Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Benitec Biopharma Inc. | d59574dex992.htm |
| 8-K - 8-K - Benitec Biopharma Inc. | d59574d8k.htm |

Exhibit 99.1 Interim Results of IND-Enabling Large Animal Study for BB-301 February 2021 Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 1 www.benitec.comExhibit 99.1 Interim Results of IND-Enabling Large Animal Study for BB-301 February 2021 Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 1 www.benitec.com

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of section 27A of the US Securities Act of 1933 and section 21E of the US Securities Exchange Act of 1934. Benitec has tried to identify such forward-looking statements by use of such words as expects, intends, hopes, anticipates, believes, could, may, evidences and estimates, and other similar expressions, but these words are not the exclusive means of identifying such statements. Such statements include, but are not limited to, any statements relating to Benitec's pipeline of ddRNAi-based therapeutics, including the initiation, progress and outcomes of clinical trials and any other statements that are not historical facts. Such forward-looking statements involve risks and uncertainties, including, but not limited to, risks and uncertainties relating to the difficulties or delays in our plans to develop and potentially commercialize our product candidates, the timing of the initiation and completion of preclinical and clinical trials, the timing of patient enrolment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, potential future out-licenses and collaborations, our intellectual property position and duration of our patent portfolio, the ability to procure additional sources of financing and other risks detailed from time to time in filings that Benitec makes with US Securities and Exchange Commission, including our most recent annual report on Form 20-F and our reports on Form 6-K. Such statements are based on management's current expectations, but actual results may differ materially due to various factors, including those risks and uncertainties mentioned or referred to in this presentation. Accordingly, you should not rely on those forward-looking statements as a prediction of actual future results. Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 2 www.benitec.comSafe Harbor Statement This presentation contains forward-looking statements within the meaning of section 27A of the US Securities Act of 1933 and section 21E of the US Securities Exchange Act of 1934. Benitec has tried to identify such forward-looking statements by use of such words as expects, intends, hopes, anticipates, believes, could, may, evidences and estimates, and other similar expressions, but these words are not the exclusive means of identifying such statements. Such statements include, but are not limited to, any statements relating to Benitec's pipeline of ddRNAi-based therapeutics, including the initiation, progress and outcomes of clinical trials and any other statements that are not historical facts. Such forward-looking statements involve risks and uncertainties, including, but not limited to, risks and uncertainties relating to the difficulties or delays in our plans to develop and potentially commercialize our product candidates, the timing of the initiation and completion of preclinical and clinical trials, the timing of patient enrolment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, potential future out-licenses and collaborations, our intellectual property position and duration of our patent portfolio, the ability to procure additional sources of financing and other risks detailed from time to time in filings that Benitec makes with US Securities and Exchange Commission, including our most recent annual report on Form 20-F and our reports on Form 6-K. Such statements are based on management's current expectations, but actual results may differ materially due to various factors, including those risks and uncertainties mentioned or referred to in this presentation. Accordingly, you should not rely on those forward-looking statements as a prediction of actual future results. Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 2 www.benitec.com

Gene Silencing: A Validated Approach to the Treatment of Some Genetic Diseases • Mutation of a single gene can cause a chronic disease via the resulting intracellular production of a disease- causing protein (i.e. an abnormal form of the protein of interest) • Genetic disorders of this type can often be treated exclusively by “silencing” the intracellular production of the disease-causing protein through well-validated biological approaches like RNA interference (“RNAi”) – RNAi employs small nucleic acid molecules to activate an intracellular enzyme complex, and this biological pathway temporarily reduces the production of the disease-causing protein – In the absence of the disease-causing protein, normal cellular function is restored, and the chronic disease improves or resolves • However, many genetic disorders are not amenable to gene silencing approaches, as the diseased cells produce a mixture of the normal protein of interest and the disease-causing variant of the protein, and the underlying genetic mutation does not allow for selective targeting of the disease-causing variant – In these cases it is impossible to exclusively silence the disease-causing protein without simultaneously silencing the normal intracellular protein of interest whose presence is vital to normal cellular functions Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 3 www.benitec.comGene Silencing: A Validated Approach to the Treatment of Some Genetic Diseases • Mutation of a single gene can cause a chronic disease via the resulting intracellular production of a disease- causing protein (i.e. an abnormal form of the protein of interest) • Genetic disorders of this type can often be treated exclusively by “silencing” the intracellular production of the disease-causing protein through well-validated biological approaches like RNA interference (“RNAi”) – RNAi employs small nucleic acid molecules to activate an intracellular enzyme complex, and this biological pathway temporarily reduces the production of the disease-causing protein – In the absence of the disease-causing protein, normal cellular function is restored, and the chronic disease improves or resolves • However, many genetic disorders are not amenable to gene silencing approaches, as the diseased cells produce a mixture of the normal protein of interest and the disease-causing variant of the protein, and the underlying genetic mutation does not allow for selective targeting of the disease-causing variant – In these cases it is impossible to exclusively silence the disease-causing protein without simultaneously silencing the normal intracellular protein of interest whose presence is vital to normal cellular functions Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 3 www.benitec.com

“Silence and Replace”: Permanent Gene Silencing and Tissue-Specific Restoration of Biological Function • Our proprietary DNA-directed RNA interference (ddRNAi) platform combines RNAi with classical AAV-based gene therapy • Through the use of the ddRNAi platform Benitec creates genetic medicines that, following a single administration, will enable target tissues to perpetually produce siRNA molecules which facilitate the sustained silencing of disease-causing genes • Importantly, the ddRNAi platform also allows for concomitant delivery of wild type replacement genes, and these distinct genetic elements work in concert to silence the expression of disease-causing mutant genes and to simultaneously replace the mutant genes with normal (wild type) genes to restore the natural underlying physiology of the diseased tissues • BB-301, the most advanced genetic medicine currently under development by Benitec, employs this “Silence and Replace” approach for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD) Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 4 www.benitec.com“Silence and Replace”: Permanent Gene Silencing and Tissue-Specific Restoration of Biological Function • Our proprietary DNA-directed RNA interference (ddRNAi) platform combines RNAi with classical AAV-based gene therapy • Through the use of the ddRNAi platform Benitec creates genetic medicines that, following a single administration, will enable target tissues to perpetually produce siRNA molecules which facilitate the sustained silencing of disease-causing genes • Importantly, the ddRNAi platform also allows for concomitant delivery of wild type replacement genes, and these distinct genetic elements work in concert to silence the expression of disease-causing mutant genes and to simultaneously replace the mutant genes with normal (wild type) genes to restore the natural underlying physiology of the diseased tissues • BB-301, the most advanced genetic medicine currently under development by Benitec, employs this “Silence and Replace” approach for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD) Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 4 www.benitec.com

Platform Enables Gene Therapy and Permanent Gene Silencing: DNA-Directed RNA Interference (ddRNAi) Transient gene silencing - siRNA Permanent gene silencing - ddRNAi • Combines RNA interference with gene therapy delivery • Long-term therapeutic potential from a single administration • Constant, steady-state levels of shRNA expression • Silence a single gene or target multiple genes simultaneously • Simultaneous silencing of disease-causing genes and co-expression of normal genes to restore biological function Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 5 www.benitec.comPlatform Enables Gene Therapy and Permanent Gene Silencing: DNA-Directed RNA Interference (ddRNAi) Transient gene silencing - siRNA Permanent gene silencing - ddRNAi • Combines RNA interference with gene therapy delivery • Long-term therapeutic potential from a single administration • Constant, steady-state levels of shRNA expression • Silence a single gene or target multiple genes simultaneously • Simultaneous silencing of disease-causing genes and co-expression of normal genes to restore biological function Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 5 www.benitec.com

BB-301 for Oculopharyngeal Muscular Dystrophy • LATE-STAGE NON-CLINICAL ASSET WITH CATEGORY-LEADING BIOLOGICAL EFFICACY • GLOBAL PREVALENCE OF OPMD EXCEEDS 15,000 PATIENTS AND COMMERCIAL OPPORTUNITY EXCEEDS $1 BILLION OVER THE LIFE OF THE PRODUCT Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 6 www.benitec.comBB-301 for Oculopharyngeal Muscular Dystrophy • LATE-STAGE NON-CLINICAL ASSET WITH CATEGORY-LEADING BIOLOGICAL EFFICACY • GLOBAL PREVALENCE OF OPMD EXCEEDS 15,000 PATIENTS AND COMMERCIAL OPPORTUNITY EXCEEDS $1 BILLION OVER THE LIFE OF THE PRODUCT Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 6 www.benitec.com

Oculopharyngeal Muscular Dystrophy Lead Candidate BB-301: Product Overview • Rare, autosomal dominant, monogenic disease Oculopharyngeal • Estimated prevalence of 15,000 patients in Western countries Muscular • Characterized by eyelid drooping, swallowing difficulties, proximal limb Dystrophy weakness, death due to aspiration pneumonia and malnutrition • Designed to treat dysphagia associated with OPMD • ‘Silence and Replace’ represents a unique gene therapy mechanism BB-301 Product • ‘Silence’: Inhibits mutant and wildtype PABPN1 gene expression Profile/Milestones • ‘Replace’: Simultaneously reintroduces normal PABPN1 gene to restore function • Clinical trial to begin enrollment over the next 18-to-22 months • Orphan Drug Designation received in the US and EU provides commercial exclusivity and expeditious development path Value / • Commercial scale manufacturing process has been optimized and reproducibly Commercial executed Opportunity • Commercial opportunity in excess of $1 billion over the life of the product Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 7 www.benitec.comOculopharyngeal Muscular Dystrophy Lead Candidate BB-301: Product Overview • Rare, autosomal dominant, monogenic disease Oculopharyngeal • Estimated prevalence of 15,000 patients in Western countries Muscular • Characterized by eyelid drooping, swallowing difficulties, proximal limb Dystrophy weakness, death due to aspiration pneumonia and malnutrition • Designed to treat dysphagia associated with OPMD • ‘Silence and Replace’ represents a unique gene therapy mechanism BB-301 Product • ‘Silence’: Inhibits mutant and wildtype PABPN1 gene expression Profile/Milestones • ‘Replace’: Simultaneously reintroduces normal PABPN1 gene to restore function • Clinical trial to begin enrollment over the next 18-to-22 months • Orphan Drug Designation received in the US and EU provides commercial exclusivity and expeditious development path Value / • Commercial scale manufacturing process has been optimized and reproducibly Commercial executed Opportunity • Commercial opportunity in excess of $1 billion over the life of the product Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 7 www.benitec.com

Genetic Basis of OPMD: Expansion of the Poly-Alanine Tract Within PABPN1 PABPN1: • Ubiquitous factor that promotes interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage In OPMD: • Genetic mutation results in trinucleotide repeat expansion within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1 Non-affected • Mutation generates a protein with an N-terminal expanded poly- alanine tract of up to 18 contiguous alanine residues prone to form aggregates called intranuclear inclusions (INIs) • INIs that also sequester wild type PABPN1 could contribute to the “loss of function” phenotype associated with OPMD Affected WT ATG (GCG) -------------(GCA) GCG GGG GCT GCG.. 6 3 MUT ATG (GCG) (GCG) (GCA) GCG GGG GCT GCG…-- 6 1-7 3 Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 8 www.benitec.comGenetic Basis of OPMD: Expansion of the Poly-Alanine Tract Within PABPN1 PABPN1: • Ubiquitous factor that promotes interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage In OPMD: • Genetic mutation results in trinucleotide repeat expansion within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1 Non-affected • Mutation generates a protein with an N-terminal expanded poly- alanine tract of up to 18 contiguous alanine residues prone to form aggregates called intranuclear inclusions (INIs) • INIs that also sequester wild type PABPN1 could contribute to the “loss of function” phenotype associated with OPMD Affected WT ATG (GCG) -------------(GCA) GCG GGG GCT GCG.. 6 3 MUT ATG (GCG) (GCG) (GCA) GCG GGG GCT GCG…-- 6 1-7 3 Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 8 www.benitec.com
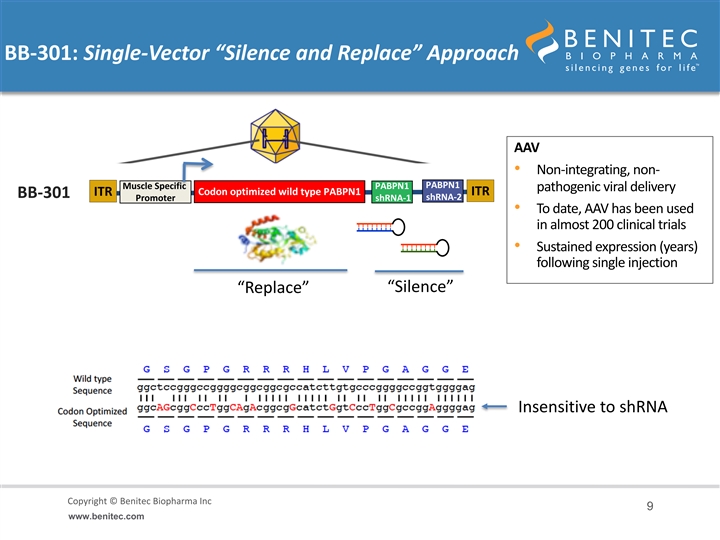
BB-301: Single-Vector “Silence and Replace” Approach AAV • Non-integrating, non- PABPN1 Muscle Specific PABPN1 pathogenic viral delivery Codon optimized wild type PABPN1 ITR ITR BB-301 shRNA-2 Promoter shRNA-1 REP/CAP removed and • To date, AAV has been used replaced with expression cassette in almost 200 clinical trials • Sustained expression (years) following single injection “Silence” “Replace” Insensitive to shRNA Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 9 www.benitec.comBB-301: Single-Vector “Silence and Replace” Approach AAV • Non-integrating, non- PABPN1 Muscle Specific PABPN1 pathogenic viral delivery Codon optimized wild type PABPN1 ITR ITR BB-301 shRNA-2 Promoter shRNA-1 REP/CAP removed and • To date, AAV has been used replaced with expression cassette in almost 200 clinical trials • Sustained expression (years) following single injection “Silence” “Replace” Insensitive to shRNA Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 9 www.benitec.com

IND-Enabling Studies for BB-301 to be Completed Over the Next 12-to-18 Months • The IND-enabling non-clinical studies outlined below are being carried out under the guidance of the scientific team at Benitec in close collaboration with a team of Thought Leaders in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for several decades • 8-week Pilot Dosing study in Beagle dogs to confirm the transduction efficiency of BB-301 following direct intramuscular injection into the pharyngeal muscles via the use of an open surgical approach (Beagle dog dosing has been completed in this study, and tissue analyses are currently ongoing) – Direct injection of BB-301 into the tibialis anterior muscle of A17 mice achieved robust transduction of the targeted skeletal muscle cells – This follow-up study in Beagle dogs is being conducted to optimize the proprietary dosing and surgical administration procedures for BB-301 injection into the pharyngeal muscle tissues that underlie the morbidity and mortality of OPMD and to refine the core analytical methods employed following the completion of dosing • 12-week GLP Toxicology and Biodistribution study in Beagle dogs Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 10 www.benitec.comIND-Enabling Studies for BB-301 to be Completed Over the Next 12-to-18 Months • The IND-enabling non-clinical studies outlined below are being carried out under the guidance of the scientific team at Benitec in close collaboration with a team of Thought Leaders in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for several decades • 8-week Pilot Dosing study in Beagle dogs to confirm the transduction efficiency of BB-301 following direct intramuscular injection into the pharyngeal muscles via the use of an open surgical approach (Beagle dog dosing has been completed in this study, and tissue analyses are currently ongoing) – Direct injection of BB-301 into the tibialis anterior muscle of A17 mice achieved robust transduction of the targeted skeletal muscle cells – This follow-up study in Beagle dogs is being conducted to optimize the proprietary dosing and surgical administration procedures for BB-301 injection into the pharyngeal muscle tissues that underlie the morbidity and mortality of OPMD and to refine the core analytical methods employed following the completion of dosing • 12-week GLP Toxicology and Biodistribution study in Beagle dogs Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 10 www.benitec.com

8-Week BB-301 Pilot Dosing Study in Beagle Dogs: Study Rationale and Background • BB-301 is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality characterizing the natural history of OPMD, and large animals (e.g. canine subjects) possess anatomical architecture in the pharyngeal region similar to that of Human subjects with OPMD and were, therefore, identified by global regulatory agencies and our key collaborators as ideal subjects for the IND-enabling studies • Against this backdrop, the BB-301 Pilot Dosing Study in Beagle dogs was conducted to demonstrate that direct intramuscular injection of BB-301 via the use of a proprietary dosing device in an open surgical procedure could safely achieve the following goals: – Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) – Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct (i.e. siRNA13, siRNA17, and codon optimized PABPN1) – Durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 11 www.benitec.com8-Week BB-301 Pilot Dosing Study in Beagle Dogs: Study Rationale and Background • BB-301 is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality characterizing the natural history of OPMD, and large animals (e.g. canine subjects) possess anatomical architecture in the pharyngeal region similar to that of Human subjects with OPMD and were, therefore, identified by global regulatory agencies and our key collaborators as ideal subjects for the IND-enabling studies • Against this backdrop, the BB-301 Pilot Dosing Study in Beagle dogs was conducted to demonstrate that direct intramuscular injection of BB-301 via the use of a proprietary dosing device in an open surgical procedure could safely achieve the following goals: – Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) – Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct (i.e. siRNA13, siRNA17, and codon optimized PABPN1) – Durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 11 www.benitec.com

8-Week BB-301 Pilot Dosing Study in Beagle Dogs: Study Design and Background • The 8-week Pilot Dosing Study evaluated the safety and biological activity of two concentrations of BB-301 (1.0+E13 vg/mL and 3.0+E13 vg/mL) across three distinct doses (1.0+E13 vg/mL, 3.0+E13 vg/mL with a low injection volume, and 3.0+E13 vg/mL with a high injection volume) following direct intramuscular injection into the Hypopharyngeus (HP) muscles and the Thyropharyngeus (TP) muscles of Beagle dogs via the use of a proprietary delivery device employed in an open surgical procedure – The HP muscle in Beagle dogs corresponds to the Middle Pharyngeal Constrictor muscle in Human subjects, and the TP muscle in Beagle dogs corresponds to the Inferior Pharyngeal Constrictor muscle in Human subjects • BB-301 was injected only on Day 1 of the Pilot Dosing Study, and the corresponding canine pharyngeal muscles were harvested for analysis after 8 weeks on study • BB-301 dosing was carried out by both a veterinary surgeon and a practicing Otolaryngologist who has extensive experience with the provision of palliative surgical care for OPMD patients • Data analyses are ongoing for the canine subjects treated in BB-301 Pilot Dosing Study, and the interim data- points highlighted in this presentation are derived from the completed analyses of pharyngeal muscle tissues isolated from the 6 Beagle dog subjects to date (of the 24-subject study population) – The data-set and the initial conclusions will be updated as additional subjects are analyzed Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 12 www.benitec.com8-Week BB-301 Pilot Dosing Study in Beagle Dogs: Study Design and Background • The 8-week Pilot Dosing Study evaluated the safety and biological activity of two concentrations of BB-301 (1.0+E13 vg/mL and 3.0+E13 vg/mL) across three distinct doses (1.0+E13 vg/mL, 3.0+E13 vg/mL with a low injection volume, and 3.0+E13 vg/mL with a high injection volume) following direct intramuscular injection into the Hypopharyngeus (HP) muscles and the Thyropharyngeus (TP) muscles of Beagle dogs via the use of a proprietary delivery device employed in an open surgical procedure – The HP muscle in Beagle dogs corresponds to the Middle Pharyngeal Constrictor muscle in Human subjects, and the TP muscle in Beagle dogs corresponds to the Inferior Pharyngeal Constrictor muscle in Human subjects • BB-301 was injected only on Day 1 of the Pilot Dosing Study, and the corresponding canine pharyngeal muscles were harvested for analysis after 8 weeks on study • BB-301 dosing was carried out by both a veterinary surgeon and a practicing Otolaryngologist who has extensive experience with the provision of palliative surgical care for OPMD patients • Data analyses are ongoing for the canine subjects treated in BB-301 Pilot Dosing Study, and the interim data- points highlighted in this presentation are derived from the completed analyses of pharyngeal muscle tissues isolated from the 6 Beagle dog subjects to date (of the 24-subject study population) – The data-set and the initial conclusions will be updated as additional subjects are analyzed Copyright © Benitec Biopharma Inc Copyright © Benitec Biopharma Ltd 12 www.benitec.com
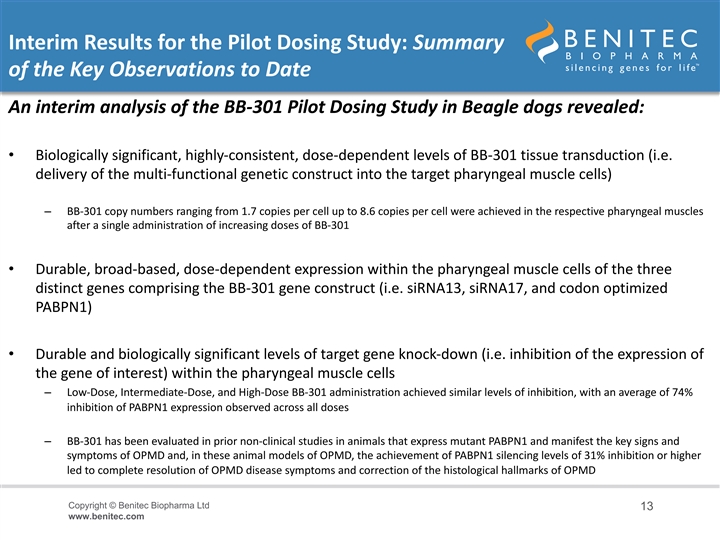
Interim Results for the Pilot Dosing Study: Summary of the Key Observations to Date An interim analysis of the BB-301 Pilot Dosing Study in Beagle dogs revealed: • Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) – BB-301 copy numbers ranging from 1.7 copies per cell up to 8.6 copies per cell were achieved in the respective pharyngeal muscles after a single administration of increasing doses of BB-301 • Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct (i.e. siRNA13, siRNA17, and codon optimized PABPN1) • Durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells – Low-Dose, Intermediate-Dose, and High-Dose BB-301 administration achieved similar levels of inhibition, with an average of 74% inhibition of PABPN1 expression observed across all doses – BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and manifest the key signs and symptoms of OPMD and, in these animal models of OPMD, the achievement of PABPN1 silencing levels of 31% inhibition or higher led to complete resolution of OPMD disease symptoms and correction of the histological hallmarks of OPMD Copyright © Benitec Biopharma Ltd 13 www.benitec.comInterim Results for the Pilot Dosing Study: Summary of the Key Observations to Date An interim analysis of the BB-301 Pilot Dosing Study in Beagle dogs revealed: • Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) – BB-301 copy numbers ranging from 1.7 copies per cell up to 8.6 copies per cell were achieved in the respective pharyngeal muscles after a single administration of increasing doses of BB-301 • Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct (i.e. siRNA13, siRNA17, and codon optimized PABPN1) • Durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells – Low-Dose, Intermediate-Dose, and High-Dose BB-301 administration achieved similar levels of inhibition, with an average of 74% inhibition of PABPN1 expression observed across all doses – BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and manifest the key signs and symptoms of OPMD and, in these animal models of OPMD, the achievement of PABPN1 silencing levels of 31% inhibition or higher led to complete resolution of OPMD disease symptoms and correction of the histological hallmarks of OPMD Copyright © Benitec Biopharma Ltd 13 www.benitec.com

Interim Results for the Pilot Dosing Study: Pharyngeal Muscle Tissue Transduction Levels for BB-301 • An interim analysis of the BB-301 pharyngeal muscle tissue transduction levels revealed biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved a vector copy number of 1.7 copies per cell – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved a vector copy number of 3.5 copies per cell – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved a vector copy number of 7.2 copies per cell • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved a vector copy number of 2.0 copies per cell – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved a vector copy number of 2.3 copies per cell – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved a vector copy number of 8.6 copies per cell Copyright © Benitec Biopharma Ltd 14 www.benitec.comInterim Results for the Pilot Dosing Study: Pharyngeal Muscle Tissue Transduction Levels for BB-301 • An interim analysis of the BB-301 pharyngeal muscle tissue transduction levels revealed biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction (i.e. delivery of the multi-functional genetic construct into the target pharyngeal muscle cells) • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved a vector copy number of 1.7 copies per cell – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved a vector copy number of 3.5 copies per cell – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved a vector copy number of 7.2 copies per cell • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved a vector copy number of 2.0 copies per cell – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved a vector copy number of 2.3 copies per cell – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved a vector copy number of 8.6 copies per cell Copyright © Benitec Biopharma Ltd 14 www.benitec.com

Interim Results for the Pilot Dosing Study: Pharyngeal Muscle Tissue Transduction Levels for BB-301 Avg. Reported BB-301 copies per cell 10.0 9.0 8.6 8.0 7.0 7.2 6.0 5.0 4.0 3.5 3.0 2.0 2.3 2.0 1.7 1.0 0.0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 15 www.benitec.comInterim Results for the Pilot Dosing Study: Pharyngeal Muscle Tissue Transduction Levels for BB-301 Avg. Reported BB-301 copies per cell 10.0 9.0 8.6 8.0 7.0 7.2 6.0 5.0 4.0 3.5 3.0 2.0 2.3 2.0 1.7 1.0 0.0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 15 www.benitec.com

Interim Results for the Pilot Dosing Study: Gene Expression Levels for BB-301 within Pharyngeal Muscles • BB-301 encodes two distinct siRNA species: – siRNA13 – siRNA17 • BB-301 encodes 2 distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. “silencing”) the expression of the mutant form of the PABPN1 protein and the wild type (i.e. endogenous) form of the PABPN1 protein • Importantly, the mutant form of the PABPN1 protein underlies the development and progression of OPMD • BB-301 also codes for a wild type version of the PABPN1 protein whose intracellular expression is unaffected by the inhibitory activities of siRNA13 and siRNA17, and this codon optimized PABPN1 protein (i.e. coPABPN1) serves to replenish the endogenous form of the PABPN1 protein and to replace the mutant form of PABPN1 that underlies the development and progression of OPMD in diseased tissues – For comparative purposes, is should be noted that the average level of expression for wild type PABPN1 within the pharyngeal muscle cells of Beagle dogs is 4.5 copies per cell to 7.8 copies per cell Copyright © Benitec Biopharma Ltd 16 www.benitec.comInterim Results for the Pilot Dosing Study: Gene Expression Levels for BB-301 within Pharyngeal Muscles • BB-301 encodes two distinct siRNA species: – siRNA13 – siRNA17 • BB-301 encodes 2 distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. “silencing”) the expression of the mutant form of the PABPN1 protein and the wild type (i.e. endogenous) form of the PABPN1 protein • Importantly, the mutant form of the PABPN1 protein underlies the development and progression of OPMD • BB-301 also codes for a wild type version of the PABPN1 protein whose intracellular expression is unaffected by the inhibitory activities of siRNA13 and siRNA17, and this codon optimized PABPN1 protein (i.e. coPABPN1) serves to replenish the endogenous form of the PABPN1 protein and to replace the mutant form of PABPN1 that underlies the development and progression of OPMD in diseased tissues – For comparative purposes, is should be noted that the average level of expression for wild type PABPN1 within the pharyngeal muscle cells of Beagle dogs is 4.5 copies per cell to 7.8 copies per cell Copyright © Benitec Biopharma Ltd 16 www.benitec.com

Interim Results for the Pilot Dosing Study: Gene Expression Levels for BB-301 within Pharyngeal Muscles • An interim analysis of the BB-301 expression levels within the target pharyngeal muscle tissues revealed biologically significant, highly-consistent, dose-dependent levels of gene expression • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 161,358 copies per cell, 26,652 copies per cell, and 21 copies per cell, respectively – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 256,928 copies per cell, 47,944 copies per cell, and 24 copies per cell, respectively – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 374,324 copies per cell, 57,126 copies per cell, and 52 copies per cell, respectively • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 195,182 copies per cell, 40,106 copies per cell, and 15 copies per cell, respectively – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 293,597 copies per cell, 57,969 copies per cell, and 43 copies per cell, respectively – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 751,484 copies per cell, 173,211 copies per cell, and 100 copies per cell, respectively Copyright © Benitec Biopharma Ltd 17 www.benitec.comInterim Results for the Pilot Dosing Study: Gene Expression Levels for BB-301 within Pharyngeal Muscles • An interim analysis of the BB-301 expression levels within the target pharyngeal muscle tissues revealed biologically significant, highly-consistent, dose-dependent levels of gene expression • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 161,358 copies per cell, 26,652 copies per cell, and 21 copies per cell, respectively – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 256,928 copies per cell, 47,944 copies per cell, and 24 copies per cell, respectively – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 374,324 copies per cell, 57,126 copies per cell, and 52 copies per cell, respectively • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 195,182 copies per cell, 40,106 copies per cell, and 15 copies per cell, respectively – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 293,597 copies per cell, 57,969 copies per cell, and 43 copies per cell, respectively – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved siRNA13, siRNA17, and coPABPN1 copy numbers of 751,484 copies per cell, 173,211 copies per cell, and 100 copies per cell, respectively Copyright © Benitec Biopharma Ltd 17 www.benitec.com

Interim Results for the Pilot Dosing Study: siRNA13 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported siRNA13 copies per cell 800,000 700,000 600,000 500,000 400,000 300,000 200,000 100,000 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 18 www.benitec.comInterim Results for the Pilot Dosing Study: siRNA13 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported siRNA13 copies per cell 800,000 700,000 600,000 500,000 400,000 300,000 200,000 100,000 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 18 www.benitec.com

Interim Results for the Pilot Dosing Study: siRNA17 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported siRNA17 copies per cell 200,000 180,000 160,000 140,000 120,000 100,000 80,000 60,000 40,000 20,000 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 19 www.benitec.comInterim Results for the Pilot Dosing Study: siRNA17 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported siRNA17 copies per cell 200,000 180,000 160,000 140,000 120,000 100,000 80,000 60,000 40,000 20,000 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 19 www.benitec.com

Interim Results for the Pilot Dosing Study: coPABPN1 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported coPABPN1 copies per cell 120.00 100.00 80.00 60.00 40.00 20.00 0.00 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 20 www.benitec.comInterim Results for the Pilot Dosing Study: coPABPN1 Expression Levels for BB-301 within Pharyngeal Muscles Avg. Reported coPABPN1 copies per cell 120.00 100.00 80.00 60.00 40.00 20.00 0.00 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 20 www.benitec.com

Interim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles • As noted above, BB-301 encodes 2 distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. “silencing”) the expression of all forms of the PABPN1 protein – siRNA13 and siRNA17 silence the expression of both wild type PABPN1 [wtPABPN1] and mutant PABPN1 • While the Beagle dog subjects treated in the current BB-301 Pilot Dosing Study do not express mutant PABPN1, the level of BB-301-driven gene silencing for the PABPN1 target can be accurately assessed due to the equivalent inhibitory effects of siRNA13 and siRNA17 on both wtPABPN1 and mutant PABPN1 – Thus, the wtPABPN1 silencing activity observed in the current BB-301 Pilot Dosing Study serves as a surrogate for the activity that would be anticipated in the presence of mutant PABPN1 • BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and manifest the key signs and symptoms of OPMD and, in these animal models of OPMD, the achievement of PABPN1 silencing levels of 31% inhibition or higher led to complete resolution of OPMD disease symptoms and correction of the histological hallmarks of OPMD Copyright © Benitec Biopharma Ltd 21 www.benitec.comInterim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles • As noted above, BB-301 encodes 2 distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. “silencing”) the expression of all forms of the PABPN1 protein – siRNA13 and siRNA17 silence the expression of both wild type PABPN1 [wtPABPN1] and mutant PABPN1 • While the Beagle dog subjects treated in the current BB-301 Pilot Dosing Study do not express mutant PABPN1, the level of BB-301-driven gene silencing for the PABPN1 target can be accurately assessed due to the equivalent inhibitory effects of siRNA13 and siRNA17 on both wtPABPN1 and mutant PABPN1 – Thus, the wtPABPN1 silencing activity observed in the current BB-301 Pilot Dosing Study serves as a surrogate for the activity that would be anticipated in the presence of mutant PABPN1 • BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and manifest the key signs and symptoms of OPMD and, in these animal models of OPMD, the achievement of PABPN1 silencing levels of 31% inhibition or higher led to complete resolution of OPMD disease symptoms and correction of the histological hallmarks of OPMD Copyright © Benitec Biopharma Ltd 21 www.benitec.com

Interim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles • An interim analysis of the level of PABPN1 silencing achieved at 8-weeks revealed durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved 74% inhibition of wtPABPN1 expression – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved 80% inhibition of wtPABPN1 expression – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved 78% inhibition of wtPABPN1 expression • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved 72% inhibition of wtPABPN1 expression – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved 61% inhibition of wtPABPN1 expression – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved 79% inhibition of wtPABPN1 expression Copyright © Benitec Biopharma Ltd 22 www.benitec.comInterim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles • An interim analysis of the level of PABPN1 silencing achieved at 8-weeks revealed durable and biologically significant levels of target gene knock-down (i.e. inhibition of the expression of the gene of interest) within the pharyngeal muscle cells • In the HP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved 74% inhibition of wtPABPN1 expression – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved 80% inhibition of wtPABPN1 expression – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved 78% inhibition of wtPABPN1 expression • In the TP muscle: – Low-Dose BB-301 (1.0E+13 vg/mL) achieved 72% inhibition of wtPABPN1 expression – Intermediate-Dose BB-301 (3.0E+13 vg/mL, low volume) achieved 61% inhibition of wtPABPN1 expression – High-Dose BB-301 (3.0E+13 vg/mL, high volume) achieved 79% inhibition of wtPABPN1 expression Copyright © Benitec Biopharma Ltd 22 www.benitec.com
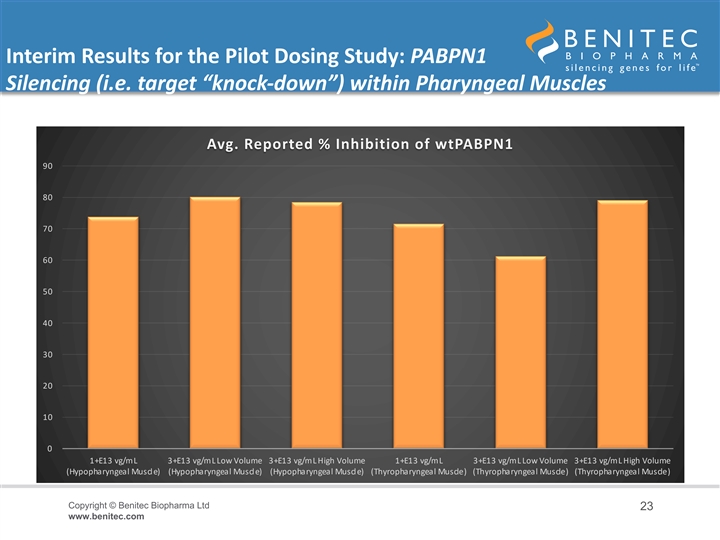
Interim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles Avg. Reported % Inhibition of wtPABPN1 90 80 70 60 50 40 30 20 10 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 23 www.benitec.comInterim Results for the Pilot Dosing Study: PABPN1 Silencing (i.e. target “knock-down”) within Pharyngeal Muscles Avg. Reported % Inhibition of wtPABPN1 90 80 70 60 50 40 30 20 10 0 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume 1+E13 vg/mL 3+E13 vg/mL Low Volume 3+E13 vg/mL High Volume (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Hypopharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) (Thyropharyngeal Muscle) Copyright © Benitec Biopharma Ltd 23 www.benitec.com

BB-301 Pilot Dosing Study: Key Methodological Improvements to the Study Design and Analytical Procedures • It is critical to highlight the key methodological distinctions between the current BB-301 Pilot Dosing Study in Beagle dogs conducted by Benitec and the prior Beagle dog dosing study carried out independently by the previous BB-301 Licensee of Benitec • The BB-301 dosing study conducted by the prior BB-301 Licensee employed non-ideal routes and methods of BB-301 administration to the target pharyngeal muscle tissues and employed similarly limited analytical methods at the completion of the dosing phase of the study • The Benitec team worked to optimize the route and method of administration of BB-301 and to refine the core analytical methods employed following the completion of dosing • Following these methodological improvements, Benitec demonstrated a +24,650% improvement in BB-301 transduction of the HP muscle and a +11,027% improvement in BB-301 transduction of the TP muscle relative to the levels of BB-301 transduction observed by the previous BB-301 Licensee Copyright © Benitec Biopharma Ltd 24 www.benitec.comBB-301 Pilot Dosing Study: Key Methodological Improvements to the Study Design and Analytical Procedures • It is critical to highlight the key methodological distinctions between the current BB-301 Pilot Dosing Study in Beagle dogs conducted by Benitec and the prior Beagle dog dosing study carried out independently by the previous BB-301 Licensee of Benitec • The BB-301 dosing study conducted by the prior BB-301 Licensee employed non-ideal routes and methods of BB-301 administration to the target pharyngeal muscle tissues and employed similarly limited analytical methods at the completion of the dosing phase of the study • The Benitec team worked to optimize the route and method of administration of BB-301 and to refine the core analytical methods employed following the completion of dosing • Following these methodological improvements, Benitec demonstrated a +24,650% improvement in BB-301 transduction of the HP muscle and a +11,027% improvement in BB-301 transduction of the TP muscle relative to the levels of BB-301 transduction observed by the previous BB-301 Licensee Copyright © Benitec Biopharma Ltd 24 www.benitec.com
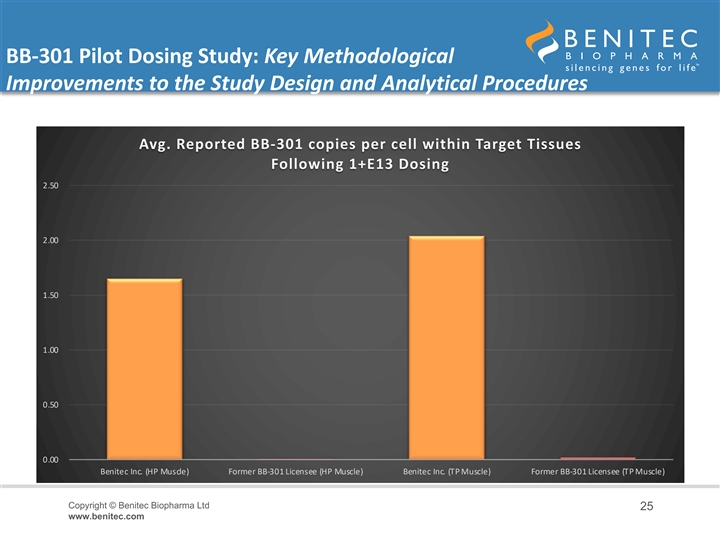
BB-301 Pilot Dosing Study: Key Methodological Improvements to the Study Design and Analytical Procedures Avg. Reported BB-301 copies per cell within Target Tissues Following 1+E13 Dosing 2.50 2.00 1.50 1.00 0.50 0.00 Benitec Inc. (HP Muscle) Former BB-301 Licensee (HP Muscle) Benitec Inc. (TP Muscle) Former BB-301 Licensee (TP Muscle) Copyright © Benitec Biopharma Ltd 25 www.benitec.comBB-301 Pilot Dosing Study: Key Methodological Improvements to the Study Design and Analytical Procedures Avg. Reported BB-301 copies per cell within Target Tissues Following 1+E13 Dosing 2.50 2.00 1.50 1.00 0.50 0.00 Benitec Inc. (HP Muscle) Former BB-301 Licensee (HP Muscle) Benitec Inc. (TP Muscle) Former BB-301 Licensee (TP Muscle) Copyright © Benitec Biopharma Ltd 25 www.benitec.com

Interim Results for the Pilot Dosing Study: Summary of the Key Conclusions An interim analysis of the BB-301 Pilot Dosing Study in Beagle dogs revealed: • Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction – BB-301 copy numbers ranging from 1.7 copies per cell up to 8.6 copies per cell were achieved in the respective pharyngeal muscles after a single administration of increasing doses of BB-301 • Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct • Durable and biologically significant levels of target gene knock-down within the pharyngeal muscle cells – Low-Dose, Intermediate-Dose, and High-Dose BB-301 administration achieved similar levels of inhibition, with an average of 74% inhibition of PABPN1 expression observed across all doses • Robust validation of Benitec’s proprietary “Silence and Replace” approach has been achieved Copyright © Benitec Biopharma Ltd 26 www.benitec.comInterim Results for the Pilot Dosing Study: Summary of the Key Conclusions An interim analysis of the BB-301 Pilot Dosing Study in Beagle dogs revealed: • Biologically significant, highly-consistent, dose-dependent levels of BB-301 tissue transduction – BB-301 copy numbers ranging from 1.7 copies per cell up to 8.6 copies per cell were achieved in the respective pharyngeal muscles after a single administration of increasing doses of BB-301 • Durable, broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct • Durable and biologically significant levels of target gene knock-down within the pharyngeal muscle cells – Low-Dose, Intermediate-Dose, and High-Dose BB-301 administration achieved similar levels of inhibition, with an average of 74% inhibition of PABPN1 expression observed across all doses • Robust validation of Benitec’s proprietary “Silence and Replace” approach has been achieved Copyright © Benitec Biopharma Ltd 26 www.benitec.com

BB-301 Development Program and Benitec Pipeline Updates: Key Upcoming Milestones • Benitec has scheduled a Scientific Advice Meeting in France in May 2021 to review the interim data and the Phase 1 clinical trial design • Benitec continues to plan for the initiation of the first-in-human clinical study of BB-301 in OPMD patients in 2022 • The interim data for the BB-301 Pilot Dosing Study validate the promise of the “Silence and Replace” approach to disease management, and Benitec plans to provide additional pipeline updates in 2H2021 Copyright © Benitec Biopharma Ltd 27 www.benitec.comBB-301 Development Program and Benitec Pipeline Updates: Key Upcoming Milestones • Benitec has scheduled a Scientific Advice Meeting in France in May 2021 to review the interim data and the Phase 1 clinical trial design • Benitec continues to plan for the initiation of the first-in-human clinical study of BB-301 in OPMD patients in 2022 • The interim data for the BB-301 Pilot Dosing Study validate the promise of the “Silence and Replace” approach to disease management, and Benitec plans to provide additional pipeline updates in 2H2021 Copyright © Benitec Biopharma Ltd 27 www.benitec.com
