Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Cara Therapeutics, Inc. | tm2114554d1_ex99-1.htm |
| 8-K - FORM 8-K - Cara Therapeutics, Inc. | tm2114554d1_8k.htm |
Exhibit 99.2

KARE Phase 2 Topline Data: Oral KORSUVA Πfor Pruritus in Atopic Dermatitis APRIL 2021 KARE Phase 2 study evaluated the efficacy and safety of oral difelikefalin for moderate to severe pruritus in adult subjects wit h atopic dermatitis (AD). The FDA has conditionally accepted KORSUVA Πas the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and efficacy have not been fully evaluated by any regulatory auth ori ty.

2 Confidential. For internal use only. Disclaimers This presentation is confidential and is being provided subject to the terms of the Confidentiality Agreement between Cara Th era peutics and the recipient. This presentation contains certain forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward - looking statements by the words “anticipate,” “believe,” “continue,” “estimate,” “expect ,” “objective,” “ongoing,” “plan,” “propose,” “potential,” “projected”, or “up - coming” and/or the negative of these terms, or other comparable terminology intended to identify statements about the future. Examples of these forward - looking statements in this presentation include, among other things, statements concerning plans, strategies and expectations for the future, including statements regarding t he expected timing of our planned clinical trials and regulatory submissions; the potential results of ongoing and planned clini cal trials; future regulatory and development milestones for the Company's product candidates; the size of the potential markets that are potent ial ly addressable for the Company’s product candidates, including the pruritus market and the potential commercialization of Korsuv a Œ . These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels o f a ctivity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statem ents. Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we cautio n you that these statements are based on a combination of facts and factors currently known by us and our expectations of the futur e, about which we cannot be certain. Factors that may cause actual results to differ materially from any future results expressed or i mpl ied by any forward - looking statements include the risks described in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K for the year ended December 31, 2020, as well as those set forth from time to time in the Company’s other SEC filings, available at http://www.sec.gov. Any forward - looking statements speak only as of the date of this presentation. The Company undertakes no obligation to publicly update any forward - looking statements, whether as a result of new information, future events or otherwise except as required by law.

3 Confidential. For internal use only. Cara Therapeutics Pipeline *Cara Therapeutics has investigated KORSUVA Œ for post - operative pain. †Vifor has commercial rights in Non - US Fresenius Medical Care dialysis clinics under a profit - share arrangement. ‡Commercialization rights to KORSUVA Œ in defined indications — Japan: Maruishi Pharma; South Korea: CKD Pharma. § PDUFA date is August 23, 2021. ||VFMCRP and Cara have rights to promote in Fresenius clinics in the US under a profit - share agreement. CKD - HD: Chronic Kidney Disease - Hemodialysis; NDD — CKD: Non - Dialysis Dependent - Chronic Kidney Disease; AD: Atopic Dermatitis; PBC: Primary Biliary Cholangitis; NP: Notalgia Paresthetica. STAGE OF DEVELOPMENT Program Indication * Phase I Phase II Phase III NDA Review Commercialization Rights † (ex - Japan and S. Korea) ‡ KORSUVA TM Injection Pruritus CKD - HD § US - Vifor EU / Other - VFMCRP || Oral KORSUVA Œ Pruritus NDD - CKD Cara Oral KORSUVA Œ Pruritus AD Cara Oral KORSUVA Œ Pruritus PBC Cara Oral KORSUVA Œ Pruritus NP Cara FDA Priority Review The FDA has conditionally accepted KORSUVA Œ as the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and efficacy have not been fully evaluated by any regulatory authority.

4 Confidential. For internal use only. Oral KORSUVA TM (Difelikefalin) For Atopic Dermatitis - Associated Pruritus 30 million US patients ~80% Mild - Moderate Disease* ~20% Severe Disease* Moderate - Severe Pruritus Approved Therapies Injectable Biologic Topical Steroids & Immunomodulators *Silverberg JI. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin. 2017;35(3):283 - 289. Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: A Cross - Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J Invest Dermatol. 2019;139(3):583 - 590. Barbarot S et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018; 1284 - 1293. Chovatiya R et al. Clinical phenotyping of atopic dermatitis using combined itch and lesional severity: A prospective observational study. Annals of Allergy, Asthma Immunology 2021.The FDA has conditionally accepted KORSUVA Πas the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and ef ficacy have not been fully evaluated by any regulatory authority.

5 Confidential. For internal use only. KARE: Phase 2 Study Design 12 Weeks TREATMENT (Twice Daily) RUN - IN 7 Days END OF TREATMENT 1:1:1:1 Randomization SCREEN Placebo BID KORSUVA : 0.25 mg BID Baseline Mean I - NRS > 5 KORSUVA : 0.5 mg BID KORSUVA : 1.0 mg BID Primary Endpoint Change from baseline in the weekly mean of the daily 24 - hr Itch - Numeric Rating Scale (I - NRS) at Week 12 Key Secondary Endpoint Proportion of subjects achieving ≥4 - point improvement in I - NRS at Week 12 4 Week Active Extension Ongoing The FDA has conditionally accepted KORSUVA Œ as the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and efficacy have not been fully evaluated by any regulatory authority.

6 Confidential. For internal use only. KARE: Patient Disposition Placebo (N=123*) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124*) DFK 1.0 mg (N=77) Total Randomized (N =401 ) Completed 97 (79%) 63 (82%) 102 (82%) 61 (79%) Discontinued 26 (21%) 14 (18%) 22 (18%) 16 (21%) Adverse event 4 3 1 9 Subject withdrew consent 5 3 8 4 Subject non - compliance 6 2 7 0 Lost to follow - up 5 2 1 2 Lack of therapeutic efficacy 3 1 2 0 Other 3 3 3 1 Use of Rescue Medication 2 (1.6%) 4 (5.2%) 1 (0.8%) 1 (1.3%) *The sample size for DFK 0.5 mg and placebo was increased based on the results of the Interim Assessment for sample size re - esti mation

7 Confidential. For internal use only. KARE: Patient Demographics Female, n (%) 80 (65) 54 (70) 83 (67) 53 (69) Age - Mean (SD) 40 (15.6) 43 (16.2) 42 (15.4) 41 (14.0) Race, n (%) White 71 (58) 44 (57) 74 (60) 40 (52) Black 42 (34) 31 (40) 40 (32) 33 (43) Asian 5 (4) 1 (1) 5 (4) 2 (3) BMI – Mean (SD) 29 (7) 30 (8) 32 (9) 31 (8) Placebo (N=123) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124) DFK 1.0 mg (N=77)

8 Confidential. For internal use only. KARE: Baseline Disease Characteristics Duration of AD ( yrs ) – Mean (SD) 20 (16) 25 (14) 21 (15) 23 (16) Baseline BSA (%) – Mean (SD) 8.4 (6.9) 8.3 (6.0) 8.4 (6.4) 9.5 (6.9) Baseline BSA Group – n (%) < 10 % 79 (64) 50 (65) 82 (66) 46 (60) ≥ 10 % 44 (36) 27 (35) 42 (34) 31 (40) Baseline EASI – Mean (SD) 5.9 (4.9) 6.9 (5.4) 5.9 (4.3) 6.5 (4.5) Baseline IGA 2 56 (46) 33 (43) 57 (46) 33 (43) 3 64 (52) 40 (52) 64 (52) 43 (56) 4 3 (2) 4 (5) 3 (2) 1 (1) Baseline I - NRS – Mean (SD) 7.7 (1.3) 7.8 (1.3) 7.8 (1.2) 7.9 (1.2) BSA=Body Surface Area & <10% is mild/moderate AD; EASI scores ranges from 0 to 72; IGA scores range from 0 to 4; I - NRS: Worst Itching Numeric Rating Scale (0 to 10) where 0 = no itch and 10 = worst itching imaginable. Placebo (N=123) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124) DFK 1.0 mg (N=77)

9 Confidential. For internal use only. -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 Placebo (N = 123) DFK 0.25 mg (N=77) DFK 0.5 mg (N=124) DFK 1.0 mg (N=77) DFK All Doses (N = 278) * * ** * * Primary Endpoint: Change from Baseline in Daily I - NRS at Week 12 (ITT) Significant improvement observed in 1.0 mg DFK vs placebo in majority of timepoints, starting at week 1 * P < 0.05, ** P < 0.01 LS Means over time Weeks in Placebo - Controlled Treatment Period Change from Baseline Baseline 1 2 3 4 5 6 7 8 9 10 11 12 * * * P=0.073 DFK 1.0 mg LS Means from MMRM with terms for treatment, week, week by treatment interaction, baseline score, and AD severity. Missing da ta imputed using multiple imputation (MI) under missing at random (MAR) assumption. I - NRS scores after use of rescue are set to missing and then imputed with MI P=0.144 DFK All Doses

10 Confidential. For internal use only. Key Secondary Endpoint: ≥ 4 - point Improvement in I - NRS at Week 12 (ITT) Estimated Percent Responder 29% 38% 32% 33% Odds Ratio 1.5 1.2 1.2 P - value p=0.18 p=0.55 p=0.59 Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline I - NRS score, and AD disease severity Patients who d/c early or have missing data in a specific week are included as “non - responders” Placebo (N=123) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124) DFK 1.0 mg (N=77)

11 Confidential. For internal use only. KARE Trial Patient Population ~64% Mild - Moderate Disease BSA < 10% (N=257) Moderate - Severe Pruritus ~36% Moderate - Severe Disease BSA ≥ 10% (N=144)
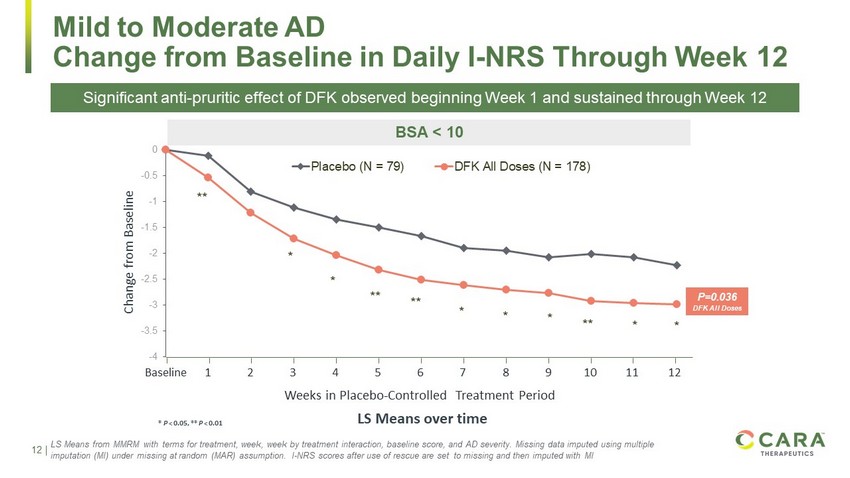
12 Confidential. For internal use only. * P < 0.05, ** P < 0.01 -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 Placebo (N = 79) DFK All Doses (N = 178) * * * ** ** * * Mild to Moderate AD Change from Baseline in Daily I - NRS Through Week 12 LS Means over time Weeks in Placebo - Controlled Treatment Period Change from Baseline Baseline 1 2 3 4 5 6 7 8 9 10 11 12 ** ** * * BSA < 10 P=0.036 DFK All Doses LS Means from MMRM with terms for treatment, week, week by treatment interaction, baseline score, and AD severity. Missing da ta imputed using multiple imputation (MI) under missing at random (MAR) assumption. I - NRS scores after use of rescue are set to missing and then imputed with MI Significant anti - pruritic effect of DFK observed beginning Week 1 and sustained through Week 12

13 Confidential. For internal use only. -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 Placebo (N = 79) DFK 0.25 mg (N=50) DFK 0.5 mg (N=82) DFK 1.0 mg (N=46) DFK All Doses (N = 178) Mild to Moderate AD Change from Baseline in Daily I - NRS through Week 12 * P < 0.05, ** P < 0.01 LS Means over time Weeks in Placebo - Controlled Treatment Period Change from Baseline Baseline 1 2 3 4 5 6 7 8 9 10 11 12 BSA < 10 LS Means from MMRM with terms for treatment, week, week by treatment interaction, baseline score, and AD severity. Missing da ta imputed using multiple imputation (MI) under missing at random (MAR) assumption. I - NRS scores after use of rescue are set to missing and then imputed with MI ** ** * * ** * * * * * ** P=0.036 DFK All Doses P=0.071 DFK 1.0 mg

14 Confidential. For internal use only. Mild to Moderate AD Subjects with ≥ 4 - point Improvement in I - NRS at Week 12 0% 10% 20% 30% 40% % of Subjects 32% Placebo DFK All Doses 19% p=0.033 N = 79 N = 178 A significantly greater proportion of patients on DFK achieved 4 - point improvement in I - NRS Estimated percentage & P - value based on a logistic regression model with terms for treatment group and baseline I - NRS score. Patients who d/c early or have missing data in a specific week are included as “non - responders”

15 Confidential. For internal use only. Mild to Moderate AD Subjects with ≥ 4 - point Improvement in I - NRS at Week 12 0% 10% 20% 30% 40% % of Subjects 19% 33% 28% 32% Placebo 0.25 mg 0.50 mg 1.0 mg 34% p=0.046 p=0.033 All Doses Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline I - NRS score, and AD disease severity. Patients who d/c early or have missing data in a specific week are included as “non - responders” Difelikefalin N = 79 N = 50 N = 82 N = 46 N = 178

16 Confidential. For internal use only. Summary of Adverse Events Subjects with at least one TEAE, n (%) 54 (43.9%) 36 (46.8%) 49 (39.5%) 42 (54.5%) Subjects with at least one serious TEAE, n (%) 0 1 (1.3%) 1 (0.8%) 2 (2.6%) Subjects with TEAE resulting in treatment discontinuation, n (%) 4 (3.3%) 3 (3.9%) 1 (0.8%) 9 (11.7%) Placebo (N=123) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124) DFK 1.0 mg (N=77)

17 Confidential. For internal use only. Most Commonly Reported TEAEs Abdominal pain* 13 (10.6%) 4 (5.2%) 11 (8.9%) 14 (18.2%) Nausea 11 (8.9%) 1 (1.3%) 6 (4.8%) 5 (6.5%) Dry Mouth 0 2 (2.6%) 2 (1.6%) 6 (7.8%) Headache 5 (4.1%) 5 (6.5%) 3 (2.4%) 2 (2.6%) Dizziness 2 (1.6%) 4 (5.2%) 3 (2.4%) 2 (2.6%) Hypertension 1 (0.8%) 1 (1.3%) 1 (0.8%) 5 (6.5%) Treatment - emergent Adverse Events at ≥5% frequency; n (%) Safety analyses performed in the safety population, defined as all randomized patients who received ≥1 dose of study drug bas ed on actual treatment received. *includes PTs abdominal pain, abdominal pain upper, abdominal discomfort Placebo (N=123) DFK 0.25 mg (N=77) DFK 0.50 mg (N=124) DFK 1.0 mg (N=77)

18 Confidential. For internal use only. KORSUVA TM Profiles: Mild to Moderate AD - aP & CKD - aP Moderate - Severe Pruritus in Atopic Dermatitis -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 Placebo (N = 236) DFK (N = 237) * * * ** * ** * * * * * ** CKD - aP: Phase 3/KALM - 2 Hemodialysis Patients -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 Placebo (N = 79) DFK All Doses (N = 178) * * * ** ** * * ** * * ** Similar anti - pruritic effects were observed in KARE and KALM - 2 trials The FDA has conditionally accepted KORSUVA Πas the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and efficacy have not been fully evaluated by any regulatory authority. * P < 0.05, ** P < 0.01 Weeks in Placebo - Controlled Treatment Period Weeks in Placebo - Controlled Treatment Period LS Means over time

19 Confidential. For internal use only. Conclusions • Oral KORSUVA TM did not meet Primary Endpoint of I - NRS change from baseline at week 12 in ITT population • However, statistically significant improvement was observed in mild - to - moderate subjects throughout Week 12 • Oral KORSUVA TM resulted in statistically significant improvement in the registration endpoint 4 - point responder analysis in subjects with mild to moderate AD at Week 12 • Oral KORSUVA TM was generally well tolerated across all doses Efficacy and safety data support further development of KORSUVA in mild - to - moderate AD patients – EOP2 FDA meeting target for 2H, 2021 The FDA has conditionally accepted KORSUVA Œ as the trade name for difelikefalin injection. Difelikefalin injection is an investigational drug product and its safety and efficacy have not been fully evaluated by any regulatory authority.
