Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SQZ Biotechnologies Co | d149445d8k.htm |
| EX-99.1 - EX-99.1 - SQZ Biotechnologies Co | d149445dex991.htm |

Exhibit 99.2 Empower Cells to Change Lives® September 2021

Forward Looking Statements and Legal Disclaimers This presentation contains forward‐looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward‐looking statements, including statements relating to our development of our product candidates, the promise and potential impact of our preclinical or clinical trial data, the timing of and plans to continue or initiate preclinical studies and clinical trials of our product candidates, the timing and results of any preclinical studies, clinical trials or readouts and the sufficiency of cash to fund operations. These forward‐looking statements are based on management's current expectations. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward‐looking statements, although not all forward‐ looking statements contain these identifying words. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward‐looking statements, including, but not limited to, the following: the impact of the COVID‐19 pandemic on our operations, including our preclinical studies and clinical trials, and the continuity of our business; we have incurred significant losses, are not currently profitable and may never become profitable; our need for additional funding; our cash runway; our limited operating history and the prospects for our future viability; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in regulatory approval; the continued relationship with our collaboration partners in our development of products, including in our SQZ™ APC oncology pipeline; the approach we are taking to discover and develop product candidates and whether it will lead to marketable products; the expense, time‐consuming nature and uncertainty of clinical trials; enrollment and retention of patients; potential side effects of our product candidates; our ability to maintain our relationships with our suppliers; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third‐party intellectual property or challenges to the ownership of our intellectual property; our ability to retain key personnel and to manage our growth; the potential volatility of our common stock; costs and resources of operating as a public company; unfavorable or no analyst research or reports; and securities class action litigation against us. These and other important factors discussed in our filings with the US Securities and Exchange Commission could cause actual results to differ materially from those indicated by the forward‐looking statements made in this presentation. Any such forward‐looking statements represent management's estimates as of the date of this presentation. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. While we may elect to update such forward‐ looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Although we believe the expectations reflected in such forward‐looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. These forward‐looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third‐party sources and our own internal estimates and research. While we believe these third‐party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third‐party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of the data included in this presentation or undertake to update such data after the date of this presentation. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. 2

SQZ Biotech: Unlocking the Full Potential of Cell Therapies üSQZ Technology addresses fundamental challenge of engineering diverse cell functions • Multiple cell therapy platforms with expansive potential across oncology, autoimmune and infectious diseases • Partnership with Roche and potential for additional BD opportunities across portfolio üThree clinical programs expected to have data readouts throughout 2022 • Lead APC program demonstrated safety, manufacturability and immune activation in solid tumors • Additional INDs targeted over the next 18 months üDemonstrated scalable cell therapy manufacturing • Fast, reliable and cost‐efficient with production times < 24hr • Path to potential point‐of‐care implementation 3

® Unique Cell Squeeze Technology Enables Differentiated Capabilities Cells and target cargo Cell membranes are Membranes 1 3 5 together in suspension temporarily disrupted reseal Cells are squeezed through SQZ Target cargo enters the 2 4 chip at high speeds cytosol of the cells Applicable to diverse Robust across material Preservation of cell ++ cell types classes function EP SQZ Proteins and peptides PBMCs Gene editing complexes RBCs Nanomaterials Nucleic Acids HSC/iPSCs Small molecules Electroporation (EP) results in substantial gene misregulation vs. Squeezing 4

Manufacturing: Fast, Reliable and Potential to Expand Cell Therapy Access Manufacturing Process and Patient Experience Approach Strengths and Considerations ~24‐113 days vein‐to‐vein • High cost • Lymphodepletion Autologous • Bridging chemotherapy• Long turn‐around times ~10‐12 days enrollment to infusion • Off‐the‐shelf • Potential Efficacy/Safety limitations due to Allogeneic • Lymphodepletion mismatch ~1 week vein to vein SQZ • No preconditioning • Rapid production, potential cost‐efficiency Cell Therapy • No planned • Many doses available from single production run hospitalizations Today Possible same day treatment SQZ • On‐site production without a clean room Point‐of‐Care • Could enable much broader accessibility Vision 5

Addressing Challenges That Could Unlock Broader Cell Therapy Universe Universe of Possible Cell Therapies Hematology Oncology Viral Cell Squeeze Solid Tumors Electroporation Transduction Technology Ability to retain cell ++ +++ health and function Flexibility in cell Autoimmune ++ + +++ types and cargo Breadth of disease ++ +++ applicability Infectious Manufacturing speed Disease ++ +++ and reliability Almost all cell therapies today are produced with Regenerative electroporation or viral transduction techniques Medicine 6

Directing Immune System to Drive Antigen Specific Activation and Tolerization 1. Antigen (What to target) 2. Context (Activation vs. Tolerance) Determinants Antigen presenting cells (APCs) Once a T cell’s TCR is engaged, it looks for show peptide antigen on MHC‐I clues via APC surface receptors and of an Immune and MHC‐II that T cells look at cytokines for whether to become activated Reaction using their T cell receptors (TCR) or tolerized Immune Activation Immune Tolerance Antigen: T Cells T Cells APC Context …activation against the target …tolerization against the target Indications: Outcome: CD8 Killer T cells CD4 Helper T cells CD8 Killer T cells Regulatory T cells CD8 Killer T cells are activated to attack desired target Activating cells (e.g., CD8, CD4) are deleted or and CD4 T cells are activated to support a broader anergized and Tregs are upregulated, blocking reaction (e.g., antibodies) unwanted immune response against the target 7 T Cell APC Surface receptors + Cytokines

Strategy: Leverage Flexible Technology To Quickly Expand Indications From Each Platform Autoimmune Disease Chronic Infectious Disease Oncology Annual incidence (‘000s) Prevalence (M) Prevalence (M) 3 3 400 KRAS 300 2 2 Potential Patient 200 Impact: HPV 1 1 (Patient numbers, US) 100 0 0 0 HBV HIV Other Celiac T1D Other SQZ Cell Therapy Platform: SQZ APCs SQZ eAPCs SQZ AACs SQZ TAC SQZ eAPCs Initial proof of concept in HPV Initial proof of concept in Celiac Initial proof of concept in HBV Strategy: Leverage SQZ Identify benefits of each platform Expand into additional indications Expand into additional indications cargo flexibility (e.g., HIV) (e.g., T1D) Expand into additional indications (e.g., KRAS G12D and G12V) 8

Diverse Pipeline: Synergistic in Execution yet Independent in Mechanism CELL SOURCE/ PLATFORM INDICATION PRE‐CLINICAL PHASE 1/2 CARGO H&N, Cervical, Anal PBMC + Peptide Other Solid Tumors SQZ APCs RBC H&N, Cervical, Anal + Peptide KRAS mut Solid Tumors + SQZ AACs Adjuvant Other Solid Tumors PBMC + Solid Tumors mRNA SQZ eAPCs Celiac disease RBC + Protein SQZ TACs Type 1 Diabetes Chronic Hepatitis B Virus PBMC + mRNA Rapid Response Vaccines SQZ eAPCs 9 INFECTIOUS AUTO‐ ONCOLOGY DISEASE IMMUNITY
Oncology SQZ™ Approach SQZ eAPCs SQZ APCs SQZ AACs 10
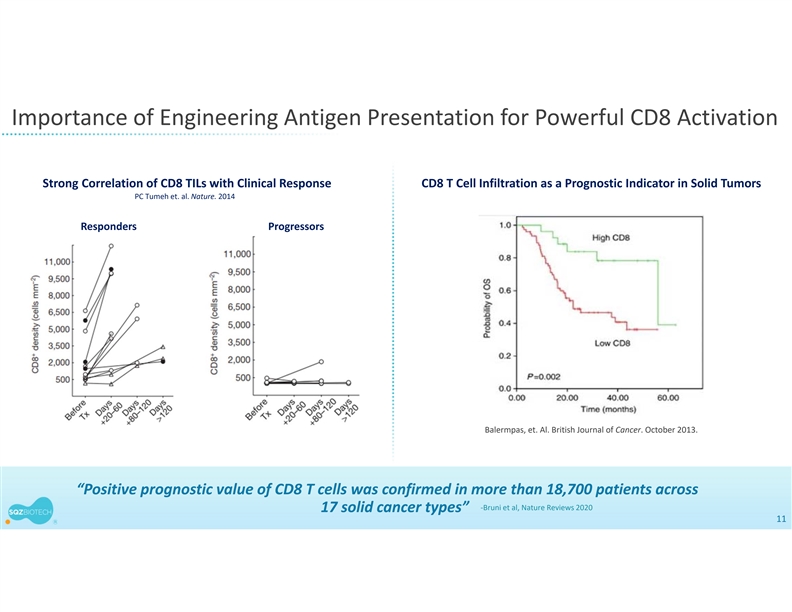
Importance of Engineering Antigen Presentation for Powerful CD8 Activation Strong Correlation of CD8 TILs with Clinical Response CD8 T Cell Infiltration as a Prognostic Indicator in Solid Tumors PC Tumeh et. al. Nature. 2014 Responders Progressors Balermpas, et. Al. British Journal of Cancer. October 2013. “Positive prognostic value of CD8 T cells was confirmed in more than 18,700 patients across ‐Bruni et al, Nature Reviews 2020 17 solid cancer types” 11
Oncology SQZ™ Approach SQZ AACs SQZ APCs SQZ eAPCs 12

Overcoming the Challenge of Cancer Vaccines TM Typical Cancer Vaccine Mechanism: SQZ APC Mechanism: Cross‐Presentation Direct Presentation MHC‐I = CD8 T Cell MHC‐I = CD8 Activation Endocytosis Squeezed Antigen MHC‐II = CD4 + antibodies • Primarily generates MHC‐II presentation for CD4 and • Primarily generates MHC‐I presentation to CD8 T cells antibody responses• CD8 T cell responses in the tumor are highly correlated • Suited for prophylactic vaccines with patient outcomes Prioritizes creation of Prioritizes creation of CD4 helper cells Antibodies CD8 Killer T cells 13
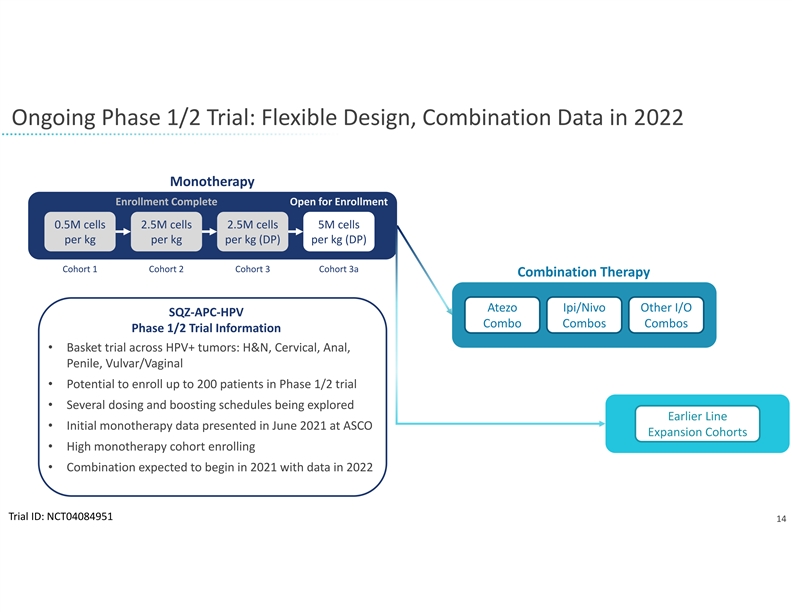
Ongoing Phase 1/2 Trial: Flexible Design, Combination Data in 2022 Monotherapy Enrollment Complete Open for Enrollment 0.5M cells 2.5M cells 2.5M cells 5M cells per kg per kg per kg (DP) per kg (DP) Cohort 1 Cohort 2 Cohort 3 Cohort 3a Combination Therapy Atezo Ipi/Nivo Other I/O SQZ‐APC‐HPV Combo Combos Combos Phase 1/2 Trial Information • Basket trial across HPV+ tumors: H&N, Cervical, Anal, Penile, Vulvar/Vaginal • Potential to enroll up to 200 patients in Phase 1/2 trial • Several dosing and boosting schedules being explored Earlier Line • Initial monotherapy data presented in June 2021 at ASCO Expansion Cohorts • High monotherapy cohort enrolling • Combination expected to begin in 2021 with data in 2022 Trial ID: NCT04084951 14

* Ph 1 Mono Data: SQZ™ APCs Demonstrated Safety and Reliable Manufacturing Safety • No patient met pre‐specified DLT criteria Total • No related Grade ≥3 SAEs reported: Patient Demographics (N=12) Age, years • Grade 2 (related) – CRS (1 pt) Median (Min, Max) 62.5 (47, 68) • Unrelated SAEs ‐ Grade 3 (7 pts), Grade 4 (1 pt) and Grade 5 (1 pt) Baseline RMH score, n (%) • No dose reduction High (≥2) 6 (50.0) • No treatment‐related deaths Site of primary tumor, n (%) Anus 6 (50.00) Cervix 2 (16.7) Head & Neck 3 (25.0) Manufacturing: Robust, consistent production in <24hrs Site of Metastases, n (%) 12 (100.0) Liver Mets 7 (58.3) Viability End‐to‐End Process Time Lung Mets 5 (41.6) 25 Other sites* 6 (50.0) Prior Systemic Therapy, n (%) 20 100% Chemotherapy 12 (100.0) IFN‐γ secretion 15 Checkpoint Inhibitor 11 (91.7) Refractory to ICI (PD as 9/11 10 BOR) 5 (41.6) 5 Other All manufactured patient APCs induced IFN‐γ secretion by T cells 0 Processing Time 15 * Phase 1 monotherapy data presented at ASCO June 2021 Viability (%)
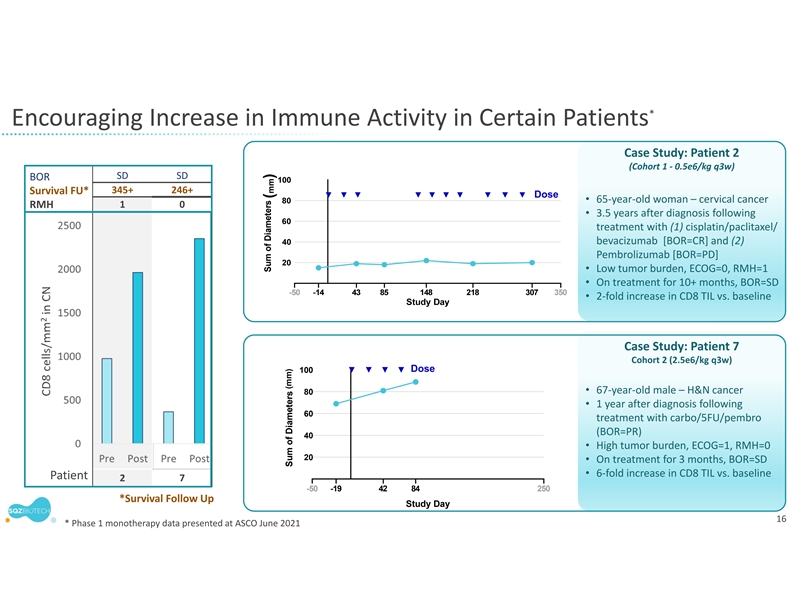
* Encouraging Increase in Immune Activity in Certain Patients Case Study: Patient 2 (Cohort 1 ‐ 0.5e6/kg q3w) SD SD BOR 345+ 246+ Survival FU* Dose • 65‐year‐old woman – cervical cancer RMH 10 • 3.5 years after diagnosis following treatment with (1) cisplatin/paclitaxel/ bevacizumab [BOR=CR] and (2) Pembrolizumab [BOR=PD] • Low tumor burden, ECOG=0, RMH=1 • On treatment for 10+ months, BOR=SD • 2‐fold increase in CD8 TIL vs. baseline Case Study: Patient 7 Case Study: Patient 7 Cohort 2 (2.5e6/kg q3w) Dose 100 • 67‐year‐old male – H&N cancer 80 • 1 year after diagnosis following 60 treatment with carbo/5FU/pembro (BOR=PR) 40 • High tumor burden, ECOG=1, RMH=0 20 • On treatment for 3 months, BOR=SD • 6‐fold increase in CD8 TIL vs. baseline Patient 27 -50 -19 42 84 250 *Survival Follow Up Study Day 16 * Phase 1 monotherapy data presented at ASCO June 2021 2 CD8 cells/mm in CN (mm) (mm)

Next Step: Combinations ‐ Potential Synergies with Other I/O Compounds Antigen‐Specific Responses could be Preclinical data demonstrated synergistic potential of SQZ APCs in combination with Roche PD1‐IL2v Amplified with Combination TM SQZ APCs drive I/O drugs tumor‐specific T enhance activity cell activation downstream 17 Presented at SITC 2020 in SQZ Poster and Roche Oral Presentation 3 Tumor Volume (mm )

Oncology SQZ™ Approach SQZ eAPCs SQZ AACs SQZ APCs 18
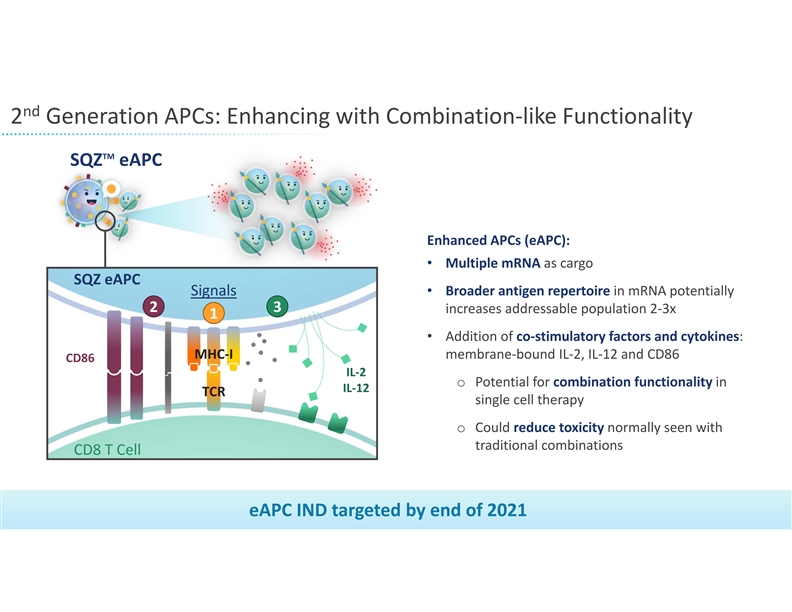
nd 2 Generation APCs: Enhancing with Combination‐like Functionality TM SQZ eAPC Enhanced APCs (eAPC): • Multiple mRNA as cargo SQZ eAPC • Broader antigen repertoire in mRNA potentially Signals 2 3 increases addressable population 2‐3x 1 • Addition of co‐stimulatory factors and cytokines: MHC‐I membrane‐bound IL‐2, IL‐12 and CD86 CD86 IL‐2 o Potential for combination functionality in IL‐12 TCR single cell therapy o Could reduce toxicity normally seen with traditional combinations CD8 T Cell eAPC IND targeted by end of 2021 19

Oncology SQZ™ Approach SQZ APCs SQZ AACs SQZ eAPCs 20

Creating Antigen Carriers from RBCs to Direct Immune Response Antigen Lymphoid Organ Presentation Cargo SQZ™ Antigen Carriers • Antigen Carriers generated by squeezing RBCs deliver antigen to guide a specific immune response • Leverage the natural process by which RBCs are regularly cleared by the body • Professional APCs in lymphoid organs are primarily responsible for RBC clearance • Antigen Carriers Act as “Trojan horses” to deliver antigen to these endogenous professional APCs 21

SQZ™ AACs Demonstrated Robust Activation in Preclinical Studies Lymphoid Organ Activation Antigen + Activating Adjuvant T cell activation SQZ™ AACs 1. High Quantities of CD8+ T Cells 3. High Quality T Cell Responses 2. Specific T Cell Responses As presented at SITC 2020 22 Data in HPV Tumor Model

Flexible AAC Candidate Trial Design | Monotherapy & Combination with ICIs Monotherapy 2022 IND 50 M Dose determined by Additional KRAS‐Mutant AACs/kg Cohort 1 observations Cohorts Tumors Cohort 1 Cohort 2 Combination Therapy SQZ‐AAC‐HPV Multiple I/O Phase 1/2 Trial Information Combo Cohorts • Basket trial across HPV+ tumors: H&N, Cervical, Anal, Penile, Vulvar/Vaginal • Safety‐adjusted dose escalation allowing for data driven Earlier Line escalation Expansion Cohorts • No need for escalation in combo cohorts, starting with the Mono or Combo recommended phase 2 dose • Monotherapy start in 2021 with data in 2022 Trial ID: NCT04892043 23

Autoimmune Diseases SQZ™ Approach SQZ TACs 24

SQZ™ TACs ‐ Directing Tolerogenic Immune Response SQZ™ Tolerizing Antigen Carriers (SQZ TACs) Leveraging antigen carriers from RBCs squeezed with antigen only (no adjuvant) tolerize the immune response to that antigen Activation SQZ Cargo Antigen + Activating Adjuvant Lymphoid Organ T cell activation SQZ AACs (Activating Antigen Carrier) Engulfment by APCs Tolerization Anergy T reg upregulation Antigen SQZ TACs T cell deletion (Tolerizing Antigen Carrier) Bystander Suppression 25
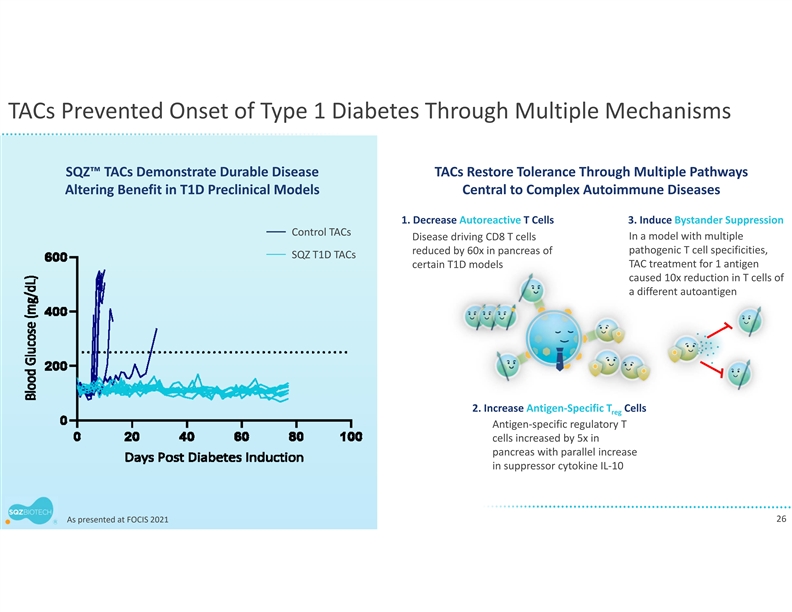
TACs Prevented Onset of Type 1 Diabetes Through Multiple Mechanisms SQZ™ TACs Demonstrate Durable Disease TACs Restore Tolerance Through Multiple Pathways Altering Benefit in T1D Preclinical Models Central to Complex Autoimmune Diseases 1. Decrease Autoreactive T Cells 3. Induce Bystander Suppression Control TACs Disease driving CD8 T cells In a model with multiple reduced by 60x in pancreas of pathogenic T cell specificities, SQZ T1D TACs TAC treatment for 1 antigen certain T1D models caused 10x reduction in T cells of a different autoantigen 2. Increase Antigen‐Specific T Cells reg Antigen‐specific regulatory T cells increased by 5x in pancreas with parallel increase in suppressor cytokine IL‐10 26 As presented at FOCIS 2021

SQZ™ TAC Opportunity in Celiac Disease Celiac Disease: Lead SQZ TAC Indication with High Unmet Need for a Tolerizing Therapy Potential Year Diagnosed CeD, US expansion SQZ‐TAC‐CeD Target Product Profile 2020 1.06M populations Initial target Long‐acting tolerization for release population: 2025 1.55M of dietary gluten restrictions ~60% of total Initial clinical population: well‐ CeD cases No approved treatments controlled and symptomatic patients with Celiac disease TM Compelling Case for SQZ TAC Potential in Celiac Disease: ü Well‐defined exogenous antigen and strong evidence of T cell driven pathology ü Rapid read‐out potential: Phase 1 trial design incorporates gluten challenge to enable early clinical PoC data ü Anticipated CeD IND in Q3 2022 27

Infectious Diseases and beyond 28

Potential for Multiple Clinical Readouts In 2022 Milestones through end of 2022 Investment Highlights • Phase 1/2 Add’l Monotherapy data • Phase 1/2 Combination data SQZ APCs ü Proprietary technology with potential to expand • Phase 1/2 data application of cell therapies • KRAS IND submission SQZ AACs ü Fast, reliable and cost‐efficient manufacturing potential • IND submission ü Unique cell therapy engine applicable across diseases • Phase 1/2 data SQZ eAPCs ü Clinical stage pipeline with multiple platform readouts expected across 2022 • Celiac IND submission SQZ TACs ü Additional INDs leverage de‐risked manufacturing backbone to expand potential patient impact • HBV IND submission ü Existing Roche partnership; potential additional BD SQZ eAPCs opportunities • Point‐of‐care non‐clinical testing data 29 INFECTIOUS AUTO‐ CORPORATE ONCOLOGY DISEASE IMMUNITY
