Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Century Therapeutics, Inc. | tm2124698d1_ex99-1.htm |
| 8-K - FORM 8-K - Century Therapeutics, Inc. | tm2124698d1_8k.htm |
Exhibit 99.2

CORPORATE OVERVIEW August 2021

2 2 FORWARD - LOOKING STATEMENTS This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbour provisions of , The Private Securities Litigation Reform Act of 1995. All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to , s tatements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, market opportunity, competitive position and potential growth opportunities are forward - looking statement s. These statements involve known and unknown risks, uncertainties and other important factors that may cause the our actual results, performance or achievements to be materially different from any future results, performance or achievemen ts expressed or implied by the forward - looking statements. In some cases, you can identify forward - looking statements by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target, ” “ project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward - looking statements in this presentation are only predictions. We have based thes e forward - looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect the our business, financial condition and results of operations. These forward - looking stateme nts speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others: our abilit y t o successfully advance our current and future product candidates through development activities, preclinical studies, and clinical trials; our reliance on the maintenance on certain key collaborative relationships for the manufacturin g a nd development of our product candidates; the timing, scope and likelihood of regulatory filings and approvals, including final regulatory approval of our product candidates; the impact of the COVID - 19 pandemic on our business and operations; the per formance of third parties in connection with the development of our product candidates, including third parties conducting our future clinical trials as well as third - party suppliers and manufacturers; our ability to successfully commercial ize our product candidates and develop sales and marketing capabilities, if our product candidates are approved; and our ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainti es are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward - looking statements as predictions of future events . The events and circumstances reflected in the our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not pl an to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

3 3 CENTURY THERAPEUTICS - EMERGING LEADER IN i PSC CELL THERAPIES EMPLOYEES INCLUDING EXPERIENCED LEADERS AND ENTREPRENEURS ~120 COMPREHENSIVE iPSC CELL PLATFORM FOR IMMUNE EFFECTOR CELLS LEAD PROGRAM EXPECTED TO FILE IND IN MID 2022 STATE - OF - THE ART GMP MANUFACTURING FACILITY EXPECTED TO BE OPERATION BY YEAR - END 2021 IN CASH, CASH EQUIVALENTS AND MARKETABLE SECURITIES AS OF 6/30/2021 $440M GENETIC & PROTEIN ENGINEERING, PROCESS DEVELOPMENT, AND IMMUNO - ONCOLOGY EXPERTISE WITH CENTERS OF EXCELLENCE IN SEATTLE AND ONTARIO HEADQUARTERED IN PHILADELPHIA PRODUCT CANDIDATE ENGINE WITH PIPELINE IN SOLID AND HEMATOLOGIC MALIGNANCIES

4 4 Osvaldo (Lalo) Flores, CEO Hy Levitsky , President R&D Adrienne Farid, COO Greg Russotti , CTO Luis Borges, CSO Michael Diem, CBO PROVEN LEADERSHIP TEAM

5 5 Allogeneic, donor - derived Multiple donors Fewer doses per batch • Complex manufacturing, heterogeneous product, limited scale • Limited genetic engineering options Allogeneic, i PSC - derived Greater doses per batch • Efficient manufacturing, homogeneous product, greater scale • Likely unlimited genetic engineering options Single Donor iPSC TECHNOLOGY CAN OVERCOME LIMITATIONS OF DONOR DERIVED PLATFORMS

6 6 FOUNDATIONAL INVESTMENTS IN iPSC KNOW - HOW AND MANUFACTURING 6 In - House Manufacturing accelerates learnings and enables faster product iteration • Century facility expected to be operational by the end of 2021 • 53,000 ft 2 facility • Designed to produce multiple immune cell types • Two sites provides optionality and maximizes flexibility Significant acceleration of platform and product development iPSC License and collaboration agreement established in 2018 • Access to clinical grade iPSC lines • Exclusive IP and know - how to generate immune effector cells using feeder - free methods (NK, T, Mac, DC) • Dedicated FCDI GMP manufacturing capacity for Century’s product candidates • Leveraging two decades of research & investment at University of Wisconsin and FCDI

7 7 Sequential gene edits Engineered iPSC iNK cell iT cell iPSC bank Cell Engineering Manufacturing INTEGRATED CELL ENGINEERING AND MANUFACTURING ENABLES RAPID PROD UCT ITERATION ITERATIVE ENHANCEMENT OF PRODUCT FITNESS AND FUNCTIONALITY Engineered iPSC MCB Differentiation

8 8 ALLO - EVASION TM TECHNOLOGY b 2M KO (HLA - I) HLA - E CIITA KO (HLA - II) CD8 + T Cell CD4 + T Cell NK cell Evading the dominant mediators of allo - rejection

9 9 Not for further distribution ILLUSTRATIVE POTENTIAL OF ALLO - EVASION TM ON CELLULAR PHARMACOKINETICS Repeat doses With Allo - Evasion TM engineering Without Allo - Evasion TM engineering Time Cell count Initial dose

10 10 CENTURY’S APPROACH TO OPTIMIZING ANTI - TUMORAL RESPONSE Potentially maximizing durability of clinical responses and efficacy Genetic Engineering (To improve fitness) Manufacturing Optimization (To improve product quality) Engineered CAR - iNK or CAR - iT cells Allo - Evasion TM To avoid rejection Repeat Dosing To replenish cell product

11 11 iNK CELL PLATFORM IS OUR MOST ADVANCED PLATFORM CNTY - 101, our first product candidate is a CAR - iNK cell engineered with multiple features Allo - Evasion Persistence Safety switch CD19 - CAR 0 5 10 15 20 0 2.0×10 8 4.0×10 8 6.0×10 8 8.0×10 8 1.0×10 9 1.2×10 9 Day Post-tumor Implantation A v e r a g e R a d i a n c e ( p / s / c m ² / s r ) S E M C A R - i N K C A R - i N K C A R - i N K Tumor only CAR - iNK NALM - 6 tumor xenograft model CNTY - 101 iNK cell CAR - iNK Cells Have Significant* Anti - Tumor Activity In Vivo *Statistically significant (p=0.0027)

12 12 OUR i T CELL PLATFORM IS CLOSELY BEHIND AND MAKING DEMONSTRABLE PROGRESS Developing ab and gd iT platforms with Trusted TCRs that are not expected to cause GvHD CAR TCR 0 5 10 15 20 0 5.0×10 8 1.0×10 9 1.5×10 9 2.0×10 9 2.5×10 9 Day Post-tumor Implantation A v e r a g e R a d i a n c e ( p / s / c m ² / s r ) S E M C A R - i T Tumor only CAR - iT Century CAR - iT with Trusted TCR iT cell NALM - 6 tumor xenograft model CAR - iT Cells Have Significant* Anti - Tumor Activity In Vivo *Statistically significant (p<0.0001)
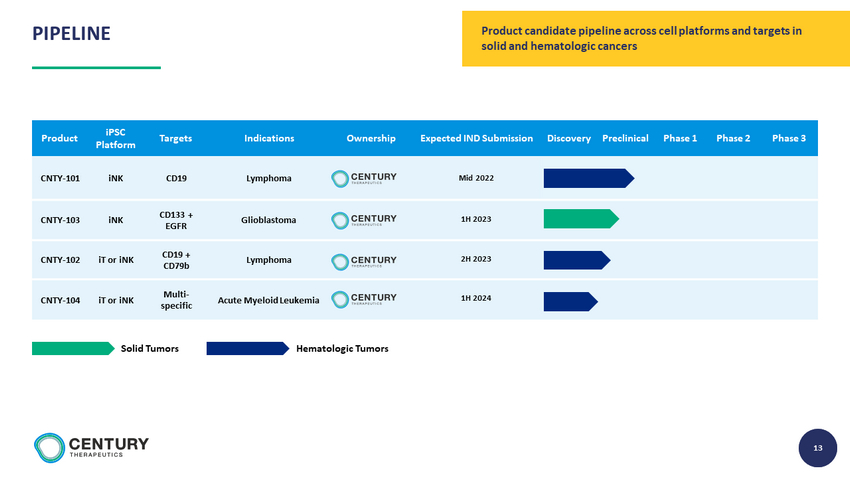
13 13 Product iPSC Platform Targets Indications Ownership Expected IND Submission Discovery Preclinical Phase 1 Phase 2 Phase 3 CNTY - 101 iNK CD19 Lymphoma Mid 2022 CNTY - 103 iNK CD133 + EGFR Glioblastoma 1H 2023 CNTY - 102 iT or iNK CD19 + CD79b Lymphoma 2H 2023 CNTY - 104 iT or iNK Multi - specific Acute Myeloid Leukemia 1H 2024 Hematologic Tumors Solid Tumors Product candidate pipeline across cell platforms and targets in solid and hematologic cancers PIPELINE

14 14 CENTURY’S NEXT GENERATION i PSC TECHNOLOGY PLATFORM Broad product pipeline with first 1 st IND submission targeting M id - 2022 Precision gene editing Advanced manufacturing Allo - Evasion Ρ Protein engineering iPSC - derived NK cells iPSC - derived T cells Comprehensive allogeneic iPSC - based cell platform

THANK YOU
