Attached files
| file | filename |
|---|---|
| 8-K - 8-K - F-star Therapeutics, Inc. | d148084d8k.htm |
| EX-99.1 - EX-99.1 - F-star Therapeutics, Inc. | d148084dex991.htm |
Exhibit 99.2
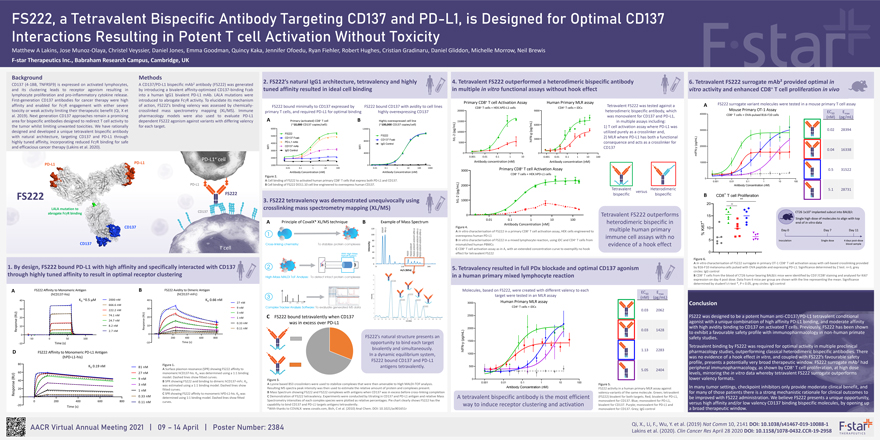
FS222, a Tetravalent Bispecific Antibody Targeting CD137 and PD-L1, is Designed for Optimal CD137 Interactions Resulting in Potent T cell Activation Without Toxicity Matthew A Lakins, Jose Munoz-Olaya, Christel Veyssier, Daniel Jones, Emma Goodman, Quincy Kaka, Jennifer Ofoedu, Ryan Fiehler, Robert Hughes, Cristian Gradinaru, Daniel Gliddon, Michelle Morrow, Neil Brewis F-star Therapeutics Inc., Babraham Research Campus, Cambridge, UK Background Methods 2 2. FS222’s natural IgG1 architecture, tetravalency and highly 4. Tetravalent FS222 outperformed a heterodimeric bispecific antibody 6. Tetravalent FS222 surrogate mAb² provided optimal in CD137 (4-1BB, TNFRSF9) is expressed on activated lymphocytes, A CD137/PD-L1 bispecific mAb antibody (FS222) was generated tuned affinity resulted in ideal cell binding in multiple in vitro functional assays without hook effect + and its clustering leads to receptor agonism resulting in by introducing a bivalent affinity-optimised CD137-binding Fcab vitro activity and enhanced CD8 T cell proliferation in vivo lymphocyte proliferation and pro-inflammatory cytokine release. into a human IgG1 bivalent PD-L1 mAb. LALA mutations were First-generation CD137 antibodies for cancer therapy were high introduced to abrogate FcgR activity. To elucidate its mechanism + Primary CD8 T cell Activation Assay Human Primary MLR assay FS222 surrogate variant molecules were tested in a mouse primary T cell assay affinity and enabled for FcgR engagement with either severe of action, FS222’s binding valency was assessed by chemically- Tetravalent FS222 was tested against a A FS222 bound minimally to CD137 expressed by FS222 bound CD137 with avidity to cell lines CD8+ T cells + HEK.hPD-L1 cells CD4+ T cells + iDCs Mouse Primary OT-1 Assay toxicity or weak activity limiting their therapeutic benefit (Qi, X et crosslinked mass spectrometry mapping (XL/MS). Immune primary T cells, and required PD-L1 for optimal binding highly overexpressing CD137 20000 9000 heterodimeric bispecific antibody, which EC E 40000 + 50 max al. 2019). Next generation CD137 approaches remain a promising pharmacology models were also used to evaluate PD-L1 was monovalent for CD137 and PD-L1, CD8 T cells + OVA-pulsed B16-F10 cells (nM) (pg/mL) A Primary (activated) CD8+ T cell B Highly overexpressed cell line area for bispecific antibodies designed to redirect T cell activity to dependent FS222 agonism against variants with differing valency L) 15000 in multiple assays including: (~10,000 CD137 copies/cell): (~100,000 CD137 copies/cell) 6000 the tumor whilst limiting unwanted toxicities. We have rationally for each target. 6000 m 1) T cell activation assay where PD-L1 was 12000 g/ (pg/mL) 30000 0.02 28394 designed and developed a unique tetravalent bispecific antibody p ( 10000 utilized purely as a crosslinker and, FS222 g with natural architecture, targeting CD137 and PD-L1 through 5000 FS222 2 — 2) MLR where PD-L1 has both a functional CD137 Fcab I L L) CD137 Fcab 3000 8000 h hIFN highly tuned affinity, incorporating reduced FcgR binding for safe PD-L1 mAb 5000 consequence and acts as a crosslinker for g/m 4000 IgG Control ( p FI CD137 mAb 20000 and efficacious cancer therapy (Lakins et al. 2020). M MFI CD137 y - 3000 IgG Control N 0.04 16338 4000 0 0 m IF 0.001 0.01 0.1 1 10 0.001 0.01 0.1 1 10 100 PD-L1+ cell 2000 PD-L1 Antibody concentration (nM) Antibody concentration (nM) 10000 PD 1000 0 0.01 0.1 1 10 100 0.01 0.1 1 10 100 1000 Primary CD8+ T cell Activation Assay 0.5 31522 3000 Antibody Concentration (nM) Antibody Concentration (nM) CD8+ T cells + HEK.hPD-L1 cells Figure 2. 0 A Cell binding of FS222 to activated human primary CD8+ T cells that express both PD-L1 and CD137. L) 0.001 0.01 0.1 1 10 100 PD-L1 B Cell binding of FS222 DO11.10 cell line engineered to overexpress human CD137. 2000 Antibody Concentration (nM) Tetravalent Heterodimeric 5.1 28731 (pg/m versus FS222 bispecific bispecific B CD8+ T cell Proliferation - 2 FS222 3. FS222 tetravalency was demonstrated unequivocally using IL h 1000 20 ✱✱ crosslinking mass spectrometry mapping (XL/MS) CD137 CT26 1x105 implanted subcut into BALB/c a 0 Tetravalent FS222 outperforms 15 0.01 0.1 1 10 100 Single high dose of molecules to align with top A Principle of CovalX* XL/MS technique B Example of Mass Spectrum Antibody Concentration [nM] heterodimeric bispecific in + end of in vitro data L1 Figure 4. 10 2—L1 L1 Ki67 - L1 CD137 PD — A In vitro characterisation of FS222 in a primary CD8+ T cell activation assay, HEK cells engineered to multiple human primary Day 0 Day 7 Day 11 overexpress human PD-L1 immune cell assays with no % + B In vitro characterisation of FS222 in a mixed lymphocyte reaction, using iDC and CD4 T cells from 5 Inoculation Single dose 4 days post-dose ity mismatched human PBMCs evidence of a hook effect blood sample T cell Intens C CD8+ T cell activation assay as in A, with an extended concentration curve to exemplify no hook FS222 FS222+1CD137 FS222+1CD137+1CD137+1PD FS222+1CD137+2CD137+1PD FS222+1CD137+2CD137+2PD effect for tetravalent FS222 0 2 Figure 6. A In vitro characterisation of FS222 surrogate in primary OT-1 CD8+ T cell activation assay with cell-based crosslinking provided 1. By design, FS222 bound PD-L1 with high affinity and specifically interacted with CD13 5. Tetravalency resulted in full PDx blockade and optimal CD137 agonism by B16-F10 melanoma cells pulsed with OVA peptide and expressing PD-L1. Significance determined by Z test. n=3, grey circles: IgG control through highly tuned affinity to result in optimal receptor clustering in a human primary mixed lymphocyte reaction B CD8+ T cells from the blood of CT26 tumor bearing BALB/c mice were identified by CD3+/CD8+ staining and analysed for Ki67 expression on day 4 post dose. Data from 6 mice per group are shown with the line representing the mean. Significance determined by student’s t-test *, P < 0.05, grey circles: IgG control A FS222 Affinity to Monomeric Antigen B FS222 Avidity to Dimeric Antigen Molecules, based on FS222, were created with different valency to each EC E (hCD137-his) (hCD137-mFc) target were tested in an MLR assay 50 max (nM) (pg/mL) 40 K ~0.5 µM 2000 nM 40 K 0.66 nM D D Human Primary MLR assay 666.6 nM 27 nM 3000 Conclusion CD4+ T cells + iDCs 30 9 nM 30 222.2 nM 0.03 2062 U) 3 nM (RU) 74.1 nM (R 2500 FS222 was designed to be a potent human anti-CD137/PD-L1 tetravalent conditional 20 20 C FS222 bound tetravalently when CD137 1 nM e nse 24.7 nM agonist with a unique combination of high affinity PD-L1 binding, and moderate affinity pons 0.33 nM was in excess over PD-L1 10 8.2 nM s 10 with high avidity binding to CD137 on activated T cells. Previously, FS222 has been shown espo Re 0.11 nM 2000 R 2.7 nM 0.03 1428 0 L) m to exhibit a favourable safety profile with immunopharmacology in non-human primate 0 0 200 400 600 800 FS222’s natural structure presents an / g safety studies. -50 0 50 100 p ( 1500 Time (s) -10 Time (s) opportunity to bind each target y -10 N—Tetravalent binding by FS222 was required for optimal activity in multiple preclinical bivalently and simultaneously. F I 1.13 2283 D FS222 Affinity to Monomeric PD-L1 Antigen In a dynamic equilibrium system, h 1000 pharmacology studies, outperforming classical heterodimeric bispecific antibodies. There (hPD-L1-his) FS222 bound CD137 and PD-L1 was no evidence of a hook effect in vitro, and coupled with FS222’s favourable safety profile, presents a potentially very broad therapeutic window. FS222 surrogate mAb2 had 80 Figure 1. K 0.19 nM 81 nM antigens tetravalently. 500 + D peripheral immunopharmacology, as shown by CD8 T cell proliferation, at high dose A Surface plasmon resonance (SPR) showing FS222 affinity to 5.05 2404 60 27 nM monomeric hCD137-his. KD was determined using a 1:1 binding levels, mirroring the in vitro data whereby tetravalent FS222 surrogate outperforms model. Dashed lines show fitted curves. (RU) 9 nM Figure 3. 0 lower valency formats. 40 B SPR showing FS222 avid binding to dimeric hCD137-mFc. K e s D 0.001 0.01 0.1 1 10 100 3 nM A Lysine based BS3 crosslinkers were used to stabilize complexes that were then amenable to High MALDI-TOF analysis. Figure 5. n was estimated using a 1:1 binding model. Dashed lines show Antibody Concentration (nM) In many tumor settings, checkpoint inhibitors only provide moderate clinical benefit, and po 20 Resulting MS spectra peak intensity was then used to estimate the relative amount of protein and complexes present. FS222 activity in a human primary MLR assay against 1 nM fitted curves. B Mass Spectrum showing FS222 and FS222 complexes with antigens when CD137 was in excess before cross-linking completion C SPR showing FS222 affinity to monomeric hPD-L1-his. K was valency-variants of the same molecule. Green; tetravalent for many of those patients there is a strong mechanistic rationale for clinical outcomes to Res D 0 0.33 nM C Demonstration of FS222 tetravalency. Experiments were conducted by titrating in CD137 and PD-L1 antigen and relative Mass (FS222) bivalent for both targets. Red; bivalent for PD-L1, determined using 1:1 binding model. Dashed lines show fitted Spectrometry intensities of each complex species were plotted as relative percentages. Pie chart clearly shows FS222 has the A tetravalent bispecific antibody is the most efficient be improved with FS222 administration. We believe FS222 presents a unique opportunity, 0 200 400 600 800 curves. monovalent for CD137. Blue; monovalent for PD-L1, 0.11 nM versus high affinity and/or low valency CD137 binding bispecific molecules, by opening up -20 capability to bind CD137 and PD-L1 targets antigens tetravalently. way to induce receptor clustering and activation bivalent for CD137. Purple; monovalent for PD-L1 and Time (s) *With thanks to COVALX: www.covalx.com, Bich, C et al. (2010) Anal Chem. DOI: 10.1021/ac901651r monovalent for CD137. Grey; IgG control a broad therapeutic window. AACR Virtual Annual Meeting 2021 | 09 – 14 April | Poster Number: 2384 Qi, X., Li, F., Wu, Y. et al. (2019) Nat Comm 10, 2141 DOI: 10.1038/s41467-019-10088-1 Lakins et al. (2020). Clin Cancer Res April 28 2020 DOI: 10.1158/1078-0432.CCR-19-2958
