%
2-2.9%
>3%
Not tested
HCV Prevalence in Core and Emerging Markets (M)
Global Prevalence of HCV is Estimated to be
160 Million Individuals
(1)
HCV Prevalence
(% of population)
Region
US
2
Japan
2
Europe
Other
3
Asia
3
Latin
America
3
All GT
2.9
3.7
0.6
6.7
70.8
5.4
GT1 (%)
73%
63%
70%
65%
39%
68%
EU-Core 5
2
Sources: 1) Lavanchy, et al. 2011. 2) Gilead Forecast (2011 prevalence estimate). 3)
Cornberg, Sievert, and Kershenobich, et al 2011. Country populations from 2009 World
Bank estimates |
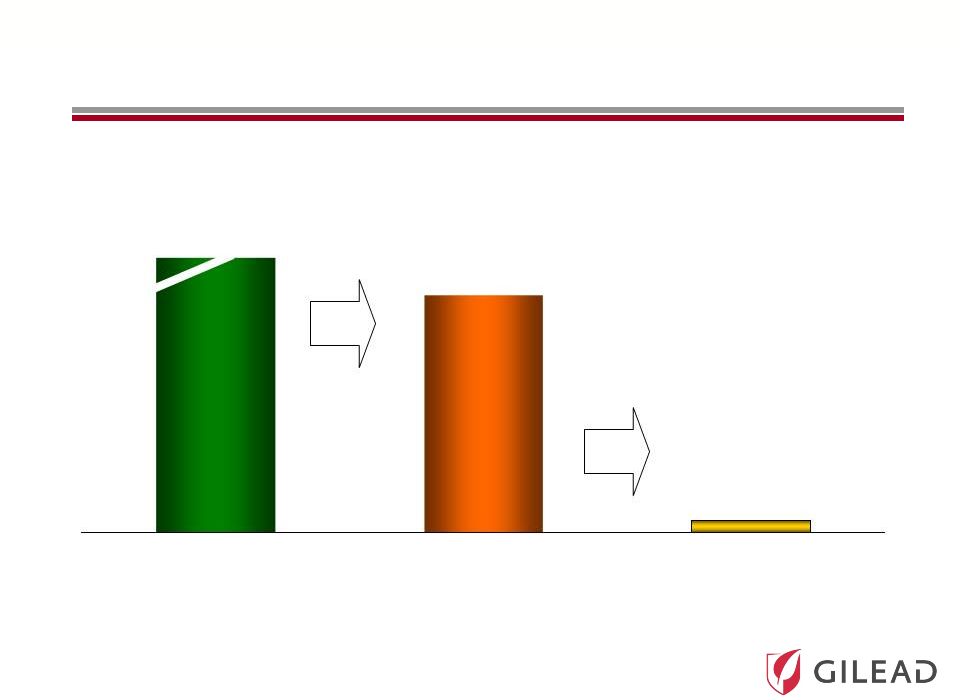 Slide 10
Over
12
Million
Infected
Individuals
in
Major
Markets
(1)
with Fewer than 200,000 Treated per Year
Treated/
Year
182
Diagnosed
Prevalence
12,601
Patients in thousands
36%
4,524
1. Major markets include US, EU-5, Japan, Australia, Austria, Brazil, Denmark,
Finland, Greece, Ireland, Norway, Poland, Portugal, Sweden, Switzerland, Turkey, Canada
Sources:
Prevalence
–
KantarHealth
Core-5
EU
epidemiology
analysis
(2010),
NHANES
(1999-2006),
Armstrong
(2004),
Hepatology,
40(4:S1):176a,
Chak
(2011),
Liver
Intl.,
8,
1090-1101,
Cornberg
(2011),
Liver
Intl.,
31
(s2),
31-60,
Kershenobich
(2011),
Liver
Intl.,
31
(s2),
18-29
Diagnosed
–
KantarHealth
Core-5
EU
epidemiology
analysis
(2010),
Armstrong
(2004)
Hepatology,
40(4:S1):176a,
Culver
(2000),
Transfusion
40:1176
Treated
–
IMS
MIDAS
(2004
–
2009),
Synovate
chart
audits
(2007),
Roche
and
Schering
Plough
annual
reports
(2009)
HCV: A Significant Unmet Medical Need
4% |
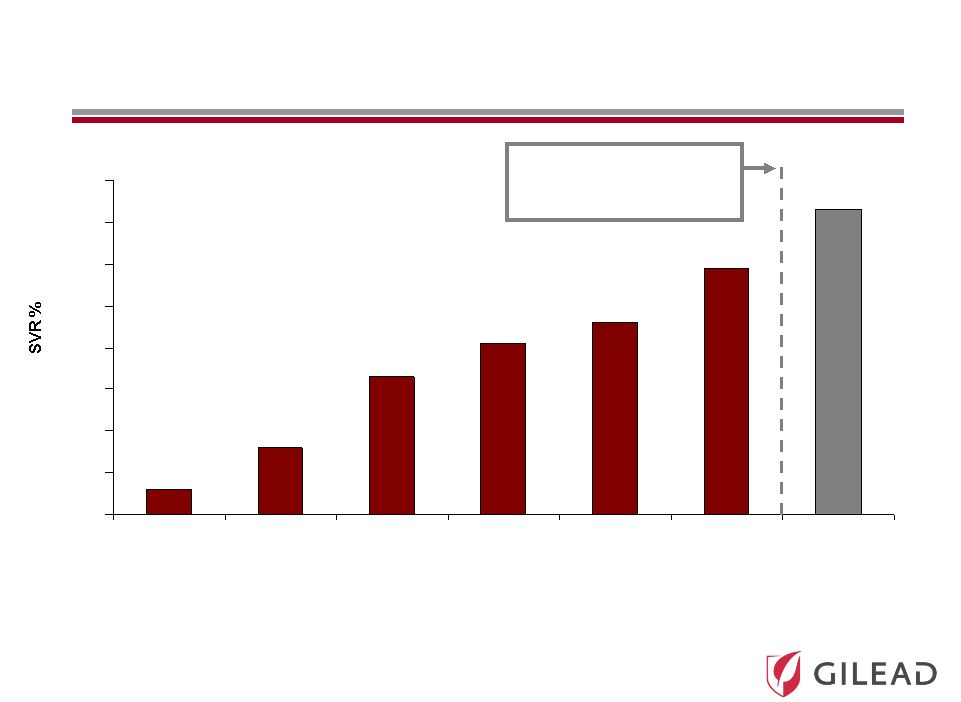 Slide 11
0
10
20
30
40
50
60
70
80
IFN 24 Wk
IFN 48 Wk
IFN/RBV 24 Wk
IFN/RBV 48 Wk
(1998)
IFN/RBV 48 Wk
(2001)
PegIFN/RBV 48
Wk
PI +
PegIFN/RBV 48
Wk (2011)
6%
13-19%
31-35%
38-43%
45-47%
54-63%
66-79%
IFN
24 Weeks
IFN
48 Weeks
IFN/RBV
24 Weeks
IFN/RBV
48 Weeks
1998
IFN/RBV
48 Weeks
2001
PegIFN/RBV
48 Weeks
Protease
Inhibitor +
PegIFN/RBV
24-48 Weeks
2011
HCV Treatment Evolution
IFN = Interferon
RBV = Ribavirin
PegIFN = Pegylated Interferon
FDA Approval of
Protease Inhibitors (PIs)
in May 2011 |
 Slide 12
Challenges of Current PI-based Treatment Remains
Additional safety issues and exacerbation of side effects
(rash and anemia)
–
Discontinuation rates significantly increased on PIs
Lower response rates in sub-populations
(previous IFN/RBV non-responders, cirrhotics)
Burdensome for patients and practitioners
–
PIs dosed 2-3 times per day, plus ribavirin and a
self-administered injection
–
Time intensive adverse event management, complexity of
response guided therapy |
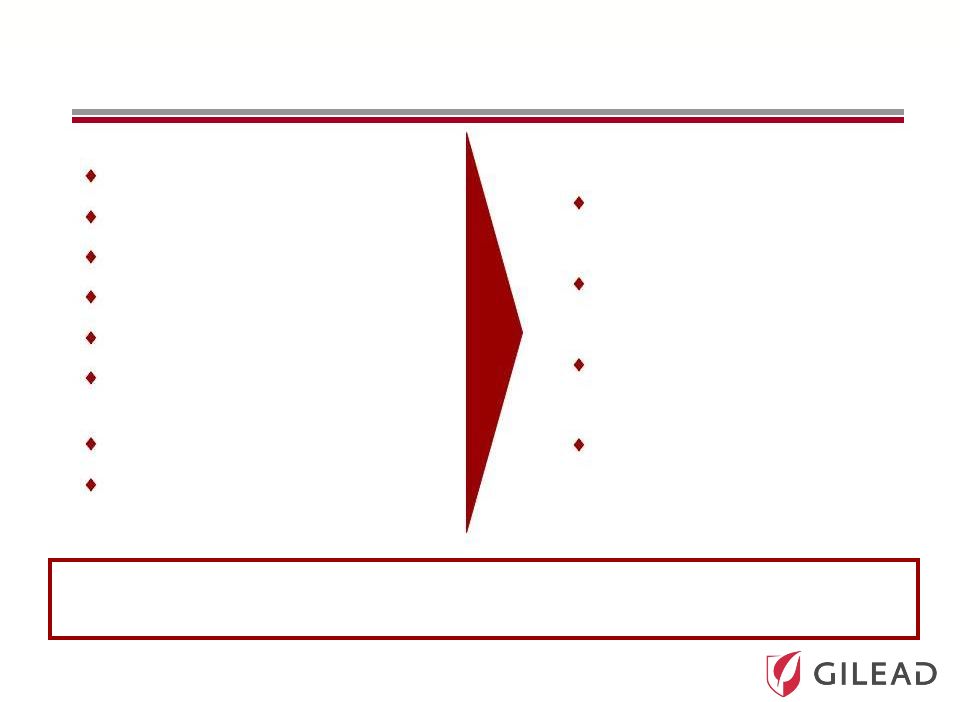 Slide 13
Ultimate Goal in HCV Therapy
Once a day
Pan-genotype
No clinical resistance
No response guided therapy
Short duration
Safe with manageable
side effects
High cure rates
Suitable for all populations
Ideal HCV Therapy Most Likely Achieved
Ideal HCV Therapy Most Likely Achieved
through a Combination of Two or More Drugs
through a Combination of Two or More Drugs
Easier for doctors and
patients
Leads to increased
diagnosis and treatment
Potential to cure many
more patients
Lowers cost to healthcare
system due to prevention
of end-stage liver disease |
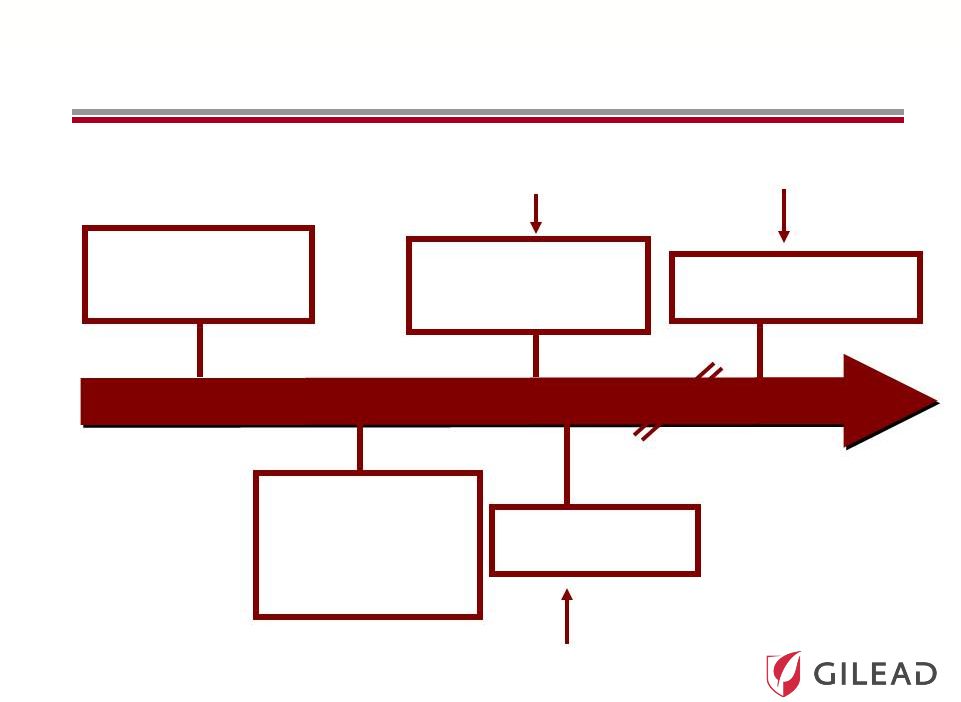 Slide 14
2010
2010
2011
2011
2020
2020
IFN Free Strategy
Ultimate Goal
The Evolution of HCV Therapy: 2010
Current Standard of
Care (SOC):
PEG + RBV
Combination
Oral Antivirals +
Shorter Course IFN
Broad Genotypic Oral
Antiviral Regimen
The First Antivirals
+ SOC to Improve
Response Rates
and Reduce
Duration
All Oral Antiviral
Regimen
IFN Sparing Strategy |
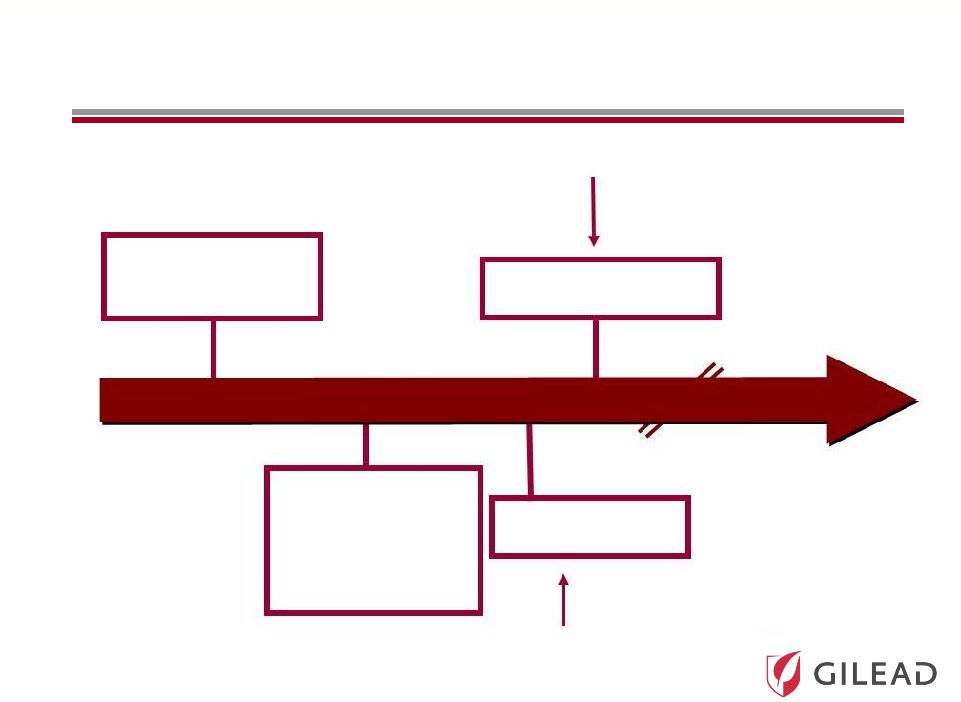 Slide 15
Current Standard of
Care (SOC):
PEG + RBV
The First Antivirals
+ SOC to Improve
Response Rates
and Reduce
Duration
The Competitive Nature and Speed of Development
Have Significantly Accelerated the Timelines
Ultimate Goal
All Oral Antiviral
Regimen
IFN Free Strategy
2010
2010
2011
2011
2020
2020
Broad Genotypic Oral
Antiviral Regimen
2014
2014 |
 Slide 16
Overview of Pharmasset HCV Portfolio |
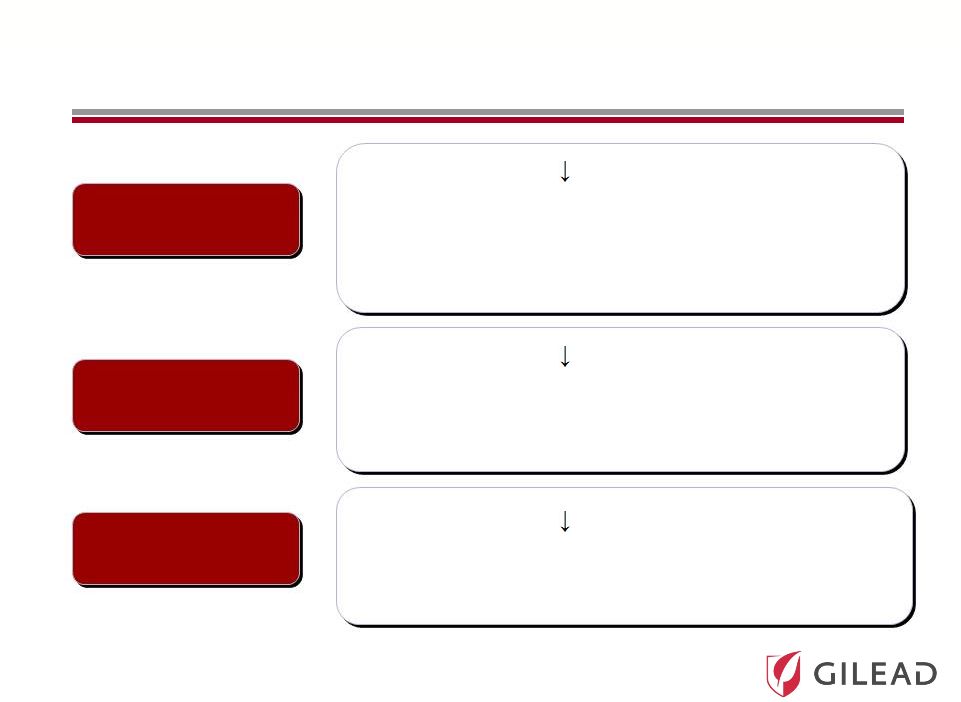 Slide 17
Pharmasset’s HCV Nucleotide Portfolio
PSI-7977
PSI-938
RG 7128
•
3.6 log HCV RNA
with 400 mg QD over 3 days
•
Phase 3 in combination with ribavirin
open to enrollment
•
Highest on treatment and SVR rates to date
•
Unpartnered with worldwide rights
•
3.6 log HCV RNA
with 300 mg QD over 3 days
•
Phase 2
•
14 day data, with ongoing 12 week studies
•
Unpartnered with worldwide rights
•
0.6 log HCV RNA
with 1gm BID
•
Phase 2b
•
Partnered with Roche |
 Slide 18
PSI-7977: Pharmasset’s Lead Pyrimidine
Nucleotide Analogue
Excellent safety profile
–
250 patients at 8 weeks or more
–
1,500 patients by May 2014
High rates of cure in genotype 2/3
–
100% (10/10) with ribavirin
–
60% (6/10) without ribavirin
Studies in genotype 1 ongoing
–
Expecting Phase 2 SVR12 data early 2012
Multiple expanding data sets from studies in different
patient populations |
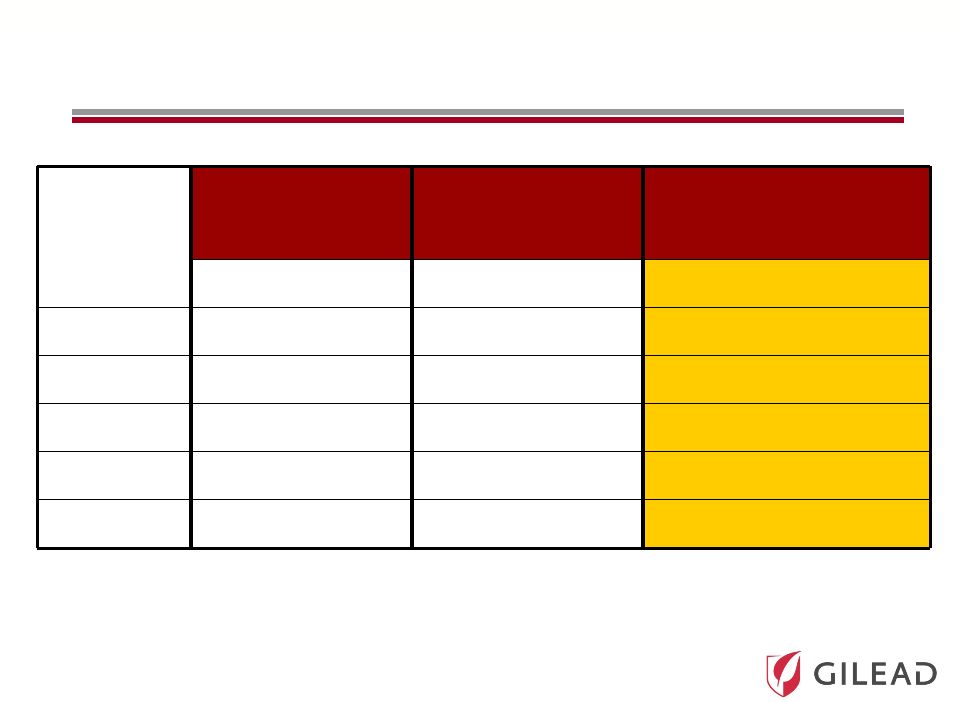 Slide 19
PSI-7977: Genotype 2/3 Phase 2 Results
(ELECTRON)
Time
(Weeks)
PSI-7977
RBV
12 weeks PEG
PSI-7977
RBV
NO PEG
PSI-7977
NO PEG
NO RBV
n/n (%) < LOD
n/n (%) < LOD
n/n (%) < LOD
2
8/11 (82%)
8/10 (80%)
8/10 (80%)
4
11/11 (100%)
10/10 (100%)
10/10 (100%)
12
11/11 (100%)
10/10 (100%)
10/10 (100%)
SVR4
11/11 (100%)
10/10 (100%)
6/10 (60%)
SVR12
11/11 (100%)
10/10 (100%)
n/a
n/a –
not yet available |
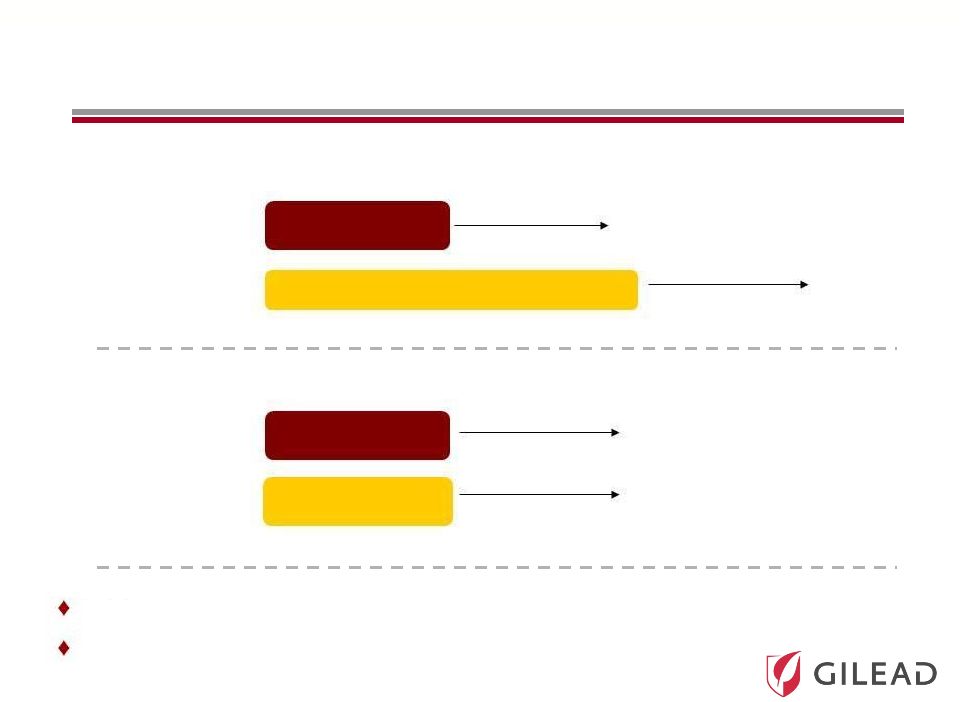 Slide 20
Pharmasset’s Phase 3 Program
(Announced November 1, 2011)
Genotype 2/3
(naïve)
Genotype 2/3
(IFN intolerant)
N=~250
N=~250
N=~150
N=~75
SVR12
SVR12
0
12
24
36
SVR12
SVR12
0
12
24
POSITRON
FISSION
36
PSI-7977 400mg
+ RBV
Peg-IFN + RBV
PSI-7977 400mg
+ RBV
PSI-7977 placebo
+ RBV placebo
A
third
Phase
3
study
to
be
initiated
in
genotype
1
patients
in
1H
12
Targeted U.S. FDA approval in 2014 |
 Slide 21
Key Remaining Questions in the Treatment of HCV
Will one regimen work for all genotypes or will different
regimens be required for different genotypes?
How many molecules or mechanisms do you need?
Can ribavirin be replaced?
What is the minimum duration of therapy?
Can HCV be cured with once-daily therapy? |
 Slide 22
Gilead’s Combined HCV Opportunity |
 Slide 23
Pharmasset and Gilead Combined HCV Efforts
Position Us to Answer These Questions
High barrier to resistance/pan-genotypic compounds will be an
important component for any all-oral regimen
–
Pharmasset’s PSI-7977 is the most advanced, pan-genotypic compound
with a high barrier to resistance
–
Ongoing efforts at Gilead to identify other pan-genotypic compounds
Complementary portfolio accelerates potential for oral regimens
–
Includes
direct
acting
antivirals
with
four
different
mechanisms
in
Phase
2/3 clinical development
–
Potential to pursue different combinations to then answer the question of
duration of treatment and necessity of ribavirin
Complementary portfolios cover a number of opportunities for
once-daily regimens
–
Potential for co-formulation |
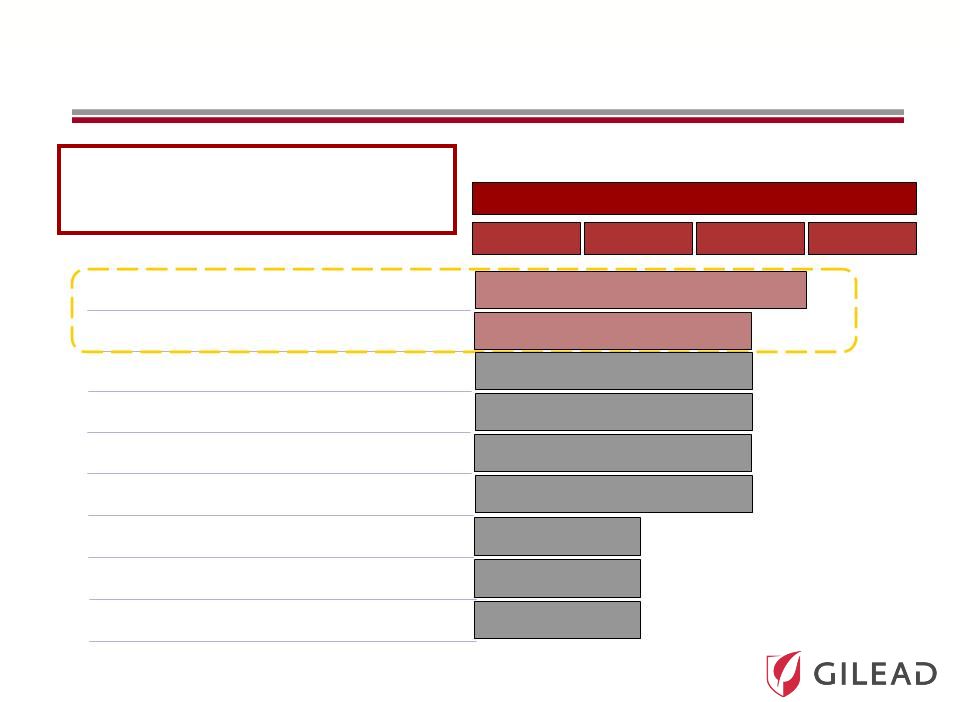 Slide 24
Phase
PC
GS
9451
(NS3
protease
inhibitor)*
GS
5885
(NS5A
inhibitor)*
GS
9620
(TLR-7
agonist)*
GS
6620
(nucleotide
NS5B
inhibitor)**
GS
9669
(non-nuc
NS5B
site
2
inhibitor)*
Combined HCV Clinical Portfolio
*
Once-daily dosing
I
II
III
PSI-7977
(nucleotide
NS5B
inhibitor)*
PSI-938
(nucleotide
NS5B
inhibitor)*
Pharmasset Acquisition
Complements and Accelerates
Gilead’s HCV Portfolio
GS
9256
(NS3
protease
inhibitor)**
Tegobuvir/GS
9190
(non-nuc
NS5B
inhibitor)
**
No further clinical trials planned |
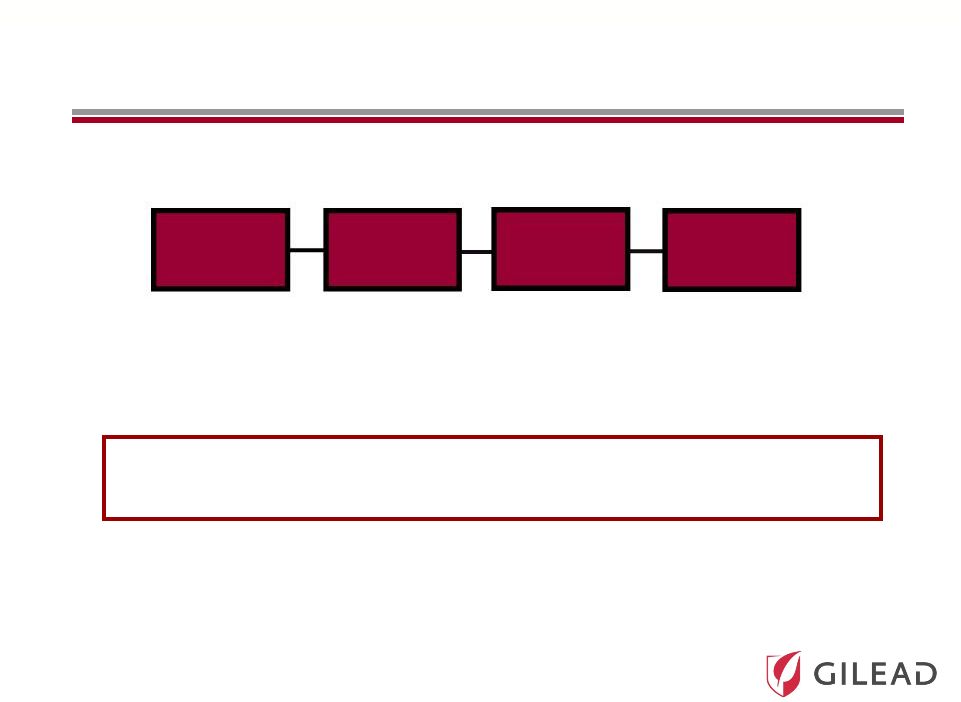 Slide 25
All-Gilead Portfolio Increases the Chance of
Developing Oral Therapies across Genotypes
Nuc
PI
NS5A
Non-Nuc
GS 5885
PSI-7977
PSI-938
GS 9451
GS 9190
GS 9669
Combination Therapies with High Potency May Further
Combination Therapies with High Potency May Further
Shorten Duration and Eliminate Ribavirin
Shorten Duration and Eliminate Ribavirin |
 Slide 26
2012 Key HCV Milestones
Initiate and integrate Pharmasset’s Phase 3 programs
–
GT 2/3 programs: no change
–
GT 1: Phase 2 data will determine strategy
Initiate clinical trial with PSI-7977 to define minimum
treatment duration in GT 2/3 patients
Initiate multiple enabling drug-drug interaction studies with
Gilead and Pharmasset assets
Explore combinations that exclude ribavirin
Initiate programs in pre-transplant and HIV co-infected
populations
SVR data from Gilead’s all-oral Phase 2 Study 120 at EASL
|
 Slide 27
In Summary
Accelerates Gilead’s strategy to develop the first all-oral
regimen for the treatment of HCV
Since HCV can be cured, it will be important to be first to
market with an all-oral, well-tolerated regimen
Pharmasset is a strategic fit with Gilead’s areas of
operational excellence
Opportunity to change revenue trajectory starting in 2014
|
 Slide 28
Gilead’s Business Prospects
Strong confidence in our core business
–
LTM HIV revenues of ~$6.4B in a total ~$13.0B market
–
LTM ~$1B in revenues coming from products outside HIV
Anticipate major HIV product launches in 2012
–
New revenue sources through partnerships to follow
More Phase 2/3 programs across therapeutic areas than
ever before
–
HIV, liver disease, oncology/inflammation/fibrosis, respiratory and
cardiovascular
Note: LTM = last twelve months |
 Slide 29
Financial Impact of Pharmasset Acquisition
Cash flows from core business will allow us to finance transaction
Expect to use existing cash and $6.2 billion in incremental debt
Committed to retaining investment grade credit rating
–
Debt/EBITDA ratio to return to
1.5x at closing + 1 year
Transaction expected to be dilutive initially and
significantly accretive beyond 2015
–
Increased interest expense associated with transaction debt
–
Share repurchases to offset dilution as debt is repaid
|
 Slide 30
Transaction Summary
Unique opportunity to dramatically change HCV disease
burden worldwide
Potential to extend leadership in liver disease to HCV
Opportunity to parallel the commercial success in HIV
Redefined potential for revenue growth in 2014 and beyond
|
 Gilead to Acquire Pharmasset
November 21, 2011 |
