Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Yubo International Biotech Ltd | yubo_ex231.htm |
| EX-10.17 - EX-10.17 - Yubo International Biotech Ltd | yubo_ex1017.htm |
| EX-10.16 - EX-10.16 - Yubo International Biotech Ltd | yubo_ex1016.htm |
| EX-2.1 - EX-2.1 - Yubo International Biotech Ltd | yubo_ex21.htm |
As filed with the Securities and Exchange Commission on July 12 , 2021
Registration No. 333 - 255805
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
Under
THE SECURITIES ACT OF 1933
___________________
| YUBO INTERNATIONAL BIOTECH LIMITED |
| (Exact Name of Registrant as Specified in its Charter) |
| New York | 0-21320 | 11-3074326 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
Room 105, Building 5, 31 Xishiku Avenue
Xicheng District, Beijing, China
+86 (010) 6615-5141
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Jun Wang
Chief Executive Officer
Yubo International Biotech Limited
Room 105, Building 5, 31 Xishiku Avenue
Xicheng District, Beijing, China
+86 (010) 6615-5141
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all correspondence to:
| Lina Liu |
| Mark C. Lee, Esq. |
| Chief Financial Officer Yubo International Biotech Limited Room 105, Building 5, 31 Xishiku Avenue Xicheng District, Beijing, China +86 (010) 6615-5141 |
| Greenberg Traurig, LLP 1201 K Street, Suite 1100 Sacramento, CA 95814 (916) 868.063 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered (1) |
| Proposed Maximum Aggregate Offering Price (2) |
|
| Amount of Registration Fee |
| ||
|
|
|
|
|
|
|
| ||
| Class A Common Stock, par value $0.001 per share |
| $ | 2,500,000.00 |
|
| $ | 272.75 |
|
| Class A Common Stock, par value $0.001 per share, issued to selling stockholders(3) |
|
| 6,125,550.00 |
|
|
| 668.30 |
|
| Total |
|
| 8,625,550.00 |
|
|
| 941.05 |
|
_____________
| (1) | In accordance with Rule 416(a), this Registration Statement also covers an indeterminate number of shares that may be issued and resold resulting from stock splits, stock dividends or similar transactions. |
| (2) | Estimated solely for the purpose of calculating the registration fee under Rule 457(o) of the Securities Act of 1933, as amended (“Securities Act”). |
| (3) | Represents an aggregate of 12,251,100 shares of Class A common stock offered for resale by the selling stockholders. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the SEC, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. Neither we nor the selling stockholders may sell these securities until the registration statement filed with the U.S. Securities and Exchange Commission is declared effective. This preliminary prospectus is not an offer to sell these securities, nor a solicitation of an offer to buy these securities, in any jurisdiction where the offer, solicitation, or sale is not permitted.
SUBJECT TO COMPLETION, DATED JULY 12, 2021
PRELIMINARY PROSPECTUS
YUBO INTERNATIONAL BIOTECH LIMITED
5,000,000 Shares of Class A Common Stock Offered by the Company
12,251,100 Shares of Class A Common Stock Offered by the Selling Stockholders
We are offering, on a “best efforts” basis, up to an aggregate of 5,000,000 shares of our Class A common stock, par value 0.001 per share , at a fixed price of $0.50 per share. Our shares of Class A common stock are quoted on the OTC Marketplace under the symbol “YBGJ”. On July 9, 2021, the closing price of our Class A common stock on the OTC Marketplace was $0.65 per share.
Additionally, the selling stockho lders are offering an additional 12,251,100 shares of Class A common stock, par value 0.001 per share, at a fixed price of $0.50 per share . The selling stockholders include: (i) Focus Draw Group Limited (“Focus”), which is selling up to 4,728,000 shares of our Class A common stock held by Focus (the “Focus Shares”), (ii) Dragoncloud Technology Limited (“Dragoncloud”), which is selling up to 5,768,100 shares of our Class A common stock held by Dragoncloud (the “Dragoncloud Shares”), and (iii) Cheung Ho Shun (“Cheung”), who is selling up to 1,755,000 shares of our Class A common stock held by Cheung (the “Cheung Shares”). The Focus Shares, the Dragoncloud Shares and the Cheung Shares are referred to collectively as the “Resale Securities”.
We are offering the shares on a self-underwritten basis which means our officers and directors will attempt to sell the shares in reliance on the safe harbor from broker-dealer registration under Rule 3a4-1 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All funds that we raise from the offering will be immediately available for our use and will not be returned to investors. We do not have any arrangements to place the funds received from the sale of shares in the offering in an escrow, trust or similar account.
The offering will end on December 31, 2021, unless all of the shares are sold before that date, we extend the offering another 30 days or we otherwise decide to terminate the offering early or cancel it, in each case in our sole discretion. If we extend the offering, we will provide that information in an amendment to this prospectus. If we close the offering early or cancel it, including during any extended offering period, we may do so without notice to investors, although if we cancel the offering we will promptly return any funds investors may already have paid. We will bear the expenses relating to the registration of the shares. The selling stockholder will sell the Resale Securities at a fixed price of $0.50 per share.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 4 of this prospectus, and under similar headings in any amendments or supplements to this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
| Per share of Common Stock |
|
| Total (100%) |
|
|
| 75% |
|
| 50% |
|
| 25% | |||||
| Public offering price |
| $ | 0.50 |
|
| $ | 2,500,000.00 |
|
| $ | 1,857,000.00 |
|
| $ | 1,250,000.00 |
|
| $ | 625,000.00 |
|
| Proceeds to us, before expenses |
| $ | 0.50 |
|
| $ | 2,415,058.95 |
|
| $ | 1,790,058.95 |
|
| $ | 1,165,058.95 |
|
| $ | 540,058.95 |
|
Because there is no minimum offering amount required as a condition to closing in this offering, the actual public offering amount and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above.
The date of this prospectus is , 2021
|
|
| PAGE |
| |
|
|
|
|
| |
|
|
| 1 |
| |
|
|
| 4 |
| |
|
|
| 4 |
| |
|
|
| 22 |
| |
|
|
| 23 |
| |
|
|
| 24 |
| |
|
|
| 24 |
| |
|
|
| 25 |
| |
|
|
| 26 |
| |
|
|
| 28 |
| |
|
|
| 29 |
| |
|
|
| 47 |
| |
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
| 48 |
|
|
|
| 55 |
| |
|
|
| 57 |
| |
|
|
| 58 |
| |
| Certain Relationships and Related Transactions and Director Independence |
|
| 60 |
|
|
|
| 62 |
| |
|
|
| 62 | ||
|
|
| 62 |
| |
|
|
| 62 |
| |
|
| F-1 |
| ||
You should rely only on the information contained in this prospectus. Neither we nor the selling stockholders have authorized any other person to provide you with information that is different from that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. Neither we nor the selling stockholders take any responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
| i |
| Table of Contents |
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in our securities. You should carefully read the entire prospectus including “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our Financial Statements, before making an investment decision.
In this prospectus, unless otherwise specified, the terms “we,” “our,” “us,” the “Company,” or the “Registrant” refer to Yubo International Biotech Limited, a New York corporation formerly known as Magna-Lab, Inc. and its wholly owned subsidiaries, including without limitation, Platinum International Biotech Co., Ltd., a company organized under the laws of the Cayman Islands, and Yubo International Biotech (Beijing) Limited, a company organized under the laws of the People’s Republic of China.
Corporate Overview
Name Change
Effective December 4, 2020, we changed our corporate name from Magna-Lab, Inc. to Yubo International Biotech Limited under the stock symbol “YBGJ” .
Reverse Merger with Platinum International Biotech Co., Ltd.
On January 14, 2021 (the “Closing Date”), we entered into a voluntary share exchange transaction with Platinum International Biotech Co., Ltd., a company organized under the laws of the Cayman Islands (“Platinum”), pursuant to that certain Agreement and Plan of Share Exchange, dated January 14, 2021 (the “Exchange Agreement”), by and among us, Platinum, Yubo International Biotech (Beijing) Limited, a company organized under the laws of the People’s Republic of China (“Yubo”), and certain selling stockholders named therein.
In accordance with the terms of the Exchange Agreement, on the Closing Date, we issued a total of 117,000,000 shares of our Class A common stock to the then stockholders of Platinum (the “Selling Stockholders”), in exchange for 100% of the issued and outstanding capital stock of Platinum (the “Exchange Transaction”). As a result of the Exchange Transaction, the Selling Stockholders acquired more than 99% of our issued and outstanding capital stock, Platinum became our wholly-owned subsidiary, and we acquired the business and operations of Platinum and Yubo.
Platinum was incorporated on April 7, 2020 under the laws of the Cayman Islands as a holding company. Commencing April 2020, its consolidated variable interest entity Yubo is a supplier of innovative products that process, store and administer therapeutic doses of endometrial stem cells for treatment of disease and injuries in the PRC.
Immediately prior to the Exchange Transaction, we had 117,875,323 shares of Class A common stock and 4,447 shares of Class B common stock issued and outstanding. Immediately after the Exchange Transaction and the surrender and cancellation of 116,697,438 shares of Class A common stock previously held by Lina Liu, and as of the date hereof, our authorized capital stock consists of 120,000,000 shares of common stock, par value $.001 per share, of which 118,177,885 Class A common plus 4,447 Class B common) are issued and outstanding, and 5,000,000 shares of Preferred Stock, $0.001 par value, none of which shares are issued or outstanding. Each share of Class A common stock is entitled to one vote with respect to all matters to be acted on by the stockholders; and each share of Class B common stock is entitled to five votes per share, and is convertible into one share of Class A common stock.
| 1 |
| Table of Contents |
Recent Deve lopment
Platinum International Biotech (Hong Kong) Limited, a wholly-owned subsidiary of Platinum, formed two new wholly-owned subsidiaries: Yubo Jingzhi Biotechnology (Chengdu) Co. Ltd. (“Yubo Jingzhi”) and Yubo Global Biotechnology (Chengdu) Co. Ltd (“Yubo Global”) in December 2020 and January 2021, respectively.
Common Stock
Our authorized common stock is divided into Class A common stock and Class B common stock. Holders of Class A common stock are entitled to one vote per share, while holders of Class B common stock are entitled to five votes per share. Each share of Class B is convertible into one share of Class A common stock upon notice of the holder , while Class A common stock is not convertible into Class B common stock under any circumstances. As of Ju ly 12, 2021, we have authorized (i) 1,000,000,000 shares of Class A common stock, of which 118,177,885 shares were issued and outstanding, and (ii) 3,750,000 shares of Class B common stock, of which 4,447 shares were issued and outstanding. See “Description of Securities-Common Stock”.
Summary Risk Factors
Investing in our common stock involves significant risks. You should carefully consider all of the information in this prospectus before making an investment in our common stock. Below please find a summary of the principal risks we face, organized under relevant headings. These risks are discussed more fully in the section titled “Risk factors”.
Risks Related to Shares of our Common Stock and this Offering
|
| · | You will experience immediate and substantial dilution in the book value per share of the common stock you purchase. |
| · | Shares of our common stock that have not been registered under the Securities Act of 1933, as amended, regardless of whether such shares are restricted or unrestricted, are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a “shell company.” | |
|
| · | The relative lack of public company experience of our management team may put us at a competitive disadvantage. |
|
| · | Our common stock is not listed on any stock exchange and there is a limited market for shares of our common stock. Even if a market for our common stock develops, our common stock could be subject to wide fluctuations. |
|
| · | Because we became public by means of a “reverse merger,” we may not be able to attract the attention of major brokerage firm or investors in general. |
|
| · | Our common stock may be subject to penny stock rules, which may make it more difficult for our stockholders to sell their common stock. |
|
| · | FINRA sales practice requirements may also limit a stockholder’s ability to buy and sell our stock. |
|
| · | We do not anticipate paying any cash dividends. |
|
| · | We may need additional capital, and the sale of additional shares or other equity securities, including our Class B common stock, could result in additional dilution to our stockholders. |
|
| · | Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval. |
|
| · | We have a substantial number of authorized common shares available for future issuance that could cause dilution of our stockholders’ interest and adversely impact the rights of holders of our common stock. |
| 2 |
| Table of Contents |
Risks R elated to our Business and Industry
|
| · | The commercial success of our products depends upon the degree of their market acceptance among the medical community. If our products do not attain market acceptance among the medical community, our operations and profitability would be adversely affected. |
|
| · | We have a history of losses and may continue to incur losses in the future, which raises substantial doubt about our ability to continue as a going concern. |
|
| · | Our proprietary, next-generation stem cell derived technologies, our approach for stem cell storage facilities and our manufacturing platform for our stem cell based product candidates, represent emerging approaches to medical treatments that face significant challenges and hurdles. |
|
| · | We may not be able to successfully create our own manufacturing infrastructure and stem cell storage facilities for supply and maintenance of our requirements of programmed stem cell products for use in clinical trials and for commercial sale. |
|
| · | We may not be able to timely identify or otherwise effectively respond to changing customer preferences, and we may fail to optimize our product offering and inventory position. |
|
| · | We face significant competition, and if we do not compete successfully against existing and new competitors, our revenue and profitability would be materially and adversely affected. |
|
| · | We rely on third-party manufacturers to supply our products. |
|
| · | Our certificates, permits, and licenses related to our business are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated. |
|
| · | If we are unable to protect our intellectual property from infringement, our business and prospects may be harmed. |
Risks R elated to our Corporate Structure
|
| · | Transactions among our affiliates are subject to scrutiny by the PRC tax authorities and a finding that we or any of our consolidated entities owe additional taxes could have a material adverse impact on our net income and the value of an investment in our common stock. |
|
| · | We currently conduct our business primarily through a contractually controlled PRC operating entity, and our control of the day-to-day operations of such PRC entity pursuant to contracts, to comply with Chinese law, may not be as effective as conducting business through direct equity ownership of such PRC entity due to uncertainties with respect to the PRC legal system which could materially and adversely affect our results of operations. |
|
| · | Our contractual arrangements with Yubo and its shareholders may not be as effective in providing control over these entities as direct ownership. |
Risks related to Doing Business in China
|
| · | The medical industry in China is highly regulated and such regulations are subject to change which may affect approval and commercialization of our drugs. |
|
| · | Our contractual arrangements with Yubo and its shareholders may not be as effective in providing control over these entities as direct ownership. |
|
| · | You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our management named in this Report based on foreign laws. |
Corporate Information
Our principal executive offices are located at Room 105, Building 5, 31 Xishiku Avenue, Xicheng District, Beijing, PRC. Our telephone number is +86 (010) 6615-5141. Our website address is http://www.yubogroup.com/. The information contained in, or that can be accessed through, our website is not incorporated by reference into, and is not a part of, this prospectus. You should not consider any information on our website to be part of this prospectus or in decides whether to purchase our securities. We have included our website address in this prospectus solely for informational purposes.
| 3 |
| Table of Contents |
The SEC allows us to “incorporate by reference” into this prospectus the information in other documents that we file with it, including at a subsequent date. The information incorporated by reference is considered to be a part of this prospectus, and information in documents that we file later with the SEC will automatically update and supersede information contained in documents filed earlier with the SEC or contained in this prospectus. In addition to documents referenced as incorporated by reference elsewhere in this prospectus, we incorporate by reference herein all documents subsequently filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the termination of the offering; provided, however, that we are not incorporating, in each case, any documents or information deemed to have been furnished and not filed in accordance with SEC rules.
Any investment in our securities involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our securities. Our business, financial condition or results of operations could be materially adversely affected by these risks if any of them actually occur. Our common stock is quoted on the OTC Marketplace under the symbol “YBGJ.” This market is extremely limited and the prices quoted are not a reliable indication of the value of our common stock. As of the date of this prospectus, there has been very limited trading of shares of our common stock. If and when our common stock is traded, the trading price could decline due to any of these risks, and an investor may lose all or part of his or her investment. Some of these factors have affected our financial condition and operating results in the past or are currently affecting us. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described below and elsewhere in this prospectus.
Risks Related to Shares of o ur C ommon S tock and this Offering:
You will experience immediate and substantial dilution in the book value per share of the common stock you purchase.
Because the price per unit being offered will be higher than the book value per share of our common stock, you will suffer substantial dilution in the net tangible book value of the common stock you acquire in this offering. See the section titled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase units in this offering.
Shares of our common stock that have not been registered under the Securities Act of 1933, as amended, regardless of whether such shares are restricted or unrestricted, are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a “shell company.” In addition, any shares of our common stock that are held by affiliates, including any received in a registered offering, will be subject to the resale restrictions of Rule 144(i).
Pursuant to Rule 144 of the Securities Act of 1933, as amended (“Rule 144”), a “shell company” is defined as a company that has no or nominal operations; and, either no or nominal assets; assets consisting solely of cash and cash equivalents; or assets consisting of any amount of cash and cash equivalents and nominal other assets. As such, we were a “shell company” pursuant to Rule 144 prior to the consummation of the Exchange Transaction, and as such, sales of our securities pursuant to Rule 144 are not able to be made until a period of at least twelve months has elapsed from January 14, 2021, which is the date on which the Company’s Current Report on Form 8-K has been filed with the Commission reflecting the Company’s status as a non-“shell company.” Therefore, any restricted securities we sell in the future or issue to consultants or employees, in consideration for services rendered or for any other purpose will have no liquidity until and unless such securities are registered with the Commission and/or until a year after January 14, 2021, and we have otherwise complied with the other requirements of Rule 144. As a result, it may be harder for us to fund our operations and pay our consultants with our securities instead of cash. Furthermore, it will be harder for us to raise funding through the sale of debt or equity securities unless we agree to register such securities with the Commission, which could cause us to expend additional resources in the future. Our previous status as a “shell company” could prevent us from raising additional funds, engaging consultants, and using our securities to pay for any acquisitions (although none are currently planned), which could cause the value of our securities, if any, to decline in value or become worthless. Lastly, any shares held by affiliates, including shares received in any registered offering, will be subject to the resale restrictions of Rule 144(i).
| 4 |
| Table of Contents |
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience and is generally unfamiliar with the requirements of the United States securities laws and U.S. Generally Accepted Accounting Principles, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management team have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
Our common stock is not listed on any stock exchange and there is a limited market for shares of our common stock. Even if a market for our common stock develops, our common stock could be subject to wide fluctuations.
Our common stock is not listed on any stock exchange. Although our common stock is listed on the OTC Marketplace, there is a limited public market for shares of our common stock, and limited trades of our common stock have taken place on the OTC Marketplace. Even if the shares of our common stock may in the future trade greater volume on the OTC Marketplace, the liquidity and price of our common stock is expected to be more limited than if such securities were quoted or listed on a national exchange. No assurances can be given that an active public trading market for our common stock will develop or be sustained. Trading volume may be limited by the fact that many major institutional investment funds, including mutual funds, as well as individual investors follow a policy of not investing in over the counter stocks and certain major brokerage firms restrict their brokers from recommending over the counter stocks because they are considered speculative, volatile and thinly traded. Lack of liquidity will limit the price at which stockholders may be able to sell our common stock.
Even if our common stock will in the future trade more actively on the OTC Marketplace, the price of such common stock could be subject to wide fluctuations, in response to quarterly variations in our operating results, announcements by us or others, developments affecting us, and other events or factors. In addition, the stock market has experienced extreme price and volume fluctuations in recent years. These fluctuations have had a substantial effect on the market prices for many companies, often unrelated to the operating performance of such companies, and may adversely affect the market prices of the securities. Such risks could have an adverse effect on the stock’s future liquidity.
| 5 |
| Table of Contents |
Because we became public by means of a “reverse merger,” we may not be able to attract the attention of major brokerage firm or investors in general.
Additional risks may exist because we became a public company through a “reverse merger.” Securities analysts of major brokerage firms may not provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of our company in the future. In addition, the SEC has recently issued an investor bulletin warning to investors about the risks of investing in companies that enter the U.S. capital markets through a “reverse merger.” The release of such information from the SEC may have the effect of reducing investor interest in companies, such as us, that enter the U.S. capital markets through a “reverse merger.”
We cannot assure you that our common stock will become eligible for listing or quotation on any exchange and the failure to do so may adversely affect your ability to dispose of our common stock in a timely fashion.
In order for our common stock to become eligible for listing or quotation on any exchange, reverse merger companies must have had their securities traded on an over-the-counter market for at least one year, maintained a certain minimum closing price for not less than 30 of the most recent 60 days prior to the filing of an initial listing application and prior to listing, and timely filed with the SEC all required reports since consummation of the reverse merger, including one annual report containing audited consolidated financial statements for a full fiscal year commencing after the date of filing of the Current Report on Form 8-K which discloses the reverse merger. We may not be able to meet all of the filing requirements above and may not be able to satisfy the initial standards for listing or quotation on any exchange in the foreseeable future or at all. Even if we are able to become listed or quoted on an exchange, we may not be able to maintain a listing of the common stock on such stock exchange.
Our common stock may be subject to penny stock rules, which may make it more difficult for our stockholders to sell their common stock.
Broker-dealer practices in connection with transactions in “penny stocks” are regulated by certain penny stock rules adopted by the SEC. Penny stocks generally are equity securities with a price of less than $5.00 per share. The penny stock rules require a broker-dealer, prior to a purchase or sale of a penny stock not otherwise exempt from the rules, to deliver to the customer a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, the penny stock rules generally require that prior to a transaction in a penny stock the broker-dealer make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules.
FINRA sales practice requirements may also limit a stockholder’s ability to buy and sell our stock.
In addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
We do not anticipate paying any cash dividends.
We presently do not anticipate that we will pay any dividends on any of our capital stock in the foreseeable future. The payment of dividends, if any, would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any dividends will be within the discretion of our Board of Directors (the “Board”). We presently intend to retain all earnings, if any, to implement our business plan; accordingly, we do not anticipate the declaration of any dividends in the foreseeable future.
| 6 |
| Table of Contents |
We may need additional capital, and the sale of additional shares or other equity securities, including our Class B common stock, could result in additional dilution to our stockholders.
We expect our existing cash will be sufficient to fund our capital requirements through the third quarter of 2021 , and we current plan to raise additional capital in the f ourth quarter of 2021 . In addition, we require additional capital for the development and commercialization of our product candidates and may require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our resources are insufficient to satisfy our cash requirements, we will seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities could result in additional dilution to our stockholders. In addition, you may experience further dilution to the extent that shares of our Class B common stock are issued and sold to raise capital . As of Ju ly 12, 2021, we have authorized 3,750,000 shares of Class B common stock, at a par value of $0.001 per share, of which 4,447 shares of Class B common stock were issued and outstandin g. See “Description of Securities-Com mon Stock ” . The incurrence of additional indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
Our dual-class voting structure will limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A common stock may view as beneficial.
Our authorized common stock is d ivided into Class A common stock and Class B common stock. Holders of Class A common stock are entitled to one vote per share, while holders of Class B common stock are entitled to five votes per share. Each share of Class B is convertible into one share of Class A common stock upon notice of the holder , while Class A common stock is not convertible into Class B common stock under any circumstances. As of Ju ly 12, 2021, we have authorized (i) 1,000,000,000 shares of Class A common stock, of which 118,177,885 shares were issued and outstanding, and (ii) 3,750,000 shares of Class B common stock, of which 4,447 shares were issued and outstanding . See “Description of Securities-Common Stock”. After this offering, the holder of Class A common stock will have the ability to control matters requiring shareholders’ approval, including any amendment of our memorandum and articles of association. However, an y futur e issuances of Class B common stock may be dilutive to the voting power of holders of Class A common stock . Any conversions of Class B common stock into Class A common stock may dilute the percentage ownership of the existing holders of Class A common stock within their class of ordinary shares.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Certain of our executive officers, directors and large stockholders own a significant percentage of our outstanding capital stock. As of July 12, 2021, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates owned approximately 80.4% of our outstanding shares of common stock. Accordingly, our directors and executive officers have significant influence over our affairs due to their substantial ownership coupled with their positions on our management team and have substantial voting power to approve matters requiring the approval of our stockholders. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This concentration of ownership may prevent or discourage unsolicited acquisition proposals or offers for our common stock that some of our stockholders may believe is in their best interest.
We have a substantial number of authorized common shares available for future issuance that could cause dilution of our stockholders’ interest and adversely impact the rights of holders of our common stock.
We have a total of 1,000,000,000 shares of Class A common stock and 3,750,000 shares of Class B common stock authorized for issuance and up to 5,000,000 shares of preferred stock with the rights, preferences and privileges that our Board may determine from time to time. As of July 12, 2021, we had 118,177,885 shares of issued and outstanding Class A common stock, 4,447 shares of issued and outstanding Class B common stock, and no outstanding preferred stock. We may seek financing that could result in the issuance of additional shares of our capital stock and/or rights to acquire additional shares of our capital stock. We may also make acquisitions that result in issuances of additional shares of our capital stock. Those additional issuances of capital stock would result in a significant reduction of your percentage interest in us. The addition of a substantial number of shares of our common stock into the market or by the registration of any of our other securities under the Securities Act of 1933, as amended (the “Securities Act”), may significantly and negatively affect the prevailing market price for our common stock.
| 7 |
| Table of Contents |
Risks Related to our Business and Industry
The commercial success of our products depends upon the degree of their market acceptance among the medical community. If our products do not attain market acceptance among the medical community, our operations and profitability would be adversely affected.
The commercial success of our products depends, in large part, upon the degree of market acceptance they achieve among the medical community, particularly among physicians, pharmacists, administrators of hospitals, clinics and other health care institutions. Physicians may not prescribe or recommend our products to patients and pharmacies, procurement departments of hospitals, clinics and other health care institutions may not purchase our products if physicians or pharmacists do not find our products attractive. The acceptance and use of our products among the medical community will depend upon a number of factors including:
| · | perceptions by physicians, pharmacists, patients and others in the medical community about the safety and effectiveness of our products; | |
|
| · | the prevalence and severity of any side effects; |
|
| · | pharmacological benefit of our products relative to competing products and products under development; |
|
| · | the efficacy and potential advantages relative to competing products and products under development; |
|
| · | relative convenience and ease of administration; |
|
| · | effectiveness of our education, marketing and distribution efforts; |
|
| · | publicity concerning our products or competing products and treatments; and |
|
| · | the price for our products and competing products. |
If our products fail to attain market acceptance among the medical community, or if our currently marketed products cannot maintain market acceptance, our results of operations and profitability would be adversely affected.
We have a history of losses and may continue to incur losses in the future, which raises substantial doubt about our ability to continue as a going concern.
We have a history of losses and may continue to incur losses in the future, which could negatively impact the trading value of our common stock. We may continue to incur operating losses in future periods. These losses may increase and we may never achieve or sustain profitability on a quarterly or annual basis in the future for a variety of reasons, including increased competition, decreased growth in the automotive industry and other factors described elsewhere in this “Risk Factors” section.
Further, we may incur significant losses in the future due to unforeseen expenses, difficulties, complications and delays and other unknown events. If we cannot continue as a going concern, our stockholders may lose their entire investment.
These circumstances raise substantial doubt about our ability to continue as a going concern as described in an explanatory paragraph to our independent registered public accounting firm’s report on our audited financial statements as of and for the year ended December 31, 2021. If we are unable to continue as a going concern, investors will likely lose all of their investment in our company. The consolidated financial statements included in this report do not include any adjustments that might result from the outcome of this uncertainty.
| 8 |
| Table of Contents |
Our proprietary, next-generation stem cell derived technologies, our approach for stem cell storage facilities and our manufacturing platform for our stem cell based products, represent emerging approaches to medical treatments that face significant challenges and hurdles.
We currently have two commercialized products: respiratory atomization products and cell basidiomycetes compound drink. We plan to market and commercialize our other planned light application products soon. Because stem cell-based healthcare products and therapies represent a relatively new field of, developing and commercializing our products subjects us to a number of risks and challenges, including:
| • | obtaining regulatory approval for our products, as the regulatory authorities may have limited experience with stem cell based healthcare products and therapies; | |
|
| • | developing and deploying consistent and reliable processes for engineering a customer’s stem cells ex vivo and infusing the engineered stem cells back into the patient; |
|
| • | sourcing clinical and, if approved, commercial supplies of the materials used to manufacture our product candidates; |
|
| • | developing programming modules with the desired properties, while avoiding adverse reactions; |
|
| • | developing a reliable and consistent vector and cell manufacturing process; |
|
| • | establishing manufacturing capacity suitable for the manufacture of our products; |
|
| • | developing protocols for the safe administration of our products; |
|
| • | educating medical personnel regarding our stem cell technologies and the potential side effect profile of our products; |
|
| • | establishing sales and marketing capabilities to successfully launch and commercialize our product candidates if and when we obtain any required regulatory approvals, and risks associated with gaining market acceptance of a novel therapy if we receive approval; and |
|
| • | the availability of coverage and adequate reimbursement from third-party payors for our novel and personalized therapies in connection with commercialization of any approved products. |
We may not be able to successfully develop our stem cell derived products, our technology or our other products in a manner that will yield products that are safe, effective, scalable or profitable.
We may not be able to successfully create our own manufacturing infrastructure and stem cell storage facilities for supply and maintenance of our requirements of programmed stem cell products for use in clinical trials and for commercial sale.
We currently have manufacturing and storage facilities in China supplying clinical materials for our trials and commercial production through agreements with third parties. We intend to expand the capacities at these sites as we begin to expand the production of our products. We are also in the process of establishing manufacturing capability in Beijing, which will provide a regional product supply as well as add to our global manufacturing ability.
Our manufacturing and commercialization strategy is based on establishing a fully integrated vein-to-vein product delivery cycle. Over time, we expect to establish regional or zonal manufacturing hubs to service major markets to meet projected needs for commercial sale quantities. However, we are still in the process of constructing manufacturing and storage facilities that will allow us to meet commercial sale quantities.
The implementation of this plan is subject to many risks. For example, the establishment of a stem cell-therapy manufacturing facility is a complex endeavor requiring knowledgeable individuals. Expanding our internal manufacturing infrastructure will rely upon finding personnel with an appropriate background and training to staff and operate the facility. Should we be unable to find these individuals, we may need to rely on external contractors or train additional personnel to fill the needed roles. There are a small number of individuals with experience in stem cell therapy and the competition for these individuals is high.
We expect that operating our own commercial stem cell manufacturing and storage facilities will provide us with enhanced control of material supply for both clinical trials and the commercial market, enable the more rapid implementation of process changes, and allow for better long-term cost margins. However, we have limited experience as a company in designing and operating a commercial manufacturing and storage facility and may never be successful in developing our own manufacturing capability. We may establish additional manufacturing and storage sites as we expand our commercial footprint to multiple geographies, which may lead to regulatory delays or prove costly. Even if we are successful, our operations could be affected by cost-overruns, unexpected delays, equipment failures, labor shortages, natural disasters, power failures and numerous other factors, or we may not be successful in establishing sufficient capacity to produce our product candidates in sufficient quantities to meet the requirements for the potential launch or to meet potential future demand, all of which could prevent us from realizing the intended benefits of our manufacturing strategy and have a material adverse effect on our business.
| 9 |
| Table of Contents |
We may not be able to timely identify or otherwise effectively respond to changing customer preferences, and we may fail to optimize our product offering and inventory position.
The medical industry in China is rapidly evolving and is subject to rapidly changing customer preferences that are difficult to predict. We believe that our success depends on our ability to anticipate and identify customer preferences and adapt our product selection to these preferences. In particular, we believe that we must optimize our product selection and inventory positions based on sales trends. No assurances can be given that our product selection, especially our selections of nutritional supplements and food products, will accurately reflect customer preferences at any given time. If we fail to anticipate accurately either the market for our products or customers’ purchasing habits or fail to respond to customers’ changing preferences promptly and effectively, we may not be able to adapt our product selection to customer preferences or make appropriate adjustments to our inventory positions, which could significantly reduce our revenue and have a material adverse effect on our business, financial condition and results of operations.
We face significant competition, and if we do not compete successfully against existing and new competitors, our revenue and profitability would be materially and adversely affected.
The medical industry in China is highly competitive, and we expect competition to intensify. In addition, there is a trend towards consolidation of the medical industry in the future. Our primary competitors are other medical distributors. We compete for customers and revenue primarily on the basis of product selection, price, and timely delivery of products. Moreover, we may be subject to additional competition from new entrants to the medical industry in China. If the PRC government removes the barriers for foreign companies to operate majority-owned medical distributors in China, we could face increased competition from foreign companies. Some of our larger competitors may enjoy competitive advantages, such as:
| · | greater financial and other resources; | |
| · | larger variety of products; | |
| · | more extensive and advanced supply chain management systems; | |
| · | greater pricing flexibility; | |
| · | larger economies of scale and purchasing power; | |
| · | more extensive advertising and marketing efforts; | |
| · | greater knowledge of local market conditions; | |
| · | larger sales and distribution networks. |
As a result, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, market our products as effectively as our competitors or otherwise respond successfully to competitive pressures. In addition, our competitors may be able to offer larger discounts on the same or competing products, and we may not be able to profitably match those discounts. Furthermore, our competitors may offer products that are more attractive to our customers or that render our products uncompetitive. Our failure to compete successfully could materially and adversely affect our business, financial condition, results of operation and prospects.
| 10 |
| Table of Contents |
We rely on third-party manufacturers to supply all of our light application products.
We rely on third-party manufactures to supply all of our light application products. Reliance on such third-party manufacturers involves a number of risks, including a lack of control over the manufacturing process and the potential absence or unavailability of adequate capacity. If any of our third-party manufacturers cannot or will not manufacture our products in required volumes in compliance with applicable regulations, on a cost-effective basis, in a timely manner, or at all, we will have to secure alternative manufacturers. Maintaining relationships with existing manufacturers and replacing such manufacturers may be difficult and time consuming. Any disruption of our network of manufacturers, including failure to renew existing distribution agreements with desired manufacturers, could negatively affect our product selection and our ability to effectively sell our products and could materially and adversely affect our business, financial condition and results of operations .
Our certificates, permits, and licenses related to our business are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
We are subject to various PRC laws and regulations pertaining to the medical industry. Although we do not currently own any licenses or permits for the production of our light application products, we are permitted under the PRC law to rely on the certificates, permits, and licenses that belong to and are maintained by our third-party manufacturers to market and sell our light application products. We are also in the process of applying for a product license for one of our subsidiaries located in Chengdu, China. In the event that we, or our third-party manufacturers, are not able to meet any new requirements imposed on our business by the appropriate regulatory authorities or are unable to renew our certificates, permits and licenses, all or part of our operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of our operations, it may adversely affect our operation and profitability.
If we are unable to protect our intellectual property from infringement, our business and prospects may be harmed.
As sales of our private label products increasingly account for a substantial portion of our revenue, we consider our brand name, trade names and trademarks to be valuable assets. Under PRC law, we have the exclusive right to use a trademark for products for which such trademark has been registered with the PRC Trademark Office of State Administration for Industry and Commerce (“SAIC”). In addition, no assurances can be given that we will be able to obtain any trademarks for which we may apply in the future.
Moreover, we may be unable to prevent third parties from using our brand name or trademarks without authorization and we may not have adequate remedies for such violations. Unauthorized use of our brand name or trademarks by third parties may adversely affect our business and reputation, including the perceived quality and reliability of our products.
We also currently own two invention patents in the PRC, and we rely on trade secrets to protect our know-how and other proprietary information, including pricing, purchasing, promotional strategies, customer lists and/or suppliers lists. However, i t is often difficult to register, maintain and enforce intellectual property rights in the PRC . Confidentiality, invention assignment and non-compete agreements may be breached by counterparties, and there may not be adequate remedies available to us for any such breach. Accordingly, we may not be able to effectively protect our intellectual property rights or to enforce our contractual rights in the PRC . Policing any unauthorized use of our intellectual property is difficult and costly and the steps we take may be inadequate to prevent the infringement or misappropriation of our intellectual property. In the event that we resort to litigation to enforce our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and financial resources, and could put our intellectual property at risk of being invalidated or narrowed in scope. We can provide no assurance that we will prevail in such litigation, and even if we do prevail, we may not obtain a meaningful recovery. In addition, our trade secrets may be leaked or otherwise become available to, or be independently discovered by, our competitors. Any failure in maintaining, protecting or enforcing our intellectual property rights could have a material adverse effect on our business, financial condition and results of operations.
In addition, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors or advisors may unintentionally or willfully disclose our information to competitors. In addition, confidentiality agreements, if any, executed by the foregoing persons may not be enforceable or provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts could be expensive and time-consuming, and the outcome is unpredictable. In addition, if our competitors independently develop information that is equivalent to our trade secrets or other proprietary information, it will be even more difficult for us to enforce our rights and our business and prospects could be harmed. Litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the intellectual property rights of others. However, because the validity, enforceability and scope of protection of intellectual property rights in the PRC are uncertain and still evolving, we may not be successful in prosecuting these cases. In addition, any litigation or proceeding or other efforts to protect our intellectual property rights could result in substantial costs and diversion of our resources and could seriously harm our business and operating results. Furthermore, the degree of future protection of our proprietary rights is uncertain and may not adequately protect our rights or permit us to gain or keep our competitive advantage. If we are unable to protect our trade names, trademarks, trade secrets and other propriety information from infringement, our business, financial condition and results of operations may be materially and adversely affected.
| 11 |
| Table of Contents |
We may acquire other businesses, license rights to products or form alliances with third-parties, which could cause us to incur significant expenses and could negatively affect our profitability.
We may pursue acquisitions, licensing arrangements, and strategic alliances, as part of our business strategy. We may not complete these transactions in a timely manner, on a cost-effective basis, or at all, and may not realize the expected benefits. If we are successful in making an acquisition, the products that are acquired may not be successful or may require significantly greater resources and investments than originally anticipated. We may not be able to integrate acquisitions successfully into our existing business and could incur or assume significant debt and unknown or contingent liabilities. This may result in increased borrowing costs and interest expense.
We have identified material weaknesses in our internal control over financial reporting, and we cannot assure you that additional material weaknesses or significant deficiencies will not occur in the future. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results or prevent fraud, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price .
We have historically had a small internal accounting and finance staff with limited experience in public reporting. This lack of adequate accounting resources has resulted in the identification of material weaknesses in our internal controls over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our consolidated financial statements will not be prevented or detected on a timely basis. As of March 31, 2021, our management team identified material weaknesses relating to, among other matters:
|
| · | We have insufficient quantity of dedicated resources and experienced personnel involved in reviewing and designing internal controls. As a result, a material misstatement of the interim and annual financial statements could occur and not be prevented or detected on a timely basis. |
|
|
|
|
|
| · | We do not have an audit committee. While not being legally obligated to have an audit committee, it is the management’s view that to have an audit committee, comprised of independent board members, is an important entity-level control over our financial statements. |
|
|
|
|
|
| · | We did not perform an entity level risk assessment to evaluate the implication of relevant risks on financial reporting, including the impact of potential fraud-related risks and the risks related to non-routine transactions, if any, on our internal control over financial reporting. Lack of an entity-level risk assessment constituted an internal control design deficiency which resulted in more than a remote likelihood that a material error would not have been prevented or detected, and constituted a material weakness. |
We have taken steps, including implementing a plan to seek to remediate these material weaknesses and to improve our financial reporting systems and implement new policies, procedures and controls. If we do not successfully remediate the material weaknesses described above, or if other material weaknesses or other deficiencies arise in the future, we may be unable to accurately report our financial results on a timely basis, which could cause our reported financial results to be materially misstated and require restatement which could result in the loss of investor confidence, delisting and/or cause the market price of our common stock to decline .
Our executive officers and directors have existing responsibilities and, in the future, may have additional responsibilities, to provide management and services to other entities in addition to us. As a result, conflicts of interest between us and the other activities of those persons may occur from time to time and could have an adverse effect on us .
Certain of o ur executive officers and directors have existing responsibilities and, in the future, may have additional responsibilities, to provide management and services to other entities in addition to us. As a result, conflicts of interest between us and the other activities of those persons may occur from time to time , including that:
|
| · | we may enter into contracts between us, on the one hand, and other entities for which our executive dire ctors or officers hold positions , on the other, that are not the result of arm’s-length transactions; |
|
|
|
|
|
| · | our executive officers and directors that hold positions of responsibility with other entities may be aware of certain business opportunities that are appropriate for presentation to us as well as to such other entities and may present such business opportunities to such other entities ; and |
|
|
|
|
|
| · | our executive officers and directors that hold positions of responsibility with other entities may have significant duties with, and spend significant time serving, other entities and may have conflicts of interest in allocating time. |
We will attempt to resolve any such conflicts of interest in our favor. Our officers and directors are accountable to us and our shareholders as fiduciaries, which requires that such officers and directors exercise good faith and integrity in handling our affairs. Nevertheless, such conflicts could cause an individual in our management to seek to advance his or her economic interests or the economic interests of certain related parties above ours. Further, the appearance of conflicts of interest created by related party transactions could impair the confidence of our investors. It is possible that a conflict of interest could have a material adverse effect on our liquidity, results of operations and financial condition.
| 12 |
| Table of Contents |
Risks Related to our Corporate Structure
Transactions among our affiliates are subject to scrutiny by the PRC tax authorities and a finding that we or any of our consolidated entities owe additional taxes could have a material adverse impact on our net income and the value of an investment in our common stock.
Under PRC law, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities. If any of the transactions we have entered into among our consolidated entities are challenged by the PRC tax authorities to be not on an arm’s-length basis, or to result in an unreasonable reduction in our PRC tax obligations, the PRC tax authorities have the authority to disallow our tax deduction claims, adjust the profits and losses of our respective PRC consolidated entities and assess late payment fees and other penalties. Our net income may be materially reduced if our tax liabilities increase or if we are otherwise assessed late payment fees or other penalties.
We currently conduct our business primarily through a contractually controlled PRC operating entity, and our control of the day-to-day operations of such PRC entity pursuant to contracts, to comply with Chinese law, may not be as effective as conducting business through direct equity ownership of such PRC entity due to uncertainties with respect to the PRC legal system which could materially and adversely affect our results of operations.
We currently conduct a substantial portion of our business primarily through our contractually controlled PRC operating entity. PRC laws and regulations govern our operations in the PRC. Our contractually controlled PRC operating entity is generally subject to laws and regulations applicable to foreign investments in the PRC and, in particular, laws applicable to wholly foreign-owned enterprises (“WFOEs”). Although members of our executive management team and our shareholders include the executive officers and owners of our contractually controlled PRC operating entity, because we do not directly own our contractually controlled PRC operating entity, we may encounter problems enforcing our rights to control the business affairs and day-to-day operations of such entity. If we find it necessary to take legal action in the PRC to enforce our rights under our contracts with the PRC operating entity, we will be subject to the uncertainties of the PRC legal system, where prior court decisions have limited precedential value. Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in PRC. However, the PRC has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in the PRC. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their non-binding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation, if any, of these policies and rules until sometime after the violation. In addition, any litigation in the PRC, regardless of outcome, may be protracted and result in substantial costs and diversion of resources and management attention. Accordingly, notwithstanding our contractual control over our PRC operating entity, such control may not be as effective as if we conducted our business through direct equity owned PRC entities which could materially and adversely affect our results of operations.
| 13 |
| Table of Contents |
Our contractual arrangements with Yubo and its shareholders may not be as effective in providing control over these entities as direct ownership.
We have no equity ownership interest in Yubo as we rely on the contractual arrangements of the VIE agreements to control and operate Yubo. These contractual arrangements may not be as effective in providing control over Yubo as direct ownership. For example, Yubo could fail to take actions required for our business or fail to pay dividends to Yubo WFOE despite its contractual obligation to do so. If Yubo fails to perform its obligation under the VIE agreements, we may have to rely on legal remedies under PRC law, which may not be effective.
If the PRC government finds that the contractual arrangements that establish the structure for operating our business in China do not comply with PRC laws and regulations, or if these regulations or their interpretations change in the future, we could be subjected to severe consequences, including the nullification of such agreements and the relinquishment of our interest in our VIE.
Current PRC laws and regulations impose certain restrictions or prohibitions on foreign ownership of companies that engage in medical institutions which our stem cell bank relates to, and in the development and application of technologies for diagnosis and treatment of human stem cells and genes, which our stem cell bank and endometrial stem cell bank business relates to. Pursuant to the Special Administrative Measures (Negative List) issued by the NDRC and MOFCOM on June 23, 2020, which came into force on July 23, 2020, foreign investment are allowed in PRC medical institutions only through joint venture entities, and the foreign shareholding in these entities is limited to 70.0%, which percentage was stipulated in the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions, or the JV Interim Measures. Additionally, certain industries are specifically prohibited for foreign investment, including the development and application of technologies for diagnosis and treatment of human stem cells and genes.
Considering the foreign investment restrictions that may be applicable to our business, we conduct our stem cell bank and endometrial stem cell bank business in China through VIE. The VIE structure through contractual arrangements has been adopted by many PRC-based companies, including us, such that if our businesses are determined to be subject to the foreign investment restrictions, we can obtain necessary license, for example, the Practice License of Medical Institutions, in the industry currently subject to foreign investment restrictions in China.
On March 15, 2019, the Foreign Investment Law was formally passed by the thirteenth National People’s Congress and it became effective on January 1, 2020. The Foreign Investment Law replaced the Law on Sino-Foreign Equity Joint Ventures, the Law on Sino-Foreign Cooperative Joint Ventures and the Law on Foreign-Capital Enterprises and became the legal foundation for foreign investment in the PRC. The Foreign Investment Law stipulates certain forms of foreign investment and defines enterprises incorporated within PRC with complete or partial foreign investment as foreign-invested enterprises or “FIEs”. However, the Foreign Investment Law does not explicitly stipulate contractual arrangements such as those we rely on as a form of foreign investment.
The 2019 PRC Foreign Investment Law further specifies that foreign investments shall be conducted in line with the "negative list" to be issued by or approved to be issued by the State Council. This means that an FIE would not be allowed to make investments in prohibited industries in the "negative list," while the FIE must satisfy certain conditions stipulated in the "negative list" for investment in restricted industries. As pursuant to the negative list issued by the NDRC and MOFCOM taking effect on July 23, 2020, medical services is a restricted industry and foreign investment are allowed in PRC medical institutions only through joint venture entities, i.e., an FIE in which foreign ownership is limited to 70.0%. Additionally, certain industries are specifically prohibited for foreign investment, including the development and application of technologies for diagnosis and treatment of human stem cells and genes, which means, pursuant to the Foreign Investment Law, FIEs are prohibited from practicing such businesses in China.
Considering the above, Yubo International Biotech Limited, Platinum and Platinum HK are considered as foreign investors under the 2019 PRC Foreign Investment Law, Yubo WOFE is deemed as an FIE. Accordingly, none of such entities are eligible to provide such restricted services related to our businesses, such as medical services. As a result, we will conduct such business activities through our variable interest entity, Yubo Beijing. Since Yubo Beijing is not an FIE, Yubo Beijing will be able to apply and hold license as medical institution as other PRC companies and provide medical services which are otherwise restricted to FIEs.
Notwithstanding the above, the Foreign Investment Law stipulates that foreign investment includes “foreign investors investing through any other methods under laws, administrative regulations or provisions prescribed by the State Council.” Future laws, administrative regulations or provisions prescribed by the State Council may possibly regard contractual arrangements as a form of foreign investment. If this happens, it is uncertain whether our contractual arrangements with our VIE, its subsidiaries and shareholders would be recognized as foreign investment, or whether our contractual arrangements would be deemed to be in violation of the foreign investment access requirements. Therefore, there is no guarantee that our contractual arrangements, the business of our VIE will not be adversely affected.
To comply with PRC laws and regulations, we conduct our stem cell bank and endometrial stem cell bank business in China through VIE. We, through Yubo WFOE, our wholly owned subsidiary in China, entered into a series of contractual arrangements with our VIE and its ultimate shareholders, in order to (i) exercise effective control over our VIE, (ii) receive substantially all of the economic benefits of our VIE, and (iii) have an exclusive option to purchase all or part of the equity interests in our VIE when and to the extent permitted by PRC law. As a result of these contractual arrangements, we have control over and are the primary beneficiary of our VIE and hence consolidate its financial results under GAAP. Although the structure we have adopted is consistent with long-standing practice in certain industries, and is also adopted by some of our peers in China, the PRC government may not agree that these arrangements comply with PRC license, registration or other regulatory requirements, with existing policies, or with requirements or policies that may be adopted in the future. Our VIE hold the licenses, approvals and key assets that are essential for the operations of our precision oncology service businesses.
| 14 |
| Table of Contents |
We believe: (i) the ownership structures of our VIE in China, currently do not, and immediately after giving effect to this offering, will not result in any violation of the applicable PRC laws or regulations currently in effect, and (ii) subject to the risks as disclosed in the section headed “Risk Factors—Risks Relating to Our Corporate Structure”, the contractual arrangements between WFOE, our VIE and its respective equity holders governed by PRC laws are valid, binding and enforceable in accordance with their terms and applicable PRC laws and regulations currently in effect and do not violate any applicable PRC laws, rule or regulation currently in effect. There are, however, substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulations. The relevant PRC regulatory authorities have broad discretion in determining whether a particular contractual structure violates PRC laws and regulations. Thus, we cannot assure you that the PRC government will ultimately take a view that our VIE structure does not violate PRC laws or regulations. If we are found in violation of any PRC laws or regulations or if the contractual arrangements among WFOE, our VIE and its respective equity holders are determined as illegal or invalid by any PRC court, arbitral tribunal or regulatory authorities, the relevant governmental authorities would have broad discretion in dealing with such violation, including, without limitation:
|
| · | revoking the agreements constituting the contractual arrangements; |
|
| · | revoking our business and operating licenses; |
|
| · | requiring us to discontinue or restrict operations; |
|
| · | restricting our right to collect revenue; |
|
| · | shutting down all or part of our websites or services; |
|
| · | levying fines on us and/or confiscating the proceeds that they deem to have been obtained through non-compliant operations; |
|
| · | requiring us to restructure the operations in such a way as to compel us to establish a new enterprise, re-apply for the necessary licenses or relocate our businesses, staff and assets; |
|
| · | imposing additional conditions or requirements with which we may not be able to comply; |
|
| · | restricting or prohibiting our use of proceeds from public offering or other financing activities to finance our business and operations in China; or |
|
| · | taking other regulatory or enforcement actions that could be harmful to our business. |
Furthermore, any of the assets under the name of any record holder of equity interest in VIE, including such equity interest, may be put under court custody in connection with litigation, arbitration or other judicial or dispute resolution proceedings against that record holder. We cannot be certain that the equity interest will be disposed of in accordance with the contractual arrangements. In addition, new PRC laws, rules and regulations may be introduced to impose additional requirements that may impose additional challenges to our corporate structure and contractual arrangements. The occurrence of any of these events or the imposition of any of these penalties may result in a material and adverse effect on our ability to conduct our precision oncology service business. In addition, if the imposition of any of these penalties causes us to be unable to direct the activities of such VIE and its subsidiaries or the right to receive their economic benefits, we would no longer be able to consolidate such VIE into our financial statements, thus adversely affecting our results of operation.
Risks Related to Doing Business in China
The medical industry in China is highly regulated and such regulations are subject to change which may affect approval and commercialization of our drugs.
A material portion of our research and development operations and manufacturing facilities are in China, which we believe confers clinical, commercial and regulatory advantages. The medical industry in China is subject to comprehensive government regulation and supervision, encompassing the approval, registration, manufacturing, packaging, licensing and marketing of new drugs. See “Business—Government Regulation—PRC Regulation” for a discussion of the regulatory requirements that are applicable to our current and planned business activities in China. For example, under PRC law, before we enter into a clinical trial agreement with a PRC partner, the parties are required to obtain an approval for projects of international collaboration in respect of human genetic resources in order to utilize genetic material contained in biological samples collected from Chinese human subjects. Furthermore, under relevant PRC laws, a license for use of laboratory animals is required for performing experimentation on animals. Any failure of fully comply with such requirement may result in the invalidation of our experimental data. In recent years, the regulatory framework in China regarding the medical industry has undergone significant changes, and we expect that it will continue to undergo significant changes. Any such changes or amendments may result in increased compliance costs on our business or cause delays in or prevent the successful development or commercialization of our products in China and reduce the current benefits we believe are available to us from developing and manufacturing drugs in China. PRC authorities have become increasingly vigilant in enforcing laws in the medical industry and any failure by us or our partners to maintain compliance with applicable laws and regulations or obtain and maintain required licenses and permits may result in the suspension or termination of our business activities in China. We believe our strategy and approach are aligned with the PRC government’s regulatory policies, but we cannot ensure that our strategy and approach will continue to be aligned.
We face risks associated with uncertainties relating to Regulation for the Administration of Human Genetic Resources.
The collection, preservation, usage and outbound provision of human genetic resources in the PRC are governed by Regulation for the Administration of Human Genetic Resources, or HGR Regulation, except for activities relating to human genetic resources conducted for some specific purposes including clinical diagnosis and treatment. We believe that our stem cell bank is both for the purpose of clinical diagnosis and treatment, so that such activities relating to human genetic resources in our diagnosis business or early screening business may not be governed by HGR Regulation. However, we cannot assure you that our stem cell bank will be continuously deemed as conducted for the purpose of clinical diagnosis and treatment by the relevant government authority. Meanwhile, our endometrial stem cell bank in our development services are governed by HGR Regulation.
Pursuant to HGR Regulation, there are some limitations for foreign entities, individuals and such entities established or actually controlled thereby (“Restricted Entities”, and each, a “Restricted Entity”) to engage in activities relating to human genetic resources. For example, the Restricted Entity is not allowed to collect or preserve human genetic resources of China, while it is prohibited from using human genetic resources of China unless that such Restricted Entity have obtained an approval from relevant government authority or have filed with relevant government authority for international cooperation with a domestic entity. Taking into consideration of our consultation with a competent government authority, among others, although an entity controlled, directly or indirectly, by foreign persons through shareholding ownership would be deemed as a Restricted Entity, HGR Regulation remains unclear as to whether a VIE entity controlled by a wholly foreign owned enterprise through contractual arrangements would be deemed as a Restricted Entity. We cannot assure you that our VIE entities will not be deemed as Restricted Entities in the future, given the lack of clear statutory interpretation regarding HGR Regulation. If our VIE entities engaging in development services are deemed as the Restricted Entities by relevant government authority, our cooperation with foreign entities, among others, would be adversely affected and we may have to cooperate with domestic entities and be required to obtain approvals or file with relevant government authority for such cooperation which could result in additional cost and our business, financial condition and results of operations will be adversely affected.
| 15 |
| Table of Contents |
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our management named in this Report based on foreign laws.
We conduct a material portion of our operations in China and a material portion of our assets are located in China. In addition, many of our senior executive officers and directors reside within China for a significant portion of the time and some of them are PRC nationals. As a result, it may be difficult for you to effect service of process upon us or those persons inside China. It may also be difficult for you to enforce in U.S. courts judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors. In addition, there is uncertainty as to whether the courts of the Cayman Islands or the PRC would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of written arrangement with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or the public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
It may be difficult for overseas regulators to conduct investigations or collect evidence within China.
It may be difficult for you or overseas regulators, such as the U.S., Department of Justice, the Commission, and other authorities of the United States, to conduct investigations or collect evidence within China. For example, in China, there are significant legal and other obstacles to obtaining information, documents and materials needed for regulatory investigations or litigation outside China or otherwise with respect to foreign entities. Although the authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the Unities States may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, according to Article 177 of the PRC Securities Law, which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no entity or individual may provide the documents and materials relating to securities business activities to overseas parties. While detailed interpretation of or implementing rules under Article 177 have yet to be promulgated, the inability for an overseas securities regulator to directly conduct investigation or evidence collection activities within China may further increase difficulties faced by you in protecting your interests.
Dividends we receive from our subsidiaries located in the PRC may be subject to PRC withholding tax, which could materially and adversely affect the amount of dividends, if any, we may pay our shareholders.
The PRC Enterprise Income Tax Law classifies enterprises as resident enterprises and non-resident enterprises. The PRC Enterprise Income Tax Law provides that an income tax rate of 20% may be applicable to dividends payable to non-resident investors, which (i) do not have an establishment or place of business in the PRC, or (ii) have an establishment or place of business in the PRC but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC. The State Council of the PRC reduced such rate to 10% through the implementation regulations of the PRC Enterprise Income Tax Law. Further, pursuant to the Double Tax Avoidance Arrangement between Hong Kong and Mainland China, or the Double Tax Avoidance Arrangement, and the Notice on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties issued in February 2009 by the State Administration of Taxation of the PRC, or the SAT, if a Hong Kong resident enterprise owns more than 25% of the equity interest in a company in China at all times during the 12-month period immediately prior to obtaining a dividend from such company, the 10% withholding tax on dividends is reduced to 5% provided that certain other conditions and requirements under the Double Tax Avoidance Arrangement and other applicable PRC laws are satisfied at the discretion of relevant PRC tax authority.
| 16 |
| Table of Contents |
If our Cayman Islands subsidiary and our Hong Kong subsidiary are considered as non-resident enterprises and our Hong Kong subsidiary is considered as a Hong Kong resident enterprise under the Double Tax Avoidance Arrangement and is determined by the competent PRC tax authority to have satisfied relevant conditions and requirements, then the dividends paid to our Hong Kong subsidiary by its PRC subsidiary may be subject to the reduced income tax rate of 5% under the Double Tax Avoidance Arrangement. However, based on the Notice on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties, if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment. In addition, based on the Announcement of the State Administration of Taxation on Issues Relating to Beneficial Owner in Tax Treaties, effective from April 1, 2018, under certain conditions a company cannot be defined as a beneficial owner under the treaty and thus are not entitled to the abovementioned reduced income tax rate of 5% under the Double Tax Avoidance Arrangement. If we are required under the PRC Enterprise Income Tax Law to pay income tax for any dividends we receive from our subsidiaries in China, or if our Hong Kong subsidiary is determined by PRC government authority as receiving benefits from reduced income tax rate due to a structure or arrangement that is primarily tax-driven, it would materially and adversely affect the amount of dividends, if any, we may pay to our shareholders.
If we are classified as a “resident enterprise” of China under the PRC Enterprise Income Tax Law, we and our non-PRC shareholders could be subject to unfavorable tax consequences, and our business, financial condition and results of operations could be materially and adversely affected.
Under the PRC Enterprise Income Tax Law and its implementation rules, an enterprise established outside the PRC with “de facto management body” within the PRC is considered a “resident enterprise” and will be subject to the enterprise income tax on its global income at the rate of 25%. The implementation rules define the term “de facto management body” as the body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In 2009, SAT issued a circular, known as SAT Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position on how the “de facto management body” text should be applied in determining the tax resident status of all offshore enterprises. According to SAT Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China and will be subject to PRC enterprise income tax on its global income only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in the PRC; and (iv) at least 50% of board members with voting rights or senior executives habitually reside in the PRC.
We believe that we are not a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.” If the PRC tax authorities determine that we are a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% tax from dividends we pay to our shareholders that are non-resident enterprises, including the holders of the common stock. In addition, non-resident enterprise shareholders, including our common stock holders, may be subject to PRC tax at a rate of 10% on gains realized on the sale or other disposition of common stock or ordinary shares, if such income is treated as sourced from within the PRC. Furthermore, if we are deemed a PRC resident enterprise, dividends paid to our non-PRC individual shareholders, including our common stock holders, and any gain realized on the transfer of common stock or ordinary shares by such shareholders may be subject to PRC tax at a rate of 20%, which in the case of dividends may be withheld at source. Any PRC tax liability may be reduced by an applicable tax treaty. However, it is unclear whether non-PRC shareholders of our company would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. Any such tax may reduce the returns on your investment in our common stock or ordinary shares.
In addition to the uncertainty as to the application of the “resident enterprise” classification, we cannot assure you that the PRC government will not amend or revise the taxation laws, rules and regulations to impose stricter tax requirements or higher tax rates. Any of such changes could materially and adversely affect our financial condition and results of operations.
| 17 |
| Table of Contents |
Governmental control of currency conversion may affect the value of your investment.
Currently, the RMB cannot be freely converted into any foreign currency. The PRC government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. Shortages in the availability of foreign currency may restrict the ability of our PRC subsidiary to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, for most capital account items, approval from or registration with appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The PRC government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our shareholders, including holders of the common stock.
Fluctuation in exchange rates could have a negative effect on our results of operations and the value of your investment.
The value of the RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions in China and by China’s foreign exchange policies. Since June 2010, the RMB has fluctuated against the U.S. dollar, at times significantly and unpredictably. On November 30, 2015, the Executive Board of the International Monetary Fund, or IMF, completed the regular five-year review of the basket of currencies that make up the Special Drawing Right, or the SDR, and decided that with effect from October 1, 2016, the RMB is determined to be a freely usable currency and will be included in the SDR basket as a fifth currency, along with the U.S. dollar, the euro, the Japanese yen and the British pound. Since the fourth quarter of 2016, the RMB has depreciated significantly in the backdrop of a surging U.S. dollar and persistent capital outflows of China. With the development of the foreign exchange market and progress toward interest rate liberalization and RMB internationalization, the PRC government may in the future announce further changes to the exchange rate system, and we cannot assure you that the RMB will not appreciate or depreciate significantly in value against the U.S. dollar in the future. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the RMB and the U.S. dollar in the future.
Very limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. As of the date of this Report, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currency or to convert foreign currency into RMB.
| 18 |
| Table of Contents |
PRC regulations relating to offshore investment activities by PRC residents and enterprises may increase our administrative burden and restrict our overseas and cross-border investment activity. If our PRC resident and enterprise shareholders fail to make any required applications and filings under such regulations, we may be unable to distribute profits to such shareholders and may become subject to liability under PRC law.
In July 2014, SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, which replaces the Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Round-tripping Investment via Overseas Special Purpose, or SAFE Circular 75. SAFE Circular 37 requires PRC residents, including PRC individuals and PRC corporate entities, to register with SAFE or its local branches in connection with their direct or indirect offshore investment activities. SAFE Circular 37 is applicable to our shareholders who are PRC residents and may be applicable to any offshore acquisitions that we may make in the future.
Under SAFE Circular 37, PRC residents who make, or have prior to the implementation of SAFE Circular 37 made, direct or indirect investments in offshore special purpose vehicles, or SPVs, are required to register such investments with SAFE or its local branches. In addition, any PRC resident who is a direct or indirect shareholder of an SPV, is required to update its registration with the local branch of SAFE with respect to that SPV, to reflect any change of basic information or material events. If any PRC resident shareholder of such SPV fails to make the required registration or to update the registration, the subsidiary of such SPV in China may be prohibited from distributing its profits or the proceeds from any capital reduction, share transfer or liquidation to the SPV, and the SPV may also be prohibited from making additional capital contributions into its subsidiaries in China. In February 2015, SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration Policy on Direct Investment, or SAFE Notice 13. Under SAFE Notice 13, applications for foreign exchange registration of inbound foreign direct investments and outbound direct investments, including those required under SAFE Circular 37, shall be filed with qualified banks instead of SAFE. Qualified banks should examine the applications and accept registrations under the supervision of SAFE.
We may not be aware of the identities of all of our beneficial owners who are PRC residents. To our knowledge, some of our beneficial owners have not complied with SAFE registration requirements under SAFE Circular 37 and subsequent implementation rules on time or at all, sometimes due to reasons beyond their control. However, we do not have control over our beneficial owners and cannot compel them to comply with SAFE Circular 37 and subsequent implementation rules. Therefore, we cannot assure you that any required registration under SAFE Circular 37 and any amendment will be completed in a timely manner, or at all. The failure of our beneficial owners who are PRC residents to register or amend their foreign exchange registrations pursuant to SAFE Circular 37 and subsequent implementation rules, or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in SAFE Circular 37 and subsequent implementation rules, may subject such beneficial owners or our PRC subsidiary to fines and legal sanctions. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to our PRC subsidiary and limit our PRC subsidiary’s ability to distribute dividends to us. These risks may have a material adverse effect on our business, financial condition and results of operations.
Furthermore, as these foreign exchange and outbound investment related regulations and their interpretation and implementation have been constantly evolving, it is unclear how these regulations, and any future regulation concerning offshore or cross-border investments and transactions, will be interpreted, amended and implemented by the relevant government authorities. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange activities, such as remittance of dividends and foreign-currency-denominated borrowings, which may adversely affect our financial condition and results of operations. We cannot assure you that we have complied or will be able to comply with all applicable foreign exchange and outbound investment related regulations. In addition, if we decide to acquire a PRC domestic company, we cannot assure you that we or the owners of such company, as the case may be, will be able to obtain the necessary approvals or complete the necessary filings and registrations required by the foreign exchange regulations. This may restrict our ability to implement our acquisition strategy and could adversely affect our business and prospects.
| 19 |
| Table of Contents |
The M&A Rules and certain other PRC regulations establish complex procedures for some acquisitions of PRC companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
The M&A Rules and relevant regulations and rules concerning mergers and acquisitions established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex. The M&A Rules require that the Ministry of Commerce, or the MOFCOM, be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise, if (i) any important industry is concerned, (ii) such transaction involves factors that have or may have an impact on the national economic security; or (iii) such transaction will lead to a change in control of a domestic enterprise which holds a famous trademark or PRC time-honored brand. The approval from MOFCOM shall be obtained in circumstances where overseas companies established or controlled by PRC enterprises or residents acquire affiliated domestic companies.
The Anti-Monopoly Law promulgated by the Standing Committee of the National People’s Congress, or NPC, which became effective in August 2008, requires that when a concentration of undertakings occurs and reaches statutory thresholds, the undertakings concerned shall file a prior notification with MOFCOM. Without the clearance from MOFCOM, no concentration of undertakings shall be implemented and effected. Mergers, acquisitions or contractual arrangements that allow one market player to take control of or to exert decisive impact on another market player must also be notified in advance to the MOFCOM when the threshold under the Provisions on Thresholds for Prior Notification of Concentrations of Undertakings, or the Prior Notification Rules, issued by the State Council in August 2008 is triggered. If such prior notification is not obtained, MOFCOM may order the concentration to cease its operations, dispose of shares or assets, transfer the business of the concentration within a time limit, take any other necessary measures to restore the situation as it was before the concentration, and may impose administrative fines.
In addition, the Implementing Rules Concerning Security Review on the Mergers and Acquisitions by Foreign Investors of Domestic Enterprises, issued by the MOFCOM in August 2011, specify that mergers and acquisitions by foreign investors involved in “an industry related to national security” are subject to strict review by the MOFCOM, and prohibit any activities attempting to bypass such security review, including by structuring the transaction through a proxy or contractual control arrangement. In the future, we may grow our business by acquiring complementary businesses. Complying with the requirements of the abovementioned regulations and other relevant rules to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the MOFCOM or its local counterparts may delay or inhibit our ability to complete such transactions.
We cannot preclude the possibility that the MOFCOM or other government agencies may publish explanations contrary to our understanding or broaden the scope of such security reviews in the future, in which case our future acquisitions in the PRC, including those by way of entering into contractual control arrangements with target entities, may be closely scrutinized or prohibited. Our ability to expand our business or maintain or expand our market share through future acquisitions would as such be materially and adversely affected.
We and our shareholders face uncertainty with respect to indirect transfers of equity interests in PRC resident enterprises, assets attributed to a PRC establishment of a non-PRC company or immovable properties located in China owned by non-PRC companies.
In February 2015, SAT issued a Public Notice Regarding Certain Corporate Income Tax Matters on Indirect Transfer of Properties by Non-Tax Resident Enterprises, or SAT Public Notice 7. SAT Public Notice 7 extends its tax jurisdiction to transactions involving transfer of other taxable assets through offshore transfer of a foreign intermediate holding company. In addition, SAT Public Notice 7 provides clear criteria for assessment of reasonable commercial purposes and has introduced safe harbors for internal group restructurings and the purchase and sale of equity through a public securities market. SAT Public Notice 7 also brings challenges to both foreign transferor and transferee (or other person who is obligated to pay for the transfer) of taxable assets. In October 2017, SAT issued the Announcement of the State Administration of Taxation on Issues Concerning the Withholding of Non-resident Enterprise Income Tax at Source, or SAT Bulletin 37, which came into effect on December 1, 2017. The Bulletin 37 further clarifies the practice and procedure of the withholding of nonresident enterprise income tax. Where a non-resident enterprise transfers taxable assets indirectly by disposing of the equity interests of an overseas holding company, which is an indirect transfer, the non-resident enterprise as either transferor or transferee, or the PRC entity that directly owns the taxable assets, may report such Indirect Transfer to the relevant tax authority. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such indirect transfer other than transfer of shares of our common stock acquired and sold on public markets may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes.
We face uncertainties as to the reporting and other implications of certain past and future transactions that involve PRC taxable assets, such as offshore restructuring, sale of the shares in our offshore subsidiaries and investments. Our company may be subject to filing obligations or taxed if our company is the transferor in such transactions, and may be subject to withholding obligations if our company is the transferee in such transactions, under SAT Public Notice 7 or Bulletin 37, or both.
| 20 |
| Table of Contents |
General Risk Factors
Because our management will have broad discretion and flexibility in how the net proceeds from this offering are used, we may use the net proceeds in ways in which you disagree.
We currently intend to use the net proceeds from this offering for general corporate purposes, including working capital. We have not allocated specific amounts of the net proceeds from this offering for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
We are subject to the reporting obligation and internal control requirements of federal securities laws, which is expensive and time-consuming .
We are a public reporting company in the United States and, accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders causes our expenses to be higher than they would be if we remained a privately-held company.
We are a reporting company with the SEC and therefore must comply with Sarbanes-Oxley Act and SEC rules concerning internal controls. It is time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. In order to expand our operations, we will need to hire additional financial reporting, internal control, and other finance staff in order to develop and implement appropriate internal controls and reporting procedures.
Volatility in our common stock price may subject us to securities litigation.
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
If securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by industry or financial analysts. If no or few analysts commence coverage of us, the trading price of our stock would likely decrease. Even if we do obtain analyst coverage, if one or more of the analysts who cover us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We are subject to critical accounting policies and actual results may vary from our estimates.
We follow generally accepted accounting principles in the United States in preparing our financial statements. As part of the preparation of such financial reports, we must make many estimates and judgments concerning future events, which affect the value of the assets and liabilities, contingent assets and liabilities, and revenue and expenses reported in our financial statements. We believe that these estimates and judgments are reasonable, and we make them in accordance with our accounting policies based on information available at the time. However, actual results could differ from our estimates, and this could require us to record adjustments to expenses or revenues that could be material to our financial position and results of operations in the future.
We may need additional capital and may not be able to obtain it on acceptable terms or at all, which could adversely affect our liquidity and financial position; the issuance of additional equity would result in dilution to our shareholders.
We may need to raise additional capital if our expenditures exceed our current expectations due to changed business conditions or other future developments. Our future liquidity needs and other business reasons may require us to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities or securities convertible or exchangeable to our equity securities would result in additional dilution to our stockholders. The incurrence of additional indebtedness would result in increased debt service obligations and could result in operating and financing covenants that restrict our operational flexibility. Our ability to raise additional funds in the future is subject to a variety of uncertainties, including:
| • | our future financial condition, results of operations and cash flows; | |
| • | general market conditions for capital-raising activities by medical companies; and | |
| • | economic, political and other conditions in China and elsewhere. |
No assurances can be given that we will be able to obtain additional capital in a timely manner or on commercially acceptable terms or at all.
| 21 |
| Table of Contents |
Statements in this prospectus that are not descriptions of historical facts are forward-looking statements that are based on management’s current expectations and assumptions and are subject to risks and uncertainties. If such risks or uncertainties materialize or such assumptions prove incorrect, our business, operating results, financial condition and stock price could be materially negatively affected. In some cases, you can identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would” or the negative of these terms or other comparable terminology. Factors that could cause actual results to differ materially from those currently anticipated include those set forth in the section titled “Risk Factors” including, without limitation, risks relating to:
| · | the results of our research and development activities relating, in particular, to stem cell related technologies; | |
| · | the early stage of our product candidates presently under development; | |
| · | our need for substantial additional funds in order to continue our operations, and the uncertainty of whether we will be able to obtain the funding we need; | |
| · | our ability to obtain and, if obtained, maintain regulatory approval of our current product candidates, and any of our other future product candidates, and any related restrictions, limitations, and/or warnings in the label of any approved product candidate; | |
| · | our ability to retain or hire key scientific or management personnel; |
| · | our ability to protect our intellectual property rights that are valuable to our business, including patent and other intellectual property rights; | |
| · | our dependence on third-party manufacturers, suppliers, research organizations, testing laboratories and other potential collaborators; | |
| · | our ability to develop successful sales and marketing capabilities in the future as needed; | |
| · | the size and growth of the potential markets for any of our approved product candidates, and the rate and degree of market acceptance of any of our approved product candidates; | |
| · | competition in our industry; and | |
| · | regulatory developments in China. |
We operate in a rapidly-changing environment and new risks emerge from time to time. As a result, it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. The forward-looking statements included in this prospectus speak only as of the date hereof, and except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
| 22 |
| Table of Contents |
We estimate that the net proceeds of this offering will be approximately $2.42 million, from the sale of our securities in this offering after deducting estimated offering expenses payable by us.
The selling stockholders may sell the Resale Securities from time to time. We will not receive any proceeds from the sale of shares of our common stock by the selling stockholders.
The table below depicts the amount of gross and net proceeds to us if 25%, 50%, 75% and 100% of the shares of our Class A common stock being offered by us are sold , respectively, at a fixed price of $0.50 per share in this offering:
|
|
| 100% |
| 75% |
| 50% |
| 25% | ||||||||
| Gross proceeds to us |
| $ | 2,500,000.00 |
|
|
| 1,875,000.00 |
|
|
| 1,250,000.00 |
|
|
| 625,000.00 |
|
| Net proceeds to us |
| $ | 2,415,058.95 |
|
|
| 1,790,058.95 |
|
|
| 1,165,058.95 |
|
|
| 540,058.95 |
|
The public offering price, and the resulting number of shares offered hereby as reflected in this prospectus, has been determined and is based on a fixed price of $0.50 per share. We intend to use the net proceeds from this offering to expand our operations in the PRC and fund the operations of our stem cell storage facilities . The net proceeds will be used for purchasing production equipment, renting storage facilities, building improvement , hiring new personnel, marketing, research and development and working capital. While we do not have any current agreements, commitments or understandings for any specific acquisitions or any specific targets in connection with which we intend to use a portion of the net proceeds from this offering, we may in the future use a portion of the net proceeds from this offering for such purposes.
This expected use of our net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our product development, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our products, and any unforeseen cash needs.
As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds from this offering. The timing and amount of our actual expenditures will be based on many factors, including cash flows from operations and the anticipated growth of our business.
| 23 |
| Table of Contents |
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to finance the development and expansion of our businesses and, therefore, do not expect to pay any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our Board, subject to applicable laws, and will depend on a number of factors, including our financial condition, results of operations, capital requirements, contractual restrictions, general business conditions, and other factors that our Board may deem relevant.
If you purchase securities in this offering, your interest will be diluted to the extent of the difference between the public offering price and the net tangible book value per share of our common stock after this offering. Our net tangible book value (deficit) as of March 31, 2021 was $874,107, or $0.01 per share of Class A common stock (based upon 118,177,885 outstanding shares of Class A common stock). “Net tangible book value (deficit)” is total assets minus the sum of liabilities and intangible assets. “Net tangible book value (deficit) per share” is net tangible book value divided by the total number of shares of common stock outstanding.
After giving effect to the sale by us in this offering of 5,000,000 shares of Class A common stock at a public offering price of $0.50 per share of Class A common stock, and after deducting estimated offering expenses that we will pay, our net tangible book value (deficit) as of March 31, 2021 would have been approximately $3,289,165.95, or $0.03 per share of common stock. This amount represents an immediate increase in net tangible book value (deficit) of $0.02 per share to existing stockholders and an immediate dilution of $0.47 per share to purchasers in this offering.
The following table illustrates the dilution:
| Public offering price per share |
|
|
|
| $ | 0.50 |
| |
| Net tangible book value (deficit) per share as of March 31, 2021 |
| $ | 0.01 |
|
|
|
|
|
| Increase in net tangible book value (deficit) per share attributable to this offering |
| $ | 0.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Pro forma, net tangible book value (deficit) per share after this offering |
|
|
|
|
| $ | 0.03 |
|
| Dilution per share to new investors |
|
|
|
|
| $ | 0.47 |
|
The following table sets forth the total number of shares previously sold to existing stockholders, the total consideration paid for the foregoing and the respective percentages applicable to such purchased shares and consideration paid , based on (i) an average price of $0.02 per share paid by our stockholders immediately prior to the reverse merger, (ii) $2,271,931 paid by the stockholders, including certain of our current officers and directors and affiliated persons in connection with the reverse merger, and (iii) $0.50 per share paid by new investors in the Offering.
|
|
| Shares Purchased |
|
| Total Consideration |
| ||||||||||
|
| Number |
|
| Percentage |
|
| Amount |
| Percentage |
| ||||||
| Shareholders prior to the reverse merger |
|
| 1,177,885 |
|
|
| 0.96 | % |
| $ | 11,779 |
|
|
| 0.25 | % |
| Shareholders in the reverse merger |
|
| 117,000,000 |
|
|
| 94.98 | % |
| $ | 2,271,931 |
|
|
| 47.49 | % |
| New investors in this offering |
|
| 5,000,000 |
|
|
| 4.06 | % |
| $ | 2,500,000 |
|
|
| 52.26 | % |
| Total |
|
| 123,177,885 |
|
|
| 100 | % |
| $ | 4,783,710 |
|
|
| 100 | % |
| 24 |
| Table of Contents |
The following table sets forth our cash and our capitalization as of March 31, 2021 on:
| · | an actual basis; and | |
| · | an as adjusted basis giving effect to the sale by us in this offering of 5,000,000 shares of Class A common stock, at the fixed public offering price of $0.50 per share, after deducting estimated offering expenses payable by us. |
The information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. Cash and cash equivalents are not components of our total capitalization. You should read these tables together with the other information contained in this prospectus, including “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical financial statements and related notes thereto included elsewhere in this prospectus.
|
|
| As of March 31, 2021 |
| |||||
|
|
| Actual |
|
| As Adjusted |
| ||
| Cash |
| $ | - |
|
| $ | 2,415,059 |
|
| Stockholders’ deficit: |
|
|
|
|
|
|
|
|
| Preferred Stock, $0.001 par value; 5,000,000 shares authorized; 0 shares issued and outstanding actual or as adjusted |
|
| - |
|
|
| - |
|
| Class A Common Stock, par value $0.001; 1,000,000,000 shares authorized; 118,177,885 shares issued and outstanding, actual; 123,177,885 shares issued and outstanding as adjusted |
|
| 118,178 |
|
|
| 123,178 |
|
| Class B Common Stock, par value $0.001; 3,750,000 shares authorized; 4,447 shares issued and outstanding, actual; 4,447 shares issued and outstanding as adjusted |
|
| 4 |
|
|
| 4 |
|
| Additional paid-in capital |
|
| 2,117,599 |
|
|
| 4,612,599 |
|
| Accumulated deficit |
|
| (1,381,669 | ) |
|
| (1,466,610 | ) |
| Total stockholders’ equity |
|
| 926,521 |
|
|
| 3,269,171 |
|
| Total capitalization |
|
| 926,521 |
|
|
| 3,269,171 |
|
| 25 |
| Table of Contents |
This is a self-underwritten (“best efforts”) offering of up to 5,000,000 shares of our Class A common stock at a price per share of $0.50. This prospectus is part of a registration statement that permits our officers and directors to sell the shares being offered by the Company directly to the public. There are no plans or arrangements to enter into any contracts or agreements to sell the shares with a broker or dealer. In offering the securities on our behalf, they will rely on the safe harbor from broker dealer registration set out in Rule 3a4-1 under the Exchange Act.
If, after the Effective Date of this prospectus, we enter into an agreement to sell our shares to a broker-dealer as principal and the broker-dealer is acting as an underwriter, we will need to file an amendment to the registration statement. We will need to identify the broker-dealer, provide required information on the plan of distribution, and revise the disclosures in that amendment, and file the agreement as an exhibit to the registration statement. Also, the broker-dealer would have to seek and obtain clearance of the underwriting compensation and arrangements from the FINRA Corporate Finance Department.
We estimate the total expenses of this offering, which will be payable by us will be approximately $84,941.05. After deducting the fees due to our estimated offering expenses, we expect the net proceeds from this offering to be approximately $2.42 million.
This prospectus also registers the resale by the selling stockholders the Resale Shares. We will not receive any proceeds from the sale of the Resale Securities.
The selling stockholders or any of their respective pledges, donees, assignees and other successors-in-interest may, from time to time, sell any or all of the Resale Securities, respectively, on any stock exchange, market or trading facility on which the Resale Securities are traded or in private transactions. The selling stockholders may use any one or more of the following methods when selling the Resale Securities:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| • | block trades in which the broker-dealer will attempt to sell the Resale Securities as agent, but may position and resell a portion of the block as principal to facilitate the transaction; | |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
| • | an exchange distribution in accordance with the rules of the applicable exchange; | |
| • | privately negotiated transactions; | |
| • | broker-dealers may agree with the selling stockholders to sell a specified number of Resale Securities at a stipulated price per share; | |
| • | through the writing of options on the Resale Securities; | |
| • | a combination of any such methods of sale; and | |
| • | any other method permitted pursuant to applicable law. |
The selling stockholders, or any of their respective pledgees, donees, transferees or other successors in interest, may also sell the Resale Securities, respectively, directly to market makers acting as principals and/or broker-dealers acting as agents for themselves or their customers. Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholders, and/or the purchasers of Resale Securities for whom such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing the Resale Securities will do so for their own account and at their own risk. It is possible that the selling stockholders will attempt to sell the Resale Securities in block transactions at a fixed offering price of $0.50 per share. The selling stockholders cannot assure that all or any of the Resale Securities offered in this prospectus will be issued to, or sold by, the selling stockholders. The selling stockholders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the Resale Securities if liabilities are imposed on that person under the Securities Act.
| 26 |
| Table of Contents |
The selling stockholders may from time to time pledge or grant a security interest in some or all of the Resale Securities owned by them respectively, and, if it defaults in the performance of its secured obligations, the pledgees or secured parties may offer and sell such Resale Securities from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or any other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
The selling stockholders also may transfer the Resale Securities, respectively, in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell such Resale Securities from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as a selling stockholders under this prospectus.
The selling stockholders have each acquired or will acquire the Resale Securities offered hereby in the ordinary course of business. The selling stockholders have advised us that they have not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the sale of such Resale Securities, nor is there an underwriter or coordinating broker acting in connection with a proposed sale of the Resale Securities owned by the selling stockholders. We will file a supplement to this prospectus if the selling stockholders enter into a material arrangement with a broker-dealer for sale of the Resale Securities being registered. If the selling stockholders use this prospectus for any sale of such shares of common stock, they will be subject to the prospectus delivery requirements of the Securities Act.
Determination of Offering Price
The public offering price of the shares of Class A common stock is $0.50 per share. The public offering price is a fixed price determined by our Board of Directors based on the trading of our Class A common stock prior to the offering, among other things. Other factors considered in determining the public offering price of the securities we are offering include our history and prospects, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
The selling stockholders will sell their respective Resale Securities at a fixed price of $0.50 per share.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock and preferred stock is Securities Transfer Corporation. The transfer agent’s address is 2901 N. Dallas Parkway, Suite 380, Plano, Texas 75093 and its phone number is (469) 633-0101.
Stock Market Listing
Our Class A common stock is not listed on any stock exchange. Our Class A common stock is currently quoted on the OTC Marketplace under the symbol “YBGJ”.
| 27 |
| Table of Contents |
We are offering, on a “best efforts” basis, up to 5,000,000 shares of Class A common stock at a price per share of $0.50.
The following description is qualified in its entirety by the provisions of our articles of incorporation, as amended (including all certificates of designation relating to our preferred stock), and our bylaws, all of which are incorporated by reference as exhibits to our registration statement, of which this prospectus forms a part.
General
As of July 12, 2021, our authorized capital stock consists of (i) 1,000,000,000 shares of Class A common stock, at a par value of $0.001 per share, of which 118,177,885 shares of Class A common stock are issued and outstanding, (ii) 3,750,000 shares of Class B common stock, at a par value of $0.001 per share, of which 4,447 shares of Class B common stock are issued and outstanding, and (iii) 5,000,000 shares of Preferred Stock at a par value of $.001 per share, none of which shares are issued or outstanding are issued and outstanding.
Common Stock
Voting Rights
Holders of our Class A common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors.
Holders of Class B common stock are entitled to five votes for each share on all matters submitted to a shareholder vote. Each share of Class B is convertible into one share of Class A common stock at all times, at the election of the holder, by delivery of a written notice by such holder to us, or to the transfer agent, together with the certificates representing such shares of Common B common stock to be converted. Each share of Class B common stock will be automatically converted into one share of Class A common stock in connection with the sale or transfer of such Class B common stock to a non-Class B holder,
Our bylaws provide that the presence in person or by proxy of the holders of a majority of the votes entitled to be cast on a matter at a meeting shall constitute a quorum of stockholders for that matter. If a quorum exists, and unless a higher proportion of the votes is required pursuant to our articles of incorporation or applicable law, an action of the stockholders entitled to vote on a particular matter shall be approved if the votes cast in favor such action exceed the votes cast against such action. However, our bylaws further provide that directors are elected by a plurality of the votes cast by the shares entitled to vote in the election at a meeting at which a quorum is present. The holders of our common stock do not have cumulative voting rights in the election of directors. The rights, powers, preferences and privileges of holders of our common stock are subject to those of the holders of our outstanding preferred stock and will be subject to those of the holders of any shares of our preferred stock we may authorize and issue in the future.
Our bylaws also permit stockholders to take action without a meeting or notice by a consent in writing, setting forth the action so taken, and duly signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted with respect to the subject matter thereof, which consent shall have the same force and effect as a vote of shareholders taken at such a meeting.
Dividend Rights
The holders of our common stock are entitled to receive such dividends as may be declared by our Board out of funds legally available for dividends. Our Board is not obligated to declare a dividend. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, future earnings, the operating and financial condition of our company, its capital requirements, general business conditions and other pertinent factors. We do not anticipate that dividends will be paid in the foreseeable future.
Miscellaneous Rights and Provisions
Holders of our common stock have no conversion, preemptive or other subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights of the holders of common stock are subject to any rights that may be fixed for holders of preferred stock, when and if any preferred stock is authorized and issued.
In the event of our liquidation, dissolution or winding up, subject to preferences that may be applicable to any then-outstanding preferred stock, each outstanding share entitles its holder to participate in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over Class A common stock.
Preferred Stock
Our articles of incorporation authorized the issuance of up to 5,000,000 shares of preferred stock in one or more series with such designations, voting powers, if any, preferences and relative, participating, optional or other special rights, and such qualifications, limitations and restrictions, as are determined by our Board in accordance with New York law. No shares of preferred stock are outstanding as of the date of this prospectus.
| 28 |
| Table of Contents |
History
On January 13, 2021 (the “Closing Date”), we closed a voluntary share exchange transaction with Platinum International Biotech Co., Ltd., a company organized under the laws of the Cayman Islands (“Platinum”), pursuant to that certain Agreement and Plan of Share Exchange, dated January 13, 2021 (the “Exchange Agreement”), by and among us, Platinum, Yubo International Biotech (Beijing) Limited, a company organized under the laws of the People’s Republic of China (“PRC”) (“Yubo”), and the shareholders of Platinum named therein.
In accordance with the terms of the Exchange Agreement, on the Closing Date, we issued a total of 117,000,000 shares of our Class A common stock to the then stockholders of Platinum (the “Platinum Stockholders”), in exchange for 100% of the issued and outstanding capital stock of Platinum (the “Exchange Transaction”). As a result of the Exchange Transaction, the Platinum Stockholders acquired more than 99% of our then issued and outstanding capital stock, Platinum became our wholly-owned subsidiary, and we acquired the business and operations of Platinum and Yubo.
Prior to the Exchange Transaction, we were a public reporting “shell company,” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). From and after the Closing Date, our primary operations will consist of the business and operations of Platinum and Yubo.
Yubo was founded on June 14, 2016 under the laws of the PRC, and has its headquarters at Room 105, Building 5, 31 Xishiku Avenue, Xicheng District, Beijing, PRC.
Platinum was established on April 22, 2020 under the laws of Cayman Islands as a limited liability company. Platinum acquired all of the outstanding stock of Platinum HK on May 4, 2020. Subsequently, the sole stockholder of Platinum sold 100% of the outstanding shares capital of Platinum to the Platinum Stockholders.
Platinum HK was established on May 4, 2020 under the laws of Hong Kong as a limited liability company. Platinum HK acquired all of the outstanding stock of Yubo WFOE on September 11, 2020.
Yubo WFOE was established on September 4, 2020, under the laws of the PRC. Yubo WFOE is a wholly-owned subsidiary of Platinum HK, and therefore, Yubo WFOE is a wholly foreign owned enterprise. The advantages of this structure include:
| · | Independence and freedom to implement the worldwide strategies of its parent company without having to consider the involvement of Chinese law; | |
| · | Ability to formally carry out business and the ability to issuing invoices to customers in RMB and receive revenues in RMB; | |
| · | Capable of converting RMB profits to US dollars or other foreign currency for remittance to their parent company outside China; and | |
| · | Greater protection of intellectual property rights, know-how and technology since no partner required and therefore more control of intellectual property. |
As discussed below, Yubo and/or its shareholders have entered into various agreements with Yubo WFOE to allow Yubo WFOE’s effective control over Yubo. We acquired 100% of the issued and outstanding capital stock of Platinum, which, in turn, holds a 100% equity interest in Yubo WFOE, in exchange for the issuance of 117,000,000 shares of our common stock to the shareholders of Platinum, which constituted more than 99% of our issued and outstanding common stock. Through our ownership of Platinum, we effectively acquired the business and operations of Yubo, Yubo WFOE and Platinum HK.
Effective December 4, 2020, we changed our corporate name from Magna-Lab, Inc. to Yubo International Biotech Limited under the stock symbol “YBGJ”.
| 29 |
| Table of Contents |
Corporate Structure
As a result of the Exchange Transaction, the organizational structure of the Registrant is as follows:

Business Overview
We are a technology company focused on the research and development and application of endometrial stem cells. We are committed to building the first public endometrial stem cell repository in the world. We offer our products and services under the brand “VIVCELL”. Our product offerings include healthcare products for respiratory system, skincare products, hair care products, healthy beverages and male and female personal care products. We also offer stem cell related services including cell testing and health management consulting services.
Our Strategy
We intend to build a first-class stem cell storage facility, which we believe, will be the first global bank of endometrial stem cells. We also intend to develop and expand our current product line, to include a series of endometrial stem cells light application technology and medical-grade cell therapeutic products for health management, innovative medicine, anti-aging treatments, clinical transformation and other application fields. We believe our existing technology and leadership position in stem cell processing will drive significant future growth for the Company.
Stem Cell Storage Facility
Our endometrial stem cell bank is divided into a public resources library and private repositories. The public resources library will meet strict testing standards. We will enter into standard donation agreements with the donor customers who meet the public resources library standards, and store the collection of stem cell samples in the public resources library. Such public resources library biological samples can be widely used in research and development and subsequent commercial development.
Effective March 1, 2021, we, through Yubo Global, entered into a lease agreement with Chengdu Liangkang Investment Co. to rent approximately 6,960 square meters of laboratory space in Chengdu, China for the use of our endometrial stem cell bank and subsequent research and development laboratory. See “– Description of Property” below. We have completed the design for our stem cell bank, and expect to complete tenant improvement and equipment procurement by October 2021. Upon completion, our stem cell bank is expected to store biological samples of more than 5,000 people in the early stage. We currently expect to open our stem cell bank to the public for commercial use in 2022.
| 30 |
| Table of Contents |
Our Business Model
Our business model leverages our ability to integrate the upstream, middle stream and downstream of our stem cell based product cycle. With respect to the upstream of the industrial chain, we intend to build a public resource library of endometrial stem cells to meet the demand for high-quality cells from stem cell application and treatment centers in China. See “– Our Strategy – Stem Cell Storage Facility” above and “– Our Service and Products – Endometrial Stem Cell Bank” below. With respect to the middle and downstream of the industrial chain, we intend to open and operate biological experience center stores where we integrate the functions of display, trial and sale of our light application products under the brand “VIVCELL”, and at the same time, can train and develop our sales personnel. By continuing to build and develop our “VIVCELL” brand, we plan to expand the biological experience center stores into a nation-wide franchise. We believe our integrated approach provides us with a competitive advantage.
Our Service and Products
Endometrial Stem Cell Bank
We currently plan to complete the first stage of building our stem cell bank by October 2021. See “– Our Strategy – Stem Cell Storage Facility” above. We, through Yubo, have entered into an Entrustment Technical Service Agreement with Beijing Zhenhuikang Biotechnology Co., Ltd., a company organized under the laws of the PRC, entrusting it to prepare, store and manage endometrial stem cell samples for the operations of our stem cell bank in exchange for services fees paid by us. Beijing Zhenhuikang Biotechnology Co., Ltd is an affiliate of a shareholder of Yubo. Pursuant to the entrustment agreement, we are responsible for supplying the endometrial stem cell samples and we may terminate the agreement at any time. See “Certain Relationships and Related Transactions and Director Independence.”
We have obtain ed a business license for the stem cell bank from the Market Supervision Administration of Chengdu Municipality. The establishment of the s tem c ell bank has been submitted for filing with the Chengdu Municipal Development and Reform Commission, the Science and Technology Bureau and the Health Commission. The blue print design of our stem cell bank has been approved by Health Commission and Fire and Rescue department.
In the future, we may re cruit young, healthy adult females as our donors of endometrial stem cells . We plan to target these donors via several channels , including from our clients for cell testing and health management consulting services, from colleges and universities where we promote the knowledge and awareness of the application of endometrial stem cells , and from our customers of light application products.
| 31 |
| Table of Contents |
Light Application Products
Our current commercialized light application products include the following:
|
| · | Respiratory atomization products (nebulizers): include lung regulating essence, which is applied through an atomizer and formulated on the basis of multiple growth factors rich in the polypeptide of cell technology such as FGF, EGF, VEGF, etc. to repair and cure the lungs. Our products can enhance the regeneration of lung stem cells, inhibit apoptosis of normal lung cells, improve immunity, relieve dry and astringent foreign body sensation, and relieve chest tightness, chest pain, wheezing, breathing difficulties and other symptoms. We launched our respiratory atomization products in April 2020 and the sales of these products have accumulated to approximately $1.7 million since launch. Our respiratory atomization products are sold in Guangdong, Shandong, Hebei, Henan, Chongqing and Sichuan provinces. The customer ba se for these products mainly includes middle class and abov e population with respiratory system diseases or weaknesses, or low immunity . |
|
| · | Cell basidiomycetes compound drink/beverage: This product is registered as beverage. Based on cellular immunology, combined with traditional Chinese medicine theories and natural plant extracts such as basidiomycetes, red ginseng and polygonatum, it has the benefits of rapid activation of immune cells, regulation of immune system, anti-tumor, anti-virus, hypoglycemic and anti-oxidation. We launched our cell basidiomycetes compound drink in February 2021 and the sales of this product have accumulated to approximately $400,000 by end of May 2021. We are still in the process of promoting this product to cover more regional markets. The customer base for th is drink mainly includes middle class and above population who are recovering from major epidemic diseases or with low immunity. |
|
|
|
|
| Our other light application products whi ch we plan to launch in the near future include the following: | ||
|
| ||
|
| · | Facial care series: this line of products include renewal essence mask powder, which is formulated with optimal active substances derived from stem cells, can significantly improve skin’s self-repair ability, and has the benefits of anti-wrinkle, tightening, water locking and brightening, and skin delicate essence, which is applied through a beauty instrument to promote the absorption and formulated based on multiple growth factors rich in the polypeptide of cell technology such as FGF and EGF to repair skin barrier, improve skin gloss and restore skin elasticity. The customer base for these products mainly includes high-income population with dry skin, lack of skin elasticity or damaged skin barrier . |
|
|
|
|
|
| · | Eye care series: include eye moist essence, which is applied through eye warm import instrument to enhance absorption and formulated based on multiple growth factors rich in the polypeptide of cell technology such as FGF, EGF, VEGF, etc., to deeply cleanse and wash out impurities, 360 degrees nourish eyes, relieve dry and scorching sensation, and strengthen self-repair of corneal cells. The customer base for these products mainly includes people who overuse their eyes, suffer from dry eyes from long-term use of electronic products, cover from eye-operati ons or surgeries . |
|
|
|
|
|
| · | Hair care series: include tough curing and solid hair follicles essence, which is applied through a micro type electric nanometer crystal injector and formulated based on multiple growth factors rich in the polypeptide of cell technology such as FGF, EGF, VEGF, etc. to strengthen hair follicle, prevent hair loss from the roots, nourish the scalp, repair damaged scalp, and enhance scalp barrier. The customer base for these products mainly includes high-income population suffering from hair thinning, damaged scalp follicles, severe hair loss . |
|
|
|
|
|
| · | Male and female private protection series: include bacteriostatic spray and intimate wash for men and women to balance pH levels in the groin and genital areas for prevention of infections, eliminate odor, and inhibit bacteria; and nourishing vagina essence, which is applied through a restorer of pelvic floor muscle and formulated based on multiple growth factors rich in the polypeptide of cell technology such as FGF, EGF, VEGF, etc. to repair and cure vagina mucosa and pelvic floor muscle, strengthen the restoration of vagina mucosa, mucous secretion, reinforce the pelvic floor muscle, and improve the sexual experience. The customer base for these products mainly includes middle and high -income population who suffer from itching and bacterial infection in their private areas, adult females who need pelvic floor repair after giving birth. |
All of our light application products target and are offered in the PRC market.
Research and Development
We are principally focused on the development of new products that support the stem cell therapy market. Our future research and development activities will be devoted to the development and launch of additional new products, line extensions, or significant upgrades to existing products and our current stem cell storage facility. Research and Development expense reflects the cost of these activities, as well as the costs to obtain regulatory approvals of new products and processes and to maintain the highest quality standards with respect to existing products.
We, through Yubo, have also entered into a Joint Research and Development Agreement with Beijing Zhenxigu Medical Research Center (L.P.) (“Beijing Zhengxigu”), which is an affiliate of a shareholder of Yubo. P ursuant to this agreement, the parties have agreed to carry out filing application and market cooperation of certain medical devices developed by Beijing Zhenxigu (the “Application”) and we agreed to provide Beijing Zhenxigu Medical Research Center (L.P.) with aggregate R&D expenses of RMB241,880. Under this agreement, Beijing Zhenxigu authorized Yubo to be the nationwide exclusive distributor of supporting products for Platinum-specific atomization inhaler applications. After the filing of the Application, Beijing Zhenxigu shall continue to develop other application fields, and the parties shall continue cooperation in the form of entrustment or joint research and development. This agreement will terminate automatically upon the completion of the subject matters of the agreement. See “Certain Relationships and Related Transactions and Director Independence.”
Intellectual Property
Intellectual property is of vital importance in our field and in biotechnology generally. We seek to protect and enhance proprietary technology, inventions, and improvements that are commercially important to the development of our business by seeking, maintaining, and defending patent rights, whether developed internally, acquired or licensed from third parties. We will also seek to rely on regulatory protection afforded through orphan drug designations, inclusion in expedited development and review, data exclusivity, market exclusivity and patent term extensions where available.
We currently own two invention patents in the PRC. Our owned patents in China are for endometrial collection and a protective solution for endometrial stem cells which will be used in connection with the operations of our stem cell bank in the future . The details of our owned patents are listed below:
|
| · | “ Menstrual blood collection kit ” patent (No. CN106264688B ) for co llection of endometrial stem cells, which is an invention patent and will expire on Oct ober 21, 2039. |
|
|
|
|
|
| · | “ Endometrial stem cell protective solution ” patent (No. CN107668025A) for storage of endometrial stem cells, which is an invention patent and will expire on February 8, 2038. |
| 32 |
| Table of Contents |
Manufacturing
We rely on third parties to manufacture our light application products and certain of the medical devices used in our collection and testing of stem cells. We have entered into a Cooperation Agreement with Beijing Zhenxigu Medical Research Center (L.P.), and Huailai Huayue Hengsheng Medical Device Co., Ltd., pursuant to which Huailai Huayue Hengsheng Medical Device Co., Ltd. has agreed to conduct sample trial production of our liquid dressing products. The Cooperation Agreement is attached hereto as Exhibit 10.9 and is incorporated herein by reference.
Industry
The PRC medical industry has evolved in the past 30 years from a complex, multi-tiered system that was subject to strict control at every governmental level to a competitive and increasingly market-oriented industry. From 1950 to 1979, all Chinese medical companies were state-owned and categorized into national, provincial and municipal-level distributors. The price markup at each level, from medical manufacturer to end-consumer, was subject to a total markup cap of 28%. During the 1980s, the rigid three-level distribution system gave way to a more open and decentralized network. Driven by increasing demand for medical products in the past three decades, the PRC medical industry has experienced rapid growth. The numbers of medical manufacturers and distributors have also increased significantly until recent years, when competition and government regulations and policies started to drive consolidation in the industry. As a result of these developments, the market volume of the PRC medical distribution market has steadily increased.
Market Drivers
The significant growth of China’s population aged 60 or above is expected to drive demand for healthcare and medical products in China. According to the PRC National Bureau of Statistics, the proportion of the population aged 60 or above in China has increased from 11.9% in 2003, or approximately 150.0 million people, to 13.6%, or approximately 162.2 million people in 2007. Rising life expectancy is also expected to contribute to the growth of China’s aging population, both as an absolute number and as a percentage of the total population. We believe that the aging population in China, which historically spends the most on healthcare, will drive the growth of the PRC healthcare and medical industries. The prevalence of chronic health problems, such as arthritis, cardiovascular diseases and cancer, is expected to increase with the growth of China’s population aged 60 or above. In addition, as living standards continue to improve and health consciousness grows in China, many lifestyle-related diseases are also increasing and becoming more widespread. For example, Business Monitor International estimates that sales of prescription cardiovascular medicines increased by 87% from ¥19.0 billion, or approximately US$2.8 billion, in 2003 to ¥35.3 billion, or approximately US$5.2 billion, in 2007, primarily as a result of the rising prevalence of heart disease in an aging population and increasingly unhealthy lifestyles in the population at large.
According to the China Statistical Yearbook 2008 (the “Yearbook”), from 2003 to 2007, the average per capita annual disposable income of China’s urban residents increased from approximately ¥8,472, or approximately US$1,250, to ¥13,785, or approximately US$2,025, representing a compound annual growth rate (“CAGR”) of approximately 12.9%. According to the Yearbook, China’s GDP grew at a CAGR of 16.4% from 2003 to 2007, and its per capita GDP grew from ¥10,542, or approximately US$1,550, in 2003 to approximately ¥18,934, or approximately US$2,780, in 2007, representing a CAGR of 15.8%. During this period, national income and disposable income levels increased significantly.
With rising living standards and increasing disposable income, people in China have become more health conscious. These developments have resulted in both Chinese urban and rural residents spending more on healthcare. According to the PRC National Bureau of Statistics, consumer expenditures on healthcare in China’s urban and rural areas increased from approximately ¥476.0, or approximately US$70, and ¥117.8, or approximately US$17, per person in 2003, respectively, to approximately ¥699.0, or approximately US$100, and ¥210.2, or approximately US$30, per person in 2007, respectively.
| 33 |
| Table of Contents |
National Medical Insurance Program
The National Medical Insurance Program (“National Program”), which was introduced in 1999, is the largest medical insurance program in China. The National Program is funded with varying levels of contributions from the PRC Government, individual program participants and their employers.
In 1999, the National Program was originally launched as the Urban Worker Basic Medical Insurance Program (“Urban Worker Program”), a mandatory scheme covering urban workers and their minor children. In 2007, a voluntary component called the Urban Resident Basic Medical Insurance Program (“Urban Resident Program”) was further implemented as part of the National Program, to cover the rest of the urban residents that are not covered by the Urban Workers Program. The National Program provides guidance on which prescription and over-the-counter medicines are included in the National Program and to what extent the purchases of these medicines are reimbursable. See the section headed “Government Regulation - Reimbursement Under the National Medical Insurance Program” below for further information.
We believe that only a small percentage of the Chinese population can afford commercial insurance plans. Therefore, the National Program coverage is expected to expand in the future. According to the PRC National Bureau of Statistics, the percentage of PRC urban residents grew from approximately 37.7% of the total population to 44.9% from 2001 to 2007. The number of people covered by the National Program increased from approximately 37.9 million in 2000 to 180.2 million in 2007, representing a CAGR of 25%. This trend is anticipated to continue as the Eleventh Five-Year Plan government development initiative projects that the PRC urban population will increase from 45% to 47% of China’s total population between 2007 to 2010. Furthermore, the provincial and municipal authorities who are responsible for administering social medical insurance funds to cover such reimbursements have been gradually increasing funding in recent years. According to the PRC Ministry of Labor and Social Security, total funding under the national insurance program reached ¥225.7 billion, or approximately US$28.9 billion, in 2008, representing an increase of 29.2% from 2007. The availability of funding is expected to increase significantly in the near future, primarily as a result of increased financial and policy support from various levels of the PRC government.
PRC Healthcare Reform
In September 2008, the State Council published a draft plan to ease the difficulties and minimize the costs for PRC citizens to obtain proper healthcare treatment. On March 17, 2009, the PRC Government issued the Opinion on Deepening the Healthcare System Reform (the “Opinion”). The State Council subsequently released the Notice on Important Implementing Plans for the Healthcare System Reform 2009-2011 (the “Implementing Plan”). The goal of the healthcare reform plan is to establish a basic, universal healthcare framework to provide Chinese citizens with safe, efficient, convenient and affordable healthcare. The Opinion calls for healthcare reform to be carried out in two steps:
|
| · | Step One, which will be completed by 2011, aims to increase the accessibility while reducing the cost of healthcare. During this phase, the PRC Government will build up a network of basic healthcare facilities, expand coverage of the public medical insurance system to cover 90% or more of the population, and reform the drug supply and public hospital system. |
|
|
|
|
|
| · | Step Two, which will take place between 2011 and 2020, envisions the establishment of a universal healthcare system. The entire population should be covered by public medical insurance; drugs and medical services should be accessible and affordable to citizens in all public healthcare facilities. |
While the PRC Government has neither provided a concrete timetable nor steps to implement certain tasks, such as the public hospital reform, it has released execution guidance for other tasks. Most notably, the PRC Government has announced it will spend an additional approximately RMB 850 billion, or US$125 billion from 2009 to 2011 on the healthcare industry. A significant portion will be expended to establish a basic healthcare medical insurance regime, which aims to cover over 90% of the national population by 2011, mainly through the Urban Worker Program, Urban Resident Program and the New Rural Insurance Scheme. The PRC Government further announced that the annual subsidy for each participant will be increased from approximately RMB 40, or US$5.90 to approximately RMB120, or US$17.60 for Urban Resident Program participants, and from approximately RMB 80, or US$11.76 to approximately RMB120 RMB, or US$17.60 for New Rural Insurance Scheme participants, starting from 2010. The reform plan will also raise the cap on claim payments from four times the local average annual income to six times such income. Another significant part of the spending plan focuses on healthcare facilities. The PRC Government plans to build 29,000 rural clinics in 2009. In the next three years, the PRC government plans to build an additional 5,000 rural clinics, 2,000 county-level hospitals and 2,400 urban community clinics in under-developed areas. This substantial increase in healthcare spending is expected to expedite the growth of the healthcare industry in China.
| 34 |
| Table of Contents |
Under the healthcare reform plan, the additional funding for the healthcare industry will primarily target four fundamental healthcare systems in China:
|
| · | The public health services system. This system focuses on preventing disease and promoting health as a complementary alternative to medical treatment. The public health services system will provide services such as immunizations, regular physical check-ups (for senior citizens over 65 years of age and children under three years of age), pre-natal and post-natal check-ups for women, prevention of infectious or chronic diseases and other preventative and fitness activities. |
|
|
|
|
|
| · | The public medical insurance system. This system covers drugs and medical treatments for the majority of the population. The healthcare reform plan will retain the framework of the current public medical insurance schemes under the National Program, but will expand them to cover more of the population and increase the scope of treatments, raise the cap on claim payments and cover more claims at higher percentages. |
|
|
|
|
|
| · | The public healthcare delivery system. One of the primary goals of the Implementing Plan is to build more healthcare facilities and to improve the training of healthcare professionals. Beyond additional public wellness centers, the reform plan aims to place a medical clinic in every village and a hospital in every county by 2011. In addition, the PRC Government will encourage private investors to establish public non-profit hospitals. |
|
|
|
|
|
| · | The drug supply system. This system regulates pricing and how drugs will be procured prescribed and dispensed in healthcare facilities. The healthcare reform plan will focus on pricing, procurement, prescription and dispensing of essential drugs. |
The Opinion and the Implementing Plan direct relevant governmental authorities, including the Ministry of Health, SFDA and the National Development Reform Commission, or NDRC, to adopt implementing regulations for the reforms outlined in the healthcare reform plan.
Industry Opportunities
The preservation of cell resources is the basis for the development of the growing cell therapy industry. At present, in the existing cell resource preservation business, the cord blood preservation is the most mature one, and mesenchymal stem cells, as a new type of cell resource, its preservation business has shown a good momentum of development. The momentum of development is expected to grow stronger in the future development and make positive contributions to human health. Up to now, the global cord blood bank has grown to more than 400, and there are more than 50 umbilical cord mesenchymal stem cell banks. Take the United States as an example, an additional stem cell bank capable of storing umbilical cord mesenchyme will be added every three months. In addition, with the promotion and application of immune cells, during the treatment of tumor patients with immune cells, it is found that some patients have very low immune system ability. It is difficult for the immune cells in the body to activate and expand outside the body. If the immune cells can be stored when the person is healthy, they can be backed up as a seed of life and health, and can be used to prevent or treat cancer. So many immune cell companies are targeting the immune cell storage market.
The report “Global Cell Therapy Market 2017-2021” released by the internationally renowned consulting company Technavio pointed out that from 2017 to 2021, the global cell therapy market is expected to grow at a compound annual growth rate of 23.27%.
| 35 |
| Table of Contents |
Stem Cells
Stem cells have the remarkable potential to develop into many different cell types in the body. They serve as a repair system for the body and they can theoretically divide without limit to replenish other cells as long as the person is alive. When a stem cell divides, each new cell has the potential to either remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell.
There are two main types of stem cells: embryonic and adult stem cells. Embryonic stem cells are primitive cells derived from a 5-day pre implantation embryo that have the potential to become a wide variety of specialized cell types. Adult stem cells are cells found in human tissue that can renew themselves, and can differentiate to yield the major specialized cell types of that tissue. Adult stem cells are thought to reside in a specific area of each tissue where they may remain quiescent (non-dividing) for many years until they are activated by disease or tissue injury. The tissues reported to contain stem cells include umbilical cord blood, bone marrow, brain, peripheral blood, adipose, blood vessels, skeletal muscle, skin, and liver.
Stem Cell Therapy
Stem cell therapies are treatments in which stem cells are inducted to differentiate into the specific cell type required to repair damaged or destroyed cells or tissues.
Since the first successful cord blood transplant performed in 1988, awareness of the potential therapeutic value of cord blood stem cells has increased and collection and storage has grown rapidly. These cord blood stem cells are harvested at no risk or pain to the donor and can be preserved in a cord blood bank for clinical use with a matched patient on short notice. Their use also results in a lower incidence of post-transplant immune complications than transplants with adult bone marrow stem cells.
Stem cell therapy is used to:
|
| · | Replace bone marrow damaged by high-dose chemotherapy or radiation therapy used to treat patients with a variety of cancers such as leukemia and lymphoma; |
|
|
|
|
|
| · | Provide genetically healthy and functioning bone marrow to treat patients with more than 60 life threatening genetic diseases such as sickle cell anemia and immunodeficiency; and |
|
|
|
|
|
| · | Regenerate and repair tissue including the treatment of myocardial infarction, peripheral limb ischemia and non-union bone fractures. |
We believe the number of stem cell units stored will continue to grow, due in part to the following factors:
|
| · | Increased awareness about the availability and benefits of preserving endometrial stem cells; |
|
|
|
|
|
| · | Improved technology to harvest the stem cells in a sterile environment and maintain their viability for many years; |
|
|
|
|
|
| · | Clinical evidence that cell dose and cell viability are critical to a successful transplant; and |
|
|
|
|
|
| · | Increased government support. |
The global cell therapy is developing rapidly. At the beginning of 2019, the US FDA announced its development plan for future cell therapy. The FDA predicts that by 2025, 10-20 new drugs in this field will be approved each year. In recent years, the increase in the number of clinical trials, strengthened government and capital support, and increased corporate cooperation are driving the development of CDMO business in the global cell therapy industry.
Additionally, the major developed countries in the world all regard cell therapy as a key support and development direction in the field of medicine. Our country has also formulated a series of guidelines and policies to accelerate the development of the biomedical industry to meet the urgent needs of patients for new technologies and new therapies.
With the continuous strengthening of our scientific research investment and technical strength, the number of cell therapy clinical research carried out in China has been increasing year by year, and the calls for the industrialization and clinical application of related products are also increasing. In our country, cell therapy and clinical transformation have become a major issue in the development of health protection.
With the gradual improvement of supervision, approval and payment methods, as well as the popularization of new generation cell manufacturing processes, the release of cell therapy products will be faster and the market penetration rate will also be greater. This means that more patients will be able to use cell therapy products.
| 36 |
| Table of Contents |
Competition
Our products will compete with novel therapies developed by biomedical companies, academic research institutions, governmental agencies and public and private research institutions, in addition to standard of care treatments.
At present, there are more than 2,000 companies engaged in stem cell research and development and application in China, but most of them focus on the storage and anti-aging application of umbilical cord blood stem cells and basic cosmetics companies. Due to our unique core technology, we believe there is no company in China whose main business is endometrial stem cell storage. At present, we believe no companies mainly engage in cell light application products and have product lines similar to ours.
Some of our key competitors are:
|
| · | China Cord Blood (NYSE: CO) currently focuses on private storage of neonatal cord blood and is the largest company engaged in such business in China, with annual revenue of more than RMB1 billion. |
|
|
|
|
|
| · | VCANBIO CELL&GENE: (SHA: 600645) its main products include cell preparation and storage, genetic testing, etc. Its revenue in 2019 was RMB1.386 billion. |
Government Regulation
In the People’s Republic of China, or PRC, we operate in an increasingly complex legal and regulatory environment. We are subject to a variety of PRC laws, rules and regulations affecting many aspects of our business. This section summarizes the principal PRC laws, rules and regulations that we believe are relevant to our business and operations.
PRC Drug Regulation
Introduction
China heavily regulates the development, approval, manufacturing and distribution of drugs, including biologics. The specific regulatory requirements applicable depend on whether the drug is made and finished in China, which is referred to as a domestically manufactured drug, or made abroad and imported into China in finished form, which is referred to as an imported drug, as well as the approval or “registration” category of the drug. For both imported and domestically manufactured drugs, China typically requires regulatory approval for a CTA to conduct clinical trials in China and submit China clinical trial data, prior to submitting an application for marketing approval. For a domestically manufactured drug, there is also a requirement to have a drug manufacturing license for a facility in China.
In 2017, the drug regulatory system entered a new and significant period of reform. The General Office of the State Council and the General Office of the Central Committee of the China Communist Party jointly issued the Opinion on Deepening the Reform of the Evaluation and Approval System to Encourage Innovation in Drugs and Medical Devices, or the Innovation Opinion in October 2017. The expedited programs and other advantages under this and other recent reforms encourage drug manufacturers to seek marketing approval in China first, manufacture domestically, and develop drugs in high priority disease areas, such as oncology.
To implement the regulatory reform introduced by the Innovation Opinion, the NPC and the NMPA has been revising the fundamental laws, regulations and rules regulating medical products and the industry, which include the framework law known as the PRC Drug Administration Law, or DAL. The DAL was promulgated by the Standing Committee of the NPC on September 20, 1984 and last amended on August 26, 2019 and took effect as of December 1, 2019. The DAL is implemented by a high-level regulation issued by the State Council referred to as the DAL Implementing Regulation. The NMPA has its own set of regulations further implementing the DAL; the primary one governing CTAs, marketing approval, and post-approval amendment and renewal is known as the Drug Registration Regulation, or DRR. The DRR was promulgated by the NMPA on February 28, 2005 and the last amended DRR will take effect from July 1, 2020. Although the NMPA has issued several notices and proposed regulations in 2018 and 2019 to implement the reforms, the implementing regulations for many of the reforms in the Innovation Opinion have not yet been finalized and issued, and therefore, the details regarding the implementation of the regulatory changes remained uncertain in some respects.
| 37 |
| Table of Contents |
Regulatory Authorities and Recent Government Reorganization
In the PRC, the NMPA is the primary regulatory agency for medical products and businesses. The agency was formed from the prior China Food and Drug Administration, or CFDA, in 2018 as part of a government reorganization. Pursuant to the Decision of the First Session of the Thirteenth National People’s Congress on the State Council Institutional Reform Proposal made by the NPC on March 17, 2018, NMPA is one of the two half-ministry level agencies under the State Administration for Market Regulation, or SAMR, which are responsible for consumer protection, advertising, anticorruption, pricing and fair competition matters. The National Intellectual Property Administration is the other half-ministry level agency under the SAMR.
Like the CFDA, the NMPA is still the primary drug regulatory agency and implements the same laws, regulations, rules, and guidelines as the CFDA, and it regulates almost all of the key stages of the life-cycle of medical products, including nonclinical studies, clinical trials, marketing approvals, manufacturing, advertising and promotion, distribution, and pharmacovigilance (i.e., post-marketing safety reporting obligations). The Center for Drug Evaluation, or CDE, which remains under the NMPA, conducts the technical evaluation of each drug and
biologic application to assess safety and efficacy.
The NHC (formerly known by the names: the Ministry of Health (MOH) and National Health and Family Planning Commission (NHFPC)), is China’s primary healthcare regulatory agency. It is responsible for overseeing the operation of medical institutions, some of which also serve as clinical trial sites, and regulating the licensure of hospitals and other medical personnel. NHC plays a significant role in drug reimbursement. Furthermore, the NHC and its local counterparts at or below the provincial-level of local government also oversee and organize public medical institutions’ centralized bidding and procurement process for medical products, through which public hospitals and their pharmacies acquire drugs.
Also, as part of the 2018 reorganization, the PRC government formed the National Healthcare Security Administration which focuses on regulating reimbursement under the state-sponsored insurance plans.
Non-Clinical Research
The NMPA requires preclinical data to support registration applications for imported and domestic drugs. According to the DRR, nonclinical safety studies must comply with the Administrative Measures for Good Laboratories Practice of Non-clinical Laboratory. On August 6, 2003, the NMPA promulgated the Administrative Measures for Good Laboratories Practice of Non-clinical Laboratory, which was revised on July 27, 2017, to improve the quality of non-clinical research, and began to conduct the Good Laboratories Practice. Pursuant to the Circular on Administrative Measures for Certification of Good Laboratory Practice for Non-clinical Laboratory issued by the NMPA on April 16, 2007, the NMPA is responsible for the certification of non-clinical research institutions nationwide and local provincial medical products administrative authorities is in charge of the daily supervision of non-clinical research institution. The NMPA decides whether an institution is qualified for undertaking medical non-clinical research by evaluating such institution’s organizational administration, its research personnel, its equipment and facilities, and its operation and management of non-clinical medical projects. A Good Laboratory Practice Certification will be issued by the NMPA if all the relevant requirements are satisfied, which will also be published on the NMPA’s website.
Pursuant to the Regulations for the Administration of Affairs Concerning Experimental Animals promulgated by the State Science and Technology Commission on November 14, 1988 and amended on January 8, 2011, July 18, 2013 and March 1, 2017, respectively, by the State Council, the Administrative Measures on Good Practice of Experimental Animals jointly promulgated by the State Science and Technology Commission and the State Bureau of Quality and Technical Supervision on December 11, 1997, and the Administrative Measures on the Certificate for Experimental Animals (Trial) promulgated by the Ministry of Science and Technology and other regulatory authorities on December 5, 2001, using and breeding experimental animals shall be subject to some rules and performing experimentation on animals requires a Certificate for Use of Laboratory Animals.
| 38 |
| Table of Contents |
Registration Categories
Prior to engaging with the NMPA on research and development and approval, an applicant will need to determine the registration category for its drug candidate (which will ultimately need to be confirmed with the NMPA), which will determine the application requirements for its clinical trial and marketing application. There are five categories for small molecule drugs: Category 1, or innovative drugs, refers to drugs that have a new chemical entity that has not been marketed anywhere in the world, Category 2, or improved new drugs, refers to drugs with a new indication, dosage form, route of administration, combination, or certain formulation changes not approved in the world, Category 3 is for domestic generics that reference an innovator drug marketed abroad but not in China, Category 4 is for domestic generics that reference an innovator drug marked in China, and Category 5 refers to an application to import into China innovative or generic drugs that have already been marketed abroad.
Therapeutic biologics follow a somewhat similar categorization, with three out of the 15 categories depending on marketing approval status: Category 1 is for innovative biologics that have not been approved inside or outside of China, Category 7 for biologics that have been marked abroad but not in China, and Category 15 for biologics that have been marketed in China, and the rest of the 15 categories depending on products characteristics. All biologics follow the new drug application pathway, but a tentative guideline on the development and evaluation of biosimilar drugs was issued by the NMPA in 2015.
Expedited Programs
Priority Evaluation and Approval Programs to Encourage Innovation
The NMPA has adopted several expedited review and approval mechanisms since 2009 and created additional expedited programs in recent years that are intended to encourage innovation. Applications for these expedited programs can be submitted together with the registration package or after the registration submission is admitted for review by the CDE. The Opinions on Encouraging the Prioritized Evaluation and Approval for Drug Innovation promulgated by the NMPA on December 21, 2017 clarified that fast track CTAs or drug registration pathways will be available to the innovative drugs.
If admitted to one of these expedited programs, an applicant will be entitled to more frequent and timely communication with reviewers at the CDE, expedited review and approval, and more agency resources throughout the review approval process.
NMPA also permits conditional approval of certain medicines based on early phase China clinical trial data or only on foreign approval clinical data. Post-approval the applicant may need to conduct one or more post-market studies. The agency has done this for drugs that meet unmet clinical needs for life-threatening illnesses and also for drugs that treat orphan indications. In 2018, NMPA established a conditional approval program for drugs designated by the CDE that have been approved in the US, EU and Japan within the last 10 years and that meet one of three criteria (1) orphan indications, (2) drugs that treat life threatening illnesses for which there are not effective treatment or preventive methods, and (3) drugs that treat life threatening illnesses and that have a clear clinical advantage over other approved therapies.
Clinical Trials and Marketing Approval
Upon completion of preclinical studies, a sponsor typically needs to conduct clinical trials in China for registering a new drug. The materials required for this application and the data requirements are determined by the registration category. The NMPA has taken a number of steps to increase efficiency for approving CTAs, and it has also significantly increased monitoring and enforcement of the Administrative Regulations of Quality of Drug Clinical Practice, or the PRC’s GCP to ensure data integrity.
| 39 |
| Table of Contents |
Trial Approval
All clinical trials conducted in China for new drug registration purposes must be approved and conducted at medical clinical trial institutions which shall be under the filing administration. For imported drugs, proof of foreign approval is required prior to the trial, unless the drug has never been approved anywhere in the world.
In addition to a standalone China trial to support development, imported drug applicants may establish a site in China that is part of an international multicenter trial, or IMCT, at the outset of the global trial. Domestically manufactured drugs are not subject to foreign approval requirements, and in contrast to prior practice, the NMPA has recently decided to permit those drugs to conduct development via an IMCT as well.
In 2015, the NMPA began to issue an umbrella approval for all phases (typically three) of a new drug clinical trial, instead of issuing approval phase by phase. For certain types of new drug candidates, CTAs may be prioritized over other applications and put in a separate expedited queue for approval.
The NMPA has now adopted a system for clinical trials of new drugs where trials can proceed if after 60 business days, the applicant has not received any objections from the CDE. China is also expanding the number of trial sites by changing from a clinical trial site certification procedure into a notification procedure.
Drug Clinical Trial Registration
Pursuant to the DRR, upon obtaining the clinical trial approval and before commencing a clinical trial, the applicant shall file a registration with the NMPA containing various details of the clinical trial, including the clinical study protocol, the name of the principal researcher of the leading institution, names of participating institutions and researchers, an approval letter from the ethics committee, and a sample of the Informed Consent Form, with a copy sent to the competent provincial administration departments where the trial institutions will be located. On September 6, 2013, the NMPA released the Announcement on Drug Clinical Trial Information Platform, providing that for all clinical trials approved by the NMPA and conducted in China, instead of the aforementioned registration filed with the NMPA, clinical trial registration shall be completed and trial information shall be published through the Drug Clinical Trial Information Platform. The applicant shall complete trial pre-registration within one month after obtaining the clinical trial approval to obtain the trial’s unique registration number and shall complete registration of certain follow-up information before the first subject’s enrollment in the trial. If approval of the foregoing pre-registration and registration is not obtained within one year after obtaining the clinical trial approval, the applicant shall submit an explanation, and if the procedure is not completed within three years, the clinical trial approval shall automatically be annulled.
Human Genetic Resources Approval
According to the Interim Measures for the Administration of Human Genetic Resources, promulgated by the Ministry of Science and Technology and the MOH jointly on June 10, 1998, an additional approval is required for any foreign companies or foreign affiliates that conduct trials in China. Prior to beginning a trial, the foreign sponsor and the Chinese clinical trial site are required to obtain approval from the Human Genetic Resources Administration of China, or HGRAC, which is an agency under the Ministry of Science and Technology, to collect any biological samples that contain the genetic material of Chinese human subjects, and to transfer any cross-border transfer of the samples or associated data. Furthermore, one of the key review points for the HGRAC review and approval process is the IP sharing arrangement between Chinese and foreign parties. The parties are required to share patent rights to inventions arising from the samples. Conducting a clinical trial in China without obtaining the relevant HGRAC preapproval will subject the sponsor and trial site to administrative liability, including confiscation of HGRAC samples and associated data, and administrative fines.
| 40 |
| Table of Contents |
On July 2, 2015, the Ministry of Science and Technology issued the Service Guide for Administrative Licensing Items concerning Examination and Approval of Sampling, Collecting, Trading, Exporting Human Genetic Resources, or Taking Such Resources out of the PRC, which provides that foreign-invested sponsors that sample and collect human genetic resources in clinical trials shall be required to file with the China Human Genetic Resources Management Office through its online system. On October 26, 2017, the Ministry of Science and Technology issued the Circular on Optimizing the Administrative Examination and Approval of Human Genetic Resources, which simplified the approval for sampling and collecting human genetic resources for the purpose of commercializing a drug in the PRC. On May 28, 2019, the State Council of PRC issued the Administration Regulations on Human Genetic Resources, which became effective on July 1, 2019. The Administration Regulations on Human Genetic Resources formalized the approval requirements pertinent to research collaborations between Chinese and foreign-owned entities. Pursuant to the new rule, a new notification system (as opposed to the advance approval approach originally in place) is put in place for clinical trials using China’s human genetic resources at clinical institutions without involving the export of human genetic resources outside of China.
Trial Exemptions and Acceptance of Foreign Data
The NMPA may reduce requirements for clinical trials and data, depending on the drug and the existing data. The NMPA has granted waivers for all or part of trials and has stated that it will accept data generated abroad (even if not part of a global study), including early phase data, that meets its requirements. On July 6, 2018, the NMPA issued the Technical Guidance Principles on Accepting Foreign Drug Clinical Trial Data, or the Guidance Principles, as one of the implementing rules for the Innovation Opinion. According to the Guidance Principles, the data of foreign clinical trials must meet the authenticity, completeness, accuracy and traceability requirements and such data must be obtained consistent with the relevant requirements under the GCP of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, or ICH. Sponsors must be attentive to potentially meaningful ethnic differences in the subject population.
The NMPA now officially permits, and its predecessor agencies have permitted on a case-by-case basis in the past, drugs approved outside of China to be approved in China on a conditional basis without the need for pre-approval clinical trials inside China. Specifically, on October 23, 2018, the NMPA issued the Procedures for Reviewing and Approval of Clinical Urgently Needed Overseas New Drugs, which established a program permitting drugs that have been approved within the last ten years in the United States, EU or Japan and that i) treat orphan diseases, ii) prevent or treat serious life-threatening illnesses for which there is either no effective therapy or prevention in China, or iii) prevent or treat serious life-threatening illnesses and the foreign-approved drug would have clear clinical advantages. Applicants will be required to establish a risk mitigation plan and may be required to complete trials in China after the drug is marketed. By May 29, 2019, the CDE has developed two lists of qualifying drugs that meet these criteria.
Clinical Trial Process and Good Clinical Practices
Typically drug clinical trials in China have four phases. Phase 1 refers to the initial clinical pharmacology and human safety evaluation studies. Phase 2 refers to the preliminary evaluation of a drug candidate’s therapeutic efficacy and safety for target indication(s) in patients. Phase 3 (often the pivotal study) refers to clinical trials to further verify the drug candidate’s therapeutic efficacy and safety in patients with target indication(s) and ultimately provide sufficient evidence for the review of a drug registration application. Phase 4 refers to a new drug’s post-marketing study to assess therapeutic effectiveness and adverse reactions when the drug is widely used to evaluate overall benefit-risk relationships of the drug when used among the general population or specific groups and to adjust the administration dose, etc. The NMPA requires that the different phases of clinical trials in China receive ethics committee approval and comply with the PRC’s GCP. The NMPA conducts inspections to assess the PRC’s GCP compliance and will cancel the CTA if it finds substantial issues.
On August 6, 2003, the NMPA promulgated the PRC’s GCP to improve the quality of clinical trials. According to the PRC’s GCP, the sponsor shall provide insurance to the subjects participating in the clinical trial and bear the cost of the treatment and the corresponding financial compensation for the subjects who suffer harm or death related to the trial. The sponsor shall provide legal and economic guarantee to the investigator, but harm or death caused by the medical accident shall be excluded. Pursuant to the Innovation Opinion, the accreditation of the institutions for drug clinical trials shall be subject to record-filing administration. The conduct of clinical trials must adhere to the PRC’s GCP, and the protocols must be approved by the ethics committees of each study site. Pursuant to the newly amended DAL, and the Regulations on the Administration of Drug Clinical Trial
Institution jointly promulgated by NMPA and NHC on November 29, 2019 and effective from December 1, 2019, drug clinical trial institutions shall be under filing administration. Entities that only conduct analysis of biological samples related to clinical trials of drugs do not need to be filed.
| 41 |
| Table of Contents |
New Drug Application (NDA) and Approval
Upon completion of clinical trials, a sponsor may submit clinical trial data to support marketing approval for the drug. For imported drugs, this means issuance of an import license. Again, the applicant must submit evidence of foreign approval, unless it is an innovative drug that has never been approved anywhere in the world.
NDA sponsors must submit data derived from domestically manufactured drugs in support of a drug approval. Under the current regime, upon approval of the registration application, the NMPA will first issue a new drug certificate to the applicant. Only when the applicant is equipped with relevant manufacturing capability will the NMPA issue a Drug Approval Serial Number, which is effectively the marketing approval allowing the holder to market/commercialize the drug in China.
Pursuant to the Opinions on the Reform of Evaluation and Approval System for Drugs and Medical Devices and Equipment promulgated on August 9, 2015, the State Council published the policy for carrying out a pilot plan for the drug marketing authorization holder mechanism.
Pursuant to the newly amended DAL, under the drug marketing authorization holder mechanism, an enterprise obtained drug registration certificate and a research and development institution are eligible to be a medical marketing authorization holder, and this medical marketing authorization holder shall be responsible for nonclinical laboratory studies, clinical trials, production and distribution, post-market studies, and the monitoring, reporting, and handling of adverse reactions in connection with medicals in accordance with the provisions of the DAL. The medical marketing authorization holder may engage contract manufacturers for manufacturing, provided that the contract manufacturers are licensed and may engage medical distribution enterprises with drug distribution license for the distribution activities. Upon the approval of the medical products administrative department under the State Council, a drug marketing authorization holder may transfer the drug marketing license and the transferee shall have the capability of quality management, risk prevention and control, and liability compensation to ensure the safety, effectiveness and quality controllability of drugs, and fulfill the obligations of the drug marketing license holder.
Manufacturing and Distribution
According to the newly amended DAL and the implementing Measures of the DAL, all facilities that manufacture drugs in China must receive a Drug Manufacturing License with an appropriate “scope of manufacturing” from the local drug regulatory authority. This license must be renewed every five years.
Similarly, to conduct sales, importation, shipping and storage, or distribution activities, a company must obtain a Drug Distribution License with an appropriate “scope of distribution” from the local drug regulatory authority, subject to renewal every five years.
China has formed a “Two Invoice System” to control distribution of drugs. The “Two-Invoice System” generally requires that no more than two invoices may be issued throughout the distribution chain, with one from the manufacturer to a distributor and another from the distributor to the end-user hospital. This excludes the sale of products invoiced from the manufacturer to its wholly owned or controlled distributors, or for imported drugs, to their exclusive distributor, or from a distributor to its wholly owned or controlled subsidiary (or between the wholly owned or controlled subsidiaries). However, the system still significantly limits the options for companies to use multiple distributors to reach a larger geographic area in China. Compliance with the Two-Invoice System will become a prerequisite for medical companies to participate in procurement processes with public hospitals, which currently provide most of China’s healthcare. Manufacturers and distributors that fail to implement the Two-Invoice System may lose their qualifications to participate in the bidding process. Non-compliant manufacturers may also be blacklisted from engaging in drug sales to public hospitals in a locality.
The Two-Invoice System was first implemented in 11 provinces that are involved in pilot comprehensive medical reforms, but the program has expanded to nearly all provinces, which have their own individual rules for the program.
Human Cell Therapy
On March 20, 2003, the NMPA published the Technical Guidelines for Research on Human Cell Therapy and Quality Control of Preparations, which set some principles for the research of human cell therapy.
Pursuant to the DRR promulgated by the NMPA on July 10, 2007 and effective from October 1, 2007, human cell therapy and its products belong to biological products and the application for biological products shall be submitted as the process of new drug application.
On March 2, 2009, the MOH published the Management Measures for Clinical Application of Medical Technology, which came into effect on May 1, 2009 and prescribed that cell immunotherapy belongs to the Category 3 medical technology of which the clinical application shall be subject to the additional provisions of the MOH. In May, 2009, the MOH published the First List of Category 3 Medical Technologies Allowed for Clinical Application, or the Category 3 Medical Technologies which prescribed cell immunotherapy technology as Category 3 medical technologies were allowed for clinical application, and was abolished by the Notice on the Relevant Work Concerning Cancellation of the Category Three of Medical Technology Entry Approval of Clinical Application on June 29, 2015. The Notice on the Relevant Work Concerning Cancellation of the Category Three of Medical Technology Entry Approval of Clinical Application also cancelled the approval of Category 3 medical technology clinical application.
On November 30, 2017, the CFDA promulgated the Notice of Guidelines for Acceptance and Examination of Drug Registration (Trial), the application of clinical trials of therapeutic biological products and the production and listing application of therapeutic biological products shall be subject to the provisions thereof. On December 18, 2017, the CFDA promulgated the Technical Guiding Principles for Research and Evaluation of Cell Therapy Products (Trial) to regulate and guide the research and evaluation of cell therapy products that are researched on, developed and registered as drugs.
| 42 |
| Table of Contents |
Post-Marketing Surveillance
Pursuant to the newly amended DAL, the drug marketing authorization holder shall be responsible for the monitoring, reporting and handling of adverse reactions in connection with medicals in accordance with the provisions of the DAL. Marketing authorization holders, medical manufacturer, medical distributors and medical institutions shall regularly inspect the quality, efficacy and adverse reactions of drugs manufactured, distributed and used by them. Cases of suspected adverse reactions shall be promptly reported to the drug administrative authorities and the competent health administrative authority. The drug marketing authorization holder shall forthwith stop selling, notify the relevant medical distributors and medical institutions to stop sales and use, recall sold drugs, promptly announce recall information if the drugs have quality issues or other safety hazards.
Advertising and Promotion of Medical Products
China has a strict regime for the advertising of approved drugs. No unapproved drugs may be advertised. The definition of an advertisement is very broad and it can be any media that directly or indirectly introduces the product to end users. There is no clear line between advertising and any other type of promotion.
Each advertisement for drugs requires an approval from a local drug regulatory authority, and the content of an approved advertisement may not be altered without filing a new application for approval. An enterprise seeking to advertise a prescription drug may do so only in medical journals jointly approved by NMPA and the NHC, and the advertisement for a prescription drug shall tag “this advertisement is for medical and medical professionals reading only.”
Drug advertisements are subject to strict content restrictions, which prohibit recommendations by doctors and hospitals and guarantees of effectiveness. Advertising that includes content that is outside of the drug’s approval documentation, off-label content, is prohibited. False advertising can result in civil suits from end users and administrative liability, including fines. In addition to advertisements, non-promotional websites that convey information about a drug must go through a separate approval process by a local drug regulatory authority.
| 43 |
| Table of Contents |
Product Liability
The Product Quality Law of the PRC, or the Product Quality Law promulgated by the Standing Committee of the NPC on February 22, 1993 and amended on July 8, 2000, August 27, 2009 and December 29, 2018, respectively, is the principal governing law relating to the supervision and administration of product quality. According to the Product Quality Law, manufacturers shall be liable for the quality of products produced by them, and sellers shall take measures to ensure the quality of the products sold by them. A manufacturer shall be liable for compensating for any bodily injuries or property damages, other than the defective product itself, resulting from the defects in the product, unless the manufacturer is able to prove that (1) the product has never been distributed; (2) the defects causing injuries or damages did not exist at the time when the product was distributed; or (3) the science and technology at the time when the product was distributed was at a level incapable of detecting the defects. A seller shall be liable for compensating for any bodily injuries or property damages of others caused by the defects in the product if such defects are attributable to the seller. A seller shall pay compensation if it fails to indicate either the manufacturer or the supplier of the defective product. A person who is injured or whose property is damaged by the defects in the product may claim for compensation from the manufacturer or the seller.
Pursuant to the General Principles of the Civil Law of the PRC promulgated by the NPC on April 12, 1986 and amended on August 27, 2009, both manufacturers and sellers shall be held liable where the defective products result in property damages or bodily injuries to others. Pursuant to the Tort Liability Law of the PRC promulgated by the Standing Committee of the NPC on December 26, 2009 and effective from July 1, 2010, manufacturers shall assume tort liabilities where the defects in products cause damages to others. Sellers shall assume tort liabilities where the defects in products that have caused damages to others are attributable to the sellers. The aggrieved party may claim for compensation from the manufacturer or the seller of the defected product that has caused damage.
Commercial Bribery
Medical companies involved in a criminal investigation or administrative proceedings related to bribery are listed in the Adverse Records of Commercial Briberies by their respective provincial health and family planning administrative department. Pursuant to the Provisions on the Establishment of Adverse Records of Commercial Briberies in the Medicine Purchase and Sales Industry which were promulgated by the NHFPC on December 25, 2013 and became effective on March 1, 2014, provincial health and family planning administrative departments formulate the implementing measures for establishment of Adverse Records of Commercial Briberies. Where a medical company or its agent is listed in the Adverse Records of Commercial Briberies on one occasion, it will be prohibited from participating in the procurement bidding process or selling its products to public medical institutions located in the local provincial-level region for two years from the publication of the adverse records. Where a medical company or its agent is listed in the Adverse Records of Commercial Briberies on two or more occasions within five years, it will be prohibited from participating in the procurement bidding process or selling its products to all public medical institutions in the PRC for two years from the publication of these adverse records.
Foreign Exchange Regulation
Pursuant to the Foreign Currency Administration Rules promulgated in 1996 and amended in 1997 and various regulations issued by the State Administration of Foreign Exchange (“SAFE”), and other relevant PRC government authorities, the Renminbi is freely convertible only to the extent of current account items, such as trade-related receipts and payments, interest and dividends. Capital account items, such as direct equity investments, loans and repatriation of investments, require the prior approval from the SAFE or its local counterpart for conversion of Renminbi into a foreign currency, such as U.S. dollars, and remittance of the foreign currency outside the PRC.
Payments for transactions that take place within the PRC must be made in Renminbi. Unless otherwise approved, PRC companies must repatriate foreign currency payments received from abroad. Foreign-invested enterprises may retain foreign exchange in accounts with designated foreign exchange banks subject to a cap set by the SAFE or its local counterpart. Unless otherwise approved, domestic enterprises must convert all of their foreign currency receipts into Renminbi.
| 44 |
| Table of Contents |
Pursuant to the SAFE’s Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Inbound Investment via Overseas Special Purpose Vehicles, or the SAFE Circular No. 75, issued on October 21, 2005:
|
| (i) | a PRC citizen residing in the PRC, or PRC resident, shall register with the local branch of the SAFE before it establishes or controls an overseas special purpose vehicle (“overseas SPV”), for the purpose of overseas equity financing (including convertible debts financing); |
|
|
|
|
|
| (ii) | when a PRC resident contributes the assets of or its equity interests in a domestic enterprise into an overseas SPV, or engages in overseas financing after contributing assets or equity interests into an overseas SPV, such PRC resident shall register his or her interest in the overseas SPV and the change thereof with the local branch of the SAFE; and |
|
|
|
|
|
| (iii) | when the overseas SPV undergoes a material event outside of China, such as change in share capital or merger and acquisition, the PRC resident shall, within 30 days from the occurrence of such event, register such change with the local branch of the SAFE. |
On May 29, 2007, the SAFE issued relevant guidance to its local branches for the implementation of the SAFE Circular No. 75. This guidance standardizes more specific and stringent supervision on the registration requirement relating to the SAFE Circular No. 75 and further requires PRC residents holding any equity interests or options in SPVs, directly or indirectly, controlling or nominal, to register with the SAFE.
Our beneficial owners are PRC residents who have registered with the local branch of the SAFE as required under SAFE Circular No. 75.
Under the Implementing Rules of Measures for the Administration of Individual Foreign Exchange, or the Implementation Rules, issued by the SAFE on January 5, 2007, PRC citizens who are granted shares or share options by an overseas listed company according to its share incentive plan are required, through a qualified PRC agent or the PRC subsidiary of such overseas listed company, to register with the SAFE and complete certain other procedures related to the share incentive plan. Foreign exchange income received from the sale of shares or dividends distributed by the overseas listed company must be remitted into a foreign currency account of such PRC citizen or be exchanged into Renminbi.
Taxation
Under the Enterprise Income Tax Law (“EIT”), effective January 1, 2008, China will adopt a uniform tax rate of 25.0% for all enterprises (including foreign-invested enterprises) and revoke the current tax exemption, reduction and preferential treatments applicable to foreign-invested enterprises. However, there will be a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatment granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25.0% may continue to enjoy the lower rate and gradually transition to the new tax rate within five years after the effective date of the EIT Law. Enterprises that are currently entitled to exemptions or reductions from the standard income tax rate for a fixed term may continue to enjoy such treatment until the fixed term expires. However, the two-year exemption from enterprise income tax for foreign-invested enterprise will begin from January 1, 2008 instead of from when such enterprise first becomes profitable. Preferential tax treatments will continue to be granted to industries and projects that are strongly supported and encouraged by the state, and enterprises otherwise classified as “new and high technology enterprises strongly supported by the state” will be entitled to a 15.0% enterprise income tax rate even though the EIT Law does not currently define this term.
| 45 |
| Table of Contents |
Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors
On August 8, 2006, six PRC regulatory agencies, including the Chinese Securities Regulatory Commission (“CSRC”), promulgated a rule entitled Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (“the new M&A rule”) to regulate foreign investment in PRC domestic enterprises. The new M&A rule provides that the Ministry of Commerce must be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise and any of the following situations exists:
| (I) | the transaction involves an important industry in China; | |
|
|
|
|
| (ii) | the transaction may affect national “economic security;” or | |
|
|
|
|
| (iii) | the PRC domestic enterprise has a well-known trademark or historical Chinese trade name in China. |
The new M&A rule also contains a provision requiring overseas SPVs, formed for listing purposes through acquisitions of PRC domestic companies and controlled by PRC individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange.
On September 21, 2006, the CSRC issued a clarification that sets forth the criteria and process for obtaining any required approval from the CSRC. To date, the application of this new M&A rule is unclear.
Anti-Corruption.
The substantial majority of hospitals in China are owned and operated by the government, and revenue from hospital pharmacies constitutes a significant portion of hospitals’ revenue. Hospitals procure their supplies of medical products in bulk from manufacturers or distributors of medical products, and generally decide whether to include a particular medicine on their formulary based upon a number of factors, including doctors’ preference in prescribing the medicine, the cost of the medicine, the perceived efficacy of the medicine and the hospital’s budget. Decisions by hospitals regarding whether to include a particular medicine in their pharmacies could be affected by corrupt practices, including illegal kickbacks and other benefits offered by manufacturers or distributors of medical products. These corrupt practices may also affect doctors’ decisions regarding which types of medicine to prescribe.
The PRC government has strengthened its anti-corruption measures and has organized a series of government-sponsored anti-corruption campaigns in recent years. In particular, China amended its criminal code in 2006, increasing the penalties for corrupt business practices. The amendment of the criminal code is expected to make medical product suppliers compete for the hospitals’ business on fair and equal terms, and thus is expected to result in more growth opportunities for drugstores that are not affiliated with hospitals.
Medical Product Labeling and Prescription Management
The PRC SFDA promulgated medical product labeling regulations in March 2006, which require that medical product labels state the generic ingredients of the medical products and which bar the registration of any brand name for any medical product which does not contain active ingredients. In addition, effective May 1, 2007, doctors are not permitted to include brand names in their prescriptions and required to specify the chemical ingredients of the medicines they prescribe in their prescription. These requirements are expected to have the following positive impacts on the business of non-hospital drugstores:
|
| · | help curb corrupt practices by medical product manufacturers and doctors; |
|
| · | ensure that patients are given better information on the medicines they purchase; and |
|
| · | weaken the hospitals’ monopoly on prescriptions and prescription medical products. |
Description of Property
Our headquarters are located at Room 105, Building 5, 31 Xishiku Avenue, Xicheng District, Beijing, PRC and consist of approximately 746 square meters of office space. The lease for our headquarters has an initial term of two years and four months from August 2, 2019 to November 30, 2021 with a right to renew for an additional term of two years and eight months from December 1, 2021 to July 31, 2024. The lease also provides for quarterly rent and management fees totaling RMB4,756,649 (approximately $728,586) during the initial term of the lease, subject to increase thereafter.
Effective March 1, 2021, we, through Yubo Global, entered into a lease agreement with Chengdu Liangkang Investment Co. to rent approximately 6,960 square meters of laboratory space in Chengdu China. The lease provides for a lease term of five years from March 1, 2021 to February 28, 2026. The lease also provides for payments of monthly rent to the landlord of approximately RMB 300,000 ( approximately $45,658 at the 6.5706 exchange rate as of March 31, 2021).
Employees
We currently employ 20 employees at our headquarters, located at Room 105, Building 5, 31 Xishiku Avenue, Xicheng District, Beijing, PRC. Our employees include three executive officers, two financial department personnel, two administrative management personnel, six R&D and product personnel, six planning and marketing personnel, and one person in IT management. With the expansion of our business and establishment of our stem cell bank in Chengdu, China, we plan to increase our recruitment efforts in the fields of technology, research and development and marketing, and we expect to employ approximately 60 employees in 2022. In order to attract, retain and reward top talents in the industry, we are considering establishing various incentive program for our employees, and we may establish an equity incentive plan in the future.
| 46 |
| Table of Contents |
Legal Proceedings
We are currently not a party to any material legal or administrative proceedings. We may from time to time be subject to various legal or administrative claims and proceedings arising in the ordinary course of our business, including matters involving our franchisees, among others. Any litigation or other legal or administrative proceedings, regardless of the outcome, are likely to result in substantial costs and a diversion of our resources, including our management’s time and attention.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information. Our Class A common stock has been quoted on the OTC Marketplace under the symbol “YBGJ”. Previously, it traded under the symbol “MAGAA” until December 4, 2020. There can be infrequent trading volume, which precipitates wide spreads in the “bid” and “ask” quotes of our common stock, on any given day. On July 9, 2021, the last reported sale price of our Class A common stock on the OTC Marketplace was $0.65 per share.
The following table sets forth, for the quarters indicated, the high and low bid prices per share of our Class A common stock on the OTC Marketplace, reported by the Financial Industry Regulatory Authority Composite Feed or other qualified interdealer quotation medium. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
| Quarter Ended |
| High |
|
| Low |
| ||
| July 1, 2021 through July 12, 2021 |
| $ | 1.00 |
|
| $ | 0.50 |
|
| June 30, 2021 |
| $ | 1.30 |
|
| $ | 0.23 |
|
| March 31, 2021 |
| $ | 3.98 |
|
| $ | 1.23 |
|
| December 31, 2020 |
| $ | 3.47 |
|
| $ | 0.40 |
|
| September 30, 2020 |
| $ | 0.72 |
|
| $ | 0.25 |
|
| June 30, 2020 |
| $ | 0.37 |
|
| $ | 0.11 |
|
| March 31, 2020 |
| $ | 0.15 |
|
| $ | 0.11 |
|
| December 31, 2019 |
| $ | 0.28 |
|
| $ | 0.11 |
|
| September 30, 2019 |
| $ | 0.25 |
|
| $ | 0.15 |
|
| June 30, 2019 |
| $ | 0.30 |
|
| $ | 0.12 |
|
| March 31, 2019 |
| $ | 0.80 |
|
| $ | 0.30 |
|
| December 31, 2018 |
| $ | 0.11 |
|
| $ | 0.28 |
|
| September 30, 2018 |
| $ | 0.80 |
|
| $ | 0.30 |
|
| June 30, 2018 |
| $ | 0.70 |
|
| $ | 0.20 |
|
| March 31, 2018 |
| $ | 0.70 |
|
| $ | 0.20 |
|
Holders. As of July 12, 2021, there were approximately 462 Class A common stockholders and 65 Class B common stockholders of record. The number of stockholders of record does not include beneficial owners of our common stock, whose shares are held in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Dividends. We have never declared or paid a cash dividend on our Class A common stock. We do not expect to pay cash dividends on our common stock in the foreseeable future. We currently intend to retain our earnings, if any, for use in our business. Any dividends declared in the future will be at the discretion of our Board and subject to any restrictions that may be imposed by our lenders.
Penny Stock Regulation. Shares of our Class A common stock will probably be subject to rules adopted by the SEC that regulate broker-dealer practices in connection with transactions in “penny stocks.” Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in those securities is provided by the exchange or system). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the SEC, which contains the following:
| · | a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; | |
| · | a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to violation to such duties or other requirements of securities’ laws; | |
| · | a brief, clear, narrative description of a dealer market, including “bid” and “ask” prices for penny stocks and the significance of the spread between the “bid” and “ask” price; | |
| · | a toll-free telephone number for inquiries on disciplinary actions; | |
| · | definitions of significant terms in the disclosure document or in the conduct of trading in penny stocks; and | |
| · | such other information and is in such form (including language, type, size and format), as the SEC shall require by rule or regulation. |
| 47 |
| Table of Contents |
Prior to effecting any transaction in penny stock, the broker-dealer also must provide the customer the following:
| · | the bid and offer quotations for the penny stock; | |
| · | the compensation of the broker-dealer and its salesperson in the transaction; | |
| · | the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and | |
| · | monthly account statements showing the market value of each penny stock held in the customer’s account. |
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for a stock that becomes subject to the penny stock rules. Holders of shares of our common stock may have difficulty selling those shares because our common stock will probably be subject to the penny stock rules.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with Platinum’s unaudited financial statements for the three months ended March 31, 2021 and 2020 and audited financial statements for the years ended December 31, 2019 and 2020 together with notes thereto. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited, to those set forth under “Risk Factors” and elsewhere in this prospectus. The audited financial statements for the period from March 1, 2020 to December 31,2020 and the years ended February 29, 2020 and February 28, 2019 of Yubo International Biotech Limited can be found in the Transition Report on Form 10-KT filed with the Commission on April 19, 2021.
Unless otherwise provided in this section,, the terms “we,” “our,” “us,” or “the “Company” refer to Platinum International Biotech Co., Ltd., a company organized under the laws of the Cayman Islands, and its wholly-owned subsidiaries, including without limitation, Yubo International Biotech (Beijing) Limited, a company organized under the laws of the People’s Republic of China.
Overview
We are a technology company focused on the research and development and application of endometrial stem cells. We are committed to building the first public endometrial stem cell repository in the world. We offer our products and services under the brand “VIVCELL”. Our product offerings include healthcare products for respiratory system, skincare products, hair care products, healthy beverages and male and female personal care products. We also offer stem cell related services including cell testing and health management consulting services.
Our previous shell company’s results of operations are immaterial and will not be included in the discussion below. Key factors affecting our results of operations include revenues, cost of revenues, operating expenses and income and taxation.
Critical Accounting Policy and Estimates
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations section discusses our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of income and expenses during the reporting period. On an on-going basis, management evaluates its estimates and judgments, including those related to accrued expenses, financing operations, and contingencies and litigation. Management bases its estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. The most significant accounting estimates inherent in the preparation of our financial statements include estimates as to the appropriate carrying value of certain assets and liabilities which are not readily apparent from other sources. We consider certain accounting policies related to fair value measurements and earnings per share to be critical accounting policies that require the use of significant judgments and estimates relating to matters that are inherently uncertain and may result in materially different results under different assumptions and conditions.
| 48 |
| Table of Contents |
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Principles of Consolidation
The consolidated financial statements include our accounts, our wholly owned subsidiaries, and its consolidated VIE for which we are the primary beneficiary.
All transactions and balances among us, our subsidiaries and consolidated VIE have been eliminated upon consolidation.
See Note 2: Summary of Significant Accounting Policies , in the notes to our consolidated financial statements includ ed elsewhere in this prospectus.
Leases
We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liabilities - current, and operating lease liabilities - noncurrent on the balance sheets. The initial lease liability is equal to the future fixed minimum lease payments discounted using our incremental borrowing rate, on a secured basis. The initial measurement of the right-of-use asset is equal to the initial lease liability plus any initial direct costs.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term.
Revenue Recognition
We derive our revenue from the sale of nebulizers containing frozen tubes with medical fluid, which are sold as one unit to our customers, and cell basidiomycetes compound drink, which was launched in early 2021. Our nebulizer s and cell basidiomycetes compound drink have a shelf life of 12 months and 18 months, respectively, if kept under regular room temperature. The nebulizers are sold directly to consumers on our online e-commerce platform. We recognize product revenues when the following four revenue recognition criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the selling price is fixed or determinable, and (iv) collectability is reasonably assured. We do not allow sales returns or exchanges. Revenue is recorded net of value-added tax (“VAT”).
Recently Issued and Adopted Accounting Pronouncements
In February 2016, the FASB issued Accounting Standards Update No. 2016-02 (ASU 2016-02) “Leases (Topic 842)”. ASU 2016-02 requires a lessee to recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term. ASU 2016-02 is effective for interim and annual reporting periods beginning after December 15, 2018. Early adoption is permitted.
Results of Operations
For the Three Months Ended March 31, 2021 Compared to the Three Months Ended March 31, 2020
Sales
We generated sales of $462,329 for the three months ended March 31, 2021, as compared to $0 for the three months ended March 31, 2020. Such increase was primarily due to sale of nebulizers and the launch of our cell basidiomycetes compound drink .
| 49 |
| Table of Contents |
Cost of Goods Sold
Our cost of goods sold was $146,738 for the three months ended March 31, 2021, as compared to $0 for the three months ended March 31, 2020. Such increase was primarily due to cost of nebulizers sold and the launch of our cell basidiomycetes compound drink .
Gross Profit
As a result, our gross profit increased from $0 for the three months ended March 31, 2020 to $315,591 for the three months ended March 31, 2021. Our gross profit margin d ecreased as compared to that for the year ended December 31, 2020 , primarily due to the launch of our cell basidiomycetes compound drink, which has a lower gross profit margin than our nebulizers.
Operating Expenses
Our operating expenses were $754,161 for the three months ended March 31, 2021, as compared to $140,902 for the three months ended March 31, 2020. The increase in operating expenses was primarily due to sales commissions, employee compensation, occupancy, and other operating expenses.
Loss from Operations
Our loss from operations was $(438,570) for the three months ended March 31, 2021, as compared to $(140,902) for the three months ended March 31, 2020. The increase in loss from operations was due to the $613,259 increase in operating expenses offset by the $315,591 increase in gross profit.
Other Income (Expense)
Our other income (expense) was $(105) for the three months ended March 31, 2021, as compared to $(46) for the three months ended March 31, 2020. The increase in other expense was primarily due to an increase in bank charge s .
Net Loss
Our net loss was $(438,675) for the three months ended March 31, 2021, as compared to $(140,948) for the three months ended March 31, 2020. The increase in net loss was primarily due to the $613,259 increase in operating expenses offset by the $315,591 increase in gross profit.
For Years ended December 31, 2020 and 2019
Sales
Our sales were $1,353,868 for the year ended December 31, 2020, as compared to $0 for the year ended December 31, 2019. The increase in sales was due to sale of nebulizers.
| 50 |
| Table of Contents |
Cost of Goods Sold
Our cost of goods sold was $114,272 for the year ended December 31, 2020, as compared to $0 for the year ended December 31, 2019. The increase in cost of goods sold was due to cost of nebulizers sold, which mainly consists of costs of the medical fluid, nebulizer and packaging materials.
Gross Profit
Our gross profit was $1,239,596 for the year ended December 31, 2020, as compared to $0 for the year ended December 31, 2019. The increase in gross profit was due to sale of nebulizers.
Operating Expenses
Our operating expenses were $1,951,394 for the year ended December 31, 2020, as compared to $231,066 for the year ended December 31, 2019. The increase in operating expenses was primarily due to increases in sales commissions, employee compensation, occupancy, and other operating expenses in connection with the sale of our nebulizers, which product was launched in 2020. We did not have any commercialized products in 2019 and our operating expenses in 2019 mainly consisted of employees’ salaries, rent and other operating expense. We currently record our research and development expenses under other operating expense, and our research and development expenses were $40,000 and $0 for the years ended December 31, 2020 and 2019, respectively. Our operating expenses increased significantly in 2020 as we launched our first commercialized product. The following table sets for t h a breakdown of our operating expenses for the years ended December 31, 2020 and 2019:
|
|
| 2020 |
|
| 2019 |
| ||
| Sales commissions |
| $ | 660,963 |
|
| $ | - |
|
| Employee compensation |
|
| 260,689 |
|
|
| 4,951 |
|
| Occupancy |
|
| 279,191 |
|
|
| 101,702 |
|
| Depreciation and amortization of property and equipment |
|
| 8,966 |
|
|
| 91 |
|
| Amortization of intangible assets |
|
| 4,096 |
|
|
| - |
|
| Other operating expenses |
|
| 737,489 |
|
|
| 124,322 |
|
| Total Operating Expenses |
| $ | 1,951,394 |
|
| $ | 231,066 |
|
Income (Loss) from Operations
Our income (loss) from operations was $(711,798) for the year ended December 31, 2020, as compared to $(231,066) for the year ended December 31, 2019. due to the $1,720,328 increase in operating expenses, partially offset by the $1,239,596 increase in gross profit.
Other Income (expense)
Our other expense was $3 for the year ended December 31, 2020, as compared to $127 for the year ended December 31, 2019. The decrease in other expense was primarily due to a decrease in bank charges.
Net Loss
Our net loss was $(711,801) for the year ended December 31, 2020, as compared to $(231,193) for the year ended December 31, 2019. The increase in net loss was primarily due to the $1,720,328 increase in operating expenses, partially offset by the $1,239,596 increase in gross profit.
For the years ended December 31, 2019 and 2018
Sales
Our sales were $0 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018.
Cost of Goods Sold
Our cost of goods sold was $0 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018.
Gross Profit
Our gross profit was $0 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018.
Operating Expenses
Our operating expenses were $231,066 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in operating expenses was primarily due to increases in occupancy (resulting from the Beijing China office space lease commencing in August 2019) and other operating expenses.
| 51 |
| Table of Contents |
Loss from Operations
Our loss from operations was $(231,066) for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in loss from operations was due to the increase in operating expenses.
Other Income (Expense)
Our other income (expense) was $(127) for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in other expense was primarily due to an increase in bank charges.
Net Loss
Our net loss was $(231,193) for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in net loss was primarily due to the increase in operating expenses.
Liquidity and Capital Resources
As of March 31, 2021, we had cash and cash equivalents on hand of $126,313 and working capital of $(287,273). Generally, the primary sources of our funds have been cash from operations and capital contributions. We estimate that the net proceeds of this offering will be approximately $2.42 million. We believe that our cash on hand and working capital will be sufficient to meet our anticipated cash requirements through the third quarter of 2021. We intend to continue working toward identifying and obtaining new sources of financing and we intend to raise additional capital in the fourth quarter of 2021. No assurances can be given that we will be successful in obtaining additional financing in the future. Any future financing that we may obtain may cause significant dilution to existing stockholders. Any debt financing or other financing of securities senior to common stock that we are able to obtain will likely include financial and other covenants that will restrict our flexibility. Any failure to comply with these covenants would have a negative impact on our business, prospects, financial condition, results of operations and cash flows.
If adequate funds are not available, we may be required to delay, scale back or eliminate portions of our operations, cease operations or obtain funds through arrangements with strategic partners or others that may require us to relinquish rights to certain of our assets. Accordingly, the inability to obtain such financing could result in a significant loss of ownership and/or control of our assets and could also adversely affect our ability to fund our continued operations and our expansion efforts.
During the next 12 months, we expect to incur significant research and development expenses with respect to our products. The majority of our research and development activity is focused on development of our stem cell bank.
We also expect to incur significant legal and accounting costs in connection with being a public company. We expect those fees will be significant and will continue to impact our liquidity. Those fees will be higher as our business volume and activity increases.
Net cash provided by (used in) operating activities
Net cash used in operating activities was $1,002,578 for the three months ended March 31, 2021, as compared to $491,589 for the three months ended March 31, 2020. The increase in net cash used in operating activities was primarily due to increase s in receivables and prepaid expenses .
Net cash provided by (used) in operating activities was $101,107 for the year ended December 31, 2020, as compared to $(682,121) for the year ended December 31, 2019. The increase in net cash provided by (used) in operating activities was primarily due to advances from prospective customers/distributors
Net cash used in operating activities was $682,121 for the year ended December 31, 2019, as compared to cash provided in operating activities of $145 for the year ended December 31, 2018. The increase in net cash used in operating activities was primarily due to the $231,193 net loss in 2019 and the $399,251 increase in due from related parties.
| 52 |
| Table of Contents |
Net cash provided by (used in) investing activities
Net cash used in investing activities was $451,555 for the three months ended March 31, 2021, as compared to $48,284 for the three months ended March 31, 2020. The increase in net used in investing activities was primarily due to purchases of property and equipment .
Net cash used in investing activities was $97,468 for the year ended December 31, 2020, as compared to $36,983 for the year ended December 31, 2019. The increase in net cash used in investing activities was primarily due to purchase of intangible assets.
Net cash used in investing activities was $36,983 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in net cash used in investing activities was due to purchases of property and equipment in 2019.
Net cash provided by financing activities
Net cash provided by financing activities was $127,164 for the three months ended March 31, 2021, as compared to $504,892 for the three months ended March 31, 2020. The decrease in net cash provided by financing activities was primarily due to the decrease of capital contributions to Yubo.
Net cash provided by financing activities was $1,384,756 for the year ended December 31, 2020, as compared to $723,861 for the year ended December 31, 2019. The increase in net cash provided by financing activities was primarily due to the sale of ordinary shares on September 11, 2020.
Net cash provided by financing activities was $723,861 for the year ended December 31, 2019, as compared to $0 for the year ended December 31, 2018. The increase in net cash provided by financing activities was due to capital contributions to Yubo.
Going Concern
The accompanying interim unaudited condensed consolidated financial statements for the three months ended March 31, 2021 and 2020 and our audited financial statements for the fiscal years ended December 31, 2020 and 2019 included an explanatory paragraph referring to our recurring operating losses and expressing substantial doubt in our ability to continue as a going concern. Our consolidated financial statements have been prepared on a going concern basis, which assumes the realization of assets and settlement of liabilities in the normal course of business. Our ability to continue as a going concern is dependent upon our ability to generate profitable operations in the future and/or to obtain the necessary financing to meet our obligations and repay our liabilities arising from normal business operations when they become due. The outcome of these matters cannot be predicted with any certainty at this time and raise substantial doubt that we will be able to continue as a going concern. Our consolidated financial statements do not include any adjustments to the amount and classification of assets and liabilities that may be necessary should we be unable to continue as a going concern.
Current Liabilities
In December 2020, Yubo received advances totaling ¥4,948,000 (approximately $757,896) from nine PRC entities who are our prospective customers and distributors. The related verbal agreements provide for the nine entities to purchase inventory from Yubo or enter into such other arrangements with Yubo as the parties mutually agree. Pending formal approval of any such arrangements, all of the eight PRC entities have the right to request the return of their advances.
We also had certain short-term borrowings from our directors totaling $243,556 and $91,951 as of March 31, 2021 and December 31, 2020, respectively. See “Certain Relationships and Related Transactions and Director Independence-Related Party Transaction.”
| 53 |
| Table of Contents |
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
Our principal commitments consist of obligations under our operating leases. The following table sets forth Platinum’s principal commitments as of March 31, 2021:
|
|
| Payments due by period |
| |||||||||||||||||
| ($ in millions) |
| Total |
|
| Less than 1 year |
|
| 1-3 years |
|
| 4-5 years |
|
| More than 5 years |
| |||||
| Operating lease obligations |
| $ | 2.99 |
|
| $ | 0.65 |
|
| $ | 1.09 |
|
| $ | 1.15 |
|
| $ | 0.10 |
|
The commitment amounts in the table above are associated with contracts that are enforceable and legally binding and that specify all significant terms. The table above does not include obligations under agreements that we can cancel without a significant penalty.
Off-Balance Sheet Arrangements
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
| 54 |
| Table of Contents |
The following table sets forth certain information as of the date of this prospectus, with respect to our directors, executive officers and significant employees.
| Name | Age | Position | ||
| Jun Wang | 54 | Director, President and Chief Executive Officer | ||
| Lina Liu | 41 | Chief Financial Officer, Treasurer and Secretary | ||
| Yang Wang |
| 41 |
| Director |
| Zhihui Bai |
| 36 |
| Director |
Biographies of Directors, Executive Officers and Significant Employees
Jun Wang. Mr. Wang was appointed as our Chief Executive Officer, President and Director in 2020. He continues to serve as President of Yubo from 2019 to present. From 2015 to 2019, Mr. Wang served as President of Borongtai Asset Management (Beijing) Co., Ltd. He graduated with a Bachelor’s degree from Tianjin Commercial University, Department of Business Management. In making the decision to appoint Mr. Wang to serve as a director, the Board considered, in addition to the criteria referred to above, his extensive marketing experience in the healthcare industry, current service as our Chief Executive Officer and his comprehensive knowledge of Yubo, its business and operations.
Yang Wang. Mr. Wang was appointed as our Director in 2020. He has served as General Manager of Yubo from 2019 to present. From 2015 to 2019, Mr. Wang served as General Manager of Beijing Zunsheng Investment Consulting Co., Ltd. Additionally, he has worked for Horwath Financing Asia Limited, Mingli CHINA Growth Fund, Peking University Shangshuai Alumni Industry Investment Fund and Zhonsheng Capital Partners. He graduated with an MBA from New York Institute of Technology. Mr. Wang’s experience in the capital markets and mergers and acquisitions were the primary qualifications that the Board considered in appointing him as a director of the Company.
Zhihui Bai. Mr. Bai was appointed as our Director in 2020. He has served as General Manager of Beijing Zhenhuikang Biotech Co.LTD from 2015 to present. He graduated with a Master’s degree from Sofia University. Mr. Bai’s experience in the stem cell industry, including in product design, selection and production were the primary qualifications that the Board considered in appointing him as a director of the Company.
Lina Liu. Ms. Liu was appointed as our Chief Financial Officer, Treasurer and Secretary in 2020. She has served as Chief Financial Officer of Yubo from 2019 to present. From 2015 to 2019, she served as Chief Financial Officer of Borongtai Asset Management (Beijing) Co., Ltd. Additionally, she has over ten years of experience working for Ernst & Young. Ms. Liu graduated with a Master of Accounting from the Central University of Finance and Economics.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers, and any persons who own more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. SEC regulation requires executive officers, directors and greater than 10% stockholders to furnish us with copies of all Section 16(a) forms they file. Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during the year ended December 31, 2020, our executive officers, directors, and greater than 10% stockholders complied with all applicable filing requirements, with the exception of a Form 3 for Ms. Lina Liu, our Chief Financial Officer, Treasurer and Secretary, which was filed 3 days later than the required reporting deadline.
Family Relationships
There are no family relationships among our directors or executive officers.
| 55 |
| Table of Contents |
Terms of Office of Directors
The Company’s directors are appointed for a one-year term to hold office until the next annual general meeting of the Company’s stockholders or until removed from office in accordance with the Company’s bylaws and the provisions of the New York Business Corporation Law (the “NYBCL”). The Company’s directors hold office after the expiration of his or her term until his or her successor is elected and qualified, or until he or she resigns or is removed in accordance with the Company’s bylaws and the provisions of the NYBCL.
The Company’s officers are appointed by the Company’s Board of Directors and hold office until removed by the Board.
Involvement in Certain Legal Proceedings
No director, executive officer, significant employee or control person of the Company has been involved in any legal proceeding listed in Item 401(f) of Regulation S-K in the past 10 years.
Committees of the Board
Our Board of Directors held no formal meetings during the 12-month period ended December 31, 2020. All proceedings of the Board of Directors were conducted by resolutions consented to in writing by the directors and filed with the minutes of the proceedings of the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the NYBCL and the bylaws of our company, as valid and effective as if they had been passed at a meeting of the directors duly called and held. We do not presently have a policy regarding director attendance at meetings.
We do not currently have standing audit, nominating or compensation committees, or committees performing similar functions. Due to the size of our board, our Board of Directors believes that it is not necessary to have standing audit, nominating or compensation committees at this time because the functions of such committees are adequately performed by our Board of Directors. We do not have an audit, nominating or compensation committee charter as we do not currently have such committees. We do not have a policy for electing members to the board. Neither our current nor proposed directors are independent directors as defined in the NASDAQ listing standards.
We intend to form separate compensation, nominating and audit committees, with the audit committee including an audit committee financial expert, after the completion of this offering.
Audit Committee
Our Board of Directors has not established a separate audit committee within the meaning of Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Instead, the entire Board of Directors acts as the audit committee within the meaning of Section 3(a)(58)(B) of the Exchange Act and will continue to do so upon the appointment of the proposed directors until such time as a separate audit committee has been established.
Nominations to the Board of Directors
Our directors take a critical role in guiding our strategic direction and oversee the management of the Company. Board candidates are considered based upon various criteria, such as their broad-based business and professional skills and experiences, a global business and social perspective, concern for the long-term interests of the stockholders, diversity, and personal integrity and judgment.
In addition, directors must have time available to devote to Board activities and to enhance their knowledge in the growing business. Accordingly, we seek to attract and retain highly qualified directors who have sufficient time to attend to their substantial duties and responsibilities to the Company.
In carrying out its responsibilities, the Board will consider candidates suggested by stockholders. If a stockholder wishes to formally place a candidate’s name in nomination, however, he or she must do so in accordance with the provisions of the Company’s Bylaws. Suggestions for candidates to be evaluated by the proposed directors must be sent to the Board of Directors, c/o Yubo International Biotech Limited, Room 105, Building 5, 31 Xishiku Avenue, Xicheng District, Beijing, PRC.
| 56 |
| Table of Contents |
Board Leadership Structure and Role on Risk Oversight
Mr. Jun Wang currently serves as the Company’s principal executive officer and a director. The Company determined this leadership structure was appropriate for the Company due to our small size and limited operations and resources. The Board of Directors will continue to evaluate the Company’s leadership structure and modify as appropriate based on the size, resources and operations of the Company.
Subsequent to the closing of this offering, it is anticipated that the Board of Directors will establish procedures to determine an appropriate role for the Board of Directors in the Company’s risk oversight function.
Compensation Committee Interlocks and Insider Participation
No interlocking relationship exists between our board of directors and the board of directors or compensation committee of any other company, nor has any interlocking relationship existed in the past.
Board Compensation
Except as described under “- Employment Agreements” below with respect to the employment agreement with Yang Wang, our director, we have no standard arrangement to compensate directors for their services in their capacity as directors. Directors are not paid for meetings attended. However, we intend to review and consider future proposals regarding board compensation. All travel and lodging expenses associated with corporate matters are reimbursed by us, if and when incurred.
Executive Compensation
| Name and Principal Position |
| Year |
| A nnual Salary ($US)* |
|
| Bonus ($US)* |
|
| Total ($US)* |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
| |||
| Jun Wang, CEO and President |
| 2020 |
| $ | 14,610.54 |
|
|
| 14,610.54 |
|
|
| 29,221.08 |
|
|
|
| 2019 |
| $ | 1,217.54 |
|
|
| - |
|
|
| 1,217.54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lina Liu, CFO |
| 2020 |
| $ | 3,652.63 |
|
|
| 6,696.50 |
|
|
| 10,349.13 |
|
|
|
| 2019 |
| $ | - |
|
|
| - |
|
|
| - |
|
* Translated from RMB at the 6.5706 exchange rate as of March 31, 2021.
None of our executive officers or directors received, nor do we have any arrangements to pay out, any bonus, stock awards, option awards, non-equity incentive plan compensation, or non-qualified deferred compensation.
Potential Payments Upon Termination or Change-in-Control
SEC regulations state that we must disclose information regarding agreements, plans or arrangements that provide for payments or benefits to our executive officers in connection with any termination of employment or change in control of the company. We currently have no employment agreements with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s responsibilities following a change-in-control. As a result, we have omitted this table.
Employment Agreements
The Company is party to employment agreements with Jun Wang, Yang Wang and Lina Liu, providing for monthly salaries of RMB8,800 (or approximately $1,339 at the 6.5706 exchange rate as of March 31, 2021), RMB8,800 (or approximately $1,339 at the 6.5706 exchange rate as of March 31, 2021) and RMB8,000 (or approximately $1,217 at the 6.5706 exchange rate as of March 31, 2021), respectively. Jun Wang’s employment agreement commenced on December 1, 2019 and will terminate on November 30, 2021 after renewal. Each of Yang Wang’s and Lina Liu’s employment agreements commenced on October 10, 2020 and will terminate on October 9, 2021. The employment agreements each provide for the Company to arrange social insurance, housing insurance and medical insurances for the executive officers and the termination by the Company or executive officer upon 30-day notice upon the occurrence of a limited number of circumstances.
| 57 |
| Table of Contents |
PRINCIPAL AND SELLING STOCKHOLDERS
The following table and accompanying footnotes set forth certain information with respect to the beneficial ownership of our common stock, immediately prior to and immediately after the completion of this offering, by:
|
| · | each of our directors and named executive officers; |
|
|
|
|
|
| · | all of our directors and named executive officers as a group; |
|
|
|
|
|
| · | each person or entity (or group of affiliated persons or entities) known by us to be the beneficial owner of 5% or more of our common shares; and |
|
|
|
|
|
| · | the selling stockholders. |
To our knowledge, each shareholder named in the table has sole voting and investment power with respect to all of the common shares shown as beneficially owned by such shareholder, except as otherwise set forth in the footnotes to the table. The number of common shares shown represents the number of shares the person “beneficially owns,” as determined by the rules of the SEC. The SEC has defined “beneficial” ownership of a security to mean the possession, directly or indirectly, of voting power and/or investment power.
The percentages reflect beneficial ownership (as determined in accordance with Rule 13d-3 under the Exchange Act) immediately prior to and immediately after the completion of this offering, and are based on 118,177,885 shares of Class A common stock and 4,447 shares of Class B common stock outstanding as of the date immediately prior to the completion of this offering, and 123,177,885 shares of Class A common stock and 4,447 shares of Class B common stock outstanding as of the date immediately following the completion of this offering.
|
|
| Beneficially ownership |
|
| Beneficially ownership |
| ||||||||||
| Name and Address of Beneficial Owner |
| Share (1)(2) |
|
| Percentage (3) |
|
| Share (1)(2) |
|
| Percentage (3) |
| ||||
| Directors and Executive Officers |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Jun Wang Room 105, Building 5, 31 Xishiku Avenue Xicheng District, Beijing, PRC |
|
| 39,943,800 |
|
|
| 33.80 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Yang Wang Room 105, Building 5, 31 Xishiku Avenue Xicheng District, Beijing, PRC |
|
| 19,211,400 |
|
|
| 16.26 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Zhihui Bai Room 105, Building 5, 31 Xishiku Avenue Xicheng District, Beijing, PRC |
|
| 2,496,780 |
|
|
| 2.11 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lina Liu Room 105, Building 5, 31 Xishiku Avenue Xicheng District, Beijing, PRC |
|
| 5,098,439 |
|
|
| 4.31 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| All Officers and Directors as a Group |
|
| 66,750,419 |
|
|
| 56.48 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5% Stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| FlyDragon International Limited(7) Wickham’s Cay II,P.O.Box 2221 Road Town, Tortol a, British Virgin Islands |
|
| 39,943,800 |
|
|
| 33.80 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ChinaOne Technology Limited(8) Wickham’s Cay II, P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 19,211,400 |
|
|
| 16.26 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Boao Biotech Limited(9) Wickham’s Cay II, P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 24,967,800 |
|
|
| 21.13 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Focus Draw Group Limited(4) Wickham’s Cay II, P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 13,829,400 |
|
|
| 11.70 | % |
|
| n/a |
|
|
| n/a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| FocusOne Technology Group Limited(5) Wickham’s Cay II,P. O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 11,524,500 |
|
|
| 9.75 | % |
|
| n/a |
|
|
| n/a |
|
| 58 |
| Table of Contents |
| Selling Stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Focus Draw Group Limited(4) Wickham’s Cay II, P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 4,728,000 |
|
|
| 4.00 | % |
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DragonCloud Technology Limited(6) Wickham’s Cay II, P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 5,768,100 |
|
|
| 4.88 | % |
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cheung Ho Shun Wickham’s Cay II,P.O.Box 2221 Road Town, Tortola, British Virgin Islands |
|
| 1,755,000 |
|
|
| 1.49 | % |
|
| 0 |
|
|
| 0 |
|
______________
| (1) | Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act. Pursuant to the rules of the SEC, shares of common stock which an individual or group has a right to acquire within 60 days pursuant to the exercise of options or warrants are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be beneficially owned and outstanding for the purpose of computing the percentage ownership of any other person shown in the table. |
| (2) | Excludes any of our Class B common stock. |
| (3) | Represents the percentage of ownership of our Class A common stock only. |
| (4) | Lina Liu has voting and investment power in respect of the shares of our common stock owned of record or beneficially by Focus Draw Group Limited. |
| (5) | Wei Jin has voting and investment power in respect of the shares of our common stock owned of record or beneficially by FocusOne Technology Group Limited. |
| (6) | Yang Wang has voting and investment power in respect of the shares of our common stock owned of record or beneficially by DragonCloud Technology Limited. |
| (7) | Jun Wang has voting and investment power in respect of the shares of our common stock owned of record or beneficially by FlyDragon International Limited. |
| (8) | Yang Wang has voting and investment power in respect of the shares of our common stock owned of record or beneficially by ChinaOne Technology Limited. |
| (9) | Yulin Cao has voting and investment power in respect of the shares of our common stock owned of record or beneficially by Boao Biotech Limited. |
Selling Stockholders’ Information
The selling stockholders acquired their shares of our Class A common stock on January 14, 2021, when we entered into the Exchange Agreement. As a result of the Exchange Transaction, the shareholders of Platinum (which included the selling stockholders) received 117,000,000 shares of our Class A common stock, representing approximately 99.00% of our Class A common stock, in exchange for 100% of the issued and outstanding common stock of Platinum.
| 59 |
| Table of Contents |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Certain Relationships and Transactions
Other than Ms. Lina Liu, who was our controlling shareholder prior to the closing of the Exchange Transaction, and is currently our Chief Financial Officer, Treasurer and Secretary, and the appointment of our directors and executive officers, none of our officers and directors have been involved in any material proceeding adverse to the Company or any transactions with the Company or any of its directors, executive officers, affiliates or associates that are required to be disclosed pursuant to the rules and regulations of the SEC.
Although we have not adopted a Code of Ethics, we rely on our board to review related party transactions on an ongoing basis to prevent conflicts of interest. Our board reviews a transaction in light of the affiliations of the director, officer or employee and the affiliations of such person’s immediate family. Transactions are presented to our board for approval before they are entered into or, if this is not possible, for ratification after the transaction has occurred. If our board finds that a conflict of interest exists, then it will determine the appropriate remedial action, if any. Our board approves or ratifies a transaction if it determines that the transaction is consistent with the best interests of the Company. These policies and procedures are not evidenced in writing. We intend to adopt a Code of Ethic after the offering.
Related Party Transactions
Exchange Transaction
On January 13, 2021, we entered into the Exchange Agreement. As a result of the Exchange Transaction, the shareholders of Platinum received 117,000,000 shares of our Class A common stock, representing approximately 99.00% of our Class A common stock, in exchange for 100% of the issued and outstanding common stock of Platinum. Mr. Jun Wang, our President, Chief Executive Officer and a director, Mr. Yang Wang, a director, Mr. Zhihui Bai, a director, and Ms. Lina Liu, our CFO, Treasurer and Secretary, were beneficial shareholders of Platinum prior to the Closing of the Exchange Transaction, through their ownership of Flydragon International Limited, Chinaone Technology Limited, Boao Biotech Limited and Focus Draw Group Limited as well as Focusone Technology Group Limited, each a company organized under the laws of British Virgin Islands, respectively. Accordingly, Mr. Jun Wang, Mr. Yang Wang, Mr. Zhihui Bai and Ms. Lina Liu were beneficial recipients of certain shares of our common stock issued in connection with the Exchange Transaction.
In addition, our wholly owned subsidiary, Yubo WFOE has entered into variable interest entity control agreements over Yubo. Mr. Jun Wang is the President of Yubo.
Employment Agreements
As detailed above under “Executive Compensation-Employment Agreements,” each of Jun Wang, Yang Wang and Lina Liu have each entered into Employment Agreements with the Company, pursuant to which they will be compensated for their services provided to the Company as executives.
Re search and Development Agreements
We , through Yubo, have entered into an Entrustment Technical Service Agreement with Beijing Zhenhuikang Biotechnology Co., Ltd. (“Zhenhuikang”) , entrusting it to prepare, store and manage endometrial stem c ell samples for the operations of our stem cell bank in exchange for services fees paid by us. Pursuant to the agreement, we are responsible for supplying the endometrial stem cell samples and we may terminate the agreement at any time. We have also entered into a Patent Transfer Agreement with Zhenhuikang , which a greement provided for the assignment of two patents owned by Zhenhuikang to Yubo for total consideration of RMB 140,000.
We, through Yubo, have also entered into a Joint Research and Development Agreement with Beijing Zhenxigu Medical Research Center (L.P.) (“Beijing Zhengxigu”) , pursuant to which the parties have agreed to carry out filing application and market cooperation of certain medical devices developed by Beijing Zhenxigu (the “Application”) and we agreed to provide Beijing Zhenxigu Medical Research Center (L.P.) with aggregate R&D expenses of RMB241,880. Under this agreement, Beijing Zhenxigu authorized Yubo to be the nationwide exclusive distributor of supporting products for Platinum-specific atomization inhaler applications. After the filing of the Application, Beijing Zhenxigu shall continue to develop other application fields, and the parties shall continue cooperation in the form of entrustment or joint research and development. This agreement will terminate automatically upon the completion of the subject matters of the agreement.
Both of Zhenhuikang and Beijing Zhenxigu are affiliates of a shareholder of Yubo.
Shareholder Loans
On May 11 , 2021, we ent ered into a verbal loan agreement with World Precision Medicine Technology Inc., a company owned and controlled by Cheung Ho Shun, one of our existing shareholders , which provided the Company with a working capital loan in the principal amount of $600,000. The loan is due on demand and non-interest bearing.
As of December 31, 2019 and 2020, we had certain borrowings from Mr. Jun Wang in the aggregate amount of $88,941 and $0, respectively. As of December 31, 2019 and 2020, we had certain borrowings from Mr. Yang Wang in the aggregate amount of $4,911 and $91,951, respectively. All such borrowings are non-interest bearing and due upon demand.
Other than as set forth above, none of our current officers or directors have been involved in any material proceeding adverse to the Company or any transactions with the Company or any of its directors, executive officers, affiliates or associates that are required to be disclosed pursuant to the rules and regulations of the SEC.
| 60 |
| Table of Contents |
Review, Approval and Ratification of Related Party Transactions
Given our small size and limited financial resources, we have not adopted formal policies and procedures for the review, approval or ratification of transactions, such as those described above, with our executive officers, directors and significant stockholders. However, all of the transactions described above were approved and ratified by our Board. In connection with the approval of the transactions described above, our Board took into account several factors, including their fiduciary duties to the Company, the relationships of the related parties described above to the Company, the material facts underlying each transaction, the anticipated benefits to the Company and related costs associated with such benefits, whether comparable products or services were available, and the terms the Company could receive from an unrelated third party.
We intend to establish formal policies and procedures in the future, once we have sufficient resources and have appointed additional directors, so that such transactions will be subject to the review, approval or ratification of our Board, or an appropriate committee thereof. On a moving forward basis, our Board will continue to approve any related party transaction based on the criteria set forth above.
Conflicts Related to Other Business Activities
The persons serving as our officers and directors have existing responsibilities and, in the future, may have additional responsibilities, to provide management and services to other entities in addition to us. As a result, conflicts of interest between us and the other activities of those persons may occur from time to time.
We will attempt to resolve any such conflicts of interest in our favor. Our officers and directors are accountable to us and our shareholders as fiduciaries, which requires that such officers and directors exercise good faith and integrity in handling our affairs. A shareholder may be able to institute legal action on our behalf or on behalf of that shareholder and all other similarly situated shareholders to recover damages or for other relief in cases of the resolution of conflicts in any manner prejudicial to us.
Director Independence
During the twelve-month ended December 31, 2020, we did not have any independent directors on our board. We evaluate independence by the standards for director independence established by applicable laws, rules, and listing standards including, without limitation, the standards for independent directors established by The New York Stock Exchange, Inc., the NASDAQ Stock Market, and the SEC.
Subject to some exceptions, these standards generally provide that a director will not be independent if (a) the director is, or in the past three years has been, an employee of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct compensation from us other than for service as a director (or for a family member, as a non-executive employee); (d) the director or a member of the director’s immediate family is, or in the past three years has been, employed in a professional capacity by our independent public accountants, or has worked for such firm in any capacity on our audit; (e) the director or a member of the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate family is an executive officer of a company that makes payments to, or receives payments from, us in an amount which, in any twelve-month period during the past three years, exceeds the greater of $1,000,000 or two percent of that other company’s consolidated gross revenues.
| 61 |
| Table of Contents |
Michael T Studer CPA P.C., our independent registered public accounting firm, has audited our consolidated balance sheets as of December 31, 2020 and 2019, and the related consolidated statements of operations and comprehensive loss, stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2020 and 2019, and the related notes, as set forth in their report, which report expresses an unqualified opinion and includes an explanatory paragraph relating to our ability to continue as a going concern. Such financial statements have been included in this prospectus and in this Registration Statement in reliance on the report of Michael T Studer CPA P.C. given on their authority as experts in accounting and auditing.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
As previously disclosed on our Current Report on Form 8-K filed with the SEC on October 16, 2020, RBSM LLP was dismissed as our independent accountant, effective October 13, 2020. On October 13, 2020, we engaged Michael T Studer CPA P.C. as our new independent registered public accounting firm. There were no disagreements (as that term is used in Item 304(a)(1)(iv) of Regulation S-K) or reportable events (as described in Item 304(a)(1)(v) of Regulation S-K) in connection with such changes in accountants.
The validity of our Class A common stock offered hereby will be passed upon for us by Greenberg Traurig, LLP.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed for such purpose on a contingency basis, or had, or is to receive, in connection with this offering, a substantial interest, direct or indirect, in us or any of our parents or subsidiaries, nor was any such person connected with us or any of our parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Such filings are available to the public over the internet at the SEC’s website at http://www.sec.gov.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered under this prospectus. This prospectus, which forms a part of that registration statement, does not contain all information included in the registration statement. Certain information is omitted and you should refer to the registration statement and its exhibits.
You may review a copy of the registration statement at the SEC’s public reference room at 100 F Street, N.E. Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. You may also read and copy any materials we file with the SEC at the SEC’s public reference room. Our filings and the registration statement can also be reviewed by accessing the SEC’s website at http://www.sec.gov.
| 62 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD AND
SUBSIDIARIES AND VARIABLE INTEREST ENTITY
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
For the years ended December 31, 2020 and December 31, 2019
|
| F-2 |
| |
|
|
|
|
|
|
| F-3 |
| |
|
|
|
|
|
| Consolidated Statements of Operations and Comprehensive Loss |
| F-4 |
|
|
|
|
|
|
|
| F-5 |
| |
|
|
|
|
|
|
| F-6 |
| |
|
|
|
|
|
|
| F-7 |
|
For the three-month periods ended March 31, 2021 and 2020
Table of Contents
| F-21 |
| ||
| Consolidated Statements of Operations and Comprehensive Loss | F-22 | ||
| F-23 | |||
| F-24 | |||
| F-25 |
| F-1 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To: The Board of Directors and Stockholders of Platinum International Biotech Co., LTD
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Platinum International Biotech Co., LTD and subsidiaries and variable interest entity (collectively, the “Company”) as of December 31, 2020 and December 31, 2019, and the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity, and cash flows for the years ended December 31, 2020 and December 31, 2019 and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and December 31, 2019, and the results of its operations and cash flows for the years ended December 31, 2020 and December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the consolidated financial statements, the Company’s present financial situation raises substantial doubt about its ability its ability to continue as a going concern. Management’s plans in regard to this matter are also described in Note 3. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Operating lease right of use asset and operating lease liability - Refer to Note 8 to the consolidated financial statements.
Critical Audit Matter Description
The consolidated balance sheet at December 31, 2020 includes a right of use asset (and an operating lease liability) in the amount of $315,207. This asset and liability relates to the Beijing office space lease signed by Yubo International Biotech (Beijing) Limited (“Yubo Beijing"), a consolidated variable interest entity of the Company's subsidiary Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”), in August 2019. We were advised by the Company that the $315,207 carrying value of this asset and liability at December 31, 2020 represented the discounted (at a 4.75% estimated incremental borrowing rate) value of the future minimum lease payments at December 31, 2020.
How the Critical Audit Matter was Addressed in the Audit
Our principal audit procedures related to the Company’s operating lease right of use asset and operating lease liability included:
|
| (1) | We obtained the Company prepared schedule of future minimum lease payments as of December 31, 2020 and compared amounts to the August 1, 2019 lease and subsequent amendments. |
|
|
|
|
|
| (2) | We verified the Company prepared calculation of the $315,207 discounted value (at a 4.75% estimated incremental borrowing rate) of the future minimum lease payments of $322,806 as of December 31, 2020. |
/s/ Michael T. Studer CPA P.C.
Michael T. Studer CPA P.C.
Freeport, New York
April 19, 2021
We have served as the Company’s auditor since 2020.
| F-2 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD AND SUBSIDIARIES AND
VARIABLE INTEREST ENTITY
CONSOLIDATED BALANCE SHEETS
(Expressed in US Dollars)
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
|
|
|
|
|
| ||
| ASSETS |
|
|
|
|
|
| ||
| Current assets |
|
|
|
|
|
| ||
| Cash |
| $ | 1,382,525 |
|
| $ | 1,262 |
|
| Receivables |
|
| 2,316 |
|
|
| - |
|
| Prepaid expenses |
|
| 27,160 |
|
|
| 62,089 |
|
| Inventory |
|
| 67,144 |
|
|
| - |
|
| Due from related parties |
|
| 429,648 |
|
|
| 399,251 |
|
| Total Current Assets |
|
| 1,908,793 |
|
|
| 462,602 |
|
|
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
| 79,153 |
|
|
| 36,597 |
|
| Intangible assets, net |
|
| 54,912 |
|
|
| - |
|
| Operating lease right of use asset |
|
| 315,207 |
|
|
| 546,350 |
|
| Lease security deposit |
|
| 86,811 |
|
|
| 83,386 |
|
| Total Assets |
| $ | 2,444,876 |
|
| $ | 1,128,935 |
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities |
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses (including accounts payable and accrued expenses of VIE without recourse to the Company of $101,175 and $0 as of December 31, 2020 and December 31, 2019, respectively) |
| $ | 101,175 |
|
| $ | - |
|
| Customer deposits (including customer deposits of VIE without recourse to the Company of $11,028 and $0 as of December 31, 2020 and December 31, 2019, respectively) |
|
| 11,028 |
|
|
| - |
|
| Advances from prospective customers/distributors (including advances from prospective customers/distributors of VIE without recourse to the Company of $757,896 and $0 as of December 31 2020 and December 31, 2019, respectively) |
| $ | 757,896 |
|
|
| - |
|
| Due to related parties (including due to related parties without recourse to the Company of $91,951 and $93,852 as of December 31, 2020 and December 31, 2019, respectively) |
|
| 91,951 |
|
|
| 93,852 |
|
| Operating lease liability – current (including operating lease liability -current of VIE without recourse to the Company of $315,207 and $262,928 as of December 31, 2020 and December 31, 2019, respectively) |
|
| 315,207 |
|
|
| 262,928 |
|
| Total Current Liabilities |
|
| 1,277,257 |
|
|
| 356,780 |
|
|
|
|
|
|
|
|
|
|
|
| Non-current liabilities |
|
|
|
|
|
|
|
|
| Operating lease liability - non-current (including operating lease liability – non- current of VIE without recourse to the Company of $0 and $283,422 as of December 31, 2020 and December 31, 2019, respectively) |
|
| - |
|
|
| 283,422 |
|
| Total Liabilities |
|
| 1,277,257 |
|
|
| 640,202 |
|
|
|
|
|
|
|
|
|
|
|
| Commitments and contingencies |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| Shareholders' Equity: |
|
|
|
|
|
|
|
|
| Common stock, par value $ 0.0001 per share; authorized 500,000,000 shares, issued and outstanding 10,152,284 and 0 shares, respectively. |
|
| 1,015 |
|
|
| - |
|
| Additional Paid in Capital |
|
| 2,107,602 |
|
|
| 723,861 |
|
| Accumulated deficit |
|
| (942,994 | ) |
|
| (231,193 | ) |
| Accumulated other comprehensive income (loss) |
|
| 1,996 |
|
|
| (3,935 | ) |
| Total Shareholders' Equity |
|
| 1,167,619 |
|
|
| 488,733 |
|
| Total Liabilities and Shareholders' Equity |
| $ | 2,444,876 |
|
| $ | 1,128,935 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD AND SUBSIDIARIES AND
VARIABLE INTEREST ENTITY
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Expressed in US Dollars)
|
|
| For the year ended December 31, |
| |||||
|
|
| 2020 |
|
| 2019 |
| ||
| Revenue |
|
|
|
|
|
| ||
| Sales |
| $ | 1,353,868 |
|
| $ | - |
|
| Cost of Goods Sold |
|
| (114,272 | ) |
|
| - |
|
| Gross Profit |
|
| 1,239,596 |
|
|
| - |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Sales commissions |
|
| 660,963 |
|
|
| - |
|
| Employee compensation |
|
| 260,689 |
|
|
| 4,951 |
|
| Occupancy |
|
| 279,191 |
|
|
| 101,702 |
|
| Depreciation and amortization of property and equipment |
|
| 8,966 |
|
|
| 91 |
|
| Amortization of intangible assets |
|
| 4,096 |
|
|
| - |
|
| Other operating expenses |
|
| 737,489 |
|
|
| 124,322 |
|
| Total Operating Expenses |
|
| 1,951,394 |
|
|
| 231,066 |
|
| Income (loss) from operations |
|
| (711,798 | ) |
|
| (231,066 | ) |
|
|
|
|
|
|
|
|
|
|
| Other Income (Expenses) |
|
|
|
|
|
|
|
|
| Interest expenses |
|
| (3 | ) |
|
| (127 | ) |
| Total Other Income (Expenses) |
|
| (3 | ) |
|
| (127 | ) |
|
|
|
|
|
|
|
|
|
|
| Loss before Provision for Income Tax |
|
| (711,801 | ) |
|
| (231,193 | ) |
|
|
|
|
|
|
|
|
|
|
| Provision for Income Tax |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
| $ | (711,801 | ) |
| $ | (231,193 | ) |
| Net loss per share basic and diluted |
| $ | (0.07 | ) |
| $ | (0.02 | ) |
| Weighted average common shares outstanding basic and diluted |
|
| 10,046,601 |
|
|
| 10,000,000 |
|
|
|
|
|
|
|
|
|
|
|
| Comprehensive income (loss) |
|
|
|
|
|
|
|
|
| Net loss |
| $ | (711,801 | ) |
| $ | (231,193 | ) |
| Foreign currency translation adjustment |
|
| 5,931 |
|
|
| (3,935 | ) |
| Total comprehensive income (loss) |
| $ | (705,870 | ) |
| $ | (235,128 | ) |
The accompanying notes are an integral part of these consolidated financial statement
| F-4 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD AND SUBSIDIARIES AND
VARIABLE INTEREST ENTITY
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(Expressed in US Dollars)
|
|
| Common Stock |
|
| Additional Paid-In |
|
| Accumulated |
|
| Accumulated Other Comprehensive Income |
|
|
| ||||||||||
|
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| (loss) |
|
| Total |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
| Balance, December 31, 2018 |
|
| - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| Capital contributions to Yubo Beijing |
|
| - |
|
|
| - |
|
|
| 723,861 |
|
|
| - |
|
|
| - |
|
|
| 723,861 |
|
| Foreign currency translation adjustment |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (3,935 | ) |
|
| (3,935 | ) |
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (231,193 | ) |
|
|
|
|
|
| (231,193 | ) |
| Balance, December 31, 2019 |
|
| - |
|
|
| - |
|
|
| 723,861 |
|
|
| (231,193 | ) |
|
| (3,935 | ) |
|
| 488,733 |
|
| Issuance of ordinary shares and capital contributions to Yubo Beijing |
|
| 10,000,000 |
|
|
| 1,000 |
|
|
| 633,756 |
|
|
| - |
|
|
| - |
|
|
| 634,756 |
|
| Sale of ordinary shares on September 11, 2020 |
|
| 152,284 |
|
|
| 15 |
|
|
| 749,985 |
|
|
|
|
|
|
|
|
|
|
| 750,000 |
|
| Foreign currency adjustment |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,931 |
|
|
| 5,931 |
|
| Net Loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (711,801 | ) |
|
| - |
|
|
| (711,801 | ) |
| Balance, December 31, 2020 |
|
| 10,152,284 |
|
| $ | 1,015 |
|
| $ | 2,107,602 |
|
| $ | (942,994 | ) |
| $ | 1,996 |
|
| $ | 1,167,619 |
|
The accompanying notes are an integral part of these consolidated financial statements
| F-5 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD AND SUBSIDIARIES AND
VARIABLE INTEREST ENTITY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Expressed in US Dollars)
|
|
| For the year ended December 31, |
| |||||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
|
|
|
|
|
| ||
| Cash flows from operating activities: |
|
|
|
|
|
| ||
| Net loss |
| $ | (711,801 | ) |
| $ | (231,193 | ) |
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
| 13,063 |
|
|
| 91 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Receivables |
|
| (2,316 | ) |
|
| - |
|
| Prepaid expense |
|
| 34,929 |
|
|
| (62,089 | ) |
| Inventory |
|
| (67,144 | ) |
|
| - |
|
| Due from related parties |
|
| (30,397 | ) |
|
| (399,251 | ) |
| Lease security deposit |
|
| (3,425 | ) |
|
| (83,386 | ) |
| Accounts payable and accrued expenses |
|
| 101,175 |
|
|
| - |
|
| Customer deposits |
|
| 11,028 |
|
|
| - |
|
| Advances from prospective customers/distributors |
|
| 757,896 |
|
|
| - |
|
| Due to related parties |
|
| (1,901 | ) |
|
| 93,707 |
|
| Net cash provided by (used in) operating activities |
|
| 101,107 |
|
|
| (682,121 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
| Purchases of property and equipment |
|
| (42,556 | ) |
|
| (36,983 | ) |
| Purchases of intangible assets |
|
| (54,912 | ) |
|
| - |
|
| Net cash used in investing activities |
|
| (97,468 | ) |
|
| (36,983 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
| Capital Contributions to Yubo Beijing |
|
| 634,756 |
|
|
| 723,861 |
|
| Sale of ordinary shares on September 11, 2020 |
|
| 750,000 |
|
|
| - |
|
| Net cash provided by financing activities |
|
| 1,384,756 |
|
|
| 723,861 |
|
|
|
|
|
|
|
|
|
|
|
| Effect of exchange rate changes |
|
| (7,133 | ) |
|
| (3,640 | ) |
|
|
|
|
|
|
|
|
|
|
| Net increase (decrease) in cash |
|
| 1,381,262 |
|
|
| 1,117 |
|
| Cash at beginning of period |
|
| 1,262 |
|
|
| 145 |
|
| Cash at end of period |
| $ | 1,382,524 |
|
| $ | 1,262 |
|
| Supplemental Cash Flow Information: |
|
|
|
|
|
|
|
|
| Income taxes paid |
| $ | - |
|
| $ | - |
|
| Interest paid |
| $ | - |
|
| $ | - |
|
| Non-cash Investing Activities: |
|
|
|
|
|
|
|
|
| Operating lease right of use asset acquired |
| $ | - |
|
| $ | 688,631 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
| Table of Contents |
PLATINUM INTERNATIONAL BIOTECH CO., LTD
AND SUBSIDIARIES AND VARIABLE INTEREST ENTITY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the years ended December 31, 2020 and December 31, 2019
NOTE 1 – ORGANIZATION
Platinum International Biotech Co., LTD (“Platinum”) was incorporated on April 7, 2020 under the laws of the Cayman Islands as a holding company. On May 4, 2020, Platinum incorporated a wholly owned subsidiary Platinum International Biotech (Hong Kong) Limited (“Platinum HK”) in Hong Kong. On September 4, 2020, Platinum HK incorporated a wholly foreign owned enterprise (“WFOE”) Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”) in Chengdu, China.
On September 11, 2020, Yubo Chengdu entered into a series of Variable Interest Entity (“VIE”) agreements with the owners of Yubo International Biotech (Beijing) Limited (“Yubo Beijing”). Pursuant to the VIE agreements, Yubo Beijing became Yubo Chengdu’s contractually controlled affiliate. The purpose and effect of the VIE Agreements is to provide Yubo Chengdu with all management control and net profits earned by Yubo Beijing.
Yubo Beijing was incorporated on June 14, 2016. For the year ended December 31, 2020 (commencing April 2020), Yubo Beijing sold approximately 850 nebulizers to customers in the People’s Republic of China (“PRC”).
Upon executing the series of VIE agreements in September 2020, Yubo Beijing has been considered a Variable Interest Entity (“VIE”) of Yubo Chengdu, its primary beneficiary. Accordingly, Yubo Beijing has been consolidated under the guidance of FASB Accounting Standards Codification (“ASC”) 810, Consolidation.
The officers, directors, and controlling beneficial owners of Yubo Beijing from its inception on June 14, 2016 are also officers, directors, and controlling beneficial owners of Platinum. Accordingly, the accompanying consolidated financial statements include Yubo Beijing’s operations from its inception on June 14, 2016.
Platinum and its consolidated subsidiaries and VIE are collectively referred to herein as the “Company” unless specific reference is made to an entity.
A flow chart of the Company follows:
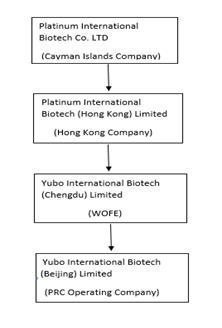
| F-7 |
| Table of Contents |
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
The accompanying consolidated financial statements for the years ended December 31, 2020 and December 31, 2019 comprise the following periods for each entity:
| Name |
| Periods |
| Platinum |
| April 7, 2020 (Inception) – December 31, 2020 |
| Yubo HK |
| May 4, 2020 (Inception) – December 31, 2020 |
| Yubo Chengdu |
| September 4, 2020 (Inception) – December 31, 2020 |
| Yubo Beijing |
| January 1, 2019 – December 31, 2020 |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its two wholly owned subsidiaries, and its consolidated VIE for which the Company is the primary beneficiary.
All transactions and balances among the Company, its subsidiaries and consolidated VIE have been eliminated upon consolidation.
The accompanying consolidated financial statements reflect the activities of the following entities:
| Name |
|
Background |
| Ownership | |
| Platinum International Biotech Co. LTD (“Platinum”) |
| · · · | A Cayman Island company Incorporated on April 7, 2020 A holding company |
|
|
| Platinum International Biotech (Hong Kong) Limited. (“Platinum HK”) |
| · · · | A Hong Kong company Incorporated on May 4, 2020 A holding company |
| 100% owned by Platinum |
| Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”) |
| · · · · | A PRC company and deemed a wholly foreign owned enterprise Incorporated on September 4, 2020 Subscribed capital of $1,500,000 A holding company |
| 100% owned by Platinum HK |
| Yubo International Biotech (Beijing) Limited (“Yubo Beijing”) |
| · · · · | A PRC limited liability company Incorporated on June 14, 2016 Subscribed capital of $1,531,722 (RMB 10,000,000) Stem cell storage and bank |
| VIE of Yubo Chengdu WFOE |
On September 11, 2020, our wholly-owned subsidiary, Yubo Chengdu, entered into the following contractual arrangements with Yubo Beijing and the shareholders of Yubo Beijing (the “Yubo Shareholders”), as applicable, each of which is enforceable and valid in accordance with the laws of the PRC:
| F-8 |
| Table of Contents |
Exclusive Consulting Services Agreement
Pursuant to the Exclusive Consulting Services Agreement among Yubo, Yubo WFOE, and the Yubo Shareholders, Yubo WFOE agrees to provide, and Yubo agrees to accept, exclusive management services provided by Yubo WFOE. Such management services include but are not limited to financial management, business management, marketing management, human resource management and internal control of Yubo. The Exclusive Consulting Services Agreement will remain in effect until the acquisition of all assets or equity of Yubo by Yubo WFOE is complete (as more fully described in the Exclusive Purchase Option Agreement below).
Exclusive Purchase Option Agreement
Under the Exclusive Option Agreement among Yubo, Yubo WFOE, and the Yubo Shareholders, the Yubo Shareholders granted Yubo WFOE an irrevocable and exclusive purchase option to acquire Yubo’s equity and/or assets at a nominal consideration. Yubo WFOE may exercise the purchase option at any time.
Equity Pledge Agreement
Under the Equity Pledge Agreement among Yubo WFOE and the Yubo Shareholders, the Yubo Shareholders pledged all of their equity interests in Yubo, including the proceeds thereof, to guarantee all of Yubo WFOE’s rights and benefits under the Exclusive Consulting Services Agreement and the Exclusive Option Agreement. Prior to termination of this Equity Pledge Agreement, the pledged equity interests cannot be transferred without Yubo WFOE’s prior consent. The Yubo Shareholders covenants to Yubo WFOE that among other things, it will only appoint/elect the candidates for the directors of Yubo nominated by Yubo WFOE.
Financial Statements of Yubo Beijing (VIE)
Except for $635,912 cash at December 31, 2020, all assets and liabilities included in the accompanying Consolidated Balance Sheets at December 31, 2020 and December 31, 2019 represent assets and liabilities of Yubo Beijing.
Except for $114,088 other operating expenses for the ended December 31, 2020, all revenues and expenses included in the accompanying Consolidated Statements of Operations for the years ended December 31, 2020 and December 31, 2019 represent revenues and expenses of Yubo Beijing.
Foreign Currency Translation
The accompanying consolidated financial statements are presented in United States dollars (“$”), which is the reporting currency of the Company. The functional currency of Platinum and Platinum HK is the United States dollar. The functional currency of the Company’s subsidiary and VIE located in the PRC is the Renminbi (“RMB”). For the entities whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period ($1=6.7473 RMB for the year ended December 31, 2020 and $1=6.9074 RMB for the year ended December 31, 2019), assets and liabilities are translated at the current exchange rate at the end of the period ($1=6.5286 RMB at December 31, 2020 and $1=6.9630 RMB at December 31, 2019), and equity is translated at historical exchange rates. The resulting translation adjustments are included in determining other comprehensive income (loss). Transaction gains and losses, which were not significant for the periods presented, are reflected in the consolidated statements of operations.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities on the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company bases its estimates and judgments on historical experience and on various other assumptions and information that are believed to be reasonable under the circumstances. Estimates and assumptions of future events and their effects cannot be perceived with certainty and, accordingly, these estimates may change as new events occur, as more experience is acquired, as additional information is obtained and as our operating environment changes. Significant estimates and assumptions by management include, among others, useful lives and impairment of long-lived assets, and income taxes including the valuation allowance for deferred tax assets. While the Company believes that the estimates and assumptions used in the preparation of the financial statements are appropriate, actual results could differ from those estimates. Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the financial statements in the period they are determined to be necessary.
| F-9 |
| Table of Contents |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, cash in bank accounts, cash in time deposits, certificates of deposit and all highly liquid instruments with original maturities of three months or less.
Inventories
Inventories, mainly consisting of nebulizers and components, are stated at the lower of cost utilizing the weighted average method or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business less the estimated selling costs.
The valuation of inventory requires the Company to estimate excess and slow-moving inventories. The Company evaluates the recoverability of the inventory based on expected demand and market conditions. No inventory write downs were recorded in the periods presented.
Property and Equipment
Property and equipment consist of leasehold improvements, air conditioning equipment, and office equipment. All property and equipment are stated at historical cost net of accumulated depreciation. Repairs and maintenance are expensed as incurred. Property and equipment are depreciated on a straight-line basis over the following periods:
| Leasehold improvements |
| Remaining term of lease |
|
| Air conditioning equipment |
| 5 years |
|
| Office equipment |
| 3 years |
|
Intangible Assets
Intangible assets consist of distribution software and patents and are stated at historical cost less accumulated amortization. Amortization of intangible assets is calculated on a straight-line basis over the shorter of the contractual terms or the expected useful lives of the respective assets. The amortization period by major asset classes is as follows:
| Distribution software |
| 5 years |
|
| Patents |
| 20 years |
|
Impairment of Long-Lived Assets
The Company’s long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of the asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Impairment evaluations involve management’s estimates on asset useful lives and future cash flows. Actual useful lives and cash flows could be different from those estimated by management which could have a material effect on our reporting results and financial position. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary.
Fair Value of Financial Instruments
The Company adopted ASC 820 “Fair Value Measurements,” which defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures.
| F-10 |
| Table of Contents |
The three levels are defined as follows:
Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liabilities, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 — inputs to the valuation methodology are unobservable and significant to the fair value.
Financial instruments include cash, receivables, due from related parties, accounts payable and accrued expenses, and due to related parties. The carrying values of these financial instruments approximate their fair values due to the short-term maturities of these instruments.
For the periods presented, there were no financial assets or liabilities measured at fair value.
Leases
We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liabilities - current, and operating lease liabilities - noncurrent on the balance sheets. The initial lease liability is equal to the future fixed minimum lease payments discounted using the Company’s incremental borrowing rate, on a secured basis. The initial measurement of the right-of-use asset is equal to the initial lease liability plus any initial direct costs.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term.
Revenue Recognition
The Company derives its revenue from the sale of nebulizers containing frozen tubes with medical fluid. The nebulizers are sold directly to consumers on the Company’s online e-commerce platform. The Company recognizes product revenues when the following four revenue recognition criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the selling price is fixed or determinable, and (iv) collectability is reasonably assured. The Company does not allow sales returns or exchanges.
Revenue is recorded net of value-added tax (“VAT”).
Advertising Costs
Advertising costs are expensed as incurred.
Income Taxes
The Company follows the liability method in accounting for income taxes in accordance with ASC topic 740 (“ASC 740”), Income Taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more-likely than not that some portion, or all, of the deferred tax assets will not be realized.
The Company applies the provisions of ASC 740 to account for uncertainty in income taxes. ASC 740 clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the consolidated financial statements.
The Company will classify interest and penalties related to unrecognized tax benefits, if and when required, as part of income tax expense in the consolidated statements of operations.
| F-11 |
| Table of Contents |
Net Loss per Share
Basic loss per ordinary share is computed by dividing net loss attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period.
Diluted loss per ordinary share reflects the potential dilution that could occur if dilutive securities (such as stock options and convertible securities) were exercised or converted into ordinary shares. For the periods presented, the Company had no dilutive securities outstanding.
Comprehensive Loss
Comprehensive loss is defined as the decrease in equity of the Company during a period from transactions and other events and circumstances excluding transactions resulting from investments by owners and distributions to owners. Comprehensive loss is reported in the consolidated statements of operations and comprehensive loss, including net loss and foreign currency translation adjustments, presented net of tax.
New Accounting Pronouncements
In February, 2016, the FASB issued Accounting Standards Update No. 2016-02 (ASU 2016-02) “Leases (Topic 842)”. ASU 2016-02 requires a lessee to recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term. ASU 2016-02 is effective for interim and annual reporting periods beginning after December 15, 2018. Early adoption is permitted.
For finance leases, a lessee is required to do the following:
|
| · | Recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in the balance sheet. |
|
|
|
|
|
| · | Recognize interest on the lease liability separately from amortization of the right-of-use asset in the statement of comprehensive income. |
|
|
|
|
|
| · | Classify repayments of the principal portion of the lease liability within financing activities and payments of interest on the lease liability and variable lease payments within operating activities in the statement of cash flows. |
For operating leases, a lessee is required to do the following:
|
| · | Recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in the balance sheet. |
|
|
|
|
|
| · | Recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term on a generally straight-line basis. |
|
|
|
|
|
| · | Classify all cash payments within operating activities in the statement of cash flows. |
Other than increasing assets and liabilities at the inception of Yubo Beijing’s office lease on August 1, 2019 (See Note 8), ASU 2016-02 has not had a significant effect on the Company’s financial position or results of operations.
The Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material impact on its consolidated financial position, statements of operations or cash flows.
| F-12 |
| Table of Contents |
NOTE 3 – GOING CONCERN
The Company’s financial statements as of December 31, 2020 and December 31, 2019 have been prepared using generally accepted accounting principles in the United States of America applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has not yet established an ongoing source of revenues and cash flows sufficient to cover its operating costs and allow it to continue as a going concern. For the years ended December 31, 2020 and December 31, 2019, the Company had losses of $711,801 and $231,193, respectively. These factors among others raise substantial doubt about the ability of the company to continue as a going concern for a reasonable period of time.
In order to continue as a going concern, the Company will need, among other things, additional capital resources. Management’s plan is to obtain such resources for the Company by obtaining capital from management and significant shareholders sufficient to meet its minimal operating expenses and seeking third party equity and/or debt financing. However, management cannot provide any assurances that the Company will be successful in accomplishing any of its plans. These financial statements do not include any adjustments related to the recoverability and classification of assets or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE 4 – INVENTORY
Inventory consisted of the following:
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Product components raw materials: |
|
|
|
|
|
| ||
| Frozen tubes to be attached to the nebulizer product |
| $ | 40,499 |
|
| $ | - |
|
| Unassembled nebulizers |
|
| 4,733 |
|
|
| - |
|
| Fluids to be inserted in the frozen tubes |
|
| 11,470 |
|
|
| - |
|
| Total product components raw materials |
|
| 56,702 |
|
|
| - |
|
| Refrigerated boxes |
|
| 3,310 |
|
|
| - |
|
| Delivery boxes |
|
| 7,132 |
|
|
| - |
|
| Total Inventory |
| $ | 67,144 |
|
| $ | - |
|
NOTE 5 – DUE FROM RELATED PARTIES
Due from related parties consisted of:
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Beijing Zhenhuikang Biotechnology Co., Ltd (“Zhenhuikang”) (1) |
| $ | 404,288 |
|
| $ | 399,251 |
|
| Yubo Global Biotechnology (Chengdu) Co., Ltd. (2) |
|
| 25,360 |
|
|
| - |
|
| Total Due From Related Parties |
| $ | 429,648 |
|
| $ | 399,251 |
|
|
| (1) | Zhenhuikang is controlled by Zhenxigu. |
|
|
|
|
|
| (2) | Yubo Global Biotechnology (Chengdu) Co., Ltd. is controlled by Mr. Jun Wang. |
The due from related parties receivables are noninterest bearing and are due on demand.
| F-13 |
| Table of Contents |
NOTE 6 – PROPERTY AND EQUIPMENT
Property and equipment, net, consisted of the following:
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Leasehold improvements |
| $ | 44,777 |
|
| $ | 23,231 |
|
| Air conditioning equipment |
|
| 21,496 |
|
|
| - |
|
| Office equipment |
|
| 22,241 |
|
|
| 13,457 |
|
| Total property and equipment |
|
| 88,514 |
|
|
| 36,688 |
|
| Less accumulated depreciation and amortization |
|
| (9,361 | ) |
|
| (91 | ) |
| Property and equipment, net |
| $ | 79,153 |
|
| $ | 36,597 |
|
For the years ended December 31, 2020 and 2019, depreciation and amortization of property and equipment was $8,966 and $91, respectively.
NOTE 7 – INTANGIBLE ASSETS
Intangible assets, net, consisted of the following:
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Distribution software |
| $ | 37,906 |
|
| $ | - |
|
| Patents acquired from related party (Note 11) |
|
| 21,239 |
|
|
| - |
|
| Total intangible assets |
|
| 59,145 |
|
|
| - |
|
| Less: Accumulated amortization |
|
| (4,233 | ) |
|
| - |
|
| Intangible assets, net |
| $ | 54,912 |
|
| $ | - |
|
For the years ended December 31, 2020 and 2019, amortization of intangible assets expense was $4,096 and $0, respectively.
| F-14 |
| Table of Contents |
At December 31, 2020, the expected future amortization of intangible assets expense was:
| Year ending December 31, 2021 |
|
| 8,632 |
|
| Year ending December 31, 2022 |
|
| 8,632 |
|
| Year ending December 31, 2023 |
|
| 8,632 |
|
| Year ending December 31, 2024 |
|
| 8,632 |
|
| Year ending December 31, 2025 |
|
| 4,846 |
|
| Thereafter |
|
| 15,538 |
|
| Total |
| $ | 54,912 |
|
NOTE 8 – OPERATING LEASE RIGHT OF USE ASSET AND OPERATING LEASE LIABILITY
On August 1, 2019 Yubo Beijing executed a lease agreement with Jiu Si Cheng Investment Management (the “Landlord”) to rent approximately 746 square meters of office space in Beijing China. The lease provided for an initial term of 2 years and 4 months from August 2, 2019 to November 30, 2021 with a right to renew for an additional term of 2 years and 8 months from December 1, 2021 to July 31, 2024. The lease also provided for payments of quarterly rent and management fees to the landlord for the initial term of a total of RMB 4,756,649 ($728,586 at the 6.5286 current exchange rate at December 31, 2020) and the payment of a security deposit to the Landlord of RMB 566,754 ($86,811 at the 6.5286 current exchange rate at December 31, 2020).
At December 31, 2020 the future undiscounted minimum lease payments under this noncancellable lease are as follows:
|
|
| As of December 31, 2020 |
| |
| Year ending December 31, 2021 |
| $ | 322,806 |
|
The operating lease liabilities totaling $315,207 at December 31, 2020 as presented in the Consolidated Balance Sheet represents the discounted (at a 4.75% estimated incremental borrowing rate) value of the future lease payments of $322,806 at December 31, 2020.
For the years ended December 31, 2020 and December 31, 2019, occupancy expense attributable to this lease was $213,185 and $101,702, respectively.
NOTE 9 – ADVANCES FROM PROSPECTIVE CUSTOMERS/DISTRIBUTORS
In December 2020, Yubo Beijing received a total of RMB ¥ 4,948,000 ($757,896) from eight PRC entities in amounts of RMB ¥ 348,000, RMB ¥50,000, RMB ¥50,000, RMB ¥500,000, RMB ¥250,000, RMB ¥500,000, RMB ¥3,000,000 and RMB ¥250,000. The related verbal agreements provide for the eight entities to purchase inventory from Yubo Beijing or enter into such other arrangements with Yubo Beijing as the parties mutually agree. Pending formal approval of any such arrangements, all of the eight PRC entities have the right to request the return of their advances.
NOTE 10 – DUE TO RELATED PARTIES
Due to related parties consisted of the following:
|
|
| December 31, |
|
| December 31, |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Mr. Jun Wang (1) |
| $ | - |
|
| $ | 88,941 |
|
| Mr. Yang Wang (2) |
|
| 91,951 |
|
|
| 4,911 |
|
| Total |
| $ | 91,951 |
|
| $ | 93,852 |
|
___________
|
| (1) | Mr. Jun Wang owns 37.81% of Yubo Beijing capital stock and is the chief executive officer and a director of Platinum and Yubo Beijing. |
|
|
|
|
|
| (2) | Mr. Yang Wang owns 23.64% of Yubo Beijing capital stock and is a director of Platinum and Yubo Beijing. |
The due to related parties payables are noninterest bearing and are due on demand.
| F-15 |
| Table of Contents |
NOTE 11 – SHAREHOLDERS’ EQUITY
Platinum International Biotech Co., LTD (Cayman Islands) (“Platinum”)
Platinum has authorized 500,000,000 ordinary shares with a par value of $0.0001 per share with 10,152,284 shares issued and outstanding at December 31, 2020.
On April 7, 2020 Platinum issued a total of 10,000,000 ordinary shares to six entities as follows:
| Entity |
| Shares |
| |
| 1. Flydragon International Limited (controlled by Mr. Jun Wang) |
|
| 3,466,000 |
|
| 2. Chinaone Technology Limited (controlled by Mr. Yang Wang) |
|
| 1,667,000 |
|
| 3. Boao Biotech Limited (controlled by Mr. Yulin Cao) |
|
| 2,167,000 |
|
| 4. Dragoncloud Technology Limited (controlled by Mr. Yang Wang) |
|
| 500,000 |
|
| 5. Focus Draw Group Limited (controlled by Ms. Lina Liu) |
|
| 1,200,000 |
|
| 6. Focusone Technology Group Limited (controlled by Mr. Jin Wei) |
|
| 1,000,000 |
|
| Total |
|
| 10,000,000 |
|
On September 11, 2020 Platinum sold 152,284 ordinary shares to an investor for $750,000 cash.
Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”)
Yubo Chengdu has subscribed capital of $1,500,000 which has not yet been paid by its shareholder. The subscribed capital is due for payment on January 1, 2040.
Yubo International Biotech (Beijing) Limited (“Yubo Beijing”)
Yubo Beijing has subscribed capital of $1,531,722 (RMB 10,000,000), of which $127,592 (RMB 833,000) has not yet been paid by its shareholders as of December 31, 2020.
In 2019, a total of RMB 5,000,000 ($723,861 at the 6.9074 average exchange rate for the year ended December 31, 2019) was received from Mr. Jun Wang (RMB 2,000,000), Mr. Yang Wang ($1,250,000), Zhenxigu Medical Research Center LP, (RMB 1,250,000) and Borong Hongtai Asset Management LP (RMB 500,000).
In the year ended December 31, 2020, a total of RMB 4,167,000 ($617,580 at the 6.7473 average exchange rate for the year ended December 31, 2020) was received from Mr. Jun Wang (RMB 1,466,000), Mr. Yang Wang (RMB 917,000), Zhengxigu Medical Research Center LP (RMB 417,000), Borong Hongtai Asset Management LP (RMB 700,000), and Platinum Health Management (Tianjin) Center (LP) (RMB 667,000)
Restricted net assets
The Company’s ability to pay dividends is primarily dependent on the Company receiving distributions of funds from its subsidiaries or its VIE. Relevant PRC statutory laws and regulations permit payments of dividends by Yubo Chengdu and Yubo Beijing only out of its retained earnings, if any, as determined in accordance with PRC accounting standards and regulations and after it has met the PRC requirements for appropriation to statutory reserves. Paid in capital of the PRC subsidiary and VIE included in the Company’s consolidated net assets are also non-distributable for dividend purposes. The results of operations reflected in the accompanying consolidated financial statements prepared in accordance with U.S. GAAP differ from those reflected in the statutory financial statements of Yubo Chengdu and Yubo Beijing.
| F-16 |
| Table of Contents |
Yubo Chengdu and Yubo Beijing are required to set aside at least 10% of their after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of its registered capital. In addition, Yubo Chengdu and Yubo Beijing may allocate a portion of its after-tax profits based on PRC accounting standards to an enterprise expansion fund and a staff bonus and welfare fund at its discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends.
For the years ended December 31, 2020 and 2019, Yubo Beijing did not generate any profit and had negative retained earnings as of December 31, 2020. As a result, the Company has not accrued statutory reserve funds.
The ability of the Company’s PRC subsidiary and its VIE to make dividends and other payments to the Company may also be restricted by changes in applicable foreign exchange and other laws and regulations. Foreign currency exchange regulation in China is primarily governed by the following rules:
|
| · | Foreign Exchange Administration Rules (1996), as amended in August 2008, or the Exchange Rules; |
|
| · | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Currently, under the Administration Rules, Renminbi is freely convertible for current account items, including the distribution of dividends, interest payments, trade and service related foreign exchange transactions, but not for capital account items, such as direct investments, loans, repatriation of investments and investments in securities outside of China, unless the prior approval of the State Administration of Foreign Exchange (the “SAFE”) is obtained and prior registration with the SAFE is made. Foreign-invested enterprises that need foreign exchange for the distribution of profits to its shareholders may affect payment from their foreign exchange accounts or purchase and pay foreign exchange rates at the designated foreign exchange banks to their foreign shareholders by producing board resolutions for such profit distribution. Based on their needs, foreign-invested enterprises are permitted to open foreign exchange settlement accounts for current account receipts and payments of foreign exchange along with specialized accounts for capital account receipts and payments of foreign exchange at certain designated foreign exchange banks.
Although the current Exchange Rules allow the convertibility of Chinese Renminbi into foreign currency for current account items, conversion of Chinese Renminbi into foreign exchange for capital items, such as foreign direct investment, loans or securities, requires the approval of SAFE, which is under the authority of the People’s Bank of China. These approvals, however, do not guarantee the availability of foreign currency conversion. The Company cannot be sure that it will be able to obtain all required conversion approvals for its operations or that the Chinese regulatory authorities will not impose greater restrictions on the convertibility of Chinese Renminbi in the future. Currently, all of the Company’s revenues are generated in Renminbi. Any future restrictions on currency exchanges may limit the Company’s ability to use its retained earnings generated in Renminbi to make dividends or other payments in U.S. dollars or fund possible business activities outside China.
| F-17 |
| Table of Contents |
NOTE 12 – RELATED PARTY TRANSACTIONS
On February 17, 2020, Yubo Beijing executed an Agreement of Joint Research and Development with Beijing Zhenxigu Medical Research Center LP (“Zhenxigu”), an entity that owns 18.18% of Yubo Beijing Capital stock and is controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). Pursuant to the agreement, Yubo Beijing paid RMB 241,880 ($35,848 at the 6.7473 average exchange rate for the year ended December 31, 2020) to Zhenxigu for research and development relating to the medical fluid to be included with the nebulizers to be sold to customers. Such expense has been included with other operating expenses in the accompanying Consolidated Statement of Operations and Comprehensive Loss for the year ended December 31, 2020.
On February 27, 2020, Yubo Beijing executed a Patent Transfer Agreement with Beijing Zhenhuikang Biotechnology Co. LTD (“Zhenhuikang”), an entity controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). The Agreement provided for the assignment of two patents owned by Zhenhuikang to Yubo Beijing for consideration of RMB 140,000 ($21,444 at the 6.5286 current exchange rate at December 31, 2020) (See Note 7).
On February 27, 2020, Yubo Beijing executed an Entrustment Technical Service Agreement with Beijing Zhenhuikang Biotechnology Co. LTD (“Zhenhuikang”), an entity controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). The Agreement provides for Zhenhuikang to, among other things, assist Yubo Beijing in the preparation of 300 sets of endometrial stem cell harvesting packages. As amended July 2, 2020, the Agreement provides for Yubo Beijing to pay Zhenhuikang at the rate of RMB 666 per set or RMB 199,800 total ($30,604 at the 6.5286 current exchange rate at December 31, 2020). As of December 31, 2020, preparation of the stem cell harvesting packages has not yet commenced, no payments to Zhenhuikang have been made, and no expense or liability has been recorded.
NOTE 13 – INCOME TAX
Cayman Islands
Under the current laws of the Cayman Islands, Platinum is not subject to tax on income or capital gains. In addition, payments of dividends by Platinum to its shareholders are not subject to withholding tax in the Cayman Islands.
Hong Kong
Platinum HK was incorporated under the Hong Kong tax law where the statutory income tax rate is 16.5%. Platinum HK has had no taxable income or loss from May 4, 2020 (inception) to December 31, 2020.
People’s Republic of China
Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”) and Yubo International Biotech (Beijing) Limited were incorporated in the PRC and are subject to PRC Enterprise Income Tax (“EIT”) on their taxable income in accordance with the relevant PRC income tax laws. On March 16, 2007, the National People’s Congress enacted a new enterprise income tax law, which took effect on January 1, 2008. The law applies a uniform 25% enterprise income tax rate to both foreign invested enterprises and domestic enterprises.
Yubo Chengdu has had no taxable income or loss from September 4, 2020 (inception) to December 31, 2020.
Yubo Beijing has had taxable losses of $231,193 for the year ended December 31, 2019 and $597,713 for year ended December 31, 2020. These losses can be carried forward for five years to reduce future years’ taxable income through year 2024 and year 2025, respectively. Based on management’s present assessment, the Company has not yet determined it to be more likely than not that future utilization of the net operating loss carryforwards will be realized. Accordingly, the Company has recorded a 100% valuation allowance against the deferred tax asset at December 31, 2020 and December 31, 2019.
| F-18 |
| Table of Contents |
The components of deferred tax assets were as follows:
|
|
| December 31, 2020 |
|
| December 31, 2019 |
| ||
|
|
|
|
|
|
|
| ||
| Net operating losses carry forward |
| $ | 207,227 |
|
| $ | 57,798 |
|
| Valuation allowance |
|
| (207,227 | ) |
|
| (57,798 | ) |
| Deferred tax assets, net |
| $ | — |
|
| $ | — |
|
The reconciliation of the provisions for (benefits from) income tax by applying the PRC tax rate to income (loss) before provisions for income tax and the actual provisions for income tax is as follows:
|
|
| For the year ended December 31, 2020 |
|
| For the year ended December 31, 2019 |
| ||
|
|
|
|
|
|
|
| ||
| Income tax (benefits) at 25% |
| $ | (177,950 | ) |
| $ | (57,798 | ) |
| Net loss of Platinum |
|
| 28,522 |
|
|
| - |
|
| Increase in valuation allowance |
|
| 149,428 |
|
|
| 57,798 |
|
| Provision for income taxes |
| $ | — |
|
| $ | — |
|
Accounting for Uncertainty in Income Taxes
The tax authority of the PRC government conducts periodic and ad hoc tax filing reviews on business enterprises operating in the PRC after those enterprises complete their relevant tax filings. Therefore, the Company’s PRC entities’ tax filings results are subject to change and may lead to tax liabilities.
ASC 740 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The management evaluated the Company’s tax positions and concluded that no liability for uncertainty in income taxes was necessary as of December 31, 2020 and December 31, 2019.
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Freelancer Service Contract
On March 30, 2020, Yubo Beijing executed an agreement with Hainan Huiyonggong Service Ltd. (“HHS”). The agreement provided for HHS to engage sales representatives (often Yubo Beijing customers) to refer new customers to Yubo Beijing and for Yubo Beijing to pay fees to HHS based on the amount of sales generated from HHS’s sales representatives. The term of the agreement was for one year expiring March 29, 2021. For the year ended December 31, 2020, the Company expensed $502,352 pursuant to this agreement which is included in “Sales Commissions” in the accompanying statement of operations.
Website Platform Maintenance Agreement
On April 29, 2020, Yubo Beijing executed an agreement with Hainan Haifu Technology Ltd. (“HHT”). The agreement provided for HHT to provide certain website maintenance services for Yubo Beijing and provided for Yubo Beijing to pay a monthly fee of RMB 150,000 ($22,231 using the December 31, 2020 average rate of 6.7473) to HHT. The term of the agreement, which originally was for one year expiring April 28, 2021, was mutually terminated on October 30, 2020. For the year ended December 31, 2020, the Company expensed $125,985 pursuant to this agreement which is included in “Other Operating Expenses” in the accompanying statement of operations.
Credit risk
Cash deposits with banks are held in financial institutions in the PRC, which are insured with deposit protection up to RMB 500,000 (approximately $76,586 at December 31, 2020). Accordingly, the Company has a concentration of credit risk related to the uninsured part of bank deposits. The Company has not experienced any losses in such accounts and believes it is not exposed to significant credit risk.
| F-19 |
| Table of Contents |
Risks of Variable Interest Entity Structure
Although the structure the Company has adopted is consistent with longstanding industry practice, and is commonly adopted by comparable companies in China, the PRC government may not agree that these arrangements comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. There are uncertainties regarding the interpretation and application of PRC laws and regulations including those that govern the Company’s contractual arrangements, which could limit the Company’s ability to enforce these contractual arrangements. If the Company or its variable interest entity is found to be in violation of any existing or future PRC laws, rules or regulations, or fail to obtain or maintain any of the required permits or approvals, the relevant PRC regulatory authorities would have broad discretion to take action in dealing with such violations or failures, including levying fines, revoking business and other licenses of the Company’s variable interest entity, requiring the Company to discontinue or restrict its operations, restricting its right to collect revenue, requiring the Company to restructure its operations or taking other regulatory or enforcement actions against the Company. In addition, it is unclear what impact the PRC government actions would have on the Company and on its ability to consolidate the financial results of its variable interest entity in the consolidated financial statements, if the PRC government authorities were to find the Company’s legal structure and contractual arrangements to be in violation of PRC laws, rules and regulations. If the imposition of any of these government actions causes the Company to lose its right to direct the activities of Yubo Beijing or the right to receive their economic benefits, the Company would no longer be able to consolidate Yubo Beijing.
NOTE 14 - SUBSEQUENT EVENTS
On January 14, 2021, Platinum and its 7 shareholders (the “Shareholders”) entered into a Share Exchange Agreement (the “Exchange Agreement”) with Yubo International Biotech Limited (“YBGJ”), formerly Magna-Lab Inc. Pursuant to the terms of the Exchange Agreement, the Shareholders received a total of 117,000,000 shares of YBGJ Class A common stock (representing approximately 99.00% of YBGJ common stock after the transaction) in exchange for their delivery of 100% of the 10,152,284 ordinary shares of Platinum issued and outstanding making Platinum a wholly owned subsidiary of YBGJ.
| F-20 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
CONSOLIDATED BALANCE SHEETS
(Expressed in US Dollars)
(Unaudited)
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
|
| ||
| ASSETS | ||||||||
| Current assets |
|
|
|
|
|
| ||
| Cash |
| $ | 126,313 |
|
| $ | 1,382,525 |
|
| Receivables |
|
| 296,049 |
|
|
| 2,316 |
|
| Prepaid expenses |
|
| 302,573 |
|
|
| 27,160 |
|
| Inventory |
|
| 82,947 |
|
|
| 67,144 |
|
| Due from related parties |
|
| 399,507 |
|
|
| 429,648 |
|
| Total Current Assets |
|
| 1,207,389 |
|
|
| 1,908,793 |
|
|
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
| 527,936 |
|
|
| 79,153 |
|
| Intangible assets, net |
|
| 52,414 |
|
|
| 54,912 |
|
| Operating lease right of use assets |
|
| 1,942,351 |
|
|
| 315,207 |
|
| Lease security deposit |
|
| 177,289 |
|
|
| 86,811 |
|
| Total Assets |
| $ | 3,907,379 |
|
| $ | 2,444,876 |
|
| LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Current liabilities |
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses (including accounts payable and accrued expenses of VIE without recourse to the Company of $0 and $101,175 as of March 31, 2021 and December 31, 2020, respectively) |
| $ | - |
|
| $ | 101,175 |
|
| Customer deposits (including customer deposits of VIE without recourse to the Company of $0 and $11,028 as of March 31, 2021 and December 31, 2020, respectively) |
|
| - |
|
|
| 11,028 |
|
| Advances from prospective customers/distributors (including advances from prospective customers/distributors of VIE without recourse to the Company of $794,951 and $757,896 as of March 31 2021 and December 31, 2020, respectively) |
|
| 794,951 |
|
|
| 757,896 |
|
| Due to related parties (including due to related parties without recourse to the Company of $243,556, and $91,951 as of March 31, 2021 and December 31, 2020 respectively) |
|
| 243,556 |
|
|
| 91,951 |
|
| Operating lease liabilities – current (including operating lease liabilities - current of VIE without recourse to the Company of $231,264 and $315,207 as of March 31, 2021 and December 31, 2020, respectively) |
|
| 456,155 |
|
|
| 315,207 |
|
| Total Current Liabilities |
|
| 1,494,662 |
|
|
| 1,277,257 |
|
|
|
|
|
|
|
|
|
|
|
| Non-current liabilities |
|
|
|
|
|
|
|
|
| Operating lease liabilities - non-current |
|
| 1,486,196 |
|
|
| - |
|
| Total Liabilities |
|
| 2,980,858 |
|
|
| 1,277,257 |
|
|
|
|
|
|
|
|
|
|
|
| Commitments and contingencies |
|
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| Shareholders' Equity: |
|
|
|
|
|
|
|
|
| Preferred stock, par value $.01 per share, 5,000,000 shares authorized, none issued |
|
| - |
|
|
|
|
|
| Common stock, Class A par value $ 0.001 per share; authorized 1,000,000,000 shares, 118,177,885 and 117,000,000 shares issued and outstanding at March 31, 2021 and December 31, 2020, respectively |
|
| 118,178 |
|
|
| 117,000 |
|
| Common stock, Class B, par value $.001 per share, 3,750,000 shares authorized, 4,447 and 0 shares issued and outstanding at March 31, 2021 and December 31, 2020, respectively |
|
| 4 |
|
|
| - |
|
| Additional Paid in Capital |
|
| 2,117,599 |
|
|
| 1,991,617 |
|
| Accumulated deficit |
|
| (1,381,669 | ) |
|
| (942,994 | ) |
| Accumulated other comprehensive income (loss) |
|
| 72,409 |
|
|
| 1,996 |
|
| Total Shareholders' Equity |
|
| 926,521 |
|
|
| 1,167,619 |
|
| Total Liabilities and Shareholders' Equity |
| $ | 3,907,379 |
|
| $ | 2,444,876 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-21 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Expressed in US Dollars)
(Unaudited)
|
|
| For the three months ended March 31, |
| |||||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
| (Unaudited) |
| ||
| Revenue |
|
|
|
|
|
| ||
| Sales |
| $ | 462,329 |
|
| $ | - |
|
| Cost of Goods Sold |
|
| (146,738 | ) |
|
| - |
|
| Gross Profit |
|
| 315,591 |
|
|
| - |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Sales commissions |
|
| 138,348 |
|
|
| - |
|
| Employee compensation |
|
| 202,832 |
|
|
| 19,481 |
|
| Occupancy |
|
| 107,734 |
|
|
| 75,933 |
|
| Depreciation and amortization of property and equipment |
|
| 2,772 |
|
|
| 1,824 |
|
| Amortization of intangible assets |
|
| 2,154 |
|
|
| - |
|
| Other operating expenses |
|
| 300,321 |
|
|
| 43,664 |
|
| Total Operating Expenses |
|
| 754,161 |
|
|
| 140,902 |
|
| Income (loss) from operations |
|
| (438,570 | ) |
|
| (140,902 | ) |
|
|
|
|
|
|
|
|
|
|
| Other Income (Expenses) |
|
|
|
|
|
|
|
|
| Interest expenses |
|
| (105 | ) |
|
| (46 | ) |
| Total Other Income (Expenses) |
|
| (105 | ) |
|
| (46 | ) |
|
|
|
|
|
|
|
|
|
|
| Loss before Provision for Income Tax |
|
| (438,675 | ) |
|
| (140,948 | ) |
|
|
|
|
|
|
|
|
|
|
| Provision for Income Tax |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
| $ | (438,675 | ) |
| $ | (140,948 | ) |
| Net loss per share basic and diluted |
|
| (0.00 | ) |
|
| (0.00 | ) |
| Weighted average common shares outstanding basic and diluted |
|
| 118,130,820 |
|
|
| 115,245,000 |
|
|
|
|
|
|
|
|
|
|
|
| Comprehensive income (loss) |
|
|
|
|
|
|
|
|
| Net loss |
| $ | (438,675 | ) |
| $ | (140,948 | ) |
| Foreign currency translation adjustment |
|
| 70,413 |
|
|
| (24,516 | ) |
| Total comprehensive income (loss) |
| $ | (368,262 | ) |
| $ | (165,464 | ) |
The accompanying notes are an integral part of these consolidated financial statement
| F-22 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(Expressed in US Dollars)
(Unaudited)
|
|
| Common Stock |
|
| Additional |
|
|
|
| Accumulated Other Comprehensive |
|
| Total |
| ||||||||||||||||||
|
|
| Class A |
|
| Class B |
|
| paid in |
|
| Accumulated |
|
| Income |
|
| Stockholders' |
| ||||||||||||||
|
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| capital |
|
| Deficit |
|
| (loss) |
|
| Deficit |
| ||||||||
| BALANCE, December 31, 2020 |
|
| 117,000,000 |
|
| $ | 117,000 |
|
|
| - |
|
| $ | - |
|
| $ | 1,991,617 |
|
| $ | (942,994 | ) |
| $ | 1,996 |
|
| $ | 1,167,619 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Capital contributions to Yubo Beijing |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 127,164 |
|
|
| - |
|
|
| - |
|
|
| 127,164 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Reverse acquisition of Yubo International Biotech Limited by Platinum International Biotech Co. Ltd. |
|
| 1,177,885 |
|
|
| 1,178 |
|
|
| 4,447 |
|
|
| 4 |
|
|
| (1,182 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss for the three months ended March 31, 2021 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (438,675 | ) |
|
| - |
|
|
| (438,675 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation adjustment |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 70,413 |
|
|
| 70,413 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE, March 31, 2021 |
|
| 118,177,885 |
|
| $ | 118,178 |
|
|
| 4,447 |
|
| $ | 4 |
|
| $ | 2,117,599 |
|
| $ | (1,381,669 | ) |
| $ | 72,409 |
|
| $ | 926,521 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE, December 31, 2019 |
|
| 115,245,000 |
|
| $ | 115,245 |
|
|
| - |
|
| $ | - |
|
| $ | 608,616 |
|
| $ | (231,193 | ) |
| $ | (3,935 | ) |
| $ | 488,733 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Capital contributions to Yubo Beijing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 504,892 |
|
|
|
|
|
|
|
|
|
|
| 504,892 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss for the three months ended March 31, 2020 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (140,948 | ) |
|
| - |
|
|
| (140,948 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation adjustment |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (24,516 | ) |
|
| (24,516 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE, March 31, 2020 |
|
| 115,245,000 |
|
| $ | 115,245 |
|
|
| - |
|
| $ | - |
|
| $ | 1,113,508 |
|
| $ | (372,141 | ) |
| $ | (28,451 | ) |
| $ | 828,161 |
|
The accompanying notes are an integral part of these consolidated financial statements
| F-23 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Expressed in US Dollars)
(Unaudited)
|
|
| For the three months ended March 31, |
| |||||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
| (Unaudited) |
| ||
| Cash flows from operating activities: |
|
|
|
|
|
| ||
| Net loss |
| $ | (438,675 | ) |
| $ | (140,948 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
| 4,926 |
|
|
| 1,824 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Receivables |
|
| (293,733 | ) |
|
| (34,806 | ) |
| Prepaid expense |
|
| (275,413 | ) |
|
| (145,230 | ) |
| Inventory |
|
| (15,803 | ) |
|
| (86,216 | ) |
| Due from related parties |
|
| 30,141 |
|
|
| 7,639 |
|
| Lease security deposit |
|
| (90,478 | ) |
|
| - |
|
| Accounts payable and accrued expenses |
|
| (101,175 | ) |
|
| - |
|
| Customer deposits |
|
| (11,028 | ) |
|
| - |
|
| Advances from prospective customers/distributors |
|
| 37,055 |
|
|
| - |
|
| Due to related parties |
|
| 151,605 |
|
|
| (93,852 | ) |
| Net cash used in operating activities |
|
| (1,002,578 | ) |
|
| (491,589 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
| Purchases of property and equipment |
|
| (451,555 | ) |
|
| (48,284 | ) |
| Purchases of intangible assets |
|
| - |
|
|
| - |
|
| Net cash used in investing activities |
|
| (451,555 | ) |
|
| (48,284 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
| Capital Contributions to Yubo Beijing |
|
| 127,164 |
|
|
| 504,892 |
|
| Net cash provided by financing activities |
|
| 127,164 |
|
|
| 504,892 |
|
|
|
|
|
|
|
|
|
|
|
| Effect of exchange rate changes |
|
| 70,757 |
|
|
| 58,869 |
|
|
|
|
|
|
|
|
|
|
|
| Net increase (decrease) in cash |
|
| (1,256,212 | ) |
|
| 23,888 |
|
| Cash at beginning of period |
|
| 1,382,525 |
|
|
| 1,262 |
|
| Cash at end of period |
| $ | 126,313 |
|
| $ | 25,150 |
|
| Supplemental Cash Flow Information: |
|
|
|
|
|
|
|
|
| Income taxes paid |
|
| - |
|
|
| - |
|
| Interest paid |
|
| - |
|
|
| - |
|
| Non-cash Investing Activities: |
|
|
|
|
|
|
|
|
| Operating lease right of use asset acquired |
| $ | 1,930,350 |
|
| $ | - |
|
The accompanying notes are an integral part of these consolidated
| F-24 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the three-months periods ended March 31, 2021 and 2020
(Unaudited)
NOTE 1 – ORGANIZATION
Yubo International Biotech Limited (formerly Magna-Lab Inc.) (the “Company”), a New York corporation, acquired Platinum International Biotech Co. Ltd. (“Platinum”) in a “reverse merger” transaction on January 14, 2021.
On January 14, 2021 (the “Closing Date”), the Company closed a voluntary share exchange transaction with Platinum International Biotech Co., Ltd., a company organized under the laws of the Cayman Islands (“Platinum”), pursuant to that certain Agreement and Plan of Share Exchange, dated January 14, 2021 (the “Exchange Agreement”), by and among the Company, Platinum, Yubo International Biotech (Beijing) Limited, a company organized under the laws of the People’s Republic of China (“PRC”) (“Yubo”), and certain selling stockholders named therein.
In accordance with the terms of the Exchange Agreement, on the Closing Date, the Company issued a total of 117,000,000 shares of its Class A common stock to the Selling Stockholders, who were then stockholders of Platinum (the “Selling Stockholders”), in exchange for 100% of the issued and outstanding capital stock of Platinum (the “Exchange Transaction”). As a result of the Exchange Transaction, the Selling Stockholders acquired more than 99% of the Company’s issued and outstanding capital stock, Platinum became the Company’s wholly-owned subsidiary, and the Company acquired the business and operations of Platinum and Yubo. Immediately prior to the Exchange Transaction, the Company had 117,875,323 shares of Class A common stock and 4,447 shares of Class B common stock issued and outstanding. Immediately after the Exchange Transaction and the surrender and cancellation of 116,697,438 shares held by Lina Liu, the controlling shareholder, Chief Financial Officer, Treasurer and Secretary of the Company, the Company has 118,177,885 shares of Class A common stock and 4,447 shares of Class B common stock issue and outstanding.
Platinum was incorporated on April 7, 2020 under the laws of the Cayman Islands as a holding company. On May 4, 2020, Platinum incorporated a wholly owned subsidiary Platinum International Biotech (Hong Kong) Limited (“Platinum HK”) in Hong Kong. On September 4, 2020, Platinum HK incorporated a wholly foreign owned enterprise (“WFOE”) Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”) in Chengdu, China.
On September 11, 2020, Yubo Chengdu entered into a series of Variable Interest Entity (“VIE”) agreements with the owners of Yubo International Biotech (Beijing) Limited (“Yubo Beijing”). Pursuant to the VIE agreements, Yubo Beijing became Yubo Chengdu’s contractually controlled affiliate. The purpose and effect of the VIE Agreements is to provide Yubo Chengdu with all management control and net profits earned by Yubo Beijing.
Yubo Beijing was incorporated on June 14, 2016. For the year ended December 31, 2020 (commencing April 2020), Yubo Beijing sold approximately 850 nebulizers to customers in the People’s Republic of China (“PRC”).
Upon executing the series of VIE agreements in September 2020, Yubo Beijing has been considered a Variable Interest Entity (“VIE”) of Yubo Chengdu, its primary beneficiary. Accordingly, Yubo Beijing has been consolidated under the guidance of FASB Accounting Standards Codification (“ASC”) 810, Consolidation.
The officers, directors, and controlling beneficial owners of Yubo Beijing from its inception on June 14, 2016 are also officers, directors, and controlling beneficial owners of Platinum. Accordingly, the accompanying consolidated financial statements include Yubo Beijing’s operations from its inception on June 14, 2016.
Commencing in the quarterly period ended March 31, 2021 Yubo Beijing started also selling certain oral liquid health products. For the three months ended March 31, 2021, sales consisted of:
| Oral liquid health products |
| $ | 345,607 |
|
| Nebulizers |
|
| 116,722 |
|
| Total |
| $ | 462,329 |
|
| F-25 |
| Table of Contents |
In December 2020 and January 2021, Platinum HK formed two new wholly owned subsidiaries: Yubo Jingzhi Biotechnology (Chengdu) Co. Ltd. (“Yubo Jingzhi”) and Yubo Global Biotechnology (Chengdu) Co. Ltd (“Yubo Global”).
Yubo International Biotech Limited and its consolidated subsidiaries and VIE are collectively referred to herein as the “Company” unless specific reference is made to an entity.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Interim Financial Information
The unaudited financial statements have been prepared in accordance with generally accepted accounting principles (GAAP) applicable to interim financial information and the requirements of Form 10-Q and Rule 8-03 of Regulation S-X of the Securities and Exchange Commission. Accordingly, they do not include all of the information and disclosure required by accounting principles generally accepted in the United States of America for complete financial statements. Interim results are not necessarily indicative of results for a full year. In the opinion of management, all adjustments considered necessary for a fair presentation of the financial position and the results of operations and cash flows for the interim periods have been included. These financial statements should be read in conjunction with the audited financial statements as of and for the year ended December 31, 2020, as not all disclosures required by generally accepted accounting principles for annual financial statements are presented. The interim financial statements follow the same accounting policies and methods of computations as the audited financial statements as of and for the year ended December 31, 2020.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and its consolidated VIE for which the Company is the primary beneficiary.
All transactions and balances among the Company, its subsidiaries and consolidated VIE have been eliminated upon consolidation.
The accompanying consolidated financial statements reflect the activities of the following entities:
| Name |
| Background |
| Ownership |
| Yubo International Biotech Limited (“Yubo New York”) |
| • A holding company • Incorporated in New York |
|
|
| Platinum International Biotech Co. LTD (“Platinum”) |
| • A Cayman Island company • Incorporated on April 7, 2020 • A holding company |
| 100% owned by Yubo New York |
| Platinum International Biotech (Hong Kong) Limited. (“Platinum HK”) |
| • A Hong Kong company • Incorporated on May 4, 2020 • A holding company |
| 100% owned by Platinum |
| Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”) |
| • A PRC company and deemed a wholly foreign owned enterprise • Incorporated on September 4, 2020 • Subscribed capital of $1,500,000 • A holding company |
| 100% owned by Platinum HK |
| Yubo International Biotech (Beijing) Limited (“Yubo Beijing”) |
| • A PRC limited liability company • Incorporated on June 14, 2016 • Subscribed capital of $1,531,722 (RMB 10,000,000) • Stem cell storage and bank |
| VIE of Yubo Chengdu WFOE |
| Yubo Jingzhi Biotechnology (ChengDu) Co. Ltd. (“Yubo Jingzhi”) |
| • A PRC company incorporated on January 21, 2021 |
| 100% owned by Platinum HK |
| Yubo Global Biotechnology (Chengdu) Co. Ltd (“Yubo Global) |
| • A PRC company incorporated on December 20, 2020 |
| 100% owned by Platinum HK |
| F-26 |
| Table of Contents |
On September 11, 2020, our wholly-owned subsidiary, Yubo Chengdu, entered into the following contractual arrangements with Yubo Beijing and the shareholders of Yubo Beijing (the “Yubo Shareholders”), as applicable, each of which is enforceable and valid in accordance with the laws of the PRC:
Exclusive Consulting Services Agreement
Pursuant to the Exclusive Consulting Services Agreement among Yubo, Yubo WFOE, and the Yubo Shareholders, Yubo WFOE agrees to provide, and Yubo agrees to accept, exclusive management services provided by Yubo WFOE. Such management services include but are not limited to financial management, business management, marketing management, human resource management and internal control of Yubo. The Exclusive Consulting Services Agreement will remain in effect until the acquisition of all assets or equity of Yubo by Yubo WFOE is complete (as more fully described in the Exclusive Purchase Option Agreement below).
Exclusive Purchase Option Agreement
Under the Exclusive Option Agreement among Yubo, Yubo WFOE, and the Yubo Shareholders, the Yubo Shareholders granted Yubo WFOE an irrevocable and exclusive purchase option to acquire Yubo’s equity and/or assets at a nominal consideration. Yubo WFOE may exercise the purchase option at any time.
Equity Pledge Agreement
Under the Equity Pledge Agreement among Yubo WFOE and the Yubo Shareholders, the Yubo Shareholders pledged all of their equity interests in Yubo, including the proceeds thereof, to guarantee all of Yubo WFOE’s rights and benefits under the Exclusive Consulting Services Agreement and the Exclusive Option Agreement. Prior to termination of this Equity Pledge Agreement, the pledged equity interests cannot be transferred without Yubo WFOE’s prior consent. The Yubo Shareholders covenants to Yubo WFOE that among other things, it will only appoint/elect the candidates for the directors of Yubo nominated by Yubo WFOE.
Financial Statements of Yubo Beijing (VIE)
The assets and liabilities of Yubo Beijing (VIE) at March 31, 2021 and December 31, 2020 consist of:
|
|
| March 31, 2021 |
|
| December 31, 2020 |
| ||
| Cash |
| $ | 48,278 |
|
| $ | 746,613 |
|
| Receivables |
|
| 296,049 |
|
|
| 2,316 |
|
| Prepaid Expenses |
|
| 58,399 |
|
|
| 27,160 |
|
| Inventory |
|
| 82,947 |
|
|
| 67,144 |
|
| Due from related parties |
|
| 399,507 |
|
|
| 429,648 |
|
| Property and equipment (net) |
|
| 80,337 |
|
|
| 79,153 |
|
| Intangible assets (net) |
|
| 52,414 |
|
|
| 54,912 |
|
| Operating lease right of use assets |
|
| 231,264 |
|
|
| 315,207 |
|
| Lease security deposits |
|
| 86,255 |
|
|
| 86,811 |
|
| Investment in Yubo Jingzhi (A) |
|
| 228,290 |
|
|
| - |
|
| Receivables from other consolidating entities (A) |
|
| 250,624 |
|
|
| - |
|
| Total assets |
|
| 1,814,364 |
|
|
| 1,808,964 |
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued expense |
|
| - |
|
|
| 101,175 |
|
| Customer deposits |
|
| - |
|
|
| 11,028 |
|
| Advances from prospective customers/distributors |
|
| 794,951 |
|
|
| 757,896 |
|
| Due to related partis |
|
| 243,556 |
|
|
| 91,951 |
|
| Operating lease liabilities |
|
| 231,264 |
|
|
| 315,207 |
|
| Total liabilities |
|
| 1,269,771 |
|
|
| 1,277,257 |
|
|
|
|
|
|
|
|
|
|
|
| Shareholders' equity |
| $ | 544,593 |
|
| $ | 531,707 |
|
| F-27 |
| Table of Contents |
Except for $149,769 other operating expenses for the three months ended March 31, 2021, all revenues and expenses included in the accompanying Consolidated Statements of Operations for the three months ended March 31, 2021 and March 31, 2020 represent revenues and expenses of Yubo Beijing.
Foreign Currency Translation
The accompanying consolidated financial statements are presented in United States dollars (“$”), which is the reporting currency of the Company. The functional currency of Platinum and Platinum HK is the United States dollar. The functional currency of the Company’s subsidiaries and VIE located in the PRC is the Renminbi (“RMB”). For the entities whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period ($1=6.5506 RMB for the three months ended March 31, 2021 and $1=7.0312 RMB for the three months ended March 31, 2020), assets and liabilities are translated at the current exchange rate at the end of the period ($1=6.5706 RMB at March 31, 2021 and $1=6.5286 RMB at December 31, 2020), and equity is translated at historical exchange rates. The resulting translation adjustments are included in determining other comprehensive income (loss). Transaction gains and losses, which were not significant for the periods presented, are reflected in the consolidated statements of operations.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities on the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company bases its estimates and judgments on historical experience and on various other assumptions and information that are believed to be reasonable under the circumstances. Estimates and assumptions of future events and their effects cannot be perceived with certainty and, accordingly, these estimates may change as new events occur, as more experience is acquired, as additional information is obtained and as our operating environment changes. Significant estimates and assumptions by management include, among others, useful lives and impairment of long-lived assets, and income taxes including the valuation allowance for deferred tax assets. While the Company believes that the estimates and assumptions used in the preparation of the financial statements are appropriate, actual results could differ from those estimates. Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the financial statements in the period they are determined to be necessary.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, cash in bank accounts, cash in time deposits, certificates of deposit and all highly liquid instruments with original maturities of three months or less.
Inventories
Inventories, mainly consisting of nebulizers and components, are stated at the lower of cost utilizing the weighted average method or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business less the estimated selling costs.
| F-28 |
| Table of Contents |
The valuation of inventory requires the Company to estimate excess and slow-moving inventories. The Company evaluates the recoverability of the inventory based on expected demand and market conditions. No inventory write downs were recorded in the periods presented.
Property and Equipment
Property and equipment consist of leasehold improvements, air conditioning equipment, and office equipment. All property and equipment are stated at historical cost net of accumulated depreciation. Repairs and maintenance are expensed as incurred. Property and equipment are depreciated on a straight-line basis over the following periods:
| Leasehold improvements |
| Remaining term of lease |
| Air conditioning equipment |
| 5 years |
| Office equipment |
| 3 years |
Intangible Assets
Intangible assets consist of distribution software and patents and are stated at historical cost less accumulated amortization. Amortization of intangible assets is calculated on a straight-line basis over the shorter of the contractual terms or the expected useful lives of the respective assets. The amortization period by major asset classes is as follows:
| Distribution software |
| 5 years |
| Patents |
| 20 years |
Impairment of Long-Lived Assets
The Company’s long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of the asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Impairment evaluations involve management’s estimates on asset useful lives and future cash flows. Actual useful lives and cash flows could be different from those estimated by management which could have a material effect on our reporting results and financial position. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary.
Fair Value of Financial Instruments
The Company adopted ASC 820 “Fair Value Measurements,” which defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures.
The three levels are defined as follows:
Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liabilities, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 — inputs to the valuation methodology are unobservable and significant to the fair value.
| F-29 |
| Table of Contents |
Financial instruments include cash, receivables, due from related parties, accounts payable and accrued expenses, advances from prospective customers/distributors and due to related parties. The carrying values of these financial instruments approximate their fair values due to the short-term maturities of these instruments.
For the periods presented, there were no financial assets or liabilities measured at fair value.
Leases
We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liabilities - current, and operating lease liabilities - noncurrent on the balance sheets. The initial lease liability is equal to the future fixed minimum lease payments discounted using the Company’s incremental borrowing rate, on a secured basis. The initial measurement of the right-of-use asset is equal to the initial lease liability plus any initial direct costs.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term.
Revenue Recognition
The Company derives its revenue from the sale of nebulizers containing frozen tubes with medical fluid and from the sale of oral liquids health products. The nebulizers are sold directly to consumers on the Company’s online e-commerce platform. The Company recognizes product revenues when the following four revenue recognition criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the selling price is fixed or determinable, and (iv) collectability is reasonably assured. The Company does not allow sales returns or exchanges.
Revenue is recorded net of value-added tax (“VAT”).
Advertising Costs
Advertising costs are expensed as incurred.
Income Taxes
The Company follows the liability method in accounting for income taxes in accordance with ASC topic 740 (“ASC 740”), Income Taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more-likely than not that some portion, or all, of the deferred tax assets will not be realized.
The Company applies the provisions of ASC 740 to account for uncertainty in income taxes. ASC 740 clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the consolidated financial statements.
The Company will classify interest and penalties related to unrecognized tax benefits, if and when required, as part of income tax expense in the consolidated statements of operations.
Net Loss per Share
Basic loss per ordinary share is computed by dividing net loss attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period.
| F-30 |
| Table of Contents |
Diluted loss per ordinary share reflects the potential dilution that could occur if dilutive securities (such as stock options and convertible securities) were exercised or converted into ordinary shares. For the periods presented, the Company had no dilutive securities outstanding.
Comprehensive Loss
Comprehensive loss is defined as the decrease in equity of the Company during a period from transactions and other events and circumstances excluding transactions resulting from investments by owners and distributions to owners. Comprehensive loss is reported in the consolidated statements of operations and comprehensive loss, including net loss and foreign currency translation adjustments, presented net of tax.
New Accounting Pronouncements
In February 2016, the FASB issued Accounting Standards Update No. 2016-02 (ASU 2016-02) “Leases (Topic 842)”. ASU 2016-02 requires a lessee to recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term. ASU 2016-02 is effective for interim and annual reporting periods beginning after December 15, 2018. Early adoption is permitted.
For finance leases, a lessee is required to do the following:
| · | Recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in the balance sheet. |
|
|
|
| · | Recognize interest on the lease liability separately from amortization of the right-of-use asset in the statement of comprehensive income. |
|
|
|
| · | Classify repayments of the principal portion of the lease liability within financing activities and payments of interest on the lease liability and variable lease payments within operating activities in the statement of cash flows. |
|
|
|
| For operating leases, a lessee is required to do the following: | |
|
|
|
| · | Recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in the balance sheet. |
|
|
|
| · | Recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term on a generally straight-line basis. |
|
|
|
| · | Classify all cash payments within operating activities in the statement of cash flows. |
Other than increasing assets and liabilities at the inception of Yubo Beijing’s office lease on August 1, 2019 and Yubo Global’s laboratory space lease on March 1, 2021 (See Note 8), ASU 2016-02 has not had a significant effect on the Company’s financial position or results of operations.
The Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material impact on its consolidated financial position, statements of operations or cash flows.
NOTE 3 – GOING CONCERN
The Company’s financial statements as of March 31, 2021 and December 31, 2020 have been prepared using generally accepted accounting principles in the United States of America applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has not yet established an ongoing source of revenues and cash flows sufficient to cover its operating costs and allow it to continue as a going concern. For the three-months ended March 31, 2021 and March 31, 2020, the Company had losses of $438,675 and $140,948, respectively. These factors among others raise substantial doubt about the ability of the company to continue as a going concern for a reasonable period of time.
| F-31 |
| Table of Contents |
In order to continue as a going concern, the Company will need, among other things, additional capital resources. Management’s plan is to obtain such resources for the Company by obtaining capital from management and significant shareholders sufficient to meet its minimal operating expenses and seeking third party equity and/or debt financing. However, management cannot provide any assurances that the Company will be successful in accomplishing any of its plans. These financial statements do not include any adjustments related to the recoverability and classification of assets or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE 4 – INVENTORY
Inventory consisted of the following:
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
| |||
| Product components raw materials: |
|
|
|
|
|
| ||
| Frozen tubes to be attached to the nebulizer product |
| $ | 39,317 |
|
| $ | 40,499 |
|
| Unassembled nebulizers |
|
| 19,403 |
|
|
| 4,733 |
|
| Fluids to be inserted in the frozen tubes |
|
| 10,327 |
|
|
| 11,470 |
|
| Total product components raw materials |
|
| 69,047 |
|
|
| 56,702 |
|
| Refrigerated boxes |
|
| 7,455 |
|
|
| 3,310 |
|
| Delivery boxes |
|
| 6,445 |
|
|
| 7,132 |
|
| Total Inventory |
| $ | 82,947 |
|
| $ | 67,144 |
|
NOTE 5 – DUE FROM RELATED PARTIES
Due from related parties consisted of:
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
|
| ||
| Beijing Zhenhuikang Biotechnology Co., LTD (“Zhenhuikang”) (1) |
| $ | 399,507 |
|
| $ | 404,288 |
|
| Yubo Global Biotechnology (Chengdu) Co., Ltd. (2) |
|
| - |
|
|
| 25,360 |
|
| Total Due From Related Parties |
| $ | 399,507 |
|
| $ | 429,648 |
|
|
| (1) | Zhenhuikang is controlled by Zhenxigu. |
|
| (2) | Yubo Global Biotechnology (Chengdu) Co., Ltd. is controlled by Mr. Jun Wang. |
The due from related parties receivables are noninterest bearing and are due on demand.
| F-32 |
| Table of Contents |
NOTE 6 – PROPERTY AND EQUIPMENT
Property and equipment, net, consisted of the following:
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
|
| ||
| Leasehold improvements |
| $ | 41,396 |
|
| $ | 44,777 |
|
| Construction in progress |
|
| 411,721 |
|
|
| - |
|
| Air conditioning equipment |
|
| 57,241 |
|
|
| 21,496 |
|
| Office equipment |
|
| 29,645 |
|
|
| 22,241 |
|
| Total property and equipment |
|
| 540,003 |
|
|
| 88,514 |
|
| Less accumulated depreciation and amortization |
|
| (12,067 | ) |
|
| (9,361 | ) |
| Property and equipment, net |
| $ | 527,936 |
|
| $ | 79,153 |
|
For the three-months ended March 31, 2021 and 2020, depreciation and amortization of property and equipment was $2,772 and $1,824, respectively.
NOTE 7 – INTANGIBLE ASSETS
Intangible assets, net, consisted of the following:
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
| |||
| Distribution software |
| $ | 37,672 |
|
| $ | 37,907 |
|
| Patents acquired from related party (Note 12) |
|
| 21,096 |
|
|
| 21,239 |
|
| Total intangible assets |
|
| 58,768 |
|
|
| 59,146 |
|
| Less: Accumulated amortization |
|
| (6,354 | ) |
|
| (4,234 | ) |
| Intangible assets, net |
| $ | 52,414 |
|
| $ | 54,912 |
|
For the three-months ended March 31, 2021 and 2020, amortization of intangible assets expense was $2,154 and $0, respectively.
At March 31, 2021, the expected future amortization of intangible assets expense was:
| Year ending December 31, 2021 |
| $ | 6,134 |
|
| Year ending December 31, 2022 |
|
| 8,632 |
|
| Year ending December 31, 2023 |
|
| 8,632 |
|
| Year ending December 31, 2024 |
|
| 8,632 |
|
| Year ending December 31, 2025 |
|
| 4,846 |
|
| Thereafter |
|
| 15,538 |
|
| Total |
| $ | 52,414 |
|
NOTE 8 – OPERATING LEASE RIGHT OF USE ASSET AND OPERATING LEASE LIABILITY
On August 1, 2019, Yubo Beijing executed a lease agreement with Jiu Si Cheng Investment Management (the “Landlord”) to rent approximately 746 square meters of office space in Beijing China. The lease provided for an initial term of 2 years and 4 months from August 2, 2019 to November 30, 2021 with a right to renew for an additional term of 2 years and 8 months from December 1, 2021 to July 31, 2024. The lease also provided for payments of quarterly rent and management fees to the landlord for the initial term of a total of RMB 4,756,649 ($728,586 at the 6.5706 current exchange rate at March 31, 2021) and the payment of a security deposit to the Landlord of RMB 566,754 ($86,257 at the 6.5706 current exchange rate at March 31, 2021).
| F-33 |
| Table of Contents |
Effective March 1, 2021, Yubo Global executed a lease agreement with Chengdu Liangkang Investment Co. to rent approximately 6,960 square meters of laboratory space in Chengdu China. The lease provides for a lease term of 5 years from March 1, 2021 to February 28, 2026. The lease also provides for payments of monthly rent to the landlord of approximately RMB 300,000 ($45,658 at the 6.5706 current exchange rate at March 31, 2021) and the payment of a security deposit to the Landlord of RMB $598,553 ($91,096 at the 6.5706 current exchange rate at March 31, 2021).
At March 31, 2021, the future undiscounted minimum lease payments under the two noncancellable leases are as follows:
|
|
| As of March 31, 2021 |
| |
| Year ending December 31, 2021 |
| $ | 644,319 |
|
| Year ending December 31, 2022 |
|
| 546,573 |
|
| Year ending December 31, 2023 |
|
| 546,573 |
|
| Year ending December 31, 2024 |
|
| 573,902 |
|
| Year ending December 31, 2025 |
|
| 579,368 |
|
| Thereafter |
|
| 96,561 |
|
| Total |
| $ | 2,987,296 |
|
The operating lease liabilities totaling $1,942,351 at March 31, 2021 as presented in the Consolidated Balance Sheet represents the discounted (at a 4.75% estimated incremental borrowing rate) value of the future lease payments of $2,987,296 March 31, 2021.
For the three-months ended March 31, 2021 and March 31, 2020, occupancy expense attributable to these two leases was $86,555 and $60,257, respectively.
NOTE 9 – ADVANCES FROM PROSPECTIVE CUSTOMERS/DISTRIBUTORS
As of March 31, 2021, Yubo Beijing received a total of RMB ¥ 5,229,012 ($794,951) from nine PRC entities in amounts of RMB ¥ 348,000, RMB ¥50,000, RMB ¥50,000, RMB ¥500,000, RMB ¥500,000, RMB ¥500,000, RMB ¥3,000,000, RMB¥ 31,012, and RMB ¥250,000. The related verbal agreements provide for the nine entities to purchase inventory from Yubo Beijing or enter into such other arrangements with Yubo Beijing as the parties mutually agree. Pending formal approval of any such arrangements, all of the nine PRC entities have the right to request the return of their advances.
NOTE 10 – DUE TO RELATED PARTIES
Due to related parties consisted of the following:
|
|
| March 31, |
|
| December 31, |
| ||
|
|
| 2021 |
|
| 2020 |
| ||
|
|
| (Unaudited) |
|
|
|
| ||
| Mr. Jun Wang (1) |
| $ | - |
|
|
| - |
|
| Mr. Yang Wang (2) |
|
| 243,556 |
|
|
| 91,951 |
|
| Total |
| $ | 243,556 |
|
|
| 91,951 |
|
|
| (1) | Mr. Jun Wang controls 33.80% of the outstanding Class A common stock of Yubo New York and is the chief executive officer and a director of Yubo New York and Yubo Beijing. |
|
| (2) | Mr. Yang Wang controls 21.14% of the outstanding Class A common stock of Yubo New York and is a director of the Company and Yubo Beijing. |
| F-34 |
| Table of Contents |
The due to related parties payables are noninterest bearing and are due on demand.
NOTE 11 – SHAREHOLDERS’ EQUITY
Yubo Biotech International Limited
The Company has three types of stocks:
Preferred stock – par value 0.01 per share, 5,000,000 shares authorized, none issued.
Common Stock Class A – par value 0.001 per share, 1,000,000,000 shares authorized, 118,177,885 shares issued and outstanding at March 31, 2021.
Common Stock Class B – par value 0.001 per share, 3,750,000 shares authorized, 4,447 shares issued and outstanding at March 31, 2021.
On January 14, 2021, Lina Liu, Company CFO, cancelled 116,697,438 shares of Class A common stock acquired by her on October 2, 2020.
The Company issued 117,000,000 shares of Class A common stock issued in connection with the acquisition of Platinum.
| Name of Selling Shareholder |
| Number of Exchange Shares |
|
| Percentage of Exchange Shares |
| ||
| FLYDRAGON INTERNATIONAL LIMITED (controlled by Mr. Jun Wang) |
|
| 39,943,800 |
|
|
| 34.14 | % |
| CHINAONE TECHNOLOGY LIMITED (controlled by Mr. Yang Wang) |
|
| 19,211,400 |
|
|
| 16.42 | % |
| BOAO BIOTECH LIMITED (controlled by Mr. Yulin Cao) |
|
| 24,967,800 |
|
|
| 21.34 | % |
| FOCUS DRAW GROUP LIMITED (controlled by Ms. Lina Liu) |
|
| 13,829,400 |
|
|
| 11.82 | % |
| FOCUSONE TECHNOLOGY GROUP LIMITED (controlled by Mr. Jin Wei) |
|
| 11,524,500 |
|
|
| 9.85 | % |
| DRAGONCLOUD TECHNOLOGY LIMITED (Controlled by Mr. Yang Wang) |
|
| 5,768,100 |
|
|
| 4.93 | % |
| CHEUNG HO SHUN |
|
| 1,755,000 |
|
|
| 1.50 | % |
| TOTAL |
|
| 117,000,000 |
|
|
| 100.00 | % |
Platinum International Biotech Co., LTD (Cayman Islands) (“Platinum”)
Platinum has authorized 500,000,000 ordinary shares with a par value of $0.0001 per share with 10,152,284 shares issued and outstanding at March 31, 2021.
On April 7, 2020, Platinum issued a total of 10,000,000 ordinary shares to six entities as follows:
| Entity |
| Shares |
| |
| 1. Flydragon International Limited (controlled by Mr. Jun Wang) |
|
| 3,466,000 |
|
| 2. Chinaone Technology Limited (controlled by Mr. Yang Wang) |
|
| 1,667,000 |
|
| 3. Boao Biotech Limited (controlled by Mr. Yulin Cao) |
|
| 2,167,000 |
|
| 4. Dragoncloud Technology Limited (controlled by Mr. Yang Wang) |
|
| 500,000 |
|
| 5. Focus Draw Group Limited (controlled by Ms. Lina Liu) |
|
| 1,200,000 |
|
| 6. Focusone Technology Group Limited (controlled by Mr. Jin Wei) |
|
| 1,000,000 |
|
| Total |
|
| 10,000,000 |
|
| F-35 |
| Table of Contents |
On September 11, 2020, Platinum sold 152,284 ordinary shares to an investor for $750,000 cash.
On January 21, 2021, Yubo New York acquired all 10,152,284 ordinary shares of Platinum outstanding.
Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”)
Yubo Chengdu has subscribed capital of $1,500,000 which has not yet been paid by its shareholder. The subscribed capital is due for payment on January 1, 2040.
Yubo International Biotech (Beijing) Limited (“Yubo Beijing”)
Yubo Beijing has subscribed capital of $1,521,931 (RMB 10,000,000), all of which have been paid by its shareholders as of March 31, 2021.
Restricted net assets
The Company’s ability to pay dividends is primarily dependent on the Company receiving distributions of funds from its subsidiaries or its VIE. Relevant PRC statutory laws and regulations permit payments of dividends by Yubo Chengdu, Yubo Jingzhi, Yubo Global, and Yubo Beijing only out of its retained earnings, if any, as determined in accordance with PRC accounting standards and regulations and after it has met the PRC requirements for appropriation to statutory reserves. Paid in capital of the PRC subsidiary and VIE included in the Company’s consolidated net assets are also non-distributable for dividend purposes. The results of operations reflected in the accompanying consolidated financial statements prepared in accordance with U.S. GAAP differ from those reflected in the statutory financial statements of Yubo Chengdu, Yubo Jingzhi, Yubo Global, and Yubo Beijing.
Yubo Chengdu, Yubo Jingzhi, Yubo,Global and Yubo Beijing are required to set aside at least 10% of their after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of its registered capital. In addition, Yubo Chengdu, Yubo Jingzhi, Yubo Global and Yubo Beijing may allocate a portion of its after-tax profits based on PRC accounting standards to an enterprise expansion fund and a staff bonus and welfare fund at its discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends.
Since inception to March 31, 2021, Yubo Chengdu, Yubo Jingzhi, Yubo Global, and Yubo Beijing have not generated any profit and had negative retained earnings as of March 31, 2021. As a result, these entities have not accrued statutory reserve funds.
The ability of the Company’s PRC subsidiary and its VIE to make dividends and other payments to the Company may also be restricted by changes in applicable foreign exchange and other laws and regulations. Foreign currency exchange regulation in China is primarily governed by the following rules:
|
| · | Foreign Exchange Administration Rules (1996), as amended in August 2008, or the Exchange Rules; |
|
| · | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Currently, under the Administration Rules, Renminbi is freely convertible for current account items, including the distribution of dividends, interest payments, trade and service related foreign exchange transactions, but not for capital account items, such as direct investments, loans, repatriation of investments and investments in securities outside of China, unless the prior approval of the State Administration of Foreign Exchange (the “SAFE”) is obtained and prior registration with the SAFE is made. Foreign-invested enterprises that need foreign exchange for the distribution of profits to its shareholders may affect payment from their foreign exchange accounts or purchase and pay foreign exchange rates at the designated foreign exchange banks to their foreign shareholders by producing board resolutions for such profit distribution. Based on their needs, foreign-invested enterprises are permitted to open foreign exchange settlement accounts for current account receipts and payments of foreign exchange along with specialized accounts for capital account receipts and payments of foreign exchange at certain designated foreign exchange banks.
| F-36 |
| Table of Contents |
Although the current Exchange Rules allow the convertibility of Chinese Renminbi into foreign currency for current account items, conversion of Chinese Renminbi into foreign exchange for capital items, such as foreign direct investment, loans or securities, requires the approval of SAFE, which is under the authority of the People’s Bank of China. These approvals, however, do not guarantee the availability of foreign currency conversion. The Company cannot be sure that it will be able to obtain all required conversion approvals for its operations or that the Chinese regulatory authorities will not impose greater restrictions on the convertibility of Chinese Renminbi in the future. Currently, all of the Company’s revenues are generated in Renminbi. Any future restrictions on currency exchanges may limit the Company’s ability to use its retained earnings generated in Renminbi to make dividends or other payments in U.S. dollars or fund possible business activities outside China.
NOTE 12 – RELATED PARTY TRANSACTIONS
On February 17, 2020, Yubo Beijing executed an Agreement of Joint Research and Development with Beijing Zhenxigu Medical Research Center LP (“Zhenxigu”), an entity that owns 18.18% of Yubo Beijing Capital stock and is controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). Pursuant to the agreement, Yubo Beijing paid RMB 241,880 ($35,848 at the 6.7473 average exchange rate for the year ended December 31, 2020) to Zhenxigu for research and development relating to the medical fluid to be included with the nebulizers to be sold to customers. Such expense has been included with other operating expenses in the accompanying Consolidated Statement of Operations and Comprehensive Loss for the three months ended March 31, 2020.
On February 27, 2020, Yubo Beijing executed a Patent Transfer Agreement with Beijing Zhenhuikang Biotechnology Co. LTD (“Zhenhuikang”), an entity controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). The Agreement provided for the assignment of two patents owned by Zhenhuikang to Yubo Beijing for consideration of RMB 140,000 ($21,307 at the 6.5706 current exchange rate at March 31, 2021) (See Note 7).
On February 27, 2020, Yubo Beijing executed an Entrustment Technical Service Agreement with Beijing Zhenhuikang Biotechnology Co. LTD (“Zhenhuikang”), an entity controlled by Mr. Yulin Cao (who is a director of Platinum and Yubo Beijing). The Agreement provides for Zhenhuikang to, among other things, assist Yubo Beijing in the preparation of 300 sets of endometrial stem cell harvesting packages. As amended July 2, 2020, the Agreement provides for Yubo Beijing to pay Zhenhuikang at the rate of RMB 666 per set or RMB 199,800 total ($30,408 at the 6.5706 current exchange rate at March 31, 2021). As of March 31, 2021, preparation of the stem cell harvesting packages has not yet commenced, no payments to Zhenhuikang have been made, and no expense or liability has been recorded.
NOTE 13 – INCOME TAX
Cayman Islands
Under the current laws of the Cayman Islands, Platinum is not subject to tax on income or capital gains. In addition, payments of dividends by Platinum to its shareholders are not subject to withholding tax in the Cayman Islands.
Hong Kong
Platinum HK was incorporated under the Hong Kong tax law where the statutory income tax rate is 16.5%. Platinum HK has had no taxable income or loss from May 4, 2020 (inception) to March 31, 2021.
People’s Republic of China
Yubo International Biotech (Chengdu) Limited (“Yubo Chengdu”), Yubo Jingzhi Biotechnology (Chengdu) Co. LTD. (“Yubo Jingzhi”), Yubo Global Biotechnology (Chengdu) Co. Ltd (“Yubo Global”) and Yubo International Biotech (Beijing) Limited were incorporated in the PRC and are subject to PRC Enterprise Income Tax (“EIT”) on their taxable income in accordance with the relevant PRC income tax laws. On March 16, 2007, the National People’s Congress enacted a new enterprise income tax law, which took effect on January 1, 2008. The law applies a uniform 25% enterprise income tax rate to both foreign invested enterprises and domestic enterprises.
Yubo Chengdu and Yubo Jingzhi have had no taxable income or loss from September 4, 2020 (inception) to March 31, 2021.
| F-37 |
| Table of Contents |
Yubo Beijing has had net losses of $231,193 for the year ended December 31, 2019, $597,713 for the year ended December 31, 2020, and $192,087 for the three months ended March 31, 2021. These losses can be carried forward for five years to reduce future years’ taxable income through year 2024 to year 2026. Based on management’s present assessment, the Company has not yet determined it to be more likely than not that future utilization of the net operating loss carryforwards will be realized. Accordingly, the Company has recorded a 100% valuation allowance against the deferred tax asset at March 31, 2021 and December 31, 2010.
The components of deferred tax assets were as follows:
|
|
| March 31, 2021` |
|
| December 31, 2020 |
| ||
|
|
|
|
|
|
|
| ||
| Net operating losses carry forward |
| $ | 279,392 |
|
| $ | 207,227 |
|
| Valuation allowance |
|
| (279,392 | ) |
|
| (207,227 | ) |
| Deferred tax assets, net |
| $ | — |
|
| $ | — |
|
The reconciliation of the provisions for (benefits from) income tax by applying the PRC tax rate to income (loss) before provisions for income tax and the actual provisions for income tax is as follows:
|
|
| For the three months ended March 31, 2021 |
|
| For the three months ended March 31, 2020 |
| ||
|
|
|
|
|
|
|
| ||
| Income tax (benefits) at 25% |
| $ | (109,669 | ) |
| $ | (35,237 | ) |
| Net loss of Platinum |
|
| 37,504 |
|
|
| - |
|
| Increase in valuation allowance |
|
| 72,165 |
|
|
| 35,237 |
|
| Provision for income taxes |
| $ | — |
|
| $ | — |
|
Accounting for Uncertainty in Income Taxes
The tax authority of the PRC government conducts periodic and ad hoc tax filing reviews on business enterprises operating in the PRC after those enterprises complete their relevant tax filings. Therefore, the Company’s PRC entities’ tax filings results are subject to change and may lead to tax liabilities.
ASC 740 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The management evaluated the Company’s tax positions and concluded that no liability for uncertainty in income taxes was necessary as of March 31, 2021 and December 31, 2020.
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Freelancer Service Contract
On March 30, 2020, Yubo Beijing executed an agreement with Hainan Huiyonggong Service Ltd. (“HHS”). The agreement provided for HHS to engage sales representatives (often Yubo Beijing customers) to refer new customers to Yubo Beijing and for Yubo Beijing to pay fees to HHS based on the amount of sales generated from HHS’s sales representatives. The term of the agreement was for one year expiring March 29, 2021. For the three months ended March 31, 2021, the Company expensed $138,349 pursuant to this agreement which is included in “Sales Commissions” in the accompanying statement of operations.
| F-38 |
| Table of Contents |
Website Platform Maintenance Agreement
On April 29, 2020, Yubo Beijing executed an agreement with Hainan Haifu Technology Ltd. (“HHT”). The agreement provided for HHT to provide certain website maintenance services for Yubo Beijing and provided for Yubo Beijing to pay a monthly fee of RMB 150,000 ($22,231 using the December 31, 2020 average rate of 6.7473) to HHT. The term of the agreement, which originally was for one year expiring April 28, 2021, was mutually terminated on October 30, 2020.
Credit risk
Cash deposits with banks are held in financial institutions in the PRC, which are insured with deposit protection up to RMB 500,000 (approximately $76,097 at March 31, 2020). Accordingly, the Company has a concentration of credit risk related to the uninsured part of bank deposits. The Company has not experienced any losses in such accounts and believes it is not exposed to significant credit risk.
Risks of Variable Interest Entity Structure
Although the structure the Company has adopted is consistent with longstanding industry practice, and is commonly adopted by comparable companies in China, the PRC government may not agree that these arrangements comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. There are uncertainties regarding the interpretation and application of PRC laws and regulations including those that govern the Company’s contractual arrangements, which could limit the Company’s ability to enforce these contractual arrangements. If the Company or its variable interest entity is found to be in violation of any existing or future PRC laws, rules or regulations, or fail to obtain or maintain any of the required permits or approvals, the relevant PRC regulatory authorities would have broad discretion to take action in dealing with such violations or failures, including levying fines, revoking business and other licenses of the Company’s variable interest entity, requiring the Company to discontinue or restrict its operations, restricting its right to collect revenue, requiring the Company to restructure its operations or taking other regulatory or enforcement actions against the Company. In addition, it is unclear what impact the PRC government actions would have on the Company and on its ability to consolidate the financial results of its variable interest entity in the consolidated financial statements, if the PRC government authorities were to find the Company’s legal structure and contractual arrangements to be in violation of PRC laws, rules and regulations. If the imposition of any of these government actions causes the Company to lose its right to direct the activities of Yubo Beijing or the right to receive their economic benefits, the Company would no longer be able to consolidate Yubo Beijing.
NOTE 14 - SUBSEQUENT EVENTS
On May 11, 2021, World Precision Medicine Technology Inc., a company owned and controlled by Cheung Ho Shun, a shareholder of Yubo International Biotech Limited, provided the Company $600,000 in working capital loan. The loan is due on demand and non-interest bearing.
| F-39 |
| Table of Contents |
YUBO INTERNATIONAL BIOTECH LIMITED
5,000,000 Shares of Class A Common Stock Offered by the Company
12,251,100 Shares of Class A Common Stock Offered by the Selling Stockholders
_________
PROSPECTUS
_________
_____________
, 2021
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following is an itemized statement of the estimated amounts of all expenses payable by us in connection with the registration of the common stock, other than underwriting discounts and commissions. All amounts are estimates except the SEC registration fee.
| SEC Registration Fee |
| $ | 941.05 |
|
| Accounting Fees and Expenses |
| $ | 39,000.00 |
|
| Legal Fees and Expenses |
| $ | 35,000.00 |
|
| Miscellaneous Expenses |
| $ | 10,000.00 |
|
| Total |
| $ | 84,941.05 |
|
Item 14. Indemnification of Directors and Officers.
The NYBCL permits a corporation to indemnify its current and former directors and officers against expenses, judgments, fines and amounts paid in connection with a legal proceeding. To be indemnified, the person must have acted in good faith and in a manner the person reasonably believed to be in, and not opposed to, the best interests of the corporation. With respect to any criminal action or proceeding, the person must not have had reasonable cause to believe the conduct was unlawful.
The NYBCL permits a present or former director or officer of a corporation to be indemnified against certain expenses if the person has been successful, on the merit or otherwise, in defense of any proceeding brought against such person by virtue of the fact that the person is or was an officer or director of the corporation. In addition, the NYBCL permits the advancement of expenses relating to the defense of any proceeding to directors and officers contingent upon the person's commitment to repay advances for expenses in the case he or she is ultimately found not to be entitled to be indemnified.
The NYBCL provides that the indemnification provisions contained in the NYBCL are not exclusive of any other right that a person seeking indemnification may have or later acquire under any provision of a corporation's certification of incorporation or by-laws, or, when authorized by the corporation's certificate of incorporation or by-laws, by any agreement, by any vote of shareholders or disinterested directors or otherwise. The NYBCL also provides that a corporation may maintain insurance, at its expense, to protect its directors and officers in instances in which they may not otherwise be indemnified by the corporation under the provisions of the NYBCL provided the contract of insurance covering the directors and officers provides, in a manner acceptable to the New York superintendent of insurance, for a retention amount and for co-insurance.
Our amended and restated bylaws provide that, to the maximum extent permitted by NYBCL and the federal securities laws, we must indemnify and, upon request advance, expenses to a director or officer made, or threatened to be made, a party to any action or proceeding (other than a shareholder derivative action) by reason of such person being a director or officer, if such director or officer acted in good faith for a purpose which he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, in criminal actions or proceedings, in addition, had no reasonable cause to believe that his or her conduct was unlawful. Indemnification would cover reasonable expenses, including attorneys’ fees, judgments, fines, amounts paid in settlement and reasonable expenses (including attorneys’ fees).
We have entered into indemnification agreements with each of our directors and executive officers that require us to indemnify these persons against expenses, judgments, fines, settlements and other amounts actually and reasonably incurred (including expenses of a derivative action) in connection with any proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that the person is or was a director or officer of our Company or any of our affiliated enterprises.
| II-1 |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities
On January 13, 2021, we entered into the Exchange Agreement. As a result of the Exchange Transaction, the shareholders of Platinum received 117,000,000 shares of our Class A common stock, representing approximately 99.00% of our Class A common stock, in exchange for 100% of the issued and outstanding common stock of Platinum. The issuance of the Class A common stock pursuant to the Exchange Agreement was exempt from registration under the Securities Act pursuant to Section 4(2) and Regulation D and Regulation S thereof.
Item 16. Exhibits
(a) The exhibits listed in the following Exhibit Index are filed as part of this Registration Statement.
_______________
| + | Certain portions of this exhibit containing personally identifiable information have been redacted. |
| * | Previously filed. |
| II-2 |
Item 17. Undertakings
(a) The undersigned Registrant hereby undertakes:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the Registration Statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement or any material change to such information in the Registration Statement;
provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in the Registration Statement.
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
| II-3 |
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(c) The undersigned Registrant hereby undertakes that:
(1) for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(d) that, for the purpose of determining liability under the Securities Act to any purchaser:
(1) if the issuer is relying on Rule 430B:
(i) each prospectus filed by the undersigned issuer pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
(2) if the issuer is relying on Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-4 |
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Amendment No. 1 to Registration Statement to be signed on its behalf by the undersigned thereunto duly authorized in the City of Beijing, China on July 12, 2021.
| Yubo International Biotech Limited, | |||
| a New York corporation | |||
| By: | /s/ Jun Wang | ||
|
| Jun Wang | ||
| Its: | Chief Executive Officer, President and Director | ||
| (Principal Executive Officer) | |||
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| By: | /s/ Jun Wang | July 12, 2021 | |
| Jun Wang | |||
| Its: | Chief Executive Officer, President and Director | ||
| (Principal Executive Officer) | |||
| By: | /s/ Lina Liu | July 12, 2021 | |
| Lina Liu | |||
| Its: | Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | |||
| By: | /s/ Yang Wang | July 12, 2021 | |
| Yang Wang | |||
| Its: | Director | ||
| By: | /s/ Zhihui Bai | July 12, 2021 | |
| Zhihui Bai | |||
| Its: | Director | ||
|
|
|
|
|
| *By: | /s/ Jun Wang |
| July 12, 2021 |
|
| Jun Wang |
|
|
|
| Attorney-in-fact |
|
|
| II-5 |
