Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - AGILITI, INC. \DE | d59213dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on April 21, 2021
No. 333-253947
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Agiliti, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 7350 | 83-1608463 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
6625 West 78th Street, Suite 300
Minneapolis, MN 55439
Telephone: (952) 893-3200
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Thomas J. Leonard
Chief Executive Officer
6625 West 78th Street, Suite 300
Minneapolis, MN 55439
Telephone: (952) 893-3200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
| Robert M. Hayward, P.C. Alexander M. Schwartz Kirkland & Ellis LLP 300 North LaSalle Chicago, IL 60654 (312) 862-2000 |
Alexander D. Lynch Barbra J. Broudy Weil, Gotshal & Manges LLP 767 Fifth Avenue New York, NY 10153 (212) 310-8000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated Filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller Reporting Company | ☐ | |||
| Emerging Growth Company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) | ||||
| Common Stock, par value $0.0001 per share |
30,263,157 | $20.00 | $605,263,140 | $66,034.21 | ||||
|
| ||||||||
|
| ||||||||
| (1) | Includes the aggregate offering price of shares of common stock subject to the underwriters’ option to purchase additional shares. |
| (2) | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(a) under the Securities Act of 1933, as amended. |
| (3) | The registrant has previously paid the registration fee. |
The registrant hereby amends this Registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities act of 1933 or until this Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. The preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer and sale is not permitted.
Subject to Completion.
Preliminary Prospectus dated April 21, 2021
26,315,789 Shares
PROSPECTUS

Common Stock
This is an initial public offering of shares of common stock of Agiliti, Inc.
Prior to this offering, there has been no public market for the common stock. It is currently estimated that the initial public offering price per share will be between $18.00 and $20.00. We have been approved to list our common stock on the New York Stock Exchange (“NYSE”) under the symbol “AGTI.”
See “Risk Factors” beginning on page 24 to read about factors you should consider before buying shares of our common stock.
Immediately after this offering, assuming an offering size as set forth above, funds controlled by our principal stockholder, Thomas H. Lee Partners, L.P., will own approximately 78.4% of our outstanding common stock (or 76.0% of our outstanding common stock if the underwriters’ option to purchase additional shares is exercised in full). As a result, we expect to be a “controlled company” within the meaning of the corporate governance standards of the NYSE. See “Management—Corporate Governance—Controlled Company Status.”
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discount (1) |
$ | $ | ||||||
| Proceeds, before expenses, to Agiliti, Inc. |
$ | $ | ||||||
| (1) | See “Underwriting (Conflicts of Interest)” for a description of compensation payable to the underwriters. |
To the extent that the underwriters sell more than 26,315,789 shares of common stock, the underwriters have the option to purchase up to an additional 3,947,368 shares of our common stock at the initial public offering price less the underwriting discount.
| BofA Securities | Goldman Sachs & Co. LLC | Morgan Stanley | BMO Capital Markets |
| Citigroup | Jefferies | UBS Investment Bank |
| KeyBanc Capital Markets |
Raymond James |
MUFG |
SMBC Nikko |
| Mischler Financial Group, Inc. |
Siebert Williams Shank |
Prospectus dated , 2021
Table of Contents
| 1 | ||||
| 18 | ||||
| 24 | ||||
| 46 | ||||
| 48 | ||||
| 49 | ||||
| 50 | ||||
| 53 | ||||
| 56 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
58 | |||
| 72 | ||||
| 90 | ||||
| 98 | ||||
| 123 | ||||
| 125 | ||||
| 128 | ||||
| 132 | ||||
| 141 | ||||
| MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS |
143 | |||
| 148 | ||||
| 154 | ||||
| 154 | ||||
| 154 | ||||
| F-1 | ||||
Neither we nor any of the underwriters have authorized anyone to provide any information or make any representations other than those contained in this prospectus or in any free writing prospectus filed with the Securities and Exchange Commission (the “SEC”). We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the common stock. Our business, financial condition, results of operations, and prospects may have changed since such date.
For investors outside of the United States, neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about, and to observe any restrictions relating to, this offering and the distribution of this prospectus outside of the United States.
Table of Contents
BASIS OF PRESENTATION
Unless we state otherwise or the context otherwise requires, the terms “Agiliti,” the “Company,” “our company,” “we,” “us,” and “our” in this prospectus refer to Agiliti, Inc. and, where appropriate, its consolidated subsidiaries. The term “THL” refers to Thomas H. Lee Partners, L.P., our principal stockholder, and the term “THL Stockholder” refers to THL Agiliti LLC, an affiliate of Thomas H. Lee Partners, L.P.
Agiliti Health, Inc. is the predecessor of Agiliti, Inc. for financial reporting purposes. The financial data presented in this prospectus for the year ended December 31, 2020 (Successor), the period from January 1 through January 3, 2019 (Predecessor) and for the period from January 4 through December 31, 2019 (Successor) is derived from the audited consolidated financial statements of Agiliti, Inc. and the related notes thereto included elsewhere in this prospectus. The financial data for the year ended 2018 is derived from the audited consolidated financial statements of Agiliti Health, Inc. and the related notes thereto included elsewhere in this prospectus.
MARKET AND INDUSTRY DATA
Unless otherwise indicated, information in this prospectus concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from independent industry analysts and publications, as well as our own estimates and research. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we believe the information presented in this prospectus is generally reliable, forecasts, assumptions, expectations, beliefs, estimates and projects involve risk and uncertainties and are subject to change based on various factors, including those described under “Forward-Looking Statements” and “Risk Factors”.
Certain information in the text of this prospectus relating to the size of the U.S. medical equipment services market is contained in independent industry publications produced by Frost & Sullivan, Prescient & Strategic Intelligence, Zion Market Research and iData Research.
This prospectus includes references to our Net Promoter Score. A Net Promoter Score is a metric used for measuring customer satisfaction and loyalty. We calculate our Net Promoter Score by asking customers the following question: “How likely are you to recommend Agiliti to another organization?” Customers are then given a scale from 0 (labeled as “Not at all likely”) to 10 (labeled as “Extremely Likely”). Customers rating us 6 or below are considered “Detractors”, 7 or 8 are considered “Passives”, and 9 or 10 are considered “Promoters”. To calculate our Net Promoter Score, we subtract the total percentage of Detractors from the total percentage of Promoters. For example, if 50% of overall respondents were Promoters and 10% were Detractors, our Net Promoter Score would be 40. The Net Promoter Score gives no weight to customers who decline to answer the survey question. This method is substantially consistent with how businesses across our industry and in other industries typically calculate their Net Promoter Score. Our most recent Net Promoter Score as of December 31, 2020 was 55. We use our Net Promoter Score results to anticipate and provide more attention to customers who may be in the Detractor category and, for those in the Promoter category, as a predictive indicator of a customer’s desire to remain a customer for the long-term.
TRADEMARKS AND TRADENAMES
This prospectus includes our trademarks and service marks such as “Agiliti®” and the Agiliti logo, “Asset360®,” “BioMed360®,” “Universal Hospital Services, Inc.,” “UHS®” and the UHS logo, “OnCare,” “Harmony” and “Quartet” which are protected under applicable intellectual property laws and are the property of us or our subsidiaries. This prospectus also contains trademarks, service marks, trade names and copyrights of
ii
Table of Contents
other companies which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names.
iii
Table of Contents
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in our common stock. For a more complete understanding of us and this offering, you should read and carefully consider the entire prospectus, including the more detailed information set forth under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes. Some of the statements in this prospectus are forward-looking statements. See “Forward-Looking Statements.”
Unless the context otherwise requires, the terms “Agiliti,” the “Company,” “our company,” “we,” “us” and “our” in this prospectus refer to Agiliti, Inc. and, where appropriate, its consolidated subsidiaries. The term “THL” refers to Thomas H. Lee Partners, L.P., our principal stockholder, and the term “THL Stockholder” refers to THL Agiliti LLC, an affiliate of Thomas H. Lee Partners, L.P.
Our Mission
Agiliti is an essential service provider to the U.S. healthcare industry with solutions that help support a more efficient, safe and sustainable healthcare delivery system. We ensure healthcare providers have the critical medical equipment they need to care for patients—wherever and whenever it’s needed—with a service model that helps lower costs, reduce waste and maintain the highest quality standard of medical device management in the industry. We are motivated by a belief that every interaction has the power to change a life, which forms the cornerstone of how we approach our work and frames the lens through which we view our responsibility to make a difference for the customers, patients and communities we serve.
Overview
We believe we are one of the leading experts in the management, maintenance and mobilization of mission-critical, regulated, reusable medical devices. We offer a comprehensive suite of medical equipment management and service solutions that help providers reduce capital and operating expenses, optimize medical equipment utilization, reduce waste, enhance staff productivity and bolster patient safety.
We commenced operations in 1939, originally incorporated in Minnesota in 1954 and reincorporated in Delaware in 2001. Since January 2019, we have been controlled by the THL Stockholder.
In our more than 80 years serving healthcare providers, we’ve built an at-scale, strong nationwide operating footprint allowing us to reach customers across the entire healthcare continuum—from individual facilities to the largest and most complex healthcare systems. Our ability to rapidly mobilize, track, repair and redeploy equipment during times of peak need or emergent events has made us a service provider of choice for city, state and federal governments to manage emergency equipment stockpiles.
1
Table of Contents
Agiliti at-a-glance: Powered by an at-scale, nationwide logistics and service infrastructure
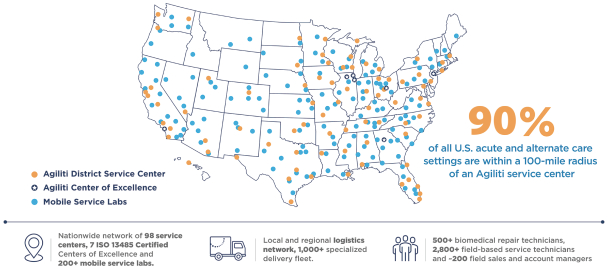
Our diverse customer base includes approximately 7,000 active national, regional and local acute care hospitals, health system integrated delivery networks and alternate site providers (such as surgery centers, specialty hospitals, home care providers, long-term acute care hospitals and skilled nursing facilities). We serve the federal government as well as a number of city and state governments providing management and maintenance of emergency equipment stockpiles, and we are an outsourced service provider to medical device manufacturers supporting critical device remediation and repair services. We deliver our solutions through our nationwide network of 98 service centers and seven Centers of Excellence, employing a team of more than 500 specialized biomed repair technicians, more than 2,800 field-based service operators who work onsite within customer facilities or in our local service centers, and approximately 200 field sales and account managers. Our fees are paid directly by our customers rather than by direct reimbursement from third-party payors, such as private insurers, Medicare or Medicaid.
Industry Challenges
Across the healthcare system, providers face compounding financial and operational challenges, including cost pressure from payors, nursing and clinical staff shortages, increasing regulatory oversight, and advances in medical technology that generally result in higher prices for newer equipment and a higher cost of managing that equipment over its lifecycle. In our experience, one area that most hospitals and health systems identify for operational and cost improvement is the management and maintenance of medical equipment.
Healthcare facilities have been shown to own large quantities of reusable capital equipment ranging from multi-million dollar highly technical devices (e.g. MRIs) to lower cost, high volume devices (e.g. infusion pumps) required for patient care, treatment and diagnosis. In our experience, providers often face challenges effectively managing their medical equipment inventory. Hospitals typically utilize roughly 42% of their owned medical equipment inventory at any given time, yet caregivers report that they routinely lack access to readily available patient-ready equipment. Nurses report spending an average of 20 minutes per shift searching for equipment, and no more than 37% of their time on direct patient care. Operational silos that naturally occur among hospital departments create inadvertent breakdowns within equipment management workflows, from the administrators who order equipment, to the support staff who clean/reprocess and deliver the equipment, to the nurses and doctors who use the equipment.
2
Table of Contents
The repair and maintenance of this highly technical equipment continues to increase in complexity and cost. Over 15 years there has been a 62% increase in the number of medical devices per hospital bed and a 90% increase in costs related to maintaining this equipment (between 1995 and 2010). Most healthcare facilities struggle to maintain in-house capabilities to ensure timely maintenance, repair and turnaround of their medical inventory which may impact time-to-therapy and patient safety, while driving up capital replacement costs on equipment that could have otherwise been kept operational with proper maintenance.
Finally, the healthcare system experiences seasonality in patient volumes, resulting in peak-need demand for specialized medical equipment (e.g. ventilators, specialty beds, infusion pumps). Given the common breakdowns in managing and maintaining their inventory during times of normal operation, hospitals face additional burden on equipment availability during times of peak need and will procure supplemental equipment through additional acquisition channels to fill this gap.
Critical gaps and costly challenges for providers at the intersection of care and technology
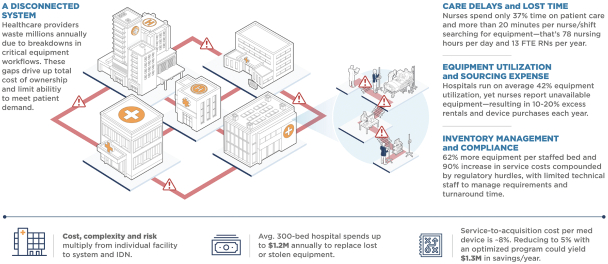
These challenges drive up significant costs and time delays within individual hospital facilities, but when multiplied across several hospitals and alternate site facilities within an IDN, the losses increase significantly. An average 2,500 bed IDN has been shown to waste more than $11 million annually on inefficient equipment maintenance and unnecessary capital purchases, while clinicians lose valuable patient time and productivity hours managing equipment needs.
These dynamics, supported by the following trends, further support the essential nature of our work:
Focus on reducing costs and increasing operational efficiency. Hospitals and other healthcare facilities face substantial pressure to conserve capital, reduce costs and become more efficient. Our solutions offer customers a way to realize costs savings while enhancing operational improvements for medical equipment access and availability, improving organizational efficiency and financial viability.
Demand for better patient safety and outcomes. Hospitals turn to Agiliti to assist in effectively managing equipment to help minimize incidents of hospital-acquired conditions (e.g. infections, patient falls and pressure injuries), and to ensure equipment is available when and where it is needed for patient care, helping improve time to therapy and support optimal patient outcomes.
3
Table of Contents
Caregiver retention and satisfaction. As healthcare organizations experience ongoing pressures around nursing retention and clinician job satisfaction, we expect providers will increasingly turn to our programs to outsource clinical equipment management processes to allow nurses more time to spend on patient care.
Increased capital and operating expense pressures and regulatory compliance. Hospitals continue to experience restricted capital and operating budgets, while the cost and complexity of medical equipment and associated recordkeeping and regulatory scrutiny increases. We expect providers will increasingly look to us to support the management and maintenance of their equipment inventory to achieve capital and operating expense savings, operating efficiencies and regulatory compliance.
Our Value Proposition
As a critical outsource partner to more than 7,000 U.S. healthcare customers, including most leading providers nationwide, we’ve tailored our solution offering and service model to address the unique challenges and opportunities we witness among our customers related to the effective management of medical equipment.
By partnering with Agiliti, providers have the benefits of:
| ➣ | Cost savings and lower total costs of equipment ownership |
| • | Increased utilization of both customer-owned and supplemental equipment |
| • | Lower overall total cost of equipment ownership by combining our solutions to solve challenges across the end-to-end equipment management process |
| • | Optimized management and logistics of provider-owned equipment through tracking, monitoring, reprocessing, maintaining, and ensuring equipment is safety-tested and redeployed for use |
| • | Reduced maintenance and repair costs through the use of our proprietary technology, flexible staffing models, parts pool, equipment capabilities, diverse skill mix of knowledgeable equipment technicians and our commitment to quality |
| • | Benefits of specialized technician labor to augment clinical biomed staff, having been shown to help reduce service costs and provide required technical proficiency to address more complex equipment types |
| • | Access to our extensive data and expertise on the cost, performance, features and functions of all major items of medical equipment |
| • | Assistance with capital planning, vendor management and regulatory compliance |
| ➣ | More time to spend with patients and confidence in the availability of patient-ready medical equipment |
| • | Increased productivity and satisfaction among nursing staff achieved by eliminating certain non-clinical work tasks and saving an average 300-bed hospital over 28,000 caregiver hours annually |
| • | Improved time-to-therapy for patients at risk for falls, skin breakdown and bariatric safety by expediting delivery of therapeutic equipment direct to the patient room |
| • | Access to supplemental moveable medical equipment, surgical equipment and next generation technology without the expense of acquisition on a pay-per-procedure basis |
| ➣ | Improved regulatory compliance, risk management and extended use life |
| • | Optimal maintenance intervals and parts replacement to extend equipment use life, reduce waste and lower obsolescence risk |
4
Table of Contents
| • | Compliance with regulatory and recordkeeping requirements and adherence to manufacturers’ specifications on the reprocessing and maintenance of medical equipment |
| • | Equipment quality assurance through the use of our comprehensive QMS based on the quality standards recognized worldwide for medical devices |
| • | Risk mitigation and lower costs associated with product recalls or device modifications |
| ➣ | Technical expertise and supplemental staffing to sustain optimal equipment workflow |
| • | Reduced administrative and time burdens on clinical staff related to managing and locating available equipment and coordinating among multiple vendors |
| • | Specialized technical and clinical specialists that directly interact with and work alongside customers to optimize equipment outsourcing solutions |
Our Market Opportunities
We participate in a $14 billion U.S. medical equipment services market comprised of the services we offer through our onsite managed services, clinical engineering services and equipment solutions service lines. We believe that this market will grow at mid-single digits annually.
There is a fundamental shift in the needs of health systems, hospitals and alternate site providers to move from supplemental and peak need sourcing of medical equipment toward more comprehensive onsite inventory management and maintenance solutions. As healthcare facilities look to balance the challenge of providing better care at lower costs, they are more open to third party partnerships that outsource critical but non-core support functions. The move toward full outsourcing is not unlike trends in similar services at hospitals including food service, laundry, professional staffing and technology.
We believe there are several key macro trends that will drive increased demand for our products and services:
Favorable demographic trends. According to the U.S. Census Bureau, individuals aged 65 and older in the United States comprise the fastest growing segment of the population and are expected to grow to approximately 81 million individuals by 2040. The aging population and increasing life expectancy are driving demand for healthcare services.
Increase in chronic disease and obesity. According to the Centers for Disease Control and Prevention (“CDC”), six in ten Americans live with at least one chronic disease and 42% of the U.S. population is obese. These populations demand greater access to specialty equipment to support care and minimize the incidence of injury during a hospital stay.
Increased mergers & acquisitions. We have seen that hospitals and healthcare systems continue to expand their covered network and acquire alternate care delivery settings in order to care for patient populations in the most cost-effective way. In our experience, providers are increasingly seeking partners that provide comprehensive services. Working with one vendor that can operate at a nationwide and system-wide scale has shown to be attractive to cities, states, and integrated delivery networks (“IDNs”) who maintain equipment inventories across multiple locations.
Centralizing shared services across the IDN. Health systems with duplicate services across multiple facilities in close proximity have an increased risk of unnecessary variation, higher costs, and suboptimal outcomes. In our experience, because most health systems do not currently have the storage, technical or transportation resources for managing a shared equipment management function, they will seek third party support to optimize equipment utilization, redeploy equipment where needed and reduce overall equipment costs.
5
Table of Contents
Increase in infection control risks. Infection control remains an essential priority for hospitals and health systems and has further escalated as a top priority due to the COVID-19 pandemic. We expect increased demand for onsite equipment management programs to address proper reprocessing of devices in order to help lower infection risks and allow clinicians more time at the patient bedside and less time cleaning equipment.
Our Solutions
We provide comprehensive medical device management solutions based on a proven framework to help providers reduce the cost and complexity of acquiring, managing and maintaining critical medical equipment inventories. The integrated nature of our service offerings within this end-to-end framework ensures we maximize value to customers as we address more aspects of the equipment lifecycle continuum.
Proven framework for end-to-end management of FDA-regulated, reusable medical devices
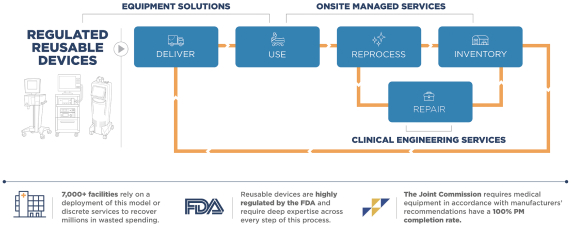
While customers may initially engage with us across one aspect of our service lines within this framework, we employ a variety of land-and-expand tactics to grow our relationships and customer share-of-wallet over time. These tactics include:
| • | Gateway solutions offer an entry point to the economic buyer and include peak needs equipment, surgical lasers and equipment, specialty beds and surfaces and supplemental clinical engineering services; |
| • | Vertical solutions provide a deeper level of service with clinical offerings tailored to specific patient needs (e.g. bariatrics, wound management) and clinical engineering programs for broad equipment categories (general biomedical devices, diagnostic imaging equipment, surgical instruments); |
| • | Comprehensive, connected solutions through onsite managed services and outsourced clinical engineering services that connect previously fragmented customer workflow processes to drive operational efficiencies, realize improved clinician and equipment productivity, lower total cost of ownership, ensure regulatory compliance, reduce waste, improve time to therapy and allow customers to effectively lower costs; and |
| • | Comprehensive logistics, management and clinical engineering solutions that allow IDNs to manage equipment inventories across multiple locations, and supports city, state and federal government agencies in managing and maintaining equipment stockpiles. |
We deploy our solution offering across three primary service lines:
Onsite Managed Services: Comprehensive programs that assume full responsibility for the management, reprocessing and logistics of medical equipment at individual facilities and IDNs. Our more than 1,700 onsite
6
Table of Contents
employees work 24/7 in customer facilities, augmenting clinical support by integrating proven equipment management processes, utilizing our proprietary management software and conducting daily rounds and unit-based training to ensure equipment is used and managed properly, overall helping to optimize day-to-day operations, adjust for fluctuations in patient census and acuity and support better care outcomes. We have over 225 onsite managed service customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 28% and 28% of our total revenue, respectively.
Clinical Engineering Services: Maintenance, repair and remediation solutions for all types of medical equipment, including general biomedical equipment and diagnostic imaging technology, through supplemental and fully outsourced offerings. Our supplemental offering helps customers manage their equipment repair and maintenance backlog, assist with remediation and regulatory reporting and temporarily fill open biotechnical positions. Our more than 500 technical repair staff flex in and out of customer facilities on an as-needed basis. We contract our Clinical Engineering Services with acute care and alternate site facilities across the U.S., as well as with the federal government and any medical device manufacturers that require a broad logistical footprint to support their large-scale service needs. We have over 6,000 clinical engineering service customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 33% and 31% of our total revenue, respectively.
Equipment Solutions: Supplemental, peak need and per-case rental of general biomedical, specialty, and surgical equipment, contracted directly with customers at approximately 7,000 U.S. acute care hospitals and alternate site facilities. We consistently achieve high customer satisfaction ratings, as evidenced by our Net Promoter Score (“NPS”) of 55 for the year ended December 31, 2020, by delivering patient-ready equipment within our contracted equipment delivery times and in response to our technical support and educational in-servicing for equipment within clinical departments, including the emergency room, operating room, intensive care, rehabilitation and general patient care areas. We are committed to providing the highest quality of equipment to our customers, supported by our comprehensive QMS which is based on the quality standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016. We have over 5,000 equipment solution customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 38% and 41% of our total revenue, respectively.
Many of our customers have multiple contracts and have revenue reported in multiple service lines. Our contracts vary based upon service offering, including with respect to term (with most being multi-year contracts), pricing (daily, monthly and fixed fee arrangements) and termination (termination for convenience to termination for cause only). Many of our contracts contain customer commitment guarantees and annual price increases tied to the consumer price index. Standard contract terms include payment terms, limitation of liability, force majeure provisions and choice of law/venue.
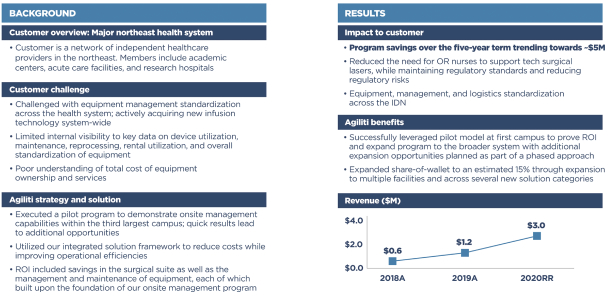
Because we work closely with customers to provide a long-term, value-based solution vs. a product-based, transactional approach, they are motivated to expand their relationships with us over time. We have approximately 82% white space within our current customer base. As indicated by the Customer Case Study presented below, we have demonstrated an ability to grow revenue up to 5-6x with an existing customer. We believe that this case study demonstrates the potential of our “land and expand” strategy of efficiently increasing revenue from our existing customers as they move toward our full suite of highly complementary services. From the year ended December 31, 2015 to the year ended December 31, 2020, our top 50 customers that experienced the largest growth in revenue over the same period increased in revenue from an aggregate of approximately $21.3 million to approximately $117.3 million (with increases at each customer ranging from $1.0 million to $8.8 million and an average increase of $1.9 million, and with consistent growth across our three primary service lines), primarily driven by our efforts to expand our share of wallet within our existing customer base. During the same period, our average existing customer growth rate was approximately 4.3%.
7
Table of Contents
Further, the infrastructure and capabilities required to provide connected, responsive equipment lifecycle management is typically cost-prohibitive, even for large IDNs. Our nationwide network of clinical engineers, storage and repair facilities, vehicles and analytics tools gives us scale to provide cost-effective services for individual facilities, systems, regional IDNs, governments and device manufacturers.
Customer Case Study:
Competitive Strengths
Strong value proposition. Comprehensive, end-to-end medical equipment management and service solutions, and ability to respond quickly to customer needs with reliable, high quality service expertise helps us to:
| • | lower total cost of device ownership by reducing capital and operating costs related to owning and managing medical equipment; |
| • | enhance operational productivity and staff satisfaction by ensuring equipment is available when and where needed; and |
| • | maintain high standards of quality and regulatory compliance related to medical equipment use, maintenance and end-of-life disposal. |
Large, nationwide infrastructure. Our extensive network of 98 service centers and seven Centers of Excellence with round-the-clock service capabilities enables us to compete effectively for large, national contracts as well as drive growth regionally and locally. Our more than 500 biomedical repair technicians, more than 2,800 field-based service operators, and approximately 200 field sales and account managers engage directly with our customers to drive improved cost, efficiency and clinical outcomes. Our specialized teams, large equipment fleet, and quality assurance programs have been built over 80 years and provide the scale to serve the most complex acute care hospitals that demand access to current and preferred technologies to meet the complex needs of their patients.
8
Table of Contents
Proprietary software and asset management tools. Our software technology and management tools enable us to meet unique customer demands and support sophisticated onsite managed services which drive cost efficiencies and equipment productivity for caregivers.
Commitment to quality. Though not required by the FDA, we’ve committed to doing the right thing for our customers and their patients by staffing a dedicated Quality team and implementing a Quality Management System (QMS) based on the standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016. We believe our robust QMS policies set us apart in our industry from those who may use less stringent quality practices on the equipment they own or maintain.
Superior customer service. We believe we have a long-standing reputation among our customers for outstanding service and quality, and we strive to seamlessly integrate our employees and solutions into the operations of our customers. We believe that our aggressive focus on the overall customer experience has helped us achieve high customer satisfaction ratings, as evidenced by our NPS of 55 for the year ended December 31, 2020.
No direct third-party payor reimbursement risk. Our fees are paid directly by our customers, rather than by third-party payors. Accordingly, our exposure to uncollectible receivables or reimbursement changes is reduced, as evidenced by our bad debt expense of approximately 0.3%, 0.2% and 0.4% of total revenue for the years ended December 31, 2020, 2019 and 2018, respectively.
Values driven culture centered on doing the right thing for our many stakeholders. Our team operates on a set of shared aspirations that underpin our culture, strategy and service model to help contribute to a safer and more sustainable healthcare system:
WE ARE BUILDING THE PREMIER CLINICAL EQUIPMENT SERVICES COMPANY. We ensure clinicians have the equipment they need, when they need it, with the confidence it is maintained to the highest industry standards.
WE ARE ESSENTIAL TO CUSTOMERS. We deliver a unique and valuable offering that helps customers improve their business and prioritize patient care.
WE ARE EMPOWERED AND ENGAGED. We lead by example, inspiring one another to be at our best, to be accountable, and to develop with purpose. We value our diversity, knowing different perspectives lead to better outcomes.
WE ARE OPERATIONALLY EXCELLENT. We demonstrate a tireless commitment to quality, reliability, and continuous improvement.
WE ARE CREATING A CATEGORY OF ONE. Together, we are building a highly differentiated, leading service company that is the vendor of choice for customers and an employer of choice nationwide.
Highly engaged team. We believe a strong and sustainable company begins with an engaged and empowered team. We are committed to investing in our team’s development and to fostering a culture of diversity, inclusion, trust and transparency. In 2020, we achieved a 77 employee engagement score rating, which places us nearing the extraordinary company benchmark according to third-party engagement indices.
Proven management team. Our diverse and industry leading management team brings decades of executive-level healthcare expertise from across the sector and has successfully supervised the development of our competitive strategy and furthered our reputation as an industry leader in our category.
Key Elements of our Growth Strategy
Retain and expand existing customer relationships. While our overall market opportunity is large, there is also significant expansion opportunity within our existing customers. We believe there is approximately
9
Table of Contents
$1.7 billion of white space among our current contracted customer base. Capturing more of our customer share-of-wallet is core to our growth strategy.
Grow our customer base among customers that outsource. We believe there is a significant opportunity to further grow our business by winning new customer contracts within the $5.85 billion that is contracted annually for medical equipment management services in the U.S. This is less than half of the total addressable market, and with increasing pressures on providers, we expect outsourcing to significantly accelerate.
Grow our serviceable market by contracting with those that insource today. Currently, we estimate that $14 billion is spent annually in the U.S. for medical equipment services and functionality, but less than half of the total market is currently outsourced to an equipment management service, while the rest is done in-house in facilities. We believe that as we reach additional potential customers with demonstrated value both in improved patient care and reduced costs, we can grow our total addressable market by contracting with new clients that were not previously outsourcing device management services.
Invest in complementary offerings that enhance customer relationships. As the medical device field becomes increasingly complex, we are constantly evaluating additional services and methods of approaching service delivery that may increase value for our clients. As an example, we recently expanded our work with federal, state, and local governments to help maintain and mobilize strategic stockpiles of ventilators and other critical medical equipment.
Opportunistically pursue accretive M&A. We believe that pursuing opportunistic M&A will drive increasing returns through embedded customer relationships. From 2015-2020, we successfully integrated seven acquisitions and will continue to opportunistically pursue additional inorganic growth.
COVID-19 Update
As COVID-19 drove demand for emergent acute care around the country and illuminated the importance of resilient supply chains and service networks, the importance of our services were also magnified. We are proud to have rapidly developed and deployed a response plan to ensure the safety of our team, while continuing to meet our customers’ evolving needs for patient-ready medical equipment when and where it was needed; notably, doing so without service interruptions.
We believe our value proposition now resonates with an even broader audience of customers as providers, IDNs and governments prepare for potential future surges in demand for acute care and the required equipment necessary to care for patients.
Specifically, during the COVID-19 pandemic, we have:
| • | fully and rapidly deployed our fleet of medical devices and accessories across the U.S. to ensure they are reaching the maximum number of patients; |
| • | leveraged our logistics, inventory management, and maintenance/repair infrastructure to work with medical device brokers and manufacturers to make thousands of additional critical medical devices available to healthcare facilities; |
| • | deployed our local biomedical repair teams to augment teams at hospitals around the country to ensure their owned medical equipment remains fully operational and available for patient needs; |
| • | redeployed teams from our 98 local service centers to support surge medical capacity in parks, gymnasiums, and hotel rooms across the country; |
10
Table of Contents
| • | been awarded a new contract to manage the maintenance and field repair of the national strategic ventilator stockpile; we are likewise working with various state and municipal governments to manage and mobilize their centralized and local medical device stockpiles; and |
| • | prioritized the care and safety of our employees who are essential to helping our customers meet patient care needs. We committed to avoid COVID-19 related layoffs or furloughs and bridge the income of our team members with variable net pay for the duration of the pandemic. We provided 100% coverage for COVID-19 testing and telemedicine, extended short-term medical leave and disability coverage related to COVID-19, and granted additional time-off benefits for COVID-19 related needs, so that our teams were able to safely focus on our customers and their patients as we served alongside them in front-line response efforts. |
Recent Developments
Recent Operating Results (Preliminary and Unaudited)
We are in the process of finalizing our results for the three months ended March 31, 2021. We have presented below certain preliminary results representing our estimates for the three months ended March 31, 2021, which are based only on currently available information and do not present all necessary information for an understanding of our financial condition as of March 31, 2021 or our results of operations for the three months ended March 31, 2021. We have provided ranges, rather than specific amounts, for the preliminary estimates for the unaudited financial data described below primarily because our financial closing procedures for the three months ended March 31, 2021 are not yet complete and, as a result, our final results upon completion of our closing procedures may vary from the preliminary estimates. Our independent registered public accounting firm has not audited, reviewed or performed any procedures with respect to this preliminary financial data or the accounting treatment thereof and does not express an opinion or any other form of assurance with respect thereto. We expect to complete our interim financial statements for the three months ended March 31, 2021 subsequent to the completion of this offering. While we are currently unaware of any items that would require us to make adjustments to the financial information set forth below, it is possible that we or our independent registered public accounting firm may identify such items as we complete our interim financial statements. Accordingly, undue reliance should not be placed on these preliminary estimates. These preliminary estimates are not necessarily indicative of any future period and should be read together with “Risk Factors”, “Forward-Looking Statements”, and our consolidated financial statements and related notes included in this Registration Statement. Adjusted EBITDA is a supplemental measure that is not calculated and presented in accordance with GAAP. See “Prospectus Summary—Summary Consolidated Financial Data—Adjusted EBITDA.”
| Three months ended March 31, 2021 |
Three months ended March 31, 2020 |
|||||||||||
| Low (estimated) |
High (estimated) |
|||||||||||
| (in thousands) | ||||||||||||
| Consolidated Statements of Operations Data (unaudited): |
||||||||||||
| Total Revenue |
$ | 230,000 | $ | 235,000 | $ | 179,240 | ||||||
| Operating income (1) |
$ | 32,000 | $ | 37,000 | $ | 1,241 | ||||||
| Non-GAAP Financials Data (unaudited): |
||||||||||||
| Adjusted EBITDA (2) |
$ | 81,000 | $ | 86,000 | $ | 48,716 | ||||||
| (1) | Excludes the remeasurement of the tax receivable agreement for 2021 and 2020. |
11
Table of Contents
| (2) | We define Adjusted EBITDA as net income (loss) before interest expense, income taxes, depreciation and amortization, excluding non-cash share-based compensation expense, management fees and non-recurring gains, expenses or losses, transaction expenses and tax receivable agreement remeasurement. However, we cannot reconcile our estimated range of Adjusted EBITDA to net income (loss), the most directly comparable GAAP measure, without unreasonable efforts because we are unable to estimate our income tax (expense) benefit for the period, as we are in the process of evaluating our tax attributes. In addition, we are in the process of remeasuring the fair value of our tax receivable agreement and finalizing the purchase accounting related to the Northfield acquisition. See “Prospectus Summary—Summary Consolidated Financial Data—Adjusted EBITDA” for a discussion of Adjusted EBITDA, why we believe this measure is important and certain limitations regarding this measure. |
For the three months ended March 31, 2020, interest expense, depreciation and amortization, non-cash share-based compensation expense, management fees and other expense, and transaction costs was $17.8 million, $40.2 million, $2.4 million, $4.1 million and $0.8 million, respectively. For the three months ended March 31, 2021, interest expense, depreciation and amortization, non-cash share-based compensation expense, management fees and other expense, and transaction costs are estimated to have been $18 million $43 million, $2 million, $1 million, and $3 million, respectively. The depreciation and amortization estimate does not include an estimate for the purchase accounting impacts of the Northfield acquisition as we are in the process of finalizing the fair value opening balance sheet for fixed assets and intangibles.
We expect total revenue to increase 28% to 31% for the three months ended March 31, 2021 compared to the three months ended March 31, 2020 primarily driven by new contracts signed over the past year including the new contract signed in 2020 for the comprehensive maintenance and management services of medical ventilator equipment with the U.S. Department of Health and Human Services, increased demand for equipment needed in connection with the COVID-19 pandemic as further described in “Prospectus Summary—COVID-19 Update” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Impact of COVID-19 on our Business” and increased revenue from our acquisitions on January 31, 2020 and March 19, 2021 of surgical equipment repair and maintenance service providers. For more information on the acquisition on January 31, 2020, see Note 4 to our audited consolidated financial statements for the year ended December 31, 2020 included elsewhere in this prospectus. For more information on the acquisition on March 19, 2021, see “—Northfield Acquisition.”
We expect cost of revenue and selling, general and administrative expenses to increase 9% to 13% for the
three months ended March 31, 2021 compared to the three months ended March 31, 2020, primarily driven by the increase in revenue. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations—Consolidated Results of Operations for the year ended December 31, 2020 compared to the period from January 4 through December 31, 2019” for a discussion of the trends driving increases in costs and expenses for the year ended December 31, 2020, which we expect to remain the drivers for increases in costs and expenses through March 31, 2021.
We expect income from operations of $32 million to $37 million for the three months ended March 31, 2021 compared to income from operations of $1.2 million for the three months ended March 31, 2020, as we continue to see positive leverage from our revenue volume growth related to the new contracts signed and the higher utilization of our equipment placed at customer locations needed to fight the COVID-19 pandemic.
We expect Adjusted EBITDA of $81 million to $86 million for the three months ended March 31, 2021 as compared to Adjusted EBITDA of $48.7 million for the three months ended March 31, 2020.
Northfield Acquisition
On October 28, 2020, Agiliti Health, Inc., an indirect subsidiary of the Company, entered into a Stock Purchase Agreement (the “Northfield SPA”) with Northfield Medical Holdings LLC (the “Northfield Seller”) and Northfield
12
Table of Contents
Medical, Inc. (“Northfield Medical”), a nationwide provider of surgical equipment repair services, to purchase 100% of the issued and outstanding capital stock of Northfield Medical from the Northfield Seller for $475.0 million, subject to adjustments (the “Northfield Acquisition”). Northfield Medical provides service and repair of medical devices, specializing in the repair of endoscopes, surgical instruments and other operating room equipment. We believe the acquisition of Northfield will enable the Company to: (i) offer a broader array of clinical engineering repair and maintenance services, extending its end-to-end service offering to customers; (ii) expand the Company’s reach and breadth within the operating room and procedural care space; and (iii) serve more customers by overlaying Northfield’s operations within the Company’s nationwide geographic footprint.
The acquisition closed on March 19, 2021. A portion of the financing for the acquisition was in the form of a $200 million first lien incremental term loan facility under the existing First Lien Term Loan Facility. Additionally, in connection with the entry by the parties into the Northfield SPA, certain members of the Northfield Medical management team delivered commitment letters to reinvest a portion of the transaction proceeds to be received by such executives in connection with the Northfield Acquisition to acquire our common stock on the closing date of the Northfield Acquisition. The aggregate commitment amount is expected to result in the issuance of 752,328 shares of common stock. In connection with this offering, the Company also intends to issue restricted stock units, performance restricted stock units and options to acquire shares of our common stock to certain members of the Northfield Medical management team in an aggregate amount of approximately 71,269 shares of our common stock under the 2018 Omnibus Incentive Plan. The restricted stock unit and stock option awards will vest ratably over a three-year period and the performance restricted stock unit awards vest based on the achievement of performance metrics over a three-year period, subject to the recipient’s continued employment through each vesting date. The acquisition is considered “significant” under Regulation S-X and requires the filing within 75 days of the closing date of audited financial statements of Northfield Medical for the year ended December 31, 2020.
For the year ended December 31, 2020, Northfield Medical generated approximately $111.1 million of revenue, and operating earnings were a nominal amount for 2020.
Summary of Risks Associated with Our Business, Our Indebtedness, this Offering and Our Common Stock
There are a number of risks related to our business, our indebtedness, this offering and our common stock that you should consider before you decide to participate in this offering. You should carefully consider all the information presented in the section entitled “Risk Factors” in this prospectus. Some of the principal risks related to our business include the following:
| • | Political and policy changes could materially limit our growth opportunities. Geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries have contributed to increased volatility and uncertain expectations for the global economy. Additionally, healthcare costs have risen significantly over the past decade, and there continue to be proposals by legislators, regulators and third-party payors to keep these costs down. We cannot predict which healthcare initiatives, if any, will be implemented at the federal or state level, or the effect that any future regulation or legislation would have on us. However, an expansion of the government’s role in the U.S. healthcare industry may lower industry reimbursements for our products, reduce medical procedure volumes and may thereby materially adversely affect our business and our ability to execute our growth strategy. |
| • | The COVID-19 pandemic could materially and adversely affect our business, operating results, financial condition and prospects. We source equipment from different parts of the world that have been affected by COVID-19, which could have an adverse impact on our supply chain operations and the ability of manufacturers to obtain materials needed to assemble the products that we offer. Government shutdown orders or a change to our business classification as an “essential business” may result in a closure of operations for an uncertain duration, impacting our business results. In addition, in |
13
Table of Contents
| response to the COVID-19 pandemic, the federal government and certain state and local governments have purchased significant amounts of medical equipment of the type that we offer in our rental fleet. These purchases of medical equipment that previously would have been rented may reduce the demand for our rental equipment and may thereby materially adversely affect our ability to grow our customer base and retain and expand our existing customer relationships. |
| • | We may be unable to maintain existing contracts or contract terms or enter into new contracts with our customers. Our ability to retain and expand existing customer relationships depends on continuing contracts with customers, including through group purchasing organizations (“GPOs”) and IDNs. If we are unable to maintain our contracts, or if the GPOs or IDNs seek additional discounts or more beneficial terms on behalf of their members, we may lose a portion or all of our existing business with, or revenues from, customers that are members of such GPOs and IDNs. In addition, certain of our customers account for large portions of our revenue, including the U.S. government, and to the extent that contracts with significant customers are terminated or are not renewed, our revenue and operating results would be significantly impacted. |
| • | A substantial portion of our revenues come from customers with which we do not have long-term commitments, and cancellations by or disputes with customers could decrease the amount of revenue we generate, thereby reducing our ability to operate and expand our business. Our customers are generally not obligated to outsource our equipment under long-term commitments. The short-term services we provide could be terminated by the customer without notice or payment of any termination fee. A large number of such terminations may adversely affect our ability to generate revenue growth and sufficient cash flows to support our growth strategies. |
| • | If we fail to maintain our reputation, including by adequately protecting our intellectual property, our sales and operating results may decline. We believe that our ability to execute our growth strategies depends on our ability to maintain and grow the value of our brand. Challenges or reactions to action or inaction by us on certain issues could harm our reputation, as could any failure to maintain the high-quality customer support that underpins our reputation for having outstanding service and quality. Our ability to protect our brand also depends on our ability to protect our confidential information, including with respect to our proprietary software and asset management tools, and if we fail to do so we may be subject to payment of monetary damages, the loss of valuable intellectual property rights or the loss of personnel. If we are unable to maintain our reputation, our ability to grow our serviceable market, grow our customer base and opportunistically pursue acquisitions will be materially adversely impacted. |
| • | If our customers’ patient census or services decrease, the revenue generated by our business could decrease. Our operating results are dependent in part on the amount and types of equipment necessary to service our customers’ needs, which are heavily influenced by patient census and the services those patients receive. At times of lower patient census, such as during severe economic downturns, our customers have a decreased need for our services on a supplemental or peak needs basis, causing our revenue to decrease. |
| • | Our competitors may engage in significant competitive practices, which could cause us to lose market share, reduce prices or increase expenditures. For example, competitors may sell significant amounts of surplus equipment or sell capital equipment at a lower gross margin to obtain the future repeat sales of disposables for a higher gross margin, thereby decreasing the demand for our equipment solutions. Any actions we may be required to take as a result of increased competitive pressure, including decreasing our prices, renegotiating contracts with customers on more favorable terms or increasing our sales and marketing expenses, could have a material adverse effect on our results of operations, curtail our ability to invest in complementary offerings that enhance our customer relationships and limit our opportunities to pursue accretive M&A. |
14
Table of Contents
| • | Consolidation in the healthcare industry may lead to a reduction in the prices we charge, thereby decreasing our revenue. Numerous initiatives and reforms initiated to combat rising healthcare costs, in addition to other economic factors, have contributed to a consolidation trend in the healthcare industry. Consolidation has resulted in increased competition to provide products and services to industry participants, and this competition is likely to grow increasingly intense. Competitive bidding also emphasizes the importance of relationships with both payors and others in the industry that impact reimbursement of our clients and customers. Further consolidation may reduce competition among our existing and prospective customers and exert further downward pressure on the prices of our products, potentially decreasing our revenue, which would limit our ability to pursue our growth strategies. |
| • | We have substantial indebtedness. As of December 31, 2020, we had approximately $922.2 million and $240.0 million in borrowings outstanding under our First Lien Term Loan Facility (as defined herein) and Second Lien Term Loan Facility (as defined herein), respectively, and $6.3 million of letters of credit outstanding under our Revolving Credit Facility. The proceeds from the borrowings under the Second Lien Term Loan Facility were used to pay a dividend in an amount equal to approximately $240.0 million (including transaction costs) to our equityholders as a means of providing our equityholders with a return on their investment. Our substantial amount of indebtedness may require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, research and development efforts and other general corporate purposes, and increase our vulnerability to general adverse economic, industry and competitive conditions. |
| • | If we are unable to fund our significant cash needs, including capital expenditures, we may be unable to expand our business as planned or to service our debt. We currently estimate that over the next 12 months, we will make net investments of approximately $60 million to $70 million in new and pre-owned medical equipment, leasehold improvements and other capital expenditures. In addition, a substantial portion of our cash flow from operations must be dedicated to servicing our debt. To the extent that we cannot fund our cash needs from our operating cash flow, we will be unable to pursue our growth strategies. |
| • | THL controls us, and its interests may conflict with yours or ours in the future. Immediately after this offering, assuming an offering size as set forth herein, the THL Stockholder will beneficially own approximately 78.4% of our outstanding common stock (or 76.0% of our outstanding common stock if the underwriters’ option to purchase additional shares is exercised in full). For as long as the THL Stockholder continues to own a significant portion of our stock, THL will be able to significantly influence the composition of our board of directors, including the approval of actions requiring shareholder approval. Accordingly, for such period of time, THL will have significant influence with respect to our management, business plans and policies, including the appointment and removal of our officers, decisions on whether to raise future capital and amending our charter and bylaws, which govern the rights attached to our common stock, and their interest in such matters may conflict with yours or ours. |
| • | We may fail to realize all of the anticipated benefits of the Northfield Acquisition or those benefits may take longer to realize than expected. We may also encounter significant difficulties in integrating the business of Northfield Medical. The failure to meet the challenges involved in the integration process and realize the anticipated benefits of the Northfield Acquisition could cause an interruption of, or a loss of momentum in, our operations and could have a material adverse effect on our business, financial condition and results of operations. |
| • | We are not providing audited historical financial information for Northfield Medical or pro forma financial statements reflecting the impact of the Northfield Acquisition on our historical operating results. We will not file a Current Report on Form 8-K until after the closing of this offering |
15
Table of Contents
| with the required financial information and, we are not currently in a position to include this information in this prospectus. As a result, investors in this offering will be required to determine whether to participate in this offering without the benefit of this historical and pro forma financial information. |
| • | An active, liquid trading market for our common stock may not develop, which may limit your ability to sell your shares. The initial public offering price will be determined by negotiations between us and the underwriters and may not be indicative of market prices of our common stock that will prevail in the open market after the offering. The failure of an active and liquid trading market to develop and continue would likely have a material adverse effect on the value of our common stock, which may impair our ability to raise capital to pursue our growth strategies, to continue to fund operations and to pursue acquisitions using our shares as consideration. |
These and other risks are more fully described in the section entitled “Risk Factors” in this prospectus. If any of these risks actually occurs, our business, financial condition, results of operations, cash flows and prospects could be materially and adversely affected. As a result, you could lose all or part of your investment in our common stock.
Our Principal Stockholder
THL is a premier private equity firm that invests in middle market growth companies, headquartered primarily in North America, exclusively in three sectors: Financial Services, Healthcare and Technology & Business Solutions. The firm couples its deep sector expertise with dedicated internal operating resources to transform and build great companies of lasting value in partnership with company management. Since 1974, THL has raised more than $25 billion of equity capital, invested in over 150 companies and completed more than 400 add-on acquisitions representing an aggregate enterprise value at acquisition of over $200 billion.
General Corporate Information
We commenced operations in 1939, originally incorporated in Minnesota in 1954 and reincorporated in Delaware in 2001. Since the Business Combination (as defined below), we have been controlled by THL Stockholder, an affiliate of THL.
Agiliti, Inc. was formed on August 1, 2018 in order to consummate a merger with Federal Street Acquisition Corp., a special purpose acquisition company affiliated with THL (“FSAC”) pursuant to the Amended and Restated Agreement and Plan of Merger, dated as of December 19, 2018 (the “A&R Merger Agreement”), by and among Agiliti, FSAC, Umpire SPAC Merger Sub, Inc., Umpire Cash Merger Sub, Inc., Agiliti Holdco, Inc. (“Agiliti Holdco”), solely in their capacities as Majority Stockholders, IPC/UHS, L.P. and IPC/UHS Co-Investment Partners, L.P., solely in its capacity as the Stockholders’ Representative (as defined in the A&R Merger Agreement), IPC/UHS and, solely for the purposes stated therein, Umpire Equity Merger Sub, Inc. Pursuant to the A&R Merger Agreement, (i) FSAC became a wholly owned subsidiary of Agiliti and the holders of Class A common stock, par value $0.0001 per share, of FSAC (the “FSAC Class A Common Stock”) received shares of common stock, par value $0.0001 per share, of Agiliti (our “common stock”); and (ii) Agiliti Holdco became a wholly owned subsidiary of FSAC and the equityholders of Agiliti Holdco received cash and/or shares of our common stock and/or fully-vested options to purchase shares of our common stock as merger consideration (the transactions contemplated by the A&R Merger Agreement are referred to herein as the “Business Combination”).
16
Table of Contents
Our principal executive offices are located at 6625 West 78th Street, Suite 300, Minneapolis, Minnesota 55439-2604. Our telephone number is (952) 893-3200. Our website address is www.agilitihealth.com. The information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock. We are a holding company and all of our business operations are conducted through our subsidiaries.
17
Table of Contents
| Common stock offered |
26,315,789 shares. |
| Option to purchase additional shares |
3,947,368 shares. |
| Common stock to be outstanding after this |
125,299,085 shares (or 129,246,453 shares if the underwriters’ option to purchase additional shares is exercised in full). |
| Use of proceeds |
We estimate that our net proceeds from this offering will be approximately $460.3 million, or approximately $530.9 million if the underwriters’ option to purchase additional shares is exercised in full, assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us. |
| The principal purposes of this offering are to increase our capitalization and financial flexibility, create a public market for our common stock and enable access to the public equity markets for us and our shareholders. We expect to use approximately $450.0 million of net proceeds of this offering (or $520.0 million of the net proceeds of this offering if the underwriters exercise their option to purchase additional shares in full) to repay outstanding borrowings and related fees and expenses, under our Credit Facilities (as defined herein). See “Use of Proceeds” for additional information. |
| Conflicts of interest |
Certain affiliates of Goldman Sachs & Co. LLC currently hold 100% of our Second Lien Term Loan Facility and, as such, will receive 5% or more of the net proceeds of this offering due to the repayment of outstanding borrowings and related fees and expenses, under our Credit Facilities. Therefore, Goldman Sachs & Co. LLC is deemed to have a conflict of interest within the meaning of Rule 5121 of the Financial Industry Regulatory Authority, Inc. (“Rule 5121”). Accordingly, this offering is being conducted in accordance with Rule 5121, which requires, among other things, that a “qualified independent underwriter” as described in Rule 5121 participate in the preparation of, and exercise the usual standards of “due diligence” with respect to, the registration statement and this prospectus. BofA Securities, Inc., one of the managing underwriters of this offering, has agreed to act as a qualified independent underwriter for this offering and to undertake the legal responsibilities and liabilities of an underwriter under the Securities Act of 1933, as amended (the “Securities Act”), specifically including those inherent in Section 11 thereof. BofA Securities, Inc. will not receive any additional fees for serving as a qualified independent underwriter in connection with this offering. We have agreed to indemnify BofA Securities, Inc. against liabilities incurred in connection with acting as a qualified |
18
Table of Contents
| independent underwriter, including liabilities under the Securities Act. For more information, see “Underwriting (Conflicts of Interest)—Conflicts of Interest.” |
| Controlled company |
After this offering, assuming an offering size as set forth in this section, the THL Stockholder will own approximately 78.4% of our common stock (or 76.0% of our common stock if the underwriters’ option to purchase additional shares is exercised in full). As a result, we expect to be a controlled company within the meaning of the corporate governance standards of the NYSE. See “Management—Corporate Governance—Controlled Company Status.” |
| Directed share program |
At our request, the underwriters have reserved up to 1,315,789 shares of our common stock, or 5% of the shares of our common stock to be offered by this prospectus for sale, at the initial public offering price, to certain individuals through a directed share program, including certain employees and certain other individuals identified by management. Shares purchased through the directed share program will not be subject to a lock-up restriction, except in the case of shares purchased by any of our officers and certain of our employees and existing equityholders. The number of shares of our common stock available for sale to the general public will be reduced to the extent these individuals or entities purchase such reserved shares. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered by this prospectus. See “Certain Relationships and Related Party Transactions” and “Underwriting (Conflicts of Interest)”. |
| Risk factors |
Investing in our common stock involves a high degree of risk. See “Risk Factors” elsewhere in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
| Proposed trading symbol |
“AGTI.” |
The number of shares of common stock to be outstanding following this offering is based on 98,983,296 shares of common stock outstanding as of March 1, 2021, and excludes:
| • | 6,690,308 shares of common stock issuable upon the exercise of options outstanding as of March 1, 2021, with a weighted average exercise price of $4.86 per share; |
| • | 2,537,619 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units, as of March 1, 2021 (assuming vesting at 150% for performance restricted stock units vesting as of March 6, 2021 and assuming 100% vesting for the performance restricted stock units thereafter); |
| • | 752,328 shares of common stock issued in connection with the Northfield Acquisition; |
| • | 190,226 shares of common stock issuable upon the exercise of warrants outstanding as of March 1, 2021, with a weighted average exercise price of $9.27 per share; |
| • | 14,474 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to members of the Northfield management team at an assumed exercise |
19
Table of Contents
| price of $19.00 (which is the midpoint of the estimated price range set forth on the cover page of this prospectus); |
| • | 56,795 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in conjunction with this offering to members of Northfield management team (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); |
| • | 497,868 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to employees of Agiliti at an assumed exercise price of $19.00, which is the midpoint of the estimated price range set forth on the cover page of this prospectus; |
| • | 643,416 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in connection with this offering (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); and |
| • | 8,961,227 shares of common stock reserved for future issuance under the 2018 Omnibus Incentive Plan (after giving effect to the anticipated issuance of 1,212,553 shares expected to be issued in conjunction with this transaction). |
Unless otherwise indicated, all information in this prospectus assumes:
| • | the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws, each in connection with the closing of this offering; |
| • | no exercise of outstanding options or warrants, or issuance of shares of restricted stock units, or performance-based restricted stock units after March 1, 2021; and |
| • | no exercise by the underwriters of their option to purchase up to 3,947,368 additional shares of common stock. |
20
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables present the summary consolidated financial data for Agiliti and its subsidiaries and the summary consolidated financial data for Agiliti Health, Inc. Agiliti Health, Inc. is the predecessor of Agiliti for financial reporting purposes. We derived the summary consolidated statements of operations data and balance sheet data for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 (Successor) from the audited consolidated financial statements of Agiliti and related notes thereto included elsewhere in this prospectus. We derived the summary consolidated statements of operations data and balance sheet data for the period from January 1 through January 3, 2019 (Predecessor) and for the year ended 2018 from the audited consolidated financial statements of Agiliti Health, Inc. and related notes thereto included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results to be expected in any future period. The information set forth below should be read together with the “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
You should read the information set forth below together with “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Capitalization,” and our consolidated financial statements and the related notes thereto included elsewhere in this prospectus.
| Successor | Predecessor | |||||||||||||||||||||||
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year ended December 31, 2018 |
||||||||||||||||||||
| Consolidated Statements of Operation Data: |
||||||||||||||||||||||||
| Revenue |
$ | 773,312 | $ | 613,073 | $ | — | $ | 565,246 | ||||||||||||||||
| Cost of revenue |
486,965 | 423,812 | — | 367,837 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Gross margin |
286,347 | 189,261 | — | 197,409 | ||||||||||||||||||||
| Selling, general and administrative |
250,289 | 187,156 | 17,147 | 137,210 | ||||||||||||||||||||
| Gain on settlement |
|
— |
|
— | — | (26,391 | ) | |||||||||||||||||
| Intangible asset impairment charge |
|
— |
|
— | — | 131,100 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating income (loss) |
36,058 | 2,105 | (17,147 | ) | (44,510 | ) | ||||||||||||||||||
| Interest expense |
61,530 | 48,199 | — | 53,390 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss before income taxes and noncontrolling interest |
(25,472 | ) | (46,094 | ) | (17,147 | ) | (97,900 | ) | ||||||||||||||||
| Income tax benefit |
(3,234 | ) | (14,857 | ) | (13,281 | ) | (66,348 | ) | ||||||||||||||||
| Net income attributable to noncontrolling interest |
240 | 171 | — | 327 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss attributable to Agiliti, Inc. and Subsidiaries |
$ | (22,478 | ) | $ | (31,408 | ) | $ | (3,866 | ) | $ | (31,879 | ) | ||||||||||||
| Pro Forma Per Share Data (1): |
||||||||||||||||||||||||
| Pro forma net (loss) income per share: |
||||||||||||||||||||||||
| Basic |
$ | (0.18 | ) | $ | (0.25 | ) | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Diluted |
$ | (0.17 | ) | $ | (0.24 | ) | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Pro forma weighted-average shares used in computing net (loss) income per share: |
||||||||||||||||||||||||
| Basic |
125,299,085 | 125,299,085 | ||||||||||||||||||||||
| Diluted |
132,821,738 | 132,821,738 | ||||||||||||||||||||||
21
Table of Contents
| Successor | Predecessor | |||||||||||||||||||
| (in thousands) |
December 31, 2020 |
December 31, 2019 |
December 31, 2018 |
|||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 206,505 | — | $ | 7,340 | |||||||||||||||
| Working Capital (2) |
$ | 82,102 | $ | 39,360 | $ | 15,756 | ||||||||||||||
| Total assets |
$ | 1,903,356 | $ | 1,619,416 | $ | 744,958 | ||||||||||||||
| Total debt |
$ | 1,161,099 | $ | 922,998 | $ | 690,999 | ||||||||||||||
| Equity (Deficit) |
$ | 441,945 | $ | 456,969 | $ | (67,659 | ) | |||||||||||||
| (1) | Unaudited pro forma per share information gives effect to our sale of 26,315,789 shares of common stock in this offering at an assumed initial public offering price of $19.00 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us and the application of the net proceeds of this offering to repay approximately $450.0 million in principal amount of outstanding borrowings under our Credit Facilities as set forth under “Use of Proceeds.” |
| (2) | Represents total current assets (excluding cash and cash equivalents) less total current liabilities (excluding current portion of long-term debt, current portion of operating lease liability and current portion of obligation under tax receivable agreement). |
Adjusted EBITDA
Adjusted earnings before interest, taxes, depreciation and amortization (“Adjusted EBITDA”) was $234.2 million, $173.1 million and $152.7 million for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and the year ended December 31, 2018, respectively. Adjusted EBITDA for the year ended December 31, 2020 was higher than the period from January 4 through December 31, 2019 primarily due to the increase in revenue. Adjusted EBITDA for the period from January 4 through December 31, 2019 was higher than the year ended December 31, 2018 primarily due to the increase in revenue.
EBITDA is defined as earnings attributable to Agiliti before interest expense, income taxes, depreciation and amortization. Adjusted EBITDA is defined as EBITDA excluding, non-cash share-based compensation expense, Management Fees (as defined below) and non-recurring gains, expenses or losses. In addition to using EBITDA and Adjusted EBITDA internally as measures of operational performance, we disclose them externally to assist analysts, investors and lenders in their comparisons of operational performance, valuation and debt capacity across companies with differing capital, tax and legal structures. We believe the investment community frequently uses EBITDA and Adjusted EBITDA in the evaluation of similarly situated companies. Adjusted EBITDA is also used by us as a factor to determine the total amount of incentive compensation to be awarded to executive officers and other employees. EBITDA and Adjusted EBITDA, however, are not measures of financial performance under accounting principles generally accepted in the United States of America (“GAAP”) and should not be considered as alternatives to, or more meaningful than, net income as measures of operating performance or to cash flows from operating, investing or financing activities or as measures of liquidity. Since EBITDA and Adjusted EBITDA are not measures determined in accordance with GAAP and are thus susceptible to varying interpretations and calculations, EBITDA and Adjusted EBITDA, as presented, may not be comparable to other similarly titled measures of other companies. EBITDA and Adjusted EBITDA do not represent amounts of funds that are available for management’s discretionary use. EBITDA and Adjusted
22
Table of Contents
EBITDA presented below may not be the same as EBITDA and Adjusted EBITDA calculations as defined in the Credit Facilities. A reconciliation of net loss attributable to Agiliti to Adjusted EBITDA is included below:
| Successor | Predecessor | |||||||||||||||||||||||
| (in thousands) | Year Ended December 31, 2020 |
From January 4 Through December 31, 2019 |
From January 1 Through January 3, 2019 |
Year Ended December 31, 2018 |
||||||||||||||||||||
| Net loss attributable to Agiliti, Inc. and Subsidiaries |
$ | (22,478 | ) | $ | (31,408 | ) | $ | (3,866 | ) | $ | (31,879 | ) | ||||||||||||
| Interest expense |
61,530 | 48,199 | — | 53,390 | ||||||||||||||||||||
| Income tax benefit |
(3,234 | ) | (14,857 | ) | (13,281 | ) | (66,348 | ) | ||||||||||||||||
| Depreciation and amortization of intangibles and contract costs |
169,241 | 161,703 | — | 79,199 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| EBITDA |
205,059 | 163,637 | (17,147 | ) | 34,362 | |||||||||||||||||||
| Gain on Settlement (1) |
— | — | — | (26,391 | ) | |||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | 131,100 | ||||||||||||||||||||
| Non-cash share-based compensation expense |
10,334 | 6,011 | 5,900 | 3,010 | ||||||||||||||||||||
| Management and other expenses (2) |
671 | 6,713 | — | 2,850 | ||||||||||||||||||||
| Transaction costs (3) |
3,837 | 4,720 | 11,247 | 7,772 | ||||||||||||||||||||
| Tax receivable agreement remeasurement |
14,300 | (7,970 | ) | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Adjusted EBITDA |
$ | 234,201 | $ | 173,111 | $ | — | $ | 152,703 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Other Financial Data: |
||||||||||||||||||||||||
| Net cash provided by operating activities |
$ | 137,927 | $ | 69,998 | $ | — | $ | 86,323 | ||||||||||||||||
| Net cash used in investing activities |
(151,732 | ) | (761,973 | ) | — | (51,034 | ) | |||||||||||||||||
| Net cash provided by (used in) financing activities |
220,310 | (42,887 | ) | — | (27,949 | ) | ||||||||||||||||||
| (1) | Gain on settlement represents a litigation settlement with Hill-Rom. |
| (2) | Management and other expenses represents (a) management fees under the Advisory Services Agreement (the “Advisory Services Fees”), which will terminate in connection with this offering, (b) legacy management fees under a professional services agreement with affiliates of Irving Place Capital (the “Legacy Management Fees” and, together with the Advisory Services Fees, the “Management Fees”), which was terminated in connection with the Business Combination and (c) non-recurring expenses primarily comprising two legal settlements and a credit received under the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”). |
| (3) | Transaction costs represents costs associated with potential mergers and acquisitions and are primarily related to the Business Combination completed on January 4, 2019. See Note 4 to our audited consolidated financial statements for the year ended December 31, 2020 included elsewhere in this prospectus for more information. |
23
Table of Contents
This offering and an investment in our common stock involve a high degree of risk. You should carefully consider the risks described below, together with the financial and other information contained in this prospectus, before you decide to purchase shares of our common stock. If any of the following risks actually occurs, our business, financial condition, results of operations, cash flows and prospects could be materially and adversely affected. As a result, the trading price of our common stock could decline and you could lose all or part of your investment in our common stock.
Risks Related to Our Business and Industry
Political and policy changes could materially limit our growth opportunities.
Our business may be impacted by political and policy changes. Geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries have contributed to increased volatility and uncertain expectations for the global economy. Additionally, political changes in the United States and elsewhere in the world have created a level of uncertainty in the markets. If the markets experience any economic slowdown, recession or prolonged stagnation, there may be a profound impact on the financial condition of our suppliers and our customers, resulting in a negative impact on our business, financial condition and results of operations.
Healthcare costs have risen significantly over the past decade. There have been and continue to be proposals by legislators, regulators and third-party payors to keep these costs down. Certain proposals, if passed, would impose limitations on the prices the Company will be able to charge for the Company’s products, or the amounts of reimbursement available for its products from governmental agencies or third-party payers. These limitations could have a material adverse effect on our financial position and results of operations. Changes in the healthcare industry in the U.S. and elsewhere could adversely affect the demand for our products as well as the way in which we conduct business. The 2010 Affordable Care Act provides that most individuals must have health insurance, establishes new regulations on health plans, and creates insurance pooling mechanisms and other expanded public health care measures. The Company anticipates that the healthcare reform legislation will further reduce Medicare spending on services provided by hospitals and other providers and further forms of sales or excise tax on the medical device sector. Various healthcare reform proposals have also emerged at the federal and state level. We cannot predict what healthcare initiatives, if any, will be implemented at the federal or state level, or the effect any future legislation or regulation will have on the Company. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for the Company’s products, reduce medical procedure volumes and may thereby materially adversely affect the Company’s business, financial condition and results of operations.
The COVID-19 pandemic could materially and adversely affect our business, operating results, financial condition and prospects.
We are closely monitoring the outbreak and spread of COVID-19 (and any evolutions thereof or related or associated epidemics, pandemics or disease outbreaks, “COVID-19”). COVID-19 has spread to many countries and has been declared by the World Health Organization to be a pandemic, resulting in action from federal, state and local governments that has significantly affected virtually all facets of the U.S. and global economies. The U.S. government has implemented enhanced screenings, quarantine requirements and travel restrictions in connection with the COVID-19 outbreak.
Our business may be more adversely impacted by the effects of COVID-19 in the future. We source equipment from different parts of the world that have been affected by the virus, which could have an adverse impact on our supply chain operations and ability for manufacturers to obtain materials needed to assemble the products we offer. The current outbreak and continued spread of COVID-19 is likely to cause an economic
24
Table of Contents
slowdown and potentially could lead to a global recession. There is a significant degree of uncertainty and lack of visibility as to the extent and duration of any such slowdown or recession. Given the significant economic uncertainty and volatility created by the COVID-19 pandemic, it is difficult to predict the nature and extent of impacts on demand for our products.
The extent of the impact of COVID-19 on our operational and financial performance will depend on future developments, including, but not limited to, the duration and spread of the outbreak and related travel advisories and restrictions, all of which are highly uncertain and cannot be predicted. Government shutdown orders or a change to our business classification as an “essential business” may result in a closure of operations for an uncertain duration impacting our business results. Preventing the effects from and responding to this market disruption or any other public health threat, related or otherwise, may further increase our costs of doing business and may have a material adverse effect on our business, financial condition and results of operations.
While we have taken steps to minimize the potential for COVID-19 exposure in the workplace, the potential for a COVID-19 outbreak within our facilities occurring and significantly disrupting operations remains possible. Increased infection rates in geographic locations in which we operate have the potential to result in disruptions to our operations at a greater rate than we currently experience.
The spread of COVID-19 has caused us to modify our business practices (including employee travel, employee work locations, cancellation of physical participation in meetings (including in-person sales activities), events and conferences and social distancing measures), and we may take further actions as may be required by government authorities or that we determine are in the best interests of our employees, customers, partners, vendors and suppliers. Work-from-home and other measures introduce additional operational risks, including cybersecurity risks, which could have an adverse effect on our operations. Similarly, work-from-home and other measures may lead to increased absenteeism or cause workplace disruption. There is no certainty that such measures will be sufficient to mitigate the risks posed by the COVID-19 virus, and illness and workforce disruptions could lead to unavailability of key personnel and harm our ability to perform critical functions.
Additionally, in response to the COVID-19 pandemic, the federal government and certain state and local governments have purchased significant amounts of medical equipment of the type we offer in our rental fleet. These purchases by federal, state and local governments of medical equipment that previously would have been rented may reduce the demand for our rental equipment.
The severity, magnitude and duration of the current COVID-19 pandemic is uncertain, rapidly changing and hard to predict and depends on events beyond our knowledge or control. These and other impacts of the COVID-19 pandemic could have the effect of heightening many of the other risks described in this “Risk Factors” section, such as those relating to our reputation, sales, results of operations or financial condition. We might not be able to predict or respond to all impacts on a timely basis to prevent near- or long-term adverse impacts to our results. As a result, we cannot at this time predict the impact of the COVID-19 pandemic, but it could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may be unable to maintain existing contracts or contract terms or enter into new contracts with our customers.
Our revenue maintenance and growth depend, in part, on continuing contracts with customers, including through GPOs and IDNs, with which certain of our customers are affiliated. In the past, we have been able to maintain and renew the majority of such contracts and expand the solutions we offer under such contracts. If we are unable to maintain our contracts, or if the GPOs or IDNs seek additional discounts or other more beneficial terms on behalf of their members, we may lose a portion or all of existing business with, or revenues from, customers that are members of such GPOs and IDNs. In addition, certain of our customers account for large portions of our revenue. From time to time, a single customer, depending on the current status and volumes of a number of separate contracts, may account for 10% or more of our total revenue. As a result, the actions of even
25
Table of Contents
a single customer can expose our business and operating results to greater volatility. For the year ended December 31, 2020, we did not have sales to a single customer that exceeded 10% of our total revenue.
On July 21, 2020, we entered into a one-year agreement with the U.S. Department of Health and Human Services (“HHS”) and the Assistant Secretary for Preparedness and Response (the “HHS Agreement”) for the comprehensive maintenance and management services of medical ventilator equipment in exchange for up to $193.0 million. As a result, we expect that the U.S. government will be our largest customer during the duration of the HHS Agreement, which is set to expire on July 21, 2021. Although we expect to have the opportunity to complete a request for proposal for continuing contracts with HHS following the expiration of the HHS Agreement, to the extent the HHS Agreement or other contracts with significant customers are not renewed or are terminated, our revenue and operating results would be significantly impacted.
A substantial portion of our revenues come from customers with which we do not have long-term commitments, and cancellations by or disputes with customers could decrease the amount of revenue we generate, thereby reducing our ability to operate and expand our business.
For the year ended December 31, 2020, approximately 49% of our total revenue was derived from customers that purchased equipment or services from us through a GPO that contracted with us on behalf of its members. The remaining 51% of revenue was derived from customers that contract with us directly. Our customers are generally not obligated to outsource our equipment under long-term commitments. The short-term services we provide could be terminated by the customer without notice or payment of any termination fee. A large number of such terminations may adversely affect our ability to generate revenue growth and sufficient cash flows to support our growth plans. In addition, those customers with long-term commitments may have contracts that do not permit us to raise our prices, yet our cost to serve may increase. Any of these risks could have a material adverse impact on our ability to operate and expand our business.
If we fail to maintain our reputation, including by adequately protecting our intellectual property, our sales and operating results may decline.
We believe our continued success depends on our ability to maintain and grow the value of our brand. Brand value is based in large part on perceptions of subjective qualities. Even isolated incidents can erode the trust and confidence of our customers and damage the strength of our brand, if such incidents result in adverse publicity or litigation. Challenges or reactions to action (or inaction) or perceived action (or inaction), by us on issues such as social policies, compliance related to social, product, labor and environmental standards or other sensitive topics, and any perceived lack of transparency about such matters, could harm our reputation. The increasing use of social media platforms and online forums may increase the chance that an adverse event could negatively affect the reputation of our brands. The online dissemination of negative information about our brand, including inaccurate information, could harm our reputation, business, competitive advantage and goodwill. Damage to our reputation could result in declines in customer loyalty and sales, relationships with our suppliers, business development opportunities, divert attention and resources from management, including by requiring responses to inquiries or additional regulatory scrutiny, and otherwise materially adversely affect our results. Any failure to offer and maintain high-quality customer support, or a market perception that we do not maintain high-quality customer support, could similarly adversely affect our reputation, our ability to sell our products and services, and in turn our business, financial condition and results of operations. In addition, we are currently implementing a new information technology business systems platform. The implementation process could result in systemwide delays or failure. Because we depend on information technology systems to operate our business, failure or delay of any or all information technology systems could impact our ability to operate and meet customer demand, resulting in reputational harm.
Further, our ability to protect our brand depends in part on our ability to protect our confidential information, including unpatented know-how, technology and other proprietary information, maintaining, defending and enforcing our intellectual property rights. We rely on our agreements with our customers,
26
Table of Contents
non-disclosure and confidentiality agreements with employees and third parties, and our trademarks and copyrights to protect our intellectual property rights. However, any of these parties may breach such agreements and disclose our proprietary information, and we may not be able to obtain adequate remedies for such breaches. In addition, third parties may allege that our products and services, or the conduct of our business, infringe, misappropriate or otherwise violate such third party’s intellectual property rights. Moreover, although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property of any third parties, including such individual’s former employer. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management. Furthermore, any of our trademarks may be challenged, opposed, infringed, cancelled, circumvented or declared generic, or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks, which we need in order to maintain name recognition by potential collaborators or clients in our markets of interest.
A global economic downturn could adversely affect our customers and suppliers or have new, additional adverse effects on them, which could have further adverse effects on our operating results and financial position.
We believe our customers could be adversely affected by further global economic downturn. The impact of further downturn on our customers may result in, among other things, a decreased number of patients our customers serve at any time (which we refer to as “patient census”), decreased number of non-essential patient services, increased uncompensated care and bad debt, increased difficulty obtaining financing on favorable terms and tighter capital and operating budgets. Many of our customers depend on investment income to supplement inadequate third-party payor reimbursement. Further disruption in the capital and credit markets could adversely affect the value of many investments, reducing our customers’ ability to access cash reserves to fund their operations. If economic conditions worsen, our customers may seek to further reduce their costs and may be unable to pay for our solutions, resulting in reduced orders, slower payment cycles, increased bad debt and customer bankruptcies.
Our suppliers also may be negatively impacted by further economic downturn and tighter capital and credit markets. If our key suppliers experience financial difficulty and are unable to deliver to us the equipment we require, we could be forced to seek alternative sources of medical equipment or to purchase equipment on less favorable terms, or we could be unable to fulfill our requirements. A delay in procuring equipment or an increase in the cost to purchase equipment could limit our ability to provide equipment to customers on a timely and cost-effective basis (e.g., supply chain issues arising out of the COVID-19 pandemic). Any of these occurrences, all of which are out of our control, could have a material adverse effect on our financial condition.
If our customers’ patient census or services decrease, the revenue generated by our business could decrease.
Our operating results are dependent in part on the amount and types of equipment necessary to service our customers’ needs, which are heavily influenced by patient census and the services those patients receive. At times of lower patient census, our customers have a decreased need for our services on a supplemental or peak needs basis. During severe economic downturns, the number of hospital admissions and inpatient surgeries declines as consumers reduce their use of non-essential healthcare services. Our operating results can also vary depending on the timing and severity of the cold and flu season, local, regional or national epidemics and the impact of national catastrophes, as well as other factors affecting patient census and service demand.
Our competitors may engage in significant competitive practices, which could cause us to lose market share, reduce prices or increase expenditures.
Our competitors may engage in competitive practices that could cause us to lose market share, reduce our prices, or increase our expenditures. For example, competitors may sell significant amounts of surplus equipment
27
Table of Contents
or sell capital equipment at a lower gross margin to obtain the future repeat sales of disposables for a higher gross margin, thereby decreasing the demand for our equipment solutions. Our competitors also may choose to offer their products and services to customers on a combined or bundled basis with reduced prices, and if we are unable to offer comparable products or prices, we may experience reduced demand for our solutions. Additionally, the overall market for our services is very competitive and our competitors often compete by lowering prices, thus impacting our ability to maintain our gross margins. Any actions we may be required to take as a result of increased competitive pressure, including decreasing our prices, renegotiating contracts with customers on more favorable terms or increasing our sales and marketing expenses, could have a material adverse effect on our business, financial condition and results of operations.
Consolidation in the healthcare industry may lead to a reduction in the prices we charge, thereby decreasing our revenue.
Numerous initiatives and reforms initiated by legislators, regulators and third-party payers to curb rising healthcare costs, in addition to other economic factors, have resulted in a consolidation trend in the healthcare industry to create new companies with greater market power, including hospitals. As the healthcare industry consolidates, competition to provide products and services to industry participants has become, and will likely continue to become, more intense. In addition, competitive bidding also emphasizes the importance of relationships with both the payors and others in the space that impact reimbursement of our clients and customers. All of this in turn has resulted, and will likely continue to result in, greater pricing pressures and the exclusion of certain suppliers from various market segments as GPOs, IDNs, and large single accounts continue to use their market power to consolidate purchasing decisions for some of our existing and prospective customers. We expect the market demand, government regulation, and third-party reimbursement policies, among other potential factors, will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers and prospective customers, which may reduce competition among our existing and prospective customers, exert further downward pressure on the prices of our implants and may adversely impact our business, financial condition or results of operations.
We have relationships with certain key medical equipment manufacturers and suppliers, and adverse developments concerning these manufacturers or suppliers could delay our ability to procure equipment or provide certain services or increase our cost of purchasing equipment.
We purchased medical equipment from over 100 manufacturers in 2020, ten of which accounted for approximately 71% of our direct medical equipment purchases in 2020. Additionally, we purchase repair parts, supplies and disposables from medical equipment manufacturers and suppliers that are necessary to our business. Adverse developments concerning key suppliers or our relationships with them could force us to seek alternative sources for our medical equipment or repair parts or to purchase such equipment or repair parts on less favorable terms. A delay in procuring equipment or repair parts or an increase in our cost to purchase equipment or repair parts could limit our ability to provide equipment and/or services to our customers on a timely and cost-effective basis. In addition, if we do not have access to certain parts, or if manufacturers do not provide access to the appropriate equipment manuals or training, we may not be able to provide certain clinical engineering services.
If we are unable to change the manner in which healthcare providers traditionally procure medical equipment, we may not be able to achieve significant revenue growth.
We believe the direct purchase or capital lease of medical equipment, and self-management of that equipment, by hospitals and alternate site providers significantly competes with our solution offerings. Many hospitals and alternate site providers view equipment rental primarily as a means of meeting short-term or peak supplemental needs, rather than as a long-term, effective and cost-efficient alternative to purchasing or leasing equipment. Many healthcare providers may continue to purchase or lease a substantial portion of their medical equipment and to manage and maintain it on their own. If we are unable to influence healthcare providers to increase the proportion of medical equipment they rent rather than purchase, our ability to achieve significant revenue growth will be materially impaired.
28
Table of Contents
We depend on key personnel and our inability to attract and retain key personnel could harm our business.
Our financial performance is dependent in significant part on our ability to hire, develop and retain key personnel, including our senior executives, sales professionals, sales specialists, hospital management employees and other qualified workers. We have experienced and will continue to experience intense competition for these resources. The loss of the services of one or more of our senior executives or other key personnel could significantly undermine our management expertise, key relationships with customers and suppliers, and our ability to provide efficient, quality healthcare solutions, which would have a material adverse effect on our business, financial condition and results of operations.
We may be unable to make attractive acquisitions or successfully integrate acquired businesses, and any inability to do so may disrupt our business and hinder our ability to grow.
From time to time, we may evaluate acquisition candidates or other strategic relationships within the healthcare industry that may strategically fit our business objectives, as opportunistic acquisitions are part of our growth strategy. However, there is no guarantee we will be able to identify attractive acquisition opportunities. In the event we are able to identify attractive acquisition opportunities, we may not be able to complete the acquisition or do so on commercially acceptable terms. We may not be successful in acquiring other businesses, and the businesses we do acquire in the future may not ultimately produce returns that justify our related investment.
Acquisitions may involve numerous risks, including:
| • | difficulties assimilating personnel and integrating distinct business cultures; |
| • | diversion of management’s time and resources from existing operations; |
| • | potential loss of key employees or customers of acquired companies; |
| • | exposure to unforeseen liabilities of acquired companies; and |
| • | liabilities that may exceed indemnification caps provided in acquisition agreements. |
We may fail to realize all of the anticipated benefits of the Northfield Acquisition or those benefits may take longer to realize than expected. We may also encounter significant difficulties in integrating the business of Northfield Medical.
The success of the Northfield Acquisition will depend, in part, on our ability to integrate Northfield Medical’s business in an effective and efficient manner, which is a complex, costly and time-consuming process. The integration process may disrupt business and, if we are unable to successfully integrate Northfield Medical’s business, we could fail to realize the anticipated benefits of the Northfield Acquisition. The failure to meet the challenges involved in the integration process and realize the anticipated benefits of the Northfield Acquisition could cause an interruption of, or a loss of momentum in, our operations and could have a material adverse effect on our business, financial condition and results of operations.
In addition, the integration of Northfield Medical may result in material unanticipated challenges, expenses, liabilities, competitive responses and losses of customers and other business relationships. Additional integration challenges may include:
| • | diversion of management’s attention to integration matters; |
| • | difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects from the Northfield Acquisition; |
| • | difficulties in the integration of operations and systems; |
| • | difficulties in conforming standards, controls, procedures and accounting and other policies, business cultures and compensation structures; |
29
Table of Contents
| • | difficulties in the assimilation of employees; |
| • | difficulties in managing the expanded operations of a materially larger and more complex company; |
| • | challenges in attracting and retaining key personnel; and |
| • | the impact of potential liabilities we may be inheriting from Northfield Medical. |
Many of these factors are outside of our control and could result in increased costs, decreases in the amount of anticipated revenues and diversion of management’s time and energy, each of which could adversely affect our business, financial condition and results of operations.
In addition, even if the integration of Northfield Medical’s business is successful, we may not realize all of the anticipated benefits of the Northfield Acquisition, including the synergies, cost savings, or sales or growth opportunities. These benefits may not be achieved within the anticipated time frames, or at all. Further, additional unanticipated costs may be incurred in the integration process. All of these factors could cause reductions in earnings per share, decrease or delay the expected accretive effect of the transaction and negatively impact the price of shares of our common stock. As a result, it cannot be assured that the Northfield Acquisition will result in the realization of the anticipated benefits and potential synergies.
We may incur increased expenses related to our pension plan, which could impact our financial position.
We have a defined benefit pension plan covering certain current and former employees. Although benefits under the pension plan were frozen in 2002, funding obligations under the pension plan continue to be impacted by the performance of the financial markets. If the financial markets do not provide the long-term returns we have assumed, the likelihood of us being required to make additional contributions will increase. The equity and debt markets can be, and recently have been, volatile, and therefore our estimate of future contribution requirements can change dramatically in relatively short periods of time.
Our cash flow fluctuates during the year.
Our results of operations have been and can be expected to be subject to quarterly fluctuations. We may experience increased revenue in the first and fourth quarters of the year, depending upon the timing and severity of the cold and flu season and the related increased hospital census and medical equipment usage during that season. Because a significant portion of our expenses are relatively fixed over these periods, our operating income as a percentage of revenue tends to increase during the first and fourth quarter of each year. If the cold and flu season is delayed by as little as one month, or is less severe than in prior periods, our quarterly operating results for a current period can vary significantly from prior periods. Our quarterly results can also fluctuate as a result of such other factors as the timing of acquisitions, new on-site managed solution agreements or new service center openings.
A portion of our revenues are derived from home care providers and nursing homes, and these healthcare providers may pose additional credit risks.
Our nursing home and home care customers may pose additional credit risks since they are generally less financially sound than hospitals. In addition, such cost pressures have increased due to temporary and permanent closure of nursing homes and home care agencies caused by the spread of COVID-19. These customers continue to face cost pressures. We may incur losses in the future due to the credit risks, including potential bankruptcy filings, associated with any of these customers.
Although we do not manufacture any medical equipment, our business entails the risk of claims related to the medical equipment that we outsource and service. We may not have adequate insurance to cover a claim, and it may be more expensive or difficult for us to obtain adequate insurance in the future.
We may be liable for claims related to the use of our medical equipment or to our maintenance or repair of a customer’s medical equipment. Any such claims, if made and upheld, could make our business more expensive
30
Table of Contents
to operate and therefore less profitable. We may be subject to claims exceeding our insurance coverage or we may not be able to continue to obtain liability insurance at acceptable levels of cost and coverage. If we are found liable for any significant claims that are not covered by insurance, our liquidity and operating results could be materially adversely affected. In addition, litigation relating to a claim could adversely affect our existing and potential customer relationships, create adverse public relations and divert management’s time and resources from the operation of the business.
We may incur increased costs that we cannot pass through to our customers.
Our customer contracts may include limitations on our ability to increase prices over the term of the contract. On the other hand, we rely on third parties, including subcontractors, to provide some of our services and supplies and we do not always have fixed pricing contracts with these subcontractors. Therefore, we are at risk of incurring increased costs that we are unable to pass through to our customers.
Any failure of our management information systems could harm our business and operating results.
We depend on our management information systems to actively manage our medical equipment fleet, control capital spending and provide fleet information, including equipment usage history, condition and availability of our medical equipment. These functions enhance our ability to optimize fleet utilization and redeployment. The inability of our management information systems to operate as we anticipate could damage our reputation with our customers, disrupt our business or result in, among other things, decreased revenue and increased overhead costs. Any such failure could harm our business and results of operations. Our results of operations could be adversely affected if these systems, or our customers’ access to them, are interrupted, damaged by unforeseen events, cyber security incidents or other actions of third parties, or fail for any extended period of time. In addition, data security breaches could adversely impact our operations, results of operations or our ability to satisfy legal requirements, including those related to patient-identifiable health information.
There are inherent limitations in all internal control systems over financial reporting, and misstatements due to error or fraud may occur and not be detected.
While we have taken actions designed to address compliance with the internal control over financial reporting and disclosure controls and other requirements of the Sarbanes-Oxley Act of 2002 and the rules and regulations promulgated by the SEC implementing these requirements, there are inherent limitations in our ability to control all circumstances. We do not expect that our internal control over financial reporting and disclosure controls will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. In addition, the design of a control system must reflect the fact that there are resource constraints and the benefit of controls must be relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, in our Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Further, controls can be circumvented by individual acts of some persons, by collusion of two or more persons, or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may be inadequate because of changes in conditions, such as our growth or increased transaction volume, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Social unrest may materially and adversely impact our business.
In recent months, there has been increasing social unrest throughout the United States (including looting, protests, strikes and street demonstrations). We have over 98 offices located in cities across the country, and such
31
Table of Contents
social unrest could materially affect the ability of certain of these offices to operate. Prolonged disruptions because of such social unrest in the markets in which we operate could disrupt our relationships with customers, employees and referral sources located in affected areas and, in the case of our corporate office, our ability to provide administrative support services, including billing and collection services. Future civil insurrection, social unrest, protests, looting, strikes or street demonstrations may adversely affect our reputation, business and consolidated financial condition, results of operations and cash flows.
If we do not respond to technological changes, our products and services could become obsolete, and we could lose customers.
To remain competitive, we must continue to enhance and improve the functionality and features of the technology that forms part of our service offering. The healthcare industry is rapidly changing, and if competitors introduce new products and services using new technologies or if new industry standards and practices emerge, our existing products and services and our systems and our proprietary software may become obsolete. Our failure to respond to technological change or to adequately maintain, upgrade and develop our products, services and systems used to process customers’ orders and payments could harm our business, prospects, financial condition and results of operations.
If we do not successfully coordinate the management of our equipment, we could lose sales.
Our business requires that we coordinate the management of our equipment over a significant geographic range. If we do not successfully coordinate the timely and efficient management of our equipment (for example, if equipment is lost, missing or misplaced), our costs may increase, we may experience a build-up or shortage in inventory, we may not be able to deliver sufficient quantities to meet customer demand and we could lose sales, each of which could seriously harm our business.
Challenges to our tax positions, the interpretation and application of recent U.S. tax legislation or other changes in taxation of our operations could harm our business, revenue and financial results.
We operate in a number of tax jurisdictions, including at the U.S. federal, state and local levels, and we therefore are subject to review and potential audit by tax authorities in these various jurisdictions. Significant judgment is required in determining our provision for income taxes and other tax liabilities, and tax authorities may disagree with tax positions we take and challenge our tax positions. Successful unilateral or multi-jurisdictional actions by various tax authorities, including in the context of our current or future corporate operating structure and third-party and intercompany arrangements, may increase our effective tax rate, result in additional taxes or other costs or have other material consequences, which could harm our business, revenue and financial results. Our effective tax rate may also change from year to year or vary materially from our expectations based on changes or uncertainties in the mix of activities and income allocated or earned among various jurisdictions, changes in tax laws and the applicable tax rates in these jurisdictions (including future tax laws that may become material) and the valuation of deferred tax assets and liabilities.
We may from time to time be subject to litigation, which may be extremely costly to defend, could result in substantial judgment or settlement costs or subject us to other remedies.
We are currently not a party to any material legal proceedings. From time to time, however, we may be involved in various legal proceedings, including, but not limited to, actions relating to breach of contract, employment-related proceedings, anti-competition-related matters and intellectual property infringement, misappropriation or other violation. Claims may be expensive to defend, may divert management’s time away from our operations, and may impact the availability and premiums of our liability insurance coverage, regardless of whether they are meritorious or ultimately lead to a judgment against us. We cannot assure you that we will be able to successfully defend or resolve any current or future litigation matters, in which case those litigation matters could have a material and adverse effect on our business, financial condition, operating results, cash flows, and prospects.
32
Table of Contents
Risks Related to Health Care and Other Legal Regulation Affecting Us
Uncertainty surrounding healthcare reform initiatives remains. Depending on the scope, form, and implementation of final healthcare reform legislation, our business may be adversely affected.
The healthcare industry is undergoing significant change. In March 2010, the Congress adopted and President Obama signed into law the Patient Protection and Affordable Care Act (the “Affordable Care Act”). The Affordable Care Act increased the number of Americans with health insurance and employer mandates and subsidies offered to lower income individuals. While the increase in coverage could translate into increased utilization of our products and services, healthcare reform and political uncertainty have historically resulted in changes in how our customers purchase our services and have adversely affected our revenue. In addition to healthcare reform, Medicare, Medicaid and managed care organizations, such as health maintenance organizations and preferred provider organizations, traditional indemnity insurers and third-party administrators are under increasing pressure to control costs and limit utilization, while improving quality and healthcare outcomes. Provider revenue per service may decline with reductions in Medicare and Medicaid reimbursement. Furthermore, the implementation of the Affordable Care Act may impose changes in healthcare delivery, reimbursement, operations or record keeping that are not compatible with our current offerings, which could force us to incur additional compliance costs. So far, starting in 2013, our business, along with that of some of our suppliers and customers that are manufacturers, came under direct regulation of the Open Payments Law, specifically the Physician Payments Sunshine Act. The Open Payments Law requires the annual reporting and publishing of all transfers of value to physicians and teaching hospitals to give greater transparency to financial relationships between manufacturers, physicians and teaching hospitals. Federal and state governments also continue to enact and consider various legislative and regulatory proposals that could materially impact certain aspects of the healthcare system. We cannot predict with certainty what additional healthcare initiatives, if any, will be implemented at the federal or state levels or what the ultimate effect of federal healthcare reform (including, but not limited to, the Affordable Care Act) or any future legislation or regulation will have on our operating results or financial condition. We cannot predict with any certainty the result of proposed regulation in the healthcare space, such as the Department of Health and Human Services initiative to accelerate a transformation of the healthcare system, with a focus on removing “unnecessary obstacles” to coordinated care (the “Sprint to Coordinated Care”). Finally, we cannot quantify the repeal of the individual mandate, effective in 2019, under the Affordable Care Act and predict with any certainty the impact of the election or composition of the U.S. Supreme Court on our business model, prospects, financial condition or results of operations.
We are subject to federal and state privacy and data security laws and regulations in connection with our collection and use of personal information, including recently enacted amendments to federal privacy laws which make us subject to more stringent penalties in the event we improperly use or disclose protected health information regarding our customers’ patients.
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) regulations apply national standards for some types of electronic health information transactions and the data elements used in those transactions to ensure the integrity, security and confidentiality of health information and standards to protect the privacy of individually identifiable health information businesses receive, maintain or transmit. The Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH Act”) expanded the scope of the privacy and security requirements under HIPAA and increased penalties for violations to “Business Associates” such as Agiliti Health, who are required to comply with certain of the HIPAA privacy standards and are required to implement administrative, physical and technical security standards. In addition, the HITECH Act enacted federal breach notification rules requiring notification to affected individuals and the Department of Health and Human Services (and in some cases, relevant media outlets) whenever a breach of protected health information occurs. In addition, the HIPAA rules now involve increased penalties, including mandatory penalties for “willful neglect” violations, starting at $100 per violation subject to a cap of $1.5 million for violations of the same standard in a single calendar year. To meet these requirements, as well as the requirements of other federal laws and regulations governing the collection and use of personal information, we may need to expend additional
33
Table of Contents
capital, software development and other resources, including to modify our products and services. Furthermore, our failure to maintain confidentiality of sensitive protected health information or other personal information in accordance with the applicable regulatory requirements could damage our reputation and expose us to claims, fines and penalties. Our operations could also be negatively impacted by a violation of the HIPAA privacy or security rules or any other applicable privacy or data security law.
Many states in which we operate also have state laws that protect the privacy and security of confidential, protected health information or other personal information that have similar or even more protection than the federal provisions. State attorneys general are also authorized to enforce federal HIPAA privacy and security rules. Furthermore, state data breach notification laws continue to expand the type of protected health information and other personal information they encompass, and in many cases are more burdensome than the HIPAA/HITECH breach reporting requirements. Some state laws impose fines and penalties upon violators in addition to allowing a private right of action to sue for damages for those who believe their protected health information or other personal information has been misused.
Our relationships with healthcare facilities and marketing practices are subject to the federal Anti-Kickback Statute and similar state laws.
Although we do not receive direct reimbursement from the U.S. federal government in the normal course of our business, we are subject to the federal Anti-Kickback Statute, which prohibits the knowing and willful offer, payment, solicitation or receipt of any form of “remuneration” in return for, or to induce, the referral of business or ordering of services paid for by Medicare or other federal programs. “Remuneration” has been broadly defined to include anything of value, including gifts, discounts, credit arrangements, and in-kind goods or services. Certain federal courts have held that the Anti-Kickback Law can be violated if “one purpose” of a payment is to induce referrals. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside the healthcare industry. Violations can result in imprisonment, civil or criminal fines or exclusion from Medicare, Medicaid and other governmental programs. The Office of Inspector General (“OIG”) issued a series of safe harbors that if met will help assure healthcare providers and other parties will not be prosecuted under the Anti-Kickback Law. Contracts with healthcare facilities and other marketing practices or transactions may implicate the Anti-Kickback Statute. We have attempted to structure our contracts and marketing practices to comply with the Anti-Kickback Statute along with providing training to our employees. However, we cannot ensure that we will not have to defend against alleged violations from private entities or that OIG or other authorities will not find that our practices violate the Anti-Kickback Statute.
Our contracts with the federal government subject us to additional oversight.
On July 21, 2020 we entered into the HHS Agreement for the comprehensive maintenance and management services of medical ventilator equipment in exchange for up to $193.0 million. As a result of this contract, we expect that the U.S. government will be our largest customer during the duration of the HHS Agreement, which is set to expire on July 21, 2021. In addition to the HHS Agreement, we have other agreements with the U.S. government. For the year ended December 31, 2020, we derived approximately 12% of our revenue from multiple contracts with agencies of the federal government. As such, we must comply with and are affected by laws and regulations relating to the award, administration and performance of U.S. government contracts. Government contract laws and regulations affect how we do business with our customers and impose certain risks and costs on our business. A violation of specific laws and regulations, by us, our employees, others working on our behalf, a supplier or a venture partner, could harm our reputation and result in the imposition of fines and penalties, the termination of our contracts, suspension or debarment from bidding on or being awarded contracts, loss of our ability to export products or services and civil or criminal investigations or proceedings. In some instances, these laws and regulations impose terms or rights that are different from those typically found in commercial transactions.
For example, the U.S. government may terminate any of our government contracts and subcontracts either at its convenience or for default based on our performance, which may result in a loss. In addition, as funds are
34
Table of Contents
typically appropriated on a fiscal year basis and as the costs of a termination for convenience may exceed the costs of continuing a program in a given fiscal year, occasionally programs do not have sufficient funds appropriated to cover the termination costs were the government to terminate them for convenience. Under such circumstances, the U.S. government could assert that it is not required to appropriate additional funding.
A termination arising out of our default may expose us to liability and have a material adverse effect on our ability to compete for future contracts and orders. In addition, the U.S. government could terminate a prime contract under which we are a subcontractor, notwithstanding the quality of our services as a subcontractor. In the case of termination for default, the U.S. government could make claims to reduce the contract value or recover its procurement costs and could assess other special penalties.
Additionally, the U.S. government may not exercise an option period for various reasons, or, alternatively, the U.S. government may exercise option periods, even for contracts for which it is expected that our costs may exceed the contract price or ceiling.
U.S. government agencies routinely audit and investigate government contractors. These agencies review a contractor’s performance under its contracts, its cost structure, its business systems and compliance with applicable laws, regulations and standards. The U.S. government has the ability to decrease or withhold certain payments when it deems systems subject to its review to be inadequate. Additionally, any costs found to be misclassified may be subject to repayment.
Changes in third-party payor reimbursement for healthcare items and services, as well as economic hardships faced by other parties from which our customers obtain funding, may affect our customers’ ability to pay for our services, which could cause us to reduce our prices or adversely affect our ability to collect payments.
Most of our customers are healthcare providers that pay us directly for the services we deliver, and these customers rely on third-party payor reimbursement for a substantial portion of their operating revenue. Third-party payors include government payors like Medicare and Medicaid and private payors like insurance companies and managed care organizations. Third-party payors continue to engage in widespread efforts to control healthcare costs. Their cost containment initiatives include efforts to control utilization of services and limit reimbursement amounts. Reimbursement limitations can take many forms, including discounts, non-payment for certain care (for example, care associated with certain hospital-acquired conditions) and fixed payment rates for particular treatment modalities or plans, regardless of the provider’s actual costs in caring for a patient. Reimbursement policies have a direct effect on our customers’ ability to pay us for our services and an indirect effect on the prices we charge. Ongoing concerns about rising healthcare costs may cause more restrictive reimbursement policies to be implemented in the future. Restrictions on the amounts or manner of reimbursements or funding to healthcare providers may affect the financial strength of our customers and the amount our customers are able to pay for our solutions.
In addition, a portion of our customers derive funding from state and local government sources, some of which are facing financial hardships, including decreased funding. Any limitation or elimination of funding to our customers by these sources could also affect the financial strength of our customers and the amount they are able to pay for our services.
Our customers operate in a highly regulated environment. Regulations affecting them could cause us to incur additional expenses associated with compliance and licensing. We could be assessed fines and face possible exclusion from participation in state and federal healthcare programs if we violate laws or regulations applicable to our business.
The healthcare industry is required to comply with extensive and complex laws and regulations at the federal, state and local government levels. While the majority of these regulations do not directly apply to us, there are some that do, including the FDCA and certain state pharmaceutical licensing requirements. Although we believe we are in
35
Table of Contents
compliance with the FDCA, if the Food and Drug Administration (“FDA”) expands the reporting requirements under the FDCA, we may be required to comply with the expanded requirements and may incur substantial additional expenses in doing so. With respect to state requirements, we are currently licensed in 20 states and may be required to obtain additional licenses, permits and registrations as state requirements change. Our failure to possess such licenses for our existing operations may subject us to certain additional expenses.
Our success depends on the ability to service medical equipment safely and effectively. We are required to comply with the Food and Drug Administration Reauthorization Act (“FDARA”), which requires us to evaluate quality, safety and effectiveness of medical devices with respect to servicing. Our quality management system has not been fully extended to all of our programs and services, and the lack of controls may result in issues related to compliance and patient safety. In addition, our suppliers may not be able to fulfill service or product commitments, resulting in delays or failure to repair medical devices, and our manufacturers may be reluctant to provide the service manuals, training, equipment or parts needed to repair medical devices. The use of inadequate or substandard parts during the repair of medical devices may also result in the inoperability of medical equipment and malfunction that results in harm to patients and employees.
In addition to the FDCA, FDARA and state licensing requirements, we are impacted by federal and state laws and regulations aimed at protecting the privacy of individually identifiable protected health information, among other things, and detecting and preventing fraud, abuse and waste with respect to federal and state healthcare programs. Some of these laws and regulations apply directly to us. Additionally, many of our customers require us to abide by their policies relating to patient privacy, state and federal anti-kickback acts, and state and federal false claim acts and whistleblower protections. Since the Affordable Care Act provides for further oversight over and detection of fraud and abuse activities, we expect many of our customers to continue to require us to abide by such policies.
Given that our industry is heavily regulated, we may be subject to additional regulatory requirements. If our operations are found to be in violation of any governmental regulations to which we, or our customers, are subject, we may be subject to the applicable penalty associated with the violation. While we believe that our practices materially comply with applicable state and federal requirements, the requirements might be interpreted in a manner inconsistent with our interpretation. Also, if we are found to have violated certain federal or state laws or regulations regarding Medicare, Medicaid or other governmental funding sources, we could be subject to fines and possible exclusion from participation in federal and state healthcare programs. Penalties, damages, fines, or curtailment of our operations could significantly increase our cost of doing business, leading to difficulty generating sufficient income to support our business.
In addition, although our business is not currently extensively regulated under healthcare laws, we are subject to certain regulatory requirements that continue to come under greater scrutiny and regulation. Our customers are subject to direct regulation under the Federal False Claims Act, the Stark Law, the Anti-Kickback Statute, rules and regulations of the Centers for Medicare and Medicaid Services (“CMS”) and other federal and state healthcare laws and regulations. Promulgation of new laws and regulations, or changes in or re-interpretations of existing laws or regulations as they relate to our customers and our business, could affect our business, operating results or financial condition. Our operations may be negatively impacted if we have to comply with additional complex government regulations.
Although we do not manufacture any medical equipment, we own a large fleet of medical equipment, which may be subject to equipment recalls or obsolescence.
We incur significant expenditures to maintain a large and modern equipment fleet. Our equipment may be subject to recalls that could be expensive to implement and could result in revenue loss while the associated equipment is removed from service. We may be required to incur additional costs to repair or replace the equipment at our own expense or we may choose to purchase incremental new equipment from a supplier not affected by the recall. Additionally, our relationship with our customers may be damaged if we cannot promptly
36
Table of Contents
replace the equipment that has been recalled. We depend on manufacturers and other third parties to properly obtain and maintain FDA clearance for their equipment and products and their failure to maintain FDA clearance could have a material adverse effect on our business.
Our success depends, in part, on our ability to respond effectively to changes in technology. Because we maintain a large fleet of equipment, we are subject to the risk of equipment obsolescence. If advancements in technology render a substantial portion of our equipment fleet obsolete, or if a competing technology becomes available that our customers prefer, we may experience a decrease in demand for our products, which could adversely affect our operating results and cause us to invest in new technology to maintain our market share and operating margins.
Risks Related to Our Indebtedness
We have substantial indebtedness.
As of December 31, 2020, we had approximately $922.2 million and $240.0 million in borrowings outstanding under our First Lien Term Loan Facility (as defined herein) and Second Lien Term Loan Facility (as defined herein) (together with the Revolving Credit Facility (as defined herein), the “Credit Facilities”), respectively, and $6.3 million of letters of credit outstanding under our Revolving Credit Facility.
This is a significant amount of indebtedness which could have important consequences. For example, it could:
| • | make it more difficult for us to satisfy our debt obligations; |
| • | increase our vulnerability to general adverse economic, industry and competitive conditions; |
| • | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, research and development efforts and other general corporate purposes; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| • | place us at a competitive disadvantage compared to our competitors that have less indebtedness; |
| • | limit our ability to borrow additional funds; |
| • | limit our ability to make investments in technology and infrastructure improvements; and |
| • | limit our ability to make significant acquisitions. |
Our ability to satisfy our debt obligations will depend on our future operating performance. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. Our business may not continue to generate sufficient cash flow from operations and future borrowings may not be available to us in an amount sufficient to enable us to repay our indebtedness or to fund our other liquidity needs. If we are unable to make our interest payments or to repay our debt at maturity, we may have to obtain alternative financing, which may not be available to us.
If we are unable to fund our significant cash needs, including capital expenditures, we may be unable to expand our business as planned or to service our debt.
We require substantial cash to operate our healthcare technology solutions and service our debt. Our healthcare technology solutions require us to invest a significant amount of cash in medical equipment purchases. To the extent that such expenditures cannot be funded from our operating cash flow, borrowings under our Credit Facilities or other financing sources, we may not be able to grow as currently planned. We
37
Table of Contents
currently expect that over the next 12 months, we will make net investments of approximately $60 to $70 million in new and pre-owned medical equipment, leasehold improvements and other capital expenditures. This estimate is subject to numerous assumptions, including revenue growth, the number of on-site managed solution signings, and any significant changes in customer contracts. In addition, a substantial portion of our cash flow from operations must be dedicated to servicing our debt and there are significant restrictions on our ability to incur additional indebtedness under the credit agreements governing our credit facilities.
Primarily because of our debt service obligations and debt refinancing charges and elevated depreciation and amortization charges we have incurred, we have had a history of net losses. If we consistently incur net losses, it could result in our inability to finance our business in the future. We had net loss of $22.5 million, $31.4 million, $3.9 million and $31.9 million for the year ended December 31, 2020, the period from January 4 through December 31, 2019, the period from January 1 through January 3, 2019 and during the year ended December 31, 2018, respectively. Our ability to use our United States federal income tax net operating loss carryforwards to offset our future taxable income may be limited. If we are limited in our ability to use our net operating loss carryforwards in future years in which we have taxable income, we will pay more current taxes than if we were able to utilize our net operating loss carryforwards without limitation, which could harm our results of operations and liquidity.
We may not be able to obtain funding on acceptable terms or at all as a result of the credit and capital markets. Thus, we may be unable to expand our business or to service our debt.
Depending on the global financial markets and economic conditions, the cost of raising money in the debt and equity capital markets may increase while the availability of funds from those markets may diminish. Without adequate funding, we may be unable to execute our growth strategy, complete future acquisitions, or take advantage of other business opportunities or respond to competitive pressures, any of which could have a material adverse effect on our revenues and results of operations.
Risks Related to Ownership of our Securities
THL controls us, and its interests may conflict with ours or yours in the future.
Immediately after this offering, assuming an offering size as set forth above, the THL Stockholder will beneficially own approximately 78.4% of our outstanding common stock (or 76.0% of our outstanding common stock if the underwriters’ option to purchase additional shares is exercised in full). As a result, we expect to be a “controlled company” within the meaning of the corporate governance standards of the NYSE. See “Management—Corporate Governance—Controlled Company Status.”
For so long as the THL Stockholder continues to own a significant portion of our stock, THL will be able to significantly influence the composition of our board of directors (our “Board”), including the approval of actions requiring shareholder approval. Accordingly, for such period of time, THL will have significant influence with respect to our management, business plans and policies, including the appointment and removal of our officers, decisions on whether to raise future capital and amending our charter and bylaws, which govern the rights attached to our common stock. In particular, for so long as the THL Stockholder continues to own a significant percentage of our stock, THL will be able to cause or prevent a change of control of us or a change in the composition of our Board and could preclude any unsolicited acquisition of us. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of us and ultimately might affect the market price of our common stock.
In connection with this offering, we will enter into an amended and restated director nomination agreement (the “director nomination agreement”) with the THL Stockholder whereby, so long as the THL Stockholder beneficially owns at least 5% of our common stock then outstanding, the THL Stockholder has the right to designate: (i) all of the nominees for election to our Board for so long as the THL Stockholder beneficially owns
38
Table of Contents
40% or more of the total number of shares of our common stock beneficially owned by the THL Stockholder upon completion of this offering, as adjusted for any reorganization, recapitalization, stock dividend, stock split, reverse stock split or similar changes in our capitalization (the “Original Amount”); (ii) a number of directors (rounded up to the nearest whole number) equal to 40% of the total directors for so long as the THL Stockholder beneficially owns at least 30% and less than 40% of the Original Amount; (iii) a number of directors (rounded up to the nearest whole number) equal to 30% of the total directors for so long as the THL Stockholder beneficially owns at least 20% and less than 30% of the Original Amount; (iv) a number of directors (rounded up to the nearest whole number) equal to 20% of the total directors for so long as beneficially owns at least 10% and less than 20% of the Original Amount; and (v) one director for so long as the THL Stockholder beneficially owns at least 5% and less than 10% of the Original Amount. In each case, the THL Stockholder’s nominees must comply with applicable law and stock exchange rules. In addition, the THL Stockholder shall be entitled to designate the replacement for any of its board designees whose board service terminates prior to the end of the director’s term regardless of the THL Stockholder’s beneficial ownership at such time. The THL Stockholder shall also have the right to have its designees participate on committees of our Board proportionate to its stock ownership, subject to compliance with applicable law and stock exchange rules. The director nomination agreement will also prohibit us from increasing or decreasing the size of our Board without the prior written consent of the THL Stockholder. This agreement will terminate at such time as the THL Stockholder owns less than 5% of the Original Amount.
THL and its affiliates engage in a broad spectrum of activities, including investments in the information and business services industry generally. In the ordinary course of their business activities, THL and its affiliates may engage in activities where their interests conflict with our interests or those of our other shareholders, such as investing in or advising businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Our certificate of incorporation to be effective in connection with the closing of this offering will provide that none of THL, any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his director and officer capacities) or its affiliates will have any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. THL also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. In addition, THL may have an interest in pursuing acquisitions, divestitures and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to you.
Upon listing of our shares on the NYSE, we will be a “controlled company” within the meaning of the rules of the NYSE and, as a result, we will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections as those afforded to shareholders of companies that are subject to such governance requirements.
After completion of this offering, the THL Stockholder will continue to control a majority of the voting power of our outstanding common stock. As a result, we will be a “controlled company” within the meaning of the corporate governance standards of the NYSE. Under these rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
| • | the requirement that a majority of our Board consist of independent directors; |
| • | the requirement that we have a nominating and corporate governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; |
| • | the requirement that we have a compensation, nominating and governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| • | the requirement for an annual performance evaluation of the compensation, nominating and governance committee. |
39
Table of Contents
Following this offering, we intend to utilize these exemptions. As a result, we may not have a majority of independent directors on our Board and our Compensation, Nominating and Governance Committee may not consist entirely of independent directors and our Compensation, Nominating and Governance Committee may not be subject to annual performance evaluations. Accordingly, you will not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of the NYSE.
An active, liquid trading market for our common stock may not develop, which may limit your ability to sell your shares.
Prior to this offering, there was no public market for our common stock. Although we have been approved to list our common stock on the NYSE under the symbol “AGTI,” an active trading market for our shares may never develop or be sustained following this offering. The initial public offering price will be determined by negotiations between us and the underwriters and may not be indicative of market prices of our common stock that will prevail in the open market after the offering. A public trading market having the desirable characteristics of depth, liquidity and orderliness depends upon the existence of willing buyers and sellers at any given time, such existence being dependent upon the individual decisions of buyers and sellers over which neither we nor any market maker has control. The failure of an active and liquid trading market to develop and continue would likely have a material adverse effect on the value of our common stock. The market price of our common stock may decline below the initial public offering price, and you may not be able to sell your shares of our common stock at or above the price you paid in this offering, or at all. An inactive market may also impair our ability to raise capital to continue to fund operations by issuing shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business.
As a public company, we will incur legal, accounting and other expenses that we did not previously incur. We will become subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Sarbanes-Oxley Act, the listing requirements of the NYSE and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business, financial condition and results of operations. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal controls and procedures for financial reporting. Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert our management’s attention from implementing our growth strategy, which could prevent us from improving our business, financial condition and results of operations. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to satisfy our obligations as a public company. In addition, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These additional obligations could have a material adverse effect on our business, financial condition and results of operations.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this
40
Table of Contents
investment may result in increased general and administrative expenses and a diversion of our management’s time and attention from sales-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and could have a material adversely effect on our business, financial condition and results of operations.
Affiliates of Goldman Sachs & Co. LLC, an underwriter in this offering, will have an interest in this offering beyond customary underwriting discounts and commissions.
Certain affiliates of Goldman Sachs & Co. LLC, an underwriter in this offering, currently hold 100% of our Second Lien Term Loan Facility and, as such, will receive 5% or more of the net proceeds of this offering due to the repayment of outstanding borrowings and related fees and expenses, under our Credit Facilities with the net proceeds of this offering. As such, Goldman Sachs & Co. LLC is deemed to have a “conflict of interest” under Rule 5121. Accordingly, this offering will be made in compliance with the applicable provisions of Rule 5121. This rule requires, among other things, that a “qualified independent underwriter” has participated in the preparation of, and has exercised the usual standards of “due diligence” with respect to, the registration statement and this prospectus. BofA Securities, Inc., one of the managing underwriters of this offering, has agreed to act as qualified independent underwriter for this offering and to undertake the legal responsibilities and liabilities of an underwriter under the Securities Act. BofA Securities, Inc. will not receive any additional fees for serving as qualified independent underwriter in connection with this offering. Although BofA Securities, Inc. has, in its capacity as qualified independent underwriter, participated in due diligence and the preparation of this prospectus and the registration statement of which this prospectus forms a part, this may not adequately address all potential conflicts of interest. We have agreed to indemnify BofA Securities, Inc. against liabilities incurred in connection with acting as qualified independent underwriter, including liabilities under the Securities Act. Pursuant to Rule 5121, Goldman Sachs & Co. LLC will not confirm sales of securities to any account over which it exercises discretionary authority without the prior written approval of the customer. See “Underwriting (Conflicts of Interest)—Conflicts of Interest” for additional information.
Our internal control over financial reporting does not currently meet the standards required by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could harm our business and stock price.
As a privately held company, we have not been required to maintain internal control over financial reporting in a manner that meets the standards of publicly traded companies as required by Section 404(a) of the Sarbanes-Oxley Act (“Section 404(a)”). As a public company, we will be required to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report on Form 10-K. Additionally, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting on an annual basis. The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation.
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. GAAP. We are currently in the process of reviewing, documenting and testing our internal control over financial reporting, but we are not currently in compliance with, and we cannot be certain when we will be able to implement the requirements of, Section 404(a). We may encounter problems or delays in implementing any changes necessary to make a favorable assessment of our internal control over financial reporting. In addition, we may encounter problems or delays in completing the implementation of any requested improvements and receiving a favorable attestation in connection with the attestation provided by our independent registered public accounting firm. If we cannot favorably assess the effectiveness of our internal control over financial reporting, or if our independent registered public accounting firm is unable to provide an unqualified attestation report on our internal controls, investors could lose confidence in our financial information and the price of our common stock could decline.
41
Table of Contents
Our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could harm our business, financial condition, and results of operations, and could cause a decline in the price of our common stock.
Provisions of our corporate governance documents could make an acquisition of us more difficult and may prevent attempts by our shareholders to replace or remove our current management, even if beneficial to our shareholders.
In addition to the THL Stockholder’s beneficial ownership of 78.4% of our common stock after this offering (or 76.0%, if the underwriters exercise in full their option to purchase additional shares from us), our certificate of incorporation and bylaws to be effective in connection with the closing of this offering and the Delaware General Corporation Law (the “DGCL”) contain provisions that could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our shareholders. Among other things:
| • | these provisions allow us to authorize the issuance of undesignated preferred stock, the terms of which may be established and the shares of which may be issued without shareholder approval, and which may include supermajority voting, special approval, dividend, or other rights or preferences superior to the rights of shareholders; |
| • | these provisions provide for a classified board of directors with staggered three-year terms; |
| • | these provisions provide that, at any time when the THL Stockholder beneficially owns, in the aggregate, less than 40% in voting power of our stock entitled to vote generally in the election of directors, directors may only be removed for cause, and only by the affirmative vote of holders of at least 662/3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; |
| • | these provisions prohibit shareholder action by written consent from and after the date on which the THL Stockholder beneficially owns, in the aggregate, less than 35% in voting power of our stock entitled to vote generally in the election of directors; |
| • | these provisions provide that for as long as the THL Stockholder beneficially owns, in the aggregate, at least 50% in voting power of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of a majority in voting power of the outstanding shares of our stock and at any time when the THL Stockholder beneficially owns, in the aggregate, less than 50% in voting power of all outstanding shares of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of the holders of at least 662/3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; and |
| • | these provisions establish advance notice requirements for nominations for elections to our Board or for proposing matters that can be acted upon by shareholders at shareholder meetings; provided, however, at any time when the THL Stockholder beneficially owns, in the aggregate, at least 10% in voting power of our stock entitled to vote generally in the election of directors, such advance notice procedure will not apply to it. |
Our certificate of incorporation to be effective in connection with the closing of this offering will contain a provision that provides us with protections similar to Section 203 of the DGCL, and will prevent us from engaging in a business combination with a person (excluding THL and any of its direct or indirect transferees and any group as to which such persons are a party) who acquires at least 15% of our common stock for a period of three years from the date such person acquired such common stock, unless Board or shareholder approval is obtained prior to the acquisition. See “Description of Capital Stock—Anti-Takeover Effects of Our Certificate of
42
Table of Contents
Incorporation and Our Bylaws”. These provisions could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other shareholders to elect directors of your choosing and cause us to take other corporate actions you desire, including actions that you may deem advantageous, or negatively affect the trading price of our common stock. In addition, because our Board is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our shareholders to replace current members of our management team.
These and other provisions in our certificate of incorporation, bylaws and Delaware law could make it more difficult for shareholders or potential acquirers to obtain control of our Board or initiate actions that are opposed by our then-current Board, including delay or impede a merger, tender offer or proxy contest involving our company. The existence of these provisions could negatively affect the price of our common stock and limit opportunities for you to realize value in a corporate transaction.
For information regarding these and other provisions, see “Description of Capital Stock”.
Our certificate of incorporation will designate the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation that may be initiated by our shareholders, which could limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our certificate of incorporation to be effective in connection with the closing of this offering, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of fiduciary duty owed by, or other wrongdoing by, any of our directors, officers or other employees or agents to us or our shareholders, or a claim of aiding and abetting any such breach of fiduciary duty, (3) any action asserting a claim against us or any director, officer, employee or agent of the Company arising pursuant to any provision of the DGCL, our certificate of incorporation or our bylaws, (4) any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws, (5) any action asserting a claim against us or any director, officer, employee or agent governed by the internal affairs doctrine or (6) any action asserting an “internal corporate claim” as that term is defined in Section 115 of the DGCL; provided that for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action”, will not apply to suits to enforce a duty or liability created by Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation will also provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Our certificate of incorporation will further provide that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have notice of and consented to the provisions of our certificate of incorporation described above. See “Description of Capital Stock—Exclusive Forum”. The forum selection clause in our certificate of incorporation may have the effect of discouraging lawsuits against us or our directors and officers and may limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us. Alternatively, if a court were to find the choice of forum provision contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business, financial condition and results of operations.
We are not providing audited historical financial information for Northfield Medical or pro forma financial statements reflecting the impact of the Northfield Acquisition on our historical operating results.
We are required to file a Current Report on Form 8-K after the closing of this offering that includes audited financial statements for Northfield Medical for its most recent fiscal year and, based on that financial statement data, pro forma financial statement information reflecting the estimated pro forma impact of the Northfield
43
Table of Contents
Acquisition. We are not currently in a position to include this information in this prospectus. As a result, investors in this offering will be required to determine whether to participate in this offering without the benefit of this historical and pro forma financial information. See “Summary—Recent Developments—Northfield Acquisition.”
If you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of your investment.
The initial public offering price of our common stock is substantially higher than the pro forma net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our pro forma net tangible book value per share after this offering. You will experience immediate dilution of $21.61 per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and the initial public offering price. In addition, purchasers of common stock in this offering will have contributed 37% of the aggregate price paid by all purchasers of our common stock but will own only approximately 21% of our common stock outstanding after this offering. See “Dilution” for more detail.
Our management will have significant flexibility in using the net proceeds of this offering, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately.
The principal purposes of this offering are to increase our capitalization and financial flexibility, create a public market for our common stock and enable access to the public equity markets for us and our shareholders. We expect to use approximately $450.0 million of the net proceeds of this offering (or $520.0 million of the net proceeds of this offering if the underwriters exercise their option to purchase additional shares in full) to repay outstanding borrowings and related fees and expenses, under our Credit Facilities, with the remaining net proceeds to be used for general corporate purposes. We may also use a portion of the net proceeds to acquire or invest in businesses, products or technologies that we believe will complement our business. However, depending on future developments and circumstances, we may use some of the proceeds for other purposes. We do not have more specific plans for the net proceeds from this offering, other than the repayment of outstanding borrowings under our Credit Facilities as described above. Therefore, our management will have significant flexibility in applying most of the net proceeds we receive from this offering. The net proceeds could be applied in ways that do not improve our operating results. The actual amounts and timing of these expenditures will vary significantly depending on a number of factors, including the amount of cash used in or generated by our operations. See “Use of Proceeds.”
Our operating results and stock price may be volatile, and the market price of our common stock after this offering may drop below the price you pay.
Our quarterly operating results are likely to fluctuate in the future. In addition, securities markets worldwide have experienced, and are likely to continue to experience, significant price and volume fluctuations. This market volatility, as well as general economic, market or political conditions, could subject the market price of our shares to wide price fluctuations regardless of our operating performance. Our operating results and the trading price of our shares may fluctuate in response to various factors, including the factors mentioned throughout this section.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares,
44
Table of Contents
could reduce the market price of our common stock. After this offering, we will have 125,299,085 (or 129,246,453 if the underwriters’ option to purchase additional shares is exercised in full) outstanding shares of common stock based on the number of shares outstanding as of March 1, 2021. This includes the shares that we are selling in this offering, which may be resold in the public market immediately. Following the consummation of this offering, substantially all shares that are not being sold in this offering will be subject to a 180-day lock-up period provided under lock-up agreements executed in connection with this offering described in “Underwriting (Conflicts of Interest)” and restricted from immediate resale under the federal securities laws as described in “Shares Eligible for Future Sale”. All of such shares will, however, be able to be resold after the expiration of the lock-up period, as well as pursuant to customary exceptions thereto or upon the waiver of the lock-up agreement by BofA Securities, Inc. and Goldman Sachs & Co. LLC on behalf of the underwriters. We also intend to register shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to the lock-up agreements. As restrictions on resale end, the market price of our stock could decline if the holders of currently restricted shares sell them or are perceived by the market as intending to sell them.
Because we have no current plans to pay regular cash dividends on our common stock following this offering, you may not receive any return on investment unless you sell your common stock for a price greater than that which you paid for it.
We do not anticipate paying any regular cash dividends on our common stock following this offering. Any decision to declare and pay dividends in the future will be made at the discretion of our Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our Board may deem relevant. In addition, our ability to pay dividends is, and may be, limited by covenants of existing and any future outstanding indebtedness we or our subsidiaries incur, including under the credit agreements governing our credit facilities. Therefore, any return on investment in our common stock is solely dependent upon the appreciation of the price of our common stock on the open market, which may not occur. See “Dividend Policy” for more detail.
If securities or industry analysts do not publish research or reports about our business, if they adversely change their recommendations regarding our shares or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
The trading market for our shares will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have any control over these analysts. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. Moreover, if one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our stock price could decline.
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our common stock, which could depress the price of our common stock.
Our certificate of incorporation will authorize us to issue one or more series of preferred stock. Our Board will have the authority to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our shareholders. Our preferred stock could be issued with voting, liquidation, dividend and other rights superior to the rights of our common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discouraging bids for our common stock at a premium to the market price, and materially adversely affect the market price and the voting and other rights of the holders of our common stock.
45
Table of Contents
This prospectus contains forward-looking statements that are subject to risks and uncertainties. All statements other than statements of historical fact included in this prospectus are forward-looking statements. Forward-looking statements give our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “anticipate”, “estimate”, “expect”, “project”, “plan”, “intend”, “believe”, “may”, “will”, “should”, “can have”, “likely” and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events. For example, all statements we make relating to our estimated and projected costs, expenditures, cash flows, growth rates and financial results or our plans and objectives for future operations, growth initiatives, or strategies are forward-looking statements. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected, including:
| • | effects from political and policy changes that could limit our growth opportunities; |
| • | effects from the continued COVID-19 pandemic on our business and the economy; |
| • | our potential inability to maintain existing contracts or contract terms with, or enter into new contracts with, our customers; |
| • | cancellations by or disputes with customers; |
| • | our potential failure to maintain our reputation, including by protecting intellectual property; |
| • | effects of a global economic downturn on our customers and suppliers; |
| • | a decrease in our customers’ patient census or services; |
| • | competitive practices by our competitors that could cause us to lose market share, reduce our prices or increase our expenditures; |
| • | the bundling of products and services by our competitors, some of which we do not offer; |
| • | consolidation in the healthcare industry, which may lead to a reduction in the prices we charge; |
| • | adverse developments with supplier relationships; |
| • | the potential inability to change the manner in which healthcare providers traditionally procure medical equipment; |
| • | our potential inability to attract and retain key personnel; |
| • | our potential inability to make attractive acquisitions or successfully integrate acquire businesses; |
| • | an increase in expenses related to our pension plan; |
| • | the fluctuation of our cash flow; |
| • | credit risks relating to home care providers and nursing homes; |
| • | potential claims related to the medical equipment that we outsource and service; |
| • | the incurrence of costs that we cannot pass through to our customers; |
| • | a failure of our management information systems; |
| • | limitations inherent in all internal controls systems over financial reporting; |
| • | social unrest; |
| • | our failure to keep up with technological changes; |
46
Table of Contents
| • | our failure to coordinate the management of our equipment; |
| • | challenges to our tax positions or changes in taxation laws; |
| • | litigation that may be costly to defend; |
| • | uncertainty surrounding healthcare reform initiatives; |
| • | federal privacy laws that may subject us to more stringent penalties; |
| • | our relationship with healthcare facilities and marketing practices that are subject to federal Anti-Kickback Statute and similar state laws; |
| • | our contracts with the federal government that subject us to additional oversight; |
| • | the impact of changes in third-party payor reimbursement for healthcare items and services on our customers’ ability to pay for our services; |
| • | the highly regulated environment our customers operate in; |
| • | potential recall or obsolescence of our large fleet of medical equipment; and |
| • | other factors disclosed in the section entitled “Risk Factors” and elsewhere in this prospectus. |
We derive many of our forward-looking statements from our operating budgets and forecasts, which are based on many detailed assumptions. While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and it is impossible for us to anticipate all factors that could affect our actual results. Important factors that could cause actual results to differ materially from our expectations, or cautionary statements, are disclosed under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this prospectus. All written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by these cautionary statements as well as other cautionary statements that are made from time to time in our other SEC filings and public communications. You should evaluate all forward-looking statements made in this prospectus in the context of these risks and uncertainties.
We caution you that the important factors referenced above may not contain all of the factors that are important to you. In addition, we cannot assure you that we will realize the results or developments we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our operations in the way we expect. The forward-looking statements included in this prospectus are made only as of the date hereof. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
47
Table of Contents
We estimate that our net proceeds from this offering will be approximately $460.3 million (or approximately $530.9 million if the underwriters’ option to purchase additional shares is exercised in full), assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting the underwriting discount and estimated offering expenses payable by us.
The principal purposes of this offering are to increase our capitalization and financial flexibility, create a public market for our common stock and enable access to the public equity markets for us and our shareholders. We expect to use approximately $450.0 million of the net proceeds of this offering (or $520.0 million of the net proceeds of this offering if the underwriters exercise their option to purchase additional shares in full) to repay outstanding borrowings and related fees and expenses, under our Credit Facilities. As of December 31, 2020, we had $922.2 million in borrowings outstanding under our First Lien Term Loan Facility and $6.3 million of letters of credit outstanding under our Revolving Credit Facility. As of December 31, 2020, the interest rate on our First Lien Term Loan Facility was 2.9375% on the $772.2 million and 3.5% on the $150.0 million. As of December 31, 2020, we had $240.0 million in borrowings outstanding under the Second Lien Term Loan Facility. As of December 31, 2020, the interest rate on our Second Lien Term Loan Facility was 7.96838%. In addition, on March 19, 2021, in conjunction with the completion of the Northfield acquisition, we funded an incremental $200.0 million term loan under our existing First Lien Term Loan Facility. We intend to use the remaining net proceeds for general corporate purposes. At this time, other than repayment of our indebtedness, we have not specifically identified a large single use for which we intend to use the net proceeds, and, accordingly, we are not able to allocate the net proceeds among any of these potential uses in light of the variety of factors that will impact how such net proceeds are ultimately utilized by us. Pending use of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, investment-grade and interest-bearing instruments. Each $1.00 increase or decrease in the assumed initial public offering price of $19.00 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, would increase or decrease the net proceeds to us from this offering by approximately $24.8 million, assuming the number of shares offered, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discount and estimated offering expenses payable by us.
Certain affiliates of Goldman Sachs & Co. LLC, an underwriter in this offering, will receive 5% or more of the net proceeds of this offering as a result of currently holding 100% of our Second Lien Term Loan Facility and the expected repayment in full of that facility. Therefore, Goldman Sachs & Co. LLC is deemed to have a conflict of interest within the meaning of Rule 5121. Accordingly, this offering is being conducted in accordance with Rule 5121. See “Underwriting (Conflicts of Interest)—Conflicts of Interest.”
Each 1,000,000 increase or decrease in the number of shares offered would increase or decrease the net proceeds to us from this offering by approximately $17.9 million, assuming that the assumed initial public offering price per share for the offering remains at $19.00, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us.
The foregoing represents our current intentions with respect to the use and allocation of the net proceeds of this offering based upon our present plans and business conditions, but our management will have significant flexibility and discretion in applying the net proceeds. The occurrence of unforeseen events or changed business conditions could result in application of the net proceeds of this offering in a manner other than as described in this prospectus.
48
Table of Contents
We currently intend to retain all available funds and any future earnings to fund the development and growth of our business and to repay indebtedness and, therefore, we do not anticipate paying any cash dividends in the foreseeable future. Additionally, our ability to pay dividends on our common stock is limited by restrictions on the ability of our subsidiaries to pay dividends or make distributions to us. Any future determination to pay dividends will be at the discretion of our Board, subject to compliance with covenants in current and future agreements governing our and our subsidiaries’ indebtedness, and will depend on our results of operations, financial condition, capital requirements and other factors that our Board may deem relevant.
49
Table of Contents
The following table describes our cash and cash equivalents and capitalization as of December 31, 2020, as
follows:
| • | on an actual basis; and |
| • | on a pro forma basis to give effect to the Northfield Acquisition as if it was consummated on December 31, 2020; and |
| • | on a pro forma, as adjusted basis, after giving effect to the sale of shares of common stock in this offering and the application of the net proceeds from this offering as set forth under “Use of Proceeds”, assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us and after giving effect to the termination fee associated with the termination of our Advisory Services Agreement and the loss on extinguishment of debt associated with the Use of Proceeds, each as described herein. See “Certain Relationships and Related Party Transactions—Related Party Transactions—Advisory Services Agreement” and “Use of Proceeds.” |
The pro forma information set forth in the table below is illustrative only and will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with our consolidated financial statements and the related notes, “Use of Proceeds” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| As of December 31, 2020 | ||||||||||||
| Actual | Pro forma (4) | Pro forma, as Adjusted |
||||||||||
| (in thousands) | ||||||||||||
| Cash and cash equivalents |
$ | 206,505 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Total debt: |
||||||||||||
| First Lien Term Loan Facility (1) |
906,624 | 1,104,676 | 943,543 | |||||||||
| Second Lien Term Loan Facility (2) |
232,361 | 232,361 | — | |||||||||
| Revolving Credit Facility (3) |
(2,481 | ) | 54,236 | — | ||||||||
| Finance Lease Liability |
24,595 | 26,973 | 26,973 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total debt |
1,161,099 | 1,418,246 | 970,516 | |||||||||
| Equity: |
||||||||||||
| Common stock, $0.0001 par value, 350,000,000 shares authorized, 98,983,296 shares issued and outstanding, actual; 350,000,000 shares authorized, 99,735,624 shares issued and outstanding, pro forma; 350,000,000 shares authorized, 126,051,413 shares issued and outstanding, pro forma, as adjusted |
10 | 10 | 13 | |||||||||
| Additional paid-in capital |
513,902 | 525,202 | 992,449 | |||||||||
| Accumulated deficit |
(68,492 | ) | (78,963 | ) | (98,483 | ) | ||||||
| Accumulated other comprehensive loss |
(3,619 | ) | (3,619 | ) | (3,619 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total shareholders’ equity |
441,801 | 442,630 | 890,360 | |||||||||
|
|
|
|
|
|
|
|||||||
| Noncontrolling interests |
144 | 144 | 144 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 1,603,044 | $ | 1,861,020 | $ | 1,861,020 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | The actual carrying value of the borrowings under the First Lien Term Loan Facility is net of unamortized deferred financing costs of $12.8 million and unamortized debt discount of $2.8 million as of December 31, 2020; on a pro forma and pro forma as adjusted basis, the carrying value of the borrowings under the First Lien Term Loan Facility is net of unamortized deferred financing costs of $12.8 million and unamortized debt discount of $4.7 million as of December 31, 2020. |
50
Table of Contents
| (2) | The actual carrying value of the borrowings under the Second Lien Term Loan Facility is net of unamortized deferred financing costs of $0.8 million and unamortized debt discount of $6.8 million as of December 31, 2020; on a pro forma and pro forma as adjusted basis, there are no unamortized deferred financing or unamortized debt discount as of December 31, 2020. |
| (3) | The actual and pro forma carrying value of the borrowings under the Revolving Credit Facility is net of unamortized deferred financing costs of $2.5 million as of December 31, 2020; on a pro forma, as adjusted basis, the unamortized deferred financing costs is $0.0 as of December 31, 2020. |
| (4) | The pro forma calculations set forth herein assume that the $475.0 million purchase price for the Northfield Acquisition is funded through the following sources: $206.5 million of cash on hand, $200.0 million first lien incremental term loan facility under our existing First Lien Term Loan Facility, $2.4 million of capital leases assumed by us as part of the acquisition, $11.3 million in issuances of our common stock and $56.7 million from our existing Revolving Credit Facility. Further, the common stock outstanding reflects the issuance of 752,328 shares of common stock in conjunction with the Northfield Acquisition and includes approximately $10.5 million in transaction fees, of which approximately $6.4 million relates to the incremental term loan with the remainder associated with legal, accounting, tax and other advisor services. |
A $1.00 increase or decrease in the assumed initial public offering price of $19.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase or decrease each of cash and cash equivalents, additional paid-in capital, total shareholders’ equity and total capitalization on a pro forma basis by approximately $24.8 million, assuming the number of shares offered, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discount and estimated offering expenses payable by us.
Similarly, each 1,000,000 increase or decrease in the number of shares of common stock offered in this offering would increase or decrease each of cash and cash equivalents, additional paid-in capital, total shareholders’ equity (deficit) and total capitalization on a pro forma basis by approximately $17.9 million, based on an assumed initial public offering price of $19.00 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us.
Except as otherwise indicated the number of shares of common stock to be outstanding following this offering is based on 98,983,296 shares of common stock outstanding as of March 1, 2021, and excludes:
| • | 6,690,308 shares of common stock issuable upon the exercise of options outstanding as of March 1, 2021, with a weighted average exercise price of $4.86 per share; |
| • | 2,537,619 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units, as of March 1, 2021 (assuming vesting at 150% for performance restricted stock units vesting as of March 6, 2021 and assuming 100% vesting for the performance restricted stock units thereafter); |
| • | 752,328 shares of common stock issued in connection with the Northfield Acquisition; |
| • | 190,226 shares of common stock issuable upon the exercise of warrants outstanding as of March 1, 2021, with a weighted average exercise price of $9.27 per share; |
| • | 14,474 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to members of the Northfield management team at an assumed exercise price of $19.00 (which is the midpoint of the estimated price range set forth on the cover page of this prospectus); |
| • | 56,795 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in conjunction with this offering to members of Northfield management team (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); |
51
Table of Contents
| • | 497,868 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to employees of Agiliti at an assumed exercise price of $19.00, which is the midpoint of the estimated price range set forth on the cover page of this prospectus; |
| • | 643,416 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in connection with this offering (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); and |
| • | 8,961,227 shares of common stock reserved for future issuance under the 2018 Omnibus Incentive Plan (after giving effect to the anticipated issuance of 1,212,553 shares expected to be issued in conjunction with this transaction). |
52
Table of Contents
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock in this offering and the pro forma net tangible book value per share of our common stock immediately after this offering.
As of December 31, 2020, we had a net tangible book value of $(777.3) million, or $(7.85) per share of common stock. Net tangible book value per share is equal to our total tangible assets, less total liabilities, divided by the number of outstanding shares of our common stock.
After giving effect to the sale of shares of common stock in this offering, after deducting the underwriting discount and estimated offering expenses payable by us, and the application of the net proceeds of this offering to as set forth under “Use of Proceeds”, at an assumed initial public offering price of $19.00 per share, which is the midpoint of the price range set forth on the cover of this prospectus, our pro forma net tangible book value as of December 31, 2020 would have been $(327.3) million, or $(2.61) per share of common stock. This represents an immediate increase in net tangible book value of $5.24 per share to our existing shareholders and an immediate dilution in net tangible book value of $21.61 per share to investors participating in this offering at the assumed initial public offering price. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | 19.00 | ||||||
| Historical net tangible book value per share as of December 31, 2020 |
(7.85 | ) | ||||||
| Increase in net tangible book value per share attributable to the investors in this offering |
5.24 | |||||||
|
|
|
|||||||
| Pro forma net tangible book value per share after giving effect to this offering |
(2.61 | ) | ||||||
|
|
|
|||||||
| Dilution in net tangible book value per share to the investors in this offering |
$ | 21.61 | ||||||
|
|
|
|
|
A $1.00 increase or decrease in the assumed initial public offering price of $19.00 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, would increase or decrease our pro forma net tangible book value per share after this offering by $0.20, and would increase or decrease the dilution per share to the investors in this offering by $0.20, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discount and estimated offering expenses payable by us. Similarly, each increase or decrease of one million shares in the number of shares of common stock offered by us would increase or decrease our pro forma net tangible book value per share after this offering by $0.16 and would increase or decrease dilution per share to investors in this offering by $0.84, assuming the assumed initial public offering price of $19.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and estimated offering expenses payable by us.
If the underwriters exercise their option to purchase additional shares in full, the pro forma net tangible book value per share after this offering would be $(1.99), and the dilution in pro forma net tangible book value per share to new investors in this offering would be $20.99.
The following table presents, on a pro forma basis as described above, as of December 31, 2020, the differences between our existing shareholders and the investors purchasing shares of our common stock in this offering, with respect to the number of shares purchased, the total consideration paid to us, and the average price per share paid by our existing shareholders or to be paid to us by investors purchasing shares in this offering at an
53
Table of Contents
assumed offering price of $19.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting the underwriting discount and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||||||
| Existing Shareholders |
98,983,296 | 79 | % | $ | 841,041,013 | 63 | % | $ | 8.50 | |||||||||||
| New Investors |
26,315,789 | 21 | % | 500,000,000 | 37 | % | $ | 19.00 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total |
125,299,085 | $ | 1,341,041,013 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
A $1.00 increase or decrease in the assumed initial public offering price of $19.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase or decrease the total consideration paid by new investors by $26.3 million and increase or decrease the percent of total consideration paid by new investors by 1%, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and before deducting the underwriting discount and estimated offering expenses payable by us.
Similarly, each 1,000,000 increase or decrease in the number of shares offered would increase or decrease the net proceeds to us from this offering by approximately $17.9 million, assuming that the assumed initial public offering price per share for the offering remains at $19.00, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us.
Except as otherwise indicated, the above discussion and tables assume no exercise of the underwriters’ option to purchase additional shares. After giving effect to sales of shares in this offering, assuming the underwriters’ option to purchase additional shares is exercised in full (and excluding any shares of common stock that may be purchased in this offering or pursuant to our directed share program), our existing shareholders would own 77% and our new investors would own 23% of the total number of shares of our common stock outstanding after this offering.
In addition, to the extent we issue any additional stock options or any stock options are exercised, or we issue any other securities or convertible debt in the future, investors participating in this offering may experience further dilution.
Except as otherwise indicated, the above discussion and tables are based on 98,983,296 shares of our common stock outstanding as of December 31, 2020 and excludes:
| • | 6,690,308 shares of common stock issuable upon the exercise of options outstanding as of March 1, 2021, with a weighted average exercise price of $4.86 per share; |
| • | 2,537,619 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units, as of March 1, 2021 (assuming vesting at 150% for performance restricted stock units vesting as of March 6, 2021 and assuming 100% vesting for the performance restricted stock units thereafter); |
| • | 752,328 shares of common stock issued in connection with the Northfield Acquisition; |
| • | 190,226 shares of common stock issuable upon the exercise of warrants outstanding as of March 1, 2021, with a weighted average exercise price of $9.27 per share; |
| • | 14,474 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to members of the Northfield management team at an assumed exercise price of $19.00 (which is the midpoint of the estimated price range set forth on the cover page of this prospectus); |
54
Table of Contents
| • | 56,795 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in conjunction with this offering to members of Northfield management team (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); |
| • | 497,868 shares of common stock issuable upon the exercise of options expected to be issued in conjunction with this offering to employees of Agiliti at an assumed exercise price of $19.00, which is the midpoint of the estimated price range set forth on the cover page of this prospectus; |
| • | 643,416 shares of common stock issuable upon vesting and settlement of restricted stock units, or performance restricted stock units expected to be issued in connection with this offering (assuming performance-based units vest at 100%, and assuming an initial public offering price of $19.00 per share, which is the midpoint of the estimated range set forth on the cover of this prospectus); and |
| • | 8,961,227 shares of common stock reserved for future issuance under the 2018 Omnibus Incentive Plan (after giving effect to the anticipated issuance of 1,212,553 shares expected to be issued in conjunction with this transaction). |
55
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following tables present the selected consolidated financial data for Agiliti and its subsidiaries and the consolidated financial data for Agiliti Health, Inc. Agiliti is the successor of Agiliti Health, Inc. for financial reporting purposes. We derived the summary consolidated statements of operations data and balance sheet data for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 (Successor) from the audited consolidated financial statements of Agiliti and related notes thereto included elsewhere in this prospectus. We derived the summary consolidated statements of operations data and balance sheet data for the period from January 1 through January 3, 2019 (Predecessor) and for the years ended 2018, 2017 and 2016, from the audited consolidated financial statements of Agiliti Health, Inc. and related notes thereto included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results to be expected in any future period. The information set forth below should be read together with the “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
| Successor | Predecessor | |||||||||||||||||||||||||||||||
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Years Ended December 31, | ||||||||||||||||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||||||||||||||||||||
| Consolidated Statements of Operation Data: |
||||||||||||||||||||||||||||||||
| Revenue |
$ | 773,312 | $ | 613,073 | $ | — | $ | 565,246 | $ | 514,783 | $ | 479,501 | ||||||||||||||||||||
| Cost of revenue |
486,965 | 423,812 | — | 367,837 | 343,028 | 322,649 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Gross margin |
286,347 | 189,261 | — | 197,409 | 171,755 | 156,852 | ||||||||||||||||||||||||||
| Selling, general and administrative (1) |
250,289 | 187,156 | 17,147 | 137,210 | 125,910 | 119,389 | ||||||||||||||||||||||||||
| Gain on settlement |
— | — | — | (26,391 | ) | — | (3,074 | ) | ||||||||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | 131,100 | — | — | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Operating income (loss) |
36,058 | 2,105 | (17,147 | ) | (44,510 | ) | 45,845 | 40,537 | ||||||||||||||||||||||||
| Interest expense (1) |
61,530 | 48,199 | — | 53,390 | 53,762 | 53,043 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Loss before income taxes and noncontrolling interest |
(25,472 | ) | (46,094 | ) | (17,147 | ) | (97,900 | ) | (7,917 | ) | (12,506 | ) | ||||||||||||||||||||
| Income tax (benefit) expense |
(3,234 | ) | (14,857 | ) | (13,281 | ) | (66,348 | ) | (17,159 | ) | 946 | |||||||||||||||||||||
| Net income attributable to noncontrolling interest |
240 | 171 | — | 327 | 414 | 308 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Net (loss) income attributable to Agiliti, Inc. and Subsidiaries |
$ | (22,478 | ) | $ | (31,408 | ) | $ | (3,866 | ) | $ | (31,879 | ) | $ | 8,828 | $ | (13,760 | ) | |||||||||||||||
| Pro Forma Per Share Data (2): |
||||||||||||||||||||||||||||||||
| Pro forma (loss) net income per share: |
||||||||||||||||||||||||||||||||
| Basic |
$ | (0.18 | ) | $ | (0.25 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Diluted |
$ | (0.17 | ) | $ | (0.24 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Pro forma weighted-average shares used in computing (loss) net income per share: |
||||||||||||||||||||||||||||||||
| Basic |
125,299,085 | 125,299,085 | ||||||||||||||||||||||||||||||
| Diluted |
132,821,738 | 132,821,738 | ||||||||||||||||||||||||||||||
56
Table of Contents
| Successor | Predecessor | |||||||||||||||||||||||||||
| December 31, 2020 |
December 31, 2019 |
December 31, (1) | ||||||||||||||||||||||||||
| (in thousands) | 2018 | 2017 | 2016 | |||||||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 206,505 | — | $ | 7,340 | $ | — | $ | — | |||||||||||||||||||
| Working capital (3) |
$ | 82,102 | $ | 39,360 | $ | 15,756 | $ | 6,245 | $ | 1,650 | ||||||||||||||||||
| Total assets |
$ | 1,903,356 | $ | 1,619,416 | $ | 744,958 | $ | 805,445 | $ | 818,123 | ||||||||||||||||||
| Total debt |
$ | 1,161,099 | $ | 922,998 | $ | 690,999 | $ | 703,108 | $ | 707,317 | ||||||||||||||||||
| Equity (deficit) |
$ | 441,945 | $ | 456,969 | $ | (67,659 | ) | $ | (44,378 | ) | $ | (59,485 | ) | |||||||||||||||
| (1) | We adopted ASU No. 2017-07 Improving the Presentation of Net Periodic Pension Cost and Net Periodic Postretirement Benefit Cost in 2018 and retrospectively applying the ASU 2017-07 to all periods presented. |
| (2) | Unaudited pro forma per share information gives effect to our sale of 26,315,789 shares of common stock in this offering at an assumed initial public offering price of $19.00 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discount and estimated offering expenses payable by us and the application of the net proceeds of this offering to repay approximately $450.0 million in principal amount of outstanding borrowings under our Credit Facilities as set forth under “Use of Proceeds.” |
| (3) | Represents total current assets (excluding cash and cash equivalents) less total current liabilities (excluding current portion of long-term debt, current portion of operating lease liability and current portion of obligation under tax receivable agreement). |
57
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The following discussion and analysis summarizes the significant factors affecting the consolidated operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with our consolidated financial statements and the related notes thereto included elsewhere in this prospectus. The discussion contains forward-looking statements that are based on the beliefs of management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this prospectus, particularly in the sections entitled “Risk Factors” and “Forward-Looking Statements”. For purposes of this section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, “we”, “us”, “our” and the “Company” refer to Agiliti Health.
Overview
We believe we are one of the leading experts in the management, maintenance and mobilization of mission-critical, regulated, reusable medical devices. We offer healthcare providers a comprehensive suite of medical equipment management and service solutions that help reduce capital and operating expenses, optimize medical equipment utilization, reduce waste, enhance staff productivity and bolster patient safety.
We commenced operations in 1939, originally incorporated in Minnesota in 1954 and reincorporated in Delaware in 2001. Since the Business Combination in January 2019, we have been controlled by the THL Stockholder.
In our more than 80 years of experience ensuring healthcare providers have high-quality, expertly maintained equipment to serve their patients, we’ve built an at-scale, strong nationwide operating footprint. This service and logistics infrastructure positions us to reach customers across the entire healthcare continuum—from individual facilities to the largest and most complex healthcare systems. Likewise, our ability to rapidly mobilize, track, repair and redeploy equipment during times of peak need or emergent events has made us a service provider of choice for city, state and federal governments to manage emergency equipment stockpiles.
Impact of COVID-19 on our Business
Health and Safety
From the earliest signs of the outbreak, we have taken proactive action to protect the health and safety of our employees, customers, partners and suppliers. We enacted safety measures in all of our sites, including implementing social distancing protocols, requiring working from home for those employees that do not need to be physically present to perform their work, suspending travel, implementing temperature checks at the entrances to our facilities, extensively and frequently disinfecting our workspaces and providing masks to those employees who must be physically present. We expect to continue to implement these measures until we determine that the COVID-19 pandemic is adequately contained for purposes of our business, and we may take further actions as government authorities require or recommend or as we determine to be in the best interests of our employees, customers, partners and suppliers.
Operations
As a result of the COVID-19 pandemic, governmental authorities have implemented and are continuing to implement numerous and constantly evolving measures to try to contain the virus, such as travel bans and restrictions, limits on gatherings, quarantines, shelter-in-place orders and business shutdowns. Measures providing for business shutdowns generally exclude certain essential services, and those essential services
58
Table of Contents
commonly include critical infrastructure and the businesses that support that critical infrastructure. While all of our facilities currently remain operational, these measures have impacted and may further impact our workforce and operations, as well as those of our customers, vendors and suppliers. In connection with the COVID-19 pandemic, we have experienced limited absenteeism from those employees who are required to be on-site to perform their jobs, and we do not currently expect that our operations will be adversely affected by significant absenteeism.
Liquidity
Although there is uncertainty related to the anticipated impact of the recent COVID-19 outbreak on our future results, we believe our business model, our current cash reserves and available borrowings under our Revolving Credit Facility leave us well-positioned to manage our business through this crisis. We believe our existing balances of cash and cash equivalents and our currently anticipated operating cash flows will be sufficient to meet our cash needs arising in the ordinary course of business for the next twelve months.
We continue to monitor the rapidly evolving situation and guidance from federal, state and local public health authorities and may take additional actions based on their recommendations. In these circumstances, there may be developments outside our control requiring us to adjust our operating plan. As such, given the dynamic nature of this situation, we cannot reasonably estimate the impacts of COVID-19 on our financial condition, results of operations or cash flows in the future.
Principles of Consolidation
The consolidated financial statements included elsewhere in this prospectus include the accounts of Agiliti Health and Agiliti Surgical, Inc. (formerly known as UHS Surgical Services, Inc.) and Agiliti Imaging, Inc. (formerly known as Radiographic Equipment Services, Inc.), its 100%-owned subsidiaries. In addition, in accordance with guidance issued by the Financial Accounting Standards Board, we have accounted for our equity investments in entities in which we are the primary beneficiary under the full consolidation method. All intercompany transactions and balances have been eliminated through consolidation. As the primary beneficiary, we consolidate the limited liability companies referred to in Note 13, Principles of Consolidation to our audited consolidated financial statements for the year ended December 31, 2020 included elsewhere in this prospectus, as we effectively receive the majority of the benefits from such entities and we provide equipment lease guarantees for such entities.
We report our financial information under one reporting segment, which is equivalent to our one operating segment and conforms to the way we currently manage and execute the strategy and operations of our business. Specifically, the chief operating decision maker (“CODM”) makes operating decisions and assesses performance using discrete financial information from one reportable segment, as our resources and infrastructure are shared, and as our go-to-market strategy has evolved with the introduction and validation of our framework for the end-to-end management of FDA-regulated, reusable medical devices. Further, we maintain a similar complement of services, type of customer for our services and method of distribution for our services and operate within a similar regulatory environment in the United States.
Critical Accounting Policies and Estimates
The preparation of consolidated financial statements in conformity with GAAP requires us to make decisions that impact the reported amounts of assets, liabilities, revenue and expenses and the related disclosures. Such decisions include the selection of the appropriate accounting principles to be applied and the assumptions on which to base accounting estimates. In reaching such decisions, we apply judgments based on our understanding and analysis of relevant circumstances, historical experience and actuarial valuations. Actual amounts could differ from those estimated at the time the consolidated financial statements are prepared.
59
Table of Contents
Some of our critical accounting policies require us to make difficult, subjective or complex judgments or estimates. An accounting estimate is considered to be critical if it meets both of the following criteria: (i) the estimate requires assumptions about matters that are highly uncertain at the time the accounting estimate is made, and (ii) different estimates reasonably could have been used, or changes in the estimate that are reasonably likely to occur from period to period may have a material impact on the presentation of our financial condition, changes in financial condition or results of operations. Our most critical accounting policies and estimates include the following:
| • | revenue recognition; |
| • | fair value measurements in business combinations; |
| • | valuation of long-lived assets, including goodwill and definite-lived intangible assets; and |
| • | valuation of obligations under the tax receivable agreement. |
Revenue Recognition
We recognize revenue when we satisfy performance obligations by transferring promised goods or services to customers in an amount that reflects the consideration we expect to receive in exchange for those goods or services.
Customer arrangements typically have multiple performance obligations to provide equipment solutions, clinical engineering and/or on-site equipment managed services on a per use and/or over time basis. Consideration paid by the customer for each performance obligation is billed within the month the service is performed, and contractual prices are established within our customer arrangements that are representative of the stand-alone selling price. Taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction, that we collect from a customer, are excluded from revenue. Our performance obligations that are satisfied at a point in time are recognized when the service is performed or equipment is delivered to the customer. For certain performance obligations satisfied over time, we use a straight-line method to recognize revenue ratably over the contract period, as this coincides with our stand-ready performance obligation under the contract.
Business Combinations
We account for the acquisition of a business in accordance with the accounting standards codification guidance for business combinations, whereby the total consideration transferred is allocated to the assets acquired and liabilities assumed, including amounts attributable to non-controlling interests, when applicable, based on their respective estimated fair values as of the date of acquisition. Goodwill represents the excess of consideration transferred over the estimated fair value of the net assets acquired in a business combination.
Assigning estimated fair values to the assets acquired and liabilities assumed requires the use of significant estimates, judgments, inputs and assumptions regarding the fair value of the assets and liabilities. Such significant estimates, judgments, inputs and assumptions may include, but would not be limited to, selection of an appropriate valuation model, applying an appropriate discount rate, assumptions related to projected financial information and estimates of customer attrition.
Recoverability and Valuation of Long-Lived Assets Including Goodwill and Indefinite Lived Intangible Assets
For long-lived assets and definite lived intangible assets, impairment is evaluated when a triggering event is indicated. If there is an indication of impairment, an evaluation of undiscounted cash flow versus carrying value is conducted. If necessary, an impairment is measured based on the estimated fair value of the long-lived or amortizable asset in comparison to its carrying value. This evaluation is conducted at the lowest level of
60
Table of Contents
identifiable cash flows. Our amortizable intangible assets consist of customer relationships and non-compete agreements. For property and equipment, primarily movable medical equipment, we continuously monitor individual makes and models for potential triggering events such as product recalls or technological obsolescence.
For goodwill we review for impairment annually at the reporting unit level and upon the occurrence of certain events that might indicate the asset may be impaired. A qualitative review is conducted to determine whether it is more likely than not that the fair value is less than its carrying amount. If it is determined that it is more likely than not that the carrying value is greater than the fair value of the asset, a quantitative impairment test is performed. We then review goodwill for impairment by comparing the fair value of a reporting unit with its carrying amount and recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value. We operate under one reporting unit and do not aggregate any components into our one reporting unit. There are no known trends or uncertainties that we reasonably expect will have an unfavorable impact on revenue or income from operations. We have not performed a quantitative impairment test since January 4, 2019, the date of the Business Combination, in which the balance sheet was fair valued.
Tax Receivable Agreement Obligation Valuation
We entered into the Tax Receivable Agreement (the “Tax Receivable Agreement”) on January 4, 2019 with our former owners to pay 85% of certain U.S. federal, state and local tax benefits realized or deemed realized by Agiliti from certain tax attributes including federal, state and local net operating losses in existence at the closing date of the Business Combination, certain deductions generated by the consummation of the Business Combination, certain deductions arising from the rollover options and other items.
The fair value of the liability is estimated using a Monte Carlo simulation model, peer company cost of capital, discount rates and projected financial information. Most of the information utilized in determining the obligation is not observable in the market and thus the measurement of the liability represents a Level 3 fair value measurement. The obligation can increase or decrease significantly based on the data inputs of our estimates and the current market conditions.
Results of Operations
The following discussion addresses:
| • | our financial condition as of December 31, 2020, December 31, 2019 and December 31, 2018; |
| • | the results of operations for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and the year ended December 31, 2018. The period from January 1 through January 3, 2019 represented an Irving Place Capital fee, non-cash share-based compensation expense and the related tax impact on the predecessor. |
61
Table of Contents
The following table provides information on the percentages of certain items of selected financial data compared to total revenue for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and the year ended December 31, 2018.
| Percent of Total Revenue | ||||||||||||||||
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Year Ended December 31, 2018 |
||||||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||||||
| Revenue |
100.0 | % | 100.0 | % | 100.0 | % | ||||||||||
| Cost of revenue |
63.0 | 69.1 | 65.1 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross margin |
37.0 | 30.9 | 34.9 | |||||||||||||
| Selling, general and administrative |
32.4 | 30.5 | 24.3 | |||||||||||||
| Gain on legal settlement |
— | — | (4.7 | ) | ||||||||||||
| Intangible asset impairment charge |
— | — | 23.2 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
4.6 | 0.4 | (7.9 | ) | ||||||||||||
| Interest expense |
8.0 | 7.9 | 9.4 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss before income taxes and noncontrolling interest |
(3.4 | ) | (7.5 | ) | (17.3 | ) | ||||||||||
| Income tax benefit |
(0.4 | ) | (2.4 | ) | (11.7 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Consolidated net loss |
(3.0 | )% | (5.1 | )% | (5.6 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
Results of Operations for the year ended December 31, 2020 compared to the period from January 4 through December 31, 2019
Total Revenue
The following table presents revenue by service solution for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 (in thousands).
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
% Change | ||||||||||
| (Successor) | (Successor) | |||||||||||
| Equipment Solutions |
$ | 296,267 | $ | 253,597 | 16.8 | % | ||||||
| Clinical Engineering |
256,874 | 188,752 | 36.1 | |||||||||
| Onsite Managed Services |
220,171 | 170,724 | 29.0 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Revenue |
$ | 773,312 | $ | 613,073 | 26.1 | % | ||||||
|
|
|
|
|
|
|
|||||||
Total revenue for the year ended December 31, 2020 was $773.3 million compared to $613.1 million for the period from January 4 through December 31, 2019, an increase of $160.2 million or 26.1%. Equipment Solutions revenue increased 16.8% primarily driven by increased demand for equipment needed to fight the COVID-19 pandemic. This was partially offset by declines in our surgical solutions due to the reduction in non-emergency elective procedures as a result of COVID-19. Although difficult to determine, we have estimated the positive impact from COVID-19 within Equipment Solutions to be in the $30 million to $40 million range. Clinical Engineering revenue increased 36.1% due to our acquisition on January 31, 2020 of certain assets of a surgical equipment repair and maintenance service provider, as well as continued strong growth as a result of our success in signing and implementing new business contracts over the last several quarters. Finally, our Onsite Managed Services revenue increased 29.0% with the majority of growth coming from a new contract signed in the third
62
Table of Contents
quarter of 2020 for the comprehensive maintenance and management services of medical ventilator equipment and from our managed only solution where we manage equipment owned by the customer.
Cost of Revenue
Total cost of revenue for the year ended December 31, 2020 was $487.0 million compared to $423.8 million for the period from January 4 through December 31, 2019, an increase of $63.2 million or 14.9%. On a percentage of revenue basis, cost of revenue decreased from 69.1% of revenue in 2019 to 63.0% in 2020. The decrease as a percentage of revenue was driven primarily from revenue growth as we were able to leverage our fixed cost infrastructure resulting in our expenses growing at a lower rate than revenue growth.
Gross Margin
Total gross margin for the year ended December 31, 2020 was $286.3 million, or 37.0% of total revenue, compared to $189.3 million, or 30.9% of total revenue for the period from January 4 through December 31, 2019, an increase of $97.0 million or 51.2%. The increase in gross margin as a percentage of revenue was primarily impacted by positive leverage from volume growth.
Selling, General and Administrative and Interest Expense
| (in thousands) |
Year
Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Change | % Change | ||||||||||||
| (Successor) | (Successor) | |||||||||||||||
| Selling, general and administrative |
$ | 250,289 | $ | 187,156 | $ | 63,133 | 33.7 | % | ||||||||
| Interest expense |
61,530 | 48,199 | 13,331 | 27.7 | ||||||||||||
Selling, General and Administrative
Selling, general, and administrative expenses for the year ended December 31, 2020 increased by $63.1 million, or 33.7%, to $250.3 million as compared to the period from January 4 through December 31, 2019. The increase of $63.1 million was primarily due to an increase in the remeasurement of the tax receivable agreement of $22.3 million, incentive expense related to our strong financial performance and an increase in payroll related costs associated with the growth of our business. Selling, general and administrative expense as a percentage of total revenue was 32.4% and 30.5% for the year ended December 31, 2020 and the period from January 4 through December 31, 2019, respectively.
Interest Expense
Interest expense for the year ended December 31, 2020 increased by $13.3 million, or 27.7%, to $61.5 million as compared to the period from January 4 through December 31, 2019 primarily due to our Second Lien Term Loan entered into in November 2019.
Income Taxes
Income taxes were a benefit of $3.2 million and $14.9 million for the year ended December 31, 2020 and the period from January 4 through December 31, 2019, respectively. The tax benefit for the year ended December 31, 2020 was primarily related to operating losses and amended state income tax filings. The tax benefit for the period from January 4 through December 31, 2019 was primarily related to operating losses.
Consolidated Net Loss
Consolidated net loss was $22.2 million and $31.2 million for the year ended December 31, 2020 and the period from January 4 through December 31, 2019, respectively. The decrease in net loss was impacted primarily by the increase in revenue.
63
Table of Contents
Results of Operations for the period from January 4 through December 31, 2019 compared to the Year Ended December 31, 2018
Total Revenue
The following table presents revenue by service solution from January 4 through December 31, 2019 compared to the Year Ended December 31, 2018.
| Successor | Predecessor | |||||||||||||||||||
| (in thousands) | From January 4 Through December 31, 2019 |
Year Ended December 31, 2018 |
% Change | |||||||||||||||||
| Equipment Solutions |
$ | 253,597 | $ | 248,059 | 2.2 | % | ||||||||||||||
| Clinical Engineering |
188,752 | 161,492 | 16.9 | % | ||||||||||||||||
| On-Site Managed Services |
170,724 | 155,695 | 9.7 | % | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Revenue |
$ | 613,073 | $ | 565,246 | 8.5 | % | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
Total revenue for the period from January 4 through December 31, 2019 was $613.1 million, compared with $565.2 million for the year ended December 31, 2018, an increase of $47.9 million or 8.5%. Equipment Solutions revenue increased 2.2% primarily driven by new customer contracts signed and implemented in 2019. These new contract signings include our traditional supplemental rental services as well as our surgical solutions. Clinical Engineering revenue increased 16.9% as we continue to see strong growth within this solution as a direct result of our continued success in signing and implementing new business contracts over the last several quarters. Finally, our On-Site Managed Services revenue increased 9.7% with the majority of growth coming from our managed only solution where we manage equipment owned by the customer.
Cost of Revenue
Total cost of revenue for the period from January 4 through December 31, 2019 was $423.8 million compared to $367.8 million for the year ended December 31, 2018, an increase of $56.0 million or 15.2%. On a percentage of revenue basis, cost of revenue increased from 65.1% of revenue in 2018 to 69.1% in 2019. The increase as a percentage of revenue was driven primarily from the increase in depreciation expense due to the asset revaluation related to the Business Combination.
Gross Margin
Total gross margin for the period from January 4 through December 31, 2019 was $189.3 million, or 30.9% of total revenues compared to $197.4 million, or 34.9% of total revenues for the year ended December 31, 2018, a decrease of $8.1 million or 4.1%. The decrease in gross margin as a percent of revenue was primarily impacted by increases in depreciation expense due to the asset revaluation related to the Business Combination.
Selling, General and Administrative, Gain on Settlement, Intangible Asset Impairment Charge and Interest Expense
| Successor | Predecessor | |||||||||||||||||||||||
| (in thousands) | From January 4 Through December 31, 2019 |
Year Ended December 31, 2018 |
Change | % Change | ||||||||||||||||||||
| Selling, general and administrative |
$ | 187,156 | $ | 137,210 | $ | 49,946 | 36.4 | % | ||||||||||||||||
| Gain on settlement |
— | (26,391 | ) | 26,391 | * | |||||||||||||||||||
| Intangible asset impairment charge |
— | 131,100 | (131,100 | ) | * | |||||||||||||||||||
| Interest Expense |
48,199 | 53,390 | (5,191 | ) | (9.7 | ) | ||||||||||||||||||
| * | Not meaningful. |
64
Table of Contents
Selling, general, and administrative expenses for the period from January 4 through December 31, 2019 increased by $49.9 million, or 36.4%, to $187.2 million as compared to the year ended December 31, 2018. The increase was due to increased amortization expense of $55.5 million primarily related to the revaluation of intangible assets as part of the Business Combination. Selling, general and administrative expense as a percentage of total revenue was 30.5% and 24.3% for the period from January 4 through December 31, 2019 and the year ended December 31, 2018, respectively.
Gain on Settlement
Gain on settlement of $26.4 million in 2018 was related to a confidential litigation settlement agreement, which resolved all disputes and legal claims of the parties associated with a suit filed by us against Hill-Rom Holdings, Inc., Hill-Rom Company, Inc. and Hill-Rom Services, Inc. The amount of the gain recorded was based on the fair value of the assets received of $23.4 million and $3.0 million of cash. The fair value of the assets was measured using inputs of replacement cost and market data, which are considered a Level 3 fair value measurement.
Intangible Asset Impairment Charge
Intangible asset impairment charge of $131.1 million in 2018 was related to the UHS trade name write-off due to the change of our legal name to Agiliti Health, Inc. and the brand identity to Agiliti.
Interest Expense
Interest expense for the period from January 4 through December 31, 2019 decreased by $5.2 million, or 9.7%, to $48.2 million as compared to the year ended December 31, 2018 due to new financing in 2019 resulting in lower interest rates.
Income Taxes
Income taxes were a benefit of $14.9 million and $66.3 million for the period from January 4 through December 31, 2019 and the year ended December 31, 2018, respectively. The tax benefit for the period from January 4 through December 31, 2019 was primarily related to operating losses. The tax benefit for the year ended 2018 was primarily related to the trade name impairment and the reversal of our valuation allowance.
Consolidated Net Loss
Consolidated net loss was $31.2 million and $31.6 million for the period from January 4 through December 31, 2019 and the year ended December 31, 2018, respectively.
Adjusted EBITDA
Adjusted Earnings Before Interest, Taxes, Depreciation and Amortization (“Adjusted EBITDA”) was $234.2 million, $173.1 million, and $152.7 million for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and the year ended December 31, 2018, respectively. Adjusted EBITDA for the year ended December 31, 2020 was higher than the period from January 4 through December 31, 2019 primarily due to the increase in revenue. Adjusted EBITDA for the period from January 4 through December 31, 2019 was higher than the year ended December 31, 2018 primarily due to the increase in revenue.
EBITDA is defined as earnings attributable to Agiliti before interest expense, income taxes, depreciation and amortization. Adjusted EBITDA is defined as EBITDA excluding, non-cash share-based compensation expense, Management Fees and non-recurring gains, expenses or losses. In addition to using EBITDA and Adjusted EBITDA internally as measures of operational performance, we disclose them externally to assist
65
Table of Contents
analysts, investors and lenders in their comparisons of operational performance, valuation and debt capacity across companies with differing capital, tax and legal structures. We believe the investment community frequently uses EBITDA and Adjusted EBITDA in the evaluation of similarly situated companies. Adjusted EBITDA is also used by us as a factor to determine the total amount of incentive compensation to be awarded to executive officers and other employees. EBITDA and Adjusted EBITDA, however, are not measures of financial performance under accounting principles generally accepted in the United States of America (“GAAP”) and should not be considered as alternatives to, or more meaningful than, net income as measures of operating performance or to cash flows from operating, investing or financing activities or as measures of liquidity. Since
EBITDA and Adjusted EBITDA are not measures determined in accordance with GAAP and are thus susceptible to varying interpretations and calculations, EBITDA and Adjusted EBITDA, as presented, may not be comparable to other similarly titled measures of other companies. EBITDA and Adjusted EBITDA do not represent amounts of funds that are available for management’s discretionary use. EBITDA and Adjusted EBITDA presented below may not be the same as EBITDA and Adjusted EBITDA calculations as defined in the Credit Facilities. A reconciliation of net loss attributable to Agiliti to Adjusted EBITDA is included below:
| Successor | Predecessor | |||||||||||||||||||||||
| (in thousands) | Year Ended December 31, 2020 |
From January 4 Through December 31, 2019 |
From January 1 Through January 3, 2019 |
Year Ended December 31, 2018 |
||||||||||||||||||||
| Net loss income attributable to Agiliti, Inc. and Subsidiaries |
$ | (22,478 | ) | $ | (31,408 | ) | $ | (3,866 | ) | $ | (31,879 | ) | ||||||||||||
| Interest expense |
61,530 | 48,199 | — | 53,390 | ||||||||||||||||||||
| Income tax benefit |
(3,234 | ) | (14,857 | ) | (13,281 | ) | (66,348 | ) | ||||||||||||||||
| Depreciation and amortization of intangibles and contract costs |
169,241 | 161,703 | — | 79,199 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| EBITDA |
205,059 | 163,637 | (17,147 | ) | 34,362 | |||||||||||||||||||
| Gain on Settlement (1) |
— | — | — | (26,391 | ) | |||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | 131,100 | ||||||||||||||||||||
| Non-cash share-based compensation expense |
10,334 | 6,011 | 5,900 | 3,010 | ||||||||||||||||||||
| Management and other expenses (2) |
671 | 6,713 | — | 2,850 | ||||||||||||||||||||
| Transaction costs (3) |
3,837 | 4,720 | 11,247 | 7,772 | ||||||||||||||||||||
| Tax receivable agreement remeasurement |
14,300 | (7,970 | ) | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Adjusted EBITDA |
$ | 234,201 | $ | 173,111 | $ | — | $ | 152,703 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Other Financial Data: |
||||||||||||||||||||||||
| Net cash provided by operating activities |
$ | 137,927 | $ | 69,998 | $ | — | $ | 86,323 | ||||||||||||||||
| Net cash used in investing activities |
(151,732 | ) | (761,973 | ) | — | (51,034 | ) | |||||||||||||||||
| Net cash provided by (used in) financing activities |
220,310 | (42,887 | ) | — | (27,949 | ) | ||||||||||||||||||
| (1) | Gain on settlement represents a litigation settlement with Hill-Rom. |
| (2) | Management and other expenses represents (a) the Advisory Services Fees, which will terminate in connection with this offering, (b) the Legacy Management Fees, which were terminated in connection with the Business Combination and (c) non-recurring expenses primarily comprising two legal settlements and a credit received under the CARES Act. |
| (3) | Transaction costs represent costs associated with potential mergers and acquisitions and are primarily related to the Business Combination completed on January 4, 2019. See Note 4 to our audited consolidated financial statements for the year ended December 31, 2020 included elsewhere in this prospectus for more information. |
Seasonality
Quarterly operating results are typically affected by seasonal factors. Historically, our first and fourth quarters are the strongest, reflecting increased customer utilization during the fall and winter months.
66
Table of Contents
Quarterly Revenue Table
The following table sets forth our unaudited condensed consolidated statement of operations data on a quarterly basis for the 2019 predecessor period, the 2019 successor period and for the successor year ended December 31, 2020. The information set forth below should be read together with the “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
| Successor | Predecessor | |||||||||||||||||||||||||||||||||||||||||||
| (in thousands) | Three Months Ended | |||||||||||||||||||||||||||||||||||||||||||
| December 31, 2020 |
September 30, 2020 |
June 30, 2020 |
March 31, 2020 |
Dec. 31, 2019 |
Sept. 30, 2019 |
June 30, 2019 |
Jan. 4 to March 31, 2019 |
Jan. 1 to Jan. 3, 2019 |
||||||||||||||||||||||||||||||||||||
| Consolidated Statements of Operation Data: |
||||||||||||||||||||||||||||||||||||||||||||
| Revenue |
$ | 214,191 | $ | 194,721 | $ | 185,161 | $ | 179,240 | $ | 157,328 | $ | 153,332 | 150,439 | $ | 151,974 | $ | — | |||||||||||||||||||||||||||
| Cost of revenue |
127,726 | 120,115 | 117,691 | 121,433 | 111,416 | 106,900 | 102,755 | 102,741 | — | |||||||||||||||||||||||||||||||||||
| Gross margin |
86,465 | 74,606 | 67,470 | 57,807 | 45,912 | 46,432 | 47,684 | 49,233 | — | |||||||||||||||||||||||||||||||||||
| Selling, general and administrative |
69,451 | 71,732 | 52,540 | 56,566 | 41,047 | 48,288 | 51,351 | 46,470 | 17,147 | |||||||||||||||||||||||||||||||||||
| Gain on settlement |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
| Operating income (loss) |
17,014 | 2,874 | 14,930 | 1,241 | 4,865 | (1,856 | ) | (3,667 | ) | 2,763 | (17,147 | ) | ||||||||||||||||||||||||||||||||
| Interest Expense |
14,998 | 13,560 | 15,156 | 17,817 | 13,038 | 10,894 | 11,137 | 13,130 | — | |||||||||||||||||||||||||||||||||||
| (Loss) income before income taxes and noncontrolling interest |
2,016 | (10,686 | ) | (226 | ) | (16,576 | ) | (8,173 | ) | (12,750 | ) | (14,804 | ) | (10,367 | ) | (17,417 | ) | |||||||||||||||||||||||||||
| Income tax (benefit) expense |
2,444 | (573 | ) | (1,077 | ) | (4,028 | ) | (6,005 | ) | (3,196 | ) | (3,238 | ) | (2,418 | ) | (13,281 | ) | |||||||||||||||||||||||||||
| Net income attributable to noncontrolling interest |
42 | 95 | 29 | 74 | 27 | 27 | 51 | 66 | — | |||||||||||||||||||||||||||||||||||
| Net (loss) income attributable to Agiliti, Inc. and subsidiaries |
$ | (470 | ) | $ | (10,208 | ) | $ | 822 | $ | (12,622 | ) | $ | (2,195 | ) | $ | (9,581 | ) | $ | (11,617 | ) | $ | (8,015 | ) | $ | (3,866 | ) | ||||||||||||||||||
Liquidity and Capital Resources
General
Our principal sources of liquidity are expected to be cash flows from operating activities and borrowings under our Revolving Credit Facility, which provides for loans in an amount of up to $190 million. It is anticipated that our principal uses of liquidity will be to fund capital expenditures related to purchases of medical equipment, provide working capital, meet debt service requirements and finance our strategic plans.
There is uncertainty related to the anticipated impact of the COVID-19 outbreak on our future results, and although we believe our business model, our current cash reserves and our available borrowings under our Revolving Credit Facility, will help us to manage our business through this crisis as it continues to unfold. We believe our existing balances of cash and cash equivalents and our currently anticipated operating cash flows will be sufficient to meet our cash needs arising in the ordinary course of business for the next twelve months. If new financing is necessary, there can be no assurance that any such financing would be available on commercially acceptable terms, or at all. To date, we have not experienced difficulty accessing the credit market; however, future volatility in the credit market may increase costs associated with issuing debt instruments or affect our ability to access those markets. In addition, it is possible that our ability to access the credit market could be limited at a time when we would like, or need to do so, which could have an adverse impact on our ability to refinance debt and/or react to changing economic and business conditions. See “Risk Factors—Risks Related to Our Indebtedness—We may not be able to obtain funding on acceptable terms or at all as a result of the credit and capital markets. Thus, we may be unable to expand our business or to service our debt.”
Net cash provided by operating activities was $137.9 million, $70.0 million and $86.3 million for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and for the year ended December 31, 2018, respectively. Net cash provided by operating activities during 2020 compared to the period of January 4 through December 31, 2019 was favorably impacted by our improved financial performance, the timing of incentive and interest payments and large transaction costs related to the Business Combination, which was completed in 2019. Our
67
Table of Contents
improved financial performance in 2020 was driven by the 26.1% increase in revenue. Higher incentive and interest payments were made in 2019, positively impacting our cash flow comparisons in 2020. In addition, large transaction costs were paid for in 2019, positively impacting our cash flow comparisons in 2020. Net cash provided by operating activities decreased in 2019 compared to 2018 primarily due to the decrease in working capital, offset by an increase in net income before depreciation, amortization, gain on settlement, intangible asset impairment charge and removal of a valuation allowance in 2018. Consolidated net income for 2019 was $129.8 million, adjusted for depreciation and amortization of intangible assets, compared to net income of $85.8 million for 2018, adjusted for depreciation, amortization of intangible assets, non-cash portion of the gain on settlement, intangible asset impairment charge, net of tax and the removal of a valuation allowance in 2018. The increase in net income was primarily due to an 8.5% increase in revenue. The decrease in working capital was impacted by higher incentive payments and the timing of interest payments during 2019, which resulted in a decrease in net cash provided by operating activities of $21.4 million and $14.9 million, respectively, offset by the timing of accounts receivable collections, which resulted in an increase in net cash provided by operations of $16.9 million.
Net cash used in investing activities was $151.7 million, $762.0 million, and $51.0 million for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and for the year ended December 31, 2018, respectively. The decrease in net cash used in investing activities during 2020 was primarily due to the business combination completed in January 2019. The increase in net cash used in investing activities in 2019 compared to 2018 was primarily due to the business combination completed in January 2019.
Net cash provided by (used in) financing activities was $220.3 million, ($42.9) million, and ($27.9) million for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and for the year ended December 31, 2018, respectively. The increase in net cash provided by financing activities during 2020 was primarily due to the new term loan to fund the acquisition on January 31, 2020. See Note 4 to our audited consolidated financial statements for the year ended December 31, 2020, included elsewhere in this prospectus for more information on the acquisition. The increase in net cash used in financing activities in 2019 compared to 2018 was primarily due to the dividend payment, redemption of warrants and payments of deferred financing costs, offset by higher net borrowings.
First Lien Credit Facilities
On January 4, 2019, Agiliti Health, Inc., as borrower, Agiliti Holdco, Inc. as holdings, and certain subsidiaries of Agiliti Health, Inc. acting as guarantors, entered into a credit agreement (the “First Lien Credit Agreement”), with JPMorgan Chase Bank, N.A., as administrative agent, collateral agent and letter of credit issuer and the lenders from time to time party thereto, comprised of a $150.0 million five-year senior secured revolving credit facility (the “Revolving Credit Facility”) and $660.0 million seven-year senior secured term loan facility (the “First Lien Term Loan Facility” and, together with the Revolving Credit Facility, the “First Lien Credit Facilities”). Pursuant to the Amendment No. 1 to First Lien Credit Agreement, dated as of February 6, 2020 (the “First Lien Credit Agreement Amendment No. 1”), the Revolving Credit Facility was increased to $190.0 million and the First Lien Term Loan Facility was increased to $785.0 million. Pursuant to the Amendment No. 2 to First Lien Credit Agreement, dated as of October 16, 2020 (the “First Lien Credit Agreement Amendment No. 2”), the First Lien Term Loan Facility was increased to $935.0 million. Pursuant to the Amendment No. 3 to First Lien Credit Agreement, dated as of March 19, 2021 (the “First Lien Credit Agreement Amendment No. 3”), the First Lien Term Loan Facility was increased to $1,135.0 million. A portion of the proceeds from the initial borrowings under the First Lien Credit Agreement were used to prepay certain existing indebtedness of the borrower and its subsidiaries and the proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 1 were used to finance working capital needs and general corporate purposes, including to finance acquisitions and to refinance the loans then outstanding under the Revolving Credit Facility. The proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 2 were used to finance working capital needs and general corporate purposes. The proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 3 were used to finance the Northfield Acquisition and related transactions. As of December 31, 2020, we had $922.2 million in borrowings outstanding under our First Lien Term Loan Facility and $6.3 million of letters of
68
Table of Contents
credit outstanding under our Revolving Credit Facility. As of December 31, 2020, the interest rate on our First Lien Term Loan Facility was 2.9375% on the $772.2 million and 3.5% on the $150.0 million.
Second Lien Credit Facilities
On November 15, 2019, Agiliti Health, Inc., as borrower, Agiliti Holdco, Inc., as holdings, and certain subsidiaries of Agiliti Health, Inc. acting as guarantors, entered into a second lien credit agreement (the “Second Lien Credit Agreement”), with Wilmington Trust, National Association, as administrative agent and collateral agent, the lenders from time to time party thereto and Goldman Sachs Lending Partners LLC, as bookrunner and lead arranger, comprised of a $240.0 million loan (the “Second Lien Term Loan Facility”). The proceeds from the borrowings under the Second Lien Credit Agreement were used to pay a dividend or distribution payment to our equityholders in an amount equal to approximately $240.0 million and certain transaction costs in connection therewith. As of December 31, 2020, we had $240.0 million in borrowings outstanding under the Second Lien Term Loan Facility. As of December 31, 2020, the interest rate on our Second Lien Term Loan Facility was 7.96838%.
Interest Rates and Fees
Borrowings under the First Lien Credit Agreement and Second Lien Credit Agreement bear interest at a rate per annum, at the borrower’s option, equal to an applicable margin, plus (a) a base rate determined by reference to the highest of (i) the prime lending rate published in The Wall Street Journal, (ii) the federal funds rate in effect on such day plus 1/2 of 1.00% and (iii) the LIBOR rate for a one-month interest period on such day plus 1.00% or (b) a LIBOR rate determined by reference to the LIBOR rate as set forth by the ICE Benchmark Administration for the interest period relevant to such borrowing subject to a LIBOR floor of 0.00%.
The applicable margin for borrowings under the First Lien Credit Agreement is (a)(1) prior to March 31, 2019 and (2) on or after March 31, 2019 (so long as the first lien leverage ratio is greater than 3.75 to 1.00), (1) 2.00% for alternate base rate borrowings and (ii) 3.00% for Eurodollar borrowings and (b) on or after June 30, 2020 (so long as the first lien leverage ratio is less than or equal to 3.75 to 1.00), subject to step downs to (i) 1.75% for alternate base rate borrowings and (ii) 2.75% for Eurodollar borrowings. Under the First Lien Credit Agreement, the borrower is also required to pay a commitment fee on the average daily undrawn portion of the Revolving Credit Facility of (i) 0.50% per annum if the first lien leverage ratio is greater than 4.00:1.00, (ii) 0.375% per annum if the first lien leverage ratio is less than or equal to 4.00:1.00 but greater than 3.50:1.00 and (iii) 0.250% if the first lien leverage ratio is less than or equal to 3.50:1.00, and a letter of credit participation fee equal to the applicable margin for Eurodollar revolving loans on the actual daily amount of the letter of credit exposure.
The applicable margin for borrowings under the Second Lien Credit Agreement is (a) 6.75% for alternate base rate borrowings and (b) 7.75% for Eurodollar borrowings.
Interest Rate Swap
In May 2020, we entered into an interest rate swap agreement for a total notional amount of $500.0 million, which has the effect of converting the interest rate applicable to $350.0 million and $150.0 million of our First Lien Term Loan Facility to fixed interest rates. The effective date for the interest rate swap agreement was June 2020 and the expiration date is June 2023.
The interest rate swap agreement qualifies for cash flow hedge accounting under ASC Topic 815, “Derivatives and Hedging.” Both at inception and on an on-going basis, we must perform an effectiveness test. The fair value of the interest rate swap agreement at December 31, 2020 was ($1.9) million, of which ($1.0) million are included in other accrued expenses and ($0.9) million are included in other long-term liabilities on our consolidated balance sheet. The change in fair value was recorded as a component of accumulated other comprehensive loss on our consolidated balance sheet, net of tax, since the instrument was determined to be an effective hedge at December 31, 2020. We have not recorded any amounts due to ineffectiveness for any periods presented.
69
Table of Contents
As a result of our interest rate swap agreement, we expect the effective interest rate on $350.0 million and $150.0 million of our First Lien Term Loan Facility to be 0.3396% and 0.3290%, respectively, through June 2023.
Contractual Obligations
The following is a summary, as of December 31, 2020, of our future contractual obligations:
| Payments due by period | ||||||||||||||||||||
| (in thousands) |
Total | Less than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
|||||||||||||||
| Other Financial Data: |
||||||||||||||||||||
| Long-term debt principal obligations |
$ | 1,162,190 | $ | 9,350 | $ | 18,700 | $ | 18,700 | $ | 1,115,440 | ||||||||||
| Interest on notes |
267,115 | 46,774 | 92,699 | 91,567 | 36,076 | |||||||||||||||
| Principal and interest on finance leases |
26,895 | 7,353 | 9,289 | 5,025 | 5,228 | |||||||||||||||
| Principal and interest on operating leases |
57,741 | 15,411 | 22,066 | 15,404 | 4,860 | |||||||||||||||
| Pension obligations (1) |
650 | 650 | — | — | — | |||||||||||||||
| Tax receivable obligations (2) |
50,600 | |
— |
|
— | — | — | |||||||||||||
| Unrecognized tax positions (3) |
1,340 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 1,566,531 | $ | 79,538 | $ | 142,754 | $ | 130,696 | $ | 1,161,604 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other commercial commitments: |
||||||||||||||||||||
| Stand by letter of credit |
$ | 6,325 | $ | — | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | While our net pension liability at December 31, 2020 was approximately $8.4 million, we cannot reasonably estimate required payments beyond 2020 due to changing actuarial and market conditions. |
| (2) | The total amount represents the fair value of the obligation. We cannot reasonably determine the exact timing of payments related to the Tax Receivable Agreement obligation. See “Certain Relationships and Related Party Transactions—Tax Receivable Agreement.” |
| (3) | We cannot reasonably determine the exact timing of payments related to our unrecognized tax positions. |
Based on the level of operating performance in 2020, we believe our cash from operations and additional borrowings under our Revolving Credit Facility will meet our liquidity needs for the foreseeable future, exclusive of any borrowings that we may make to finance potential acquisitions. However, if during that period or thereafter we are not successful in generating sufficient cash flows from operations or in raising additional capital when required in sufficient amounts and on terms acceptable to us, our business could be adversely affected. See “Risk Factors—Risks Related to Our Indebtedness—We may not be able to obtain funding on acceptable terms or at all as a result of the credit and capital markets. Thus, we may be unable to expand our business or to service our debt.”
Our levels of borrowing are further restricted by the financial covenants set forth in our Revolving Credit Facility. See “Description of Certain Indebtedness.”
As of December 31, 2020, we were in compliance with all covenants for all years presented.
Our expansion and acquisition strategy may require substantial capital. Sufficient funding for future acquisitions may not be available under our Revolving Credit Facility, and we may not be able to raise any necessary additional funds through bank financing or the issuance of equity or debt securities on terms acceptable to us, if at all. See “Risk Factors—Risks Related to Our Indebtedness—We may not be able to obtain funding on acceptable terms or at all as a result of the credit and capital markets. Thus, we may be unable to expand our business or to service our debt.”
70
Table of Contents
Off-Balance Sheet Arrangements
As part of our ongoing business, we do not participate in transactions that generate relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities (“SPEs”), which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As of December 31, 2020, we did not have any unconsolidated SPEs.
Recent Accounting Pronouncements
For a description of our recently adopted accounting pronouncements and recently issued accounting standards not yet adopted, see Note 2 to our consolidated financial statements: “Recent Accounting Pronouncements” appearing elsewhere in this prospectus.
Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risk arising from adverse changes in fuel costs and pension valuation. We do not enter into derivatives or other financial instruments for speculative purposes.
Interest Rates
We use both fixed and variable rate debt as sources of financing. As of December 31, 2020, we had approximately $1,186.8 million of total debt outstanding before netting with deferred financing costs and unamortized debt discount, of which $662.2 million was bearing interest at variable rates. Based on variable debt levels at December 31, 2020, a 1.0 percentage point change in interest rates on variable rate debt would have resulted in annual interest expense fluctuating by approximately $6.6 million.
Fuel Costs
We are exposed to market risks related to changes in the price of gasoline used to fuel our fleet of delivery and sales vehicles. A hypothetical 10% increase or decrease in the average 2020 prices of unleaded gasoline, assuming normal gasoline usage levels for the year, would lead to an annual increase or decrease in fuel costs of approximately $0.3 million.
Pension
Our pension plan assets, which were approximately $24.6 million at December 31, 2020, are subject to volatility that can be caused by fluctuations in general economic conditions. Continued market volatility and disruption could cause further declines in asset values, and if this occurs, we may need to make additional pension plan contributions and our pension expense in future years may increase. A hypothetical 10% decrease in the fair value of plan assets at December 31, 2020 would lead to a decrease in the funded status of the plan of approximately $2.5 million.
Other Market Risk
As of December 31, 2020, we have no other material exposure to market risk.
71
Table of Contents
Our Mission
Agiliti is an essential service provider to the U.S. healthcare industry with solutions that help support a more efficient, safe and sustainable healthcare delivery system. We ensure healthcare providers have the critical medical equipment they need to care for patients—wherever and whenever it’s needed—with a service model that helps lower costs, reduce waste and maintain the highest quality standard of medical device management in the industry. We are motivated by a belief that every interaction has the power to change a life, which forms the cornerstone of how we approach our work and frames the lens through which we view our responsibility to make a difference for the customers, patients and communities we serve.
Overview
We believe we are one of the leading experts in the management, maintenance and mobilization of mission-critical, regulated, reusable medical devices. We offer healthcare providers a comprehensive suite of medical equipment management and service solutions that help reduce capital and operating expenses, optimize medical equipment utilization, reduce waste, enhance staff productivity and bolster patient safety.
We commenced operations in 1939, originally incorporated in Minnesota in 1954 and reincorporated in Delaware in 2001. Since the Business Combination in January 2019, we have been controlled by the THL Stockholder.
In our more than 80 years of experience ensuring healthcare providers have high-quality, expertly maintained equipment to serve their patients, we’ve built an at-scale, strong nationwide operating footprint. This service and logistics infrastructure positions us to reach customers across the entire healthcare continuum—from individual facilities to the largest and most complex healthcare systems. Likewise, our ability to rapidly mobilize, track, repair and redeploy equipment during times of peak need or emergent events has made us a service provider of choice for city, state and federal governments to manage emergency equipment stockpiles.
Agiliti at-a-glance: Powered by an at-scale, nationwide logistics and service infrastructure

Our diverse customer base includes approximately 7,000 active national, regional and local acute care hospitals, health system integrated delivery networks and alternate site providers (such as surgery centers,
72
Table of Contents
specialty hospitals, home care providers, long-term acute care hospitals and skilled nursing facilities). We serve the federal government as well as a number of city and state governments providing management and maintenance of emergency equipment stockpiles, and we are an outsourced service provider to medical device manufacturers supporting critical device remediation and repair services. We deliver our solutions through our nationwide network of 98 service centers and seven Centers of Excellence, among which we employ a team of more than 500 specialized biomed repair technicians, more than 2,800 field-based service operators who work onsite within customer facilities or in our local service centers, and approximately 200 field sales and account managers. Our fees are paid directly by our customers rather than by direct reimbursement from third-party payors, such as private insurers, Medicare or Medicaid.
Industry Challenges
The U.S. healthcare industry continues to face transformative pressure that affects how provider organizations conduct business and serve their patients. Across the healthcare system, providers face compounding financial and operational challenges, including cost pressure from payors, nursing and clinical staff shortages, rising costs of drugs and supplies, increasing regulatory oversight, and advances in medical technology that generally result in higher prices for newer equipment and a higher cost of managing that equipment over its lifecycle. Given there is little that providers can do to change external dynamics, there is increased focus on areas within their enterprise that they can control. In our experience, one area that most hospitals and health systems identify for operational and cost improvement is the management and maintenance of medical equipment.
Healthcare facilities have been shown to own large quantities of reusable capital equipment ranging from multi-million dollar highly technical devices (e.g. MRIs) to lower cost, high volume devices (e.g. infusion pumps) required for patient care, treatment and diagnosis. In our experience, providers often face challenges in managing their medical equipment inventory effectively. For example, hospitals typically utilize roughly 42% of their owned medical equipment inventory at any given time, yet caregivers report that they routinely lack access to readily available patient-ready equipment. Nurses report spending an average of 20 minutes per shift searching for equipment, and no more than 37% of their time on direct patient care. Operational silos that naturally occur among hospital departments create inadvertent breakdowns within equipment management workflows, from the administrators who order equipment, to the support staff who clean/reprocess and deliver the equipment, to the nurses and doctors who use the equipment.
Further, the repair and maintenance of this highly technical equipment continues to increase in complexity and cost. Over 15 years there has been a 62% increase in the number of medical devices per hospital bed and a 90% increase in costs related to maintaining this equipment (between 1995 and 2010). Given the increasingly complex nature of these devices and stringent regulatory mandates guiding their upkeep, specialized technical knowledge is required to repair and maintain them. Most healthcare facilities struggle to employ the in-house capabilities and resources needed to ensure timely, routine maintenance and rapid testing, repair and turnaround of their medical inventory which may impact time-to-therapy and patient safety, while driving up capital replacement costs on equipment that could have otherwise been kept operational with proper maintenance.
Finally, the healthcare system experiences seasonality in patient volumes, resulting in peak-need demand for specialized medical equipment (e.g. ventilators, specialty beds, infusion pumps). Given the common breakdowns in managing and maintaining their inventory during times of normal operation, hospitals face additional burden on equipment availability during times of peak need and will procure supplemental equipment through additional acquisition channels to fill this gap.
73
Table of Contents
Critical gaps and costly challenges for providers at the intersection of care and technology

These challenges drive up significant costs and time delays within individual hospital facilities, but when multiplied across several hospitals and alternate site facilities within an IDN, the losses increase significantly. An average 2,500 bed IDN has been shown to waste more than $11 million annually on inefficient equipment maintenance and unnecessary capital purchases, while clinicians lose valuable patient time and productivity hours managing equipment needs.
These dynamics, supported by the following trends, further support the essential nature of our work:
Focus on reducing costs and increasing operational efficiency. Hospitals and other healthcare facilities continue to experience substantial pressure to conserve capital, reduce operating expenses and become more operationally efficient. We expect these pressures to continue in the future and believe that we will always be on the right side of healthcare reform. Our comprehensive, end-to-end solutions offer customers a way to realize costs savings while enhancing operational improvements for medical equipment access and availability, thereby improving their organizational efficiency and financial viability.
Demand for better patient safety and outcomes. Hospitals across the U.S. are focused on improving patient safety and outcomes, which includes efforts to minimize hospital-acquired conditions (e.g. infections, patient falls and pressure injuries) and increase nursing time at the patient bedside. Hospitals turn to us to assist them in managing their equipment to help them to minimize these incidents and ensure equipment is available when and where it is needed for patient care, thereby improving patient safety and time to therapy, and supporting optimal patient outcomes.
Caregiver retention and satisfaction. Hospitals continue to experience nursing and other caregiver retention and job satisfaction pressures. According to reports from the World Health Organization, the United States is expected to have a nurse shortage of 500,000 nurses by 2025. Adding non-patient care duties, such as searching for, cleaning and managing equipment, adds to nurse workload and contributes to clinician dissatisfaction and turnover. We expect that with these internal pressures, hospitals will increasingly turn to our programs to outsource healthcare technology management duties and related management processes to allow nurses more time to spend on patient care, resulting in improved job satisfaction.
Increased capital and operating expense pressures and regulatory compliance. Hospitals continue to experience restricted capital and operating budgets, while the cost and complexity of medical equipment increases. Furthermore, the increasing complexity and sophistication of medical equipment brings with it more
74
Table of Contents
recordkeeping requirements and regulatory scrutiny in its use and maintenance. We expect that hospitals will increasingly look to us to support the management and maintenance of their capital equipment inventory to achieve capital and operating expense savings, operating efficiencies and regulatory compliance.
Our Value Proposition
As a critical outsource partner to more than 7,000 U.S. healthcare customers, including most leading providers nationwide, we’ve tailored our solution offering and service model to address the unique challenges and opportunities we witness among our customers related to the effective management of medical equipment.
Our services help eliminate significant capital and operating costs associated with the ownership and lifecycle management of mission-critical medical equipment. In addition to optimizing use of providers’ owned equipment, we provide ready access and increase the on-patient utilization of supplemental medical equipment to address fluctuations in patient census and patient acuity. By partnering with Agiliti, providers have the benefits of:
| ➣ | Cost savings and lower total costs of equipment ownership |
| • | Increased utilization of both customer-owned and supplemental equipment |
| • | Lower overall total cost of equipment ownership by combining our solutions to solve challenges across the end-to-end equipment management process |
| • | Optimized management and logistics of provider-owned equipment through tracking, monitoring, reprocessing, maintaining, and ensuring equipment is safety-tested and redeployed for use |
| • | Reduced maintenance and repair costs through the use of our proprietary technology, flexible staffing models, parts pool, equipment capabilities and diverse skill mix of knowledgeable equipment technicians and our commitment to quality |
| • | Benefits of specialized technician labor to augment clinical biomed staff, having been shown to help reduce service costs and provide required technical proficiency to address more complex equipment types |
| • | Access to our extensive data and expertise on the cost, performance, features and functions of all major items of medical equipment |
| • | Assistance with capital planning, vendor management and regulatory compliance |
| ➣ | More time to spend with patients and confidence in the availability of patient-ready medical equipment |
| • | Increased productivity and satisfaction among nursing staff achieved by eliminating certain non-clinical work tasks and saving an average 300-bed hospital over 28,000 caregiver hours annually, allowing more time to focus on patient care responsibilities |
| • | Improved time-to-therapy for patients at risk for falls, skin breakdown and bariatric safety by expediting delivery of therapeutic equipment direct to the patient room |
| • | Access to supplemental moveable medical equipment, surgical equipment and next generation technology without the expense of acquisition on a pay-per-procedure basis |
| ➣ | Improved regulatory compliance, risk management and extended use life |
| • | Optimal maintenance intervals and parts replacement to extend equipment use life, reduce waste and lower obsolescence risk |
| • | Compliance with regulatory and recordkeeping requirements and adherence to manufacturers’ specifications on the reprocessing and maintenance of medical equipment |
| • | Equipment quality assurance through the use of our comprehensive QMS based on the quality standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016 |
| • | Risk mitigation and lower costs associated with product recalls or device modifications |
75
Table of Contents
| ➣ | Technical expertise and supplemental staffing to sustain optimal equipment workflow |
| • | Reduced administrative and time burdens on clinical staff related to managing and locating available equipment and coordinating among multiple vendors |
| • | Specialized technical and clinical specialists that directly interact with and work alongside customers to optimize equipment outsourcing solutions |
Our Market Opportunities
We participate in a $14 billion U.S. medical equipment services market comprised of the services we offer through our onsite managed services, clinical engineering services and equipment solutions service lines. We believe that this market will grow at mid-single digits annually.
Per the CMS, as of 2018, healthcare spending reached $3.6 trillion, or $11,172 per person and accounted for 17.7% of the U.S. GDP. Spending is expected to grow at an average annual rate of 5.4% from 2019-2028, due to secular tailwinds including an aging population, rising acuity, and prevalence of chronic conditions. Within healthcare, hospital care, physician and clinical services accounted for 53% of total healthcare spend in 2018.
There is a fundamental shift in the needs of health systems, hospitals and alternate site providers to move from supplemental and peak need sourcing of medical equipment toward more comprehensive onsite inventory management and maintenance solutions. As healthcare facilities look to balance the challenge of providing better care at lower costs, they are more open to third party partnerships that outsource critical but non-core support functions. The move toward full outsourcing is not unlike trends in similar services at hospitals including food service, laundry, professional staffing and technology.
We believe there are several key macro trends that will drive increased demand for our products and services:
Favorable demographic trends. According to the U.S. Census Bureau, individuals aged 65 and older in the United States comprise the fastest growing segment of the population. This segment is expected to grow to approximately 81 million individuals by 2040. This represents a 44% increase in the 65-and-older segment of the population over the next 20 years. As a result, over time, the number of patients and the volume of hospital admissions are expected to grow. The aging population and increasing life expectancy are driving demand for healthcare services.
Increase in chronic disease and obesity. According to the CDC, six in ten Americans live with at least one chronic disease, like heart disease and stroke, cancer, or diabetes. These conditions often require specialty equipment to support therapeutic intervention in inpatient and outpatient care settings. In addition, obesity in the U.S. increased to 42% of the population between 2017-2018, up from 30.5% in 2000 (CDC). This population demands greater access to specialty bariatric equipment to support care and minimize the incidence of injury during a hospital stay.
Increased mergers & acquisitions. We have seen that hospitals and healthcare systems continue to expand their covered network and acquire alternate care delivery settings in order to care for patient populations in the most cost-effective way. In our experience, providers are increasingly seeking partners that provide comprehensive services and that can quickly adapt to changing health system infrastructure and growth. Working with one vendor that can operate at a nationwide and system-wide scale is attractive to cities, states, and IDNs who operate, manage, and maintain equipment inventories across multiple locations.
Centralizing shared services across the IDN. Health systems with duplicate services across multiple facilities in close proximity have an increased risk of unnecessary variation, greater costs, and suboptimal outcomes. Many health systems have centralized and consolidated non-clinical services as a shared service, including billing, reimbursement, supply chain, human resources, IT, etc. We have witnessed a growing trend among IDNs to centralize and consolidate equipment maintenance and logistics among member facilities. In our experience, because most health systems do not currently have the storage, technical or transportation resources
76
Table of Contents
for managing a shared equipment management function, they will seek third party support to optimize equipment utilization, redeploy equipment where needed and reduce overall equipment costs.
Increase in infection control risks. Infection control remains an essential priority for hospitals and health systems as a way to limit the spread of hospital-acquired infections. This has further escalated as a top priority due to the COVID-19 pandemic. Most focus in this area is around hand hygiene, the proper use of personal protective equipment (PPE) and the reprocessing and sterilization of critical and semi-critical medical devices (e.g. surgical instruments, endoscopes). Often overlooked is the reprocessing of non-critical medical devices, such as infusion pumps and ventilators, that are commonly touched by caregivers and patients. If not properly cleaned and sanitized between patient use, these devices can pose increased infection control risks. We expect an increase in demand of onsite equipment management programs to address proper reprocessing of these types of devices and help lower infection risks and allow clinicians to spend more time at the patient bedside and less time cleaning equipment.
Our Solutions
We provide comprehensive medical device management solutions based on a proven framework for optimizing use of reusable, regulated medical devices. Within this framework, we engage with healthcare providers to help reduce the cost and complexity of acquiring, managing and maintaining critical medical equipment inventories. The integrated nature of our service offerings within this end-to-end framework ensures we maximize value to customers as we address more aspects of the equipment lifecycle continuum.
Proven framework for end-to-end management of FDA-regulated, reusable medical devices

While customers may initially engage with us across one aspect of our service lines within this framework, we employ a variety of land-and-expand tactics to grow our relationships and customer share-of-wallet over time. These tactics include:
| • | Gateway solutions which offer an entry point to the economic buyer and include peak needs equipment, surgical lasers and equipment, specialty beds and surfaces and supplemental clinical engineering services; |
| • | Vertical solutions which provide a deeper level of service with clinical offerings tailored to specific patient needs (e.g. bariatrics, wound management, falls management) and clinical engineering programs for broad equipment categories (general biomedical devices, diagnostic imaging equipment, surgical instruments); |
| • | Comprehensive, connected solutions through onsite managed services and outsourced clinical engineering services that connect previously fragmented customer workflow processes to drive |
77
Table of Contents
| operational efficiencies, realize improved clinician and equipment productivity, lower total cost of ownership, ensure regulatory compliance, reduce waste, improve time to therapy and allow customers to effectively lower costs; and |
| • | Comprehensive logistics, management and clinical engineering solutions that allow IDNs to manage equipment inventories across multiple locations, and supports city, state and federal government agencies in managing and maintaining equipment stockpiles. |
We deploy our solution offering across three primary service lines:
Onsite Managed Services: Onsite Managed Services are comprehensive programs that assume full responsibility for the management, reprocessing and logistics of medical equipment at individual facilities and IDNs, with the added benefit of enhancing equipment utilization and freeing more clinician time for patient care. This solution monitors and adjusts equipment quantities and availability to address fluctuations in patient census and acuity. Our more than 1,700 onsite employees work 24/7 in customer facilities, augmenting clinical support by integrating proven equipment management processes, utilizing our proprietary management software and conducting daily rounds and unit-based training to ensure equipment is being used and managed properly, overall helping to optimize day-to-day operations and care outcomes. We assume full responsibility for ensuring equipment is available when and where it is needed, removing equipment when no longer in use, and decontaminating, testing and servicing equipment as needed between each patient use. We have over 225 onsite managed service customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 28% and 28% of our total revenue, respectively.
Clinical Engineering Services: Clinical Engineering Services provides maintenance, repair and remediation solutions for all types of medical equipment, including general biomedical equipment and diagnostic imaging equipment, through supplemental and outsourced offerings. Our supplemental offering helps customers manage their equipment repair and maintenance backlog, assist with remediation and regulatory reporting and temporarily fill open biotechnical positions. With our outsourced offering, we assume full management, staffing and clinical engineering service responsibilities for individual or system-wide customer sites. The outsourced model deploys a dedicated, on-site team to coordinate the management of customer-owned equipment utilizing our proprietary information systems, third party vendors of services and parts, and a broad range of professional services for capital equipment planning and regulatory compliance. We leverage more than 500 technical resources from our 98 local market service centers and seven Centers of Excellence to flex staff in and out of customer facilities on an as-needed basis, ensuring customers pay only for time spent directly servicing their equipment by an appropriately qualified technician. We use flex staffing for our supplemental clinical engineering solution and to augment support when additional technicians are needed to supplement our outsourced services during peak workload. We contract our Clinical Engineering Services with acute care and alternate site facilities across the U.S., as well as with the federal government and any medical device manufacturers that require a broad logistical footprint to support their large-scale service needs. We have over 6,000 clinical engineering service customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 33% and 31% of our total revenue, respectively.
Equipment Solutions: Equipment Solutions primarily provides supplemental, peak need and per-case rental of general biomedical, specialty, and surgical equipment to approximately 7,000 acute care hospitals and alternate site providers in the U.S., including some of the nation’s premier healthcare institutions and integrated delivery networks. We contract for Equipment Solutions services directly with customers or through our contractual arrangements with hospital systems and alternate site providers. We consistently achieve high customer satisfaction ratings, as evidenced by our NPS of 55 for the year ended December 31, 2020, by delivering patient-ready equipment within our contracted equipment delivery times and by providing technical support and educational in-servicing for equipment as-needed in clinical departments, including the emergency room, operating room, intensive care, rehabilitation and general patient care areas. We are committed to providing the highest quality of equipment to our customers, and we do so through the use of our comprehensive
78
Table of Contents
QMS which is based on the quality standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016. This commitment ensures that customers have access to patient-ready equipment with the confidence of knowing it has been prepared and maintained to the highest industry standard to deliver optimal patient safety and outcomes. We have over 5,000 equipment solution customers. Revenue attributable to such customers for the years ended December 31, 2020 and 2019 represented 38% and 41% of our total revenue, respectively.
Many of our customers have multiple contracts and have revenue reported in multiple service lines. Our contracts vary based upon service offering, including with respect to term (with most being multi-year contracts), pricing (daily, monthly and fixed fee arrangements) and termination (termination for convenience to termination for cause only). Many of our contracts contain customer commitment guarantees and annual price increases tied to the consumer price index. Standard contract terms include payment terms, limitation of liability, force majeure provisions and choice of law/venue.
Because we work closely with customers to provide a long-term, value-based solution vs. a product-based, transactional approach, they are motivated to expand their relationships with us over time. We have approximately 82% white space within our current customers base. As indicated in the Customer Case Study presented below, we have demonstrated an ability to grow revenue up to 5-6x with an existing customer. We believe that this case study demonstrates the potential of our “land and expand” strategy of efficiently increasing revenue from our existing customers as they move toward our full suite of highly complementary services. From the year ended December 31, 2015 to the year ended December 31, 2020, our top 50 customers that experienced the largest growth in revenue over the same period increased in revenue from an aggregate of approximately $21.3 million to approximately $117.3 million (with increases at each customer ranging from $1.0 million to $8.8 million and an average increase of $1.9 million, and with consistent growth across our three primary service lines), primarily driven by our efforts to expand our share of wallet within our existing customer base. During the same period, our average existing customer growth rate was approximately 4.3%.
Further, the infrastructure and capabilities required to provide connected, responsive equipment lifecycle management is typically cost-prohibitive, even for large IDNs. Our nationwide network of clinical engineers, storage and repair facilities, vehicles and analytics tools gives us scale to provide cost-effective services for individual facilities, systems, regional IDNs, governments and device manufacturers.
Customer Case Study:

79
Table of Contents
Competitive Strengths
We believe our business model presents an attractive value proposition to our customers and that our comprehensive medical device management solutions and ability to work in partnership with and across OEMs as a device-agnostic service provider have contributed to our growth in recent years. Our unique framework for end-to-end medical equipment management, delivered through our nationwide service and logistics infrastructure, differentiates us in the marketplace and is without comparable peer. We believe our more than 80 years of experience, extensive employee base of trained technicians and our reputation for service excellence has earned us a leading position in our industry. We attribute our historical success to our:
Strong value proposition. With our focus and expertise in connected, end-to-end medical equipment management and service solutions, we offer a compelling customer value proposition. We believe that many of our customers have come to rely on our ability to respond quickly to their needs with reliable, high quality service expertise. We believe our ability to provide this level of service distinguishes us from our competitors. It also requires us to maintain inventories and infrastructure that we do not believe our competitors currently maintain. Our comprehensive solutions focus on helping customers:
| • | lower total cost of device ownership by reducing capital and operating costs related to owning and managing medical equipment; |
| • | enhance operational productivity and staff satisfaction by ensuring equipment is available when and where needed; and |
| • | maintain high standards of quality and regulatory compliance related to medical equipment use, maintenance and end-of-life disposal. |
Large, nationwide infrastructure. We have a broad and specialized nationwide staff, facility, and vehicle service network coupled with focused and customized operations at the local level. Our extensive network of service centers and Centers of Excellence and our 24-hours-a-day, 365 days-a-year service capabilities enable us to compete effectively for large, national contracts as well as drive growth regionally and locally.
We employ a number of technical, clinical and surgical specialists that engage directly with our customers to drive improved cost, efficiency and clinical outcomes. These include over 500 biomedical repair technicians, more than 2,800 field-based service operators, and approximately 200 field sales and account managers. Our specialized teams, large equipment fleet, and quality assurance programs have been built over 80 years serving provider customers and represent a significant investment in infrastructure over time. This places us in a unique and hard-to-replicate position with the scale to serve the most complex acute care hospitals, such as teaching, research or specialty institutions, that demand access to current and preferred technologies to meet the complex needs of their patients.
Proprietary software and asset management tools. We have used our more than 80 years of experience and our extensive database of equipment management information to develop sophisticated software technology and management tools. These tools enable us to meet unique customer demands by supporting sophisticated onsite managed services that help drive cost efficiencies and equipment productivity for caregivers. We believe that our ongoing investment in new tools and technology will help continue to distinguish our offerings to the healthcare industry.
Commitment to quality. Third-party service providers like Agiliti are not required to register with the FDA; therefore, there are no regulations that specifically apply to our maintenance of medical devices. We’ve made a commitment, however, to do the right thing for our customers and their patients by staffing a dedicated Quality team and implementing a Quality Management System (“QMS”) based on the quality standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016. This commitment to quality ensures that patient safety and risk management are at the center of every product decision, and that our equipment is serviced to the highest standards in the industry. All 98 local market service centers and seven Centers of Excellence follow an ISO 13485 compliant QMS, and we have elected to hire outside independent accredited registrars to audit our quality system. British Standards Institute (“BSI”) has certified five of our Centers of Excellence to
80
Table of Contents
ISO 13485:2016, and the remaining two Centers of Excellence are certified by Deutscher Kraftfahrzeug-Überwachungs-Verein e.V. (“Dekra”) and National Quality Assurance (“NQA”). We believe that ISO 13485:2016 provides the stringent guidelines specific to medical devices to ensure that our fleet of equipment, as well as the equipment we service, is maintained to the highest quality standards. Our commitment to quality extends to our exclusive use of Original Equipment Manufacturer (“OEM”) parts to repair FDA registered medical devices that we own, whenever available. Implementing optimal maintenance intervals and parts replacement extends equipment use life, thereby reducing waste and lowering risk of obsolescence. We believe that our robust QMS policies set us apart in our industry from those who may use less stringent quality practices on the equipment they own or maintain.
Superior customer service. We believe we have a long-standing reputation among our customers for outstanding service and quality. This reputation is largely attributable to our strong customer service culture, which is continuously reinforced by management’s commitment to, and significant investment in, hiring and training resources. We strive to seamlessly integrate our employees and solutions into the operations of our customers. We believe that our aggressive focus on the overall customer experience has helped us achieve high customer satisfaction ratings, as evidenced by our NPS of 55 for the year ended December 31, 2020.
No direct third-party payor reimbursement risk. Many healthcare providers rely on direct payment from patients or reimbursement from third-party payors. Our fees are paid directly by our customers, rather than by third-party payors, such as Medicare, Medicaid, managed care organizations or indemnity insurers. Accordingly, our exposure to uncollectible patient or reimbursement receivables or Medicare or Medicaid reimbursement changes is reduced, as evidenced by our bad debt expense of approximately 0.3%, 0.2%, and 0.4% of total revenue for the years ended December 31, 2020, 2019, and 2018, respectively.
Values driven culture centered on doing the right thing for our many stakeholders. Our team operates on a set of shared aspirations that reflect the manner in which we approach our work and serve the needs of our customers, team members, shareholders and local communities. We believe these aspirations that underpin our culture, strategy and service model help contribute to a safer and more sustainable healthcare system and frame the cornerstone of our success:
WE ARE BUILDING THE PREMIER CLINICAL EQUIPMENT SERVICES COMPANY. We ensure clinicians have the equipment they need, when they need it, with the confidence it is maintained to the highest industry standards. We never waver from doing what is right for our customers, our team members, and our shareholders.
WE ARE ESSENTIAL TO CUSTOMERS. We are dependable, trusted advisors—steadfast in our commitments and ready to serve. We deliver a unique and valuable offering that helps customers improve their business and prioritize patient care.
WE ARE EMPOWERED AND ENGAGED. We lead by example, inspiring one another to be at our best, to be accountable, and to develop with purpose. We value our diversity, knowing different perspectives lead to better outcomes. We share a common drive to make a difference and take pride in being part of something bigger than ourselves.
WE ARE OPERATIONALLY EXCELLENT. We demonstrate a tireless commitment to quality, reliability, and continuous improvement. We demand of ourselves the highest degree of accuracy, efficiency and integrity in order to deliver exceptional service to our customers and their patients.
WE ARE CREATING A CATEGORY OF ONE. Together, we are building a highly differentiated service company that is the vendor of choice for customers and an employer of choice nationwide.
Highly engaged team. We believe a strong and sustainable company begins with an engaged and empowered team. We are committed to investing in our team’s development and to fostering a culture of diversity, inclusion, trust and transparency. Approximately 52% of our total work force is comprised of minorities and approximately 31% of our team members are female. Since 2018, we have consistently had an
81
Table of Contents
annual internal promotion rate of approximately 28%, compared to the national average of 8.9%, as reported by ADP Research Institute. We offer competitive compensation and benefits programs, and we ensure our team members share in the success of our business with a companywide annual bonus program and, following the completion of this offering, an Employee Stock Purchase Plan. We strive to ensure Agiliti is a place where our people are proud to work, and we achieve that by listening to feedback and taking active steps to improve. In 2020, we achieved a 77 employee engagement score rating, which places us nearing the extraordinary company benchmark according to third-party engagement indices.
Proven management team. Our diverse and industry leading management team brings decades of executive-level healthcare expertise from across the sector and has successfully supervised the development of our competitive strategy, continually enhanced and expanded our service and product offerings, reinforced our nationwide operating footprint and furthered our reputation as an industry leader in our category.
Key Elements of our Growth Strategy
Retain and expand existing customer relationships. While our overall market opportunity is large, there is also significant expansion opportunity within our existing customers. We currently have 18% wallet share within our existing contracted customers and have demonstrated the ability to grow our wallet share up to 5-6x with an existing customer, as indicated in the Customer Case Study presented above, by expanding the services we provide to them over time. From the year ended December 31, 2015 to the year ended December 31, 2020, our top 50 customers that experienced the largest growth in revenue over the same period increased in revenue from an aggregate of approximately $21.3 million to approximately $117.3 million (with increases at each customer ranging from $1.0 million to $8.8 million and an average increase of $1.9 million, and with consistent growth across our three primary service lines), primarily driven by our efforts to expand our share of wallet within our existing customer base. During the same period, our average existing customer growth rate was approximately 4.3%. We believe there is approximately $1.7 billion of white space among our current contracted customer base and capturing more of our customer share-of-wallet is core to our growth strategy.
Grow our customer base among customers that outsource. We believe there is a significant opportunity to further grow our business by winning new customer contracts within the $5.85 billion that is contracted annually for medical equipment management services in the U.S. This is less than half of the total addressable market and, due to increasing pressures that providers are facing, we expect outsourcing to significantly accelerate. As a leader in our industry, we believe we are poised to take advantage of this continued shift.
Grow our serviceable market by contracting with those that insource today. Currently, we estimate that $14 billion is spent annually in the U.S. for medical equipment services and functionality, but less than half of the total market is currently being outsourced to an equipment management service, while the rest is done in-house in facilities. We believe that as we reach additional potential customers with demonstrated value both in improved patient care and reduced costs, we can grow our total addressable market by contracting with new clients that were not previously outsourcing device management services. Further, this market is also experiencing tailwinds that make the total addressable market, the total contracted market, and our own contracts with ongoing customers poised to continue to expand. These tailwinds include increasing overall provider volumes, increasing use and complexity of medical devices, increasing outsourcing by hospitals, and additional factors that we believe will continue to drive growth.
Invest in complementary offerings that enhance customer relationships. As the medical device field becomes increasingly complex and the number of devices used per patient on average increases over time, we are constantly evaluating additional services and methods of approaching service delivery that increase value for our clients. As an example, this has recently taken the form of expanding our work with federal, state, and local governments to help them maintain and mobilize strategic stockpiles of ventilators and other critical medical equipment.
82
Table of Contents
Opportunistically pursue accretive M&A. Due to our high and sustained value creation for customers and significant white space with existing customer relationships; we believe that pursuing opportunistic M&A will drive increasing returns through embedded customer relationships. From 2015-2020, we have successfully integrated seven acquisitions and will continue to opportunistically pursue additional inorganic growth.
COVID-19 Update
COVID-19 has placed our customers, business, teams and communities in uncharted waters. We consider the impact of the pandemic on our business by evaluating the health of our operations, changes to our revenue outlook, and the degree to which perceptions of and need for Agiliti solutions have evolved during this unprecedented time. As demand for emergent acute care increases around the country and as the global pandemic highlights the importance of resilient supply chains and service networks, the importance of our services has been magnified. We are proud to have rapidly developed and deployed a response plan to ensure the safety of our team, while continuing to meet our customers’ evolving needs for patient-ready medical equipment when and where it was needed; notably, doing so without service interruptions.
We believe our value proposition now resonates with an even broader audience of customers as providers, IDNs and governments prepare for potential future surges in demand for acute care and the required equipment necessary to care for patients.
Specifically, during the COVID-19 pandemic, we have:
| • | fully and rapidly deployed our fleet of medical devices and accessories across the U.S. to ensure they are reaching the maximum number of patients; |
| • | leveraged our logistics, inventory management, and maintenance/repair infrastructure to work with medical device brokers and manufacturers to make thousands of additional critical medical devices available to healthcare facilities; |
| • | deployed our local biomedical repair teams to augment teams at hospitals around the country to ensure their owned medical equipment remains fully operational and available for patient needs; |
| • | redeployed teams from our 98 local service centers to support surge medical capacity in parks, gymnasiums, and hotel rooms across the country; |
| • | been awarded a new contract to leverage our unique scale and capabilities to manage the maintenance and field repair of the national strategic ventilator stockpile; we are likewise working with various state and municipal governments to manage and mobilize their centralized and local medical device stockpiles; and |
| • | prioritized the care and safety of our employees who are essential to helping our customers meet patient care needs. We committed to avoid COVID-19 related layoffs or furloughs and bridge the income of our team members with variable net pay for the duration of the pandemic. As many of our team members entered high-exposure environments each day, we ensured all had the necessary PPE, guidelines, training and support from the highest levels of the company. We provided 100% coverage for COVID-19 testing and telemedicine, extended short-term medical leave and disability coverage related to COVID-19, and granted additional time-off benefits for COVID-19 related needs, so that our teams were able to safely focus on our customers and their patients as we served alongside them in front-line response efforts. |
Due in part to our ability to service clients rapidly and effectively during COVID-19, our revenue increased 26.1% from 2019 to 2020.
83
Table of Contents
Business Operations
Service Centers
As of December 31, 2020, we operated 98 local market service centers which allow us to provide our end-to-end healthcare technology management and service solutions to customers in virtually all markets throughout the United States. Each service center is responsible for supporting the equipment management needs of its local healthcare market across all sites of care. Each service center maintains an inventory of locally demanded equipment, parts, supplies and other items tailored to accommodate the needs of individual customers within its geographical area. Should additional or unusual equipment be required by one of our customers, a local service center can draw upon the resources of our other service centers. With access to more than a million owned or managed units of medical equipment (over 250,000 owned) available for customer use as of December 31, 2020, we believe we can most often obtain the necessary equipment within 24 hours.
Depending on market size and demands, our service centers are staffed by multi-disciplined teams of sales professionals, service representatives, customer service technicians, clinical engineering (biomedical) equipment technicians and surgical services technologists trained to deliver on our complete portfolio of customer solutions. Employees providing resident-based services through our on-site managed programs are supported by local site managers and/or the service centers in the markets where those customers are located.
Centers of Excellence
Our local market service center network is supported by seven strategically located Centers of Excellence. These centers focus on providing highly specialized clinical engineering service and support. The Centers of Excellence also provide overflow support, technical expertise, training programs and specialized service functions for our local service centers. All specialized depot work required by our manufacturer customers resides within these Centers of Excellence. Five of our Centers of Excellence are certified as being in compliance with the BSI, which has certified the five Centers of Excellence to ISO 13485:2016 as a quality commitment to our customers, while the remaining two are certified by Dekra and NQA.
Centralized Functions
Our corporate office is located in Minneapolis, Minnesota. We have centralized many of the key elements of our equipment and service offerings in order to create standardization and to maximize our operating efficiencies and uniformity of service. Some of the critical aspects of our business centralized within our corporate office include contract administration, marketing, purchasing, pricing, logistics, accounting and information technology.
Medical Equipment Fleet
We acquire or manage medical equipment to meet our customers’ needs in some of the following product areas: respiratory therapy, infusion therapy, newborn care, critical care, patient monitors, specialty beds and therapy surfaces (which includes fall management equipment, bariatrics equipment, pressure area management and wound therapy equipment, stretchers and wheelchairs) and surgical equipment. We believe we maintain one of the most technologically advanced and comprehensive equipment fleets in the industry, routinely acquiring new and certified pre-owned equipment to enhance our fleet. Our specialized equipment portfolio managers evaluate new products each year to keep abreast of current market technology and to determine whether to add new products to our equipment fleet. In making equipment purchases, we consider a variety of factors, including manufacturer credibility, repair and maintenance costs, anticipated user demand, equipment mobility and anticipated obsolescence. We generally do not enter into long-term fixed price contracts with suppliers of our equipment. As of December 31, 2020, we owned or managed more than a million units of medical equipment available for use by our customers of which over 250,000 were owned.
84
Table of Contents
In 2020, our ten largest manufacturers of medical equipment supplied approximately 71% (measured in dollars spent) of our direct medical equipment purchases. In 2019, four of our largest medical equipment suppliers accounted for approximately 53% of our medical equipment purchases (measured in dollars spent).
Employees
We believe a strong and sustainable company begins with an engaged and empowered team. We are committed to investing in our team’s training and development, and to open, two-way communication. Our culture is underpinned by our core belief, our Code of Conduct and our strong commitment to diversity, equity and inclusion.
We had 3,852 employees as of December 31, 2020, including 3,497 full-time and 355 part-time employees. Of such employees, 163 were sales representatives, 3,356 were operations personnel and 333 were employed in corporate support functions.
None of our employees are covered by a collective bargaining agreement, and we have experienced no work stoppages to date. We believe that our relations with our employees are good.
Intellectual Property
We have registrations with the United States Patent and Trademark Office for the following marks: Asset360® and BioMed360®; “Universal Hospital Services, Inc.,” ‘‘UHS®’’ and the UHS logo; “OnCare,” “Harmony,” “Quartet,” “Agiliti” and the Agiliti logo. We have applications pending with the United States Patent and Trademark Office for the following marks: “Vityl.” United States service mark registrations are generally for a term of 10 years, renewable every 10 years if the mark is used in the regular course of business.
We have a domain name registration for agilitihealth.com, which serves as our main website, and UHS.com. In 2011, we registered the domain name OnCareMedical.com featuring our OnCare™ sub-brand for patient handling products. In 2012, we registered UHSSurgicalServices.com. In 2016, we acquired resxray.com. We do not own any patents.
We have developed a number of proprietary software programs to directly service or support our customers including “inCare™” which is a medical equipment inventory management system that allows us to track the location and usage of equipment we are managing at a customer’s location in our 360 Solutions. “MyAgiliti™” is our online ordering and reporting site which accesses our proprietary programs specifically designed to help customers meet medical equipment documentation and reporting needs under applicable regulations and standards, such as those promulgated by the FDA and The Joint Commission. Additionally, this tool provides detailed reporting on utilization, compliance, and analytics for management. “inService™” is our equipment maintenance and planning system which houses our work order system and assists in our customers regulatory compliance recordkeeping. “Scheduler™” is our web-based scheduling, tracking, reporting and physician preference system for Agiliti Surgical solutions. “inCommand™” encompasses the proprietary software tools that allow our employees to manage and maintain our extensive equipment fleet and serve our customers more effectively and efficiently. We primarily rely on trade secret, copyright and other similar laws for the protection of our proprietary software. Our employees who access such proprietary software sign confidentiality agreements and receive training on protecting the security of our data systems, and any independent contractors who assist with development of our proprietary software are required to sign non-disclosure and work product assignment agreements.
Marketing
We market our programs primarily through our direct sales force, which consisted of 163 sales representatives as of December 31, 2020. We support our direct sales force with technical, clinical, surgical and
85
Table of Contents
financial specialists, who lead new business selling efforts to deliver comprehensive solutions for our customers. Our national accounts team also supports our direct sales force through its focus on securing national and regional contracts.
Our sales force uses a structured and consistent process to target customers where we can deliver significant financial and operational value over time. Each sales team member is responsible for identifying and prioritizing customer opportunities in their territory through the use of segmentation tools and market intelligence, leading to short- and long-term sales pipelines balanced across our comprehensive solutions. The sales force then engages customers directly with insights and tailored solutions that address specific customer challenges while using tools to demonstrate financial and operational savings. Our goal with this approach is to help customers with their most pressing challenges first and measure their return on value for each solution. We then work to connect additional solutions that add incremental and synergistic value for our customers, leading to an end-to-end approach to medical equipment management. Each activity our sales force initiates is aligned to our customer’s buying process and is designed to move the opportunity quickly through the sales process. Every step in the process is documented in a customer relationship management (CRM) system, where we continually monitor and manage sales pipelines, balanced opportunity mix and sales forecasts.
The members of the sales force are compensated with a combination of base pay and variable incentive pay. The percentage of each individual’s overall compensation that is comprised of base pay versus variable incentive pay is dependent on the individual’s position. Sales force members whose primary responsibility is account management receive a higher percentage of base pay, while sales force members whose primary responsibility is the generation of new business receive a higher percentage of variable incentive pay. The actual variable incentive pay received by an individual is based on his or her achievement of certain performance metrics, including revenue, earnings and/or new business milestones.
We also market our end-to-end solutions through our website at www.agilitihealth.com and various social media and digital marketing channels, including a variety of trade publications and organizations with subscribers and members who are key decision makers for our solutions. In addition, we participate in numerous national and regional conventions where we interact with industry groups and opinion leaders. Information presented on our website is not incorporated by reference and should not be considered a part of this Registration Statement.
In our marketing efforts, we primarily target key decision makers such as administrators, chief executive officers, chief financial officers, chief technology officers, chief medical officers and chief nursing officers as well as physicians, directors and managers of functional departments, such as supply chain, materials management, surgery, purchasing, pharmacy, biomedical services, and clinical engineering. We also promote comprehensive solutions to IDNs, hospitals, surgery centers, manufacturers and alternate site provider groups and associations.
Seasonality and Business Interruption
Quarterly operating results are typically affected by seasonal factors. Historically, our first and fourth quarters are the strongest, reflecting increased hospital census and patient acuity during the fall and winter months. Our business can also be impacted by natural disasters, such as hurricanes and earthquakes, which affect our ability to transfer equipment to and from our customers, and equipment recalls, which can cause equipment to be removed from market use. We also see declines in our business in down economic cycles with high levels of unemployment. Our customers typically see weaker census and higher levels of indigent patients during these times, causing them to use fewer of our solutions.
86
Table of Contents
Regulatory Matters
Regulation of Medical Equipment
Our customers are subject to documentation and safety reporting regulations and standards with respect to the medical equipment they use, including those established by the FDA, CMS and the National Fire Protection Association (“NFPA”). Various states and municipalities may also have similar regulations.
We monitor changes in regulations and standards to accommodate the needs of customers by providing specific product and manufacturer information upon request. Manufacturers of medical equipment are subject to regulation by agencies and organizations such as the FDA, Underwriters Laboratories and the NFPA. We believe that all medical equipment we outsource conforms to these regulations.
The Safe Medical Devices Act of 1990 (“SMDA”), which amended the Food, Drug and Cosmetic Act (“FDCA”), requires manufacturers, user facilities and importers of medical devices to report whenever they believe there is a probability that a medical device has caused or contributed to a death, illness, or injury. In addition, the SMDA requires the establishment and maintenance of adverse safety and effectiveness data and various other FDA reports. Manufacturers and importers are also required to report certain device malfunctions. We also work with our customers to assist them in meeting their reporting obligations under the FDCA, including those requirements added by the SMDA.
Besides the FDA, a number of states regulate medical device distributors and wholesalers either through pharmacy or device distributor licensure. Currently, we hold such licenses in 20 states. Some licensure regulations and statutes in additional states may apply to our activities. Although our failure to possess such licenses in these states for our existing operations may subject us to certain monetary fines, we do not believe the extent of such fines, in the aggregate, would be material to our liquidity, financial condition or results of operations.
In addition, we are required to provide information to manufacturers regarding the permanent disposal or any change in ownership of certain categories of medical outsourcing equipment. While we believe our medical equipment tracking systems are in compliance with these regulations, these regulations are subject to change and such changes could have an impact on how we conduct our business.
HIPAA applies to certain covered entities, including health plans, healthcare clearinghouses and healthcare providers, as well as to business associates such as us. HIPAA regulations protect individually identifiable health information, including information in an electronic format, by, among other things, setting forth specific standards under which such information may be used and disclosed, providing patients’ rights to obtain and amend their health information, requiring notification to individuals, federal and state agencies and media outlets in the event of a breach of health information and establishing certain administrative requirements for covered entities. The HITECH Act created legal obligations for business associates and extended criminal and civil sanctions to business associates for violations of HIPAA requirements.
Because of our self-insured health plans, we are also a covered entity under the HIPAA regulations. Also, we may be obligated to comply with certain HIPAA requirements as a business associate of various healthcare providers. In addition, various state legislatures have enacted and may continue to enact additional privacy legislation that is not preempted by the federal law, which may impose additional burdens on us. Moreover, other federal privacy legislation may be enacted. Accordingly, we have made and, as new standards go into effect, we expect to continue to make administrative, operational and information infrastructure changes in order to comply with these requirements.
The Affordable Care Act (have and will result in significant reforms to the U.S. healthcare system and the structure of the healthcare provider delivery system. The Affordable Care Act calls for additional transparency around payments made by the pharmaceutical and medical device industries to doctors and teaching hospitals,
87
Table of Contents
which may include gifts, food, travel and speaking or consultancy fees. All U.S. manufacturers of drugs, devices, biologics or medical supplies, including distributors who hold title to such drugs, devices, biologics, or medical supplies, for which payment is available under government-funded health insurance programs (i.e., Medicare, Medicaid and the State Children’s Health Insurance Program) must report annually to the U.S. Department of Health and Human Services any payment or gift, which represents a “transfer of value,” to a physician or teaching hospital, including detailed information about the nature and value of remuneration provided, and the identity of the receiving physician or teaching hospital. Additionally, states may require manufacturers to report information that is not required or is exempted under the federal reporting requirements. For example, a state may require manufacturers to report advertising expenditures, loans of medical devices, in-kind gifts to charities and payments to other recipients, group purchasing organizations and retailers. We identify applicable state reporting requirements as they become effective.
We are subject to the federal Anti-Kickback Statute, which prohibits the knowing and willful offer, payment, solicitation or receipt of any form of “remuneration” in return for, or to induce, the referral of business or ordering of services paid for by Medicare or other federal programs. ”Remuneration” has been broadly defined to include anything of value, including gifts, discounts, credit arrangements, and in-kind goods or services. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside the healthcare industry. Violations can result in imprisonment, civil or criminal fines or exclusion from Medicare, Medicaid and other governmental programs. Contracts with healthcare facilities and other marketing practices or transactions may implicate the Anti-Kickback Statute. We have attempted to structure our contracts and marketing practices to comply with the Anti-Kickback Statute along with providing training to our employees. However, we cannot ensure that we will not have to defend against alleged violations from private entities or that OIG or other authorities will not find that our practices violate the Anti-Kickback Statute.
Although our business is not currently extensively regulated under healthcare laws, we are subject to certain regulatory requirements as discussed above and our customers are subject to direct regulation under the Federal False Claims Act, the Stark Law, the Anti-Kickback Law, rules and regulations of the CMS, and other federal and state healthcare laws and regulations. Promulgation of new laws and regulations, or changes in or re-interpretations of existing laws and regulations, could affect our business, operating results or financial condition. Our operations may be negatively impacted if we have to comply with additional complex government regulations.
Third-Party Reimbursement
Our fees are paid directly by our customers rather than through direct reimbursement from third-party payors, such as Medicare or Medicaid. We do not bill the patient, the insurer or other third-party payors directly for services provided for hospital or alternate site provider inpatients or outpatients. Sometimes our customers are eligible to receive third-party reimbursement for our services. Consequently, the reimbursement policies of such third-party payors have a direct effect on the ability of healthcare providers to pay for our services and an indirect effect on our level of charges. Also, in certain circumstances, third-party payors may take regulatory or other action against service providers even though the service provider does not receive direct reimbursement from third-party payors.
Hospitals and alternate site providers face cost containment pressures from public and private insurers and other managed care providers, such as health maintenance organizations, preferred provider organizations and managed fee-for-service plans, as these organizations continue to place controls on the reimbursement and utilization of healthcare services. We believe that these payors have followed or will follow the government in limiting the services that are reimbursed and in exerting downward pressure on prices. In addition to promoting managed care plans, employers are increasingly self-funding their benefit programs and shifting costs to employees through increased deductibles, co-payments and employee contributions. Hospitals and healthcare facilities are also experiencing an increase in uncompensated care or “charity care,” which causes increased economic pressures on these organizations. We believe that these cost reduction efforts will place additional
88
Table of Contents
pressures on healthcare providers’ operating margins and will encourage efficient equipment management practices such as the use of our outsourcing and 360 on-site managed solutions.
Liability and Insurance
Although we do not manufacture any medical equipment, our business entails the risk of claims related to the outsourcing, sale and service of medical equipment. In addition, our instruction of hospital and alternate site provider employees with respect to the use of equipment and our professional consulting services are sources of potential claims. We have not suffered a material loss due to a claim. However, any such claim, if made, could have a material adverse effect on our business. While we do not currently provide any services that require us to work directly with patients, expansion of services in the future could involve such activities and subject us to claims from patients.
We maintain a number of insurance policies, including commercial general liability coverage (product and premises liability insurance), automobile liability insurance, worker’s compensation insurance and professional liability insurance. We also maintain excess liability coverage. Our policies are subject to annual renewal. We believe that our current insurance coverage is adequate. Claims exceeding such coverage may be made and we may not be able to continue to obtain liability insurance at acceptable levels of cost and coverage.
Facilities
Our corporate headquarters are in Minneapolis, Minnesota, where we lease 55,197 square feet of office space as of December 31, 2020. We also have domestic offices in Alabama, Arkansas, Arizona, California, Colorado, Connecticut, Florida, Georgia, Iowa, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Massachusetts, Maryland, Michigan, Missouri, Mississippi, North Carolina, North Dakota, Nebraska, New Jersey, New Mexico, Nevada, New York, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, Wisconsin and West Virginia.
We lease all of our facilities. We believe that our facilities are adequate for our current needs and anticipate that suitable additional space will be readily available to accommodate any foreseeable expansion of our operations.
89
Table of Contents
Below is a list of the names, ages, positions and a brief account of the business experience of the individuals who serve as our executive officers and directors.
| Name |
Age | Title | ||
| Thomas J. Leonard |
53 | Chief Executive Officer and Director | ||
| James B. Pekarek |
52 | Executive Vice President and Chief Financial Officer | ||
| Thomas W. Boehning |
55 | President | ||
| Bettyann Bird |
61 | Senior Vice President, Strategy and Solution Management | ||
| Robert L. Creviston |
54 | Senior Vice President, Human Resources | ||
| Scott A. Christensen |
56 | Vice President, Controller and Chief Accounting Officer | ||
| Matthew E. McCabe |
40 | Vice President of Finance and Treasurer | ||
| Lee M. Neumann |
45 | Senior Vice President and General Counsel | ||
| John L. Workman |
69 | Director and Chairman of the Board of Directors | ||
| Michael A. Bell |
65 | Director | ||
| Darren M. Friedman |
52 | Director | ||
| Gary L. Gottlieb |
65 | Director | ||
| Joshua M. Nelson |
48 | Director | ||
| Megan M. Preiner |
37 | Director | ||
| Scott M. Sperling |
63 | Director | ||
| Diane B. Patrick |
69 | Director Nominee |
Thomas J. Leonard joined us as Chief Executive Officer and has been a member of our Board of Directors since April 2015. Prior to joining us, Mr. Leonard served as the President of Medical Systems for CareFusion Corporation, where he led the $2.4 billion revenue global medical device segment, which includes Alaris® infusion pumps, Pyxis® automated dispensing and patient identification systems, and AVEA® and LTV® series ventilators and respiratory diagnostics products. Prior to joining CareFusion in 2008, Mr. Leonard served as the Senior Vice President and General Manager of Ambulatory Solutions for McKesson Provider Technologies, and prior to that, Executive Vice President of Operations for Picis, Inc. Mr. Leonard has a bachelor’s degree in Engineering from the United States Naval Academy and an MBA from S.C Johnson Graduate School of Management at Cornell University. Mr. Leonard is an experienced senior executive with extensive operating experience leading businesses focused on medical devices, services and healthcare IT. His experience across these closely related business segments, combined with his years leading us as our Chief Executive Officer, make him a valuable member of our Board.
James B. Pekarek joined us in April 2013 as Executive Vice President and Chief Financial Officer. Prior to joining us, Mr. Pekarek was the Chief Financial Officer for Cornerstone Brands since 2005. Cornerstone Brands is a leading home and apparel lifestyle retailer. Before that, Mr. Pekarek held executive level finance positions with The Spiegel Group, Montgomery Wards, Inc. and Outboard Marine Corporation. Mr. Pekarek has an MBA from Northwestern University and a Bachelor of Science in Accounting from Indiana University.
Thomas W. Boehning was appointed as President in January 2020. Prior to joining us, Mr. Boehning spent 13 years at Optum, serving in various positions, culminating in his role as Chief Executive Officer of their joint venture Optum360—the nation’s largest independent revenue cycle services organization. Prior to Optum, Mr. Boehning was vice president and general manager of McKesson’s Group Practice Solutions business. Mr. Boehning has a bachelor’s degree in Economics and an MBA from Pennsylvania State University.
Bettyann Bird joined us in January 2016 as the Senior Vice President of Strategy and Solution Management. Prior to joining us, Ms. Bird was Vice President of Marketing for the Global Dispensing business at CareFusion from 2012 to 2015. She has held numerous executive leadership roles within the healthcare industry, including Executive at eHealth Portal from 2009 to 2011, President and Chief Executive Officer of eStudySite from 2007
90
Table of Contents
to 2008 and President of the Consulting and Services business of Cardinal Health from 2004 to 2006. Prior to that, Bettyann held leadership positions with Deloitte Consulting and Ernst & Young. She spent her early years in healthcare as a trauma and intensive care nurse. Ms. Bird earned a bachelor’s degree in Nursing from Texas Christian University and an MBA from Baylor University.
Robert L. Creviston was named Senior Vice President, Human Resources effective February 2013. From 2007 until 2013, Mr. Creviston was the Vice President of Human Resources—Midwest Group at Waste Management, Inc., a leading North America provider of integrated environmental solutions. From 2000 to 2007 he held a variety of human resources management roles at Pactiv Corporation, a packaging business focused in the food service and consumer products space. Mr. Creviston holds a B.S. in Industrial and Labor Relations from Cornell University.
Scott A. Christensen joined us in 2012 as Controller and Chief Accounting Officer. In 2013, Mr. Christensen was named Vice President. Prior to joining us, from 1992 to 2012 Mr. Christensen served in several executive capacities at Supervalu, Inc., one of the largest companies in the U.S. grocery channel, including Vice President of Finance and Vice President of Accounting. From 1987 to 1992, Mr. Christensen served as a supervising accountant at KPMG Peat Marwick. Mr. Christensen is a graduate of St. Cloud State University and holds a B.S. in Accounting.
Matthew E. McCabe joined Agiliti, Inc. in 2006. In April 2017, Mr. McCabe was named Vice President of Finance and Treasurer. From 2014 to 2017, Mr. McCabe served as Vice President of Finance and Business Intelligence. From 2006 to 2014, Mr. McCabe held several roles with Agiliti including Business Unit CFO, Director of Strategy & Business Development and several other roles within the accounting and finance organization. Prior to Agiliti, Mr. McCabe served as an auditor at Mahoney, Ulbrich, Christensen and Russ. Mr. McCabe holds an MBA from Metropolitan State University and a B.S. in Accounting and Business Administration from Winona State University.
Lee M. Neumann became our General Counsel in January 2011. From 2009 to 2011, Ms. Neumann was our Associate General Counsel. Prior to joining us, Ms. Neumann was an attorney at Faegre Drinker Biddle & Reath LLP, formerly Faegre & Benson LLP, from 2002 to 2009. Ms. Neumann holds a Juris Doctorate degree from Hamline University School of Law and a B.A. in Biology from Gustavus Adolphus College.
John L. Workman joined us in November 2014 as a director and Chair of the Audit Committee. From February to April 2015, Mr. Workman was our Interim Executive Chairman of the Board. In April 2015, Mr. Workman was appointed as non-executive Chairman of the Board. From 2012 to June 2014, Mr. Workman served as Chief Executive Officer and Director at OmniCare, Inc. Prior to that, Mr. Workman served as Chief Financial Officer of OmniCare, Inc. from 2009 to 2012, and President from 2011 to 2012. Prior to joining OmniCare, Mr. Workman served as Chief Financial Officer of HealthSouth Corporation from 2004 to 2009. Prior to that, he spent six years serving in executive officer roles at U.S. Can Corporation, including Chief Executive Officer and Chief Operating Officer. Mr. Workman also spent more than 14 years with Montgomery Ward & Company, Inc., serving in various financial leadership capacities, including Chief Financial Officer and Chief Restructuring Officer. Mr. Workman began his career with the public accounting firm KPMG LLP. Mr. Workman is also a Director of Federal Signal Corporation (NYSE:FSS), an international manufacturing company that specializes in environmental and safety solutions where he chairs the Audit Committee. Mr. Workman also serves on the board of directors of ConMed (NASDAQ:CNMD), an international manufacturer of equipment and supplies for orthopedic and general surgery and was a director of CareCapital Properties (NYSE:CCP), which, prior to merging into a public company, was a REIT involved with long-term healthcare facilities from, August 2015 until August 2017. Mr. Workman holds a doctorate degree in education in ethical leadership from Olivet Nazarene University, an MBA from the University of Chicago and a B.S. degree from Indiana University. Mr. Workman is a valuable member of our Board because of his extensive business and financial background and his multiyear service in executive roles of companies in the healthcare sector.
91
Table of Contents
Michael A. Bell became a member of our Board in January 2019. Mr. Bell is a Managing Director at THL. Most recently, he was an executive advisor to THL. Prior to that, Mr. Bell was executive chairman of Syneos Health, Inc. (formerly INC Research Holdings, Inc./inVentiv Health, Inc.). From August 1, 2017 to December 1, 2017, Mr. Bell served as Executive Chairman and President of Syneos Health Commercial Solutions (formerly inVentiv Health Commercial Solutions). From September 2014 until he joined Syneos Health, Inc., Mr. Bell served as Chairman and Chief Executive Officer of Inventiv Health, Inc. Mr. Bell served as inVentiv Health, Inc.’s Chief Operating Officer from May 2014 until his appointment as Chairman and Chief Executive Officer. From 1998 until he joined inVentiv Health, Inc., Mr. Bell was a Managing Partner and Founder of Monitor Clipper Partners, a private equity firm where he invested in companies in the professional services, procurement outsourcing and biopharmaceutical services industries. Mr. Bell also served as Senior Executive Vice President of John Hancock Financial Services, Inc., reporting to the Chairman and Chief Executive Officer, from 2001 until the business was sold to Manulife Financial Corporation in 2004. In 1983, Mr. Bell was a founder of Monitor Group, a management consulting firm engaged across the healthcare delivery system, and was a member of the firm’s leadership team until 1998 when he joined Monitor Clipper Partners, LLC. In addition to Agiliti, Mr. Bell currently sits on the boards of AmeriLife Group, LLC, Autism Home Care Holdings, Inc., Healthcare Staffing Services, Professional Physical Therapy and Senior Home Care Holdings, Inc. Mr. Bell holds a B.S. in Economics from the Wharton School at the University of Pennsylvania and an MBA from Harvard Business School. Mr. Bell is a valuable member of our Board because of his experience serving as an executive in healthcare and related companies for several decades, experience in the private equity industry analyzing, investing in and serving on the board of directors of companies in the healthcare industry, as well as his perspective as a representative of our largest shareholder.
Darren M. Friedman became a member of our Board in March 2019. Mr. Friedman is a Partner of StepStone Group LP, a global private markets firm that oversees approximately $240 billion of private capital allocations. Prior to joining StepStone in 2010, Mr. Friedman was a Managing Partner of Citi Private Equity, where he worked from 2001 to 2010, managing over $10 billion of capital across various private equity investing activities. Mr. Friedman sits or has sat on the boards or advisory boards of multiple portfolio companies, general partners and a number of Investment Committees. Prior to joining Citi Private Equity, Mr. Friedman worked in the Investment Banking division at Salomon Smith Barney. Mr. Friedman has an MBA from the Wharton School at the University of Pennsylvania and a B.S. in Finance from the University of Illinois. Mr. Friedman is an experienced senior executive with extensive accounting and financial experience. He is a valuable member of our Board because of his accounting, financial and private equity experience and his experience on the boards of directors of other companies.
Dr. Gary L. Gottlieb became a member of our Board in January 2019. Dr. Gottlieb is a professor of psychiatry at Harvard Medical School and a member of the National Academy of Medicine. Dr. Gottlieb served as Chief Executive Officer of Partners In Health from March 2015 until June 2019. From January 2010 until February 2015, he served as President and Chief Executive Officer of Partners HealthCare (now MassGeneral Brigham), the parent of the Brigham and Women’s and Massachusetts General Hospitals and a number of the nation’s other leading academic medical centers, community hospitals and physician organizations. He served as President of Brigham and Women’s Hospital, as President of North Shore Medical Center, and as Chairman of Partners Psychiatry. Dr. Gottlieb served as a member of the Board of Directors of the Federal Reserve Bank of Boston from 2012 to 2016 and as its Chair from 2016 to 2018. Dr. Gottlieb serves as Executive Chair of Cohere, Inc. and as a Director of Kyruus, Inc., OM1, Inc. and, previously, inVentiv Health, Inc. He is an Executive Partner of Flare Capital Partners. Dr. Gottlieb earned an MBA from the Wharton School at the University of Pennsylvania and an M.D. from the Albany Medical College of Union University in a six-year accelerated biomedical program. Dr. Gottlieb is a valuable member of our Board because of his extensive experience in the healthcare and medical industries and his multiyear service in executive roles of companies in such industries.
Joshua M. Nelson became a member of our Board in January 2019. Mr. Nelson is a Managing Director and Head of Healthcare at THL, which he joined in 2003. Prior to joining THL, he worked at JPMorgan Partners, the private equity affiliate of JPMorgan Chase. Mr. Nelson currently serves on the board of directors of Adare
92
Table of Contents
Pharma Solutions, Autism Home Care Holdings, Inc., CSafe Global, Healthcare Staffing Services, Hospice Care Holdings, L.P., Professional Physical Therapy, Senior Home Care Holdings, Inc. and Syneos Health, Inc., and he previously served on the boards of 1-800 CONTACTS, Inc., Curo Health Services, Hawkeye Energy Holdings, Intermedix Corporation and Party City Holdco. Mr. Nelson holds a B.A. in political science, summa cum laude, from Princeton University and an M.B.A., with honors, from Harvard Business School. Mr. Nelson is a valuable member of our Board because of his experience investing in and serving on the boards of various healthcare companies, his skills related to analyzing and understanding a company’s financial performance, and his broad perspective related to strategic planning is valuable to our Company.
Megan M. Preiner became a member of our Board in January 2019. Ms. Preiner is a Managing Director at THL, which she joined in 2008. Prior to joining THL, she worked in the Media and Telecommunications Group at Credit Suisse. Ms. Preiner currently serves on the board of directors of Adare Pharma Solutions, Autism Home Care Holdings, Inc., CSafe Global, Healthcare Staffing Services, Hospice Care Holdings, L.P. and Senior Home Care Holdings, Inc., and she previously served on the board of directors of Intermedix Corporation and Phillips Pet Food & Supplies. Ms. Preiner holds a B.A. in Political Economy, cum laude, from Georgetown University and an MBA from Harvard Business School. Ms. Preiner is a valuable member of our Board because of her experience in the private equity industry analyzing, investing in and serving on the board of directors of companies in the healthcare industry, as well as her perspective as a representative of our largest shareholder.
Scott M. Sperling became a member of our Board in January 2019. Mr. Sperling is currently a Co-Chief Executive Officer of THL, which he has led for over 20 years. Prior to joining THL, Mr. Sperling was, for more than ten years, managing partner of The Aeneas Group, Inc., the private capital affiliate of Harvard Management Company. Before that he was a senior consultant with the Boston Consulting Group. Mr. Sperling’s current and prior directorships include Experian Information Solutions, Fisher Scientific, Front Line Management Companies, Inc., Houghton Mifflin Co., iHeartMedia, Inc., The Learning Company, Madison Square Garden Company, PriCellular Corp, ProcureNet, ProSiebenSat.1, Thermo Fisher Scientific, Univision Communications, Inc., Warner Music Group and Wyndham Hotels and several other private companies. Mr. Sperling is also the Chairman of MassGeneral Brigham. Mr. Sperling holds a B.S. from Purdue University and an MBA from Harvard Business School. Mr. Sperling is a valuable member of our Board because of his experience in the private equity industry analyzing, investing in and serving on the board of directors of companies across various industries, including healthcare, as well as his perspective as a representative of our largest shareholder.
Diane B. Patrick is expected to join our Board prior to completion of this offering. Ms. Patrick is a Senior Counsel at Ropes & Gray LLP and has practiced labor and employment law for over 35 years. Prior to serving as Senior Counsel at Ropes & Gray LLP, Ms. Patrick served as a Co-Managing Partner and as a member of several committees, including the Diversity Committee, of Ropes & Gray LLP, as an Associate at O’Melveny & Myers, as an Associate at Hogan Hartson (n/k/a Hogan Lovells US LLP), as University Attorney at Harvard University, and as Director and Associate Vice President of Human Resources at Harvard University. Ms. Patrick also serves on the boards of Massachusetts General Hospital (as Co-Vice Chair, and as Chair and member of several committees, including the Nominating and Governance Committee), MassGeneral Brigham (as Chair of the United Against Racism Task Force and member of several committees, including the Committee on Institutional Conflicts), Epiphany School (as Chair of the Academic and School Life Committee and member of several other committees, including the Committee on Trustees), and WBUR-FM, a National Public Radio station (as a member of the Community Advisory Board). Ms. Patrick also served as First Lady of the Commonwealth of Massachusetts while her husband was Governor from 2007 to 2015. Ms. Patrick will be a valuable member of our Board because of the unique perspective she brings as a legal expert, her dedication to diversity initiatives and her experience serving on the boards of directors of companies in the healthcare sector.
Family Relationships
There are no family relationships between any of our executive officers or directors.
93
Table of Contents
Corporate Governance
Board Composition and Director Independence
Our business and affairs are managed under the direction of our Board. Following completion of this offering, our Board will be composed of nine directors. Our certificate of incorporation provides that the authorized number of directors may be changed only by resolution of our Board. Our certificate of incorporation also provides that our Board is divided into three classes of directors, with the classes as nearly equal in number as possible. Subject to any earlier resignation or removal in accordance with the terms of our certificate of incorporation, and bylaws, our Class I directors are John L. Workman, Darren F. Friedman and Megan M. Preiner and will serve until the first annual meeting of shareholders following the completion of this offering, our Class II directors will be Gary G. Gottlieb, Diane B. Patrick and Scott M. Sperling and will serve until the second annual meeting of shareholders following the completion of this offering and our Class III directors will be Thomas J. Leonard, Michael A. Bell and Joshua M. Nelson and will serve until the third annual meeting of shareholders following the completion of this offering. Upon completion of this offering, we expect that each of our directors will serve in the classes as indicated above. In addition, our certificate of incorporation provide that our directors may be removed with cause upon the affirmative vote of at least a majority of the voting power of our outstanding shares of stock entitled to vote thereon. Our bylaws provide that the majority of the Board will have the right to designate the Chairman of the Board. Following this offering, John L. Workman will remain as the Chairman of our Board.
The listing standards of the NYSE require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act. As described in the sub-section entitled “Board Committees”, we believe we meet these requirements.
We anticipate that, prior to our completion of this offering, our Board will determine that John L. Workman, Michael A. Bell, Darren M. Friedman, Gary L. Gottlieb, Joshua M. Nelson, Megan M. Preiner, Diane B. Patrick and Scott M. Sperling meet the NYSE requirements to be independent directors. In making this determination, our Board considered the relationships that each such non-employee director has with us and all other facts and circumstances that our Board deemed relevant in determining their independence, including beneficial ownership of our common stock.
Controlled Company Status
After completion of this offering, THL will continue to control a majority of our outstanding common stock. As a result, we will be a “controlled company”. Under NYSE rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain NYSE corporate governance requirements, including the requirements that, within one year of the date of the listing of our common stock:
| • | our Board is composed of a majority of “independent directors”, as defined under the rules of such exchange; and |
| • | we have a compensation, nominating and governance committee that is composed entirely of independent directors. |
Following this offering, we intend to rely on this exemption. As a result, we may not have a majority of independent directors on our Board. In addition, our Compensation, Nominating and Governance Committee may not consist entirely of independent directors or be subject to annual performance evaluations. Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to all of the NYSE corporate governance requirements.
94
Table of Contents
Board Committees
Our Board has an Audit Committee and a Compensation, Nominating and Governance Committee. The composition, duties and responsibilities of these committees will be as set forth below. In the future, our Board may establish other committees, as it deems appropriate, to assist it with its responsibilities.
| Board Member |
Audit Committee | Compensation, Nominating and Governance Committee | ||
| Thomas J. Leonard |
||||
| John L. Workman |
X (Chair) | X | ||
| Michael A. Bell |
X (Chair) | |||
| Darren M. Friedman |
X | |||
| Gary L. Gottlieb |
X | |||
| Joshua M. Nelson |
X | |||
| Megan M. Preiner |
X | X | ||
| Scott M. Sperling |
||||
| Diane B. Patrick |
Audit Committee
Following this offering, our Audit Committee will remain composed of John L. Workman, Darren M. Friedman, Gary L. Gottlieb and Megan M. Preiner, with John L. Workman serving as chair of the committee. We intend to comply with the audit committee requirements of the SEC and the NYSE, which require that the Audit Committee be composed of at least one independent director at the closing of this offering, a majority of independent directors within 90 days following this offering and all independent directors within one year following this offering. We anticipate that, prior to the completion of this offering, our Board will determine that each of John L. Workman, Gary L. Gottlieb and Darren M. Friedman meets the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of the NYSE. We anticipate that, prior to our completion of this offering, our Board will determine that John L. Workman is an “audit committee financial expert” within the meaning of SEC regulations and applicable listing standards of the NYSE. The Audit Committee’s responsibilities upon completion of this offering will include:
| • | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
| • | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| • | review our policies on risk assessment and risk management; |
| • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| • | reviewing the adequacy of our internal control over financial reporting; |
| • | establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; |
| • | recommending, based upon the Audit Committee’s review and discussions with management and the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K; |
| • | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
95
Table of Contents
| • | preparing the Audit Committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing and discussing with management and our independent registered public accounting firm our earnings releases and scripts. |
Compensation, Nominating and Governance Committee
Following this offering, our Compensation, Nominating and Governance Committee will remain composed of Michael A. Bell, Joshua M. Nelson, Megan M. Preiner and John L. Workman with Michael A. Bell serving as chair of the committee. We believe that Michael A. Bell, Joshua M. Nelson and Megan M. Preiner are independent under NYSE independence standards. The Compensation, Nominating and Governance Committee’s responsibilities upon completion of this offering will include:
| • | annually reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer; |
| • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining and approving the compensation of our chief executive officer; |
| • | reviewing and approving the compensation of our other executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the Compensation, Nominating and Governance Committee; |
| • | conducting the independence assessment outlined in NYSE rules with respect to any compensation consultant, legal counsel or other advisor retained by the Compensation, Nominating and Governance Committee; |
| • | annually reviewing and reassessing the adequacy of the committee charter in its compliance with the listing requirements of the NYSE; |
| • | reviewing and establishing our overall management compensation, philosophy and policy; |
| • | overseeing and administering our compensation and similar plans; |
| • | reviewing and making recommendations to our Board with respect to director compensation; |
| • | reviewing and discussing with management the compensation discussion and analysis to be included in our annual proxy statement or Annual Report on Form 10-K; |
| • | developing and recommending to our Board criteria for board and committee membership; |
| • | subject to the rights of THL under the director nomination agreement as described in “Certain Relationships and Related Party Transactions—Related Party Transactions—Director Nomination Agreement”, identifying and recommending to our Board the persons to be nominated for election as directors and to each of our Board’s committees; |
| • | developing and recommending to our Board best practices and corporate governance principles; |
| • | developing and recommending to our Board a set of corporate governance guidelines; and |
| • | reviewing and recommending to our Board the functions, duties and compositions of the committees of our Board. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board or Compensation Committee of any entity that has one or more executive officers serving on our Board or Compensation, Nominating and Governance Committee.
96
Table of Contents
Code of Business Conduct and Ethics
We have adopted a code of conduct and ethics for all employees, directors and officers, including our chief executive officer, chief financial officer and chief accounting officer. Our code of conduct and ethics can be found at our website, www.agilitihealth.com.
ESG Oversight
Our Board oversees our approach to environmental, social and governance (“ESG”) matters, including: our governance-related policies and practices; our systems of risk management and controls; our human capital strategy; the manner in which we serve our customers and support our communities; and how we advance sustainability in our businesses and operations. The principal standing committees of our Board oversee a range of ESG matters in accordance with the scope of their charters. We know that the long-term success of our company requires a continued focus on these evolving topics and a commitment to regularly evaluating how we are doing and challenging ourselves to improve.
97
Table of Contents
Compensation Discussion and Analysis
The executive compensation discussion and analysis set forth herein reflects our executive compensation for the year ended December 31, 2020.
Introduction
In this compensation discussion and analysis, we discuss our compensation program, including our compensation philosophy and objectives and each component of compensation for our Chief Executive Officer, Chief Financial Officer, and the other individuals included with respect to 2020 compensation in the Summary Compensation Table below (collectively, the “named executive officers”).
The base salary for our named executive officers for fiscal 2020 was determined by the Compensation, Nominating and Governance Committee in March 2020 and recommended to our Board for final approval, which occurred in March 2020. The annual performance-based incentive compensation paid to our named executive officers for fiscal 2020 is expected to be determined by the Compensation, Nominating and Governance Committee and recommended to our Board for final approval in March 2021 after our audited financial results are prepared. Based on performance results from the year ended December 31, 2020, we anticipate payouts of 200% of our annual performance-based incentive compensation.
Compensation Philosophy and Objectives
We strive to ensure that we are able to attract and retain talented employees and reward performance. We also believe that the most effective executive compensation program is one that is designed to reward the achievement of our specific annual and long-term strategic goals. Accordingly, our Compensation, Nominating and Governance Committee (and our Board, in ratifying the Compensation, Nominating and Governance Committee’s determination) evaluates both performance and compensation to ensure that the compensation provided to key executives is fair and reasonable and that it remains competitive using a compilation of broad- based industry studies. Additionally, following this offering, the Compensation, Nominating and Governance Committee expects to determine a group of peer companies in the same or similar industries, adjusted for our size and ownership model, against which to benchmark our executive compensation. To these ends, our Compensation, Nominating and Governance Committee and Board have determined that our executive compensation program for our named executive officers should include base salary, annual performance-based incentive and long-term equity (stock option) compensation that rewards performance as measured against established goals, and competitive health, dental and other benefits.
Overview of Compensation Program and Process
We have structured our compensation program to motivate the named executive officers to achieve the business goals set by us and to reward them for achieving these goals. In furtherance of these aims, our Compensation, Nominating and Governance Committee conducts an annual review of our total compensation program to achieve the following goals:
| • | provide fair, reasonable and competitive compensation; |
| • | link compensation with our business plans; |
| • | reward achievement of both company and individual performance; and |
| • | attract and retain talented executives who are critical to our success. |
We believe that these goals reflect the importance of pay for performance by providing our named executive officers with an opportunity to earn compensation for above average performance. The compensation for each
98
Table of Contents
named executive officer includes (1) a base salary that we believe is competitive with salary levels for similarly situated executives of our peer companies, adjusted for our size and ownership model, and (2) incentive compensation that is contingent upon achievement of specific corporate and individual objectives.
Prior to this offering, the Compensation, Nominating and Governance Committee reviewed compensation elements and amounts for named executive officers in connection with a third-party consulting firm to assist us with determining compensation levels appropriate for a public company of our size and market. In connection with this analysis, the Compensation, Nominating and Governance Committee determined a company peer group for purposes of benchmarking our compensation packages and comparing our pay practices and overall pay levels with other leading healthcare organizations when establishing our pay guidelines. The following companies were included in this peer group: STERIS plc, Hill-Rom Holdings, Inc., Healthcare Services Group, Inc., Premier, Inc., ICU Medical, Inc., R1 RCM Inc., Integer Holdings Corporation, Masimo Corporation, Cantel Medical Corp., Merit Medical Systems, Inc., Haemonetics Corporation, Omnicell, Inc., Invacare Corporation, CONMED Corporation, Varex Imaging Corporation, AdaptHealth Corp., Avanos Medical, Inc., Natus Medical Incorporated, AngioDynamics, Inc. and AtriCure, Inc.
Based on the above analysis, the Chief Executive Officer provided compensation recommendations to the Compensation, Nominating and Governance Committee for executives other than himself based on this data and the other considerations mentioned in this compensation discussion and analysis. The Compensation, Nominating and Governance Committee recommended a compensation package for our Chief Executive Officer and determined compensation packages for our other named executive officers that are consistent with our compensation philosophy.
We expect that following this offering, the Compensation, Nominating and Governance Committee will review compensation elements and amounts for named executive officers on an annual basis, at the time of a promotion or other change in level of responsibilities and when competitive circumstances or business needs may require. We also expect that, following this offering, the Compensation, Nominating and Governance Committee will consider input from our Chief Executive Officer and Chief Financial Officer when setting financial objectives for our incentive plans. We also expect that the Compensation, Nominating and Governance Committee, in determining compensation, will consider input from our Chief Executive Officer, with the assistance of our Chief Human Resources Officer (for officers other than themselves) regarding benchmarking and recommendations for base salary, annual incentive targets and other compensation awards. The Compensation, Nominating and Governance Committee will likely give significant weight to our Chief Executive Officer’s judgment when assessing each of the other officers’ performance and determining appropriate compensation levels and incentive awards. The members of our Board (other than the Chief Executive Officer), meeting in executive session, will determine the compensation of the Chief Executive Officer, including his annual incentive targets.
2020 Executive Compensation Components
For fiscal 2020, the principal components of compensation for our named executive officers were base salary and annual performance-based incentive compensation, each of which is addressed in greater detail below. Each named executive officer also has severance and/or change of control benefits and is eligible to participate in our long-term savings plan and the broad-based benefit and welfare plans that are available to our employees in general. In addition, the named executive officers were eligible to receive equity awards. Our Compensation, Nominating and Governance Committee considers the equity awards in assessing each named executive officer’s compensation package and whether such package is consistent with our compensation program philosophy and objectives.
We do not have an established formula or target for allocating between cash and non-cash compensation or between short-term and long-term incentive compensation. Instead, our goal is to ensure that the compensation we pay is sufficient to attract and retain executive officers and to reward them for performance that meets the goals set by our Compensation, Nominating and Governance Committee.
99
Table of Contents
Setting Executive Compensation
The Compensation, Nominating and Governance Committee determines the total compensation for our named executive officers based on a consideration of the following factors:
| • | the scope of responsibility of each named executive officer; |
| • | market data from peer group companies; |
| • | an assessment of the positions of similarly situated executives within the peer group and internal comparisons to the compensation received by those executives; |
| • | internal review of each named executive officer’s compensation, both individually and relative to other named executive officers; |
| • | individual performance of each named executive officer, which is assessed based on factors such as fulfillment of job responsibilities, the financial and operating performance of the activities directed by each named executive officer, experience and potential; |
| • | the total compensation paid to each named executive officer in past years (including long-term equity (stock option) compensation awarded); and |
| • | performance evaluations for each named executive officer. |
Base Salary
We provide our named executive officers with a base salary to compensate them for services rendered. Salary levels are typically considered in March of each year as part of our performance review process and upon a promotion or other change in job responsibility. Merit-based increases to salaries of the named executive officers are based on the Compensation, Nominating and Governance Committee’s assessment of the individual’s performance.
Base salary ranges are determined for each named executive officer based on his or her position and responsibility and utilizing our Compensation, Nominating and Governance Committee’s knowledge and expertise regarding the market, with reference to market data regarding peer group compensation compiled from public sources, compensation consultants and independent analysts. Base salary ranges are designed so that salary opportunities for a given position will be targeted at the midpoint of the base salary established for each range.
Goals and objectives for our senior management team include:
| • | setting the appropriate tone at the top framework for our ethics, policies and business practices; |
| • | driving growth of revenue and EBITDA; |
| • | being present in the field across our diverse geographical footprint with our localized employee base driving culture and best practices; and |
| • | supporting the roll out of our products to customers by helping to communicate the key customer benefits of reducing cost, improving efficiency and improving patient outcomes. |
Each of the named executive officers is expected to participate in the above activities, with particular emphasis on their areas of expertise. Thus, Mr. Leonard has emphasis areas in setting our strategy and direction; Mr. Pekarek has emphasis areas in controls, reporting, budgeting and capital attraction; Mr. Boehning has emphasis areas of growth and revenue, operational excellence and customer service; Ms. Bird has the emphasis areas of market development, growth and research; and Mr. Anbari has the emphasis areas of growth and revenue, operational excellence and customer service for our surgical equipment repair service offerings.
100
Table of Contents
Finding that the named executive officers achieved the objectives discussed above, on March 3, 2020, the Compensation, Nominating and Governance Committee determined to increase the base salaries of Mr. Pekarek from $472,500 to $491,400 and Ms. Bird from $363,000 to $372,075. These increases reflected the increasing complexity of our business and the increased responsibilities these executives took on to meet growth objectives. These include increased strategic partnership activity, product development, expanded geographic markets and design and delivery of more complex customer-focused service solutions. Mr. Boehning and Mr. Anbari joined the company in 2020 and therefore were not considered for annual merit increases. On December 9, 2020, the Compensation, Nominating and Governance Committee and the Board of Directors determined to increase the base salaries of Mr. Leonard from $750,000 to $850,000 and Ms. Bird from $372,075 to $400,000.
Annual Performance-Based Incentive Compensation
The annual performance-based incentive compensation component is offered through the Executive Incentive Program (the “EIP”), an annual cash incentive program that provides our executive officers with an opportunity to earn annual incentive awards based on our financial performance. Under the EIP, our named executive officers can earn incentive awards that are based on a percentage of base salary, with the percentage for Messrs. Leonard, Pekarek and Boehning as specified in their respective employment agreements and for Mr. Anbari and Ms. Bird set at a level the Compensation, Nominating and Governance Committee has determined is consistent with such officer’s level of accountability and impact on our operations. In setting this percentage of base salary, the Compensation, Nominating and Governance Committee also considers the incentive compensation paid to executives in the peer group. The percentage of base salary for our named executive officers (other than the Chief Executive Officer) varies from 50% to 80% of base salary. The target award under the EIP for Mr. Leonard, our Chief Executive Officer, was 100% of base salary, as specified in his employment agreement. The 2020 EIP targets for each of our named executive officers were as follows:
| Named Executive Officer |
2020 Executive Incentive Plan Target as a Percent of 2020 Base Salary |
|||
| Thomas J. Leonard |
100 | % | ||
| James B. Pekarek |
70 | % | ||
| Thomas W. Bohening |
80 | % | ||
| Bettyann Bird |
70 | % | ||
| David L. Anbari |
50 | % | ||
The corporate financial performance objectives under the EIP relate to total revenue and Adjusted EBITDA, defined as earnings attributable to Agiliti Health before interest expense, income taxes, depreciation and amortization and excluding non-cash share-based compensation expense, management, board and other nonrecurring gain, expenses or loss. The threshold levels for achievement of the corporate financial objectives and the annual performance matrix for payment of awards under the EIP are determined by the Compensation, Nominating and Governance Committee. In March 2020, the threshold levels for achievement of the corporate financial objectives for 2020 EIP awards were determined by our Compensation, Nominating and Governance Committee. Our named executive officers’ eligibility to earn an incentive award is based on our achievement of those corporate financial objectives for the current year, calculated by comparing actual and target revenue and Adjusted EBITDA for that fiscal year.
Methodology
The Compensation, Nominating and Governance Committee develops an annual performance matrix that assigns a performance target between 0 and 200% based on performance to certain established metrics achieved during the fiscal year. For fiscal 2020, the Board established a defined revenue target of $664.1 million and a defined Adjusted EBITDA target of $167.9 million. The revenue target was defined using an established subset of our total revenue for fiscal 2020. The Adjusted EBITDA target was established using a subset of our operational performance for fiscal 2020. The Compensation, Nominating and Governance Committee believes
101
Table of Contents
that by using these financial metrics, the Company is encouraging profitable growth and operational excellence for the Company and its stockholders. The Compensation, Nominating and Governance Committee further believes that the financial metrics should measure comparable operating performance, as those measures provide a clearer view into the Company’s underlying performance. Consequently, the financial measures for defined revenue and defined Adjusted EBITDA exclude the effects of certain non-core items such as specific revenue streams related to certain customers and associated costs impacting comparability in our operating plan to facilitate year-to-year comparisons. As a result of the budgeted assumptions, performance reported in our audited financial statements may differ from performance against our EIP performance targets. In setting defined revenue and defined Adjusted EBITDA targets in the first quarter of 2020 for purposes of award amounts for 2020 performance under the EIP, the Company used “stretch” goals that were generally aggressive and represented internal financial and operational goals beyond the normal level of performance. The Company set these aggressive goals in an effort to motivate its officers to grow the Company and build shareholder value.
The matrix establishes a percentage achievement of zero to 200% based on our achievement of the defined Adjusted EBITDA target. The objective of the matrix is to provide significantly more weight to Adjusted EBITDA, allowing it to be the primary accelerator and decelerator while allowing revenue to be a modifier. Based on performance results from the year ended December 31, 2020, Adjusted EBITDA will be weighted approximately 90%, while revenue will be weighted approximately 10%. The following description of the matrix provides insight into how this functions by displaying deviations for various examples. While the performance matrix does range from zero to 200%, the performance matrix also provides that any achievement that falls below 50% earned on the performance matrix will result in no payout. However, the Compensation, Nominating and Corporate Governance Committee, in its discretion, may determine to pay the percentage achieved in accordance with the performance matrix as a discretionary bonus, but such discretionary bonus cannot exceed the amount as determined under the performance matrix. The range used for the revenue target for 2020 was $644.1 million to $684.1 million and the range used for Adjusted EBITDA for 2020 was $158.9 million to $174.9 million. In general, the matrix is designed to stabilize near 100% for achievement that is plus or minus $3 million from the midpoint for defined Adjusted EBITDA and plus or minus $10 million from the midpoint for defined revenue. The matrix is designed to reward near-target achievement while minimizing volatility driven by unanticipated events that are outside of management’s control, such as seasonal or market-related fluctuations. Conversely, positive or negative achievement accelerates when outside of these bands, in line with the extraordinary nature, whether positive or negative, of such performance.
For fiscal 2020, the Compensation, Nominating and Governance Committee will review our actual total revenue and Adjusted EBITDA performance for the year ended December 31, 2020 and will determine the level of achievement in accordance with annual performance matrix determined by the Compensation, Nominating and Governance Committee. Based on performance results from the year ended December 31, 2020, we anticipate payouts of 200% of our annual performance-based incentive compensation. Assuming payouts at 200%, Mr. Leonard will receive $1,510,382 for his award, Mr. Pekarek will receive $682,104 for his award, Mr. Boehning will receive $720,546 for his award, Mr. Anbari will receive $252,083 for his award and Ms. Bird will receive $520,123 for her award.
We anticipate that award amounts for 2020 performance under the EIP will be approved for the named executive officers by the Compensation, Nominating and Governance Committee in March 2021 and will be paid in April 2021. The anticipated 2020 EIP award amounts are reflected in the “Non-equity Incentive Plan Compensation” column of the Summary Compensation Table.
During the past five years (not including fiscal 2020), we achieved performance of the target five times and achieved the maximum performance level twice. The payout percentage in the past five years ranged between approximately 100.4% and 200% of the participant’s target award opportunity. Generally, the minimum, target and maximum levels for achievement of the corporate financial objective are set so that the relative difficulty of achieving the target is consistent from year to year.
102
Table of Contents
Severance and/or Change of Control Benefits
On January 4, 2019, we assumed the Agiliti Health Executive Severance Pay Plan, which provides for severance benefits for certain of our senior executive officers, including Mr. Anbari and Ms. Bird, who are named executive officers. In addition, the employment agreements with Mr. Leonard, our Chief Executive Officer, Mr. Pekarek, our Chief Financial Officer, and Mr. Boehning, our President, effective throughout 2020 provided for severance and/or change of control benefits. Severance and/or change in control benefits are meant to serve several purposes and are designed to:
| • | aid in the attraction and retention of the executives as a competitive practice; |
| • | keep the executives focused on running the business and impartial and objective when confronted with transactions that could result in a change of control; and |
| • | encourage our executives to act in the best interest of our shareholders in evaluating transactions. |
Additionally, pursuant to his employment agreement, Mr. Leonard has agreed to be subject to two-year noncompetition and two-year non-solicitation covenants. Pursuant to their employment agreement, Mr. Pekarek and Mr. Boehning have agreed to be subject to one-year noncompetition and one-year non-solicitation covenants.
For a detailed discussion of the foregoing as of December 31, 2020, please refer to the caption “Potential Payments Upon Termination or Change in Control” below.
Long-Term Savings Plan and Other Benefits
We have adopted a Long-Term Savings Plan, which is a tax-qualified retirement savings plan pursuant to which all employees, including the named executive officers, are able to contribute to the plan the lesser of up to 60% of their annual salary or the limit prescribed by the Internal Revenue Service (“IRS”) on a pre-tax basis. We will match 50% of up to 6% of base pay that is contributed to the Long-Term Savings Plan, subject to the limits prescribed by the IRS, excluding any catch-up contributions. Matching contributions and any earnings on the matching contributions are vested in accordance with the following schedule:
| Years of Service |
Vesting Percentage | |||
| Less than 1 |
— | |||
| 1 |
33 | % | ||
| 2 |
66 | % | ||
| 3 |
100 | % | ||
Long-Term Equity Incentive (Stock Option) Compensation
The 2007 Stock Option Plan of Agiliti Holdco (“2007 Stock Option Plan”) provided for the award of stock options to our named executive officers and was designed to enhance the link between the creation of shareholder value and long-term executive incentive compensation, provide an opportunity for increased equity ownership of Agiliti Holdco by executives and maintain competitive levels of total compensation. On May 9, 2018, Agiliti Holdco adopted the 2018 Executive Management Stock Option Plan (the “2018 Stock Option Plan”). On January 4, 2019, concurrently with the consummation of the Business Combination and in accordance with the terms of the A&R Merger Agreement, Agiliti assumed the 2007 Stock Option Plan and the 2018 Stock Option Plan. In accordance with the A&R Merger Agreement, approximately 75% of the outstanding stock option awards issued under these plans were terminated and exchanged for cash and the remaining 25% of the outstanding stock option awards were rolled over into fully-vested options to purchase our common stock subject to the same terms and conditions of the original awards. These options have a weighted average exercise price of $2.13 per share.
In addition, on January 4, 2019, the Agiliti, Inc. 2018 Omnibus Incentive Plan (the “2018 Omnibus Incentive Plan”) became effective. The 2018 Omnibus Incentive Plan was approved by FSAC’s shareholders at
103
Table of Contents
the special meeting held on January 3, 2019 to approve the Business Combination. The purpose of the 2018 Omnibus Incentive Plan is to provide incentives that will attract, retain and motivate our high performing officers, directors, employees and consultants. The 2018 Omnibus Incentive Plan provides for grants of stock options, stock appreciation rights, restricted stock, restricted stock units, bonus stock, dividend equivalents, other stock-based awards and other substitute awards, annual incentive awards and performance awards. Our directors, officers and other employees, as well as others performing consulting or advisory services for us and our affiliates, are eligible for grants under the 2018 Omnibus Incentive Plan. We have reserved a total of 10,356,000 shares of our common stock for issuance pursuant to the 2018 Omnibus Incentive Plan, subject to certain adjustments set forth therein. For a detailed discussion of the 2018 Omnibus Incentive Plan, please refer to the caption “2018 Omnibus Incentive Plan” below.
On March 6, 2020, we granted long-term incentive equity awards to our named executive officers. Similar to base salary determinations, the aggregate target value of equity awards is determined for each named executive officer based on his or her position and responsibility and utilizing our Compensation, Nominating and Governance Committee’s knowledge and expertise regarding the market, with reference to market data regarding the Company’s peer group. For 2020, the Compensation, Nominating and Governance Committee determined that our named executive officers would receive approximately one-third of their long-term incentive opportunity in performance restricted stock unit awards, one-third in stock options, and the remaining one-third in time-based restricted stock units, each granted pursuant to the 2018 Omnibus Incentive Plan. Options and time-based restricted stock units granted to the named executive officers during 2020 will vest in equal installments over three or four years on the anniversaries of the grant date. Performance restricted stock units granted under the 2018 Omnibus Incentive Plan for our named executive officers vest ratably on an annual basis over the three-year period following the grant date. The performance restricted stock unit awards vest annually at or between the threshold (50% of target) and maximum (150% of target) levels depending on achievement of the target annual/cumulative Adjusted EBITDA over the 36-month period commencing on January 1, 2020 and ending on December 31, 2022. There is not a discretionary component to the determination of target cumulative Adjusted EBITDA for the performance restricted stock unit awards. The target cumulative Adjusted EBITDA for the performance restricted stock unit awards granted in 2020 is based on our long-range operating plan and represents internal financial and operational goals. Our long-term incentive equity awards are intended to motivate our officers to grow the Company and build shareholder value.
Other Benefits
Our named executive officers are eligible to participate in the same broad-based benefit and welfare plans that are made available to our employees in general.
Tax and Accounting Implications
Deductibility of Executive Compensation
The Compensation, Nominating and Governance Committee reviews and considers our deductibility of executive compensation. We believe that compensation paid under our EIP is generally deductible for federal income tax purposes. Section 280G of the IRS Code provides that companies may not deduct compensation paid in connection with a change of control that is treated as an excess parachute payment. In certain circumstances, the Compensation, Nominating and Governance Committee may elect to approve compensation that is not fully deductible to ensure competitive levels of compensation for our executive officers. The Tax Act limited our deductibility to $1 million for named executive officers beginning after January 1, 2018.
Accounting for Share-Based Compensation
We account for our share-based compensation, namely stock options, restricted stock units and performance restricted stock units issued under the 2007 Stock Option Plan and the 2018 Omnibus Incentive Plan, as required by ASC Topic 718, “Compensation—Stock Compensation.”
104
Table of Contents
Compensation expense related to service provided by our employees, including the named executive officers, is recognized in our Statements of Operations.
Summary Compensation Table
The table below sets forth the total compensation awarded to, earned by or paid to the named executive officers for our 2020, 2019, 2018 and 2017 fiscal years, as applicable, and also sets forth information about certain executive officers whose compensation information was required to be included in prior confidential draft submissions of this registration statement.
Amounts listed under the “Non-Equity Incentive Plan Compensation” column for 2020 reflect the anticipated achievement of maximum performance results resulting in 200% payouts. The actual payments will determined in accordance with the “2020 Executive Incentive Plan Targets,” as approved by the Compensation, Nominating and Governance Committee and are anticipated to be paid in April 2021.
| Name and Principal Position |
Year | Salary $ |
Option Awards $ (1) |
Stock Awards $ (2) |
Non-Equity Incentive Plan Compensation $ (3) |
All Other Compensation $ (4) |
Total $ |
|||||||||||||||||||
| Thomas J. Leonard |
2020 | $ | 782,693 | $ | 586,694 | $ | 1,666,665 | $ | 1,510,382 | $ | 8,650 | $ | 4,555,084 | |||||||||||||
| Chief Executive Officer and Director |
2019 | $ | 746,500 | $ | 2,670,644 | $ | 3,992,603 | $ | 752,250 | $ | 2,606,271 | $ | 10,768,268 | |||||||||||||
| 2018 | $ | 679,952 | $ | — | $ | — | $ | 1,361,473 | $ | 8,100 | $ | 2,049,525 | ||||||||||||||
| 2017 | $ | 661,875 | $ | — | $ | — | $ | 697,616 | $ | 9,000 | $ | 1,368,491 | ||||||||||||||
| James B. Pekarek |
2020 | $ | 505,212 | $ | 166,328 | $ | 472,494 | $ | 682,104 | $ | 9,384 | $ | 1,835,522 | |||||||||||||
| Executive Vice President and Chief Financial Officer |
2019 | $ | 466,442 | $ | 895,031 | $ | 1,268,243 | $ | 331,742 | $ | 701,631 | $ | 3,663,089 | |||||||||||||
| 2018 | $ | 466,130 | $ | — | $ | — | $ | 625,424 | $ | 9,180 | $ | 1,080,734 | ||||||||||||||
| 2017 | $ | 432,765 | $ | — | $ | — | $ | 350,000 | $ | 9,555 | $ | 792,320 | ||||||||||||||
| Thomas W. Boehning (5) |
2020 | $ | 447,596 | $ | 825,768 | $ | 1,379,169 | $ | 720,546 | $ | 5,755 | $ | 3,378,834 | |||||||||||||
| President |
||||||||||||||||||||||||||
| Bettyann Bird |
2020 | $ | 385,016 | $ | 106,485 | $ | 302,495 | $ | 520,123 | $ | 8,552 | $ | 1,322,671 | |||||||||||||
| Senior Vice President, Strategy and Solution Management |
2019 | $ | 354,115 | $ | 632,008 | $ | 868,037 | $ | 305,835 | $ | 394,415 | $ | 2,554,410 | |||||||||||||
| 2018 | $ | 323,942 | $ | — | $ | — | $ | 447,845 | $ | 8,100 | $ | 779,887 | ||||||||||||||
| 2017 | $ | 305,481 | $ | — | $ | — | $ | 250,000 | $ | 1,349 | $ | 556,830 | ||||||||||||||
| David Anbari (6) |
2020 | $ | 316,346 | $ | 227,728 | $ | 341,674 | $ | 252,083 | $ | 5,626 | $ | 1,143,457 | |||||||||||||
| Senior Vice President, Operations |
||||||||||||||||||||||||||
| Kevin E. Ketzel (7) |
2019 | $ | 483,678 | $ | 1,059,114 | $ | 1,629,926 | $ | 366,252 | $ | 1,744,726 | $ | 5,283,696 | |||||||||||||
| Former President |
2018 | $ | 467,331 | $ | — | $ | — | $ | 702,784 | $ | 8,100 | $ | 1,178,215 | |||||||||||||
| 2017 | $ | 443,584 | $ | — | $ | — | $ | 400,000 | $ | 8,872 | $ | 852,456 | ||||||||||||||
| Robert L. Creviston (8) |
2019 | $ | 320,946 | $ | 232,220 | $ | 355,326 | $ | 210,624 | $ | 294,515 | $ | 1,413,631 | |||||||||||||
| Senior Vice President, Human Resources |
2018 | $ | 313,118 | $ | — | $ | — | $ | 407,472 | $ | 5,427 | $ | 726,017 | |||||||||||||
| 2017 | $ | 305,481 | $ | — | $ | — | $ | 209,285 | $ | 4,079 | $ | 518,845 | ||||||||||||||
| (1) | The amounts in the “Option Awards” column reflect the grant of option awards to the named executive officers under the 2018 Omnibus Incentive Plan. No option awards under the 2007 Stock Option Plan were granted in 2017 and 2018 to our named executive officers. The amounts in the “Option Awards” column reflect the grant date fair value or incremental fair value, determined in accordance with ASC Topic 718, of awards granted pursuant to the 2007 Stock Option Plan or 2018 Omnibus Incentive Plan, as applicable. Assumptions used in the calculation of these amounts are included in Note 7, Share-Based Compensation to our audited consolidated financial statements for the year ended December 31, 2019 included elsewhere in this prospectus. |
| (2) | The amounts in the “Stock Awards” column reflect the grant of restricted stock unit and performance restricted stock unit awards to the named executive officers under the 2018 Omnibus Incentive Plan. Assumptions used in the calculation of these amounts are included in Item 15, Note 11, Share-Based Compensation to our audited consolidated financial statements for the year ended December 31, 2019 included elsewhere in this prospectus. With respect to the performance |
105
Table of Contents
| restricted stock unit awards, the value of such awards at the grant date assuming that the highest level of performance conditions will be achieved along with the value for restricted stock units for 2019 for Messrs. Leonard, Pekarek, Ketzel and Creviston and Ms. Bird is: $4,462,322, $1,395,067, $1,836,604, $399,742 and $945,540, respectively, and for 2020 for Messrs. Leonard, Pekarek, Boehning and Anbari and Ms. Bird is: $2,083,331, $590,618, $1,545,836, $370,842 and $378,118, respectively. |
| (3) | The amounts in the “Non-Equity Incentive Plan Compensation” column reflect the cash awards to the named executive officers under the EIP and for 2020, includes the anticipated payments, which is discussed in detail under the caption “Annual Performance-Based Incentive Compensation.” |
| (4) | For 2020, the amounts in the “All Other Compensation” column reflect our contributions for the named executive officers to the Long-Term Savings Plan, discussed in detail under the caption “Long-Term Savings Plan and Other Benefits” and credit to healthcare premiums. For 2019, the amounts in the “All Other Compensation” column reflect our contributions for the named executive officers to the Long-Term Savings Plan, discussed in detail under the caption “Long-Term Savings Plan and Other Benefits,” credit to the healthcare premiums, gross-up of payroll taxes, and dividend payments on vested rollover options. For Messrs. Leonard, Pekarek, Creviston and Ms. Bird, the amounts in this column include dividend payments on vested rollover options in the amount of $2,586,111, $678,692, $276,453 and $373,115, respectively. For Mr. Ketzel, the amount includes dividend payments on vested rollover options of $770,349 and severance payment of $953,009, which was accrued for in 2019. Mr. Ketzel’s employment was terminated by the Company without cause on March 7, 2020; however, the Company notified Mr. Ketzel of such pending termination of employment in 2019 and accrued his severance payment in accordance with his employment agreement in 2019. |
| (5) | Mr. Boehning commenced employment with the Company during 2020. |
| (6) | Mr. Anbari commenced employment with the Company during 2020 and was an executive officer until January 2021, at which point he continued his role as Senior Vice President in a non-executive capacity. |
| (7) | Mr. Ketzel’s employment was terminated by the Company without cause on March 7, 2020. While he is not a named executive officer for purposes of this registration statement, we have continued to include the information provided with respect to Mr. Ketzel in our prior confidential draft submissions of this registration statement. |
| (8) | Mr. Creviston was considered a named executive officer for prior confidential draft submissions of this registration statement. While he is not a named executive officer for purposes of this registration statement, we have continued to include the information provided with respect to Mr. Creviston in our prior confidential draft submissions of this registration statement. |
2020 Grants of Plan-Based Awards
|
Estimated Future Payouts Awards (1) |
Estimated Future Payouts Awards (3) |
All Other Stock Awards: Number of Shares of Stock or Units (#) (4) |
All other Option Awards: Number of Securities Underlying Options (#) (5) |
Exercise or Base Price of Option Awards ($/sh) |
Grant Date Fair Value of Stock and Option Awards ($) (2) |
|||||||||||||||||||||||||||||||||||||
| Name |
Grant Date |
Threshold ($) |
Target ($) |
Maximum ($) |
Threshold (#) |
Target (#) |
Maximum (#) |
|||||||||||||||||||||||||||||||||||
| Thomas J. Leonard |
2020 | $ | 377,596 | $ | 755,191 | $ | 1,510,382 | 50,505 | 101,010 | 151,515 | 101,010 | 303,030 | $ | 8.25 | $ | 2,253,359 | ||||||||||||||||||||||||||
| James B. Pekarek |
2020 | $ | 170,526 | $ | 341,052 | $ | 682,104 | 14,318 | 28,636 | 42,954 | 28,636 | 85,909 | $ | 8.25 | $ | 638,822 | ||||||||||||||||||||||||||
| Thomas W. Boehning |
2020 | $ | 180,137 | $ | 360,273 | $ | 720,546 | 20,202 | 40,404 | 60,606 | 126,768 | 380,303 | $ | 8.25 | $ | 2,204,937 | ||||||||||||||||||||||||||
| Bettyann Bird |
2020 | $ | 130,031 | $ | 260,062 | $ | 520,123 | 9,167 | 18,333 | 27,500 | 18,333 | 55,000 | $ | 8.25 | $ | 408,980 | ||||||||||||||||||||||||||
| David Anbari |
2020 | $ | 80,209 | $ | 160,417 | $ | 252,083 | 3,536 | 7,071 | 10,607 | 34,344 | 103,030 | $ | 8.25 | $ | 569,402 | ||||||||||||||||||||||||||
| (1) | The amounts shown under “Estimated Future Payouts Under Non-Equity Incentive Plan Awards” reflect the threshold, target and maximum payment levels, respectively, under our EIP. The EIP awards, if earned, will be paid at or between the threshold (50% of target) and maximum (200% of target) levels depending on achievement of total revenue and Adjusted EBITDA targets for the year ended December 31, 2020. These amounts are based on the named executive officer’s base salary and position for the year ending and as of December 31, 2020. The 2020 EIP payout is included in the “Non-Equity Incentive Plan Compensation” column in the Summary Compensation Table. |
| (2) | The amounts in the “Grant Date Fair Value of Stock Option Awards” column reflect the grant date fair value, determined in accordance with ASC Topic 718, of awards pursuant to the 2018 Omnibus Incentive Plan. Assumptions used in the calculation of these amounts are |
106
Table of Contents
| included in our audited consolidated financial statements for the year ended December 31, 2019. The value reflected for any performance-conditioned award is the value at the grant date based upon the probable outcome of the award. |
| (3) | The amounts shown under “Estimated Future Payouts Under Equity Incentive Plan Awards” reflect performance restricted stock unit awards granted to the named executive officers during 2020 under the 2018 Omnibus Incentive Plan. The performance restricted stock unit, if earned, will vest at or between the threshold (50% of target) and maximum (150% of target) levels depending on the target cumulative Adjusted EBITDA over the 36-month period commencing on January 1, 2020, and ending on December 31, 2022. The named executive officers are also entitled to an accrual of dividend equivalents, equal to the cash amount that would have been payable on the number of performance restricted stock units held by them as of the close of business on the record date for each declared dividend, which shall be credited to them as the equivalent amount of shares that could have been purchased as of the close of business on the dividend payment date. The accrued dividend equivalents will be payable when the performance restricted stock units on which such dividend equivalents were credited have become earned, vested and payable. |
| (4) | Restricted stock units were granted to the named executive officers during 2020 under the 2018 Omnibus Incentive Plan, which will vest in equal installments over three to four years on the anniversaries of the grant date. The named executive officers are also entitled to an accrual of dividend equivalents, equal to the cash amount that would have been payable on the number of restricted stock units held by them as of the close of business on the record date for each declared dividend, which shall be credited to them as the equivalent amount of shares that could have been purchased as of the close of business on the dividend payment date. The accrued dividend equivalents will be payable when the restricted stock units on which such dividend equivalents were credited have become earned, vested and payable. |
| (5) | Stock options were granted to the named executive officers during 2020 under the 2018 Omnibus Incentive Plan. |
Outstanding Equity Awards at December 31, 2020
| OPTIONS AWARDS (1) | STOCK AWARDS (2) | |||||||||||||||||||||||||||||||
| Grant Date |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Grant Date |
Number of Securities Underlying Unvested Restricted Stock Unit |
Market Value of Securities Underlying Unvested Restricted Stock Unit (6) |
|||||||||||||||||||||||||
| Thomas J. Leonard (4) |
3/6/2020 | — | 303,030 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 202,020 | $ | 3,434,340 | ||||||||||||||||||||||
| Thomas J. Leonard (4) |
3/6/2019 | 110,522 | 221,043 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 147,362 | $ | 2,505,154 | ||||||||||||||||||||||
| Thomas J. Leonard (5) |
3/6/2019 | 186,505 | 559,516 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 186,505 | $ | 3,170,585 | ||||||||||||||||||||||
| Thomas J. Leonard (3) |
1/4/2019 | 1,159,691 | — | 2.13 | 11/4/2024 | — | — | |||||||||||||||||||||||||
| James B. Pekarek (4) |
3/6/2020 | — | 85,909 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 57,272 | $ | 973,624 | ||||||||||||||||||||||
| James B. Pekarek (4) |
3/6/2019 | 29,841 | 59,682 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 39,788 | $ | 676,396 | ||||||||||||||||||||||
| James B. Pekarek (5) |
3/6/2019 | 67,142 | 201,426 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 67,142 | $ | 1,141,414 | ||||||||||||||||||||||
| James B. Pekarek (3) |
1/4/2019 | 304,346 | — | 2.13 | 11/4/2024 | — | — | |||||||||||||||||||||||||
| Thomas W. Boehning (4) |
3/6/2020 | — | 121,212 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 80,808 | $ | 1,373,736 | ||||||||||||||||||||||
| Thomas W. Boehning (5) |
3/6/2020 | — | 259,091 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 86,364 | $ | 1,468,188 | ||||||||||||||||||||||
| Bettyann Bird (4) |
3/6/2020 | — | 55,000 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 36,666 | $ | 623,322 | ||||||||||||||||||||||
| Bettyann Bird (4) |
3/6/2019 | 18,236 | 36,472 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 24,314 | $ | 413,338 | ||||||||||||||||||||||
| Bettyann Bird (5) |
3/6/2019 | 49,238 | 147,712 | $ | 6.27 | 3/6/2029 | 3/6/2019 | 49,237 | $ | 837,029 | ||||||||||||||||||||||
| Bettyann Bird (3) |
1/4/2019 | 167,319 | — | 2.13 | 11/4/2024 | — | — | |||||||||||||||||||||||||
| David Anbari (4) |
3/6/2020 | — | 21,212 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 14,142 | $ | 240,414 | ||||||||||||||||||||||
| David Anbari (5) |
3/6/2020 | — | 81,818 | $ | 8.25 | 3/6/2030 | 3/6/2020 | 27,273 | $ | 463,641 | ||||||||||||||||||||||
| (1) | Option awards granted under the 2018 Omnibus Incentive Plan and the 2007 Stock Option Plan are discussed in detail under the caption “Long-Term Equity Incentive (Stock Option) Compensation”. Such unvested options included in the “Number of Securities Underlying Unexercised Options Unexercisable” column. |
| (2) | Stock awards granted under the 2018 Omnibus Incentive Plan is discussed in detail under the caption “Long-Term Equity Incentive (Stock Option) Compensation”. |
107
Table of Contents
| (3) | These option award grants reflect the assumption and conversion of outstanding options by Agiliti in connection with the Business Combination. |
| (4) | Options, restricted stock units and performance restricted stock units granted under the 2018 Omnibus Incentive Plan for our named executive officers vest ratably over three years on the grant anniversary date. |
| (5) | Options and restricted stock units granted under the 2018 Omnibus Incentive Plan for our named executive officers vest ratably over four years on the grant anniversary date. |
| (6) | This value is determined using a per share price of $17.00. |
Executive Deferred Compensation Plan
Our named executive officers, and certain other employees, may participate in the Agiliti Executive Deferred Compensation Plan, as amended and restated effective December 3, 2018 (the “Executive Deferred Compensation Plan”). The Executive Deferred Compensation Plan allows our named executives officers to defer up to 60% of their base salary and up to 100% of their earnings under the EIP payable upon the occurrence of a specified future event or date. Investment options available under the Executive Deferred Compensation Plan mirror the investment options available under our Long-Term Savings Plan.
2020 Nonqualified Deferred Compensation Table
The following table provides information for each of our named executive officers regarding aggregate executive contributions, aggregate earnings for 2020, and year-end account balances under the Executive Deferred Compensation Plan. Messrs. Leonard, Pekarek, Boehning and Anbari have never participated in the Executive Deferred Compensation Plan.
| Name |
Executive |
Aggregate Earnings in |
Aggregate Balance at | |||
| Bettyann Bird |
498,343 | 106,237 | 679,256 |
| (1) | Amounts reflect elective deferrals of 2020 salary. |
| (2) | Amounts reflect change in value of investment holdings. |
| (3) | Reflects year-end account balances of deferred compensation. Of the amounts in this column $498,343 was also included in the Total Compensation column of the Summary Compensation Table for 2020 and $70,823 was included in the Total Compensation column of the Summary Compensation Table for prior years. |
Potential Payments upon Termination or Change in Control
Employment Agreements of Thomas J. Leonard, James B. Pekarek and Thomas W. Boehning
The following is a description of the payments that would have been made to Mr. Leonard, Mr. Pekarek or Mr. Boehning pursuant to the employment agreement in effect as of December 31, 2020 had his employment been terminated on December 31, 2020.
Payments Made Upon Death or Disability
In the event of death or disability, the executive or his legal representative would have received the following:
| • | 12 months of his base salary in effect immediately prior to his death or disability; |
| • | pro-rata bonus for the year of termination, based on the number of days he was employed during such year and based on objective actual performance through the date of termination, payable in a lump-sum on the next regularly scheduled payroll date after the general release of claims becomes effective and irrevocable (the “Payment Date”); and |
108
Table of Contents
| • | within ten days following such termination, payment of accrued and unpaid base salary, accrued but unused vacation days, earned and unpaid bonus for the calendar year ending prior to the date of termination, all other accrued amounts or benefits in accordance with the Company’s benefit plans and reimbursement for expenses. |
Additionally, the executive would have received accrued vested benefits through any of our benefit plans, programs or policies (other than severance) at the times specified therein. “Disability” means the executive is disabled within the meaning of our long-term disability policy then in effect.
Payments Made Upon Termination Without Cause or Resignation For Good Reason
If the executive’s employment would have been terminated without Cause or he resigned for Good Reason prior to a change in control or more than two years after a change in control, the executive would have received payments consisting of the following:
| • | 12 months of his current base salary and 100% of his target bonus opportunity for the year of termination, which sum would have been payable during the twelve month period commencing on the date of termination in substantially equal installments in accordance with the Company’s regular payroll practices as in effect from time to time, provided that the first payment shall be made on the Payment Date; and |
| • | within ten days following such termination, payment of accrued and unpaid base salary, accrued but unused vacation days, earned and unpaid bonus for the calendar year ending prior to the date of termination, all other accrued amounts or benefits in accordance with the Company’s benefit plans and reimbursement for expenses. |
We also would have paid the executive an amount equal to the pro-rata portion of his annual bonus for the year of termination, based on the number of days he is employed during such year and based on objective actual performance through the date of termination (or the end of the month proceeding such termination if such performance is generally determined at the end of the month) payable in a lump-sum on the Payment Date. Additionally, the executive would have received a lump sum payment equivalent to the amount the COBRA premium would be for his health coverage prior to the termination (for the executive and his family to the extent applicable) multiplied by twelve. Finally, the executive’s options and restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program that would have otherwise vested during the twelve months following such termination of employment would have vested in full immediately upon such termination of employment. Performance restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program would have vested based on actual performance through the date of the executive’s termination of employment and projected performance from the date immediately after the executive’s termination of employment through the end of the applicable performance period based on our then current operating budget, with the shares earned pursuant to such performance restricted stock units prorated based on the number of days the executive would have been employed during the performance period if he had remained employed through the first anniversary of his termination of employment in comparison to the total number of days in the performance period.
Under Mr. Leonard’s, Mr. Pekarek’s and Mr. Boehning’s employment agreement in effect as of December 31, 2019, “Cause” meant:
| • | the commission by the executive of, or his indictment for (or pleading guilty or nolo contendere to) a felony or crime involving moral turpitude; |
| • | the executive’s repeated failure or refusal to faithfully and diligently perform the usual and customary duties of his employment or to act in accordance with any lawful direction or order of our Board, which failure or refusal is not cured within 30 days after written notice thereof; |
| • | the executive’s material breach of fiduciary duty; |
109
Table of Contents
| • | the executive’s theft, fraud, or dishonesty with regard to us or any of our affiliates or in connection with his duties; |
| • | the executive’s material violation of our code of conduct or similar written policies; |
| • | the executive’s willful misconduct unrelated to us or any of our affiliates having, or likely to have, a material negative impact on us or any of our affiliates (economically or reputationally); |
| • | an act of gross negligence or willful misconduct by the executive that relates to our affairs or the affairs of any of our affiliates; or |
| • | material breach by the executive of any of the provisions of his employment agreement. |
Mr. Leonard, Mr. Pekarek and Mr. Boehning would have had “Good Reason” for termination if, other than for Cause, any of the following occurred:
| • | we would have materially diminished his responsibilities, authorities or duties (provided that in the event of his disability, our appointment of an interim officer would not have constituted a diminution of his responsibilities, authorities or duties); |
| • | we would have reduced his base salary or target bonus opportunity, other than in connection with an across-the-board reduction of base salary or target bonus opportunity applicable to substantially all of our senior executives; |
| • | we would have relocated his place of employment by more than 50 miles; or |
| • | he were no longer a member of the Board; |
provided, that no event described above shall constitute Good Reason unless (A) the executive has given the Company written notice of the termination, setting forth the conduct of the Company that is alleged to constitute Good Reason, within thirty (30) days following the occurrence of such event, and (B) the executive has provided the Company at least sixty (60) days following the date on which such notice is provided to cure such conduct and the Company has failed to do so. Failing such cure, a termination of employment by executive for Good Reason shall be effective on the day following the expiration of such cure period.
Payments Made Upon Termination for Cause or Resignation Without Good Reason
If we had terminated Mr. Leonard’s, Mr. Pekarek’s or Mr. Boehning’s employment as of December 31, 2020 for Cause or he resigned without Good Reason, within ten days following such termination, he would have been entitled to receive his accrued and unpaid base salary, accrued but unused vacation days, earned and unpaid bonus for the calendar year ending prior to the date of termination, all other accrued amounts or benefits in accordance with the Company’s benefit plans and reimbursement for expenses, in each case, accrued through the date of termination. Additionally, if Mr. Leonard, Mr. Pekarek or Mr. Boehning had resigned without Good Reason, he would have been entitled to an amount equal to the pro-rata portion of his annual bonus for the year of termination, based on the number of days he was employed during such year and based on objective actual performance through the date of termination (or the end of the month proceeding such termination if such performance is generally determined at the end of the month), payable in a lump-sum on the Payment Date.
Payments Made Upon Termination Without Cause or For Good Reason following a Change of Control
If Mr. Leonard’s, Mr. Pekarek’s or Mr. Boehning’s employment would have been terminated without Cause or he resigned for Good Reason on or within two years after a change in control, he would have received the following:
| • | 24 months of his current base salary and 200% of his target bonus opportunity for the year of termination, which sum would have been payable in lump sum on the Payment Date; |
110
Table of Contents
| • | within ten days following such termination, payment of accrued and unpaid base salary, accrued but unused vacation days, earned and unpaid bonus for the calendar year ending prior to the date of termination and reimbursement for expenses, in each case, accrued through the date of termination; and |
| • | all other accrued amounts or accrued benefits in accordance with the Company’s benefit plans, programs or policies (other than severance). |
We also would have paid the executive an amount equal to the pro-rata portion of his annual bonus for the year of termination, based on the number of days he was employed during such year and based on objective actual performance through the date of termination (or the end of the month proceeding such termination if such performance is generally determined at the end of the month), payable in a lump-sum on the Payment Date. Additionally, the executive would have received a lump sum payment equivalent to the amount the monthly COBRA premium would be for his health coverage prior to the termination (for executive and his family to the extent applicable) multiplied by twenty four. Finally, executive’s options and restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program that would have otherwise vested during the twelve months following such termination of employment would have vested in full immediately upon such termination of employment. Performance restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program that would have vested based on actual performance through the date of executive’s termination of employment and projected performance from the date immediately after executive’s termination of employment through the end of the applicable performance period based on the then current operating budget of the Company, with the shares earned pursuant to such performance restricted stock units prorated based on the number of days executive would have been employed during the performance period if executive had remained employed through the first anniversary of his termination of employment in comparison to the total number of days in the performance period.
Under Mr. Leonard’s, Mr. Pekarek’s and Mr. Boehning’s employment agreements in effect as of December 31, 2020, “Change in Control” meant the occurrence, in a single transaction or in a series of related transactions, of any of the following events:
| • | any individual, entity or group has beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of more than 50% of either the then-outstanding shares or the combined voting power of the then-outstanding voting securities of the Company entitled to vote generally in the election of directors; |
| • | individuals who constitute the Board cease for any reason to constitute at least a majority of the Board (other than any individual whose election, or nomination for election by the Company’s shareholders, was approved by a vote of at least a majority of the directors); |
| • | consummation of a reorganization, merger, statutory share exchange or consolidation or similar corporate transaction involving the Company or any entity controlled by the Company, or a sale or other disposition of all or substantially all of the assets of the Company, or the acquisition of assets or stock of another entity by the Company or any entity controlled by the Company, in each case, unless, following such business combination, (A) all of substantially all of the individuals and entities that were the beneficial owners of the outstanding company common stock and outstanding company voting securities immediately prior to such business combination beneficially own, directly or indirectly, more than 50% of the then-outstanding shares of common stock and the combined voting power of the then-outstanding voting securities, as the case may be, of the corporation or entity resulting from such business combination in substantially the same proportions as their ownership, immediately prior to such business combination of the outstanding company common stock and outstanding company voting securities, as the case may be, (B) no person beneficially owns, directly or indirectly, more than 50% of, respectively, the then-outstanding shares of common stock of the corporation or equity of the entity resulting from such business combination or the combined voting power of the then-outstanding voting securities of such corporation or entity, and (C) at least a majority of the members of the Board were members of the incumbent board at the time of the execution of the initial agreement, or of the action of the Board, proving for such business combination; or |
| • | immediately prior to the complete liquidation or dissolution of the Company. |
111
Table of Contents
Payments Made Upon a Transition Plan
If Mr. Leonard’s employment would have been terminated in accordance with an orderly transition plan coordinated by the Board, Mr. Leonard would have received, in addition to the payments he would have been entitled to upon termination without cause, resignation for good reason or upon a change in control, Mr. Leonard’s options and restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program that would have otherwise vested during the twenty-four months following such termination of employment would have vested in full immediately upon such termination of employment. Performance restricted stock units granted under the Company’s 2018 Omnibus Incentive Plan or any other equity plan or program would vest based on actual performance through the date of Mr. Leonard’s termination of employment and projected performance from the date immediately after Mr. Leonard’s termination of employment through the end of the applicable performance period based on the then current operating budget of the Company, with the shares earned pursuant to such performance restricted stock units prorated based on the number of days Mr. Leonard would have been employed during the performance period if Mr. Leonard had remained employed through the second anniversary of his termination of employment in comparison to the total number of days in the performance period.
Executive Severance Pay Plan
On November 2, 2016, the Company amended its Executive Severance Pay Plan, previously adopted on June 1, 2007 and amended on December 31, 2008 to comply with Section 409A of the Code. This disclosure is qualified in its entirety by reference to the Amended Executive Severance Pay Plan.
Executives covered by the Executive Severance Pay Plan include the following named executive officers: Mr. Anbari and Ms. Bird. The terms of the Executive Severance Pay Plan, as they relate to the possible payments upon the terminations of the aforementioned named executive officers, are summarized below. The amounts shown assume that termination would have been effective as of December 31, 2020, include amounts earned through that date and are estimates of the amounts which would have been paid out to the named executive officer upon his or her termination. The actual amounts paid can only be determined at the time of the named executive officer’s separation from us.
Agiliti assumed the Executive Severance Pay Plan, as amended, in connection with the closing of the Business Combination.
Payments Made upon Certain Involuntary Terminations
In the event of termination of employment without Cause, by the executive with Good Reason or due to the death or disability of the executive, provided that the executive or his or her designee signed the General Release within 45 days of the date of termination, the executive or his or her designee would have received the following:
| • | current salary on a bi-weekly payment schedule for the 12-month period following termination (“Severance Period”); |
| • | lump payment of $11,350 for COBRA benefits; and |
| • | pro-rated bonus for the then-current fiscal year, calculated at the executive officer’s individual bonus target and pro-rated based upon the number of days the executive officer was actually employed that year, payable in a single lump sum payment on the 61st day following the date of termination. |
The first payments would have been made as soon as practicable following the effectiveness of the General Release and would have included any such payment(s) that otherwise would have been made prior to the time the General Release was effective. “General Release” means a written release of all claims against us and our affiliates in the form presented by us, which includes confidentiality, non-competition, non-solicitation and no-hire provisions.
112
Table of Contents
The Executive Severance Pay Plan does not provide for additional benefits to Mr. Anbari and Ms. Bird if their termination of employment is in connection with a change of control of the Company. In the event of termination of employment for Cause or voluntary resignation except for Good Reason, no severance benefits would have been payable.
Under the Executive Pay Severance Plan, as in effect on December 31, 2020, Cause, Disability and Good Reason meant the following:
“Cause” meant:
| • | the executive’s continued failure, whether willful, intentional, or grossly negligent, after written notice, to perform substantially the executive’s duties (the “duties”) as determined by the executive’s immediate supervisor, the Chief Executive Officer, or a senior vice president (other than as a result of a disability); |
| • | the executive’s dishonesty or fraud in the performance of the executive’s duties or a material breach by the executive of the executive’s duty of loyalty to us or our subsidiaries; |
| • | conviction or confession by the executive of an act or acts on the executive’s part constituting a felony under the laws of the United States or any state thereof or any misdemeanor by the executive which materially impairs such executive’s ability to perform the executive’s duties; |
| • | any willful act or omission on the executive’s part which is materially injurious to our financial condition or business reputation or that of any of our subsidiaries; or |
| • | any breach by the executive of any non-competition, non-solicitation, non-disclosure or confidentiality agreement applicable to the executive. |
“Disability” means the executive’s inability to perform the essential functions of his or her position for a period of at least six months due to illness or accident after being provided with any reasonable accommodation or leave the Company may be obligated by law to provide.
“Good Reason” meant that, other than for Cause, any of the events set forth in the bullets below would have occurred, the executive notified us in writing of such event; within 30 days of such event, we failed to cure the event within 60 days of receiving such notice; and the executive terminated employment no later than 90 days after providing such notice:
| • | the executive was demoted, as evidenced by a material reduction or reassignment of the executive’s duties (per the executive’s job description), provided, however, that any change in the executive’s position constituting a lateral move or promotion would not have been deemed to give rise to Good Reason unless the executive was required to relocate pursuant to the third bullet below; |
| • | the executive’s base salary was materially reduced other than in connection with an across-the-board reduction of approximately the same percentage in executive compensation to employees imposed by our Board in response to negative financial results or other adverse circumstances affecting us; or |
| • | we required the executive to relocate in excess of 50 miles from the location where the executive was then-currently employed. |
Payments Upon a Change in Control
Pursuant to the award agreements with each of our named executive officers for the stock option awards, restricted stock units and performance restricted stock units granted under the 2018 Omnibus Incentive Plan, upon a Change in Control, all outstanding stock option awards would have been exercisable, all outstanding restricted stock units would have vested in full and all outstanding performance restricted stock units would have vested in full with performance determined based on actual performance through the Change in Control, plus projected performance from the date immediately after the Change in Control through the end of the applicable performance period based on the then current operating budget of the Company.
113
Table of Contents
Under the 2018 Omnibus Incentive Plan, “Change in Control” meant the occurrence, in a single transaction or in a series of related transactions, of any of the following events:
| • | any individual, entity or group has beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of more than 50% of either the then-outstanding shares or the combined voting power of the then-outstanding voting securities of the Company entitled to vote generally in the election of directors; |
| • | individuals who constitute the Board cease for any reason to constitute at least a majority of the Board (other than any individual whose election, or nomination for election by the Company’s shareholders, was approved by a vote of at least a majority of the directors); |
| • | consummation of a reorganization, merger, statutory share exchange or consolidation or similar corporate transaction involving the Company or any entity controlled by the Company, or a sale or other disposition of all or substantially all of the assets of the Company, or the acquisition of assets or stock of another entity by the Company or any entity controlled by the Company, in each case, unless, following such business combination, (A) all of substantially all of the individuals and entities that were the beneficial owners of the outstanding company common stock and outstanding company voting securities immediately prior to such business combination beneficially own, directly or indirectly, more than 50% of the then-outstanding shares of common stock and the combined voting power of the then-outstanding voting securities, as the case may be, of the corporation or entity resulting from such business combination in substantially the same proportions as their ownership, immediately prior to such business combination of the outstanding company common stock and outstanding company voting securities, as the case may be, (B) no person beneficially owns, directly or indirectly, more than 50% of, respectively, the then-outstanding shares of common stock of the corporation or equity of the entity resulting from such business combination or the combined voting power of the then-outstanding voting securities of such corporation or entity, and (C) at least a majority of the members of the Board were members of the incumbent board at the time of the execution of the initial agreement, or of the action of the Board, proving for such business combination; or |
| • | immediately prior to the complete liquidation or dissolution of the Company. |
114
Table of Contents
The following table provides a summary of the potential payments to each of the named executive officers in connection with certain termination events or upon a change of control of the Company.
| Named Executive Officer |
Death or Disability |
Voluntary Termination (without Good Reason) |
For Good Reason or Without Cause Termination |
Change of Control Related Termination (1) |
||||||||||||
| Thomas J. Leonard: |
||||||||||||||||
| Non-Equity Incentive Plan (2) |
$ | 1,510,382 | $ | 1,510,382 | $ | 1,510,382 | $ | 1,510,382 | ||||||||
| Stock Options (3) |
— | — | — | $ | 11,026,911 | |||||||||||
| Restricted Stock Units (4) |
— | — | — | $ | 6,140,332 | |||||||||||
| Performance Restricted Stock Units (5) |
— | — | — | $ | 2,969,747 | |||||||||||
| Health and Welfare Benefits |
— | — | $ | 18,829 | $ | 37,659 | ||||||||||
| Severance Payments |
$ | 850,000 | — | $ | 2,305,890 | $ | 4,005,890 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 2,360,382 | $ | 1,510,382 | $ | 3,835,102 | $ | 25,690,921 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| James B. Pekarek: |
||||||||||||||||
| Non-Equity Incentive Plan (2) |
$ | 682,104 | $ | 682,104 | $ | 682,104 | $ | 682,104 | ||||||||
| Stock Options (3) |
— | — | — | $ | 3,553,393 | |||||||||||
| Restricted Stock Units (4) |
— | — | — | $ | 1,966,424 | |||||||||||
| Performance Restricted Stock Units (5) |
— | — | — | $ | 825,010 | |||||||||||
| Health and Welfare Benefits |
— | — | $ | 15,851 | $ | 31,703 | ||||||||||
| Severance Payments |
$ | 491,400 | — | $ | 929,653 | $ | 1,765,033 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,173,504 | $ | 682,104 | $ | 1,627,608 | $ | 8,823,666 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Thomas W. Boehning (6) |
||||||||||||||||
| Non-Equity Incentive Plan (2) |
$ | 720,546 | $ | 720,546 | $ | 720,546 | $ | 720,546 | ||||||||
| Stock Options (3) |
— | — | — | 3,327,651 | ||||||||||||
| Restricted Stock Units (4) |
— | — | — | $ | 2,155,056 | |||||||||||
| Performance Restricted Stock Units (5) |
— | — | — | $ | 686,868 | |||||||||||
| Health and Welfare Benefits |
— | — | $ | 17,135 | $ | 34,269 | ||||||||||
| Severance Payments |
$ | 475,000 | — | $ | 855,000 | $ | 1,710,000 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,195,546 | $ | 720,546 | $ | 1,592,681 | $ | 8,634,390 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Bettyann Bird |
||||||||||||||||
| Non-Equity Incentive Plan (2) |
$ | 260,062 | — | $ | 260,062 | $ | 260,062 | |||||||||
| Stock Options (3) |
— | — | — | $ | 2,457,544 | |||||||||||
| Restricted Stock Units (4) |
— | — | — | $ | 1,355,359 | |||||||||||
| Performance Restricted Stock Units (5) |
— | — | — | $ | 518,330 | |||||||||||
| Health and Welfare Benefits |
$ | 11,350 | — | $ | 11,350 | $ | 11,350 | |||||||||
| Severance Payments |
$ | 385,016 | — | $ | 385,016 | $ | 385,016 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 656,428 | — | $ | 656,428 | $ | 4,987,661 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| David Anbari |
||||||||||||||||
| Non-Equity Incentive Plan (2)- |
$ | 160,417 | — | $ | 160,417 | $ | 160,417 | |||||||||
| Stock Options (3) |
— | — | — | 901,513 | ||||||||||||
| Restricted Stock Units (4) |
— | — | — | $ | 583,848 | |||||||||||
| Performance Restricted Stock Units (5) |
— | — | — | $ | 120,207 | |||||||||||
| Health and Welfare Benefits |
$ | 11,350 | — | $ | 11,350 | $ | 11,350 | |||||||||
| Severance Payments |
$ | 350,000 | — | $ | 350,000 | $ | 350,000 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 521,767 | — | $ | 521,767 | $ | 2,127,334 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
115
Table of Contents
| (1) | Amounts in this column represent the potential payments upon a termination of employment without Cause or a termination with Good Reason on or within the two year period following a Change of Control; provided, however, that the amounts representing the value of accelerated vesting of stock options, restricted stock units and performance restricted stock units would have been payable upon a Change in Control (as defined under the 2018 Omnibus Incentive Plan) regardless of whether the named executive officer’s employment was terminated. |
| (2) | With respect to Messrs. Leonard, Pekarek and Boehning this row represents the value of the annual bonus payable under the EIP based on actual results for 2020 and, with respect to Mr. Anbari and Ms. Bird, this row represents the target value of the annual bonus payable under the EIP. |
| (3) | Represents the value of accelerated vesting of stock options that were unvested as of December 31, 2020, which is determined based on the difference between the exercise price of such options and the value of our common stock on December 31, 2020. Additionally, if Mr. Leonard’s employment were terminated in accordance with a transition plan coordinated by our Board, Mr. Leonard would have been entitled to accelerated vesting with respect to any stock options that would have otherwise vested during the twenty- four months following such termination of employment, and such accelerated vesting has a value of $8,141,873. |
| (4) | Represents the value of accelerated vesting of restricted stock units that were unvested as of December 31, 2020, which is determined based on the value of our common stock on December 31, 2020. Additionally, if Mr. Leonard’s employment were terminated in accordance with a transition plan coordinated by our Board, Mr. Leonard is entitled to accelerated vesting with respect to any restricted stock units that would have otherwise vested during the twenty-four months following such termination of employment, such accelerated vesting has a value of $4,511,092. |
| (5) | Represents the value of accelerated vesting of performance restricted stock units that were unvested at December 31, 2020, which is determined based on the value of our common stock on December 31, 2020. Additionally, if Mr. Leonard’s employment were terminated in accordance with a transition plan coordinated by our Board, Mr. Leonard would have been entitled to accelerated vesting with respect to any performance restricted stock units that would have otherwise vested prorated based on the ratio of the number of days Mr. Leonard would have been employed during the performance period if Mr. Leonard had remained employed through the second anniversary of his termination of employment in comparison to the total number of days in the performance period, and such accelerated vesting has a value of $2,397,363. |
Actions Taken in 2021 or in Connection with This Offering
In connection with this offering, we intend to grant to certain of the employees of Northfield Medical restricted stock units, performance restricted stock units and stock option awards under the 2018 Omnibus Incentive Plan with respect to an aggregate of approximately 71,269 shares of the Company’s common stock. The restricted stock unit and stock option awards will vest ratably over a three-year period and the performance restricted stock unit awards vest based on the achievement of performance metrics over a three-year period, subject to the recipient’s continued employment through each vesting date.
IPO Emergence Grants
In connection with this offering, we expect to grant certain of our employees, including our named executive officers, restricted stock units, performance restricted stock units, and stock options under the 2018 Omnibus Incentive Plan with respect to approximately 1,141,284 shares of the Company’s common stock. The restricted stock unit and stock option awards will vest ratably over a three-year period and the performance restricted stock unit awards vest based on the achievement of performance metrics over a three-year period, subject to the recipient’s continued employment through each vesting date.
116
Table of Contents
2018 Omnibus Incentive Plan
In connection with the Business Combination, we adopted the 2018 Omnibus Incentive Plan. In connection with this offering, we plan to amend the 2018 Omnibus Incentive Plan, as described herein. The 2018 Omnibus Incentive Plan provides for grants of stock options, stock appreciation rights, restricted stock, restricted stock units, bonus stock, dividend equivalents and other stock-based awards and other substitute awards, annual incentive awards and performance awards. Directors, officers and other employees of the Company and its subsidiaries, as well as others performing consulting or advisory services for the Company and its affiliates, are eligible for grants under the 2018 Omnibus Incentive Plan. The purpose of the 2018 Omnibus Incentive Plan is to provide incentives that will attract, retain and motivate high performing officers, directors, employees and consultants by aligning the interests of participants with those of our shareholders. Set forth below is a summary of the material terms of the 2018 Omnibus Incentive Plan. The following description is qualified in its entirety by reference to the 2018 Omnibus Incentive Plan, a copy of which has been filed as an exhibit to the registration statement of which this prospectus is a part.
Administration
The 2018 Omnibus Incentive Plan is administered by our compensation, nominating and governance committee. The compensation, nominating and governance committee will have the authority to construe and interpret the 2018 Omnibus Incentive Plan, grant awards and make all other determinations necessary or advisable for the administration of the plan. Awards under the 2018 Omnibus Incentive Plan may be made subject to “performance conditions” and other terms.
Share Reserve
A total of 16,663,956 shares of our common stock shares of our common stock will initially be reserved for issuance under the 2018 Omnibus Incentive Plan. Additionally, the number of shares of our common stock for issuance under the 2018 Omnibus Incentive Plan will be subject to an annual increase on January 1 of each calendar year during the remaining term of the plan, equal to the lesser of (a) 3% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares of common stock as is determined by the Board. In addition, the following shares of our common stock will again be available for grant or issuance under the 2018 Omnibus Incentive Plan: (i) shares subject to awards granted under the 2018 Omnibus Incentive Plan that are subsequently forfeited or cancelled; (ii) shares subject to awards granted under the 2018 Omnibus Incentive Plan that otherwise terminate without shares being issued; and (iii) shares surrendered, cancelled or exchanged for cash (but not shares surrendered to pay the exercise price or withholding taxes associated with the award).
Eligibility for Participation
Our employees, consultants and non-employee directors, and employees, consultants and non-employee directors of our affiliates, will be eligible to receive awards under the 2018 Omnibus Incentive Plan. The Board or compensation committee will determine who will receive awards, and the terms and conditions associated with such award.
Award Forms and Limitations
The 2018 Omnibus Incentive Plan authorized the award of stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, other cash-based awards and other stock-based awards. For stock options that are intended to qualify as incentive stock options (“ISOs”), under Section 422 of the Code, the maximum number of shares subject to ISO awards shall be .
Stock Options
The 2018 Omnibus Incentive Plan will provide for the grant of ISOs only to employees of the Company and its subsidiaries. All options other than ISOs may be granted to employees, directors and consultants of the
117
Table of Contents
Company and its affiliates. The exercise price of each stock option must generally be at least equal to the fair market value of our common stock on the date of grant. The exercise price of ISOs granted to 10% or more shareholders must be at least equal to 110% of that value. Options granted under the 2018 Omnibus Incentive Plan may be exercisable at such times and subject to such terms and conditions as the compensation, nominating and governance committee determines. The maximum term of options granted under the 2018 Omnibus Incentive Plan is ten (10) years (five years in the case of ISOs granted to 10% or more shareholders). None of the options may be exercised until vested.
Stock Appreciation Rights
Stock appreciation rights provide for a payment, or payments, in cash or shares of our common stock, to the holder based upon the difference between the fair market value of our common stock on the date of exercise and the stated exercise price of the stock appreciation right. The exercise price must be at least equal to the fair market value of common stock of Agiliti on the date the stock appreciation right is granted. Stock appreciation rights may vest based on time or achievement of performance conditions, as determined by the compensation, nominating and governance committee in its discretion.
Restricted Stock
The compensation, nominating and governance committee may grant awards consisting of shares of our common stock subject to restrictions on sale and transfer. The price (if any) paid by a participant for a restricted stock award will be determined by the compensation, nominating and governance committee. Unless otherwise determined by the compensation, nominating and governance committee at the time of award, vesting will cease on the date the participant no longer provides services to us and unvested shares will be forfeited to or repurchased by the Company. The compensation, nominating and governance committee may condition the grant or vesting of shares of restricted stock on the achievement of performance conditions and/or the satisfaction of a time-based vesting schedule.
Restricted Stock Units
Restricted stock units represent a right to receive shares of our common stock in the future. Restricted stock units may vest based on time or achievement of performance conditions, as determined by the compensation, nominating and governance committee in its discretion.
Other Stock-Based Awards and Other Cash-Based Awards
Stock-based awards, such as dividend equivalent rights and other awards denominated or payable in shares of our common stock, may be granted as additional compensation for services or performance. Similarly, the compensation, nominating and governance committee may grant other cash-based awards to participants in amounts and on terms and conditions determined by them in their discretion. Both other stock-based awards and other cash-based awards may be granted subject to vesting conditions or awarded without being subject to conditions or restrictions.
Performance Awards
A performance award is an award that becomes payable upon the attainment of specific performance goals. A performance award may become payable in cash or in shares of our common stock. These awards are subject to forfeiture prior to settlement due to termination of a participant’s employment or failure to achieve the performance conditions.
Non-Employee Director Compensation Limitation
All compensation paid or granted to any individual for service as a non-employee director with respect to any calendar year, including awards granted under the 2018 Omnibus Incentive Plan, shall not exceed $600,000, calculating the value of any award based on the grant date fair value for financial reporting purposes; provided
118
Table of Contents
that, for any fiscal year in which a non-employee director (i) first commences service on the Board, (ii) serves on a special committee of the Board, or (iii) serves as lead director or chairman of the Board, such limit shall be $750,000.
Change in Control
In the event of a change in control (as defined in the 2018 Omnibus Incentive Plan), the compensation, nominating and governance committee may, in its discretion, provide for any or all of the following actions: (i) awards may be continued, assumed or substituted with new rights, (ii) awards may be purchased for cash equal to the excess (if any) of the highest price per share of common stock paid in the change in control transaction over the aggregate exercise price of such awards, (iii) outstanding and unexercised stock options and stock appreciation rights may be terminated prior to the change in control (in which case holders of such unvested awards would be given notice and the opportunity to exercise such awards), or (iv) vesting or lapse of restrictions may be accelerated. All awards will be equitably adjusted in the case of stock splits, recapitalizations and similar transactions. If awards are not continued, assumed or substituted with new rights in connection with such change in control, all of the unvested awards (but only such awards that are not continued, assumed or continued) will vest. If awards are continued, assumed or substituted with new rights in connection with such change in control, such awards may be entitled to full double trigger vesting.
Transferability
Awards granted under the 2018 Omnibus Incentive Plan may not be transferred in any manner other than by will or by the laws of descent and distribution, or as determined by the compensation, nominating and governance committee. Unless otherwise restricted by the compensation, nominating and governance committee, nonqualified stock options or SARs may be exercised during the lifetime of the grantee only by the grantee, the grantee’s guardian or legal representative or a family member of the grantee who has acquired the nonqualified stock options or SARs by a permitted transfer. ISOs may be exercised during the lifetime of the grantee only by the grantee or the grantee’s guardian or legal representative.
Effective Date; Term
The 2018 Omnibus Incentive Plan is expected to terminate ten years from the date the Board approved the plan, unless it is terminated earlier by the Board.
Employee Stock Purchase Plan
In connection with the offering, we intend to adopt and ask our shareholders to approve the Employee Stock Purchase Plan (the “ESPP”), the material terms of which are summarized below. This summary is not a complete description of all of the provisions of the ESPP and is qualified in its entirety by reference to the ESPP, a copy of which will be filed as an exhibit to the registration statement of which this prospectus forms a part.
The ESPP authorizes the grant of options to employees that are intended to qualify for favorable U.S. federal tax treatment under Section 423 of the Code.
Shares Available for Awards; Administration
A total of 2,000,000 shares of our common stock will initially be reserved for issuance under the ESPP. In addition, the number of shares available for issuance under the ESPP will be increased annually on January 1 of each calendar year beginning in 2022, by an amount equal to the lesser of (A) 0.5% of the shares outstanding on the final day of the immediately preceding calendar year and (B) such smaller number of shares as is determined by our board of directors. In no event will more than 20,000,000 shares of our common stock be available for issuance under the ESPP. Our board of directors or a committee of our board of directors will administer and will have authority to interpret the terms of the ESPP and determine eligibility of participants. We expect that the compensation, nominating and governance committee will be the initial administrator of the ESPP.
119
Table of Contents
Eligibility
We expect that all of our employees will be eligible to participate in the ESPP, other than those employees whose customary employment is less than five months in any calendar year. However, an employee may not be granted rights to purchase stock under our ESPP if the employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power of all classes of our stock.
Grant of Rights
Stock will be offered under the ESPP during offering periods. Each offering will consist of a six-month offering period commencing on May 1 and November 1. Employee payroll deductions will be used to purchase shares on each purchase date during an offering period. The purchase dates for each offering period will be the final trading day in the offering period; however, the plan administrator may, in its discretion, choose a different length of future offering periods, provide for multiple purchase dates within an offering period or otherwise modify the terms of future offering periods.
The ESPP permits participants to purchase common stock through payroll deductions of up to 15% of their eligible compensation. The purchase price of the shares, in the absence of a contrary designation, will be 85% of the fair market value of our common stock on the purchase date. The maximum number of shares that may be purchased by a participant during any offering period will be 5,000 shares. In addition, no employee will be permitted to accrue the right to purchase stock at a rate in excess of $25,000 worth of shares during any calendar year during which such a purchase right is outstanding (based on the fair market value per share of our common stock as of the first day of the offering period).
In order to become a participant and participate in an offering period, an eligible employee will be required to complete an enrollment election form on or prior to the first trading day of an offering period. After becoming a participant in the ESPP such participant’s elections on the enrollment election form will carry forward to future offering periods until or unless the participant completes new elections on an enrollment election form. Participants may voluntarily end their participation in the ESPP at any time during a specified offering period prior to the end of the offering period, and will be distributed their accrued payroll deductions without interest and no shares of common stock will be purchased on the participant’s behalf at the end of the offering period. Participation ends automatically upon a participant’s termination of employment.
A participant may not transfer rights granted under the ESPP other than by will or the laws of descent and distribution, and rights granted under the ESPP are generally exercisable only by the participant.
Certain Transactions
In the event, at any time or from time to time, a stock dividend, stock split, spin off, combination or exchange of shares, recapitalization, merger, consolidation, statutory share exchange, distribution to shareholders other than a normal cash dividend, or other change in the Company’s corporate or capital structure results in (a) the outstanding shares of our common stock, being exchanged for a different number or kind of our securities or of any other company or (b) new, different or additional securities of us or of any other company being received by the holders of our common stock, then the plan administrator will make proportional adjustments in (i) the maximum number and kind of securities available for issuance under the ESPP, including without limitation. Notwithstanding the foregoing, in the event of any dissolution, liquidation, merger, consolidation, sale of all or substantially all of our outstanding securities, sale, lease, exchange or other transfer of all or substantially all of our assets, or any similar transaction as determined by the plan administrator in its sole discretion, the plan administrator may make such adjustments it deems appropriate to prevent dilution or enlargement of rights in (1) the number and class of shares which may be delivered under the ESPP, (2) in the number, class of shares or price of shares available for purchase under the ESPP and (3) in the number of shares
120
Table of Contents
which a participant is entitled to purchase and any other adjustments it deems appropriate. Additionally, in the event of any such transaction, the plan administrator may elect to (1) have the options under the ESPP assumed or converted or substituted by a successor entity, (2) terminate all outstanding options either prior to their expiration or upon completion of the purchase of shares on the next purchase date, (3) shorten the offering period by setting a new purchase date, or (4) take such other action deemed appropriate by the plan administrator.
Plan Amendment
The plan administrator may amend, suspend or terminate the ESPP at any time. However, shareholder approval will be obtained for any amendment that increases the aggregate number or changes the type of shares that may be sold pursuant to rights under the ESPP or changes the corporations or classes of corporations whose employees are eligible to participate in the ESPP.
Director Compensation
Through December 31, 2020, we paid each of our then-current independent directors cash compensation of $60,000 per year for their service as independent directors ($95,000 for the chair). All amounts are prorated for partial year directorship or membership, as appropriate. Directors who were not independent did not receive compensation for services on the board of directors in 2020.
In connection with the closing of the Business Combination, Agiliti assumed the 2007 Stock Option Plan and approximately 75% of the outstanding stock option awards were terminated and cashed out. The remaining 25% of the outstanding stock option awards were rolled over into options to purchase common stock of Agiliti subject to the same terms and conditions of the original awards.
2020 Director Compensation Table
The table below summarizes the compensation paid by us to directors for the year ended December 31, 2020:
| Director |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
Stock Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
All Other Compensation ($) |
Total ($) |
||||||||||||||||||
| Thomas J. Leonard |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Michael A. Bell |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Dr. Gary L. Gottlieb |
$ | 60,000 | $ | 56,805 | $ | — | $ | — | $ | — | $ | 116,805 | ||||||||||||
| Joshua M. Nelson |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Megan M. Preiner |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Scott M. Sperling |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| John L. Workman |
$ | 95,000 | $ | 56,805 | $ | — | $ | — | $ | — | $ | 151,805 | ||||||||||||
| Darren Friedman |
$ | 60,000 | $ | 56,805 | $ | — | $ | — | $ | — | $ | 116,805 | ||||||||||||
| (1) | This column reflects the aggregate grant date fair value of option awards granted in 2020 in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Item 15 Note 11, Share-Based Compensation to our audited consolidated financial statements for the year ended December 31, 2019 included elsewhere in this prospectus. The aggregate number of options outstanding as of December 31, 2020 was as follows: |
| Name |
Options Outstanding | |
| Dr. Gary L. Gottlieb |
106,612 | |
| John L. Workman (a) |
134,265 | |
| Darren Friedman |
106,612 |
121
Table of Contents
| (a) | Includes 27,653 options that reflect the assumption and conversion of outstanding options by Agiliti in connections with the Business Combination. |
In connection with this offering, we expect to grant each of Dr. Gottlieb and Messrs. Workman and Friedman restricted stock units with a grant date value of approximately $160,000. These restricted stock units will vest in full on the one-year anniversary of the vesting commencement date, subject to the recipient’s continued service through such vesting date. On a going forward basis, following the completion of this offering, we expect to provide our non-employee directors with an annual cash retainers as follows:
| Position |
Retainer ($) | |||
| Chairman of the Board |
125,000 | |||
| Board Member |
75,000 | |||
| Audit Committee: |
||||
| Chairperson |
25,000 | |||
| Committee Member |
12,000 | |||
| Compensation, Nominating and Governance Committee: |
||||
| Chairperson |
17,500 | |||
| Committee Member |
7,500 | |||
The non-employee directors who are employees of THL are not eligible to receive cash retainers or equity awards for their service on our board.
122
Table of Contents
The following table sets forth information about the beneficial ownership of our common stock as of March 1, 2021 and as adjusted to reflect the sale of the common stock in this offering, for
| • | each person or group known to us who beneficially owns more than 5% of our common stock immediately prior to this offering; |
| • | each of our directors, director nominees and named executive officers; and |
| • | all of our directors, director nominees and executive officers as a group. |
Each shareholder’s percentage ownership before the offering is based on common stock outstanding as of March 1, 2021. Each shareholder’s percentage ownership after the offering is based on common stock outstanding immediately after the completion of this offering. We have granted the underwriters an option to purchase up to 3,947,368 additional shares of common stock. The table below does not reflect any shares of common stock that may be purchased in this offering or pursuant to our directed share program.
Beneficial ownership for the purposes of the following table is determined in accordance with the rules and regulations of the SEC. These rules generally provide that a person is the beneficial owner of securities if such person has or shares the power to vote or direct the voting thereof, or to dispose or direct the disposition thereof or has the right to acquire such powers within 60 days. Common stock subject to options or RSUs that are currently exercisable or exercisable within 60 days of March 1, 2021 are deemed to be outstanding and beneficially owned by the person holding the options or RSUs. These shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. Except as disclosed in the footnotes to this table and subject to applicable community property laws, we believe that each shareholder identified in the table possesses sole voting and investment power over all common stock shown as beneficially owned by the shareholder.
Unless otherwise noted below, the address of each beneficial owner listed on the table is c/o 6625 West 78th Street, Suite 300, Minneapolis, Minnesota. Beneficial ownership representing less than 1% is denoted with an asterisk (*).
| Name of Beneficial Owner |
Shares Beneficially Owned Prior to this Offering |
Shares Beneficially Owned After this Offering |
||||||||||||||||||
| Number of Shares |
Percentage | Number of Shares |
No Exercise of Underwriters’ Option |
Full Exercise of Underwriters’ Option |
||||||||||||||||
| Percentage | Percentage | |||||||||||||||||||
| 5% Shareholders: |
||||||||||||||||||||
| THL Agiliti LLC (1) |
98,195,398 | 99.2 | % | 98,195,398 | 78.4 | % | 76.0 | % | ||||||||||||
| Directors, Director Nominees and Named Executive Officers: |
||||||||||||||||||||
| Scott M. Sperling |
— | — | — | — | — | |||||||||||||||
| Joshua M. Nelson |
— | — | — | — | — | |||||||||||||||
| Megan M. Preiner |
— | — | — | — | — | |||||||||||||||
| Michael A. Bell |
25,000 | * | 25,000 | * | * | |||||||||||||||
| Gary L. Gottlieb (2) |
131,612 | * | 131,612 | * | * | |||||||||||||||
| Darren M. Friedman (3) |
106,612 | * | 106,612 | * | * | |||||||||||||||
| John L. Workman (4) |
134,265 | * | 134,265 | * | * | |||||||||||||||
| Thomas J. Leonard (5) |
2,566,604 | 2.5 | % | 2,566,604 | 2.0 | % | 2.0 | % | ||||||||||||
| James B. Pekarek (6) |
640,733 | * | 640,733 | * | * | |||||||||||||||
| Bettyann Bird (7) |
396,295 | * | 396,295 | * | * | |||||||||||||||
| Thomas W. Boehning (8) |
160,438 | * | 160,438 | * | * | |||||||||||||||
| Diane B. Patrick |
— | — | — | — | — | |||||||||||||||
| Directors, director nominees and executive officers as a group (16 individuals) |
4,706,106 | 4.6 | % | 4,706,106 | 3.6 | % | 3.5 | % | ||||||||||||
123
Table of Contents
| * | Represents beneficial ownership of less than 1% |
| (1) | Voting and investment determinations with respect to the securities held of record by THL Stockholder are made by unanimous consent of its members. The members of THL Stockholder are Thomas H. Lee Equity Fund VIII, L.P. (“THL Equity VIII”), Thomas H. Lee Parallel Fund VIII, L.P. (“Parallel Fund VIII”), THL Executive Fund VIII, L.P. (“Executive Fund VIII”), THL Fund VIII Coinvestment Partners, L.P. (“Coinvestment VIII”), THL Equity Fund VIII Investors (Agiliti), L.P. (“THL Agiliti VIII”) and FS Sponsor LLC (“FS Sponsor”). Voting and investment determinations with respect to the securities beneficially owned by FS Sponsor listed herein are made by a management committee consisting of Todd M. Abbrecht, Thomas M. Hagerty, Anthony J. DiNovi and Scott M. Sperling. THL Holdco, LLC (“Holdco”) is the managing member of Thomas H. Lee Advisors, LLC, which is the general partner of Thomas H. Lee Partners, L.P., which in turn is the general partner of Coinvestment VIII, the sole member of THL Managers VIII, LLC and is the sole member of THL Equity Advisors VIII, LLC (“Equity Advisors”). Equity Advisors is the general partner of each of THL Equity VIII, Parallel Fund VIII, Executive Fund VIII and THL Agiliti VIII. Voting and investment determinations with respect to the securities beneficially owned by Holdco listed herein are made by a management committee consisting of Messrs. Abbrecht, Hagerty, DiNovi and Sperling. Each of the entities and individuals named above disclaims beneficial ownership of the securities of the Company held of record by THL Stockholder, except to the extent of its pecuniary interest therein. The address of the entities and individuals named above is c/o Thomas H. Lee Partners, L.P., 100 Federal Street, 35th Floor, Boston, MA 02110. |
| (2) | Includes (i) 25,000 shares held directly and (ii) 106,612 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021. |
| (3) | Includes (i) 106,612 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021. |
| (4) | Includes (i) 134,265 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021. |
| (5) | Includes (i) 336,081 shares held directly, (ii) 1,854,755 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021 and (iii) 375,768 shares issuable upon the settlement of restricted stock units or performance-based restricted stock units that are currently vested or that will become vested within 60 days of March 1, 2021. |
| (6) | Includes (i) 526,948 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021 and (ii) 113,785 shares issuable upon the settlement of restricted stock units or performance-based restricted stock units that are currently vested or that will become vested within 60 days of March 1, 2021. |
| (7) | Includes (i) 320,596 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021 and (ii) 75,699 shares issuable upon the settlement of restricted stock units or performance-based restricted stock units that are currently vested or that will become vested within 60 days of March 1, 2021. |
| (8) | Includes (i) 105,177 shares issuable upon the exercise of options that are currently exercisable or that will become exercisable within 60 days of March 1, 2021 and (ii) 55,261 shares issuable upon the settlement of restricted stock units or performance-based restricted stock units that are currently vested or that will become vested within 60 days of March 1, 2021. |
124
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Policies for Approval of Related Party Transactions
Prior to completion of this offering, we intend to adopt a policy with respect to the review, approval and ratification of related party transactions. Under the policy, our Audit Committee is responsible for reviewing and approving related person transactions. In the course of its review and approval of related party transactions, our Audit Committee will consider the relevant facts and circumstances to decide whether to approve such transactions. In particular, our policy requires our Audit Committee to consider, among other factors it deems appropriate:
| • | the related person’s relationship to us and interest in the transaction; |
| • | the material facts of the proposed transaction, including the proposed aggregate value of the transaction; |
| • | the impact on a director’s independence in the event the related person is a director or an immediate family member of the director; |
| • | the benefits to us of the proposed transaction; |
| • | if applicable, the availability of other sources of comparable products or services; and |
| • | an assessment of whether the proposed transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
The Audit Committee may only approve those transactions that are in, or are not inconsistent with, our best interests and those of our shareholders, as the Audit Committee determines in good faith.
Registration Rights Agreement
Agiliti is party to a registration rights agreement with THL Stockholder, Mr. Leonard and certain other holders, under which the parties thereto have been granted certain customary demand registration rights on short form and long form registration statements as well as “piggyback” registration rights, with respect to the shares of common stock of Agiliti, in each case subject to cutback provisions. The registration rights agreement does not contemplate the payment of penalties or liquidated damages to the shareholders party thereto as a result of a failure to register, or delays with respect to the registration of, Agiliti’s common stock.
Director Nomination Agreement
In connection with this offering, we will enter into an amended and restated director nomination agreement (the “director nomination agreement”) with THL Stockholder whereby so long as THL Stockholder beneficially owns at least 5% of our common stock then outstanding, THL Stockholder has the right to designate: (i) all of the nominees for election to our Board for so long as THL Stockholder beneficially owns 40% or more of the total number of shares of our common stock beneficially owned by THL Stockholder upon completion of this offering, as adjusted for any reorganization, recapitalization, stock dividend, stock split, reverse stock split or similar changes in our capitalization (the “Original Amount”); (ii) a number of directors (rounded up to the nearest whole number) equal to 40% of the total directors for so long as THL Stockholder beneficially owns at least 30% and less than 40% of the Original Amount; (iii) a number of directors (rounded up to the nearest whole number) equal to 30% of the total directors for so long as THL Stockholder beneficially owns at least 20% and less than 30% of the Original Amount; (iv) a number of directors (rounded up to the nearest whole number) equal to 20% of the total directors for so long as THL Stockholder beneficially owns at least 5% and less than 10% of the Original Amount; and (v) one director for so long as THL Stockholder beneficially owns at least 5% and less than 10% of the Original Amount. In each case, THL Stockholder’s nominees must comply with applicable law and stock exchange rules. In addition, THL Stockholder shall be entitled to designate the replacement for any of
125
Table of Contents
its board designees whose board service terminates prior to the end of the director’s term regardless of THL Stockholder’s beneficial ownership at such time. THL Stockholder shall also have the right to have its designees participate on committees of our Board proportionate to its stock ownership, subject to compliance with applicable law and stock exchange rules. The director nomination agreement will also prohibit us from increasing or decreasing the size of our Board without the prior written consent of THL Stockholder. This agreement will terminate at such time as THL Stockholder owns less than 5% of the Original Amount.
Tax Receivable Agreement
On January 4, 2019, Agiliti Holdco and IPC/UHS, as Stockholders’ Representative, entered into the Tax Receivable Agreement (the “Tax Receivable Agreement”). The Tax Receivable Agreement provides for the payment by Agiliti Holdco to the sellers of 85% of certain U.S. federal, state, and local tax benefits realized or deemed realized by Agiliti Holdco and its subsidiaries from the following tax attributes: (i) U.S. federal, state and local net operating losses (including carryforwards) of Agiliti Holdco and its subsidiaries in existence as of the closing of the Business Combination, (ii) without duplication of amounts included under clause (i), certain deductions generated by the consummation of the Business Combination, (iii) certain deductions arising from rollover options cancelled or exercised post-closing as such deductions are generated post-closing and (iv) certain deductions arising from payments made under the Tax Receivable Agreement. Agiliti Holdco and its subsidiaries will retain the tax benefit, if any, of the remaining 15% of such tax attributes. The amount and timing of any such payments may vary depending upon a number of factors. The term of the Tax Receivable Agreement will continue until all tax benefits subject to the Tax Receivable Agreement have been utilized or deemed utilized, unless Agiliti Holdco exercises its right to terminate the Tax Receivable Agreement early or there is otherwise a deemed early termination (e.g., upon the occurrence of a change of control of us, FSAC or Agiliti Holdco or certain divestitures of Agiliti Holdco’s subsidiaries) pursuant to the terms of Tax Receivable Agreement. If Agiliti Holdco elects to terminate the Tax Receivable Agreement early or there is a deemed early termination, its obligations under the Tax Receivable Agreement would accelerate and it generally would be required to make an immediate payment equal to the present value of the deemed anticipated future payments to be made by it under the Tax Receivable Agreement, calculated in accordance with certain valuation assumptions set forth in the Tax Receivable Agreement.
Notwithstanding the foregoing, after the fifth anniversary of the date of the closing of the Business Combination, a tax benefit payment due to a holder of any options or restricted stock units (RSUs) in Agiliti Holdco shall be paid only if either (i) such holder is employed by on the first day of the calendar year following the taxable year for which such tax benefit payment was calculated or (ii) Agiliti Holdco has aggregate revenue of at least $225,000,000 (which may be adjusted downward due to certain transactions) for the first two quarters of the taxable year following the taxable year for which such tax benefit payment was calculated.
Advisory Services Agreement
On January 4, 2019, we entered into an advisory services agreement (the “Advisory Services Agreement”) with Agiliti Health and THL Managers VIII, LLC (the “Advisor”). Pursuant to the Advisory Services Agreement, the Advisor provides management, consulting and other advisory services to the companies. In consideration for these services, the companies pay to the Advisor the Advisory Services Fees, comprising (i) a non-refundable periodic retainer fee in an aggregate amount per fiscal quarter equal to the greater of (a) $375,000 or (b) 1% of the consolidated Adjusted EBITDA (as defined in the Advisory Services Agreement) for the immediately preceding fiscal quarter or such other amount as may be mutually agreed, (ii) fees in amounts to be mutually agreed in connection with any financing or refinancing, dividend, recapitalization, acquisition, disposition and spin-off or split-off transaction, (iii) prior to the completion of this offering, in addition to the fees under clauses (i) and (ii), an amount equal to the net present value of the higher periodic fee that would have been payable from the date of the closing of this offering until the scheduled termination date of the Advisory Services Agreement, and (iv) fees for other management, consulting and other advisory services to be discussed in good faith among the parties. The companies pay expenses incurred by the Advisor, its consultants and certain other parties affiliated with Advisor.
126
Table of Contents
The Advisory Services Agreement terminates automatically immediately prior to the completion of this offering. We estimate that in connection with the termination of the Advisory Services Agreement, we will be required to pay to the Advisor a buyout fee of approximately $7.0 million, all of which would be expensed at the time of the buyout.
Indemnification Agreements
On January 4, 2019, we entered into indemnification agreements with each of our directors and executive officers. Each indemnification agreement provides for indemnification and advancements by us of certain expenses and costs relating to claims, suits or proceedings arising from his or her service to us or, at our request, service to other entities, as officers or directors to the maximum extent permitted by Delaware law.
Assignment and Assumption Agreement
On January 4, 2019, we, FSAC and Continental Stock Transfer & Trust Company (the “Warrant Agent”) entered into an Assignment and Assumption Agreement (the “Assignment and Assumption Agreement”) pursuant to which we agreed to accept and assume all of FSAC’s rights, interests and obligations in and under the warrants to purchase FSAC Class A Common Stock and under that certain Warrant Agreement, by and between FSAC and the Warrant Agent, dated as of July 18, 2017. Following the Business Combination and the entry into the Assignment and Assumption Agreement, each FSAC warrant became exercisable for one share of our common stock in accordance with the terms of the agreement governing such warrants, and are referred to herein as the “warrants.”
Directed Share Program
At our request, the underwriters have reserved up to 1,315,789 shares of our common stock, or 5% of the shares of our common stock to be offered by this prospectus for sale, at the initial public offering price, to certain individuals through a directed share program, including certain employees and certain other individuals identified by management.
127
Table of Contents
DESCRIPTION OF CERTAIN INDEBTEDNESS
Set forth below is a summary of the terms of the Credit Agreement governing certain of our outstanding indebtedness. This summary is not a complete description of all of the terms of the Credit Agreement. The Credit Agreement setting forth the terms and conditions of certain of our outstanding indebtedness will be filed as an exhibit to the registration statement of which this prospectus forms a part.
First Lien Credit Facilities
On January 4, 2019, Agiliti Health, Inc., as borrower, Agiliti Holdco, Inc. as holdings, and certain subsidiaries of Agiliti Health, Inc. acting as guarantors, entered into a credit agreement, with JPMorgan Chase Bank, N.A., as administrative agent, collateral agent and letter of credit issuer and the lenders from time to time party thereto, comprised of a $150.0 million five-year senior secured revolving credit facility and $660.0 million seven-year senior secured term loan facility. Pursuant to the Amendment No. 1 to First Lien Credit Agreement, dated as of February 6, 2020, the Revolving Credit Facility was increased to $190.0 million and the First Lien Term Loan Facility was increased to $785.0 million. Pursuant to the Amendment No. 2 to First Lien Credit Agreement, dated as of October 16, 2020, the First Lien Term Loan Facility was increased to $935.0 million. Pursuant to the Amendment No. 3 to First Lien Credit Agreement, dated as of March 19, 2021, the First Lien Term Loan Facility was increased to $1,135.0 million. A portion of the proceeds from the initial borrowings under the First Lien Credit Agreement were used to prepay certain existing indebtedness of the borrower and its subsidiaries and the proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 1 were used to finance working capital needs and general corporate purposes, including to finance acquisitions and to refinance the loans then outstanding under the Revolving Credit Facility. The proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 2 were used to finance working capital needs and general corporate purposes. The proceeds of the incremental term loans from the First Lien Credit Agreement Amendment No. 3 were used to finance the Northfield Acquisition and related transactions. As of December 31, 2020, we had $922.2 million in borrowings outstanding under our First Lien Term Loan Facility and $6.3 million of letters of credit outstanding under our Revolving Credit Facility. As of December 31, 2020, the interest rate on our First Lien Term Loan Facility and Revolving Credit Facility was 2.9375% on the $772.2 million and 3.5% on the $150.0 million.
Second Lien Credit Facilities
On November 15, 2019, Agiliti Health, Inc., as borrower, Agiliti Holdco, Inc., as holdings, and certain subsidiaries of Agiliti Health, Inc. acting as guarantors, entered into a second lien credit agreement, with Wilmington Trust, National Association, as administrative agent and collateral agent, the lenders from time to time party thereto and Goldman Sachs Lending Partners LLC, as bookrunner and lead arranger, comprised of a $240.0 million loan. The proceeds from the borrowings under the Second Lien Credit Agreement were used to pay a dividend or distribution payment to our equityholders as a means to provide our equityholders with a return on their investment in an amount equal to approximately $240.0 million and certain transaction costs in connection therewith. As of December 31, 2020, we had $240.0 million in borrowings outstanding under the Second Lien Term Loan Facility. As of December 31, 2020, the interest rate on our Second Lien Term Loan Facility was 7.96838%.
Interest Rates and Fees
Borrowings under the First Lien Credit Agreement and Second Lien Credit Agreement bear interest at a rate per annum, at the borrower’s option, equal to an applicable margin, plus (a) a base rate determined by reference to the highest of (i) the prime lending rate published in The Wall Street Journal, (ii) the federal funds rate in effect on such day plus 1/2 of 1.00% and (iii) the LIBOR rate for a one-month interest period on such day plus 1.00% or (b) a LIBOR rate determined by reference to the LIBOR rate as set forth by the ICE Benchmark Administration for the interest period relevant to such borrowing subject to a LIBOR floor of 0.00%.
128
Table of Contents
The applicable margin for borrowings under the First Lien Credit Agreement is (a)(1) prior to March 31, 2019 and (2) on or after March 31, 2019 (so long as the first lien leverage ratio is greater than 3.75 to 1.00), (1) 2.00% for alternate base rate borrowings and (ii) 3.00% for Eurodollar borrowings and (b) on or after June 30, 2020 (so long as the first lien leverage ratio is less than or equal to 3.75 to 1.00), subject to step downs to (i) 1.75% for alternate base rate borrowings and (ii) 2.75% for Eurodollar borrowings. Under the First Lien Credit Agreement, the borrower is also required to pay a commitment fee on the average daily undrawn portion of the Revolving Credit Facility of (i) 0.50% per annum if the first lien leverage ratio is greater than 4.00:1.00, (ii) 0.375% per annum if the first lien leverage ratio is less than or equal to 4.00:1.00 but greater than 3.50:1.00 and (iii) 0.250% if the first lien leverage ratio is less than or equal to 3.50:1.00, and a letter of credit participation fee equal to the applicable margin for Eurodollar revolving loans on the actual daily amount of the letter of credit exposure.
The applicable margin for borrowings under the Second Lien Credit Agreement is (a) 6.75% for alternate base rate borrowings and (b) 7.75% for Eurodollar borrowings.
Voluntary Prepayments
The borrower may voluntarily prepay outstanding loans under the First Lien Credit Agreement without premium or penalty, subject to certain notice and priority requirements. The borrower may voluntarily prepay outstanding loans under the Second Lien Credit Agreement (i) subject to a 102% premium with respect to prepayments made prior to November 15, 2020 and (ii) 101% premium on or after November 15, 2020 but prior to November 15, 2022.
Mandatory Prepayments
The First Lien Credit Agreement requires the borrower to prepay, subject to certain exceptions, the First Lien Term Loan Facility with:
| • | commencing with the year ending on December 31, 2019, 50% of excess cash flow for the year then ended, minus, at the borrower’s option, certain optional prepayments and permitted assignments of indebtedness, to the extent such amount is above a threshold amount and subject to step downs to (i) 25% when the first lien leverage ratio is less than or equal to 4.00 to 1.00 but greater than 3.50 to 1.00 and (ii) 0% when the first lien leverage ratio is less than or equal to 3.50 to 1.00; |
| • | 100% of the net cash proceeds of certain asset sales or casualty events above a threshold amount, subject to reinvestment rights and other exceptions; and |
| • | 100% of the net cash proceeds of any issuance or incurrence of debt other than debt permitted under the First Lien Credit Agreement. |
The Second Lien Credit Agreement also requires the borrower to prepay, subject to certain exceptions, the Second Lien Term Loan Facility with:
| • | commencing with the year ending on December 31, 2019, 50% of excess cash flow for the year then ended, minus, at the borrower’s option, certain optional prepayments and permitted assignments of indebtedness, to the extent such amount is above a threshold amount and subject to step downs to (i) 25% when first lien leverage ratio is less than or equal to 5.00 to 1.00 but greater than 4.50 to 1.00 and (ii) 0% when first lien leverage ratio is less than or equal to 4.50 to 1.00; |
| • | 100% of the net cash proceeds of certain asset sales or casualty events above a threshold amount, subject to reinvestment rights and other exceptions; and |
| • | 100% of the net cash proceeds of any issuance or incurrence of debt other than debt permitted under the Second Lien Credit Agreement; provided that, notwithstanding anything to the contrary in the Second Lien Credit Agreement, unless the borrower otherwise so elects, no mandatory prepayments are required under the Second Lien Credit Agreement until all obligations and amounts owed under the First Lien Credit Agreement are discharged in full. |
129
Table of Contents
Final Maturity and Amortization
The First Lien Term Loan Facility will mature on January 4, 2026 and the Revolving Credit Facility will mature on January 4, 2024. The term loans under the First Lien Term Loan Facility amortize in equal quarterly instalments, which commenced on June 30, 2019, in an aggregate annual amount equal to 1.00% of the original principal amount of such term loans, with the balance due at maturity unless prepaid prior thereto.
The Second Lien Term Loan Facility will mature on November 15, 2027. The Second Lien Term Loan Facility does not amortize.
Guarantors
All obligations under the First Lien Credit Agreement and Second Lien Credit Agreement are unconditionally guaranteed by substantially all of Agiliti Health, Inc.’s existing and future direct and indirect wholly-owned domestic subsidiaries, other than certain excluded subsidiaries.
Security
All obligations under the First Lien Credit Agreement are secured, subject to permitted liens and other exceptions, relative rights and priorities between the First Lien Credit Agreement and the Second Lien Credit Agreement, by first-priority perfected security interests in substantially all of the borrower’s and the guarantors’ assets. All obligations under the Second Lien Credit Agreement are secured, subject to permitted liens and other exceptions, by second-priority perfected security interests in substantially all of the borrower’s and the guarantors’ assets are governed by an intercreditor agreement.
Certain Covenants, Representations and Warranties
The First Lien Credit Agreement and the Second Lien Credit Agreement contain customary representations and warranties, affirmative covenants, reporting obligations and negative covenants. The negative covenants restrict Agiliti Health, Inc. and its subsidiaries’ ability (and, with respect to certain of the affirmative covenants and negative covenants, Agiliti Holdco’s ability), among other things, to (subject to certain exceptions set forth in the First Lien Credit Agreement and the Second Lien Credit Agreement):
| • | incur additional indebtedness and guarantee obligations; |
| • | create or incur liens; |
| • | engage in mergers or consolidations; |
| • | sell, transfer or otherwise dispose of assets; |
| • | pay dividends and distributions or repurchase capital stock; |
| • | prepay, redeem or repurchase certain indebtedness; |
| • | make investments, loans and advances; |
| • | enter into burdensome agreements with negative pledge clauses or restrictions on subsidiary distributions; |
| • | modify organizational documents in a manner that is materially adverse to the lenders under the First Lien Credit Agreement or Second Lien Credit Agreement; |
| • | with respect to Agiliti Holdco, Inc., modify our holding company status; |
| • | materially alter the business it conducts; |
130
Table of Contents
| • | change our fiscal year; and |
| • | enter into amendments to certain junior lien and subordinated indebtedness in a manner materially adverse to the lenders thereunder with negative pledge clauses or restrictions on subsidiary distributions. |
Financial Covenant
The First Lien Credit Agreement contains a springing financial covenant, solely with respect to the Revolving Credit Facility, which requires the borrower to maintain a maximum first lien leverage ratio (as calculated on a quarterly basis as of the most recently ended test period for which the covenant is being tested pursuant to the First Lien Credit Agreement) of 7.00:1.00. The financial covenant is only tested for any fiscal quarter in the event that on the last day of the such fiscal quarter, the aggregate amount of the Revolving Credit Facility that were outstanding exceeded 35% of the total commitment under the Revolving Credit Facility.
The Second Lien Credit Agreement does not include any financial covenant.
Events of Default
The lenders under the First Lien Credit Agreement and Second Lien Credit Agreement are permitted to accelerate the loans and terminate commitments thereunder or exercise other remedies upon the occurrence of certain customary events of default, including default with respect to financial and other covenants, subject to certain grace periods and exceptions. These events of default include, among others, payment defaults, cross-defaults to certain material indebtedness, covenant defaults, material inaccuracy of representations and warranties, certain events of bankruptcy, material judgments, material defects with respect to lenders’ perfection on the collateral, and changes of control.
131
Table of Contents
The following description of our capital stock is intended as a summary only and is qualified in its entirety by reference to our certificate of incorporation and bylaws to be in effect at the closing of this offering, which are filed as exhibits to the registration statement of which this prospectus forms a part, and to the applicable provisions of the DGCL.
Common Stock
Dividend Rights
Holders of our common stock are entitled to receive such dividends, if any, as may be declared from time to time by our Board in its discretion out of funds legally available therefor. In no event will any stock dividends or stock splits or combinations of stock be declared or made on our common stock unless the shares of common stock at the time outstanding are treated equally and identically.
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors. Our shareholders do not have cumulative voting rights in the election of directors. Accordingly, holders of a majority of the voting shares are able to elect all of the directors.
Preemptive or Other Rights
Our shareholders will have no preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to our common stock.
Liquidation, Dissolution and Winding Up
In the event of the voluntary or involuntary liquidation, dissolution, distribution of assets or winding-up of us, our holders of the common stock will be entitled to receive an equal amount per share of all of our assets of whatever kind available for distribution to shareholders, after the rights of the holders of the preferred stock have been satisfied.
Preferred Stock
Our Board has the authority, without further action by our shareholders, to issue up to 50,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges, and restrictions thereof. No shares of preferred stock are outstanding and we have no present plan to issue any shares of preferred stock.
Warrants
Each whole warrant entitles the registered holder to purchase one share of our common stock at a price of $11.50 per share, subject to adjustment as discussed below, and only whole warrants are exercisable. The warrants expire on January 4, 2023, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
We may call the warrants for redemption in whole and not in part at a price of $0.01 per warrant upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder if, and only if, the reported last sale price of our common stock equals or exceeds $18.00 per share as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like for any 20 trading days within a 30-trading day period ending three business days before we send the notice of redemption to warrant holders.
If and when the warrants become redeemable, we may exercise the redemption rights even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws.
132
Table of Contents
We have established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and we issue a notice of redemption of the warrants, each warrant holder will be entitled to exercise its warrant prior to the scheduled redemption date. However, the price of our common stock may fall below the $18.00 redemption trigger price as well as the $11.50 (for whole shares) warrant exercise price after the redemption notice is issued.
If we call the warrants for redemption as described above, our management will have the option to require any holder that wishes to exercise its warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their warrants on a “cashless basis,” our management will consider, among other factors, our cash position, the number of warrants that are outstanding and the dilutive effect on our shareholders of issuing the maximum number of shares of our common stock issuable upon the exercise of the warrants. If our management takes advantage of this option, all holders of warrants would pay the exercise price by surrendering their warrants for that number of shares of our common stock equal to the quotient obtained by dividing (x) the product of the number of shares of our common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” is the average reported last sale price of our common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. If our management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of our common stock to be received upon exercise of the warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption. We believe this feature is an attractive option to it if we do not need the cash from the exercise of the warrants. If we call its warrants for redemption and management does not take advantage of this option, THL and its permitted transferees would still be entitled to exercise their private placement warrants for cash or on a cashless basis using the same formula described above that other warrant holders would have been required to use had all warrant holders been required to exercise their warrants on a cashless basis, as described in more detail below.
A holder of a warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 9.8% (or such other amount as a holder may specify) of the shares of our common stock outstanding immediately after giving effect to such exercise.
If the number of outstanding shares of our common stock is increased by a stock dividend payable in shares of common stock, or by a split-up of shares of common stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of common stock issuable on exercise of each warrant will be increased in proportion to such increase in the outstanding shares of common stock. A rights offering to holders of common stock entitling holders to purchase shares of common stock at a price less than the fair market value will be deemed a stock dividend of a number of shares of common stock equal to the product of (i) the number of shares of common stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for common stock) multiplied by (ii) one (1) minus the quotient of (x) the price per share of common stock paid in such rights offering divided by (y) the fair market value. For these purposes (i) if the rights offering is for securities convertible into or exercisable for common stock, in determining the price payable for common stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) fair market value means the volume weighted average price of common stock as reported during the ten (10) trading day period ending on the trading day prior to the first date on which the shares of common stock trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if we, at any time while the warrants are outstanding and unexpired, pay a dividend or make a distribution in cash, securities or other assets to the holders of common stock on account of such shares of
133
Table of Contents
common stock (or other shares of our capital stock into which the warrants are convertible), other than (a) as described above or (b) certain ordinary cash dividends, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of our common stock in respect of such event.
If the number of outstanding shares of our common stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of common stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of common stock issuable on exercise of each warrant will be decreased in proportion to such decrease in outstanding shares of common stock.
Whenever the number of shares of our common stock purchasable upon the exercise of the warrants is adjusted, as described above, the warrant exercise price will be adjusted by multiplying the warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of common stock purchasable upon the exercise of the warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of common stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding shares of our common stock (other than those described above or that solely affects the par value of such shares of common stock), or in the case of any merger or consolidation of us with or into another corporation (other than a consolidation or merger in which we are the continuing corporation and that does not result in any reclassification or reorganization of our outstanding shares of common stock), or in the case of any sale or conveyance to another corporation or entity of its assets or other property as an entirety or substantially as an entirety in connection with which we are dissolved, the holders of the warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the warrants and in lieu of the shares of our common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the warrants would have received if such holder had exercised their warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of common stock in such a transaction is payable in the form of common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the warrant properly exercises the warrant within thirty days following public disclosure of such transaction, the warrant exercise price will be reduced as specified in the warrant agreement based on the Black-Scholes value (as defined in the warrant agreement) of the warrant.
The warrants were issued in registered form under a warrant agreement with Continental Stock Transfer & Trust Company, as warrant agent. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of a majority of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to us, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of our common stock and any voting rights until they receive the shares of our common stock issuable upon exercise of the warrants. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one (1) vote for each share held of record on all matters to be voted on by shareholders.
134
Table of Contents
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round down to the nearest whole number of shares of our common stock to be issued to the warrant holder.
Anti-Takeover Effects of Our Certificate of Incorporation and Our Bylaws
Our certificate of incorporation, bylaws and the DGCL will contain provisions, which are summarized in the following paragraphs that are intended to enhance the likelihood of continuity and stability in the composition of our Board. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our Board to maximize shareholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of us by means of a tender offer, a proxy contest or other takeover attempt that a shareholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of common stock held by shareholders.
These provisions include:
Classified Board
Our certificate of incorporation will provide that our Board will be divided into three classes of directors, with the classes as nearly equal in number as possible, and with the directors serving three-year terms. As a result, approximately one-third of our Board will be elected each year. The classification of directors will have the effect of making it more difficult for shareholders to change the composition of our Board. Our certificate of incorporation will also provide that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed exclusively pursuant to a resolution adopted by our Board. Upon completion of this offering, we expect that our Board will have nine members.
Shareholder Action by Written Consent
Our certificate of incorporation will preclude shareholder action by written consent at any time when THL beneficially owns, in the aggregate, less than 35% in voting power of our stock entitled to vote generally in the election of directors.
Special Meetings of Shareholders
Our certificate of incorporation and bylaws will provide that, except as required by law, special meetings of our shareholders may be called at any time only by or at the direction of our Board, Chief Executive Officer or the chairman of our Board; provided, however, at any time when THL beneficially owns, in the aggregate, at least 35% in voting power of our stock entitled to vote generally in the election of directors, special meetings of our shareholders shall also be called by our Board or the chairman of our Board at the request of THL. Our bylaws will prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in our control or management.
Advance Notice Procedures
Our bylaws will establish an advance notice procedure for shareholder proposals to be brought before an annual meeting of our shareholders, including proposed nominations of persons for election to our Board; provided, however, at any time when THL beneficially owns, in the aggregate, at least 10% in voting power of our stock entitled to vote generally in the election of directors, such advance notice procedure will not apply to THL. Shareholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our Board or by a shareholder who was
135
Table of Contents
a shareholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our Secretary timely written notice, in proper form, of the shareholder’s intention to bring that business before the meeting. Although the bylaws will not give our Board the power to approve or disapprove shareholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, the bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us. These provisions do not apply to nominations by THL pursuant to the director nomination agreement.
Removal of Directors; Vacancies
Our certificate of incorporation will provide that directors may be removed with or without cause upon the affirmative vote of a majority in voting power of all outstanding shares of stock entitled to vote thereon, voting together as a single class; provided, however, at any time when THL beneficially owns, in the aggregate, less than 40% in voting power of our stock entitled to vote generally in the election of directors, directors may only be removed for cause, and only by the affirmative vote of holders of at least 662⁄3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class. In addition, our certificate of incorporation will provide that, subject to the rights granted to one or more series of preferred stock then outstanding, any newly created directorship on our Board that results from an increase in the number of directors and any vacancies on our Board will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, by a sole remaining director.
Supermajority Approval Requirements
At any time when THL beneficially owns, in the aggregate, less than 50% in voting power of all outstanding shares of the stock of the Company entitled to vote generally in the election of directors, our certificate of incorporation and bylaws will provide that our Board is expressly authorized to make, alter, amend, change, add to, rescind or repeal, in whole or in part, our bylaws without a shareholder vote in any matter not inconsistent with the laws of the State of Delaware and our certificate of incorporation. For as long as THL beneficially owns, in the aggregate, at least 50% in voting power of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of a majority in voting power of the outstanding shares of our stock entitled to vote on such amendment, alteration, change, addition, rescission or repeal. At any time when THL beneficially owns, in the aggregate, less than 50% in voting power of all outstanding shares of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of the holders of at least 662⁄3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class.
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together as a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate of incorporation requires a greater percentage.
Our certificate of incorporation will provide that at any time when THL beneficially owns, in the aggregate, less than 50% in voting power of our stock entitled to vote generally in the election of directors, the following provisions in our certificate of incorporation may be amended, altered, repealed or rescinded only by the affirmative vote of the holders of at least 662⁄3% (as opposed to a majority threshold that would apply if THL beneficially owns, in the aggregate, 50% or more) in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class:
| • | the provision requiring a 662⁄3% supermajority vote for shareholders to amend our bylaws; |
| • | the provisions providing for a classified board of directors (the election and term of our directors); |
| • | the provisions regarding resignation and removal of directors; |
| • | the provisions regarding entering into business combinations with interested shareholders; |
136
Table of Contents
| • | the provisions regarding shareholder action by written consent; |
| • | the provisions regarding calling special meetings of shareholders; |
| • | the provisions regarding filling vacancies on our Board and newly created directorships; |
| • | the provisions eliminating monetary damages for breaches of fiduciary duty by a director; and |
| • | the amendment provision requiring that the above provisions be amended only with a 662⁄3% supermajority vote. |
The combination of the classification of our Board, the lack of cumulative voting and the supermajority voting requirements will make it more difficult for our existing shareholders to replace our Board as well as for another party to obtain control of us by replacing our Board. Because our Board has the power to retain and discharge our officers, these provisions could also make it more difficult for existing shareholders or another party to effect a change in management.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock and preferred stock will be available for future issuance without shareholder approval, subject to stock exchange rules. These additional shares may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. One of the effects of the existence of authorized but unissued common stock or preferred stock may be to enable our Board to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our shareholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Business Combinations
Upon completion of this offering, we will not be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested shareholder” for a three-year period following the time that the person becomes an interested shareholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested shareholder. An “interested shareholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested shareholder status, 15% or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested shareholder is prohibited unless it satisfies one of the following conditions: (1) before the shareholder became an interested shareholder, the board of directors approved either the business combination or the transaction which resulted in the shareholder becoming an interested shareholder; (2) upon consummation of the transaction which resulted in the shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or (3) at or after the time the shareholder became an interested shareholder, the business combination was approved by the board of directors and authorized at an annual or special meeting of the shareholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested shareholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a shareholders’ amendment approved by at least a majority of the outstanding voting shares.
137
Table of Contents
We will opt out of Section 203; however, our certificate of incorporation will contain similar provisions providing that we may not engage in certain “business combinations” with any “interested shareholder” for a three-year period following the time that the shareholder became an interested shareholder, unless:
| • | prior to such time, our Board approved either the business combination or the transaction which resulted in the shareholder becoming an interested shareholder; |
| • | upon consummation of the transaction that resulted in the shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding certain shares; or |
| • | at or subsequent to that time, the business combination is approved by our Board and by the affirmative vote of holders of at least 662/3% of our outstanding voting stock that is not owned by the interested shareholder. |
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested shareholder” to effect various business combinations with us for a three-year period. This provision may encourage companies interested in acquiring us to negotiate in advance with our Board because the shareholder approval requirement would be avoided if our Board approves either the business combination or the transaction which results in the shareholder becoming an interested shareholder. These provisions also may have the effect of preventing changes in our Board and may make it more difficult to accomplish transactions which shareholders may otherwise deem to be in their best interests.
Our certificate of incorporation will provide that THL, and any of its direct or indirect transferees and any group as to which such persons are a party, do not constitute “interested shareholders” for purposes of this provision.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our shareholders will have appraisal rights in connection with a merger or consolidation of us. Pursuant to the DGCL, shareholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Shareholders’ Derivative Actions
Under the DGCL, any of our shareholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the shareholder bringing the action is a holder of our shares at the time of the transaction to which the action relates or such shareholder’s stock thereafter devolved by operation of law.
Exclusive Forum
Our certificate of incorporation will provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the United States District Court for the District of Delaware) will be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of fiduciary duty owed by, or other wrongdoing by, any of our directors, officers or other employees or agents to us or our shareholders, or a claim of aiding and abetting any such breach of fiduciary duty, (3) any action asserting a claim against us or any of our directors or officers or agents arising pursuant to any provision of the DGCL, our certificate of incorporation or our bylaws, (4) any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws, (5) any action asserting a claim against us or any director, officer, employee or agent governed by the internal affairs doctrine or (6) any action asserting an “internal corporate claim” as that term is defined in Section 115 of the DGCL; provided that for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action”, will not apply to suits to enforce a duty or liability
138
Table of Contents
created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation will also provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to the provisions of our certificate of incorporation described above. Although we believe these provisions benefit us by providing increased consistency in the application of Delaware law for the specified types of actions and proceedings, the provisions may have the effect of discouraging lawsuits against us or our directors and officers.
Conflicts of Interest
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or shareholders. Our certificate of incorporation will, to the maximum extent permitted from time to time by Delaware law, renounce any interest or expectancy that we have in, or right to be offered an opportunity to participate in, specified business opportunities that are from time to time presented to certain of our officers, directors or shareholders or their respective affiliates, other than those officers, directors, shareholders or affiliates who are our or our subsidiaries’ employees. Our certificate of incorporation will provide that, to the fullest extent permitted by law, none of THL or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his director and officer capacities) or his or her affiliates will have any duty to refrain from (1) engaging in a corporate opportunity in the same or similar lines of business in which we or our affiliates now engage or propose to engage or (2) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that THL or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself or himself or its or his affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our certificate of incorporation will not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as our director or officer. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our certificate of incorporation, we have sufficient financial resources to undertake the opportunity, and the opportunity would be in line with our business.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their shareholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our certificate of incorporation will include a provision that eliminates the personal liability of directors for monetary damages for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions will be to eliminate the rights of us and our shareholders, through shareholders’ derivative suits on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation will not apply to any director if the director has acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper benefit from his or her actions as a director.
Our bylaws will provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also will be expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance will be useful to attract and retain qualified directors and officers.
The limitation of liability, indemnification and advancement provisions that will be included in our certificate of incorporation and bylaws may discourage shareholders from bringing a lawsuit against directors for
139
Table of Contents
breaches of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our shareholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company. The transfer agent’s address is 1 State Street Plaza, 30th Floor, New York, NY 10004-1561.
Listing
We have been approved to list our common stock on the NYSE under the symbol “AGTI”.
140
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Before this offering, there has been no public market for our common stock. As described below, only a limited number of shares currently outstanding will be available for sale immediately after this offering due to contractual and legal restrictions on resale. Nevertheless, future sales of substantial amounts of our common stock, including shares issued upon the exercise of outstanding options or outstanding warrants, in the public market after this offering, or the perception that those sales may occur, could cause the prevailing market price for our common stock to fall or impair our ability to raise capital through sales of our equity securities.
Upon the closing of this offering, based on the number of shares of our common stock outstanding as of March 1, 2021, we will have 125,299,085 outstanding shares of our common stock, after giving effect to the issuance of shares of our common stock in this offering, assuming no exercise by the underwriters of their option to purchase additional shares.
Of the 125,299,085 shares that will be outstanding immediately after the closing of this offering, we expect that all of the shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates”, as that term is defined in Rule 144 under the Securities Act. Shares purchased by our affiliates may not be resold except pursuant to an effective registration statement or an exemption from registration, including the safe harbor under Rule 144 of the Securities Act described below.
The remaining 98,983,296 shares of our common stock outstanding after this offering will be “restricted securities”, as that term is defined in Rule 144 of the Securities Act, and we expect that substantially all of these restricted securities will be subject to the lock-up agreements described below. These restricted securities may be sold in the public market only if the sale is registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 of the Securities Act, which are summarized below.
Lock-up Agreements
We, each of our directors and executive officers and other shareholders, optionholders and warrantholders owning substantially all of our common stock and options or warrants to acquire common stock, have agreed that, without the prior written consent of BofA Securities, Inc. and Goldman Sachs & Co. LLC on behalf of the underwriters, we and they will not, subject to limited exceptions, directly or indirectly sell or dispose of any shares of common stock or any securities convertible into or exchangeable or exercisable for shares of common stock for a period of 180 days after the date of this prospectus.
Prior to the consummation of the offering, certain of our employees, including our executive officers, and/or directors may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements relating to the offering described above.
Following the lock-up periods set forth in the agreements described above, and assuming that the representatives of the underwriters do not release any parties from these agreements, all of the shares of our common stock that are restricted securities or are held by our affiliates as of the date of this prospectus will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
Registration Rights Agreement
Pursuant to the registration rights agreement, we have granted THL the right to cause us, in certain instances, at our expense, to file registration statements under the Securities Act covering resales of our common stock held by THL (or certain transferees) and to provide piggyback registration rights to THL and certain executives, subject to the certain limitations and priorities on registration detailed therein, on registered offerings initiated by us in certain circumstances. See “Certain Relationships and Related Party Transactions—Related
141
Table of Contents
Party Transactions—Registration Rights Agreement”. These shares will represent 78.4% of our outstanding common stock after this offering, or 76.0% if the underwriters exercise their option to purchase additional shares in full.
Rule 144
In general, under Rule 144, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, any person who is not our affiliate, who was not our affiliate at any time during the preceding three months and who has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us and subject to applicable lock-up restrictions. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than one of our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
Beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act and subject to applicable lock-up restrictions, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of: (1) 1% of the number of shares of our common stock outstanding, which will equal approximately shares immediately after this offering; and (2) the average weekly trading volume of our common stock on the NYSE during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, any of our employees, directors or officers who acquired shares from us in connection with a compensatory stock or option plan or other compensatory written agreement before the effective date of this offering are, subject to applicable lock-up restrictions, eligible to resell such shares in reliance upon Rule 144 beginning 90 days after the date of this prospectus. If such person is not an affiliate and was not our affiliate at any time during the preceding three months, the sale may be made subject only to the manner-of-sale restrictions of Rule 144. If such a person is an affiliate, the sale may be made under Rule 144 without compliance with the holding period requirements under Rule 144, but subject to the other Rule 144 restrictions described above.
Equity Incentive Plans
Following this offering, we intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of common stock that are subject to outstanding options and other awards issuable pursuant to the 2018 Omnibus Incentive Plan. Shares covered by such registration statement will be available for sale in the open market following its effective date, subject to certain Rule 144 limitations applicable to affiliates and the terms of lock-up agreements applicable to those shares.
142
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of certain material U.S. federal income tax consequences to Non U.S. Holders (as defined below) of the purchase, ownership and disposition of our common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax considerations relating thereto. The effects of other U.S. federal tax laws, such as estate tax laws, gift tax laws, and any applicable state, local or non U.S. tax laws are not discussed. This discussion is based on the Internal Revenue Code of 1986 (the “Code”), Treasury regulations promulgated or proposed thereunder (the “Treasury Regulations”), judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service, or (the “IRS”), in each case as in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a Non U.S. Holder of our common stock. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to those discussed below regarding the tax consequences of the purchase, ownership and disposition of our common stock.
This discussion is limited to Non-U.S. Holders who purchase our common stock pursuant to this offering and who hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the U.S.; |
| • | persons subject to the alternative minimum tax; |
| • | persons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or traders in securities or currencies; |
| • | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| • | “controlled foreign corporations”, “passive foreign investment companies”, and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt organizations or governmental organizations; |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; |
| • | persons that own, or are deemed to own, more than five percent of our capital stock (except to the extent specifically set forth below); |
| • | “qualified foreign pension funds” (within the meaning of Section 897(1)(2) of the Code) and entities, all of the interests of which are held by qualified foreign pension funds; and |
| • | tax-qualified retirement plans. |
143
Table of Contents
If any entity or arrangement classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock and partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences of the purchase, ownership and disposition of shares of our common stock.
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS THE TAX CONSIDERATIONS RELATED TO THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, AS WELL AS ANY TAX CONSIDERATIONS RELATED TO THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE APPLICABLE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING AUTHORITY OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a Non-U.S. Holder
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of our common stock that is neither a “United States person” nor an entity or arrangement treated as a partnership for U.S. federal income tax purposes. A United States person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | an individual who is a citizen or resident of the U.S.; |
| • | a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized under the laws of the U.S. any state thereof, or the District of Columbia; |
| • | an estate whose income is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a U.S. court and the control of all substantial decisions of the trust is by one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy”, we do not anticipate declaring or paying dividends to holders of our common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a non-taxable return of capital up to (and will reduce, but not below zero) a Non-U.S. Holder’s adjusted tax basis in its common stock. Any excess amounts generally will be treated as capital gain and will be treated as described below under “Sale or Other Taxable Disposition”.
Subject to the discussion below on effectively connected income, backup withholding, and the Foreign Account Tax Compliance Act, dividends paid to a Non-U.S. Holder of our common stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided the Non-U.S. Holder furnishes to us or the applicable withholding agent prior to the payment of dividends a valid IRS Form W-8BEN or W-8BEN-E (or other applicable or successor form) certifying under penalty of perjury that such Non-U.S. Holder is not a “United States person” as defined in the Code and qualifies for a reduced treaty rate). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
144
Table of Contents
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI (or a successor form), certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S.
Any such effectively connected dividends will generally be subject to U.S. federal income tax on a net income basis at the regular graduated rates generally applicable to U.S. persons. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected dividends. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Sale or Other Taxable Disposition
Subject to the discussion below on backup withholding and the Foreign Account Tax Compliance Act, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our common stock unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such gain is attributable); |
| • | the Non-U.S. Holder is a nonresident alien individual present in the U.S. for 183 days or more during the taxable year of the sale or other taxable disposition and certain other requirements are met; or |
| • | our common stock constitutes a U.S. real property interest (a “USRPI”), by reason of our status as a U.S. real property holding corporation (a “USRPHC”), for U.S. federal income tax purposes at any time within the shorter of (1) the five-year period preceding the Non-U.S. Holder’s disposition of our common stock and (2) the Non-U.S. Holder’s holding period for our common stock. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net basis at the regular graduated rates generally applicable to U.S. persons. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected gain.
A Non-U.S. Holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the sale or other taxable disposition, which may generally be offset by U.S. source capital losses of the Non-U.S. Holder for that taxable year (even though the individual is not considered a resident of the U.S.), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Generally, a corporation is a USRPHC if the fair market value of its USRPIs equals 50% or more of the sum of the fair market value of (a) its worldwide real property interests and (b) its other assets used or held for use in a trade or business. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-USRPIs and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to U.S. federal income tax if our common stock is
145
Table of Contents
“regularly traded”, as defined by applicable Treasury Regulations, on an established securities market during the calendar year in which the sale or other taxable disposition occurs, and such Non-U.S. Holder owned, actually and constructively, five percent or less of our common stock throughout the shorter of (1) the five-year period ending on the date of the sale or other taxable disposition or (2) the Non-U.S. Holder’s holding period. If we were to become a USRPHC and our common stock were not considered to be “regularly traded” on an established securities market during the calendar year in which the relevant sale or other taxable disposition by a Non-U.S. Holder occurs, such Non-U.S. Holder (regardless of the percentage of stock owned) would be subject to U.S. federal income tax on a sale or other taxable disposition of our common stock and a 15% withholding tax would apply to the gross proceeds from such disposition.
Non-U.S. Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Payments of dividends on our common stock generally will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the Non-U.S. Holder is a United States person and the Non-U.S. Holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI (or a successor form), or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the U.S. or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such Non-U.S. Holder is a United States person, or the Non-U.S. Holder otherwise establishes an exemption. If a Non-U.S. Holder does not provide the certification described above or the applicable withholding agent has actual knowledge or reason to know that such Non-U.S. Holder is a United States person, payments of dividends or of proceeds of the sale or other taxable disposition of our common stock may be subject to backup withholding at a rate currently equal to 24% of the gross proceeds of such dividend, sale, or other taxable disposition. Proceeds of a sale or other disposition of our common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides, is established or is organized.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the Non-U.S. Holder timely files the appropriate claim with the IRS and furnishes any required information to the IRS.
Non-U.S. Holders should consult their tax advisors regarding information reporting and backup withholding.
Foreign Account Tax Compliance Act
Subject to the discussion below regarding recently issued Proposed Regulations (as defined below), withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the “Foreign Account Tax Compliance Act”, or “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a “foreign
146
Table of Contents
financial institution” or a “non-financial foreign entity” (each as defined in the Code) (including, in some cases, when such foreign financial institution or non-financial foreign entity is acting as an intermediary), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each direct and indirect substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to noncompliant foreign financial institutions and certain other account holders. Foreign financial institutions or branches thereof located in jurisdictions that have an intergovernmental agreement with the U.S. governing FATCA may be subject to different rules.
Although FATCA withholding could apply to gross proceeds on the disposition of our common stock, on December 13, 2018, the U.S. Department of the Treasury released proposed regulations (the “Proposed Regulations”) the preamble to which specifies that taxpayers may rely on them pending finalization. The Proposed Regulations eliminate FATCA withholding on the gross proceeds from a sale or other disposition of our common stock. There can be no assurance that the Proposed Regulations will be finalized in their present form.
Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock.
147
Table of Contents
UNDERWRITING (CONFLICTS OF INTEREST)
The company and the underwriters named below have entered into an underwriting agreement with respect to the shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of shares indicated in the following table. BofA Securities, Inc. and Goldman Sachs & Co. LLC are the representatives of the underwriters.
| Underwriters |
Number of Shares | |||
| BofA Securities, Inc. |
||||
| Goldman Sachs & Co. LLC |
||||
| Morgan Stanley & Co. LLC |
||||
| BMO Capital Markets Corp. |
||||
| Citigroup Global Markets Inc. |
||||
| Jefferies LLC |
||||
| UBS Securities LLC |
||||
| KeyBanc Capital Markets Inc. |
||||
| Raymond James & Associates, Inc. |
||||
| MUFG Securities Americas Inc. |
||||
| SMBC Nikko Securities America, Inc. |
||||
| Mischler Financial Group, Inc. |
||||
| Siebert Williams Shank & Co., LLC |
||||
|
|
|
|||
| Total |
26,315,789 | |||
|
|
|
|||
The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
The underwriters have an option to buy up to an additional 3,947,368 shares from the Company to cover sales by the underwriters of a greater number of shares than the total number set forth in the table above. They may exercise that option for 30 days. If any shares are purchased pursuant to this option, the underwriters will severally purchase shares in approximately the same proportion as set forth in the table above.
The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters by the Company. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase up to 3,947,368 additional shares.
Paid by the Company
| No Exercise | Full Exercise | |||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. After the initial offering of the shares, the representatives may change the offering price and the other selling terms. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
The company and its officers, directors, and holders of substantially all of the company’s common stock have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of common stock during the period from the date
148
Table of Contents
of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of BofA Securities, Inc. and Goldman Sachs & Co. LLC on behalf of the underwriters. This agreement does not apply to any existing employee benefit plans. See “Shares Available for Future Sale” for a discussion of certain transfer restrictions.
Prior to the offering, there has been no public market for the shares. The initial public offering price has been negotiated among the company and the representatives. Among the factors to be considered in determining the initial public offering price of the shares, in addition to prevailing market conditions, will be the company’s historical performance, estimates of the business potential and earnings prospects of the company, an assessment of the company’s management and the consideration of the above factors in relation to market valuation of companies in related businesses.
We have been approved to list the common stock on the NYSE under the symbol “AGTI”. In order to meet one of the requirements for listing the common stock on the NYSE, the underwriters have undertaken to sell lots of 100 or more shares to a minimum of 400 beneficial holders.
In connection with the offering, the underwriters may purchase and sell shares of common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering, and a short position represents the amount of such sales that have not been covered by subsequent purchases. A “covered short position” is a short position that is not greater than the amount of additional shares for which the underwriters’ option described above may be exercised. The underwriters may cover any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to cover the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares pursuant to the option described above. “Naked” short sales are any short sales that create a short position greater than the amount of additional shares for which the option described above may be exercised. The underwriters must cover any such naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the company’s stock, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the common stock. As a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. The underwriters are not required to engage in these activities and may end any of these activities at any time. These transactions may be effected on the NYSE, in the over-the-counter market or otherwise.
Directed Share Program
At our request, the underwriters have reserved up to 1,315,789 shares of our common stock, or 5% of the shares of our common stock to be offered by this prospectus for sale, at the initial public offering price, to certain individuals through a directed share program, including certain employees and certain other individuals identified by management. Shares purchased through the directed share program will not be subject to a lock-up restriction,
149
Table of Contents
except in the case of shares purchased by any of our officers and certain of our employees and existing equityholders. The number of shares of common stock available for sale to the general public will be reduced to the extent these individuals or entities purchase such reserved shares. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered by this prospectus. The underwriters will receive the same discount from such reserved shares as they will from other shares of our common stock sold to the public in this offering. We have agreed to indemnify the underwriters against certain liabilities and expenses, including liabilities under the Securities Act, in connection with sales of the reserved shares.
Conflicts of Interest
An affiliate of Goldman, Sachs & Co. LLC acted as bookrunner and lead arranger under our Second Lien Term Loan Facility. Certain affiliates of Goldman Sachs & Co. LLC currently hold 100% of our Second Lien Term Loan Facility and, as such, will receive 5% or more of the net proceeds of this offering due to the repayment of outstanding borrowings and related fees and expenses, under our Credit Facilities. Therefore, Goldman Sachs & Co. LLC is deemed to have a conflict of interest within the meaning of Rule 5121, which requires, among other things, that a “qualified independent underwriter” participate in the preparation of, and exercise the usual standards of “due diligence” with respect to, the registration statement and this prospectus. BofA Securities, Inc., one of the managing underwriters of this offering, has agreed to act as a qualified independent underwriter for this offering and to undertake the legal responsibilities and liabilities of an underwriter under the Securities Act, specifically including those inherent in Section 11 thereof. BofA Securities, Inc. will not receive any additional fees for serving as a qualified independent underwriter in connection with this offering. We have agreed to indemnify BofA Securities, Inc. against liabilities incurred in connection with acting as a qualified independent underwriter, including liabilities under the Securities Act.
Pursuant to Rule 5121, Goldman Sachs & Co. LLC will not confirm any sales to any account over which it exercises discretionary authority without the specific written approval of the account holder. See “Use of Proceeds” for additional information.
European Economic Area
In relation to each Member State of the European Economic Area (each, a “Member State”), no securities have been offered or will be offered pursuant to the offering to the public in that Member State prior to the publication of a prospectus in relation to the securities which has been approved by the competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent authority in that Member State, all in accordance with the Prospectus Regulation, except that offers of securities may be made to the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
| (a) | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives; or |
| (c) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of shares shall require us or any of our representatives to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the representatives and us that it is a “qualified investor” as defined in the Prospectus Regulation.
In the case of any shares being offered to a financial intermediary as that term is used in Article 5 of the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and
150
Table of Contents
agreed that the shares acquired by it in the offer have not been acquired on a nondiscretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Member State means the communication in any form and by means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase shares, the expression “Prospectus Regulation” means Regulation (EU) 2017/1129 (as amended).
United Kingdom
Each underwriter has represented and agreed that:
| (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000, (“FSMA”)), received by it in connection with the issue or sale of the shares of our common stock in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
| (b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of our common stock in, from or otherwise involving the United Kingdom. |
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption form, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this offering memorandum (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Hong Kong
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules
151
Table of Contents
made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”)
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), or the FIEA. The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or
152
Table of Contents
indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
The company estimates that the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $4.0 million. We have agreed to reimburse the underwriters for certain expenses in an amount up to $35,000. The underwriters have agreed to reimburse us for certain expenses incurred by us in connection with this offering upon the closing of the offering.
The company has agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the issuer and to persons and entities with relationships with the issuer, for which they received or will receive customary fees and expenses. For example, Goldman Sachs Lending Partners LLC, an affiliate of Goldman Sachs & Co. LLC, acted as bookrunner and lead arranger in connection with the incurrence of loans under our Second Lien Credit Agreement.
In the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the issuer. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
153
Table of Contents
The validity of the issuance of our common stock offered in this prospectus will be passed upon for us by Kirkland & Ellis LLP, Chicago, Illinois. Certain partners of Kirkland & Ellis LLP are members of a limited partnership that is an investor in one or more investment funds affiliated with THL. Kirkland & Ellis LLP represents entities affiliated with THL in connection with legal matters. Certain legal matters will be passed upon for the underwriters by Weil, Gotshal & Manges LLP, New York, New York.
The consolidated financial statements of Agiliti, Inc. and its subsidiaries as of December 31, 2020 and 2019, and for the year ended December 31, 2020, the period from January 4, 2019 through December 31, 2019, and the consolidated financial statements of Agiliti Health, Inc. and subsidiaries for the period from January 1, 2019 through January 3, 2019 and for the year ended December 31, 2018 have been included herein in reliance upon the report of KPMG LLP, an independent registered public accounting firm (“KPMG”), appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2020 financial statements refers to a change in our method of accounting for leases effective January 1, 2019, due to the adoption of Accounting Standards Update No. 2016-02, Leases (Topic 842) and all related amendments. Our Independent Registered Public Accounting Firm, KPMG, informed our Audit Committee that KPMG Legal Limited (“KPMG Vietnam”), a member firm of KPMG International Limited, provided a legal service to an entity affiliated with the Company (“Sister Affiliate”) through common control by THL during a portion of fiscal year 2019, which is not permissible under the independence rules of the SEC. THL acquired Sister Affiliate in April 2019, at which time Sister Affiliate became an affiliate of the Company. Therefore, the service provided was considered impermissible for the period from April 2019 through August 2019, when the service was terminated. The fees paid to KPMG Vietnam were immaterial to KPMG, THL and the Company. While SEC independence rules prohibit the auditor from providing legal services to the audit client or its affiliates, the legal service provided to an entity that was under common control had no impact on the Company. KPMG and our Audit Committee, in consultation with legal counsel, concluded that the service does not, did not and will not impact KPMG’s ability to exercise objective and impartial judgment on all issues encompassed within the audits of the Company.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act to register our common stock being offered in this prospectus. This prospectus, which forms part of the registration statement, does not contain all of the information included in the registration statement and the attached exhibits. You will find additional information about us and our common stock in the registration statement. References in this prospectus to any of our contracts, agreements or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or documents. The SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
On the closing of this offering, we will be subject to the information reporting requirements of the Exchange Act, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the website of the SEC referred to above.
We also maintain a website at www.agilitihealth.com. Information contained in, or accessible through, our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is only as an inactive textual reference.
154
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Audited Consolidated Financial Statements of Agiliti, Inc. and subsidiaries as of December 31, 2020 and 2019 and for the year ended December 31, 2020, the period from January 4, 2019 through December 31, 2019 and Agiliti Health, Inc. and its subsidiaries for the period January 1, 2019 through January 3, 2019 and the year ended December 31, 2018.
| F-2 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
| F-9 | ||||
| F-11 |
F-1
Table of Contents

Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Agiliti, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Agiliti, Inc. and subsidiaries (the Company) as of December 31, 2020 and 2019, the related consolidated statements of operations, comprehensive (loss) income, equity, and cash flows for the year ended December 31, 2020 and for the period January 4, 2019 through December 31, 2019 and the related notes. We have also audited the accompanying consolidated statements of operations, deficit, and cash flows of Agiliti Health, Inc. and subsidiaries for the period January 1, 2019 through January 3, 2019 and the year ended December 31, 2018 and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Agiliti, Inc. and subsidiaries as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the year ended December 31, 2020 and for the period January 4, 2019 through December 31, 2019, in conformity with U.S. generally accepted accounting principles. It is also our opinion that the consolidated financial statements referred to above present fairly, in all material respects, the results of operations and cash flows of Agiliti Health, Inc. and subsidiaries for the period January 1, 2019 through January 3, 2019 and the year ended December 31, 2018, in conformity with U.S. generally accepted accounting principles.
Change in Accounting Principle
As discussed in note 2 to the consolidated financial statements, the Company has changed its method of accounting for leases effective January 1, 2019 due to the adoption of Accounting Standards Update No. 2016-02, Leases (Topic 842), and all related amendments.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
F-2
Table of Contents
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Valuation of the obligation under the tax receivable agreement
As discussed in Note 5 to the consolidated financial statements the Company recorded an obligation payable to the former shareholders of the predecessor entity related to a tax receivable agreement (“TRA”). Amounts are payable to the former shareholders under the agreement upon realization of certain tax benefits in future periods. The TRA obligation is recorded at fair value each reporting period, which is estimated using company specific assumptions that are not observable in the market. Changes in the fair value of the TRA obligation each reporting period are presented in selling, general & administrative expenses in the statement of operations. The fair value of the TRA obligation at December 31, 2020 was $50.6 million.
We identified the evaluation of the value of the obligation under the TRA as a critical audit matter. As there was limited observable market information for the forecasted revenue growth and gross margin assumptions, subjective auditor judgment was required to assess these assumptions. In addition, evaluation of the valuation model used required specialized skills and knowledge.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and implementation of certain internal controls related to the valuation of the obligation under the TRA. These included controls over the review of the forecasted revenue growth and gross margin assumptions as well as the Company’s evaluation of the valuation model used. We evaluated the Company’s forecasted revenue growth and gross margin assumptions by comparing them to actual growth and gross margin rates experienced by the Company and actual and forecast growth and gross margin rates of the Company’s peers and within industry reports. We compared the Company’s historical estimates of forecasted revenue growth and gross margin to the Company’s actual results to assess the Company’s ability to accurately forecast. We inspected customer contracts regarding significant changes in forecasted revenue growth and gross margin. In addition, we involved valuation professionals with specialized skills and knowledge, who assisted in evaluating the valuation model used by the Company to develop the fair value of the TRA obligation by developing an estimate of the TRA obligation using the Company’s assumptions and comparing the result to the Company’s estimate.
Acquisition date fair value of customer relationship asset
As discussed in Note 4 to the consolidated financial statements, on January 31, 2020, the Company acquired certain assets of a surgical equipment repair and maintenance service provider in a business combination for consideration of approximately $88.3 million. As a result of the transaction, the Company acquired a customer relationship intangible asset associated with the generation of future income from the acquiree’s existing customers. The acquisition-date fair value for the customer relationship asset was $33.3 million.
We identified the evaluation of the initial measurement of the customer relationship intangible asset acquired as a critical audit matter. There was a high degree of subjective auditor judgment in evaluating certain assumptions included in the discounted cash flow model used to estimate the acquisition-date fair value of the customer relationship intangible asset. Specifically, there was limited observable market information for the forecasted revenue growth and gross margin assumptions, and evaluation of the discount rate assumption required specialized skills and knowledge.
F-3
Table of Contents
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design of certain internal controls related to the valuation of the customer relationship intangible asset. These included controls over the review of the forecasted revenue growth, gross margin, and discount rate assumptions. We evaluated the Company’s forecasted revenue growth and gross margin assumptions by comparing them to actual growth and gross margin rates historically experienced by the acquired company and actual and forecasted growth and gross margin rates of the Company’s peers and within industry reports. We compared the Company’s assumptions for forecasted revenue growth and gross margin of the acquired company to actual post-acquisition results to assess the Company’s ability to accurately forecast. In addition, we involved valuation professionals with specialized skills and knowledge, who assisted in evaluating the Company’s discount rate, by comparing it against a discount rate range that was independently developed using publicly available market data for comparable entities.
We have served as the Company’s auditor since 2013.
/s/ KPMG LLP
Minneapolis, Minnesota
February 16, 2021
F-4
Table of Contents
Agiliti, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share and per share information)
| December 31, 2020 |
December 31, 2019 |
|||||||
| (Successor) | (Successor) | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 206,505 | $ | — | ||||
| Accounts receivable, less allowance for doubtful accounts of $1,993 at December 31, 2020 and $1,156 at December 31, 2019 |
154,625 | 106,374 | ||||||
| Inventories |
27,062 | 13,414 | ||||||
| Other current assets |
14,175 | 10,404 | ||||||
|
|
|
|
|
|||||
| Total current assets |
402,367 | 130,192 | ||||||
| Property and equipment: |
||||||||
| Medical equipment |
285,723 | 261,477 | ||||||
| Property and office equipment |
112,646 | 73,216 | ||||||
| Accumulated depreciation |
(183,953 | ) | (91,967 | ) | ||||
|
|
|
|
|
|||||
| Total property and equipment, net |
214,416 | 242,726 | ||||||
| Other long-term assets: |
||||||||
| Goodwill |
817,113 | 780,835 | ||||||
| Operating lease right-of-use assets |
51,214 | 29,964 | ||||||
| Other intangibles, net |
402,095 | 427,193 | ||||||
| Other |
16,151 | 8,506 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 1,903,356 | $ | 1,619,416 | ||||
|
|
|
|
|
|||||
| Liabilities and Equity |
||||||||
| Current liabilities: |
||||||||
| Current portion of long-term debt |
$ | 16,044 | $ | 13,272 | ||||
| Current portion of operating lease liability |
14,155 | 9,171 | ||||||
| Current portion of obligation under tax receivable agreement |
15,572 | — | ||||||
| Book overdrafts |
— | 1,771 | ||||||
| Accounts payable |
37,215 | 39,203 | ||||||
| Accrued compensation |
38,671 | 21,066 | ||||||
| Accrued interest |
6,347 | 3,905 | ||||||
| Other accrued expenses |
31,527 | 24,887 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
159,531 | 113,275 | ||||||
| Long-term debt, less current portion |
1,145,055 | 909,726 | ||||||
| Obligation under tax receivable agreement, pension and other long-term liabilities |
53,794 | 49,187 | ||||||
| Operating lease liability, less current portion |
40,283 | 22,089 | ||||||
| Deferred income taxes, net |
62,748 | 68,170 | ||||||
| Commitments and contingencies (Note 9) |
||||||||
| Equity |
||||||||
| Common stock, $0.0001 par value; 350,000,000 shares authorized; 98,983,296 and 98,943,489 shares issued and outstanding at December 31, 2020 and December 31, 2019 |
10 | 10 | ||||||
| Additional paid-in capital |
513,902 | 503,637 | ||||||
| Accumulated deficit |
(68,492 | ) | (46,014 | ) | ||||
| Accumulated other comprehensive loss |
(3,619 | ) | (940 | ) | ||||
|
|
|
|
|
|||||
| Total Agiliti, Inc. and Subsidiaries equity |
441,801 | 456,693 | ||||||
| Noncontrolling interest |
144 | 276 | ||||||
|
|
|
|
|
|||||
| Total equity |
441,945 | 456,969 | ||||||
|
|
|
|
|
|||||
| Total liabilities and equity |
$ | 1,903,356 | $ | 1,619,416 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
Agiliti, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands)
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||||
| Revenue |
$ | 773,312 | $ | 613,073 | $ | — | $ | 565,246 | ||||||||||||
| Cost of revenue |
486,965 | 423,812 | — | 367,837 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross margin |
286,347 | 189,261 | — | 197,409 | ||||||||||||||||
| Selling, general and administrative |
250,289 | 187,156 | 17,147 | 137,210 | ||||||||||||||||
| Gain on settlement |
— | — | — | (26,391 | ) | |||||||||||||||
| Intangible asset impairment charge |
— | — | — | 131,100 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating income (loss) |
36,058 | 2,105 | (17,147 | ) | (44,510 | ) | ||||||||||||||
| Interest expense |
61,530 | 48,199 | — | 53,390 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes and noncontrolling interest |
(25,472 | ) | (46,094 | ) | (17,147 | ) | (97,900 | ) | ||||||||||||
| Income tax benefit |
(3,234 | ) | (14,857 | ) | (13,281 | ) | (66,348 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Consolidated net loss |
(22,238 | ) | (31,237 | ) | (3,866 | ) | (31,552 | ) | ||||||||||||
| Net income attributable to noncontrolling interest |
240 | 171 | — | 327 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss attributable to Agiliti, Inc. and Subsidiaries |
$ | (22,478 | ) | $ | (31,408 | ) | $ | (3,866 | ) | $ | (31,879 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
Agiliti, Inc. and Subsidiaries
Consolidated Statements of Comprehensive (Loss) Income
(in thousands)
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||
| Consolidated net loss |
$ | (22,238 | ) | $ | (31,237 | ) | $ | (3,866 | ) | $ | (31,552 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive loss: |
||||||||||||||||
| Loss on minimum pension liability, net of tax of $435, $319, $0 and $111 |
(1,275 | ) | (940 | ) | — | 5 | ||||||||||
| Loss on cash flow hedge, net of tax of $478,$0, $0 and $0 |
(1,404 | ) | — | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other comprehensive loss |
(2,679 | ) | (940 | ) | — | 5 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
(24,917 | ) | (32,177 | ) | (3,866 | ) | (31,547 | ) | ||||||||
| Comprehensive income attributable to noncontrolling interest |
240 | 171 | — | 327 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss attributable to Agiliti, Inc. and Subsidiaries |
$ | (25,157 | ) | $ | (32,348 | ) | $ | (3,866 | ) | $ | (31,874 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
Agiliti, Inc. and Subsidiaries
Consolidated Statements of Equity (Deficit)
(in thousands)
| Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Total Agiliti, Inc. and Subsidiaries |
Noncontrolling Interests |
Total Equity (Deficit) |
||||||||||||||||||||||
| Balance at December 31, 2017 (Predecessor) |
$ | — | $ | 250,018 | $ | (287,998 | ) | $ | (6,638 | ) | $ | (44,618 | ) | $ | 240 | $ | (44,378 | ) | ||||||||||
| Impact of change in accounting policy |
— | — | 6,221 | — | 6,221 | — | 6,221 | |||||||||||||||||||||
| Net (loss) income |
— | — | (31,879 | ) | — | (31,879 | ) | 327 | (31,552 | ) | ||||||||||||||||||
| Other comprehensive income |
— | — | — | 5 | 5 | — | 5 | |||||||||||||||||||||
| Share-based compensation expense |
— | 3,010 | — | — | 3,010 | — | 3,010 | |||||||||||||||||||||
| Shares forfeited for taxes |
— | (1,149 | ) | — | — | (1,149 | ) | — | (1,149 | ) | ||||||||||||||||||
| Stock options exercised |
— | 547 | — | — | 547 | — | 547 | |||||||||||||||||||||
| Cash distributions to noncontrolling interests |
— | 13 | — | — | 13 | (376 | ) | (363 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2018 (Predecessor) |
$ | — | $ | 252,439 | $ | (313,656 | ) | $ | (6,633 | ) | $ | (67,850 | ) | $ | 191 | $ | (67,659 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss |
— | — | (3,866 | ) | — | (3,866 | ) | — | (3,866 | ) | ||||||||||||||||||
| Share-based compensation expense |
— | 5,900 | — | — | 5,900 | — | 5,900 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at January 3, 2019 (Predecessor) |
$ | — | $ | 258,339 | $ | (317,522 | ) | $ | (6,633 | ) | $ | (65,816 | ) | $ | 191 | $ | (65,625 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at January 4, 2019 (Successor) |
$ | 10 | $ | 764,582 | $ | (14,606 | ) | — | 749,986 | 191 | 750,177 | |||||||||||||||||
| Net (loss) income |
— | — | (31,408 | ) | — | (31,408 | ) | 171 | (31,237 | ) | ||||||||||||||||||
| Other comprehensive loss |
— | — | — | (940 | ) | (940 | ) | — | (940 | ) | ||||||||||||||||||
| Share-based compensation expense |
— | 6,011 | — | — | 6,011 | — | 6,011 | |||||||||||||||||||||
| Stock options exercised |
— | 193 | — | — | 193 | — | 193 | |||||||||||||||||||||
| Shares repurchased |
— | (775 | ) | — | — | (775 | ) | — | (775 | ) | ||||||||||||||||||
| Redemption of warrants |
— | (35,872 | ) | — | — | (35,872 | ) | — | (35,872 | ) | ||||||||||||||||||
| Dividend and equity distribution |
— | (230,502 | ) | — | — | (230,502 | ) | — | (230,502 | ) | ||||||||||||||||||
| Contributions from new members to limited liability company |
— | — | — | — | — | 140 | 140 | |||||||||||||||||||||
| Cash distributions to noncontrolling interests |
— | — | — | — | — | (226 | ) | (226 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2019 (Successor) |
$ | 10 | $ | 503,637 | $ | (46,014 | ) | $ | (940 | ) | $ | 456,693 | $ | 276 | $ | 456,969 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net (loss) income |
— | — | (22,478 | ) | — | (22,478 | ) | 240 | (22,238 | ) | ||||||||||||||||||
| Other comprehensive loss |
— | — | — | (2,679 | ) | (2,679 | ) | — | (2,679 | ) | ||||||||||||||||||
| Share-based compensation expense |
— | 10,334 | — | — | 10,334 | — | 10,334 | |||||||||||||||||||||
| Shares forfeited for taxes |
— | (145 | ) | — | — | (145 | ) | — | (145 | ) | ||||||||||||||||||
| Dividend forfeited, net of payable |
— | 76 | — | — | 76 | — | 76 | |||||||||||||||||||||
| Contributions from members to limited liability company |
— | — | — | — | — | 25 | 25 | |||||||||||||||||||||
| Cash distributions to noncontrolling interests |
— | — | — | — | — | (397 | ) | (397 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2020 (Successor) |
$ | 10 | $ | 513,902 | $ | (68,492 | ) | $ | (3,619 | ) | $ | 441,801 | $ | 144 | $ | 441,945 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Table of Contents
Agiliti, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||||||
| Consolidated net loss |
$ | (22,238 | ) | $ | (31,237 | ) | $ | (3,866 | ) | $ | (31,552 | ) | ||||||||
| Adjustments to reconcile net loss to net cash provided by operating activities: |
||||||||||||||||||||
| Depreciation |
99,638 | 96,486 | — | 69,680 | ||||||||||||||||
| Intangible asset impairment charge |
— | — | — | 131,100 | ||||||||||||||||
| Gain on settlement |
— | — | — | (23,391 | ) | |||||||||||||||
| Remeasurement of tax receivable agreement and contingent consideration |
12,931 | (8,586 | ) | — | — | |||||||||||||||
| Amortization of intangibles, contract costs, deferred financing costs and debt |
73,456 | 68,357 | — | 9,755 | ||||||||||||||||
| Amortization of operating lease right-of use-assets and changes in operating lease liability |
101 | 350 | — | — | ||||||||||||||||
| Provision for doubtful accounts |
1,959 | 1,031 | — | 2,125 | ||||||||||||||||
| Provision for inventory obsolescence |
722 | 455 | — | 146 | ||||||||||||||||
| Non-cash share-based compensation expense |
10,334 | 6,011 | 5,900 | 3,010 | ||||||||||||||||
| Gain on sales and disposals of equipment |
(1,191 | ) | (1,715 | ) | — | (2,124 | ) | |||||||||||||
| Deferred income taxes |
(4,944 | ) | (15,302 | ) | (13,281 | ) | (67,297 | ) | ||||||||||||
| Interest on note receivable |
— | — | — | (46 | ) | |||||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
(39,763 | ) | (827 | ) | — | (17,742 | ) | |||||||||||||
| Inventories |
(9,712 | ) | (1,325 | ) | — | (1,508 | ) | |||||||||||||
| Other operating assets |
(13,597 | ) | (7,397 | ) | — | (3,027 | ) | |||||||||||||
| Accounts payable |
552 | 1,661 | — | 4,424 | ||||||||||||||||
| Other operating liabilities |
29,679 | (37,964 | ) | 11,247 | 12,770 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash provided by operating activities |
137,927 | 69,998 | — | 86,323 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash flows from investing activities: |
||||||||||||||||||||
| Medical equipment purchases |
(31,668 | ) | (45,768 | ) | — | (45,418 | ) | |||||||||||||
| Property and office equipment purchases |
(27,597 | ) | (9,974 | ) | — | (8,757 | ) | |||||||||||||
| Proceeds from disposition of property and equipment |
3,486 | 3,583 | — | 3,722 | ||||||||||||||||
| Collection (issuance) of note receivable from officer |
— | 2,585 | — | (581 | ) | |||||||||||||||
| Acquisition of Agiliti Health, Inc. by THL Shareholders |
— | (702,139 | ) | — | — | |||||||||||||||
| Acquisitions, net of cash acquired |
(95,953 | ) | (10,260 | ) | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash used in investing activities |
(151,732 | ) | (761,973 | ) | — | (51,034 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
F-9
Table of Contents
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||||
| Cash flows from financing activities: |
||||||||||||||||||||
| Proceeds under new revolver |
199,500 | 207,100 | — | — | ||||||||||||||||
| Payments under new revolver |
(233,000 | ) | (173,600 | ) | — | — | ||||||||||||||
| Proceeds under new term loan |
273,344 | 890,775 | — | — | ||||||||||||||||
| Payments under new term loan |
(7,860 | ) | (4,950 | ) | — | — | ||||||||||||||
| Proceeds under senior secured credit facility |
— | — | — | 205,967 | ||||||||||||||||
| Payments under senior secured credit facility |
— | (25,933 | ) | — | (220,233 | ) | ||||||||||||||
| Repayments of 2012 Notes |
— | (645,000 | ) | — | — | |||||||||||||||
| Payments of principal under finance lease liability |
(8,024 | ) | (7,609 | ) | — | (6,491 | ) | |||||||||||||
| Payments of deferred financing costs |
(199 | ) | (21,836 | ) | — | — | ||||||||||||||
| Distributions to noncontrolling interests |
(397 | ) | (226 | ) | — | (376 | ) | |||||||||||||
| Proceeds from exercise of parent company stock options |
— | 193 | — | 547 | ||||||||||||||||
| Shares repurchased |
— | (775 | ) | — | — | |||||||||||||||
| Redemption of warrants |
— | (35,872 | ) | — | — | |||||||||||||||
| Dividend and equity distribution payment |
(1,138 | ) | (227,065 | ) | — | — | ||||||||||||||
| Contribution from new members to limited liability company |
— | 140 | — | — | ||||||||||||||||
| Holdback and earn-out payments related to acquisitions |
— | — | — | (847 | ) | |||||||||||||||
| Shares forfeited for taxes |
(145 | ) | — | — | (1,149 | ) | ||||||||||||||
| Change in book overdrafts |
(1,771 | ) | 1,771 | — | (5,367 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash provided by (used in) financing activities |
220,310 | (42,887 | ) | — | (27,949 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net change in cash and cash equivalents |
206,505 | (734,862 | ) | — | 7,340 | |||||||||||||||
| Cash and cash equivalents at the beginning of period |
— | 734,862 | 7,340 | — | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash and cash equivalents at the end of period |
$ | 206,505 | $ | — | $ | 7,340 | $ | 7,340 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Supplemental cash flow information: |
||||||||||||||||||||
| Interest paid |
$ | 55,161 | $ | 59,737 | $ | — | $ | 52,327 | ||||||||||||
| Income taxes paid |
1,260 | 1,564 | — | 959 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-10
Table of Contents
Agiliti, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 1. | Basis of Presentation |
Agiliti, Inc. and its consolidated subsidiaries (Federal Street Acquisition Corp (“FSAC”), Agiliti Holdco, Inc. and Agiliti Health, Inc. and subsidiaries (“we”, “our”, “us”, the “Company” or “Agiliti”) is a nationwide provider of healthcare technology management and service solutions to the United States healthcare industry. Agiliti, Inc. owns 100% of FSAC. FSAC owns 100% of Agiliti Holdco, Inc. Agiliti Holdco, Inc. owns 100% of Agiliti Health, Inc. Agiliti Health, Inc. owns 100% of Agiliti Surgical, Inc. and Agiliti Imaging, Inc. Agiliti Health, Inc. and subsidiaries is the only company with operations. All other entities have no material assets, liabilities, cash flows or operations other than their investment and ownership of Agiliti Health, Inc. and subsidiaries.
The successor period reflected the financial position and result of operations of Agiliti, Inc and its consolidated subsidiaries as of December 31, 2020 and 2019 and for the year ended December 31, 2020 and the period from January 4 through December 31, 2019. The predecessor period reflected the financial position and result of operations of Agiliti Health, Inc. and its consolidated subsidiaries as of December 31, 2018 and for the period from January 1 through January 3, 2019 and the year ended December 31, 2018.
Our service solutions consist of Equipment Solutions, Clinical Engineering Services and Onsite Managed Services.
Equipment Solutions: Equipment Solutions primarily provides supplemental, peak need and per-case rental of general biomedical, specialty, and surgical equipment to approximately 7,000 acute care hospitals and alternate site providers in the U.S., including some of the nation’s premier healthcare institutions and integrated delivery networks. We contract for Equipment Solutions services directly with customers or through our contractual arrangements with hospital systems and alternate site providers. We consistently achieve high customer satisfaction ratings, as evidenced by our Net Promoter Score of 55 for the year ended December 31, 2020, by delivering patient-ready equipment within our contracted equipment delivery times and by providing technical support and educational in-servicing for equipment as-needed in clinical departments, including the emergency room, operating room, intensive care, rehabilitation and general patient care areas. We are committed to providing the highest quality of equipment to our customers, and we do so through the use of our comprehensive quality management system (QMS) which is based on the quality standards recognized worldwide for medical devices: 21 CFR 820 and ISO 13485:2016. This commitment ensures that customers have access to patient-ready equipment with the confidence of knowing it has been prepared and maintained to the highest industry standard to deliver optimal patient safety and outcomes. We have over 5,000 equipment solution customers.
Clinical Engineering Services: Clinical Engineering Services provides maintenance, repair and remediation solutions for all types of medical equipment, including general biomedical equipment and diagnostic imaging equipment, through supplemental and outsourced offerings. Our supplemental offering helps customers manage their equipment repair and maintenance backlog, assist with remediation and regulatory reporting and temporarily fill open biotechnical positions. With our outsourced offering, we assume full management, staffing and clinical engineering service responsibilities for individual or system-wide customer sites. The outsourced model deploys a dedicated, on-site team to coordinate the management of customer-owned equipment utilizing our proprietary information systems, third party vendors of services and parts, and a broad range of professional services for capital equipment planning and regulatory compliance. We leverage more than 500 technical resources from our 98 local market service centers and seven Centers of Excellence to flex staff in and out of customer facilities on an as-needed basis, ensuring customers pay only for time spent directly servicing their equipment by an appropriately qualified technician. We use flex staffing for our supplemental clinical engineering solution and to augment support when additional technicians are needed to supplement our outsourced services during peak workload. We contract our Clinical Engineering Services with acute care and
F-11
Table of Contents
alternate site facilities across the U.S., as well as with the federal government and any medical device manufacturers that require a broad logistical footprint to support their large-scale service needs. We have over 6,000 clinical engineering service customers.
Onsite Managed Services: Onsite Managed Services are comprehensive programs that assume full responsibility for the management, reprocessing and logistics of medical equipment at individual facilities and IDNs, with the added benefit of enhancing equipment utilization and freeing more clinician time for patient care. This solution monitors and adjusts equipment quantities and availability to address fluctuations in patient census and acuity. Our more than 1,700 onsite employees work 24/7 in customer facilities, augmenting clinical support by integrating proven equipment management processes, utilizing our proprietary management software and conducting daily rounds and unit-based training to ensure equipment is being used and managed properly, overall helping to optimize day-to-day operations and care outcomes. We assume full responsibility for ensuring equipment is available when and where it is needed, removing equipment when no longer in use, and decontaminating, testing and servicing equipment as needed between each patient use. We have over 225 onsite managed service customers.
On January 4, 2019, Agiliti, Inc. consummated the previously announced business combination, see Note 4, Business Combination and Acquisition. Purchase accounting has been reflected in the accompanying consolidated financial statements.
Principles of Consolidation
The consolidated financial statements include the accounts of Agiliti, Inc., FSAC (a 100%-owned subsidiary of Agiliti, Inc.), Agiliti Holdco, Inc. (a 100%-owned subsidiary of FSAC), Agiliti Health, Inc. (a 100%-owned subsidiary of Agiliti Holdco, Inc.) and its 100%-owned subsidiaries, Agiliti Surgical, Inc. and Agiliti Imaging, Inc. In addition, in accordance with guidance issued by the Financial Accounting Standards Board (“FASB”), we have accounted for our equity investments in entities in which we are the primary beneficiary under the full consolidation method. All intercompany transactions and balances have been eliminated through consolidation. As the primary beneficiary, we consolidate the limited liability companies (“LLCs”) referred to in Note 13, Limited Liability Companies, as we effectively receive the majority of the benefits from such entities and we provide equipment lease guarantees for such entities.
| 2. | Significant Accounting Policies |
Cash and Cash Equivalents
The Company considers money market accounts and other highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Changes in book overdrafts are considered financing activities in the consolidated statements of cash flows.
Accounts Receivable and Allowance for Doubtful Accounts
Trade accounts receivable are recorded at the invoiced amount. Concentrations of credit risk with respect to trade accounts receivable are limited due to the number of customers and their geographical distribution. The Company performs initial and ongoing credit evaluations of its customers and maintains allowances for potential credit losses. The allowance for doubtful accounts is based on historical loss experience and estimated exposure on specific trade receivables.
Inventories
Inventories consist of supplies and equipment held for resale and are valued at the lower of cost and net realizable value. Cost is determined by the average cost method, which approximates the first-in, first-out (“FIFO”) method.
F-12
Table of Contents
Medical Equipment
Depreciation of medical equipment is provided on the straight-line method over the equipment’s estimated useful life, generally four to seven years. The cost and accumulated depreciation of medical equipment retired or sold is eliminated from their respective accounts and the resulting gain or loss is recorded in cost of revenue in the period the asset is retired or sold.
Property and Office Equipment
Property and office equipment includes property, leasehold improvements and office equipment.
Depreciation and amortization of property and office equipment is provided on the straight-line method over the lesser of the remaining useful life or lease term for leasehold improvements and 3 to 10 years for office equipment. The cost and accumulated depreciation or amortization of property and equipment retired or sold is eliminated from their respective accounts and the resulting gain or loss is recorded in selling, general and administrative expense in the period the asset is retired or sold.
Goodwill
Goodwill represents the excess of the cost of acquired businesses over the fair value of identifiable tangible net assets and identifiable intangible assets purchased.
Goodwill is tested at least annually for impairment at the reporting unit level and more frequently if events or changes in circumstances indicate that the asset might be impaired. We review goodwill for impairment by comparing the fair value of a reporting unit with its carrying value and recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value.
At December 31, 2020, we have one reporting unit which is the same as our reporting segment. No goodwill impairments have been recognized in 2020, 2019, or 2018.
Other Intangible Assets
Other intangible assets primarily include customer relationships, trade names and non-compete agreements. Our other intangible assets are amortized over their estimated economic lives of three to fifteen years. The straight-line method of amortization generally reflects an appropriate allocation of the cost of the intangible assets to earnings in proportion to the amount of economic benefits obtained by the Company in each reporting period. However, for certain of our customer relationships, we use the sum-of-the-years-digits amortization method to more appropriately allocate the cost to earnings in proportion to the estimated amount of economic benefit obtained.
Intangible assets with indefinite lives were tested for impairment on an annual basis and more frequently if events or changes in circumstances indicated that the asset might be impaired. We reviewed indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether the existence of events and circumstances indicates that it is more likely than not that the indefinite-lived intangible asset is impaired. If, after assessing the totality of events and circumstances, we conclude that it is not more likely than not that the indefinite-lived intangible asset is impaired, then we take no further action. On December 3, 2018, the Company changed its legal name from Universal Hospital Services, Inc. to Agiliti Health, Inc. and the brand identity to Agiliti. As a result, the indefinite-lived intangible assets related to UHS trade name of $131.1 million was written off in 2018. As of December 31, 2019, we no longer have indefinite lives intangible assets.
Long-Lived Assets
The Company periodically reviews its long-lived assets for impairment and assesses whenever significant events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. A
F-13
Table of Contents
recoverability test is performed by comparing the anticipated future undiscounted cash flows to the carrying value of the assets. If impairment is identified, an impairment loss is recognized for the excess of the carrying amount of an asset over the anticipated future discounted cash flows expected to result from the use of the asset and its eventual disposition. For other long-lived assets, primarily movable medical equipment, we continuously monitor specific makes/models for events such as product recalls or obsolescence. The amount of the impairment loss to be recorded, if any, is calculated by the excess of the asset’s carrying value over its fair value.
Deferred Financing Costs
Financing costs associated with issuing debt are presented in the consolidated balance sheet as a direct deduction from the carrying amount of the debt and are deferred and amortized over the related terms using the effective interest rate method.
Purchase Accounting
We account for acquisitions by allocating the purchase price paid to effect the acquisition to the acquired assets and liabilities at fair value with excess purchase price being recorded as goodwill.
Revenue Recognition
The Company adopted ASU 2014-09, Revenue from Contracts with Customers, effective January 1, 2018, herein referred as ASC Topic 606. The Company recognizes revenue when it satisfies performance obligations by transferring promised goods or services to customers.
Customer arrangements typically have multiple performance obligations to provide equipment solutions, clinical engineering and/or onsite equipment managed services on a per use and/or over time basis. Consideration paid by the customer for each performance obligation is billed within the month the service is performed, and contractual prices are established within our customer arrangements that are representative of the stand-alone selling price. Taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction, that are collected by the Company from a customer, are excluded from revenue. The Company’s performance obligations that are satisfied at a point in time are recognized when the service is performed or equipment is delivered to the customer. For performance obligations satisfied over time, the Company uses a straight line method to recognize revenue ratably over the contract period, as this coincides with the Company’s performance under the contract.
From time to time the Company receives both non-monetary and cash refunds on equipment recalled by manufacturers. It is the Company’s practice to record such recall gains as a reduction in cost of revenue.
The Company incurs costs related to obtaining new contracts. Management expects those costs attributable to new revenue production are recoverable and therefore the Company capitalized them as contract costs in accordance with ASC Topic 340 and is amortizing them over the anticipated period of the new revenue production.
Leases
At inception, we determine whether an arrangement is a lease and the appropriate lease classification. Operating leases with terms greater than twelve months are included as operating lease right-of-use (“ROU”) assets, and lease liabilities within current portion of operating lease liability and operating lease liability less current portion on our consolidated balance sheets. Finance leases with terms greater than twelve months are included as finance ROU assets within property and office equipment, and finance lease liabilities within current portion of long-term debt and long-term debt, less current portion on our consolidated balance sheets. Leases with terms of less than twelve months, referred to as short-term leases, do not create a ROU asset or lease liability on the balance sheet.
F-14
Table of Contents
ROU assets represent our right to use an underlying asset for the lease term. Lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at the commencement date of the lease, based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The company’s incremental borrowing rate for a lease is the rate of interest it would have to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms. For both operating and finance leases, the initial ROU asset equals the lease liability, plus initial direct costs, plus favorable lease commitments, less lease incentives received. Our lease agreements may include options to extend or terminate the lease, which are included in the lease term at the commencement date when it is reasonably certain that we will exercise that option. In general, we do not consider optional periods included in our lease agreements as reasonably certain of exercise at inception.
We have lease agreements with lease and non-lease components, which are generally accounted for separately. Variable lease payments (for example, common area maintenance and real estate tax charges) are recorded separately from the determination of the ROU asset and lease liability.
Derivative Financial Instruments
The Company has an interest rate swap agreement which it uses as a derivative financial instrument to manage its interest rate exposure. The Company does not use financial instruments for trading or other speculative purposes.
ASC Topic 815, “Derivatives and Hedging,” establishes accounting and reporting standards requiring that derivative instruments be recorded on the balance sheet as either an asset or liability measured at fair value. The statement requires that changes in the derivative’s fair value be recognized currently in earnings unless specific hedge accounting criteria are met. If hedge accounting criteria are met, the changes in a derivative’s fair value (for a cash flow hedge) are deferred in stockholders’ equity as a component of accumulated other comprehensive loss. These deferred gains and losses are recognized as income in the period in which hedged cash flows occur. The ineffective portions of hedge returns are recognized as earnings.
Income Taxes
The Company accounts for deferred income taxes utilizing ASC Topic 740, “Income Taxes.” ASC Topic 740 requires the asset and liability method, whereby deferred tax assets and liabilities are recognized based on the tax effects of temporary differences between the financial statement and the tax bases of assets and liabilities, as measured at current enacted tax rates. We have assessed the need for a valuation allowance by considering whether it is more likely than not that some portion or all of our deferred tax assets will not be realized. We continue to evaluate our ability to realize the tax benefits associated with deferred tax assets by analyzing the relative impact of all the available positive and negative evidence regarding our forecasted taxable income, the reversal of existing deferred tax liabilities, taxable income in prior carry-back years (if permitted) and the availability of tax planning strategies. In future reporting periods, we will continue to assess the likelihood that deferred tax assets will be realizable.
Interest and penalties associated with uncertain income tax positions is classified as income tax expense. The Company has not recorded any material income tax related interest or penalties during any of the periods presented.
Fair Value of Other Financial Instruments
The Company considers that the carrying amount of financial instruments, including accounts receivable, accounts payable and accrued liabilities approximate fair value due to their short maturities. The fair value of our
F-15
Table of Contents
outstanding First Lien Term Loan and Second Lien Term Loan (each as defined in Note 7, Long-Term Debt), based on the quoted market price for the same or similar issues of debt, which represents a Level 2 fair value measurement, is approximately:
| December 31, 2020 (Successor) |
December 31, 2019 (Successor) |
|||||||||||||||
| (in thousands) |
Carrying Value |
Fair Value | Carrying Value |
Fair Value | ||||||||||||
| First Lien Term Loan (1) |
$ | 906,624 | $ | 911,788 | $ | 638,640 | $ | 658,325 | ||||||||
| Second Lien Term Loan (2) |
232,361 | 240,000 | 231,628 | 232,800 | ||||||||||||
| (1) | The carrying value of the First Lien Term Loan is net of unamortized deferred financing costs of $12.8 and $15.0 and unamortized debt discount of $2.8 and $1.4 million as of December 31, 2020 and 2019, respectively. |
| (2) | The carrying value of the Second Lien Term Loan is net of unamortized deferred financing costs of $0.8 and $0.9 and unamortized debt discount of $6.8 and $7.5 million as of December 31, 2020 and 2019, respectively. |
Share-Based Compensation
Share-based compensation expense related to stock options is measured by the fair value of the stock options on the date of grant, net of the estimated forfeiture rate. We determine the fair value of options using the Black-Scholes option pricing model. The estimated fair value of options is recognized as expense on a straight-line basis over the options’ vesting periods. The fair value of the stock options contain certain assumptions, such as the risk-free interest rate, expected volatility, dividend yield and expected option life.
Share-based compensation expense related to restricted stock units and performance restricted stock units is recorded based on the market value of our common stock on the date of grant, net of the estimated forfeiture rate. The expense is recognized over the requisite service period.
Our performance restricted stock units award have a graded vesting schedule. The expense is recognized for each separately vesting tranche as though each tranche of the award is, in substance, a separate award. The amount of compensation expense recognized for performance restricted stock units is dependent upon an assessment of the likelihood of achieving the performance conditions and is subject to adjustment based on management’s assessment of the Company’s performance relative to the target number of shares performance criteria.
Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of net income (loss) and other comprehensive income (loss). Other comprehensive income (loss) includes minimum pension liability adjustments and cash flow hedge. These amounts are presented in the consolidated statements of comprehensive income (loss) net of reclassification adjustments to earnings.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Examples include, but are not limited to, estimates for fair value measurements in business combination, valuation of long-lived assets, including goodwill and definite-lived intangible assets and valuation of obligation under the tax receivable agreement. Actual results could differ from those estimates.
F-16
Table of Contents
Recent Accounting Pronouncements
Standards Adopted
In June 2016, the FASB issued ASU No. 2016-13 Financial Instruments – Credit Losses (Topis 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 changes the impairment model for most financial assets and certain other instruments. For trade and other receivables, held-to-maturity debt securities, loans and other instruments, entities will be required to use a new forward-looking “expected loss” model that will replace today’s “incurred loss” model and generally will result in the earlier recognition of allowances for losses. For available-for-sale debt securities with unrealized losses, entities will measure credit losses in a manner similar to current practice, except that the losses will be recognized as an allowance. The ASU is effective for annual and interim periods in fiscal years beginning after December 15, 2019. We adopted this standard on January 1, 2020. The adoption of this standard did not have a material impact on our consolidated financial statements.
Standards Not Yet Adopted
In December 2019, the FASB issued ASU No. 2019-12 Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). ASU 2019-12 simplifies the accounting for income taxes by removing certain exceptions to the general principles in Topic 740 related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and recognition of deferred tax liabilities. This standard also simplifies the accounting for franchise taxes and enacted change in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the basis of goodwill. The ASU is effective for annual and interim periods in fiscal years beginning after December 15, 2020. Early adoption is permitted. We are evaluating the impact that ASU 2019-12 will have on our consolidated financial statements.
In March 2020, the FASB issued ASU No. 2020-04 Reference Rate Reform (Topic 848) Facilitation of the Effects of Reference Rate Reform on Financial Reporting (“ASU 2020-04”). ASU 2020-04 provides optional expedients and exceptions for applying generally accepted accounting principles (GAAP) to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate expected to be discontinued because of reference rate reform. The ASU may be applied through December 31, 2022. We will evaluate the impact of ASU 2020-04 on our consolidated financial statements.
| 3. | Revenue Recognition |
In the following table, revenue is disaggregated by service solution.
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Year Ended December 31, 2018 |
|||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||
| Equipment Solutions |
$ | 296,267 | $ | 253,597 | $ | 248,059 | ||||||
| Clinical Engineering |
256,874 | 188,752 | 161,492 | |||||||||
| Onsite Managed Services |
220,171 | 170,724 | 155,695 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 773,312 | $ | 613,073 | $ | 565,246 | |||||||
|
|
|
|
|
|
|
|||||||
The Company capitalized the estimated costs to obtain a contract in the amount of $6.2 million at January 1, 2018 with a corresponding adjustment to accumulated deficit for the “Impact of Change in Accounting Policy”. The Contract Asset included in other long-term assets in the Consolidated Balance Sheet at December 31, 2020 and 2019 was $13.4 and $7.9 million, respectively. Capitalized costs are amortized over the expected life of the related contracts which is estimated to be five years. Amortization is computed on a straight-line basis which coincides with the predominant expected life of the underlying contracts. Amortization costs are reflected in cost
F-17
Table of Contents
of revenue and selling, general and administrative expenses. The amount of amortization included in cost of revenue was $0.4 and $0.1 million for the year ended December 31, 2020 (successor) and the period from January 4 through December 31, 2019 (successor), respectively. The amount of amortization included in selling, general and administrative expense was $2.0, $0.5 and $1.9 million for the year ended December 31, 2020 (successor), the period from January 4 through December 31, 2019 (successor) and the year ended December 31, 2018 (predecessor), respectively. In connection with the business combination, the remaining predecessor contract costs at January 3, 2019 were eliminated through purchase accounting. There was no impairment loss recorded in relation to the costs capitalized during 2020, 2019 and 2018.
| 4. | Business Combination and Acquisition |
On October 28, 2020, we entered into a Stock Purchase Agreement to purchase all of the outstanding capital stock of a company specializing in the service and repair of medical equipment and instruments from the sellers for $475 million, subject to adjustments. Pending regulatory approval, the earliest the acquisition is expected to close is March 2021.
We entered into a financing commitment letter (the “Commitment Letter”) with various lenders to finance the acquisition. We expect the financing under the Commitment Letter, together with cash balances and availability under our existing $190 million Revolving Loan, to be sufficient to provide the financing necessary to consummate the transaction. The Commitment Letter provides for an additional $200 million under our First Lien Term Loan with all the same terms as our existing balances outstanding, except this additional loan is subject to an interest rate floor of 0.75%. The financing commitments are subject to certain conditions set forth in the Commitment Letter.
The completion of the acquisition is subject to the satisfaction or waiver of a number of customary closing conditions including the satisfaction of certain representations and warranties and the expiration or termination of any waiting period under the Hart-Scott Rodino Antitrust Improvements Act of 1976, as amended.
On December 11, 2020, we completed the acquisition of certain assets of a surgical laser equipment solutions provider for total consideration of approximately $8.9 million.
On January 31, 2020, we completed the acquisition of certain assets of a surgical equipment repair and maintenance service provider for total consideration of approximately $88.3 million, which was paid at closing. The result of the acquired company’s operations have been included in the consolidated financial statements since that date.
The following summarizes the fair values of assets acquired and liabilities assumed at the date of acquisition within our consolidated balance sheet:
| (in thousands) |
||||
| Cash |
$ | 51 | ||
| Accounts receivable |
10,447 | |||
| Inventories |
4,591 | |||
| Other current assets |
208 | |||
| Property and equipment |
3,534 | |||
| Goodwill |
35,554 | |||
| Operating lease right-of-use assets |
2,422 | |||
| Other intangibles |
34,714 | |||
| Accounts payable |
(1,333 | ) | ||
| Accrued compensation |
(494 | ) | ||
| Other accrued expenses |
(275 | ) | ||
| Operating lease liability |
(1,142 | ) | ||
|
|
|
|||
| Total purchase price |
$ | 88,277 | ||
|
|
|
|||
F-18
Table of Contents
The acquired intangible assets, all of which are finite-life, are comprised of trade name and customer relationships and have a weighted average useful life of approximately 14.5 years. The total amount of goodwill that is deductible for tax purpose is $35.4 million.
This acquisition was funded from the revolving loan.
On January 4, 2019, Agiliti consummated its business combination pursuant to the Amended and Restated Agreement and Plan of Merger, dated as of December 19, 2018 (“A&R Merger Agreement”), by and among FSAC, a special purpose acquisition company affiliated with Thomas H. Lee Partners, L.P., Agiliti, Umpire SPAC Merger Sub, Inc., a Delaware corporation, Umpire Cash Merger Sub, Inc., a Delaware corporation, Agiliti Holdco, Inc. (previously known as UHS Holdco, Inc.), a Delaware corporation (“Agiliti Holdco” or “Parent”), solely in their capacities as Majority Stockholders, IPC/UHS, L.P. (“IPC/UHS”) and IPC/UHS Co-Investment Partners, L.P., each a Delaware limited partnership, solely in its capacity as the Stockholders’ Representative, IPC/UHS, and solely for the purposes stated therein, Umpire Equity Merger Sub, Inc., a Delaware corporation. The A&R Merger Agreement amended and restated the Agreement and Plan of Merger dated as of August 13, 2018. Pursuant to the A&R Merger Agreement, (i) FSAC became a wholly owned subsidiary of Agiliti and the holders of Class A common stock, par value $0.0001 per share, of FSAC (the “FSAC Class A Common Stock”) received shares of common stock, par value $0.0001 per share, of Agiliti (the “Agiliti Common Stock” or “Common Stock”); and (ii) Agiliti Holdco became a wholly owned subsidiary of FSAC and the equityholders of Agiliti Holdco received cash and/or shares of Agiliti Common Stock and/or fully-vested options to purchase shares of Agiliti Common Stock as merger consideration (the transactions contemplated by the A&R Merger Agreement are referred to herein as the “Business Combination”).
On December 19, 2018, in connection with the entry into the A&R Merger Agreement, FSAC entered into the Amended and Restated Subscription Agreement (the “Backstop Agreement”) with THL Agiliti LLC (the “THL Stockholder”), pursuant to which THL Stockholder agreed to purchase a number of shares of FSAC common stock at a price of $8.50 per share necessary to cause the minimum cash condition under the A&R Merger Agreement to be satisfied, subject to a cap of $750 million. On the Closing Date, pursuant to the Backstop Agreement, THL Stockholder purchased 86,795,398 shares of FSAC Class A Common Stock for an aggregate of $737.8 million, which shares were exchanged for shares of Agiliti Common Stock on a one-for-one basis at the closing of the Business Combination.
The following summarizes the estimated fair value of the Business Combination:
| (in thousands) |
||||
| Cash paid |
$ | 703,219 | ||
| Equity consideration paid to Selling Equityholders |
22,464 | |||
| Tax receivable agreement (1) |
44,270 | |||
|
|
|
|||
| Total consideration |
769,953 | |||
| Debt assumed |
690,907 | |||
|
|
|
|||
| Estimated fair value of the Business Combination |
$ | 1,460,860 | ||
|
|
|
|||
| (1) | On the Closing Date, the Company, Agiliti Holdco and IPC/UHS, as Stockholders’ Representative, entered into the Tax Receivable Agreement (the “TRA”). The TRA provides for the payment by Agiliti Holdco to the sellers of 85% of certain U.S. federal, state, and local tax benefits realized or deemed realized by Agiliti Holdco and its subsidiaries from the following tax attributes: (i) U.S. federal, state and local net operating losses (including carryforwards) of Agiliti Holdco and its subsidiaries in existence as of the end of the Closing Date, (ii) without duplication of amounts included under clause (i), certain deductions generated by the consummation of the Business Combination, (iii) certain deductions arising from rollover options cancelled or exercised post-closing as such deductions are generated post-closing and (iv) certain deductions arising from payments made under the TRA. Agiliti Holdco and its subsidiaries will retain the tax benefit, if any, of the remaining 15% of such tax attributes. The amount and timing of any such payments may vary |
F-19
Table of Contents
| depending upon a number of factors. The term of the TRA will continue until all tax benefits subject to the TRA have been utilized or deemed utilized, unless Agiliti Holdco exercises its right to terminate the TRA early or there is otherwise a deemed early termination (e.g., upon the occurrence of a change of control of the Company, FSAC or Agiliti Holdco or certain divestitures of Agiliti Holdco’s subsidiaries) pursuant to the terms of the TRA. If Agiliti Holdco elects to terminate the TRA early or there is a deemed early termination, its obligations under the TRA would accelerate and it generally would be required to make an immediate payment equal to the present value of the deemed anticipated future payments to be made by it under the TRA, calculated in accordance with certain valuation assumptions set forth in the TRA. |
Notwithstanding the foregoing, after the fifth anniversary of the Closing Date, a tax benefit payment due to a holder of any options or restricted stock units (RSUs) in Agiliti Holdco shall be paid only if either (i) such holder is employed on the first day of the calendar year following the taxable year for which such tax benefit payment was calculated or (ii) Agiliti Holdco has aggregate revenue of at least $225 million (which may be adjusted downward due to certain transactions) for the first two quarters of the taxable year following the taxable year for which such tax benefit payment was calculated.
The Company recorded an allocation of the purchase price to Predecessor’s tangible and identified intangible assets acquired and liabilities assumed, excluding long-term debt, based on the valuation performed to determine the fair value of the net assets as of the closing date. The purchase price allocation is as follows:
| (in thousands) |
||||
| Cash |
$ | 7,340 | ||
| Accounts receivable |
105,256 | |||
| Inventories |
11,122 | |||
| Other current assets |
10,339 | |||
| Property and equipment |
273,364 | |||
| Customer relationships (1) |
468,000 | |||
| Trade name (2) |
2,406 | |||
| Non-compete agreements (3) |
16,532 | |||
| Goodwill |
775,219 | |||
| Operating lease right-of-use assets (4) |
30,426 | |||
| Other long-term assets |
3,261 | |||
| Accounts payable |
(37,649 | ) | ||
| Accrued compensation |
(33,715 | ) | ||
| Accrued interest |
(18,732 | ) | ||
| Other accrued expenses |
(31,624 | ) | ||
| Pension and other long-term liabilities |
(6,649 | ) | ||
| Operating lease liability |
(30,246 | ) | ||
| Deferred income taxes |
(83,790 | ) | ||
|
|
|
|||
| Total purchase price |
$ | 1,460,860 | ||
|
|
|
|||
| (1) | Customer relationships were valued using the multi-period excess earning method. Under this approach, the applicable cost structure was deducted from the existing customer revenue estimates to arrive at operating income. Certain adjustments were made to operating income to derive after-tax cash flows. These adjustments included applicable income tax expense, adjustment to economic depreciation and an appropriate charge for the use of contributory assets. After-tax cash flows were estimated over an explicit projection period and discounted to present value at an appropriate discount rate. The estimated useful life of fifteen years represents the approximate point in the projection period in which a majority of the asset’s cash flow are expected to be realized based on assumed attrition rates. |
| (2) | Trade name was valued using the cost approach. The valuation of the trade name involves estimating, on a replacement cost basis, the external vendor related expenses, internal employee expenses, a developer’s profit on the internal employee costs, and an entrepreneurial incentive. The trade name is estimated to have a useful life of ten years. |
F-20
Table of Contents
| (3) | Non-compete agreements were valued using the comparative method under the income approach. To estimate the value of the non-compete agreements, two different scenarios were developed: (i) after tax cash flows of the business with the non-compete agreements in place; and (ii) after-tax cash flows of the business without the non-compete agreements in place. The difference between the present values of the after-tax cash flows of the two scenarios isolates the impact of the non-compete agreement and provides an estimation of value. This value is then adjusted by the probability that the individual will compete. The assessment of the probability considers the individual’s ability, feasibility and desire to compete. The estimated useful life of three to four years represent the duration of the non-compete agreements. |
| (4) | Include favorable leases of $0.2 million. Favorable leases were valued based on the Market Approach to determine the market rental rate at the subject properties and where applicable utilized the Income Approach to determine if an above/below position exists for leasehold interest. The leased locations include office and warehouse spaces and are located in various states across the United States. The leases have varying rent payments, escalation clauses and terms. The fair value of the leasehold interest is the present value of the differences between the in-place contract rents and the market rents. The contract rents can be considered at market, above market, or below market. The estimated useful life is three years. |
The acquired intangible assets, all of which are finite-life, are comprised of customer relationships, non-compete agreements and trade name and have a weighted average useful life of approximately 14.6 years.
From January 1 through January 3, 2019 (predecessor), approximately $17.1 million of expenses were incurred directly related to the Business Combination. The impact of other activities on Predecessor financial statements from January 1 through January 3, 2019 related to revenue, cost of revenue and other operating expenses is not considered material. We have omitted certain disclosures related to the period from January 1 through January 3, 2019 since they were not material or have no impact to the consolidated financial statements. On the Closing Date, the Company repaid $25.9 million of its senior secured credit facility.
The following unaudited pro forma consolidated results of operations assumed as though the Business Combination had occurred at January 1, 2018. The unaudited pro forma consolidated financial information should not be relied upon as necessarily being indicative of the historical results that would have been obtained if the Business Combination had actually closed on that date, nor the results that may be obtained in the future.
| Year Ended December 31, | ||||||||
| (in thousands) |
2019 | 2018 | ||||||
| (Successor) | (Successor) | |||||||
| Revenue |
$ | 613,073 | $ | 565,246 | ||||
| Net loss attributable to Agiliti, Inc. and Subsidiaries |
(29,223 | ) | (85,481 | ) | ||||
Included in the determination of pro forma net loss for the years ended December 31, 2019 and 2018 are pro forma charges for various purchase accounting adjustments. These pro forma adjustments resulted in pro forma decreases to gross margin and higher selling, general and administrative expense for the year ended December, 2018 primarily from the increases in carrying value of medical equipment, property and office equipment and intangible assets. Income taxes are provided at the estimated statutory rate.
As provided for in the A&R Merger Agreement, Agiliti assumed stock warrants that were issued by FSAC as part its initial public offering and a subsequent transaction. In total, Agiliti assumed 23 million public stock warrants and 14.95 million private placement stock warrants. Each warrant provided the holders the option to purchase one share of Agiliti Class A Common Stock at a strike price of $11.50, which was adjusted to $9.27 in connection with extraordinary dividend payment in 2019 (see Note 10). Subsequent to the Business Combination, Agiliti issued a cash tender offer for all of the stock warrants at $0.95 per warrant. All of the private placement stock warrants and 22.8 million of the public stock warrants were repurchased by the Company for total cash consideration of $35.9 million through December 31, 2019. This transaction was accounted for as a reduction to additional paid-in capital. A total of 0.2 million public stock warrants remain outstanding at December 31, 2020. The warrants will expire on January 4, 2024.
F-21
Table of Contents
On July 8, 2019, we completed the acquisition of certain assets of a medical imaging equipment service provider for total consideration of approximately $12.5 million. The consideration consisted of $10.3 million of cash paid at closing, $0.2 million of liability assumed and $2.0 million of earn-out. The result of the acquired company’s operations have been included in the consolidated financial statements since that date.
The following summarizes the fair values of assets acquired and liabilities assumed at the date of acquisition within our consolidated balance sheet:
| (in thousands) |
||||
| Accounts receivable |
$ | 1,324 | ||
| Inventories |
1,422 | |||
| Property and equipment |
136 | |||
| Intangible assets |
4,810 | |||
| Goodwill |
5,615 | |||
| Accounts payable |
(476 | ) | ||
| Accrued compensation |
(174 | ) | ||
| Other accrued expenses |
(172 | ) | ||
|
|
|
|||
| Total purchase price |
$ | 12,485 | ||
|
|
|
|||
The acquired intangible assets, all of which are finite-life, are comprised of customer relationships and non-compete agreement and have a weighted average useful life of approximately 9.9 years.
The acquisition was funded from the revolving loan.
| 5. | Fair Value Measurements |
Financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2020 and 2019 are summarized in the following table by type of inputs applicable to the fair value measurements:
| Fair Value at December 31, 2020 (Successor) |
Fair Value at December 31, 2019 (Successor) |
|||||||||||||||||||||||||||||||
| (in thousands) |
Level 1 | Level 2 | Level 3 | Total | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||||||
| Assets: |
||||||||||||||||||||||||||||||||
| Deferred compensation assets |
$ | 1,104 | $ | — | $ | — | $ | 1,104 | $ | 160 | $ | — | $ | — | $ | 160 | ||||||||||||||||
| Liabilities: |
||||||||||||||||||||||||||||||||
| Contingent consideration |
$ | — | $ | — | $ | 321 | $ | 321 | $ | — | $ | — | $ | 1,369 | $ | 1,369 | ||||||||||||||||
| Obligation under tax receivable agreement |
— | — | 50,600 | 50,600 | — | — | 36,300 | 36,300 | ||||||||||||||||||||||||
| Interest rate swap |
— | 1,883 | — | 1,883 | — | — | — | — | ||||||||||||||||||||||||
| Deferred compensation liabilities |
1,104 | — | — | 1,104 | 160 | — | — | 160 | ||||||||||||||||||||||||
A description of the inputs used in the valuation of assets and liabilities is summarized as follows:
Level 1 — Inputs represent unadjusted quoted prices for identical assets or liabilities exchanged in active markets.
Level 2 — Inputs include directly or indirectly observable inputs other than Level 1 inputs such as quoted prices for similar assets or liabilities exchanged in active or inactive markets; quoted prices for identical assets or liabilities exchanged in inactive markets; other inputs that are considered in fair value determinations of the
F-22
Table of Contents
assets or liabilities, such as interest rates and yield curves that are observable at commonly quoted intervals, volatilities, prepayment speeds, loss severities, credit risks and default rates; and inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 3 — Inputs include unobservable inputs used in the measurement of assets and liabilities. Management is required to use its own assumptions regarding unobservable inputs because there is little, if any, market activity in the assets or liabilities or related observable inputs that can be corroborated at the measurement date. Measurements of non-exchange traded derivative contract assets and liabilities are primarily based on valuation models, discounted cash flow models or other valuation techniques that are believed to be used by market participants. Unobservable inputs require management to make certain projections and assumptions about the information that would be used by market participants in pricing assets or liabilities.
The deferred compensation assets are held in mutual funds. The fair value of the deferred compensation assets and liabilities is based on the quoted market prices for the mutual funds and thus represents a Level 1 fair value measurement.
In May 2020, we entered into an interest rate swap agreement to manage our interest rate exposure, see Note 7, Long-Term Debt. The carrying value of interest rate swap contracts is at fair value, which is determined based on current interest rate and forward interest rates as of the balance sheet date and is classified within Level 2.
During 2019, we recorded a contingent consideration liability, in the form of earn-out payment, related to our July 8, 2019 acquisition in the total amount of approximately $2.0 million. The contingent consideration was based on achieving certain revenue results. The fair value of the liability was estimated using a discounted cash flow approach with significant inputs that are not observable in the market and thus represents a Level 3 fair value measurement. The significant inputs in the Level 3 measurement not supported by market activity included our assessments of expected future cash flows during the earn-out period related to the assets acquired, appropriately discounted considering the uncertainties associated with the obligation, and calculated based on estimated revenue in accordance with the terms of the agreement. We made a remeasurement adjustment of $1.4 and $0.6 million during the year ended December 31, 2020 and the period from January 4 through December 31, 2019 based on the revenue results not being achieved.
During 2020, we recorded a contingent consideration liability, in the form of earn-out payment, related to our December 11, 2020 acquisition in the total amount of approximately $0.3 million. The contingent consideration is based on achieving certain revenue results. The fair value of the liability was estimated using a discounted cash flow approach with significant inputs that are not observable in the market and thus represents a Level 3 fair value measurement. The significant inputs in the Level 3 measurement not supported by market activity included our assessments of expected future cash flows during the earn-out period related to the assets acquired, appropriately discounted considering the uncertainties associated with the obligation, and calculated based on estimated revenue in accordance with the terms of the agreement. The earn-out is expected to be paid in the first quarter of 2022.
The assumptions used in preparing the discounted cash flow analyses included estimates of interest rates and the timing and amount of incremental cash flows.
During 2019, we initially recorded $44.3 million obligation under tax receivable agreement related to the Business Combination. The fair value of the liability was estimated using company specific assumptions that are not observable in the market and thus represents a Level 3 fair value measurement. Management’s estimate of the valuation of the obligation under the tax receivable agreement is based on a Monte Carlo model which involves the use of projected cash flows of the Company, a discount rate, and historical deferred tax assets subject to the agreement. We made a remeasurement adjustment to reduce the liability by $8.0 million during the period from January 4 through December 31, 2019. We made a remeasurement adjustment to increase the liability by $14.3 million for the year ended December 31, 2020.
F-23
Table of Contents
A reconciliation of the beginning and ending balance for the Level 3 measurement are as follows:
| (in thousands) |
||||
| Balance at December 31, 2018 (Predecessor) |
$ | — | ||
| Addition |
46,255 | |||
| Remeasurement adjustment |
(8,586 | ) | ||
|
|
|
|||
| Balance at December 31, 2019 (Successor) |
$ | 37,669 | ||
| Addition |
321 | |||
| Remeasurement adjustment |
12,931 | |||
|
|
|
|||
| Balance at December 31, 2020 (Successor) |
$ | 50,921 | ||
|
|
|
|||
6. Selected Financial Statement Information
Property and Equipment
Our property and equipment at December 31, 2020 and 2019 consists of the following:
| (in thousands) |
December 31, 2020 (Successor) |
December 31, 2019 (Successor) |
||||||||||
| Medical Equipment |
$ | 285,723 | $ | 261,477 | ||||||||
| Less: Accumulated depreciation |
(145,645 | ) | (71,008 | ) | ||||||||
|
|
|
|
|
|||||||||
| Medical equipment, net |
140,078 | 190,469 | ||||||||||
|
|
|
|
|
|||||||||
| Leasehold improvements |
28,415 | 15,006 | ||||||||||
| Office equipment and vehicles |
84,231 | 58,210 | ||||||||||
|
|
|
|
|
|||||||||
| 112,646 | 73,216 | |||||||||||
| Less: Accumulated depreciation and amortization |
(38,308 | ) | (20,959 | ) | ||||||||
|
|
|
|
|
|||||||||
| Property and office equipment, net |
74,338 | 52,257 | ||||||||||
|
|
|
|
|
|||||||||
| Total property and equipment, net |
$ | 214,416 | $ | 242,726 | ||||||||
|
|
|
|
|
|||||||||
There were no impairment charges on property and equipment during 2020, 2019 and 2018.
Goodwill and Other Intangible Assets
We have one reporting unit which is equal to our single reporting segment.
Goodwill and other intangible assets as of December 31, 2020 were recognized as part of purchase price allocation of the Business Combination completed on January 4, 2019 and other acquisitions during 2020 and 2019. There were no impairment losses recorded on goodwill through December 31, 2020.
F-24
Table of Contents
Our other intangible assets as of December 31, 2020 and 2019 consist of the following:
| December 31, 2020 (Successor) |
December 31, 2019 (Successor) |
|||||||||||||||||||||||||||||||
| (in thousands) |
Cost | Accumulated Amortization |
Impairment | Net | Cost | Accumulated Amortization |
Impairment | Net | ||||||||||||||||||||||||
| Finite-life intangibles |
||||||||||||||||||||||||||||||||
| Customer relationship |
$ | 513,189 | $ | (118,172 | ) | $ | — | $ | 395,017 | $ | 472,748 | $ | (58,931 | ) | $ | — | $ | 413,817 | ||||||||||||||
| Non-compete agreements |
14,613 | (9,647 | ) | — | 4,966 | 16,593 | (5,382 | ) | — | 11,211 | ||||||||||||||||||||||
| Trade names |
3,779 | (1,667 | ) | — | 2,112 | 2,406 | (241 | ) | — | 2,165 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total intangible assets |
$ | 531,581 | $ | (129,486 | ) | $ | — | $ | 402,095 | $ | 491,747 | $ | (64,554 | ) | $ | — | $ | 427,193 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
Total amortization expense related to intangible assets was approximately $67.0, $64.6 and $7.6 million for the year ended December 31, 2020 (successor), the period from January 4 through December 31, 2019 (successor) and the year ended December 31, 2018 (predecessor), respectively.
On December 3, 2018, the Company changed its legal name from Universal Hospital Services, Inc. to Agiliti Health, Inc. and the brand identity to Agiliti. As a result, the indefinite-lived intangible assets related to UHS trade name of $131.1 million was written off in 2018.
There were no impairment charges during 2020 or 2019 with respect to other intangible assets.
At December 31, 2020, future estimated amortization expense related to intangible assets for each of the years ended December 31, 2021 to 2025 is estimated as follows:
| (in thousands) |
||||
| 2021 |
60,306 | |||
| 2022 |
53,460 | |||
| 2023 |
47,856 | |||
| 2024 |
43,526 | |||
| 2025 |
39,195 | |||
Future amortization expense is an estimate. Actual amounts may change due to additional intangible asset acquisitions, impairment, accelerated amortization or other events.
Supplementary Cash Flow Information
Supplementary cash flow information are as follows (in thousands):
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||||
| Non-cash activities: |
||||||||||||||||||||
| Property and equipment purchases included in accounts payable (at end of period) |
$ | 3,141 | $ | 7,842 | $ | — | $ | 9,919 | ||||||||||||
| Finance lease additions |
10,286 | 9,968 | — | 8,412 | ||||||||||||||||
| Operating lease right-of-use assets and operating lease liability additions |
29,577 | 9,674 | 30,426 | — | ||||||||||||||||
| Non-cash equity contribution |
— | 22,464 | — | — | ||||||||||||||||
F-25
Table of Contents
Valuation and Qualifying Accounts
Our valuation and qualifying accounts are as follows (in thousands):
| Description |
Balance - Beginning of Year |
Charged to Costs and Expense |
Deductions from Reserves |
Balance - End of Year |
||||||||||||
| Allowance for Doubtful Accounts |
||||||||||||||||
| Year Ended December 31, 2020 (Successor) |
$ | 1,156 | $ | 1,959 | $ | 1,122 | $ | 1,993 | ||||||||
| Period from January 4 through December 31, 2019 (Successor) |
— | 1,031 | (125 | ) | 1,156 | |||||||||||
| Period from January 1 through January 3, 2019 (Predecessor) |
1,217 | — | 1,217 | — | ||||||||||||
| Year Ended December 31, 2018 (Predecessor) |
1,234 | 2,125 | 2,142 | 1,217 | ||||||||||||
| Income Tax Valuation Allowance |
||||||||||||||||
| Year Ended December 31, 2018 (Predecessor) |
44,403 | (44,403 | ) | — | — | |||||||||||
7. Long-Term Debt
Long-term debt at December 31, 2020 and 2019 consists of the following:
| (in thousands) |
December 31, 2020 |
December 31, 2019 |
||||||
| (Successor) | (Successor) | |||||||
| First Lien Term Loan (1) |
$ | 906,624 | $ | 638,640 | ||||
| Second Lien Term Loan (2) |
232,361 | 231,628 | ||||||
| Revolving Loan (3) |
(2,481 | ) | 30,396 | |||||
| Finance lease liability |
24,595 | 22,334 | ||||||
|
|
|
|
|
|||||
| 1,161,099 | 922,998 | |||||||
| Less: Current portion of long-term debt |
(16,044 | ) | (13,272 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt |
$ | 1,145,055 | $ | 909,726 | ||||
|
|
|
|
|
|||||
| (1) | The carrying value of the First Lien Term Loan is net of unamortized deferred financing costs of $ 12.8 and $15.0 and unamortized debt discount of $2.8 and $1.4 million as of December 31, 2020 and 2019, respectively. |
| (2) | The carrying value of the Second Lien Term Loan is net of unamortized deferred financing costs of $0.8 and $0.9 and unamortized debt discount of $6.8 and $7.5 million as of December 31, 2020 and 2019, respectively. |
| (3) | The carrying value of the Revolving Loan is net of unamortized deferred financing costs of $2.5 and $3.1 as of December 31, 2020 and 2019, respectively. |
First Lien Credit Facilities. On January 4, 2019, in connection with and substantially concurrent with the closing of the Business Combination, Agiliti entered into a credit agreement (the “First Lien Credit Facilities”) with JPMorgan Chase Bank, N.A. as administrative agent, collateral agent, and letter of credit issuer, Agiliti Holdco, certain subsidiaries of Agiliti Health acting as guarantors (the “Guarantors”), and the lenders from time to time party thereto.
The First Lien Credit Facilities provide for a seven-year senior secured delayed draw term loan facility in an aggregate principal amount of $660 million (the “First Lien Term Loan”) and a five-year senior secured revolving credit facility in an aggregate principal amount of $150 million (the “Revolving Loan”). In February 2020, we increased our principal First Lien Term Loan facility by $125 million and the revolving loan facility by $40 million. All terms to the First Lien Term Loan and Revolving Loan facilities remain the same. In October
F-26
Table of Contents
2020, we increased our principal First Lien Term Loan facility by $150 million. All terms to the First Lien Term Loan remain the same, except this additional loan is subject to a different interest rate floor of 0.75%.
The First Lien Term Loan amortizes in equal quarterly installments, commencing on June 30, 2019, in an aggregate annual amount equal to 1.00% of the original principal amount of such term loan, with the balance due and payable at maturity unless prepaid prior thereto.
Borrowings under the First Lien Credit Facilities bear interest, at Agiliti’s option, at a rate per annum equal to an applicable margin over either (a) a base rate determined by reference to the highest of (1) the prime lending rate published in the Wall Street Journal, (2) the federal funds effective rate plus 1/2 of 1% and (3) the LIBOR rate for a one-month interest period, plus 1.00%, or (b) a LIBOR rate determined by reference to the LIBOR rate as set forth by the ICE Benchmark Administration for the interest period relevant to such borrowing, in each case, subject to interest rate floors.
The First Lien Credit Facilities contain a number of negative covenants that, among other things, restrict, subject to certain exceptions, the ability of Agiliti and the guarantors thereunder to incur additional indebtedness and guarantee indebtedness; create or incur liens; engage in mergers or consolidations; sell, transfer or otherwise dispose of assets; pay dividends and distributions or repurchase capital stock; prepay, redeem or repurchase certain indebtedness; make investments, loans and advances; enter into agreements which limit the ability of Agiliti and the guarantors thereunder to incur liens on assets; and enter into amendments to certain junior lien and subordinated indebtedness in a manner materially adverse to the lenders.
Solely with respect to the Revolving Loan, commencing with the fiscal quarter ending March 31, 2019, the Company is required to maintain leverage ratio not to exceed 7.00:1.00, when the aggregate principal amount of outstanding Revolving Loans and drawn Letters of Credit, on the last day of the most recent fiscal quarter, exceeds 35% of the total revolving credit commitments.
As of December 31, 2020, we had $183.7 million of availability under our revolving loan based on a revolving credit facility of $190.0 million less $6.3 million used letters of credit.
Second Lien Term Loan. The Second Lien Term Loan provides for an eight-year term loan facility in an aggregate principal amount of $240 million (the “Second Lien Term Loan”). The proceeds of the Second Lien Term Loan were drawn on November 15, 2019 and used to return capital to shareholders.
The Second Lien Term Loan is payable at maturity unless prepaid prior thereto. We may repay some or all of the Second Lien Term Loan at any time prior to November 15, 2020 at a price equal to 102% of the principal amount thereof; from November 15, 2020 to November 14, 2021 at a price equal to 101% of the principal amount thereof; and anytime thereafter at a price equal to 100% of the principal amount thereof, in each case, plus accrued and unpaid interest, if any, to the date of redemption.
Borrowings under the Second Lien Term Loan bear interest, at Agiliti’s option, at a rate per annum equal to an applicable margin over either (a) a base rate determined by reference to the highest of (1) the prime lending rate published in the Wall Street Journal, (2) the federal funds effective rate plus 1/2 of 1% and (3) the LIBOR rate for a one-month interest period, plus 1.00%, or (b) a LIBOR rate determined by reference to the LIBOR rate as set forth by the ICE Benchmark Administration for the interest period relevant to such borrowing, in each case, subject to interest rate floors. Interest rate was LIBOR rate plus 7.75%.
The Second Lien Term Loan contains a number of negative covenants that, among other things, restrict, subject to certain exceptions, the ability of Agiliti and the guarantors thereunder to incur additional indebtedness and guarantee indebtedness; create or incur liens; engage in mergers or consolidations; sell, transfer or otherwise dispose of assets; pay dividends and distributions or repurchase capital stock; prepay, redeem or repurchase certain indebtedness; make investments, loans and advances; enter into agreements which limit the ability of Agiliti and the guarantors thereunder to incur liens on assets; and enter into amendments to certain junior lien and subordinated indebtedness in a manner materially adverse to the lenders.
F-27
Table of Contents
Interest Rate Swap. In May 2020, we entered into an interest rate swap agreement for a total notional amount of $500.0 million, which has the effect of converting a portion of our First Lien Term Loan to fixed interest rates. The effective date for the interest rate swap agreement was June 2020 and the expiration date is June 2023.
The interest rate swap agreement qualifies for cash flow hedge accounting under ASC Topic 815, “Derivatives and Hedging”. Both at inception and on an on-going basis, we must perform an effectiveness test. The fair value of the interest rate swap agreement at December 31, 2020 was ($1.9) million, of which ($1.0) million is included in other accrued expenses and ($0.9) million is included in other long-term liabilities on our consolidated balance sheet. The change in fair value was recorded as a component of accumulated other comprehensive loss on our consolidated balance sheet, net of tax, since the instrument was determined to be an effective hedge at December 31, 2020. We have not recorded any amounts due to ineffectiveness for any periods presented.
As a result of our interest rate swap agreement, we expect the effective interest rate on $350.0 million and $150.0 million of our First Lien Term Loan to be 0.3396% and 0.3290%, respectively, through June 2023.
We were in compliance with all financial debt covenants for all years presented.
Maturities of Long-Term Debt. At December 31, 2020, maturities of long-term debt for each of the years ending December 31, 2021 to 2025 and thereafter, are contractually as follows:
| (in thousands) |
||||
| 2021 |
$ | 16,044 | ||
| 2022 |
14,221 | |||
| 2023 |
12,895 | |||
| 2024 |
11,930 | |||
| 2025 |
11,228 | |||
| Thereafter |
1,120,467 | |||
|
|
|
|||
| Total payments |
1,186,785 | |||
| Unamortized deferred financing costs |
(16,033 | ) | ||
| Unamortized debt discount |
(9,653 | ) | ||
|
|
|
|||
| $ | 1,161,099 | |||
|
|
|
|||
8. Leases
We lease facilities under operating lease agreements, which include both monthly and longer-term arrangements. Our finance leases consist primarily of leased vehicles.
F-28
Table of Contents
The lease assets and liabilities at December 31, 2020 and 2019 were as follows:
| (in thousands) |
Classification |
December 31, |
December 31, |
|||||||
| Lease Assets |
||||||||||
| Operating lease assets |
Operating lease right-of-use assets |
$ | 51,214 | $ | 29,964 | |||||
| Finance lease assets |
Property and equipment(a) |
23,513 | 23,274 | |||||||
|
|
|
|
|
|||||||
| Total leased assets |
$ | 74,727 | $ | 53,238 | ||||||
|
|
|
|
|
|||||||
| Lease Liabilities |
||||||||||
| Current |
||||||||||
| Operating |
Current portion of operating lease liability | $ | 14,155 | $ | 9,171 | |||||
| Finance |
Current portion of long-term debt | 6,694 | 6,672 | |||||||
| Noncurrent |
||||||||||
| Operating |
Operating lease liability, less current portion | 40,283 | 22,089 | |||||||
| Finance |
Long-term debt, less current portion | 17,901 | 15,662 | |||||||
|
|
|
|
|
|||||||
| Total lease liabilities |
$ | 79,033 | $ | 53,594 | ||||||
|
|
|
|
|
|||||||
| (a) | Finance lease assets are recorded net of accumulated depreciation of $13,096 and $8,264 as of December 31, 2020 and 2019, respectively. |
The lease cost for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 were as follows:
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
||||||
| (Successor) | (Successor) | |||||||
| Lease Cost |
||||||||
| Finance lease cost |
||||||||
| Amortization of right-of-use assets |
$ | 9,531 | $ | 9,058 | ||||
| Interest on lease liabilities |
781 | 658 | ||||||
| Operating lease cost |
12,706 | 10,547 | ||||||
| Short-term lease cost |
715 | 41 | ||||||
| Variable lease cost |
4,388 | 3,397 | ||||||
|
|
|
|
|
|||||
| Total lease cost |
$ | 28,121 | $ | 23,701 | ||||
|
|
|
|
|
|||||
The maturity of lease liabilities at December 31, 2020 were as follows:
| (in thousands) |
Operating Leases |
Finance Leases |
Total | |||||||||
| 2021 |
$ | 15,411 | $ | 7,353 | $ | 22,764 | ||||||
| 2022 |
11,981 | 5,350 | 17,331 | |||||||||
| 2023 |
10,085 | 3,939 | 14,024 | |||||||||
| 2024 |
8,827 | 2,908 | 11,735 | |||||||||
| 2025 |
6,577 | 2,117 | 8,694 | |||||||||
| Thereafter |
4,860 | 5,228 | 10,088 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total lease payments |
$ | 57,741 | $ | 26,895 | $ | 84,636 | ||||||
|
|
|
|
|
|
|
|||||||
| Less: Interest |
3,303 | 2,300 | 5,603 | |||||||||
|
|
|
|
|
|
|
|||||||
| Present value of lease liabilities |
$ | 54,438 | $ | 24,595 | $ | 79,033 | ||||||
|
|
|
|
|
|
|
|||||||
F-29
Table of Contents
The lease term and discount rate at December 31, 2020 were as follows:
| December 31, 2020 |
||||
| Lease Term and Discount Rate |
||||
| Weighted-average remaining lease term (years) |
||||
| Operating leases |
4.6 | |||
| Finance leases |
2.7 | |||
| Weighted-average discount rate |
||||
| Operating leases |
2.8 | % | ||
| Finance leases |
2.8 | % | ||
Other information related to cash paid related to lease liabilities and lease assets obtained for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 and from January 1 through January 3, 2019 were as follows:
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
|||||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||||||
| Cash paid for amounts included in the masurement of lease liabilities |
||||||||||||||||
| Operating cash flows from finance leases |
$ | 781 | $ | 658 | $ | — | ||||||||||
| Operating cash flows from operating leases |
12,733 | 10,028 | — | |||||||||||||
| Financing cash flows from finance leases |
8,024 | 7,609 | — | |||||||||||||
| Lease asset obtained in exchange for new finance lease liabilities |
10,286 | 9,968 | — | |||||||||||||
| Lease asset obtained in exchange for new operating lease liabilities |
29,577 | 9,674 | 30,426 | |||||||||||||
| 9. | Commitments and Contingencies |
The Company, in the ordinary course of business, is subject to liability claims related to employees and the equipment that it rents and services. Asserted claims are subject to many uncertainties and the outcome of individual matters is not predictable. Certain claims where the loss is probable, a provision is recorded based on the Company’s best estimate. While the ultimate resolution of these actions may have an impact on the Company’s financial results for a particular reporting period, management believes that any such resolution would not have a material adverse effect on the financial position, results of operations or cash flows of the Company and the chance of a negative outcome on outstanding litigation is considered remote.
On January 13, 2015, the Company filed suit in the Western District of Texas against Hill-Rom Holdings, Inc., Hill-Rom Company, Inc. and Hill-Rom Services, Inc. (the “Defendants”). On March 7, 2018, the Company and the Defendants entered into a confidential settlement agreement resolving all disputes and legal claims of the parties associated with the suit filed by the Company. In consideration for this release, terms of the confidential settlement agreement called for the Company to receive legal title to certain medical equipment of the Defendants as specified in the agreement on May 1, 2018. The Company recorded a gain on settlement of approximately $26.4 million during 2018 based on the fair value of the assets received of $23.4 million and $3.0 million of cash. The fair value of the assets was measured using inputs of replacement cost and market data, which are considered a Level 3 fair value measurement.
F-30
Table of Contents
On July 29, 2019, the Company entered into a memorandum of understanding to settle all claims in a class action litigation brought in California. The Company received a release in exchange for a payment of $3.5 million. Payment of the settlement amount was made in February 2020.
| 10. | Shareholder’s Equity (Deficit) |
Common Stock
The Company has authorized and issued 98,983,296 shares of common stock with a par value of $0.0001 per share as of December 31, 2020.
In November 2019, the Company declared and paid $2.23 dividend per share to the common stock and vested stock option holders for a total amount of $227.1 million. The Company also declared $2.23 dividend per unit to the restricted stock unit and performance restricted stock unit holders which will be paid upon vesting of those units. Dividend paid on vested restricted stock unit and performance restricted stock units was $1.1 million during 2020. Dividend payable was $2.2 and $3.4 million as of December 31, 2020 and 2019, respectively, of which $0.9 and $1.0 million was included in accounts payable and $1.3 and $2.4 million was included in other long-term liabilities. In connection with the dividend payment, the exercise price of unvested stock options was adjusted from $8.50 to $6.27 per share.
Accumulated Other Comprehensive Loss
| (in thousands) |
December 31, 2020 |
December 31, 2019 |
||||||
| (Successor) | (Successor) | |||||||
| Unrealized loss on minimum pension liability adjustment, net of tax |
$ | (2,215 | ) | $ | (940 | ) | ||
| Unrealized loss on cash flow hedge, net of tax |
(1,404 | ) | — | |||||
|
|
|
|
|
|||||
| $ | (3,619 | ) | $ | (940 | ) | |||
|
|
|
|
|
|||||
Changes in Accumulated Other Comprehensive Loss are as follows:
| (in thousands) |
||||
| Minimum pension liability - balance at December 31, 2019 |
$ | (940 | ) | |
|
|
|
|||
| Net actuarial loss |
(1,759 | ) | ||
| Reclassification for amortization of net gain |
49 | |||
| Income tax expense related to pension |
435 | |||
|
|
|
|||
| Net current year other comprehensive income |
(1,275 | ) | ||
|
|
|
|||
| Minimum pension liability - balance at December 31, 2020 |
$ | (2,215 | ) | |
|
|
|
|||
| Cash flow hedge - balance at December 31, 2019 |
$ | — | ||
|
|
|
|||
| Changes in the effective portion of the fair value of cash flow hedge |
(1,882 | ) | ||
| Income tax expense related to cash flow hedge |
478 | |||
|
|
|
|||
| Net current year other comprehensive income |
(1,404 | ) | ||
|
|
|
|||
| Cash flow hedge - balance at December 31, 2020 |
$ | (1,404 | ) | |
|
|
|
|||
| Net current year other comprehensive income |
$ | (2,679 | ) | |
|
|
|
|||
Amount reclassified from accumulated other comprehensive loss is included in the selling, general and administrative expense in the Consolidated Statements of Operations.
F-31
Table of Contents
| 11. | Share-Based Compensation |
Predecessor
The 2007 Stock Option Plan provided for the issuance of 43.9 million nonqualified stock options of Parent to any of its and Parent’s executives, other key employees and to consultants and certain non-employee directors. The options allowed for the purchase of shares of common stock of Parent at prices equal to the stock’s fair market value at the date of grant. Options granted had a ten-year contractual term and vested over approximately six years.
In April 2015, Parent granted the Company’s Chief Executive Officer 7.0 million restricted stock units which vest over four years. Total compensation expense related to this grant was $1.3 million for the year ended December 31, 2018.
On May 9, 2018, Parent adopted the 2018 Executive Management Stock Option Plan (the “2018 Stock Option Plan”). Pursuant to the 2018 Stock Option Plan, awards may be in the form of Non-Qualified Stock Options. The maximum number of shares for which options may be granted was 2,500,000 under the 2018 Stock Option Plan. In 2018, 2,499,000 shares of common stock were issued to certain Agiliti Health executives, including Named Executive Officers other than the Chief Executive Officer pursuant to the 2018 Stock Option Plan.
Options granted pursuant to the 2018 Stock Option Plan have an exercise price of $0.71 per share and could only be exercised upon a change of control (as defined in the 2018 Stock Option Plan) and only if such change of control occurred no later than March 15th of the calendar year following the calendar year in which the signing of a binding agreement for Parent to undergo a change of control occurs. No expense was recorded for this plan in 2018. Additionally, all awards of options granted pursuant to the 2018 Stock Option Plan provide for a claw back in the event an award recipient voluntarily terminates his or her employment with the Company without Good Reason or has his or her employment terminated by the Company for Cause (as such terms are defined in the Company’s Executive Severance Pay Plan) within one year following a Change in Control of the Company.
On August 9, 2018, the board of directors of the Parent approved and adopted an amendment to the 2018 Stock Option Plan to confirm that any options granted under the 2018 Plan will only be exercisable upon a Change of Control of Parent.
In connection with the closing of the Business Combination, Agiliti, Inc. assumed the 2007 Stock Option Plan and approximately 75% of the outstanding stock option awards were terminated and cashed out, and the remaining 25% of the outstanding stock option awards were rolled over into options to purchase common stock of Agiliti, Inc. subject to the same terms and conditions of the original awards. The predecessor recognized approximately $5.9 million of non-cash share-based compensation expense for the period from January 1 through January 3, 2019 related to the fully vested 2007 Stock Option Plan, the 2018 Executive Management Stock Option Plan and the Restricted Stock Unit granted to an officer in April 2015.
Successor
On January 4, 2019, the 2018 Omnibus Incentive Plan (“2018 Plan”) became effective. Approximately 3.0 million shares of the 2007 Stock Option Plan with an exercise price of $2.13 per share and expiration date of November 4, 2024 were rolled into the 2018 Plan on January 4, 2019.
The 2018 Plan provides for issuance of 10.4 million nonqualified stock options, restricted stock units and performance restricted stock units to any of its executives, other key employees and certain non-employee directors. The stock options allow for the purchase of shares of common stock of the Company at prices equal to the stock’s fair market value at the date of grant. Options granted had a ten-year contractual term and vest over one to four years. The restricted stock units vest over three to four years. The performance restricted stock units vest over three years upon achievement of established performance targets as defined in the agreement.
F-32
Table of Contents
The shares issued to a grantee upon the exercise of such grantee’s options will be subject to certain restrictions on transferability as provided in the 2018 Plan. Grantees are subject to non-competition, non-solicitation and confidentiality requirements as set forth in their respective stock option grant agreements. Forfeited options are available for future issue.
In connection with the dividend payment in November 2019, the exercise price of stock options granted under the 2018 Plan was adjusted from $8.50 to $6.27 per share (see Note 10, Shareholder’s Equity (Deficit). This modification did not result in additional share-based compensation expense.
Stock Options
A summary of activity for the stock options under the 2018 Plan is detailed below:
| (in thousands, except exercise price and years) |
Number of options |
Weighted average exercise price |
Aggregate intrinsic value |
Weighted average remaining contractual term (years) |
||||||||||||
| Outstanding at January 4, 2019 |
— | $ | — | $ | — | — | ||||||||||
| Granted |
6,023 | 4.22 | ||||||||||||||
| Exercised |
(91 | ) | 2.13 | 580 | ||||||||||||
| Forfeited or expired |
(10 | ) | 6.27 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2019 |
5,922 | $ | 4.25 | $ | 23,669 | 7.1 | ||||||||||
|
|
|
|||||||||||||||
| Granted |
1,274 | 8.25 | ||||||||||||||
| Exercised |
(239 | ) | 6.27 | 473 | ||||||||||||
| Forfeited or expired |
(267 | ) | 6.38 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2020 |
6,690 | $ | 4.86 | $ | 81,235 | 6.5 | ||||||||||
|
|
|
|||||||||||||||
| Exercisable at December 31, 2020 |
3,699 | $ | 3.04 | $ | 51,634 | 4.8 | ||||||||||
|
|
|
|||||||||||||||
The exercise price of the stock option award is equal to the market value of Company’s common stock on the grant date as determined reasonably and in good faith by the Company’s Board of Directors and compensation committee and based on an analysis of a variety of factors including peer group multiples, merger and acquisition multiples, and discounted cash flow analyses.
The intrinsic value of a stock award is the amount by which the market value of the underlying stock exceeds the exercise price of the award.
We determine the fair value of options using the Black-Scholes option pricing model. The estimated fair value of options, including the effect of estimated forfeitures, is recognized as expense on a straight-line basis over the options’ vesting periods. The assumptions in the table below were used to determine the Black-Scholes fair value of stock options granted for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 and for the year ended December 31, 2018.
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Year Ended December 31, 2018 |
||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||
| Risk-free interest rate |
0.51 | % | 2.50 | % | 1.62 | |||||||
| Expected volatility |
33.95 | % | 34.13 | % | 29.0 | |||||||
| Dividend yield |
N/A | N/A | N/A | |||||||||
| Expected option life (years) |
3.03 | 3.57 | 4.60 | |||||||||
| Black-Scholes Value of options |
$ | 1.94 | $ | 2.42 | $ | 0.50 | ||||||
F-33
Table of Contents
Expected volatility is based on an independent valuation of the stock of companies within our peer group. Given the lack of a true comparable company, the peer group consists of selected public healthcare companies representing our suppliers, customers and competitors within certain product lines. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the grant date based on the expected option life. The expected option life is estimated based on foreseeable trends.
At December 31, 2020, unearned non-cash share-based compensation related to 2018 Plan that we expect to recognize as expense over a weighted average period of 2.1 years, totals approximately $4.5 million, net of our estimated forfeiture rate of 2.0%. The expense could be accelerated upon the sale of the Company.
Restricted Stock Units and Performance Restricted Stock Units
A summary of activity for restricted stock units and performance restricted stock units is detailed below:
| Number of units |
Weighted average grant date fair value |
|||||||
| Nonvested at January 4, 2019 |
— | $ | — | |||||
| Granted |
1,575 | 8.50 | ||||||
| Vested |
— | — | ||||||
| Forfeited |
(29 | ) | 8.50 | |||||
|
|
|
|||||||
| Nonvested at December 31, 2019 |
1,546 | $ | 8.50 | |||||
|
|
|
|||||||
| Granted |
1,057 | 8.25 | ||||||
| Vested |
(512 | ) | 8.50 | |||||
| Forfeited |
(147 | ) | 8.45 | |||||
|
|
|
|||||||
| Nonvested at December 31, 2020 |
1,944 | 8.37 | ||||||
|
|
|
|||||||
Future expense related to restricted stock units and performance restricted stock units that we expect to recognize as expense over a weighted average period of 2.0 years totals approximately $10.8 million, net of our estimated forfeiture rate of 2.0%.
For the year ended December 31, 2020 (successor), the period from January 4 through December 31, 2019 (successor), from January 1 through January 3, 2019 (predecessor) and the year ended December 31, 2018 (predecessor), we recognized non-cash share-based compensation expense of $10.3, $5.8, $5.9 and $3.0 million, respectively, which is primarily included in selling, general and administrative expenses.
Remaining authorized options, restricted stock units and performance restricted stock units available for future issuance was 3.9 million shares at December 31, 2020.
| 12. | Related Party Transactions |
On January 4, 2019, Agiliti entered into an advisory services agreement (the “Advisory Services Agreement”) with Agiliti Holdco, Agiliti Health and THL Managers VIII, LLC (the “Advisor”). Pursuant to the Advisory Services Agreement, the Advisor will provide management, consulting and other advisory services to the companies. In consideration for these services, the companies will pay to the Advisor (i) a non-refundable periodic retainer fee in an aggregate amount per fiscal quarter equal to the greater of (a) $375,000 or (b) 1% of the consolidated Adjusted EBITDA (as defined in the Advisory Services Agreement) for the immediately preceding fiscal quarter or such other amount as may be mutually agreed, with the first such payment to be made on April 15, 2019, (ii) fees in amounts to be mutually agreed in connection with any financing or refinancing, dividend, recapitalization, acquisition, disposition and spin-off or split-off transaction, (iii) in the case of an
F-34
Table of Contents
initial public offering (“IPO”), in addition to the fees under clauses (i) and (ii), an amount equal to the net present value of the higher periodic fee that would have been payable from the date of such IPO until the scheduled termination date of the Advisory Services Agreement, and (iv) fees for other management, consulting and other advisory services to be discussed in good faith among the parties. The companies will also pay expenses incurred by the Advisor, its consultants and certain other parties affiliated with Advisor. The initial term of the Advisory Agreement ends on January 4, 2027, and is automatically extended for successive periods of one (1) year. The Advisory Services Agreement also terminates automatically immediately prior to an IPO or a transaction involving a change of control (as defined in the Advisory Services Agreement). Total professional services fees incurred to the Advisor were $2.3 and $1.5 million for the year ended December 31, 2020 and the period from January 4 through December 31, 2019.
On May 31, 2007, the predecessor and affiliates of Irving Place Capital (together with its affiliates, “IPC”) entered into a professional services agreement pursuant to which IPC provided general advisory and management services to us with respect to financial and operating matters. IPC was a principal owner of Parent, and each of Robert Juneja, Bret Bowerman and Keith Zadourian were members of our board of directors and were associated with IPC. The professional services agreement required us to pay an annual fee for ongoing advisory and management services equal to the greater of $0.5 million or 0.75% of our Adjusted EBITDA (as defined in the professional services agreement) for the immediately preceding fiscal year, payable in quarterly installments. The professional services agreement provided that IPC was reimbursed for its reasonable out-of-pocket expenses in connection with certain activities undertaken pursuant to the professional services agreement and was indemnified for liabilities incurred in connection with its role under the professional services agreement, other than for liabilities resulting from its gross negligence or willful misconduct. The term of the professional services agreement commenced on May 31, 2007 and remained in effect until either party notified the other of its desire to terminate, we were sold to a third-party purchaser or we consummated a qualified initial public offering, as defined in the professional services agreement.
On August 9, 2018, the predecessor and IPC entered into a Second Amended and Restated Professional Services Agreement (the “Amended PSA”). Pursuant to the Amended PSA, in addition to the services previously provided under the original agreement, IPC provided services related to a possible transaction resulting in a qualified public offering or change of control and received a fee of $10 million payable upon the consummation of such transaction. The Amended PSA also removed the acceleration of the advisory fee in the event of a sale of the Company or qualified public offering. Additionally, the Amended PSA amended the agreement to terminate on the earliest of (a) consummation of a qualified public offering, (b) consummation of a change in control and (c) the tenth anniversary of the date of the Amended PSA, provided, however, that if no qualified public offering or change of control has been consummated prior to such tenth anniversary, the Amended PSA automatically extended on a year to year basis until either party provided written notice of its desire to terminate the Amended PSA or at such time as a qualified public offering or change of control has been consummated.
Total professional services agreement fees incurred to IPC were $10 million for the period from January 1 through January 3, 2019 (predecessor) in connection with the consummation of the Business Combination and $1.1 for the year ended December 31, 2018 (predecessor).
Upon the consummation of the Business Combination, the professional service agreement with IPC was terminated.
In connection with the Restricted Stock Unit Award Agreement, dated as of April 13, 2015, between an officer and the Company, we entered into a promissory note agreement with the officer dated April 13, 2016 for a total amount of $1.0 million, dated April 13, 2017 for a total amount of $0.9 million and dated April 13, 2018 for a total amount of $0.6 million which were included in other long-term assets in the Consolidated Balance Sheets. These note receivables had annual interest rates of 1.45%-2.72%. The principal and accrued interest of these notes were due on the earliest of (i) the seventh anniversary of the date of this loan, (ii) any event with respect to borrower, which, in any such case of the loan were to remain outstanding on and after such date, would result in
F-35
Table of Contents
violation of Section 402 of the Sarbanes-Oxley Act of 2002, (iii) and certain events of default or (iv) a change in control. Interest income for this note was $0.05 million for the year ended December 31, 2018 (predecessor). The notes receivable were collected upon consummation of the Business Combination.
On May 31, 2017, Agiliti Holdco sold an aggregate of 1,596,640 unregistered shares of its common stock to certain of our executive officers for an aggregate purchase price of $1.9 million. These shares are subject to the restrictions set forth in UHS Holdco, Inc.’s Securityholders Agreement, dated as of May 31, 2007 (as amended). The sales of the above securities were exempt from registration under the Securities Act of 1933, as amended (the “Securities Act”), in reliance upon Section 4(2) of the Securities Act. The purchasers of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. Proceeds from sale of the securities were contributed from UHS Holdco to the Company and used for general operating purposes and the repayment of outstanding debt obligations.
| 13. | Limited Liability Companies |
We participate with others in the formation of LLCs in which the Company becomes a partner and shares the financial interest with the other investors. The Company is the primary beneficiary of these LLCs. These LLCs acquire certain medical equipment for use in their respective business activities, which generally focus on surgical procedures. The LLCs will acquire medical equipment for rental purposes under equipment financing leases. At December 31, 2020, the LLCs had approximately $0.5 million of total assets. The third party investors in each respective LLC generally provide the lease financing company with individual proportionate lease guarantees based on their respective ownership percentages in the LLCs. In addition, the Company will provide such financing companies with its corporate guarantee based on its respective ownership interest in each LLC. In certain instances, the Company has provided such financing companies with an overall corporate guarantee in connection with equipment financing transactions. In such instances, the individual investors in each respective LLC will generally indemnify us against losses, if any, incurred in connection with its corporate guarantee. Additionally, we provide operational and administrative support to the LLCs in which it is a partner. Assets of the LLCs that can only be utilized to settle obligations are immaterial. As of December 31, 2020, we held interests in two active LLCs.
In accordance with guidance issued by the FASB, we accounted for equity investments in LLCs (in which we are the primary beneficiary) under the full consolidation method whereby transactions between the Company and the LLCs have been eliminated through consolidation.
| 14. | Employee Benefit Plans |
ASC Topic 715, “Compensation — Retirement Benefits” requires employers to recognize the under- funded or over funded status of a defined benefit post retirement plan as an asset or liability in its statements of financial position and to recognize changes in the funded status in the year in which the changes occur through accumulated other comprehensive income. Additionally, ASC Topic 715 requires employers to measure the funded status of a plan as of the date of its year-end statement of financial position.
Pension plan benefits are to be paid to eligible employees after retirement based primarily on years of credited service and participants’ compensation. The Company uses a December 31 measurement date. Effective December 31, 2002, the Company froze the benefits under the pension plan.
F-36
Table of Contents
The change in benefit obligation, pension plan assets and funded status as of and for the years ended December 31, 2020 and 2019 are as follows:
Change in Benefit Obligation
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Benefit obligations at beginning of year |
$ | 30,514 | $ | 27,011 | ||||
| Interest cost |
955 | 1,132 | ||||||
| Actuarial loss |
2,877 | 3,622 | ||||||
| Benefit paid |
(1,339 | ) | (1,251 | ) | ||||
|
|
|
|
|
|||||
| Benefit obligations at end of year |
$ | 33,007 | $ | 30,514 | ||||
|
|
|
|
|
|||||
Change in Plan Assets
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Fair value of plan assets at beginning of year |
$ | 22,616 | $ | 19,916 | ||||
| Actual return on plan assets |
2,228 | 3,436 | ||||||
| Benefits paid |
(1,339 | ) | (1,251 | ) | ||||
| Employer contribution |
1,125 | 515 | ||||||
|
|
|
|
|
|||||
| Fair value of plan assets at end of year |
$ | 24,630 | $ | 22,616 | ||||
|
|
|
|
|
|||||
Funded Status
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Funded status |
$ | (8,377 | ) | $ | (7,898 | ) | ||
| Unrecognized net actuarial loss/accumulated other comprehensive loss |
2,969 | 1,259 | ||||||
|
|
|
|
|
|||||
| Net amount recognized |
$ | (5,408 | ) | $ | (6,639 | ) | ||
|
|
|
|
|
|||||
A summary of our pension plan projected benefit obligation, accumulated obligation and fair value of pension plan assets at December 31, are as follows:
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Projected benefit obligation |
$ | 33,007 | $ | 30,514 | ||||
| Accumulated benefit obligation (“ABO”) |
33,007 | 30,514 | ||||||
| Fair value of plan assets |
24,630 | 22,616 | ||||||
| ABO less fair value of plan assets |
8,377 | 7,898 | ||||||
Amounts recognized in the consolidated balance sheets at December 31, are as follows:
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Current Liabilities |
$ | 650 | $ | 1,080 | ||||
| Noncurrent Liabilities |
7,727 | 6,818 | ||||||
|
|
|
|
|
|||||
| Total Amount Recognized |
$ | 8,377 | $ | 7,898 | ||||
|
|
|
|
|
|||||
F-37
Table of Contents
Net Periodic Benefit Cost
The components of net periodic benefit cost are as follows:
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Year Ended December 31, 2018 |
|||||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||||||
| Interest cost |
$ | 955 | $ | 1,132 | $ | 1,044 | ||||||||||
| Expected return on plan assets |
(1,110 | ) | (1,073 | ) | (1,158 | ) | ||||||||||
| Recognized net actuarial loss |
49 | — | 848 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net periodic benefit cost |
$ | (106 | ) | $ | 59 | $ | 734 | |||||||||
|
|
|
|
|
|
|
|||||||||||
Change in Accumulated Other Comprehensive Loss
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
Year Ended December 31, 2018 |
|||||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||||||
| Beginning of year |
$ | (940 | ) | $ | (9,059 | ) | $ | (9,064 | ) | |||||||
| Net actuarial loss |
(1,759 | ) | (1,259 | ) | (732 | ) | ||||||||||
| Impact of reflecting purchase accounting |
— | 9,059 | — | |||||||||||||
| Amortization of net gain |
49 | — | 848 | |||||||||||||
| Income tax expense related to pension |
435 | 319 | (111 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| End of year |
$ | (2,215 | ) | $ | (940 | ) | $ | (9,059 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
Pension Plan Assets
Our target pension plan asset allocation and actual pension plan allocation of assets at December 31, are as follows:
| Asset Category |
Target Allocation |
2020 | 2019 | |||||||||
| (Successor) | (Successor) | |||||||||||
| Equity securities |
75 | % | 80 | % | 80 | % | ||||||
| Debt securities and cash |
25 | 20 | 20 | |||||||||
|
|
|
|
|
|
|
|||||||
| 100 | % | 100 | % | 100 | % | |||||||
|
|
|
|
|
|
|
|||||||
The pension plan assets are invested with the objective of maximizing long-term returns while minimizing material losses in order to meet future benefit obligations when they come due.
The Company utilizes an investment approach with a mix of equity and debt securities used to maximize the long-term return on assets. Risk tolerance is established through consideration of pension plan liabilities, funded status and corporate financial condition. The investment portfolio consists of a diversified blend of mutual funds and fixed-income investments. Investment risk is measured and monitored on an ongoing basis through quarterly investment portfolio reviews and annual asset and liability reviews.
F-38
Table of Contents
Fair Value Measurement
The following table presents our plan assets, using the fair value hierarchy as disclosed in Note 5, Fair Value Measurements, as of December 31, 2020 and 2019.
| Assets at Fair Value as of December 31, 2020 | ||||||||||||||||
| (in thousands) |
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Cash and cash equivalents |
$ | 660 | $ | — | $ | — | $ | 660 | ||||||||
| Registered investment companies: |
||||||||||||||||
| Total international stock index fund |
9,533 | — | — | 9,533 | ||||||||||||
| Total stock market index fund |
6,400 | — | — | 6,400 | ||||||||||||
| Total return fund |
4,150 | — | — | 4,150 | ||||||||||||
| Volatility risk premium defensive fund |
3,887 | — | — | 3,887 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value |
$ | 24,630 | $ | — | $ | — | $ | 24,630 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Assets at Fair Value as of December 31, 2019 | ||||||||||||||||
| (in thousands) |
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Cash and cash equivalents |
$ | 113 | $ | — | $ | — | $ | 113 | ||||||||
| Registered investment companies: |
||||||||||||||||
| Total international stock index fund |
9,172 | — | — | 9,172 | ||||||||||||
| Total stock market index fund |
5,289 | — | — | 5,289 | ||||||||||||
| Total return fund |
4,330 | — | — | 4,330 | ||||||||||||
| Volatility risk premium defensive fund |
3,712 | — | — | 3,712 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value |
$ | 22,616 | $ | — | $ | — | $ | 22,616 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Investments in Equity and Debt Securities are valued at the net asset value of units held at the end of the period based upon the value of the underlying investments as determined by quoted market prices. These investments are classified as Level 1.
Contributions
The Company contributed $1.1, $0.5 and $0.8 million to the pension plan during the year ended December 31, 2020 (successor), the period from January 4 through December 31, 2019 (successor) and the year ended December 31, 2018 (predecessor), respectively. The Company expects to make contribution of approximately $0.7 million in 2021.
Estimated Future Benefit Payments
The following benefit payments are expected to be paid:
| (in thousands) |
||||
| 2021 |
$ | 1,400 | ||
| 2022 |
1,462 | |||
| 2023 |
1,552 | |||
| 2024 |
1,597 | |||
| 2025 |
1,644 | |||
| 2024 to 2028 |
8,800 | |||
F-39
Table of Contents
Pension Plan Assumptions
The following weighted-average assumptions were used as of each of the years ended December 31, as follows:
| 2020 | 2019 | 2018 | ||||||||||
| (Successor) | (Successor) | (Predecessor) | ||||||||||
| Weighted-average actuarial assumptions used to determine benefit obligations: |
||||||||||||
| Discount rate |
2.43 | % | 3.17 | % | 4.26 | % | ||||||
| Expected return on assets |
5.40 | % | 5.50 | % | 5.50 | % | ||||||
| Weighted-average actuarial assumptions used to determine net periodic benefit cost: |
||||||||||||
| Discount rate |
3.17 | % | 4.26 | % | 3.61 | % | ||||||
| Expected return on assets |
5.40 | % | 5.50 | % | 5.50 | % | ||||||
| Rate of compensation increase |
N/A | N/A | N/A | |||||||||
These assumptions are reviewed on an annual basis. The discount rate reflects the current rate at which the pension obligation could be effectively settled at the end of the year. The Company sets its rate to reflect the yield of a portfolio of high quality, fixed-income debt instruments that would produce cash flow sufficient in timing and amount to settle projected future benefits. In determining the expected return on asset assumption, the Company evaluates the long-term returns earned by the pension plan, the mix of investments that comprise pension plan assets and forecasts of future long-term investment returns.
Other Employee Benefits
The Company also sponsors a defined contribution plan, which qualifies under Section 401(k) of the Internal Revenue Code of 1986, as amended (the “Code”) and covers substantially all of the Company’s employees. Employees may contribute annually up to 60% of their base compensation on a pre-tax basis (subject to Internal Revenue Service limitation). The company matching contribution is 50% of the first 6% of base compensation that an employee contributes. We made matching contributions to the plan of approximately $4.0, $3.0 and $2.7 million for the year ended December 31, 2020 (successor), the period from January 4 through December 31, 2019 (successor) and the year ended December 31, 2018 (predecessor), respectively.
The Company is self-insured for employee healthcare up to $250,000 per member per plan year and aggregate claims up to 125% of expected claims per plan year. Also, the Company purchases workers’ compensation and automobile liability coverage with related deductibles. The Company is liable for up to $250,000 per individual workers’ compensation claim and up to $500,000 per accident for automobile liability claims. Self-insurance and deductible costs are included in other accrued expenses in the consolidated balance sheets and are accrued based upon the aggregate of the liability for reported claims and an actuarially determined estimated liability for claims development and incurred but not reported.
15. Income Taxes
The income tax expense (benefit) consists of the following:
| (in thousands) |
Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||
| Current - State |
$ | 1,710 | $ | 445 | $ | — | $ | 949 | ||||||||
| Deferred |
(4,944 | ) | (15,302 | ) | (13,281 | ) | (67,297 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | (3,234 | ) | $ | (14,857 | ) | $ | (13,281 | ) | $ | (66,348 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
F-40
Table of Contents
| Reconciliations between the Company’s effective income tax rate and the U.S. statutory rate are as follow:
|
| |||||||||||||||
| Year Ended December 31, 2020 |
From January 4 through December 31, 2019 |
From January 1 through January 3, 2019 |
Year Ended December 31, 2018 |
|||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||
| Statutory U.S. Federal income tax rate |
(21.0 | )% | (21.0 | )% | (21.0 | )% | (21.0 | )% | ||||||||
| State income taxes, net of U.S. Federal income tax |
(6.3 | ) | (3.5 | ) | (2.2 | ) | (4.1 | ) | ||||||||
| Permanent items |
2.0 | 0.9 | 1.7 | 1.0 | ||||||||||||
| Deferred rate change |
0.2 | (1.0 | ) | (0.6 | ) | — | ||||||||||
| Share-based compensation |
(1.4 | ) | (3.5 | ) | (58.2 | ) | — | |||||||||
| TRA fair value adjustment |
14.1 | (4.4 | ) | — | — | |||||||||||
| Transaction costs |
— | — | 2.7 | — | ||||||||||||
| Valuation allowance |
— | — | — | (43.5 | ) | |||||||||||
| Other |
(0.2 | ) | 0.4 | 0.1 | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Effective income tax rate |
(12.6 | )% | (32.1 | )% | (77.5 | )% | (67.6 | )% | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Our effective tax rate for the year ended December 31, 2020 and the period from January 4 through December 31, 2019 was primarily impacted by our tax receivable agreement. Our effective tax rate for the period from January 1 through January 3, 2019 was reduced by 55.7 percent for excess tax benefits associated with exercises of share-based compensation.
Many of the new laws included in the Tax Act became effective during 2018, including limitations on the deductibility of interest expense and executive compensation, as well as international provisions. However, these provisions did not significantly impact the Company, as the Company does not have international operations and the Company was not materially impacted under the new domestic provisions. Any impacts of future issued guidance will be appropriately accounted for in the period in which the guidance is effective.
The Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) was signed into law on March 27, 2020. Among others, the CARES Act delayed payment of employer payroll taxes and adjusted the depreciable life of qualified leasehold improvement property. We have reflected the impact of the CARES Act within our consolidated financial statements for the year ended December 31, 2020, and such impact was not material to our consolidated financial statements.
F-41
Table of Contents
The components of the Company’s overall deferred tax assets and liabilities at December 31 are as follows:
| (in thousands) |
2020 | 2019 | ||||||
| (Successor) | (Successor) | |||||||
| Deferred tax assets |
||||||||
| Accounts receivable |
$ | 507 | $ | 293 | ||||
| Accrued compensation and pension |
19,190 | 10,686 | ||||||
| Inventories |
616 | 604 | ||||||
| Other assets |
518 | 1,181 | ||||||
| Unrealized loss on pension |
754 | 320 | ||||||
| Operating lease liability |
13,826 | 7,930 | ||||||
| Unrealized loss on cash flow hedge |
478 | — | ||||||
| Net operating loss carryforwards |
44,819 | 58,209 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
80,708 | 79,223 | ||||||
| Deferred tax liabilities |
||||||||
| Accelerated depreciation and amortization |
(128,518 | ) | (138,465 | ) | ||||
| Prepaid assets |
(1,931 | ) | (1,087 | ) | ||||
| Operating lease right-of-use assets |
(13,007 | ) | (7,841 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
(143,456 | ) | (147,393 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax asset (liabilities) |
$ | (62,748 | ) | $ | (68,170 | ) | ||
|
|
|
|
|
|||||
At December 31, 2020, the Company had available unused federal net operating loss carryforwards of approximately $171.2 million. The net operating losses for tax years beginning before January 1, 2018 of $92.0 million will expire at various dates from 2027 through 2037. Net operating losses for tax years beginning after January 1, 2018 of $79.2 million will not expire. Net operating loss carryforwards of the Company are subject to review and possible adjustment by the taxing authorities.
Under the Code, certain corporate stock transactions into which the Company has entered or may enter in the future could limit the amount of the net operating loss carryforwards that can be utilized in future periods. The Company has completed a review of historical stock transactions, as well as the stock transactions completed in conjunction with the Business and concluded that there is no material limitation on the use of the net operating loss carryforwards.
In assessing the need for a valuation allowance, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. We evaluate our ability to realize the tax benefits associated with deferred tax assets by analyzing the relative impact of all the available positive and negative evidence regarding our forecasted taxable income, the reversal of existing deferred tax liabilities, taxable income in prior carry-back years (if permitted) and the availability of tax planning strategies. The Company has been generating taxable income in recent years and is projecting significant taxable income in future years due to continued growth. As such, we believe that it is more likely than not that we will be able to realize our deferred tax assets and have not recorded a valuation allowance for the year ended December 31, 2020, the period from January 4 through December 31, 2019 and the period from January 1 through January 3, 2019. During the year ended December 31, 2018, we recorded a tax benefit of $44.4 million associated with the reversal of our valuation allowance.
ASC Topic 740 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
F-42
Table of Contents
The Company files income tax returns in the U.S. federal jurisdiction and numerous state jurisdictions. With few exceptions, the Company is no longer subject to U.S. federal or state and local income tax examinations by tax authorities for taxable years before 2017.
A reconciliation of the beginning and ending amount of unrecognized tax benefit for the years ended December 31, 2020, 2019 and 2018
| (in thousands) |
||||
| Unrecognized tax benefits balance at January 1, 2018 (Predecessor) | $1,365 | |||
| Gross decreases for tax positions in 2018 |
— | |||
|
|
|
|||
| Unrecognized tax benefits balance at December 31, 2018 (Predecessor) |
1,365 | |||
| Gross decreases for rate change in 2019 |
(25 | ) | ||
|
|
|
|||
| Unrecognized tax benefits balance at December 31, 2019 (Successor) |
1,340 | |||
| Gross decreases for tax positions in 2020 |
— | |||
|
|
|
|||
| Unrecognized tax benefits balance at December 31, 2020 (Successor) | $1,340 | |||
|
|
|
|||
There are no unrecognized tax benefits as of December 31, 2020 that, if recognized, would impact the effective tax rate.
See Note 4, Business Combination and Acquisition, related to Tax Receivable Agreement
16. Concentration
On July 21, 2020, we entered into a one-year agreement with the U.S. Department of Health and Human Services and the Assistant Secretary for Preparedness and Response (the “HHS Agreement”) for the comprehensive maintenance and management services of medical ventilator equipment in exchange for up to $193 million in consideration throughout the duration of the agreement. As a result, we expect that the U.S. government will be our largest customer during the duration of the HHS Agreement.
In 2020, no individual customer represented over 10% of our revenue.
17. Subsequent Event
We evaluated subsequent events through the date of our report of independent registered public accounting firm, and except for items disclosed elsewhere in the consolidated financial statements, we have not identified any subsequent events requiring recognition or disclosure in the consolidated financial statements as of and for the year ended December 31, 2020.
F-43
Table of Contents
Through and including , 2021 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
26,315,789 Shares

Common Stock
PROSPECTUS
BofA Securities
Goldman Sachs & Co. LLC
Morgan Stanley
BMO Capital Markets
Citigroup
Jefferies
UBS Investment Bank
KeyBanc Capital Markets
Raymond James
MUFG
SMBC Nikko
Mischler Financial Group, Inc.
Siebert Williams Shank
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than the underwriting discounts and commissions payable by us, in connection with the offer and sale of the securities being registered. All amounts shown are estimates except for the Securities and Exchange Commission, or SEC, registration fee and the FINRA filing fee.
| SEC registration fee |
$ | 66,034 | ||
| FINRA filing fee |
91,290 | |||
| NYSE listing fee |
250,000 | |||
| Printing expenses |
400,000 | |||
| Legal fees and expenses |
2,500,000 | |||
| Accounting fees and expenses |
550,000 | |||
| Transfer agent fees and registrar fees |
5,000 | |||
| Miscellaneous expenses |
137,676 | |||
|
|
|
|||
| Total expenses |
$ | 4,000,000 | ||
|
|
|
Item 14. Indemnification of Directors and Officers.
Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its shareholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation will provide for this limitation of liability.
Section 145 of the DGCL, or Section 145, provides that a Delaware corporation may indemnify any person who was, is or is threatened to be made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his conduct was illegal. A Delaware corporation may indemnify any persons who are, were or are a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests, provided that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him against the expenses which such officer or director has actually and reasonably incurred.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the
II-1
Table of Contents
corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against him and incurred by him in any such capacity, or arising out of his status as such, whether or not the corporation would otherwise have the power to indemnify him under Section 145.
Our bylaws will provide that we will indemnify our directors and officers to the fullest extent authorized by the DGCL and must also pay expenses incurred in defending any such proceeding in advance of its final disposition upon delivery of an undertaking, by or on behalf of an indemnified person, to repay all amounts so advanced if it should be determined ultimately that such person is not entitled to be indemnified under this section or otherwise.
Upon completion, of this offering we intend to enter into indemnification agreements with each of our executive officers and directors. The indemnification agreements will provide the executive officers and directors with contractual rights to indemnification, expense advancement and reimbursement, to the fullest extent permitted under the DGCL.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our certificate of incorporation or bylaws, agreement, vote of shareholders or disinterested directors or otherwise.
We will maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers. The proposed form of Underwriting Agreement to be filed as Exhibit 1.1 to this Registration Statement provides for indemnification of our directors and officers by the underwriters party thereto against certain liabilities arising under the Securities Act of 1933 or otherwise.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding securities sold by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such securities and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
Since we were incorporated on August 1, 2018, we have made sales of the following unregistered securities:
| • | On March 19, 2021 (the “Closing Date”), we completed the Northfield Acquisition for a purchase price of $475.0 million, subject to adjustments. In connection with the Northfield Acquisition, certain members of the Northfield management team delivered commitment letters to reinvest a portion of the transaction proceeds to acquire our common stock on the Closing Date. The aggregate commitment amount is expected to result in the issuance of approximately 752,328 shares of our common stock. |
| • | In connection with the settlement of restricted stock units held by a former employee, we intend to issue 107,711 shares of common stock prior to the completion of this offering. |
| • | In August 2018, we sold 1,000 shares of common stock at a purchase price of $0.01 per share, for an aggregate purchase price of $10.00. |
| • | In January 2019, we sold an aggregate of 98,195,398 shares of common stock at a purchase price of $8.50 per share, for an aggregate purchase price of $834,660,883. |
| • | In January 2019, we sold 336,081 shares of common stock at a purchase price of $8.50 per share, in exchange for shares of UHS Holdco, Inc., for an aggregate purchase price of $2,856,688.50. |
| • | In January 2019, we sold 407,593 shares of common stock at a purchase price of $8.50 per share, in exchange for shares of FSAC for an aggregate purchase price of $3,464,540.50. |
II-2
Table of Contents
| • | In January 2019, we sold 37,950,000 warrants, each exercisable for one share of common stock, for $11.50, in exchange for warrants of FSAC with equivalent value. |
| • | From August 1, 2018 to December 31, 2020, we granted to our directors, officers, employees, consultants, and other service providers 6,953,528 Non-Qualified Stock Options, Performance Restricted Stock Units and/or Restricted Stock Units pursuant to our 2018 Omnibus Incentive Plan. |
| • | On January 4, 2019, we issued 2,975,618 Non-Qualified Stock Options pursuant to our 2007 Stock Option Plan. |
The offers and sales of the above securities were or will be deemed to be exempt from registration under the Securities Act of 1933 in reliance upon Section 4(a)(2) of the Securities Act of 1933 or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the above securities represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof. Appropriate legends were or will be placed upon any stock certificates issued in these transactions.
Item 16. Exhibits and Financial Statement Schedules.
| (i) | Exhibits |
II-3
Table of Contents
II-4
Table of Contents
| + | Indicates a management contract or compensatory plan or arrangement. |
| * | Previously filed. |
| (ii) | Financial statement schedules |
No financial statement schedules are provided because the information called for is not applicable or is shown in the financial statements or notes.
II-5
Table of Contents
Item 17. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this Registration Statement, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective; and
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at the time shall be deemed to be the initial bona fide offering thereof
II-6
Table of Contents
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Minneapolis, State of Minnesota, on April 21, 2021.
| AGILITI, INC. | ||
| By: |
/s/ THOMAS J. LEONARD | |
| Name: Thomas J. Leonard | ||
| Title: Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Amendment to the Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ THOMAS J. LEONARD Thomas J. Leonard |
Chief Executive Officer and Director (Principal Executive Officer) |
April 21, 2021 | ||
| /s/ JAMES B. PEKAREK James B. Pekarek |
Chief Financial Officer (Principal Financial Officer) |
April 21, 2021 | ||
| /s/ SCOTT A. CHRISTENSEN Scott A. Christensen |
Vice President, Controller and Chief Accounting Officer (Principal Accounting Officer) |
April 21, 2021 | ||
| * Michael A. Bell |
Director | April 21, 2021 | ||
| * Darren Friedman |
Director | April 21, 2021 | ||
| * Dr. Gary L. Gottlieb |
Director | April 21, 2021 | ||
| * Joshua M. Nelson |
Director | April 21, 2021 | ||
| * Megan M. Preiner |
Director | April 21, 2021 | ||
| * Scott M. Sperling |
Director | April 21, 2021 | ||
| * John L. Workman |
Director | April 21, 2021 | ||
| * | The undersigned, by signing her name hereto, signs and executes this Amendment to the Registration Statement pursuant to the Powers of Attorney executed by the above named signatures and previously filed with the Securities and Exchange Commission on March 5, 2021. |
| /s/ Lee M. Neumann |
| Lee M. Neumann |
| Attorney-in-Fact |
II-7
