Attached files
| file | filename |
|---|---|
| EX-23.1 - Save Foods Inc. | ex23-1.htm |
| EX-5.1 - Save Foods Inc. | ex5-1.htm |
As filed with the Securities and Exchange Commission on April 6, 2021
Registration Statement No. 333-254327
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
SAVE FOODS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2870 | 26-468460 | ||
| (State
or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
Save Foods, Inc.
Kibbutz Alonim, Israel, 3657700
Tel: (347) 468 9583
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
David Palach
Chief Executive Officer
Save Foods, Inc.
Kibbutz Alonim, Israel, 3657700
Tel: (347) 468 9583
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Oded Har-Even, Esq. David A. Huberman, Esq. Ron Ben-Bassat, Esq. Sullivan & Worcester LLP 1633 Broadway New York, NY 10019 (212) 660-3060 |
Leslie
Marlow, Esq. Patrick J. Egan, Esq. Gracin & Marlow, LLP The Chrysler Building 405 Lexington Avenue, 26th Floor New York, NY 10174 (212) 907- 6457 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer [ ] |
Accelerated filer [ ] |
Non-accelerated filer [X] |
Smaller reporting company [X] |
Emerging Growth Company [ ] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. [ ]
CALCULATION OF REGISTRATION FEE
| Title of Securities Being Registered | Proposed Maximum Aggregate
Offering |
Amount
of Registration Fee(3) |
||||||
| Common Stock, par value $0.0001 per share | $ | 13,800,000 | $ | 1,505.58 | ||||
| Underwriter’s warrants to purchase Common Stock (4) | — | — | ||||||
| Common Stock issuable upon exercise of the Underwriter’s warrants (5) | 750,000 | 81.83 | ||||||
| Total Registration Fee | $ | 14,550,000 | $ | 1,587.41 | (6) | |||
| (1) | Estimated solely for purposes of calculating the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). Includes the offering price of shares of Common Stock that the Underwriter have the option to purchase to cover over-allotments, if any. |
(2)
|
Pursuant to Rule 416 under the Securities Act, the shares registered hereby also include an indeterminate number of additional shares of Common Stock as may from time to time become issuable by reason of stock splits, distributions, recapitalizations or other similar transactions. |
| (3) | Calculated pursuant to Rule 457(o) under the Securities Act based on an estimate of the proposed maximum aggregate offering price. |
| (4) | No fee required pursuant to Rule 457(g). |
| (5) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. The underwriter’s warrants are exercisable at a per share exercise price equal to 125% of the public offering price. As estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the underwriter’s warrants is equal to 125% of $600,000 (which is 5% of $12,000,000). |
| (6) | Previously paid. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement related to these securities filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell or a solicitation of an offer to buy these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED APRIL 6, 2021 |
1,090,909 Shares
Common Stock

Save Foods, Inc.
We are offering up to 1,090,909 shares of our Common Stock par value $0.0001 per share (“Common Stock”). We anticipate that the initial public offering price will be between $10.00 and $12.00. We have applied to list our Common Stock on the Nasdaq Capital Market under the symbol “SVFD.” No assurance can be given that our application will be approved or that a trading market will develop.
Our Common Stock is currently traded on the OTC Markets, Pink Open Market, under the symbol “SAFO.” On March 31, 2021, the last reported sale price of our Common Stock was $15 per share.
Investing in our securities involves risks. See “Risk Factors” beginning on page 14 of this prospectus for a discussion of the risks that you should consider in connection with an investment in our securities. Neither the Securities and Exchange Commission (the “SEC”) nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ | ||||||
| (1) | Underwriting discounts and commissions do not include a non-accountable expense allowance equal to 1.0% of the initial public offering price payable to the underwriter. We have agreed to reimburse the underwriters for certain expenses and the underwriters will receive compensation in addition to underwriting discounts and commissions. We have also agreed to issue warrants to the representative of the underwriters as a portion of the underwriting compensation payable to the underwriters in connection with this offering. See the section titled “Underwriting” beginning on page 92 of this prospectus for additional disclosure regarding underwriter compensation and offering expenses. |
We have granted the underwriters an option for a period of 45 days to purchase up to 163,636 additional shares of our Common Stock.
We estimate the expenses of this offering, excluding underwriting discounts and commissions, will be approximately $325,000.
The underwriters expect to deliver the Company’s securities to the purchasers on or about April , 2021.
ThinkEquity
a division of Fordham Financial Management, Inc.
The date of this prospectus is April , 2021.

| 1 |

| 2 |
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any free writing prospectus that we have authorized for use in connection with this offering. Neither we nor the underwriters have authorized anyone to provide you with information that is different. We are offering to sell, and seeking offers to buy, the securities covered hereby only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities covered hereby. Our business, financial condition, results of operations and prospects may have changed since that date. We are not, and the underwriters are not, making an offer of these securities in any jurisdiction where the offer is not permitted. You should also read and consider the information in the documents to which we have referred you under the caption “Where You Can Find Additional Information” in the prospectus. In addition, this prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed or will be filed as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
For investors outside the United States: Neither we nor any of the underwriters have taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby and the distribution of this prospectus outside of the United States.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications and third-party research, surveys and studies are reliable. We are ultimately responsible for all disclosure included in this prospectus.
Except where the context requires otherwise, in this prospectus the “Company,” “Save Foods,” “we,” “us” and “our” refer to Save Foods, Inc., a Delaware corporation and, where appropriate, its subsidiary, Save Foods Ltd.
In June 2019, we implemented a one-for-fifteen reverse stock split of our common stock pursuant to which holders of our Common Stock received one share of our Common Stock for every fifteen shares of Common Stock held.
On February 23, 2021, we implemented a one-for-seven reverse stock split of our common stock pursuant to which holders of our Common Stock received one share of our Common Stock for every seven shares of Common Stock held. Unless the context expressly dictates otherwise, all references to share and per share amounts referred to herein reflect the reverse stock split.
| 3 |
Cautionary NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, including statements regarding the progress and timing of our product development, the goals of our development activities, estimates of the potential markets for our products, estimates of the capacity of manufacturing and other facilities to support our products, our expected future revenues, operations and expenditures and projected cash needs. The forward-looking statements are contained principally in the sections of this prospectus entitled “Prospectus Summary” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events of our financial performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. Those risks and uncertainties include, among others:
| ● | our expectations regarding our short and long-term capital requirements; | |
| ● | our ability to raise additional capital to meet our liquidity needs; | |
| ● | our ability to meet the initial listing requirements of the Nasdaq Capital Market and to comply with the continued listing standards of the Nasdaq Capital Market; | |
| ● | our ability to generate sufficient proceeds from this offering to affect our business plan; | |
| ● | our expected use of proceeds from this offering; | |
| ● | sales of our products; | |
| ● | the size and growth of our product market; | |
| ● | our marketing plans; | |
| ● | our activity in the civilian market; | |
| ● | our ability to obtain market acceptance of our technology and products; | |
| ● | our ability to satisfy U.S. (including the U.S. Food and Drug Administration, the United States Environmental Protection Agency and the California Department of Pesticide Regulation), and international regulatory requirements and obtain required approvals for sales or exports of our products; | |
| ● | our ability to compete in our respective markets; | |
| ● | our plans to continue to invest in research and development; | |
| ● | our ability to gain acceptance of packing house community and other industries for use of our products; | |
| ● | our ability to establish and maintain strategic partnerships with third parties, including for the distribution of products; | |
| ● | our ability to attract and retain sufficient, qualified personnel; | |
| ● | our ability to obtain or maintain patents or other appropriate protection for the intellectual property; | |
| ● | our ability to grow both domestically and internationally; | |
| ● | our ability to adequately support future growth; | |
| ● | potential product liability or intellectual property infringement claims; | |
| ● | the effect of COVID-19 on our business; and | |
| ● | information with respect to any other plans and strategies for our business. |
Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential,” or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus and, except as required by law, we undertake no obligation to update or review publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this prospectus. You should read this prospectus, the documents incorporated by reference in this prospectus, the documents referenced in this prospectus and the documents filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
| 4 |
This summary highlights information about us, this offering and selected information contained elsewhere in and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus and the documents incorporated by reference herein, including our financial statements and the related notes and the information set forth under the sections titled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.” Unless otherwise indicated, all share amounts and per share amounts in this prospectus have been presented on a retrospective and pro forma basis to reflect the reverse stock split of our outstanding shares of common stock at a ratio of 1-for-7 which we effected on February 23, 2021.
Our Company
We develop eco-friendly “green” solutions for the food industry. Our solutions are developed to improve the food safety and shelf life of fresh produce. We do this by controlling human and plant pathogens, thereby reducing spoilage, and in turn, reducing food loss.
Our products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of oxidizing agent-based sanitizers and fungicides at low concentrations. Our “green” products are capable of cleaning, sanitizing and controlling pathogens on fresh produce with the goal of making them safer for human consumption and extending their shelf life by reducing their decay. One of the main advantages of our products is that our active ingredients do not leave any toxicological residues on the fresh produce we treat. In contrary, by forming a temporary protective shield around the fresh produce we treat, our products make it difficult for pathogens to develop and potentially provide protection which also reduces cross-contamination.
The U.S. Food and Drug Administration (the “FDA”) Food Safety Modernization Act (the “FSMA”) is transforming the United States’ food safety system by shifting the focus from responding to foodborne illness to preventing it. According to the recent data from the Centers for Disease Control and Prevention, approximately 48 million people in the United States get sick each year from foodborne diseases. We believe this is a significant public health burden that is largely preventable. Since 2011, the FDA has had a legislative mandate to require comprehensive, science-based preventive controls across the food supply. In the context of fresh produce at packing houses, the FDA’s final produce safety rule (with an initial compliance date of January 26, 2018) provides for the use of sanitizers to ensure produce is cleaned from human pathogens.
In addition, most conventional chemical pesticides (fungicides), which are currently used to protect fresh produce from microbial spoilage and reduce food waste, are toxic, they remain on fruit peel and present health concerns, while also polluting the environment. Therefore, the use of these products is strictly regulated and their residue on food and on the environment are carefully monitored. Today’s trends led by both consumers and regulatory bodies are to significantly reduce the use of fungicides and switch to greener solutions. In a series of studies conducted in collaboration with a large post-harvest service company during the second quarter of 2020, our products have shown to extend the shelf life of fresh produce in “organic” (where no fungicides are used at the post-harvest stage) and conventional (where fungicides are being used at the post-harvest stage) settings. On average, our products may reduce the rotten fruits at the retail level by 50%.
We have a unique opportunity to make a positive difference throughout the food value chain from field to fork and address two of the major’s challenges in the food industry today — safety and waste. We target major markets that use conventional chemical pesticides and sanitizers, including the pre- and post-harvest market, the greenhouse market and the fresh-cut market, where our “green” products are used as alternatives for, or mixed with, conventional products in order to reduce (i) health and environmental concerns, and/or (ii) microbial resistance that has reduced the efficacy of conventional chemical pesticides.
Our Core Products
Our innovative products address two of the most significant challenges in the food industry: increase food safety and reduce food loss. Our main product lines consist of a proprietary blend of organic food acids applied in post-harvest applications designed to ensure food safety and increase fruit and vegetable’ shelf life by reducing microbial spoilage.
The main steps in post-harvest applications are cleaning, sanitization, and coating (wax). Our products address the cleaning and sanitization application points which are the critical first steps for preserving the quality of fresh produce by controlling microbial contamination related to food safety (e.g., Listeria, Salmonella, E. coli) and food loss due to microbial spoilage (e.g., fungi, mold and yeast).
| 5 |
One of the main advantages of our food acid blend is its non-toxic and safe residues that are providing protection to the treated produce. All the blend ingredients are recognized by the FDA as Generally Recognized as Safe (the “GRAS”) when used as intended in fruit and vegetable wash applications. Moreover, they significantly reduce or eliminate the need for additional post-harvest applications with conventional fungicide by at least 50%, and in some cases entirely, and can reduce food waste due to spoilage by up to 50% (see results below on easy peelers and mango).
Our main products are:
| ● | Processing Aids – SavePROTECT or PeroStar: post-harvest treatment added to fruit and vegetable wash water as a processing aid to increase the efficiency of the oxidizing agent present in the water tank against plant pathogens to reduce produce loss; and | |
| ● | Sanitizers - SF3HS and SF3H: post-harvest cleaning and sanitizing solution to control both plant and foodborne pathogens to ensure both food safety as well as increase produce’s shelf life. |
Our product portfolio also includes our SpuDefender, which targets and is designed to control the post-harvest potato sprout, and our FreshProtect, which targets and is designed to control spoilage-creating microorganisms on post-harvest citrus fruit and has a potential to reduce the bacterial load entering the packing house in pre-harvest applications.
Our Strengths
We believe that our main strengths include:
| ● | Strong Management Team with Commitment to Green Products. Led by a team with over 30 years of experience in developing sanitization products and solutions for the agriculture industry, we plan on becoming a significant player in providing consumers with healthy and green fresh produce from farm to fork while endeavoring to ensure food safety and reducing food waste. We believe that our proprietary blend of food acids provides protection to the treated produce and works in synergy with well-known fungicides and sanitizers. This synergy allows us to significantly reduce the concentration of the fungicides that are heavily regulated in several countries and, in certain countries, outright banned and meet the food trends of sustainable and green produce. | |
| ● | Multi-Purpose Products that Simplify Crop Treatment Routine and Save Money. While most chemicals marketed in the industry address either food safety or food waste, our multi-purpose products are intended to provide a solution for both problems, while simplifying crop treatment and achieving cost saving. Our products are capable of cleaning and controlling pathogens that would otherwise render fresh produce as unsafe for human consumption. Our proprietary blend of food acids combined with well-known sanitizers are very efficient against foodborne pathogens like E. coli, Salmonella and Listeria as well as plant pathogens in short contact time (99.999% reduction within 30 seconds of contact). In addition, with multipurpose products, there is no need to order, ship or dispose of bottles of product, resulting in less energy consumed, less CO2, less fuel, and less waste. Our focus on natural product chemistries allows us to continually drive lower costs, higher product gross margins and efficacy through longer shelf life and reduction of food waste. | |
| ● | Strong Intellectual Property Portfolio. We believe that we have built a strong intellectual property position throughout the food chain (from field to fork) as our patents claim compositions and methods that can be used to protect food and agricultural products from decay. We rely on a combination of important intellectual property assets, to protect our innovation. Our employees, consultants, customers, and vendors are subject to confidentiality agreements that protect our proprietary manufacturing processes. Our patent portfolio includes granted patents in the United States, Europe, and Israel, as well as several priority applications, across several patent families, including composition-of-matter claims, methods of use claims, including for treating edible matter, for improving the appearance of edible plant matter, and sterilization methods, as well as for articles for implementing these methods. These patents directly protect a proprietary method for extending life shelf and reducing edible matter from microbial decay. |
| 6 |
| ● | Commercially Available Products and Seamless Implementation. One of the oxidizers being used with our products is peracetic acid (“PAA”), a well-known and widely used sanitizer. Following the enforcement of the FSMA in connection with the use of sanitizers, more and more packers have been choosing this healthy and eco-friendly sanitizer over chlorine, and this choice facilitates implementation of our products. In addition, the application of our products does not require special equipment as they are used in combination with or replace existing products applied on the packing line or in the mix tank in the field. This allows a relatively cheap, seamless and fast implementation. | |
| ● | Significant Reduction of Hazardous Chemicals Food Residue. All the ingredients in our blend of food acids are recognized by the FDA as GRAS when used as intended in fruit and vegetable wash applications, while oxidizers we use, such as hydrogen peroxide, rapidly decompose into water and oxygen. The absence of toxicological residues not only improves food quality but also promotes occupational safety for the employees of packing houses, contributing to a friendlier and safer working environment. |
Our Strategy
In September 2018, the Company changed its organizational structure and management team. After reviewing the Company’s then existing strategy and results of operation, as well as examining the market opportunities, the new management team decided to update the Company’s strategy, reduce the marketing and sales of its existing products, and focus the Company’s efforts and financial resources in developing its next generation products. During the years 2019 and 2020, we developed, validated and tested the efficacy of our next generation product – a blend of food acids – on a variety of crops in small and large scale commercial pilots.
Our strategy is to develop and commercialize our products through strategic partnerships with global post-harvest service companies and with large food distributors and retailers with the intent of: (i) extending the shelf life of fresh produce while reducing (and even eliminating) the use of harmful chemicals (fungicides); (ii) ensuring food safety and shelf life by controlling foodborne pathogens and allow our customers to meet FSMA regulatory requirements; (iii) reducing food loss and the associated carbon “footprint.”
In order to achieve our goals, we intend to:
| ● | Advance our Breakthrough Technologies and Commercialization Efforts. During the first half of 2021, we plan to run a series of additional pilot studies in various commercial collaborations with post-harvest service vendors packing houses and food retailers. | |
| ● | Develop a Strong Marketing Message Around Promoting Safe Food While Avoiding Food Waste. We plan to brand our fresh produce with a “chemical residues free” or “naturally protected” seal of approval, and we believe that like-minded fruit packers around the globe will seek to differentiate themselves from their competitors by obtaining this seal. | |
| ● | Acquire or License Complementary Products and Technologies. We actively search for products and technologies that can enhance our portfolio and grow our business to address all the post-harvest treatments such as fruit coating products or technologies. | |
| ● | Expand to Additional Produce and Geographies. Our plan is to focus first on key countries and regions with the largest markets for our crops, including Mexico, Spain, Italy, Israel and key markets in the United States such as California, Florida and Texas. We also plan to increase the variety of crops that can be treated with our products, to include produce such as apples, bell peppers, tomatoes and papayas. | |
| ● | Leverage Our Products Through Collaborations. Our focus and expertise in the development of green products for the agritech industry and in post-harvest treatments allow us to be a partner of choice for other businesses looking for development partners and for larger companies wanting to leverage their product such as PAA into new combination products. For example, companies selling or owning fungicides, the maximum residue level (“MRL”) of which is being reduced, and that are working in synergy with our products are good partners. This type of collaboration could allow them to continue selling their product. |
| 7 |
Our selling and marketing strategy is twofold:
| ● | establish collaborations with food retailers; and | |
| ● | partner with service vendors to fruit and vegetable packing houses. |
Summary Risk Factors
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flows and prospects that you should consider before making a decision to invest in our Common Stock. These risks are discussed more fully in the section titled “Risk Factors” beginning on page 14 of this prospectus, and include the following:
|
● |
we have a history of operating losses and expect to incur additional losses in the future; | |
● |
we have not generated significant revenue from the sale of our products and do not believe that our current cash on hand will be sufficient to fund our growth plans or our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern; | |
● |
even if this offering is successful, we expect that we will need to raise significant additional capital, which we may be unable to obtain; | |
● |
because of our limited operating history, we may not be able to successfully operate our business or execute our business plan; | |
| ● | our products and technology require additional trials; | |
● |
the commercial success of our new generation products, as well as any future products, depends upon the degree of market acceptance by the packing house community as well as by other prospect markets and industries; | |
| ● | the COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease, may materially and adversely affect our business and operations; | |
| ● | we may face significant competition from other companies looking to develop or acquire new alternative environmentally friendly solutions for the treatment of fruits and vegetables, and other edible matter; | |
| ● | our success is dependent upon the acceptance of our environmentally friendly solutions for fruits and vegetable; | |
| ● | we may be unable to respond effectively to technological changes in our industry, which could reduce the demand for our products; |
| 8 |
| ● | we currently rely on a limited number of suppliers to produce certain key components of our products. | |
● |
if we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with third parties to perform these services, we may not be successful in commercializing our products; | |
| ● | we rely on rapidly establishing global distributorship network in order to effectively market our products; | |
● |
the results of our early tests may not be indicative of results in future tests and we cannot assure you that any planned or future tests will lead to results sufficient for the necessary regulatory approvals; | |
● |
our products are highly regulated by governmental agencies in the countries where we conduct business and into which we plan to expand. Our failure to obtain regulatory approvals and registration, to comply with registration and regulatory requirements or to maintain regulatory approvals would have an adverse impact on our ability to market and sell our products; | |
● |
our success is dependent upon our ability to achieve regulatory approvals and registration in the United States and abroad (Mexico, Israel, Spain and Italy), which might take longer periods than expected; | |
● |
the inherent dangers in production and transportation of hydrogen peroxide and highly concentrated organic acids could cause disruptions and could expose us to potentially significant losses, costs or other liabilities; | |
● |
our business and operations may be affected by climate change conditions, which could materially harm our financial results; | |
● |
conditions in the global economy may adversely affect our business, financial condition and results of operation; | |
● |
our relationship with our employees could deteriorate, and certain key employees could leave, which could adversely affect our business and results of operations; | |
| ● | we are subject to risks relating to portfolio concentration; | |
● |
our operating results may fluctuate, which makes our results difficult to predict and could cause our results to fall short of expectations; | |
● |
international expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Mexico or Israel; | |
| ● | our business depends to some extent on international transactions; | |
● |
if we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, our ability to compete will be harmed; | |
● |
if we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unprotected know-how, our ability to compete will be harmed; | |
● |
we could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products; | |
● |
we may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property; | |
● |
we may experience claims that our products infringe the intellectual property rights of others, which may cause us to incur unexpected costs or prevent us from selling our products or services; | |
● |
if we or our contractors or service providers fail to comply with laws and regulations, we or they could be subject to regulatory actions, which could affect our ability to develop, market and sell our products or future products that we may develop and may harm our reputation in our industry; | |
| ● | regulatory reforms may adversely affect our ability to sell our products profitably; | |
| ● | conditions in Israel may limit our ability to manage and market our products, which would lead to a decrease in revenues; | |
● |
we may not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products; | |
● |
it may be difficult to acquire jurisdiction and enforce liabilities against our officers and directors who are based in Israel; and | |
● |
even if we meet the initial listing requirements of the Nasdaq Capital Market, there can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq Capital Market. Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a de-listing of our Common Stock. |
| 9 |
Corporate Information
We were incorporated in the State of Delaware on April 1, 2009. Our principal executive offices are located at Kibbutz Alonim, Israel, 3657700 and our telephone number is (347) 468 9583. Our website address is www.savefoods.co. The information contained on, or that can be accessed through, our websites is not incorporated by reference into this prospectus and is intended for informational purposes only.
The SEC also maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
This prospectus contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Solely for convenience, trademarks, trade names and service marks referred to in this prospectus may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent permitted under applicable law, our rights or the right of the applicable licensor to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
| 10 |
The Offering
| Common
Stock offered by us |
1,090,909 shares | |
| Common Stock issued and outstanding | 1,606,760 shares (as of April 1, 2021) | |
Total shares of Common Stock to be outstanding after this offering |
2,743,801 shares (or 2,907,437 shares if the underwriters exercise in full their option to purchase additional 163,636 shares to cover over-allotments, if any) | |
| Over-allotment option | We have granted the underwriters an option for a period of 45 days from the date of this prospectus to purchase up to an additional 163,636 shares of Common Stock at the public offering price, less the underwriting discount. | |
| Use of proceeds |
We estimate that we will receive gross proceeds of approximately $12,000,000, assuming a public offering price of $11.00 per share, which is the midpoint of the range set forth on the cover page of this prospectus before deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for the following purposes: |
| ● | approximately $2.6 million for product research and development (which may include acquisition of technology to complement our product portfolio) and purchase of lab equipment; | |
| ● | approximately $1.2 million for gaining regulatory approvals and commercialization; | |
| ● | approximately $2.7 million for selling and marketing; and | |
| ● | the remainder for working capital and general corporate purposes. |
| See “Use of Proceeds” for additional information. |
| Risk factors | See “Risk Factors” beginning on page 14 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our Common Stock. | |
| Proposed Nasdaq Capital Market symbol | We have applied to list the Common Stock to be issued in this offering on the Nasdaq Capital Market under the symbol “SVFD.” |
| 11 |
The number of Common Stock that will be outstanding after this offering as shown above is based on 1,606,760 shares of Common Stock issued and outstanding as of the date of this prospectus, and the issuance and sale of 1,090,909 shares of our Common Stock in this offering at a public offering price of $11.00 per share, which is the midpoint of the range set forth on the cover page of this prospectus, and the issuance of 46,132 shares of Common Stock upon the automatic conversion of convertible promissory notes, assuming a public offering price of $11.00 per share, which is the midpoint of the range set forth on the cover page of this prospectus. This number excludes:
| ● | 129,984 shares of Common Stock issuable upon the exercise of warrants outstanding as of the date of this prospectus, with an exercise price of $8.40 per share; |
| ● | 206,862 shares of Common Stock issuable upon the exercise of options to directors, employees and consultants under our 2018 equity incentive plan (the “Equity Incentive Plan”) at a weighted average exercise price of $3.37, of which 119,042 vested as of the date of this prospectus; and |
| ● | 76,730 shares of Common Stock reserved for future issuance under our Equity Incentive Plan. |
Unless otherwise indicated, all information in this prospectus assumes and gives effect to:
| ● | no exercise of the underwriter’s over-allotment option; |
| ● | no exercise of the representative’s warrants; |
| ● | automatic conversion of convertible promissory notes issued in a series of convertible loan agreements into an aggregate of 46,132 shares of Common Stock, assuming an offering price of $11.00, which is the midpoint of the price range set forth on the cover page of this prospectus, which will occur immediately prior to the closing of this offering; | |
| ● | an initial public offering price of $11.00 per share, which is the midpoint of the range set forth on the cover page of this prospectus; and |
| ● | a one-for-seven reverse stock split effected on February 23, 2021. |
| 12 |
Summary Consolidated Financial Data
The following table summarizes our consolidated financial data as of, and for the periods ended on, the dates indicated. We have derived the following consolidated statements of operations data for the years ended December 31, 2020 and 2019 from our audited consolidated financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. The following summary consolidated financial data should be read in conjunction with our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus. Our consolidated financial statements are prepared and presented in accordance with U.S. generally accepted accounting principles (“GAAP”).
| Year ended | ||||||||
| U.S. dollars, except share and per share data | December 31 | |||||||
| 2020 | 2019 | |||||||
| Revenues from sales of products | 232,274 | 175,823 | ||||||
| Cost of sales | (43,405 | ) | (144,548 | ) | ||||
| Gross profit | 188,869 | 31,275 | ||||||
| Research and development expenses | (417,000 | ) | (615,623 | ) | ||||
| Selling and marketing expenses | (51,105 | ) | (342,058 | ) | ||||
| General and administrative expenses | (1,070,109 | ) | (1,004,899 | ) | ||||
| Operating loss | (1,349,345 | ) | (1,931,305 | ) | ||||
| Financing expenses, net | (270,393 | ) | (43,408 | ) | ||||
| Other expenses, net | (2,532 | ) | - | |||||
| Share in losses of affiliated company | - | (15,690 | ) | |||||
| Gain on disposal of affiliated company | 15,690 | - | ||||||
| Net loss | (1,606,580 | ) | (1,990,403 | ) | ||||
| Less: Net loss attributable to non-controlling interests | 13,441 | 18,986 | ||||||
| Net loss attributable to the Company | (1,593,139 | ) | (1,971,417 | ) | ||||
| Loss per share (basic and diluted) | (1.05 | ) | (1.38 | ) | ||||
| Basic and diluted weighted average number of shares of Common Stock outstanding | 1,519,122 | 1,424,045 | ||||||
| As of December 31, 2020 | ||||||||||||
| U.S. dollars | Actual | Pro Forma (1) | Pro
Forma As Adjusted (2) | |||||||||
| Consolidated Balance Sheet Data: | ||||||||||||
| Cash and cash equivalents | 242,900 | 516,900 | 11,231,900 | |||||||||
| Restricted cash | 22,395 | 22,395 | 22,395 | |||||||||
| Total assets | 687,649 | 961,649 | 11,676,649 | |||||||||
| Additional paid-in capital | 11,867,585 | 12,366,580 | 23,081,471 | |||||||||
| Accumulated deficit | (12,277,647 | ) | (12,244,498 | ) | (12,244,498 | ) | ||||||
| Total stockholders’ equity (deficit) | (465,453 | ) | 66,696 | 10,781,696 | ||||||||
| (1) | The pro forma data gives effect to (a) our receipt during January 2021 of aggregate of proceeds of $274,000 upon the issuance of convertible promissory notes in the aggregate principal amount of $274,000 issued in a series of convertible loan agreements; and (b) the automatic conversion of convertible promissory notes in the aggregate principal amount of $499,000, issued in a series of convertible loan agreements into an aggregate of 46,132 shares of Common Stock, assuming an offering price of $11.00, which is the midpoint of the price range set forth on the cover page of this prospectus, which will occur immediately prior to the closing of this offering. |
| (2) | The pro forma as adjusted balance sheet data give additional effect to the pro forma adjustments and the sale of 1,090,909 shares of Common Stock in this offering at the assumed initial public offering price of $11.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| 13 |
Investing in our securities involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including our financial statements and related notes, before deciding whether to purchase shares of our securities. If any of the following risks is realized, our business, operating results, financial condition and prospects could be materially and adversely affected. In that event, the price of our Common Stock could decline, and you could lose part or all of your investment.
Risks Related to Our Financial Condition and Capital Requirements
We have a history of operating losses and expect to incur additional losses in the future.
We have sustained losses in recent years, which as of December 31, 2020, accumulated to $12.3 million, including an operating net loss of $1.3 million and $1.9 million for the years ended December 31, 2020 and 2019, respectively. We are likely to continue to incur significant net losses for at least the next several years as we continue to pursue our strategy, which is currently focused on research and development. Our losses have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. Any failure to achieve and maintain profitability would continue to have an adverse effect on our stockholders’ equity and working capital and could result in a decline in our share price or cause us to cease operations.
We have not generated significant revenue from the sale of our products and do not believe that our current cash on hand will be sufficient to fund our growth plans or our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern.
Our ability to become profitable depends upon our ability to generate revenue. We have not yet generated any material revenues and we do not know when, or if, we will generate any such revenue. We currently have no sources of recurring revenue and are therefore dependent upon external sources for financing our operations. There can be no assurance that we will succeed in obtaining the necessary financing to continue our operations.
This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our audited financial statements do not include any adjustments that might result from the outcome of the uncertainty regarding our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of equity or debt securities or otherwise. Further reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern. If we cannot continue as a going concern, our investors may lose their entire investment in our Common Stock.
Even if this offering is successful, we expect that we will need to raise significant additional capital, which we may be unable to obtain.
Our capital requirements in connection with our research and development activities and transition to commercial operations have been, and will continue to be significant. We will require additional funds to continue research, development and testing of our technologies and products, to obtain intellectual property protection relating to our technologies when appropriate, and to market our products. There can be no assurance that financing will be available in amounts or on terms acceptable to us, if at all. In either of the aforementioned situations, we may not be able to fully implement its growth plans.
Additional financings that we may require in the future will dilute the percentage ownership interests of our stockholders and may adversely affect our earnings and net book value per share. In addition, we may not be able to secure any such additional financing on terms acceptable to us, if at all. Moreover, if we are unable to obtain such additional capital as discussed above, we will be required to stop our operations, and will resume our activities, only after capital is raised.
| 14 |
Risks Related to Our Business, Industry and Business Operations
Because of our limited operating history, we may not be able to successfully operate our business or execute our business plan.
In September 2018, the Company changed its organizational structure and management team. After reviewing the Company’s then existing strategy and results of operation, as well as examining the market opportunities, the new management team decided to update the Company’s strategy, reduce the marketing and sales of its existing products, and focus the Company’s efforts and financial resources in developing its next generation products. During the years 2019 and 2020, we developed, validated and tested the efficacy of our next generation product – a blend of food acids – on a variety of crops in small and large scale commercial pilots.
Given our limited operating history, it is hard to evaluate our proposed business and prospects. Our proposed business operations will be subject to numerous risks, uncertainties, expenses and difficulties associated with early-stage enterprises. Such risks include, but are not limited to, the following:
| ● | the absence of a lengthy operating history; | |
| ● | insufficient capital to fully realize our operating plan; | |
| ● | expected continual losses for the foreseeable future; | |
| ● | operating in multiple currencies; | |
| ● | our ability to anticipate and adapt to a developing market(s); | |
| ● | acceptance of our products by the pre- and post-harvest industry players and consumers; | |
| ● | limited marketing experience; | |
| ● | a competitive environment characterized by well-established and well-capitalized competitors; | |
| ● | the ability to identify, attract and retain qualified personnel; and | |
| ● | operating in an environment that is highly regulated by a number of agencies. |
Because we are subject to these risks, evaluating our business may be difficult, our business strategy may be unsuccessful and we may be unable to address such risks in a cost-effective manner, if at all. If we are unable to successfully address these risks our business could be harmed.
Our products and technology require additional trials.
The efficacy of our products has only been shown in the limited number of pathogens tested on certain produce and aforementioned climates, and therefore our products have yet to be proven against certain additional and relevant pathogens, produce and market climates to validate the efficacy and benefits of our products. However, due to COVID-19, and the current restrictions on travels, we may delay or postpone certain of our planned trials.
The commercial success of our new generation products, as well as any future products, depends upon the degree of market acceptance by the packing house community as well as by other prospect markets and industries.
In order to achieve high volume sales and attain a leading market share and become the new standard of treatment, our products must not only be approved by the regulators, but also endorsed by the major packing houses and service providers, retailers of fruits and vegetables as well as environmental organizations. Our success depends on our ability to create significant value to the growers, the packing houses and the food retailers. We are aware of this key factor and are focusing on conducting large scale trials with major fruits and vegetables packers and retail suppliers of fresh consumed goods in several countries, in order to show the efficacy of the products and our technology, and to receive the recognition of packers and retailers. However, there can be no assurances that we will succeed in such an endeavor, nor is it clear how long it will take until we receive market recognition.
| 15 |
There can be no assurance that any product that we bring to the market will gain market acceptance by prospective customers. The commercial success of our new generation products and any future product depends in part on the packing house community as well as other industries for various use cases, depending on the acceptance by such industries of our technology as a useful and cost-effective solution compared to current solutions. If our new generation products or any future product does not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of our products will depend on a number of factors, including:
| ● | the results of our large-scale trials; | |
| ● | the cost, safety, efficacy, and convenience of our new generation products; | |
| ● | the acceptance of our products as a superior solution in the fresh produce industry; | |
| ● | the ability of third parties to enter into relationships with us without violating their existing agreements; | |
| ● | the effectiveness of our selling and marketing efforts; | |
| ● | the strength of marketing and distribution support for, and timing of market introduction of, competing products; and | |
| ● | publicity concerning our products or competing products. |
Our efforts to penetrate the packing house industry and educate the marketplace on the benefits of our products may require significant resources and may never be successful.
The COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease, may materially and adversely affect our business and operations.
The outbreak of COVID-19, which originated in Wuhan, China, in late 2019, has since spread across the globe, including the United States, Israel and many European countries in which we operate. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. While COVID-19 is still spreading and the final implications of the pandemic are difficult to estimate at this stage, it is clear that it has affected the lives of a large portion of the global population. At this time, the pandemic has caused states of emergency to be declared in various countries, travel restrictions imposed globally, quarantines established in certain jurisdictions and various institutions and companies being closed. We are actively monitoring the pandemic and we are taking all necessary measures to respond to the situation in cooperation with the various stakeholders.
A COVID-19 infection outbreak among our workforce could result in a temporary or long-term disruption in our business activities, including manufacturing and other functions.
Based on guidelines provided by the Israeli Government, employers (including us) are required to prepare and increase as much as possible the capacity and arrangement for employees to work remotely. In that regard, and in compliance with all applicable Israeli rules and guidelines, our offices have remained closed since the middle of March 2020, and all of our employees currently work remotely. In addition, some of our employees, including our Chief Technology Officer, are currently on a temporary leave without pay (furlough) and we have postpend some of our planned field tests due to the current restriction on international travels.
| 16 |
We may face significant competition from other companies looking to develop or acquire new alternative environmentally friendly solutions for the treatment of fruits and vegetables, and other edible matter.
We expect to face significant competition in every aspect of our business, and particularly from other companies that seek to enter our focal market. As regulators continue to move away from current residue chemical solutions, such as chlorpropham or CIPC, existing suppliers of these solutions are continually looking to develop or acquire new alternative environment-friendly solutions that can sustain their market share and revenue streams, or to enable the continuance of CIPC at current levels in new ways of treatment. Additionally, as market opportunity becomes eminent, competitors and new players will most likely attempt to develop similar or comparable solutions. It is possible that superior or more cost-effective alternative technology will emerge that will achieve greater market acceptance and render our products less competitive. Furthermore, existing vendors can cooperate to combat new players by reducing market prices and margins or other competitive initiatives. Our future success will therefore depend, to a large extent, upon our ability to achieve market acceptance of our innovative solutions as well as develop and introduce new products and enhancements to existing products. No assurance can be given that we will be able to compete in such a marketplace.
The market for post-harvest solutions is fragmented with various regional suppliers. The market of post-harvest treatments for fruits and vegetables is dominated by five large players with wide reach across the globe. We believe that the principal factors of competition in our industry include reputation, product quality, customer service and customer intimacy, product innovation, technical service and value creation.
Our success is dependent upon the acceptance of our environmentally friendly solutions for fruits and vegetables.
Our future success is dependent upon the acceptance of our environmentally friendly, non-toxicresidual treatment solutions for fruits and vegetables. While the market is signaling that such a direction is likely, certain trends as well as the future size of this market, and other potential markets for our products, rely upon a number of factors, many of which are beyond our control. For example, both the failure to convince retailers to bear additional costs for “green” fruit and vegetables as well as the failure to persuade consumers to purchase “green” fruits and vegetables for higher prices may adversely affect our business, financial condition, operating results and cash flow going forward.
We may be unable to respond effectively to technological changes in our industry, which could reduce the demand for our products.
Our future business success will depend upon our ability to maintain and enhance our technological capabilities and develop and market products, services and applications that meet changing customer needs and market conditions in a cost-effective and timely manner. Maintaining and enhancing technological capabilities and developing new products may also require significant investments in research and development. We may not be successful in developing new products, services and technology that successfully compete or be able to anticipate changing customer needs and preferences, and our customers may not accept one or more of our new products or services. If we fail to keep pace with evolving technological innovations or fail to modify our products and services in response to customers’ needs or preferences, then our business, financial condition and results of operations could be adversely affected.
We currently rely on a limited number of suppliers to produce certain key components of our products.
We rely on unaffiliated contract manufacturers to produce certain key components of our products. In Israel, we are working with a well-known producer of chemicals, SasaTech, who is responsible for the production of our products. SasaTech is well known for its knowledge and handling of hydrogen peroxide. In the United States, we have worked for the past few years with Seeler Industries, a national leader in the marketing and handling of hydrogen peroxide. There is limited available manufacturing capacity that meets our quality standards and regulatory requirements, especially for the manufacturing of the SF3H and SF3HS with one of their active ingredient – hydrogen peroxide – as well as for FreshProtect with one of its active ingredient – PO3. If we are unable to arrange for sufficient production capacity among our contract manufacturers or if our contract manufacturers encounter production, quality, financial, or other difficulties, including labor or geopolitical disturbances, we may encounter difficulty in meeting customer demands as we seek alternative sources of supply, or we may have to make financial accommodations to such contract manufacturers or otherwise take steps to mitigate supply disruption. We may be unable to locate an additional or alternate contract manufacturer that meets our quality controls and standards and regulatory requirements in a timely manner or on commercially reasonable terms. Any such difficulties could have an adverse effect on our business, financial condition and results of operations, which could be material.
| 17 |
If we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with third parties to perform these services, we may not be successful in commercializing our products.
We have a limited selling and marketing infrastructure and have limited experience in the sale, marketing or distribution of products. To achieve commercial success for any product for which we have obtained marketing approval, we will need to enter into collaborations with third parties like post-harvest service companies and establish a selling and marketing infrastructure or to out-license our products.
In the future, we may consider building a focused selling and marketing infrastructure to market our products in the United States or elsewhere in the world. There are risks involved with establishing our own sales, marketing and distribution capabilities. For example, recruiting and training a sales force could be expensive and time consuming and could delay any product launch. This may be costly, and our investment would be lost if we cannot retain or reposition our selling and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| ● | our inability to recruit, train and retain adequate numbers of effective selling and marketing personnel; | |
| ● | the inability of sales personnel to obtain access to potential customers; | |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and | |
| ● | unforeseen costs and expenses associated with creating an independent selling and marketing organization. |
If we are unable to establish our own sales, marketing and distribution capabilities or enter into successful arrangements with third parties to perform these services, our revenues and our profitability may be materially adversely affected.
In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our products in our target markets, including first the United States, Mexico, Spain, Italy and Israel, or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We rely on rapidly establishing global distributorship network in order to effectively market our products.
We have developed initial partnerships with local partners. In order to expand selling and marketing globally, and capture leading market share before any potential reaction from the competitors, we will need to rapidly expand geographically and establish a global distribution network. This is likely to put pressure on our management, financial and operational resources. In order to mitigate this factor, once we establish a significant presence in the market, we will proceed to establish strategic partnerships with some of the leading players in the market; however, there are no assurances that we will succeed in establishing such partnerships, which may harm the marketing of our products and the development of our business.
| 18 |
The results of our early tests may not be indicative of results in future tests and we cannot assure you that any planned or future tests will lead to results sufficient for the necessary regulatory approvals.
Our products have been tested in multiple commercial and small-scale pilots on certain types of produce and during specific time of the year. We are currently in the development and optimization phases of these products. Results from our later-stage commercial tests may show lower efficacy than our early-tests conducted previously and we cannot guarantee that when commercialized, our products will be effective and stable and product improvements as well as possible changes in the application and usage protocol may be required. These factors may significantly delay receipts of regulatory approvals, and the introduction of our products into the market. Likewise, we cannot be sure these products will be commercially viable, and have no assurances that we will be able to expand upon our current product offerings or that any such expansion will generate revenue.
Our products are highly regulated by governmental agencies in the countries where we conduct business and into which we plan to expand. Our failure to obtain regulatory approvals and registration, to comply with registration and regulatory requirements or to maintain regulatory approvals would have an adverse impact on our ability to market and sell our products.
Some of our products are subject to technical review and approval by government authorities in each country where we currently conduct our business and where we intend to sell our products.
The regulatory requirements to which we are subject are complex and vary from country to country. To obtain new registrations, it is necessary to have a local registrant, and to understand the country’s regulatory requirements, both at the time an application for registration is submitted and when the registration decision is made, which may be several years later. A significant investment in registration data is required (covering all aspects from manufacturing specifications through storage and transport, use, and, finally, disposal of unwanted product and used containers) to ensure that product performance (e.g. bio efficacy), intrinsic hazards and use patterns are fully characterized. Risk assessments are conducted by government regulatory authorities who make the final decision on whether the documented risk associated with a product and active ingredient is acceptable prior to granting approval for sale. This process may be prolonged due to requirements for additional data or internal administrative processes. There is a risk that registration of a new product may not be obtained or that a product label may be severely reduced, restricting the use of the product. If these circumstances arise, there is a risk that the substantial investments made in product development will generate the projected sales that justified the investment, and our business, financial condition and results of operations may be adversely affected by failure to obtain new registrations.
Products that are already approved may be subject to periodic review by regulatory authorities in many countries. Such reviews frequently require the provision of new data and more complex risk assessments. The outcome of such reviews of existing registrations cannot be guaranteed and registrations may be modified or canceled. Since all government regulatory authorities have the right to review existing registrations at any time, the sustainability of the existing portfolio cannot be guaranteed. Existing registrations may be lost at any time, resulting in an immediate impact on sales. Furthermore, prior to expiration, it is necessary to renew registrations. The renewal period and processes vary by country and may require additional studies to support the renewal process. Failure to comply could result in cancellation of the registration, resulting in an impact on sales.
In addition, new laws and regulations may be introduced, or existing laws and regulations may be changed or may become subject to new interpretations, which could result in additional compliance costs, seizures, confiscations, recalls, monetary fines or delays that could affect us or our customers.
In the United States, to complete the registration process of our sanitizers, we will need to submit a number of studies in the form of a registration application or dossier, which has not yet been submitted to the United States Environmental Protection Agency (the “EPA”). In addition, applicable rules and regulations in California require registration of processing aids with the California Department of Pesticide Regulation (the “CDPR”). On July 31, 2020 we submitted an “Application for Registration of Adjuvant” for our SavePROTECT processing aid to the CDPR. We anticipate registration of our SavePROTECT during the second half of 2021, however, there can be no assurance that we will successfully complete any required studies or that we will obtain such registration.
Our success is dependent upon our ability to achieve regulatory approvals and registration in the United States and abroad (Mexico, Israel, Spain and Italy), which might take longer periods than expected.
We are subject to extensive national, state and local government regulation. A critical key to our success and ability to expand our business is our ability to obtain regulatory approvals and registration in the United States and in other countries for the use of our products. The regulatory approvals of some of our products are dependent on trials to show the efficacy and the non-toxicity of our products, and are time and cost consuming. We do not anticipate any significant problems in obtaining future required licenses, permits or approvals that are necessary to expand our business, however such registration filling might take longer period than expected due to various factors including the recent disruptions in regular services as a result of COVID-19, and it might delay obtaining such regulatory approvals, or might cause delays in starting operations on a large scale in these countries and other jurisdictions.
| 19 |
The inherent dangers in production and transportation of hydrogen peroxide and highly concentrated organic acids could cause disruptions and could expose us to potentially significant losses, costs or other liabilities.
Our operations are subject to significant hazards and risks inherent to the transportation of the active ingredient of one of our products – hydrogen peroxide. In high concentrations, our blend of acids has a very low pH which may lead to skin burn and hydrogen peroxide is an aggressive oxidizer and both can corrode many materials. We are working with limited low concentration of the material, however in high concentrations of H2O2 it will react violently. Hydrogen peroxide should be stored in a cool, dry, well-ventilated area and away from any flammable or combustible substances. It should be transported in special tanks and vehicles and should be stored in a container composed of non-reactive materials. These hazards and risks include, but are not limited to fires, explosions, third-party interference (including terrorism) and mechanical failure of equipment at our or third-party facilities. The occurrence of any of these events could result in production and distribution difficulties and disruptions, personal injury or wrongful death claims and other damage to properties.
Our business and operations may be affected by climate change conditions, which could materially harm our financial results.
Our business may be affected from changes in climate conditions as such events would affect the crops and their storability in those cases where there is unusually warm, dry, humid or cold weather before cropping.
In such instances, we may suffer a decrease in revenues as a result of a smaller storage volume of rooms or shorter storage period. We anticipate that once we increase our operations, and enter certain markets which experience or will experience significant climate change, such as above-common rain fall, heat waves, dry air conditions, and unusually cold or prolonged cold weather conditions, such events may materially impact our financial results.
Conditions in the global economy may adversely affect our business, financial condition and results of operation.
Although demand for fresh horticultural products is considered inelastic in developed economies, the fresh produce and citrus industries that we sell to may be affected by material changes in supply, market prices, exchange rates and general economic conditions. Delays or reductions in our customers’ purchasing or shifts to lower-cost alternatives that result from tighter economic market conditions would reduce demand for our products and services and could, consequently, have a material adverse effect on our business, financial condition and results of operations.
Our relationship with our employees could deteriorate, and certain key employees could leave, which could adversely affect our business and results of operations.
Our business involves complex operations and demands a management team to determine and implement our strategy and workforce that is knowledgeable and has expertise in many areas necessary for our operations. As a company focused on research and development in the highly-specialized horticultural post-harvest field, we rely on our ability to attract and retain skilled employees, consultants and contractors, including our specialized research and development. As of April 1, 2021, we employed two full-time employees and four part-time employees, including the employees employed by our subsidiary, Save Foods Ltd. The departure of highly skilled employees, consultants or contractors or one or more employees who hold key regional management positions could have an adverse impact on our operations, including customers choosing to follow a such regional manager to one of our competitors.
In addition, to execute our growth plan we must attract and retain highly qualified personnel. Competition for these employees exists; new members of management must have significant industry expertise when they join us or engage in significant training which, in many cases, requires significant time before they achieve full productivity. If we fail to attract, train, retain, and motivate our key personnel, our business and growth prospects could be severely harmed.
Furthermore, we are dependent upon the managers to oversee our operations. Thus, there can be no assurance that the managers’ experience will be sufficient to successfully achieve our business objectives. All decisions regarding the management of our affairs will be made exclusively by our officers and directors. In the event these persons are ineffective, our business and results of operation would likely be adversely affected.
| 20 |
We are subject to risks relating to portfolio concentration.
Our business is highly dependent on a small number of products, which are based on our main active ingredients. Our core post-harvest business includes solutions designed to improve the yields of the packing house but mainly ensure food safety and assisting packing houses to meet the new FSMA requirements. Our ability to market and sell products containing our active ingredient to key service providers for treatment in post-harvest food safety industry in order to utilize their market position is important to our future success.
Our operating results may fluctuate, which makes our results difficult to predict and could cause our results to fall short of expectations.
Our operating results may fluctuate as a result of a number of factors, many of which are outside of our control. As a result, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our quarterly, year-to-date and annual expenses as a percentage of our revenues may differ significantly from our historical or projected rates. Our operating results in future quarters may fall below expectations. Any of these events could cause our stock price to fall. Each of the following factors, among the other risks described herein, may affect our operating results:
| ● | our ability to penetrate the packing house industry with our products; | |
| ● | our ability to generate revenue from our products; | |
| ● | the amount and timing, of operating costs and capital expenditures related to the maintenance and expansion of our businesses, and operations; | |
| ● | our focus on long-term goals over short-term results; | |
| ● | the global economic situation; and | |
| ● | fluctuations in weather conditions. |
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Mexico or Israel.
Other than our headquarters and other operations which are located in Israel (as further described below), we currently have limited international operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of our product candidates. We also plan to retain sales representatives and third-party distributors, outside of the United States and Israel at a later date. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits, and licenses; | |
| ● | failure by us to obtain regulatory approvals for the use of our product candidates in various countries; | |
| ● | additional potentially relevant third-party patent or other intellectual property rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| 21 |
| ● | natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, as amended (the “FCPA”) its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
Our business depends to some extent on international transactions.
As a result of the international nature of our business, we are exposed to risks associated with changes in foreign currency exchange rates. A majority of our revenues and substantially all of our cost of sales are in USD, whilst our management, marketing, sales and R&D costs are in NIS. We are therefore exposed to foreign currency risk due to fluctuations in exchange rates. This may result in gains or losses with respect to movements in exchange rates, which may be significant and may also cause fluctuations in reported financial information that are not necessarily related to our operating results.
Risks Related to Intellectual Property
If we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, our ability to compete will be harmed.
Our commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection for the proprietary blend used in our products and our manufacturing process, as well as continuing to develop and secure trade secrets. We might in the future opt to license intellectual property from other parties. If we, or the other parties from whom we may license intellectual property, fail to obtain and maintain adequate patent or other intellectual property protection for intellectual property used in our products, or if any protection is reduced or eliminated, others could use the intellectual property used in our products, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise ways of making competitive products without infringing any patents that we own or have rights to.
U.S. patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, re-examination and opposition proceedings may be costly and time consuming, and we, or the other parties from whom we might potentially license intellectual property, may be unsuccessful in defending against such proceedings. Thus, any patents that we own or might license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. In addition, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States.
| 22 |
If we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unprotected know-how, our ability to compete will be harmed.
Proprietary trade secrets, copyrights, trademarks and unprotected know-how are also very important to our business. We rely on a combination of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable. We require our office holders, employees, consultants and distributers of our products and most third parties to execute confidentiality agreements in connection with their relationships with us. However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless, arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. Our office holders, employees, consultants and other advisors may unintentionally or willfully disclose our confidential information to competitors. In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized disclosure. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our products that we consider proprietary. As a result, other parties may be able to use our proprietary technology or information, and our ability to compete in the market would be harmed.
We could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of a patent litigation action is often uncertain. No assurance can be given that patents containing claims covering our products, parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Furthermore, our competitors or other parties may assert that our products and the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which may result in issued patents which our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications with claims that we infringe. There could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number of competitors in the post-harvest market grows, and as the number of patents issued grows, the possibility of patent infringement claims against us increases.
Infringement actions and other intellectual property claims and proceedings brought against or by us, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation. Some of our competitors may be able to sustain the costs of complex patent or intellectual property litigation more effectively than we can because they have substantially greater resources.
We cannot be certain that we will successfully defend against allegations of infringement of patents and intellectual property rights of others. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other party’s patents or other intellectual property were upheld as valid and enforceable and we were found to infringe the other party’s patents or violate the terms of a license to which we are a party, we could be required to pay damages. We could also be prevented from selling our products unless we could obtain a license to use technology or processes covered by such patents or will be able to redesign the product to avoid infringement. A license may not be available at all or on commercially reasonable terms or we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct clinical trials and to revise our filings with the applicable regulatory bodies, which would be time consuming and expensive. In these circumstances, we may be unable to sell our products at competitive prices or at all, our business and operating results could be harmed.
| 23 |
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators, or other third parties have an ownership interest in our patents or other intellectual property. Ownership disputes may arise in the future, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may experience claims that our products infringe the intellectual property rights of others, which may cause us to incur unexpected costs or prevent us from selling our products or services.
We continually seek to improve our business processes and develop new products and applications in a crowded patent space that we must continually monitor to avoid infringement. We cannot guarantee that we will not experience claims that our processes and products infringe issued patents (whether present or future) or other intellectual property rights belonging to others.
From time to time, we oppose patent applications that we consider overbroad or otherwise invalid in order to maintain the ability to operate freely in our various business lines without the risk of being sued for patent infringement. If, however, patents are subsequently issued on any such applications by other parties, or if patents belonging to others already exist that cover our products, processes or technologies, we could experience claims for infringement or have to take other remedial or curative actions to continue our manufacturing and sales activities with respect to one or more products. Likewise, our competitors may also already hold or have applied for patents in the United States or abroad that, if enforced or issued, could prevail over our patent rights or otherwise limit our ability to manufacture or sell one or more of our products in the United States or abroad. Any actions asserted against us could include payment of damages for infringement, stopping the use, require that we obtain licenses from these parties or substantially re-engineer our products or processes in order to avoid infringement. We may not be able to obtain the necessary licenses on acceptable terms, or at all, or be able to re-engineer our products successfully. Further, intellectual property litigation is expensive and time-consuming, regardless of the merits of any claim, and could divert our management’s attention from operating our business.
Risks Related to Regulatory Compliance
If we or our contractors or service providers fail to comply with laws and regulations, we or they could be subject to regulatory actions, which could affect our ability to develop, market and sell our products or future products that we may develop and may harm our reputation in our industry.
If we or our manufacturers or other third-party contractors fail to comply with applicable federal, state or foreign laws or regulations, including with respect to food treatment, we could be subject to regulatory actions, which could affect our ability to develop, market and sell our current products or any future products which we may develop in the future and could harm our reputation and lead to reduced demand for or non-acceptance of our proposed products by the market.
Regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in the United States, Mexico, Israel or other countries in which we operate, that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of our products, including in the food health industry. In addition, regulations and guidance may often be revised or reinterpreted by the regulatory authorities in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or interpretations changed, and what the impact of such changes, if any, may be.
| 24 |
Risks Related to Our Operations in Israel
Conditions in Israel may limit our ability to manage and market our products, which would lead to a decrease in revenues.
Because most of our operations is conducted in Israel and our management is located in Israel, our operations are directly affected by economic, political, geopolitical and military conditions affecting Israel. Accordingly, political, economic and military conditions in Israel directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its neighboring countries. These conflicts involved missile strikes against civilian targets in various parts of Israel including most recently, central Israel, and negatively affected business conditions in Israel as well as home starts and the building industry in Israel.
Our facilities are in range of rockets that may be fired from Lebanon, Syria or the Gaza Strip into Israel. In the event that our facilities are damaged as a result of hostile action or hostilities otherwise disrupt the ongoing operation of our facilities, our ability to deliver products to customers could be materially and adversely affected. Our commercial insurance in Israel does not cover losses that may occur as a result of acts of war; however, losses as a result of terrorist attacks to our facilities and disruption to the ongoing operations, are covered by our insurance for damages of up to $40 million, if such damages are not covered by the Israeli government. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained and will be adequate in the event we submit a claim. Even if insurance is maintained and adequate, we cannot assure you that it will reduce or prevent any losses that may occur as a result of such actions or will be exercised in a timely manner to meet our contractual obligations with customers and vendors.
In addition, popular uprisings in various countries in the Middle East and North Africa have affected the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries, such as Turkey, from which we import a significant amount of our raw materials. Moreover, some countries around the world restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. These restrictions may limit materially our ability to obtain raw materials from these countries or sell our products to companies and customers in these countries. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods. Such efforts, particularly if they become more widespread, may materially and adversely impact our ability to sell our products out of Israel.
Our employees in Israel, generally males, including executive officers, may be called upon to perform military service on an annual basis until they reach the age of 40 (and in some cases, up to 45 or 49). In emergency circumstances, they could be called to immediate and prolonged active duty. Our operations could be disrupted by the absence of a significant number of our employees related to military service or the absence for extended periods of one or more of our key employees for military service. Such a disruption could materially and adversely affect our business and results of operations. Additionally, the absence of a significant number of the employees of our Israeli suppliers and contract manufacturers related to military service may disrupt their operations, in which event our ability to deliver products to customers may be materially and adversely affected.
Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or a significant downturn in the economic or financial condition of Israel, could materially and adversely affect our operations and product development, cause our revenues to decrease and materially harm the share price of publicly traded companies with operations in Israel, such as us.
In early January 2020, certain events contributed to an increase in hostilities between the United States and Iran, and as a result Iran issued multiple public statements threatening to attack Israel and the United States. These events, coupled with the already mounting tensions between Israel and Iran, may threaten to destabilize the Middle East, the result of which may impact our ability to conduct our business effectively.
| 25 |
The Israeli Parliament (the “Knesset”), for reasons related to political instability, has failed to pass a budget for the year 2020, and certain government ministries, which may be critical to the operation of our business, are without necessary resources and may not receive sufficient funding moving forward. Given the likelihood that the current political stalemate might not be resolved during the next calendar year, our ability to conduct our business effectively may be adversely materially affected.
We may not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products.
We have non-competition agreements with all of our employees, all of which are governed by Israeli law. These agreements prohibit our employees from competing with or working for our competitors, generally during their employment and for up to 12 months after termination of their employment. However, Israeli courts are reluctant to enforce non-compete undertakings of former employees and tend, if at all, to enforce those provisions for relatively brief periods of time in restricted geographical areas, and only when the employee has obtained unique value to the employer specific to that employer’s business and not just regarding the professional development of the employee. If we are not able to enforce non-compete covenants, we may be faced with added competition.
It may be difficult to acquire jurisdiction and enforce liabilities against our officers and directors who are based in Israel.
The majority of our officers and present directors reside outside of the United States and most of our operations at the date of this prospectus are located outside the United States. As a result, it may not be possible for United States investors to enforce their legal rights, to effect service of process upon our directors or officers or to enforce judgments of United States courts predicated upon civil liabilities and criminal penalties of our directors and officers under federal securities laws. Moreover, we have been advised that Israel does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States. Further, it is unclear if extradition treaties now in effect between the United States and Israel would permit effective enforcement of criminal penalties of the federal securities laws.
Risks Related to this Offering and Ownership of Our Common Stock
Even if we meet the initial listing requirements of the Nasdaq Capital Market, there can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq Capital Market. Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a de-listing of our Common Stock.
Even if we meet the initial listing requirements of the Nasdaq Capital Market, we cannot assure you that we will be able to comply with the other standards that we are required to meet in order to maintain a listing of our common stock on the Nasdaq Capital Market. If after listing we fail to satisfy the continued listing requirements of the Nasdaq Capital Market, such as the corporate governance requirements or the minimum stockholder’s equity requirement, the Nasdaq Capital Market may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our Common Stock and would impair our shareholders’ ability to sell or purchase our Common Stock when they wish to do so. In the event of a de-listing, we would take actions to restore our compliance with the Nasdaq Capital Market’s listing requirements, but we can provide no assurance that any action taken by us would result in our common stock becoming listed again, or that any such action would stabilize the market price or improve the liquidity of our Common Stock.
If you purchase shares of our Common Stock in this offering You will experience immediate and substantial dilution in the book value per share of the common stock you purchase.
The public offering price per share of our Common Stock will be substantially higher than the net tangible book value per share of our Common Stock immediately prior to the offering. After giving effect to the sale of shares of our Common Stock at a public offering price of $11.00 per share and after deducting the estimated underwriting discount and estimated offering expenses payable by us, purchasers of our Common Stock in this offering will incur immediate dilution of $7.2 per share in the net tangible book value of the Common Stock they acquire. For a further description of the dilution that investors in this offering will experience, see “Dilution.”
In addition, to the extent that outstanding stock options have been or may be exercised or other shares issued, you may experience further dilution.
The market price of our Common Stock may be highly volatile.
The market price of our Common Stock is likely to be volatile. Our Common Stock price could be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | reports of adverse events with respect to the commercialization and distribution of our products; | |
| ● | inability to obtain additional funding; |
| 26 |
| ● | any delay in filing a regulatory submission for any of our products and any adverse development or perceived adverse development with respect to the review of that regulatory submission by the EPA, the FDA or other regulatory authority; | |
| ● | failure to successfully develop and commercialize our products; | |
| ● | failure to enter into strategic collaborations; | |
| ● | failure by us or strategic collaboration partners to prosecute, maintain or enforce our intellectual property rights; | |
| ● | changes in laws or regulations applicable to future products; | |
| ● | inability to scale up our manufacturing capabilities through third-party manufacturers, inability to obtain adequate product supply for our products or the inability to do so at acceptable prices; | |
| ● | introduction of new products or technologies by our competitors; | |
| ● | failure to meet or exceed financial projections we may provide to the public; | |
| ● | failure to meet or exceed the financial expectations of the investment community; | |
| ● | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by our competitors; | |
| ● | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our platform technologies, technologies, products or product candidates; | |
| ● | additions or departures of key scientific or management personnel; | |
| ● | significant lawsuits, including patent or stockholder litigation; | |
| ● | changes in the market valuations of similar companies; | |
| ● | sales of our securities by us or our stockholders in the future; and | |
| ● | trading volumes of our securities. |
In addition, companies trading in the stock market have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our Common Stock, regardless of our actual operating performance.
Sales of a substantial number of shares of our Common Stock in the public market by our existing stockholders could cause our share price to fall.
Sales of a substantial number of shares of our Common Stock in the public market, or the perception that these sales might occur, could depress the market price of our Common Stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our Common Stock.
| 27 |
Our principal stockholders, officers and directors beneficially own approximately 39.5% of our outstanding shares of Common Stock. They will therefore be able to exert significant control over matters submitted to our stockholders for approval.
As of April 6, 2021, our principal stockholders, officers and directors beneficially own approximately 39.5% of our outstanding Common Stock. This significant concentration of share ownership may adversely affect the trading price for our Common Stock because investors often perceive disadvantages in owning shares in companies with controlling stockholders. As a result, these stockholders, if they acted together, could significantly influence matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these stockholders may not always coincide with our interests or the interests of other stockholders.
Delaware law and provisions in our amended and restated certificate of incorporation and amended and restated bylaws could make a merger, tender offer or proxy contest difficult, thereby depressing the market price of our Common Stock.
Our status as a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, even if a change of control would be beneficial to our existing stockholders. In addition, our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that may make the acquisition of the Company more difficult, including the following:
| ● | our stockholders will only be able to take action at a meeting of stockholders and will not be able to take action by written consent for any matter; | |
| ● | our board of directors is classified into three classes of directors with staggered three-year terms; | |
| ● | a special meeting of our stockholders may only be called by a majority of our board of directors; | |
| ● | advance notice procedures apply for stockholders to nominate candidates for election as directors or to bring matters before an annual meeting of stockholders; and | |
| ● | certain litigation against us can only be brought in Delaware. |
These provisions, alone or together, could discourage, delay or prevent a transaction involving a change in control of the Company. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors of their choosing and to cause us to take other corporate actions they desire, any of which, under certain circumstances, could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock, and could also affect the price that some investors are willing to pay for our Common Stock.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our Common Stock, our stock price and trading volume could decline.
The trading market for our Common Stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our shares, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analysts who may cover us were to cease coverage of the Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 28 |
Shares of our Common Stock are an illiquid investment as there is presently a limited market for our Common Stock. We do not know whether a market for our Common Stock will be sustained or what the trading price of our Common Stock will be and as a result it may be difficult for you to sell your shares of Common Stock.
There is presently a limited market for our Common Stock. Although we intend to list our Common Stock on the Nasdaq Stock Market, an active trading market for our Common Stock may not be sustained. It may be difficult for you to sell your shares of Common Stock without depressing the market price for our Common Stock or at all. As a result of these and other factors, you may not be able to sell your shares of Common Stock at or above the offering price or at all. Further, an inactive market may also impair our ability to raise capital by selling our Common Stock and may impair our ability to enter into strategic partnerships or acquire companies, products, or services by using our equity securities as consideration.
We do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends, and we do not anticipate paying cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our Common Stock as a source for any future dividend income. Our board of directors has complete discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiary, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors.
We may need additional capital, and the sale of additional shares or equity or debt securities could result in additional dilution to our stockholders.
We believe that our current cash and cash used in operations, together with the net proceeds from this offering, will be sufficient to meet our anticipated cash needs for the next 24 months . We may, however, require additional cash resources due to changed business conditions or other future developments, including further adverse effects to our business from COVID-19 related issues. If these resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain one or more credit facilities. The sale of additional equity securities could result in additional dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. It is uncertain whether financing will be available in amounts or on terms acceptable to us, if at all.
Management will have broad discretion as to the use of the proceeds from this offering.
Our management will have broad discretion in the allocation of the net proceeds and could use them for purposes other than those contemplated at the time of this offering and as described in the section titled “Use of Proceeds.” You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our securities to decline and delay the development of our product candidates. Pending the application of these funds, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
General Risk Factors
Disruptions to our information technology systems due to cyber-attacks or our failure to upgrade and adjust our information technology systems, may materially impair our operations, hinder our growth and materially and adversely affect our business and results of operations.
We believe that an appropriate information technology (“IT”), infrastructure is important in order to support our daily operations and the growth of our business. If we experience difficulties in implementing new or upgraded information systems or experience significant system failures, or if we are unable to successfully modify our management information systems or respond to changes in our business needs, we may not be able to effectively manage our business, and we may fail to meet our reporting obligations. Additionally, if our current back-up storage arrangements and our disaster recovery plan are not operated as planned, we may not be able to effectively recover our information system in the event of a crisis, which may materially and adversely affect our business and results of operations.
| 29 |
In the current environment, there are numerous and evolving risks to cybersecurity and privacy, including criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error. High-profile security breaches at other companies and in government agencies have increased in recent years, and security industry experts and government officials have warned about the risks of hackers and cyber-attacks targeting businesses such as ours. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclose information or unwittingly provide access to systems or data. We can provide no assurance that our current IT system or any updates or upgrades thereto and the current or future IT systems of our distributors use or may use in the future, are fully protected against third-party intrusions, viruses, hacker attacks, information or data theft or other similar threats. Legislative or regulatory action in these areas is also evolving, and we may be unable to adapt our IT systems or to manage the IT systems of third parties to accommodate these changes. We have experienced and expect to continue to experience actual or attempted cyber-attacks of our IT networks. Although none of these actual or attempted cyber-attacks has had a material adverse impact on our operations or financial condition, we cannot guarantee that any such incidents will not have such an impact in the future.
Failure to comply with anti-bribery, anti-corruption and anti-money laundering laws could subject us to penalties and other adverse consequences.
We are subject to the FCPA and other anticorruption, anti-bribery and anti-money laundering laws in the jurisdictions in which we do business, both domestic and abroad. These laws generally prohibit us and our employees from improperly influencing government officials or commercial parties in order to obtain or retain business, direct business to any person or gain any advantage. The FCPA and other applicable anti-bribery and anti-corruption laws also may hold us liable for acts of corruption and bribery committed by our third-party business partners, representatives and agents. In addition, we leverage third parties to sell our products and conduct our business abroad. We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities and we may be held liable for the corrupt or other illegal activities of these third-party business partners and intermediaries, our employees, representatives, contractors, channel partners and agents, even if we do not explicitly authorize such activities. These laws also require that we keep accurate books and records and maintain internal controls and compliance procedures designed to prevent any such actions. While we have policies and procedures to address compliance with such laws, we cannot assure you that our employees and agents will not take actions in violation of our policies or applicable law, for which we may be ultimately held responsible and our exposure for violating these laws increases as our international presence is established and as we increase sales and operations in foreign jurisdictions. Any violation of the FCPA or other applicable anti-bribery, anti-corruption laws and anti-money laundering laws could result in whistleblower complaints, adverse media coverage, investigations, imposition of significant legal fees, loss of export privileges, severe criminal or civil sanctions or suspension or debarment from U.S. government contracts, substantial diversion of management’s attention, a decline in the market price of our Common Stock or overall adverse consequences to our reputation and business, all of which may have an adverse effect on our results of operations and financial condition.
We will incur significant increased costs as a result of the listing of our securities for trading on Nasdaq and our management will be required to devote substantial time to new compliance initiatives as well as compliance with ongoing U.S. requirements.
Upon the listing of securities on Nasdaq, we will become a publicly traded company in the United States. We anticipate that we will incur costs associated with corporate governance requirements of the SEC and Nasdaq, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs and resources, particularly a greater percentage of the time and efforts of our management team will be diverted to these new requirements. Any future changes in the laws and regulations affecting public companies in the United States, including Section 404 and other provisions of the Sarbanes-Oxley Act, and the rules and regulations adopted by the SEC and Nasdaq, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers.
We face risks related to compliance with corporate governance laws and financial reporting standards.
The Sarbanes-Oxley Act of 2002, as well as related new rules and regulations implemented by the SEC and the Public Company Accounting Oversight Board, require changes in the corporate governance practices and financial reporting standards for public companies. These new laws, rules and regulations, including compliance with Section 404 of the Sarbanes-Oxley Act of 2002 relating to internal control over financial reporting, have materially increased the legal and financial compliance costs of small companies and have made some activities more time-consuming and more burdensome.
We may not have effective internal controls.
In connection with Section 404 of the Sarbanes-Oxley Act of 2002, we need to assess the adequacy of our internal controls, remedy any weaknesses that may be identified, validate that controls are functioning as documented and implement a continuous reporting and improvement process for internal controls. We may discover deficiencies that require us to improve our procedures, processes and systems in order to ensure that our internal controls are adequate and effective and that we are in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. If the deficiencies are not adequately addressed, or if we are unable to complete all of our testing and any remediation in time for compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the SEC rules under it, we would be unable to conclude that our internal controls over financial reporting are designed and operating effectively, which could adversely affect investor confidence in our internal controls over financial reporting.
| 30 |
We estimate that the net proceeds from our issuance and sale of 1,090,909 shares of our Common Stock in this offering will be approximately $10,715,000 (or approximately $12,389,000 if the underwriter will exercise its over-allotment option in full), based upon an assumed public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, and after deducting underwriting discounts and commissions and offering expenses payable by us.
A $1.00 increase or decrease in the assumed public offering price of $11.00 per share would increase or decrease the net proceeds from this offering by approximately $1.0 million, assuming that the number of shares of Common Stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 100,000 in the number of shares of Common Stock offered by us would increase or decrease our proceeds by approximately $1.0 million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for:
| ● | approximately $2.6 million for product research and development (which may include acquisition of technology to complement our product portfolio) and purchase of lab equipment; | |
| ● | approximately $1.2 million for gaining regulatory approvals and commercialization; | |
| ● | approximately $2.7 million for selling and marketing; and | |
| ● | the remainder for working capital and general corporate purposes. |
Changing circumstances may cause us to consume capital significantly faster than we currently anticipate. The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our global marketing and sales efforts, with development efforts, economic and political effects of COVID-19 and government responses to it, and the overall economic environment. Therefore, our management will retain broad discretion over the use of the proceeds from this offering. We may ultimately use the proceeds for different purposes than what we currently intend. Pending any ultimate use of any portion of the proceeds from this offering, if the anticipated proceeds will not be sufficient to fund all the proposed purposes, our management will determine the order of priority for using the proceeds, as well as the amount and sources of other funds needed.
Pending our use of the net proceeds from this offering, we may invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
| 31 |
We have never declared or paid any dividends on our Common Stock. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on various factors, including applicable laws, our results of operations, financial condition, future prospects and any other factors deemed relevant by our board of directors.
| 32 |
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2020:
| ● | on an actual basis as of December 31, 2020; | |
| ● | On a pro forma basis to give effect to (a) our receipt during January 2021 of aggregate of proceeds of $274,000 upon the issuance of convertible promissory notes in the aggregate principal amount of $274,000 issued in a series of convertible loan agreements; and (b) the automatic conversion of convertible promissory notes in the aggregate principal amount of $499,000, issued in a series of convertible loan agreements into an aggregate of 46,132 shares of Common Stock, assuming an offering price of $11.00, which is the midpoint of the price range set forth on the cover page of this prospectus, which will occur immediately prior to the closing of this offering; and | |
| ● | on a pro forma as adjusted basis to give further effect to the pro forma adjustments and the issuance and sale of shares of our Common Stock in this offering at an assumed public offering price of $11.00 per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale of the Common Stock had occurred on December 31, 2020. |
The pro forma as adjusted information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
You should read this table in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
The as adjusted information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
You should read this table in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| 33 |
| As of December 31, 2020 | ||||||||||||
| U.S. dollars, except share and per share data | Actual | Pro Forma | Pro
Forma As Adjusted (1) | |||||||||
| Assets | ||||||||||||
| Current Assets | ||||||||||||
| Cash and cash equivalents | 242,900 | 516,900 | 11,231,900 | |||||||||
| Restricted cash | 22,395 | 22,395 | 22,395 | |||||||||
| Accounts receivable, net | 147,941 | 147,941 | 147,941 | |||||||||
| Inventories | 16,356 | 16,356 | 16,356 | |||||||||
| Other current assets | 65,579 | 65,579 | 65,579 | |||||||||
| Total Current assets | 495,171 | 769,171 | 11,484,171 | |||||||||
| Right-of-Use Asset Arising from Operating Lease | 14,700 | 14,700 | 14,700 | |||||||||
| Property and Equipment, Net | 55,194 | 55,194 | 55,194 | |||||||||
| Funds in Respect of Employee Rights Upon Retirement | 122,584 | 122,584 | 122,584 | |||||||||
| Total Assets | 687,649 | 961,649 | 11,676,649 | |||||||||
| Liabilities and Shareholders’ Deficit | ||||||||||||
| Current Liabilities | ||||||||||||
| Short-term loan from banking institution | 7,949 | 7,949 | 7,949 | |||||||||
| Current maturities of convertible loans | 56,250 | - | - | |||||||||
| Accounts payable | 203,323 | 203,323 | 203,323 | |||||||||
| Other accounts liabilities | 517,711 | 517,711 | 517,711 | |||||||||
| Total Current Liabilities | 785,233 | 728,983 | 728,983 | |||||||||
| Fair Value of Convertible Component in Convertible Loans | 54,970 | - | - | |||||||||
| Convertible Loans | 146,929 | - | - | |||||||||
| Long term from Banking Institution | 8,115 | 8,115 | 8,115 | |||||||||
| Liability for Employee Rights Upon Retirement | 157,855 | 157,855 | 157,855 | |||||||||
| Total Liabilities | 1,153,102 | 894,953 | 894,953 | |||||||||
| Stockholders’ Equity (Deficit) | ||||||||||||
| Common stock par value $0.0001 per
share: 495,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 1,606,760 and 1,458,567 shares as of December 31, 2020 and 2019, respectively; and pro forma, as adjusted shares issued and outstanding 2,743,801. | 161 | 166 | 275 | |||||||||
| Preferred stock par value $ 0.0001
per share: 5,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 0 shares as December 31, 2020 and 2019. | - | - | - | |||||||||
| Additional paid-in capital | 11,867,585 | 12,366,580 | 23,081,471 | |||||||||
| Foreign currency translation adjustments | (26,275 | ) | (26,275 | ) | (26,275 | ) | ||||||
| Accumulated deficit | (12,277,647 | ) | (12,244,498 | ) | (12,244,498 | ) | ||||||
| Total Company’s stockholders’ equity | (436,176 | ) | 95,973 | 10,810,973 | ||||||||
| Non-Controlling Interests | (29,277 | ) | (29,277 | ) | (29,277 | ) | ||||||
| Total Stockholders’ Equity (Deficit) | (465,453 | ) | 66,696 | 10,781,696 | ||||||||
| Total Capitalization | 687,649 | 961,649 | 11,676,649 | |||||||||
| (1) | A $1.00 increase or decrease in the assumed public offering price of $11.00 per share of Common Stock would increase or decrease the amount of each of cash and cash equivalents and total stockholders’ equity by approximately $1.0 million, assuming that the number of shares of Common Stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 100,000 in the number of shares of Common Stock offered by us would increase or decrease each of cash and cash equivalents and total stockholders’ equity by approximately $1.0 million, assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and commissions and any estimated offering expenses payable by us. |
The number of shares presented in the table above does not include:
| ● | 129,984 shares of Common Stock issuable upon the exercise of warrants outstanding as of December 31, 2020, with an exercise price of $8.40 per share; | |
| ● | 206,862 shares of Common Stock issuable upon the exercise of options to directors, employees and consultants under our 2018 equity incentive plan (the “Equity Incentive Plan”) at a weighted average exercise price of $3.27, of which 97,351 vested as of December 31, 2020; and | |
| ● | 76,730 shares of Common Stock reserved for future issuance under our Equity Incentive Plan. |
| 34 |
If you invest in our Common Stock, you will experience immediate and substantial dilution to the extent of the difference between the public offering price of our Common Stock and the as adjusted net tangible book value per share of Common Stock immediately after the offering. On December 31, 2020, we had negative net tangible book value of ($465,453), representing a net tangible book value of $0.29 per share of Common Stock. Net tangible book value per share of Common Stock represents the amount of our total tangible assets less our total liabilities, divided by 1,606,760, the total number of Common Stock outstanding on December 31, 2020.
Our pro forma negative net tangible book value as of December 31, 2020 was ($207,304), representing approximately $(0.1) per share of Common Stock. Pro forma net tangible book value per share of common stock represents the amount of our total tangible assets less our total liabilities, divided by 1,652,892, the total number of common stock outstanding on December 31, 2020, and gives effect (a) our receipt during January 2021 of aggregate of proceeds of $274,000 upon the issuance of convertible promissory notes in the aggregate principal amount of $274,000 issued in a series of convertible loan agreements; and (b) the automatic conversion of convertible promissory notes in the aggregate principal amount of $499,000, issued in a series of convertible loan agreements into an aggregate of 46,132 shares of Common Stock, assuming an offering price of $11.00, which is the midpoint of the price range set forth on the cover page of this prospectus, which will occur immediately prior to the closing of this offering.
After giving effect to the sale of the Common Stock offered by us in this offering and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, assuming no exercise of the Underwriter’s over-allotment option, our pro forma as adjusted net tangible book value estimated at December 31, 2020 would have been approximately $10,507,696, representing $3.8 per share of Common Stock. At the assumed public offering price for this offering of $11.00 per share of Common Stock, the midpoint of the estimated price range set forth on the cover page of this prospectus, this represents an immediate increase in historical net tangible book value of $3.9 per share of Common Stock to existing stockholders and an immediate dilution in net tangible book value of $7.2 per share of Common Stock to purchasers of Common Stock in this offering. Dilution for this purpose represents the difference between the price per share of Common Stock paid by these purchasers and as adjusted net tangible book value per share of Common Stock immediately after the completion of this offering.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed public offering price per share, the midpoint of the estimated price range set forth on the cover page of this prospectus | $ | 11 | ||||||
| Pro forma adjusted net tangible book value per share as of December 31, 2020 | $ | (0.1 | ) | |||||
| Increase in pro forma net tangible book value per share attributable to new investors in this offering | $ | 3.9 | ||||||
| Pro forma as adjusted net tangible book value per share after offering | $ | 3.8 | ||||||
| Dilution in pro forma tangible book value per share to new investors | $ | 7.2 |
The dilution information discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering to be determined at pricing. A $1.00 increase or decrease in the assumed initial public offering price of $11.00 per share of Common Stock would increase or decrease our as adjusted net tangible book value per share of Common Stock after this offering by $1,000,000 and the dilution per share of Common Stock to new investors by $7.8, assuming the number of Common Stock offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of Common Stock we are offering.
An increase or decrease of 100,000 in the number of Common Stock offered by us would increase or decrease our as adjusted net tangible book value after this offering by approximately $1.0 million and the as adjusted net tangible book value per share of Common Stock after this offering by $0.4 per share of Common Stock and would increase or decrease the dilution per share of Common Stock to new investors by $0.37, assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and estimated offering expenses payable by us.
The following table summarizes, on an as adjusted basis as of December 31, 2020, the differences between the number of Common Stock acquired from us, the total amount paid and the average price per share of Common Stock paid by the existing holders of our Common Stock and by investors in this offering and based upon an assumed public offering price of $11.00 per share of Common Stock, the midpoint of the estimated price range set forth on the cover page of this prospectus.
| 35 |
| Shares | Total Consideration (*) | Average Price Per Share of Common | ||||||||||||||||||
| Number | Percent | Amount | Percent | Stock | ||||||||||||||||
| Existing stockholders (**) | 1,652,892 | 60.2 | % | $ | 5,548,765 | 31.6 | % | $ | 3.36 | |||||||||||
| New investors | 1,090,909 |
39.8 | % | $ | 12,000,000 | 68.4 | % | $ | 11 | |||||||||||
| Total | 2,743,801 | 100.0 | % | 17,548,765 | $ | 100 | % | $ | 6.4 | |||||||||||
(*) Total consideration refers to all amounts received in cash.
(**) Includes the shares to be issued upon the automatic conversion of convertible promissory notes issued in a series of convertible loan agreements into an aggregate of 46,132 shares of Common Stock, which will occur immediately prior to the closing of this offering.
The total number of Common Stock that will be outstanding after this offering is based on 1,606,760 shares of Common Stock issued and outstanding as of December 31, 2020, and excludes:
| ● | 129,984 shares of Common Stock issuable upon the exercise of warrants outstanding as of December 31, 2020, with an exercise price of $8.40 per share; | |
| ● | 206,862 shares of Common Stock issuable upon the exercise of options to directors, employees and consultants under our 2018 equity incentive plan (the “Equity Incentive Plan”) at a weighted average exercise price of $3.27, of which 97,351 vested as of December 31, 2020; and | |
| ● | 76,730 shares of Common Stock reserved for future issuance under our Equity Incentive Plan. |
To the extent that new options are granted under our equity benefit plans, there will be further dilution to investors purchasing Common Stock in this offering.
If all of such issued and outstanding options, warrants and performance rights had been exercised as of December 31, 2020, the number of Common Stock held by existing stockholders would increase to 1,943,606, or 64% of the total number of shares of Common Stock outstanding after this offering, and the average price per share of Common Stock paid by the existing stockholders would be $2.85.
If the underwriter exercises its option to purchase additional shares of Common Stock in full in this offering, the number of shares of Common Stock held by new investors will increase to 1,300,677, or 44.0% of the total number of Common Stock outstanding after this offering and the percentage of Common Stock held by existing stockholders will decrease to 56.0% of the total Common Stock outstanding.
| 36 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and under “Risk Factors” elsewhere in this prospectus.
The following financial data in this narrative are expressed in thousands, except for share and share data or as otherwise noted.
Overview
We develop eco-friendly “green” solutions for the food industry. Our solutions are developed to improve the food safety and shelf life of fresh produce. We do this by controlling human and plant pathogens, thereby reducing spoilage, and in turn, reducing food loss.
Our products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of oxidizing agent-based sanitizers and fungicides at low concentrations. Our green products are capable of cleaning, sanitizing and controlling pathogens on fresh produce with the goal of making them safer for human consumption and extending their shelf life by reducing their decay. One of the main advantages of our products is that our active ingredients do not leave any toxicological residues on the fresh produce we treat. In contrary, by forming a temporary protective shield around the fresh produce we treat, our products make it difficult for pathogens to develop and potentially provide protection which also reduces cross-contamination.
Components of Results of Operation
Revenues and Cost of Revenues
Our total revenue consists of products and our cost of revenues consists of cost of products.
The following table discloses the breakdown of revenues and costs of revenues:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sale of products | $ | 232,274 | $ | 175,823 | ||||
| Cost of sales | (43,405 | ) | (144,548 | ) | ||||
| Gross profit | $ | 188,869 | $ | 31,275 | ||||
Operating Expenses
Our current operating expenses consist of three components — research and development expenses, selling and marketing expenses and general and administrative expenses.
Research and Development Expenses, net
Our research and development expenses consist primarily of salaries and related personnel expenses, share base compensation, professional fees and other related research and development expenses such as field tests.
| 37 |
The following table discloses the breakdown of research and development expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | $ | 39,021 | $ | 177,712 | ||||
| Share based compensation | 91,190 | 75,998 | ||||||
| Professional fees | 130,592 | 178,854 | ||||||
| Laboratory and field tests | 72,593 | 73,968 | ||||||
| Depreciation | 29,319 | 20,544 | ||||||
| Other expenses | 54,285 | 88,547 | ||||||
| Total | $ | 417,000 | $ | 615,623 | ||||
We expect that our research and development expenses will increase as we continue to develop our products and services, field trials and recruit additional research and development employees.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and related expenses, share based compensation and other expenses.
The following table discloses the breakdown of selling and marketing expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | $ | 30,450 | $ | 148,221 | ||||
| Share based compensation | (20,971 | ) | 64,229 | |||||
| Other expenses | 41,626 | 129,609 | ||||||
| Total | $ | 51,105 | $ | 342,059 | ||||
We expect that our selling and marketing expenses will increase as we continue to increase our selling and marketing efforts including commercial validation pilots and recruit additional employees or contractor to support our selling and marketing efforts in our targeted geographical areas.
General and Administrative Expenses
General and administrative expenses consist primarily of professional services, share based compensation and other non-personnel related expenses.
The following table discloses the breakdown of general and administrative expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Professional services | $ | 443,883 | $ | 461,840 | ||||
| Share based compensation | 416,996 | 283,910 | ||||||
| Legal expenses | 67,492 | 125,753 | ||||||
| Other expenses | 141,738 | 133,396 | ||||||
| Total | $ | 1,070,109 | $ | 1,004,899 | ||||
Comparison of the Year Ended December 31, 2020 to the Year Ended December 31, 2019
Results of Operations
The following table summarizes our results of operations for the years ended December 31, 2020 and 2019, together with the changes in those items in dollars:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sales of products | $ | 232,274 | $ | 175,823 | ||||
| Cost of sales | (43,405 | ) | (144,548 | ) | ||||
| Gross profit | 188,869 | 31,275 | ||||||
| Research and development expenses | (417,000 | ) | (615,623 | ) | ||||
| Selling and marketing expenses | (51,105 | ) | (342,058 | ) | ||||
| General and administrative expenses | (1,070,109 | ) | (1,004,899 | ) | ||||
| Operating loss | (1,349,345 | ) | (1,931,305 | ) | ||||
| Finance income (expenses), net | (270,393 | ) | (43,408 | ) | ||||
| Other income, net | (2,532 | ) | - | |||||
| Share in losses of affiliated company | - | (15,690 | ) | |||||
| Gain on disposal of affiliated company | 15,690 | - | ||||||
| Net loss | (1,606,580 | ) | (1,990,403 | ) | ||||
| Less: Net loss attributable to non-controlling interests | 13,441 | 18,986 | ||||||
| Net loss attributable to the Company | (1,593,139 | ) | (1,971,417 | ) | ||||
| Loss per share | (1.05 | ) | (1.38 | ) | ||||
| Weighted average number of shares of Common Stock outstanding attributable to shareholders | 1,519,122 | 1,424,045 | ||||||
| 38 |
Revenues
Revenues for the year ended December 31, 2020 were $232,274, an increase of $56,451, or 32%, compared to total revenues of $175,823 for the year ended December 31, 2019. The increase is mainly a result of the Company’s sales of its new products, which the Company commenced in the fourth quarter of 2020.
We do not have backlogs or firm commitments from our customers for our products. Our sales might deteriorate if we fail to achieve commercial success or obtain regulatory approval of any of our products.
Cost of Sales
Cost of sales consists primarily of salaries, materials, transportation and overhead costs of manufacturing our products. Cost of revenues for the year ended December 31, 2020 was $43,405, a decrease of $101,143, or 70%, compared to total cost of revenues of $144,548 for the year ended December 31, 2019. The decrease is mainly a result of the decrease in salaries and related expenses, due to the fact that some of our employee are currently on a temporary leave without pay (furlough), due to the effects of COVID-19 on our business, and a decrease in the overall cost of materials, mainly as a result of inventory elimination made in 2019, due to our efforts to deploy our new solutions.
Gross Profit
Gross loss for the year ended December 31, 2020 was $188,869, an increase of $157,594, or 504%, compared to gross profit of $31,275 for the year ended December 31, 2019. The increase is mainly a result of the increase in revenues and the decrease in cost of revenues, as detailed above.
Research and Development Expenses
Research and development expenses consist of salaries and related expenses, share base compensation, consulting fees, service providers’ costs, related materials and overhead expenses. Research and development expenses for the year ended December 31, 2020 were $417,000, a decrease of $198,623, or 32%, compared to total research and development expenses of $615,623 for the year ended December 31, 2019. The decrease is mainly attributable to: (1) the decrease in professional fees, share based compensation and in payroll; and (2) the decrease in expenses associated with international travel and field trials which have been postponed due to COVID-19.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and related costs for selling and marketing personnel, travel related expenses and services providers. Selling and marketing expenses for the year ended December 31, 2020 were $51,105, a decrease of $290,954, or 85%, compared to total selling and marketing expenses of $342,058 for the year ended December 31, 2019. The decrease is mainly attributable to the decrease in payroll expenses and service providers used in relation to selling and marketing activities mainly associated with the termination of the employment of our former Vice President of Sales in February 2020.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related expenses including share based compensation and other non-personnel related expenses such as legal expenses and directors and insurance costs. General and administrative expenses for the year ended December 31, 2020 were $1,070,109, an increase of $65,210, or 6%, compared to total general and administrative expenses of $1,004,899 for the year ended December 31, 2019. The increase is mainly a result of the increase in share-based compensation to our service providers and directors offset partially by a decrease in professional fees.
| 39 |
Financing Expenses, Net
Financing expenses, net for the year ended December 31, 2020 were $270,393, an increase of $226,985, or 523%, compared to total financing expenses of $43,408 for the year ended December 31, 2019. The increase is mainly a result of compensation expenses related to the exchange of our convertible loans as well as accrued interest and amortization expenses related to such convertible loans.
Total Comprehensive Loss
As a result of the foregoing, our total comprehensive loss for the year ended December 31, 2020 was $1,593,139, compared to $1,971,417 for the year ended December 31, 2019, a decrease of $378,278, or 19%.
Impact of COVID-19
The global spread of COVID-19 led many countries, including the United States, Europe and Israel (where we maintain material operations), to impose stringent limitations on movement, gatherings, transit of passengers and goods and to close the borders between countries. The responses of governments have notably impacted many economies as well as capital markets worldwide.
Based on guidelines provided by the Israeli Government, employers (including us) are required to prepare and increase as much as possible the capacity and arrangement for employees to work remotely. In that regard, and in compliance with all applicable Israeli rules and guidelines, our offices have remained closed since the middle of March 2020, and all of our employees currently work remotely. In addition, as of the date of this prospectus, some of our employees are on a temporary leave without pay (furlough), including our Chief Technology Officer, and we have postponed some of our planned field tests due to the current restriction on international travels. However, we did experience any material impact on our financial condition and results of operations due to COVID-19, and we do not expect to experience any material impact on our overall liquidity positions and outlook as a result of the outbreak. Nevertheless, it is not possible at this time to estimate the full impact that the COVID-19 pandemic, the continued spread of COVID-19, and any additional measures taken by governments, health officials or by us in response to such spread, could have on our business results of operations and financial condition.
Critical Accounting Policies and Estimates
We describe our significant accounting policies more fully in Note 2 to our consolidated financial statements included elsewhere in this prospectus. We believe that the accounting policy described in Note 2 is critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our financial statements in accordance with U.S. GAAP. At the time of the preparation of the financial statements, our management is required to use estimates, evaluations and assumptions which affect the application of the accounting policy and the amounts reported for assets, obligations, income and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting estimates are credited during the period in which the change to the estimate is made.
Going Concern Uncertainty
The development and commercialization of our product will require substantial expenditures. We have not yet generated any material revenues and have incurred substantial accumulated deficit and negative operating cash flows. We currently have no sources of recurring revenue and are therefore dependent upon external sources for financing our operations. There can be no assurance that we will succeed in obtaining the necessary financing to continue our operations. As a result, our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Stock-based Compensation
Employees and other service providers of the Company may receive benefits by way of stock-based compensation settled with company options exercised for shares of our Common Stock. The cost of transactions with employees settled with capital instruments is measured based on the fair value of the capital instruments on the granting date. The fair value is determined using an accepted options pricing model. The model is based on share price, grant date and on assumptions regarding expected volatility, expected lifespan, expected dividend, and a no risk interest rate.
The cost of the transactions settled with capital instruments is recognized in profit or loss together with a corresponding increase in the equity over the period in which the performance and/or service takes place, and ending on the date on which the relevant employees are entitled to the benefits (the “Vesting Period”). The aggregate expense recognized for transactions settled with capital instruments at the end of each reporting date and until the Vesting Period reflects the degree to which the Vesting Period has expired and our best estimate regarding the number of warrants that have ultimately vested. The expense or income in profit or loss reflects the change of the aggregate expense recognized as of the end of the reported period.
Convertible Loans
The Company entered into a series of convertible loan agreements with certain lenders to sell convertible promissory notes containing conversion feature. The conversion feature is considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations.
The Company was using a third-party appraiser to estimate the fair value of the convertible component.
| 40 |
We selected the Black-Scholes-Merton (“Black-Scholes”), as our option pricing model to estimate the fair value of our options awards. The option-pricing model requires a number of assumptions:
Expected dividend yield — The expected dividend yield assumption is based on our historical experience and expectation of no future dividend payouts. We have historically not paid cash dividends and have no foreseeable plans to pay cash dividends in the future.
Volatility — Since the Company is not traded on any stock exchange market, quoted prices of our shares are unavailable. In case of insufficient historical data for a company, the expected volatility is based on similar companies’ stock volatility.
Risk free interest rate — The risk-free interest rate is based on the yield of governmental bonds with equivalent terms.
Contractual term — An option’s contractual term is the amount of time the holder has to exercise the option, per the contract.
Share price — The share price is determined according to the last known closing price of the share of our Common Stock at the grant date.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures. Since our inception through December 31, 2020, we have funded our operations principally with approximately $11,867,746 (net of issuance expenses) from the issuance of shares of shares of our Common Stock, options and loans.
The table below presents our cash flows for the periods indicated:
Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Net cash used in operating activities | $ | (798,740 | ) | $ | (1,244,772 | ) | ||
| Net cash provided by (used in) investing activities | 9,065 | (81,655 | ) | |||||
| Net cash provided by financing activities | 741,760 | 1,177,436 | ||||||
| Decrease in cash and cash equivalents | $ | (47,915 | ) | $ | (148,991 | ) | ||
As of December 31, 2020, we had cash of $242,900, as compared to $290,815 as of December 31, 2019. As of December 31, 2020, we had a negative working capital of $290,062, as compared to a negative working capital of $199,212 as of December 31, 2019. The decrease in our cash balance is mainly attributable to our net loss described above and payments to account payables offset by our equity financing and convertible loans.
Operating Activities
Net cash used in operating activities was $798,740 for the year ended December 31, 2020, as compared to $1,244,772 for the year ended December 31, 2019.
| 41 |
Investing Activities
Net cash provided by investing activities was $9,065 for the year ended December 31, 2020, as compared to net cash used in investing activities of $81,655 for the year ended December 31, 2019. The increase is mainly attributable to the decrease in short term bank deposits in banking institutions and proceeds from investment in unconsolidated entity.
Financing Activities
Net cash provided by financing activities was $741,760 for the year ended December 31, 2020, as compared to $1,177,436 for the year ended December 31, 2019. The decrease is mainly the result of a decrease in equity financing and proceeds from convertible loans.
Financial Arrangements
Since our inception, we have financed our operation primarily through proceeds from sales of our shares of Common Stock, convertible loan agreements and grants from the IAA.
During December 2019, January 2020 and March 2020 we entered into a series of convertible loan agreements (the “December 2019 CLAs,” and each a “December 2019 CLA”) with third parties and certain existing shareholders (the “December 2019 Lenders”), pursuant to which the December 2019 Lenders agreed to provide the Company loans in the aggregate amount of $514,000 and in exchange the Company issued to the December 2019 Lenders (i) convertible promissory notes (the “December 2019 Notes”) and (ii) warrants with an exercise price of $8.40.
According to the terms of the December 2019 CLA, the December 2019 Notes bear interest at a rate of 5% per annum and the loan amount represented by the December 2019 Notes will be repaid to the December 2019 Lenders according to the following schedule: (i) the principal amount represented by the December 2019 Notes to be repaid in twenty four equal monthly installments, commencing on the twenty fifth month following the closing of each December 2019 CLA and (ii) the interest accrued on the loan amount to be paid in two bi-annual installments, commencing on the first anniversary of the first payment of the principal amount.
According to the terms of the December 2019 CLA, the outstanding loan amount matures on the earlier of (i) the third anniversary of each December 2019 CLA or (ii) a deemed liquidation event (as defined therein), and the December 2019 Lenders may convert all or any portion of the December 2019 Notes at any time prior to the one-year anniversary of each issuance into shares of Common Stock at a conversion price of $8.40 per share.
On June 24, 2020, we entered into a Securities Purchase Agreement (the “June 2020 SPA”) with the December 2019 Lenders in connection with the sale and issuance of 67,369 units, at a purchase price of $7.63 per unit. Each unit consists of: (i) one share of the Company’s Common Stock; and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.40. In connection with the June 2020 SPA, the Company issued to the December 2019 Lenders an aggregate of 67,369 shares of Common Stock and warrants to purchase an aggregate of 67,369 shares of Common Stock. The shares of Common Stock were issued on July 2, 2020.
Simultaneous with and conditioned upon the execution of the June 2020 SPA, the Company and each of the December 2019 Lenders, agreed to effectively cancel the December 2019 CLA and the equity securities issued thereunder. In connection therewith, each of the December 2019 Lenders, voluntarily waived any right to receive interest that accrued thereupon pursuant to the December 2019 CLAs.
| 42 |
On September 23, 2020, we entered into a Securities Purchase Agreement (the “September 2020 SPA”) with Medigus Ltd. (“Medigus”) in connection with the sale and issuance of 13,107 units, at a purchase price of $7.63 per unit, and for an aggregate purchase price of $100,000. Each unit consists of: (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $1.20. In connection with the September 2020 SPA, the Company issued to Medigus an aggregate of 13,107 shares of Common Stock and warrants to purchase an aggregate of 13,107 shares of Common Stock. Furthermore, the September 2020 SPA contemplates an additional investment by Medigus not to exceed $25,000 (the “Additional Investment”), which investment shall be triggered following the parties’ initiation of a proof of concept procedure to test the effectiveness of the Company’s sanitizers and its residual effects against different pathogens. In consideration for the Additional Investment, the Company has agreed to issue an additional 3,277 units at a purchase price of $7.63, which units shall contain the same composition of securities as described in the aforementioned description of the September 2020 SPA.
During September 2020, we entered into a series of convertible loan agreements (each a “September 2020 CLA”) with certain lenders (the “September 2020 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $125,000 (the “September 2020 Notes”). The September 2020 Notes will bear interest at a rate of 5% per annum. The outstanding loan amount will mature on the earlier of (i) the third anniversary of each September 2020 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the September 2020 Notes will be repaid to the September 2020 Lenders according to the following schedule: (i) the principal amount represented by the September 2020 Notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each September 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The September 2020 Lenders may convert all or any portion of the September 2020 Notes into shares of Common Stock at any time prior to the closing of an underwritten public offering (the “Mandatory Conversion Event”), at a conversion price of $7.63 per share. In addition, the September 2020 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event.
During October 2020, we entered into a series of convertible loan agreements (each a “October 2020 CLA”) with certain lenders (the “October 2020 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $100,000 (the “October 2020 Notes”). The October 2020 Notes will bear interest at a rate of 5% per annum. The outstanding loan amount will mature on the earlier of (i) the third anniversary of each October 2020 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the October 2020 Notes will be repaid to the October 2020Lenders according to the following schedule: (i) the principal amount represented by the notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each October 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The October 2020 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event. In addition, the October 2020 Lenders may convert all or any portion of the notes into shares of Common Stock at any time prior to a Mandatory Conversion Event, at a conversion price of $7.63 per share.
During January 2021, we entered into a series of convertible loan agreements (each a “January 2021 CLA”) with certain lenders (the “January 2021 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $274,000 (the “January 2021 Notes”). The January 2021 Notes bear interest at a rate of 5% per annum. The outstanding loan amount matures on the earlier of (i) the third anniversary of each January 2021 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the January 2021 Notes will be repaid to the January 2021 Lenders according to the following schedule: (i) the principal amount represented by the notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each January 2021 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The January 2021 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event. In addition, the January 2021 Lenders may convert all or any portion of the notes into shares of Common Stock at any time prior to a Mandatory Conversion Event, at a conversion price of $7.63 per share.
As part of the September 2020 CLAs, the October 2020 CLAs and the January 2021 CLAs, we entered into a registration rights agreement with each of the September 2020 Lenders, October 2020 Lenders and the January 2021 Lenders, whereby each of such lenders received piggyback registration rights for the shares issuable upon conversion of the September 2020 Notes, October 2020 Notes and the January 2021 Notes to shares of Common Stock.
Current Outlook
We have financed our operations to date primarily through proceeds from sales of our shares of Common Stock. We have incurred losses and generated negative cash flows from operations since inception in 2009. Most of our revenues are currently generated in U.S. dollars from the sale of our products and services.
As of December 31, 2020, our cash and cash equivalents were $242,900. We expect that our existing cash and cash equivalents will be sufficient to fund our current operations until June 2021, without using the net proceeds from this offering. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future capital requirements will depend on many factors, including:
| ● | the progress and costs of our studies and other research and development activities; | |
| ● | the costs of manufacturing our products; | |
| ● | the costs and timing of obtaining regulatory approval for our products; | |
| ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; | |
| ● | the potential costs of contracting with third parties to provide marketing and distribution services for us or for building such capacities internally; and | |
| ● | the magnitude of our general and administrative expenses. |
| 43 |
Until we can generate significant recurring revenues and profit, we expect to satisfy our future cash needs through debt or equity financings. We cannot be certain that additional funding will be available to us when needed, on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to our products and services. This may raise substantial doubts about our ability to continue as a going concern.
In its report on our financial statements for the year ended December 31, 2020, our independent registered public accounting firm included an explanatory paragraph stating that our recurring losses from operations since inception and our need for additional funding to finance our operations raises substantial doubt about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
We do not currently have any off-balance sheet arrangements.
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2020:
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Operating leases | $ | 15,049 | $ | - | $ | 15,049 | - | - | ||||||||||||
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our current investment policy is to invest available cash in bank deposits with banks that have a credit rating of at least A-minus. Accordingly, some of our cash and cash equivalents is held in deposits that bear interest. Given the current low rates of interest we receive, we will not be adversely affected if such rates are reduced. Our market risk exposure is primarily a result of foreign currency exchange rates, which is discussed in detail in the following paragraph.
Impact of Inflation and Currency Fluctuations
Our functional currency and reporting currency is the U.S. dollar. We incur some of our expenses in other currencies. As a result, we are exposed to the risk that the rate of inflation in countries in which we are active other than the United States will exceed the rate of devaluation of such countries’ currencies in relation to the dollar or that the timing of any such devaluation will lag behind inflation in such countries. To date, we have been affected by changes in the rate of inflation or the exchange rates of other countries’ currencies compared to the dollar, and we cannot assure you that we will not be adversely affected in the future.
The annual rate of inflation in Israel decreased by 0.7% in 2020 and 0.6% in 2019. The New Israeli Shekel (NIS) revaluated against the U.S. dollar and decreased by approximately 7% in 2020 and 8% in 2019.
| 44 |
Company Overview
We develop eco-friendly “green” solutions for the food industry. Our solutions are developed to improve the food safety and shelf life of fresh produce. We do this by controlling human and plant pathogens, thereby reducing spoilage, and in turn, reducing food loss.
Our products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of oxidizing agent-based sanitizers and fungicides at low concentrations. Our “green” products are capable of cleaning, sanitizing and controlling pathogens on fresh produce with the goal of making them safer for human consumption and extending their shelf life by reducing their decay. One of the main advantages of our products is that our active ingredients do not leave any toxicological residues on the fresh produce we treat. In contrary, by forming a temporary protective shield around the fresh produce we treat, our products make it difficult for pathogens to develop and potentially provide protection which also reduces cross-contamination.
The U.S. Food and Drug Administration (the “FDA”) Food Safety Modernization Act (the “FSMA”) is transforming the United States’ food safety system by shifting the focus from responding to foodborne illness to preventing it. According to the recent data from the Centers for Disease Control and Prevention, approximately 48 million people in the United States get sick each year from foodborne diseases. We believe this is a significant public health burden that is largely preventable. Since 2011, the FDA has had a legislative mandate to require comprehensive, science-based preventive controls across the food supply. In the context of fresh produce at packing houses, the FDA’s final produce safety rule (with an initial compliance date of January 26, 2018) provides for the use of sanitizers to ensure produce is cleaned from human pathogens.
In addition, most conventional chemical pesticides (fungicides), which are currently used to protect fresh produce from microbial spoilage and reduce food waste, are potentially toxic, they remain on fruit peel and present health concerns, while also polluting the environment. Therefore, the use of these products is strictly regulated and their residue on food and on the environment are carefully monitored. Today’s trends led by both consumers and regulatory bodies are to significantly reduce the use of fungicides and switch to greener solutions. In a series of studies conducted in collaboration with a large post-harvest service company during the second quarter of 2020, our products have shown impressive results in extending the shelf life of fresh produce in “organic” (where no fungicides are used at the post-harvest stage) and conventional (where fungicides are being used at the post-harvest stage) settings. On average, our products may reduce the rotten fruits at the retail level by 50%.
We have a unique opportunity to make a positive difference throughout the food value chain from field to fork and address two of the major’s challenges in the food industry today — safety and waste. We target major markets that use conventional chemical pesticides and sanitizers, including the pre- and post-harvest market, the greenhouse market and the fresh-cut market, where our “green” products are used as alternatives for, or mixed with, conventional products in order to reduce (i) health and environmental concerns, and/or (ii) microbial resistance that has reduced the efficacy of conventional chemical pesticides.
Industry Overview and Market Opportunity
Background
The world’s population is expected to grow to almost 10 billion people by 2050, boosting agricultural demand by some 50%. Providing healthy and safe food to feed the world’s population is one of the biggest challenges of the twenty first century, accentuated with the backdrop of a of a fragile global economy. Globally, around one-third of the food produced (estimated at circa 1.3 billion tons), is lost or wasted along the food chain – from production to consumption.
| 45 |
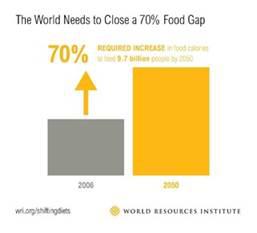
Fruits and vegetables are considered essential food commodities and demonstrate their best benefits especially when consumed fresh. Consumption as well as production of fresh fruit and vegetables is growing globally; in 2018, the global production of fresh fruit amounted to about 868 million tons, while the production of fresh vegetable amounted to about 1.09 billion tons. According to a report published by technavio in October 2020, the fresh food market size has the potential to grow by 337.76 million tons from 2020 to 2024, growing at a CAGR of almost 3% during the forecast period, and the market’s growth momentum will accelerate during the forecast period due to the steady increase in year-over-year growth. In the United States, according to a report by Grand View Research, increasing health awareness among the U.S population and potential development of secondary diseases due to obesity and unhealthy eating habits are propelling the market of fruit and vegetables to reach an estimated $1.1 billion by 2025.
Food Safety and Food Loss
Food Safety
We believe foodborne diseases are a significant public health concern globally. Hundreds of diseases are caused by eating contaminated food. Many diseases are spread through unwashed or untreated produce. With approximately 48 million people in the United States (one in six) getting sick, 128,000 hospitalized, and 3,000 die each year from foodborne diseases, according to recent data published by the FDA, and 23 million in the European region getting sick due to food borne disease, food safety is another major concern and source of waste, placing a material burden on public health and significant healthcare cost. The economic burden of foodborne illness has been estimated to be as high as $90 billion annually.
When considering the farm-to-fork chain, microbial contamination of fresh produce can occur at multiple steps. Contamination can take place during the cultivation of fresh produce, at harvest, during preparation/washing, within distribution chains and transport to shops, and even at the final step in the consumers’ kitchen. We believe this is a significant public health burden that is largely preventable. The FSMA is transforming the United States food safety system by shifting the focus from responding to foodborne illness to preventing it. The Produce Safety rule of the FSMA establishes, for the first time, science-based minimum standards for the safe growing, harvesting, packing, and holding of fruits and vegetables grown for human consumption. The final rule went into effect on January 26, 2016. Sanitization is a cornerstone of FSMA compliance, which requires preventing or eliminating food safety hazards or reducing such hazards to a minimal level.
Markets require many types of produce to be washed prior to sale in order to remove dirt and other debris. Produce can be contaminated with foodborne pathogens before it enters the packing house, and these pathogens cannot be seen with the naked eye. Inability to visually spot pathogens makes the washing step one of the most important steps in packing because, if washing process is not controlled, it can become a source of cross-contamination (when foodborne pathogens fall off contaminated produce into the water where they can contaminate more produce). These washing steps are defined by the packing house safety managers as critical point because water mixed with organic materials are good conditions for pathogens to develop. Therefore, the use of sanitizers should be introduced during the washing step because they are, most of the time, one of the last treatments applied before the produce meets with the consumer. Sanitizers are designed to inactivate/kill any bacteria in the water, drastically reducing the possibility of cross-contamination. We believe this represents a significant opportunity for us.
| 46 |
Food Loss
The Food and Agriculture Organization of United Nations predicts that about third of the food produced globally are wasted or lost every year. Approximately 644 million tons of fruits and vegetables are thrown away each year (representing 42% of the total food wasted every year). A report published in April 2020, generated by the European Innovation Partnership Agricultural Productivity and Sustainability, estimates that in Europe an estimated 9 million tons of food is lost at the production stage (farm), while up to 16.9 million tons are lost at the processing stage (packing houses, etc.).
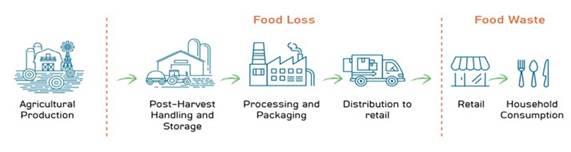
Much of this loss is caused by spoilage, which can be caused by microorganisms – primarily bacteria and mold. In addition, bacteria and fungi represent the highest numbers of incidents of post-harvest microbial diseases in fresh produce worldwide. Taken together, it is estimated that nearly a third of all food grown is lost between the time that it is grown and harvested and the time that it is packaged for retail sale. Such waste equates to roughly $680 billion in industrialized countries and $310 billion in developing countries.
Post-harvest losses due to spoilage represent a significant problem along the supply chain and lead to profit losses in the millions. The main causes of these losses are pest or disease infestation and incorrect storage conditions, which lead to rotting or loss of fresh mass due to respiration and evaporation. Fruits and vegetables are largely damaged after harvest by fungi and bacteria . It is estimated that an average of 45% of harvested fruit and vegetables are lost globally. Post-harvest diseases have been identified as the greatest cause of post-harvest losses in fruits and vegetables, causing significant economic losses. It is estimated that approximately 20 to 25% of the fruit and vegetables harvested are lost due to microbial spoilage during post-harvest handling in developed countries. Furthermore, the demand for fresh fruits and vegetables, especially exotic tropical fruit has contributed to the demand for post-harvest treatments to increase shelf life and maintain quality, resulting in more efficient export trade.
The most common way to protect fresh produce and prevent loss is the use of hazardous chemicals such as fungicides in post-harvest applications. Post-harvest diseases are generally controlled by fungicides. Systemic (non-organic) fungicides, are one of the most commonly used fungicides, for example, citrus fruits in California are completely covered by the fungicides, and the residue is persistent for the life of the fruit providing protection. However, as they tend to affect a single biochemical pathway within the pathogen, fungi may readily develop resistance to systemic fungicides. To avoid potential issues with resistance, maximum concentration of fungicides will be generally used to ensure highly efficient eradication of the targeted pathogen which leaves high residue level on the treated produce.
However, these chemical agents have been applied for many years with few or limited success due to the development of resistance. Further they have severe negative effects on human health, and the environment mainly due to the carcinogenic and/or teratogenic properties of the compounds, and by their cumulative toxic effects.
The effects of exposure to these hazardous chemicals on humans and the environment are a continuing concern as they are intrinsically toxic and pollute the environment through wastewater discharge from the packing house or a discarded fruit. Therefore, the agricultural use of certain pesticides (in the field or in the packing house) has been abandoned in some countries leaving the growers with significant challenges.
| 47 |
To control and monitor the potential negative impacts pesticides might have over time, regulatory agencies that regulate pesticides – for example, the EPA, the Pest Management Authority Agency in Canada, and the European Food Safety Authority (“EFSA”) in Europe, have defined a maximum residue level (the “MRL”) that can be present on the treated produce. Additionally, more countries require an MRL for the commodity to be imported into their country. As there is increased awareness regarding compliance with MRLs, MRLs have become a much greater concern. These changes have also impacted the market and we believe that consumers spearheaded this change by demanding organic or pesticide-free foods. Consumers have recently increasingly want to understand where and how their food is grown. Retailers and processors have capitalized on what they view as an opportunity to offer more information to consumers. It is more common now for retailers and processors to ask which products have been used on the commodities they are purchasing. There are also retailers and processors banning the use of certain products, requiring any residues to be below the established MRLs. The reduction in MRLs results in lower efficacy of fungicide and increased loss.
We believe that the rising demand for healthy food among the global population will trigger the market’s growth in the forthcoming years. Over the last decade, the organic market in Europe continued to grow and reached €40.7 billion in 2018 with 15.6 million hectares (approximately 38,548,439 acres) (including 2.2 million hectares in Spain, the largest organic area in Europe, followed by 2.0 million hectares in France and 2.0 million hectares in Italy), providing farmers with further added value on their production. The strong growth rates in both production and consumption indicate that the organic market has not yet reached its peak and further growth can still be expected. Organic farming is already responding to further emerging consumer trends such as veganism and demand for locally produced food products, turning these challenges into opportunities.
As consumer demand for organic fruits and vegetables is increasing globally and there is an increasing promotion by government organizations for the adoption of environmentally friendly pesticides, the biorational pesticides market estimated at $2.78 billion in 2017 is projected to reach a value of $5.02 billion by 2022, at a compound annual growth rate (the “CAGR”) of 12.5% from 2017. A biorational pesticide is a term used to define any pesticide material that causes relatively no harm to humans or animals and does little or no damage to the environment. We believe that our products could be defined as biorational products.
Analysts have predicted that the organic fresh food market will reach a CAGR of almost 15% by 2023. The market size will increase by $62.23 billion during the forecast period from 2019 to 2023. In addition, strict regulations have been imposed on the usage of pesticides and GMO-produced crops worldwide. This, in turn, has influenced consumer demand for organic fruits and vegetables.
Case Study – Citrus Fruit
Citrus fruit, which represent one of the main fruit produce worldwide with more than 100 million tons produced worldwide, can be infected by many fungal pathogens, and these pathogens can cause considerable losses during storage and transportation. Losses are mainly caused by Penicillium digitatum, P. italicum, Aspergillus flavus and Alternaria alternata for citrus fruit. Post-harvest treatments such as thiabendazole, imazalil, sodium ortho-phenil phenate or other active ingredients have been used for many years. They are currently the most commonly used fungicides effective for controlling post-harvest fungal pathogens in citrus and they are used in citrus packing houses to maintain fresh fruit, control post-harvest decay, and extend fruit shelf life. However, significant problems such as environmental issues and health concerns have risen in the citrus industry due to chemical residues or the occurrence of pathogenic resistant strains which require the use of even higher concentration of these post-harvest treatments. However, currently, the residues of imazalil on citrus fruits is being revised by the European Commission. The EFSA put forward a proposal in 2018 to cut the MRL for imazalil from 5 milligrams per kilogram to 0.01 milligrams per kilogram, causing worry among Europe’s main citrus producing countries and packers exporting their produce to European countries. Due to the significant impact this proposal could have on the citrus industry, the European Council has decided, in the meantime, to start reducing imazalil residues to four milligrams per kilogram for citrus fruit for a limited period of time to allow the citrus industry an extra time to find green and safe alternatives. Our product PeroStar/SaveProtect has already shown its benefits in reducing significantly the residues of imazalil while maintaining the produce shelf life.
| 48 |
Current Market Drivers and Trends
In addition to food safety and food waste concerns, the following market drivers are also shaping the food industry by setting standards and conditions on the main actors in the industry:
| ● | Focus of consumers on health characteristics: consumers are more aware and conscious of the health characteristics of the food they consume. Consumers pay more attention to the qualities of the fresh produce they buy. Particularly in the United States and Europe, products such as berries, avocados, mangoes, pomegranates, papayas and sweet potatoes are gaining popularity and considered “super foods,” and these products are showing a strong annual import growth of 10% to 20%. | |
| ● | Increasing demand for organic produce: the demand for organic products is growing rapidly particularly in Europe and North America, and is closely related to consumer interest in healthy and pure eating. While the increasing demand created potential for oversees supply, it can be challenging and expansive for exporters in tropical climates to comply with the increasingly demanding organic standards. | |
| ● | Success of retailers determined by quality of produce: a recent report by Fruit Logistica published in 2019, based on consumer surveys that involved almost 7,000 consumers in 14 different markets across Europe and North America, demonstrated the increased importance of fresh produce for the profitability of food retailers. According to the report, when choosing the place to buy their groceries, consumers focus on the quality of the stores’ fresh food, with freshness of fruits and vegetable being their top priority. The report also showed that customers who are satisfied with the store’s fresh food quality, would visit the store more frequently than those who are not. In addition, consumers are also willing to pay more for better-quality produce and their basked will be 4% larger. | |
| ● | Promoting sustainability: a large range of sustainability aspects are directly related and affected by the fresh produce industry. Food waste accounts for 8% of global greenhouse gas emissions. Both consumers and businesses, are becoming more aware of the growing importance of sustainability issues. As consumers increasingly embrace social causes, they seek products and brands that align with their values. According to a recent analysis published by Research Insights, nearly six in 10 consumers surveyed are willing to change their shopping habits to reduce environmental impact, nearly eight in 10 respondents indicated sustainability is important for them, and among those respondents that indicated that sustainability is very or extremely important, over 70% indicated that they would pay a premium of 35%, on average, for brands that are sustainable and environmentally responsible. Increasing number of companies in the fresh food sector are investing in sustainability. Survey conducted by Champions 12.3 in 2017 showed that 99% of businesses that invested in reduction of food loss and waste, received a net positive financial return. Primary production companies are investing in aspects of food losses, energy efficiency and carbon footprint, through innovations such as drying produce, on-farm and off-grid cold rooms and post-harvest treatments. | |
| ● | Food retailers seek to reduce their waste and maximize their revenues: more than eight million tons of food are wasted every year in the United States in the retail sector alone, which translates into $18 billion in lost value (cost of waste) every year. Some retailers, including Walmart, have already committed to implement a zero-waste policy by 2025. Prevention solutions across the retail value chain offer the highest returns to retailers and are growing the fastest. | |
| ● | Regulators are promoting the use of safer chemical-based product: for example, the EPA offers a “Safer Choice” label that product manufacturers may use on qualifying products to help consumers and commercial buyers identify products with safer chemical ingredients. The EPA requires that every chemical, regardless of percentage, in a Safer Choice-certified product is evaluated and only the safest ingredients are allowed. | |
| ● | Increasing investment in foodtech and agritech companies: according to a recent report published by AgFunder, a venture capital firm active in the foodtech and agritech, startups developing agrifoodtech solutions and products, raised approximately $26.1 billion in 2020, a 15.5% year-over-year increase. Reduction of food waste, extension of the shelf life of fresh produce and reduction of the use of pesticides are main focus of the industry and many companies are addressing these objectives, including: |
| ● | Apeel Sciences, a company developing an edible coating to extend the shelf life of fruit and vegetables, secured in May 2020 a $250 million investment from a leading venture capital, based on a firm valuation of more than $1 billion; | |
| ● | Lineage Logistics, an expert in cold chain management for food, raised $1.6 billion in September 2020. | |
| ● | Zymergen, an expert in biofacturing with applications also for the agricultures with the goal to develop safer crop protection and pest control using their natural products, secured in September 2020 an investment in a total amount of $300 million. | |
| ● | GreenLight Biosciences, an RNA based pest control reducing the use of toxic pesticides, secured in June 2020 an investment of $102 million. | |
| ● | Provivi, a company developing sustainable and safer pest control based on pheromones, closed a $45.5 million financing round in December 2020. | |
| ● | Enko Chem, a company using AI to develop green insecticides, fungicides, and herbicides, announced in June an investment of $45 million. | |
| ● | PeroxyChem, a manufacturer of hydrogen peroxide and peracetic acid and a well-positioned in high-margin specialty applications and applications for environmentally friendly disinfectants, was successfully acquired by Evonik for $640 million. |
The increased consumption of fruits and vegetables in combination with the current regulation and consumers’ demand for healthier food has placed a greater burden on the fresh produce industry to provide food products that are fresher in quality, demonstrate an extended shelf life and are safer to consume.
The aforementioned changes provide a unique opportunity for us to introduce our products. We are aiming to become a significant player in post-harvest green produce treatment, fully responsive to the world’s ongoing change in fruit and vegetables consumption, food safety requirements as well as regulations and consumer demand to eliminate the use of hazardous chemicals.
| 49 |
Our Core Products
Our innovative products address what we believe to be two of the most significant challenges in the food industry: increase food safety and reduce food loss. Our main product lines consist of a proprietary blend of organic food acids applied in post-harvest applications designed to ensure food safety and increase fruit and vegetable’ shelf life by reducing microbial spoilage.
The main steps in post-harvest applications are cleaning, sanitization, and coating (wax). Our products address the cleaning and sanitization application points which are the critical first steps for preserving the quality of fresh produce by controlling microbial contamination related to food safety (e.g., Listeria, Salmonella, E. coli) and food loss due to microbial spoilage (e.g., fungi, mold and yeast). In general, the current process includes an initial washing step to remove soil and other debris, which improves the product appearance and lowers the product temperature. The next step includes sanitation or disinfection methods combined with fungicides that can further reduce the presence and prevent the transfer of spoilage and pathogenic microorganisms on fresh produce surfaces. The last step usually includes application of wax sometimes combined with an additional application of fungicides to prevent or reduce physiological changes and risks of spoilage. Our main products are applied at the cleaning and sanitization steps.
One of the main advantages of our food acid blend is its non-toxic residues that are providing protection to the treated produce. And we believe that all the blend ingredients are recognized by the FDA as GRAS when used as intended in fruit and vegetable wash applications. Moreover, they significantly reduce or eliminate the need for additional post-harvest applications with conventional fungicide by at least 50%, and in some cases entirely, and can reduce food waste due to spoilage by up to 50% (see results below on easy peelers and mango). Our main products are:
| ● | Processing Aids – SavePROTECT or PeroStar: post-harvest treatment added to fruit and vegetable wash water as a processing aid to increase the efficiency of the oxidizing agent present in the water tank against plant pathogens to reduce produce loss; and | |
| ● | Sanitizers - SF3HS and SF3H: post-harvest cleaning and sanitizing solution to control both plant and foodborne pathogens to ensure both food safety as well as increase produce’s shelf life. |
Processing Aids – SavePROTECT or PeroStar
Processing aids are products that are intended to be used with other products to aid the application or enhance the effect of that product. Save Foods processing aids, which are marketed as SavePROTECT in the United States and PeroStar in Spain, Israel and Italy, are based on our proprietary blend of food acids and are added to the wash water at the cleaning and sanitization stages simultaneously with a low concentration of peracetic acid (“PAA”), a sanitizing agent. This food acid blend serves several functions:
| ● | SavePROTECT/PeroStar keeps the process wash waters at a relatively low stable pH level. We have observed that low pH levels strengthen the effectiveness of the PAA and the fungicide used which result in increased sanitation and biocide activity; | |
| ● | PAA-based products are used as disinfectant in wash water. When used with PAA-based products, SavePROTECT/PeroStar may optimize the efficacy of PAA and eliminates the strong odor of PAA, creating a more friendly and safe working environment; | |
| ● | When used with fungicides, including imidazole, imazillil, thiabendazole, etc. – most commonly used fungicides – SavePROTECT/PeroStar may optimize the efficacy of the fungicides used and prevent resistance buildup; | |
| ● | SavePROTECT/PeroStar helps to clean the fruit surface and can improve the performance of the wax applied leading to an improved appearance of treated fruit by leaving a glossy finish on the outer skin of the fruit; and | |
| ● | SavePROTECT/PeroStar helps to extend shelf life. |
| 50 |
During 2020, we ran a series of proof of concept and small trials in collaboration with commercial partners on pears, avocado, easy peelers, lime, mango, bell pepper, lemons, fresh cut vegetables and figs. In February 2021, we initiated a proof of concept in bananas.
Results on Easy Peelers
Easy peelers are citrus fruits that are easier to peel, such as tangerines, mandarins, satsumas, and clementines. As previously described, imazalil is currently one the most commonly used fungicide that is effective in controlling post-harvest fungal pathogens in citrus. Currently, the residues of imazalil on citrus fruit is being revised by the European Commission and have already been reduced, and this reduction poses challenges, especially to packing houses exporting to Europe.
Between February and June 2020, we collaborated with the Israeli branch of one of the largest worldwide post-harvest service companies to demonstrate the safety and ability of PeroStar to meet the new requirement of reduced residue level of imazalil and efficiently control decay against the most common pathogens attacking citrus fruit such as Green mold (Penecillium digitatum) and sour rot (Geotrichum candidum). The experiment simulated the applications in a packing house which tested the use of imazalil with and without our products. The reference used in the trials to compare the results was the maximum amount of imazalil allowed and the current treatment in the packing house which is a combination of PAA and imazalil as well as PAA alone to simulate treatment in organic settings.
To ensure the efficacy of the products, it is customary to deliberately infect the fruit with the target pathogen at a concentration of around 105 and to inoculate it for 16 to 24 hours before treatment. Following the treatments, the fruit was stored in cold storage for between 9 to 21 days and then stored in room temperature for shelf-life evaluation.
During these months, we ran a series of trials from small scale/lab test (between 350 to 500 fruits per trial) to semi-commercial application (more than 1000 fruits per trial). The semi-commercial pilots were run in Ashkelon, Israel on the packing line of Mehadrin in Israel, a well-know and recognized citrus packer.
The results of the trials have shown that PeroStar significantly reduced the need for additional post-harvest applications with imazalil by at least 50%, and in some cases entirely while improving the fruit shelf life, reducing waste). In addition, the use of PeroStar allows the packing house to meet the new limitations of imazalil utilization as well as meet its goal to apply greener and safer products (see graph below).
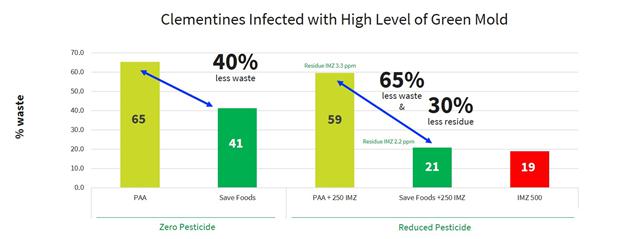
In addition, over the 2018 to 2019 and 2019 to 2020 citrus fruit seasons in the State of California, the safety of our products was demonstrated on more than 100,000 tons of fresh produce in the aggregate, which, according to the biannual report “Citrus: World Markets and Trade” published in July 2020 by the United States Department of Agriculture, represent more than 12% of the total production in the United States of mandarins/tangerines. The United States is ranked as the number six producer worldwide with around 800,000 tons, while Israel is ranked number ten with around 200,000 tons.
| 51 |
Results on Mangos
We have recently tested our PeroStar on mangos in collaboration with the Israeli-based Volcani Center for Agricultural Research. The goal of the test was to evaluate the effectiveness of PeroStar in preventing decay in harvested mangos in comparison with fludioxonil. Fludioxonil is a fungicide that is commercially available in Israel at a level of 250 to 300 parts per million. Fludioxonil is deemed to be an effective fungicide against fungi that attack the mango post-harvest, yet there is a growing need for “greener” solutions, given Fludioxonil’s level of toxicity.
Mangos was stored for three weeks after treatment at 12°C and an additional week of shelf life at 20°C in what would typically simulate a mango crate shipment to Europe and retailers in similarly distanced markets.
Results after evaluation have shown us that the treatment with PeroStar, improved the biocide activity of the PAA, which resulted in a reduction in both side decay and stem-end rot (common pathogens in mango) leading to an extended shelf life with no use of fungicide (as demonstrated in the picture below). In addition, the results also showed that the combination of PeroStar with a low concentration of fludioxonil reduced the post-harvest decay to zero. The results (as presented in the graph below) demonstrate that applying PeroStar enables mango producers to achieve an improved shelf life of produce compared to the current treatment while reducing the use of conventional chemical pesticides.

| 52 |
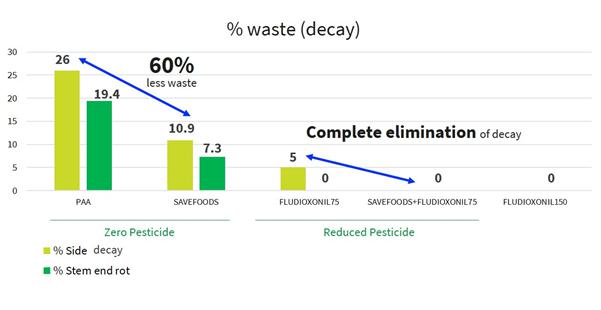
Commercialization Stage
Following a successful pilot in Mexico on Persian lime (where SavePROTECT has reduced to zero the fruit decay after 21 days as shown in the graph below), the packing house has bought a first batch to start the utilization of our product.
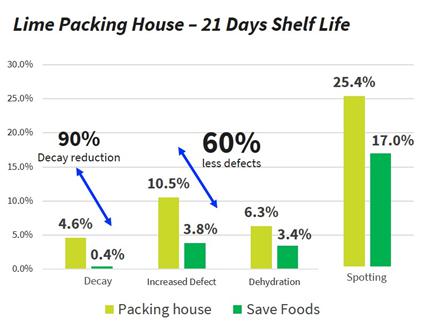
Based on these results, food retailers may benefit from additional income of up to $126 per ton of limes assuming a conservative average price of $3,000 per ton lime (based on an average price per pound lime of $1.49 in 2019), as presented in the graph below.
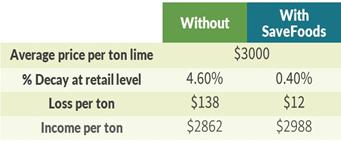
The European Union is a significant target market for our organic food acid blends because of strict regulations that are being imposed on the use of pesticides and GMO-produced crops, as well as health conscious consumers who represent a growing demand for organic fruits and vegetables. In August 2020, we submitted a regulatory dossier for our PeroStar as a processing aid to be used with PAA in Spain and Italy, two of the largest fruit and vegetables producers in Europe. See “Government Regulation and Product Approval” below.
In Israel, with the start of the citrus season, Safe-Pack (Decco Israel) is using our product under a white label name in one of the largest packing house in Israel.
| 53 |
Sanitizer – SF3HS or SF3H
Post-harvest sanitizers are considered a pesticide and regulated by the EPA in the United States. The EPA will review toxicity data and results from tests to show how well the product kills bacteria to determine if the product should be approved. See “Government Regulation and Product Approval” below.
This sub-category of products is based on our proprietary blend of food acids combined with hydrogen peroxide as the oxizider and includes SF3HS and SF3H. We believe that this category of products will be an improved sanitizer as compared to traditional sanitizers. SF3HS and SF3H are public health antimicrobial pesticide products that bear a claim to control by at least a 3 log10 reduction (99.9%) pest microorganisms that pose a threat to human health (foodborne pathogens), and whose presence cannot readily be observed by the consumer.
After we have finalized our toxicological studies, we conducted a series of microbial trials in laboratories in both the United States and Israel in non-Good Laboratory Practice (“non-GLP”) settings in order to evaluate the efficacy of SF3H as an antimicrobial agent to reduce foodborne pathogenic bacteria in “processing water” for fruit and vegetables. We used a modification of the Association of Official Agricultural Chemists Germicidal and Detergent Sanitizing Action of Disinfectants method and test protocol EN1276 (European standard for the evaluation of chemical disinfectant or antiseptic for bactericidal activity). The tested organisms are Listeria monocytogenes, Salmonella enterica and Escherichia coli O157:H7.
The last test was performed by Analytical Lab Group on a mix culture of Listeria monocytogenes with an exposure time of 30 seconds. The results showed more than 99.99999% (>7.51 Log10) reduction. In Israel, the tests were performed by the Institute for Food Microbiology and Consumer Good Health on a single strain for each pathogen (Listeria monocytogenes, Salmonella typhimurium and E. coli) with exposure time of 30 seconds and the results have shown between 99.99% to 99.9999% reduction. Exposure time is a key parameter in sanitization process, therefore allowing a short contact time is a significant advantage over the competition where the current minimum contact time available is 45 seconds.
We plan to conduct GLP efficacy studies during the first half of 2021 to complete our regulatory dossier for the EPA and the FDA to obtain the appropriate regulatory approvals for our products to be marketed and used as “sanitizers” to claim control of foodborne pathogens (food safety) as well as plant pathogens (food loss reduction). We plan to submit our regulatory dossier during the second half of 2021. It is planned that SF3HS/SF3H will be used in post-harvest to control both plant and foodborne pathogens for fruit and vegetables (including microgreens). For additional information regarding the regulatory approval process see “Government Regulation and Product Approval” below.
Results on Avocados
We have tested the efficacy of our SF3H and SF3HS products against Listeria on 40 avocados of which 10 avocados were treated with our SF3H and SF3HS products. The peel of the avocado was punctured and infected with high level of Listeria. The results have shown a 99.99% reduction within fifteen seconds of exposure time. In addition, we have also tested the efficacy of SF3H on avocado’s shelf life compared to current treatments (12 avocados per treatment). The results (demonstrated below) show that after 18 days in room temperature the treated avocados display material reduction in microbial spoilage as compared to avocados treated with water and chlorine, a well-known sanitizer.

| 54 |
Results on Microgreens
An increasing number of studies point to the growing demand for locally sourced, organic vegetables. Various types of “young vegetables,” such as sprouts, microgreens and baby greens, are becoming increasingly popular due to their high nutritional value. Microgreens are deemed premium products and command higher retail value. They also belong to a group of “functional foods” and have high levels of bioactive compounds, while requiring less water and energy to grow, which they do year-round. Currently, microgreens are largely being cultivated in major greenhouses across the United States. According to Agrilyst, an agro-intelligence platform, greenhouse cultivation of microgreens was the highest in South and North East regions, each accounting for 71% and 59% in 2017. While consumers in the United States are more focused on growing leafy greens and microgreens than any other vegetables.
We have tested the efficacy of our SF3H products to control and prevent potential pathogen contamination on microherbs (pea and sorrel) produced by Israeli-based microgreens exporter 2BFresh. Our treatment combined a post-harvest spray application and a fogging treatment to be used in the cooldown storage room. In order to determine the efficacy of the product, 25 swabs were taken across 18 trays (nine of each microgreen species). The results (as presented herein) show more than a 90% reduction of the total bacterial load post-treatment (see table).
We believe that our SF3HS and SF3H provide improved sanitization of bacteria (including E. coli, Salmonella and Listeria) while leaving no toxic residues on fruits and vegetables.
We expect the first commercialization of SF3H and SF3HS at the earliest by the end of the first half of 2022.
Other Products
Our product portfolio also includes the SpuDefender and FreshProtect.
SpuDefender
SpuDefender is one of our EPA-registered products which targets and is designed to control the post-harvest potato sprout. Due to the European Commission’s decision on January 1, 2020 to no longer allow the use of the herbicide chlorpropham (the “CIPC”), the post-harvest potato industry is looking for new solutions. For over 50 years, CIPC was widely used as a sprout suppressing agrochemical agent applied to potatoes that were stored in processing facilities.
| 55 |
Following recent discussions with post-harvest experts and potential customers, we believe our SpuDefender product may offer a successful alternative to CIPC. During 2021, we plan to initiate pilot tests with potential customers to treat potatoes pre-storage (three to six months storage in average).
FreshProtect
FreshProtect is our second EPA registered product, which targets and is designed to control spoilage-creating microorganisms on post-harvest citrus fruit. The registered label of the product only allows us to market and sell our FreshProtect in the United States (excluding California). However, we believe our FreshProtect has a significant potential in reducing the bacterial load entering the packing house in the pre-harvest market. The non-toxicity of our FreshProtect allows its application up to the day of harvest (0-day pre-harvest interval), which is critical to prolong crop protection and reduce microbial spoilage.
We recently ran a proof of concept study under a controlled group environment of different plant fungi responsible for decay which showed promising initial results.
We also conducted a series of smaller studies, consisting of laboratory tests and tests on a limited number of lemon trees whereby we demonstrated significant reduction of decay in treated fruit and reduction in bacterial populations.
The main conclusions of the trials were that FreshProtect with concentration of 1% and 2% applied at 400 gallons per acre materially reduced sour-rot on inoculated fruit. While both rates were also effective against fruit inoculated with P. digitatum, (i.e. fungus found in the soil of citrus-producing areas and major source of post-harvest decay), the 2% concentration of FreshProtect demonstrated significantly more efficacy at reducing sour-rot. Natural incidence of Penicillium spp. (a family of fungi) was also reduced on fruit inoculated with G. candidum, fungus that is a member of the human microbiome.
Furthermore, FreshProtect can be used in combination with several different kinds of pesticides and fertilizers which allows the application of more than one pesticide at once. This in turn reduces cost and facilitates implementation. The graph below summarizes these results:
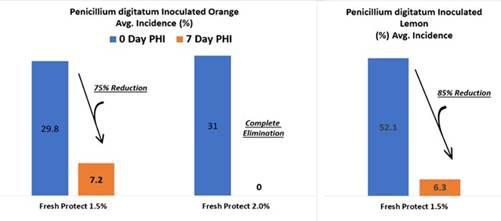
The regulation for pre-harvest (in the field) application especially in California as well as in Israel may take more time than post-harvest application due the potential impact on the environment. Therefore, we expect the product to reach the market during 2022.
| 56 |
Our Strengths
We believe that our main strengths include:
| ● | Strong Management Team with Commitment to Green Products. Led by a team with over 30 years of experience in developing sanitization products and solutions for the agriculture industry, we plan on becoming a significant player in providing consumers with healthy and green fresh produce from farm to fork while endeavoring to ensure food safety and reducing food waste. We believe that our proprietary blend of food acids provides protection to the treated produce and works in synergy with well-known fungicides and sanitizers. This synergy allows us to significantly reduce the concentration of the fungicides that are heavily regulated in several countries and, in certain countries, outright banned and meet the food trends of sustainable and green produce. | |
| ● | Multi-Purpose Products that Simplify Crop Treatment Routine and Save Money. While most chemicals marketed in the industry address either food safety or food waste, our multi-purpose products are intended to provide a solution for both problems, while simplifying crop treatment and achieving cost saving. Our products are capable of cleaning and controlling pathogens that would otherwise render fresh produce as unsafe for human consumption. Our proprietary blend of food acids combined with well-known sanitizers are very efficient against foodborne pathogens like E. coli, Salmonella and Listeria as well as plant pathogens in short contact time (99.999% reduction within 30 seconds of contact). In addition, with multipurpose products, there is no need to order, ship or dispose of bottles of product, resulting in less energy consumed, less CO2, less fuel, and less waste. We believe our focus on natural product chemistries will allow us to continually drive lower costs, higher product gross margins and efficacy through longer shelf life and reduction of food waste. | |
| ● | Strong Intellectual Property Portfolio. We believe that we have built a strong intellectual property position throughout the food chain (from field to fork) as our patents claim compositions and methods that can be used to protect food and agricultural products from decay. We rely on a combination of important intellectual property assets, to protect our innovation. Our employees, consultants, customers, and vendors are subject to confidentiality agreements that protect our proprietary manufacturing processes. Our patent portfolio includes granted patents in the United States, Europe, and Israel, as well as several priority applications, across several patent families, including composition-of-matter claims, methods of use claims, including for treating edible matter, for improving the appearance of edible plant matter, and sterilization methods, as well as for articles for implementing these methods. These patents directly protect a proprietary method for extending life shelf and reducing edible matter from microbial decay. | |
| ● | Commercially Available Products and Seamless Implementation. One of the oxidizers being used with our products is PAA, a well-known and widely used sanitizer. Following the enforcement of the FSMA in connection with the use of sanitizers, more and more packers have been choosing this healthy and eco-friendly sanitizer over chlorine, and this choice facilitates implementation of our products. In addition, the application of our products does not require special equipment as they are used in combination with or replace existing products applied on the packing line or in the mix tank in the field. This allows a relatively cheap, seamless and fast implementation. | |
| ● | Significant Reduction of Hazardous Chemicals Food Residue. All the ingredients in our blend of food acids are recognized by the FDA as GRAS when used as intended in fruit and vegetable wash applications, while oxidizers we use such as hydrogen peroxide rapidly decompose into water and oxygen. The absence of toxicological residues not only improves food quality but also promotes occupational safety for the employees of packing houses, contributing to a friendlier and safer working environment. |
| 57 |
Our Strategy
In September 2018, the Company changed its organizational structure and management team. After reviewing the Company’s then existing strategy and results of operation, as well as examining the market opportunities, the new management team decided to update the Company’s strategy, reduce the marketing and sales of its existing products, and focus the Company’s efforts and financial resources in developing its next generation products. During the years 2019 and 2020, we developed, validated and tested the efficacy of our next generation product – a blend of food acids – on a variety of crops in small and large scale commercial pilots.
Our strategy is to develop and commercialize our products through strategic partnerships with global post-harvest service companies and with large food distributors and retailers with the intent of: (i) extending the shelf life of fresh produce while reducing (and even eliminating) the use of harmful chemicals (fungicides); (ii) ensuring food safety and shelf life by controlling foodborne pathogens and allow our customers to meet FSMA regulatory requirements; (iii) reducing food loss and the associated carbon “footprint.” Our ultimate goal is to eventually gain presence in a variety of businesses compromising the food industry, including pre-harvest, post-harvest, retail and consumer businesses.
In order to achieve our goals, we intend to:
| ● | Advance our Breakthrough Technologies and Commercialization Efforts. During the first half of 2021, we plan to run a series of additional pilot studies in various commercial collaborations with post-harvest service vendors packing houses and food retailers. | |
| ● | Develop a Strong Marketing Message Around Promoting Safe Food While Avoiding Food Waste. We plan to brand our fresh produce with a “chemical residues free” seal of approval and we believe that like-minded fruit packers around the globe will seek to differentiate themselves from their competitors by obtaining this seal. | |
| ● | Acquire or License Complementary Products and Technologies. We actively search for products and technologies that can enhance our portfolio and grow our business to address all the post-harvest treatments such as fruit coating products or technologies. | |
| ● | Expand to Additional Produce and Geographies. Our plan is to focus first on key countries and regions with the largest markets for our crops, including Mexico, Spain, Italy, Israel and key markets in the United States such as California, Florida and Texas. We are also plan to increase the variety of crops that can be treated with our products, to include produce such as apples, bell peppers, tomatoes and papayas. | |
| ● | Leverage Our Products Through Collaborations. Our focus and expertise in the development of green products for the agritech industry and in post-harvest treatments allow us to be a partner of choice for other businesses looking for development partners and for larger companies wanting to leverage their product such as PAA into new combination products. For example, companies selling or owning fungicides, the MRL of which is being reduced, and that are working in synergy with our products are good partners. This type of collaboration could allow them to continue selling their product. | |
| Our selling and marketing strategy is twofold: | ||
| ● | Establish Collaborations with Food Retailers. Large food retailers play an important role in influencing the decisions of key suppliers down the food chain (i.e. they can dictate to their suppliers which cleaning solutions they will use when treating the fresh produce at their packing house). Food retailers must ensure food safety as well as reducing food loss occurring during distribution, storage and retail. In the United States alone, 4.65 million tons of fresh produce were thrown away at the retail level. This waste cost $8.9 billion and amounted to 12% of the U.S. fresh fruit supply and 10% of the fresh vegetable supply. With an average percentage of 5% of all strawberries, apples, avocadoes, tomatoes and broccoli in the United Kingdom and 12% and 10% of the fresh fruit supply and fresh vegetable supply in the United States, respectively, wasted at the retail and/or distribution level, we believe even a small reduction in fresh produce loss can generate large savings. | |
| 58 |
| ● | Partner with Service Vendors to Fruit and Vegetable Packing Houses. Post-harvest service companies provide packing houses with the necessary machinery and products, such as sanitizers and fungicides to enable the packing houses to treat the produce. Post-harvest service companies face competition due to the current regulations that dictate specific types of treatment products and set tolerable residue levels. In addition, these companies must provide their packing houses with state of the art, cost effective and green product to incentivize the packing houses to build long-term business relationship with them. Moreover, service vendors are important because they have strong influence on the decision of which cleaners, sanitizers, fungicides and waxes that the fruit and vegetable packing houses will use. Additional benefit of partnering with post-harvest service company is their strong global market presence in the relevant geography. In addition, both food retailers and post-harvest service companies have a significant interest in green and efficient post-harvest solutions. |
Selling and Marketing
We concentrate our marketing efforts on high value crops, such as avocado, mango, citrus, apples, pears, bananas, papaya, bell pepper, lettuce and tomatoes and target large producers of these crops as well as large food distributors and retailers. As of the date of this prospectus, we are exploring collaboration opportunities for our SavePROTECT or PeroStar with producers in the United States, Spain, Israel, Italy and Mexico. And we intend during the next 12 months to initiate commercial collaboration with local packers and retailers in our first targeted location previously mentioned.
The table below summarizes the market opportunities for selected produce in our target markets.
| Bananas | Apples and Pears | Avocados | Citrus | Mangos | Papayas | Tomatos | Lettuce and Chicory | Chilli and Green Peppers | Total (in milion tons) | |||||||||||||||||||||||||||||||
| Global Production of the Crop (in million ton)1 | 114.2 | 108.2 | 6.0 | 147.9 | 52.3 | 13.1 | 180.5 | 27.5 | 35.8 | 685.5 | ||||||||||||||||||||||||||||||
| Production of the Crop in the Company’s Target Markets 2 (in million ton) | 2.9 | 10.5 | 2.4 | 25.6 | 2.3 | 1.0 | 28.0 | 6.5 | 5.6 | 84.7 | ||||||||||||||||||||||||||||||
| Production of the Crop in the Company’s Target Markets (in %) | 2.5 | % | 9.7 | % | 39.8 | % | 17.3 | % | 4.3 | % | 7.6 | % | 15.5 | % | 23.6 | % | 15.7 | % | 12.4 | % | ||||||||||||||||||||
| 1. | Average global production for the years 2016, 2017 and 2018. | |
| 2. | Our target markets include Israel, Italy, Mexico, Spain and the United States. |
To support our efforts, we will increase our marketing and sales team as well as services in our target location to support all our efforts.
United States
The first market we target for the sale and distribution of SavePROTECT is the post-harvest citrus industry in the State of California, which alone accounts for approximately 80% of all fruits and vegetables in the United States.
Over the last three years, we have treated more than 180,000 tons of citrus fruit with the different version of our SavePROTECT product. Under the supervision of a world leading packing house to the citrus fruit industry, we evidenced SavePROTECT utility as having a good safety profile, ensuring food safety and in controlling microbial spoilage. We plan to leverage this collaboration in order to further penetrate the citrus based fruit packing industry, both in California and beyond.
The Post-harvest treatment market for fruits and vegetables, which is projected to grow from $1.5 billion in 2019 to $2.3 billion by 2026, growing at a CAGR of 6.5% during the forecast period, is led globally by select companies, including DECCO U.S. Post-Harvest, Inc., (United States), Pace International, (United States), Xeda International (France), John Bean Technologies (United States), and Agrofresh (United States).
Once we have completed our studies and secured the appropriate regulatory approvals, we plan to further penetrate this market. We currently focus on post-harvest treatment for the citrus industry (as well as mangos and avocados) but are conducting pilot studies with leading players in the industry to evaluate and validate our products formulations.
We plan on commencing one commercial pilot study with a global food retailer by the end of the first quarter of 2021.
On September 22, 2020, we entered into a non-exclusive commission agreement with Earthbound Technologies, LLC (“EBT”) for a period of 12 months, according to which EBT will introduce us to potential clients, pre-approved by us (the “Introduced Parties”) and will assist us in finalizing commercial agreements with the Introduced Parties. In consideration for its services, we agreed to pay EBT 12.5% of the net revenues generated from the Introduced Parties (during the agreement period and within 18 months following the termination of the agreement) up to a total aggregated amount of $2,000,000, provided that the compensation shall not exceed 25% of our gross profit under the given commercial agreement signed with the Introduced Party. In addition, in the event that the aggregated net revenues generated from the Introduced Parties exceeds $500,000, and subject to the approval of our board of directors, we will issue to EBT 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share. In the event that certain additional events detailed in the agreement occur, we will also issue to EBT, subject to the approval of our board of directors, an additional 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share.
Israel
On September 11, 2020, we signed a five-year exclusive distribution agreement (the “Distribution Agreement”) with Safe-Pack Products Ltd. (“Safe-Pack”), according to which we granted Safe-Pack an exclusive right to resell, distribute, advertise, and market ours products in the citrus industry in Israel and Palestine. In addition, we agreed to grant Safe-Pack a right of first refusal to be designated as an exclusive distributor of ours in certain agreed upon territory for additional products of ours in the post-harvest market. In consideration for the above rights granted to Safe-Pack, Safe-Pack will submit to us purchase orders of our products at a price specified in the Distribution Agreement. Commencing upon the second calendar year of the agreement, Safe-Pack is required to meet a minimum purchase quota, as shall be mutually agreed upon between the parties. In the event that the parties fail to agree on a quota, the quota shall be equal to last year quota plus 3%.
Spain
We have been collaborating with one of the world’s leading post-harvest treatment service vendors in Spain since June 2020, where we are examining our product on citrus fruit. We believe our product could be an improved alternative to current fungicides that will soon be significantly reduced in this market.
Mexico
We are planning a series of studies in which our SavePROTECT will be applied on tomatoes, bananas, lemons, bell peppers and avocados. The first study to ensure safety of avocados is planned for the first quarter of 2021.
| 59 |
Between August and October of 2020, we have conducted three successful trials in Mexico, on more than 200 kilograms of Persian limes. The results have shown that the addition of SavePROTECT to current treatments extend shelf life. Shelf life was tested for 25 days and results have shown that SavePROTECT substantially reduces decay. Mexico is the largest producer of Persian limes and is deemed to be that country’s second most important citrus fruit.
Intellectual Property
We rely on patents and trade secret protection laws to protect our proprietary products and intellectual property. We entered into confidentiality agreements with our employees, consultants, customers, service providers and vendors that cover, inter alia, our technology and proprietary manufacturing processes.
As of the date of this prospectus, we own five issued patents, one allowed patent, and seven pending patent applications, four of which may be submitted worldwide. Expiration dates of our patents, and any patents which may be granted under our pending patent applications, are from 2031 through 2041. Our patent family includes patents granted in Israel, the United States and Europe.
Compositions and Methods of Treating Edible Matter and Substrates Therefor
This patent family includes granted patents in the United States, Israel, and an allowed application in Europe and is directed to a method for protecting edible matter from decay by applying to the edible matter a disinfecting composition containing, among other things, (1) phosphonic or phosphoric acid, (2) a carboxylic acid, (3) performic acid, (4) a performic acid source (such as formic acid) and an oxidizer (such as hydrogen peroxide).
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-001-EP | Europe | Patent | Allowed | 11825901.9 | September 14, 2010 | |||||
| SVF-P-001-IL | Israel | Patent | Issued | 225247 | September 14, 2010 | |||||
| SVF-P-001-IL1 | Israel | Patent | Pending | 254909 | September 14, 2010 | |||||
| SVF-P-001-US1 | United States | Patent | Issued | 10,212,956 | September 14, 2010 | |||||
| SVF-P-001-US2 | United States | Patent | Pending | 16/278,108 | September 14, 2010 |
Methods for Improving the Appearance of Edible Plant Matter
This patent family includes a granted patent in Israel and is directed to a method of improving the appearance of edible plant matter either during the pre-harvest or post-harvest stage. The method includes applying a composition based on phosphonic acid to the edible plant matter.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-002-IL | Israel | Patent | Issued | 229724 | May 30, 2011 |
Method and Apparatus for Maintaining Fresh Produce in a Transportation Container
This patent family includes granted patents in Israel and in the United States. The patent family is related to a method of using thereof for maintaining fresh produce stored in a transportation container. The apparatus is configured to generate an aerosol of one or more liquid pesticides, thereby reducing pathogenic contamination within the transportation container. This patent family covers any liquid pesticide for use in the above-mentioned apparatus.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-003-IL | Israel | Patent | Issued | 227328 | June 23, 2013 | |||||
| SVF-P-003-US | United States | Patent | Issued | 9,487,350 | June 23, 2013 |
| 60 |
Sterilization Compositions and Methods for Use Thereof
This patent family is directed to compositions and methods for reducing pathogen load within a container or on a surface, including inter alia the surface of an edible plant matter. Furthermore, the application is directed to compositions and methods for disinfection of cooling systems.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-004-USP | United States | Patent | Pending | 63/111,197 | November 9, 2020 |
Sterilization Devices and Methods for Use Thereof
This patent family is directed to a device for controlling pathogen load within a container or on a surface by spraying a disinfecting composition in response to a trigger, such as increased pathogenic contamination.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-005-USP | United States | Patent | Pending | 63/111,220 | November 9, 2020 |
Compositions Comprising of Several Organic Acids and Use Thereof
This patent family is directed to kits and methods for controlling pathogen load within or on the surface of an edible plant matter.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-006-PCT | International application | Patent | Pending | PCT/IL2021/050229 | March 1, 2020 |
Combined Fungicidal Preparations and Methods for Use Thereof
This patent family is directed to compositions and to methods for reducing pathogen load on a substrate.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-007-USP | United States | Patent | Pending | 63/042,622 | June 23, 2020 | |||||
| SVF-P-007-USP1-07931-P0004B | United States | Patent | Pending | 63/126,649 | December 17, 2020 |
We cannot be sure that any patent will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future. There is also a significant risk that any issued patents will have substantially narrower claims than those that are currently sought.
Competition
Given that the market for the use of green and “residue free” solutions is evolving, we are continually facing growing competition. The market for post-harvest solutions is fragmented and includes various regional suppliers. The market of post-harvest treatments for fruits and vegetables is dominated by five large players with wide reach across the globe. We believe that a market edge will be given to a company that can solidify its reputation, product quality, customer service and customer intimacy, product innovation, technical service and value creation. Based on these variables, we believe that we compete favorably when compared with the global competition in this market.
Currently, our main competitors are companies providing PAA, chlorine and other sanitization solutions, such as Ozone as well as technology companies developing new biorational fungicides.
| 61 |
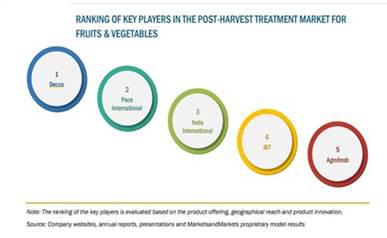
We also compete with heavily diversified multi-national chemical conglomerates, who produce various biocide formulations designed to kill or deactivate pathogenic micro-organisms. Of these, two companies are the most significant:
| ● | Peroxychem: Peroxychem is a subsidiary of Evonik Industries AG (Germany). It is a significant worldwide producer of hydrogen peroxide, persulfates and PAA. Peroxychem expected revenue of approximately $300 million in 2018; and | |
| ● | Solvay (Belgium): Similar to Evonik Industries, Solvay is a heavily diversified multinational chemical conglomerate. In fiscal year 2019, the company had approximately €10.2 billion in net sales, spread across the breadth of their product lines. Most relevant to us is their blends of PAA and hydrogen peroxide, sold in two primary formulations – OXYSTRONG for water treatment and PROXITANE for the food industry. |
In addition, we have several indirect competitors, which are companies with whom we seek to make strategic partnerships – large companies specializing in post-harvest solutions for the agricultural industry. Such companies include:
| ● | Decco US Post-Harvest: Decco is a subsidiary of Decco Worldwide, which itself is a division of United Phosphorous Ltd. Decco provides a variety of solutions, both mechanical and chemical, for the post-harvest industry. They produce conventional fungicides (imazalil, thiabendizole, etc.), as well as produce coatings; and | |
| ● | Pace International: Pace International is a subsidiary of the Sumitomo Chemical Company. Similar to Decco, it provides a variety of solutions – primarily in the realm of conventional fungicides and carnauba wax coatings for fruit. |
We also consider Xeda International, JBT and Agrofresh as our indirect competitors (and current or potential collaborators).
The organic market offers a huge trade and income potential for producers, processors and trading companies globally. The rising demand of various organic products has driven the demand of organic post-harvest treatments. Green and organic technologies are increasingly being developed in a global market and several conventional post-harvest product and equipment suppliers, such as Citrosol, Fomesa and Peroxychem, have taken the opportunity and are starting to develop natural products.
Research and Development
In the last two years we spent an aggregate of $1,032,623 on research and development. We focus on developing innovative solutions consisting of new generation, patented products that address immediate and long-term needs. Our research efforts are aimed at optimizing the application protocols of our existing core products such as PeroStar/SavePROTECT for new crops, developing new blend of acids and enhancing our SF3H/SF3HS products’ antimicrobial efficacy while taking into account costs, consumers trends and preferences, which will give the extra value needed to separate our products from those of our competitors in the marketplace.
| 62 |
Post-Harvest
We are currently working on the compatibility and synergistic effect of our processing aids products SavePROTECT/PeroStar with additional post-harvest treatments used in the packing house to provide an efficient, greener and cost-effective solution.
We are also focused on the characterization of our new sanitizers including the identification, improvement and other validations of our formulas. These products are based on a unique stabilization process that blends hydrogen peroxide and food acids to create a broad-spectrum, safe and eco-friendly solution for killing germs. The synergistic effects of combining hydrogen peroxide with food acids produce a stable yet environmentally safe and easy to handle sanitizer. We use a network of experts in related fields, such as microbiology, and food chemistry to obtain all the required regulatory approvals.
In addition, we are in the initial stages of development of natural antimicrobial edible coatings for microbial safety and food quality enhancement comprising our acid blend.
To accurately test the strength of a sanitizing solution, we are working on developing quantitative methods. Similarly, we are developing analytical methods that will enable rapid and effective monitoring of the active ingredients through a novel and improved testing kit that allows for testing at a faster pace and with greater certainty.
Pre-Harvest – FreshProtect
We are also focused on developing new eco-friendly pre-harvest products which will improve post-harvest management practices by reduction of total microbial load, before even entering the packing house. An effective pre-harvest treatment may reduce the need for post-harvest chemical fungicides while increasing profit through reduced spoilage in supply chains. In addition, the potential of addressing pre-harvest treatment might offer new opportunities for treating crops, such as rice and wheat, with large market potential.
In pre-harvest application, one of the main advantages of our products is the non-toxicity of its ingredients, allowing its application up to the day of harvest (0-day pre-harvest interval), which is critical to prolonged crop protection and reduced microbial spoilage while reducing the total bacterial load entering the packing house. Field studies are conducted by Dr. James E. Adaskeveg from University of California, Riverside and the largest grower cooperative in CA in the United States on citrus trees to determine the effectiveness, optimize use protocol and effect on the environment.
Production
In Israel, we work with SasaTech, a reputable chemical production company. Based on our formulation and guidance, SasaTech is producing our PeroStar and any other small-scale formulation that we might need for research and development purposes and trials. SasaTech is particularly regarded for its deep understanding and experience working with oxidizer like hydrogen peroxide and PAA. We also work with Zohar Dalia which we engage on a case-by-case basis.
In the United States we work with Seeler Industries, a national leader in marketing, handling, and in the termination of hydrogen peroxide. Both Seeler and SasaTech, purchase all raw materials necessary for the production of our products.
All ingredients and/or raw materials that are used in the creation of our products are commodities and are readily available for purchase off the shelf.
| 63 |
Government Regulation and Product Approval
Our products are subject to national, state and local government regulations. Based on the product claims and classification, different regulatory and registration requirements may apply at the state, provincial or federal level.
Regulation of Our Sanitizers – SF3H and SF3HS
In the United States, the primary federal laws that regulate the sale and distribution of our sanitizer products are the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”) and the Federal Food, Drug and Cosmetic Act (“FFDCA”).
FIFRA is the federal law that regulates the sale and distribution of pesticides and is administered by the EPA. Products that claim or are otherwise intended to control microorganisms on inanimate surfaces, in water and on raw agricultural commodities are regulated, under FIFRA, as pesticides. FIFRA generally requires the pre-market registration of pesticide products. To register a pesticide product, we are required to provide test data and related information to demonstrate that the product is safe and effective under the conditions of use, as specified on the product label. The cost and timeframe to achieve EPA product registration depends on the type of product and the claims made for the product. Registered products are also subject to a number of recordkeeping and reporting obligations which require constant product oversight by companies.
Pursuant to FIFRA and Section 408 of the FFDCA, the EPA establishes tolerances for pesticide chemical residues that could remain in or on food, including raw agricultural commodities. A tolerance is the EPA established maximum residue level of a specific pesticide chemical that is permitted in or on a human or animal food in the United States. Generally, any pesticide chemical residue must have either a tolerance or an exemption from the requirement to have a tolerance in order to be permitted in or on human or animal food. The FDA enforces the tolerances pursuant to its authority under the FFDCA.
The FFDCA regulates the sale and distribution of drugs, medical devices, cosmetics and foods (including substances added to and found in food such as pesticide residues) and is administered by the FDA. Under the FFDCA, the FDA does not register or approve products that are used on food commodities and certain food-contact surfaces, such as food packaging. However, all substances that are used on food or food-contact surfaces need to be subject to an FDA regulation or permitted through other clearance mechanisms, such as a Food-Contact Notification (FCN), Threshold of Regulation (TOR) opinion, by Prior Sanction or be “Generally Recognized as Safe” or “GRAS”. If all the substances or ingredients in a particular product are cleared for use on food or food-contact surfaces or are GRAS then a company can market a product without obtaining any additional clearances. GRAS substances do not require pre-market approval or clearance by the FDA although the FDA does have a notification process for GRAS substances.
At the federal level, antimicrobial agents are subject to regulation by the FDA and/or EPA, either singly or jointly, depending upon the intended use of the product. Antimicrobial products applied to processed food are solely regulated by the FDA per longstanding FDA and EPA policy outlined in an EPA Notice titled “Legal and Policy Interpretation of the Jurisdiction Under the Federal Food, Drug, and Cosmetic Act of the Food and Drug Administration and the Environmental Protection Agency Over the Use of Certain Antimicrobial Substances” (63 Fed. Reg. 54,532 at 54,536 & 54,541 (Oct. 9, 1998)) and EPA’s Pesticide Registration Manual, Chapter 18. Antimicrobial products applied to raw agricultural commodities (e.g. fruits and vegetables) are jointly regulated by the EPA and the FDA if their application takes place in a food-processing facility. If the antimicrobial product is applied to a raw agricultural commodity in a treatment facility that solely washes and packs food commodities, and the treatment does not change the status of the food as a raw agricultural commodity, then the EPA has sole federal regulatory jurisdiction.
Since our sanitizers will be and are intended to be used solely to treat raw agricultural commodities in post-harvest washing and packing facilities, at the federal level they are regulated solely by the EPA (as opposed to FDA): product registration is required under FIFRA and any food residues are regulated under the FFDCA. To complete the registration process, we will be required to submit a number of studies in the form of a registration application or dossier, which has not yet been submitted to EPA. These studies will specifically include: (i) safety studies - six acute toxicity studies (already finalized), (ii) physio-chemical properties testing (already finalized), (iii) one-year storage stability and corrosion (ongoing), and (iv) an efficacy study to demonstrate that the product is an effective sanitizer (studies conducted under non-good laboratory practices already performed and they show the product meets EPA performance standards). We have already identified and engaged with a third-party company in the United States to perform our good laboratory practices efficacy studies.
In addition, every state has its own laws that regulate pesticides and these laws require registration of pesticide products at the state level. Accordingly, products must also be registered in the states in which they are distributed prior to any sale.
Regulation of Our Processing Aid – SavePROTECT or PeroStar
In the United States, our SavePROTECT product does not make any pesticidal claims and is not intended for use as a pesticide and, therefore, is not subject to the registration requirements of FIFRA. However, since the product is used on raw agricultural commodities in food processing facilities, the product is subject to regulation under the FFDCA. We believe that the product is in compliance with the FFDCA since every ingredient in the product can be considered GRAS when used as intended.
Although SavePROTECT is not a pesticide under FIFRA, it is still required to be registered in California because the California statute requires the registration of both pesticide and adjuvant products. Based on the intended use and claims for SavePROTECT, we believe that product falls within California’s definition of a spray adjuvant.
| 64 |
On July 31, 2020 we submitted an “Application for Registration of Adjuvant” for SavePROTECT to the CDPR. The dossier submitted included the following studies: (i) acute oral toxicity and acute dermal toxicity studies, (ii) physico-chemical property testing (determination of color, physical state, odor, density, pH, viscosity and oxidation/reduction chemical incompatibility), (iii) validation of the high-performance liquid chromatography method assay, (iv) stability test, and (iv) efficacy data. We anticipate registration of SavePROTECT during the second half of 2021, however, there can be no assurance that we will obtain such registration.
In addition, based on the opinion of our U.S. regulatory experts, all SavePROTECT ingredients are GRAS when used as intended.
During the second half of 2021 we plan to submit an application for certification for our SavePROTECT to the Organic Materials Review Institute (the “OMRI”), an international nonprofit organization that determines which input products are allowed for use in organic production and processing. OMRI Listed® products are allowed for use in certified organic operations under the United States Department of Agriculture (the “USDA”) National Organic Program. OMRI reviews input products to verify that they meet the organic standards for use on organic farms or in organic processing. OMRI is recognized by the USDA National Organic Program as a reputable third-party input reviewer in Interim Instruction 3012 of the NOP Handbook. In addition, OMRI is accredited under the International Organization for Standardization (ISO) 17065 by the USDA Quality Assessment Division.
In Europe, processing aids are defined as substances that are added to exert a technological function during food processing and which may end up in the finished product. According to Regulation (EC) No. 1333/2008, processing aids means any substance which (i) is not consumed as food by itself; (ii) is intentionally used in the processing of raw materials, foods or their ingredients, to fulfil a certain technological purpose during treatment or processing; and (iii) may result in the unintentional but technically unavoidable presence in the final product as residues of the substance or its derivatives, provided they do not present any health risk and do not have any technological effect on the final product.
Processing aids are differentiated from food additives, which are substances that are added to food with the intention to exert a technological function within the final food product. Therefore, processing aids must not follow the European Food Safety Authority (the “EFSA”) guideline of “Data Requirements for the Evaluation of Food Additive Applications.”
In Europe, our PeroStar is not considered a processing aids in the enzymatic preparation category and, therefore, PeroStar is only regulated at the national level. While there are no harmonised requirements regarding the registration of a processing aids, some data (such as full composition and some toxicological data) must be disclosed and discussed with the competent authorities before the submission of a registration request.
In Spain, the guidelines for precise documentation for evaluation of technological adjuvants intended to be used in human food, state specific conditions for the assessment, authorisation and use of all other types of processing aids, which are not processing aids in the enzymatic preparation category. We have submitted during the third quarter of 2020 a regulatory dossier as a processing aid for PeroStar in Spain and Italy with very similar information as the regulatory dossier submitted in California.
In Mexico, based on the product composition and the legal status of the substances to be used as food additives, our PeroStar/SavePROTECT can be marketed and used in Mexico as a food additive (processing aid) and no registration is required (pending the final confirmation from Mexican government).
In Israel, the guidelines of the National Food Services, Ministry of Health, define the requirements for cleaning and disinfectant agents used with food. These guidelines state that such cleaning and disinfectant agents applied to the cleaning equipment which comes into direct contact with food, must not contain carcinogens. Specifically, List A and List B published by the Inter-ministerial Committee on Carcinogens, Mutagens and Teratogens of the Ministry of Health identify products and ingredients with carcinogenic, mutagenic and teratogenic properties. Our regulatory consultant in Israel has confirmed that our PeroStar does not contain carcinogens, mutagens and/or teratogens, and, therefore, is considered approved in terms of the relevant regulations of the National Food Services, Ministry of Health, and can be used as an additive to cleaning and disinfectant agents for fresh produce.
Registration of Our SpuDefender and FrehProtect
We currently have registrations for our SpuDefender (EPA Reg. No. 86381-1) and our FreshProtect (EPA Reg. No. 86381-2), at both the federal level and in the individual states where the products are sold for the use in post-harvest settings. To allow the utilization of our FreshProtect in pre-harvest settings, additional studies will need to be submitted to the EPA.
Organizational Structure
We currently have one 98.94% owned subsidiary: Save Foods Ltd., which is incorporated in the State of Israel. Save Foods Ltd. is responsible for all of our research and development and sales activities.
| 65 |
Property and Facilities
Our research and development and manufacturing operations are currently conducted at Kibbutz Alonim (Israel) where we lease approximately 70 square feet of space to run our lab studies. The lease expires on December 31, 2021. Our current monthly rent payment is NIS 4,846 (approximately $1,400) which includes taxes.
We believe that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
Employees
As of April 6, 2021, we employ two full-time employees and four part-time employees. None of our employees are members of a union or subject to the terms of a collective bargaining agreement.
Legal Proceedings
We are not currently subject to any material legal proceedings.
Company History
We were incorporated under the name Pimi Agro Cleantech, Inc. on April 1, 2009, under the laws of the state of Delaware. On April 11, 2016, we changed our name from Pimi Agro Cleantech, Inc. to Save Foods, Inc. Our subsidiary was incorporated on January 14, 2004 under the name Pimi Marion Holdings Ltd., to exploit the knowledge, intellectual property and business assets of Nir Ecology Ltd., a company founded in September 1989, focused on developing sanitizing solutions for the water and food industry. During the initial years of its activity and until 2009, Pimi Marion Holdings Ltd. focused on the development of new products and applications within the potato-growing industry. On October 5, 2008, Pimi Marion Holdings Ltd. changed its name to Pimi Agro Cleantech Ltd. In September 2018, we changed our organizational structure and leadership team to support our new strategy and objectives. The goal of the organizational change was to drive the Company towards regulatory approvals for our new generation of products. Our revamped strategy was developed following research we conducted on the applicable and potential commercial markets for our products. The results of this research demonstrated a clear and significant market for our new products to be deployed as sanitizers for the agricultural and food tech industries. On May 2, 2019, Pimi Agro Cleantech Ltd. changed its name to Save Foods Ltd.
| 66 |
Executive Officers and Directors
The following table sets forth information regarding our executive officers, key employees and directors as of the date of this prospectus:
| Name | Age | Position | ||
| Prof. Benad Goldwasser (1) (11) | 69 | Chairman of the Board of Directors | ||
| David Palach | 55 | Chief Executive Officer | ||
| Dan Sztybel | 43 | Chief Executive Officer of Save Foods Ltd. | ||
| Shlomo Zakai (2) | 51 | Chief Financial Officer | ||
| Nimrod Ben Yehuda (3) | 67 | Chief Technology Officer | ||
| Dr. Neta Matis | 37 | Vice President of Research and Development | ||
| Dr. Arthur Dawson | 78 | Business Manager of U.S. Operations | ||
| Vered Raz-Avayo (4) (5) (10) | 49 | Director | ||
| Ronen Rosenbloom (5) (6) (7) (8) (9) | 48 | Director | ||
| Israel Berenshtein (5) (6) (8) (9) | 49 | Director | ||
| Amitay Weiss (5) (7) (10) | 58 | Director | ||
| Eliahou Arbib (5) (6) (8) (11) | 54 | Director | ||
| Udi Kalifi (5) (7) | 42 | Director Nominee |
| (1) | Prof. Benad will resign from his position as Chairman of the board of directors of Save Foods, Inc. upon the closing of this offering. We expect Prof. Benad to be nominated as Chairman of the board of directors of Save Foods Ltd. following his resignation. |
| (2) | Mr. Zakai will resign from his position as Chief Financial Officer of the Company upon the closing of this offering. We expect Mr. Zakai to be appointed as Controller of the Company following his resignation. |
| (3) | Mr. Ben Yehuda has been on a temporary leave without pay (furlough) since March 2020. |
| (4) | Ms. Raz-Avayo will resign from her position as a member of the board of directors of the Company upon the closing of this offering. We expect Ms. Raz-Avayo to be appointed as Chief Financial Officer of the Company following the resignation of our current Chief Financial Officer. |
| (5) | Independent Director |
| (6) | Member of the Compensation Committee |
| (7) | Member of the Audit Committee |
| (8) | Member of the Nominating and Corporate Governance Committee. |
| (9) | Member of Class I with a term ending at the 2021 annual meeting of stockholders. |
| (10) | Member of Class II with a term ending at the 2022 annual meeting of stockholders. |
| (11) | Member of Class III with a term ending at 2023 annual meeting of stockholders. |
Prof. Benad Goldwasser, Chairman of the Board of Directors
Prof. Goldwasser has served as chairman of our board of directors since May 2018. He has also served as chairman of the board of directors of our subsidiary, Save Foods Ltd., since May 2018. Prof. Goldwasser has served as chairman of the board of directors of ScoutCam Inc (OTC: SCTC) since March 2019, and as a member of the board of directors of Innoventric Ltd. since September 2017. Prior to that, Prof. Goldwasser has served as chairman of the board of directors of Medigus Ltd. (Nasdaq and TASE: MDGS) from September 2018 to December 2019, and as a consultant to Shanghai-Israel Investment Fund from May 2016 to May 2019. In 2016, Prof. Goldwasser launched a venture capital fund partnered with Shanghai Alliance Investment Ltd (SAIL), a Shanghai Government investment company. Prof. Goldwasser has also served on the board of directors of BioCanCell Ltd. (TASE: BICL) from 2013 to 2016. Prof. Goldwasser holds an MD and MBA from Tel-Aviv University. We believe that Prof. Goldwasser is qualified to serve on our board of directors because of his leadership in conceptualizing and developing our brand and business, his entrepreneurship skills and his 27 years of experience building businesses.
| 67 |
David Palach, Chief Executive Officer
Mr. David Palach has served as our chief executive officer since January 2021. Mr. Palach has owned and served as chief executive officer of S.T. Sporting LTD and Sun Light Lightning Solutions LTD, companies operating in the environmental industry since 2009 and 2015, respectively. Mr. Palach holds a BBA in Accounting from Baruch College/City University of New York and completed a Directors Course at Bar Ilan University in Israel. Mr. Palach previously maintained a certified public accounting license in the State of Maryland.
Dan Sztybel, Chief Executive Officer of Save Foods Ltd.
Mr. Sztybel has served as the chief executive officer of Save Foods Ltd. since April 2019. Mr. Sztybel previously served as the chief executive officer of the Company from April 2019 to January 2021. Prior to this, Mr. Sztybel has served as our vice president of business development from October 2018 to March 2019. Prior to joining us, Mr. Sztybel has served as principal at Goldmed Ltd., a consulting firm from September 2016 to September 2018. Mr. Sztybel is the founder of Dan Sztybel Consulting Group, a boutique firm advising global leaders and emerging startups in the healthcare field on strategy, partnerships, and investments and has served as its managing director/ since November 2014. Mr. Sztybel is also the co-founder of MyndYou, a digital health-tech company. Prior to that, Mr. Sztybel led the life sciences and healthcare advisory team at Kost, Forer, Gabbay & Kasierer, a member firm of Ernst & Young Global from July 2007 to November 2014. Mr. Sztybel received his B.Sc. and M.Sc. in molecular biology and biotechnology from Bar-Ilan University. Mr. Sztybel also completed a special EY-Kellogg-Recanati business program for employee excellence.
Shlomo Zakai, Chief Financial Officer
Mr. Zakai has served as our chief financial officer since August 2017. Mr. Zakai has served as chief financial officer of UAS Drone Corp. (OTC: USDR) since May 2020, as chief financial officer of Sonovia Ltd. from October 2014 to August 2020, as chief financial officer of Blue Sphere Corporation (OTC: BLSP) from January 2012 to May 2016, and as chief financial officer of Todos Medical Ltd. (OTC: TOMDF) from February 2017 to December 2017. Mr. Zakai has established Shlomo Zakai CPA in in 2004, an accounting firm providing a range of services to publicly traded and private companies. He also previously worked as an accountant for nine years at Kost, Forer, Gabbay & Kasierer, an independent registered public accounting firm and a member firm of Ernst & Young Global, where he last served as a senior manager and worked with technology companies publicly traded on Nasdaq and in Israel. Mr. Zakai holds a BA in Accounting from the College of Management in Rishon Le’Zion, Israel, and is a certified public accountant in Israel.
Nimrod Ben Yehuda, Chief Technology Officer
Mr. Ben Yehuda has served as our chief technology officer since our inception in 2009. Mr. Ben Yehuda has also served as our chief executive officer from March 2014 to May 2017 and from February 2018 to September 2019 and served on our board of directors from August 2005 to October 2019. Mr. Ben Yehuda has been a leading entrepreneur in the area of environmentally friendly solutions using hydrogen peroxide in many applications. Mr. Ben Yehuda is the founder of our subsidiary, Save Foods, Inc. (formerly Pimi Marion Holdings Ltd. and Pimi Agro CleanTech Ltd.), which was established to exploit the knowledge, intellectual property and business assets of Nir Ecology Ltd.
Dr. Neta Matis, Vice President of Research and Development
Dr. Matis has served as our vice president of research and development since January 2019. Prior to joining us, Dr. Matis has served in various roles at Virdia Inc. including head of process development from January 2017 to October 2018 and research and development chemist from January 2012 to December 2017. Dr. Matis has a proven track record of leading innovation and proficiency in developing refinery-scale agricultural processes. Dr. Matis holds a PhD in Chemistry and an MBA from Tel Aviv University.
| 68 |
Dr. Arthur (Art) Dawson, Business Manager of U.S. Operations
Dr. Dawson has served as our business manager of U.S. operations since January 2020. Since March 2001, Dr. Dawson has served as the president of The Dawson Company, a boutique consulting firm with expertise in product commercialization and market development for shelf-life extension products in the post-harvest market, Previously, Dr. Dawson held several key positions in the post-harvest industry, including the general manager of Decco Worldwide from December 1989 to June 1999, one of the largest post-harvest service company worldwide, the vice president postharvest fungicides of Ecogen, Inc. from June 1999 to February 2001, as well as different managerial position at Sunkist Growers, Inc. from October 1973 to November 1983 and Dole Fresh, Inc. from November 1983 to November 1989. Dr. Dawson holds a master’s degree in Plant Science and a Ph.D. in Plant Physiology from the University of California, Riverside. Dr. Dawson also holds a license as a pest control advisor in California.
Vered Raz-Avayo, Director
Ms. Raz-Avayo has served as a member of our board of directors since August 2018. Ms. Raz-Avayo is a business strategy and a financial consultant. She has served on the board of directors of Foresight Autonomous Holdings Ltd. (TASE and Nasdaq: FRSX) since July 2017, Apollo Power Ltd. (TASE: APLP) since December 2017 and Africa Israel Residences Ltd. (TASE: AFRE) since November 2012. Prior to that, Ms. Raz-Avayo served as chief financial officer at one of the companies under the Levayev group from December 1999 to April 2010. In addition, during the last 12 years, Ms. Raz-Avayo served on the board of directors of S.R Accord LTD (TASE: SRAC) since July 2010 until July 2016, Analyst I.M.S mutual funds management (1986) LTD since May 2012 until May 2017, Safe -T group LTD ( Nasdaq: SFET) since March 2016 until March 2019, Naaman group (n.v) LTD since July 2012 until July 2017, TMDA LTD since June 2016 until June 2020. Ms. Raz-Avayo holds a BA in Business Administration – Accounting and Finance, from the College of Management, and an MFA in Film, TV and Screenwriting from the Faculty of Arts of the Tel-Aviv University. Ms. Raz-Avayo is also a certified public accountant in Israel. Ms. Raz-Avayo was selected to serve as a member of our board of directors due to her extensive managerial and consulting experience in finance encompassing a wide range of industries in Israel and overseas.
Ronen Rosenbloom, Director
Mr. Rosenbloom has served as a member of our board of directors since August 2020. Mr. Rosenbloom is an independent lawyer and has been working out of a self-owned law firm specializing in white collar offences since 2004. Mr. Rosenbloom has served on the board of directors of Medigus Ltd. (Nasdaq and TASE: MDGS) since September 2018 and ScoutCam Inc. (OTC: SCTC) since December 2019. Prior to that, Mr. Rosenbloom served as chairman of the Israeli Money Laundering Prohibition committee and the Prohibition of Money Laundering Committee of the Tel Aviv District, both of the Israel Bar Association from November 2015 to December 2019. Mr. Rosenbloom holds an LL.B. from the Ono Academic College, an Israeli branch of University of Manchester. We believe that Mr. Rosenbloom is qualified to serve on our board of directors because of his business experience and expertise and background with regard to legal matters.
Israel Berenshtein, Director
Mr. Berenshtein has served as a member of our board of directors since August 2020. Mr. Berenshtein has also served on the board of directors of Chrion Refineries Ltd. (TASE: CHR) since May 2019 and recently started working as a lawyer in Ben Yakov, Shvimer , Dolv – Law Office. He previously served in the legal department of Sonol Israel Ltd. since April 2010 to December 2020. Before that, Mr. Berenshtein worked as a commercial lawyer and litigator for a leading Israeli law firm from July 2000 to April 2010. Mr. Berenshtein earned an LL.B. in law and an M.A. in political science from Bar Ilan University, Israel. Mr. Berenshtein was admitted to the Israel Bar Association in 2000. We believe that Mr. Berenshtein is qualified to serve on our board of directors due to his extensive legal experience.
Amitay Weiss, Director
Mr. Weiss has served as a member of our board of directors since August 2020. Mr. Weiss has also served as chairman of the board of directors of P.L.T Financial Services Ltd. (TASE: PLTP) since April 2016 and Matomy Media Group Ltd. (LSE: MTMY, TASE: MTMY.TA) since May 2020, as a member of the board of directors of Cofix Group Ltd. (TASE: CFCS) since August 2015, Ziron Ltd. since June 2019, Algomizer Ltd. (TASE: GIX) since March 2019, and Cyntar Ventures Inc. since August 2019, and as a member of the board of directors and chief executive officer of Therapix Biosciences Ltd. (OTC: TRPXY) since August 2020. In April 2016, Mr. Weiss founded Amitay Wiss Management Ltd., an economic consulting company and now serves as its chief executive officer. Mr. Weiss holds a B.A in economics from New England College, M.B.A. in business administration from Ono Academic College in Israel, an Israeli branch of University of Manchester and LL.B from the Ono Academic College. We believe that Mr. Weiss is qualified to serve on our board of directors because of his diverse business, management and leadership experience.
| 69 |
Eliahou Arbib, Director
Mr. Arbib has served as a member of our board of directors since January 2021. Mr. Arbib has also served as chairman of the board of directors of Chiron Refineries Ltd. (TASE: CHR) since September 2016. He has also the current owner and manager of Eliahou Arbib Law Offices, since May 2013. Prior to that, from 1993 until 2000, Mr. Arbib was the managing director of AA Arbib Agriculture Supply Ltd. Mr. Arbib holds an LLB from the Law and Business Academic Center of Ramat Gan, Israel. Mr. Arbib has been an active member of the Israeli Bar Association since 2013, and served as deputy chairman of the Security and Defense Committee of the Israeli Bar Association since 2014. We believe that Mr. Weiss is qualified to serve on our board of directors because of his legal experience as well as experience in the field of agriculture.
Udi Kalifi, Director Nominee
Mr. Klifi has agreed to serve on our board of directors subject to the consummation of this offering. Mr. Kalifi is the owner and manager of Udi Kalifi Law Officer since 2006. He has also served as a member of the board of directors of Matomi Media Group Ltd. (TASE: MTMY) since May 2020. Mr. Kalifi holds an LLB, BSc in Accounting and LLM from the Tel Aviv University, Israel and a master’s degree in law and economics from the University of Bologna, Humbourg and Roterdam. Mr. Kalifi has been an active member of the Israeli Bar Association since 2006. Mr. Kalifi was selected to serve as a member of our board of directors due to his legal and finance experience.
Family Relationships
There are no family relationships among any of our directors or executive officers.
Composition of Board of Directors
After this offering, our board of directors will consist of 6 directors. Our directors are appointed by the board of directors at the annual general meeting. Each director’s term will continue until the annual meeting of the stockholders held following his or her election and the election and qualification of his or her successor, or his or her earlier death, disqualification, resignation or removal.
When considering whether directors have the experience, qualifications, attributes or skills, taken as a whole, to enable our board of directors to satisfy its oversight responsibilities effectively in light of our business and structure, the board of directors focuses primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business.
Arrangements for Election of Directors and Members of Management
There are no arrangements or understandings with major stockholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected.
Director Independence
Our board of directors has determined that Vered Raz-Avayo, Ronen Rosenbloom, Israel Berenshtein, Amitay Weiss, Eliahou Arbib and Udi Kalifi do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the rules of The Nasdaq Stock Market LLC (the “Nasdaq Rules”).
Committees of the Board of Directors
Our board of directors directs the management of our business and affairs, as provided by Delaware law, and conducts its business through meetings of the board of directors and standing committees. We will have a standing audit committee, nominating and corporate governance committee and compensation committee following the consummation of this offering. In addition, from time to time, special committees may be established under the direction of the board of directors when necessary to address specific issues.
Audit Committee
Our audit committee will be responsible for, among other things:
| ● | appointing, compensating, retaining, evaluating, terminating and overseeing our independent registered public accounting firm; | |
| ● | discussing with our independent registered public accounting firm their independence from management; | |
| ● | reviewing with our independent registered public accounting firm the scope and results of their audit; | |
| ● | approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm; | |
| ● | overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the quarterly and annual consolidated financial statements that we file with the SEC; | |
| ● | overseeing our financial and accounting controls and compliance with legal and regulatory requirements; |
| 70 |
| ● | reviewing our policies on risk assessment and risk management; | |
| ● | reviewing related person transactions; and | |
| ● | establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters. |
Upon the consummation of this offering, our audit committee will consist of Udi Kalifi, Amitay Weiss and Ronen Rosenbloom, with Udi Kalifi serving as chair. Rule 10A-3 of the Exchange Act and Nasdaq Rules require that our audit committee have at least one independent member upon the listing of our Common Stock, have a majority of independent members within 90 days of the date of this prospectus and be composed entirely of independent members within one year of the date of this prospectus. Our board of directors has affirmatively determined that Udi Kalifi, Amitay Weiss and Ronen Rosenbloom each meet the definition of “independent director” for purposes of serving on the audit committee under Rule 10A-3 under the Exchange Act and Nasdaq rules. Each member of our audit committee also meets the financial literacy requirements of Nasdaq listing standards. In addition, our board of directors has determined that Udi Kalifi will qualify as an “audit committee financial expert,” as such term is defined in Item 407(d)(5) of Regulation S-K. Our board of directors will adopt a written charter for the audit committee, which will be available on our principal corporate website at www.savefoods.co. substantially concurrently with the consummation of this offering. The information on any of our websites is deemed not to be incorporated in this prospectus or to be part of this prospectus.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee will be responsible for, among other things:
| ● | identifying individuals qualified to become members of our board of directors, consistent with criteria approved by our board of directors; | |
| ● | overseeing our succession plan for the CEO and other executive officers; | |
| ● | overseeing the evaluation of the effectiveness of our board of directors and its committees; and | |
| ● | developing and recommending to our board of directors a set of corporate governance guidelines. |
Upon the consummation of this offering, our nominating and corporate governance committee will consist of Ronen Rosenbloom, Israel Berenshtein and Eliahou Arbib, with Ronen Rosenbloom serving as chair. Our board of directors will adopt a written charter for the nominating and corporate governance committee, which will be available on our principal corporate website at www.savefoods.co. substantially concurrently with the consummation of this offering. The information on any of our websites is deemed not to be incorporated in this prospectus or to be part of this prospectus.
Compensation Committee
Our compensation committee will be responsible for, among other things:
| ● | reviewing and approving the compensation of our chief executive officer and other executive officers; | |
| ● | reviewing and making recommendations to the board of directors regarding director compensation; and | |
| ● | appointing and overseeing any compensation consultants. |
Upon the consummation of this offering, our compensation committee will consist of Ronen Rosenbloom, Israel Berenshtein and Eliahou Arbib, with Israel Berenshtein serving as chair. Our board has determined that Ronen Rosenbloom, Israel Berenshtein and Eliahou Arbib meet the definition of “independent director” for purposes of serving on the compensation committee under Nasdaq rules, including the heightened independence standards for members of a compensation committee, and are “non-employee directors” as defined in Rule 16b-3 of the Exchange Act. Our board of directors will adopt a written charter for the compensation committee, which will be available on our principal corporate website at www.savefoods.co. substantially concurrently with the consummation of this offering. The information on any of our websites is deemed not to be incorporated in this prospectus or to be part of this prospectus.
| 71 |
Risk Oversight
Our board of directors is responsible for overseeing our risk management process. Our board of directors focuses on our general risk management strategy, the most significant risks facing us, and oversees the implementation of risk mitigation strategies by management. Our audit committee is also responsible for discussing our policies with respect to risk assessment and risk management. Our board of directors believes its administration of its risk oversight function has not negatively affected our board of directors’ leadership structure.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is or has been an officer or employee of the Company. None of our executive officers serves as a member of the board of directors or compensation committee (or other committee performing equivalent functions) of any entity that has one or more of its executive officers serving on our board of directors or compensation committee.
Code of Business Conduct and Ethics
Prior to the completion of this offering, we will adopt a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the code will be posted on our website, www.savefoods.co. In addition, we intend to post on our website all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the code. The information on any of our websites is deemed not to be incorporated in this prospectus or to be part of this prospectus.
| 72 |
EXECUTIVE AND DIRECTOR COMPENSATION
Executive Compensation
Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers for the years ended December 31, 2020 and 2019.
| Name and principal position | Fiscal Year | Salary ($) | Bonus ($) | Stock awards ($) | Option awards ($) | All other compensation ($) | Total ($) | |||||||||||||||||||||
| David Palach | 2020 | 14,667 | - | - | - | - | 14,667 | |||||||||||||||||||||
| Chief Executive Officer (1) | - | - | - | - | - | - | - | |||||||||||||||||||||
| Dan Sztybel | 2020 | 120,282 | - | - | 135,778 | - | 256,060 | |||||||||||||||||||||
| Former co-Chief Executive Officer, Chief Executive Officer of Save Foods Ltd (2) | 2019 | 137,688 | - | - | 124,081 | - | 261,769 | |||||||||||||||||||||
| Shlomo Zakai | 2020 | 55,721 | - | - | 9,986 | - | 65,707 | |||||||||||||||||||||
| Chief Financial Officer | 2019 | 64,310 | - | - | 32,291 | - | 96,601 | |||||||||||||||||||||
| Nimrod Ben Yehuda | 2020 | 25,304 | - | - | - | - | 25,304 | |||||||||||||||||||||
| Chief Technology Officer | 2019 | 117,014 | - | - | - | - | 117,014 | |||||||||||||||||||||
| Neta Matis | 2020 | 75,202 | - | - | 94,474 | - | 169,676 | |||||||||||||||||||||
| VP Research and Development | 2019 | 44,444 | - | - | 30,235 | - | 74,679 | |||||||||||||||||||||
| (1) | Mr. Palach was appointed as the Company’s co-Chief Executive Officer on November 5, 2020, and as the Company’s Chief Executive Officer of the Company on January 11, 2021, upon the resignation of Mr. Sztybel. |
| (2) | Mr. Sztybel resigned from his position as co-Chief Executive Officer of the Company effective January 11, 2021 and continue serving as the Chief Executive Officer of the subsidiary Save Foods Ltd. |
| 73 |
Outstanding Equity Awards at Fiscal Year-End
The following table provides information about the number of outstanding equity awards held by each of our named executive officers as of December 31, 2020:
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name | Number
of Securities Underlying Unexercised Options (Exercisable) | Number
of Securities Underlying Unexercised Options (Unexercisable) | Option Exercise Price | Option Expiration Date | Equity Incentive Plan Awards: Number of Unearned Shares That Have Not Vested | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares That Have Not Vested | ||||||||||||||||||
| David Palach | - | - | - | - | - | - | ||||||||||||||||||
| Chief Executive Officer | - | - | - | - | - | - | ||||||||||||||||||
| Dan Sztybel | 10,715 | 3,571 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| Former co- Chief Executive Officer | 16,667 | 11,905 | 3.15 | 4/2/2029 | ||||||||||||||||||||
| 7,143 | 21,429 | 3.78 | 7/1/2030 | |||||||||||||||||||||
| Shlomo Zakai | 6,349 | 3,175 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| Chief Financial Officer | ||||||||||||||||||||||||
| Nimrod Ben Yehuda | - | - | - | - | - | - | ||||||||||||||||||
| Chief Technology Officer | ||||||||||||||||||||||||
| Neta Matis | 4,167 | 2,976 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| VP Research and Development | 2,976 | 4,167 | 3.15 | 10/31/2029 | ||||||||||||||||||||
| 5,286 | 15,857 | 3.15 | 6/23/2030 | |||||||||||||||||||||
| Arthur Dawson | - | - | - | - | - | - | ||||||||||||||||||
| Business Manager of U.S. Operations | - | - | - | - | - | - | ||||||||||||||||||
| 74 |
Employment Agreements with Executive Officers
We, and through our Israeli subsidiary, have entered into written employment agreements with certain of our executive officers. Upon the closing of this offering, we intend to enter into written employment and service agreement with each of our executive officers. Such employment and service agreement will contain customary provisions and representations, including confidentiality, non-competition, non-solicitation and inventions assignment undertakings by the executive officers. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we entered into agreements with each executive officer and director pursuant to which we will indemnify each of them up to a certain amount and to the extent that these liabilities are not covered by directors and officers insurance.
Services Agreement with Dan Sztybel as Chief Executive Officer of Save Foods Ltd.
On October 10, 2018, we entered into a service agreement (as amended on March 28, 2019, the “Save Foods Ltd. CEO Services Agreement”), with Mr. Sztybel and Dan Sztybel Consulting Group Ltd., a consulting services company owned and controlled by Mr. Sztybel, pursuant to which Mr. Sztybel provides us with services as the chief executive officer of Save Foods Ltd. Pursuant to the terms of the Save Foods Ltd. CEO Services Agreement, Mr. Sztybel is currently entitled to a monthly fee in the amount of NIS 47,125 (approximately $14,500) plus value added tax and car allowance in the amount of NIS 3,250 (approximately $1,000) plus value added tax per month. In addition, under the Save Foods Ltd. CEO Services Agreement, Mr. Sztybel was granted options to purchase shares of our Common Stock, including the following:
| (a) | Options to purchase up to 14,286 shares of Common Stock, under our Equity Incentive Plan, in the event that we will receive EPA and FDA approvals by the end of the second quarter of 2020. Such conditions were not met as of June 30, 2020. | |
| (b) | Options to purchase up to 28,572 shares of Common Stock, under our Equity Incentive Plan. |
Both parties may terminate the Save Foods Ltd. CEO Services Agreement at any time for any reason upon a 30-day prior written notice.
Commencing April 2020, the Company and Mr. Sztybel agreed to temporarily reduce the monthly fixed fee to $9,000 per month.
Consulting Agreement with David Palach as Chief Executive Officer
On November 6, 2020, we entered into a consulting agreement with S.T Sporting (1996) Ltd., for the services of David Pallach (the “CEO Consulting Agreement”). Pursuant to the terms of the CEO Consulting Agreement, Mr. Palach provides us services as chief executive officer. Pursuant to the terms of the CEO Consulting Agreement, Mr. Palach is currently entitled to a monthly fee in the amount of $8,000 plus value added tax per month and a grant of options to purchase shares of our Common Stock, which amount shall be determined by good faith negotiations by the board of directors on a future date.
| 75 |
Engagement with Shlomo Zakai for Accounting Services
Mr. Zakai is acting as our chief financial officer and our engagement with him is based on a price offer dated September 28, 2017, pursuant to which Mr. Zakai provides us accountant services. Pursuant to the terms of such price offer, Mr. Zakai is entitled to a fixed fee for several services as well as compensation according to an hourly rate. For 2019, the scope of our engagement with Mr. Zakai was in an amount of approximately $64,310.
Commencing January 2020, the Company and Mr. Zakai agreed on a monthly fixed fee of $6,500 per month. Commencing April 2020, the Company and Mr. Zakai Agreed to temporarily reduce the monthly fixed fee to $4,000 per month. For the year ended December 31, 2020, the scope of our engagement with Mr. Zakai was in an amount of approximately $55,721.
Services Agreement with Neta Matis as Vice President of Research and Development
On January 15, 2019, we entered into a service agreement (the “VP Research and Development Services Agreement”), with NSNC Consulting Ltd. (the “Contractor”), pursuant to which Ms. Neta Matis, on behalf of the Contractor, provides us with services as our VP of research and development. Pursuant to the terms of the VP Research and Development Service Agreement, Ms. Matis provides the services in a part time position scope of 50% and is entitled to a monthly fee in the amount of NIS 15,000 (approximately $4,615) plus value added tax. In addition, Ms. Matis will be granted options to purchase up to 7,143 shares of our Common Stock. Such options shall vest over a 36-month period commencing in January 2019 as follows: (i) options to purchase up to 2,381 shares of Common Stock shall vest following the initial 12-month period as of the date of grant, (ii) options to purchase up to 4,762 shares of Common Stock shall vest following the lapse of each additional three-month period thereafter. All other terms and conditions of the options shall be consistent to those applicable under our Equity Incentive Plan. Commencing January 2020, Ms. Matis became a full-time employee and is entitled to a monthly fee in the amount of NIS 30,000 (approximately $9,230) plus value added tax. Commencing April 2020, the Company and Ms. Matis agreed to temporarily reduce the monthly fixed fee to $6,200 per month.
Director Compensation
Summary Compensation Table
The following table sets forth the compensation we paid our non-executive directors during the fiscal year ended December 31, 2020.
| Name | Fees earned or paid in
cash | Stock awards ($) | Option awards ($) | All other compensation ($) | Total ($) | |||||||||||||||
| Prof. Benad Goldwasser | 131,764 | - | 232,530 | - | 364,294 | |||||||||||||||
| Vered Raz-Avayo | - | - | 16,462 | - | 16,462 | |||||||||||||||
| Israel Berenshtein | - | - | - | - | - | |||||||||||||||
| Ronen Rosenbloom | - | - | - | - | - | |||||||||||||||
| 76 |
Service Contracts with Non-Executive Directors
Except for the following agreements, we do not have any written agreements with any of non-officer directors.
Corporate Advisory Consulting Agreement with Goldmed Ltd.
On August 30, 2017, we entered into a corporate advisory consulting agreement (the “Goldmed Agreement”), with Goldmed Ltd., a company wholly owned by Prof. Benad Goldwasser, who currently serves as our active chairman of the board of directors. Pursuant to the Goldmed Agreement, Prof. Goldwasser provides us with services since May 1, 2018. Under the terms of the Goldmed Agreement, Prof. Goldwasser is entitled to a monthly fee in the amount of $5,000, of which $2,000 will acrrue as debt of the Company to Prof. Goldwasser until the Company raises $1,000,000, and to a monthly fee in the amount of $10,000 after the Company will raise more than $2,000,000. The Goldmed Agreement will continue to be in effect unless terminated by either party by providing a 90-day prior written notice. In addition. The Goldmed Agreement provides that for its services as director of the Company’s subsidiary, Save Foods Ltd., the Company will issue to Prof. Goldwasser, under the Equity Incentive Plan, warrants to purchase up to 14,286 shares of our Common Stock at an exercise price of $2.1. Such warrants will vest quarterly over a period of 36 months and will be accelerated in full if we terminate the Goldmed Agreement for no cause prior to the end of the vesting period.
On January 3, 2019, we updated the terms and conditions of the Goldmed Agreement, which now provides that Prof. Goldwasser is entitled to a monthly fee in the amount of $10,000 and a monthly car allowance of $2,000, which will be triggered when the Company raises $1 million, and of which will commence on the date Prof. Goldwasser was elected to serve as a member of the board of directors of the Company. The fee is included in the fees payable to Prof. Goldwasser as a director.
Equity Incentive Plan
We maintain one equity incentive plan – the 2018 Equity Incentive Plan. As of December 31, 2020, the number of shares of Common Stock reserved for the exercise of options granted under Equity Incentive Plan was 289,942.
Our Equity Incentive Plan was adopted by our board of directors in October 2018, and became effective immediately thereafter. The 2018 Equity Incentive Plan permits the grant of incentive stock options to employees of the Company, including officers and directors, and non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units, performance units, performance shares and other stock or cash awards as the administrator of the plan may determine, to the Company’s employees and service providers.
The 2018 Equity Incentive Plan may be administered by the board of directors or by different committees that may be established with respect to different groups of service providers; in that event, the committee established with respect to a group of service providers shall administer the 2018 Equity Incentive Plan with respect to awards granted to members of such group.
Subject to the provisions of the 2018 Equity Incentive Plan, and in the case of a committee, subject to the specific duties delegated by the board to such committee, the administrator will have the authority, in its sole discretion to determine subject to Israeli law and section 16 of the Exchange Act, the grantees of awards and the terms of the grant, including, exercise prices, vesting schedules, acceleration of vesting and conditions and restrictions applicable to an award, as well other matters necessary in the administration of the 2018 Equity Incentive Plan. The 2018 Equity Incentive Plan enables us to issue awards under various tax regimes, including, without limitation, pursuant to Section 102 of the Ordinance, and under Section 3(i) of the Ordinance and Section 422 of the United States Internal Revenue Code of 1986, as amended (the “Code”).
| 77 |
The 2018 Equity Incentive Plan provides that awards granted to our employees, directors and officers who are not controlling shareholders and who are not considered Israeli residents are intended to qualify for special tax treatment under the “capital gain track” provisions of Section 102(b) of the Ordinance as detailed above. Our Israeli non-employee service providers and controlling shareholders may only be granted awards under Section 3(i) of the Ordinance, which does not provide for similar tax benefits.
Awards granted under the 2018 Equity Incentive Plan to U.S. residents may qualify as “incentive stock options” within the meaning of Section 422 of the Code. The exercise price for “incentive stock options” must not be less than the fair market value on the date on which an option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital. Notwithstanding the foregoing provisions, options may be granted with a per share exercise price of less than 100% of the fair market value per share on the date of grant pursuant to the issuance or assumption of an option in a transaction to which Section 424(a) of the Code applies in a manner consistent with said Section 424(a).
The vesting schedule of options granted under the 2018 Equity Incentive Plan is set forth in each grantee’s award agreement.
Awards terminate upon the date set out in the grantee’s specific award agreement or at the end of an extended period following the termination of the grantee’s employment or service. In the event of the death of a grantee while employed by or performing service for us or an affiliate, or in the event of termination of a grantee’s employment or services for reasons of disability, the grantee or his or her estate or legal successor (in the case of death), may exercise awards that have vested prior to termination within a period of nine (9) months from the date of disability or death but in any event no later than the expiration date of the awards. If a participant ceases to be a service provider, other than upon the participants termination as the result of the participant’s death or disability, may exercise his or her option within such period of time as is specified in the award agreement to the extent that the option is vested on the date of termination (but in no event later than the expiration of the term of such option as set forth in the award agreement). In the absence of a specified time in the award agreement, the option will remain exercisable for three (3) months following the participant’s termination. Unless otherwise provided by the administrator, if on the date of termination, the participant is not vested as to his or her entire option, the Common Stock covered by the unvested portion of the option will revert to the 2018 Equity Incentive Plan. If after termination, the participant does not exercise his or her option within the time specified by the award agreement or by operation of this paragraph, the option will terminate, and the Common Stock covered by such option will revert to the 2018 Equity Incentive Plan.
Israeli options may not be assigned or transferred other than by will or laws of descent, unless otherwise determined by the committee.
In the event of our proposed dissolution or liquidation, the administrator will notify each participant as soon as practicable prior to the effective date of such proposed transaction. To the extent it has not been previously exercised, an award will terminate immediately prior to the consummation of such proposed action.
In the event of a merger or change in control, each outstanding award will be treated as the administrator determines, including, without limitation, that each award will be assumed or an equivalent option or right substituted by the successor corporation or a parent or subsidiary of the successor corporation. The administrator will not be required to treat all awards similarly in the transaction.
In the event that the successor corporation does not assume or substitute for the award, the participant will fully vest in and have the right to exercise all of his or her outstanding options and stock appreciation rights, including Common Stock as to which such awards would not otherwise be vested or exercisable. All restrictions on restricted stock will lapse, and, with respect to restricted stock units, performance shares and performance units, all performance goals or other vesting criteria will be deemed achieved at target levels and all other terms and conditions met. In addition, if an option or stock appreciation right is not assumed or substituted for in the event of a change in control, the administrator will notify the participant in writing or electronically that the option or stock appreciation right will be fully vested and exercisable for a period of time determined by the administrator in its sole discretion, and the option or stock appreciation right will terminate upon the expiration of such period.
In the year ended December 31, 2020, we granted to our directors and officers options to purchase a maximum aggregated number of 92,574 of the Company’s Common Stock, under our equity incentive plan. 92,574 options were granted at an exercise price of $3.15 to $3.78 per share, and the latest expiration date for such options is July 1, 2030.
| 78 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Employment Agreements
Certain of our executive officers have employment and service agreements with us. Upon the closing of this offering, we intend to enter into written employment and service agreement with each of our executive officers. Such employment and service agreement will contain customary provisions and representations, including confidentiality, non-competition, non-solicitation and inventions assignment undertakings by the executive officers. However, the enforceability of the noncompetition provisions may be limited under applicable law. See “Management—Employment Agreements with Executive Officers” and “— Service Contracts with Non-Executive Directors.”
Indemnification Agreements
We entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us or will require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer. For further information, see “Executive and Director Compensation—Limitations of Liability and Indemnification.”
Consulting and Service Agreements
Consulting Agreement with Amir Uziel
On March 2, 2021, we entered into a consulting agreement with Amir Uziel (the “Amir Uziel Consulting Agreement”), effective as of January 1, 2021, pursuant to which, Mr. Amir Uziel, a holder of greater than 5% of our Common Stock, provides us with consulting services. Pursuant to the terms of the Amir Uziel Consulting Agreement, Amir Uziel is entitled to a fee in the amount of $100 per hour up to a maximum of 60 hours. The Amir Uziel Consulting Agreement will terminate on December 31, 2021.
Corporate Advisory Consulting Agreement with Nir Ecology Ltd.
On August 30, 2017, we entered into a corporate advisory consulting agreement with Nir Ecology Ltd. (the “Nir Ecology Agreement”), pursuant to which, Mr. Ben Yehuda, our chief technology officer, provided us with services since September 1, 2017. Pursuant to the terms of the Nir Ecology Agreement, Nir Ecology Ltd. was entitled to a monthly fee in the amount of $3,000. The Nir Ecology Agreement remained in effect until August 31, 2019.
Loan Agreements
Loan Agreement with Nimrod Ben Yehuda
On February 26, 2019, we entered into a loan agreement (the “Ben Yehuda Loan Agreement”), with Mr. Nimrod Ben Yehuda, our founder and former director of Nir Ecology Ltd., a company wholly owned by Mr. Ben Yehuda, pursuant to which we extended Nir Ecology Ltd. with a loan in an aggregate amount of NIS 50,000 (approximately $14,021). The loan amount bears interest at an annual compounded rate of four percent from the date the loan was actually provided and until the repayment date.
On March 18, 2019, we entered into a settlement agreement (the “Settlement Agreement”), pursuant to which we agreed to repay Mr. Ben Yehuda NIS 263,929 (the “Ben Yehuda Debt”) that we owed him for his services rendered as chief executive officer of Nir Ecology Ltd. from 2014 through the date of the Settlement Agreement. Pursuant to the Settlement Agreement, we agreed to offset an amount of NIS 50,000 from the Ben Yehuda Loan Agreement and to repay the remaining balance of the Ben Yehuda Debt in 12 monthly payments starting in August 2021. The Ben Yehuda Debt will not bear interest and may be prepaid in whole or in part.
On January 12, 2021, our board of directors approved the prepayment of an additional $5,000 to Mr. Ben Yehuda, ahead of the designated date of August 2021, pursuant to the Company’s right under the Settlement Agreement.
| 79 |
Convertible Loan Agreements
During December 2019, we entered into the December 2019 CLAs, with certain lenders, including three of our holders of greater than 5% of our Common Stock, pursuant to which the December 2019 Lenders agreed to provide us loans in the aggregate amount of $379,000 in exchange for convertible promissory notes and warrants. The following table sets forth the aggregate amount provided by our related parties in the December 2019 CLAs:
| Participant | Loan Amount | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | $ | 100,000 | ||
| L.I.A Pure Capital Ltd. | $ | 35,000 | ||
| YAAD Consulting & Management Services (1995) Ltd | $ | 100,000 | ||
Simultaneous with and conditioned upon the execution of the June 2020 SPA, the Company and each of the December 2019 Lenders, January 2020 Lender and February agreed to effectively cancel the December 2019 CLA and the equity securities issued thereunder. In connection therewith, each of the December 2019 Lenders voluntarily waived any right to receive interest that accrued thereupon pursuant to the December 2019 CLA.
Lease Agreement with YAAD Consulting & Management Services (1995) Ltd.
On September 1, 2019, we entered into a lease agreement, through our Israeli subsidiary, with YAAD Consulting & Management Services (1995) Ltd., a holder of greater than 5% of our Common Stock (the “YAAD Lease Agreement”). Pursuant to the YAAD Lease Agreement, we paid YAAD Consulting & Management Services (1995) Ltd. a monthly payment of NIS 5,000 (approximately $1,430) plus value added tax in consideration for office space located at 20 Raul Wallenberg, Tel Aviv, Israel. The YAAD Lease Agreement remained in effect until September 1, 2019.
Private Placements of our Securities
On June 24, 2020, we entered into the June 2020 SPA with certain investors, including three of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 67,369 shares of Common Stock and warrants to purchase an aggregate of 67,369 shares of Common Stock for an aggregate purchase price of $565,868. The following table sets forth the aggregate number of Common Stock issued to our related parties in the June 2020 SPA:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 13,106 | |||
| L.I.A Pure Capital Ltd. | 4,588 | |||
| YAAD Consulting & Management Services (1995) Ltd | 13,107 | |||
During July 2020, we entered into securities purchase agreements (the “July 2020 SPAs”) with certain investors, including two of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 6,554 shares of Common Stock and warrants to purchase an aggregate of 6,554 shares of Common Stock for an aggregate purchase price of $55,046. The following table sets forth the aggregate number of Common Stock issued to our related parties in the July 2020 SPAs:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 3,277 | |||
| YAAD Consulting & Management Services (1995) Ltd. | 3,277 | |||
| Nir Reinhold / Buffalo Investments Ltd. | 13,107 | |||
| 80 |
During August 2020, we entered into securities purchase agreements (the “August 2020 SPAs”) with certain investors, including three of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 13,108 shares of Common Stock and warrants to purchase an aggregate of 13,108 shares of Common Stock for an aggregate purchase price of $110,093. The following table sets forth the aggregate number of Common Stock issued to our related parties in the August 2020 SPAs:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 6,554 | |||
| L.I.A Pure Capital Ltd. | 3,277 | |||
| YAAD Consulting & Management Services (1995) Ltd | 3,277 | |||
Stock Options
Since our inception, we have granted options to purchase shares of our Common Stock to our officers and certain of our directors. Such option agreements may contain acceleration provisions upon certain merger, acquisition, or change of control transactions. We describe our option plans under “Executive and Director Compensation—Equity Incentive Plan.” If the relationship between us and an executive officer or a director is terminated, except for cause (as defined in the various option plan agreements), options that are vested will generally remain exercisable for three months after such termination.
Policies and Procedures for Related Person Transactions
We intend to adopt a written related person transaction policy, to be effective immediately prior to the effectiveness of this registration statement of which this prospectus forms a part, setting forth the policies and procedures for the review and approval or ratification of “related person transactions.” For purposes of this policy, a “related person transaction” is any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any “related person” are participants involving an amount that exceeds $120,000 including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. Transactions involving compensation for services provided to us as an employee, consultant or director will not be considered related-person transactions under this policy. A “related person” is any executive officer, director or a holder of more than five percent of our Common Stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under this policy, where a transaction has been identified as a related person transaction, management must present information regarding the proposed related-person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our board of directors) for review. In reviewing and approving any such transactions, our audit committee or other independent body of our board of directors, is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval. All of the transactions described in this section occurred prior to the adoption of this policy.
| 81 |
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information regarding beneficial ownership of shares of our Common Stock as of April 6, 2021 by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our issued and outstanding share of Common Stock; | |
| ● | each of our directors and executive officers; and | |
| ● | all of our current directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC, and includes voting or investment power with respect to shares of Common Stock. Shares of Common Stock issuable under share options that are exercisable within 60 days after the date of this prospectus, are deemed issued and outstanding for the purpose of computing the percentage ownership of the person holding the options but are not deemed issued and outstanding for the purpose of computing the percentage ownership of any other person. Percentage of shares beneficially owned before this offering is based on 1,606,760 shares of Common Stock issued and outstanding as of April 6, 2021. The number of shares of Common Stock deemed outstanding after this offering is 2,743,801 and includes the shares of Common Stock being offered for sale in this offering but assumes no exercise by the underwriter of the over-allotment option.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such stockholders. Unless otherwise indicated below, the address for each beneficial owner listed in the table below is c/o Save Foods, Inc., Kibbutz Alonim, Israel, 3657700.
| No.
of Shares Beneficially Owned Prior to this Offering | Percentage
Owned Before this Offering(1) | Percentage Owned After this Offering | ||||||||||
| 5% or more shareholders: | ||||||||||||
| Amir Uziel / Amir Uziel Economic Consultant Ltd.(2) | 115,512 | 7.1 | % | 4.2 | % | |||||||
| L.I.A Pure Capital Ltd. (3) | 104,938 | 6.5 | % | 3.8 | % | |||||||
| Nir Reinhold / Buffalo Investments Ltd.(4) | 111,453 | 6.9 | % | 4.1 | % | |||||||
| YAAD Consulting & Management Services (1995) Ltd.(5) | 115,831 | 7.2 | % | 4.2 | % | |||||||
| Directors and named executive officers | ||||||||||||
| Amitay Weiss (*) | - | - | % | - | % | |||||||
| Arthur Dawson | - | - | % | - | % | |||||||
| Prof. Benad Goldwasser (*)(6) | 87,302 | 5.3 | % | 3.1 | % | |||||||
| Dan Sztybel (7) | 44,843 | 2.8 | % | 1.6 | % | |||||||
| David Palach | - | - | % | - | % | |||||||
| Israel Berenshtein (*) | - | - | % | - | % | |||||||
| Nimrod Ben Yehuda (8) | 99,890 | 6.2 | % | 3.6 | % | |||||||
| Ronen Rosenbloom(*) | - | - | % | - | % | |||||||
| Shlomo Zakai (9) | 7,143 | ** | % | ** | % | |||||||
| Vered Raz-Avayo (*)(10) | 5,179 | ** | % | ** | % | |||||||
| Eliahou Arbib (*) | - | - | ||||||||||
| All directors and named executive officers as a group (11 persons) | 244,357 | 14.9 | % | 8.6 | % | |||||||
| (*) | Indicates director of the Company. |
| (**) | Less than 1%. |
| (1) | The percentages shown are based on 1,606,760 Common Stock issued and outstanding as of the date of April 6, 2021. Under the rules of the SEC, a person is deemed to be the beneficial owner of a security if such person has or shares the power to vote or direct the voting of such security or the power to dispose or direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities if that person has the right to acquire beneficial ownership within 60 days of April 6, 2021. |
| (2) | Includes 9,830 shares issuable upon the exercise of warrants that are exercisable within 60 days of April 6, 2021 and 6,981 shares held by Amir Uziel, and 13,107 shares issuable upon the exercise of warrants that are exercisable within 60 days of April 6, 2021 and 85,594 shares held by Amir Uziel Economic Consultant Ltd. Amir Uziel is the owner of Amir Uziel Economic Consultant Ltd. and has voting and dispositive power over the shares held by Amir Uziel Economic Consultant Ltd. |
| (3) | Includes 7,865 shares issuable upon the exercise of warrants that are exercisable within 60 days of April 6, 2021 and 97,073 shares. Kfir Zilberman is the Owner of L.I.A Pure Capital Ltd. and has the voting and dispositive power over the shares held by L.I.A Pure Capital Ltd. |
| (4) | Includes 16,191 shares held by Nir Reinhold, and 13,107 shares issuable upon the exercise of warrants that are exercisable within 60 days of April 6, 2021 and 82,155 shares held by Buffalo Investments Ltd. Nir Reinhold is the owner of Buffalo Investments Ltd. and has voting and dispositive power over the shares held by Buffalo Investments Ltd. |
| (5) | Includes 3,175 shares held by Itschak Shrem, and 19,661 shares issuable upon the exercise of warrants that are exercisable within 60 days of April 6, 2021 and 92,995 shares held by YAAD Consulting & Management Services (1995) Ltd. Itschak Shrem is the owner of YAAD Consulting & Management Services (1995) Ltd. and has the voting and dispositive power over the shares held by YAAD Consulting & Management Services (1995) Ltd. |
| (6) | Includes 30,179 options held by Prof. Benad Goldwasser that are exercisable within 60 days of April 6, 2021. and 57,143 shares held by Anat Tamir Lotan, a member of Prof. Benad’s immediate family sharing the same household. |
| (7) | Includes 3,175 shares and 41,668 options that are exercisable within 60 days of April 6, 2021. |
| (8) | Includes 14,146 shares held by Nimrod Ben Yehuda, and 85,744 shares held by Nir Ecology Ltd. Nimrod Ben Yehuda is the owner of Nir Ecology Ltd. and has voting and dispositive power over the shares held by Nir Ecology Ltd. |
| (9) | Includes 7,143 options that are exercisable within 60 days of April 6, 2021. |
| (10) | Includes 5,179 options that are exercisable within 60 days of April 6, 2021. |
| 82 |
The following description summarizes important terms of our capital stock and the provisions of our certificate of incorporation, our bylaws and Delaware law are summaries. The following descriptions of our capital stock and provisions of our certificate of incorporation, as amended, and bylaws are summaries and are qualified by reference to the certificate of incorporation and the bylaws which are filed as exhibits to our registration statement of which this prospectus forms a part.
General
We are currently authorized to issue up to 495,000,000 shares of Common Stock, of which 1,606,760 shares were issued and outstanding as of the date of this prospectus, and 5,000,000 shares of preferred stock, par value $0.0001 per share, of which none were issued and outstanding as of the date of this prospectus. All of our issued and outstanding shares have been validly issued, fully paid and non-assessable.
Our bylaws permit the board of directors to issue the whole or any part of any unissued balance of the authorized capital stock. Our certificate of incorporation permits us to increase or decrease the number of authorized shares of Common Stock by the affirmative vote of the holders of a majority of the stock of the Company entitled to vote.
Common Stock
In the last three years, since January 31, 2018, we issued an aggregate of 856,267 shares of Common Stock of which 467,862 shares were issued in several private placements for aggregate net proceeds of $2,160,033; 67,369 shares were issued as conversion of several convertible loans for aggregate net proceeds of $620,627; 11,128 shares were issued as stock based compensation; and 309,908 shares were issued in other equity transactions.
Voting Rights
Holders of our Common Stock are entitled to one vote for each share held on all matters submitted to a vote of our stockholders. Holders of our Common Stock have no cumulative voting rights. Further, holders of our Common Stock have no preemptive, conversion, redemption or subscription rights and there are no sinking fund provisions applicable to our Common Stock.
Liquidation Rights
In the event of our liquidation, dissolution or winding-up, holders of our Common Stock have the right under Section 281 of the Delaware General Corporation Law to a ratable portion of assets remaining after satisfaction in full of the prior rights of our creditors, all liabilities and the total liquidation preferences of any outstanding shares of preferred stock.
Dividends
Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of our Common Stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors, or board, out of our assets which are legally available.
Preferred Stock
Our Certificate of Incorporation authorizes 5,000,000 shares of preferred stock, par value $0.0001 per share, of which none were issued and outstanding as of the date of this registration statement. The board of directors is authorized to provide for the issuance of these unissued shares of preferred stock in one or more series, and to fix the number of shares and to determine the rights, preferences and privileges thereof. Accordingly, the board of directors may issue preferred stock which may convert into large numbers of shares of Common Stock and consequently lead to further dilution of other shareholders.
| 83 |
Warrants and Options
In the last three years, since January 31, 2018, we issued warrants to purchase an aggregate of 187,128 shares of Common Stock to investors and service providers, with exercise prices ranging from $2.10 to $8.40 per share, of which 28,572 warrants were exercised and 28,572 were forfeited, and granted options to purchase an aggregate of 261,625 shares of Common Stock to directors, officers, employees and service providers with exercise prices ranging from $3.15 to $3.78 per share, of which 6,350 options were exercised and 43,651 options were forfeited.
Convertible Promissory Notes
In the last three years, we issued convertible promissory notes in an aggregate principal amount of $1,013,000 in a series of convertible loan agreements. As of the date of this prospectus we issued an aggregate of 67,369 shares of Common Stock as a result of conversion of certain convertible promissory notes, at a conversion price of $7.63 per share. The convertible promissory notes will be converted into shares of Common Stock immediately prior to the closing of this offering.
Anti-Takeover Provisions
The provisions of Delaware law, our Certificate of Incorporation and our Bylaws may have the effect of delaying, deferring or discouraging another person from acquiring control of the Company. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaw Provisions
Our amended and restated certificate of incorporation and our amended and restated bylaws include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of our management team, including the following:
| ● | Board of directors’ vacancies. Our Certificate of Incorporation provides that vacancies on the board of directors may be filled only by the affirmative vote of a majority of the directors then in office, irrespective of whether there is a quorum, or by a sole remaining director. Additionally, the number of directors to serve on our board of directors is fixed solely and exclusively by resolution duly adopted by our board of directors. This would prevent a stockholder from increasing the size of our board of directors and then gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of our board of directors but promotes continuity of management. |
| ● | Special meetings of stockholders. Our Certificate of Incorporation currently provides that special meetings of our stockholders may be called by the board of directors acting pursuant to a resolution approved by the affirmative vote of a majority of the directors then in office, and special meetings of stockholders may not be called by any other person or persons. |
| ● | No cumulative voting. The Delaware General Corporation Law provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation does not provide for cumulative voting. |
| ● | Amendment of charter provisions. Any amendment of our Certificate of Incorporation requires the affirmative vote of the majority of the outstanding shares of capital stock entitled to vote on such amendment, and the affirmative vote of the majority of the outstanding shares of each class entitled to vote thereon as a class. Amendments to the Bylaws may be executed pursuant to a resolution by the board of directors pursuant to an affirmative vote of a majority of the directors then in office, or by the affirmative vote of at least 75% of the outstanding shares of capital stock entitled to vote. |
| 84 |
| ● | Issuance of undesignated preferred stock. Our board of directors has the authority, without further action by the stockholders, to issue up to 5,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock, which may be converted into large numbers of shares of Common Stock, would enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or other means. |
| ● | Delaware Business Combination Statute. The Company is subject to the “business combination” provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years following the date such person becomes an interested stockholder, unless the business combination or the transaction in which such person becomes an interested stockholder is approved in a prescribed manner. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. Generally, an “interested stockholder” is a person that, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by our board of directors, and the anti-takeover effect includes discouraging attempts that might result in a premium over the market price for the shares of our Common Stock. |
| ● | Exclusive forum. Unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our amended and restated certificate of incorporation or our amended and restated bylaws, or (iv) any action asserting a claim against us governed by the internal affairs doctrine. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. |
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Action Stock Transfer. The transfer agent and registrar’s address is 2469 E. Fort Union Blvd, Suite 214, Salt Lake City, UT 84121. The transfer agent’s telephone number is (801) 274-1088.
Listing
Our Common Stock is quoted on the OTC, Pink Tier under the symbol “SAFO.” We have applied to list our Common Stock on the Nasdaq Capital Market under the symbol “SVFD.” No assurance can be given that our application will be approved or that a trading market will develop.
| 85 |
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering our Common Stock was traded only on the OTC Markets, Pink Tier. In connection with this offering, we have applied to apply to list Our Common Stock on the Nasdaq Capital Market. No assurance can be given that our application will be approved. Sales of substantial amounts of our Common Stock in the public market, or the perception that such sales could occur, could adversely affect prevailing market prices of our Common Stock. Upon completion of this offering, we will have an aggregate of 2,697,669 shares of Common Stock issued and outstanding, assuming the underwriter does not exercise its over-allotment option. All of the Common Stock sold in this offering will be freely transferable without restriction or further registration under the Securities Act by persons other than by our affiliates.
Certain of the shares of Common Stock after the offering will be held by our existing shareholders. Upon consummation of the offering, approximately 19.8% of our issued and outstanding Common Stock will be subject to such lock-up agreements.
Lock-up Agreements
We, all of our directors and executive officers and holders of all of our outstanding Common Stock signed lock-up agreements in connection with this offering, pursuant to which, subject to certain exceptions, they agreed not to sell or otherwise dispose of their shares of Common Stock or any securities convertible into or exchangeable for Common Stock for a period of at least 180 days after the date of the pricing of our initial public offering without the prior written consent of the underwriter.
Rule 144
Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our Common Stock for at least 180 days would be entitled to sell in “broker’s transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three-month period that does not exceed the greater of:
| ● | 1% of the number of shares of our Common Stock then outstanding; and | |
| ● | the average weekly trading volume in our Common Stock on the during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC and Nasdaq concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities
Under Rule 144, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the 90 days preceding a sale, and who has beneficially owned shares of our Common Stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
| 86 |
Rule 701
In general, under Rule 701, any of our employees, directors, officers, consultants or advisors who purchases shares from us in connection with a compensatory stock or option plan or other written agreement before the effective date of the registration statement of which this prospectus forms a part is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. Our affiliates can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The SEC has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Form S-8 Registration Statement
Following the completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register up the Common Stock issued or reserved for issuance under our 2018 Equity Incentive Plan. The registration statement on Form S-8 will become effective automatically upon filing. Common Stock issued upon exercise of a share option and registered under the Form S-8 registration statement will, subject to vesting and lock-up provisions and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately unless they are subject to a lock-up, in which case, immediately after the term of the lock-up expires.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL SHARE TRANSFER RESTRICTION MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN LEGAL ADVISOR REGARDING THE PARTICULAR SECURITIES LAWS AND TRANSFER RESTRICTION CONSEQUENCES OF PURCHASING, HOLDING, AND DISPOSING OF THE COMMON STOCK INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
| 87 |
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a general discussion of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined herein) with respect to their ownership and disposition of shares of our Common Stock and warrants issued pursuant to this offering. All prospective non-U.S. holders of our Common Stock should consult their tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the purchase, ownership and disposition of our Common Stock. In general, a non-U.S. holder means a beneficial owner of our Common Stock (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; | |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust if (1) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), existing U.S. Treasury Regulations promulgated thereunder, published administrative pronouncements and rulings of the U.S. Internal Revenue Service (the “IRS”), and judicial decisions, all as in effect as of the date of this prospectus. These authorities are subject to change and to differing interpretation, possibly with retroactive effect. Any change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus.
We assume in this discussion that a non-U.S. holder holds shares of our Common Stock and warrants as a capital asset within the meaning of Section 1221 of the Code (generally, for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances, nor does it address any alternative minimum, Medicare contribution, estate or gift tax consequences, or any aspects of U.S. state, local or non-U.S. taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as holders that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below), corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt organizations, banks, financial institutions, insurance companies, brokers, dealers or traders in securities, commodities or currencies, tax-qualified retirement plans, holders who hold or receive our Common Stock pursuant to the exercise of employee stock options or otherwise as compensation, holders holding our Common Stock as part of a hedge, straddle or other risk reduction strategy, conversion transaction or other integrated investment, holders deemed to sell our Common Stock under the constructive sale provisions of the Code, controlled foreign corporations, passive foreign investment companies and certain former U.S. citizens or former long-term residents.
In addition, this discussion does not address the tax treatment of partnerships (or entities or arrangements that are treated as partnerships for U.S. federal income tax purposes) or persons that hold their Common Stock through such partnerships. If a partnership, including any entity or arrangement treated as a partnership for U.S. federal income tax purposes, holds shares of our Common Stock, the U.S. federal income tax treatment of a partner in such partnership will generally depend upon the status of the partner and the activities of the partnership. Such partners and partnerships should consult their tax advisors regarding the tax consequences of the purchase, ownership and disposition of our Common Stock or warrants.
There can be no assurance that a court or the IRS will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain, a ruling with respect to the U.S. federal income tax consequences to a non-U.S. holder of the purchase, ownership or disposition of our Common Stock.
| 88 |
Distributions
As discussed in the section entitled “Dividend Policy,” we do not anticipate paying any dividends on our Common Stock in the foreseeable future. If we make distributions on our Common Stock or on the warrants (as described below under “Constructive Dividends on Warrants”), those payments will constitute dividends for U.S. federal income tax purposes to the extent we have current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our Common Stock or the warrants, as applicable, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “Gain on Sale or Other Disposition of Common Stock or warrants.” Any such distributions would be subject to the discussions below regarding back-up withholding and the Foreign Account Tax Compliance Act (“FACTA”).
Subject to the discussion below on effectively connected income, any dividend paid to a Non-U.S. Holder generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a Non-U.S. Holder must provide us or our agent with an IRS Form W-8BEN, IRS Form W-8 BEN-E or another appropriate version of IRS Form W-8 (or a successor form), which must be updated periodically, and which, in each case, must certify qualification for the reduced rate. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and that are not eligible for relief from U.S. (net basis) income tax under an applicable income tax treaty generally are exempt from the (gross basis) withholding tax described above. To obtain this exemption from withholding tax, the Non-U.S. Holder must provide the applicable withholding agent with an IRS Form W-8ECI or successor form or other applicable IRS Form W-8 certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States. Such effectively connected dividends, if not eligible for relief under a tax treaty, would not be subject to a withholding tax, but would be taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits and if, in addition, the Non-U.S. Holder is a corporation, may also be subject to a branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
If you are eligible for a reduced rate of withholding tax pursuant to a tax treaty, you may be able to obtain a refund of any excess amounts withheld if you timely file an appropriate claim for refund with the IRS.
Constructive Dividends on Warrants
Under Section 305 of the Code, an adjustment to the number of shares of Common Stock that will be issued on the exercise of the warrants, or an adjustment to the exercise price of the warrants, may be treated as a constructive distribution to a U.S. Holder of the warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our stockholders). Adjustments to the exercise price of a warrant made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the warrants should generally not result in a constructive distribution. Any constructive distributions would generally be subject to the tax treatment described above under “Distributions.”
Exercise or Expiration of Warrants
In general, a Non-U.S. Holder will not be required to recognize income, gain or loss upon the exercise of a Warrant by payment of the exercise price, except possibly to the extent of cash paid in lieu of a fractional share. However, no discussion is provided herein regarding the U.S. federal income tax treatment on the exercise of a Warrant on a cashless basis, and Non-U.S. Holders are urged to consult their tax advisors as to the exercise of a Warrant on a cashless basis.
| 89 |
If a Warrant expires without being exercised, a Non-U.S. Holder that is engaged in a U.S. trade or business to which any income from the Warrant would be effectively connected or who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the expiration occurs (and certain other conditions are met) will recognize a capital loss in an amount equal to such Non-U.S. Holder’s tax basis in the Warrant. The amount paid to purchase our Common Stock and warrants will be apportioned between them in proportion to the respective fair market values of the Common Stock and warrants, and the apportioned amount will be the tax basis of the Common Stock and warrants respectively. The fair market value of our Common Stock for this purpose will generally be its trading value immediately after issuance.
Gain on Sale, Exchange or Other Disposition of Our Common Stock or Warrants
Subject to the discussion below regarding backup withholding and FATCA, a Non-U.S. Holder generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our Common Stock or the warrants unless:
| ● | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and not eligible for relief under an applicable income tax treaty, in which case the Non-U.S. Holder will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and for a Non-U.S. Holder that is a corporation, such Non-U.S. Holder may be subject to the branch profits tax at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items; | |
| ● | the Non-U.S. Holder is an individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case the Non-U.S. Holder will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses (even though the Non-U.S. Holder is not considered a resident of the United States) (subject to applicable income tax or other treaties); or | |
| ● | we are a “U.S. real property holding corporation” for U.S. federal income tax purposes (“USRPHC”), at any time within the shorter of the five-year period preceding the disposition or the Non-U.S. Holder’s holding period for our Common Stock or the warrants. We believe we are not currently and do not anticipate becoming a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property interests relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our Common Stock will not be subject to United States federal income tax if (A) in the case of our Common Stock, (a) shares of our Common Stock are “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, such as Nasdaq, and (b) the Non-U.S. Holder owns or owned, actually and constructively, 5% or less of the shares of our Common Stock throughout the five-year period ending on the date of the sale or exchange; and (B) in the case of the warrants, either (a)(i) shares of our Common Stock are “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, such as Nasdaq, (ii) the warrants are not considered regularly traded on an established securities market and (iii) the Non-U.S. Holder does not own, actually or constructively, warrants with a fair market value greater than the fair market value of 5% of the shares of our Common Stock, determined as of the date that such Non-U.S. Holder acquired its warrants, or (b)(i) the warrants are considered regularly traded on an established securities market, and (ii) the Non-U.S. Holder owns or owned, actually and constructively, 5% or less of the warrants throughout the five-year period ending on the date of the sale or exchange. The warrants are not expected to be regularly traded on an established securities market. If the foregoing exception does not apply, and we are a USRPHC, such Non-U.S. Holder’s proceeds received on the disposition of shares will generally be subject to withholding at a rate of 15% and such Non-U.S. Holder will generally be taxed on any gain in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax generally will not apply. |
| 90 |
Backup Withholding and Information Reporting
Information returns may be filed with the IRS in connection with distributions on our Common Stock or constructive dividends on the warrants, and the proceeds of a sale or other disposition of the Common Stock or the warrants. A non-exempt U.S. Holder may be subject to U.S. backup withholding on these payments if it fails to provide its taxpayer identification number to the withholding agent and comply with certification procedures or otherwise establish an exemption from backup withholding.
A Non-U.S. Holder may be subject to U.S. information reporting and backup withholding on these payments unless the Non-U.S. Holder complies with certification procedures to establish that it is not a U.S. person (within the meaning of the Code). The certification requirements generally will be satisfied if the Non-U.S. Holder provides the applicable withholding agent with a statement on the applicable IRS Form W-8BEN or IRS Form W-8BEN-E (or suitable substitute or successor form), together with all appropriate attachments, signed under penalties of perjury, stating, among other things, that such Non-U.S. Holder is not a U.S. Person. Applicable Treasury Regulations provide alternative methods for satisfying this requirement. In addition, the amount of distributions on Common Stock or constructive dividends on Common Stock paid to a Non-U.S. Holder, and the amount of any U.S. federal tax withheld therefrom, must be reported annually to the IRS and the holder. This information may be made available by the IRS under the provisions of an applicable tax treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides.
Payment of the proceeds of the sale or other disposition of the Common Stock or the warrants to or through a non-U.S. office of a U.S. broker or of a non-U.S. broker with certain specified U.S. connections generally will be subject to information reporting requirements, but not backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or an exemption otherwise applies. Payments of the proceeds of a sale or other disposition of the Common Stock or the warrants to or through a U.S. office of a broker generally will be subject to information reporting and backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or otherwise establishes an exemption.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment generally will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Account Tax Compliance Act
FATCA imposes withholding tax on certain types of payments made to foreign financial institutions and certain other non-U.S. entities. The legislation imposes a 30% withholding tax on dividends on, or, subject to the discussion of certain proposed Treasury Regulations below, gross proceeds from the sale or other disposition of, our Common Stock or the warrants paid to a “foreign financial institution” or to certain “non-financial foreign entities” (each as defined in the Code), unless (i) the foreign financial institution undertakes certain diligence and reporting obligations, (ii) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (iii) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (i) above, it must enter into an agreement with the U.S. Treasury requiring, among other things, that it undertake to identify accounts held by “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. If the country in which a payee is resident has entered into an “intergovernmental agreement” with the United States regarding FATCA, that agreement may permit the payee to report to that country rather than to the U.S. Department of the Treasury. The U.S. Treasury recently released proposed Treasury Regulations which, if finalized in their present form, would eliminate the federal withholding tax of 30% applicable to the gross proceeds of a sale or other disposition of our Common Stock or the warrants. In its preamble to such proposed Treasury Regulations, the U.S. Treasury stated that taxpayers may generally rely on the proposed regulations until final regulations are issued. Prospective investors should consult their own tax advisors regarding the possible impact of these rules on their investment in our Common Stock or the warrants, and the possible impact of these rules on the entities through which they hold our Common Stock or the warrants, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of this 30% withholding tax under FATCA.
THE PRECEDING DISCUSSION IS FOR GENERAL INFORMATION ONLY. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK AND WARRANTS, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
| 91 |
ThinkEquity, a division of Fordham Financial Management, Inc., is acting as representative of the underwriters. Subject to the terms and conditions of an underwriting agreement between us and the representative, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of Common Stock listed next to its name in the following table:
| Underwriter | Number Shares of Common Stock | |||
| ThinkEquity, a division of Fordham Financial Management, Inc. | ||||
| Total | ||||
The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the shares of Common Stock offered by this prospectus are subject to various conditions and representations and warranties, including the approval of certain legal matters by their counsel and other conditions specified in the underwriting agreement. The shares of Common Stock are offered by the underwriters, subject to prior sale, when, as and if issued to and accepted by them. The underwriters reserve the right to withdraw, cancel or modify the offer to the public and to reject orders in whole or in part. The underwriters are obligated to take and pay for all of the shares of Common Stock offered by this prospectus if any such shares of Common Stock are taken, other than those shares of Common Stock covered by the over-allotment option described below.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
Over-Allotment Option
We have granted a 45-day option to the representative of the underwriters to purchase up to additional shares of our Common Stock at a public offering price of $ per share, solely to cover over-allotments, if any. The underwriters may exercise this option for 45 days from the date of this prospectus solely to cover sales of shares of Common Stock by the underwriters in excess of the total number of shares of Common Stock set forth in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
Discounts and Commissions; Expenses
The underwriters propose initially to offer the shares of Common Stock to the public at the public offering price set forth on the cover page of this prospectus and to dealers at those prices less a concession not in excess of $ per share of Common Stock. If all of the shares of Common Stock offered by us are not sold at the public offering price, the underwriters may change the offering price and other selling terms by means of a supplement to this prospectus.
| 92 |
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise of the over-allotment option we granted to the representative of the underwriters.
| Per Share | Total
Without Over-allotment Option | Total
With Over-allotment Option | ||||||||||
| Public offering price | $ | |||||||||||
| Underwriting discount (7%) | $ | |||||||||||
| Proceeds, before expenses, to us | $ | |||||||||||
| Non-accountable expense allowance (1%)(1) | $ | |||||||||||
| (1) | The non-accountable expense allowance will not payable with respect to representative’s exercise of the over-allotment option. |
We have agreed to pay a non-accountable expense allowance to the representative of the underwriters equal to 1% of the gross proceeds received at the closing of the offering. The non-accountable expense allowance of 1% is not payable with respect to the shares sold upon exercise of the underwriters’ over-allotment option. We have paid an expense deposit of $20,000 to the representative, which will be applied against the out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering, and will be reimbursed to us to the extent not actually incurred in compliance with applicable FINRA rules.
We have also agreed to pay certain of the representative’s expenses relating to the offering, including (a) filing fees associated with the review of the offering by FINRA; (b) all fees and expenses relating to the listing of the Common Stock on the Nasdaq Capital Market, including any fees charges by The Depository Trust for new securities; (c) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $15,000 in the aggregate; (d) all fees, expenses and disbursements relating to the registration or qualification of the shares of our Common Stock under the “blue sky” securities laws of such states and other jurisdictions as the representative may reasonably designate; (e) all fees, expenses and disbursements relating to the registration, qualification or exemption of the shares of our Common Stock under the securities laws of such foreign jurisdictions as the representative may reasonably designate; (f) the costs associated with post-closing advertising the offering in the national editions of the Wall Street Journal and New York Times; (g) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and Lucite tombstones, each of which the Company or its designee shall provide within a reasonable time after the closing of this offering in such quantities as the representative may reasonably request in an amount not to exceed $3,000 in the aggregate; (h) the fees and expenses of the Company’s accountants, transfer agents and public relations firm; (i) fees and expenses of the underwriters’ legal counsel not to exceed $85,000; (j) a $29,500 cost associated with the underwriters use of Ipreo’s book-building, prospectus tracking and compliance software for the offering; (k) $10,000 for data services and communications expenses; and (l) up to $20,000 of the underwriters’ actual accountable “road show” expenses for the offering. The total reimbursement amount, inclusive if the expense deposit of $20,000 to the representative in connection with the offering shall not be more than an aggregate of $100,000.
Our total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $325,000.
Representative’s Warrants
Upon closing of this offering, we have agreed to issue to the representative as compensation warrants to purchase up to shares of Common Stock (5% of the aggregate number of shares of Common Stock sold in this offering exclusive of the over-allotment option, or the representative’s warrants). The representative’s warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share in this offering (excluding the over-allotment option). The representative’s warrants are exercisable at any time and from time to time, in whole or in part, during the four and one half year period commencing 180 days from the effective date of the registration statement of which this prospectus is a part.
| 93 |
The representative’s warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1)(A) of FINRA. The representative (or permitted assignees under Rule 5110(e)(1)(A)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the effective date of the registration statement of which this prospectus is a part. In addition, the warrants provide for registration rights upon request, in certain cases. The sole demand registration right provided will not be greater than five years from the effective date of the registration statement in compliance with Rule 5110(g)(8)(C) of FINRA. The piggyback registration rights provided will not be greater than seven years from the effective date of the registration statement in compliance with Rule 5110(g)(8)(D) of FINRA. We will bear all fees and expenses attendant to registering the shares of Common Stock issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of Common Stock at a price below the warrant exercise price.
Lock-Up Agreements
Pursuant to “lock-up” agreements, we, our executive officers and directors, and holders of 5% or more of the outstanding shares of Common Stock, have agreed, without the prior written consent of the representative not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of our Common Stock (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of our Common Stock), enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our Common Stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of Common Stock or securities convertible into or exercisable or exchangeable for Common Stock or any other securities of ours or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of six months after the date of this prospectus in the case of our directors, executive officers, the Company and any successor of the Company and certain stockholders.
Pricing of this Offering
Prior to this offering, there has not been an active market for our Common Stock. The public offering price for our Common Stock will be determined through negotiations between us and the underwriters. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
We offer no assurances that the public offering price of our Common Stock will correspond to the price at which our Common Stock will trade in the public market subsequent to this offering or that an active trading market for our Common Stock and warrants will develop and continue after this offering.
Right of First Refusal
Until thirty six (36) months from the closing date of this offering, the representative will have an irrevocable right of first refusal, in its sole discretion, to act as sole investment banker, sole book-runner, and/or sole placement agent, at the representative’s sole discretion, for each and every future public and private equity and debt offering, including all equity linked financings, during such thirty six (36) month period, on terms customary to the representative. The representative will have the sole right to determine whether or not any other broker-dealer will have the right to participate in any such offering and the economic terms of any such participation. The representative will not have more than one opportunity to waive or terminate the right of first refusal in consideration of any payment or fee.
| 94 |
Discretionary Accounts
The underwriters do not intend to confirm sales of the shares of Common Stock offered hereby to any accounts over which they have discretionary authority.
Trading; Nasdaq Capital Market Listing
Our Common Stock is presently quoted on the OTC, Pink Tier under the symbol “SAFO.” We have applied to list our Common Stock on the Nasdaq Capital Market under the symbol “SVFD.” No assurance can be given that our application will be approved or that a trading market will develop.
Other
From time to time, certain of the underwriters and/or their affiliates may in the future provide, various investment banking and other financial services for us for which they may receive customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans. Except for services provided in connection with this offering, no underwriter has provided any investment banking or other financial services to us during the 180-day period preceding the date of this prospectus and we do not expect to retain any underwriter to perform any investment banking or other financial services for at least 90 days after the date of this prospectus.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our Common Stock. Specifically, the underwriters may over-allot in connection with this offering by selling more shares than are set forth on the cover page of this prospectus. This creates a short position in our Common Stock for its own account. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of Common Stock over-allotted by the underwriters is not greater than the number of shares of Common Stock that they may purchase in the over-allotment option. In a naked short position, the number of shares of Common Stock involved is greater than the number of shares Common Stock in the over-allotment option. To close out a short position, the underwriters may elect to exercise all or part of the over-allotment option. The underwriters may also elect to stabilize the price of our Common Stock or reduce any short position by bidding for, and purchasing, Common Stock in the open market.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing shares of Common Stock in this offering because the underwriter repurchases the shares of Common Stock in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, shares of our Common Stock in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our Common Stock at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on the national securities exchange on which our shares of Common Stock are traded, in the over-the-counter market, or otherwise.
| 95 |
Indemnification
We have agreed to indemnify the underwriters against liabilities relating to this offering arising under the Securities Act and the Exchange Act, liabilities arising from breaches of some or all of the representations and warranties contained in the underwriting agreement, and to contribute to payments that the underwriters may be required to make for these liabilities.
Electronic Distribution
This prospectus in electronic format may be made available on websites or through other online services maintained by one or more of the underwriters, or by their affiliates. Other than this prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Selling Restrictions
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of our Common Stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or our Common Stock in any jurisdiction where action for that purpose is required. Accordingly, our Common Stock may not be offered or sold, directly or indirectly, and this prospectus or any other offering material or advertisements in connection with our Common Stock may be distributed or published, in or from any country or jurisdiction, except in compliance with any applicable rules and regulations of any such country or jurisdiction.
European Economic Area and United Kingdom
In relation to each Member State of the European Economic Area and the United Kingdom (each a “Relevant State”), no Common Stock has been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the Common Stock which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that Relevant State at any time under the following exemptions under the Prospectus Regulation:
| ● | to legal entities which are qualified investors as defined under the Prospectus Regulation; | |
| ● | by the underwriters to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives of the underwriters for any such offer; or | |
| ● | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of Common Stock shall result in a requirement for us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer of Common Stock to the public” in relation to any Common Stock in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any Common Stock to be offered so as to enable an investor to decide to purchase or subscribe for our Common Stock, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
| 96 |
United Kingdom
This prospectus has only been communicated or caused to have been communicated and will only be communicated or caused to be communicated as an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act of 2000, or the FSMA) as received in connection with the issue or sale of our Common Stock in circumstances in which Section 21(1) of the FSMA does not apply to us. All applicable provisions of the FSMA will be complied with in respect to anything done in relation to our Common Stock in, from or otherwise involving the United Kingdom.
Canada
The shares of Common Stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts, or NI 33-105, the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
| 97 |
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code Monétaire et Financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 ;and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1; and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
| 98 |
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israel Securities Authority (the “ISA”), nor have such securities been registered for sale in Israel. The securities may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus that has been approved by the ISA. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing this prospectus, nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered.
This document does not constitute a prospectus under the Israeli Securities Law and has not been filed with or approved by the ISA. In the State of Israel, this document may be distributed only to, and may be directed only at, and any offer of the securities may be directed only at, (i) to the extent applicable, a limited number of persons in accordance with the Israeli Securities Law and (ii) investors listed in the first addendum to the Israeli Securities Law (the “Addendum”) consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange Ltd., underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals,” each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ — $$ — Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
| 99 |
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
| 100 |
Sullivan & Worcester LLP of New York, New York will pass upon the validity of the securities offered hereby. Certain legal matters in connection with this offering will be passed upon for the underwriter by Gracin & Marlow, LLP, New York, New York.
The consolidated financial statements of Save Foods, Inc. as of December 31, 2020 and 2019 and for each of the two years in the period ended December 31, 2020, included in this prospectus and in the Registration Statement have been so included in reliance on the report of Halperin CPA, Financial Consulting & Management, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed with the registration statement. For further information about us and the securities offered hereby, we refer you to the registration statement and the exhibits filed with the registration statement. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The SEC also maintains an internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
Upon the closing of this offering, we will be required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Exchange Act. These reports, proxy statements, and other information will be available on the website of the SEC referred to above.
We also maintain a website at www.savefoods.co., through which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. However, the information contained in or accessible through our website is not part of this prospectus or the registration statement of which this prospectus forms a part, and investors should not rely on such information in making a decision to purchase our Common Stock in this offering.
| 101 |
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2020
TABLE OF CONTENTS
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND STOCKHOLDERS OF
SAVE FOODS, INC.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Save Foods, Inc (the “Company”) and its subsidiary, Save Foods Ltd. as of December 31, 2020 and 2019, the related consolidated statements of operations and comprehensive loss, changes in stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1C to the consolidated financial statements, the Company has not yet generated material revenues from its operations to fund its activities and is therefore dependent upon external sources for financing its operations. As of December 31, 2020, the Company has incurred accumulated deficit of $12,277,647 and negative operating cash flows. These factor among others, as discussed in Note 1C to the consolidated financial statements raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 1C to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of’ these uncertainties.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
| /s/ Halperin Ilanit. | |
| Certified Public Accountants (Isr.) |
Tel Aviv, Israel
March 9, 2021
We have served as the Company’s auditor since 2018

| F-2 |
CONSOLIDATED BALANCE SHEETS
(U.S. dollars except share and per share data)
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | 242,900 | 290,815 | ||||||
| Restricted cash (Note 2D) | 22,395 | 38,194 | ||||||
| Accounts receivable, net | 147,941 | 64,003 | ||||||
| Inventories | 16,356 | 16,302 | ||||||
| Other current assets (Note 3) | 65,579 | 15,300 | ||||||
| Total Current assets | 495,171 | 424,614 | ||||||
| Right Of Use asset arising from operating lease | 14,700 | 48,982 | ||||||
| Property and Equipment, Net (Note 4) | 55,194 | 81,119 | ||||||
| Funds in Respect of Employee Rights Upon Retirement | 122,584 | 109,955 | ||||||
| Total assets | 687,649 | 664,670 | ||||||
| Liabilities and Shareholders’ Deficit | ||||||||
| Current Liabilities | ||||||||
| Short-term loan from banking institution (Note 7) | 7,949 | 7,230 | ||||||
| Current maturities of convertible loans | 56,250 | |||||||
| Accounts payable | 203,323 | 235,864 | ||||||
| Other accounts liabilities (Note 5) | 517,711 | 380,732 | ||||||
| Total current liabilities | 785,233 | 623,826 | ||||||
| Fair Value of Convertible Component in Convertible Loans (Note 6) | 54,970 | - | ||||||
| Convertible Loans (Notes 6) | 146,929 | 285,917 | ||||||
| Long term from banking institution (Note 7) | 8,115 | 14,955 | ||||||
| Liability for employee rights upon retirement | 157,855 | 142,091 | ||||||
| Total liabilities | 1,153,102 | 1,066,789 | ||||||
| Stockholders’ Deficit (Note 9) | ||||||||
| Common stocks of
US$ 0.0001 par value each (“Common Stocks”): 495,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 1,606,760 and 1,458,593 shares as of December 31, 2020 and 2019, respectively. | 161 | 146 | ||||||
| Preferred stocks
of US$ 0.0001 par value (“Preferred stocks”): 5,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 0 shares as of December 31, 2020 and 2019. | - | - | ||||||
| Additional paid-in capital | 11,867,585 | 10,329,571 | ||||||
| Foreign currency translation adjustments | (26,275 | ) | (26,275 | ) | ||||
| Accumulated deficit | (12,277,647 | ) | (10,684,508 | ) | ||||
| (436,176 | ) | (381,066 | ) | |||||
| Non-controlling interests | (29,277 | ) | (21,053 | ) | ||||
| Total stockholders’ deficit | (465,453 | ) | (402,119 | ) | ||||
| Total liabilities and stockholders’ deficit | 687,649 | 664,670 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-3 |
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(U.S. dollars except share and per share data)
| Year ended | ||||||||
| December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sales of products | 232,274 | 175,823 | ||||||
| Cost of sales (Note 11) | (43,405 | ) | (144,548 | ) | ||||
| Gross profit (loss) | 188,869 | 31,275 | ||||||
| Research and development expenses (Note 12) | (417,000 | ) | (615,623 | ) | ||||
| Selling and marketing expenses | (51,105 | ) | (342,058 | ) | ||||
| General and administrative expenses (Note 13) | (1,070,109 | ) | (1,004,899 | ) | ||||
| Operating loss | (1,349,345 | ) | (1,931,305 | ) | ||||
| Financing expenses, net (Note 14) | (270,393 | ) | (43,408 | ) | ||||
| Other expenses, net | (2,532 | ) | - | |||||
| Shares in losses of affiliated company | - | (15,690 | ) | |||||
| Gain on disposal of affiliated company | 15,690 | - | ||||||
| Net loss | (1,606,580 | ) | (1,990,403 | ) | ||||
| Less: Net loss attributable to non-controlling interests | 13,441 | 18,986 | ||||||
| Net loss attributable to the Company | (1,593,139 | ) | (1,971,417 | ) | ||||
| Loss per share (basic and diluted) (Note 16) | (1.05 | ) | (1.38 | ) | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(U.S. dollars, except share and per share data)
| Number of Shares | Amount | Additional paid-in capital | Accumulated other comprehensive income (loss) | Proceeds
on account of shares | Accumulated deficit | Total
Company’s stockholders’ equity | Non- controlling interest | Total
stockholders’ deficit | ||||||||||||||||||||||||||||
| BALANCE AT JANUARY 1, 2019 | 1,318,422 | 132 | 8,852,461 | (26,275 | ) | 105,000 | (8,713,091 | ) | 218,227 | (6,712 | ) | 211,515 | ||||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2019: | ||||||||||||||||||||||||||||||||||||
| Issuance of shares for cash | 140,171 | 14 | 945,679 | - | (105,000 | ) | - | 840,693 | - | 840,693 | ||||||||||||||||||||||||||
| Value of warrant issued in convertible loans | - | - | 97,406 | - | - | - | 97,406 | - | 97,406 | |||||||||||||||||||||||||||
| Stock based compensation | - | - | 434,025 | - | - | - | 434,025 | 4,645 | 438,670 | |||||||||||||||||||||||||||
| Comprehensive loss for the year | - | - | - | - | - | (1,971,417 | ) | (1,971,417 | ) | (18,986 | ) | (1,990,403 | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2019 | 1,458,593 | 146 | 10,329,571 | (26,275 | ) | - | (10,684,508 | ) | (381,066 | ) | (21,053 | ) | (402,119 | ) | ||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2020: | ||||||||||||||||||||||||||||||||||||
| Issuance of shares for cash | 45,876 | 5 | 349,995 | - | - | - | 350,000 | - | 350,000 | |||||||||||||||||||||||||||
| Value of warrant issued in convertible loans | - | - | 34,696 | - | - | - | 34,696 | - | 34,696 | |||||||||||||||||||||||||||
| Conversion of convertible loans | 67,369 | 7 | 585,924 | - | - | - | 585,931 | - | 585,931 | |||||||||||||||||||||||||||
| Exercise of warrants | 28,572 | 2 | 59,998 | - | - | - | 60,000 | - | 60,000 | |||||||||||||||||||||||||||
| Exercise of options | 6,350 | 1 | 19,999 | - | - | - | 20,000 | - | 20,000 | |||||||||||||||||||||||||||
| Stock based compensation | - | - | 487,402 | - | - | - | 487,402 | 5,217 | 492,619 | |||||||||||||||||||||||||||
| Comprehensive loss for the year | - | - | - | - | - | (1,593,139 | ) | (1,593,139 | ) | (13,441 | ) | (1,606,580 | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2020 | 1,606,760 | 161 | 11,867,585 | (26,275 | ) | - | (12,277,647 | ) | (436,176 | ) | (29,277 | ) | (465,453 | ) | ||||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars)
| Year ended | ||||||||
| December 31 | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Loss for the period | $ | (1,606,580 | ) | $ | (1,990,403 | ) | ||
| Adjustments required to reconcile net loss for the period to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 45,205 | 27,351 | ||||||
| Loss from sales of Property and Equipment | 2,382 | - | ||||||
| Share in losses (gain on disposal) of affiliated company | (15,690 | ) | 15,690 | |||||
| Increase (decrease) in liability for employee rights upon retirement | 15,764 | 16,019 | ||||||
| Stock based compensation | 492,619 | 438,670 | ||||||
| Expenses on convertible loans | 176,216 | 6,147 | ||||||
| Compensation expenses in exchange of instruments | 57,793 | - | ||||||
| Decrease (increase) in accounts receivable | (83,938 | ) | 71,294 | |||||
| Decrease (increase) in inventory | (54 | ) | 21,783 | |||||
| Decrease (increase) in other current assets | (47,575 | ) | 49,739 | |||||
| Increase (decrease) in accounts payable | (32,541 | ) | 39,313 | |||||
| Increase in other accounts payable | 197,659 | 59,625 | ||||||
| Net cash used in operating activities | (798,740 | ) | (1,244,772 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Proceeds from (payments on) investment in unconsolidated entity | 4,864 | (7,567 | ) | |||||
| Decrease (increase) in Short term deposits in banking institutions | 15,799 | (38,194 | ) | |||||
| Increase in funds in respect of employee rights upon retirement | (12,629 | ) | (12,567 | ) | ||||
| Proceeds from sales of Property and Equipment | 1,031 | - | ||||||
| Purchase of Property and Equipment | - | (23,327 | ) | |||||
| Net cash provided by (used in) investing activities | 9,065 | (81,655 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from Secured promissory notes | 135,000 | - | ||||||
| Proceeds from convertible loans | 225,000 | 379,000 | ||||||
| Repayments of long-term loans from banking institutes | (7,272 | ) | (42,257 | ) | ||||
| Repayments of right of use asset arising from operating lease | (40,968 | ) | - | |||||
| Proceeds from stock issued for cash | 350,000 | 840,693 | ||||||
| Exercise of options | 20,000 | - | ||||||
| Exercise of warrants | 60,000 | - | ||||||
| Net cash provided by financing activities | 741,760 | 1,177,436 | ||||||
| INCREASE IN CASH AND CASH EQUIVALENTS | (47,915 | ) | (148,991 | ) | ||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR | 290,815 | 439,806 | ||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR | $ | 242,900 | $ | 290,815 | ||||
| F-6 |
SAVE FOODS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars)
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | 408 | $ | 836 | ||||
| Non cash transactions: | ||||||||
| Disposal of affiliated company | 2,704 | - | ||||||
| Termination of lease agreement | 11,590 | - | ||||||
| Issuance of warrants in convertible loans | 53,388 | 97,406 | ||||||
| Conversion of convertible loans | 528,138 | - | ||||||
| Initial recognition of operating lease right-of-use assets | - | 48,982 | ||||||
| Initial recognition of operating lease liability | - | (48,982 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
| F-7 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL
| A. | Operations |
Save Foods, Inc. (the “Company”) was incorporated on April 1, 2009, under the laws of the State of Delaware. On April 27, 2009, the Company acquired from its stockholders 98.94% of the issued and outstanding shares of Save Foods Ltd., including preferred and common stock.
Save Foods Ltd. was incorporated in 2004 and commenced its operations in 2005. Save Foods Ltd. develops, produces, and focuses on delivering innovative solutions for the food industry aimed at improving food safety and prolonging shelf life of fresh produce.
The Company’s common stock is quoted on the OTC, Pink Tier, under the symbol “SAFO”.
| B. | Reverse stock split |
On April 23, 2019, the Company amended and restated its Certificate of Incorporation to effect a 15 to 1 reverse stock split of the Company’s outstanding Common Stock.
As a result of the reverse stock split, which became effective on June 11, 2019, every 15 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of outstanding shares of the Company’s Common Stock and par value of the shares remained unchanged.
On February 23, 2021, the Company amended its Certificate of Incorporation to effect a 7 to 1 reverse stock split of the Company’s outstanding Common Stock.
As a result of the reverse stock split, every 7 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of authorized capital of the Company’s Common Stock and par value of the shares remained unchanged.
All share, stock option and per share information in these condensed consolidated financial statements have been restated to reflect the stock split on a retroactive basis.
| C. | Going concern uncertainty |
Since its incorporation (April 1, 2009), the Company has not had any operations other than those carried out by Save Foods Ltd. The development and commercialization of Save Foods Ltd.’s products will require substantial expenditures. Save Foods Ltd. and the Company have not yet generated sufficient revenues from their operations to fund the Group’s (as defined below) activities and are therefore dependent upon external sources for financing their operations. There can be no assurance that the Company will succeed in obtaining the necessary financing to continue its operations. As of December 31, 2020, the Company had $242,900 in cash, a negative working capital of $290,062 and an accumulated deficit of $12,277,647.
The Company will need to secure additional capital in the future in order to meet its anticipated liquidity needs primarily through the sale of additional Common Stock or other equity securities and/or debt financing. Funds from these sources may not be available to the Company on acceptable terms, if at all, and the Company cannot give assurance that it will be successful in securing such additional capital.
The Company focuses its solutions towards vegetables and fruits which are considered the largest in terms of worldwide consumption. Among other things, the Company tries to cooperate with major fruit packing houses in Israel and abroad.
These factors raise substantial doubt about Save Foods Ltd. and the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| F-8 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL (continue)
| D. | In December 2019, a novel strain of coronavirus, COVID-19, was identified in Wuhan, China. This virus continues to spread globally and, has spread to over 180 countries, including the United States and Israel. The spread of COVID-19 from China to other countries has resulted in the World Health Organization declaring the outbreak of COVID-19 as a “pandemic,” or a worldwide spread of a new disease, on March 11, 2020. Many countries around the world have imposed quarantines and restrictions on travel and mass gatherings to slow the spread of the virus, including in the United States and in Israel. Governments may divert spending from other budgeted resources as they seek to reduce and/or stop the spread of an infectious disease, such as COVID-19. Such events may result in a period of business and manufacturing disruption, and in reduced operations, any of which could materially affect the Company’s business, financial condition and results of operations. The extent to which COVID-19 impacts the Company’s business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. The Company believes it is taking appropriate actions to mitigate the negative impacts. However, the full impact of COVID-19 is unknown and cannot be reasonably estimated as these events occurred subsequent to year end and are still developing. |
| E. | Risk factors |
The Company and Save Foods Ltd. (collectively, the “Group”) face a number of risks, including uncertainties regarding finalization of the development process, demand and market acceptance of the Group’s products, the effects of technological changes, competition and the development of products by competitors. Additionally, other risk factors also exist, such as the ability to manage growth and the effect of planned expansion of operations on the Group’s future results. In addition, the Group expects to continue incurring significant operating costs and losses in connection with the development of its products and increased marketing efforts. As mentioned above, the Group has not yet generated significant revenues from its operations to fund its activities, and therefore the continuance of its activities as a going concern depends on the receipt of additional funding from its current stockholders and investors or from third parties.
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES
The financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (US GAAP).
| A. | Use of estimates in the preparation of financial statements |
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates. As applicable to these financial statements, the most significant estimates and assumptions relate to the going concern assumptions, share based compensation and convertible loans.
| B. | Functional currency |
A majority of the Group’s revenues is generated in U.S. dollars. In addition, most of the Group’s costs are denominated and determined in U.S. dollars and in new Israeli shekels. Management believes that the dollar is the currency in the primary economic environment in which the Group operates. Thus, the functional and reporting currency of the Group is the U.S. dollar.
Accordingly, monetary accounts maintained in currencies other than the dollar are remeasured into dollars in accordance with Accounting Standards Codification (ASC) 830, “Foreign Currency Matters”. All transaction gains and losses of the remeasured monetary balance sheet items are reflected in the statements of operations as financial income or expenses, as appropriate.
| F-9 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| C. | Principles of consolidation |
The consolidated financial statements include the accounts of the Company and its subsidiary, Save Foods Ltd. All significant intercompany balances and transactions have been eliminated on consolidation.
| D. | Cash and cash equivalents, and Restricted cash |
Cash equivalents are short-term highly liquid investments which include short term bank deposits (up to three months from date of deposit), that are not restricted as to withdrawals or use that are readily convertible to cash with maturities of three months or less as of the date acquired.
Restricted cash as of December 31, 2020 and 2019 included a NIS 72,000 ($22,395) and NIS 132,000 ($38,194), respectively collateral account for the Company’s corporate credit cards and a loan and is classified in current assets.
| E. | Accounts receivables |
Accounts receivables are stated at their net realizable value. The allowance against gross accounts receivables reflects the best estimate of losses inherent in the receivables portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other currently available information. As of December 31, 2020, and 2019, an allowance for doubtful debts in the amount of $26,553 and $24,702, respectively, is reflected in net accounts receivables. Accounts receivables are written off after all reasonable means to collect the full amount have been exhausted.
| F. | Property, plant and equipment, net |
| 1. | Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. When an asset is retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition is reflected in the Statements of Operations and Comprehensive Loss. | |
| 2. | Rates of depreciation: |
| % | ||||
| Furniture and office equipment | 7-15 | |||
| Machines | 10-15 | |||
| Computers | 33 | |||
| Vehicle | 15 |
| G. | Impairment of long-lived assets |
The Group’s long-lived assets are reviewed for impairment in accordance with Accounting Standards Codification (“ASC”) Topic 360, “Property, Plant and Equipment”, whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. No impairment expenses were recorded during the years ended December 31, 2020 or 2019.
| F-10 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| H. | Deferred income taxes |
The Group accounts for income taxes in accordance with ASC Topic 740, “Income Taxes”. Accordingly, deferred income taxes are determined utilizing the asset and liability method based on the estimated future tax effects of differences between the financial accounting and the tax bases of assets and liabilities under the applicable tax law. Deferred tax balances are computed using the enacted tax rates expected to be in effect when these differences reverse. Valuation allowances in respect of deferred tax assets are provided for, if necessary, to reduce deferred tax assets to amounts more likely than not to be realized.
The Group accounts for uncertain tax positions in accordance with ASC Topic 740-10, which prescribes detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in an enterprise’s financial statements. According to ASC Topic 740-10, tax positions must meet a more-likely-than-not recognition threshold. The Company’s accounting policy is to classify interest and penalties relating to uncertain tax positions under income taxes, however the Company did not recognize such items in its fiscal 2020 and 2019 financial statements and did not recognize any liability with respect to an unrecognized tax position in its balance sheets.
| I. | Liability for employee rights upon retirement |
Save Foods Ltd’s liability for employee rights upon retirement with respect to its Israeli employees is calculated, pursuant to Israeli Severance Pay Law, based on the most recent salary of each employee multiplied by the number of years of employment, as of the balance sheet date. Employees are entitled to one month’s salary for each year of employment, or a portion thereof. Save Foods Ltd. makes monthly deposits to insurance policies and severance pay funds. The liability of the Company is fully provided for.
The deposited funds include profits accumulated up to the balance sheet date. The deposited funds may be withdrawn upon the fulfillment of the obligation pursuant to Israeli severance pay laws or labor agreements. The value of the deposited funds is based on the cash surrender value of these policies, and includes immaterial profits/losses.
Severance expenses for the years ended December 31, 2020 and 2019, amounted to $7,419 and $24,000, respectively.
| J. | Revenue recognition |
Revenues are recognized when delivery has occurred and there is persuasive evidence of an agreement, the fee is fixed or determinable and collection of the related receivables is reasonably assured and no further obligations exist.
Revenues from sales of products are recognized when title and risk and rewards for the products are transferred to the customer.
| K. | Research and development expenses |
Research and development expenses are charged to operations as incurred.
| F-11 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| L. | Royalty-bearing grants |
Royalty-bearing grants from the Israeli Innovation Authority (the “IIA”) for funding approved research and development projects are recognized at the time Save Foods Ltd. is entitled to such grants (i.e. at the time that there is reasonable assurance that the Company will comply with the conditions attached to the grant and that there is reasonable assurance that the grant will be received), on the basis of the costs incurred and reduce research and development costs. The cumulative research and development grants received by the Company from inception through December 2020 amounted to NIS 484,429 (US$150,678).
As of December 31, 2020, and 2019, the Company did not accrue for or pay any royalties to the IIA since no revenues were recognized in respect of the funded projects.
| M. | Inventories |
Inventories are valued at the lower of cost or net realizable value. Cost of raw and packaging materials, purchased products, manufactured finished products and products in process are determined on the average costs basis.
The Company regularly reviews its inventories for impairment and reserves are established when necessary.
| N. | Basic and diluted loss per common stock |
Basic loss per common stock is computed by dividing the loss for the period applicable to shareholders, by the weighted average number of shares of common stock outstanding during the period. Securities that may participate in dividends with the shares of common stock (such as the convertible preferred) are considered in the computation of basic loss per share under the two-class method. However, in periods of net loss, only the convertible preferred shares are considered, since such shares have a contractual obligation to share in the losses of the Company.
In computing diluted loss per share, basic loss per share is adjusted to reflect the potential dilution that could occur upon the exercise of potential shares. Accordingly, in periods of net loss, no potential shares are considered.
| O. | Stock-based compensation |
The Company measures and recognizes the compensation expense for all equity-based payments to employees based on their estimated fair values in accordance with ASC 718, “Compensation-Stock Compensation”. Share-based payments including grants of stock options are recognized in the statement of comprehensive loss as an operating expense based on the fair value of the award at the date of grant. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model. The Company has expensed compensation costs, net of estimated forfeitures, applying the accelerated vesting method, over the requisite service period or over the implicit service period when a performance condition affects the vesting, and it is considered probable that the performance condition will be achieved.
Share-based payments awarded to consultants (non-employees) are accounted for in accordance with ASC Topic 505-50, “Equity-Based Payments to Non-Employees”.
| F-12 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| P. | Fair Value Measurements |
Fair value of certain of the Company’s financial instruments including cash, accounts receivable, account payable, accrued expenses, notes payables, and other accrued liabilities approximate cost because of their short maturities. The Company measures and reports fair value in accordance with ASC 820, “Fair Value Measurements and Disclosure” defines fair value, establishes a framework for measuring fair value in accordance with generally accepted accounting principles and expands disclosures about fair value investments.
Fair value, as defined in ASC 820, is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value of an asset should reflect its highest and best use by market participants, principal (or most advantageous) markets, and an in-use or an in-exchange valuation premise. The fair value of a liability should reflect the risk of non-performance, which includes, among other things, the Company’s credit risk.
Valuation techniques are generally classified into three categories: the market approach; the income approach; and the cost approach. The selection and application of one or more of the techniques may require significant judgment and are primarily dependent upon the characteristics of the asset or liability, and the quality and availability of inputs. Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 also provides fair value hierarchy for inputs and resulting measurement as follows:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities.
Level 2: Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3: Unobservable inputs for the asset or liability that are supported by little or no market activity, and that are significant to the fair values.
Fair value measurements are required to be disclosed by the Level within the fair value hierarchy in which the fair value measurements in their entirety fall. Fair value measurements using significant unobservable inputs (in Level 3 measurements) are subject to expanded disclosure requirements including a reconciliation of the beginning and ending balances, separately presenting changes during the period attributable to the following: total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings, and a description of where those gains or losses included in earning are reported in the statement of income.
The Company records a debt discount related to the issuance of convertible debts that have conversion features at adjustable rates. The debt discount for the convertible instruments is recognized and measured by allocating a portion of the proceeds as an increase in additional paid-in capital and as a reduction to the carrying amount of the convertible instrument equal to the fair value of the conversion features. The debt discount will be accreted by recording additional non-cash gains and losses related to the change in fair values of derivative liabilities over the life of the convertible notes.
The Company’s financial assets and liabilities that are measured at fair value on a recurring basis by level within the fair value hierarchy are as follows:
| Balance as of December 31, 2020 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Fair Value of convertible component in convertible loan | - | - | 54,970 | 54,970 | ||||||||||||
| Total liabilities | - | - | 54,970 | 54,970 | ||||||||||||
The following table presents the changes in fair value of the level 3 liabilities for the Year ended December 31, 2020:
| Fair value of Convertible component | ||||
| Outstanding at January 1, 2020 | - | |||
| Fair value of issued level 3 liability | 27,762 | |||
| Changes in fair value | 27,208 | |||
| Outstanding at December 31, 2020 | 54,970 | |||
| Q. | Concentrations of credit risk |
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents as well as certain other current assets that do not amount to a significant amount. Cash and cash equivalents, which are primarily held in Dollars and New Israeli Shekels, are deposited with major banks in Israel and United States. Management believes that such financial institutions are financially sound and, accordingly, minimal credit risk exists with respect to these financial instruments. The Company does not have any significant off-balance-sheet concentration of credit risk, such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
| R. | Contingencies |
The Company records accruals for loss contingencies arising from claims, litigation and other sources when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. Legal costs incurred in connection with loss contingencies are expensed as incurred.
| F-13 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| S. | New Accounting Pronouncements |
In June 2016, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2016-13, “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments.” In November 2018, FASB issued ASU No. 2018-19, “Codification Improvements to Topic 326, Financial Instruments-Credit Losses”, which amends the scope and transition requirements of ASU 2016-13. Topic 326 requires a financial asset (or a group of financial assets) measured at amortized cost basis to be presented at the net amount expected to be collected. The measurement of expected credit losses is based on relevant information about past events, including historical experience, current conditions and reasonable and supportable forecasts that affect the collectability of the reported amount. Topic 326 will originally become effective for the Company beginning January 1, 2020, with early adoption permitted, on a modified retrospective approach. As a smaller reporting company, the effective date for the Company has been delayed until fiscal years beginning after December 15, 2022, in accordance with ASU 2019-10, although early adoption is still permitted. This standard did not have a material impact to the Company’s consolidated financial statements after evaluation.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. The amendments in this ASU simplify the accounting for income taxes, eliminates certain exceptions to the general principles in Topic 740 and clarifies certain aspects of the current guidance to improve consistent application among reporting entities. ASU 2019-12 is effective for fiscal years beginning after December 15, 2021 and interim periods within annual periods beginning after December 15, 2022, though early adoption is permitted, including adoption in any interim period for which financial statements have not yet been issued. This standard is not expected to have a material impact to the Company’s consolidated financial statements.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement. This standard will require entities to disclose the amount of total gains or losses for the period recognized in other comprehensive income that is attributable to fair value changes in assets and liabilities held as of the balance sheet date and categorized within Level 3 of the fair value hierarchy. This ASU will be effective for the Company for annual and interim periods beginning after December 31, 2020. Early adoption of this standard is permitted. This standard did not have a material impact to the Company’s consolidated financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging Contracts in Entity s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity s Own Equity. ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models results in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective for public companies for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. Management has not yet evaluated the impact that the adoption of ASU 2020-06 will have on the Company’s consolidated financial statement presentation or disclosures.
Other new pronouncements issued but not effective as of December 31, 2020 are not expected to have a material impact on the Company’s consolidated financial statements.
NOTE 3 – OTHER CURRENT ASSETS
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Prepaid expenses and advances to vendors | 51,020 | 4,811 | ||||||
| Receivables from sale of subsidiary (Note 5) | 2,704 | - | ||||||
| Government Institutions | 11,855 | 10,489 | ||||||
| 65,579 | 15,300 | |||||||
| F-14 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – PROPERTY AND EQUIPMENT, NET
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Computers | 10,328 | 10,328 | ||||||
| Furniture and office equipment | 5,002 | 5,002 | ||||||
| Machines | 130,797 | 134,665 | ||||||
| Vehicles | 85,149 | 85,149 | ||||||
| 231,276 | 235,144 | |||||||
| Less - accumulated depreciation | (139,382 | ) | (117,325 | ) | ||||
| Less – Impairment of long lived assets | (36,700 | ) | (36,700 | ) | ||||
| Total property and equipment, net | 55,194 | 81,119 | ||||||
In the years ended December 31, 2020 and 2019, depreciation expenses were US$ 22,512 and US$ 27,351 respectively, and additional property and equipment were purchased in an amount of US$ 23,327 for the years ended December 31, 2019 (none for the year ended December 31, 2020).
NOTE 5 – OTHER ACCOUNTS LIABILITIES
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Employees and related institutions | 110,220 | 135,901 | ||||||
| Accrued expenses | 392,442 | 184,616 | ||||||
| Right Of Use liability arising from operating lease | 15,049 | 52,093 | ||||||
| Affiliated company (*) | - | 8,122 | ||||||
| 517,711 | 380,732 | |||||||
| (*) | On April 2, 2019, the Company invested 10,000 Canadian Dollars for 20% of the outstanding shares of Savecann Solutions Inc. (“Savescann”) a newly formed company registered in Canada. Savecann intended to market the Company’s solutions to the Cannabis market. | |
| On April 21, 2020, the Company sold its entire holdings in Savecann for total consideration of 10,000 Canadian Dollars ($7,000), of which $2,704 were not paid yet and are presented as part of other current assets. |
| F-15 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – CONVERTIBLE LOANS
| A. | In December 2019, the Company entered into a series of Convertible Loan Agreements (each a “CLA”) with third parties and certain existing shareholders (the “Lenders”), pursuant to which the Lenders agreed to provide the Company loans in the aggregate amount of $379,000 and in exchange the Company issued to the Lenders (i) convertible promissory notes (the “Notes”) and (ii) warrants with an exercise price of $8.40. In January and March 2020, the Company entered into two additional CLA agreements for an aggregate amount of $135,000, consisting of the same terms. | |
| According to the terms of the CLA, the Notes bear interest at a rate of 5% per annum and the loan amount represented by the Notes is to be repaid to the Lenders according to the following schedule: (i) the principal amount represented by the Notes to be repaid in twenty four equal monthly installments, commencing on the twenty fifth month following the closing of each CLA, and (ii) the interest accrued on the loan amount to be paid in two bi-annual installments, commencing on the first anniversary of the first payment of the principal amount. | ||
| In addition, according to the terms of the CLA, the outstanding loan amount matures on the earlier of (i) the third anniversary of each CLA or (ii) a deemed liquidation event (as defined therein), and the Lenders may convert all or any portion of the Notes at any time prior to the one-year anniversary of each issuance into shares of the Company’s Common Stock at a conversion price of $8.40 per share. | ||
| In accordance with ASC 815-15-25, the conversion feature was considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations. | ||
As a result of the above issuances, the Company recorded in the periods ended March 31, 2020 and December 31, 2019, a total amount of $34,696 and $97,406, respectively, in respect of the detachable warrants, as a credit to stockholders’ equity (additional paid in capital). The fair value of the Warrants was determined using the Black-Scholes pricing model, assuming a risk free rate of 1.6%, a volatility factor of 54.00%, dividend yields of 0% and an expected life of 3 years.
| ||
| On June 24, 2020, the Company entered into a Securities Purchase Agreement (the “SPA”) with the Lenders in connection with the sale and issuance of 69,332 units (“Units”), at a purchase price of $7.63 per Unit. Each Unit consists of: (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.4 (the “Warrant”). In connection with the SPA, the Company issued to the Lenders an aggregate of 67,369 shares of Common Stock and Warrants to purchase an aggregate of 67,369 shares of Common Stock. The shares of Common Stock were issued on July 2, 2020. | ||
| Simultaneous with and conditioned upon the execution of the SPA, the Company and each of the Lenders agreed to effectively cancel the CLA and the equity securities issued thereunder. In connection therewith, each of the Lenders voluntarily waived any right to receive interest that accrued thereupon pursuant to the CLA. | ||
| The Company evaluated the transaction as an exchange of instruments and as a result of the above conversion, recorded a compensation expenses in a total amount of $57,793, as of the exchange date, and as a credit to stockholders’ equity (additional paid in capital). The fair value of the additional shares granted in the conversion was calculated based on the Company’s share price as of the date of the conversion. The fair value of the additional warrants granted in the conversion was determined using the Black-Scholes pricing model, assuming a risk-free rate of 0.21%, a volatility factor of 51.96%, dividend yields of 0% and an expected life of 2.45-2.71 years. | ||
| During the years ended December 31, 2020 and 2019, the Company recorded net interest and amortization expenses in the amount of $199,709 and $4,323, respectively, in respect of the discounts recorded on the CLAs. | ||
| B. | On September 21, 2020, the Company entered into a series of additional convertible loan agreements (each a “2020 CLA”) with certain lenders (the “2020 Lenders”) to sell convertible promissory notes with an aggregate principal amount of $125,000 (each a “2020 Note”). The outstanding loan amount under the 2020 CLA will mature on the earlier of (i) the third anniversary of each 2020 CLA or (ii) a deemed liquidation event (as defined therein), and the 2020 Lenders may convert all or any portion of the 2020 Notes into shares of Common Stock at any time prior to a mandatory conversion event (as defined therein) at a conversion price of $7.63 per share. The 2020 Notes will bear interest at a rate of 5% per annum. The loan amount represented by the 2020 Notes will be repaid to the 2020 Lenders according to the following schedule: (i) the principal amount represented by the 2020 Notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. |
| F-16 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – CONVERTIBLE LOANS (continue)
| During October 2020, the Company entered into a series of additional 2020 CLAs with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $100,000. | ||
| During January 2021, the Company entered into a series of additional 2020 CLAs with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $274,000. | ||
| As part of the 2020 CLA, the Company entered into a registration rights agreement with each of the 2020 Lenders, whereby each 2020 Lender received piggyback registration rights for the shares issuable upon conversion of the 2020 Notes to shares of Common Stock. |
The loans are convertible into common Stock upon (i) a completion of underwritten public offering (“Mandatory Conversion”) convert the outstanding loan amount at a share price as shall be determined in the offering, or (ii) at the lender’s discretion (“Optional Conversion”) convert the outstanding loan amount at a share price per share of $7.63.
In accordance with ASC 815-15-25, the conversion feature was considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations.
The fair value of the convertible component was estimated by third party appraiser as weighted average of the two possible scenarios of the total loan amount conversion: as of September 21, 2020 and October 23, 2020, 70% probability for the Mandatory Conversion and 30% probability for the Optional Conversion and as of December 31, 2020, 75% probability for the Mandatory Conversion and 25% probability for the Optional Conversion.
The Mandatory Conversion (scenario 1) was estimated by the appraiser using the Black-Scholes option pricing model, to compute the fair value of the derivative and to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of issuance dates and as of the balance sheet date:
| September 21, 2020 | October 23, 2020 | December 31, 2020 | ||||||||||
| Dividend yield | 0 | 0 | 0 | |||||||||
| Risk-free interest rate | 0.19 | % | 0.11 | % | 0.09 | % | ||||||
| Expected term (years) | 0.775 | 0.685 | 0.417 | |||||||||
| Volatility | 51.96 | % | 51.96 | % | 48.06 | % | ||||||
| Share price | 6.72 | 5.88 | 8.61 | |||||||||
| Exercise price | 7.63 | 7.63 | 7.63 | |||||||||
| Fair value | 15,208 | 6,457 | 47,499 | |||||||||
The Optional Conversion (scenario 2) was estimated by the appraiser using binomial option pricing model and simulating and waiver of the lender as an exercise price, to compute the fair value of the derivative and to mark to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of the issuance dates and as of balance sheet date:
| September 21, 2020 | October 23, 2020 | December 31, 2020 | ||||||||||
| Dividend yield | 0 | 0 | 0 | |||||||||
| Risk-free interest rate | 0.12-0.16 | % | 0.12-0.2 | % | 0.10-0.14 | % | ||||||
| Volatility | 51.96 | % | 51.96 | % | 48.06 | % | ||||||
| Share price | 6.72 | 5.88 | 8.61 | |||||||||
| Fair value | 26,824 | 15,167 | 77,381 | |||||||||
The fair value of the convertible component was estimated by the third-party appraiser after giving effect to the weighted average of the two possible scenarios as of issuance dates was $27,762 and as of December 31, 2020 was $54,970.
The fair value allocated to the convertible loan was estimated by third party appraiser as the residual value of the proceeds net of the convertible component and was estimated at a value of $203,179 as of December 31, 2020 of which $56,250 is presented under current liabilities and $146,929 is presented under long term liabilities.
| F-17 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars)
NOTE 7 - LONG-TERM LOANS FROM BANKING INSTITUTIONS
| A. | Composition |
Interest
rate at 2020 | December 31, | |||||||||||
| % | 2020 | 2019 | ||||||||||
| Long-term loans | 2.1 | 16,064 | 22,185 | |||||||||
| Less current maturities | (7,949 | ) | (7,230 | ) | ||||||||
| 8,115 | 14,955 | |||||||||||
| B. | Maturity dates: |
| First year | 7,949 | |||
| Second year | 8,115 | |||
| 16,064 |
NOTE 8 – COMMITMENT AND CONTINGENT LIABILITIES
| A. | Save Foods Ltd. is committed to pay royalties to the IIA on the proceeds from sales of products resulting from research and development projects in which the IIA participates by way of grants. In the first 3 years of sales the Company shall pay 3% of the sales of the product which was developed under IIA research and development projects. In the fourth, fifth and sixth years of sales, the Company shall pay 4% of such sales and from the seventh year onwards the Company shall pay 5% of up to 100% of the amount of grants received plus interest at LIBOR. Save Foods Ltd. was entitled to the grants only upon incurring research and development expenditures. There were no future performance obligations related to the grants received from the IIA. As of December 31, 2020, the contingent liabilities with respect to grants received from the IIA, subject to repayment under these royalty agreements on future sales is NIS 484,429 (US$ 150,678), not including interest. | |
| B. | The Company and its subsidiary currently lease office space at Kibbutz Alonim under a short-term operating lease agreement ends at December 31, 2020 with an option to extend the agreement with additional year ended at December 31, 2021. During the years 2020 and 2019, the Company paid an annual rent of $14,967 and $10,605, respectively under the above agreement. The agreement was automatically renewed (option) until December 31, 2021. |
In addition, the Company and Save Foods Ltd. entered into a short term lease agreement for the period ended at May 31, 2019, with a shareholder for the lease of an office and related services for a monthly fee of NIS 5,000 (approximately $1,400).
On May 1, 2019 the Company and Save Foods Ltd. entered into a lease agreement for its offices in Tel Aviv for the period ending December 31, 2020, for the lease of an office and management fees for a monthly fee of NIS 11,214 (approximately $3,450). On August 9, 2020, the Company and the lessor agreed that the Company would pay the lessor a one-time NIS10,000 ($3,100) and the agreement would be terminate as of August 13, 2020
On September 1, 2017, the Company entered into a lease agreement for office space in New York, hereinafter the New York Lease. The New York Lease will expire on September 30, 2020, unless terminated earlier by either party by providing 30 days prior written notice to the other party. The New York Lease rent amount, $7,200, was fully paid for through an issuance of 720,000 shares of our Common Stock on November 5, 2017.
| F-18 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars)
NOTE 8 – COMMITMENT AND CONTINGENT LIABILITIES (continue)
| C. | In July 2011, Save Foods, Ltd. filed with the Commissioner of Patent of the Israeli Patent Office (the “Commissioner”) a claim stating its opposition to a patent application made by Xeda International S.A, (“Xeda”), which would have restricted Save Foods Ltd’s operations. |
In June 2018, the Commissioner accepted Save Foods Ltd’s claims against Xeda’s patent application and, accordingly, rejected Xeda’s application. The Commissioner awarded Save Foods Ltd. with expenses and legal fees in the aggregate amount of approximately NIS 165,000 (approximately $46,000)
In September 2018, Xeda filed an appeal with the District Court in Jerusalem (the “Court”), with respect to the Commissioner’s decision, and in January 2019, the Court dismissed Xeda’s appeal and awarded Save Foods Ltd. expenses and legal fees in the aggregate amount of approximately NIS 50,000 (approximately $13,000).
In February 2019, Xeda filed a request to appeal the Court’s decision with the Israeli Supreme Court. In May 2019, the Israeli Supreme Court rejected Xeda’s request to appeal and awarded Save Foods Ltd. expenses and legal fees in the aggregate amount of NIS 8,000.
| D. | On September 22, 2020, the Company entered into a non-exclusive Commission Agreement with Earthbound Technologies, LLC (“EBT”) for a period of 12 months, according to which EBT shall introduce the Company to potential clients, pre-approved by the Company (“Introduced Parties”) and shall assist the Company in finalizing commercial agreements with the Introduced Parties. In consideration for its services, the Company agreed to pay EBT 12.5% of the net revenues generated from Introduced Parties (during the agreement period and within 18 months following the termination of the agreement) up to a total aggregated amount of $2,000,000, provided that the compensation shall not exceed 25% of the Company’s gross profit under the given commercial agreement signed with the Introduced Party. In addition, in the event that the aggregated net revenues generated from Introduces Parties exceeds $500,000, and subject to the approval of the Board, the Company shall issue to EBT 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share. In the event that certain additional events detailed in the agreement occur, the Company will also issue to EBT, subject to the approval of the Board, an additional 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share. |
| E. | On September 22, 2020, the Company entered into a Distribution Agreement (the “Distribution Agreement”), with Safe-Pack Products Ltd (“Safe-Pack”) according to which the Company granted Safe-Pack an exclusive right to resell, distribute, advertise, and market Company’s products related to the citrus industry in Israel and other territories, as well as additional products as shall be mutually agreed upon in the future. In addition, the Company agreed to grant Safe-Pack a right of first refusal to be designated as an exclusive distributor of the Company in certain agreed upon territory for additional products of the Company as they relate to the field of post-harvest. In consideration for the above rights granted to Safe-Pack, Safe-Pack will submit to the Company purchase orders of its products at a price specified in the Distribution Agreement. Commencing upon the second calendar year of the agreement, Safe-Pack is required to meet a minimum purchase quota, as shall be mutually agreed upon between the parties. In the event that the parties fail to agree on a quota, the quota shall be equal to last year quota plus 3%. |
NOTE 9 – SHAREHOLDERS’ EQUITY
Description of the rights attached to the Shares in the Company:
Common stock:
Each share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively. Accordingly, the stockholders of the Company’s common stock who hold, in the aggregate, more than fifty percent of the total voting rights can elect all of the directors and, in such event, the holders of the remaining minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding shares of common stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except as otherwise provided by law.
Transactions:
During January 2019 the Company issued total of 19,050 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $120,000.
During February 2019 the Company issued total of 35,717 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $225,000.
During March 2019 the Company issued total of 15,874 shares of Common Stock of the Company $0.0001 par value, to accredited investor for total consideration of $100,000.
In addition, during March 2019 the Company issued 7,937 shares of Common Stock of the Company $0.0001 par value, to an accredited investor for total consideration of $50,000 and at the same time issued him 7,937 shares of Common Stock $0.0001 par value for total consideration of $66,666 and 7,937 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
During June 2019, the Company issued total of 31,747 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $200,000. In addition, During June 2019 the Company issued 10,004 shares of Common Stock of the Company $0.0001 par value, to an accredited investor for total consideration of $84,034 and at the same time issued that accredited investor 10,004 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
During August 2019, the Company signed a subscription agreement with an investor according to which the Company will issue total of 11,905 shares of Common Stock of the Company $0.0001 par value, to accredited investor for total consideration of $100,000. In addition, and at the same time issued that accredited investor 11,905 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
| F-19 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – SHAREHOLDERS’ EQUITY (continue)
On May 9, 2020, the Company entered into a Securities Purchase Agreement (the “May Agreement”) with an existing shareholder (the “Investor”), pursuant to which the Company sold to the Investor for an aggregated amount of $100,000, 13,107 units at a price per unit of $7.63 (the “2019 Units”), each 2019 Unit consists of (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.40 for a period of 36 months following the issuance date. The shares of Common Stock were issued on July 2, 2020.
On July 2, 2020, the Company issued 67,369 shares of Common Stock in respect of the conversion of convertible loans as detailed in Note 3A above.
During July and August 2020, the Company entered into additional Securities Purchase Agreements with existing shareholders (the “Additional Investors”), pursuant to which the Company sold to the Additional Investors for an aggregate amount of $150,000, 19,662 units, based substantially upon the same terms as in the May Agreement.
On September 23, 2020, the Company entered into a Securities Purchase Agreement (the “Medigus SPA”) with Medigus Ltd. (“Medigus”) in connection with the sale and issuance of 13,107 units for total consideration of $100,000, based substantially upon the same terms as in the May Agreement.
The Medigus SPA contemplates an additional investment by Medigus not to exceed $25,000 (the “Additional Medigus Investment”), which shall be triggered following the parties’ initiation of a proof of concept procedure to test the effectiveness of the Company’s sanitizers and its residual effects on surfaces against different pathogens including COVID-19. In consideration for the Additional Medigus Investment, the Company has agreed to issue an additional 3,277 units at a purchase price of $7.63, which units shall contain the same composition of securities as described in the foregoing description of the Medigus SPA.
On September 22, 2020 and September 24, 2020, the Chairman of the Board of Directors of the Company (the “Board”), exercised a warrant to purchase an aggregate of 28,572 shares of Common Stock, which warrants were granted to him on June 15, 2020 by the Board as a replacement for his recently expired options, which were previously granted to him in April 2018.
During December 2020, two directors on Save Food Ltd exercised 6,350 options under the 2018 Equity Incentive Plan into 6,350 shares of common stock of the Company total consideration of $20,000.
| F-20 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – STOCK OPTIONS
On October 18, 2018, the Company adopted the 2018 Share Incentive Plan (the “2018 Equity Incentive Plan”), pursuant to which the Company’s Board of Directors is authorized to grant up to 190,477 options, exercisable into 190,477 shares of Common Stock of the Company. The purpose of the 2018 Equity Incentive Plan is to offer attract and retain the best available personnel, provide incentive to individuals who perform services for the Company and promote the success of the Company’s business.
On January 3, 2019, the Company granted of 80,954 options under the 2018 Equity Incentive Plan of which 9,524 options are vested quarterly over three years commencing May 15, 2018, 35,715 options are vested 1/3 after a year commencing October 1, 2018 and the remaining 2/3 are vested quarterly over additional two years, 14,286 options are vested quarterly over three years commencing October 1, 2018 and 21,429 options are vested 1/3 after a year commencing January 1, 2019 and the remaining 2/3 are vested quarterly over additional two years
On April, 2019, the Board of Directors of the Company approved the issuance of 28,572 options to purchase 28,572 Company’s Common Stock 0.0001 par value, to Mr. Dan Sztybel, under the Company’s 2018 Equity Incentive Plan. The options shall vest quarterly over three years, commencing April 1, 2019, and shall be exercisable for an exercise price of $3.15 per share. In addition, the Board of Directors of the Company approved the issuance of 14,286 options to purchase 14,286 Company’s Common Stock 0.0001 par value, to Mr. Dan Sztybel, subject to Save Foods Ltd’s obtainment of certain EPA and FDA approvals by the end of the second quarter of 2020. Such conditions did not met as of June 30, 2020.
On November 12, 2019, the Board of Directors of the Company approved the grant of 45,239 options under the 2018 Equity Incentive Plan of which 28,572 options are vested quarterly over three years commencing January 3, 2019, 9,524 options are vested 1/3 after a year commencing January 3, 2019 and the remaining 2/3 are vested quarterly over additional two years, and 7,143 options are vested 1/3 after a year commencing October 1, 2019 and the remaining 2/3 are vested quarterly over additional two years.
In addition, the Board of Directors approved the agreement with a consultant, according to which the consultant would receive 2,858 fully vested options to purchase Company’s shares at exercise price of $6.3 per option for certain “closed” introduction made by the consultant in Chile. No options were granted under this agreement as of December 31, 2020.
On June 23, 2020, the Company granted 21,143 options to purchase its Common Stock under the 2018 Equity Incentive Plan (the “Plan”). The options shall vest quarterly over two years commencing June 23, 2020, whereby 12.50% of the shares covered by the options will vest on the three month anniversary of June 23, 2020, and 12.50% of the shares covered by the options will vest at the end of each subsequent three month period thereafter over the course of the subsequent 21 months.
On July 1, 2020, the Company granted 71,431 options to purchase its Common Stock under the 2018 Equity Incentive Plan. The options shall vest quarterly over two years commencing June 1, 2020, whereby 12.50% of the shares covered by the options will vest on the three month anniversary of June 1, 2020, and 12.50% of the shares covered by the options will vest at the end of each subsequent three month period thereafter over the course of the subsequent 21 months. The fair value of the options was estimated at a value of $344,767 at the date of issuance using the Black-Scholes option pricing model.
In addition, on July 1, 2020, the Board approved an increase to the share option pool under the Plan by 99,466 shares of Common Stock, such that after the increase the total number of shares of Common Stock issuable under the Plan is 289,942 shares of Common Stock.
On September 22, 2020, the Board approved an amendment of the terms of the outstanding options granted to certain employees and directors of the Company. According to the new terms, subject to the consummation of equity financing in excess of $1,000,000 and the completion of listing of the Company’s Common Stock for trade on the Nasdaq, and in the event that the employment or engagement of such grantee is either terminated (not for cause) or otherwise changed thereby resulting in the conclusion of such engagement (including voluntary resignation), all outstanding options of such grantee shall vest immediately and shall be exercisable for a period of three years following the termination date.
| F-21 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 10 – STOCK OPTIONS (continue)
The following table presents the Company’s stock option activity for employees and directors of the Company for the year ended December 31, 2020 and 2019:
Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2019 | 59,525 | 3.15 | ||||||
| Granted (*) | 109,526 | 3.15 | ||||||
| Exercised | - | - | ||||||
| Forfeited | (4,762 | ) | 3.15 | |||||
| Outstanding at January 1, 2020 | 164,289 | 3.15 | ||||||
| Granted | 92,574 | 3.64 | ||||||
| Exercised | (6,350 | ) | 3.15 | |||||
| Forfeited | (29,365 | ) | 3.15 | |||||
| Expired | (14,286 | ) | 3.15 | |||||
| Outstanding at December 31, 2020 | 206,862 | 3.15 | ||||||
| Number of options exercisable at December 31, 2020 | 97,351 | 3.27 | ||||||
(*) Options that were granted on January 3, 2019 with vesting that commences before December 31, 2018.
The aggregate intrinsic value of the awards outstanding as of December 31, 2020 is US$1,084,465. These amounts represent the total intrinsic value, based on the Company’s stock price of US$ 8.61 as of December 31, 2020, less the weighted exercise price. This represents the potential amount received by the option holders had all option holders exercised their options as of that date.
The fair value of options granted was estimated at the dates of grant using the Black-Scholes option pricing model. The following are the data and assumptions used:
| 2020 | 2019 | |||||||
| Dividend yield | 0 | 0 | ||||||
| Expected volatility (%) (*) | 52 | % | 54 | % | ||||
| Risk-free interest rate (%) (**) | 0.23 | % | 1.56-2.39 | % | ||||
| Expected term of options (years) (***) | 5 | 5 | ||||||
| Exercise price (US dollars) | 3.15-3.78 | 3.15 | ||||||
| Share price (US dollars) | 7.63 | 6.3 | ||||||
| Fair value (US dollars) | 4.83-5.17 | 4.19 | ||||||
| (*) | Due to the low trading volume of the Company’s Common Stock, the expected volatility was based on the historical volatility of the share price of other public companies that operate in the same industry sector as the Company (agricultural chemical industry). | |
| (**) | The risk-free interest rate represented the risk-free rate of US$ zero – coupon US Government Loans. | |
| (***) | Due to the fact that the Company does not have sufficient historical exercise data, the expected term was determined based on the “simplified method” in accordance with SEC Staff Accounting Bulletin No. 110. |
The total fair value estimation of the non-cash compensation of the grant at 2020 and 2019 was approximately $453,976 and $440,848, respectively. Costs incurred in respect of stock-based compensation for employees and directors, for the year ended December 31, 2020 and 2019 were $492,619 and $438,670, respectively.
As of December 31, 2020, there are 76,730 options available for future grants under the 2018 Equity Incentive Plan.
| F-22 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 11 – COST OF SALES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | 8,074 | 35,142 | ||||||
| Share based compensation | 5,402 | 14,531 | ||||||
| Materials | 16,692 | 69,390 | ||||||
| Vehicle maintenance | 2,063 | 8,455 | ||||||
| Travel expenses | 978 | 3,408 | ||||||
| Transportation and storage | 5,632 | 13,299 | ||||||
| Other expenses | 4,564 | 323 | ||||||
| 43,405 | 144,548 | |||||||
NOTE 12 – RESEARCH AND DEVELOPMENT EXPENSES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | 39,021 | 177,712 | ||||||
| Share based compensation | 91,190 | 75,998 | ||||||
| Professional fees | 130,592 | 178,854 | ||||||
| Depreciation | 29,319 | 20,544 | ||||||
| Travel expenses | 7,190 | 26,138 | ||||||
| Vehicle maintenance | 13,657 | 26,227 | ||||||
| Rent and asset management | - | 10,582 | ||||||
| Laboratory and Field tests | 72,593 | 73,968 | ||||||
| Other expenses | 33,438 | 25,600 | ||||||
| 417,000 | 615,623 | |||||||
| F-23 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 13 – GENERAL AND ADMINISTRATIVE EXPENSES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Professional services | 443,883 | 461,840 | ||||||
| Share based compensation | 416,996 | 283,910 | ||||||
| Legal expenses | 67,492 | 125,753 | ||||||
| Insurance | 63,380 | 54,367 | ||||||
| Rent and office maintenance | 11,135 | 38,080 | ||||||
| Levies and tolls | 28,477 | 8,601 | ||||||
| Communications | 1,679 | 1,910 | ||||||
| Depreciation | 13,914 | 2,133 | ||||||
| Travel expenses | 5,305 | 15,310 | ||||||
| Other expenses | 17,848 | 12,995 | ||||||
| 1,070,109 | 1,004,899 | |||||||
NOTE 14 – FINANCING EXPENSES, NET
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Interest and amortization expenses | 202,917 | 4,323 | ||||||
| Currency exchange differences | 34,037 | 28,266 | ||||||
| Changes in fair value of convertible loans | 27,208 | - | ||||||
| Bank charges and other finance expenses, net | 6,231 | 10,819 | ||||||
| 270,393 | 43,408 | |||||||
NOTE 15 – INCOME TAX
| A. | US resident companies are taxed on their worldwide income for corporate income tax purposes at a statutory rate of 21% this reflect certain effects of the Act which includes a reduction in the corporate tax rate from 35% to 21% as well as other changes. No further taxes are payable on this profit unless that profit is distributed. If certain conditions are met, income derived from foreign subsidiaries is tax exempt in the US under applicable tax treaties to avoid double taxation.
| |
Income of the Israeli company is taxable from 2018 and onwards, at corporate tax rate of 23%.
| ||
The Company and Save Foods Ltd. has not received final tax assessments since its inception.
| ||
As of December 31, 2020, the Company and Save Foods Ltd. has estimated carry forward losses for tax purposes of approximately $1,264,000 and $9,412,000, respectively, which can be offset against future taxable income, if any.
|
| F-24 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 15 – INCOME TAX (continue)
| B. | The following is reconciliation between the theoretical tax on pre-tax income, at the tax rate applicable to the Company (federal tax rate) and the tax expense reported in the financial statements: |
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Pretax loss | 1,593,139 | 1,971,417 | ||||||
| Federal tax rate | 21 | % | 21 | % | ||||
| Income tax computed at the ordinary tax rate | 334,559 | 413,998 | ||||||
| Non-deductible expenses | (63,565 | ) | (2,278 | ) | ||||
| Stock-based compensation | (109,772 | ) | (93,970 | ) | ||||
| Tax in respect of differences in corporate tax rates | 13,480 | 26,688 | ||||||
| Losses and timing differences in respect of which no deferred taxes were generated | (174,702 | ) | (344,438 | ) | ||||
| - | - | |||||||
| C. | Deferred taxes result primarily from temporary differences in the recognition of certain revenue and expense items for financial and income tax reporting purposes. Significant components of the Company’s future tax assets are as follows: |
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Composition of deferred tax assets: | ||||||||
| Provision for employee related obligation | 31,627 | 29,353 | ||||||
| Non capital loss carry forwards | 2,350,367 | 2,171,821 | ||||||
| Valuation allowance | (2,381,994 | ) | (2,201,174 | ) | ||||
| - | - | |||||||
| F-25 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 16 – LOSS PER COMMON STOCK
Basic loss per share is computed by dividing net loss by the weighted average number of shares outstanding during the year. The weighted average number of shares of Common Stock used in computing basic and diluted loss per common stock for the years ended December 31, 2020 and 2019, are as follows:
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Number of shares | ||||||||
| Weighted average number of shares of Common Stock outstanding attributable to shareholders | 1,519,122 | 1,424,045 | ||||||
| Total weighted average number of shares of Common Stock related to outstanding options, excluded from the calculations of diluted loss per share (*) | 206,862 | 164,289 | ||||||
(*) The effect of the inclusion of option and convertible loans in 2020 and 2019 is anti-dilutive.
NOTE 17 – RELATED PARTIES
A. Transactions and balances with related parties
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| General and administrative expenses: | ||||||||
| Directors compensation | 380,756 | 295,088 | ||||||
| Salaries and fees to officers | 336,433 | 358,370 | ||||||
| Consultants and other fees | 52,331 | - | ||||||
| (*) 769,520 | (*) 653,458 | |||||||
| (*) share based compensation | 394,756 | 272,077 | ||||||
| Research and development expenses: | ||||||||
| Salaries and fees to officers | 25,301 | 116,692 | ||||||
B. Balances with related parties and officers:
| Other accounts payables | 424,515 | 199,983 |
C. Other information:
| A. | On November 5, 2020, the board of directors of the Company appointed Mr. David Palach, to serve as co-Chief Executive Officer of the Company, effective as of the same date. In connection with Mr. Palach’s appointment, the parties entered into a Consulting Agreement pursuant to which the Company and Mr. Palach agreed upon, inter alia, the following engagement terms: (a) a monthly retainer of $8,000, and (b) a grant of options to purchase shares of the Company’s common stock, which amount shall be determined by the Board on a future date. |
| B. | On October 10, 2018 the Board of directors of Save Foods Ltd. approved to engage in consulting agreements with Amir Uziel Economic Consultant Ltd (a company controlled by Amir Uziel) and with L.A Pure Capital Ltd (a company controlled by Kfir Zilberman) at a monthly fee of $1,500. |
| C. | On October 10, 2018 the Board of directors of Save Foods Ltd. approved a monthly directors fee of $1,500 to Itzhak Shrem. |
| F-26 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 17 – SUBSEQUENT EVENTS
| A. | On February 23, 2021, the Company amended its Certificate of Incorporation to effect a 7 to 1 reverse stock split of the Company’s outstanding Common Stock. As a result of the reverse stock split, which became effective on February 23, 2021, every 7 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of authorized capital of the Company’s Common Stock and par value of the shares remained unchanged. |
All share, stock option and per share information in these condensed consolidated financial statements have been restated to reflect the stock split on a retroactive basis.
| B. | During January 2021, the Company entered into a series of additional 2020 CLAs (See note 6B above) with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $274,000. |
| F-27 |
1,090,909 Shares of Common Stock

Save Foods, Inc.
PROSPECTUS
ThinkEquity
a division of Fordham Financial Management, Inc.
, 2021
Through and including, 2021 (25 days after the date of this prospectus), all dealers that effect transactions in our securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotment or subscription.
| 99 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable in connection with the sale and distribution of the securities being registered. All amounts are estimated except the SEC registration fee and the FINRA filing fee. Except as otherwise noted, all the expenses below will be paid by us.
| Amount | ||||
| SEC registration fee | $ | 1,588 | ||
| FINRA filing fee | $ | 2,183 | ||
| Initial listing fee | $ | 75,000 | ||
| Accountants’ fees and expenses | $ | 15,000 | ||
| Legal fees and expenses | $ | 200,000 | ||
| Transfer Agent’s fees and expenses | $ | 1,000 | ||
| Printing and engraving expenses | $ | 10,000 | ||
| Miscellaneous | $ | 20,229 | ||
| Total expenses | $ | 325,000 | ||
Item 14. Indemnification of Directors and Officers
Section 145 of the DGCL (“Section 145”) provides that a Delaware corporation may indemnify any person who was, is or is threatened to be made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who are, were or are a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests, provided that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him against the expenses which such officer or director has actually and reasonably incurred.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against him and incurred by him in any such capacity, or arising out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify him under Section 145.
Our bylaws provide that we must indemnify our directors and officers to the fullest extent permitted by the DGCL and must also pay expenses incurred in defending any such proceeding in advance of its final disposition upon delivery of an undertaking, by or on behalf of an indemnified person, to repay all amounts so advanced if it should be determined ultimately that such person is not entitled to be indemnified.
| II-1 |
We entered into indemnification agreements with certain of our executive officers and directors pursuant to which we have agreed to indemnify such persons against all expenses and liabilities incurred or paid by such person in connection with any proceeding arising from the fact that such person is or was an officer or director of the Company, and to advance expenses as incurred by or on behalf of such person in connection therewith.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our certificate of incorporation, as amended, our bylaws, agreement, vote of stockholders or disinterested directors or otherwise.
We maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers.
In any underwriting agreement we enter into in connection with the sale of Common Stock being registered hereby, the underwriter will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us, within the meaning of the Securities Act, against certain liabilities.
Item 15. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by the Company since April 2018, which were not registered under the Securities Act. The Company believes that each of such issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act.
On April 29, 2018, we issued 28,572 shares of Common Stock for total consideration of $30,000.
In August 2018, we issued: (i) 11,128 shares of Common Stock to several existing shareholders of the Company who were owed a sum of $35,000; (ii) 274,986 shares of Common Stock to accredited investors who held shares in Save Foods Ltd. in exchange for 7,218,129 shares of Save Foods Ltd., at the rate of 0.038 shares of Common Stock of the Company for each share of Save Foods Ltd; and (iii) 160,643 shares of Common Stock of the Company to several investors, for total consideration of $506,000 reflecting a price of $3.15 per share of Common Stock.
On September 4, 2018, we issued 25,398 shares of Common Stock to two accredited investors for total consideration of $80,000.
On October 8, 2018, we issued 36,513 shares of Common Stock to an accredited investor for total consideration of $115,000.
During November 2018, we issued an aggregate of 30,689 shares of Common Stock to accredited investors for total consideration of $133,333.
During January 2019, we issued an aggregate of 19,050 shares of Common Stock to accredited investors for total consideration of $120,000.
During February 2019, we issued an aggregate of 35,717 shares of Common Stock to accredited investors for total consideration of $225,000.
During March 2019, we issued (i) 31,748 shares of Common Stock to an accredited investors for total consideration of $216,666, and (iii) warrants to purchase 7,937 shares of Common Stock at an exercise price of $12.60 per share.
| II-2 |
During June 2019, we issued: (i) 31,747 shares of Common Stock to accredited investors for total consideration of $200,000; and (ii) 10,004 shares of Common Stock to an accredited investor for total consideration of $84,034 and at the same time issued that accredited investor 10,004 warrants to purchase shares of Common Stock at an exercise price of $12.60.
During August 2019, we issued 11,905 shares of Common Stock to an accredited investor for total consideration of $100,000. In addition, we issued him 11,905 warrants to purchase shares of Common Stock at an exercise price of $12.60.
During July and August 2020, we issued: (i) 67,369 shares of Common Stock in respect of the conversion of convertible loans; (ii) 32,769 units, for an aggregate amount of $250,000, at a price of $7.63 per unit, where each unit consists of one share of Common Stock and one warrant to purchase one share of Common Stock with an exercise price of $8.40 per share.
During September 2020, we issued 13,107 units, for an aggregate amount of $100,000, at a purchase price of $7.63 per unit to Medigus Ltd., where each unit consists of one share of Common Stock and one warrant to purchase one share of Common Stock with an exercise price of $8.40 per share.
During December 2020, we issued 6,350 shares of Common Stock following the exercise of options.
Since April 2018, we issued warrants to purchase an aggregate of 187,128 shares of Common Stock to investors and service providers, with exercise prices ranging from $2.1 to $8.4 per share. As of the date of this prospectus, 28,572 warrants were exercised and 28,572 warrants were forfeited, such that the total outstanding amount of warrants as of the date of this prospectus is 129,984.
Since April 2018, we granted to our directors, officers, employees and service providers options to purchase an aggregate of 261,625 shares of Common Stock under our Equity Incentive Plan, with exercise prices ranging from $3.15 to $3.78 per share. As of the date of this prospectus, 6,350 options were exercised and 43,651 options were forfeited, such that the total outstanding number of options as of the date of this prospectus is 206,862.
Since April 2018, we issued convertible promissory notes in an aggregate principal amount of $1,013,000 in a series of convertible loan agreements. As of the date of this prospectus, we issued an aggregate of 67,369 shares of Common Stock as a result of conversion of certain convertible promissory notes, at a conversion price of $7.63 per share.
| II-3 |
Item 16. Exhibits and Financial Statement Schedules
| (a) | Exhibits. |
| * | Filed herewith. | |
| ** | Previously filed. | |
| *** | To be filed by amendment. | |
| + | Management contract or compensatory plan or arrangement. | |
^ |
Portions of this exhibit have been omitted under rules of the U.S. Securities and Exchange Commission permitting the confidential treatment of select information. |
| II-4 |
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”);
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission (the “Commission”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
Provided, however, that Paragraphs (a)(1)(i), (ii), and (iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser: If the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
| II-5 |
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) The undersigned registrant hereby undertakes to supplement the prospectus, after the expiration of the subscription period, to set forth the results of the subscription offer, the transactions by the underwriter during the subscription period, the amount of unsubscribed securities to be purchased by the underwriter, and the terms of any subsequent reoffering thereof. If any public offering by the underwriter is to be made on terms differing from those set forth on the cover page of the prospectus, a post-effective amendment will be filed to set forth the terms of such offering.
(d) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(e) For the purpose of determining any liability under the Securities Act, the registrant will treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant under Rule 424(b)(1), or (4), or 497(h) under the Securities Act as part of this registration statement as of the time the Commission declared it effective.
(f) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-6 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, we have duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Rishon L’zion State of Israel, on the day of April 6, 2021.
| SAVEFOODS, INC. | ||
| By: | /s/ David Palach | |
| David Palach | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature | Title | Date | ||
| /s/ David Palach | Chief Executive Officer (Principal Executive Officer) |
April 6, 2021 | ||
| David Palach | ||||
| /s/ Shlomo Zakai | Chief Financial Officer | April 6, 2021 | ||
| Shlomo Zakai | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ * | Director, Chairman of the Board of Directors | April 6, 2021 | ||
| Benad Goldwasser | ||||
| /s/ * | Director | April 6, 2021 | ||
| Vered Raz-Avayo | ||||
| /s/ * | Director | April 6, 2021 | ||
| Ronen Rosenbloom | ||||
| /s/ * | Director | April 6, 2021 | ||
| Israel Berenshtein | ||||
| /s/ * | Director | April 6, 2021 | ||
| Amitay Weiss | ||||
| /s/ * | Director | April 6, 2021 | ||
| Eliahou Arbib |
| By: | /s/ David Palach | |
| David Palach | ||
| Attorney-in-fact |
| II-7 |
