Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Relay Therapeutics, Inc. | d110953d8k.htm |
| EX-99.1 - EX-99.1 - Relay Therapeutics, Inc. | d110953dex991.htm |
| EX-2.1 - EX-2.1 - Relay Therapeutics, Inc. | d110953dex21.htm |

Conference Call April 2021 Exhibit 99.2

This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include express or implied statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates; the therapeutic potential and clinical benefits of our product candidates; the combination potential of our product candidates, including RLY-1971 in combination with Genentech’s KRAS G12C inhibitor, GDC-6036; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; the market opportunities for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform, including the potential synergies with ZebiAI's platform; the expected strategic benefits of acquiring ZebiAI; the achievement of certain platform and program-related milestones or entry into partnering or collaboration agreements related to ZebiAI’s platform; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our Annual Report on Form 10-K for the year ended December 31, 2020, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Disclaimer
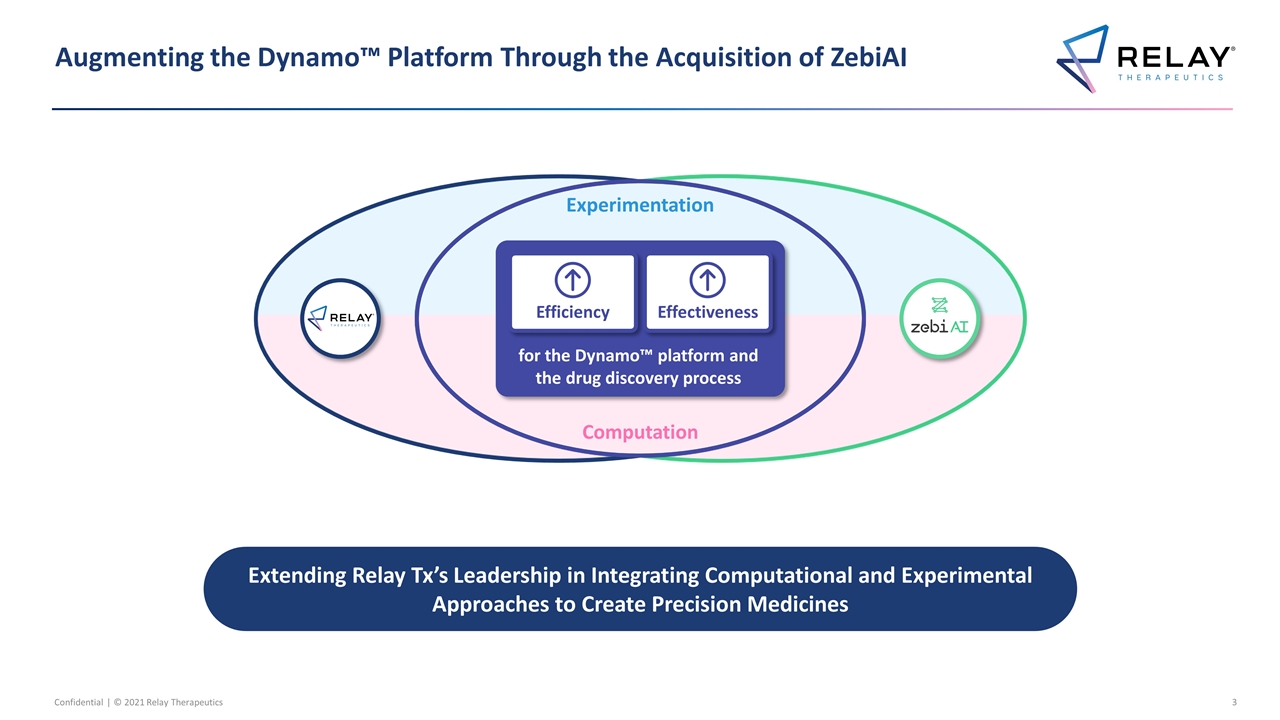
Augmenting the Dynamo™ Platform Through the Acquisition of ZebiAI Extending Relay Tx’s Leadership in Integrating Computational and Experimental Approaches to Create Precision Medicines Experimentation Computation for the Dynamo™ platform and the drug discovery process Efficiency Effectiveness
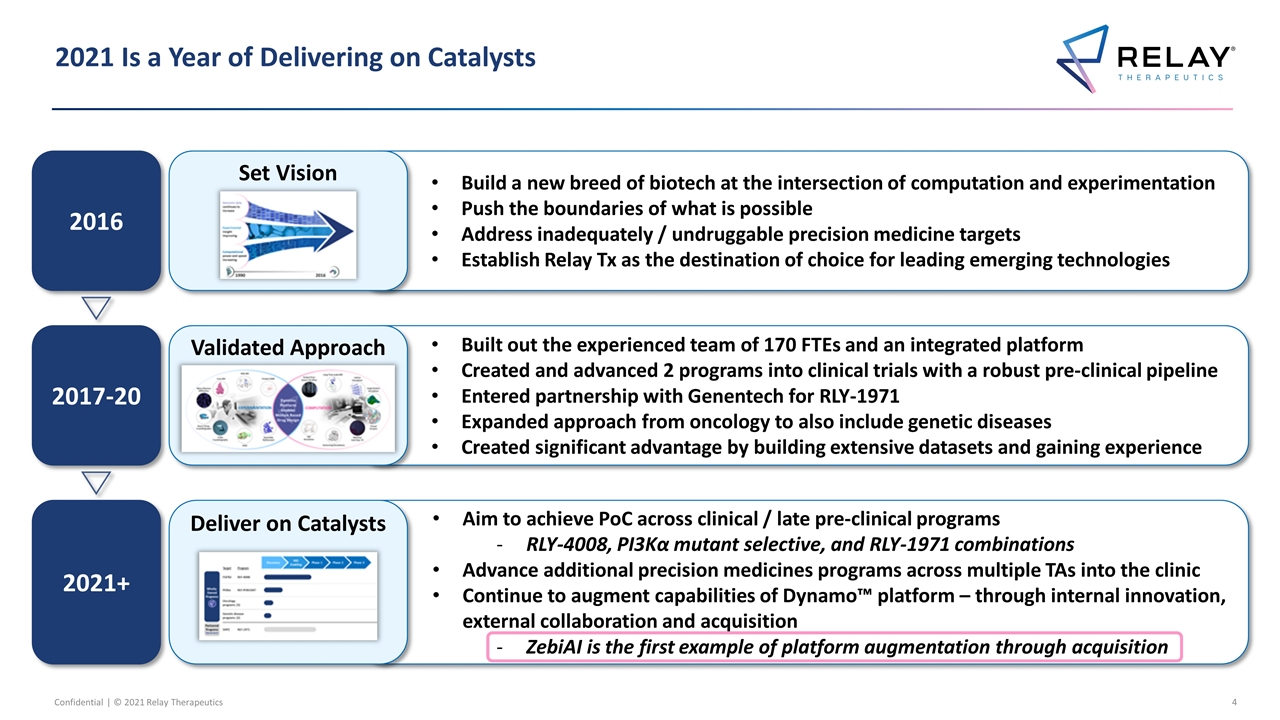
2021 Is a Year of Delivering on Catalysts 2016 2017-20 Built out the experienced team of 170 FTEs and an integrated platform Created and advanced 2 programs into clinical trials with a robust pre-clinical pipeline Entered partnership with Genentech for RLY-1971 Expanded approach from oncology to also include genetic diseases Created significant advantage by building extensive datasets and gaining experience Validated Approach Build a new breed of biotech at the intersection of computation and experimentation Push the boundaries of what is possible Address inadequately / undruggable precision medicine targets Establish Relay Tx as the destination of choice for leading emerging technologies Set Vision 2021+ Aim to achieve PoC across clinical / late pre-clinical programs RLY-4008, PI3Kα mutant selective, and RLY-1971 combinations Advance additional precision medicines programs across multiple TAs into the clinic Continue to augment capabilities of Dynamo™ platform – through internal innovation, external collaboration and acquisition ZebiAI is the first example of platform augmentation through acquisition Deliver on Catalysts
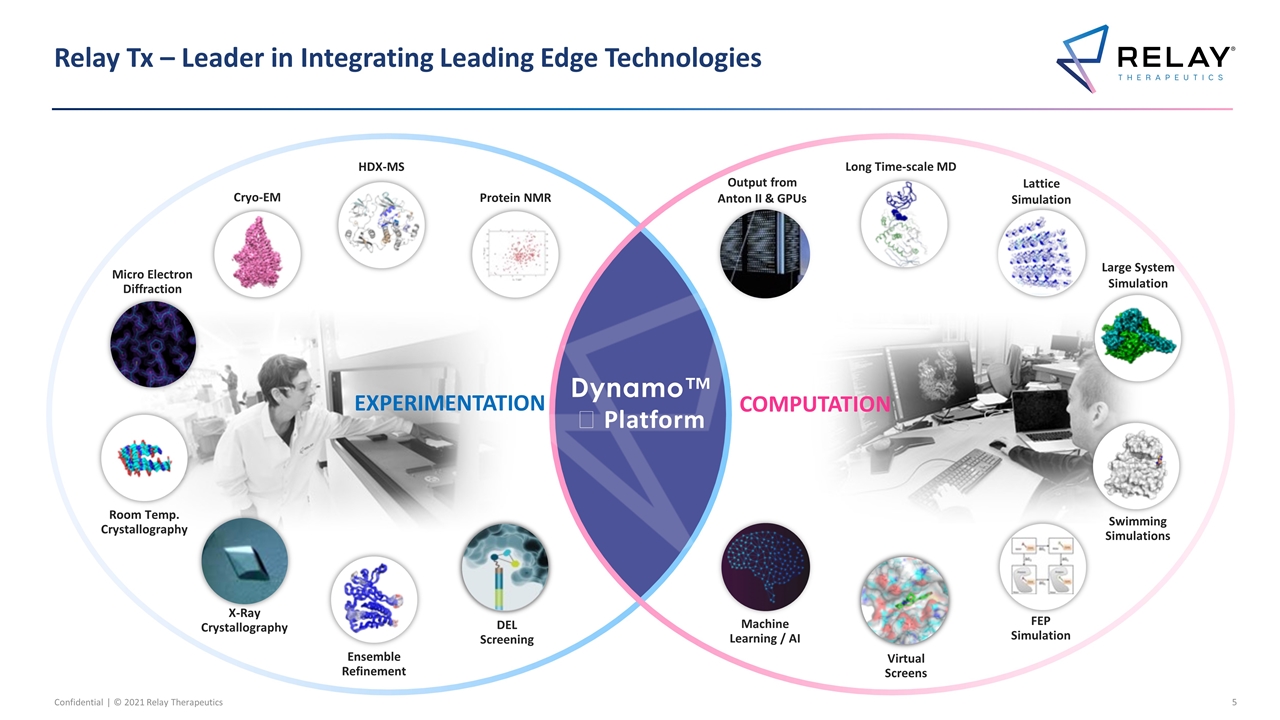
Relay Tx – Leader in Integrating Leading Edge Technologies Dynamo™️ Platform Protein NMR Cryo-EM Micro Electron Diffraction HDX-MS Output from Anton II & GPUs Long Time-scale MD Large System Simulation Lattice Simulation EXPERIMENTATION X-Ray Crystallography Virtual Screens Swimming Simulations FEP Simulation COMPUTATION Machine Learning / AI Room Temp. Crystallography Ensemble Refinement DEL Screening
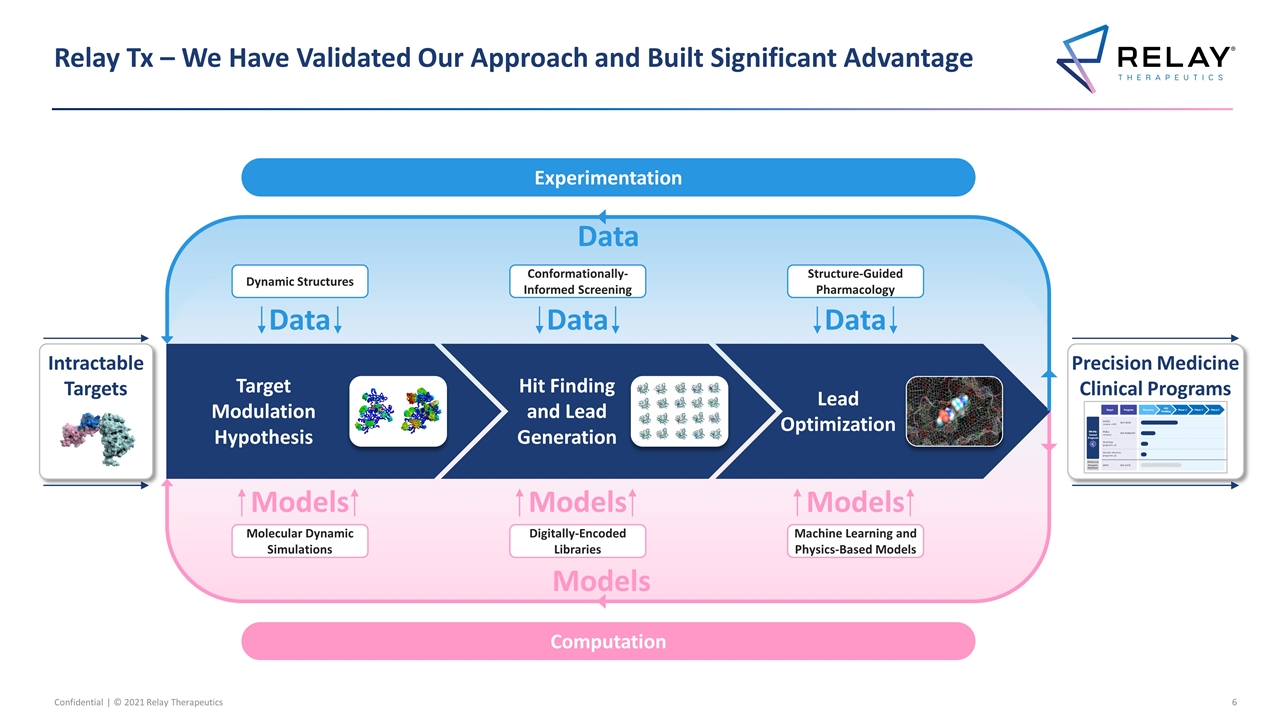
Relay Tx – We Have Validated Our Approach and Built Significant Advantage Precision Medicine Clinical Programs Dynamic Structures Conformationally-Informed Screening Structure-Guided Pharmacology Molecular Dynamic Simulations Digitally-Encoded Libraries Machine Learning and Physics-Based Models Target Modulation Hypothesis Hit Finding and Lead Generation Lead Optimization Data Data Data Models Data Models Experimentation Computation Intractable Targets Models Models
ZebiAI – Novel Platform Based on a Big Data Approach 101001110010010010010010001001010010010010010011010100011100101100010110001000001001001001010 111001001001001001000100101001001001001001101010001110010110001011000100000100100100101010100 100100100100100010010100100100100100110101000111001011000101100010000010010010010101010011100 010010010001001010010010010010011010100011100101100010110001000001001001001010101001110010010 001000100101001001001001001101010001110010110001011000100000100100100101010100111001001001001 010010100100100100100110101000111001011000101100010000010010010010101010011100100100100100100 010010010010010011010100011100101100010110001000001001001001010101001110010010010010010001001 001001001001101010001110010110001011000100000100100100101010100111001001001001001000100101001 100100110101000111001011000101100010000010010010010101010011100100100100100100010010100100100 011010100011100101100010110001000001001001001010101001110010010010010010001001010010010010010 010001110010110001011000100000100100100101010100111001001001001001000100101001001001001001101 111001011000101100010000010010010010101010011100100100100100100010010100100100100100110101000 101100010110001000001001001001010101001110010010010010010001001010010010010010011010100011100 001011000100000100100100101010100111001001001001001000100101001001001001001101010001110010110 100010000010010010010101010011100100100100100100010010100100100100100110101000111001011000101 000001001001001010101001110010010010010010001001010010010010010011010100011100101100010110001 100100100101010100111001001001001001000100101001001001001001101010001110010110001011000100000
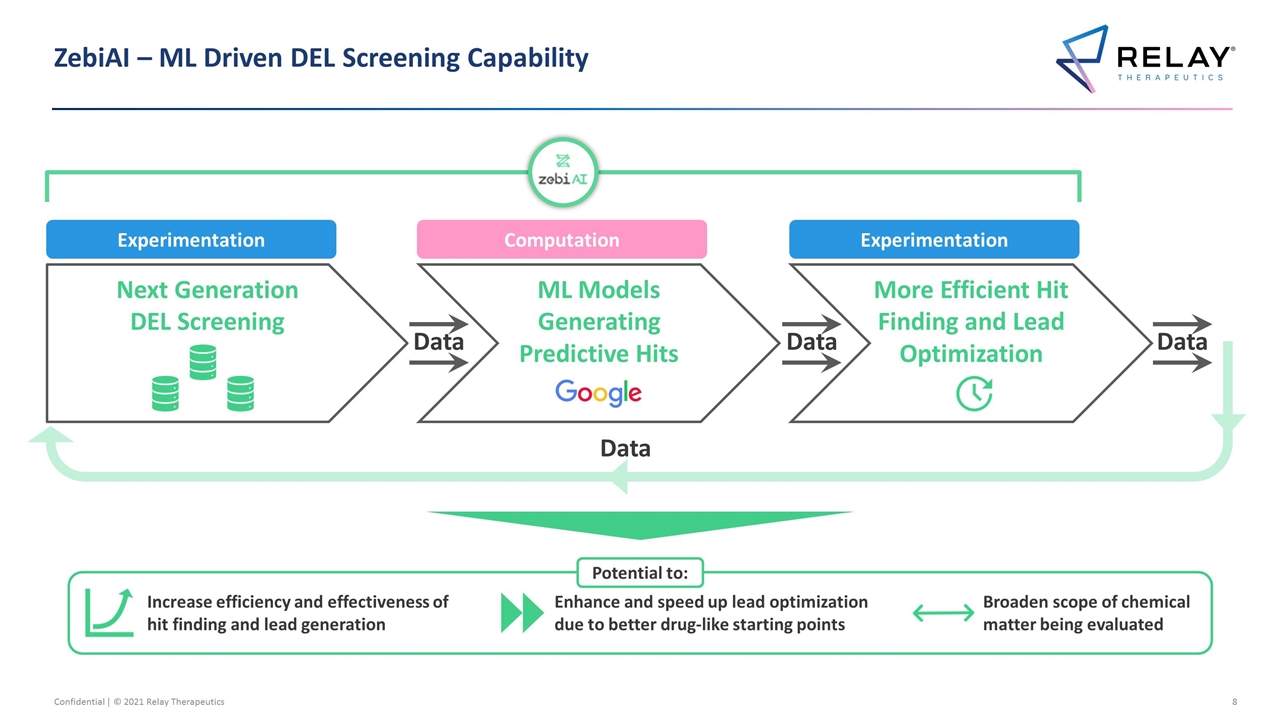
ZebiAI – ML Driven DEL Screening Capability Data Next Generation DEL Screening ML Models Generating Predictive Hits More Efficient Hit Finding and Lead Optimization Experimentation Computation Experimentation Data Data Data Increase efficiency and effectiveness of hit finding and lead generation Enhance and speed up lead optimization due to better drug-like starting points Broaden scope of chemical matter being evaluated Potential to:

ZebiAI – Validated Approach
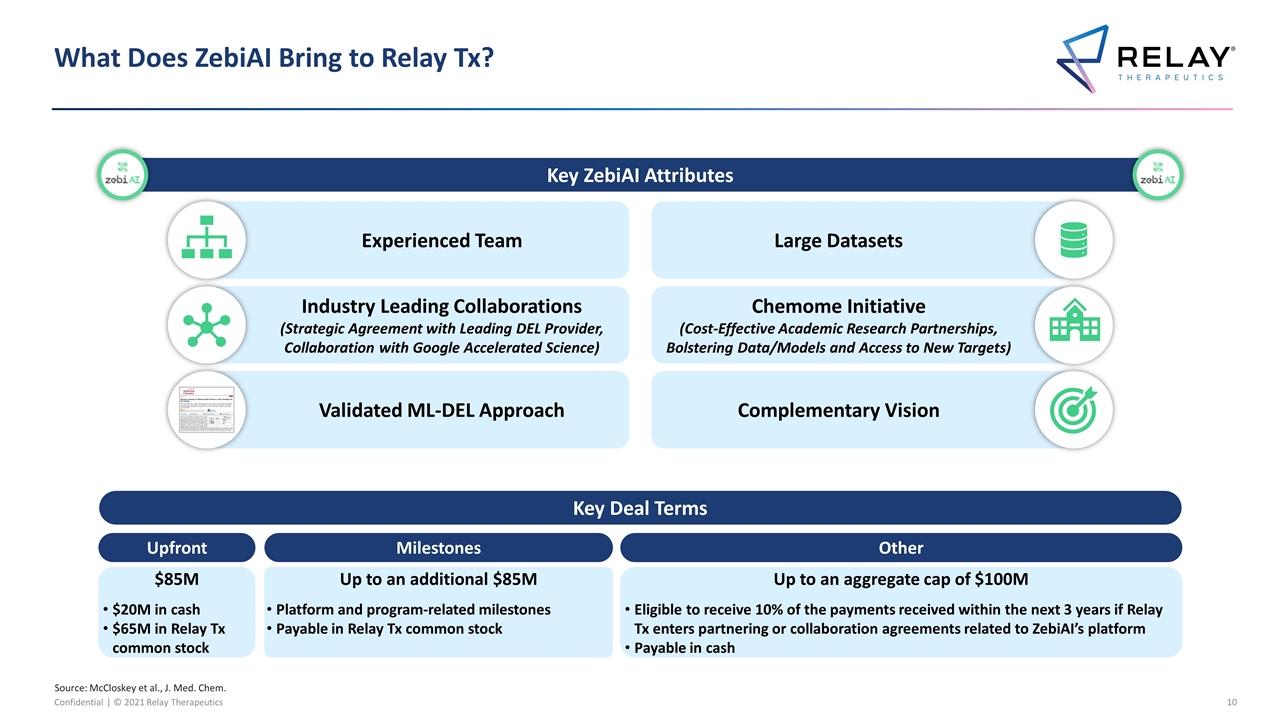
What Does ZebiAI Bring to Relay Tx? Key ZebiAI Attributes Experienced Team Large Datasets Validated ML-DEL Approach Complementary Vision Source: McCloskey et al., J. Med. Chem. Industry Leading Collaborations (Strategic Agreement with Leading DEL Provider, Collaboration with Google Accelerated Science) Chemome Initiative (Cost-Effective Academic Research Partnerships, Bolstering Data/Models and Access to New Targets) $85M $20M in cash $65M in Relay Tx common stock Upfront Up to an additional $85M Platform and program-related milestones Payable in Relay Tx common stock Milestones Up to an aggregate cap of $100M Eligible to receive 10% of the payments received within the next 3 years if Relay Tx enters partnering or collaboration agreements related to ZebiAI’s platform Payable in cash Other Key Deal Terms
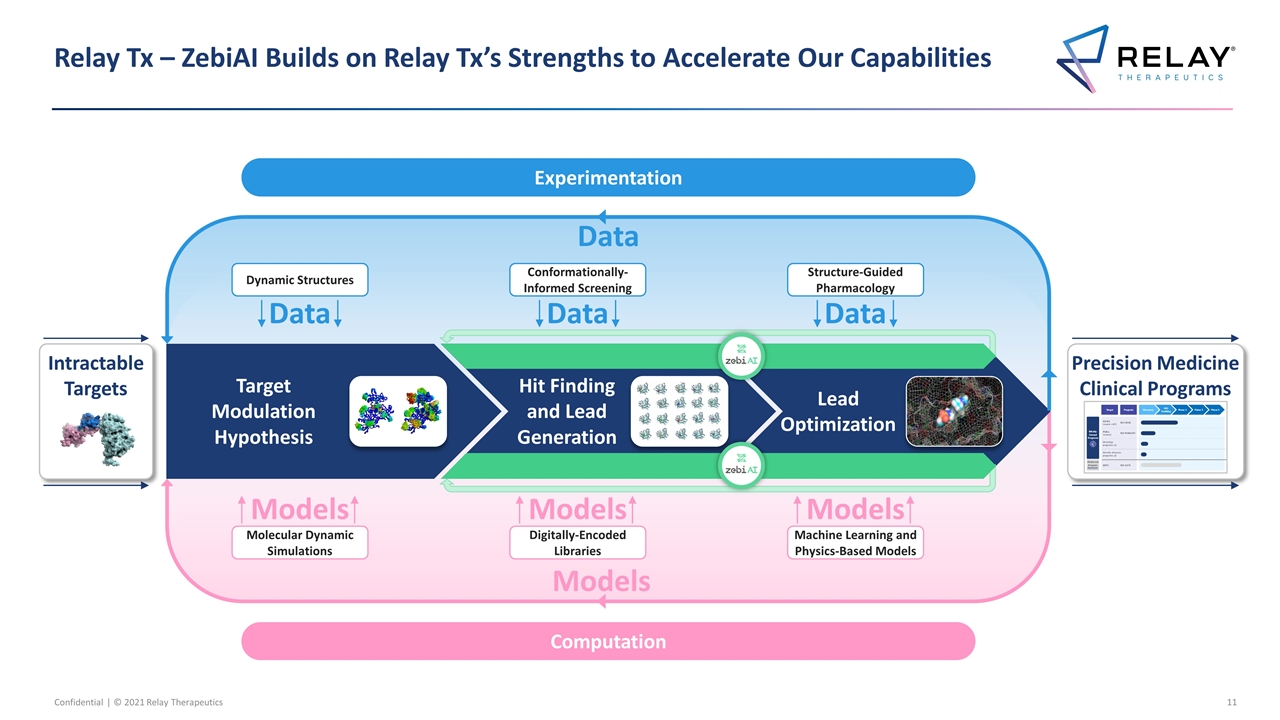
Relay Tx – ZebiAI Builds on Relay Tx’s Strengths to Accelerate Our Capabilities Precision Medicine Clinical Programs Dynamic Structures Conformationally-Informed Screening Structure-Guided Pharmacology Molecular Dynamic Simulations Digitally-Encoded Libraries Machine Learning and Physics-Based Models Target Modulation Hypothesis Hit Finding and Lead Generation Lead Optimization Data Data Data Data Experimentation Computation Intractable Targets Models Models Models Models
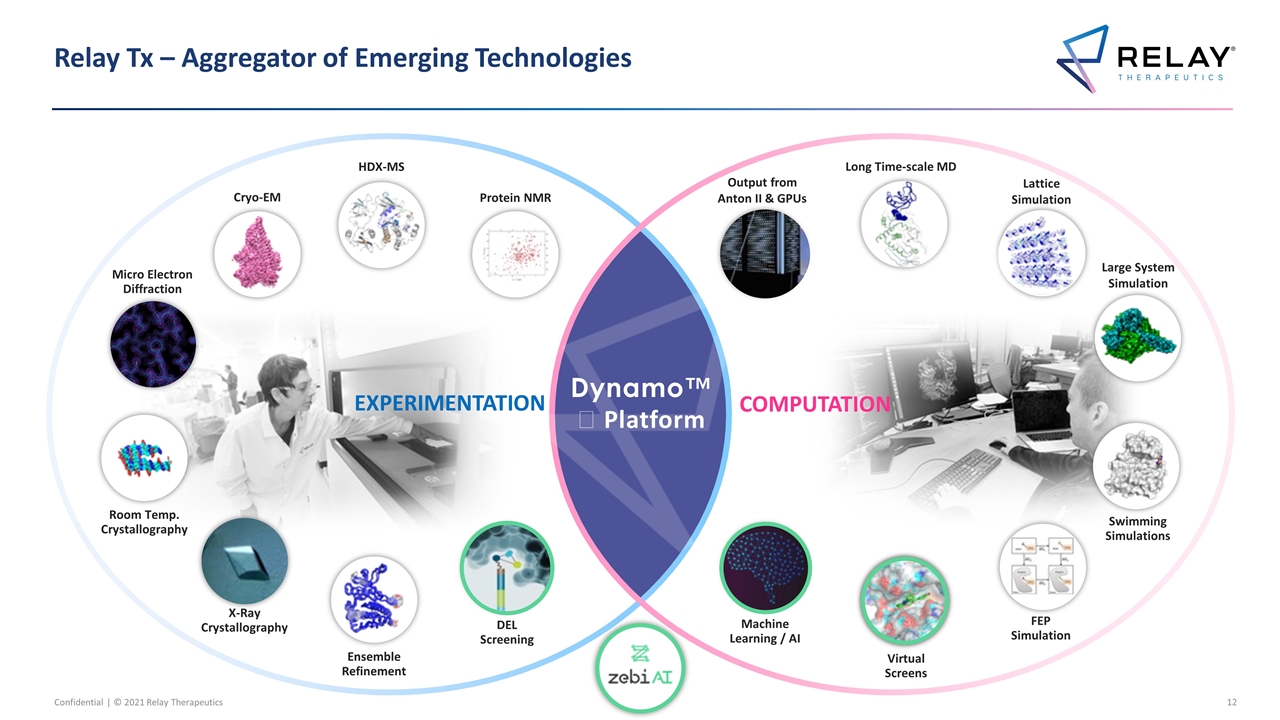
Relay Tx – Aggregator of Emerging Technologies Dynamo™️ Platform Protein NMR Cryo-EM Micro Electron Diffraction HDX-MS Output from Anton II & GPUs Long Time-scale MD Large System Simulation Lattice Simulation EXPERIMENTATION X-Ray Crystallography Virtual Screens Swimming Simulations FEP Simulation COMPUTATION Machine Learning / AI Room Temp. Crystallography Ensemble Refinement DEL Screening
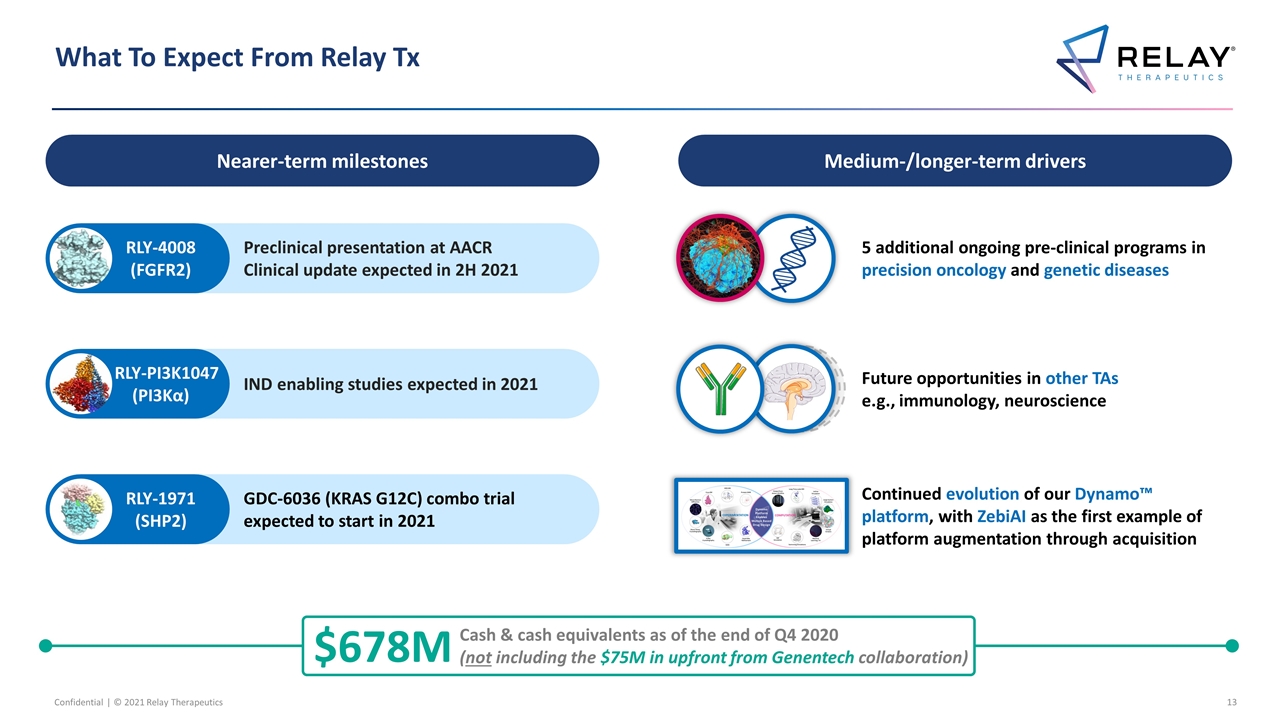
What To Expect From Relay Tx Nearer-term milestones Medium-/longer-term drivers 5 additional ongoing pre-clinical programs in precision oncology and genetic diseases Future opportunities in other TAs e.g., immunology, neuroscience $678M Cash & cash equivalents as of the end of Q4 2020 (not including the $75M in upfront from Genentech collaboration) IND enabling studies expected in 2021 RLY-PI3K1047 (PI3Kα) Preclinical presentation at AACR Clinical update expected in 2H 2021 RLY-4008 (FGFR2) GDC-6036 (KRAS G12C) combo trial expected to start in 2021 RLY-1971 (SHP2) Continued evolution of our Dynamo™ platform, with ZebiAI as the first example of platform augmentation through acquisition

