Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE OF BRISTOL-MYERS SQUIBB COMPANY DATED JULY 28, 2021 - BRISTOL MYERS SQUIBB CO | q22021ex991.htm |
| EX-99.2 - CERTAIN SUPPLEMENTAL INFORMATION - BRISTOL MYERS SQUIBB CO | q22021ex992.htm |
| 8-K - 8-K - BRISTOL MYERS SQUIBB CO | bmy-20210728.htm |

Q2 2021 Results July 28, 2021

Q2 2021 Results Not for Product Promotional Use Forward Looking Statement and Non-GAAP Financial Information 2 This presentation contains statements about the Company’s future plans and prospects that constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated as a result of various important factors, including those discussed in the Company’s most recent annual report on Form 10-K and reports on Form 10-Q and Form 8-K. These documents are available on the SEC’s website, on the Bristol-Myers Squibb website or from Bristol-Myers Squibb Investor Relations. In addition, any forward-looking statements represent our estimates only as of the date hereof and should not be relied upon as representing our estimates as of any subsequent date. While we may elect to update forward-looking statements at some point in the future, we specifically disclaim any obligation to do so, even if our estimates change. This presentation includes certain non-generally accepted accounting principles (GAAP) financial measures that we use to describe our company’s performance. The non-GAAP information presented provides investors with additional useful information but should not be considered in isolation or as substitutes for the related GAAP measures. Moreover, other companies may define non-GAAP measures differently, which limits the usefulness of these measures for comparisons with such other companies. We encourage investors to review our financial statements and publicly-filed reports in their entirety and not to rely on any single financial measure. An explanation of these non-GAAP financial measures and a reconciliation to the most directly comparable GAAP financial measure are available on our website at bms.com/investors. Also note that a reconciliation of certain forward-looking statements, however, is not provided due to no reasonably accessible or reliable comparable GAAP measures for such statements and the inherent difficulty in forecasting and quantifying such statements that are necessary for such reconciliation.

Not for Product Promotional Use Q2 2021 Results Giovanni Caforio Board Chair and Chief Executive Officer 3

Q2 2021 Results Not for Product Promotional Use Operational Performance Strong commercial execution • Sales of $11.7B in Q2; +16% YoY, +13% ex-FX • Strong momentum with launches for I-O and new product portfolio Pipeline Execution Significant milestones • Solid Tumors: Opdivo U.S. approval in adj. EC/GEJ & positive CHMP opinion; Opdivo in adj. MIBC - PDUFA Sep 3, 2021 • Hematology: Positive data for Breyanzi in 2L TE LBCL; Abecma positive CHMP opinion in 4L+ MM; iberdomide + dex in 4L+ MM data in-house • Immunology: Zeposia U.S. approval in moderate-to-severe UC • CV: Milvexian (FXIa inhibitor) Ph 2 TKR data in-house • ] Financial Strength Strong financial results and outlook • Continued revenue and EPS growth • Reaffirm 2021 Revenue and Non-GAAP EPS guidance • Balance sheet strength and strong cash flow generation; debt repayments of ~$5.7B & executed share repurchases of ~$3B YTD Business Development • Licensed anti-TIGIT bispecific antibody program w/ Agenus & FRα ADC collab w/ Eisai Q2 2021 Performance 4

Q2 2021 Results Not for Product Promotional Use Opdivo (+/- Yervoy) U.S./EU expected approvals: 1L RCC (9ER) , 1L GC (649, O+Chemo), adj Eso (577) adj MIBC (274) 1L Esophageal (CM-648) Opdivo return to annual growth Relatlimab 1L Melanoma w/ Opdivo Ph3 Breyanzi 3L+ DLBCL U.S. / EU approval 2L TE and TNE DLBCL 3L+ CLL Abecma 4L+ MM U.S.1 / EU approval Iberdomide + dex 4L+ MM Ph 1b/2a Deucravacitinib PsO (2nd study) Ph3 & U.S. filing UC Ph2 (POC) Zeposia UC U.S. / EU approval Cendakimab Initiation of Ph3 Factor XIa inh. Total Knee Replacement VTEp Ph2 (POC) Mavacamten oHCM U.S. filing & approval2 Opdivo (+/- Yervoy) Metastatic 1L HCC (CM-9DW) Adjuvant Neo-adj Lung EFS (CM-816) Peri-adj Lung (CM-77T) Bempeg 1L melanoma3 & 1L renal Breyanzi 3L+ Follicular lymphoma Abecma 3L+ MM (KarMMa-3) Ph3 2L+ MM (KarMMa-2) POC CC-92480 4L+ MM Ph1/2 CC-93269 (TCE) Initiation of pivotal trial Deucravacitinib PsO U.S./EU approval CD & Lupus Ph2 (POC) Zeposia CD Ph3 Factor XIa inh. Secondary Stroke Prevention Ph2 (POC) Reblozyl 1L MDS (ESA naïve) COMMANDS Ph3 Ph 1/2 Pipeline >20 POC decisions 5 2021 Key Milestones Execution Scorecard • 2020-2025: Low to mid-single digit revenue CAGR* Low double-digit revenue CAGR for Continuing business* • Operating margins low to mid 40%s** • ~$3B of synergies by end of 2022 • $45B - $50B of free- cash flow 2021-2023** Financial Expectations 2022/2023 Key Milestones To be expanded to include regulatory milestones pending future registrational successes On track based on 2021 guidance 1Approved after 4 prior lines of therapy 2 PDUFA January 28, 2022 3 Expected in 2022 *At constant exchange rates – Non-GAAP: there is no reliable or reasonable estimable comparable GAAP metric for this Non-GAAP forward-looking information; **Non-GAAP: there is no reliable or reasonable estimable comparable GAAP metric for this forward-looking information

Q2 2021 Results Not for Product Promotional Use Continued portfolio renewal into 2H of decade 6 *Non-risk adjusted revenue potential is subject to positive registrational trials & health authority approval for Reblozyl, Zeposia, Onureg, Inrebic, Breyanzi, Abecma, Deucravacitinib, and Mavacamten Anticipated Launches deucravacitinib mavacamten Launch Portfolio Best / first-in-class assets $20B-$25B NRA revenue potential in 2029* relatlimab Future growth opportunities • Additional mid-to late-stage pipeline, including: • Diverse early-stage pipeline • Balance Sheet strength for additional BD opportunities iberdomide milvexian + relatlimab

Not for Product Promotional Use David Elkins Chief Financial Officer 7 Q2 2021 Results

Q2 2021 Results Not for Product Promotional Use Net Sales $ in Billions Vs. Prior Year $3.2 11% $2.8 29% $1.9 16% $0.9 15% $0.8 9% $0.5 6% $0.5 38% $0.3 4% Strong performance in key franchises 8 Net Sales $ in Billions Vs. Prior Year $6.1 6% $5.7 18% $3.6 6% $1.6 12% $1.6 7% $1.0 2% $1.0 26% $0.6 0% YTD SalesQ2 Sales ▲ ▲ ▲ ▲ ▲ ▲ ▲ ▲ ▲ ▲ - Q2 2021 Total Sales: $11.7B, up 16% & 13% ex-FX ▲ ▲ ▲ ▲ ▲ YTD 2021 Total Sales: $22.8B, up 9% & 7% ex-FX
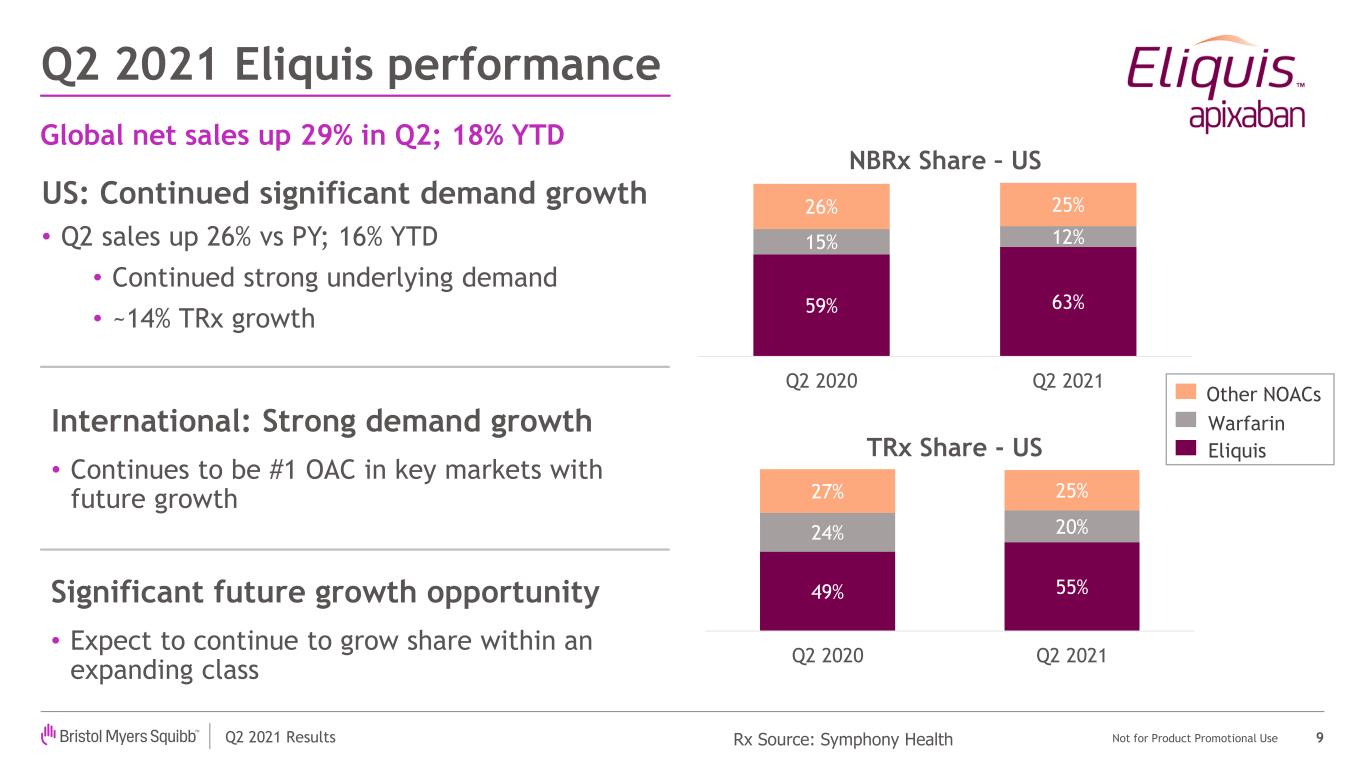
Q2 2021 Results Not for Product Promotional Use US: Continued significant demand growth • Q2 sales up 26% vs PY; 16% YTD • Continued strong underlying demand • ~14% TRx growth Q2 2021 Eliquis performance 9Rx Source: Symphony Health Significant future growth opportunity • Expect to continue to grow share within an expanding class International: Strong demand growth • Continues to be #1 OAC in key markets with future growth Global net sales up 29% in Q2; 18% YTD 49% 55% 24% 20% 27% 25% Q2 2020 Q2 2021 TRx Share - US Other NOACs Warfarin Eliquis 59% 63% 15% 12% 26% 25% Q2 2020 Q2 2021 NBRx Share – US

Q2 2021 Results Not for Product Promotional Use Global net sales up 16% in Q2 U.S. • Return to growth (+13% vs. PY) • 1L lung* shares in low double-digits • Leadership position in 1L renal enabled by O+Cabo • Strong early launches in Upper GI International • Momentum from new launches, FX, improved COVID dynamics vs. PY Q2 2021 Opdivo performance 10 24% 24%29% 23% Approx. U.S. Sales Mix Note: percentages approximate based on tumor ranges 33% 20% 25% 22% Approx. Ex-U.S. Sales Mix NSCLC RCC Melanoma All others Near term growth drivers • Momentum from recent launches • Potential next launches: adjuvant bladder (CM-274), 1L esophageal (CM-648) *excluding EGFR/ALK patients

Q2 2021 Results Not for Product Promotional Use Q2 2021 Multiple Myeloma performance 11 $522 $567 $223 $287 Q2 2020 Q2 2021 Global sales growth of 11% • US sales growth of 6% • Increased use of triplet regimens & longer treatment duration • Recovery to pre-COVID levels in NRx • International sales growth of 24% • Demand from triplets & maintenance use • Improved COVID dynamics & FX Global sales growth of 15% • Demand growth from new triplet regimens and use in earlier lines Global Net Sales $2,048 $2,164 $836 $1,038 Q2 2020 Q2 2021 US Ex-US

Q2 2021 Results Not for Product Promotional Use • Continued transition from initial bolus to underlying incidence demand • Remain focused on new patients earlier in their treatment journey & dose optimization • Expansion in global markets Advancing new product launches 12 • #1 S1P modulator in written Rx in MS • Acceleration of Rx to commercial demand • UC approved in the U.S. - positive physician receptivity • MAA under review • Strong demand growth sequentially • Establishing profile in 1L response maintenance setting in AML • Approved by EC June 2021 $96 $115 $112 $128 $2 $9 $18 $28 $3 $14 $15 $12 Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q2 2021 Global Net Sales Reblozyl Zeposia Onureg

Q2 2021 Results Not for Product Promotional Use Cell Therapy Performance 13 • Rapid site activation (>65 sites) • Strong demand and physician awareness Best-in-class CD19 CAR T Global net sales: $17M First-in-class BCMA CAR T Global net sales: $24M Two differentiated CAR T products, now in the marketplace
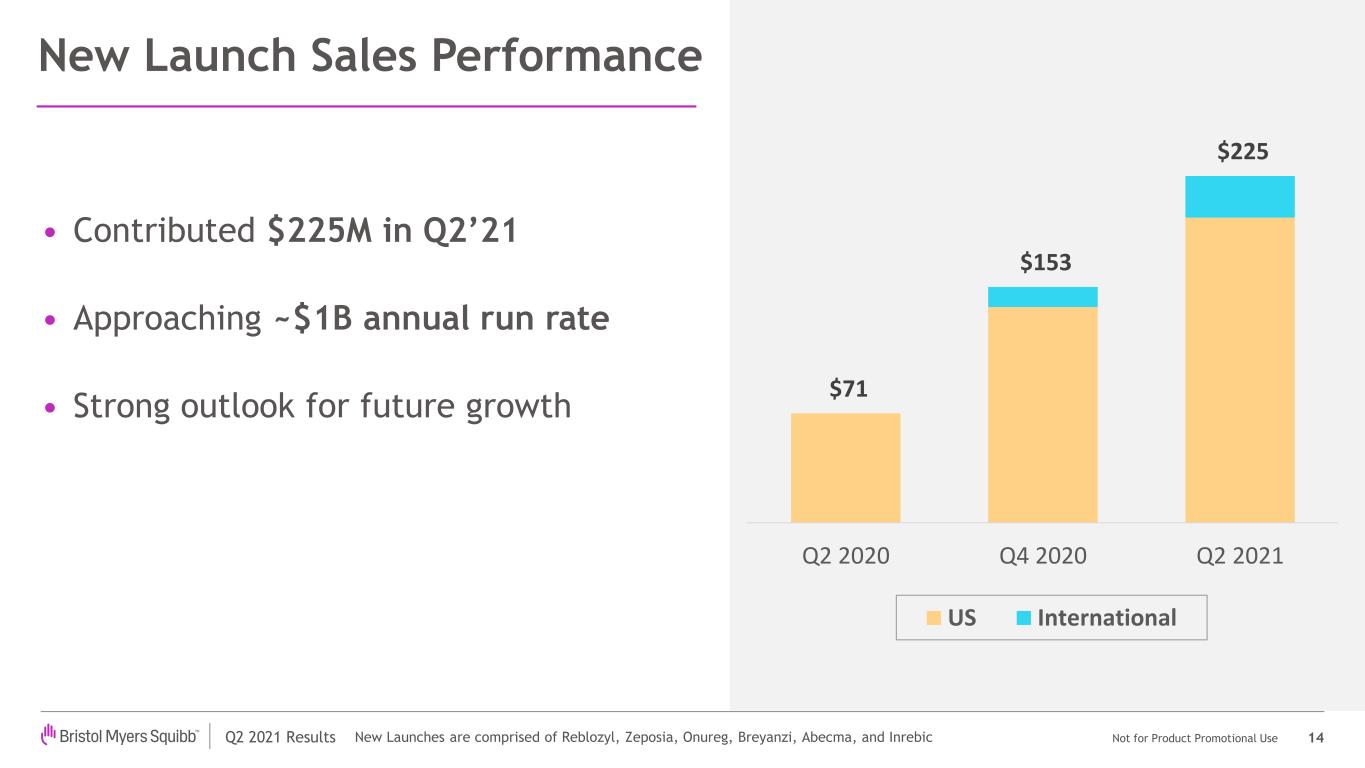
Q2 2021 Results Not for Product Promotional Use New Launch Sales Performance • Contributed $225M in Q2’21 • Approaching ~$1B annual run rate • Strong outlook for future growth 14 $71 $153 $225 Q2 2020 Q4 2020 Q2 2021 US International New Launches are comprised of Reblozyl, Zeposia, Onureg, Breyanzi, Abecma, and Inrebic

Q2 2021 Results Not for Product Promotional Use US GAAP Non-GAAP $ in billions, except EPS Q2 2021 Q2 2020 Q2 2021 Q2 2020 Total Revenues, net 11.7 10.1 11.7 10.1 Gross Margin % 79.0% 73.4% 79.8% 80.5% MS&A 1.9 1.6 1.9 1.6 R&D 3.3 2.5 2.3 2.2 Effective Tax Rate 31.7% 104.9% 16.9% 13.8% Diluted EPS 0.47 (0.04) 1.93 1.63 Diluted Shares Outstanding (# in millions) 2,252 2,263 2,252 2,297 Q2 2021 Financial Performance 15

Q2 2021 Results Not for Product Promotional Use Significant financial flexibility to support a balanced approach to capital allocation 16 $B Q2 2021 Total Cash** $13.1B Total Debt $45.2B Net Debt Position $32.0B *Subject to Board approval **Cash includes cash, cash equivalents and marketable debt securities Committed to reducing debt • ~$5.7B in debt reduction YTD • Maintain strong investment-grade credit ratings Returning capital to shareholders • Continued dividend growth* • $3B - $4B total share repurchase planned; executed ~$3B YTD & remain opportunistic in 2H Future innovation through business development • Strategically Aligned • Scientifically Sound • Financially Attractive

Q2 2021 Results Not for Product Promotional Use 2021 Guidance Details 17 GAAP Non-GAAP Net Sales High single-digit increase High single-digit increase Gross Margin % ~79% ~80% MS&A Expense In line with 2020 Low single-digit increase R&D Expense Low single-digit decrease Mid single-digit increase Tax Rate ~23% ~16% Diluted EPS $2.77-$2.97 $7.35 - $7.55 Refer to separate reconciliation of GAAP to these non-GAAP measures on company’s website Non-GAAP EPS reaffirmed

Q2 2021 Results Not for Product Promotional Use Q&A Giovanni Caforio, M.D. Board Chair, Chief Executive Officer David Elkins Executive VP, Chief Financial Officer 18 Chris Boerner, Ph.D. Executive VP, Chief Commercialization Officer Samit Hirawat, M.D. Executive VP, Chief Medical Officer, Global Drug Development
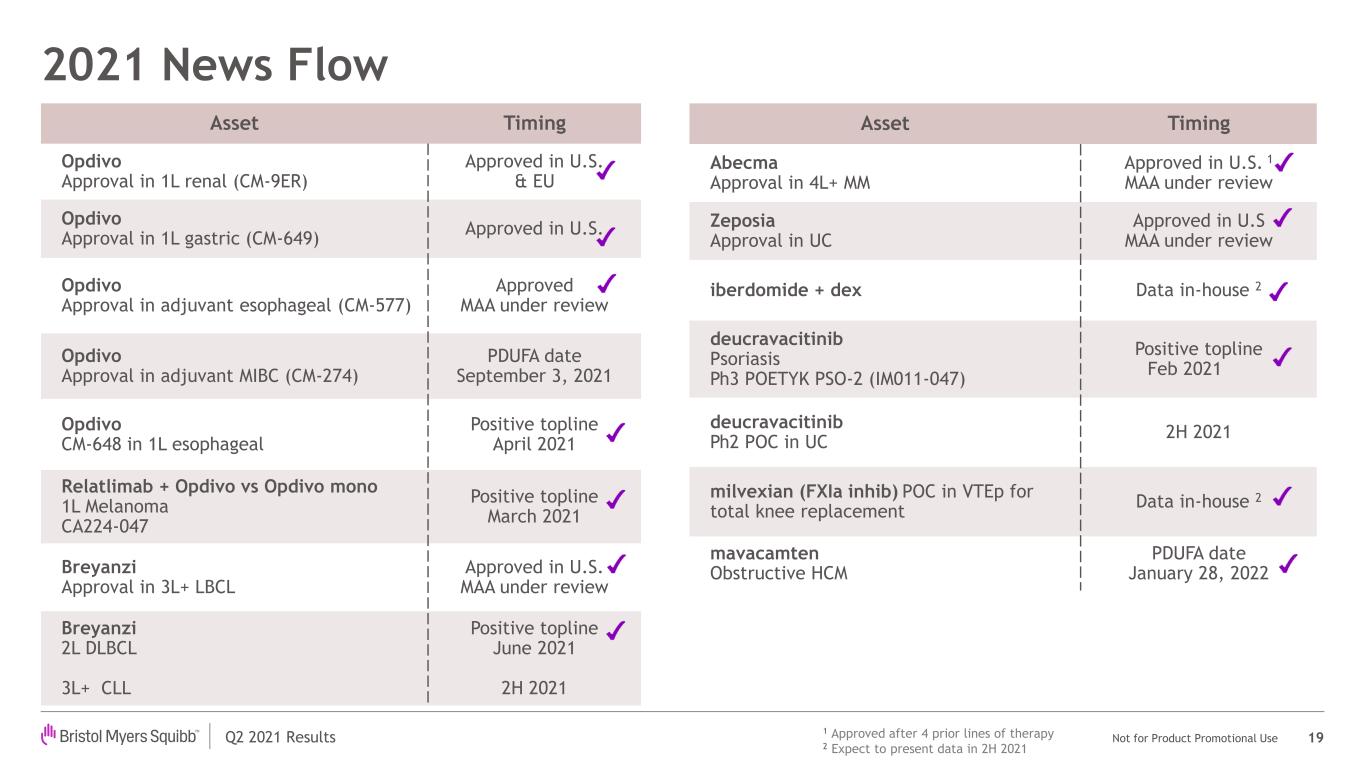
Q2 2021 Results Not for Product Promotional Use 2021 News Flow 19 Asset Timing Opdivo Approval in 1L renal (CM-9ER) Approved in U.S. & EU Opdivo Approval in 1L gastric (CM-649) Approved in U.S. Opdivo Approval in adjuvant esophageal (CM-577) Approved MAA under review Opdivo Approval in adjuvant MIBC (CM-274) PDUFA date September 3, 2021 Opdivo CM-648 in 1L esophageal Positive topline April 2021 Relatlimab + Opdivo vs Opdivo mono 1L Melanoma CA224-047 Positive topline March 2021 Breyanzi Approval in 3L+ LBCL Approved in U.S. MAA under review Breyanzi 2L DLBCL 3L+ CLL Positive topline June 2021 2H 2021 Asset Timing Abecma Approval in 4L+ MM Approved in U.S. 1 MAA under review Zeposia Approval in UC Approved in U.S MAA under review iberdomide + dex Data in-house 2 deucravacitinib Psoriasis Ph3 POETYK PSO-2 (IM011-047) Positive topline Feb 2021 deucravacitinib Ph2 POC in UC 2H 2021 milvexian (FXIa inhib) POC in VTEp for total knee replacement Data in-house 2 mavacamten Obstructive HCM PDUFA date January 28, 2022 1 Approved after 4 prior lines of therapy 2 Expect to present data in 2H 2021
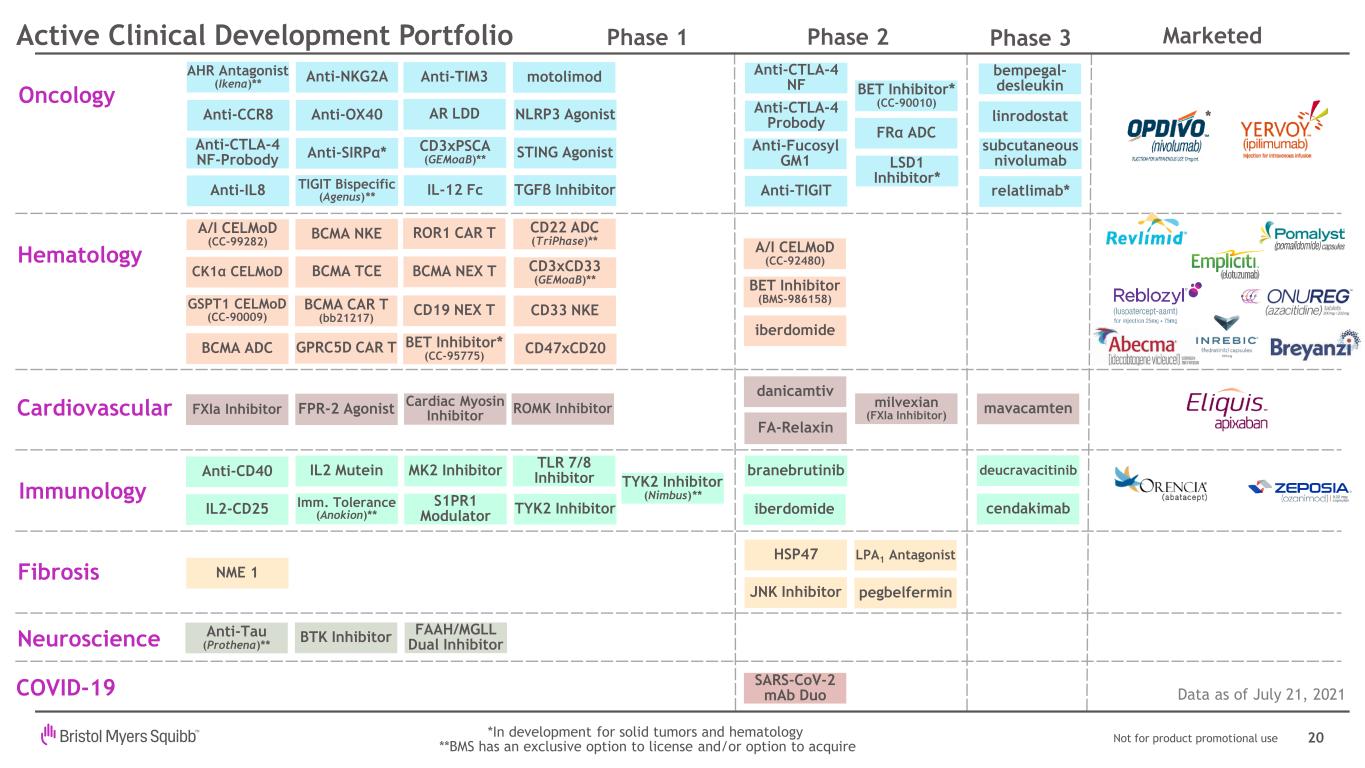
Not for product promotional use Hematology Fibrosis pegbelfermin HSP47 LPA1 Antagonist JNK Inhibitor Immunology Oncology Data as of July 21, 2021 Anti-Fucosyl GM1 Anti-OX40 STING Agonist Anti-NKG2A Anti-SIRPα* Anti-TIM3 Anti-CTLA-4 NF Anti-CCR8 Anti-CTLA-4 Probody BCMA TCE AR LDD CD3xPSCA (GEMoaB)** Anti-IL8 Anti-TIGIT relatlimab* bempegal- desleukin linrodostat BET Inhibitor (BMS-986158) CD19 NEX T BCMA ADC BCMA CAR T (bb21217) TGFβ Inhibitor LSD1 Inhibitor* iberdomide *In development for solid tumors and hematology **BMS has an exclusive option to license and/or option to acquire iberdomide MK2 InhibitorAnti-CD40 IL2-CD25 Imm. Tolerance (Anokion)** IL2 Mutein cendakimab branebrutinib deucravacitinib Cardiovascular FA-Relaxin FXIa Inhibitor 20 Phase 1 Phase 2 Phase 3 A/I CELMoD (CC-92480) A/I CELMoD (CC-99282) GSPT1 CELMoD (CC-90009) BET Inhibitor* (CC-90010) CD33 NKE CD47xCD20 Neuroscience Anti-Tau (Prothena)** AHR Antagonist (Ikena)** S1PR1 Modulator TLR 7/8 Inhibitor CD22 ADC (TriPhase)** BET Inhibitor* (CC-95775) NME 1 FPR-2 Agonist Active Clinical Development Portfolio motolimod NLRP3 Agonist danicamtiv BCMA NEX T IL-12 Fc Cardiac Myosin Inhibitor milvexian (FXIa Inhibitor) mavacamten CD3xCD33 (GEMoaB)** TYK2 Inhibitor TYK2 Inhibitor (Nimbus)** GPRC5D CAR T COVID-19 SARS-CoV-2 mAb Duo Anti-CTLA-4 NF-Probody CK1α CELMoD ROMK Inhibitor BTK Inhibitor * BCMA NKE ROR1 CAR T TIGIT Bispecific (Agenus)** FRα ADC FAAH/MGLL Dual Inhibitor subcutaneous nivolumab Marketed
