Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - PDS Biotechnology Corp | brhc10028828_ex99-1.htm |
| 8-K - 8-K - PDS Biotechnology Corp | brhc10028828_8k.htm |
Exhibit 99.2

Nasdaq: PDSBDeveloping powerful, safe, versatile immunotherapies CORPORATE OVERVIEW Frank Bedu-Addo
Ph.D. President & CEO SEPTEMBER 2021

2 Forward-Looking Statements This presentation contains forward-looking statements about PDS
Biotechnology Corporation (“PDSB”), and its businesses, business prospects, strategies and plans, including but not limited to statements regarding anticipated pre-clinical and clinical drug development activities and timelines and market
opportunities. All statements other than statements of historical facts included in this presentation are forward-looking statements. The words “anticipates,” “may,” “can,” “plans,” “believes,” “estimates,” “expects,” “projects,” “intends,”
“likely,” “will,” “should,” “to be,” and any similar expressions or other words of similar meaning are intended to identify those assertions as forward-looking statements. These forward-looking statements involve substantial risks and
uncertainties that could cause actual results to differ materially from those anticipated.Factors that may cause actual results to differ materially from such forward-looking statements include those identified under the caption “Risk Factors”
in the documents filed with the Securities and Exchange Commission (“SEC”) from time to time, including its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. You are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of the date of this presentation. Except to the extent required by applicable law or regulation, PDSB undertakes no obligation to update the forward-looking statements included
in this presentation to reflect subsequent events or circumstances.
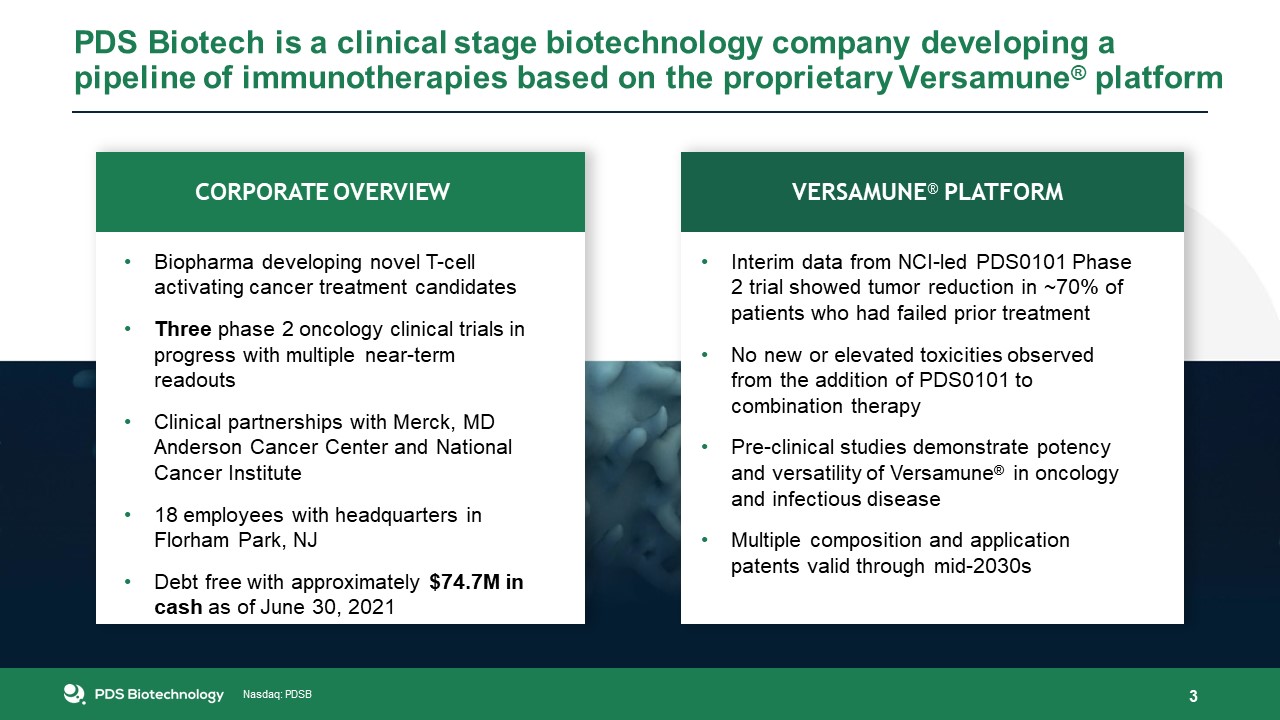
Nasdaq: PDSB Nasdaq: PDSB PDS Biotech is a clinical stage biotechnology company developing a
pipeline of immunotherapies based on the proprietary Versamune® platform 3 Interim data from NCI-led PDS0101 Phase 2 trial showed tumor reduction in ~70% of patients who had failed prior treatmentNo new or elevated toxicities observed from
the addition of PDS0101 to combination therapyPre-clinical studies demonstrate potency and versatility of Versamune® in oncology and infectious diseaseMultiple composition and application patents valid through mid-2030s Biopharma developing
novel T-cell activating cancer treatment candidatesThree phase 2 oncology clinical trials in progress with multiple near-term readoutsClinical partnerships with Merck, MD Anderson Cancer Center and National Cancer Institute18 employees with
headquarters in Florham Park, NJDebt free with approximately $74.7M in cash as of June 30, 2021 VERSAMUNE® PLATFORM CORPORATE OVERVIEW

4 PDS Biotech’s Versamune®-based immunotherapies are designed to promote a powerful in vivo
tumor-specific CD8+ killer T-cell response A significant barrier to effective immunotherapy has been the inability to promote adequate CD8+ killer T-cell responses in vivo 70-90% of cancer patients fail check point inhibitor
therapy Versamune®-based therapies also show promising potential to:Generate the right type and quantity of effective CD8+ killer T-cellsGenerate memory T-cells, to enhance durability of responseGenerate potency without systemic side effects

Nasdaq: PDSB Nasdaq: PDSB PDS Biotech executive team has demonstrated success in the development
and commercialization of leading pharmaceutical products 5 Senior executive experience with management of strategy and execution at both large pharma and biotechsNotable drug development:Abelcet® (Liposome Company/ Elan) PEG-Intron®
(Schering-Plough/ Merck) Frank Bedu-Addo, PhDChief Executive Officer Co-founder>35 years of drug development experienceIn-depth experience with biotech drug discovery, product development and manufacturing Gregory Conn, PhDChief
Scientific Officer >30 years of translational clinical research experienceFormer Director of Clinical Research at National Cancer Institute Center for Cancer Research (Cancer Vaccine Branch) Lauren V. Wood, MDChief Medical
Officer Senior executive experience with over 20 years of experience in high tech companiesIn-depth experience with M&A transactions, capital markets, business development and investor relations Seth Van Voorhees, PhDChief Financial
Officer

Nasdaq: PDSB PDS Biotech’s robust Versamune® -based pipeline is being developed in partnership
with leaders in immuno-oncology and infectious disease 6 Reference: Data on file. * *Consortium of PDS Biotech, Farmacore Biotechnology and Blanver Farmoquimica. Funding provided by The Ministry of Science, Technology and Innovation of
Brazil (“MCTI”)
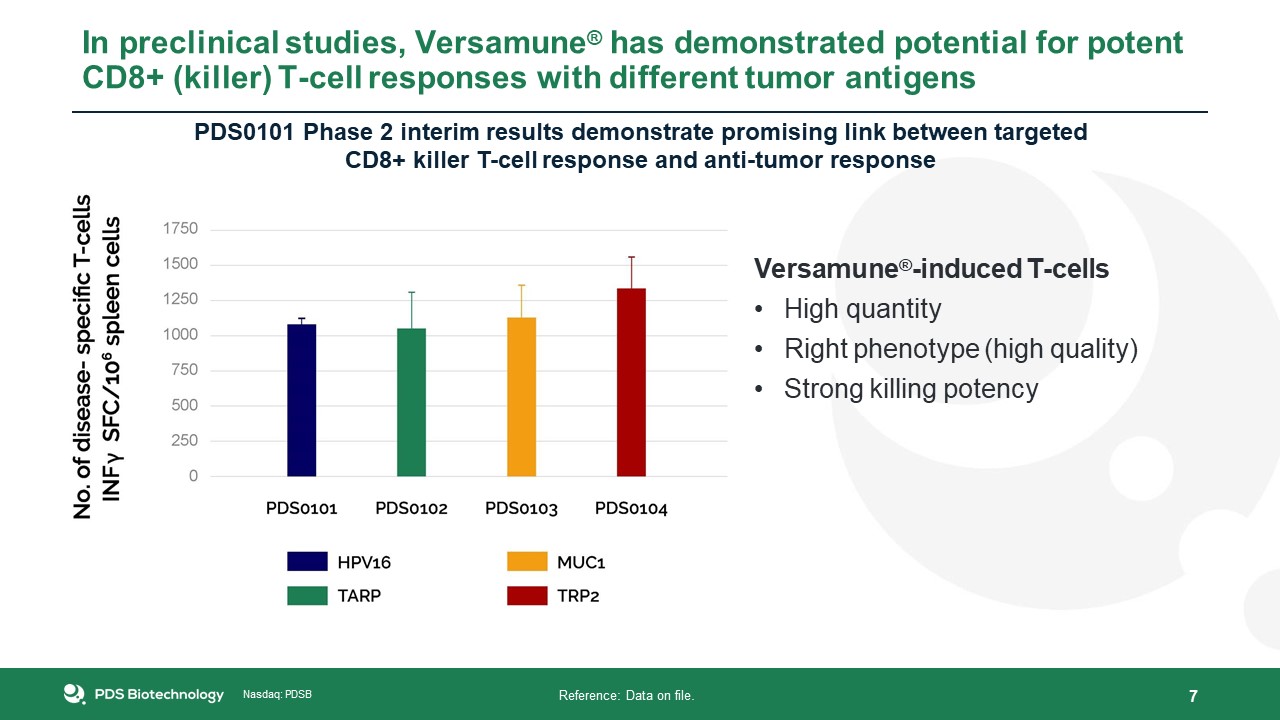
Nasdaq: PDSB Reference: Data on file. In preclinical studies, Versamune® has demonstrated
potential for potent CD8+ (killer) T-cell responses with different tumor antigens 7 PDS0101 Phase 2 interim results demonstrate promising link between targetedCD8+ killer T-cell response and anti-tumor response Versamune®-induced T-cellsHigh
quantityRight phenotype (high quality)Strong killing potency

Introduction to PDS0101

Nasdaq: PDSB Nasdaq: PDSB 9 Approximately 43,000 patients are diagnosed with HPV-associated
cancers annually in the US alone1 Cancers caused by HPV include anal, cervical, head and neck, penile vaginal and vulvar cancers Incidence rate of HPV-related head and neck and anal cancer is growing and remains a significant unmet medical
need Existing immunotherapies cost$120,000+ annually per patient References: Markowitz et al. 2016. Centers for Disease Control and Prevention. 2018. Hernandez et al. 2018. American Journal of Managed Care Volume 24, Issue 2; Company
Research, Strauss J. et al. 2021 ASCO Annual Meeting Abstract: 2501. PDS0101 is designed to treat advanced human papillomavirus (HPV)-16 cancers which represents 70-80% of the HPV-associated cancer market 20-30% of patients either
progress or have a recurrence of cancer and are considered advanced cancer FIRST LINERadiation and/or Chemotherapy Objective response rate (ORR) ranges from 12-24%75-80% of patients fail treatment with CIs and are considered CI
Refractory ADVANCED CANCERCheckpoint Inhibitors (CPI) Objective response rate (ORR) rangesfrom 5-12%Historical median survival is 3-4 months CPI REFRACTORYFew Treatment Options

Nasdaq: PDSB 10 Sub-cutaneous injection of PDS0101 monotherapy induced high quantity of potent
HPV16-specific CD8+T-cells in Phase 1 clinical trial 0Patient 50 100 150 200 250 2-4 2-5 3-1 3-2 5-7 2-7 A2 HPV-specific T-cell Response IFN-γ
ELISPOT 0Patient 200 400 600 800 1000 1200 1400 1600 1800 2000 5-1 5-4 2-1 2-3 Low Medium HighHLA Type A2 A1, A2, A3, 30 HLA Type A2 A2 A3 A74 HPV-specificT-cell
Response IFN-γ ELISPOT Responses were evaluated on Days 14-19 after SC injectionPredominant CD8+ T-cell responses confirmed by Granzyme-b ELISPOT Pre-treatment Post-treatment Lesion
regression in 8/10 CIN patients within 3 months of treatment (Retrospective analysis)No recurrence within 2-year evaluation period may suggest durable immune responses 10

Indication Patients with advanced HPV-associated cancer who have failed prior treatment Clinical
Agents Bintrafusp alfa: Bifunctional checkpoint inhibitor-“TGF-β trap” fusion proteinM9241: Antibody-conjugated immuno-cytokinePDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ T-cells Study goals Group 1: Objective
response rate (ORR) in checkpoint inhibitor (CPI) naïve patients Group 2: ORR in patients who have failed checkpoint inhibitor therapy (CPI refractory) Timing Full enrollment of 56 patientsComplete enrollment expected by Q1 2022 Trial
Sponsor Nasdaq: PDSB Phase 2 NCI-led clinical trial evaluating the triple combination of PDS0101, Bintrafusp alfa and M9241 in advanced HPV-associated cancer 11 The objective of this trial is to evaluate the potential of the triple
combination to provide aneffective therapy for patients with advanced and untreatable cancer
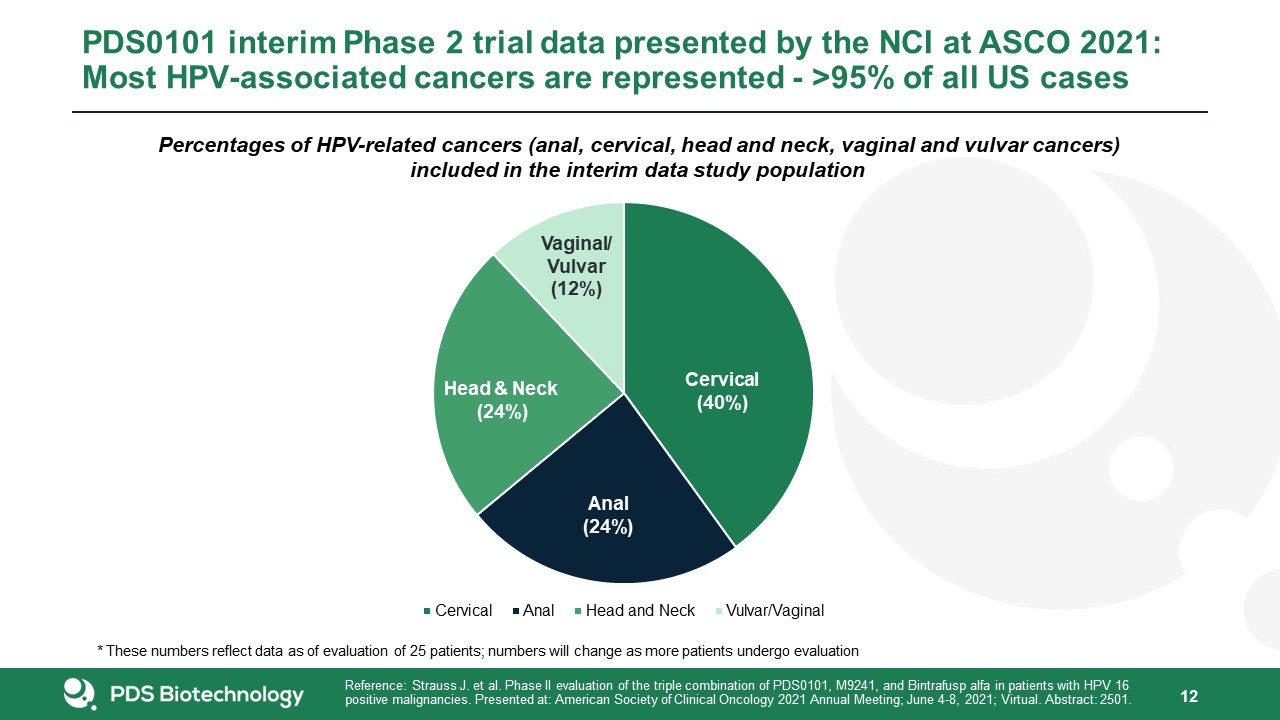
PDS0101 interim Phase 2 trial data presented by the NCI at ASCO 2021: Most HPV-associated cancers
are represented - >95% of all US cases 12 Cervical (40%) Anal (24%) Percentages of HPV-related cancers (anal, cervical, head and neck, vaginal and vulvar cancers) included in the interim data study
populationVaginal/ Vulvar (12%) Head & Neck (24%) Cervical Anal Head and Neck Vulvar/Vaginal* These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Reference: Strauss J. et
al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract:
2501.

12 – 24% 83% (5/6)1 patient had no evidence of
disease by ASCO 2021 (complete response) 0 10 20 80 90 100 30 40 50 60 70Percentage of patients who experienced >30% tumor reduction Historical standard of care (checkpoint inhibitor) PDS0101 +Bintafusp alfa + M9241 Percentage of
CPI Naïve Patients Who Experienced Objective Response with Treatment ASCO 2021: PDS0101 triple combination achieved 83% ORR among six advanced HPV16-positive CPI naive patients, suggesting potential efficacy 13 Reference: Strauss J. et al.
Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract:
2501. * These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation

58% (7/12)1 patient had no evidence of disease by ASCO 2021 (complete
response) 5 – 12% 0 10 70 80 90 100 20 30 40 50 60Percentage of patients who experienced tumor reduction Historical standard of care (checkpoint inhibitor) PDS0101
+Bintafusp alfa + M9241 ASCO 2021: Triple combination achieved 58% tumor reduction among 12 HPV16 checkpoint inhibitor refractory patients 14 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and
Bintrafusp alfa in patients with HPV 16positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients at a
median of 8 months; numbers will change as more patients undergo evaluation 5 patients had already achieved an objective response (>30% tumor reduction)Percentage of CPI Refractory Patients WhoExperienced Tumor Reduction with Treatment
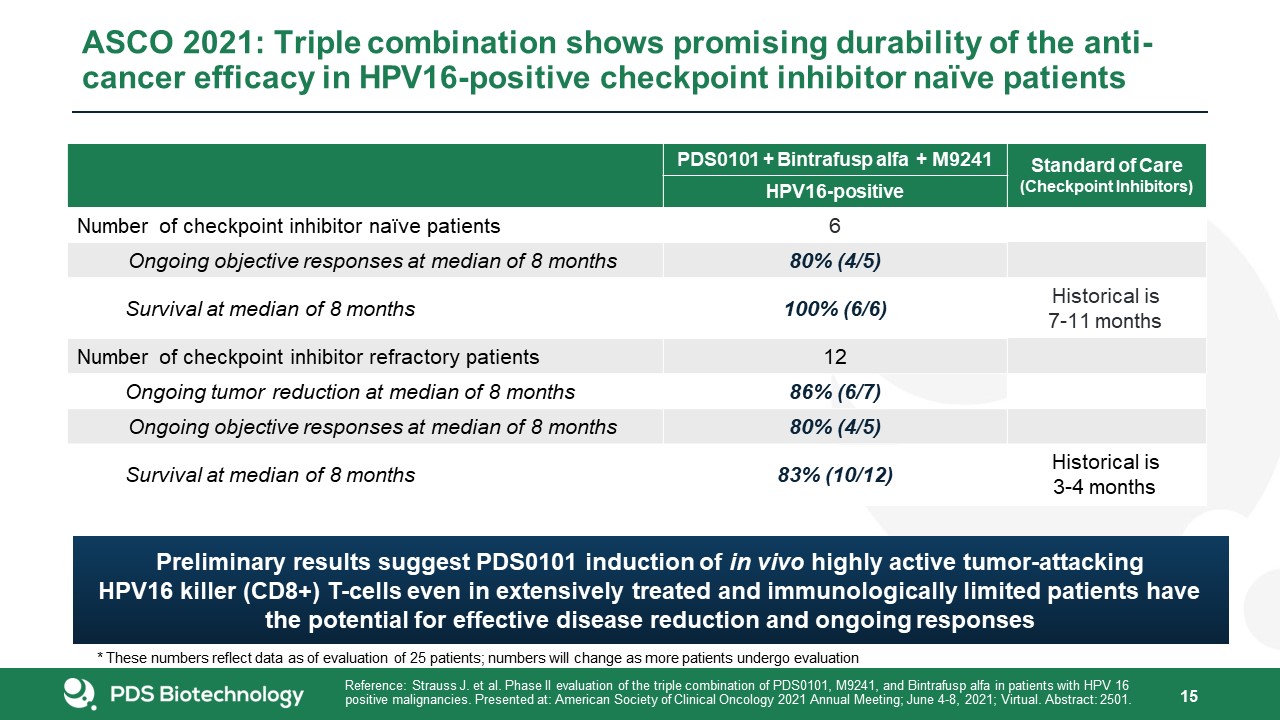
15 ASCO 2021: Triple combination shows promising durability of the anti- cancer efficacy in
HPV16-positive checkpoint inhibitor naïve patients PDS0101 + Bintrafusp alfa + M9241 Standard of Care(Checkpoint Inhibitors) HPV16-positive Number of checkpoint inhibitor naïve patients 6 Ongoing objective responses at
median of 8 months 80% (4/5) Survival at median of 8 months 100% (6/6) Historical is7-11 months Number of checkpoint inhibitor refractory patients 12 Ongoing tumor reduction at median of 8 months 86% (6/7) Ongoing objective
responses at median of 8 months 80% (4/5) Survival at median of 8 months 83% (10/12) Historical is3-4 months Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients
with HPV 16positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. Preliminary results suggest PDS0101 induction of in vivo highly active tumor-attacking HPV16
killer (CD8+) T-cells even in extensively treated and immunologically limited patients havethe potential for effective disease reduction and ongoing responses * These numbers reflect data as of evaluation of 25 patients; numbers will change as
more patients undergo evaluation

ASCO 2021: Results in HPV16-negative patients suggests critical role of PDS0101-induced
HPV16-specific CD8+ T-cells in promoting tumor reduction 16 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16positive malignancies. Presented at: American
Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Preliminary results suggest that
HPV16-specific CD8+ and CD4+ T-cell induction by PDS0101 as predicted by preclinical studies may promote enhanced clinical benefit of the triple combination 67% (12/18) 0%
(0/7) 0 80 90 100 10 20 30 40 50 60 70Percentage of patients who experienced tumor reduction at a median of 8 months HPV16-Negative (n=7) HPV16-Positive (n=18) Percentage of HPV16-Positive and HPV16-Negative Patients
Who Experienced Tumor Reduction at Median 8 Months

Nasdaq: PDSB Nasdaq: PDSB 17 Phase 2 trial evaluating the combination of PDS0101/KEYTRUDA® for
treatment of HPV16-positive metastatic/recurrent head and neck cancer (VERSATILE-002) Indication Treatment of patients with HPV16-positive head and neck cancer whose cancer hasspread or returned Clinical Agents KEYTRUDA® (Standard of Care):
Anti-PD1 checkpoint inhibitor (ORR ~20%)PDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ and CD4+ T-cells Study goals Group 1: Objective response rate (ORR) as first-line treatment in checkpoint inhibitor (CPI)naïve
patientsGroup 2: ORR in patients who have failed checkpoint inhibitor therapy (CPI refractory) Timing Preliminary data anticipated Q4 2021/Q1 2022 Trial Partner Confirmation that PDS0101 enhances the therapeutic benefit of checkpoint
inhibitorscould expand evaluation of Versamune®-based therapies in multiple cancer indications

Nasdaq: PDSB Nasdaq: PDSB 18 Phase 2 investigator-led trial evaluating the combination of
PDS0101 and chemoradiation in patients with locally advanced cervical cancer (IMMUNOCERV) Indication Treatment of patients with locally advanced cervical cancer – Stages IB3-IVA ClinicalAgents Chemoradiotherapy (CRT – Standard of Care):
Cisplatin and radiation therapy PDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ and CD4+ T-cells Study goals Safety, rate of regression and local control in patients with primary tumor ≥5cm (n=35
patients) Timing Preliminary data anticipated 1H 2022 – Rate of complete response by PET-CT at 6 months and rate of tumor volume reduction by MRI at 30-40 days from start of treatment Trial Sponsor If successful, this study could support
further investigation of Versamune®-based immunotherapies in combination with chemotherapy or CRT to treat multiple cancers

Development of PDS0102

20 Approximately 470,000 patients are diagnosed annually with AML, prostate or breast cancer, most
of which are associated with target T-cell receptor gamma alternate reading frame protein (TARP) Acute Myeloid Leukemia (AML)Almost 20,000 cases in the US annuallyTARP expressed in 100% of AML Prostate cancerAlmost 175,000 US cases
annuallyThe immunogenic TARP protein is expressed in about 90% of prostate cancers at all stages of the disease^Breast cancerMore than 270,000 US cases annuallyTARP expressed in about 50% of breast cancers at all stages of the
disease References: Fritzche FR et al. Histol Histopathol 2010 Jun; 25 (6): 733-9 doi: 10.14670/HH-25.733, Cancer Facts & Figures, American Cancer Society, 2019, LV Wood, et al. Oncoimmunology, 2016. Vol 5. No 8. e1197459. Prostate Cancer
(174,650) Breast Cancer (271,270) AML (19,970) PDS0102 is designed to treat cancers caused by T-cell receptor gamma alternate reading frame protein (TARP), including AML, prostate and breast cancers

21 PDS0102 may provide superior induction of TARP-specific tumor attacking CD8+ killer
T-cells Reference: Wood LV et al, Oncoimmunology, 2016, Vol. 5 (8)CFA – Complete Freund’s Adjuvant a highly potent immune activator not used in humans due to potentially lethal toxicity PRE-CLINICAL OPTIMIZATION STUDIES:TARP-Specific T-cell
Induction after 2 injections of PDS0102

Development of PDS0103

0 1 2 Clinical
trial design will seek to evaluate PDS0103 in tumor types with the highest expression ofMUC1 and the greatest differences in MUC1 expression between malignant and healthy tissueMUC1 Expression by Tumor Type3 PDS0103 is designed to treat
cancers caused by mucin-1 (MUC1), which is highly expressed in solid tumors and is associated with poor prognosis Reference: M. Uhlen, et al. A pathology atlas of the human cancer transcriptome. Science.18 Aug 2017. MUC1 protein expression
overview data available from https://www.proteinatlas.org/ENSG00000185499-MUC1/tissue.

24 Greater quantity and quality of Versamune®-induced CD8+ killer T-cells may result in ability to
eradicate MUC1-positive tumors Induced a >10-fold number of polyfunctional MUC1 specific CD8+ T-cells500450400350300250200150100500 Adjuvant* + MUC1 Antigen 4-Combo Adjuvant + MUC1 Antigen Versamune + MUC1 Antigen
(PDS0103) # of Antigen-Recognizing CD8+ T-CellsIFN-γ Spot Forming Cells/1X106 Spleen Cells Polyfunctional T-Cells Monofunctional T-Cells*Adjuvant = cytokine GMCSFReferences: J. Immunology, 2019 (202), 1215; Studies in TC-1 tumor model
with other immunotherapies reported in: Vaccine 2009, January 14, 27 (3): 431; Science Translational Medicine 2016, 13 April, Vol 8 Issue 334; Vaccine 2009, September 25, 27 (42): 5906.

PDS0101 Near-Term Milestones and Market Opportunities

Nasdaq: PDSB Nasdaq: PDSB Projected milestones through 2022* *Based on
current enrollment and forecast modeling as of September 2021. Subject to change. 4Q22 3Q22 2Q22 1Q22 4Q21 3Q21 2Q21 1Q21 PDS0101 26 PDS Biotech Funded Clinical Trials Partner Co-Funded Clinical
Trials PDS0103 PDS0102 Preliminary efficacy data from advanced HPV-associated cancer trial (NCI)Interim data from HPV-associated cancer trial (NCI)Expected completion of HPV-associated cancer trial (NCI)Preliminary data from
VERSATILE- 002 (KEYTRUDA® combo) expectedPreliminary data from ImmunoCerv (MD Anderson) expectedPlanned initiation of Phase 1/2 clinical trial in TARP-related cancers Planned initiation of Phase 1/2 clinical trial in MUC1-related cancers

Nasdaq: PDSBDeveloping powerful, safe, versatile immunotherapies

Appendix

Nasdaq: PDSB PDS Biotech’s robust Versamune® -based oncology pipeline is being developed in
partnership with the leaders in immuno-oncology 29 Reference: Data on file.

Nasdaq: PDSB Nasdaq: PDSB Versamune® is designed to induce a robust and targeted anti-tumor response
in vivo when administered with a tumor-associated antigen 30 References: Gandhapudi SK, et al. 2019. Antigen priming with enantiospecific cationic lipid nanoparticles induces potent antitumor CTL responses through novel induction of a Type I
IFN response. J Immunol. 202 (12): 3524-3536. Smalley Rumfield C et al. 2020.Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. for ImmunoTherapy of Cancer 8:e000612. Promotes uptake of vaccine or immunotherapy and
entry into lymph nodes Promotes antigen processing and presentation to T-cells via MHC I and II pathways Activates Type I Interferon pathway, enabling a powerful anti- tumor killer CD8+ T-cell response Versamune® + Tumor- associated
proteins (antigens)
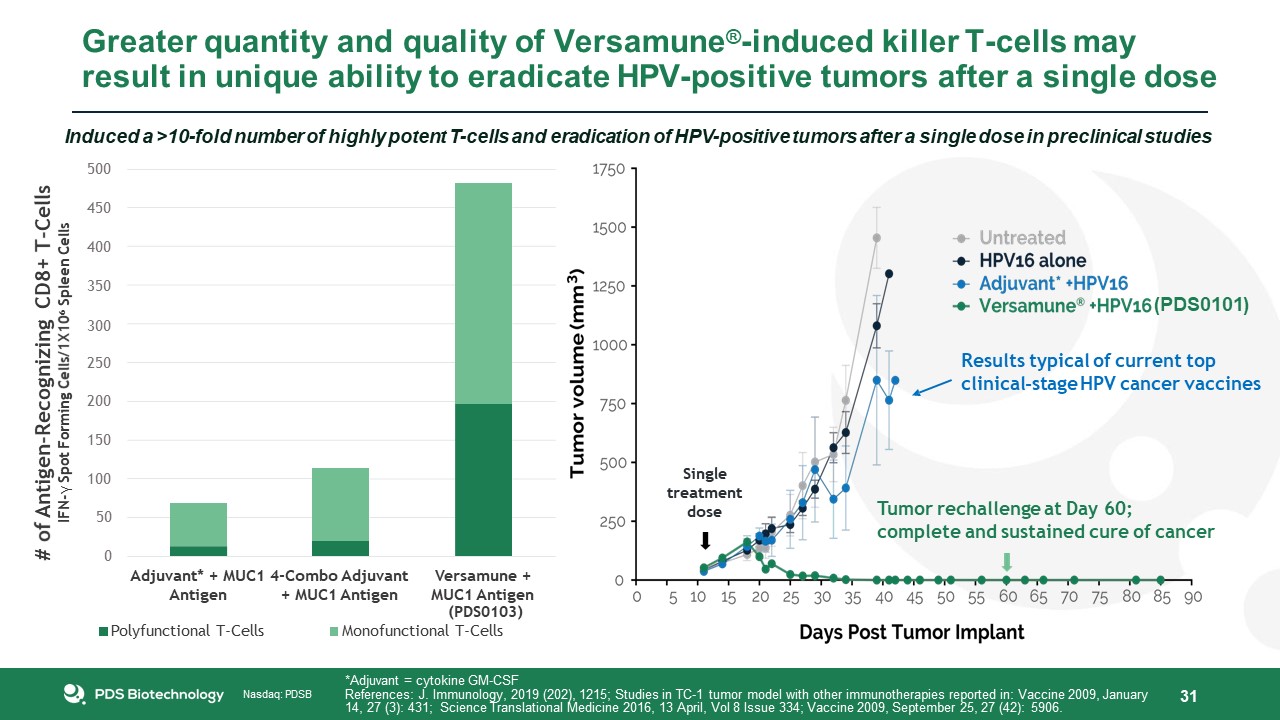
Nasdaq: PDSB Nasdaq: PDSB Greater quantity and quality of Versamune®-induced killer T-cells may
result in unique ability to eradicate HPV-positive tumors after a single dose 31 Single treatment dose Results typical of current top clinical-stage HPV cancer vaccines Tumor rechallenge at Day 60; complete and sustained cure of
cancer *Adjuvant = cytokine GM-CSFReferences: J. Immunology, 2019 (202), 1215; Studies in TC-1 tumor model with other immunotherapies reported in: Vaccine 2009, January 14, 27 (3): 431; Science Translational Medicine 2016, 13 April, Vol 8
Issue 334; Vaccine 2009, September 25, 27 (42): 5906. 0 50 100 200150 250 Adjuvant* + MUC1 4-Combo Adjuvant Antigen + MUC1 Antigen Versamune + MUC1 Antigen # of Antigen-Recognizing CD8+ T-CellsIFN-γ Spot Forming
Cells/1X106 Spleen Cells Induced a >10-fold number of highly potent T-cells and eradication of HPV-positive tumors after a single dose in preclinical studies500450400350(PDS0101)300 (PDS0103)Polyfunctional T-Cells Monofunctional
T-Cells

Nasdaq: PDSB Nasdaq: PDSB 32 Bintrafusp alfa (M7824 -bi-functional checkpoint
inhibitor)Tumor Regression: 0/16 (0%)T-cell Clones: 22 PDS0101 + Bintrafusp alfa + M9241 (NHS IL-12)Tumor Regression: 13/16 (81%)T-cell Clones: 3 *Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of
Cancer 2020; 8:e000612. doi: 10.1136/jitc-2020-000612 Red – CD8+ (killer) T-cells Green – CD4 + (helper) T-cells T-cell clones per 25% of TCR repertoire (Average) Combination of PDS0101 with M9241 or Bintrafusp alfa generated superior
targeted T-cell response; triple combination demonstrated superior efficacy T-cell induction levels Preclinical study: Triple combination of PDS0101, Bintrafusp alfa (M7824) and M9241 (NHS-IL12) demonstrated higher targeted T-cell response

Nasdaq: PDSB Nasdaq: PDSB 33 Reference: Data on file. Versamune® induces high quantity and
quality of CD8+ killer T-cells that infiltrate the tumors and make them more susceptible to killing Minimizes the presence of immune suppressive regulatory T-cells (Treg) within the tumor microenvironment PDS0101 treatment alters the tumor
from having >250-fold more immune repressive Treg cells than CD8+ (killer) T-cells to having about 10-fold higher CD8+ T-cells than Treg cells within 10 days of treatment A-antigen, R-Versamune® (R-DOTAP); G-GM-CSF; S-Sucrose; N-Naive

Nasdaq: PDSB Nasdaq: PDSB Reference: Data on file. PDS0104 preclinical studies: Potent
TRP2-specific CD8+ killer T-cells break immune tolerance, with efficacy in difficult-to-treat B16 melanoma 34 Black stainsdueto tumors Healthy animals Versamune® Formulation 1Versamune® Formulation 2 Negative Control Untreated 14
days after singleinjection treatment Potent activity with different tumor antigens
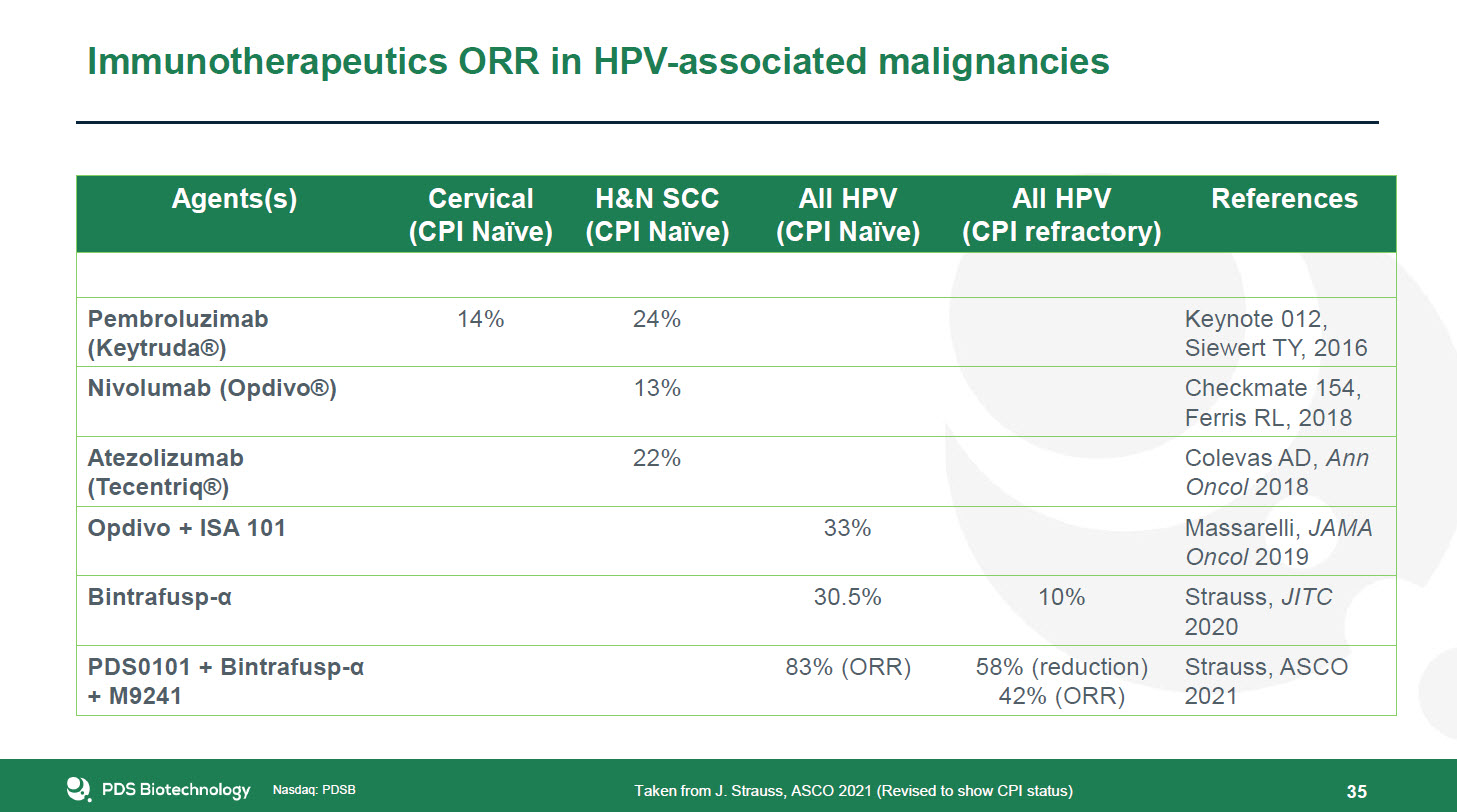
Nasdaq: PDSB Nasdaq: PDSB Immunotherapeutics ORR in HPV-associated malignancies 35 Taken from
J. Strauss, ASCO 2021 (Revised to show CPI status) Agents(s) Cervical (CPI Naïve) H&N SCC(CPI Naïve) All HPV (CPI Naïve) All HPV (CPI refractory) References Pembroluzimab (Keytruda®) 14% 24% Keynote 012,Siewert TY,
2016 Nivolumab (Opdivo®) 13% Checkmate 154,Ferris RL, 2018 Atezolizumab (Tecentriq®) 22% Colevas AD, Ann Oncol 2018 Opdivo + ISA 101 33% Massarelli, JAMA Oncol 2019 Bintrafusp-α 30.5% 10% Strauss,
JITC2020 PDS0101 + Bintrafusp-α+ M9241 83% (ORR) 58% (reduction)42% (ORR) Strauss, ASCO 2021

Nasdaq: PDSB Nasdaq: PDSB Adverse Event Summary All patients N=25* Grade
≥2 Treatment-related adverse events (TRAEs) 23 (92%) TRAEs leading to discontinuation of ≥ 1 drug(s) 5 (20%) Treatment-related serious AEs 7 (28%) TRAEs in ≥5% of patients Anemia 12 (48%) Lymphocyte decrease 7 (28%) Flu like
symptoms 6 (24%) Injection site reactions 5 (20%) Hematuria 4 (16%) AST/ ALT/ Alk phos elevation 4 (16%) Keratoacanthomas 4 (16%) Leukocyte decrease 3 (12%) Maculopapular rash 3 (12%) Pruritis 3 (12%) Nausea/ vomiting 3
(12%) Mucositis 3 (12%) Hypothyroidism 3 (12%) Peripheral motor neuropathy 2 (8%) Fatigue 2 (8%) Grade 3 TRAEs occurred in 10 (40%) patientsAnemia due to gross hematuria (n=4), AST/ALT elevation (n=2); flu like symptoms (n=1), nausea/
vomiting (n=1), leukopenia (n=1), lymphopenia (n=2), HLH (n=1)One patient with transient grade 3 leukopenia and lymphopenia also had transient grade 4 neutropenia4 patients who originally had grade 3 toxicities with the triple combo including
M9241 at 16.8 mcg/kg tolerated the triple combo with M9241 at 8 mcg/kg w/o any further grade ≥3 toxicitiesNo treatment-related deaths occurred No new or elevated toxicities observed from the addition of PDS0101 to the combination; PDS0101 only
caused transient injection site reactions 36 * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Reference: Strauss J. et al. Phase II evaluation of the triple combination of
PDS0101, M9241, and bintrafusp alfa in patients with HPV 16positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501.
