Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HEAT BIOLOGICS, INC. | htbx_8k.htm |
EXHIBIT 99.1

Heat Biologics N asdaq : HTBX CORPORATE PRESENTATION AUGUST 2021

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer , non - oncology and infectious diseases , our planned discovery and development of a COVID - 19 vaccine, our planned biosecurity/biodefense initiative, our planned bioanalytics , process development and manufacturing activities, our biologics drug discovery, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 2020 , our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent filings with the Securities and Exchange Commission (collectively, our “SEC Filings”) . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2
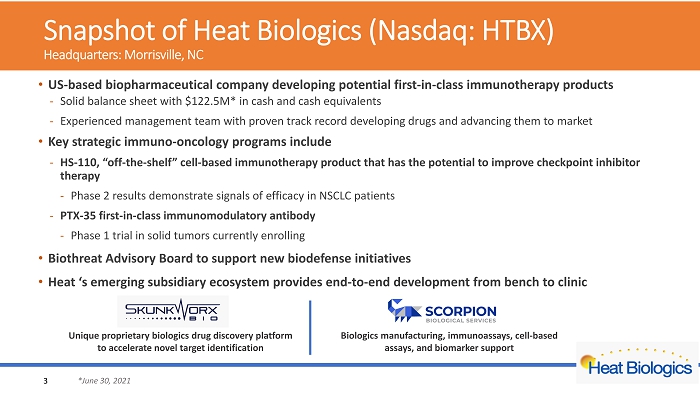
Snapshot of Heat Biologics (Nasdaq: HTBX) Headquarters: Morrisville, NC • US - based biopharmaceutical company developing potential first - in - class immunotherapy products - Solid balance sheet with $122.5M* in cash and cash equivalents - Experienced management team with proven track record developing drugs and advancing them to market • Key strategic immuno - oncology programs include - HS - 110, “off - the - shelf” cell - based immunotherapy product that has the potential to improve checkpoint inhibitor therapy - Phase 2 results demonstrate signals of efficacy in NSCLC patients - PTX - 35 first - in - class immunomodulatory antibody - Phase 1 trial in solid tumors currently enrolling • Biothreat Advisory Board to support new biodefense initiatives • Heat ‘s emerging subsidiary ecosystem provides end - to - end development from bench to clinic 3 *June 30, 2021 Unique p roprietary biologics drug discovery platform to accelerate novel target identification Biologics manufacturing, immunoassays, cell - based assays, and biomarker support
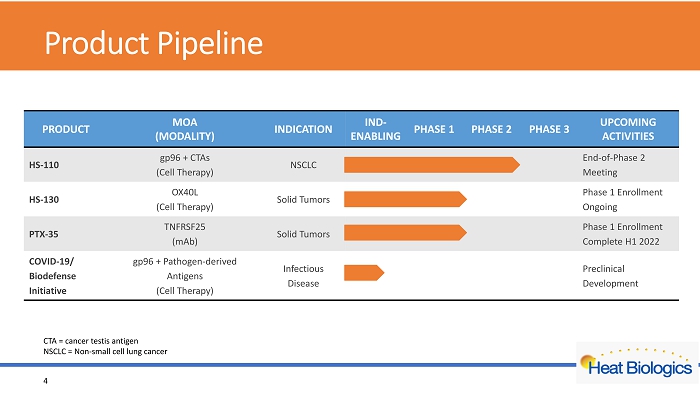
P roduct Pipeline 4 CTA = cancer testis antigen NSCLC = Non - small cell lung cancer PRODUCT MOA (MODALITY) INDICATION IND - ENABLING PHASE 1 PHASE 2 PHASE 3 UPCOMING ACTIVITIES HS - 110 gp96 + CTAs (Cell Therapy) NSCLC End - of - Phase 2 Meeting HS - 130 OX40L (Cell Therapy) Solid Tumors Phase 1 Enrollment Ongoing PTX - 35 TNFRSF25 ( mAb ) Solid Tumors Phase 1 Enrollment Complete H1 2022 COVID - 19/ Biodefense Initiative gp96 + Pathogen - derived Antigens (Cell Therapy) Infectious Disease Preclinical Development
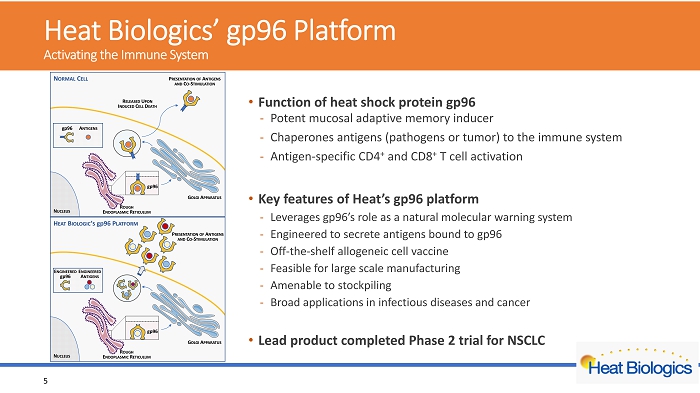
H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96 - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Antigen - specific CD4 + and CD8 + T cel l activation • Key features of Heat’s gp96 platform - Leverages gp96’s role as a natural molecular warning system - Engineered to secrete antigens bound to gp96 - Off - the - shelf allogeneic cell vaccine - Feasible for large scale manufacturing - Amenable to stockpiling - Broad applications in infectious diseases and cancer • Lead product completed Phase 2 trial for NSCLC 5
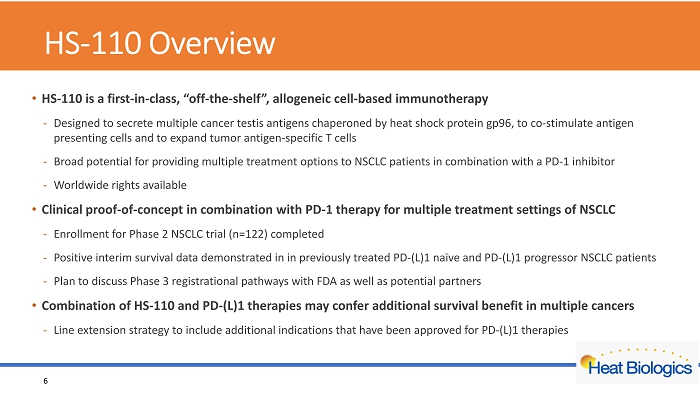
HS - 110 Overview • HS - 110 is a first - in - class, “off - the - shelf”, allogeneic cell - based immunotherapy - Designed to secrete multiple cancer testis antigens chaperoned by heat shock protein gp96, to co - stimulate antigen presenting cells and to expand tumor antigen - specific T cells - Broad potential for providing multiple treatment options to NSCLC patients in combination with a PD - 1 inhibitor - Worldwide rights available • Clinical proof - of - concept in combination with PD - 1 therapy for multiple treatment settings of NSCLC - Enrollment for Phase 2 NSCLC trial (n=122) completed - Positive interim survival data demonstrated in in previously treated PD - (L)1 naïve and PD - (L)1 progressor NSCLC patients - Plan to discuss Phase 3 registrational pathways with FDA as well as potential partners • Combination of HS - 110 and PD - (L)1 therapies may confer additional survival benefit in multiple cancers - Line extension strategy to include additional indications that have been approved for PD - (L)1 therapies 6
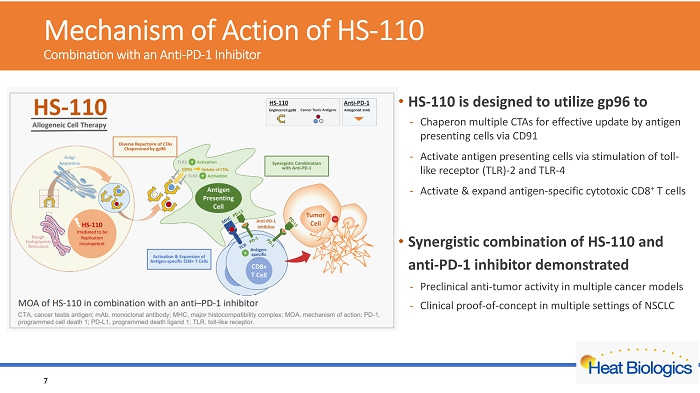
Mechanism of Action of HS - 110 Combination with an Anti - PD - 1 Inhibitor 7 • HS - 110 is designed to utilize gp96 to - Chaperon multiple CTAs for effective update by antigen presenting cells via CD91 - Activate antigen presenting cells via stimulation of toll - like receptor (TLR) - 2 and TLR - 4 - Activate & expand antigen - specific cytotoxic CD8 + T cells • Synergistic combination of HS - 110 and anti - PD - 1 inhibitor demonstrated - Preclinical anti - tumor activity in multiple cancer models - Clinical proof - of - concept in multiple settings of NSCLC
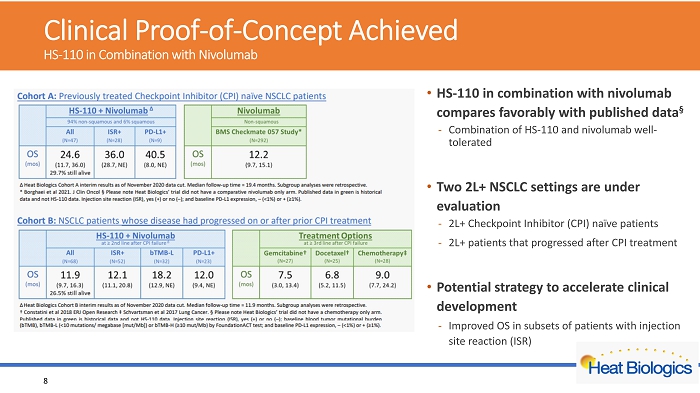
Clinical Proof - of - Concept Achieved HS - 110 in Combination with Nivolumab 8 • HS - 110 in combination with nivolumab compares favorably with published data Α - Combination of HS - 110 and nivolumab well - tolerated • Two 2L+ NSCLC settings are under evaluation - 2L+ Checkpoint Inhibitor (CPI) naïve patients - 2L+ patients that progressed after CPI treatment • Potential strategy to accelerate clinical development - Improved OS in subsets of patients with injection site reaction (ISR)
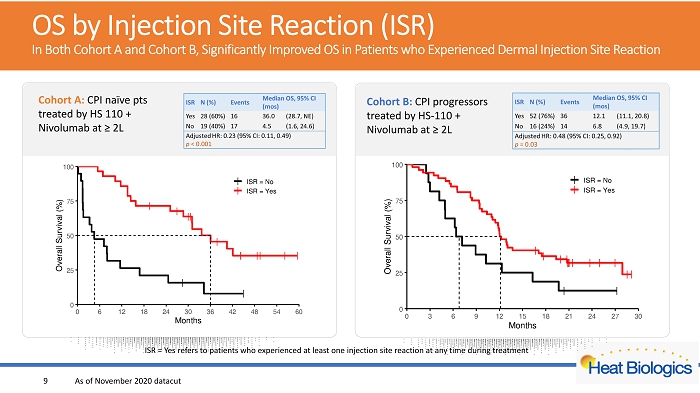
OS by Injection Site Reaction (ISR) In Both Cohort A and Cohort B, Significantly Improved OS in Patients who Experienced Dermal Injection Site Reaction ISR = Yes refers to patients who experienced at least one injection site reaction at any time during treatment 9 As of November 2020 datacut Cohort A: CPI naïve pts treated by HS 110 + Nivolumab at ≥ 2L Cohort B: CPI progressors treated by HS - 110 + Nivolumab at ≥ 2L
HS - 130 Overview • HS - 130 is the first “off - the - shelf”, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance immune signals - Leverage HS - 110 clinical experience and manufacturing insights - Addition of OX40L fusion protein to promote the extension and expansion of T cell memory - Worldwide rights available • Mechanism of action offers broad market potential • Phase I oncology trial ongoing 10
PTX - 35 Overview • PTX - 35 offers a unique opportunity to modulate T effector or regulatory T - cells - Context driven depending on specific disease settings - Broad applications in oncology and non - oncology • Potential first - in - class antibody targeting TNFRSF25 for oncology - Phase 1 trial in solid tumors currently enrolling - Anti - tumor activity demonstrated in multiple preclinical in vivo colon, lung and breast cancer models - Preclinical data demonstrate anti - tumor activity, expansion of antigen - specific CD8 + T cells and decreased Treg suppression in the presence of tumor antigen - Awarded a $15.2M CPRIT grant to fund Phase 1 clinical development • Worldwide rights licensed by Heat Biologics 11
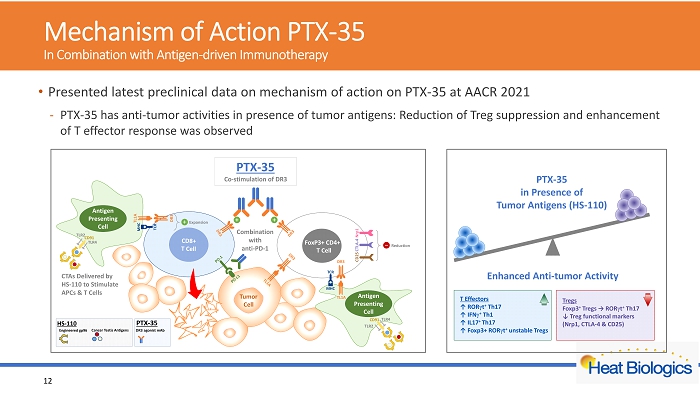
Mechanism of Action PTX - 35 In Combination with Antigen - driven Immunotherapy 12 • Presented latest preclinical data on mechanism of action on PTX - 35 at AACR 2021 - PTX - 35 has anti - tumor activities in presence of tumor antigens: Reduction of Treg suppression and enhancement of T effector response was observed
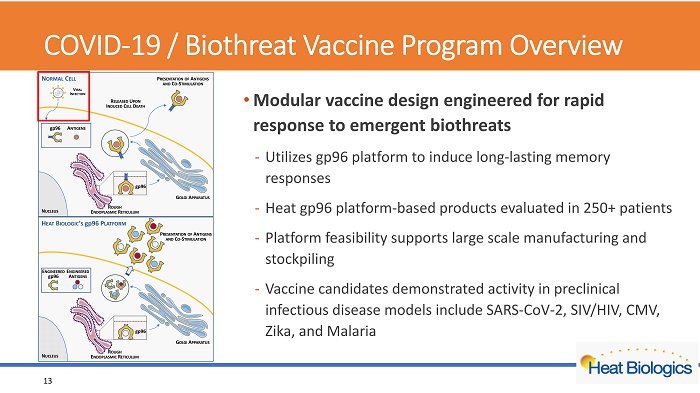
COVID - 19 / Biothreat Vaccine Program Overview • Modular vaccine design engineered for rapid response to emergent biothreats - Utilizes gp96 platform to induce long - lasting memory responses - Heat gp96 platform - based products evaluated in 250+ patients - Platform feasibility supports large scale manufacturing and stockpiling - Vaccine candidates demonstrated activity in preclinical infectious disease models include SARS - CoV - 2, SIV/HIV, CMV, Zika, and Malaria 13

Biological Threat Advisory Board of Heat Biologics 14 Bipartisan Advisory Board provid ing counsel and guidance on Heat’s biodefense initiatives David Lasseter Andrew Weber Jack Kingston Dr. Gregory Koblentz Mark Pryor Former Deputy Asst. Sec. of Defense for Countering Weapons of Mass Destruction Former Asst. Sec. of Defense for Nuclear, Chemical & Biological Defense Programs Former US Representative, Secretariat of the Alliance for Biosecurity (current) Professor of Biodefense at George Mason University, Expert on Chemical and Biological Weapons Former US Senator, AR
