Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sesen Bio, Inc. | sesn-20210910.htm |

Sandeep Gurram, Sonia Bellfield, Rebecca Dolan, Beatriz Walter, Maria Merino, Scot Niglio, Andrea Apolo, Piyush Agarwal, Vladimir Valera NCT03258593 @SandeepGurramMD Interim Analysis of a Phase I Single-Arm Study of the Combination of Durvalumab and Vicineum (oportuzumab monatox, VB4-845) in Subjects with High-Grade Non-Muscle-Invasive Bladder Cancer Previously Treated with BCG

Disclosures • No disclosures to present • Study drugs were provided by Sesen Bio (Vicineum) and AstraZeneca (Durvalumab)

Background • Vicineum is a recombinant fusion protein comprised of an anti-EpCAM linked to a truncated form of Pseudomonas exotoxin A • 98% of BCG-refractory pts with EpCAM overexpression • Phase III VISTA trial has demonstrated efficacy • Duration of response in CR subjects: 287 days • PD-1/PD-L1 inhibitor activity in BCG- unresponsive population was demonstrated in Keynote 057 Timepoints Cohort 1 Recurrent CIS ± papillary within 6 mo of BCG n = 82 Cohort 2 Recurrent CIS ± papillary within >6 but <12 mo of BCG n = 7 Combined Cohort 1 and 2 All CIS subjects s n= 89 3-mo CR rate 39% 57% 40% 12-mo CR rate 17% 14% 17% VISTA Phase III Results

T cells recognize neoantigen and kill cancer cells T cell proliferation DAMPs Neoantigen releaseVicineum causes immunogenic cell death by triggering damage- associated molecular patterns (DAMPs) Vicineum selectively targets EpCAM on urothelial carcinoma cells Neoantigen presentation and T cell activation Memory T cell M e c h a n i sm 1 : K i l l s c e l l d i r e c t l y APC Activation Exotoxin A Payload Non-cleavable peptide Linker Antibody Fragment Vicinium M e c h a n i sm 2 : A c t i v a t e s i m m u n e s y s t e m V i c i ne um

Study Design Eligibility Criteria • CIS ± papillary tumor or HG Ta or T1 UC • BCG unresponsive* • Last dose of BCG within 1 year • ECOG PS 0-1 • No prior use of PD-1/PD-L1 inhibitor • No history of upper tract or urethral UC within the last 2 years • Adequate hepatic and renal function Safety Run- in Cohort N = 6 - 12 Treatment Vicineum 30 mg intravesically Induction: weekly for 12 weeks Maintenance: every other week up to week 52 Optional: every other week for an additional 52 weeks + Durvalumab 1500 mg IV monthly Required: q4 weeks for 52 weeks Optional: every 12 weeks for an additional 52 weeks Expansion Cohort N = 18 Primary Endpoint: Safety and Tolerability Secondary Endpoints: Efficacy, Pharmacokinetics, & Biomarker Analysis CIS, Carcinoma in situ. HG, High grade. BCG, Bacillus Calmette-Guérin. UC, urothelial carcinoma. ECOG PS, Eastern Cooperative Oncology Group performance status. PD, Programmed death. IV, intravenous. *Per FDA and SUO definitions
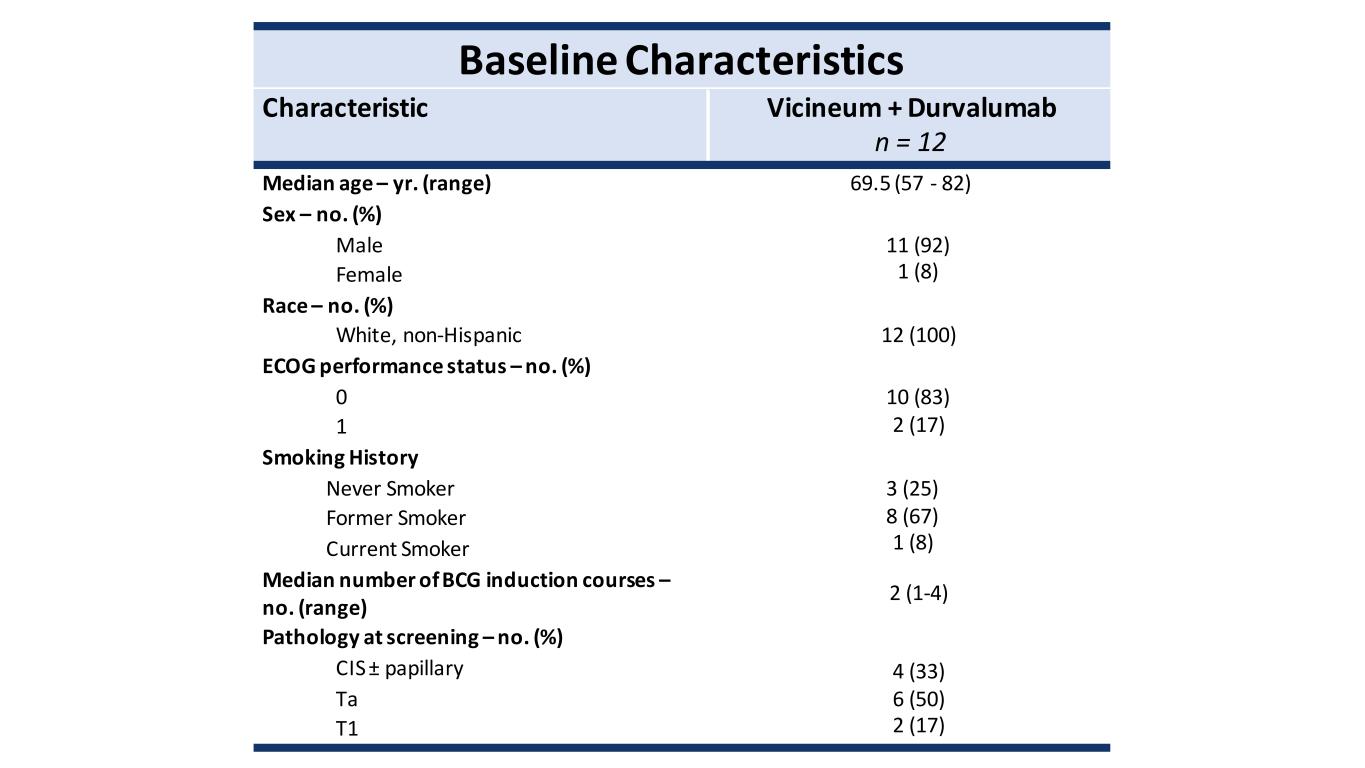
Baseline Characteristics Characteristic Vicineum + Durvalumab n = 12 Median age – yr. (range) 69.5 (57 - 82) Sex – no. (%) Male 11 (92) 1 (8) Female Race – no. (%) White, non-Hispanic 12 (100) ECOG performance status – no. (%) 0 10 (83) 2 (17) 1 Smoking History Never Smoker 3 (25) 8 (67) 1 (8) Former Smoker Current Smoker Median number of BCG induction courses – no. (range) 2 (1-4) Pathology at screening – no. (%) CIS ± papillary 4 (33) 6 (50) 2 (17) Ta T1

Baseline Characteristics Characteristic Vicineum + Durvalumab n = 12 Median age – yr. (range) 69.5 (57 - 82) Sex – no. (%) Male 11 (92) 1 (8) Female Race – no. (%) White, non-Hispanic 12 (100) ECOG performance status – no. (%) 0 10 (83) 2 (17) 1 Smoking History Never Smoker 3 (25) 8 (67) 1 (8) Former Smoker Current Smoker Median number of BCG induction courses – no. (range) 2 (1-4) Pathology at screening – no. (%) CIS ± papillary 4 (33) 6 (50) 2 (17) Ta T1

Baseline Characteristics Characteristic Vicineum + Durvalumab n = 12 Median age – yr. (range) 69.5 (57 - 82) Sex – no. (%) Male 11 (92) 1 (8) Female Race – no. (%) White, non-Hispanic 12 (100) ECOG performance status – no. (%) 0 10 (83) 2 (17) 1 Smoking History Never Smoker 3 (25) 8 (67) 1 (8) Former Smoker Current Smoker Median number of BCG induction courses – no. (range) 2 (1-4) Pathology at screening – no. (%) CIS ± papillary 4 (33) 6 (50) 2 (17) Ta T1
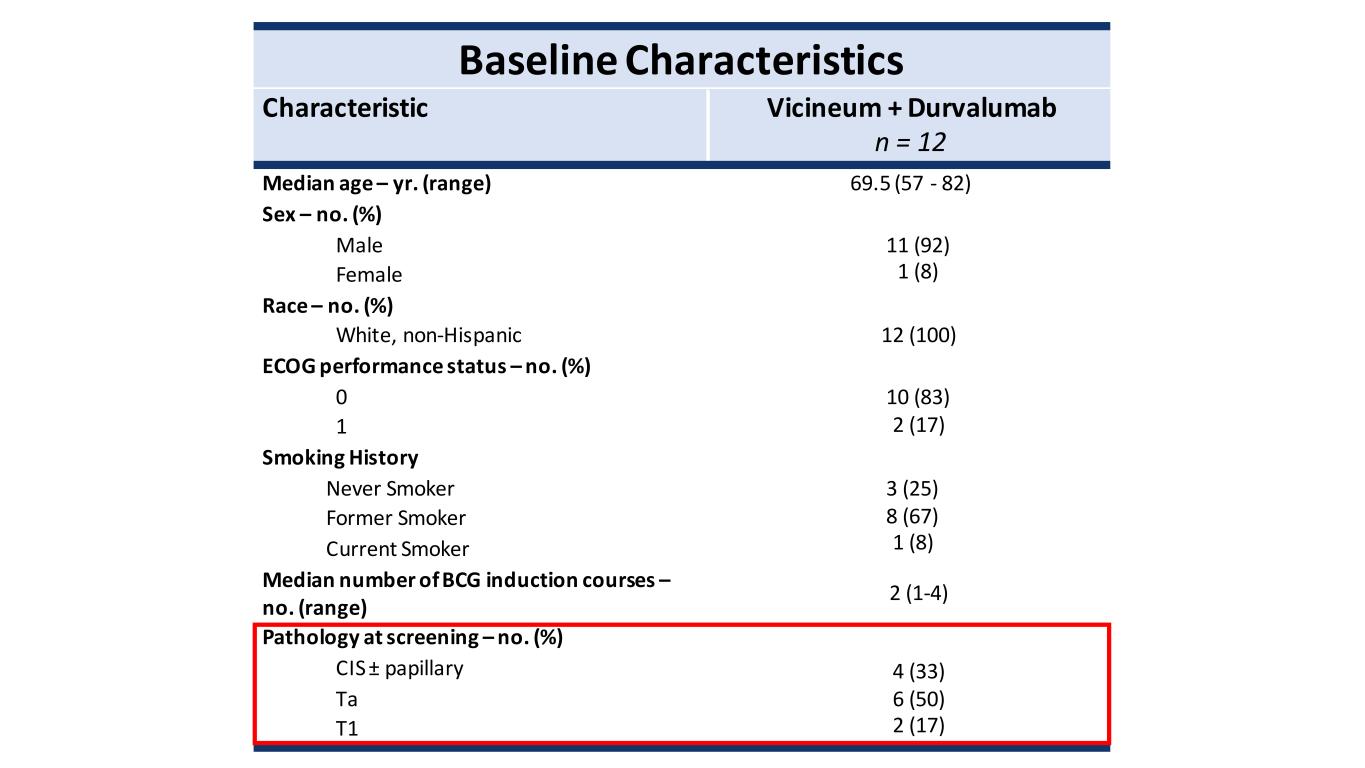
Baseline Characteristics Characteristic Vicineum + Durvalumab n = 12 Median age – yr. (range) 69.5 (57 - 82) Sex – no. (%) Male 11 (92) 1 (8) Female Race – no. (%) White, non-Hispanic 12 (100) ECOG performance status – no. (%) 0 10 (83) 2 (17) 1 Smoking History Never Smoker 3 (25) 8 (67) 1 (8) Former Smoker Current Smoker Median number of BCG induction courses – no. (range) 2 (1-4) Pathology at screening – no. (%) CIS ± papillary 4 (33) 6 (50) 2 (17) Ta T1
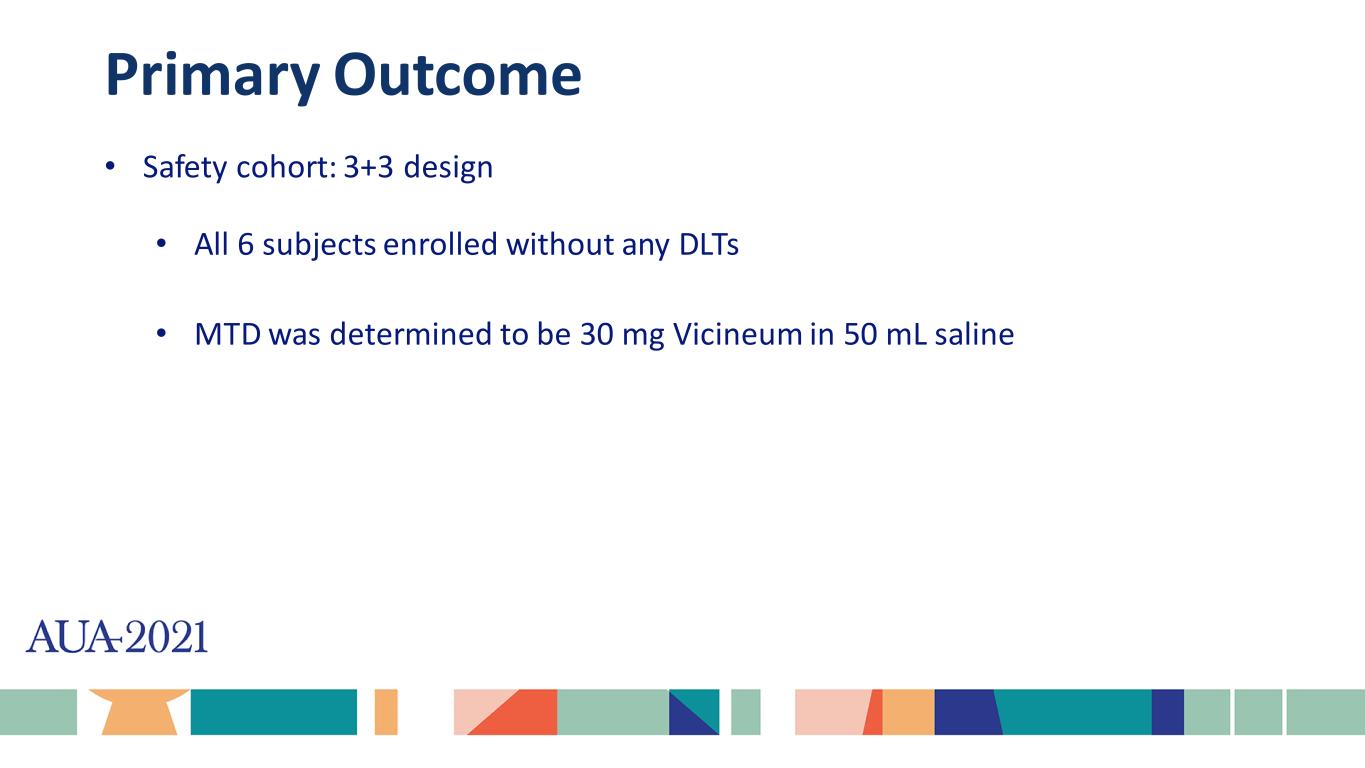
Primary Outcome • Safety cohort: 3+3 design • All 6 subjects enrolled without any DLTs • MTD was determined to be 30 mg Vicineum in 50 mL saline

Tolerability • No dose reductions or discontinuation of any agent • No subjects were taken off study due to treatment related adverse events • Two subjects were taken off study due to unrelated medical issues • Worsening of baseline peripheral vascular disease • Cognitive decline secondary to worsening vascular dementia

Treatment Related Adverse Events • Number of patients experiencing any TrAE: 12 (100%) • Number of patients experiencing grade ≥3 TrAE*: 3 (25%) • 1 subject required systemic corticosteroids for a persistent grade 2 diarrhea due to checkpoint associated colitis *No grade 4 or 5 TrAE’s were noted Treatment Related Adverse Events of Any Grade in ≥10% of Patients CTCAE Term All Grades (%) Grade ≥3 (%) Hematuria 5 (41.7) 1 (8.3) Renal and urinary disorders - Other 5 (41.7) 0 (0) Urinary tract infection 5 (41.7) 0 (0) Urinary frequency 5 (41.7) 0 (0) Pruritus 4 (33.3) 0 (0) Diarrhea 3 (25) 0 (0) Rash maculo-papular 3 (25) 0 (0) Dry skin 2 (16.7) 0 (0) Bladder spasm 2 (16.7) 0 (0) Hypotension 2 (16.7) 0 (0) Fatigue 2 (16.7) 0 (0) Hyperthyroidism 2 (16.7) 0 (0) Creatinine increased 2 (16.7) 0 (0) Bladder infection 2 (16.7) 1 (8.3) Insomnia 2 (16.7) 0 (0) Serum amylase increased 2 (16.7) 0 (0) Headache 2 (16.7) 0 (0) Dysuria 2 (16.7) 0 (0) Proteinuria 2 (16.7) 0 (0) Urinary urgency 2 (16.7) 0 (0) Chills 2 (16.7) 0 (0) Thromboembolic event 1 (8.3) 1 (8.3) Hyponatremia 1 (8.3) 1 (8.3)
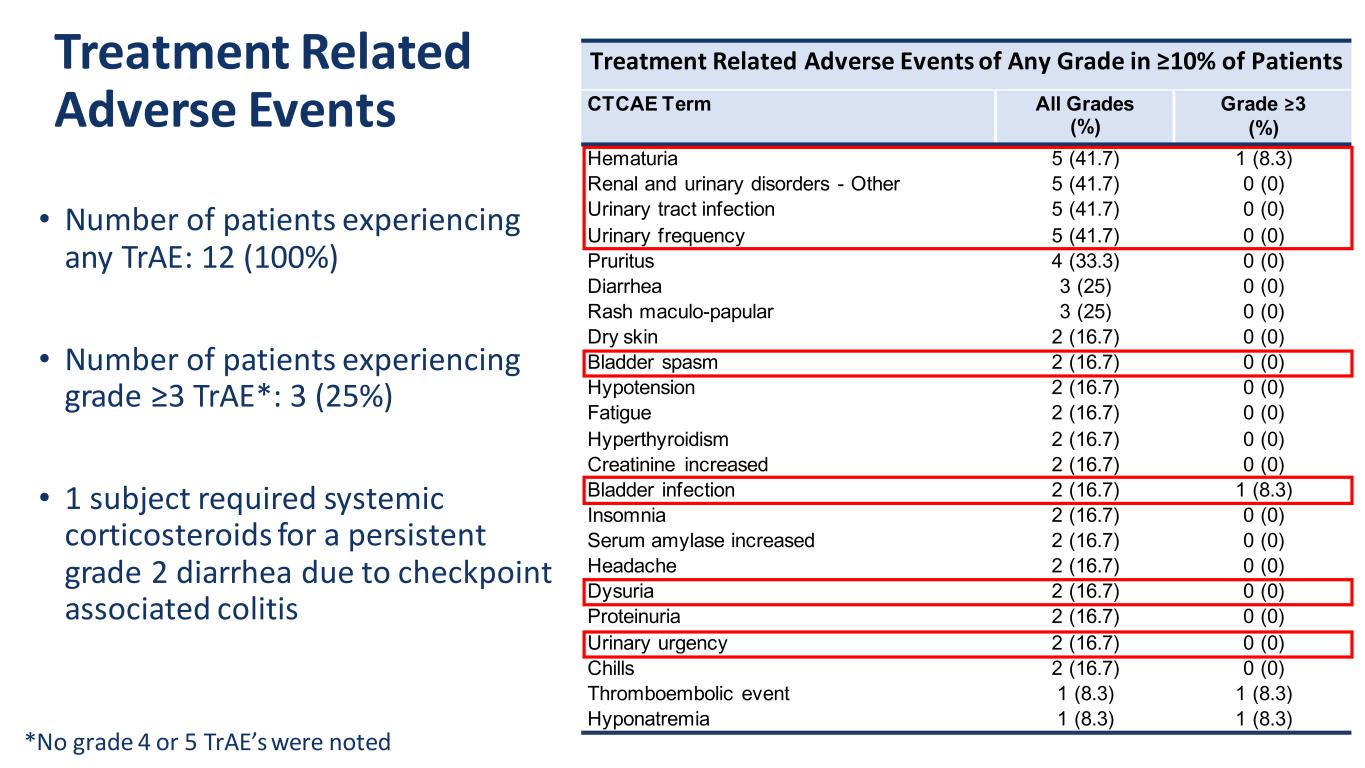
Treatment Related Adverse Events • Number of patients experiencing any TrAE: 12 (100%) • Number of patients experiencing grade ≥3 TrAE*: 3 (25%) • 1 subject required systemic corticosteroids for a persistent grade 2 diarrhea due to checkpoint associated colitis *No grade 4 or 5 TrAE’s were noted Treatment Related Adverse Events of Any Grade in ≥10% of Patients CTCAE Term All Grades (%) Grade ≥3 (%) Hematuria 5 (41.7) 1 (8.3) Renal and urinary disorders - Other 5 (41.7) 0 (0) Urinary tract infection 5 (41.7) 0 (0) Urinary frequency 5 (41.7) 0 (0) Pruritus 4 (33.3) 0 (0) Diarrhea 3 (25) 0 (0) Rash maculo-papular 3 (25) 0 (0) Dry skin 2 (16.7) 0 (0) Bladder spasm 2 (16.7) 0 (0) Hypotension 2 (16.7) 0 (0) Fatigue 2 (16.7) 0 (0) Hyperthyroidism 2 (16.7) 0 (0) Creatinine increased 2 (16.7) 0 (0) Bladder infection 2 (16.7) 1 (8.3) Insomnia 2 (16.7) 0 (0) Serum amylase increased 2 (16.7) 0 (0) Headache 2 (16.7) 0 (0) Dysuria 2 (16.7) 0 (0) Proteinuria 2 (16.7) 0 (0) Urinary urgency 2 (16.7) 0 (0) Chills 2 (16.7) 0 (0) Thromboembolic event 1 (8.3) 1 (8.3) Hyponatremia 1 (8.3) 1 (8.3)

Secondary Outcomes • Complete Responses • 3 month: 5/12 (42%) • 12 month: 2/12 (17%) • Two subjects who came off study for unrelated medical reasons were 3- month responders High-grade recurrence No high-grade recurrence Currently on therapy Su bj ec ts

Efficacy Outcomes Number of Patients (%) Progression/recurrence of high grade NMIBC 8 (67) Progression to MIBC† 1 (8.3) 12-month cystectomy-free survival 9 (75) Death from bladder cancer 0 (0) † includes at final on-study TUR or occult disease on cystectomy pathology
Conclusions • The combination of Vicineum and Durvalumab in BCG unresponsive NMIBC is tolerated well with no new safety signals • The doublet has a similar safety profile compared to both agents used individually • Interim analysis shows a 3-month CR rate of 42% (5/12) • Further biomarker analysis may help predict responders

Acknowledgements Patients and their Families @SandeepGurramMD Investigators Vladimir Valera Romero MD PhD – PI Sandeep Gurram MD Piyush Agarwal MD Andrea Apolo MD Scot Niglio MD Collaborators Rebecca Dolan PA Sonia Bellfield RN Jacqueline Cadena NP Liang Cao PhD Beatriz Walter MD PhD Maria Merino MD Antoun Toubaji MD Sesen Bio AstraZeneca
