Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | h9142108k.htm |
Exhibit 99.1

Humanigen September 2021

2 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation are forward - looking statements . Forward - looking statements reflect management's current knowledge, assumptions, judgment, and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward - looking statements . Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without limitation, statements regarding the sufficiency of the data from the ACTIV - 5 /BET - B study to warrant a future submission of a new EUA request ; statements regarding our efforts to request and receive Marketing Authorization or Conditional Marketing Authorization for lenzilumab in COVID - 19 in the U . K . and other territories ; our expectations for the duration and severity of COVID - 19 in the United States and around the world and our projections for COVID - 19 hospitalizations in 2021 and future years ; our projections regarding the need for lenzilumab as a therapeutic if authorized or approved ; the commercial potential of lenzilumab and our ability to maintain a single worldwide price in the multiple jurisdictions in which we are seeking marketing authorizations or approvals or otherwise working to sell product prior to formal approvals ; and our other plans to initiate or participate in planned clinical trials and otherwise explore the effectiveness of lenzilumab and other candidates in our development portfolio as therapies for other inflammation and immune - oncology indications . Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability and need for additional capital to grow our business ; our dependence on partners to further the development of our product candidates ; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product ; the outcome of pending or future litigation ; and the various risks and uncertainties described in the "Risk Factors" sections of our latest annual and quarterly reports and other filings with the SEC . All forward - looking statements are expressly qualified in their entirety by this cautionary notice . You should not rely upon any forward - looking statements as predictions of future events . We undertake no obligation to revise or update any forward - looking statements made in this presentation to reflect events or circumstances after the date hereof, to reflect new information or the occurrence of unanticipated events, to update the reasons why actual results could differ materially from those anticipated in the forward - looking statements, in each case, except as required by law .

3 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Company Overview Leading expertise in Hyper - inflammation and Cytokine Storm across multiple therapeutic applications Filed for for Conditional Marketing Authorization in UK, commercial readiness in the UK underway; preparing EMA submission for EU 520 patient Phase 3 study (‘LIVE - AIR’) in hospitalized COVID - 19 patients Ph 1b CAR - T study completed with Yescarta ; aGvHD and CMML studies planned to start Q4; ~250 patient CAR - T study 2022 NIH - sponsored Phase 2/3 ACTIV - 5/BET - B: hospitalized COVID - 19 patients targeting 400 patients in baseline CRP<150mg/L and up to 550 total 1 2 3 4 5

4 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Business Update: September 2021 Lenzilumab for COVID - 19 • September 8, 2021 FDA response to EUA submission: • Declined EUA at this time - invited Humanigen to submit additional data as it becomes available • No safety issues identified by FDA, noted relatively limited size of safety database • Additional data may be be derived both from LIVE - AIR and ACTIV - 5/BET - B • UK Rolling Rapid - 19 Review of Marketing Authorization Application (MAA) underway • Final module submissions expected before end of September 2021 Other key programs • CMML and aGvHD studies target FPD Q4, 2021 • CAR - T study protocol to be submitted to FDA for review and approval, target FPD 1H, 2022
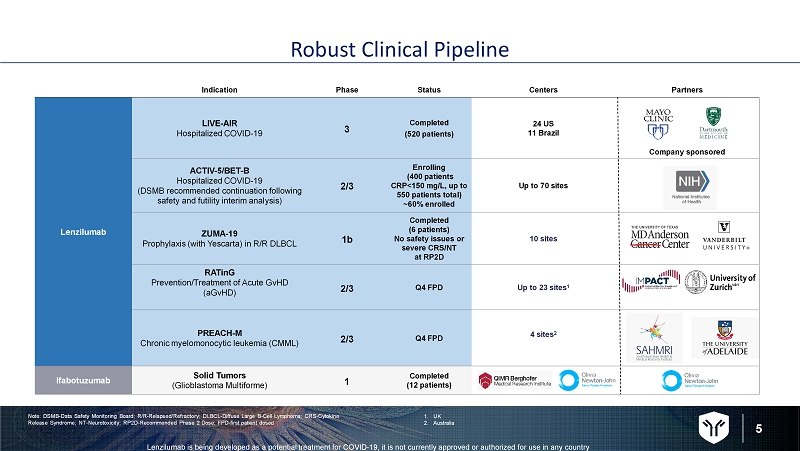
5 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Robust Clinical Pipeline Indication Phase Status Centers Partners Lenzilumab LIVE - AIR Hospitalized COVID - 19 3 Completed (520 patients) 24 US 11 Brazil Company sponsored ACTIV - 5/BET - B Hospitalized COVID - 19 (DSMB recommended continuation following safety and futility interim analysis) 2/3 Enrolling (400 patients CRP<150 mg/L, up to 550 patients total) ~60% enrolled Up to 70 sites ZUMA - 19 Prophylaxis (with Yescarta ) in R/R DLBCL 1b Completed (6 patients) No safety issues or severe CRS/NT at RP2D 10 sites RATinG Prevention/Treatment of Acute GvHD ( aGvHD ) 2/3 Q4 FPD Up to 23 sites 1 PREACH - M Chronic myelomonocytic leukemia (CMML) 2/3 Q4 FPD 4 sites 2 Ifabotuzumab Solid Tumors (Glioblastoma Multiforme) 1 Completed (12 patients) Note: DSMB - Data Safety Monitoring Board; R/R - Relapsed/Refractory; DLBCL - Diffuse Large B - Cell Lymphoma; CRS - Cytokine Release Syndrome; NT - Neurotoxicity; RP2D - Recommended Phase 2 Dose; FPD - first patient dosed 1. UK 2. Australia

6 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry LIVE - AIR Demographics and Baseline Characteristics ( mITT Population) Characteristics Lenzilumab (N=236) Placebo (N=243) Overall (N=479) Age Mean (SD) 60.5 (13.5) 60.5 (14.3) 60.5 (13.9) Median (Min - Max) 62.0 (28 - 98) 62.0 (22 - 96) 62.0 (22 - 98) < 65 years old (%) 60.2 58.4 59.3 ≥65 years old (%) 39.8 41.6 40.7 >80 years old (%) 7.6 5.3 6.5 Gender Male (%) 64.8 64.6 64.7 Race American Indian/Alaskan Native, % 1.7 0.0 0.8 Asian, % 4.2 2.1 3.1 Black/African American, % 16.1 13.6 14.8 White, % 69.9 73.3 71.6 Multiple, % 0.4 0.0 0.2 Other, % 7.6 11.1 9.4 Ethnicity Hispanic or Latino, % 35.2 42.0 38.6 Not Hispanic or Latino, % 64.0 56.8 60.3 Body Mass Index Mean (SD) 33.1 (8.4) 31.0 (7.9) 32.5 (8.2) ≥30 Kg/m², % 57.6 52.7 55.1 Clinical Status at Baseline SpO 2 ≤94% or low - flow oxygen 61.9 57.6 59.5 High - flow oxygen or NIPPV 38.1 42.8 40.5 CRP Median 77.0 82.0 79.0 CRP <150 mg/L, % 75.8 79.9 77.9 CRP >150 mg/L, % 24.2 20.1 22.1

7 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Study Primary Endpoint Met Kaplan - Meier Estimates For IMV and/or Death Failure to achieve survival without ventilation was defined as mortality or the requirement for IMV Fewer patients required IMV or died with lenzilumab treatment compared to placebo 15.6 22.1 0 5 10 15 20 25 Lenzilumab Placebo 95% CI: 17.4 - 27.9 95% CI: 11.5 - 20.9 Invasive Mechanical Ventilation (IMV) and/or death mITT population (n=479) P =0.0403 Hazard Ratio: 1.54 (95% CI: 1.02 - 2.32); P=0.0403 Kaplan - Meier Estimate (percentage of subjects)

8 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Patients With Baseline CRP<150 mg/L Required IMV Or Died *Study was not powered to demonstrate a difference in mortality 4.0 29.3 0 10 20 30 40 Lenzilumab Placebo 95% CI: 15.1 - 51.9 95% CI: 0.6 - 25.2 SWOV in Black/African - American Patients: CRP<150 mg/L* mITT population (n=51) P =0.0418 Hazard Ratio: 8.90 (95% CI: 1.08 - 73.09) Kaplan - Meier Estimate (percentage of patients) 9.8 21.2 0 10 20 30 40 Lenzilumab Placebo 95% CI: 15.9 - 27.9 95% CI: 6.1 - 15.4 SWOV CRP<150 mg/L* mITT population (n=351) P =0.0009 Hazard Ratio: 2.54 (95% CI: 1.46 - 4.41) Kaplan - Meier Estimate (percentage of patients)

9 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Safety Balanced Between Treatment Arms System Organ Class Preferred Term n (%) Lenzilumab (N=255) Placebo (N=257) Overall (N=512) Any AE ≥ Grade 3 68 (26.7) 84 (32.7) 152 (29.7) Respiratory, thoracic, and mediastinal disorders 64 (25.1) 71 (27.6) 135 (26.4) Cardiac disorders 15 (5.9) 14 (5.4) 29 (5.7) Infections and infestations 10 (3.9) 16 (6.2) 26 (5.1) Vascular disorders 10 (3.9) 15 (5.8) 25 (4.9) Renal and urinary disorders 5 (2.0) 11 (4.3) 16 (3.1) General disorders and administration site conditions 4 (1.6) 11 (4.3) 15 (2.9) No Suspected Unexpected Serious Adverse Reactions (SUSARS) or SAEs attributed to lenzilumab were reported No increased risk of infections, cases of PAP, or serious infusion related reactions were reported with lenzilumab FDA noted relatively limited size of safety database but did not identify any safety issues

10 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Cardiac SAEs from LIVE - AIR Preferred Term n (%) Lenzilumab (N=255) Placebo (N=257) Overall (N=512) All cardiac SAEs 12 (4.7) 13 (5.1) 25 (4.9) Cardiac arrest/cardio - respiratory arrest 11 (4.3) 8 (3.1) 19 (3.7) AMI 0 (0.0) 3 (1.2) 3 (0.6) The overall incidence of cardiac SAEs was equivalent between the treatment groups All subjects had significant underlying conditions FDA noted relatively limited size of safety database but did not identify any safety issues
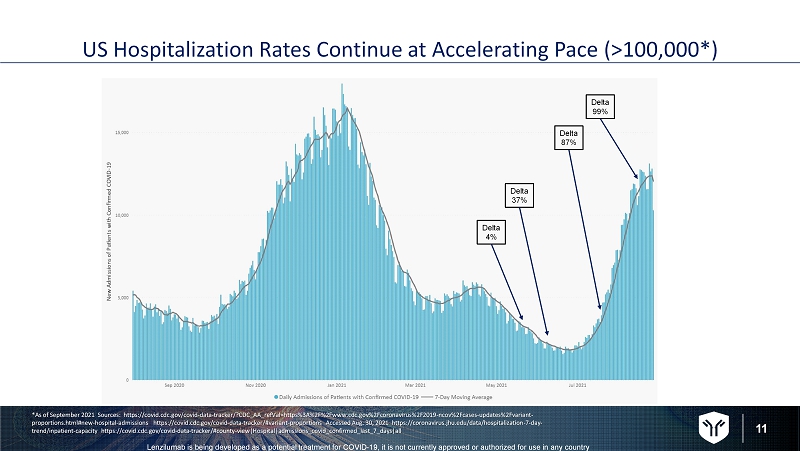
11 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry US Hospitalization Rates Continue at Accelerating Pace (>100,000*) *As of September 2021 Sources: https://covid.cdc.gov/covid - data - tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus %2F2019 - ncov%2Fcases - updates%2Fvariant - proportions.html#new - hospital - admissions https://covid.cdc.gov/covid - data - tracker/#variant - proportions Accessed Aug. 30, 2021 https://coronavirus.jhu.edu/data/hospitalization - 7 - day - trend/inpatient - capacity https://covid.cdc.gov/covid - data - tracker/#county - view|Hospital|admissions_covid_confirmed_last_7_days| all Delta 4% Delta 37% Delta 87% Delta 99%

Lenzilumab in the United Kingdom

13 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Multiple Agency Interactions x Introductory meeting held with Rapid C - 19 multi - agencies (MHRA, Therapeutics Task Force (TTF), NICE, NHSE, DHSC) x MAA accepted for rolling review July 7, classified as “COVID rolling review” x Information request provided to NICE, multiple interactions □ All modules to expected to be submitted by end of September 2021 □ Additional data from LIVE - AIR being furnished to MHRA, similar to additional data to be furnished to FDA following EUA assessment letter □ Supply chain and local UK distributor agreements at or close to completion □ Additional meetings scheduled with DHSC, NHSE and NICE 1. https://www.gov.uk/government/collections/mhra - guidance - on - coronavirus - covid - 19 4. https://www.gov.uk/government/groups/the - covid - 19 - therapeutics - taskforce 5. https://www.pulsetoday.co.uk/news/clinical - areas/infectious - diseases/antiviral - drug - remdesivir - approved - for - covid - 19 - treatment - in - the - uk/ 2. https://www.gov.uk/guidance/apply - for - a - licence - to - market - a - medicine - in - the - ukf Approved Hospital Therapeutics *Tocilizumab Dexamethasone Remdesivir Sarilumab *Tocilizumab authorized by MHRA prior to FDA
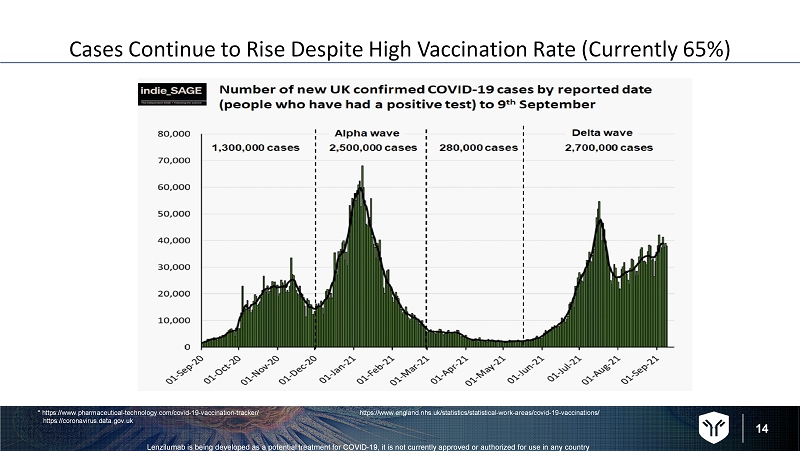
14 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Cases Continue to Rise Despite High Vaccination Rate (Currently 65%) * https://www.pharmaceutical - technology.com/covid - 19 - vaccination - tracker/ https://coronavirus.data.gov.uk https://www.england.nhs.uk/statistics/statistical - work - areas/covid - 19 - vaccinations/

15 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Key UK Statistics (as of September 12, 2021) • >134,000 deaths • Daily death rate 30% vs. September 5 • 30,000 people tested positive September 11 • 7.2 million cases to date • > 8,000 people hospitalized ( 14% from prior 11 days) Potential Demand based on current: • Hospitalizations > 8,000 • Concentration of cases in NorthWest England https://www.msn.com/en - gb/news/uknews/uk - covid - death - toll - exceeds - 134000 - as - hospitalisation - numbers - rise/ar - AAOla8Q https://www.independent.co.uk/topic/death - toll

16 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry UK Commercial Preparation UK launch plan developed in parallel with US preparation and leverages multiple tools already created in anticipation of US launch • Market research shows potentially positive acceptance of lenzilumab value proposition by physicians • Importation and distribution channels identified and in preparation • Budget impact model developed, collaborating with NICE to refine • Local resources in place, key account managers recruitment underway • US MSLs already hired and trained to be deployed to UK account • Scientific Advisory Board scheduled this month

Clinical Development Pipeline
18 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry ZUMA - 19 Phase 1b Results • Lenzilumab in combination with CAR - T in DLBCL demonstrated a 100% objective response rate (ORR) at the recommended Phase 2 dose • No severe cytokine release syndrome or severe neurotoxicity • Lenzilumab reduced IL - 6, CRP, ferritin, MCP - 1, IL - 8, and IP - 10 (CXCL - 10) in a dose - dependent fashion • Plans to conduct a potentially registrational Phase 2 study with all 3 currently commercially available CAR - T therapies in DLBCL Note: DLBCL - Diffuse Large B - Cell Lymphoma; CRP - C - Reactive Protein

19 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Significant CAR - T Market Growth Expected DLBCL patients receiving commercial CAR - T Therapy Lenzilumab + CAR - T CAR - T Primary outcome : Efficacy and Toxicity At 6 months N≈250 2H23 Follow up : 2 years Randomize Phase 2 trial intended to address these barriers FPI 1Q22 1. “Global CAR - T Therapy Market Report 2020” Research And Markets https://www.prnewswire.com/news - releases/global - car - t - therapy - mar ket - report - 2020 - market - is - expected - to - stabilize - and - reach - 3 - 150 - million - in - 2025 --- covid - 19 - impact - and - recovery - forecast - to - 2030 -- 301218802.html Feb. 1, 2021 2. Journal of Clinical Pathways. 2017;3(7):31 - 35 The C AR - T market is expected reach $6.1 billion by 2030 1 Multiple barriers to stronger uptake of CAR - T therapy 2 1/3 of physicians surveyed think toxicity might hinder their use of CAR - T Over 60% suggested that patients should be monitored for 1 to 2 weeks after administration

20 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Acute Graft vs. Host Disease ( aGvHD ): Growing Market, High Unmet Need Steroids used for initial treatment of aGvHD 2 - 6 ~50% of patients do not respond adequately to steroids 2 - 6 Steroid - resistant aGvHD patients have a very poor prognosis >90% mortality 7 Allogeneic Stem Cell Transplant aGvHD Diagnosis Outcome prediction using “MAGIC” algorithm SOC Lenzilumab + SOC V Placebo + SOC Low Risk Intermediate/ High Risk The “ RATinG ” Study 1 . Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2020 2. First and second - line s ystemic treatment of acute graft - versus - host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2021;18(8):1150 - 1163 3. Diagnosis and management of acute graft - versus - host disea se. Br J Haematol . 2012;158(1):30 - 45. 4. Response of 443 patients to steroids as primary therapy for acute graft - versus - host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387 - 394. 5. EBMT - NIH - CIBMTR Task Force posi tion statement of standardized terminology & guidance for graft - versus - host disease assessment. Bone Marrow Transplant. 2018;53(11):1401 - 1415. 6. New and emerging therapies for acute and chronic graft - versus - host disease. Ther Adv Hermatol . 2018;9(1):21 - 46. 7. Steroid - refractory acute GVHD: Predictions and outcomes. Advances in Hematology 2011
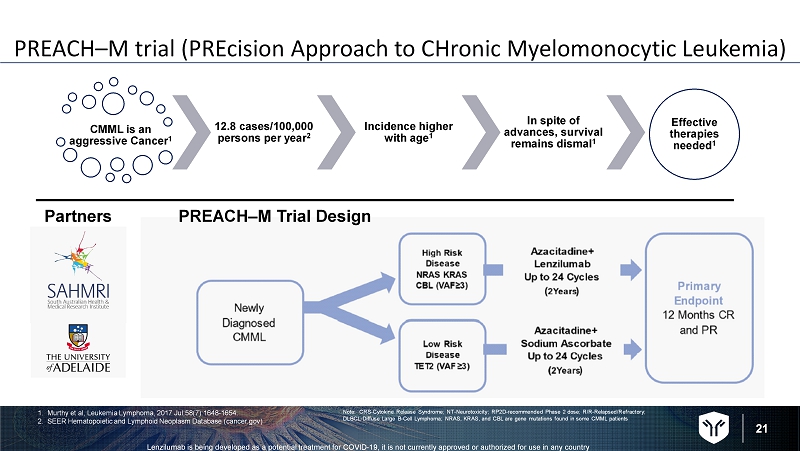
21 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry PREACH – M trial ( PREcision Approach to CHronic Myelomonocytic Leukemia) CMML is an aggressive Cancer 1 12.8 cases/100,000 persons per year 2 Incidence higher with age 1 In spite of advances, survival remains dismal 1 Effective therapies needed 1 PREACH – M Trial Design Note: CRS - Cytokine Release Syndrome; NT - Neurotoxicity; RP2D - recommended Phase 2 dose; R/R - Relapsed/Refractory; DLBCL - Diffuse Large B - Cell Lymphoma; NRAS, KRAS, and CBL are gene mutations found in some CMML patients Partners 1. Murthy et al, Leukemia Lymphoma, 2017 Jul;58(7):1648 - 1654. 2. SEER Hematopoietic and Lymphoid Neoplasm Database (cancer.gov)

22 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry MRI ( T1 + C) 89 Zr - ifabotuzumab PET 18 F - FDG PET Phase 1 study: multiple tumors Breast Lung Colorectal Pancreas No normal tissue uptake of 89 Zr - ifabotuzumab Radio - labelled ifabotuzumab showed rapid, specific targeting of GBM tumor Activity in Glioblastoma Multiforme Phase 1 Ifabotuzumab Data in GBM and Development Plan

23 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Financial Overview Balance (based on 10 - Q filing as of June 30, 2021) • Cash and cash equivalents $120MM • Q2 manufacturing scale - up, advance purchasing of raw materials and production slots ~$57MM • Future spending expected to be significantly reduced • ACTIV - 5 paid for by NIH • aGvHD , CMML trials majority paid for by partners • HGEN infrastructure remains minimal Hercules Loan Facility • Total credit available $80MM • Initial draw (3 - 29 - 21) $25MM • Interest only period for 18 months

24 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry 12 Month Goals □ Complete regulatory submission for lenzilumab in hospitalized COVID - 19 in the UK in September and commence commercialization if authorized □ Short - term: Provide additional data from LIVE - AIR to support EUA application in US □ Medium - term: Provide additional data from ACTIV - 5/BET - B to support EUA application in US □ Initiate Phase 2 potential registrational CAR - T, Phase 2/3 acute GvHD, Phase 2/3 CMML studies □ Exploring other revenue - generating opportunities □ Carefully manage cash
