Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - F-star Therapeutics, Inc. | d216605dex991.htm |
| 8-K - 8-K - F-star Therapeutics, Inc. | d216605d8k.htm |

Q3 2021 Next Generation Immunotherapies. Overcoming Cancer. Exhibit 99.2

Certain statements contained in this communication regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. F-star undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. In some cases, you can identify forward-looking statements by terminology such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” or the negative of these terms or other comparable terminology, which are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, those provided in F-star’s reports to the SEC. Risks and uncertainties related to F-star that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to F-star’s status as a clinical stage immuno-oncology company and its need for substantial additional funding in order to complete the development and commercialization of its product candidates, that F-star may experience delays in completing, or ultimately be unable to complete, the development and commercialization of its product candidates, that F-star’s clinical trials may fail to adequately demonstrate the safety and efficacy of its product candidates, that preclinical drug development is uncertain, and some of F-star’s product candidates may never advance to clinical trials, that results of preclinical studies and early stage clinical trials may not be predictive of the results of later stage clinical trials, that F-star relies on patents and other intellectual property rights to protect its product candidates, and the enforcement, defense and maintenance of such rights may be challenging and costly, and that F-star faces significant competition in its drug discovery and development efforts. New factors emerge from time to time and it is not possible for us to predict all such factors, nor can we assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These risks are more fully discussed in F-star’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other documents filed from time to time with the SEC. Forward-looking statements included in this communication are based on information available to F-star as of the date of this communication. F-star does not assume any obligation to update such forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Cautionary Note Regarding Forward-Looking Statements
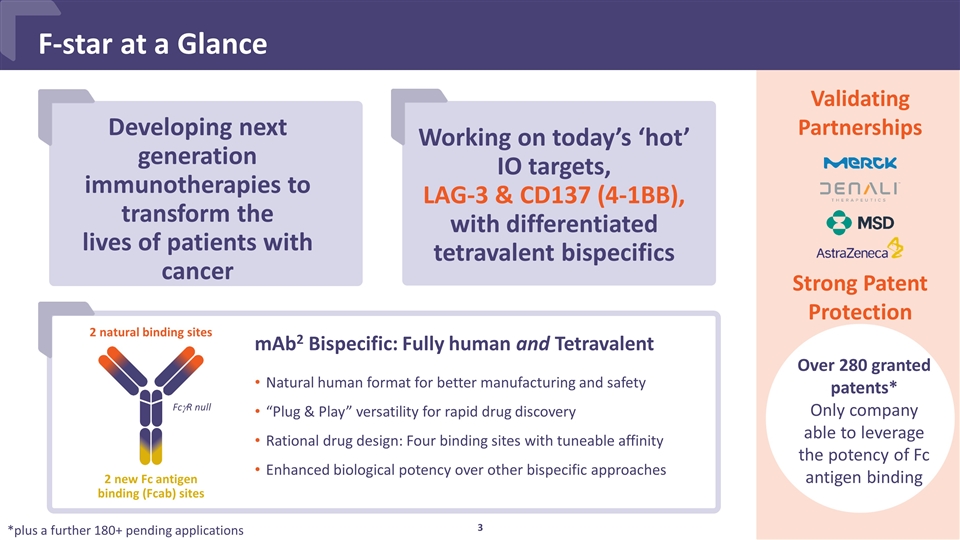
Developing next generation immunotherapies to transform the lives of patients with cancer F-star at a Glance Validating Partnerships Natural human format for better manufacturing and safety “Plug & Play” versatility for rapid drug discovery Rational drug design: Four binding sites with tuneable affinity Enhanced biological potency over other bispecific approaches mAb2 Bispecific: Fully human and Tetravalent Over 280 granted patents* Only company able to leverage the potency of Fc antigen binding FcgR null 2 new Fc antigen binding (Fcab) sites 2 natural binding sites Working on today’s ‘hot’ IO targets, LAG-3 & CD137 (4-1BB), with differentiated tetravalent bispecifics Strong Patent Protection *plus a further 180+ pending applications
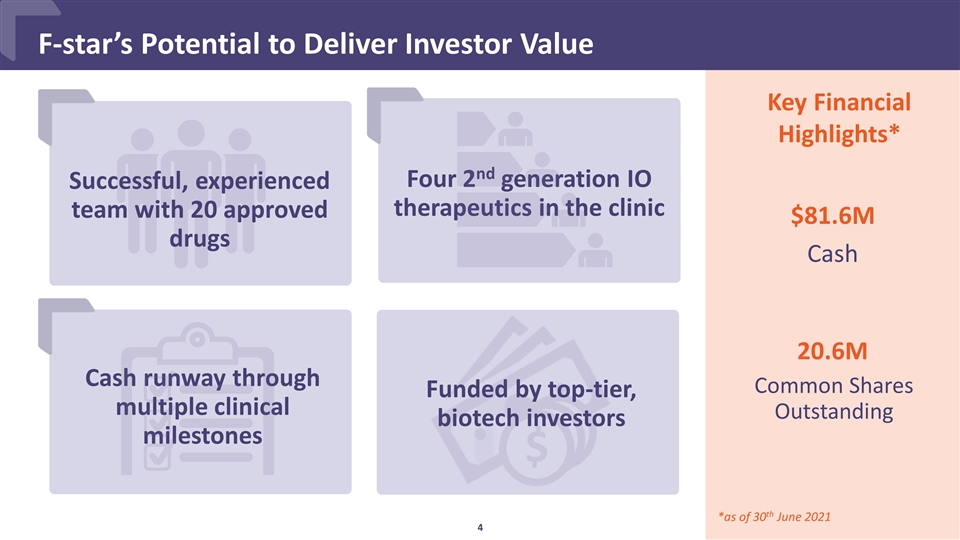
Successful, experienced team with 20 approved drugs F-star’s Potential to Deliver Investor Value Cash runway through multiple clinical milestones Key Financial Highlights* $81.6M Cash 20.6M Common Shares Outstanding *as of 30th June 2021 Four 2nd generation IO therapeutics in the clinic Funded by top-tier, biotech investors
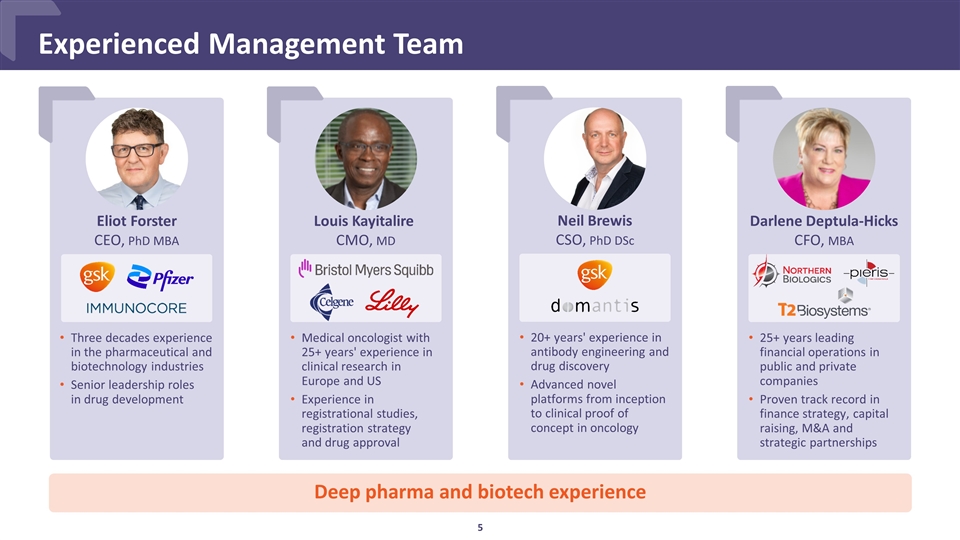
Experienced Management Team Medical oncologist with 25+ years' experience in clinical research in Europe and US Experience in registrational studies, registration strategy and drug approval Louis Kayitalire CMO, MD 25+ years leading financial operations in public and private companies Proven track record in finance strategy, capital raising, M&A and strategic partnerships Darlene Deptula-Hicks CFO, MBA Three decades experience in the pharmaceutical and biotechnology industries Senior leadership roles in drug development Eliot Forster CEO, PhD MBA 20+ years' experience in antibody engineering and drug discovery Advanced novel platforms from inception to clinical proof of concept in oncology Neil Brewis CSO, PhD DSc Deep pharma and biotech experience
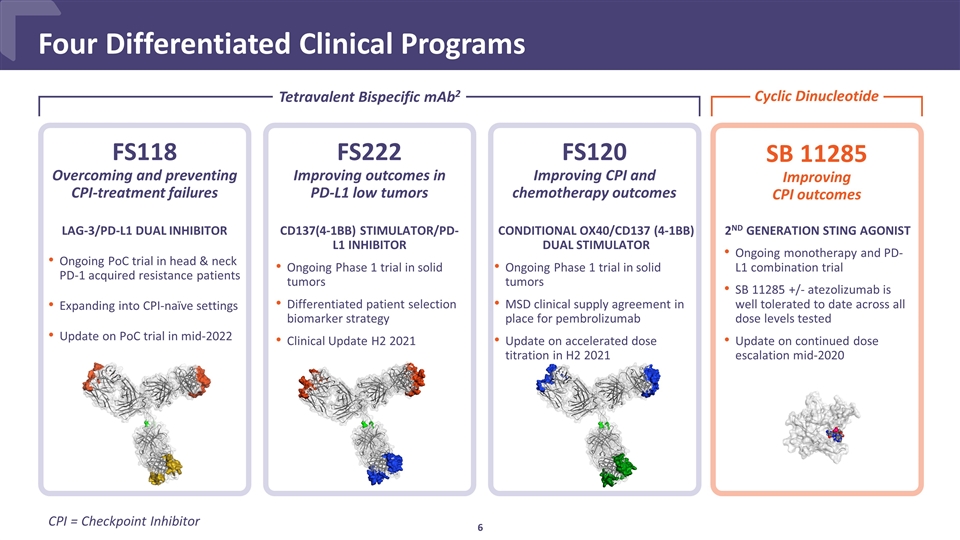
Four Differentiated Clinical Programs FS120 Improving CPI and chemotherapy outcomes CONDITIONAL OX40/CD137 (4-1BB) DUAL STIMULATOR Ongoing Phase 1 trial in solid tumors MSD clinical supply agreement in place for pembrolizumab Update on accelerated dose titration in H2 2021 FS118 Overcoming and preventing CPI-treatment failures LAG-3/PD-L1 DUAL INHIBITOR Ongoing PoC trial in head & neck PD-1 acquired resistance patients Expanding into CPI-naïve settings Update on PoC trial in mid-2022 Tetravalent Bispecific mAb2 FS222 Improving outcomes in PD-L1 low tumors CD137(4-1BB) STIMULATOR/PD-L1 INHIBITOR Ongoing Phase 1 trial in solid tumors Differentiated patient selection biomarker strategy Clinical Update H2 2021 CPI = Checkpoint Inhibitor Cyclic Dinucleotide SB 11285 Improving CPI outcomes 2ND GENERATION STING AGONIST Ongoing monotherapy and PD-L1 combination trial SB 11285 +/- atezolizumab is well tolerated to date across all dose levels tested Update on continued dose escalation mid-2020
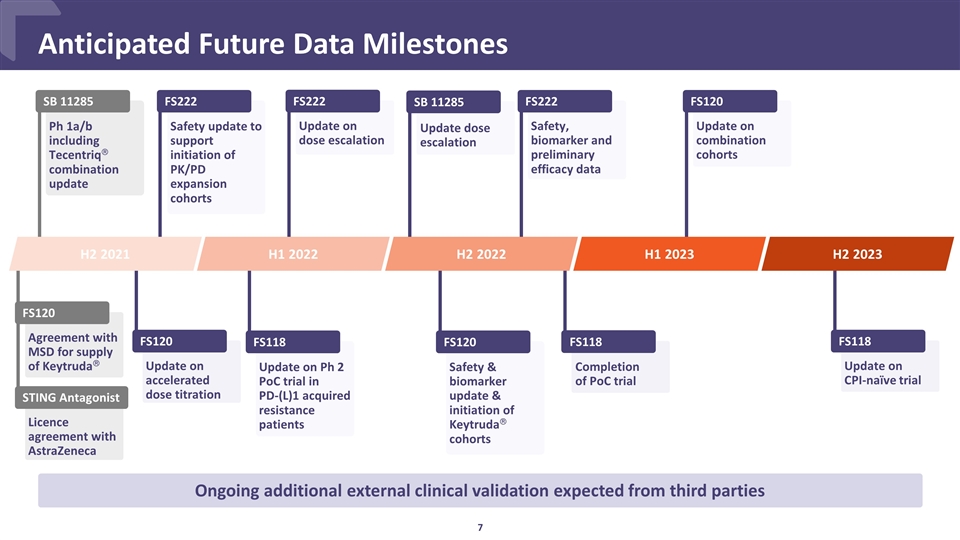
Update on dose escalation FS222 Update on accelerated dose titration FS120 Safety, biomarker and preliminary efficacy data FS222 Update dose escalation SB 11285 FS120 Safety & biomarker update & initiation of Keytrudaâ cohorts FS222 Safety update to support initiation of PK/PD expansion cohorts Anticipated Future Data Milestones Ongoing additional external clinical validation expected from third parties SB 11285 H2 2021 H1 2022 H2 2022 Ph 1a/b including Tecentriqâ combination update FS118 Update on Ph 2 PoC trial in PD-(L)1 acquired resistance patients Agreement with MSD for supply of Keytrudaâ FS120 Licence agreement with AstraZeneca STING Antagonist FS118 Update on CPI-naïve trial H2 2023 Update on combination cohorts FS120 Completion of PoC trial FS118 H1 2023

FS118 Overcoming and preventing CPI-treatment failures LAG-3 PD-L1
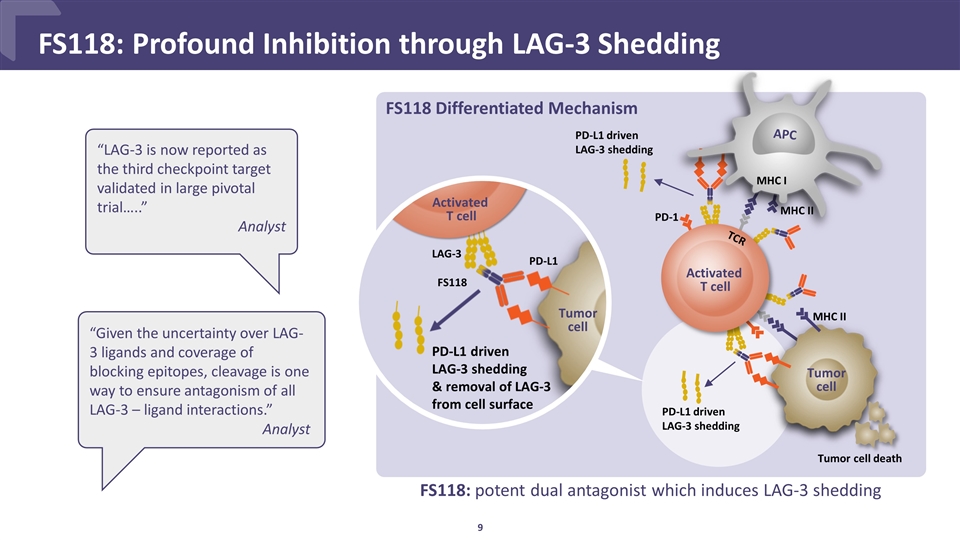
FS118: potent dual antagonist which induces LAG-3 shedding CONFIDENTIAL FS118: Profound Inhibition through LAG-3 Shedding “Given the uncertainty over LAG-3 ligands and coverage of blocking epitopes, cleavage is one way to ensure antagonism of all LAG-3 – ligand interactions.” Analyst FS118 Differentiated Mechanism “LAG-3 is now reported as the third checkpoint target validated in large pivotal trial…..” Analyst Activated T cell APC Tumor cell PD-1 TCR MHC II MHC I PD-L1 driven LAG-3 shedding Tumor cell death PD-L1 driven LAG-3 shedding PD-L1 driven LAG-3 shedding & removal of LAG-3 from cell surface Activated T cell Tumor cell MHC II FS118 LAG-3 PD-L1
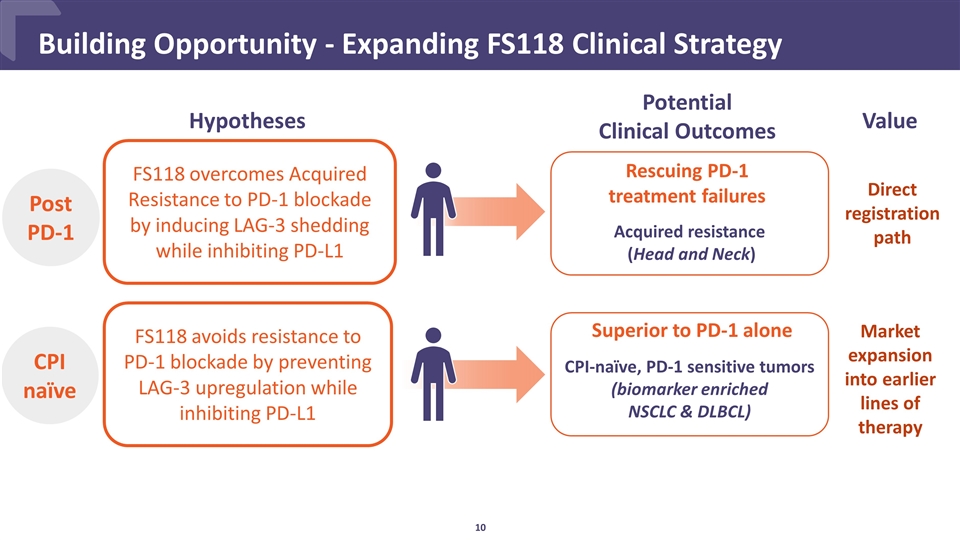
Building Opportunity - Expanding FS118 Clinical Strategy FS118 overcomes Acquired Resistance to PD-1 blockade by inducing LAG-3 shedding while inhibiting PD-L1 FS118 avoids resistance to PD-1 blockade by preventing LAG-3 upregulation while inhibiting PD-L1 Hypotheses CPI naïve Post PD-1 CPI-naïve, PD-1 sensitive tumors (biomarker enriched NSCLC & DLBCL) Acquired resistance (Head and Neck) Rescuing PD-1 treatment failures Superior to PD-1 alone Potential Clinical Outcomes Direct registration path Value Market expansion into earlier lines of therapy
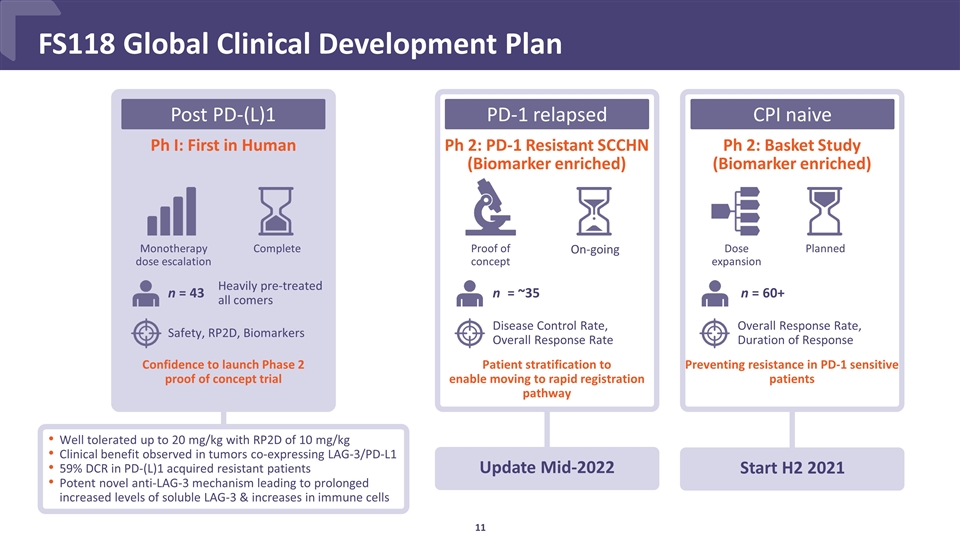
FS118 Global Clinical Development Plan Ph I: First in Human Well tolerated up to 20 mg/kg with RP2D of 10 mg/kg Clinical benefit observed in tumors co-expressing LAG-3/PD-L1 59% DCR in PD-(L)1 acquired resistant patients Potent novel anti-LAG-3 mechanism leading to prolonged increased levels of soluble LAG-3 & increases in immune cells Ph 2: PD-1 Resistant SCCHN (Biomarker enriched) Ph 2: Basket Study (Biomarker enriched) Start H2 2021 Patient stratification to enable moving to rapid registration pathway Monotherapy dose escalation Complete Proof of concept On-going n = 43 n = ~35 n = 60+ PD-1 relapsed Planned Dose expansion Preventing resistance in PD-1 sensitive patients Heavily pre-treated all comers Update Mid-2022 Confidence to launch Phase 2 proof of concept trial Safety, RP2D, Biomarkers Disease Control Rate, Overall Response Rate Overall Response Rate, Duration of Response CPI naive Post PD-(L)1
CD137 PD-L1 FS222 Improving outcomes in PD-L1 low tumors
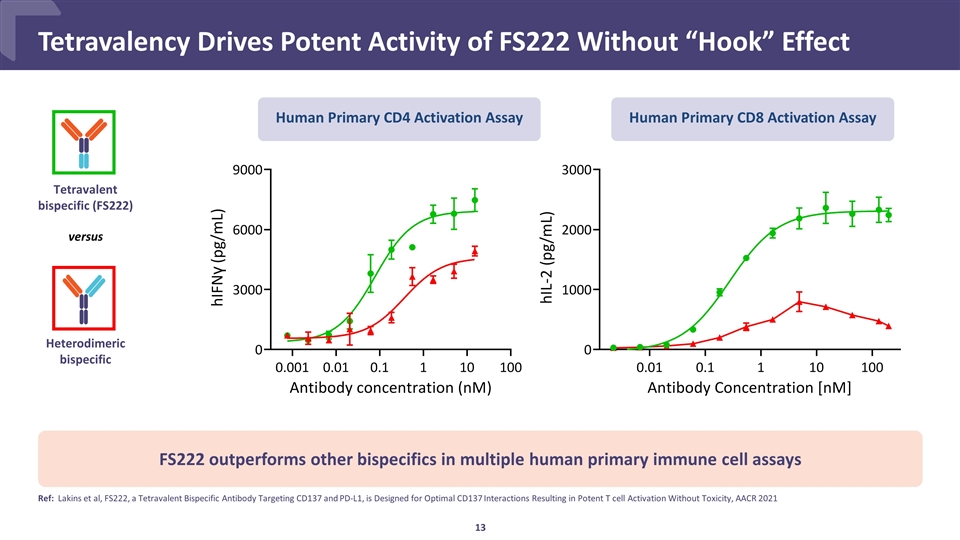
FS222 outperforms other bispecifics in multiple human primary immune cell assays Ref: Lakins et al, FS222, a Tetravalent Bispecific Antibody Targeting CD137 and PD-L1, is Designed for Optimal CD137 Interactions Resulting in Potent T cell Activation Without Toxicity, AACR 2021 Tetravalent bispecific (FS222) versus Heterodimeric bispecific Human Primary CD4 Activation Assay Human Primary CD8 Activation Assay Tetravalency Drives Potent Activity of FS222 Without “Hook” Effect
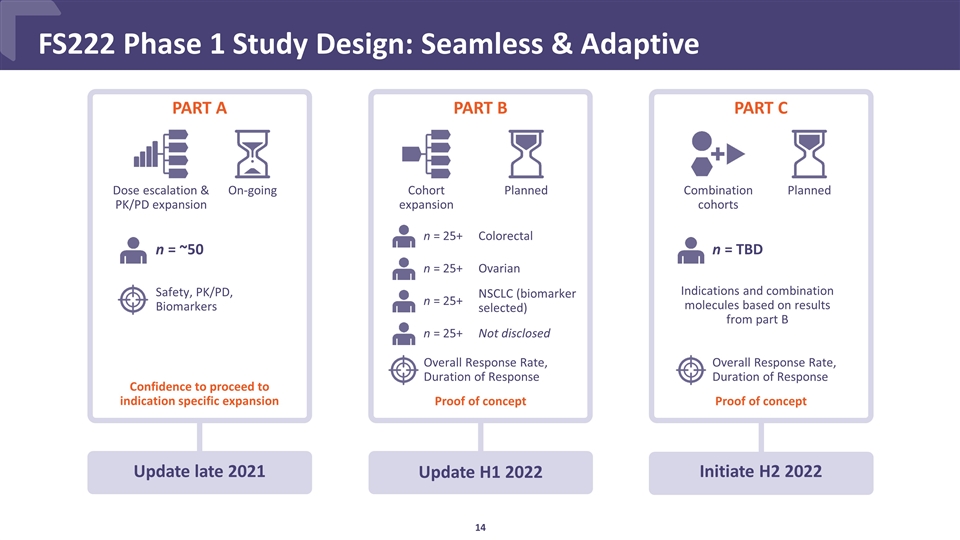
FS222 Phase 1 Study Design: Seamless & Adaptive PART B Update H1 2022 Cohort expansion Planned PART A Update late 2021 Dose escalation & PK/PD expansion Confidence to proceed to indication specific expansion n = ~50 On-going Safety, PK/PD, Biomarkers n = 25+ Colorectal n = 25+ Ovarian n = 25+ NSCLC (biomarker selected) n = 25+ Not disclosed Overall Response Rate, Duration of Response Proof of concept PART C Combination cohorts Planned n = TBD Initiate H2 2022 Overall Response Rate, Duration of Response Proof of concept Indications and combination molecules based on results from part B
OX40 CD137 FS120 Improving CPI and chemotherapy outcomes
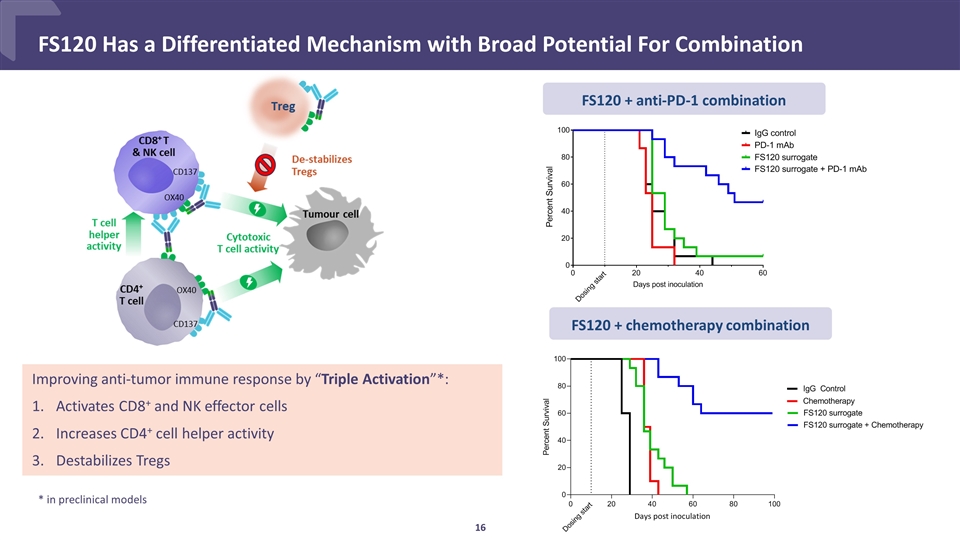
CONFIDENTIAL FS120 Has a Differentiated Mechanism with Broad Potential For Combination Improving anti-tumor immune response by “Triple Activation”*: Activates CD8+ and NK effector cells Increases CD4+ cell helper activity Destabilizes Tregs FS120 + anti-PD-1 combination FS120 + chemotherapy combination * in preclinical models
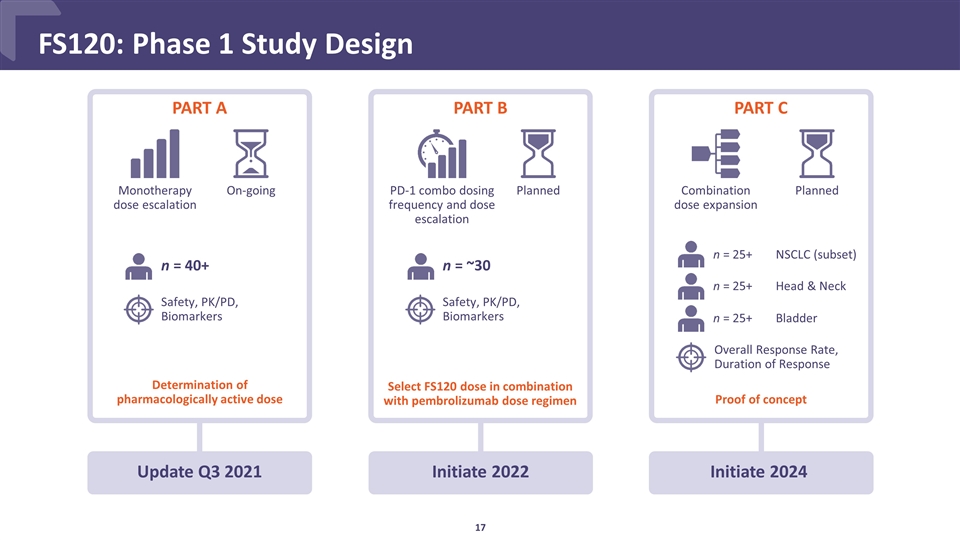
PART B Initiate 2022 PART A Update Q3 2021 PART C FS120: Phase 1 Study Design Monotherapy dose escalation On-going PD-1 combo dosing frequency and dose escalation Planned Planned Combination dose expansion Determination of pharmacologically active dose n = 40+ n = ~30 n = 25+ n = 25+ n = 25+ NSCLC (subset) Head & Neck Bladder Safety, PK/PD, Biomarkers Safety, PK/PD, Biomarkers Select FS120 dose in combination with pembrolizumab dose regimen Proof of concept Overall Response Rate, Duration of Response Initiate 2024

STING agonist SB 11285 Improving CPI outcomes
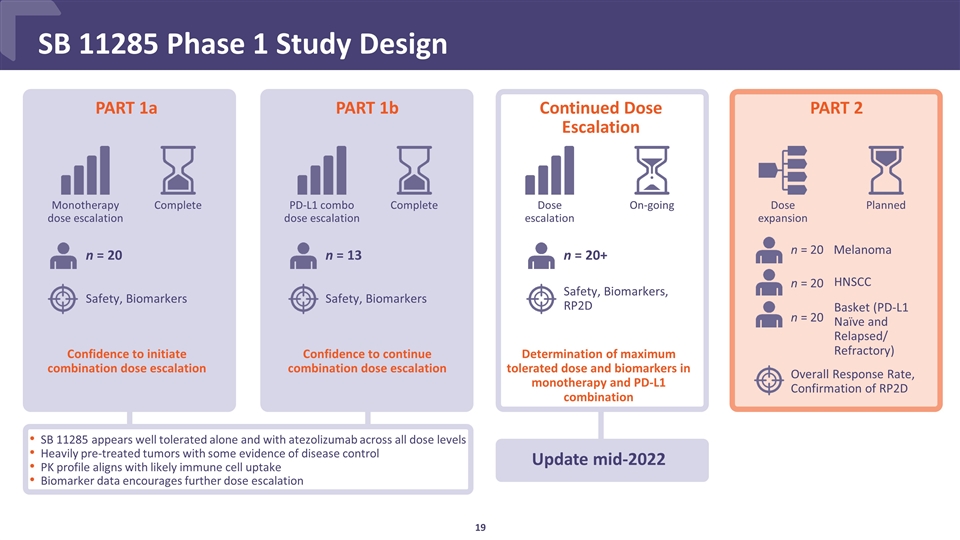
SB 11285 Phase 1 Study Design PART 1a SB 11285 appears well tolerated alone and with atezolizumab across all dose levels Heavily pre-treated tumors with some evidence of disease control PK profile aligns with likely immune cell uptake Biomarker data encourages further dose escalation PART 1b Continued Dose Escalation PART 2 Update mid-2022 Confidence to continue combination dose escalation Monotherapy dose escalation Complete Dose escalation On-going Dose expansion Planned n = 20 n = 13 n = 20+ n = 20 n = 20 n = 20 Melanoma HNSCC Basket (PD-L1 Naïve and Relapsed/ Refractory) Complete PD-L1 combo dose escalation Determination of maximum tolerated dose and biomarkers in monotherapy and PD-L1 combination Confidence to initiate combination dose escalation Safety, Biomarkers Safety, Biomarkers Safety, Biomarkers, RP2D Overall Response Rate, Confirmation of RP2D
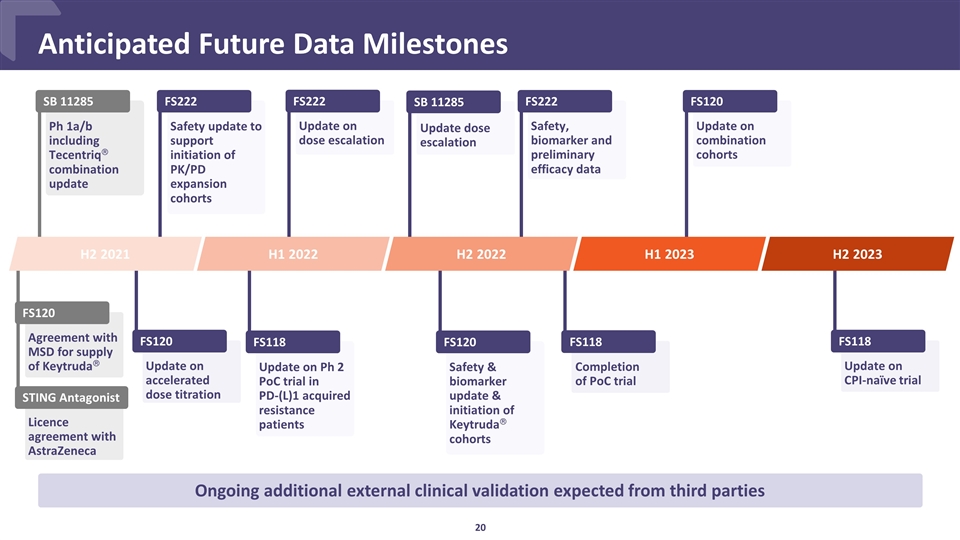
Update on dose escalation FS222 Update on accelerated dose titration FS120 Safety, biomarker and preliminary efficacy data FS222 Update dose escalation SB 11285 FS120 Safety & biomarker update & initiation of Keytrudaâ cohorts FS222 Safety update to support initiation of PK/PD expansion cohorts Anticipated Future Data Milestones Ongoing additional external clinical validation expected from third parties SB 11285 H2 2021 H1 2022 H2 2022 Ph 1a/b including Tecentriqâ combination update FS118 Update on Ph 2 PoC trial in PD-(L)1 acquired resistance patients Agreement with MSD for supply of Keytrudaâ FS120 Licence agreement with AstraZeneca STING Antagonist FS118 Update on CPI-naïve trial H2 2023 Update on combination cohorts FS120 Completion of PoC trial FS118 H1 2023
CONFIDENTIAL FSTX – A Stellar Outlook Four ongoing programs with multiple clinical data readouts in next 24 months Continued validation of platform & pipeline through big pharma alliances Strong balance sheet and support from top-tier biotech investors © 2020 F-star. All rights reserved
CONFIDENTIAL F-star Potential Next Generation Immunotherapies

Thank you.

Platform: Designing Fcabs: well-expressed and stable high affinity antigen-binding Fc fragments. DOI: 10.1093/protein/gzx042 Generation of Fcabs targeting human and murine LAG-3 as building blocks for novel bispecific antibody therapeutics. DOI:10.1016/j.ymeth.2018.09.003. Bispecific antibodies: a mechanistic review of the pipeline. DOI: 10.1038/s41573-019-0028 FS118: Kraman et al, Clinical Cancer Research, (2020) DOI:10.1158/1078-0432.CCR-19-3548 FS120 Gaspar et al, Cancer Immunology Research, (2020) DOI: 10.1158/2326-6066.CIR-19-0798 FS222 Lakins et al, Clinical Cancer Research, (2020): DOI: 10.1158/1078-0432.CCR-19-2958 References
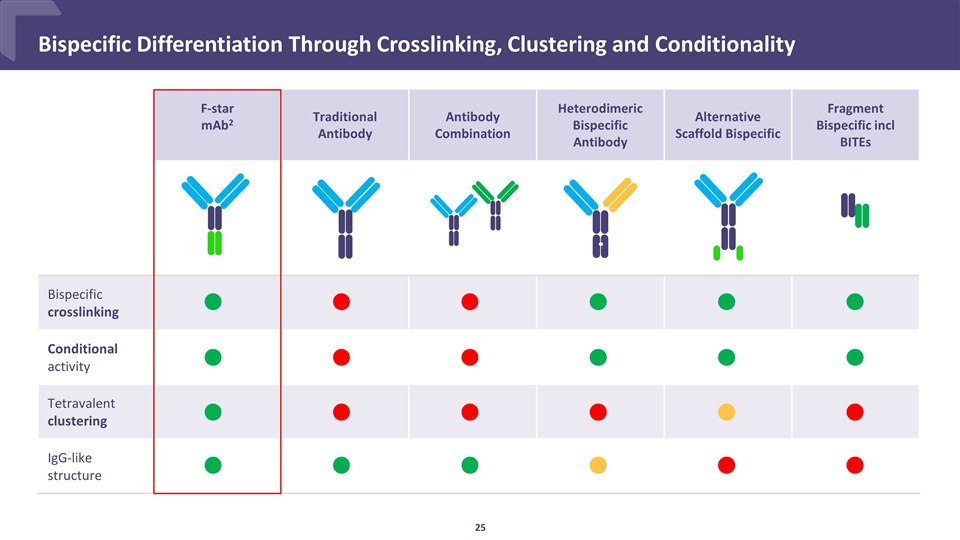
Bispecific Differentiation Through Crosslinking, Clustering and Conditionality F-star mAb2 Traditional Antibody Antibody Combination Heterodimeric Bispecific Antibody Alternative Scaffold Bispecific Fragment Bispecific incl BITEs Bispecific crosslinking Conditional activity Tetravalent clustering IgG-like structure
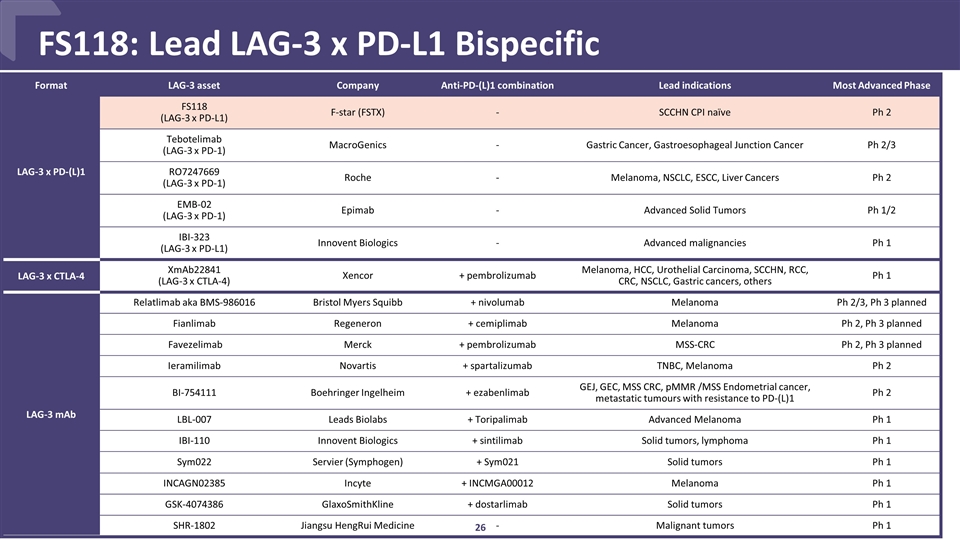
CONFIDENTIAL FS118: Lead LAG-3 x PD-L1 Bispecific Format LAG-3 asset Company Anti-PD-(L)1 combination Lead indications Most Advanced Phase LAG-3 x PD-(L)1 FS118 (LAG-3 x PD-L1) F-star (FSTX) - SCCHN CPI naïve Ph 2 Tebotelimab (LAG-3 x PD-1) MacroGenics - Gastric Cancer, Gastroesophageal Junction Cancer Ph 2/3 RO7247669 (LAG-3 x PD-1) Roche - Melanoma, NSCLC, ESCC, Liver Cancers Ph 2 EMB-02 (LAG-3 x PD-1) Epimab - Advanced Solid Tumors Ph 1/2 IBI-323 (LAG-3 x PD-L1) Innovent Biologics - Advanced malignancies Ph 1 LAG-3 x CTLA-4 XmAb22841 (LAG-3 x CTLA-4) Xencor + pembrolizumab Melanoma, HCC, Urothelial Carcinoma, SCCHN, RCC, CRC, NSCLC, Gastric cancers, others Ph 1 LAG-3 mAb Relatlimab aka BMS-986016 Bristol Myers Squibb + nivolumab Melanoma Ph 2/3, Ph 3 planned Fianlimab Regeneron + cemiplimab Melanoma Ph 2, Ph 3 planned Favezelimab Merck + pembrolizumab MSS-CRC Ph 2, Ph 3 planned Ieramilimab Novartis + spartalizumab TNBC, Melanoma Ph 2 BI-754111 Boehringer Ingelheim + ezabenlimab GEJ, GEC, MSS CRC, pMMR /MSS Endometrial cancer, metastatic tumours with resistance to PD-(L)1 Ph 2 LBL-007 Leads Biolabs + Toripalimab Advanced Melanoma Ph 1 IBI-110 Innovent Biologics + sintilimab Solid tumors, lymphoma Ph 1 Sym022 Servier (Symphogen) + Sym021 Solid tumors Ph 1 INCAGN02385 Incyte + INCMGA00012 Melanoma Ph 1 GSK-4074386 GlaxoSmithKline + dostarlimab Solid tumors Ph 1 SHR-1802 Jiangsu HengRui Medicine - Malignant tumors Ph 1
