Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Scopus BioPharma Inc. | tm2127337d1_8k.htm |
Exhibit 99.1

Launch of Duet Therapeutics, a wholly - owned immuno - oncology subsidiary of Scopus BioPharma September 2021

DISCLAIMERS This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . All statements regarding Scopus BioPharma Inc . , “Scopus” or the “Company” that are not historical fact, including, but not limited to, statements regarding expected future financial position, results of operations, cash flows, business strategy, budgets, competitive position, growth opportunities, plans and objectives of management for future operations, as well as statements containing words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “intends,” “may” or “will” or variations of such words and similar expressions, are forward - looking statements . By their nature, forward - looking statements are subject to a number of risks, uncertainties and assumptions . It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make . In addition, we operate in a very competitive and rapidly changing environment . In light of these risks, uncertainties and assumptions, any forward - looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements . Forward - looking statements speak only as of the date they were made, and we undertake no obligation to update or revise any forward - looking statements for any reason, except as may be required by law . This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . The information contained in this presentation or otherwise provided to you is provided for informational purposes only, does not recommend the purchase or sale of any security, is not complete and does not contain all material information about Scopus, including important information and risk factors associated with an investment in Scopus, and is subject to change without notice .
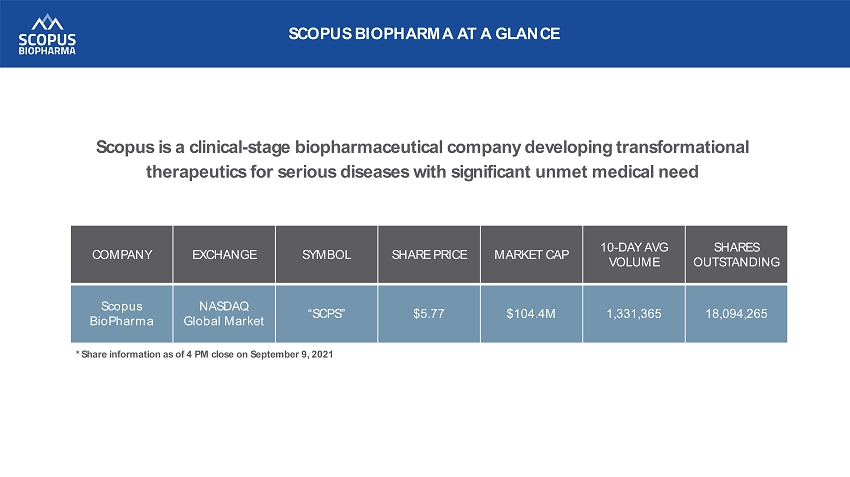
SCOPUS BIOPHARMA AT A GLANCE Scopus is a clinical - stage biopharmaceutical company developing transformational therapeutics for serious diseases with significant unmet medical need COMPANY EXCHANGE SYMBOL SHARE PRICE MARKET CAP 10 - DAY AVG VOLUME SHARES OUTSTANDING Scopus BioPharma NASDAQ Global Market “SCPS” $5.77 $104.4M 1,331,365 18,094,265 *Share information as of 4 PM close on September 9, 2021

DUET THERAPEUTICS, A SCOPUS BIOPHARMA COMPANY A Scopus BioPharma company On September 2, 2021, Scopus announced the launch of Duet Therapeutics to integrate the management and clinical development of the immuno - oncology assets of Scopus and Olimmune, acquired by Scopus in June 2021
A Scopus BioPharma company Creating targeted oligonucleotide therapies for treatment - resistant cancers

Investment highlights 6 Duet’s platform provides a unique approach to treating hematological malignancies and solid tumor cancers Strong IP position Duet has 4 issued patents and 4 submitted PCT applications that cover the technologies that make up Duet’s platform. Bi - functional mode of action Duet’s platform stimulates the immune activation and releases immunosuppression, both of which are required for durable therapeutic responses. Addresses solid tumors Duet’s platform targets a fundamental mechanism that underlies hematological malignancies and solid tumor cancers. World - class team & partners Duet is working with world class researchers and clinicians to bring the technology through the clinic and to patients. Targeted delivery platform Duet’s method of targeted delivery works by activating specific immune cells in the tumor microenvironment against the cancer. Clinical stage Duet has a Phase 1 clinical trial that is actively recruiting patients for B - cell non - Hodgkin Lymphoma, and two additional Phase 1 trials targeted to begin in Q1 2023.

Technology portfolio 7 The Duet technologies create a powerful portfolio of bi - functional molecules that hold broad potential across multiple tumor types Molecule name CpG - STAT3siRNA (DUET - 01) CpG - STAT3ASO (DUET - 02) CpG - STAT3decoy (DUET - 03) Structure STAT3 mechanism of action Bi - functional effect resulting from synergy of CpG - mediated immunostimulation and STAT3 inhibition through RNA silencing Bi - functional effect resulting from synergy of CpG - mediated immunostimulation and STAT3 inhibition through antisense Bi - functional effect resulting from synergy of CpG - mediated immunostimulation and DNA - binding inhibition Optimal delivery / targets Local delivery Systemic or local delivery Systemic or local delivery Publications Nature Biotechnology, 2009; Blood, 2013, 2014; Cancer Research, 2010, 2013; Clinical Cancer Research, 2015 Clinical Cancer Research (2018) Journal of Clinical Investigation (2021) Blood, 2015; Journal of Leukemia Biology, 2017; Molecular Therapy, 2018 Patents US 9,688,982 US 10,758,624: broad coverage for all STATs (STAT1/2/3/4/5/6) for cancer/inflammation US 9,976,147 US 10,829,765 Clinical trials initiation / indication Q3 2021 / B - cell non - Hodgkin lymphoma Q1 2023 / Genitourinary and Head & Neck cancers Acute Myeloid Leukemia CpG ODN STAT3siRNA CpG ODN STAT3ASO CpG ODN STAT3dODN

Duet pipeline 8 Duet’s pipeline of STAT3 inhibitors includes a suite of technologies and several expansion indications in immuno - oncology Candidate Program / indication Discovery / optimization IND enabling Phase 1 Development DUET - 01 B - cell non - Hodgkin lymphoma DUET - 01 + ICI B - cell non - Hodgkin lymphoma DUET - 02 Genitourinary cancers DUET - 02 Head & neck cancers Research DUET - 01 + ICI Cutaneous T - cell lymphoma DUET - 03 Acute myeloid leukemia Target milestone: Dosing first patient Q4 2021 Target milestone: Dosing first patient Q3 2022 Target milestone: IND Q4 2022 Target milestone: IND Q4 2022 Discovery Discovery
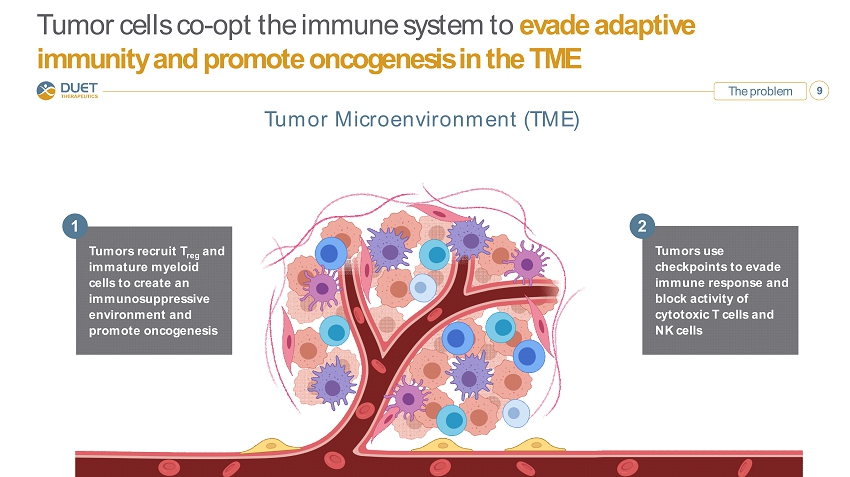
The problem 9 Tumor cells co - opt the immune system to evade adaptive immunity and promote oncogenesis in the TME Tumors use checkpoints to evade immune response and block activity of cytotoxic T cells and NK cells 2 Tumors recruit T reg and immature myeloid cells to create an immunosuppressive environment and promote oncogenesis 1 Tumor Microenvironment (TME)

STAT3 as an I - O target 10 STAT3 is a master regulator of the immune system and a high - value immuno - oncology target, but requires cell - specific modulation Tumor cell STAT3 • Immune evasion • Proliferation • Angiogenesis • Metastasis STAT3 plays a major role in maintaining immunosuppression in the TME, however, it is important for cytotoxic T cell function MDSC STAT3 • Expansion • Immune tolerance • Angiogenesis • PD - L1 expression Cytotoxic T cell STAT3 • CD8+ T - cell expansion • Generation of memory T cells Dendritic cell STAT3 • TLR9 activation • Maturation • Antigen presentation • T - cell activation

The Duet solution 11 Duet strategy: bi - functional oligonucleotide activates immune system (TLR9) and turns off master checkpoint (STAT3) Scavenger receptors, highly expressed in antigen - presenting cells, have a natural affinity for phosphorothioated CpG oligos, leading to rapid uptake of Duet’s therapeutic molecule into myeloid cells 1 The bi - functional oligonucleotide is internalized into early endosomes, where it binds to TLR9 and is released into the cytoplasm 2 The activated TLR9 triggers the signalling cascade that results in enhanced antigen presentation, pro - inflammatory cytokine release, and T - cell stimulation 3 STAT3 inhibition releases the brakes on immune activation, allowing for the full potential of TLR9 - driven innate and adaptive immune responses 4
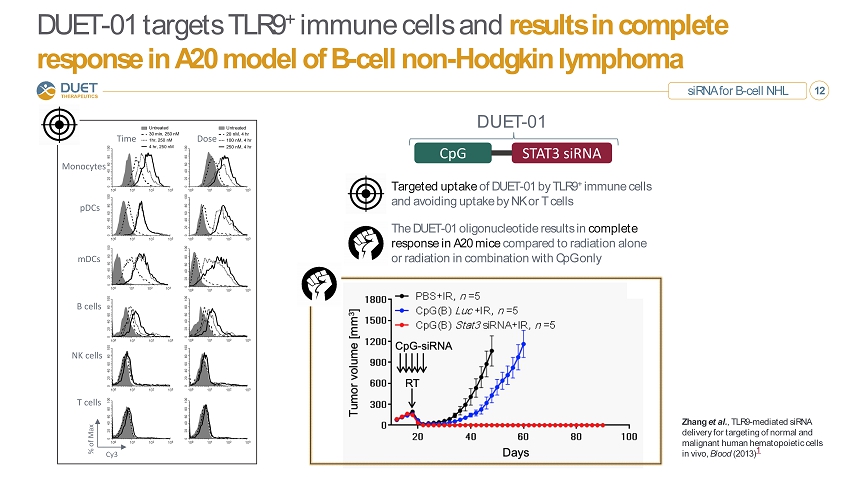
siRNA for B - cell NHL 12 DUET - 01 targets TLR9 + immune cells and results in complete response in A20 model of B - cell non - Hodgkin lymphoma Zhang et al. , TLR9 - mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo, Blood (2013) 1 Targeted uptake of DUET - 01 by TLR9 + immune cells and avoiding uptake by NK or T cells The DUET - 01 oligonucleotide results in complete response in A20 mice compared to radiation alone or radiation in combination with CpG only CpG STAT3 siRNA DUET - 01 Monocytes B cells NK cells T cells Dose Time pDCs mDCs Cy3 % of Max

Phase 1 clinical trial 13 A Phase I study of intratumoral injections of DUET - 01 with local radiation in patients with relapsed/refractory B - cell NHL ELIZABETH BUDDE, MD, PHD Associate Professor, Division of Lymphoma, Department of Hematology & Hematopoietic Cell Transplantation at City of Hope PRIMARY OBJECTIVES: • Determine the recommended Phase 2 dose of DUET - 01 when given in combination with local radiation therapy. • Evaluate safety and feasibility of intratumoral injections when combined with radiation, by evaluation of toxicities. SECONDARY OBJECTIVES: • Characterize the clinical activity of DUET - 01 through the assessment of disease response, and response duration. (Clinical) • Characterize the biologic activity when combined with radiation by assessing through immunologic correlative studies. (Biologic) • Characterize silencing of the STAT3 gene and its key downstream genes through correlative studies. (Biologic) • Characterize local and systemic immune responses , including evidence/extent of immune cell intratumoral infiltration, immune cell activation within the tumor and in the peripheral blood, and changes in proinflammatory mediators in plasma.

Phase 1 clinical trial 14 The Phase 1 clinical trial is evaluating three different dose levels of DUET - 01 in combination with radiation therapy for B cell NHL DLT assessment 1 2 3 4 5 9 11 16 18 19 23 25 42 56 Primary tumor site Secondary tumor sites Peripheral blood draw Radiation (200 cGy ) CpG - STAT3siRNA Lymph node biopsy CT PET DL3 ONLY DL3 ONLY
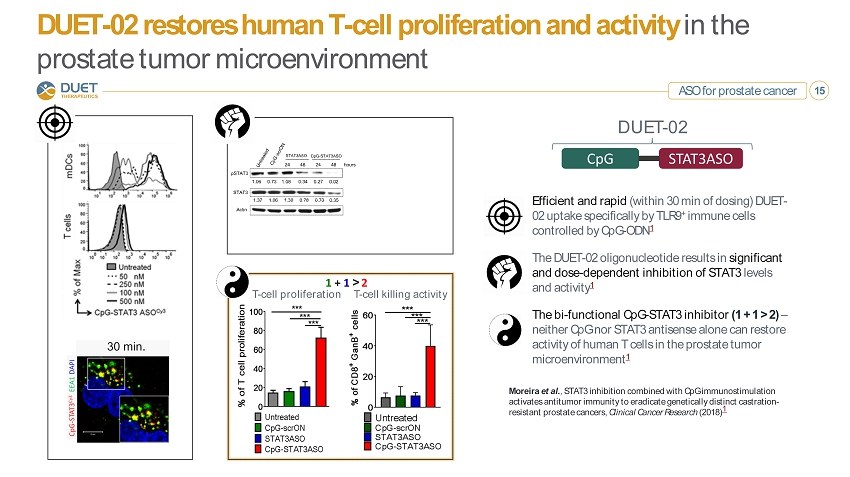
ASO for prostate cancer 15 DUET - 02 restores human T - cell proliferation and activity in the prostate tumor microenvironment Moreira et al. , STAT3 inhibition combined with CpG immunostimulation activates antitumor immunity to eradicate genetically distinct castration - resistant prostate cancers, Clinical Cancer Research (2018) 1 Efficient and rapid (within 30 min of dosing) DUET - 02 uptake specifically by TLR9 + immune cells controlled by CpG - ODN 1 The DUET - 02 oligonucleotide results in significant and dose - dependent inhibition of STAT3 levels and activity 1 The bi - functional CpG - STAT3 inhibitor (1 + 1 > 2) – neither CpG nor STAT3 antisense alone can restore activity of human T cells in the prostate tumor microenvironment 1 CpG STAT3ASO DUET - 02 T - cell proliferation T - cell killing activity 1 + 1 > 2

ASO for prostate cancer 16 DUET - 02 induces T - cell mediated rejection of bone - localized and ICI - resistant prostate cancers in mice Six intravenous injections of DUET - 02 are sufficient to unleash potent antitumor effects and increase animal survival in genetically different prostate tumor models The bi - functionality of DUET - 02 is key for efficacy (CpG and STAT3ASO co - injected as unconjugated oligonucleotides fail to improve the survival) DUET - 02 breaks tumor immune tolerance and generates cancer - specific CD8 + and CD4 + T cell - mediated immune responses A C CD8 :T reg ratio CD8 T-cell:Treg ratio in prostate tumors Ras/Myc - driven tumors (RM9) 1 + 1 > 2 Ras/Myc - driven (RM9) prostate cancer Ras/Myc-driven tumors (RM9) 5 mg/kg q2d Moreira et al. , STAT3 inhibition combined with CpG immunostimulation activates antitumor immunity to eradicate genetically distinct castration - resistant prostate cancers, Clinical Cancer Research (2018) 1 PTEN - deficient (PPS) prostate cancer

ASO for head & neck cancer 17 DUET - 02 generates effective CD8 + T - cell immune responses against human HPV - head and neck cancer (HNC) in humanized mice CpG - STAT3ASO (DUET - 02) results in regression of xenotransplanted human head and neck tumors (SCC1) in humanized mice, with evidence of immune activation, CD8 + T - cell recruitment and reduction of regulatory T cells in tumors . Humanized mice (NSG - SGM3/hCD45 + ) Immunodeficient mice (NSG - SGM3/hCD45 - ) PBS CpG - scrON STAT3ASO DUET - 02 CD8 CD4 FOXP3 CD8 CD4 FOXP3 CD8 T cells > Tregs! Tregs > CD8 T cells Low T cells High CD4/Tregs Moreira et al. , Myeloid cell - targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell - mediated immunity, The Journal of Clinical Investigation (2021) 2

Planned clinical trials 18 Duet is planning two clinical Phase 1 trials to begin in Q1 2023 for Genitourinary and Head & Neck Cancers Genitourinary Cancers Tanya Dorff MD Associate Clinical Professor, Medical Oncology & Therapeutics Research, Head of the Genitourinary Cancers Program at City of Hope Sumanta Pal MD Clinical Professor, Medical Oncology & Therapeutics Research; Director of the Kidney Cancer Program at City of Hope Systemic intravenous infusions of DUET - 02 as a monotherapy for treatment of metastatic prostate, kidney, and bladder cancers Head & Neck Cancers Ermina Massarelli , MD, PhD Associate Clinical Professor, Medical Oncology & Therapeutics Research, specializing in lung and head and neck cancers Sagus Sampath MD Associate Clinical Professor, Radiation Oncology, specializing in head and neck, lung, skin, bladder cancers Intratumoral injection of DUET - 02 in combination with radiotherapy for squamous cell carcinoma of head & neck cancers

Target milestones that will culminate in Phase 1 clinical trials in three unique clinical indications Q3 2021 Q1 2022 Q3 2022 Q1 2023 Q4 2021 Q2 2022 Q4 2022 • Launch of Duet Therapeutics through Scopus/Olimmune integration • Completion of GMP manufacturing for IND - enabling studies for DUET - 02 • Dose 1 st patient with DUET - 01 in Phase 1 clinical trial • Initiation of IND - enabling studies for DUET - 02 • Completion of IND - enabling studies for DUET - 02 • Begin combination study of DUET - 01 + ICI • Initial clinical readout from clinical trial for DUET - 01 • Completion of GMP manufacturing for DUET - 02 Phase 1 clinical trials • IND submission for head & neck and genitourinary cancers using DUET - 02 • Begin recruiting for genitourinary and head & neck cancers using DUET - 02 Timeline & milestones 19

20 ALAN HORSAGER, PH.D. President, CEO & Director Duet is building a leadership team that has extensive company building and clinical development experience MARCIN KORTYLEWSKI, PH.D. Senior Scientific Advisor Other team members to be announced in the coming weeks Leadership team

Contact Alan Horsager, Ph.D. President – Immuno - Oncology, Scopus BioPharma President & CEO, Duet Therapeutics horsager@duettx.com A Scopus BioPharma company
