Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Sorrento Therapeutics, Inc. | tm2127470d1_8k.htm |

Saving Life TM Medicine September 20 21 NASDAQ : SRNE © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 1 Exhibit 99.1

Certain statements contained in this presentation or in other documents of Sorrento Therapeutics, Inc . (the “Company” or “Sorrento”) and of any of its affiliates, along with certain statements that may be made by management of the Company orally in presenting this material, are or may be considered “forward - looking statements” as defined in the Private Securities Litigation Reform Act of 1995 . These statements can be identified by the fact that they do not relate strictly to historic or current facts . They use words such as "estimate," "expect," "intend," "believe," "plan," "anticipate," “potential,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition . Sorrento cautions that these statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties . Statements regarding future action, future perfo rmance and/or future results including, without limitation, those relating to the timing fo r completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same, and receipt by the Company of milestone and royalty payments may differ from those set forth in the forward - looking statements . Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate . The Company assumes no obligation to update forward - looking statements as circumstances change . Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company ’ s most recent periodic reports filed with the Securities and Exchange Commission, including Sorrento’s Annual Report on Form 10 - K for the year ended December 31 , 2020 and subsequent Quarterly Reports on Form 10 - Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings . In presenting this material or responding to inquiries in connection with a presentation, management may refer to results, projections or performance measures that are not prepared in accordance with U . S . Generally Accepted Accounting Principles (“GAAP”) as reported in the Company’s SEC filings . These results, projections or performance measures are non - GAAP measures and are not intended to replace or substitute for results measured under GAAP and are supplement al to GAAP reported results . Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement . It is not possible to predict or identify all such risks, contingencies and uncertainties . The Company identifies some of these factors in its SEC filings on Forms 10 - K, 10 - Q and 8 - K, including Sorrento’s Annual Report on Form 10 - K for the year ended December 31 , 2020 and subsequent Quarterly Reports on Form 10 - Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings . I nvestors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial performance . Sorrento® and the Sorrento logo are registered trademarks of Sorrento Therapeutics, Inc . Forward - Looking Statements and Non - GAAP Financial Information © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 2

About the COMPANY • Funded and Operational in 2009 • NASDAQ: SRNE • HQ in San Diego, CA ~ 700 Employees worldwide ~100 Employees with PhDs & MDs ~5 00,000 SF Research and cGMP Manufacturing Facilities • 7 cGMP Manufacturing Sites (5 USA, 2 China) mAbs, Small Molecule, ADC, Plasmid DNA, Lymphatic Drug Delivery Device, Cell Therapies, Oncolytic Viruses, and Fill & Finish • 1 FDA Approved Drug ZTlido® (lidocaine topical system)1.8% • Multiple Products in Late - Stage Clinical Development for Non - Opioid Pain Management, Immune - Oncology, Inflammation and Autoimmune Diseases and COVID - 19 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 3

Transforming GMAB Ρ Innovative Platforms into Saving Life Ρ Medicines 4 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
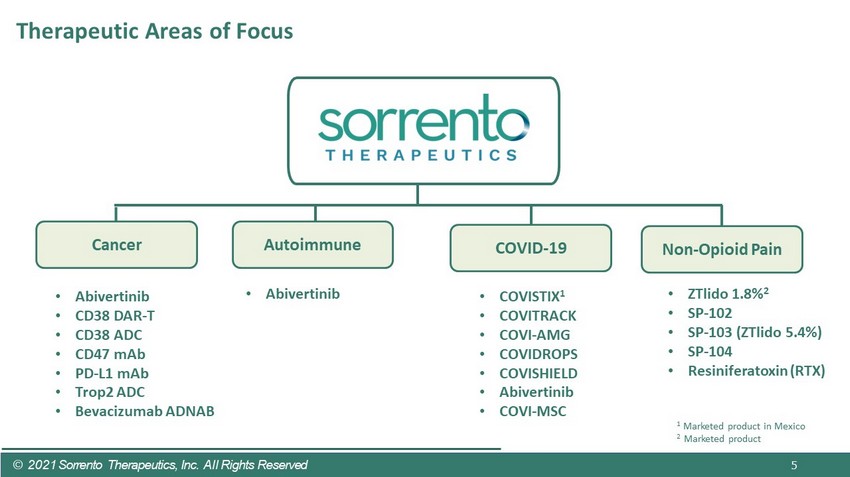
Cancer © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 5 Autoimmune COVID - 19 Non - Opioid Pain • Abivertinib • CD38 DAR - T • CD38 ADC • CD47 mAb • PD - L1 mAb • Trop2 ADC • Bevacizumab ADNAB • Abivertinib • COVISTIX 1 • COVITRACK • COVI - AMG • COVIDROPS • COVISHIELD • Abivertinib • COVI - MSC • ZTlido 1.8% 2 • SP - 102 • SP - 103 ( ZTlido 5.4%) • SP - 104 • Resiniferatoxin (RTX) 1 Marketed product in Mexico 2 Marketed product Therapeutic Areas of Focus

Transforming Platforms to Saving Life Ρ Medicines Platforms to Innovative Products G - MAB • PD - L1 mAb • CD - 47 mAb • Anti - COVID nAbs COVISHIELD nAbs • ROR1 • Sofusa Lymphatic Delivery o TNF, PD1, CTLA4 • CD38 ADC • BCMA ADC • Trop2 ADC • VEGF ADNAB • CD20 ADNAB • PD - L1 ADNAB • CD38 DAR - T • BCMA DAR - T • PD - L1 (C/DAR - T) • CyCART - 19 (Partnered) • COVI - MSC • Seprehvec • ZTlido • SP - 102 • SP - 103 • SP - 104 • Resiniferatoxin (RTX) • Abivertinib • Cannabidiol (CBD) © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 6

Cancer Programs 7 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

Cancer Pipeline © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 8 Key Programs Indication Preclinical FDA Approved Abivertinib NSCLC Abivertinib B Cell Lymphomas PD-L1* SCLC * In China PD-L1* Cervical Cancer * In China CD47 Solid Tumors CD38 DAR-T Multiple Myeloma CD38 ADC Amyloidosis and Multiple Myeloma TROP2 ADC* Solid Tumors * In China Seprehvec™ oncolytic virus Solid Tumors; CNS Tumors BCMA ADC Liquid Tumors Bevacizumab-ADNAB™ Endometrial Cancer In partnership with Mayo Clinic Bevacizumab-ADNAB™ Ovarian Cancer In partnership with Mayo Clinic Rituximab-ADNAB™ B-cell Lymphomas In partnership with Mayo Clinic Phase III/PivotalPhase I Phase II

9 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved ABIVERTINIB (STI - 5656): NSCLC and B Cell Lymphomas • Abivertinib maleate (STI - 5656) is a small molecule third - generation tyrosine kinase inhibitor (TKI) for both mutant epidermal growth factor receptor (EGFR) and Bruton tyrosine kinase (BTK) receptor. • Abivertinib inhibits the gatekeeper mutation of EGFR; T790M, as well as the common activating mutations (L858R, 19del), and has minimal inhibitory activity against the wild type (WT) EGFR, contributing to its observed safety profile. • Abivertinib has good tolerability at oral doses up to 600 mg daily. • Pivotal study in NSCLC has been completed and is pending final review of 227 patients with a longer follow - up period. • Phase 1/2 study in patients with mantle cell lymphoma has been completed. • Phase 2 study in hairy cell leukemia is in startup.

Abivertinib Efficacy in EGFR T790M + NSCLC Patients (Interim Data Analyses Presented at ASCO 2019) © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 10 Among 209 response evaluable patients, per investigator assessment: 93.3% (n/N: 195/209) subjects achieved tumor shrinkage at target lesions 68.4% (n/N: 143/209) subjects achieved target lesion PR (tumor shrinkage ≥ 30%) 26 months OS

COVID - 19 Programs 11 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

COVID - 19 Portfolio © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 12 Key Programs Indication Preclinical FDA Approved COVISTIX™ (diagnostic) Antigen Test Emergency Use Authorization (EUA) Approval by COFEPRIS in Mexico and cleared for commercialization COVIDROPS™ (treatment) Neutralizing Antibody (Intranasal) in Outpatients COVI-AMG™ (treatment) Neutralizing Antibody (IV) in Outpatients COVISHIELD™ (treatment) Neutralizing Antibody (IV and IN) in Outpatients and Inpatients ABIVERTINIB (treatment) Severe or Critical COVID-19 in ICU Patients COVI-MSC (treatment) ARDS due to COVID-19 in ICU Patients MPI8 (treatment) Anti-viral SALICYN - 30 (treatment) Anti-viral Multi-Valent RBD Vaccine Multi-Valent mRNA Vaccine Phase III/PivotalPhase IIPhase I

13 Largest Pipeline of Innovative COVID - 19 Solutions PREVENT DETECT TREAT MultiValent mRNA Vaccine COVISTIX Ρ COVITRACK Ρ SARS - CoV - 2 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved COVIDROPS Ρ COVI - AMG Ρ Antivirals RESCUE Abivertinib Ρ COVI - MSC Ρ Potent neutralizing antibodies for outpatient use BTK Inhibitor Mesenchymal Stem Cells Platinum Colloid Based Diagnostics (PtC) MultiValent RBD Vaccine Protein mRNA Antigen Antibody Lymphatic Drug Delivery Microneedle

14 DETECT EARLY – TREAT TIMELY © 202 1 Sorrento Therapeutics, Inc. All Rights Reserved … to save lives Abivertinib (STI - 5656) “When given early, neutralizing antibodies given IV have shown a > 70% reduction in hospitalizations” 1 CDC, July 23, 2021 COVID cases are on the rise now in 90% of US juristictions 1

Diagnostics 15 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

• Simple: Eliminates laborious extraction step and is designed for potential point - of - care (moderately trained technicians) or at - home use • Accurate: Rapid and highly sensitive platinum colloid - based lateral flow immunoassay to detect SARS - CoV - 2 virus antigens • Rapid: Produces results in less than 15 minutes • Scalable: Designed to be ideally suited for both single user and high - traffic point - of - care testing or at - home use • Convenient: Simple nasal swab and free COVISTIX App for IFU instructions and reporting • Offered at Low Cost: Expected to be offered at low cost for mass consumption • Versatile: Platform may be adapted to detect multiple pathogens simultaneously (COVITRACK IgG/IgM, RESPISTIX Multiplex) EUA Clearance by COFEPRIS in Mexico (2 June 2021) COVISTIX Ρ - Sensitive and Simple Virus Antigen Test © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 16

Therapeutics 17 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
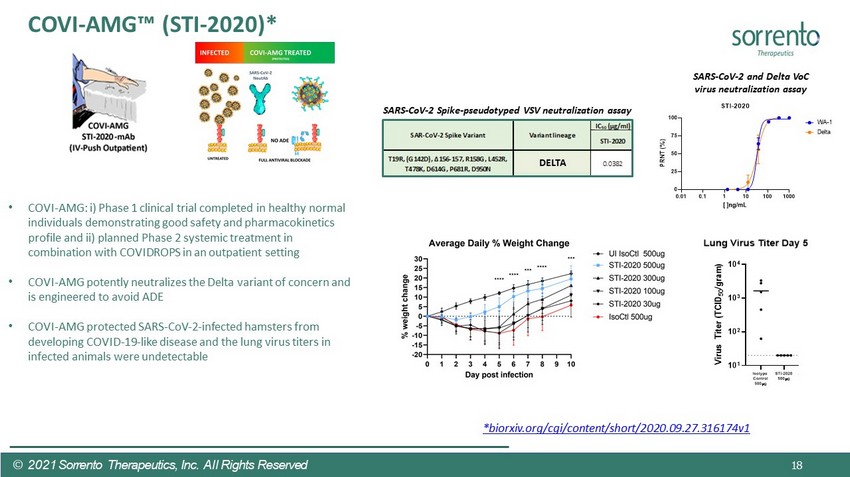
COVI - AMG Ρ (STI - 2020)* 18 • COVI - AMG: i) Phase 1 clinical trial completed in healthy normal individuals demonstrating good safety and pharmacokinetics profile and ii) planned Phase 2 systemic treatment in combination with COVIDROPS in an outpatient setting • COVI - AMG potently neutralizes the Delta variant of concern and is engineered to avoid ADE • COVI - AMG protected SARS - CoV - 2 - infected hamsters from developing COVID - 19 - like disease and the lung virus titers in infected animals were undetectable © 2021 Sorrento Therapeutics, Inc. All Rights Reserved *biorxiv.org/cgi/content/short/2020.09.27.316174v1 SARS - CoV - 2 Spike - pseudotyped VSV neutralization assay SARS - CoV - 2 and Delta VoC virus neutralization assay DELTA

COVIDROPS Ρ (STI - 2099)* 19 • COVIDROPS is an intranasal formulation of the highly potent neutralizing antibody STI - 2020 (COVI - AMG) • COVIDROPS: completed a Phase 1 safety and pharmacokinetic study in healthy volunteers and a Phase 2 study in outpatients with mild COVID - 19 disease is in process in the United Kingdom and the US. Separate studies are planned in Mexico. • A single intranasal administration of COVIDROPS prevented disease - associated weight loss in treated hamsters. The impact of the treatment was observed within 24 hours of STI - 2099 treatment, demonstrating unique disease treatment properties as compared to intravenously administered antibodies • Remarkably, animals treated with intranasal COVIDROPS showed evidence of prevention of disease progression with limited weight loss and reduced duration of disease symptoms as compared to animals treated with intravenous COVI - AMG • The intranasal route is expected to be enabled by the high potency of the antibody and is potentially promising against this highly contagious respiratory pathogen • STI - 2099 has the potential to be broadly deployable for early treatment in an outpatient setting and administered immediately upon detection * https://www.biorxiv.org/content/10.1101/2020.10.28.359836v1 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

COVISHIELD Antibody: STI - 9167 Potently Neutralizes Multiple SARS - CoV - 2 Variants 20 • STI - 9167 developed at Sorrento in collaboration with scientists at the Mt. Sinai Center for Therapeutic Antibody Development • STI - 9167 displays potent in vivo neutralization of SARS - CoV - 2 and Variants of Concern, including Alpha, Beta, Gamma, Delta, and Lambda • As with COVI - AMG, the STI - 9167 Fc region has been engineered with the LALA modification to mitigate the risk of ADE • STI - 9167 is being developed for use as an IV or IN administered therapeutic for i) outpatients and ii) hospitalized COVID - 19 patients © 2021 Sorrento Therapeutics, Inc. All Rights Reserved Antibody IC 50 ( m g/ml) STI-9167 Δ69/70, Δ144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H ALPHA 0.0029 D80A, D215G, Δ242/244, K417N, E484K, N501Y, D614G, A701V BETA 0.0195 L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I GAMMA 0.0063 T19R, (G142D), Δ156-157, R158G, L452R, T478K, D614G, P681R, D950N DELTA 0.0054 T19R, (G142D), Δ156-157, R158G, K417N, L452R, T478K, D614G, P681R, D950N DELTA PLUS 0.0033 D614G, S13I, W152C, L452R EPSILON 0.0040 E484Q, F565L, D614G, V1176F ZETA 0.0034 G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H, H1101D KAPPA 0.0090 G75V, T76I, R246N, Δ247-253, L452Q, F490S, D614G, T859N LAMBDA 0.0027 T95I, Y144T, Y145S, ins146N, R346K, E484K, N501Y, D614G, P681H, D950N MU 0.0202 SAR-CoV-2 Spike Variant Variant lineage SARS - CoV - 2 Spike - pseudotyped VSV neutralization assay LUNG VIRUS TITER DATA DAY 5 LUNG VIRUS TITER DATA DAY 5 0 2 4 6 Alpha S A R S - C o V - 2 t i t e r s ( l o g 1 0 P F U / l u n g ) 500mg IsoCtl 500mg STI-9167 0 1 2 3 4 5 Beta S A R S - C o V - 2 t i t e r s ( l o g 1 0 P F U / l u n g ) 500mg IsoCtl 500mg STI-9167 Protection Against the Evolving Pandemic Threat

Vaccines 21 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
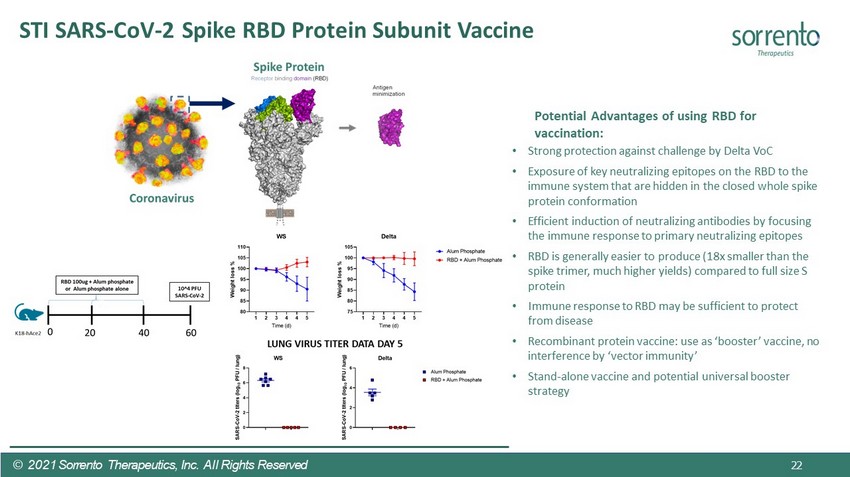
STI SARS - CoV - 2 Spike RBD Protein Subunit Vaccine Potential Advantages of using RBD for vaccination: • Strong protection against challenge by Delta VoC • Exposure of key neutralizing epitopes on the RBD to the immune system that are hidden in the closed whole spike protein conformation • Efficient induction of neutralizing antibodies by focusing the immune response to primary neutralizing epitopes • RBD is generally easier to produce (18x smaller than the spike trimer, much higher yields) compared to full size S protein • Immune response to RBD may be sufficient to protect from disease • Recombinant protein vaccine: use as ‘booster’ vaccine, no interference by ‘vector immunity’ • Stand - alone vaccine and potential universal booster strategy © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 22
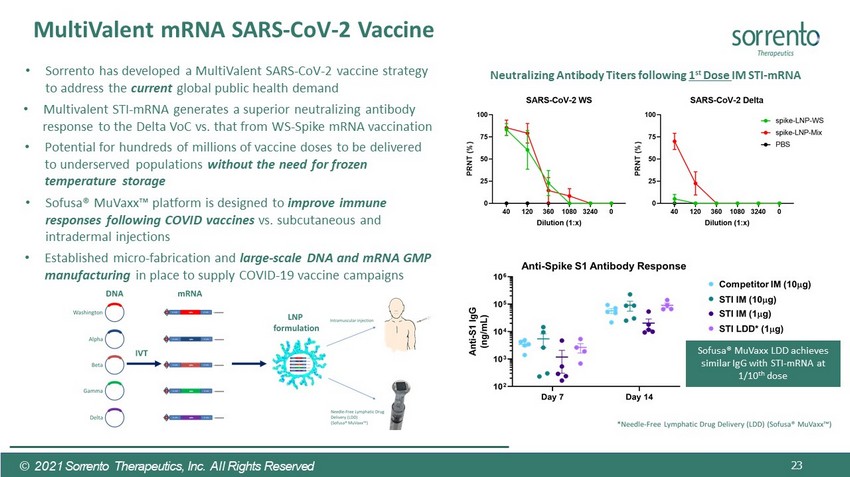
23 MultiValent mRNA SARS - CoV - 2 Vaccine © 2021 Sorrento Therapeutics, Inc. All Rights Reserved • Sorrento has developed a MultiValent SARS - CoV - 2 vaccine strategy to address the current global public health demand • Potential for hundreds of millions of vaccine doses to be delivered to underserved populations without the need for frozen temperature storage • Sofusa® MuVaxx Ρ platform is designed to improve immune responses following COVID vaccines vs. subcutaneous and intradermal injections • Established micro - fabrication and large - scale DNA and mRNA GMP manufacturing in place to supply COVID - 19 vaccine campaigns Sofusa® MuVaxx LDD achieves similar IgG with STI - mRNA at 1/10 th dose Neutralizing Antibody Titers following 1 st Dose IM STI - mRNA • Multivalent STI - mRNA generates a superior neutralizing antibody response to the Delta VoC vs. that from WS - Spike mRNA vaccination

Antivirals 24 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
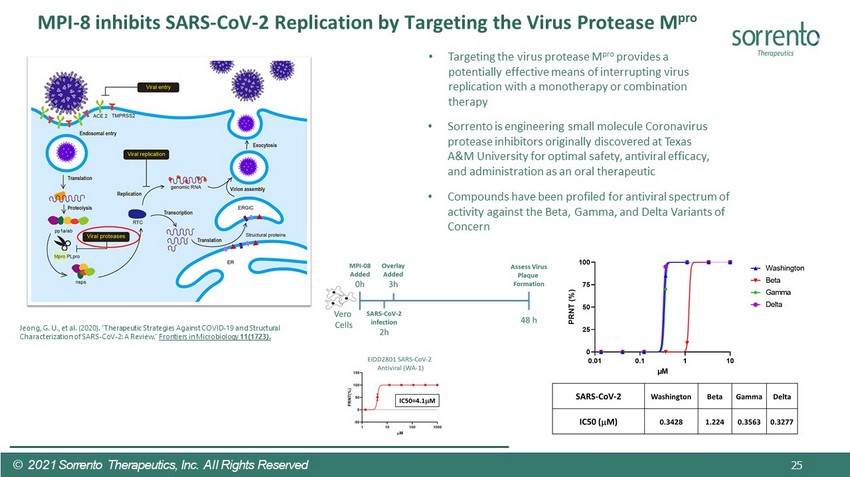
MPI - 8 inhibits SARS - CoV - 2 Replication by Targeting the Virus Protease M pro © 2021 Sorrento Therapeutics, Inc. All Rights Reserved • Sorrento is engineering small molecule Coronavirus protease inhibitors originally discovered at Texas A&M University for optimal safety, antiviral efficacy, and administration as an oral therapeutic • Compounds have been profiled for antiviral spectrum of activity against the Beta, Gamma, and Delta Variants of Concern • Targeting the virus protease M pro provides a potentially effective means of interrupting virus replication with a monotherapy or combination therapy Jeong, G. U., et al. (2020). "Therapeutic Strategies Against COVID - 19 and Structural Characterization of SARS - CoV - 2: A Review." Frontiers in Microbiology 11(1723). 25

Rescue Treatments 26 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
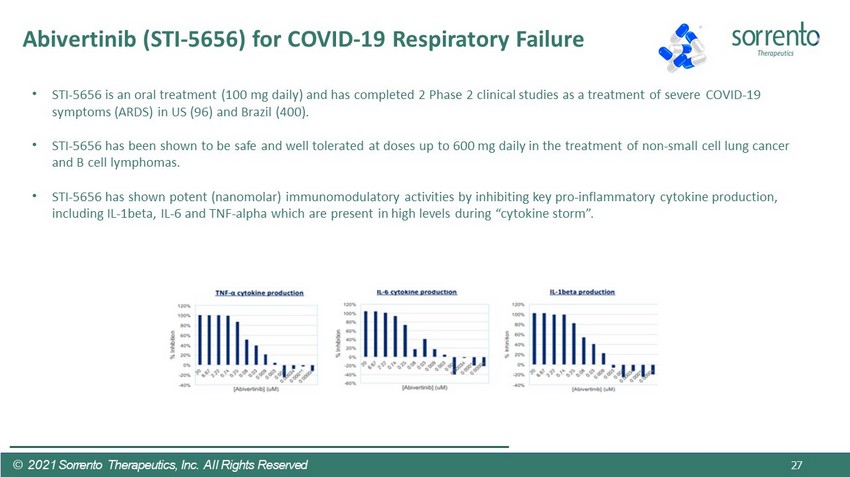
27 • STI - 5656 is an oral treatment (100 mg daily) and has completed 2 Phase 2 clinical studies as a treatment of severe COVID - 19 symptoms (ARDS) in US (96) and Brazil (400). • STI - 5656 has been shown to be safe and well tolerated at doses up to 600 mg daily in the treatment of non - small cell lung cancer and B cell lymphomas. • STI - 5656 has shown potent (nanomolar) immunomodulatory activities by inhibiting key pro - inflammatory cytokine production, including IL - 1beta, IL - 6 and TNF - alpha which are present in high levels during “cytokine storm”. Abivertinib (STI - 5656) for COVID - 19 Respiratory Failure © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
28 • COVI - MSC (STI - 8282): Allogeneic culture - expanded adipose - derived Mesenchymal stromal cells for the rescue of patients with lung damage and acute respiratory distress syndrome (ARDS) due to COVID - 19 • Preclinical studies have demonstrated that COVI - MSCs may produce a reduction in lung inflammation, fibrosis and edema • Very promising early data - A Phase 1b study with 3 x COVI - MSC IV infusions in subjects with severe COVID - 19 - related acute respiratory distress and ARDS has completed enrollment with 10/10 patients being discharged within days of the last infusion. • A Phase 2 pivotal study is starting in Brazil for COVID - 19 - induced ARDS • A separate Phase 2 waiting for clearance in Brazil for COVID - induced pulmonary long haulers. COVI - MSC TM (STI - 8282) for Patients with Acute Lung Damage © 202 1 Sorrento Therapeutics, Inc. All Rights Reserved

Non - Opioid Pain Management Programs 29 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

Non - Opioid Pain Therapeutics © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 30 Key Programs Indication Preclinical FDA Approved ZTlido™ 1.8% Postherpetic Neuralgia - PHN SP-102 Lumbar Radicular/Sciatica Pain SP-103 Acute Back Pain SP-104 Fibromyalgia RTX (resiniferatoxin) – Epidural Intractable Pain in Advanced Cancer Orphan designation RTX (resiniferatoxin) – Intra- Moderate to Severe Knee OA Pain Phase III/PivotalPhase I Phase II

US FDA APPROVAL – FEBRUARY 2018 COMMERCIAL LAUNCH – OCTOBER 2018 Marketed Product: ZTlido® 1.8% (FDA approved for relief of pain associated with PHN ) Lidocaine Patch Market Overview >3 .5 million prescriptions per year More than 129 million prescription lidocaine patches were sold in the US in 2020 according to IMS Benefits vs. other lidocaine pain patches Superior adhesion vs. other lidocaine patches in various head - to - head studies Only lidocaine patch proven during moderate exercise Properties ZTlido 1.8% Lidoderm 5% Bioavailability ~45% ~3 + 2% Weight 2 g 14 g Thickness 0.8 mm 1.7 mm Lidocaine content 36 mg 700 mg Adhesive Non - aqueous Water - based © 2021 Sorrento Therapeutics, Inc. All Rights Reserved 31

Developing SP - 102 as a non - opioid injectable therapeutic for low back pain Novel viscous gel formulation, optimized for epidural injection Novel biocompati ble excipient enables extended local effect On track to be the first and only FDA - approved epidural steroid product Currently used products are off - label and contain potentially neurotoxic preservatives, particulates, surfactants or solvents. Compounded epidural steroids led to >70 deaths in 2012 due to fungal contamination Large market over 1 2 million epidural steroid injections per year in U.S. Bigger opportunity than knee intra - articular OA injections, with no direct competition Established reimbursement Part B route for most frequently performed pain procedure Phase 3 CLEAR study enrollment complet ed in July 2021. Top line results by January 2022 F a s t T ra c k st a t u s g r an t e d b y FD A , File for Breakthrough status & Priority Review post Phase 3 data Significant barriers to entry for competitors or generics Method of use patent granted (2036 expiry) and formulation patent approved (2036 expiry) Complex manufacturing process and know - how for excipient and sterile viscous gel products 32 SP - 102 – Opportunity Summary © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

Well - tolerated. Key viscous excipient, long history of use including safety and safer repeat injections Fast acting onset of effect with less spread Potent non - particulate steroid (injectable dexamethasone sodium phosphate viscous gel) Pre - filled syringe for epidural use Gel formulation for extended local release and substantial magnitude of pain relief No preservatives, no surfactants, no particulates. Non - opioid and non - addictive Projected 24 month shelf life 33 SP - 102 – On Track to be the First Steroid Formulation With FDA - Approved Label to Treat Sciatica © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

• Triple strength dose of lidocaine • S uperior adhesion and efficient formulation • Expect to initiate Phase 2 trial in 2 H 2021 • For the treatment of low back pain – a substantially larger opportunity than PHN 34 • Innovative technology delivering equivalent therapeutic dose to current lidocaine 5% patches • Superior adhesion and drug formulation efficiency • Safe, convenient, and functional pain treatment • Indicated for relief of pain associated with post - herpetic neuralgia (shingles pain) SP - 103 is a Next - Generation, Triple Strength Formulation of ZTlido SP - 103 Next - Generation, ZTlido 5.4% Lidocaine Topical System © 2021 Sorrento Therapeutics, Inc. All Rights Reserved
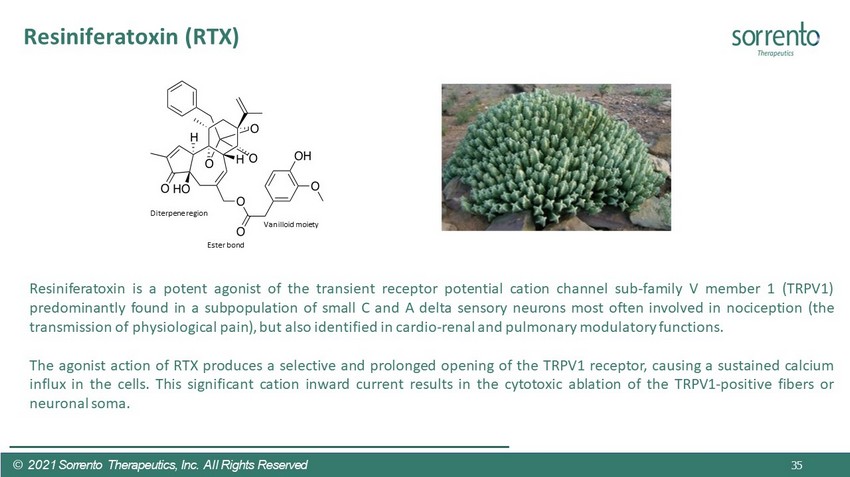
Resiniferatoxin (RTX) 35 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved Resiniferatoxin is a potent agonist of the transient receptor potential cation channel sub - family V member 1 (TRPV 1 ) predominantly found in a subpopulation of small C and A delta sensory neurons most often involved in nociception (the transmission of physiological pain), but also identified in cardio - renal and pulmonary modulatory functions . The agonist action of RTX produces a selective and prolonged opening of the TRPV 1 receptor, causing a sustained calcium influx in the cells . This significant cation inward current results in the cytotoxic ablation of the TRPV 1 - positive fibers or neuronal soma . Vanilloid moiety Ester bond Diterpene region
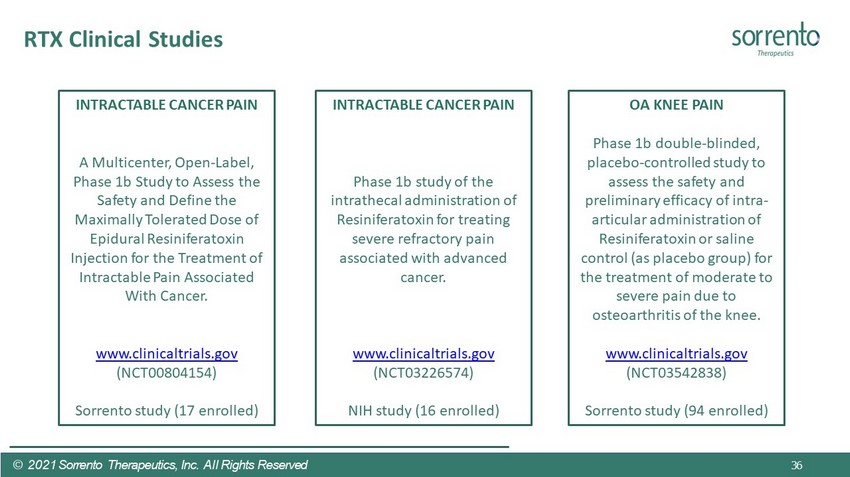
RTX Clinical Studies OA KNEE PAIN Phase 1b double - blinded, placebo - controlled study to assess the safety and preliminary efficacy of intra - articular administration of Resiniferatoxin or saline control (as placebo group) for the treatment of moderate to severe pain due to osteoarthritis of the knee. www.clinicaltrials.gov (NCT03542838) Sorrento study (94 enrolled) INTRACTABLE CANCER PAIN Phase 1b study of the intrathecal administration of Resiniferatoxin for treating severe refractory pain associated with advanced cancer. www.clinicaltrials.gov (NCT03226574) NIH study (16 enrolled) INTRACTABLE CANCER PAIN A Multicenter, Open - Label, Phase 1b Study to Assess the Safety and Define the Maximally Tolerated Dose of Epidural Resiniferatoxin Injection for the Treatment of Intractable Pain Associated With Cancer. www.clinicaltrials.gov (NCT00804154) Sorrento study (17 enrolled) 36 © 2021 Sorrento Therapeutics, Inc. All Rights Reserved

37
