Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HELIUS MEDICAL TECHNOLOGIES, INC. | hsdt-8k_20210917.htm |

Empowering Neuroplasticity Disruptive Technology for Healthcare NASDAQ:HSDT Exhibit 99.1

Legal Disclaimers This presentation contains forward-looking statements, including statements about: statements regarding the Company’s future strategic and operational execution, the next phase of the Company’s market development activities, clinical and regulatory development plans for the PoNS device, and the timing and success of the Company’s commercialization efforts in the United States. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Risk Factors: Factors that may cause actual results to differ materially from any future results expressed or implied by any forward looking statements uncertainties regarding the Company’s capital requirements to achieve its business objectives, the impact of the COVID-19 pandemic, the Company’s ability to secure contracts with rehabilitation clinics, the Company’s ability to obtain national Medicare coverage and to obtain a reimbursement code so that the PoNS device is covered by Medicare and Medicaid, the Company’s ability to obtain FDA clearance for stroke, the Company’s ability to build internal commercial infrastructure, market awareness of the PoNS device, future clinical trials and the clinical development process, manufacturing and supply chain risks, potential changes to the MCIT program, the product development process and FDA regulatory submission review and approval process, other development activities, ongoing government regulation, and other risks described in the “Risk Factors” section of Company’s Annual Report on Form 10-K for the year ended December 31, 2020, its Quarterly Report on Form 10-Q for the quarter ended June 30, 2021 as well as those set forth from time to time in the Company’s other filings with the securities and exchange commission and the Canadian securities regulators available at http://www.sec.gov or www.sedar.com The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Certain data in this presentation was obtained from various external sources. Neither the Company nor its affiliates, advisers or representatives have verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives make any representations as to the accuracy or completeness of that data or commits to update such data after the date of this presentation. Such data involves risks and uncertainties and is subject to change based on various factors. The Company’s first product, PoNS, is indicated for use in the United States as a short term treatment of gait deficit due to mild-to-moderate symptoms from multiple sclerosis (MS) and is to be used as an adjunct to a supervised therapeutic exercise program in patients 22 years of age and over by prescription only. The PoNS device is authorized for sale in Canada as a class II, non-implantable, medical device intended as a short term treatment (14 weeks) of gait deficit due to mild and moderate symptoms from MS, and chronic balance deficit due to mild-to-moderate traumatic brain injury (mmTBI) and is to be used in conjunction with physical therapy. The PoNS device is an investigational medical device in the European Union (“EU”) and Australia (“AUS”). It is currently under premarket review by the AUS Therapeutic Goods Administration. 2
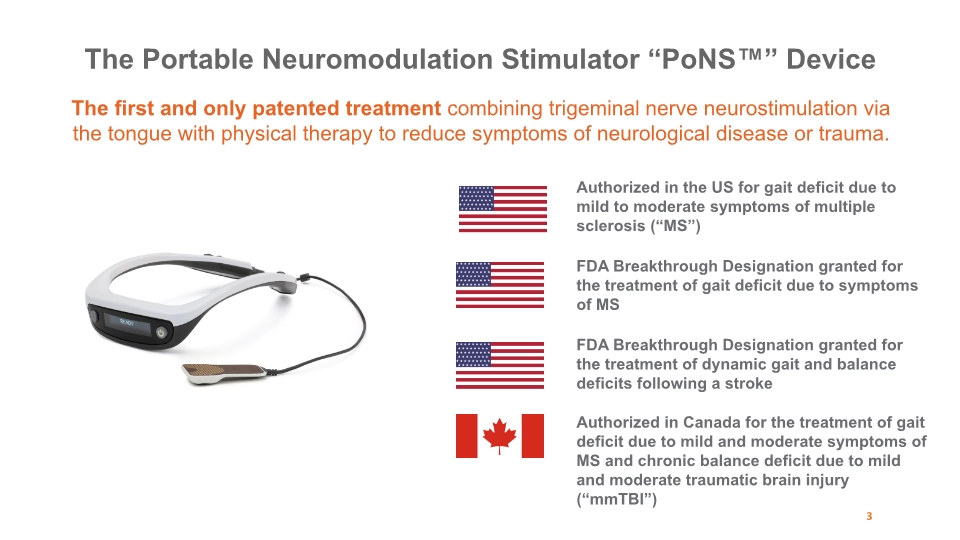
The first and only patented treatment combining trigeminal nerve neurostimulation via the tongue with physical therapy to reduce symptoms of neurological disease or trauma. The Portable Neuromodulation Stimulator “PoNS™” Device Authorized in Canada for the treatment of gait deficit due to mild and moderate symptoms of MS and chronic balance deficit due to mild and moderate traumatic brain injury (“mmTBI”) Authorized in the US for gait deficit due to mild to moderate symptoms of multiple sclerosis (“MS”) FDA Breakthrough Designation granted for the treatment of gait deficit due to symptoms of MS FDA Breakthrough Designation granted for the treatment of dynamic gait and balance deficits following a stroke 3
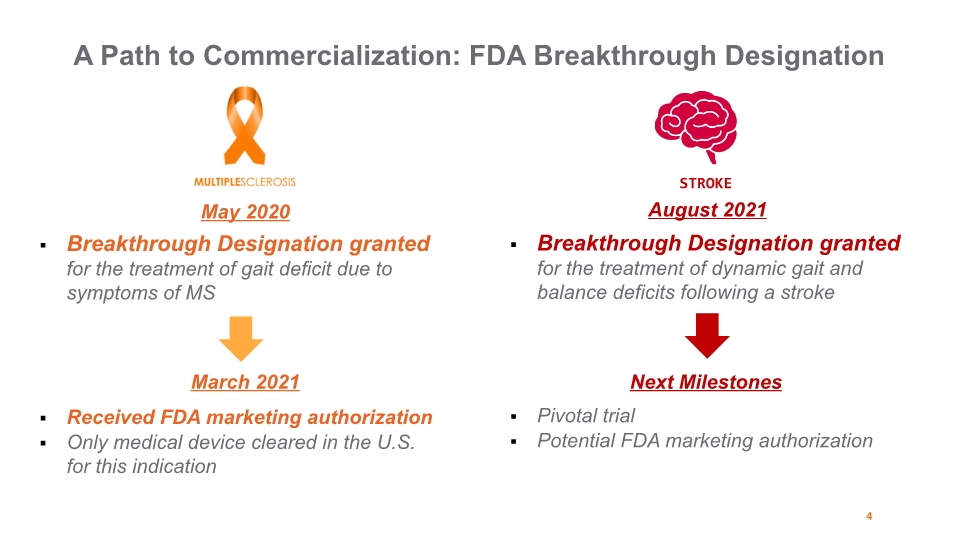
4 A Path to Commercialization: FDA Breakthrough Designation Breakthrough Designation granted for the treatment of gait deficit due to symptoms of MS May 2020 Breakthrough Designation granted for the treatment of dynamic gait and balance deficits following a stroke March 2021 Received FDA marketing authorization Only medical device cleared in the U.S. for this indication August 2021 Next Milestones Pivotal trial Potential FDA marketing authorization STROKE
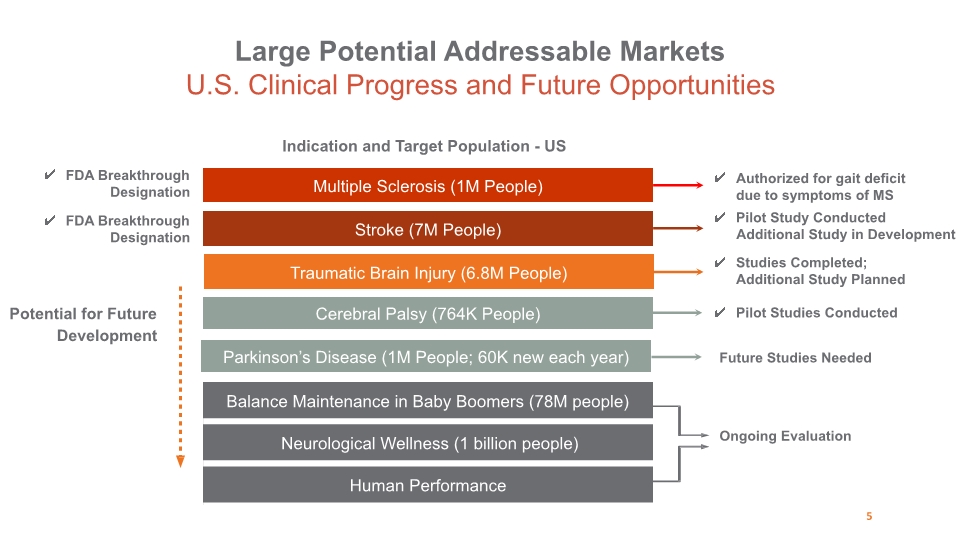
Stroke (7M People) Cerebral Palsy (764K People) Indication and Target Population - US Potential for Future Development Ongoing Evaluation Multiple Sclerosis (1M People) Pilot Studies Conducted Studies Completed; Additional Study Planned Traumatic Brain Injury (6.8M People) Parkinson’s Disease (1M People; 60K new each year) Future Studies Needed FDA Breakthrough Designation Large Potential Addressable Markets U.S. Clinical Progress and Future Opportunities 5 Authorized for gait deficit due to symptoms of MS Pilot Study Conducted Additional Study in Development FDA Breakthrough Designation
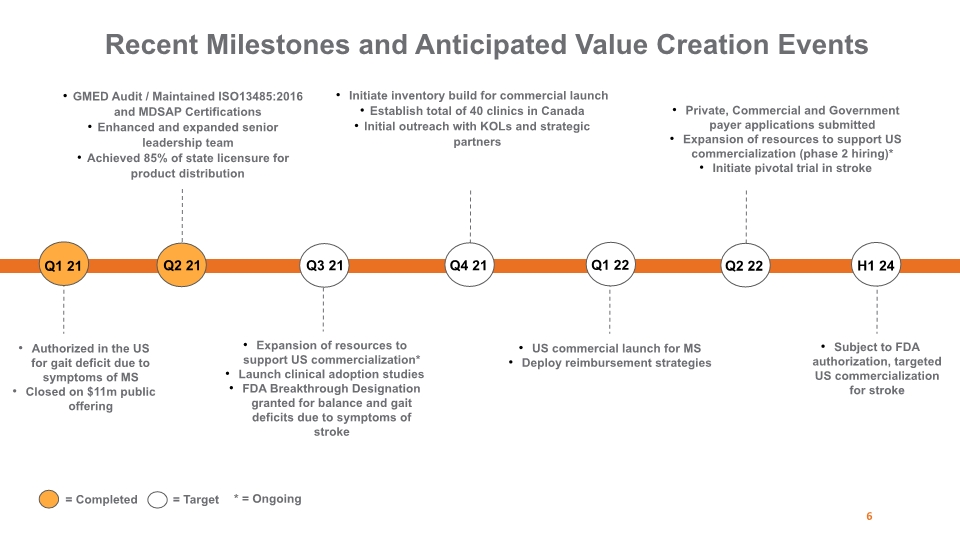
Recent Milestones and Anticipated Value Creation Events Authorized in the US for gait deficit due to symptoms of MS Closed on $11m public offering 6 Subject to FDA authorization, targeted US commercialization for stroke Q1 21 Q2 21 Q3 21 Q4 21 Q1 22 Q2 22 H1 24 GMED Audit / Maintained ISO13485:2016 and MDSAP Certifications Enhanced and expanded senior leadership team Achieved 85% of state licensure for product distribution Expansion of resources to support US commercialization* Launch clinical adoption studies FDA Breakthrough Designation granted for balance and gait deficits due to symptoms of stroke Initiate inventory build for commercial launch Establish total of 40 clinics in Canada Initial outreach with KOLs and strategic partners US commercial launch for MS Deploy reimbursement strategies Private, Commercial and Government payer applications submitted Expansion of resources to support US commercialization (phase 2 hiring)* Initiate pivotal trial in stroke = Completed = Target * = Ongoing

PoNS Treatment™
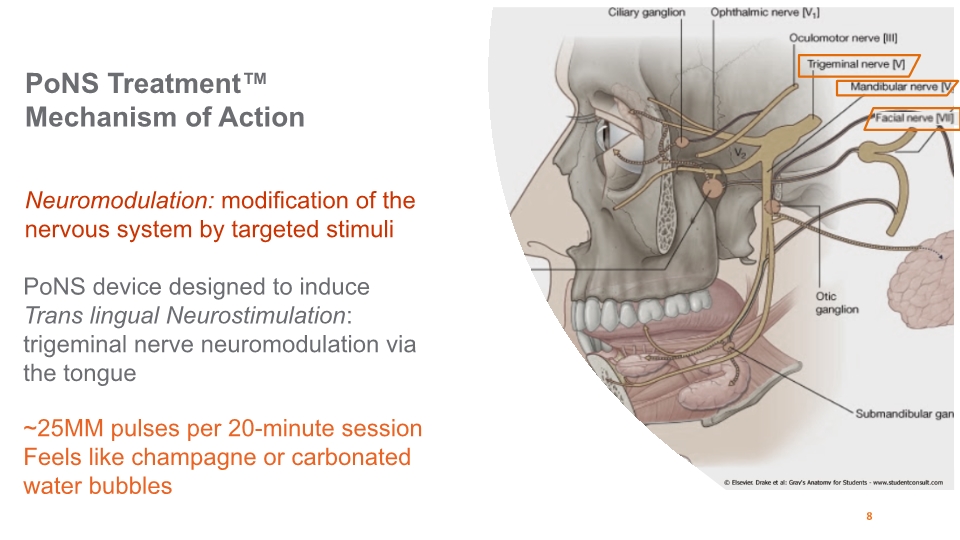
PoNS Treatment™ Mechanism of Action 8

PoNS™ Device Empowering the brain and improvement during PoNS Treatment™ Mouthpiece electrodes stimulate the tongue surface, sending signals to the brain The stimulation produced by PoNS is believed to help strengthen the neural connections associated with balance and walking when combined with physical rehabilitation 9
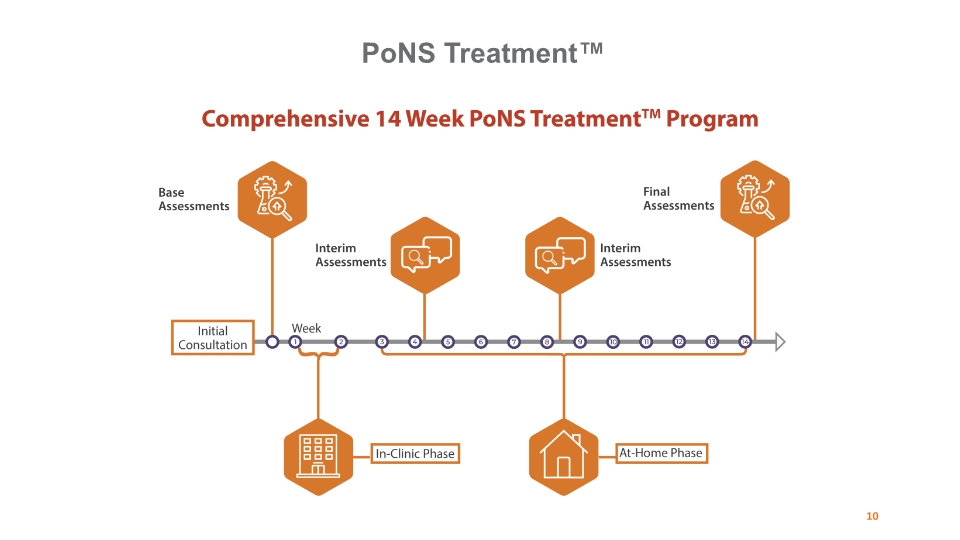
PoNS Treatment™ 10

PoNS Treatment™ for Symptoms Due to Stroke *PoNS is not authorized to treat individuals with stroke

FDA Breakthrough Designation for Treatment of Symptoms Due to Stroke Announced receipt of FDA Breakthrough Designation on August 17, 2021 Indication of use as a temporary treatment of dynamic gait and balance deficits due to symptoms from stroke PoNS Treatment to be used as an adjunct to a supervised therapeutic exercise program in patients 22 years of age and over 12 *PoNS is not authorized to treat individuals with stroke
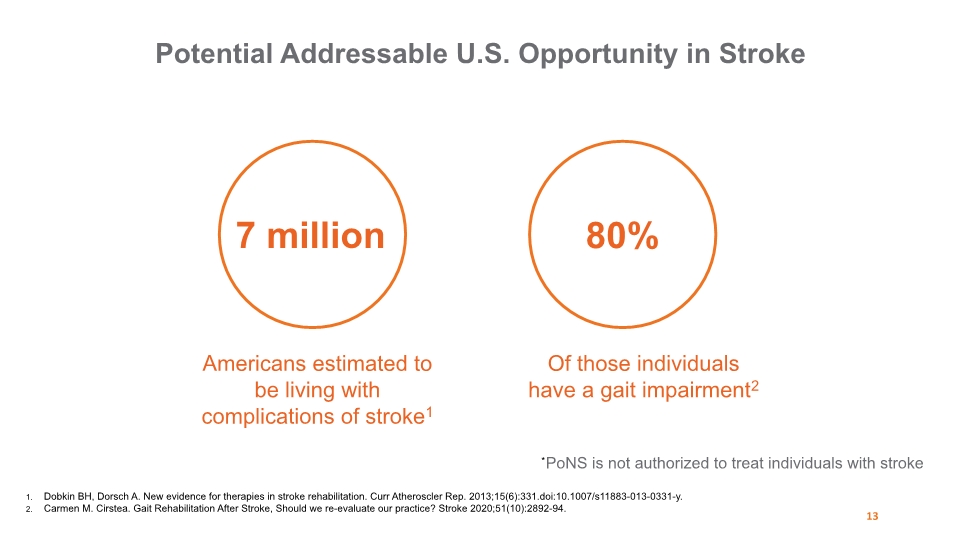
Potential Addressable U.S. Opportunity in Stroke 13 *PoNS is not authorized to treat individuals with stroke 7 million 80% Dobkin BH, Dorsch A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep. 2013;15(6):331.doi:10.1007/s11883-013-0331-y. Carmen M. Cirstea. Gait Rehabilitation After Stroke, Should we re-evaluate our practice? Stroke 2020;51(10):2892-94. Americans estimated to be living with complications of stroke1 Of those individuals have a gait impairment2

PoNS Treatment™ for Symptoms of Multiple Sclerosis
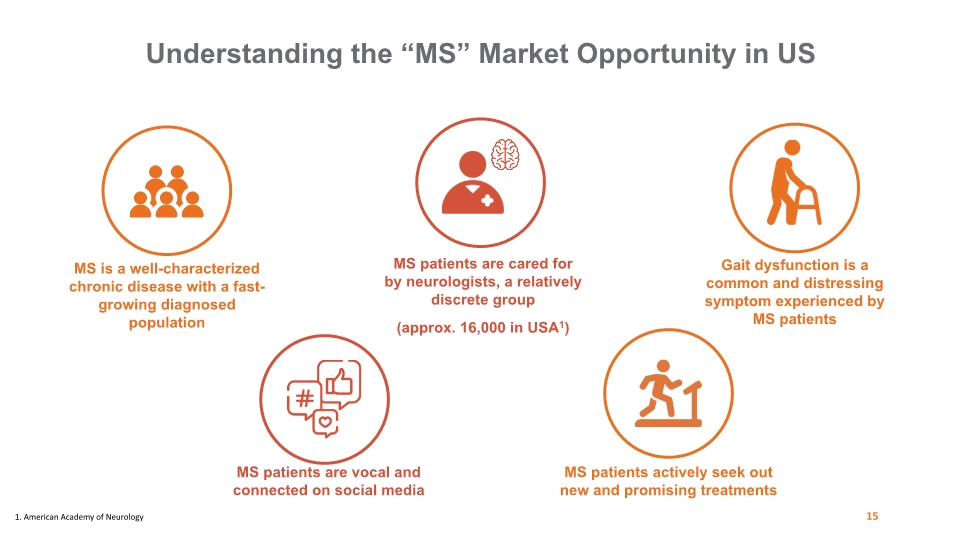
Understanding the “MS” Market Opportunity in US MS is a well-characterized chronic disease with a fast-growing diagnosed population MS patients are vocal and connected on social media MS patients are cared for by neurologists, a relatively discrete group (approx. 16,000 in USA1) Gait dysfunction is a common and distressing symptom experienced by MS patients MS patients actively seek out new and promising treatments 15 1. American Academy of Neurology
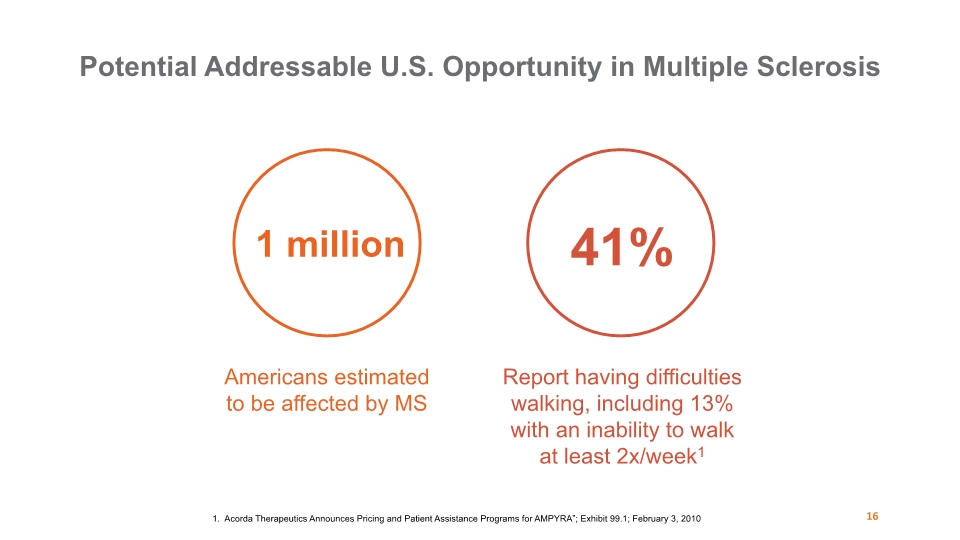
1 million 41% Americans estimated to be affected by MS Report having difficulties walking, including 13% with an inability to walk at least 2x/week1 Potential Addressable U.S. Opportunity in Multiple Sclerosis 16 1. Acorda Therapeutics Announces Pricing and Patient Assistance Programs for AMPYRA”; Exhibit 99.1; February 3, 2010
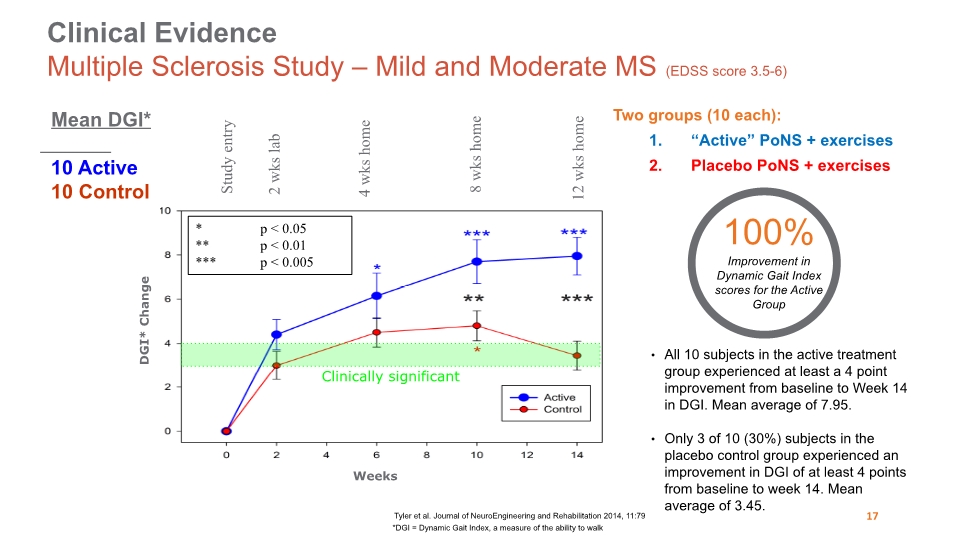
Study entry 2 wks lab 4 wks home 8 wks home 12 wks home Weeks DGI* Change * p < 0.05 ** p < 0.01 *** p < 0.005 Clinically significant Two groups (10 each): “Active” PoNS + exercises Placebo PoNS + exercises Clinical Evidence Multiple Sclerosis Study – Mild and Moderate MS (EDSS score 3.5-6) Tyler et al. Journal of NeuroEngineering and Rehabilitation 2014, 11:79 All 10 subjects in the active treatment group experienced at least a 4 point improvement from baseline to Week 14 in DGI. Mean average of 7.95. Only 3 of 10 (30%) subjects in the placebo control group experienced an improvement in DGI of at least 4 points from baseline to week 14. Mean average of 3.45. Mean DGI* 10 Active 10 Control *DGI = Dynamic Gait Index, a measure of the ability to walk 17
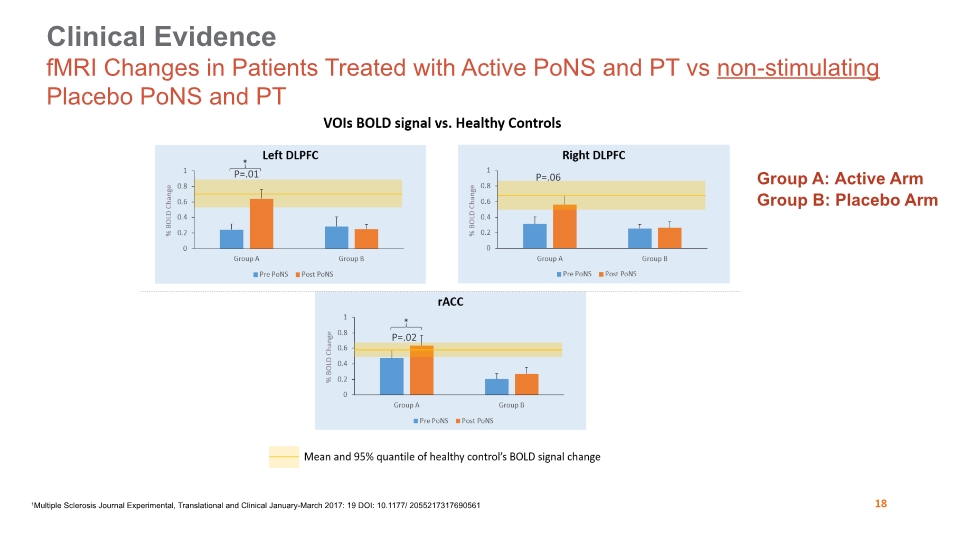
Clinical Evidence fMRI Changes in Patients Treated with Active PoNS and PT vs non-stimulating Placebo PoNS and PT 26 Group A: Active Arm Group B: Placebo Arm Active Arm Placebo Arm Active Arm Active Arm Placebo Arm 1Multiple Sclerosis Journal Experimental, Translational and Clinical January-March 2017: 19 DOI: 10.1177/ 2055217317690561 P=.01 P=.02 P=.06 18
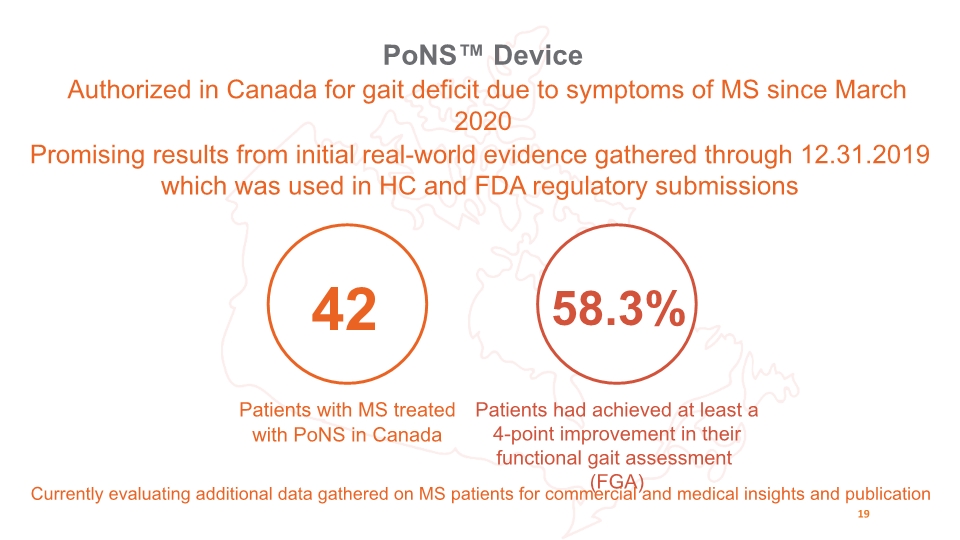
42 Patients with MS treated with PoNS in Canada 58.3% Patients had achieved at least a 4-point improvement in their functional gait assessment (FGA) PoNS™ Device Authorized in Canada for gait deficit due to symptoms of MS since March 2020 19 Promising results from initial real-world evidence gathered through 12.31.2019 which was used in HC and FDA regulatory submissions Currently evaluating additional data gathered on MS patients for commercial and medical insights and publication
20
Current Strategies for Managing Neurological Disorders Prescription Drugs Therapy Surgery Medical Devices 21

Commercialization and Reimbursement

Therapeutic Experience Program Helius sponsored open-label, interventional, observational, clinical study Evaluating PoNS on-label treatment in target population (Multiple Sclerosis) aiming to investigate patients’ adherence and compliance to PoNS therapy regimen Enrolling ~ 50-60 subjects with gait deficit due to mild-moderate MS at Centers of Excellence across the US (10-12 sites) Expected to start enrollment in Q4’ 21 and continue through Q2’ 22. 23

U.S. Pre-Commercial Activities Building out go-to-market strategy (including licensing, territory identification, KOL engagement, etc.) and supporting infrastructure Finalizing distribution model of the PoNS device Targeting commercial launch in Q1’22 with initial cash pay customers, while pursuing commercial and government reimbursement programs Creating Patient Access Programs Identifying and onboarding neuro rehab clinics currently treating MS patients to provide treatment 24

Capitalization & Ownership
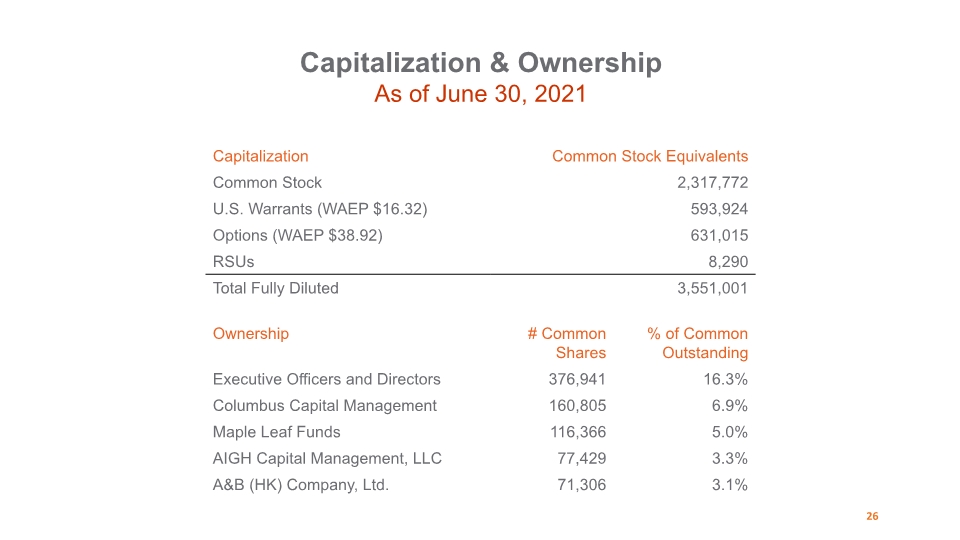
Capitalization & Ownership As of June 30, 2021 26

Dane Andreeff President & CEO Dr. Antonella Favit-Van Pelt Chief Medical Officer 20+ years at Maple Leaf Partners as the General Partner and Portfolio manager, a value-based hedge fund which grew to over $2b in assets Board member and advisor to Helius for over 3 years, Myocardial Solutions for 4 years and HDL Therapeutics, Inc. for over 15 years ~8.8% ownership of the company Board-certified neurologist with 20+ years in the health sciences industry Led U.S. Medical Strategy for the Neurology program of H. Lundbeck A/S Founded and led as President and CEO, Synaerion Therapeutics and its affiliate Thera Neuropharma, Inc. Served as Senior Director and Global Medical Lead at Shire Pharmaceuticals, Director of Medical Strategy at Bristol-Myers Squibb, and as Global Clinical Development Lead at GE Healthcare Jeff Mathiesen, CPA Chief Financial Officer Vice Chair, Lead Independent Director, and Audit Committee Chair of Panbela Therapeutics, Inc. (Nasdaq: PBLA) Director and Audit Committee Chair of NeuroOne Medical Technologies Corporation (OTCQB: NMTC) Former Board Member and Audit Committee Chair of eNeura, Inc. Former CFO at Gemphire Therapeutics and Sunshine Heart Executive Team Experienced Leadership With Healthcare and Commercialization Expertise 25+ years combined experience in Medical Device and Pharmaceutical Sales 16+ years in the medical devices industry with extensive commercialization expertise in neuromodulation sales and marketing Former VP of North America Sales and Marketing, LivaNova and legacy Cyberonics (Cyberonics experience included global responsibility) Pharma Experience - Launched and sold multiple pharmaceuticals including Tiazac, Celexa, Monourol, and Lexapro Frederick Fantazzia VP, Sales & Marketing North America 27

Sherrie Perkins Director 20 years neuromodulation medical device experience in neurology, psychiatry, and sleep medicine Former Marketing and Business Development Executive, Cyberonics, Inc. (Nasdaq: CYBX) and LivaNova PLC (Nasdaq: LIVN) Former member Board of Directors, eNeura, Inc. Former member Board of Directors, ImThera Medical, Inc. Ed Straw Director Founder, Managing Partner of Osprey Venture Partners Chairman of Odyssey Logistics Member of the Board of Directors of Performance Equity Management, Capital Teas and Document Capture Technologies, Inc. Former President, Global Operations, Estee Lauder Former SVP, Global Manufacturing and Supply Chain Management at Compaq Computer Corporation Distinguished 3-star Admiral, US Navy Blane Walter Chairman of the Board Partner, Talisman Capital Partners Vice Chair of InVentiv Health Chair of the Governor of Ohio’s Executive Workforce Board Former CEO of InVentiv Health Former Founder of InChord Communications Non-Executive Directors Experienced Leadership With Healthcare and Commercialization Expertise 28

Paul Buckman Director Founder and Co-Inventor of PoNS™ technology Co-founder of Wicab, Inc and former VP, Research and Development Lead Inventor of the BrainPort Balance Device Former University of Wisconsin Biomedical Engineering faculty member MS, Biomedical Engineering and Registered Professional Engineer Non-Executive Directors Experienced Leadership With Healthcare and Commercialization Expertise Mitch Tyler Director Thirty-nine years of medical device experience in general management, sales, marketing, finance, international and operations President, North America – LivaNova PLC Former President of the Cardiovascular Divisions of both Boston Scientific and St. Jude Medical Director on the Boards of NeuroOne (Chairman), Miromatrix (Chairman), Ablative Solutions, ActivOrtho, Inc. (Co-Founder), and Shoulder Innovations 29

Helius MS Scientific Advisory Board and Key Opinion Leaders 30

Extensive IP Portfolio Exclusively licensed from inventors (4% royalty): 9 US Medical Method Patents Issued Patents expire between 2029 and 2031 Patents owned by Helius (no royalty): 29 US Patents Issued 41 Foreign Patents Issued Patents expire between 2026 and 2040 Helius Patents Transferred to China Medical System Holdings (CMS): 3 Chinese Design Patents Independent Verification of Patents and Freedom to Operate Opinion: September 2017 31

First-in-Class Neurotech Unique and innovative treatment authorized to improve impaired function by stimulating cranial nerves via the tongue, supported by an extensive IP portfolio US authorization in gait deficit due to MS MS launch targeted Q1’22 in US FDA Breakthrough Designation granted for the treatment of balance and gait deficits due to symptoms of stroke 32

Thank you NASDAQ:HSDT
