Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Scholar Rock Holding Corp | a52406941ex99_1.htm |
| 8-K - SCHOLAR ROCK HOLDING CORPORATION 8-K - Scholar Rock Holding Corp | a52406941.htm |
Exhibit 99.2

TOPAZ Phase 2 Trial Top-Line Results April 6, 2021 Improvements in Motor Function with Apitegromab for
Patients with Spinal Muscular Atrophy (SMA)

Disclaimers Various statements in this presentation concerning the future expectations, plans and
prospects of Scholar Rock, Inc. (“Scholar Rock”), including without limitation, Scholar Rock’s expectations regarding its strategy, its product candidate selection and development timing, including timing for the initiation of and reporting
results from its clinical trials for its product candidates, its disease indication selection and timing for such selection, the ability of apitegromab to affect the treatment of patients suffering from Spinal Muscular Atrophy (SMA) either as a
monotherapy or in conjunction with the current standard of care, and the ability of SRK-181 to affect the treatment of cancer patients constitute forward-looking statements for the purposes of the safe harbor provisions under The Private
Securities Litigation Reform Act of 1995. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “target,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar
expressions are intended to identify such forward-looking statements. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, Scholar
Rock’s ability to provide the financial support and resources necessary to identify and develop multiple product candidates on the expected timelines, competition from others developing products for similar uses, the preliminary nature of
interim clinical data, the possibility that preclinical or clinical data is inconsistent with subsequent data, Scholar Rock’s ability to obtain, maintain and protect its intellectual property, Scholar Rock’s dependence on third parties for
development and manufacture of product candidates including to supply any clinical trials, and Scholar Rock’s ability to manage expenses and to obtain additional funding when needed to support its business activities and establish and maintain
strategic business alliances and new business initiatives as well as those risks more fully discussed in the section entitled "Risk Factors" in the Annual Report on Form 10-K for the year ended December 31, 2020, which is on file with
the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements
represent Scholar Rock’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Scholar Rock explicitly disclaims any obligation to update any forward-looking statements unless required by law.
© Scholar Rock, Inc. All rights reserved. April 2021.

Agenda 3 Opening Remarks Next Steps for Apitegromab Program Tony Kingsley, President & CEO Tony
Kingsley, President & CEOTed Myles, Chief Financial Officer Questions and Answers Trial Design and Baseline Characteristics Top-line Safety and Efficacy Results Yung Chyung M.D., Chief Medical Officer

4 Bringing a Revolutionary Approach to Highly Sought-After Growth Factors Implicated in Devastating
Diseases Scholar Rock’s Target Growth Factor Precursor (Latent Form) Scholar Rock’s R&D PlatformTransform Medical Practice Pursue important targets with well-validated biology but are difficult to drugApply revolutionary approach to
tough targetsLeverage deep insights into structure and function Engineer antibodies to deliver differentiated therapeutic profiles(i.e. exquisite selectivity) TOPAZ demonstrates the therapeutic potential of inhibiting the latent forms of
growth factors

Apitegromab Offers Potential to Pioneer a New Treatment Era to Improve Motor Function in Patients with
SMA 5 SMN = survival motor neuron. *Also referred to as SMN correctors. **Apitegromab (SRK-015) is an investigational product candidate under development. SMN Upregulator Therapies* + Muscle-Directed Therapy (apitegromab) Potential for
Enhanced Outcomes for Patients Apitegromab (SRK-015)** SMN Upregulator TherapiesAddress SMN deficiency to prevent further motor neuron deterioration Muscle-Directed TherapiesAct directly on muscle with aim to improve motor function

6 TOPAZ 12-Month Data Further Support the Potential of Apitegromab in Patients with Type 2 and Type 3
SMA •Cohort 2: Mean change in HFMSE score from baseline was updated to +1.1-points (from +1.4-points). Proportion of patients with ≥3-point increases was updated to 2/14 (from 3/14) and updated to 1/14 (from 2/14) for patients with ≥5-point
increases.•Cohort 3 high dose arm (20 mg/kg): Mean change in HFMSE score from baseline was revised to +5.3-points (from +5.6-points). Mean RHS decline from baselineMajority of patients maintained or improved (≥0-pt change from baseline) TOPAZ
6-month interim results* TOPAZ 12-month top-line results Mean RHS increase from baselineMajority of patients maintained or improved (≥0-pt change from baseline) Mean HFMSE increase from baseline Majority of patients improved (≥1-pt
increase from baseline)Sizeable % of patients achieved ≥3-pt increase (29%) Mean HFMSE increase from baselineMajority of patients improved (≥1-pt increase from baseline) Further HFMSE increases observed vs. 6-month interim analysisMajority of
patients achieved ≥5-pt increaseDose response continues to be observed Mean HFMSE increases from baselineMajority of patients achieved ≥3-pt increaseDose response observed Adverse events were consistent with the underlying patient population
and background therapy Cohort 1Ambulatory Type 3 Cohort 2Type 2 & non-ambulatory Type 3 (initiated nusinersen ≥5 yrs) Cohort 3Type 2(initiated nusinersen <5 yrs) *Database for HFMSE and RHS scores for the 12-month topline analysis
are locked. The 6-month interim analysis was a snapshot and subsequent adjustments by sites investigators resulted in the following changes to the 6-month interim results:

Phase 2 Trial Design and 12-Month Top-Line Results Yung Chyung, M.D. Chief Medical Officer

Apitegromab Phase 2 Trial Design Design Patients Primary Objectives HFMSE=Hammersmith Functional
Motor Scale Expanded; RHS=Revised Hammersmith ScaleData on file. Scholar Rock, Inc. Cambridge, MA Evaluate potential of apitegromab in improving motor function in patients with Type 2 and Type 3 SMA 8 Ambulatory Patients(Revised
Hammersmith Scale) Non-Ambulatory Patients(Hammersmith Functional Motor Scale Expanded) Cohort 1 Cohort 2 Cohort 3 N= 23; ages 5-21Open-label, single-arm20 mg/kg apitegromab IV Q4W12-month treatment period N= 15; ages 5-21 Open-label,
single-arm20 mg/kg apitegromab IV Q4W12-month treatment period N= 20; ages ≥2Double-blind, randomized (1:1) to 2 mg/kg or 20 mg/kg apitegromab IV Q4W12-month treatment period Ambulatory Type 3 SMATwo subgroups: Receiving background nusinersen
Apitegromab monotherapy Type 2 or non-ambulatory Type 3 SMAReceiving background nusinersen (initiated ≥5 years of age) Type 2 SMA Receiving background nusinersen (initiated before 5 years of age) SafetyMean change from baseline in
RHS SafetyMean change from baseline in HFMSE SafetyMean change from baseline in HFMSE

Considerations in the Conduct and Design of TOPAZ Proof-of-Concept Study 9 Main focus of TOPAZ was to
assess the potential additive therapeutic benefit of apitegromab on top of background SMN upregulator therapy.*While the protocol allowed the use of any approved SMN upregulator as background therapy, only nusinersen had widespread use during
TOPAZ trial enrollment. Specifically designed with 3 distinct cohorts to assess apitegromab’s potential across patient populations with varying disease severity and different background expectations for disease course.Clinical data for
nusinersen and natural history data help inform our background expectations for disease course for the different populations evaluated in TOPAZ.These insights further our understanding as we continue to investigate apitegromab in SMA.Cohort 3
evaluated two dose arms as we recognized that complete target saturation may not be necessary to achieve therapeutic effect.Low dose of 2 mg/kg was selected to explore this question by aiming for a high level of target engagement but lower than
that of the 20 mg/kg dose. *An apitegromab monotherapy subgroup was included in Cohort 1.

Baseline Characteristics 10 Ambulatory Patients Non-Ambulatory Patients Cohort
1 Cohort 2 Cohort 3 20 mg/kgmonotherapy 20 mg/kg +nusinersen Pooled 20 mg/kg +nusinersen 20 mg/kg +nusinersen 2 mg/kg +nusinersen Pooled N 11 12 23 15 10 10 20 Mean age (min, max) 12.1 (7, 19) 13.1 (7, 21) 12.6
(7, 21) 11.7 (8, 19) 3.8 (2, 6) 4.1 (2, 6) 4.0 (2, 6) Female (%) 73% 58% 65% 53% 50% 30% 40% SMN2 Gene Copy* (#, %) 2 1 (9%) 0 (0%) 1 (4%) 1 (10%) 1 (10%) 2 (10%) 3 4 (36%) 9 (75%) 13 (57%) 11 (73%) 8
(80%) 8 (80%) 16 (80%) 4 4 (36%) 1 (8%) 5 (22%) 2 (13%) 0 (0%) 1 (10%) 1 (5%) Mean # of nusinersen maintenance doses (min, max) N/A 5.6 (2, 8) N/A 5.1 (2, 9) 5.4 (3, 8) 5.5 (2, 9) 5.5 (2,
9) Discontinuation(s) 0 1** 1** 0 0 0 0 Mean RHS score (min, max) 47.6 (26, 63) 51.3 (43, 62) 49.6 (26, 63) Mean HFMSE score (min, max) 22.7 (13, 39) 23.5 (14, 42) 26.1 (12, 44) 24.8 (12, 44) *Data not available
for all patients**Patient who discontinued study for reasons unrelated to study drugHFMSE=Hammersmith Functional Motor Scale Expanded; RHS=Revised Hammersmith Scale Data on file. Scholar Rock, Inc. Cambridge, MA

Safety Results from TOPAZ 12-Month Top-Line Analysis Support Evaluation of Apitegromab in Phase 3
Trial 11 Treatment-emergent adverse events (TEAEs) Apitegromab 2 mg/kg (n=10) Apitegromab 20 mg/kg (n=48) Total(n=58) Any TEAE 9 (90.0%) 44 (91.7%) 53 (91.4%) Any Serious TEAE 1 (10.0%) 4 (8.3%) 5 (8.6%) Any TEAE leading to study
drug discontinuation 0 (0.0%) 1 (2.1%) 1 (1.7%) Any Grade 3 (severe) or higher TEAE 0 (0.0%) 3 (6.2%) 3 (5.2%) Treatment-emergent adverse events (TEAEs) are defined as AEs that start after the first dose of study drug or start prior to
the administration of study drug and worsen in severity/grade or relationship to investigational medication after the administration of study drug.*TEAE rates are across all patients in TOPAZ trialData on file. Scholar Rock, Inc. Cambridge,
MA Five most frequently reported TEAEs*: Headache (24%), pyrexia (22%), upper respiratory tract infection (22%), cough (22%), and nasopharyngitis (21%).Anti-drug antibodies (ADA) were present at low titers following apitegromab treatment in 3
out of 58 enrolled patients. No apparent impact on drug exposure was observed and was not associated with any hypersensitivity reactions. No safety signals identified as of the TOPAZ 12-month top-line analysis Incidence and severity of AEs
were consistent with the underlying patient population and background therapy

Serious and Severe Treatment-Emergent Adverse Events (TEAEs) 12 Treatment-emergent adverse events
(TEAEs) are defined as AEs that start after the first dose of study drug or start prior to the administration of study drug and worsen in severity/grade or relationship to investigational medication after the administration of study drug.Data
on file. Scholar Rock, Inc. Cambridge, MA 2 mg/kg: Cohort 3: 1 patient hospitalized due to adenoidal and tonsillar hypertrophy and scheduled adenotonsillectomy (Grade 2). Resolved without sequelae.20 mg/kg:Cohort 1: 2 patients with gait
inability considered a significant disability (both Grade 3). Events remain ongoing. Cohort 1: 1 patient hospitalized with post lumbar puncture syndrome (Grade 2). Resolved without sequelae.Cohort 1: 1 patient hospitalized due to viral upper
respiratory infection (Grade 2/prior history). Resolved without sequelae. Serious TEAEs; All Assessed by Trial Investigators as Unrelated to Apitegromab Other Severe TEAE; Assessed by Trial Investigator as Unrelated to Apitegromab Study
Discontinuation; Assessed by Trial Investigator as Unrelated to Apitegromab Cohort 1: 1 patient presented with post lumbar puncture syndrome (non-serious Grade 3). Resolved without sequelae. Cohort 1: 1 patient withdrew consent after ~2
months in the trial. Grade 2 leg muscle fatigue (developed prior to enrollment).

13 Cohort 1 data suggest potential clinical effect in certain patients in this patient populationMean
decline in RHS from baselineMajority (57%) maintained or improved in RHS (≥0-point change from baseline) and 22% achieved ≥3-point increaseCohort 2 observed improvement of motor function from baselineMean improvement in HFMSE from baseline;
potential durability of improvement apparent up to 12-monthsMajority (64%) achieved ≥1-point increase in HFMSE and sizeable subset (29%) achieved ≥3-point increaseCohort 3 observed further improvements in motor function and continued dose
response vs. 6-month interim analysisLarge mean improvement in HFMSE from baseline in both dose arms; high dose numerically outperformed low doseMajority (59%) achieved ≥5-point increase and sizeable subset (35%) achieved >10-point increase
in HFMSE TOPAZ 12-Month Top-line Results Demonstrate Potential of Apitegromab for Patients with Type 2 and Type 3 SMA Ambulatory Patients(Revised Hammersmith Scale) Non-Ambulatory Patients(Hammersmith Functional Motor Scale
Expanded) (Intent-to-Treat Population) Cohort 1 Cohort 2* Cohort 3* 20 mg/kgmonotherapy (n=11) 20 mg/kg +nusinersen (n=12) Pooled(n=23) 20 mg/kg +nusinersen (n=14) 20 mg/kg +nusinersen(n=8) 2 mg/kg
+nusinersen(n=9) Pooled (n=17) Mean change from baseline (95% CI) -0.4 (-3.9, 3.1) -0.3 (-2.0, 1.4) -0.3 (-2.1, 1.4) +0.6 (-1.4, 2.7) +7.1 (1.8, 12.5) +5.3 (-1.5, 12.2) +6.2 (2.2, 10.1) # (%) pts achieving ≥1-pt increase 4/11
(36%) 5/12 (42%) 9/23 (39%) 9/14 (64%) 7/8 (88%) 7/9 (78%) 14/17 (82%) # (%) pts achieving ≥3-pt increase 3/11 (27%) 2/12 (17%) 5/23 (22%) 4/14 (29%) 5/8 (63%) 5/9 (56%) 10/17 (59%) *4 patients (1 in Cohort 2 and 3 in Cohort 3)
each missed 3 doses of apitegromab due to COVID-19-related site access restrictions and were not included in the primary (intent-to-treat) analysis. Data on file. Scholar Rock, Inc. Cambridge, MA

Cohort 1
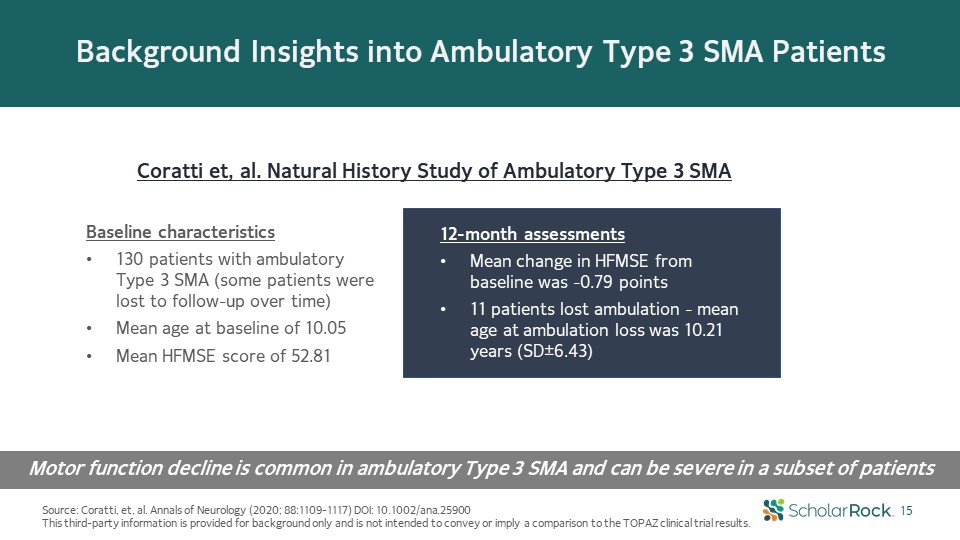
Background Insights into Ambulatory Type 3 SMA Patients Source: Coratti, et, al. Annals of Neurology
(2020; 88:1109-1117) DOI: 10.1002/ana.25900This third-party information is provided for background only and is not intended to convey or imply a comparison to the TOPAZ clinical trial results. 15 Coratti et, al. Natural History Study of
Ambulatory Type 3 SMA Motor function decline is common in ambulatory Type 3 SMA and can be severe in a subset of patients 12-month assessmentsMean change in HFMSE from baseline was -0.79 points11 patients lost ambulation - mean age at
ambulation loss was 10.21 years (SD±6.43) Baseline characteristics130 patients with ambulatory Type 3 SMA (some patients were lost to follow-up over time)Mean age at baseline of 10.05 Mean HFMSE score of 52.81

Cohort 1: Mean Decline in RHS at 12-Months but Majority of Patients Maintained or Improved in RHS
Score from Baseline 16 Individual RHS responses Ambulatory Type 3 SMA (Intent-to-Treat Population) Apitegromab (20 mg/kg)monotherapy (n=11) Apitegromab (20 mg/kg) + nusinersen (n=12) Pooled (n=23) Mean change from baseline in RHS (95%
CI) -0.4 (-3.9, 3.1) -0.3 (-2.0, 1.4) -0.3 (-2.1, 1.4) # (%) patients achieving ≥1-pt increase in RHS 4/11 (36%) 5/12 (42%) 9/23 (39%) # (%) patients achieving ≥3-pt increase in RHS 3/11 (27%) 2/12 (17%) 5/23 (22%) # (%) patients
achieving ≥5-pt increase in RHS 1/11 (9%) 0/12 (0%) 1/23 (4%) Mean (±SEM) change from baseline in RHS scores *Includes 2 patients in monotherapy and 2 patients in apitegromab + nusinersen subgroup who maintained RHS score (0-point change
from baseline)Per protocol and sensitivity (all patients) analyses showed similar results to primary intent-to-treat analysisapitegromab = SRK-015 Data on file. Scholar Rock, Inc. Cambridge, MA 13/23* (57%) maintained or improved in RHS
(≥0-point change from baseline)

Cohort 2

18 Nusinersen CHERISH Trial in Later-Onset SMA (15-month treatment period) *Mercuri E, et.al.
Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625-635.**Mercuri E. et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials.
https://doi.org/10.1016/j.nmd.2015.10.006This third-party information is provided for background only and is not intended to convey or imply a comparison to the TOPAZ clinical trial results. CHERISH clinical trial data*After 15 months of
treatment in patients who started on nusinersen at age >5....Mean HFMSE decline of >0.5-points<15% with ≥3-point increaseNatural history study**After 12-month follow-up in patients age >5...Mean HFMSE decline <5%
with ≥3-point increase Majority of patients in this age range do not experience HFMSE improvements and rarely achieve a ≥3-point increase Background Insights Into Non-Ambulatory Later-Onset SMA ≥5 Years of Age

Cohort 2: Improvement in Mean HFMSE at 12-Months with Majority of Patients Achieving ≥1-point
Increase Individual HFMSE responses Type 2 and Non-Ambulatory Type 3 SMA Apitegromab (20 mg/kg) + nusinersen Intent-to-Treat Population (n=14) Per Protocol Population* (n=13) Mean change from baseline in HFMSE (95% CI) +0.6 (-1.4,
2.7) +1.2 (-0.5, 2.9) # (%) patients achieving ≥1-pt increase in HFMSE 9/14 (64%) 9/13 (69%) # (%) patients achieving ≥3-pt increase in HFMSE 4/14 (29%) 4/13 (31%) # (%) patients achieving ≥5-pt increase in HFMSE 2/14 (14%) 2/13
(15%) 19 Mean (±SEM) change from baseline in HFMSE scores *Patient had concomitant exposure to an acetylcholinesterase inhibitor, which is not permitted per the TOPAZ trial protocol Sensitivity analysis (all patients) showed similar
results to primary intent-to-treat analysisapitegromab = SRK-015. Data on file. Scholar Rock, Inc. Cambridge, MA Per protocol analysis* excludes patient (-7 pts).

Cohort 3

Background Insights Into Non-Ambulatory Later-Onset SMA with Early Initiation of Nusinersen
Therapy 21 Nusinersen SHINE Trial in Later-Onset SMA* Source: Darras, B., et.al. Nusinersen in later-onset spinal muscular atrophy. Neurology. May 2019; 92 (21) e2492-e2506.“Longer-term treatment with nusinersen: results in later-onset
spinal muscular atrophy from the SHINE study” P.257, World Muscle Society Congress 2020This third-party information is provided for background only and is not intended to convey or imply a comparison to the TOPAZ clinical trial results. *Most
nusinersen-treated patients in CHERISH were under age 5 years at time of therapy initiation TOPAZ Cohort 3Patients on average had received ~2 years of treatment with nusinersen at baseline and ~3 years by the 12-month analysis
timepoint. Nusinersen SHINE TrialSHINE data suggest nusinersen-treated patients primarily stabilize or experience modest and gradual improvement beyond the initial 15 months of therapy Mean (+ SE) Change in HFMSETotal Score From Baseline 1
92 169 253 350 450 690 930 1170 1410 1650 Analysis Visit, d n = 84 82 84 84 83 76 83 83 79 61 20 n = 42 40 18 24 37 35 n = 42 41 41 41 42 39 -1-2-3-4 8765 43210 Nusinersen in CHERISH and SHINENusinersen in SHINE (sham control in
CHERISH)Sham control in CHERISH 4.6 1.7 -0.4 Long-term follow-up of nusinersen therapy

Cohort 3: Sizeable Continued Improvements in Mean HFMSE Observed Across 12 Months 22 Individual HFMSE
responses Type 2 SMA (Intent-to-Treat Population) Apitegromab 20 mg/kg + nusinersen (n=8) Apitegromab 2 mg/kg + nusinersen (n=9) Pooled (n=17) Mean change from baseline in HFMSE (95% CI) +7.1 (1.8, 12.5) +5.3 (-1.5, 12.2) +6.2 (2.2,
10.1) # (%) patients achieving ≥1-pt increase in HFMSE 7/8 (88%) 7/9 (78%) 14/17 (82%) # (%) patients achieving ≥3-pt increase in HFMSE 5/8 (63%) 5/9 (56%) 10/17 (59%) # (%) patients achieving ≥5-pt increase in HFMSE 5/8 (63%) 5/9
(56%) 10/17 (59%) Per protocol and sensitivity (all patients) analyses showed similar results to primary intent-to-treat analysis apitegromab = SRK-015 Data on file. Scholar Rock, Inc. Cambridge, MA Mean (±SEM) change from baseline in
HFMSE scores 6/17 (35%) with >10-point increase in HFMSE

Dose-proportional and sustained drug exposure following chronic administration of
apitegromab Pharmacokinetic and Pharmacodynamic Data are Supportive of Clinically Observed Effects Both 2 mg/kg and 20 mg/kg doses yielded high levels of target engagement (>100-fold increase from baseline)20 mg/kg dose offers relatively
higher magnitude of target engagement than 2 mg/kg dose High levels of target engagement achieved by both doses, with relatively higher absolute levels with high dose 23 *Starting at day 28, measures are pre-dose trough levels Data on file.
Scholar Rock, Inc. Cambridge, MA Pharmacokinetics* (PK) Pharmacodynamics (PD)

24 12-Month Top-line Results Support the Therapeutic Potential of Apitegromab and Further
Development Data on file. Scholar Rock, Inc. Cambridge, MA 57 Patients* Completed 12-Month TOPAZ Trial All Elected to Opt Into Extension Period *Excludes one patient from Cohort 1 that discontinued from the trial Cohort 1 Mean RHS
decline from baseline, but majority of patients maintained or improved (≥0-pt change in RHS)Potential subset of patients with more pronounced effect (22% with ≥3-pt increase) Cohort 2 Mean HFMSE improvement from baseline Majority (64%) of
patients improved (≥1-pt increase in HFMSE) and sizeable subset (29%) attained ≥3-pt increase in HFMSE Cohort 3 Large HFMSE improvements from baseline, with dose response observed Majority (59%) of patients attained ≥5-pt increase and
sizeable subset (35%) attained >10-pt increase in HFMSEPK/PD results support observed dose response Safety No safety signals identified as of the 12-month top-line analysisIncidence and severity of AEs were consistent with the underlying
patient population and background therapy
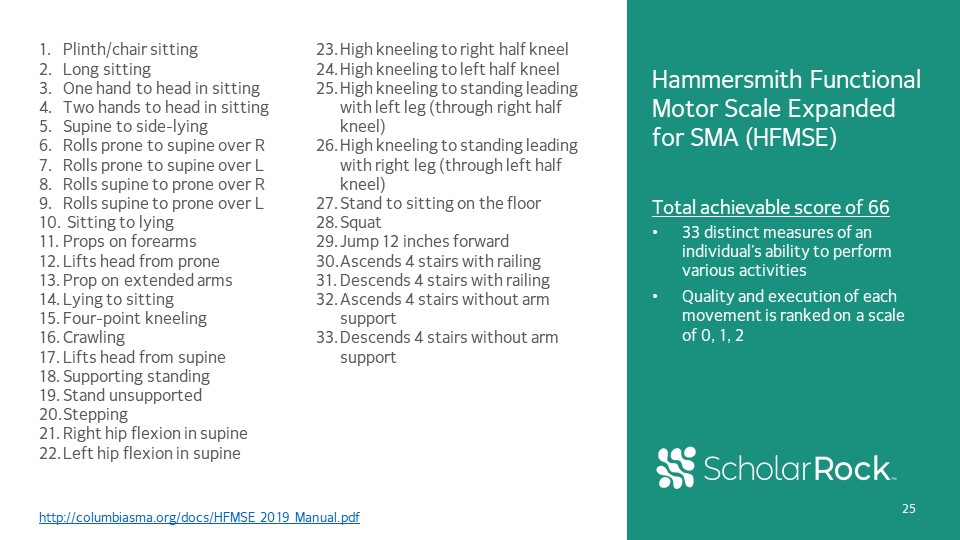
Hammersmith Functional Motor Scale Expanded for SMA (HFMSE)Total achievable score of 6633 distinct
measures of an individual’s ability to perform various activitiesQuality and execution of each movement is ranked on a scale of 0, 1, 2 http://columbiasma.org/docs/HFMSE_2019_Manual.pdf Plinth/chair sittingLong sittingOne hand to head in
sittingTwo hands to head in sittingSupine to side-lyingRolls prone to supine over RRolls prone to supine over LRolls supine to prone over RRolls supine to prone over L Sitting to lyingProps on forearmsLifts head from proneProp on extended
armsLying to sittingFour-point kneelingCrawlingLifts head from supineSupporting standingStand unsupportedSteppingRight hip flexion in supineLeft hip flexion in supineHigh kneeling to right half kneelHigh kneeling to left half kneelHigh kneeling
to standing leading with left leg (through right half kneel)High kneeling to standing leading with right leg (through left half kneel)Stand to sitting on the floorSquatJump 12 inches forwardAscends 4 stairs with railing Descends 4 stairs with
railingAscends 4 stairs without arm support Descends 4 stairs without arm support 25

26 Next Steps for Apitegromab Program Tony Kingsley - President & CEOTed Myles - CFO & Head of
Business Ops

27 Additional TOPAZ Data and Analyses Will Further Our Understanding of Apitegromab’s Potential in SMA
Exploratory analyses, including patient-level dataAdditional outcome measuresAdditional safety data Plan to present 12-month top-line data and additional analyses at upcoming medical congresses

28 Apitegromab Has Broad Potential in SMA…Global Disease with Overall Prevalence of 30,000-35,000 in
U.S. and Europe Alone High unmet medical need; benefits of SMN upregulators not well establishedTOPAZ data suggest potential clinical benefit; possibly more pronounced in subset of patientsOpportunity for additional exploration of apitegromab,
both as monotherapy and in conjunction with SMN upregulators Most prevalent population TOPAZ has shown potential to improve motor functionMany patients already treated with or eligible to be treated with SMN upregulators Highest
incidence population and growing prevalence due to increased survivalTOPAZ Cohort 3 data points to potential benefit of treating at an early agePotential to evaluate apitegromab with all SMN upregulators, including gene therapy Ambulatory
Patients with SMA Patients with Type 1 SMA Non-Ambulatory Patients with Type 2 and Type 3 SMA Subject to discussions with regulatory authorities; planned Phase 3 trial expected to initiate by
year-end http://www.smafoundation.org/wp-content/uploads/2012/03/SMA-Overview.pdfhttps://www.curesma.org/wp-content/uploads/2018/01/SMA-VoP-for-publication-1-22-2018.pdf

29 Spinal Muscular Atrophy Additional Indications Glucocorticoid induced myopathy Potential
benefit for subset of patients unable to discontinue steroid therapy Post-cancer muscle recovery in pediatrics*** Some children may develop severe muscle wasting from chemotherapy Late-onset Pompe Disease** Large percentage of patients
treated with enzyme replacement therapies (ERTs)Existing ERTs may address underlying pathology, but muscle strength remains ongoing challenge Muscular Dystrophies …as well as Broad Potential Beyond SMA Becker Muscular Dystrophy* Prevalence
of 15,000-25,000, substantially under-diagnosed in earliest stagesYounger population with less severe dystrophin deficiency and slower progressing muscle damage Non-AmbulatoryLater-Onset Type 1 AmbulatoryLater-Onset Leverage TOPAZ findings
to conduct further explorations in Type 1 and other subpopulations Duchenne Muscular Dystrophy* Other Dystrophies Potential for add-on muscle-directed therapy in other rare dystrophies with less severe phenotypes or upon availability of
disease-stabilizing therapies Prevalence of 30,000-40,000 with very severe symptoms and high unmet needProgress in the development of next-generation disease-stabilizing therapies may enable add-on muscle-directed approach *“Muscular
Dystrophy: Disease Landscape and Forecast.” DRG Reports, June 19, 2020**Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review, Journal of Neuology. 2013***A Systematic Review of Selected Musculoskeletal Late
Effects in Survivors of Childhood Cancer, Current Pediatric Reviews. 2014

30 Potential for Apitegromab in Becker Muscular Dystrophy (BMD); Aim to Initiate Clinical Trial in
2022 Strong fit for a selective inhibitor of latent myostatin… Key Scientific Question BMD Fit Genetic disorder present at birth, with majority of patients identified at young age (before 18) Is patient population young? Less severe
dystrophin deficiency and muscle disease than DMD with slower progression Are muscles structurally intact? BMD causes a substantial deficit in fast-twitch muscle fibers Does disease impact fast-twitch fibers? Several endpoints(1) (NSAA,
TTSTAND) dependent on fast-twitch fibers have been used in past pivotal studies of muscular dystrophy therapies Is there an established endpoint that relies on fast-twitch fibers? Source: KOL Interviews; Dystrophin levels and clinical
severity in Becker muscular dystrophy patients, J Neurol Neurosurg Psychiatry. 2014; Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies, Scientific Reports, 2016 (1)NSAA: North Star
Ambulatory Assessment. TTSTAND: Time To Stand … with a sizeable unmet need to be addressed Estimated Prevalence of 15,000-25,000 ≤18 Years of Age Adults Severe Moderate Mild Source: KOL Interviews; Practicing neurologist survey (N=21),
‘Muscular Dystrophy: Disease Landscape & Forecast’ published Jun 2020 Natural adjacencies to current program in SMAPositions apitegromab program for evaluation in a range of other muscular dystrophies (e.g. Duchenne Muscular
Dystrophy) ~55% % of diagnosed population by age (estimated)

Rare Pediatric Disease for SMA granted by FDAOrphan Drug Designation for SMA granted by FDA Priority
Medicines (PRIME) Designation for SMA granted by EMAOrphan Medicinal Product Designation for SMA granted by EMA Broad Patent Portfolio Protecting Apitegromab Into Late 2030s; Multiple Designations Granted by FDA/EMA 31 Highlights of
apitegromab patent portfolio:US 10,751,413 (2037): Composition of matter and methods of use for apitegromabUS 9,758,576 (2034): Composition of matter claims to mAbs that inhibit the activation of myostatin precursorUS 10,307,480 (2035):
Antibodies that selectively inhibit myostatin activationUS 10,287,345 (2037): Treatment methods for various myostatin-related conditions US 10,946,036 (2037): Covers both add-on and combination therapy with a myostatin inhibitor and a neuronal
corrector therapyUS 10,882,904 (2036): Broadly directed to use of apitegromab to achieve certain therapeutic effects; without limiting to specific indicationsUS 9,399,676 (2034): Methods of producing antibodies that bind pro/latent
myostatin Multiple designations granted by FDA/EMA recognizing the potential for apitegromab to address unmet medical needs in SMA

Gilead fibrosis-focused TGFβ collaboration DRAGON Part A: dose escalation and continued
follow-up ApitegromabSpinal Muscular Atrophy (SMA) SRK-181 Immuno-Oncology and Oncology Preclinical/ Platform TOPAZ 12M topline data Planned apitegromab Phase 3 program in SMA by year-end 2021: Potential for Another Transformative
Year TOPAZ extension Continue to discover and advance preclinical programs Multiple opportunities for additional myostatin-related indications beyond SMA and muscular dystrophies Multiple additional opportunities: 1) SRK-181 in
oncology; 2) latent TGFβ1 immune cell in IO; 3) latent TGFβ1 immune cell in oncology Gilead Fibrosis collaboration 2022 and Beyond 2021 Q3 Q4 Q2 Latent TGFβ1 Immune Cell Immuno-Oncology and Oncology 32 DRAGON Part B to initiate
mid-year: multiple tumor typesMelanomaNSCLCUrothelial CarcinomaOther Solid Tumor Types ApitegromabOther Indications Multiple opportunities in muscular dystrophies, including Becker Muscular Dystrophy

