Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OCULAR THERAPEUTIX, INC | tm216360d1_8k.htm |
Exhibit 99.1
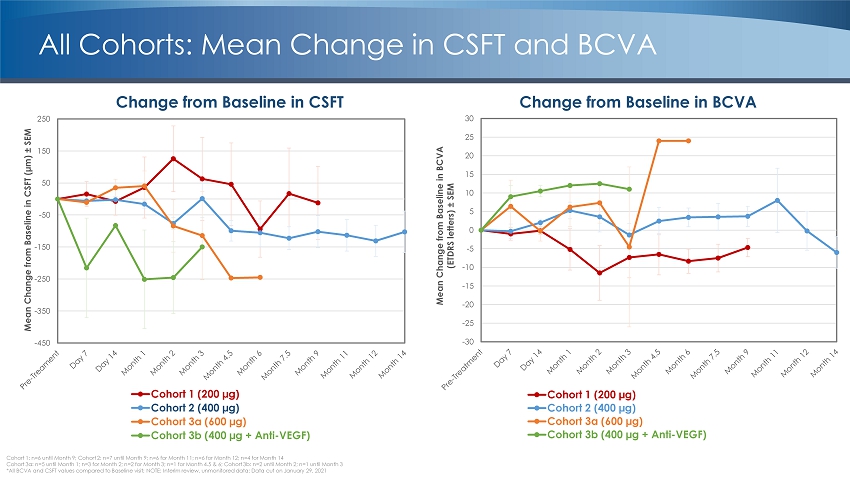
Cohort 1: n=6 until Month 9; Cohort 2: n=7 until Month 9; n=6 for Month 11; n=6 for Month 12; n=4 for Month 14 Cohort 3a: n=5 until Month 1; n=3 for Month 2; n=2 for Month 3; n=1 for Month 4.5 & 6; Cohort 3b: n=2 until Month 2; n=1 unti l M onth 3 *All BCVA and CSFT values compared to Baseline visit; NOTE: Interim review, unmonitored data; Data cut on January 29, 2021 Change from Baseline in BCVA Change from Baseline in CSFT -30 -25 -20 -15 -10 -5 0 5 10 15 20 25 30 Mean Change from Baseline in BCVA (ETDRS letters) “ SEM Cohort 1 (200 µg) Cohort 2 (400 µg) Cohort 3a (600 µg) Cohort 3b (400 µg + Anti-VEGF) All Cohorts: Mean Change in CSFT and BCVA -450 -350 -250 -150 -50 50 150 250 Mean Change from Baseline in CSFT (µm) “ SEM Cohort 1 (200 µg) Cohort 2 (400 µg) Cohort 3a (600 µg) Cohort 3b (400 µg + Anti-VEGF)

Duration of Effect Percentage of Subjects Without Needing Rescue Medications Cohorts At 3 months % (n/N) At 6 months % (n/N) At 7.5 months % (n/N) At 9 months % (n/N) At 11 months % (n/N) At 13.5 months % (n/N) Cohort 1 (200 µg) 66.7 (4/6) 50 (3/6) 50 (3/6) 50 (3/6) NA NA Cohort 2 (400 µg)* 71.4 (5/7) 57.1 (4/7) 42.9 (3/7) 42.9 (3/7) 33.3 (2/6)* 25 (1/4)* Cohort 3a (600 μ g)* 100 (2/2) 100 (1/1) TBD TBD TBD TBD Cohort 3b (400 μ g + anti - VEGF)* 100 (1/1) TBD TBD TBD TBD TBD NOTE: Interim review, unmonitored data; Data cut on January 29, 2021 *Follow - up ongoing Extended Follow - up
