Attached files
| file | filename |
|---|---|
| 8-K - 8-K - YUMANITY THERAPEUTICS, INC. | d175595d8k.htm |

Yumanity Corporate Overview June 2021 NASDAQ: YMTX Exhibit 99.1

Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding: future product development plans and projected timelines for the initiation and completion of preclinical and clinical trials and other activities; the potential for the results of ongoing preclinical or clinical trials and the efficacy of Yumanity’s product candidates; future product development and regulatory strategies, including with respect to specific indications; Yumanity's cash position and financial outlook; and the expected upcoming milestones described in these slides and the accompanying oral presentation. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Yumanity’s current beliefs, expectations and assumptions regarding the future of Yumanity’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to those risks detailed in Yumanity’s most recent Annual or Quarterly Report, as well as discussions of potential risks, uncertainties, and other important factors in Yumanity’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. None of Yumanity, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.

A Future Free from Neurodegenerative Diseases Our Vision: Yumanity Therapeutics, Inc. 2021
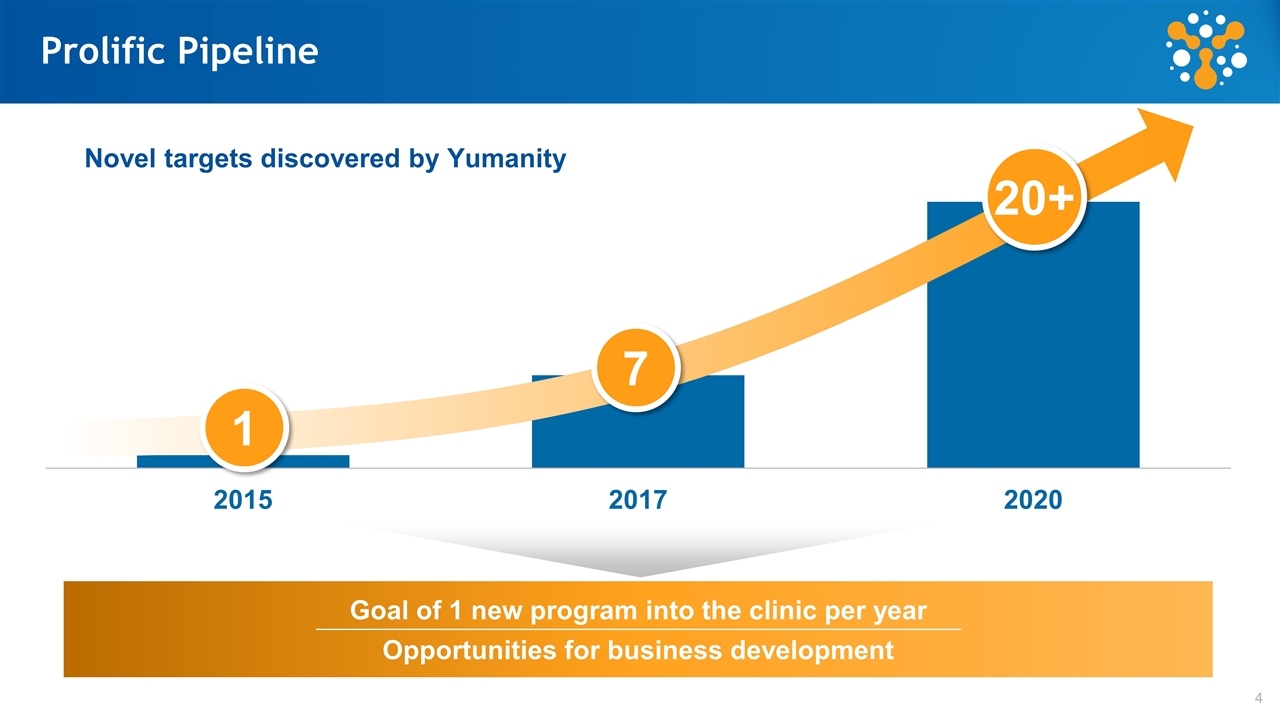
Prolific Pipeline Novel targets discovered by Yumanity Goal of 1 new program into the clinic per year Opportunities for business development 1 7 20+
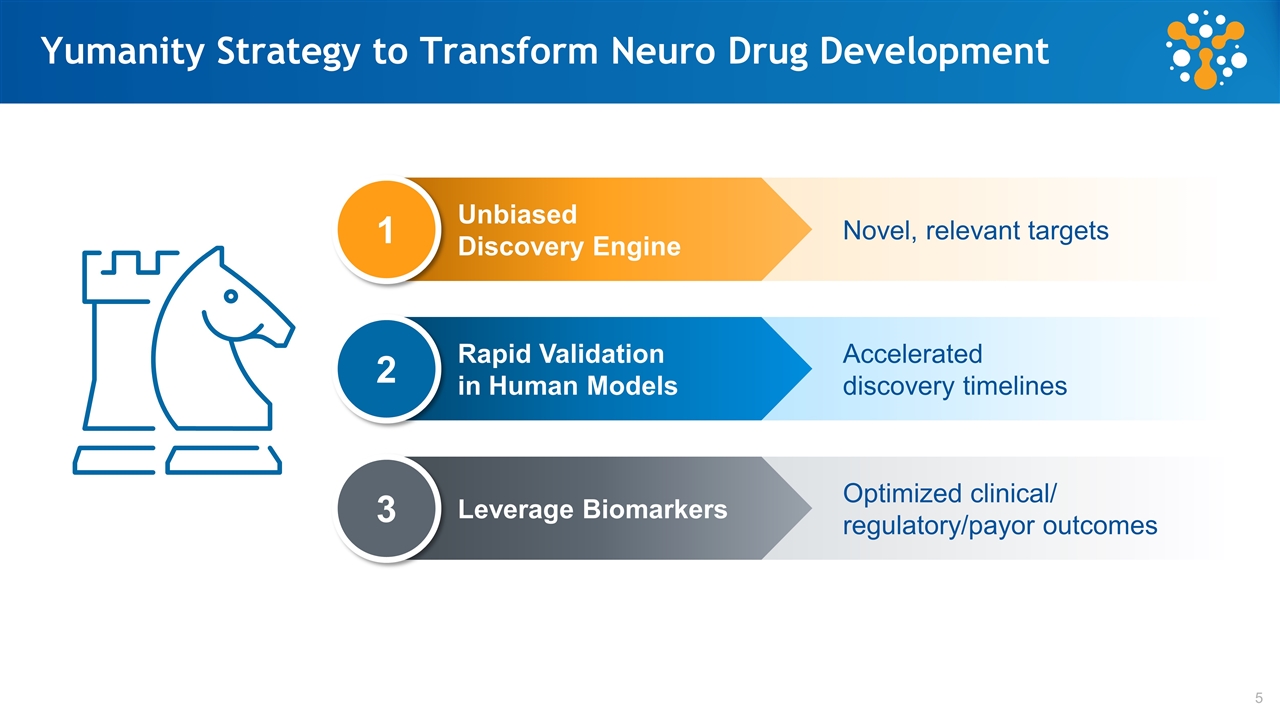
Accelerated discovery timelines Optimized clinical/ regulatory/payor outcomes Novel, relevant targets Yumanity Strategy to Transform Neuro Drug Development Unbiased Discovery Engine Rapid Validation in Human Models Leverage Biomarkers 1 2 3

Leveraging External Research Collaboration on 2 targets in ALS and FTD Supporting studies in proprietary Parkinson’s Disease animal models Emerging data in Glioblastoma
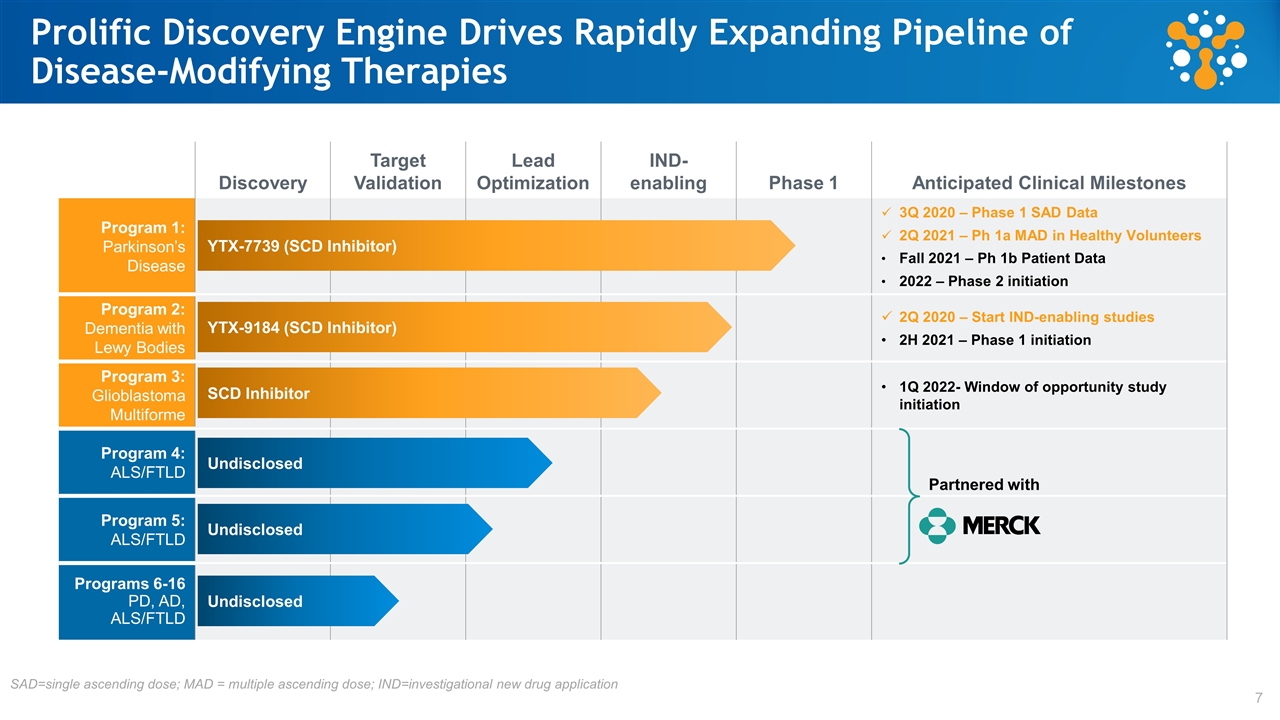
Discovery Target Validation Lead Optimization IND- enabling Phase 1 Anticipated Clinical Milestones Program 1: Parkinson’s Disease 3Q 2020 – Phase 1 SAD Data 2Q 2021 – Ph 1a MAD in Healthy Volunteers Fall 2021 – Ph 1b Patient Data 2022 – Phase 2 initiation Program 2: Dementia with Lewy Bodies 2Q 2020 – Start IND-enabling studies 2H 2021 – Phase 1 initiation Program 3: Glioblastoma Multiforme 1Q 2022- Window of opportunity study initiation Program 4: ALS/FTLD Program 5: ALS/FTLD Programs 6-16 PD, AD, ALS/FTLD YTX-7739 (SCD Inhibitor) Undisclosed Undisclosed Undisclosed YTX-9184 (SCD Inhibitor) Prolific Discovery Engine Drives Rapidly Expanding Pipeline of Disease-Modifying Therapies Partnered with SAD=single ascending dose; MAD = multiple ascending dose; IND=investigational new drug application SCD Inhibitor

Differentiating Advantages Against Parkinson’s Disease Intervene upstream from α-synuclein pathology rather than downstream Advantages of small molecule pharmacology vs. large antibodies Potential to leverage treatment biomarker to individualize therapy and enrich for likely responders
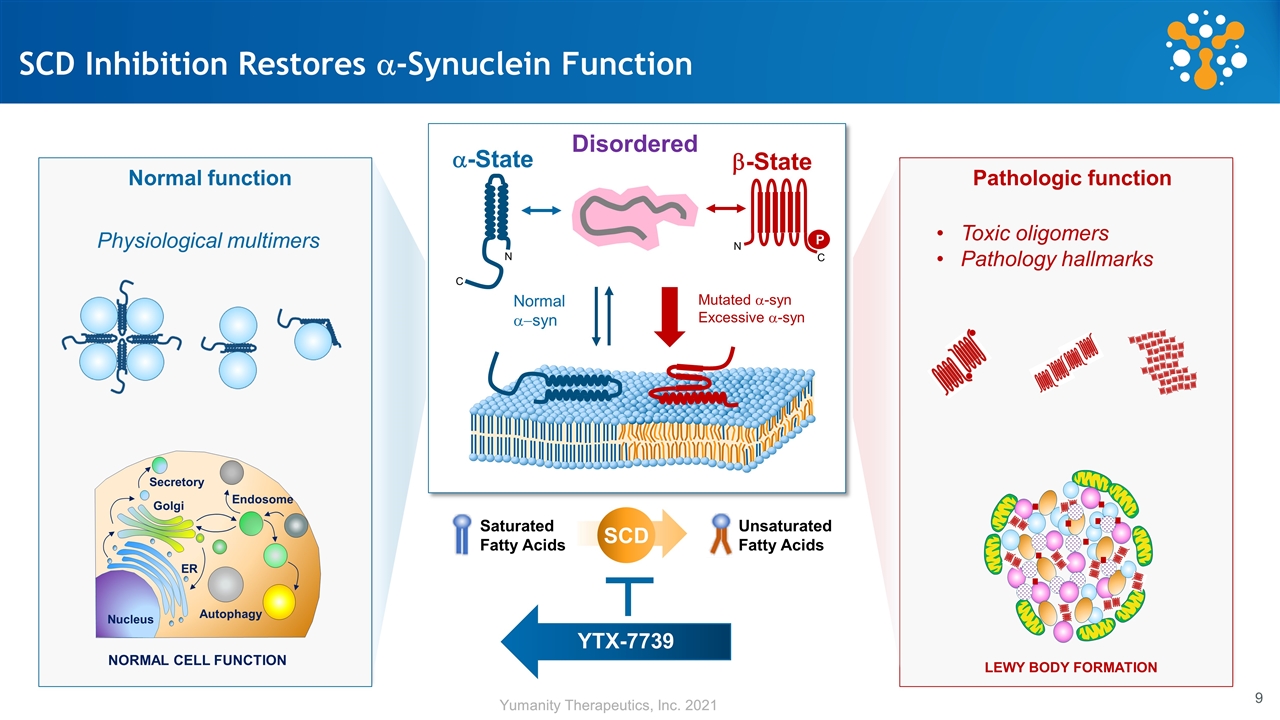
SCD Inhibition Restores a-Synuclein Function Normal a-syn Mutated a-syn Excessive a-syn a-State N C b-State P C N Disordered YTX-7739 Secretory Endosome Autophagy Nucleus ER Golgi Physiological multimers Toxic oligomers Pathology hallmarks SCD Unsaturated Fatty Acids Saturated Fatty Acids NORMAL CELL FUNCTION LEWY BODY FORMATION Normal function Pathologic function
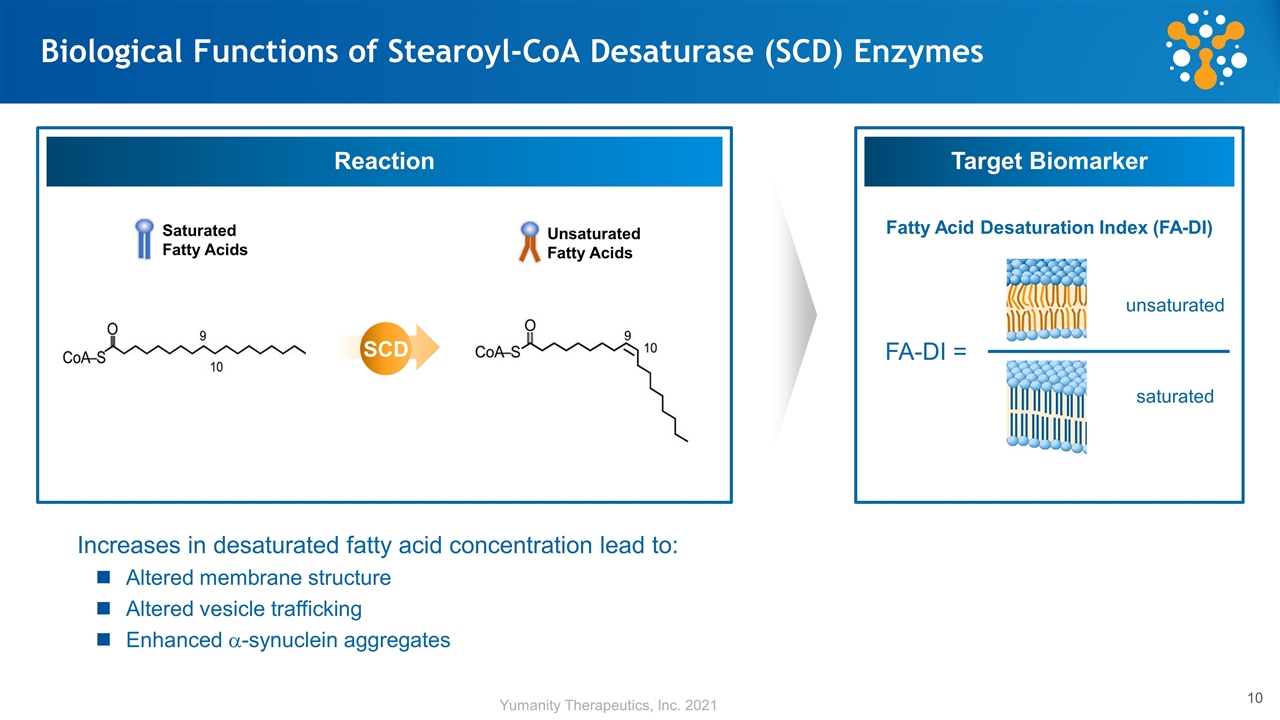
Biological Functions of Stearoyl-CoA Desaturase (SCD) Enzymes Reaction Target Biomarker Increases in desaturated fatty acid concentration lead to: Altered membrane structure Altered vesicle trafficking Enhanced a-synuclein aggregates SCD FA-DI = Fatty Acid Desaturation Index (FA-DI) unsaturated saturated Saturated Fatty Acids Unsaturated Fatty Acids
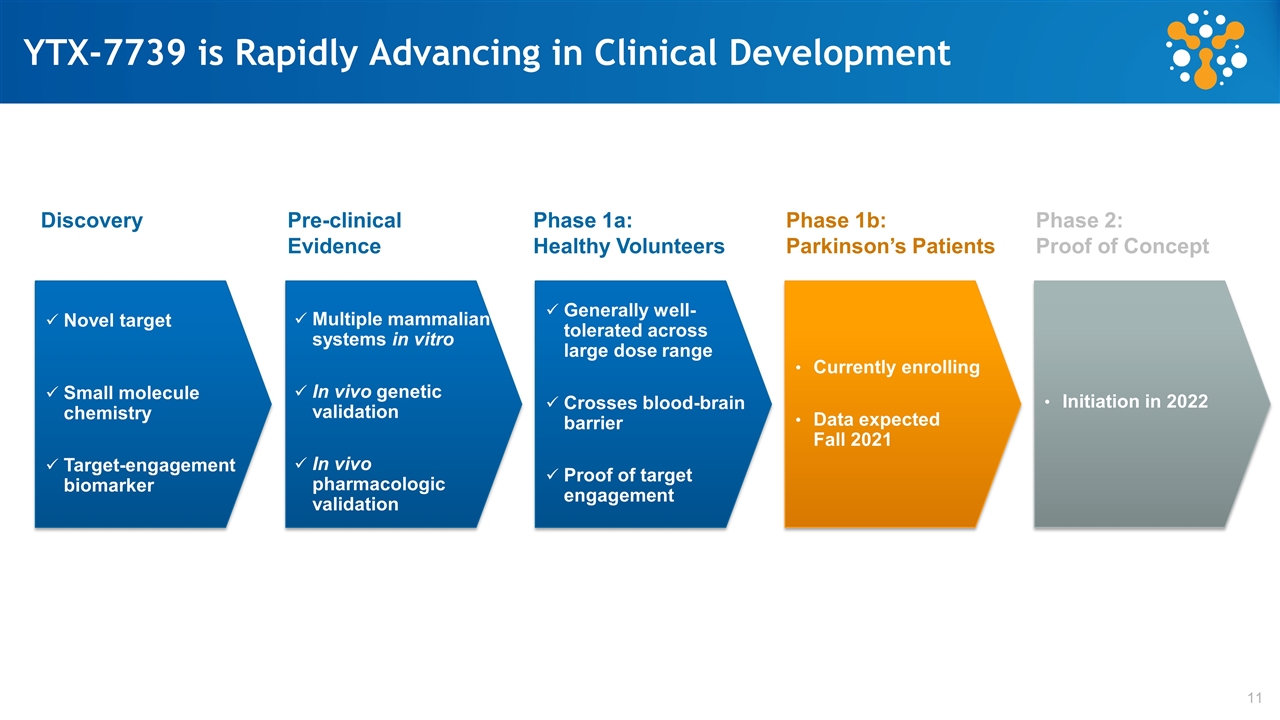
YTX-7739 is Rapidly Advancing in Clinical Development Novel target Small molecule chemistry Target-engagement biomarker Multiple mammalian systems in vitro In vivo genetic validation In vivo pharmacologic validation Generally well-tolerated across large dose range Crosses blood-brain barrier Proof of target engagement Currently enrolling Data expected Fall 2021 Discovery Pre-clinical Evidence Phase 1a: Healthy Volunteers Phase 1b: Parkinson’s Patients Phase 2: Proof of Concept Initiation in 2022
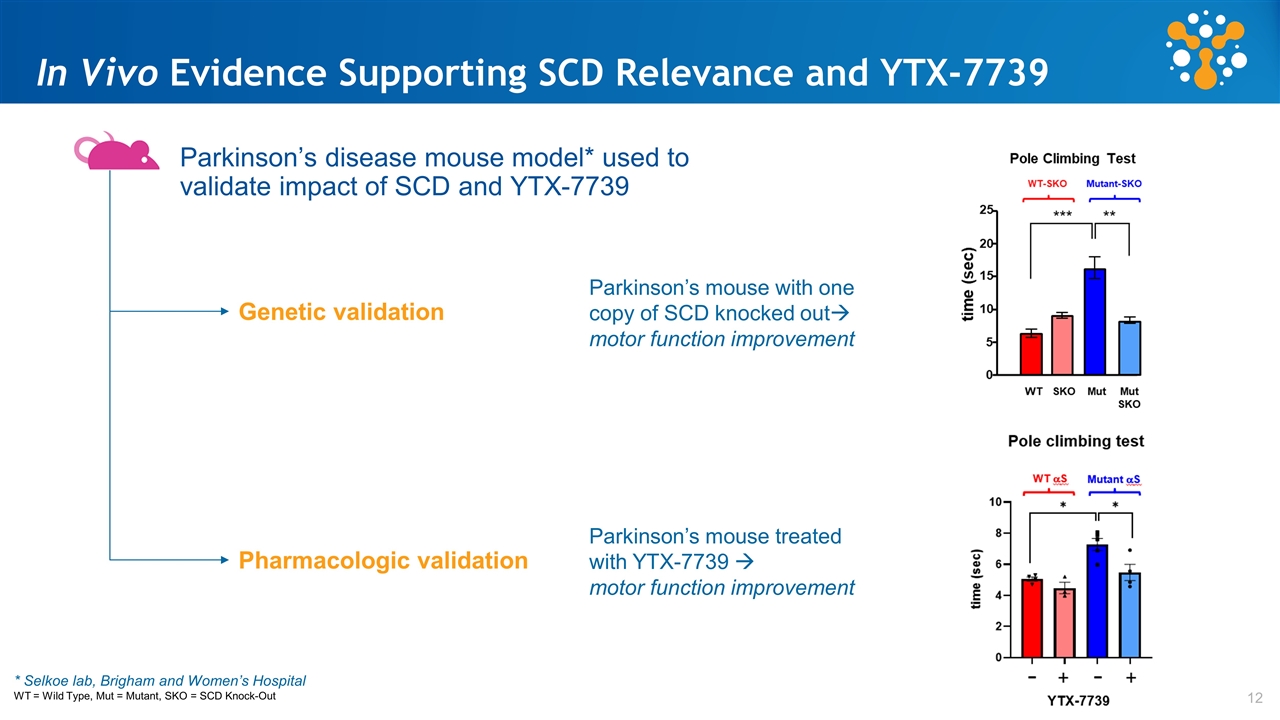
In Vivo Evidence Supporting SCD Relevance and YTX-7739 Parkinson’s disease mouse model* used to validate impact of SCD and YTX-7739 * Selkoe lab, Brigham and Women’s Hospital WT = Wild Type, Mut = Mutant, SKO = SCD Knock-Out Genetic validation Parkinson’s mouse with one copy of SCD knocked outà motor function improvement Pharmacologic validation Parkinson’s mouse treated with YTX-7739 à motor function improvement
YTX-7739 Results in Healthy Volunteers Total of 72 subjects enrolled in Single- and Multiple- Ascending Dose studies Gold standard execution: Double-blind, randomized, placebo controlled* Generally well-tolerated: across dose range for up to 28 days Crossed blood-brain barrier: relevant drug concentrations in the cerebrospinal fluid Proof of biology demonstrated: Target engagement achieved Dose-dependent decreases in biomarker at levels associated with motor function restoration in animal models Yumanity Therapeutics, Inc. 2021 * 16 Single Ascending Dose subjects were open label to inform the MAD study
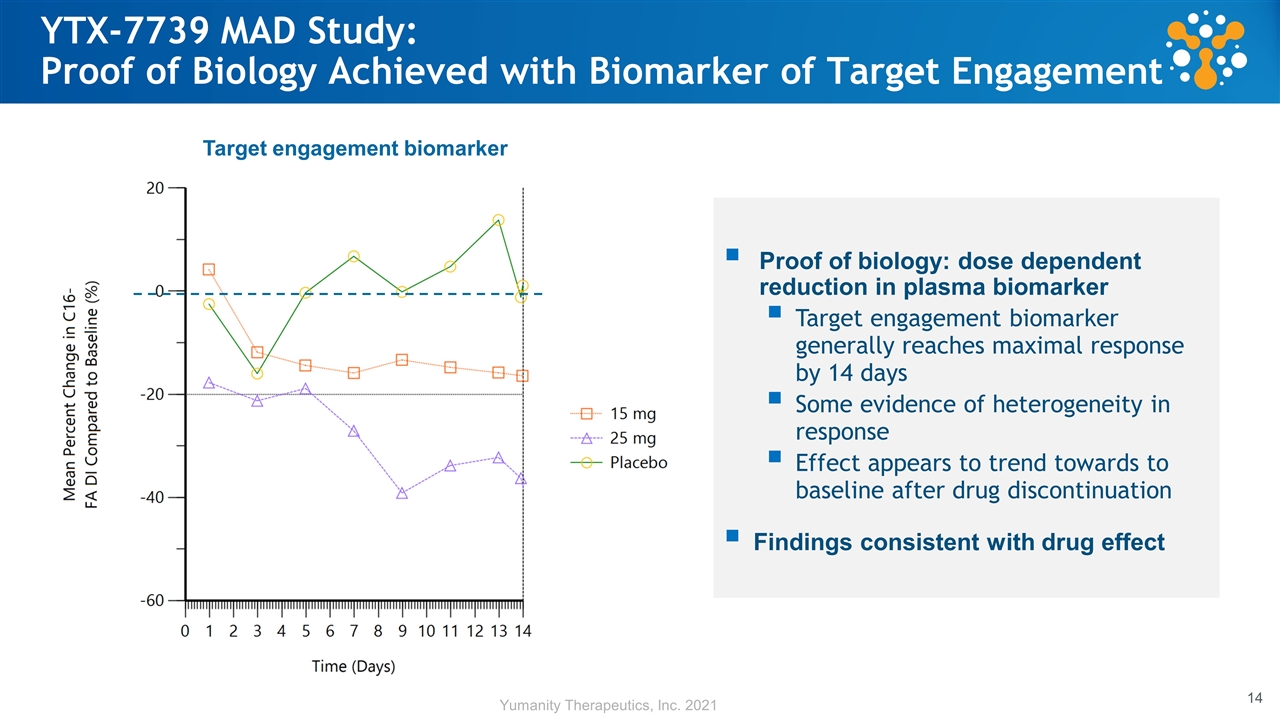
YTX-7739 MAD Study: Proof of Biology Achieved with Biomarker of Target Engagement Proof of biology: dose dependent reduction in plasma biomarker Target engagement biomarker generally reaches maximal response by 14 days Some evidence of heterogeneity in response Effect appears to trend towards to baseline after drug discontinuation Findings consistent with drug effect Target engagement biomarker
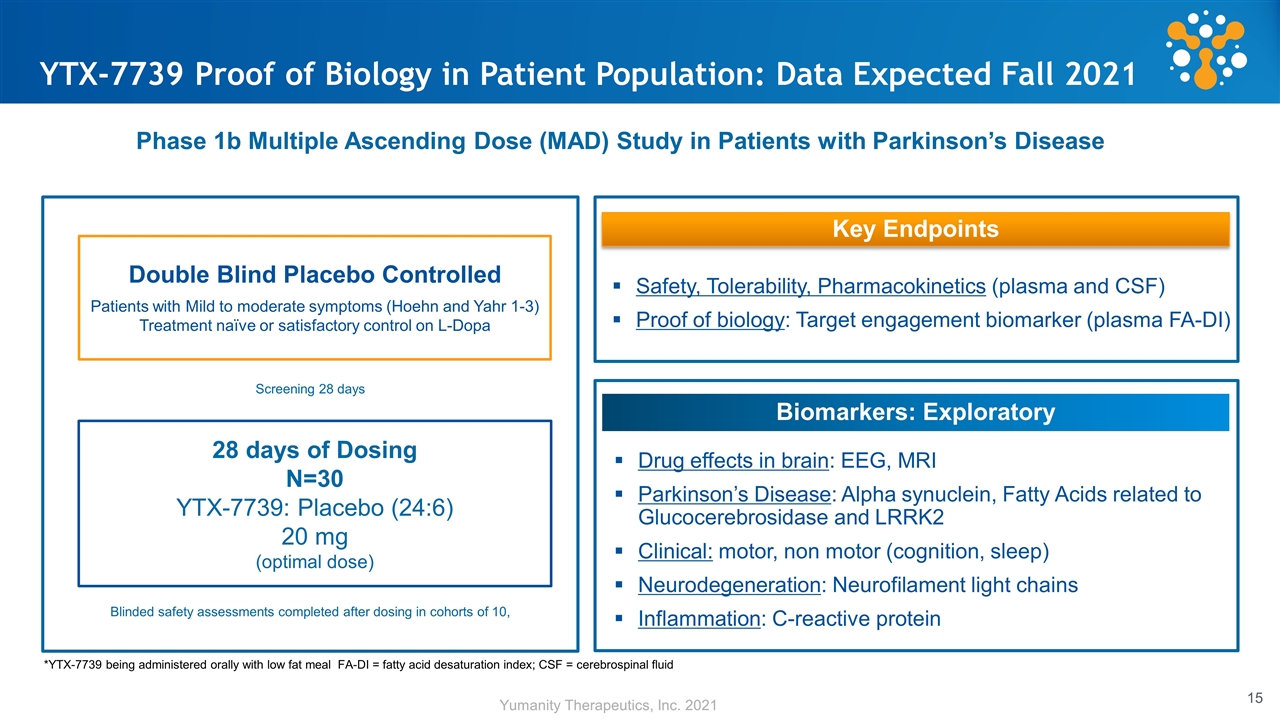
YTX-7739 Proof of Biology in Patient Population: Data Expected Fall 2021 Blinded safety assessments completed after dosing in cohorts of 10, Key Endpoints Safety, Tolerability, Pharmacokinetics (plasma and CSF) Proof of biology: Target engagement biomarker (plasma FA-DI) Biomarkers: Exploratory Drug effects in brain: EEG, MRI Parkinson’s Disease: Alpha synuclein, Fatty Acids related to Glucocerebrosidase and LRRK2 Clinical: motor, non motor (cognition, sleep) Neurodegeneration: Neurofilament light chains Inflammation: C-reactive protein Phase 1b Multiple Ascending Dose (MAD) Study in Patients with Parkinson’s Disease *YTX-7739 being administered orally with low fat meal FA-DI = fatty acid desaturation index; CSF = cerebrospinal fluid Double Blind Placebo Controlled Patients with Mild to moderate symptoms (Hoehn and Yahr 1-3) Treatment naïve or satisfactory control on L-Dopa 28 days of Dosing N=30 YTX-7739: Placebo (24:6) 20 mg (optimal dose) Screening 28 days

Three Clinical Read-outs Expected During Cash Runway Parkinson’s Disease: Phase 1b top line results anticipated in Fall 2021 Lewy Body Dementia: Phase 1 initiation in H2 2021 à Results anticipated in 2022 Glioblastoma: Window of opportunity study initiation in Q1 2022 à Results anticipated in 2022 Yumanity Therapeutics, Inc. 2021 * 16 Single Ascending Dose subjects were open label to inform the MAD study

Thank you
